Consciousness is an essential core feature of human life, so it is not surprising that disorders of consciousness have a major impact. Recent work has demonstrated that epilepsy shares many features with other disorders of consciousness. When patients lose consciousness during epileptic seizures they exhibit no meaningful responses to external stimuli; however, the eyes are usually open. In addition, although there is insufficient time to determine whether sleep-wake cycles are present, patients may exhibit orienting responses or other simple behaviors. Therefore, impaired consciousness during seizures resembles other disorders of consciousness such as vegetative state or minimally conscious state and, less so, coma. The major difference from these other disorders of consciousness is that (with the exception of status epilepticus) seizures typically last for minutes rather than days, months, or years. The transient nature of epileptic seizures provides a unique opportunity for determining the anatomic and physiologic basis of impaired consciousness and its recovery.
Seizures and other disorders of consciousness converge on a common set of cortical and subcortical structures. These structures constitute the “consciousness system,” defined in the next section as the bilateral medial and lateral fronto-parietal association cortex and subcortical arousal systems. Recent neuroimaging, intracranial EEG, and animal models demonstrate that the consciousness system forms a common anatomical substrate for all seizure types causing impaired consciousness. The main types of seizures causing transiently impaired consciousness include absence seizures, generalized tonic-clonic seizures, and temporal lobe complex partial seizures. This article will discuss the clinical and behavioral features, as well as recent neuroimaging and electrophysiology studies which have begun to shed light on the pathophysiology of impaired consciousness in these seizure types. The impact of impaired consciousness on quality of life in patients with epilepsy, and potential treatment strategies will also be discussed.
THE CONSCIOUSNESS SYSTEM
Following the tradition of Plum and Posner,1 it has been useful to separate consciousness into systems that are important for the content of consciousness, and those that control the level of consciousness. The content of consciousness can be thought of as the subject matter or substrate on which systems controlling the level of consciousness act. Thus, the content of consciousness includes all the information encoded in our hierarchically organized sensory and motor systems, as well as in the systems dedicated to memory and emotions. The level of consciousness is controlled by a specialized system of cortical and subcortical structures, which regulate alertness, attention, and awareness (mnemonic: AAA).2,3 In analogy to other nervous system networks serving specialized functions such as the motor, sensory, and limbic systems, the networks regulating the level of consciousness should logically be referred to as the “consciousness system.”3,4
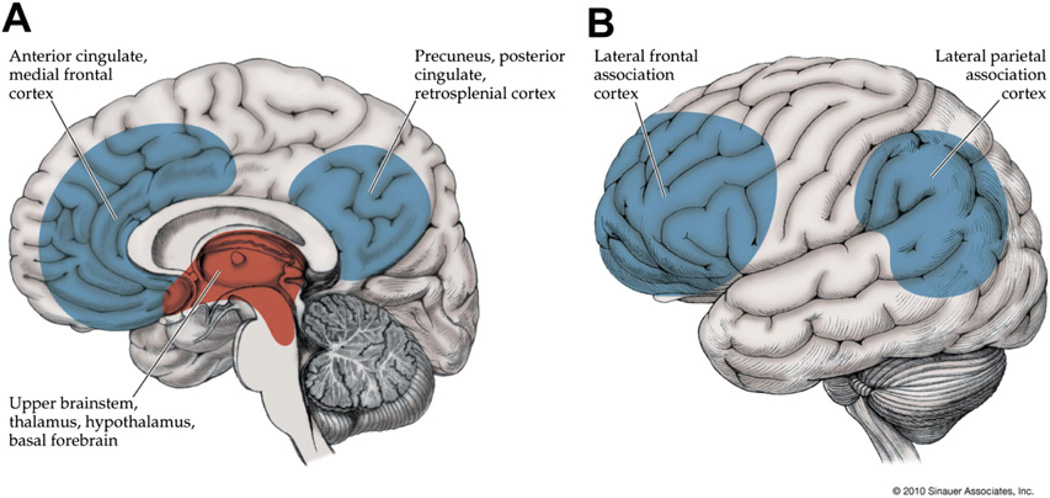
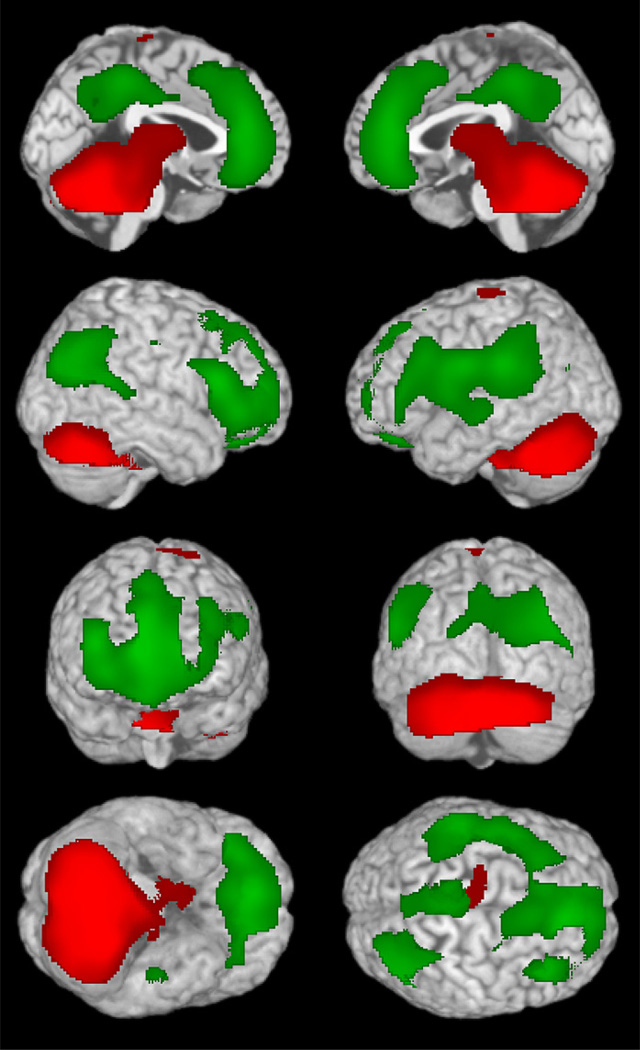
The consciousness system comprises cortical regions important for higher-order integration including the lateral frontal and parietal association cortex, as well as the medial frontal, anterior, and posterior cingulate, and medial parietal (precuneus, retrosplenial) cortex (Fig. 1). Subcortical structures participating in the consciousness system include the activating systems of the upper pons and midbrain, the thalamus, hypothalamus, and basal forebrain (see Fig. 1). Numerous studies have shown that these structures are crucial for normal alertness, attention, and awareness.5,6 In addition, disorders of consciousness including coma, vegetative state, and minimally conscious state are known to be associated with dysfunction in these cortical and subcortical brain networks7,8 (see also articles by N. Schiff and J. Giacino elsewhere in this issue). The sections that follow will provide evidence that 3 types of epileptic seizures—namely absence, generalized tonic-clonic, and temporal lobe complex partial seizures—all converge on the consciousness system when they cause impaired consciousness (see Fig. 1). Although the anatomic regions causing impaired consciousness in these 3 seizure types appear to be the same, the physiologic mechanisms may differ. Thus, different patterns of abnormal increases or decreases in activity can occur in different seizure types, but they all lead to impaired consciousness by affecting the same set of anatomic structures.
Fig. 1.
The consciousness system. Anatomic structures involved in regulating the level of alertness, attention, and awareness. (A) Medial view showing cortical (blue) and subcortical (red) components of the consciousness system. (B) Lateral cortical components of the consciousness system. Note that other circuits not pictured here, such as the basal ganglia and cerebellum, may also play a role in attention and other aspects of consciousness. (Reproduced from Blumenfeld H. Neuroanatomy through clinical cases. 2nd edition. Sunderland (MA): Sinauer Associates; 2010; with permission.)
ABSENCE SEIZURES
Absence (petit mal) seizures are typically brief 5- to 10-second events consisting of staring and unresponsiveness. Electroencephalography (EEG) during absence seizures shows widespread bilateral 3- to 4-Hz spike-wave discharges (Fig. 2A). Absence seizures usually last 5 to 10 seconds, with abrupt onset and end of EEG changes and behavioral deficits. Seen most commonly in children as part of childhood absence epilepsy, absence seizures occur less often in adolescents and adults. Before the initiation of treatment, seizures can recur up to hundreds of times per day, causing significant impairment in school and work performance.
Fig. 2.
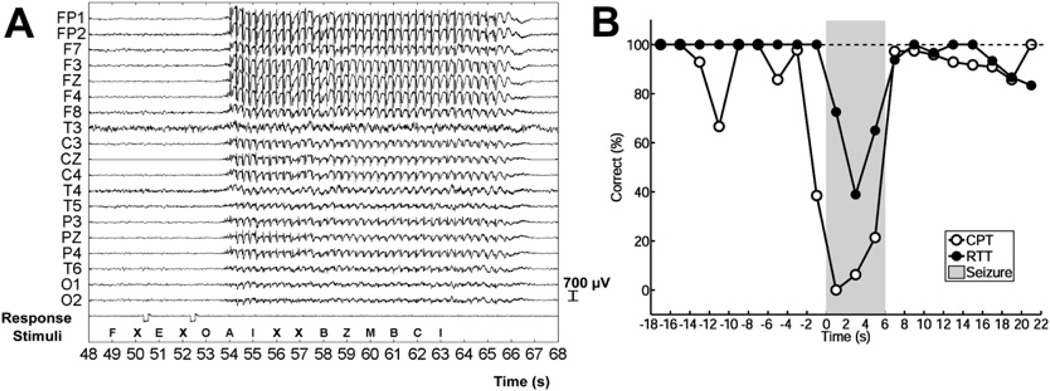
Electroencephalography (EEG) and behavior during typical absence seizures. (A) EEG showing typical 3- to 4-Hz spike-wave discharge during an absence seizure. Amplitude is maximal in frontal electrodes (FP1, FP2, F7, F3, FZ, F4, F8) and lower in more posterior occipital regions (O1, O2). A series of letters were presented to the patient (Stimuli) in a continuous performance task. Prior to the seizure the patient pushed the button (Response, voltage deflections) correctly to each target letter (X). However, when the target letter X occurred during the seizure, the patient was unable to respond. Linked-ears referential EEG recording. Functional magnetic resonance imaging (fMRI) changes for this seizure are shown in Fig. 3. (B) Average behavioral impairment during absence seizures. Percent correct responses are shown over time (2-second time bins) before, during, and after seizures (shaded region, normalized to mean seizure duration of ~6 seconds). Results are shown for 2 different tasks: in the continuous performance task (CPT) random letters appeared once per second and patients were instructed to push a button each time the target letter X appeared (see also A); in the repetitive tapping task (RTT) patients were instructed to push the button for every letter regardless of its identity. Performance on the more difficult CPT task declined rapidly for letters presented just before seizure onset and recovered quickly after seizure end. Impaired performance on the RTT task was more transient than on CPT, did not begin until after seizure onset, and was less severely impaired during seizures than the CPT task (F = 15.3, P = .017; analysis of variance). Results are based on a total of 53 seizures in 8 patients; 41 seizures in 5 patients during CPT and 12 seizures in 4 patients during RTT. ([A] Reproduced from Berman R, Negishi M, Vestal M, et al. Simultaneous EEG, fMRI, and behavioral testing in typical childhood absence seizures. Epilepsia 2010;51(10):2011–22; with permission; and [B] Bai X, Vestal M, Berman R, et al. Dynamic time course of typical childhood absence seizures: EEG, behavior, and functional magnetic resonance imaging. J Neurosci 2010;30:5884–93; with permission.)
Behavioral changes during absence seizures consist of arrest of ongoing movements, and lack of response to questions and commands. Absence seizures appear as if someone has “pushed the pause button” on the patient’s behavior and responsiveness. Episodes are commonly accompanied by minor eyelid, mouth, or finger movements, but more significant motor activity is not part of typical absence seizures. The eyelids may droop but remain open; in fact if the eyes are closed before onset they tend to open during seizures.9 Onset and end are usually abrupt, and patients do not usually show significant postictal deficits, although subtle impairment has been reported in some studies for a few seconds after seizures end.10–14 The usual duration of absence seizures is 5 to 10 seconds, and some classify episodes lasting less than 3 seconds as interictal epileptiform activity rather than seizures.15,16 However, this arbitrary cutoff is not fully supported by the available data, because even very brief episodes of spike-wave activity lasting a second or less may cause behavioral deficits when evaluated by careful testing.10,17–19 The time course of impairment during absence seizures exhibits a “trough” (see Fig. 2B) with maximal deficits occurring within about a second of seizure onset, and performance then gradually improving toward the end of the seizure.18,20–25 It has been reported that task performance is relatively spared during the last 2 to 3 seconds of longer seizures,18,22,25–27 although the exact ending time of longer seizures may be difficult to determine electrographically because the spike-wave pattern often gradually merges into a period of generalized slowing without spikes. Memory for new information presented to patients during absence seizures is usually (but not always) lost, and some studies have reported a short period of anterograde amnesia during which information from a few seconds before the seizure also cannot be retrieved.21,23,28–32
Many patients retain the capacity to perform simple tasks such as repetitive finger tapping during absence seizures.17,18,20,32–34 In general, tasks that require greater decision making, such as selecting a particular letter on visual presentation or responding to verbal questions, are most severely impaired during absence seizures, whereas simpler more automatic tasks may be relatively spared (reviewed in Ref.35) (see Fig. 2B). One patient reported that she could continue to play the violin during absence seizures as long as the passage was not very difficult or new to her, and another reported that if he had an absence seizure while swimming he would come to the surface and tread water until it was over. In addition to variations in the severity of impairment depending on the task, there is a large degree of variability in impairment on the same task both between patients and from one seizure to the next within the same patient.35 The cause of this variability is not known. Therefore, investigating the relationship between variable task performance and variable EEG and neuroimaging involvement of specific brain regions may provide crucial insights into the mechanisms of impaired consciousness in absence seizures.
Absence seizures electrographically are accompanied by 3- to 4-Hz spike-wave discharges that have a widespread distribution, but tend to have a larger amplitude in more anterior head regions (see Fig. 2A).36–38 The frequency and amplitude of the discharges gradually decreases toward the end of seizures. Recent source localization studies using high-density EEG recordings or magnetoencephalography (MEG) have reported maximal involvement of focal regions of the medial frontal, or bilateral lateral frontal cortex.39–42 Along with measurements from animal models also showing focal involvement in bilateral cortical and subcortical structures,43–46 these findings support the growing notion that “generalized” seizures are not truly generalized.47,48 Rather, absence seizures appear to involve focal brain regions most intensely while sparing others.
A few aspects of the EEG signal have been investigated in relation to behavioral impairment during absence seizures. First, as already noted, some studies report that longer EEG duration is related to more severe behavioral impairment in absence seizures.22,32,49,50 However, others have described deficits even with very brief spike-wave discharges on careful testing.10,17 Several studies have examined other features of the EEG and have found that spike-wave amplitude, rhythmicity, frontocentral distribution, or “generalization” predicted more severe behavioral impairment during absence seizures.10,32,33,51,52 Of importance, some of the variable performance from one seizure to the next may also be related to the timing of tasks relative to spike-wave onset and end because, as already noted, impairment may be maximal shortly after spike-wave onset and less severe toward the end of seizures.
Neuroimaging of absence seizures has been greatly facilitated in the past 10 years by use of functional magnetic resonance imaging (fMRI), which has better spatial and temporal resolution than methods used in earlier studies (reviewed in Ref.53). Combining EEG with fMRI has allowed the analysis of fMRI changes during absence seizures, demonstrating mainly increases in the thalamus, whereas cortical areas have shown a mixture of fMRI increases and decreases (Fig. 3). Cortical fMRI decreases during absence seizures involve mainly the so-called default mode network including the posterior cingulate/precuneus, lateral parietal, and medial frontal cortex.54–57 Meanwhile, cortical fMRI increases are variably seen mainly in primary cortical areas including primary visual, primary auditory, and primary sensorimotor cortex, as well as in the frontal association cortex (see Fig. 3).17 Recent work has demonstrated that conventional fMRI analysis methods do not adequately capture the true time course of fMRI signals during absence seizures,20,58–61 which in addition to the aforementioned areas also show biphasic fMRI changes in the lateral frontal association cortex.20 Also of note, the relationship between fMRI changes and neuronal activity is not fully known during absence seizures. Studies from animal models suggest that fMRI increases are related to increased neuronal activity.44,45,62 However, the underlying neuronal activity in regions (including the default mode network) showing decreased fMRI signals during absence seizures is not known, and will require further investigation with better models.
Fig. 3.
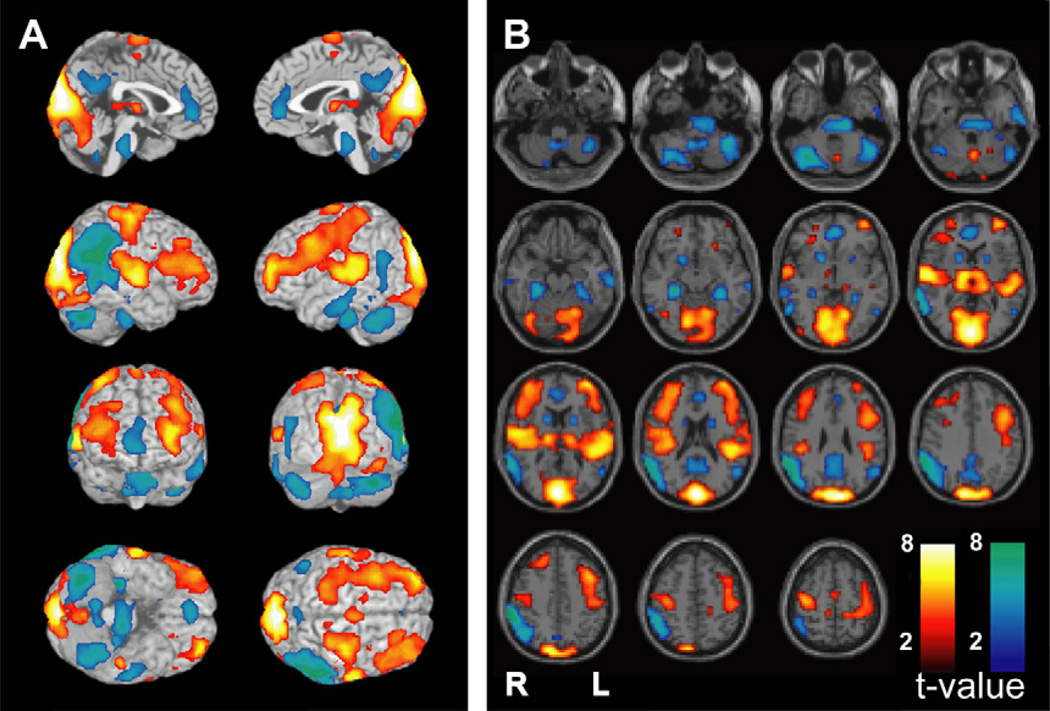
fMRI changes during a typical absence seizure involve the consciousness system and primary cortices. Blood oxygen level dependent (BOLD) fMRI changes are shown from a 12-second seizure in a 14-year-old girl with childhood absence epilepsy (EEG for this seizure is shown in Fig. 2A). The consciousness system demonstrates BOLD fMRI increases in the thalamus, decreases in the interhemispheric regions (anterior cingulate, precuneus), decreases in the lateral parietal cortex, and increases in the lateral frontal cortex. In addition, fMRI increases are present in the primary cortices including the primary visual (occipital), primary auditory (superior temporal), and primary sensorimotor (Rolandic) cortex. fMRI decreases are also seen in the pons, basal ganglia, and cerebellum. Results were analyzed in SPM2 (http://www.fil.ion.ucl.ac.uk/SPM) using a t-test to compare seizure versus baseline with uncorrected height threshold (P = .001) and extent threshold (k = 3 voxels). (Unpublished data Courtesy of R. Berman).
To summarize, fMRI studies have revealed a complex sequence of fMRI changes in absence seizures including the main structures of the consciousness system (see Fig. 1). Thus, the thalamus shows mainly fMRI increases, the medial frontoparietal and lateral parietal cortex mainly decreases, and lateral frontal cortex biphasic changes.20 Changes in primary cortical areas may also contribute to impaired consciousness during absence seizures.
fMRI has the potential to explain the anatomic basis of the variable behavioral performance from one seizure to the next, which could provide crucial insights into the specific structures involved in impaired consciousness. Variability in the fMRI pattern has been observed in different seizures and different patients.17,63 However, very few studies have directly investigated the relationship between variable performance and fMRI. Studying variable ictal behavior and fMRI in children presents substantial challenges, and many subjects are needed to obtain sufficient seizures for meaningful analysis. To date, two studies have shown that absence seizures with impaired responsiveness tend to have more fMRI changes in the cortex and thalamus than seizures without impaired responsiveness.17,64 However, both studies were limited by relatively small sample sizes, and by the lack of specific changes in defined anatomic regions to explain the impairment. An additional single case was reported with relatively spared function during absence seizures and asymmetric fMRI changes affecting the right hemisphere more strongly.65 Finally, another recent study examined the timing of behavioral impairment relative to EEG and fMRI and found that fMRI changes appeared to both precede and outlast the more transient behavioral and EEG changes.20 Despite the inherent challenges, additional work is clearly needed to relate behavior and fMRI in absence epilepsy.
Overall, the behavioral impairment and anatomic regions involved in absence seizures are similar to other more chronic disorders of consciousness.7,8 Like the minimally conscious state,66,67 patients have their eyes open and are variably capable of simple responses at times, but do not show consistent evidence of functional interactive communication or object use. Anatomic regions affected during absence seizures also resemble other disorders of consciousness, because there is dysfunction in the bilateral association cortex and upper brainstem/diencephalic arousal systems comprising the consciousness system (see Fig. 1). However, further work will be needed to identify the specific anatomic and physiologic differences between absence seizures with complete behavioral arrest and those that leave some behavioral responses intact.
GENERALIZED TONIC-CLONIC SEIZURES
Generalized tonic-clonic (grand mal) seizures are dramatic convulsive episodes. Patients exhibit sustained tonic limb extension, followed by rhythmic clonic jerking of all limbs and deep unresponsiveness both during seizures and in the postictal period. Generalized tonic-clonic seizures can occur in primary generalized epilepsy or can arise when partial seizures spread and secondarily generalize. These seizures are also provoked by a variety of physiologic derangements (electrolyte imbalance, hypoglycemia, toxin exposure, electrical shock, and so forth) in patients who do not have habitual seizures.
The behavior in generalized tonic-clonic seizures usually includes complete lack of responsiveness to questions, commands, or other stimuli, and amnesia for the both the ictal and postictal periods. As in the vegetative state, the eyes are open during generalized tonic-clonic seizures, but in most other ways the lack of meaningful response to external stimuli is similar to coma. Postictally the eyes close, and the patient is often in an unresponsive sleeplike state for a variable period of time. The motor activity in tonic-clonic seizures typically lasts about 2 minutes. This period includes a tonic phase lasting about 30 seconds, which gives way to a “vibratory” phase, followed by more discrete jerks in the clonic phase.68–71 It is interesting that many seizures do not exhibit the classic pattern of tonic followed by clonic activity. Instead patients may initially have clonic jerks then tonic activity, followed by another phase of clonic jerks, or the tonic phase may be incompletely expressed or not occur at all. These observations suggest that, like absence seizures, “generalized” tonic-clonic seizures do not homogeneously involve the whole nervous system; rather, selective networks may be most intensely involved while others are relatively spared. Work from animal models also supports relatively intense involvement of specific brain regions during generalized tonic-clonic seizures.72
Consciousness is spared in a minority of patients who have episodes that are otherwise behaviorally indistinguishable from generalized tonic-clonic seizures.73–75 Tonic-clonic seizures with spared consciousness are often described as being quite unpleasant or painful by patients unfortunate enough to experience these episodes. The mechanism for spared consciousness in these patients is not known, but it has been speculated that their seizures may arise from bilateral motor or supplementary motor regions while largely sparing the association cortex.73
The scalp EEG during generalized tonic-clonic seizures shows high-frequency polyspike activity during the tonic phase, which gives way to rhythmic polyspike and slow-wave activity in the clonic phase. Postictally, while patients usually lie flaccid and unresponsive, the EEG shows generalized suppression, consisting of relatively low-amplitude EEG activity. Of interest, intracranial EEG recordings have shown that generalized tonic-clonic seizures do not involve the whole brain, and that some regions can be relatively spared.76 One important study that has not yet been done, to the author’s knowledge, would be to examine the intracranial EEG of the rare patients who have preserved consciousness in generalized tonic-clonic seizures, and to determine how the anatomic distribution of the discharges differs from patients with impaired consciousness.
Neuroimaging of generalized tonic-clonic seizures cannot readily be done with fMRI because convulsions require close clinical attention and induce significant movement artifacts. Instead, insights have been gained from ictal single-photon emission computed tomography (SPECT) as well as positron emission tomography (PET).77 PET cerebral blood flow imaging also requires imaging during the convulsion. With SPECT, on the other hand, injection of radiopharmaceutical during the seizure is taken up within 20 to 30 seconds by the brain, providing a map of relative blood flow at the time of the injection. Therefore, imaging can be done later when the patient is clinically stable and no longer moving. Ictal SPECT is analyzed by comparison with baseline interictal SPECT in the same patients.78,79 SPECT imaging of generalized tonic-clonic seizure has been done in both electroconvulsive therapy–induced seizures80–85 and in spontaneous secondarily generalized seizures,68,71,82,86–88 with similar results.
Electroconvulsive therapy–induced seizures have the advantage of controlled timing and relatively consistent seizure onset. Seizures are induced by placement of electrodes in fixed locations either in the bilateral frontotemporal, bilateral frontal, or right unilateral regions.84 Early cerebral blood flow (CBF) increases occur near the region of the electrodes, presumably reflecting seizure onset.83 CBF maps of the whole seizure show changes that overlap with the consciousness system, including increases in the lateral frontal and mediolateral parietal cortex, decreases in the inter-hemispheric medial frontal and cingulate cortex, and increases in deep structures including the thalamus (Fig. 4).81–84 Similar regional changes were also found in a study using PET CBF imaging.77
Fig. 4.
Generalized tonic-clonic seizure induced by bilateral frontotemporal electroconvulsive therapy. Ictal single-photon emission computed tomography (SPECT) image for a single generalized tonic-clonic seizure compared with interictal baseline (red = cerebral blood flow [CBF] increases; green = CBF decreases). (A) Lateral view. (B) Medial view. Changes in the consciousness system include CBF increases in lateral frontotemporal cortex, lateral parietal and medial parietal cortex. CBF increases were also present in the thalamus (best seen in cross sections, not shown), as well as in the cerebellum. CBF decreases were present in the medial frontal and cingulate cortex, as well as in lateral cortical regions. SPM extent threshold k = 125 voxels; height threshold: P = .01. (Modified from Blumenfeld H, McNally KA, Ostroff RB, et al. Targeted prefrontal cortical activation with bifrontal ECT. Psychiatry Res 2003;123:165–70; with permission.)
SPECT imaging in epilepsy patients during secondarily generalized tonic-clonic seizures has also shown involvement of the consciousness system (see Fig. 1), including increases in the lateral frontoparietal cortex, upper brainstem, and thalamus, and decreases in the interhemispheric regions.68,71,82 As in absence seizures, these so-called generalized tonic-clonic seizures produce changes that are not homogeneous throughout the brain, but rather affect some regions more intensely than others.
Of note, some brain regions show relative CBF decreases during both spontaneous and induced generalized tonic-clonic seizures, particularly in the interhemispheric frontal and cingulate regions (see Fig. 4). In the postictal period, when patients remain deeply unresponsive, these CBF decreases become more pronounced and also include the medial and lateral frontoparietal association cortex.68 Our group has observed that there are CBF increases in the cerebellum that progressively increase at late times during generalized tonic-clonic seizures and into the postictal period (Fig. 5).68 This increased CBF in the cerebellum is correlated with CBF increases in the thalamus and upper brainstem, as well as with CBF decreases in the medial and lateral frontoparietal association cortex (see Fig. 5). These findings suggest that, in agreement with prior work in animal models,89 strong activation of inhibitory cerebellar Purkinje cells late in seizures may inhibit thalamocortical networks, contributing to seizure termination as well as to impaired cortical function and suppressed consciousness in the postictal period.
Fig. 5.
Frontoparietal CBF decreases and thalamic increases are correlated with increased CBF in the cerebellum during and following generalized tonic-clonic seizures. Network correlations are shown for spontaneous secondarily generalized tonic-clonic seizures imaged with ictal SPECT in epilepsy patients during video/EEG monitoring. Positive (red) and negative (green) correlations are shown between CBF changes in the cerebellum and the rest of the brain across patients (n = 59 seizures in 53 patients). Significant positive correlations were found between the cerebellum and the upper brainstem tegmentum and thalamus. Negative correlations were found with the bilateral frontoparietal association cortex, anterior and posterior cingulate, and precuneus. Images were analyzed with SPM extent threshold k = 125 voxels, and height threshold P = .01. (Reproduced from Blumenfeld H, Varghese G, Purcaro MJ, et al. Cortical and subcortical networks in human secondarily generalized tonic-clonic seizures. Brain 2009;132:999–1012; with permission.)
In summary, generalized tonic-clonic seizures usually cause complete unresponsiveness but the eyes are open, making the behavior resemble a transient vegetative state. Anatomic involvement of the consciousness system includes abnormal increased activity in the upper brainstem and diencephalon, decreases in the medial frontal and cingulate cortex, and increases in the lateral frontal and mediolateral parietal association cortex. Postictal depressed cortical function may have a functional relationship with increased activity in the cerebellum. Further investigations are needed to better understand the mechanisms of selective network involvement in generalized tonic-clonic seizures, and the cortical-subcortical interactions governing postictal suppression of physiology and behavior.
TEMPORAL LOBE COMPLEX PARTIAL SEIZURES: NETWORK INHIBITION HYPOTHESIS
Impaired consciousness is classically seen in disorders that involve bilateral cortical-subcortical networks.1 It is therefore not entirely surprising that absence seizures and generalized tonic-clonic seizures cause impaired consciousness, perhaps through bilateral involvement of the consciousness system as already noted. However, why focal temporal lobe seizures should so often cause impaired consciousness is more puzzling. During temporal lobe seizures, patients are typically unresponsive to questions and commands for 1 to 2 minutes, and then remain confused for a variable period of time postictally. Scalp EEG recordings during seizures show rhythmic theta frequency discharges usually of greatest amplitude over one temporal lobe. The “network inhibition hypothesis,” described in greater detail in the discussion that follows, proposes that focal temporal lobe seizures inhibit subcortical arousal systems, leading to depressed cortical function and impaired consciousness.
Onset of a temporal lobe seizure may be heralded by an aura or warning consisting of a fearful premonition, rising feeling in the stomach, or other unusual sensations such as déjà vu. Patients usually retain normal responses to question or commands, at least initially. In terminology that was adopted in 1981 and remains widely used, if consciousness is spared throughout the seizure it is called simple partial, whereas if consciousness is impaired it is called a complex partial seizure.90 (See also Ref.91 for a more recent discussion of epilepsy classification.) In complex partial temporal lobe seizures there is typically behavioral arrest, staring, and automatic repetitive behaviors called automatisms including lip smacking, chewing, and picking or rubbing movements of the hands. During this time the eyes remain open and patients do not respond to questions or commands. However, they may show simple responses to stimuli including orienting or grasping responses called reactive automatisms.92 After the seizure ends, patients usually have a period of continued decreased responsiveness, confusion, and amnesia for events around the time of the seizure. The lack of meaningful responses during complex partial seizures, but with preserved eye opening and simple orienting movements, resembles a transient form of vegetative state.93–95 However, the presence of manual automatisms may be considered more similar to behaviors seen in the minimally conscious state.66,67
Like tonic-clonic seizures, SPECT imaging has been a more practical way of performing neuroimaging in patients than fMRI during temporal lobe complex partial seizures. As expected, in temporal lobe seizures SPECT imaging shows increased CBF in the temporal lobe on the side of seizure onset.79,87,96 However, there is also decreased CBF in bilateral regions of the lateral and medial frontoparietal association cortex (Fig. 6A, B).97–101 In addition, increased CBF in the upper brainstem and medial diencephalon has been observed in temporal lobe seizures (see Fig. 6B).97,102–105 Of importance, it has been found that the CBF decreases in the frontoparietal association cortex, as well as CBF increases in the upper brainstem and medial diencephalon, are associated with impaired consciousness in partial seizures.97,103 Simple partial seizures, in which consciousness is spared, tend to exhibit more limited CBF changes confined to the temporal lobe. In addition, increased CBF in the medial thalamus was found to be correlated with decreased CBF in bilateral medial and lateral frontoparietal association cortex,97 suggesting a mechanistic link between cortical and subcortical changes in temporal lobe epilepsy. Of note, the midline subcortical structures as well as the medial and lateral frontoparietal cortex regions affected in temporal lobe complex partial seizures again correspond to the same anatomic regions involved in absence and tonic-clonic seizures, namely the consciousness system (see Fig. 1).
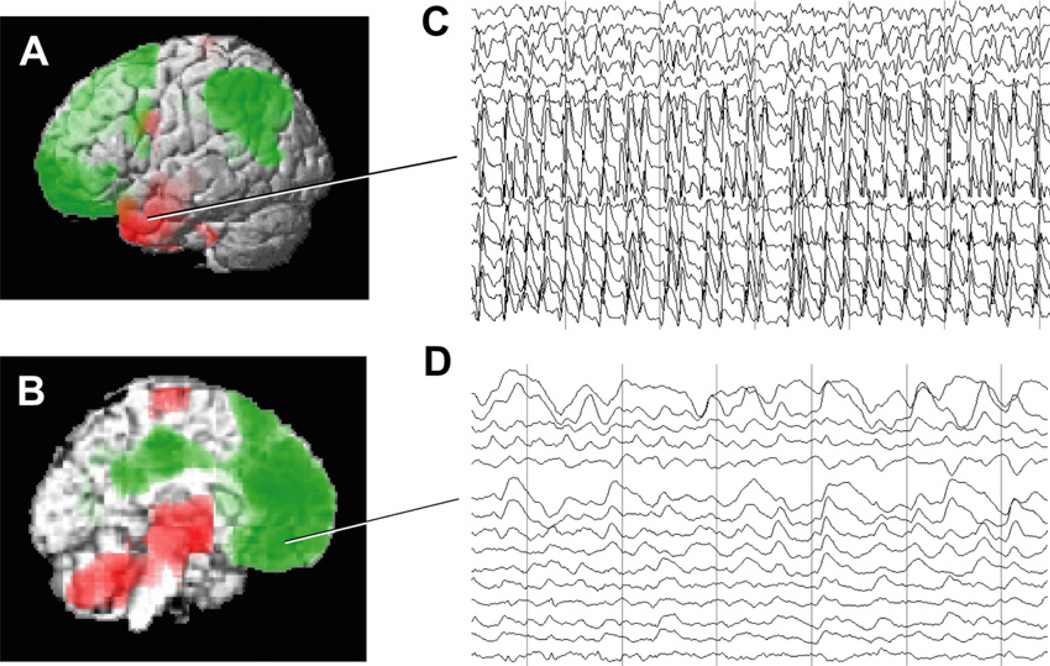
Fig. 6.
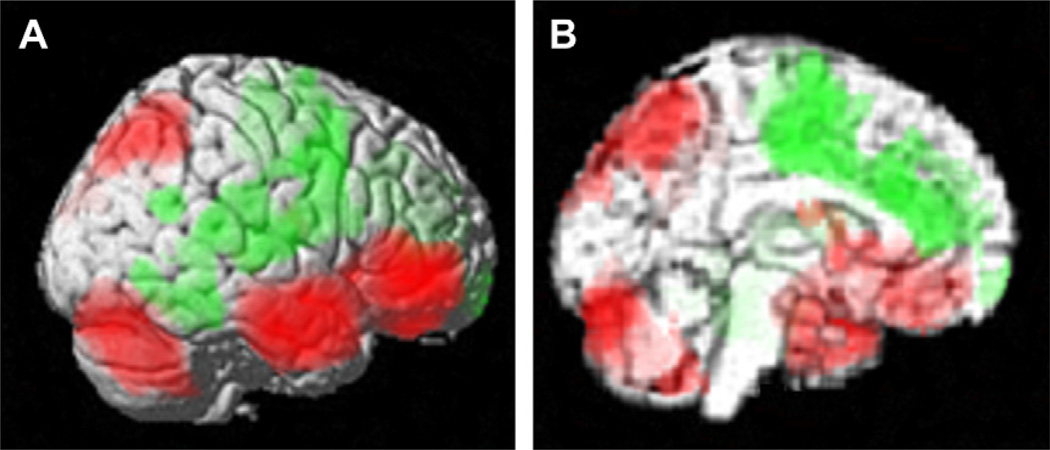
CBF and EEG changes in temporal lobe complex partial seizures. (A, B) Group analysis of SPECT ictal-interictal difference imaging during temporal lobe seizures. CBF increases are present in the temporal lobe (A) and in the medial thalamus (B). Decreases are seen in the lateral frontoparietal association cortex (A) and in the interhemispheric regions (B). (C, D) Intracranial EEG recordings from a patient during a temporal lobe seizure. High-frequency polyspike-and-wave seizure activity is seen in the temporal lobe (C). The orbital and medial frontal cortex (and other regions, EEG not shown) do not show polyspike activity, but instead large-amplitude, irregular slow rhythms resembling coma or sleep (D). Vertical lines in C and D denote 1-second intervals. Note that the EEG and SPECT data were from similar patients, but were not simultaneous, and are shown together here for illustrative purposes only. ([A, B] Modified from Blumenfeld H, McNally KA, Vanderhill SD, et al. Positive and negative network correlations in temporal lobe epilepsy. Cerebral Cortex 2004;14:892–902; with permission; and [C, D] Blumenfeld H, Rivera M, McNally KA, et al. Ictal neocortical slowing in temporal lobe epilepsy. Neurology 2004;63:1015–21; with permission.)
Intracranial EEG recordings are often performed as part of surgical planning in patients with temporal lobe epilepsy, and provide an opportunity to more directly study the physiologic changes in the association cortex during temporal lobe seizures. Although the temporal lobe shows high-frequency alpha (8–12 Hz), beta (13–25 Hz), and higher-frequency polyspike-and-wave discharges during temporal lobe seizures (see Fig. 6C), the frontoparietal association cortex shows delta (1–4 Hz) and slower oscillations (see Fig. 6D).106,107 These neocortical slow waves more closely resemble the EEG of coma, encephalopathy, or slow-wave sleep than ictal patterns on intracranial EEG.108–110 The anatomic distribution of ictal neocortical slow waves in temporal lobe seizures includes the bilateral lateral frontal, orbital frontal, medial frontal, cingulate, and lateral parietal cortex, corresponding to the same regions in which decreased CBF is observed on SPECT imaging.97,106,107 Slow wave activity and CBF decreases in the frontoparietal association cortex were significantly greater in complex partial than in simple partial seizures.97,107 Both the neocortical slow waves and CBF decreases in frontoparietal association cortex persist into the postictal period, during which time patients often remain confused.
These findings support the network inhibition hypothesis for impaired consciousness in temporal lobe epilepsy (Fig. 7).3,107,108,111–115 The network inhibition hypothesis proposes that temporal lobe seizures propagate to subcortical structures, leading to inhibition (through mechanisms still being investigated) of subcortical arousal systems (see Fig. 7A–C). This in turn removes the normal activation of the frontoparietal association cortex, leading to depressed neocortical function and impaired consciousness (see Fig. 7D). Of note, in the network inhibition hypothesis the neocortex enters a sleep-like (or minimally conscious-like) state, not due to direct seizure propagation but because of remote network effects on subcortical arousal systems. Another way of describing these long-range effects of seizures on other parts of the brain is “ictal diaschesis.”114,115
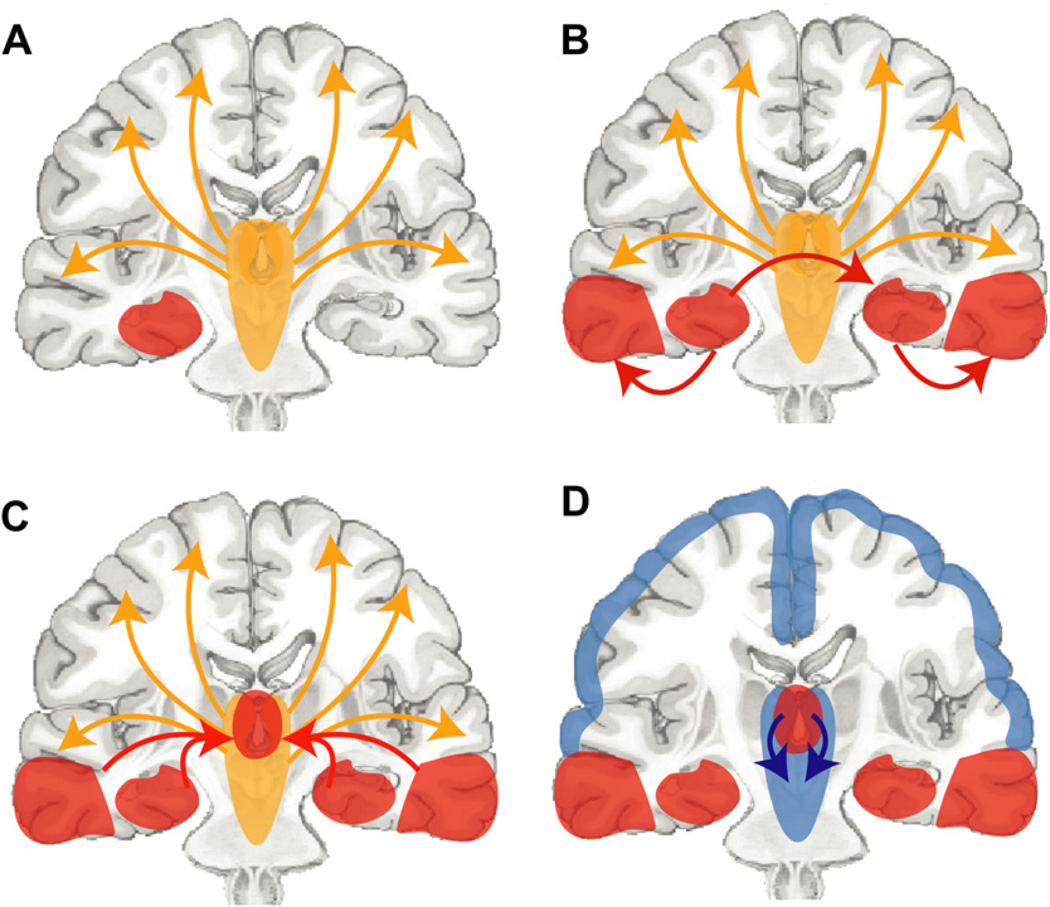
Fig. 7.
Network inhibition hypotheses for impaired consciousness during temporal lobe complex partial seizures. (A) Under normal conditions, the upper brainstem-diencephalic activating systems interact (yellow arrows) with the cerebral cortex to maintain normal consciousness. A focal seizure involving the mesial temporal lobe begins unilaterally (red region). If it remains unilateral then a simple-partial seizure will occur without impairment of consciousness. (B) Propagation (red arrows) of seizure activity from the mesial temporal lobe to the ipsilateral lateral temporal lobe and the contralateral temporal lobe. (C). Spread of seizure activity from bilateral temporal lobes to midline subcortical structures. (D). Inhibition (blue arrows) of the midline subcortical structures, together with the resulting depressed activity in bilateral frontoparietal association cortex in complex-partial seizures, leads to loss of consciousness. (Reproduced from Englot DJ, Yang L, Hamid H, et al. Impaired consciousness in temporal lobe seizures: role of cortical slow activity. Brain 2010;133(Pt 12): 3764–77; with permission.)
The fundamental changes occurring in neocortical, subcortical, and limbic networks have recently been investigated in rodent models of hippocampal seizures. As in human temporal lobe epilepsy, rats with spontaneous limbic seizures following pilocarpine-induced status epilepticus as well as acute seizures induced by hippocampal stimulation exhibit fast activity in the hippocampus but slow 1- to 3-Hz activity in the frontoparietal cortex, associated with behavioral arrest.112 This neocortical slow activity differs fundamentally from seizure activity measured in the same animals. Seizure activity in the hippocampus or neocortex is associated with increased neuronal action potential firing, CBF, cerebral blood volume, blood oxygen level–dependent fMRI signal, and cerebral metabolic rate of oxygen consumption.112 By contrast, neocortical slow activity during hippocampal seizures is associated with decreases in all of these parameters, closely resembling slow-wave activity in deep anesthesia.109,110,112
The rat model has also begun to shed important insights into the network mechanisms of ictal neocortical slow waves. High-field fMRI mapping during hippocampal seizures demonstrated increased fMRI signal in the hippocampus, decreases in orbital frontal, anterior cingulate, and retrosplenial cortex, and subcortical increases in the medial thalamus and lateral septal nuclei.112,113 Because the lateral septal nuclei contain a large population of γ-aminobutyric acid (GABA)ergic inhibitory neurons, it was hypothesized that activation of the lateral septal nuclei and other GABAergic subcortical structures during seizures could inhibit the subcortical arousal systems (network inhibition hypothesis). In support of this, local stimulation of the lateral septal nuclei produced neocortical slow waves and behavioral arrest mimicking the effects of hippocampal seizures.113 In addition, animals in which the hippocampus was disconnected from the lateral septal nuclei (by cutting the fornix) had focal seizures in the hippocampus without neocortical slow activity and without behavioral arrest.113 These findings further support the network inhibition hypothesis, and suggest that hippocampal seizures produce neocortical slow activity and impaired consciousness through network effects critically involving subcortical structures.108
In summary, temporal lobe complex partial seizures cause unresponsiveness with the eyes open and some automatic repetitive movements, most similar to a transient vegetative or minimally conscious state. Like absence and generalized tonic-clonic seizures, abnormal brain activity again converges on the anatomic structures of the consciousness system (see Fig. 1). Thus, temporal lobe complex partial seizures are associated with abnormal increased activity in the upper brainstem and diencephalon, and decreased activity in the medial frontal, cingulate, precuneus, and lateral frontoparietal association cortex, which persists into the postictal period. Both human data and animal models support a network inhibition hypothesis in which limbic seizures propagate to subcortical structures, which inhibit subcortical arousal networks, in turn causing neocortical slow waves and impaired consciousness. Additional mechanistic studies are needed to determine in greater detail how subcortical structures are inhibited during temporal lobe seizures to produce the impaired consciousness seen in temporal lobe seizures.
IMPACT OF IMPAIRED CONSCIOUSNESS ON QUALITY OF LIFE IN EPILEPSY
Impaired consciousness has a major negative impact on quality of life in patients with epilepsy. In large patient series the major factors that determined impaired quality of life in epilepsy were frequency and severity of seizures.116,117 Impaired consciousness is a crucial determinant of seizure severity and can have numerous adverse consequences including motor vehicle accidents, burns, falls, drowning, other accidental injuries, loss of productivity at work or school, and social stigmatization. One of the more challenging aspects of the sudden impaired consciousness in epilepsy is the unpredictable times at which this can occur, disrupting ongoing activities and producing fear that a seizure could happen at any time.118,119
Because many adults depend on driving to reach work or other daily activities, loss of driving privileges has a large effect on quality of life. Though laws vary based on location, in most places patients are prohibited from driving if they have uncontrolled seizures associated with impaired consciousness. Several studies have looked at the risk of driving in patients with epilepsy, and have found variable results. Risk of motor vehicle accidents in patients with epilepsy may not be higher than in other patients with chronic illnesses such as diabetes.120,121 Although more limited, there are data to suggest that patients with uncontrolled seizures are at greater risk of motor vehicle accidents,120,122–124 and that certain seizure types such as generalized tonic-clonic or complex partial seizures may pose a greater risk than simple partial or myoclonic, or perhaps absence, seizures.125,126 In examining these seizure types, it is likely that several factors including impaired consciousness, seizure duration, and motor deficits could affect driving safety. A recent study used a computer game–based driving simulator to prospectively evaluate driving performance during seizures.127 Patients were instructed to play a realistic driving game using a steering wheel and gas/brake pedal controllers during inpatient video/EEG monitoring while performance metrics were collected continuously. Collisions and other evidence of loss of control (sustained decrease in steering-wheel velocity, gas pedal use, or car velocity) were most evident for generalized tonic-clonic seizures, whereas no significant deficits were observed in subclinical seizures.127 Partial seizures (including both simple partial and complex partial seizures) and absence seizures caused impairment of driving in some but not all seizures. Additional prospective data of this kind may make it possible to determine specifically which patients are at greatest risk of motor vehicle accidents during seizures, and could be useful for advising patients and physicians.
TREATMENT STRATEGIES
Although the best way to prevent impaired consciousness in epilepsy would be to stop all seizures, in patients with medically refractory epilepsy this cannot always be achieved. For these patients it would be a welcome improvement in lifestyle to at least prevent impaired consciousness during seizures. Several treatment modalities could be considered for preventing or reducing impaired consciousness in epilepsy. For example, because cortical slow oscillations in complex partial seizures are similar to slow-wave sleep, medications that promote the awake state, such as modafinil,128,129 could be tested to determine whether they improve alertness during and following seizures. Deep brain stimulation is being investigated for the prevention of seizures,130–134 but stimulation of thalamic arousal circuits has also been used to improve alertness in disorders of consciousness,135,136 so could potentially be tested for this purpose in epilepsy as well. Disconnection procedures were found in rodent models to prevent neocortical slow waves and to improve responsiveness in limbic seizures.113 The specific disconnection procedure used was fornix transection, which may not be feasible in humans because of potential memory side effects137,138 (although see also Ref.139). However, this at least demonstrates that in principle a procedure of this kind could ultimately prevent epileptic unconsciousness when the underlying circuits and mechanisms are better understood.
Finally, it will be important to study behavioral interventions that may increase awareness by patients and families of impaired consciousness during seizures. This approach could help in the development of practical strategies for improving quality of life. In addition to impaired consciousness during seizures, patients with epilepsy commonly are unaware of the fact that they have had seizures and tend to underreport them.140–144 Improved prospective measures are needed to determine and evaluate impaired consciousness during seizures,145 and to relate impaired consciousness or other variables to patient recognition and report.146 Behavioral or educational interventions may be particularly helpful in increasing seizure recognition and report by patients, which could be highly valuable in improving their clinical care.
SUMMARY AND FUTURE DIRECTIONS
Recent human neuroimaging studies, intracranial EEG analysis, and animal model investigations have greatly increased our understanding of the fundamental mechanisms of impaired consciousness in epilepsy. The 3 seizure types causing impaired consciousness, namely absence, generalized tonic-clonic, and complex partial seizures, all converge on a final common set of anatomic structures we refer to as the consciousness system, consisting of medial and lateral frontoparietal association cortex and subcortical activating networks. These same anatomic structures in the consciousness system are also affected in other states of impaired consciousness, including sleep, anesthesia, coma, vegetative state, and minimally conscious state.7,8
In behavioral terms absence or complex partial seizures often resemble a transient vegetative state, in which patients exhibit no meaningful behavioral responses, yet have open eyes and maintain some rudimentary postural tone and orienting responses. Other absence or complex partial seizures more closely resemble a transient minimally conscious state, because patients may show automatisms or variable simple responses yet do not demonstrate consistent functional interactive communication or object use. Generalized tonic-clonic seizures resemble coma because orienting responses and postural stability are lost, yet unlike most cases of coma, the eyes do remain open.
The anatomic and physiologic changes in the consciousness system associated with seizures can be summarized as follows. Absence seizures exhibit fMRI signal increases in the thalamus, decreases in the anterior cingulate, medial frontal cortex, and precuneus, and a mixture of increases and decreases in the lateral frontoparietal association cortex. Generalized tonic-clonic seizures produce CBF increases in lateral frontal and mediolateral parietal cortex, decreases in medial frontal and cingulate cortex, and increases in the thalamus and upper brainstem during seizures. Postictally after generalized tonic-clonic seizures there is decreased CBF in medial and lateral frontoparietal association cortex. Temporal lobe complex partial seizures show increased activity in the medial diencephalon, with decreased activity in the medial frontal, cingulate, precuneus, and lateral frontoparietal association cortex.
Work to date has provided some insights into the fundamental network mechanisms for these changes, but there is much that remains to be done. For example, an elegant temporal sequence of cortical and subcortical fMRI changes are seen beginning 5 to 10 seconds before and continuing over 20 seconds after absence seizures,20,58,61 but the neurophysiologic basis of these changes is not known. In generalized tonic-clonic seizures, cerebellar CBF increases continue into the postictal period and are correlated with CBF decreases in the forebrain,68 but the role of these networks in seizure termination or postictal depression has not been thoroughly investigated. In temporal lobe seizures, evidence from human patients and animal models support the network inhibition hypothesis in which disruption of subcortical arousal systems leads to depressed neocortical function,107,112,113 but the exact cellular and neurotransmitter mechanisms for these changes require further investigation.
Additional work is also needed to relate variable deficits in consciousness with specific regions involved or spared during seizures. For example, it is not clear why profound deficits in consciousness occur in some absence seizures and some generalized tonic-clonic seizures but not in others. Further work with behavioral tasks and neuroimaging during absence seizures or intracranial recordings during tonic-clonic seizures may help clarify these unresolved questions.
Finally, improved treatments are needed to prevent impaired consciousness in epilepsy, particularly for patients in whom seizures cannot be stopped. Advances in understanding the fundamental mechanisms of impaired consciousness in epilepsy will be crucial for developing novel treatments targeting this major source of patient disability. Further work in this field will, it is hoped, lead to medications, surgical procedures, and behavioral interventions to reduce impaired consciousness and greatly improve the quality of life of patients with epilepsy.
Acknowledgments
This work was supported by NIH (R01NS055829, R01NS066974, R01NS049307, R01MH67528, P30NS052519), a Donaghue Investigator Award, and the Betsy and Jonathan Blattmachr Family.
REFERENCES
- 1.Plum F, Posner JB. The diagnosis of stupor and coma. 3rd edition. Philadelphia: Davis; 1982. [Google Scholar]
- 2.Blumenfeld H. Neuroanatomy through clinical cases. Sunderland (MA): Sinauer Assoc. Publ., Inc; 2002. [Google Scholar]
- 3.Blumenfeld H. Epilepsy and consciousness. Chapter 2. In: Laureys S, editor. The neurology of consciousness: cognitive neuroscience and neuropathology. New York: G Tononi Academic Press; 2009. pp. 15–30. [Google Scholar]
- 4.Blumenfeld H. Neuroanatomy through clinical cases. 2nd edition. Sunderland (MA): Sinauer Assoc Publ Co; 2010. [Google Scholar]
- 5.Steriade M, Jones EG, McCormick DA, editors. Thalamus. Amsterdam: Elsevier Science; 1997. [Google Scholar]
- 6.Steriade MM, McCarley RW. Brain control of wakefulness and sleep. 2nd edition. New York: Springer; 2010. [Google Scholar]
- 7.Laureys S, Tononi G. The neurology of consciousness: cognitive neuroscience and neuropathology. Amsterdam: Academic Press; 2008. [Google Scholar]
- 8.Laureys S, Schiff ND. Annals of the New York Academy of Sciences. Hoboken: Wiley-Blackwell; 2009. Disorders of consciousness. [DOI] [PubMed] [Google Scholar]
- 9.Sadleir LG, Scheffer IE, Smith S, et al. Factors influencing clinical features of absence seizures [see comment] Epilepsia. 2008;49:2100–2107. doi: 10.1111/j.1528-1167.2008.01708.x. [DOI] [PubMed] [Google Scholar]
- 10.Browne TR, Penry JK, Porter RJ, et al. Responsiveness before, during and after spike-wave paroxysms. Neurology. 1974;24:659–665. doi: 10.1212/wnl.24.7.659. [DOI] [PubMed] [Google Scholar]
- 11.Goldie L, Green JM. Spike and wave discharges and alterations of conscious awareness. Nature. 1961;191:200–201. doi: 10.1038/191200a0. [DOI] [PubMed] [Google Scholar]
- 12.Grisell JL, Levin SM, Cohen BD, et al. Effects of subclinical seizure activity on overt behavior. Neurology. 1964;14:133–135. doi: 10.1212/wnl.14.2.133. [DOI] [PubMed] [Google Scholar]
- 13.Lehmann HJ. Weckreaktionen bei pyknoleptischen absenzen. Arch Psychiatr Nervenkr. 1963;204:417–426. [PubMed] [Google Scholar]
- 14.Mallin U, Stefan H, Penin H. Psychopathometrische Studien zum Verlauf epileptischer Absencen. Z EEG EMG. 1981;12:45–49. [PubMed] [Google Scholar]
- 15.Daly D, Pedley TA. Current practice of clinical electroencephalography. 2nd edition. New York: Raven Press; 1990. [Google Scholar]
- 16.Sadleir LG, Scheffer IE, Smith S, et al. EEG features of absence seizures in idiopathic generalized epilepsy: impact of syndrome, age, and state. Epilepsia. 2009;50:1572–1578. doi: 10.1111/j.1528-1167.2008.02001.x. [DOI] [PubMed] [Google Scholar]
- 17.Berman R, Negishi M, Vestal M, et al. Simultaneous, EEG, fMRI, and behavioral testing in typical childhood absence seizures. Epilepsia. 2010;51(10):2011–2022. doi: 10.1111/j.1528-1167.2010.02652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimazono Y, Hirai T, Okuma T, et al. Disturbance of consciousness in petit mal epilepsy. Epilepsia. 1953;2:49–55. [Google Scholar]
- 19.Tuvo F. Contribution a l’etude des niveaux de conscience au cours des paroxysmes epileptiques infraclinique. Electroencephalogr Clin Neurophysiol. 1958;10:715–718. doi: 10.1016/0013-4694(58)90076-2. [DOI] [PubMed] [Google Scholar]
- 20.Bai X, Vestal M, Berman R, et al. Dynamic time course of typical childhood absence seizures: EEG, behavior, and functional magnetic resonance imaging. J Neurosci. 2010;30:5884–5893. doi: 10.1523/JNEUROSCI.5101-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bates JA. A technique for identifying changes in consciousness. Electroencephalogr Clin Neurophysiol. 1953;5:445–446. doi: 10.1016/0013-4694(53)90088-1. [DOI] [PubMed] [Google Scholar]
- 22.Goode DJ, Penry JK, Dreifuss FE. Effects of paroxysmal spike-wave on continuous visual-motor performance. Epilepsia. 1970;11:241–254. doi: 10.1111/j.1528-1157.1970.tb03888.x. [DOI] [PubMed] [Google Scholar]
- 23.Jus A, Jus C. Etude electro-clinique des alterations de conscience dans le petit mal. Studii si cercetari de Neurol. 1960;5:243–254. [Google Scholar]
- 24.Panayiotopoulos CP, Obeid T, Waheed G. Differentiation of typical absence seizures in epileptic syndromes: a video-EEG study of 124 seizures in 20 patients. Brain. 1989;112:1039–1056. doi: 10.1093/brain/112.4.1039. [DOI] [PubMed] [Google Scholar]
- 25.Tizard B, Margerison JH. Psychological functions during wave-spike discharges. Br J Soc Clin Psychol. 1963;3:6–15. [Google Scholar]
- 26.Fernandez H, Robinson R, Taylor RR. A device for testing consciousness. Am J EEG Technol. 1967;7:77–78. [Google Scholar]
- 27.Mirsky AF, Rosvold HE. Behavioral and physiological studies in impaired attention. In: Zea V, editor. Psychopharmacological methods: proceedings of a symposium on the effects of psychotropic drugs on higher nervous activity. Oxford: Pergamon Press; 1963. pp. 302–315. [Google Scholar]
- 28.Boudin G, Barbizet J, Masson S. Etude de la dissolution de la conscience dans 3 cas de petit mal avec crises prolongees. Rev Neurol. 1958;99:483–487. [Google Scholar]
- 29.Geller MR, Geller A. Brief amnestic effects of spike wave discharges. (Section on Child Neurology, abstract CN1) Neurology. 1970;20:380–381. [PubMed] [Google Scholar]
- 30.Hutt SJ, Gilbert S. Effects of evoked spike-wave discharges upon short term memory in patients with epilepsy. Cortex. 1980;16:445–457. doi: 10.1016/s0010-9452(80)80045-1. [DOI] [PubMed] [Google Scholar]
- 31.Jus A, Jus K. Retrograde amnesia in petit mal. Arch Gen Psychiatry. 1962;6:163–167. doi: 10.1001/archpsyc.1962.01710200055007. [DOI] [PubMed] [Google Scholar]
- 32.Mirsky AF, Van Buren JM. On the nature of the “absence” in centrencephalic epilepsy: a study of some behavioral, electroencephalographic, and autonomic factors. Electroencephalogr Clin Neurophysiol. 1965;18:334–348. doi: 10.1016/0013-4694(65)90053-2. [DOI] [PubMed] [Google Scholar]
- 33.Courtois GA, Ingvar DH, Jasper HH. Nervous and mental defects during petit mal attacks. Electroencephalogr Clin Neurophysiol. 1953;(Suppl 3):87. [Google Scholar]
- 34.Gastaut H. The brain stem and cerebral electrogenesis in relation to consciousness. In: Delafresnaye JF, editor. Brain mechanisms and consciousness. Springfield (IL): Thomas; 1954. pp. 249–283. [Google Scholar]
- 35.Blumenfeld H. Consciousness and epilepsy: why are patients with absence seizures absent? Prog Brain Res. 2005;150:271–286. doi: 10.1016/S0079-6123(05)50020-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coppola R. Topographic display of spike-and-wave discharges. In: Mysobodsky MS, Mirsky AF, editors. Elements of petit mal epilepsy. New York: Peter Lang; 1988. pp. 105–130. [Google Scholar]
- 37.Rodin E, Ancheta O. Cerebral electrical fields during petit mal absences. Electroencephalogr Clin Neurophysiol. 1987;66:457–466. doi: 10.1016/0013-4694(87)90092-7. [DOI] [PubMed] [Google Scholar]
- 38.Weir B. The morphology of the spike-wave complex. Electroencephalogr Clin Neurophysiol. 1965;19:284–290. doi: 10.1016/0013-4694(65)90208-7. [DOI] [PubMed] [Google Scholar]
- 39.Holmes MD, Brown M, Tucker DM. Are “generalized” seizures truly generalized? Evidence of localized mesial frontal and frontopolar discharges in absence. Epilepsia. 2004;45:1568–1579. doi: 10.1111/j.0013-9580.2004.23204.x. [DOI] [PubMed] [Google Scholar]
- 40.Sakurai K, Takeda Y, Tanaka N, et al. Generalized spike-wave discharges involve a default mode network in patients with juvenile absence epilepsy: a MEG study. Epilepsy Res. 2010;89(2–3):176–184. doi: 10.1016/j.eplepsyres.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 41.Stefan H, Paulini-Ruf A, Hopfengartner R, et al. Network characteristics of idiopathic generalized epilepsies in combined MEG/EEG. Epilepsy Res. 2009;85:187–198. doi: 10.1016/j.eplepsyres.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 42.Westmijse I, Ossenblok P, Gunning B, et al. Onset and propagation of spike and slow wave discharges in human absence epilepsy: a MEG study. Epilepsia. 2009;50(12):2538–2548. doi: 10.1111/j.1528-1167.2009.02162.x. [DOI] [PubMed] [Google Scholar]
- 43.Meeren HK, Pijn JP, Van Luijtelaar EL, et al. Cortical focus drives widespread corticothalamic networks during spontaneous absence seizures in rats. J Neurosci. 2002;22:1480–1495. doi: 10.1523/JNEUROSCI.22-04-01480.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nersesyan H, Hyder F, Rothman D, et al. Dynamic fMRI and EEG recordings during spike-wave seizures and generalized tonic-clonic seizures in WAG/Rij rats. J Cereb Blood Flow Metab. 2004;24:589–599. doi: 10.1097/01.WCB.0000117688.98763.23. [DOI] [PubMed] [Google Scholar]
- 45.Nersesyan H, Herman P, Erdogan E, et al. Relative changes in cerebral blood flow and neuronal activity in local microdomains during generalized seizures. J Cereb Blood Flow Metab. 2004;24:1057–1068. doi: 10.1097/01.WCB.0000131669.02027.3E. [DOI] [PubMed] [Google Scholar]
- 46.Vergnes M, Marescaux C, Depaulis A. Mapping of spontaneous spike and wave discharges in Wistar rats with genetic generalized non-convulsive epilepsy. Brain Res. 1990;523:87–91. doi: 10.1016/0006-8993(90)91638-w. [DOI] [PubMed] [Google Scholar]
- 47.Blumenfeld H. Cellular and network mechanisms of spike-wave seizures. Epilepsia. 2005;46(Suppl 9):21–33. doi: 10.1111/j.1528-1167.2005.00311.x. [DOI] [PubMed] [Google Scholar]
- 48.Meeren H, van Luijtelaar G, Lopes da Silva F, et al. Evolving concepts on the pathophysiology of absence seizures: the cortical focus theory. Arch Neurol. 2005;62:371–376. doi: 10.1001/archneur.62.3.371. [DOI] [PubMed] [Google Scholar]
- 49.Guey J, Tassinari CA, Charles C, et al. Variations du niveau d’efficience en relation avec des descharges epileptiques paroxystiques. Rev Neurol. 1965;112:311–317. [PubMed] [Google Scholar]
- 50.Schwab RS. Method of measuring consciousness in attacks of petit mal epilepsy. (Society Transactions: Boston Society of Psychiatry and Neurology, presented May 19, 1938) Arch Neurol Psychiatry. 1939;41:215–217. [Google Scholar]
- 51.Porter RJ, Penry JK. Responsiveness at the onset of spike-wave bursts. Electroencephalogr Clin Neurophysiol. 1973;34:239–245. doi: 10.1016/0013-4694(73)90251-4. [DOI] [PubMed] [Google Scholar]
- 52.Sellden U. Psychotechnical performance related to paroxysmal discharges in EEG. Clin Electroenceph. 1971;2 [Google Scholar]
- 53.Motelow JE, Blumenfeld H. Functional neuroimaging of spike-wave seizures. Methods Mol Biol. 2009;489(9):189–209. doi: 10.1007/978-1-59745-543-5_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Archer JS, Abbott DF, Waites AB, et al. fMRI “deactivation” of the posterior cingulate during generalized spike and wave. Neuroimage. 2003;20:1915–1922. doi: 10.1016/s1053-8119(03)00294-5. [DOI] [PubMed] [Google Scholar]
- 55.Danielson NB, Guo JN, Blumenfeld H. The default mode network and altered consciousness in epilepsy. Behav Neurol. 2011;24(1):55–65. doi: 10.3233/BEN-2011-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gotman J, Grova C, Bagshaw A, et al. Generalized epileptic discharges show thalamocortical activation and suspension of the default state of the brain. Proc Natl Acad Sci U S A. 2005;102:15236–15240. doi: 10.1073/pnas.0504935102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Salek-Haddadi A, Lemieux L, Merschhemke M, et al. Functional magnetic resonance imaging of human absence seizures. Ann Neurol. 2003;53:663–667. doi: 10.1002/ana.10586. [DOI] [PubMed] [Google Scholar]
- 58.Carney PW, Masterton RA, Harvey AS, et al. The core network in absence epilepsy. Differences in cortical and thalamic BOLD response. Neurology. 2010;75:904–911. doi: 10.1212/WNL.0b013e3181f11c06. [DOI] [PubMed] [Google Scholar]
- 59.Moeller F, Siebner HR, Wolff S, et al. Simultaneous EEG-fMRI in drug-naive children with newly diagnosed absence epilepsy. Epilepsia. 2008;49(9):1510–1519. doi: 10.1111/j.1528-1167.2008.01626.x. [DOI] [PubMed] [Google Scholar]
- 60.Moeller F, Siebner HR, Wolff S, et al. Changes in activity of striato-thalamo-cortical network precede generalized spike wave discharges. Neuroimage. 2008;39(4):1839–1849. doi: 10.1016/j.neuroimage.2007.10.058. [DOI] [PubMed] [Google Scholar]
- 61.Rathakrishnan R, Moeller F, Levan P, et al. BOLD signal changes preceding negative responses in EEG-fMRI in patients with focal epilepsy. Epilepsia. 2010;51(9):1837–1845. doi: 10.1111/j.1528-1167.2010.02643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mishra AM, Ellens DJ, Schridde U, et al. Where fMRI and electrophysiology agree to disagree: corticothalamic and striatal activity patterns in the WAG/Rij rat. J Neurosci. 2011 doi: 10.1523/JNEUROSCI.0101-11.2011. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moeller F, LeVan P, Muhle H, et al. Absence seizures: individual patterns revealed by EEG-fMRI. Epilepsia. 2010;51:2000–2010. doi: 10.1111/j.1528-1167.2010.02698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li Q, Luo C, Yang T, et al. EEG-fMRI study on the interictal and ictal generalized spike-wave discharges in patients with childhood absence epilepsy. Epilepsy Res. 2009;87:160–168. doi: 10.1016/j.eplepsyres.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 65.Moeller F, Muhle H, Wiegand G, et al. EEG-fMRI study of generalized spike and wave discharges without transitory cognitive impairment. Epilepsy Behav. 2010;18(3):313–316. doi: 10.1016/j.yebeh.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 66.Giacino J, Whyte J. The vegetative and minimally conscious states: current knowledge and remaining questions. J Head Trauma Rehabil. 2005;20:30–50. doi: 10.1097/00001199-200501000-00005. [DOI] [PubMed] [Google Scholar]
- 67.Giacino JT, Ashwal S, Childs N, et al. The minimally conscious state: definition and diagnostic criteria [see comment] Neurology. 2002;58:349–353. doi: 10.1212/wnl.58.3.349. [DOI] [PubMed] [Google Scholar]
- 68.Blumenfeld H, Varghese G, Purcaro MJ, et al. Cortical and subcortical networks in human secondarily generalized tonic-clonic seizures. Brain. 2009;132:999–1012. doi: 10.1093/brain/awp028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jobst BC, Williamson PD, Neuschwander TB, et al. Secondarily generalized seizures in mesial temporal epilepsy: clinical characteristics, lateralizing signs, and association with sleep-wake cycle. Epilepsia. 2001;42:1279–1287. doi: 10.1046/j.1528-1157.2001.09701.x. [DOI] [PubMed] [Google Scholar]
- 70.Theodore WH, Porter RJ, Albert P, et al. The secondarily generalized tonicclonic seizure: a videotape analysis. Neurology. 1994;44:1403–1407. doi: 10.1212/wnl.44.8.1403. [DOI] [PubMed] [Google Scholar]
- 71.Varghese G, Purcaro MJ, Motelow JE, et al. Clinical use of ictal SPECT in secondarily generalized tonic-clonic seizures. Brain. 2009;132(8):2102–2113. doi: 10.1093/brain/awp027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.DeSalvo MN, Schridde U, Mishra AM, et al. Focal BOLD fMRI changes in bicuculline-induced tonic-clonic seizures in the rat. Neuroimage. 2010;50:902–909. doi: 10.1016/j.neuroimage.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bell WL, Walczak TS, Shin C, et al. Painful generalised clonic and tonic-clonic seizures with retained consciousness. J Neurol Neurosurg Psychiatry. 1997;63:792–795. doi: 10.1136/jnnp.63.6.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Botez MI, Serbanescu T, Stoica I. The problem of focal epileptic seizures on both parts of the body without loss of consciousness. Psychiatr Neurol Neurochir. 1966;69:431–437. [PubMed] [Google Scholar]
- 75.Weinberger J, Lusins J. Simultaneous bilateral focal seizures without loss of consciousness. Mt Sinai J Med. 1973;40:693–696. [PubMed] [Google Scholar]
- 76.Schindler K, Leung H, Lehnertz K, et al. How generalised are secondarily “generalized” tonic clonic seizures? J Neurol Neurosurg Psychiatry. 2007;78:993–996. doi: 10.1136/jnnp.2006.108753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Takano H, Motohashi N, Uema T, et al. Changes in regional cerebral blood flow during acute electroconvulsive therapy in patients with depression: positron emission tomographic study. Br J Psychiatry. 2007;190:63–68. doi: 10.1192/bjp.bp.106.023036. [DOI] [PubMed] [Google Scholar]
- 78.Kim SH, Zubal IG, Blumenfeld H. Epilepsy localization by ictal and interictal SPECT. Chapter 10. In: Van Heertum RL, Ichise M, Tikofsky RS, editors. Functional cerebral SPECT and PET imaging 2009. Philadelpia: Lippincott Williams & Wilkins; 2009. pp. 131–148. [Google Scholar]
- 79.McNally KA, Paige AL, Varghese G, et al. Localizing value of ictal-interictal SPECT analyzed by SPM (ISAS) Epilepsia. 2005;46:1450–1464. doi: 10.1111/j.1528-1167.2005.06705.x. [DOI] [PubMed] [Google Scholar]
- 80.Bajc M, Medved V, Basic M, et al. Acute effect of electroconvulsive therapy on brain perfusion assessed by Tc99m-hexamethylpropyleneamineoxim and single photon emission computed tomography. Acta Psychiatr Scand. 1989;80:421–426. doi: 10.1111/j.1600-0447.1989.tb03000.x. [DOI] [PubMed] [Google Scholar]
- 81.Blumenfeld H, McNally KA, Ostroff RB, et al. Targeted prefrontal cortical activation with bifrontal ECT. Psychiatry Res. 2003;123:165–170. doi: 10.1016/s0925-4927(03)00073-8. [DOI] [PubMed] [Google Scholar]
- 82.Blumenfeld H, Westerveld M, Ostroff RB, et al. Selective frontal, parietal and temporal networks in generalized seizures. Neuroimage. 2003;19:1556–1566. doi: 10.1016/s1053-8119(03)00204-0. [DOI] [PubMed] [Google Scholar]
- 83.Enev M, McNally KA, Varghese G, et al. Imaging onset and propagation of ECT-induced seizures. Epilepsia. 2007;48:238–244. doi: 10.1111/j.1528-1167.2007.00919.x. [DOI] [PubMed] [Google Scholar]
- 84.McNally KA, Blumenfeld H. Focal network involvement in generalized seizures: new insights from electroconvulsive therapy. Epilepsy Behav. 2004;5:3–12. doi: 10.1016/j.yebeh.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 85.Vollmer-Haase J, Folkerts HW, Haase CG, et al. Cerebral hemodynamics during electrically induced seizures. Neuroreport. 1998;9:407–410. doi: 10.1097/00001756-199802160-00009. [DOI] [PubMed] [Google Scholar]
- 86.Lee BI, Markand ON, Wellman HN, et al. HIPDM single photon emission computed tomography brain imaging in partial onset secondarily generalized tonic-clonic seizures. Epilepsia. 1987;28:305–311. doi: 10.1111/j.1528-1157.1987.tb04223.x. [DOI] [PubMed] [Google Scholar]
- 87.Rowe CC, Berkovic SF, Sia ST, et al. Localization of epileptic foci with postictal single photon emission computed tomography. Ann Neurol. 1989;26:660–668. doi: 10.1002/ana.410260512. [DOI] [PubMed] [Google Scholar]
- 88.Shin WC, Hong SB, Tae WS, et al. Ictal hyperperfusion patterns according to the progression of temporal lobe seizures. Neurology. 2002;58:373–380. doi: 10.1212/wnl.58.3.373. [DOI] [PubMed] [Google Scholar]
- 89.Salgado-Benitez A, Briones R, Fernandez-Guardiola A. Purkinje cell responses to a cerebral penicillin-induced epileptogenic focus in the cat. Epilepsia. 1982;23:597–606. doi: 10.1111/j.1528-1157.1982.tb05074.x. [DOI] [PubMed] [Google Scholar]
- 90.ILAE. Proposal for revised clinical and electroencephalographic classification of epileptic seizures. From the Commission on Classification and Terminology of the International League Against Epilepsy. Epilepsia. 1981;22:489–501. doi: 10.1111/j.1528-1157.1981.tb06159.x. [DOI] [PubMed] [Google Scholar]
- 91.Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010;51:676–685. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- 92.Escueta AV, Kunze U, Waddell G, et al. Lapse of consciousness and automatisms in temporal lobe epilepsy: a videotape analysis. Neurology. 1977;27:144–155. doi: 10.1212/wnl.27.2.144. [DOI] [PubMed] [Google Scholar]
- 93.American Congress of Rehabilitation Medicine. Recommendations for use of uniform nomenclature pertinent to patients with severe alterations in consciousness, American Congress of Rehabilitation Medicine [see comment] [erratum appears in Arch Phys Med Rehabil 1995 Apr;76(4):397] Arch Phys Med Rehabil. 1995;76:205–209. doi: 10.1016/s0003-9993(95)80031-x. [DOI] [PubMed] [Google Scholar]
- 94.Blumenfeld H. The neurologic examination of consciousness. Chapter 2. In: Laureys S, editor. The neurology of consciousness. G Tononi Elsevier, Ltd; 2008. pp. 15–30. [Google Scholar]
- 95.Majerus S, Gill-Thwaites H, Andrews K, et al. Behavioral evaluation of consciousness in severe brain damage. Prog Brain Res. 2005;150:397–413. doi: 10.1016/S0079-6123(05)50028-1. [DOI] [PubMed] [Google Scholar]
- 96.Devous MD, Sr, Thisted RA, Morgan GF, et al. SPECT brain imaging in epilepsy: a meta-analysis. J Nucl Med. 1998;39:285–293. [PubMed] [Google Scholar]
- 97.Blumenfeld H, McNally KA, Vanderhill SD, et al. Positive and negative network correlations in temporal lobe epilepsy. Cereb Cortex. 2004;14:892–902. doi: 10.1093/cercor/bhh048. [DOI] [PubMed] [Google Scholar]
- 98.Chang DJ, Zubal IG, Gottschalk C, et al. Comparison of statistical parametric mapping and SPECT difference imaging in patients with temporal lobe epilepsy. Epilepsia. 2002;43:68–74. doi: 10.1046/j.1528-1157.2002.21601.x. [DOI] [PubMed] [Google Scholar]
- 99.Menzel C, Grunwald F, Klemm E, et al. Inhibitory effects of mesial temporal partial seizures onto frontal neocortical structures. Acta Neurol Belg. 1998;98:327–331. [PubMed] [Google Scholar]
- 100.Rabinowicz AL, Salas E, Beserra F, et al. Changes in regional cerebral blood flow beyond the temporal lobe in unilateral temporal lobe epilepsy. Epilepsia. 1997;38:1011–1014. doi: 10.1111/j.1528-1157.1997.tb01484.x. [DOI] [PubMed] [Google Scholar]
- 101.Van Paesschen W, Dupont P, Van Driel G, et al. SPECT perfusion changes during complex partial seizures in patients with hippocampal sclerosis. Brain. 2003;126:1103–1111. doi: 10.1093/brain/awg108. [DOI] [PubMed] [Google Scholar]
- 102.Hogan RE, Kaiboriboon K, Bertrand ME, et al. Composite SISCOM perfusion patterns in right and left temporal seizures. Arch Neurol. 2006;63:1419–1426. doi: 10.1001/archneur.63.10.1419. [DOI] [PubMed] [Google Scholar]
- 103.Lee KH, Meador KJ, Park YD, et al. Pathophysiology of altered consciousness during seizures: subtraction SPECT study [comment] Neurology. 2002;59:841–846. doi: 10.1212/wnl.59.6.841. [DOI] [PubMed] [Google Scholar]
- 104.Mayanagi Y, Watanabe E, Kaneko Y. Mesial temporal lobe epilepsy: clinical features and seizure mechanism. Epilepsia. 1996;37(Suppl 3):57–60. doi: 10.1111/j.1528-1157.1996.tb01823.x. [DOI] [PubMed] [Google Scholar]
- 105.Tae WS, Joo EY, Kim JH, et al. Cerebral perfusion changes in mesial temporal lobe epilepsy: SPM analysis of ictal and interictal SPECT. Neuroimage. 2005;24:101–110. doi: 10.1016/j.neuroimage.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 106.Blumenfeld H, Rivera M, McNally KA, et al. Ictal neocortical slowing in temporal lobe epilepsy. Neurology. 2004;63:1015–1021. doi: 10.1212/01.wnl.0000141086.91077.cd. [DOI] [PubMed] [Google Scholar]
- 107.Englot DJ, Yang L, Hamid H, et al. Impaired consciousness in temporal lobe seizures: role of cortical slow activity. Brain. 2010;133(Pt 12):3764–3777. doi: 10.1093/brain/awq316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Englot DJ, Blumenfeld H. Consciousness and epilepsy: why are complex-partial seizures complex? Prog Brain Res. 2009;177:147–170. doi: 10.1016/S0079-6123(09)17711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Haider B, Duque A, Hasenstaub AR, et al. Neocortical network activity in vivo is generated through a dynamic balance of excitation and inhibition. J Neurosci. 2006;26:4535–4545. doi: 10.1523/JNEUROSCI.5297-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Steriade M, Contreras D, Curro Dossi R, et al. The slow (<1 Hz) oscillation in reticular thalamic and thalamocortical neurons: scenario of sleep rhythm generation in interacting thalamic and neocortical networks. J Neurosci. 1993;13:3284–3299. doi: 10.1523/JNEUROSCI.13-08-03284.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Blumenfeld H, Taylor J. Why do seizures cause loss of consciousness? Neuroscientist. 2003;9:301–310. doi: 10.1177/1073858403255624. [DOI] [PubMed] [Google Scholar]
- 112.Englot DJ, Mishra AM, Mansuripur PK, et al. Remote effects of focal hippocampal seizures on the rat neocortex. J Neurosci. 2008;28(36):9066–9081. doi: 10.1523/JNEUROSCI.2014-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Englot DJ, Modi B, Mishra AM, et al. Cortical deactivation induced by subcortical network dysfunction in limbic seizures. J Neurosci. 2009;29(41):13006–13018. doi: 10.1523/JNEUROSCI.3846-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Norden AD, Blumenfeld H. The role of subcortical structures in human epilepsy. Epilepsy Behav. 2002;3:219–231. doi: 10.1016/s1525-5050(02)00029-x. [DOI] [PubMed] [Google Scholar]
- 115.Yu L, Blumenfeld H. Theories of impaired consciousness in epilepsy. Ann N Y Acad Sci. 2009;1157:48–60. doi: 10.1111/j.1749-6632.2009.04472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sperling MR. The consequences of uncontrolled epilepsy. CNS Spectr. 2004;9:98–101. doi: 10.1017/s1092852900008464. [DOI] [PubMed] [Google Scholar]
- 117.Vickrey BG, Berg AT, Sperling MR, et al. Relationships between seizure severity and health-related quality of life in refractory localization-related epilepsy. Epilepsia. 2000;41:760–764. doi: 10.1111/j.1528-1157.2000.tb00239.x. [DOI] [PubMed] [Google Scholar]
- 118.Elliott IM, Lach L, Smith ML. I just want to be normal: a qualitative study exploring how children and adolescents view the impact of intractable epilepsy on their quality of life. Epilepsy Behav. 2005;7:664–678. doi: 10.1016/j.yebeh.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 119.Morrell MJ. Stigma and epilepsy. Epilepsy Behav. 2002;3(6 S 2):21–25. doi: 10.1016/s1525-5050(02)00547-4. [DOI] [PubMed] [Google Scholar]
- 120.Hansotia P, Broste SK. The effect of epilepsy or diabetes mellitus on the risk of automobile accidents [see comment] N Engl J Med. 1991;324:22–26. doi: 10.1056/NEJM199101033240105. [DOI] [PubMed] [Google Scholar]
- 121.Lossius R, Kinge E, Nakken KO. Epilepsy and driving: considerations on how eligibility should be decided. Acta Neurol Scand Suppl. 2010;190:67–71. doi: 10.1111/j.1600-0404.2010.01379.x. [DOI] [PubMed] [Google Scholar]
- 122.Krauss GL, Krumholz A, Carter RC, et al. Risk factors for seizure-related motor vehicle crashes in patients with epilepsy [see comment] Neurology. 1999;52:1324–1329. doi: 10.1212/wnl.52.7.1324. [DOI] [PubMed] [Google Scholar]
- 123.Sheth SG, Krauss G, Krumholz A, et al. Mortality in epilepsy: driving fatalities vs other causes of death in patients with epilepsy [summary for patients in Neurology. 2004 Sep 28;63(6):E12–13; PMID: 15452331] Neurology. 2004;63:1002–1007. doi: 10.1212/01.wnl.0000138590.00074.9a. [DOI] [PubMed] [Google Scholar]
- 124.Taylor J, Chadwick D, Johnson T. Risk of accidents in drivers with epilepsy. J Neurol Neurosurg Psychiatry. 1996;60:621–627. doi: 10.1136/jnnp.60.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Berkovic SF. Epilepsy syndromes: effects on cognition, performance and driving ability. Med Law. 2000;19:757–761. [PubMed] [Google Scholar]
- 126.Gastaut H, Zifkin BG. The risk of automobile accidents with seizures occurring while driving: relation to seizure type. Neurology. 1987;37:1613–1616. doi: 10.1212/wnl.37.10.1613. [DOI] [PubMed] [Google Scholar]
- 127.Yang L, Morland TB, Schmits K, et al. A prospective study of loss of consciousness in epilepsy using virtual reality driving simulation and other video games. Epilepsy Behav. 2010;18:238–246. doi: 10.1016/j.yebeh.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Arias-Carrión O, Palomero-Rivero M, Millán-Aldaco D, et al. Infusion of modafinil into anterior hypothalamus or pedunculopontine tegmental nucleus at different time-points enhances waking and blocks the expression of recovery sleep in rats after sleep deprivation. Exp Neurol. 2011;229(2):358–363. doi: 10.1016/j.expneurol.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 129.Ballon JS, Feifel D. A systematic review of modafinil: potential clinical uses and mechanisms of action. J Clin Psychiatry. 2006;67:554–566. doi: 10.4088/jcp.v67n0406. [DOI] [PubMed] [Google Scholar]
- 130.Fisher R, Salanova V, Witt T, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51:899–908. doi: 10.1111/j.1528-1167.2010.02536.x. [DOI] [PubMed] [Google Scholar]
- 131.Jobst BC, Darcey TM, Thadani VM, et al. Brain stimulation for the treatment of epilepsy. Epilepsia. 2010;51(Suppl 3):88–92. doi: 10.1111/j.1528-1167.2010.02618.x. [DOI] [PubMed] [Google Scholar]
- 132.Kahane P, Depaulis A. Deep brain stimulation in epilepsy: what is next? Curr Opin Neurol. 2010;23:177–182. doi: 10.1097/WCO.0b013e3283374a39. [DOI] [PubMed] [Google Scholar]
- 133.Morrell M. Brain stimulation for epilepsy: can scheduled or responsive neurostimulation stop seizures? Curr Opin Neurol. 2006;19:164–168. doi: 10.1097/01.wco.0000218233.60217.84. [DOI] [PubMed] [Google Scholar]
- 134.Theodore WH, Fisher RS. Brain stimulation for epilepsy [erratum appears in Lancet Neurol. 2004 Jun;3(6)332] Lancet Neurol. 2004;3:111–118. doi: 10.1016/s1474-4422(03)00664-1. [DOI] [PubMed] [Google Scholar]
- 135.Schiff ND, Giacino JT, Kalmar K, et al. Behavioural improvements with thalamic stimulation after severe traumatic brain injury [see comment] [erratum appears in Nature. 2008 Mar 6;452(7183):120 Note: Biondi, T [added]] Nature. 2007;448:600–603. doi: 10.1038/nature06041. [DOI] [PubMed] [Google Scholar]
- 136.Yamamoto T, Katayama Y. Deep brain stimulation therapy for the vegetative state. Neuropsychol Rehabil. 2005;15:406–413. doi: 10.1080/09602010443000353. [DOI] [PubMed] [Google Scholar]
- 137.Easton A, Zinkivskay A, Eacott MJ. Recollection is impaired, but familiarity remains intact in rats with lesions of the fornix. Hippocampus. 2009;19:837–843. doi: 10.1002/hipo.20567. [DOI] [PubMed] [Google Scholar]
- 138.Heilman KM, Sypert GW. Korsakoff’s syndrome resulting from bilateral fornix lesions. Neurology. 1977;27:490–493. doi: 10.1212/wnl.27.5.490. [DOI] [PubMed] [Google Scholar]
- 139.Garcia-Bengochea F, Friedman WA. Persistent memory loss following section of the anterior fornix in humans. A historical review. Surg Neurol. 1987;27:361–364. doi: 10.1016/0090-3019(87)90012-7. [DOI] [PubMed] [Google Scholar]
- 140.Blum DE, Eskola J, Bortz JJ, et al. Patient awareness of seizures. Neurology. 1996;47:260–264. doi: 10.1212/wnl.47.1.260. [DOI] [PubMed] [Google Scholar]
- 141.Cavanna AE, Mula M, Servo S, et al. Measuring the level and content of consciousness during epileptic seizures: the Ictal Consciousness Inventory. Epilepsy Behav. 2008;13:184–188. doi: 10.1016/j.yebeh.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 142.Hoppe C, Poepel A, Elger CE. Epilepsy: accuracy of patient seizure counts. Arch Neurol. 2007;64:1595–1599. doi: 10.1001/archneur.64.11.1595. [DOI] [PubMed] [Google Scholar]
- 143.Kerling F, Mueller S, Pauli E, et al. When do patients forget their seizures? An electroclinical study. Epilepsy Behav. 2006;9:281–285. doi: 10.1016/j.yebeh.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 144.Tatum WO, Winters L, Gieron M, et al. Outpatient seizure identification: results of 502 patients using computer-assisted ambulatory EEG. J Clin Neurophysiol. 2001;18:14–19. doi: 10.1097/00004691-200101000-00004. [DOI] [PubMed] [Google Scholar]
- 145.Yang L, Shklyar I, Lee HW, et al. Impaired consciousness in epilepsy investigated by a prospective responsiveness in epilepsy scale (RES) Epilepsia. 2011 doi: 10.1111/j.1528-1167.2011.03341.x. In review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Detyniecki K, Yang L, Enamandram S, et al. AES meeting. San Antonio, TX: 2010. Dec 3–7, Seizure recognition during inpatient Video/EEG Monitoring. [abstracts 1382]. Available at: http://www.aesnet.org/. [Google Scholar]