Abstract
Initiator methionine tRNA from the cytoplasm of Neurospora crassa has been purified and sequenced. The sequence is: pAGCUGCAUm1GGCGCAGCGGAAGCGCM22GCY*GGGCUCAUt6AACCCGGAGm7GU (or D) - CACUCGAUCGm1AAACGAG*UUGCAGCUACCAOH. Similar to initiator tRNAs from the cytoplasm of other eukaryotes, this tRNA also contains the sequence -AUCG- instead of the usual -TphiCG (or A)- found in loop IV of other tRNAs. The sequence of the N. crassa cytoplasmic initiator tRNA is quite different from that of the corresponding mitochondrial initiator tRNA. Comparison of the sequence of N. crassa cytoplasmic initiator tRNA to those of yeast, wheat germ and vertebrate cytoplasmic initiator tRNA indicates that the sequences of the two fungal tRNAs are no more similar to each other than they are to those of other initiator tRNAs.
Full text
PDF







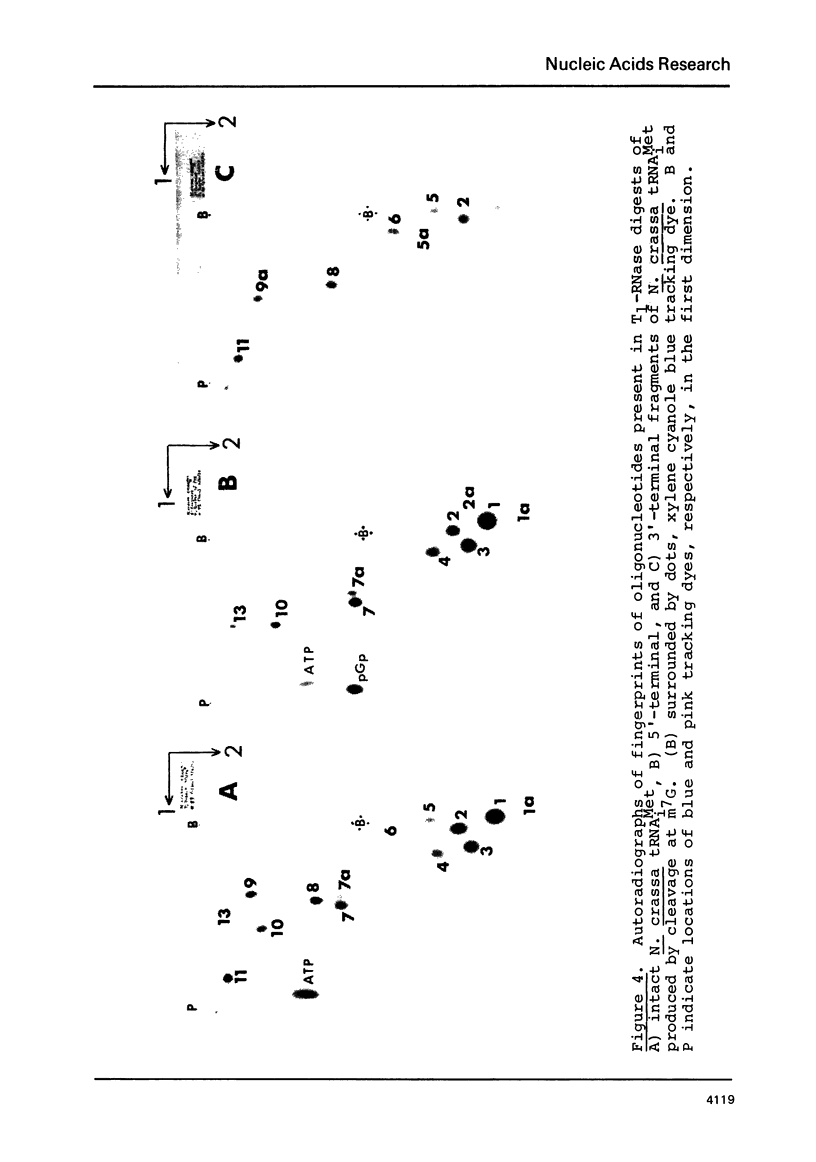
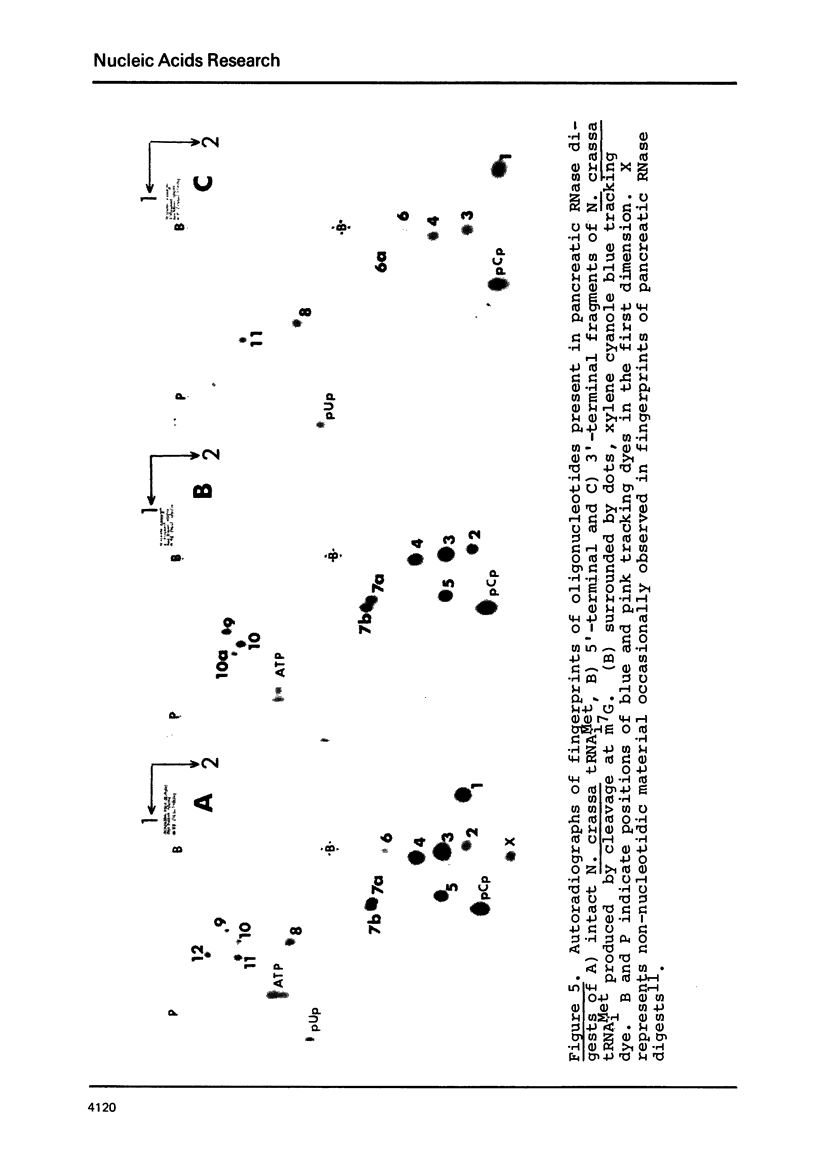











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOLLUM F. J. Thermal conversion of nonpriming deoxyribonucleic acid to primer. J Biol Chem. 1959 Oct;234:2733–2734. [PubMed] [Google Scholar]
- Barnett W. E., Brown D. H. Mitochondrial transfer ribonucleic acids. Proc Natl Acad Sci U S A. 1967 Feb;57(2):452–458. doi: 10.1073/pnas.57.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett W. E., Epler J. L. Multiple aminoacyl-RNA synthetase systems and the genetic code in neurospora. Cold Spring Harb Symp Quant Biol. 1966;31:549–555. doi: 10.1101/sqb.1966.031.01.071. [DOI] [PubMed] [Google Scholar]
- Chang S. H., Kuo S., Hawkins E., Miller N. R. The corrected nucleotide sequence of yeast leucine transfer ribonucleic acid. Biochem Biophys Res Commun. 1973 Apr 16;51(4):951–955. doi: 10.1016/0006-291x(73)90019-3. [DOI] [PubMed] [Google Scholar]
- Delk A. S., Rabinowitz J. C. Partial nucleotide sequence of a prokaryote initiator tRNA that functions in its non-formylated form. Nature. 1974 Nov 8;252(5479):106–109. doi: 10.1038/252106a0. [DOI] [PubMed] [Google Scholar]
- Dube S. K., Marcker K. A., Clark B. F., Cory S. Nucleotide sequence of N-formyl-methionyl-transfer RNA. Nature. 1968 Apr 20;218(5138):232–233. doi: 10.1038/218232a0. [DOI] [PubMed] [Google Scholar]
- Dube S. K., Rudland P. S., Clark B. F., Marcker K. A. A structural requirement for codon-anticodon interaction on the ribosome. Cold Spring Harb Symp Quant Biol. 1969;34:161–166. doi: 10.1101/sqb.1969.034.01.023. [DOI] [PubMed] [Google Scholar]
- Ecarot B., Cedergren R. J. Structure-function correlations of formylmethionine transfer RNA. Biochem Biophys Res Commun. 1974 Jul 10;59(1):400–405. doi: 10.1016/s0006-291x(74)80220-2. [DOI] [PubMed] [Google Scholar]
- Epler J. L., Shugart L. R., Barnett W. E. N-formylmethionyl transfer ribonucleic acid in mitochondria from Neurospora. Biochemistry. 1970 Sep 1;9(18):3575–3579. doi: 10.1021/bi00820a011. [DOI] [PubMed] [Google Scholar]
- Fairfield S. A., Barnett W. E. On the similarity between the tRNAs of organelles and prokaryotes. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2972–2976. doi: 10.1073/pnas.68.12.2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh K., Ghosh H. P., Simsek M., Raj Bhandary U. L. Initiator methionine transfer ribonucleic acid from wheat embryo. Purification, properties, and partial nucleotide sequences. J Biol Chem. 1974 Aug 10;249(15):4720–4729. [PubMed] [Google Scholar]
- Gillam I., Blew D., Warrington R. C., von Tigerstrom M., Tener G. M. A general procedure for the isolation of specific transfer ribonucleic acids. Biochemistry. 1968 Oct;7(10):3459–3468. doi: 10.1021/bi00850a022. [DOI] [PubMed] [Google Scholar]
- Gillum A. M., Roe B. A., Anandaraj M. P., RajBhandary U. L. Nucleotide sequence of human placenta cytoplasmic initiator tRNA. Cell. 1975 Nov;6(3):407–413. doi: 10.1016/0092-8674(75)90190-7. [DOI] [PubMed] [Google Scholar]
- Gillum A. M., Urquhart N., Smith M., RajBhandary U. L. Nucleotide sequence of salmon testes and salmon liver cytoplasmic initiator tRNA. Cell. 1975 Nov;6(3):395–405. doi: 10.1016/0092-8674(75)90189-0. [DOI] [PubMed] [Google Scholar]
- Hashimoto S., Sakai M., Muramatsu M. 2'-O-methylated oligonucleotides in ribosomal 18S and 28S RNA of a mouse hepatoma, MH 134. Biochemistry. 1975 May 6;14(9):1956–1964. doi: 10.1021/bi00680a024. [DOI] [PubMed] [Google Scholar]
- Hecker L. I., Uziel M., Barnett W. E. Comparative base compositions of chloroplast and cytoplasmic tRNAPhe's from Euglena gracilis. Nucleic Acids Res. 1976 Feb;3(2):371–380. doi: 10.1093/nar/3.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes W. M., Hurd R. E., Reid B. R., Rimerman R. A., Hatfield G. W. Separation of transfer ribonucleic acid by sepharose chromatography using reverse salt gradients. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1068–1071. doi: 10.1073/pnas.72.3.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazumi T., Yoshino G., Fujii S., Baba S. Tumorigenic action of streptozotocin on the pancreas and kidney in male Wistar rats. Cancer Res. 1978 Jul;38(7):2144–2147. [PubMed] [Google Scholar]
- Kowalski S., Yamane T., Fresco J. R. Nucleotide sequence of the "denaturable" leucine transfer RNA from yeast. Science. 1971 Apr 23;172(3981):385–387. doi: 10.1126/science.172.3981.385. [DOI] [PubMed] [Google Scholar]
- Pearson R. L., Weiss J. F., Kelmers A. D. Improved separation of transfer RNA's on polychlorotrifuoroethylene-supported reversed-phase chromatography columns. Biochim Biophys Acta. 1971 Feb 11;228(3):770–774. doi: 10.1016/0005-2787(71)90748-9. [DOI] [PubMed] [Google Scholar]
- Piper P. W., Clark B. F. Primary structure of a mouse myeloma cell initiator transfer RNA. Nature. 1974 Feb 22;247(5442):516–518. doi: 10.1038/247516a0. [DOI] [PubMed] [Google Scholar]
- RajBhandary U. L., Ghosh H. P. Studies on polynucleotides. XCI. Yeast methionine transfer ribonucleic acid: purification, properties, and terminal nucleotide sequences. J Biol Chem. 1969 Mar 10;244(5):1104–1113. [PubMed] [Google Scholar]
- RajBhandary U. L., Kumar A. A formylatable methionine transfer ribonucleic acid from yeast: comparison of coding properties and sequences around the anticodon with Escherichia coli formylatable methionine transfer RNA. J Mol Biol. 1970 Jun 28;50(3):707–711. doi: 10.1016/0022-2836(70)90095-1. [DOI] [PubMed] [Google Scholar]
- Reiner A. M. Isolation and mapping of polynucleotide phosphorylase mutants of Escherichia coli. J Bacteriol. 1969 Mar;97(3):1431–1436. doi: 10.1128/jb.97.3.1431-1436.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich A., RajBhandary U. L. Transfer RNA: molecular structure, sequence, and properties. Annu Rev Biochem. 1976;45:805–860. doi: 10.1146/annurev.bi.45.070176.004105. [DOI] [PubMed] [Google Scholar]
- Sanger F., Donelson J. E., Coulson A. R., Kössel H., Fischer D. Use of DNA polymerase I primed by a synthetic oligonucleotide to determine a nucleotide sequence in phage fl DNA. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1209–1213. doi: 10.1073/pnas.70.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberklang M., Prochiantz A., Haenni A. L., Rajbhandary U. L. Studies on the sequence of the 3'-terminal region of turnip-yellow-mosaic-virus RNA. Eur J Biochem. 1977 Feb;72(3):465–478. doi: 10.1111/j.1432-1033.1977.tb11270.x. [DOI] [PubMed] [Google Scholar]
- Simsek M., Petrissant G., Rajbhandary U. L. Replacement of the sequence G-T-phi-C-G(A)- by G-A-U-C-G- in initiator transfer RNA of rabbit-liver cytoplasm. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2600–2604. doi: 10.1073/pnas.70.9.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simsek M., RajBhandary U. L., Boisnard M., Petrissant G. Nucleotide sequence of rabbit liver and sheep mammary gland cytoplasmic initiatory transfer RNAs. Nature. 1974 Feb 22;247(5442):518–520. doi: 10.1038/247518a0. [DOI] [PubMed] [Google Scholar]
- Simsek M., RajBhandary U. L. The primary structure of yeast initiator transfer ribonucleic acid. Biochem Biophys Res Commun. 1972 Oct 17;49(2):508–515. doi: 10.1016/0006-291x(72)90440-8. [DOI] [PubMed] [Google Scholar]
- Simsek M., Ziegenmeyer J., Heckman J., Rajbhandary U. L. Absence of the sequence G-T-psi-C-G(A)- in several eukaryotic cytoplasmic initiator transfer RNAs. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1041–1045. doi: 10.1073/pnas.70.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker R. T., RajBhandary U. L. Formylatable methionine transfer RNA from Mycoplasma: purification and comparison of partial nucleotide sequences with those of other prokaryotic initiator tRNAs. Nucleic Acids Res. 1975 Jan;2(1):61–78. doi: 10.1093/nar/2.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K., Oshima T., Nishimura S. CD spectra of 5-methyl-2-thiouridine in tRNA-Met-f from an extreme thermophile. Nucleic Acids Res. 1976 Jul;3(7):1703–1713. doi: 10.1093/nar/3.7.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegnez M., Mazabraud A., Denis H., Pétrissant G., Boisnard M. Biochemical research on oogenesis. Nucleotide sequence of initiator tRNA from oocytes and from somatic cells of Xenopus laevis. Eur J Biochem. 1975 Dec 1;60(1):295–302. doi: 10.1111/j.1432-1033.1975.tb21003.x. [DOI] [PubMed] [Google Scholar]
- Wintermeyer W., Thiebe R., Zachau H. G. Amin-katalysierte Spaltung von Phenylalanin-spezifischer tRNA nach Baseneliminierung. Hoppe Seylers Z Physiol Chem. 1972 Oct;353(10):1625–1632. [PubMed] [Google Scholar]
- Yamada Y., Ishikura H. Nucleotide sequence of initiator tRNA from Bacillus subtilis. FEBS Lett. 1975 Jun 15;54(2):155–158. doi: 10.1016/0014-5793(75)80064-0. [DOI] [PubMed] [Google Scholar]