Abstract
Loss of estrogen in women following menopause is associated with increased risk for cognitive decline, dementia and depression, all of which can be prevented by estradiol replacement. The dentate gyrus plays an important role in cognition, learning and memory. The gatekeeping function of the dentate gyrus to filter incoming activity into the hippocampus is modulated by estradiol in a frequency-dependent manner and involves activation of metabotropic glutamate receptors (mGluR). In the present study, we investigated whether estradiol (EB) modulates the metaplastic effect of inducing synaptic long-term potentiation (LTP) on subsequent propensity for expression of larger LTP in the dentate gyrus. At medial perforant path-dentate granule cell synapses in hippocampal slices of ovariectomized female rats, EB replacement was critical for an initial induction of LTP to enhance the magnitude of subsequent LTP elicited by a second high-frequency stimulation, metaplasticity, which was not present in slices from oil-treated control animals. EB enhanced expression of group I mGluRs, and the metaplastic effect of EB on LTP required activation of group I mGluRs that led to Src-family tyrosine kinase-mediated phosphorylation of NR2B subunits of N-methyl-D-aspartate receptors (NMDAR) that enhanced the magnitude of NMDAR-dependent LTP. Our data show that EB effects on LTP in the hippocampal dentate gyrus require activation of group I mGluRs, which in turn leads to functional metaplastic regulation of NR2B subunit-containing NMDARs, as opposed to direct effects of EB on NMDARs.
Keywords: β-estradiol, female, dentate gyrus, LTP, metabotropic glutamate receptors, NMDA receptors, NR2B subunit
Introduction
In recent years, an increasing number of young women are receiving preventative pre-menopausal ovariectomy for benign gynecological disorders. If estrogen replacement is not given after this sudden decline in estrogen levels, the procedure is associated with serious health consequences, including increased risk for cognitive decline, dementia and mental disorders such as depression or schizophrenia (Ostlund et al., 2003; Sherwin, 2005a; Kulkarni et al., 2008; Rocca et al., 2008).
Modulatory effects of β-estradiol (EB) on cognition and depression are quite complex and not well understood (Osterlund et al., 2005; Sherwin, 2005b; Rocca et al., 2008), although the effects of EB on structural plasticity and neurophysiological function in the hippocampal CA1 region have been extensively studied during the past two decades (Dumitriu et al., 2010). In field CA1, EB induces enhancement of the magnitude of activity-dependent long-term potentiation (LTP), which involves a functional upregulation of NR2B subunit-containing N-methyl-D-aspartate receptors (NMDARs) (Smith and McMahon, 2006). However, a recent study failed to identify any changes in NMDAR protein levels or baseline phosphorylation of the NR2B subunit following EB administration (Snyder et al., 2011). Thus, was unclear how EB modulates NR2B subunit-mediated effects on Schaffer collateral-CA1 LTP in the hippocampus.
Even less is known about the effects of EB in the hippocampal dentate gyrus (Velíšková, 2007), although estrogen receptors are expressed abundantly on granule and hilar neurons (Milner et al., 2001; Milner et al., 2005). The dentate gyrus plays an important role in cognitive processing and learning and memory by filtering and encoding sensory inputs from the entorhinal cortex for delivery into the hippocampal formation (Steele and Morris, 1999; Kesner, 2007; Sahay et al., 2007). We have previously shown in ovariectomized rats that chronic administration of 17-β-estradiol, at doses that produce plasma levels within a physiological range, enhances inhibition of dentate granule cells in a frequency-dependent manner (Velíšková and Velíšek, 2007). This EB-regulated gating of information flow through the dentate gyrus could play an important role in physiological information processing leading to formation of activity-dependent long-term synaptic plasticity such as LTP. At dentate gyrus perforant path-granule cell synapses, activation of NMDARs is essential for the induction of LTP, with specific involvement of NR2B subunit-containing NMDARs (Chen et al., 2009; Foster et al., 2010).
We have identified that one of the mechanisms by which EB modulates granule cell excitability in female rats involves activation of metabotropic glutamate receptors (mGluRs) (Velíšková and Velíšek, 2007). Previous studies have shown that, in the male rat dentate gyrus, a single standard high frequency stimulation (HFS) does not lead to activation of mGluRs, while repeated strong HFS trains cause initial group I mGluR activation that enhances NMDAR-dependent LTP evoked by subsequent HFS trains (Wu et al., 2008; Welsby et al., 2006). Rather than being obligatory for induction of LTP, mGluRs appear to play a modulatory and metaplastic role, thus contributing to a “higher order form of plasticity” (Abraham and Bear, 1996; Abraham, 2008) that shifts the magnitude of NMDAR-dependent LTP in the dentate gyrus [for detailed review see (Anwyl, 2009)]. There is a physical linkage between NMDARs and group I mGluRs within the postsynaptic density. While this association can allow for mGluR-NMDAR interactions, including src tyrosine kinase-dependent phosphorylation of NR2B subunit, resulting in potentiation of NMDA currents (Tu et al., 1999; Guo et al., 2004), the role of such interactions in mGluR-dependent metaplasticity is unknown.
In this study we hypothesized that, in the female dentate gyrus, EB might be a critical molecule in enabling mGluR-dependent metaplasticity of LTP. To test this, we used an established stimulation paradigm known to enhance medial perforant path LTP by activating mGluRs in male rodents (Wu et al., 2008). We tested the hypotheses that EB-dependent upregulation of LTP could lead to selective potentiation of NMDARs by selectively altering activation of NR2B-containing NMDARs, and that this metaplasticity might be driven by mGluR-mediated activation of src kinase-dependent NR2B phosphorylation.
Methods
Animals
Experiments were carried out according to the NIH Revised Guide for the Care and Use of Laboratory Animals and approved by the Albert Einstein College of Medicine and New York Medical College Animal Care and Use Committees. We used 8–9 week old female Sprague-Dawley rats (Taconic Farms) kept on a 12-h light/dark cycle (lights on at 7:00) with food (NIH#31 Rodent Diet) and water ad libitum. Rats were ovariectomized under ketamine/xylazine anesthesia (50/7 mg/kg i.p.). Estradiol replacement started one week later. Sterile peanut oil (0.1 ml; vehicle controls) or 17β-estradiol benzoate (EB, 2 μg/0.1 ml/day; Sigma-Aldrich, St. Louis, MO) were injected once a day subcutaneously (SC) at 10:00 for 4 consecutive days (Velíšková and Velíšek, 2007). This treatment produces plasma levels of estradiol within a physiological range (Neal-Perry et al., 2005). Control animals received sterile peanut oil injections (0.1 ml SC) with the same timing. Measurements of vaginal impedance confirmed both successful ovariectomy and EB treatment (Velíšek et al., 2006).
Electrophysiology
Rats were decapitated under deep CO2 anesthesia 24 hours after the last EB or oil injection.
Extracellular recordings
Transverse combined entorhinal cortex-hippocampal slices (400 μm thick) were cut at the mid rostro-caudal level with a vibratome (Leica VT1000) in ice-cold artificial cerebrospinal fluid (aCSF; in mM: NaCl 126, KCl 5, NaH2PO4 1.25, MgCl2 2, CaCl2 2, NaHCO3 26, and glucose 10; pH 7.4). Slices were pre-incubated in an interface chamber in pre-warmed (34–35°C) and oxygenated (5% CO2/95% O2) aCSF for at least one hour before commencing recording. Picrotoxin (50 μM) was added to the aCSF to block GABAA receptor-mediated transmission (Wigstrom and Gustafsson, 1983). Medial perforant path-evoked field excitatory postsynaptic potentials (fEPSPs) were recorded with a glass micropipette (2 M NaCl; 2–5 M!) placed in the middle third of the molecular layer. Paired-pulse stimulation at 50 ms interstimulus interval was used to verify activation of the medial perforant pathway, the criterion being that they exhibit paired-pulse inhibition, with the second response being smaller than the first (Colino and Malenka, 1993). Baseline fEPSPs were recorded using a stimulus intensity that evoked ~30% of the maximal fEPSP slope. Test pulses were delivered to medial perforant path axons once each 30 seconds with a bipolar stainless-steel stimulating electrode (FHC, Inc.). In the dentate gyrus, repeated strong high-frequency burst stimulation paradigms (HFS) produce activation of group I mGluRs and lead to greater LTP than that induced by a single HFS, which does not activate mGluRs (Wu et al., 2008). Thus, we compared the effects of single and multiple HFS stimulus paradigms adapted from Wu et al. (Wu et al., 2008). The mGluR-dependent HFS paradigm (mGluR-HFS) consisted of two trains of HFS bursts (20 bursts of 8 stimuli@200 Hz; interburst interval 2 s) applied 10 min apart. The mGluR-independent HFS paradigm was a single train of HFS bursts (weak HFS; 8 bursts of 8 stimuli@200Hz; interburst interval 2 s). During either HFS, stimulus intensity was increased to evoke a fEPSP slope double the test intensity.
The following drugs were bath-applied prior to induction of LTP; (1) the broad-spectrum mGluR antagonist LY341495 (20 μM; Ascent Scientific, Princeton, NJ) [(2S)-2-amino-2-[(1S,2S)-2-carboxycycloprop-1-yl]-3-(xanth-9-yl)propanoic acid] (Fitzjohn et al., 1998), (2) the mGluR1-selective antagonist YM 202074 (4 μM; Ascent Scientific) N-Cyclohexyl-6-[[N-(2-methoxyethyl)-N-methylamino]methyl]-N-methylthiazolo[3,2-a]benzimidazole-2-carboxamide sesquifumarate (Kohara et al., 2008); (3) the mGluR5-selective antagonist MPEP (1 μM; Ascent Scientific) 2-Methyl-6-(phenylethynyl)pyridine hydrochloride (Wu et al., 2008); (4) the NR2B-selective NMDA receptor antagonist Ro25-6981 (1 μM; Tocris, Ellisville, MO) [R-(R,S)-α-(4-hydroxyphenyl)-β-methyl-4-(phenylmethyl)-1-piperidine propranol] (Foster et al., 2010); and (5) the selective Src-family tyrosine kinase inhibitor PP2 (10 μM; Tocris) 3-(4-chlorophenyl)1-(1,1-dimethylethyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine (Wu et al., 2007).
Intracellular recordings
Transverse combined entorhinal cortex-hippocampal slices (320 μm thick) were cut at the rostro-caudal level using a Leica VT1000 vibratome in ice-cold high-sucrose cutting solution (composition in mM): KCl 3, MgSO4 1.9, NaH2PO4 1.2, CaCl2 2, sucrose 187, glucose 20, and NaHCO3 26, gassed with 95% O2/5% CO2. Slices were preincubated for a minimum 1 hour recovery period in warmed (34°C) artificial cerebrospinal fluid (aCSF, later used for recordings but with omitted MgSO4); composition in mM: NaCl 124, KCl 2.5, NaH2PO4 1, MgSO4 1.3, CaCl2 2.5, glucose 10, and NaHCO3 26. Patch pipettes (4–6 M! when filled) for whole-cell recordings of NMDA currents were filled with internal solution containing (in mM): 125 CsMeSO3, 8 NaCl, 15 HEPES, 4 Mg-ATP, 0.5 Na-GTP, 0.1 QX-314; pH was adjusted to 7.25 with CsOH2, and osmolarity was 280 ± 10 mOsm.
Whole-cell patch clamp recordings were performed in dentate granule cells using standard techniques under visual control using a Zeiss upright microscope equipped with infrared differential interference contrast (DIC) optics in a submerged recording chamber (Warner Instruments) perfused with aCSF, and gassed with 95%O2/5%CO2 at room temperature (23±2°C). Granule cells in the outer blade of the DG were visualized with a water immersion lens (Zeiss 60x/0.80W) and voltage-clamped at −70 mV. A bipolar stimulating electrode (FHC, Inc.) was placed in the medial perforant pathway. Evoked excitatory postsynaptic currents (eEPSCs) were triggered with a Master-8 Pulse Generator and delivered to the bipolar stimulating electrode via a stimulus isolator (ISO-Flex; A.M.P.I., Jerusalem, Israel). Electrical signals were sampled at 10 kHz and low-pass filtered at 3 kHz. After whole cell voltage-clamp configuration was established, access resistance was constantly monitored and only cells with stable access resistance that changed <20% were included in analyses. Data were amplified with a MultiClamp 700B amplifier (Molecular Devices, Foster City, CA), acquired with a 16-bit D/A interface (Digidata 1440A, Molecular Devices), stored on a PC-computer and analyzed using Clampex software (version 10.2, Molecular Devices) and MiniAnalysis version 6.0 from Synaptosoft.
NMDA currents were pharmacologically isolated by bath application of the AMPA/kainate receptor antagonist NBQX (10 μM, Tocris) plus the GABAA receptor antagonist picrotoxin (50 μM, Ascent Scientific). Voltage-dependent block of NMDARs was removed by omitting extracellular Mg2+ from the aCSF. NMDA eEPSCs were evoked using a glass microelectrode filled with aCSF and placed in the medial perforant pathway (constant current rectangular monopolar pulses, duration 150 μs, intensity evoking 50–60% of maximal EPSC amplitudes). At the end of each experiment, 50 μM D-AP5 (Ascent Scientific) or a selective NR2A subunit receptor antagonist NVP-AAM077 (0.1 μM; a kind gift from Dr Yves Auberson, Novartis) was bath-applied to confirm that recorded currents were NMDAR-mediated. Charge transfer was determined as area under the curve of NMDAR-EPSC within 300 msec after the onset of the downward deflection. The contribution of NR2B-mediated charge transfer was determined as a percentage of charge transfer sensitive to blockade by Ro25-6981 compared to total NMDAR charge transfer.
Western blot
Dentate gyri including the hilar regions at the rostro-caudal level (same level as for the electrophysiological recordings) of the hippocampus were dissected out and harvested into ice cold PBS, pH 7.4 containing a protease inhibitor cocktail (Roche, Indianapolis, IN). Tissue was homogenized and sonicated in lysis buffer containing 15 mM Hepes, pH 7.4, 62.5 mM Tris (pH 6.8), 0.5 mM EDTA, 0.5 mM EGTA, 2% SDS, 10% sucrose, 1 mM PMSF, 1 mM Na3VO4, 1 mM NaF, and 10% protease inhibitor cocktail. Lysates were resolved on gradient 4–20% Ready Gel (Bio-Rad, Hercules, CA) and electro-transferred onto Protran nitrocellulose membranes (Whatman). Membranes were blocked in 4% nonfat milk in PBS/Tween 20 (0.5%) for 1.5 hour at room temperature, and incubated with primary antibody overnight at 4 °C. The following commercially available primary antibodies were used: monoclonal purified mouse anti-mGluR1 (1:1000; BD Biosciences Pharmingen, San Diego, CA; cat. # 610965; target MW 133 kDa) (Kurnellas et al., 2007), polyclonal affinity purified rabbit anti-mGluR5 (1:4000; Millipore, Billerica, MA; cat. # AB5675; target MW 132 kDa) (Francesconi et al., 2009). After 3 additional washes in PBS/Tween, membranes were incubated for 1 hour with appropriate horseradish peroxidase-conjugated secondary antibodies (1:10000; AbCam). Proteins were visualized by chemiluminescence with the ECL Western Blotting Detection Reagents (Millipore), using a Kodak 4000MM Pro Image System. For quantification, we used only exposures within the linear optical density range and excluded oversaturated values using the saturation time determination tool of the automated Gel Logic System. Measurements of optical densities were obtained using ImageJ software. The band densities were first normalized to β-actin levels and then to the mean of the corresponding membrane band densities from oil-injected controls.
Statistical analysis
Extracellular fEPSP slopes exhibited a Gaussian distribution (tested by a Kolmogorov-Smirnov test vs normally-distributed sample) and similar data variance in all data subsets, so parametric statistics was used. We used repeated measures ANOVA to determine differences in LTP between 30 and 45 minutes following the end of the last HFS train. If ANOVA identified overall differences, we used following post hoc comparisons: 1. When comparing between EB and oil groups, we used Bonferroni/Dunn test. 2. For pairwise comparisons of individual pharmacological manipulations within EB or oil groups, we used Dunnett’s test (comparison versus group without pharmacological manipulation). Level of significance was preset to P<0.05 and adjusted for multiple comparisons as required. Intracellularly recorded sEPSCs were sorted based on the response to the specific antagonist of the NR2B subunit. The proportions were compared using χ2-test with post hoc cell contributions. For Western blot comparisons we used two-tailed Student’s t-test. Analysis of whole cell patch clamp sEPSCs was performed using repeated measures ANOVA. Statistical significance was always set to P<0.05.
Results
Repeated but not a single HFS of the medial perforant path leads to EB-induced enhancement of LTP
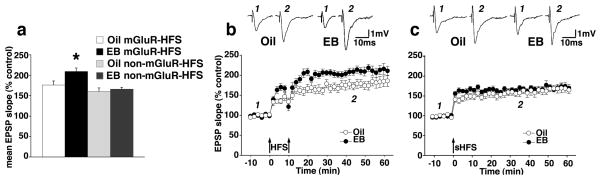
We first determined, at medial perforant pathway-dentate granule cell synapses in hippocampal slices from female ovariectomized rats, whether EB replacement affected LTP by comparing the effects of mGluR-HFS (20×8@200 Hz, interburst interval 2s; repeated twice 10 min apart) to non-mGluR-HFS paradigms (8 trains of 8 stimuli@200 Hz, interburst interval 2s) (Wu et al., 2008). We found that EB enhanced LTP only when we used the mGluR-HFS paradigm (repeated measures ANOVA F(3,35)=7.373; P=0.0006; Figure 1a). Post-hoc Bonferroni/Dunn test revealed that the magnitude of LTP in slices from EB-replaced rats stimulated with the mGluR-HFS was significantly different from all other groups (all P<0.008).
Figure 1.
EB has differential effects on LTP induced by mGluR-HFS and non-mGluR-HFS. (a) EB enhances LTP following mGluR-HFS, but not non-mGluR-HFS (repeated measures ANOVA F(3,35)=7.373; P=0.0006). (b) The time course of LTP induced by mGluR-HFS of medial perforant path synapses in the dentate gyrus showing larger LTP in slices from EB-replaced (n=11) versus oil-injected (n=9) rats (Bonferroni/Dunn post hoc test, *P<0.006). (c) The time course of LTP induced by non-mGluR-HFS showing that EB replacement (n=9) does not alter LTP in comparison to LTP in oil-injected (n=9) rats (Bonferroni/Dunn post hoc test, P=0.63). Insets are signal-averages of fEPSPs prior to HFS and 35 minutes following the last HFS.
Comparison of LTP induced by mGluR-HFS (Figure 1b) and non-mGluR-HFS (Figure 1c) show that, in slices from animals exposed to EB, mGluR-HFS significantly enhanced the magnitude of LTP produced by a second HFS. On the other hand, LTP in slices from rats without EB replacement (oil-injected) did not show the ability to further potentiate following the mGluR-HFS (P=0.2; Bonferroni/Dunn test).
EB-induced enhancement of LTP in the dentate gyrus involves mGluR-NMDAR interactions
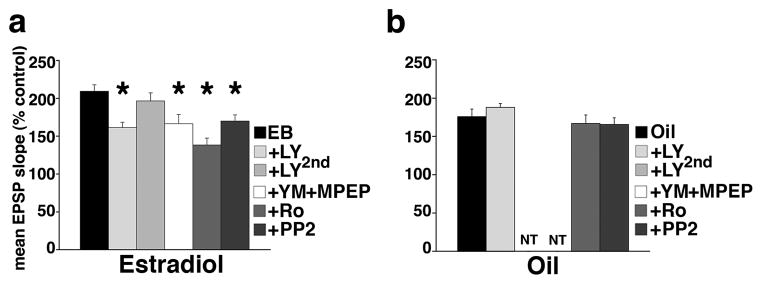
Next we examined the role of mGluRs and NR2B-containing NMDARs in the effects of EB on mGluR-dependent metaplasticity of LTP using pharmacological blockade of either mGluRs or NR2B-containing NMDARs during the first mGluR-HFS. In slices from EB-replaced ovariectomized rats, repeated measures ANOVA showed a significant main effect of pharmacological treatments on the magnitude of LTP F(5,48)=9.074; P<0.0001 (Figure 2a). In contrast, in slices from oil-injected ovariectomized rats, repeated measures ANOVA did not show any main effect of pharmacological manipulations on LTP (repeated measures ANOVA F(3,32)=0.948; P=0.429; Figure 2b). Thus, effects of blockade of mGluR and NR2B-containing NMDARs described below were evaluated using post-hoc Dunnet’s tests only in slices from EB-treated rats.
Figure 2.
EB primes medial perforant path-dentate gyrus synapses for greater LTP by up-regulating group I mGluR-dependent phosphorylation of NMDAR NR2B subunit. Mean ± SEM % LTP of medial perforant path synaptic transmission in slices from EB- (estradiol)-treated (a) and oil-injected (b) rats. Repeated measures ANOVA showed a significant main effect of pharmacological treatments on the magnitude of LTP in slices from EB-treated (a; F(5,48)=9.074; P<0.0001) rats. Post-hoc analyses (versus control slices: EB) specified that the mGluR antagonist LY341495 (20 μM) suppressed magnitude of LTP only if bath-applied prior to the first mGluR HFS (LY) but not after the first mGluR HFS (LY2nd). A cocktail of mGluR1 and mGluR5 antagonists YM202074 (4 μM) + MPEP (1 μM) also decreased magnitude of LTP (YM+MPEP). Similarly, NR2B subunit antagonist Ro25-6981 (1 μM) diminished the enhanced magnitude of LTP. The src kinase inhibitor PP2 (10 μM) had a similar suppressing effect on the magnitude of LTP. There was no effect of bath-applied drugs in slices from oil-injected ovariectomized rats (b; repeated measures ANOVA; F(3,32)=0.948; P=0.429). NT = not tested.
HFS-induced activation of mGluRs by the first HFS is required for enhancement of LTP from subsequent HFS in the dentate gyrus
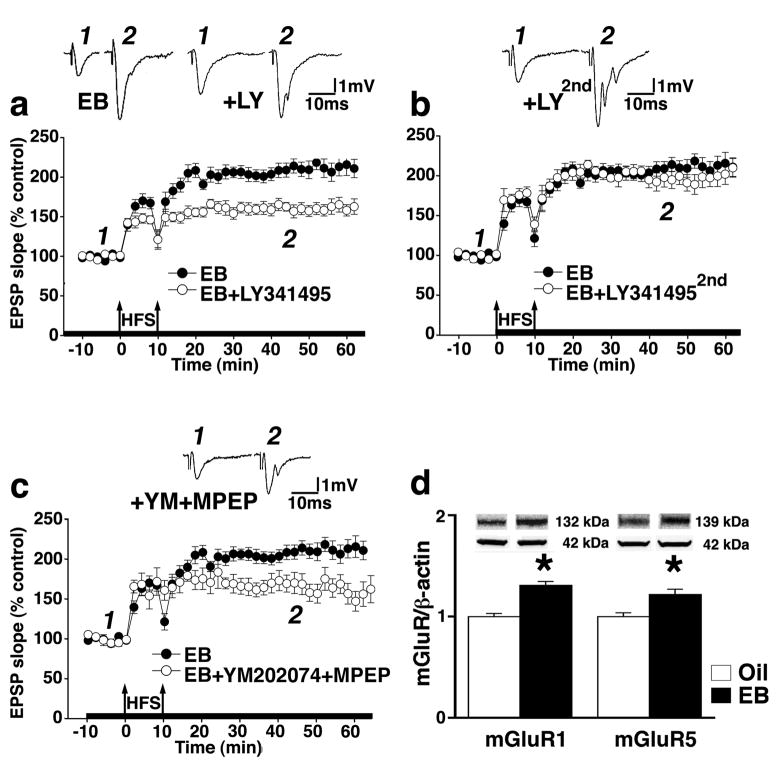
In slices from EB-replaced ovariectomized rats, we found that activation of mGluRs by the first mGluR-HFS was necessary for the enhancement in LTP magnitude observed in response to the second mGluR-HFS. The mGluR antagonist LY341495 (20 μM), at a concentration which blocks all mGluR subtypes without affecting AMPA or NMDAR (Fitzjohn et al., 1998), prevented the enhancement of LTP resulting from the first application of mGluR-HFS (post-hoc Dunnett’s test, P<0.05 compared to EB controls, Figure 3a) but not in OVX rats without EB replacement (see Figure 2b).
Figure 3.
Role of mGluR activation in EB-induced metaplasticity of LTP in the dentate gyrus. (a) The time course of LTP induced by mGluR-HFS shows that the enhancement of LTP in slices from EB replaced rats was prevented by the general mGluR-blocker LY 341495 (20 μM) only when bath applied prior to the first of two HFS trains (n=11; post-hoc Dunnett’s test P<0.05), but (b) not when applied immediately after the first HFS train (n=8; post hoc Dunnett’s test P>0.05). (c) Bath co-application of both mGluR1 and mGluR5-selective antagonists (YM202074, 4 μM and MPEP, 1 μM; n=5) starting 10 min prior to the first HFS significantly suppressed the EB-dependent metaplastic enhancement of LTP (post hoc Dunnett’s test P<0.05). (d) Western blots showing EB-dependent up-regulation of the expression of both mGluR1 and mGluR5 subunit protein levels in isolated dentate gyri (mGluR1: oil, n=6; EB (EB), n=6; Student’s t-test *P<0.0002 compared to oil-injected expression level; mGluR5: oil, n=8; EB, n=7; Student’s t-test *P<0.005 compared to oil-injected expression level). For comparison purposes, LTP in slices from EB-treated rats was added to each plot. Insets are representative mean traces of fEPSPs prior to HFS and 35 minutes following the last HFS stimulation. Thickened x-axis indicates duration of drug application.
To test whether the first mGluR-HFS stimulus train was both necessary and sufficient to initiate mGluR-mediated molecular changes that prime synapses for greater subsequent LTP, we bath-applied 20 μM LY341495 immediately after the first mGluR-HFS, i.e., 10 minutes prior to the second mGluR-HFS train. This timing of drug application did not prevent the mGluR-HFS from enhancing LTP in response to the second HFS (post hoc Dunnett’s test P>0.05 from EB control, Figure 3b).
EB-induced effects on LTP in the dentate gyrus require activation of group I mGluRs
We then tested whether group I mGluRs are essential for the metaplastic effects of mGluR-HFS, by exposing hippocampal slices from EB-replaced rats to a cocktail of mGluR1 and mGluR5 subunit (comprising group I mGluRs) selective antagonists YM202074 (4 μM) and MPEP (1 μM), 10 minutes prior to application of the first mGluR-HFS. Indeed, blocking all group I mGluRs did prevent the augmentation of LTP after mGluR-HFS (post hoc Dunnett’s test P<0.05 from EB control, Figure 3c). The cocktail of mGluR1 and mGluR5 antagonists was applied for only 10 minutes prior to the first HFS train of the mGluR-HFS to verify that the lack of LY341495 effect (see Figure 3b) is not related to insufficient blockade of relatively insensitive group I mGluRs.
The appearance of a prominent role for group I mGluRs in HFS-induced metaplasticity may indicate EB-induced up-regulation of group I mGluRs protein expression. Western blot analysis showed that indeed, EB-treatment up-regulated expression of both mGluR1 and mGluR5 subunit protein levels in isolated dentate gyri (Student’s t-test P<0.0002 and P<0.005; compared to expression levels in normalized oil controls, respectively; Figure 3d).
EB enhances LTP by augmenting group I mGluR-mediated phosphorylation of NR2B subunits
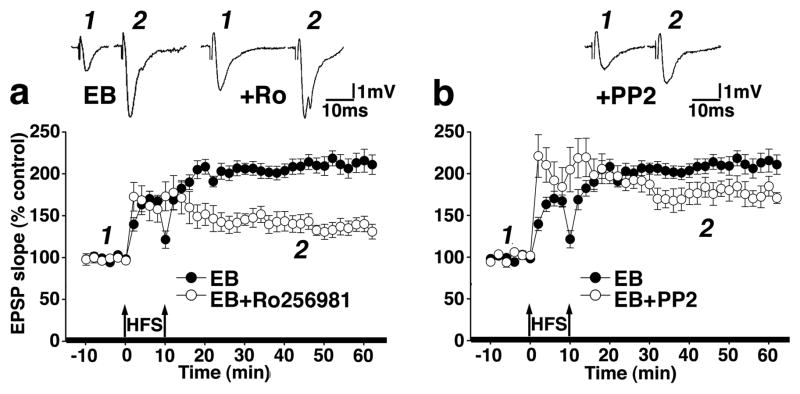
Activation of group I mGluRs may potentiate NMDAR-mediated responses by phosphorylating NMDARs containing NR2B subunits (Guo et al., 2004). It has been demonstrated that in the dentate gyrus, strong HFS paradigms lead to NR2B subunit phosphorylation without phosphorylating other NMDAR subunits (Rosenblum et al., 1996; Rostas et al., 1996). Therefore, we hypothesized that EB-induced changes in NR2B subunit phosphorylation might be involved in the metaplastic enhancement of LTP following the strong mGluR-HFS. To test this hypothesis, we bath applied the selective NR2B subunit antagonist Ro25-6981 prior to the first mGluR-HFS, and found that NR2B-NMDAR blockade significantly reduced the enhancement of LTP in slices from EB-treated rats (P<0.05, post-hoc Dunnett’s test compared to EB alone, Figure 4a) but not in slices from OVX rats without EB replacement (see figure 2b), confirming that the metaplastic enhancement of LTP by mGluR-HFS does require activation of NR2B-containing NMDARs.
Figure 4.
Role of NR2B subunit-containing NMDARs in EB-induced metaplasticity of LTP in the dentate gyrus. The time course of LTP induced by mGluR-HFS shows that (a) expression of EB-induced enhancement of LTP evoked by the mGluR-HFS was prevented by the NR2B-selective NMDAR antagonist Ro25-6981 (1 μM, n=11; post hoc Dunnett’s test P<0.05), and by (b) the tyrosine kinase inhibitor PP2 (10 μM, n=7; post-hoc Dunnett’s test P<0.05). (Insets are signal-averaged fEPSPs prior to HFS and 35 minutes following the last HFS stimulation.). Thickened x-axis indicates duration of drug application.
To differentiate between mGluR-mediated NR2B subunit phosphorylation and/or increased NR2B protein levels, we bath applied the selective Src tyrosine kinase phosphorylation inhibitor PP2 to selectively prevent phosphorylation of NMDARs (Guo et al., 2002). In slices from EB-replaced rats, PP2 also significantly suppressed the metaplastic enhancement of LTP by mGluR-HFS (P<0.05, post-hoc Dunnett’s test compared to EB alone, Figure 4b) but not in slices from OVX rats without EB replacement (see Figure 2b), indicating that mGluR-activated phosphorylation is required for EB up-regulation of NMDAR-dependent LTP.
EB has no direct effect on baseline synaptic NMDAR conductances in the dentate gyrus
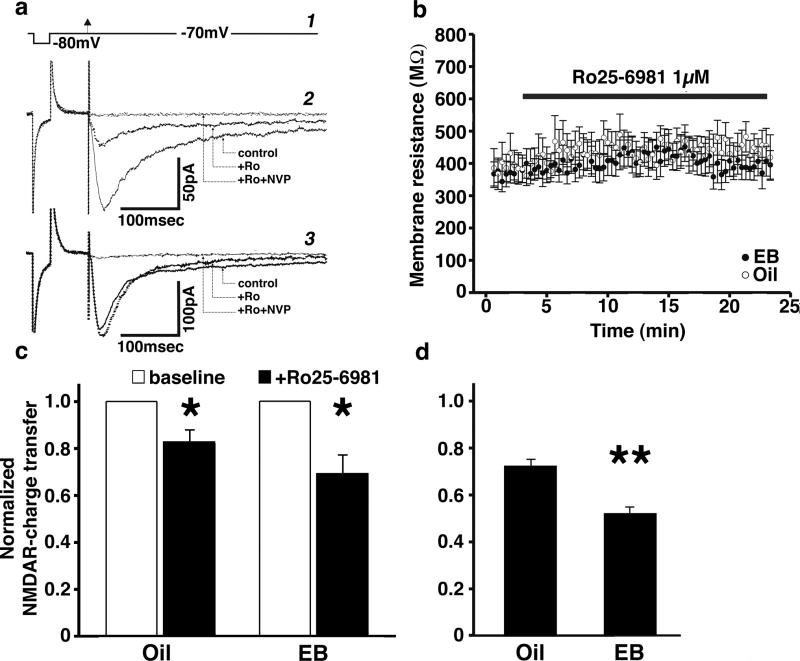
While our data indicate that the first mGluR-HFS upregulates function of NR2B-containing NMDARs in slices from EB-treated rats, it was not clear whether the baseline contribution of NR2B versus non-NR2B subunits to overall synaptic NMDAR conductance prior to HFS would differ between EB- and oil-treated rats. To answer this question, we used whole-cell patch-clamp recordings of pharmacologically isolated stimulus-evoked synaptic NMDAR-mediated currents (NMDAR-EPSCs) in granule neurons (Figure 5). Blockade of NR2B subunit-mediated currents by the NR2B subunit-specific NMDAR antagonist Ro25-6981 (1 μM) resulted in a significant decrease in NMDAR charge transfer in granule cells from both EB-treated (n=11 cells) and oil-treated (n=11 cells) rats (repeated measures, ANOVA F(1,20)=15.817; P=0.0007; Figure 5c). The contribution of NR2B subunits to NMDAR-mediated charge transfer was not different between EB-treated and oil-injected animals (main effect, ANOVA F(1,20)=1.15; P=0.3). Western blot analysis of baseline NR2B protein levels also showed no difference in dentate gyri from EB-replaced versus oil-injected rats (data not shown), in support of this conclusion.
Figure 5.
Whole-cell patch-clamp recordings of pharmacologically isolated NMDAR-mediated currents in granule neurons from EB-treated versus oil-injected rats before and after application of the NR2B-selective NMDAR antagonist Ro25-6981 (1 μM). (a) To monitor voltage resistance changes, a 10 mV rectangular hyperpolarizing pulse (50 ms) was delivered through the patch pipette, followed by a medial perforant path single-shock stimulus (arrowhead) to evoke NMDA current (1). Examples of NMDAR currents in granule cells show two different responses to NR2B subunit blockade with Ro25-6981: Above is a “strong responder” (>15% change; +Ro) (2), and below a “weak responder” (<15% change; +Ro) (3). Bath application of an NR2A blocker NVP-AAM077 (0.1 μM) at the end of each recording blocked the remaining current, confirming that the entire current is NMDAR-mediated and consisted of a combination of NR2A- and NR2B-containing NMDARs (2-3; Ro+NVP). (b) Time course of membrane resistances during the entire recording period in cells from both EB-and oil-treated rats. (c) Normalized NMDAR charge transfer before (baseline) and after (+Ro25-6981) bath application of Ro25-6981 significantly decreased NMDAR-EPSCs in granule cells from both oil-injected (n=11) and EB (EB)-treated (n=11) rats, compared to normalized pre-drug NMDAR-EPSC (*P<0.05; repeated measures ANOVA). The magnitude of NR2B subunit contribution to total NMDAR-mediated charge transfer did not differ in granule neurons from oil-and EB-treated rats. (d) Separate analysis of cells with a strong response to Ro25-6981 (>15% change) showed that the NR2B-mediated charge transfer in these cells represented a significantly larger share of NMDAR-mediated charge transfer in granule neurons from EB-treated rats (n=7), compared to oil-injected (n=8) controls (**P<0.05, repeated measures ANOVA).
We noticed that from both EB- and oil-injected rats, granule cells responses of NMDAR-EPSCs to Ro25-6981 were not uniform (Figure 5a). Some cells showed a strong response (>15% change in charge transfer), while others showed much weaker effects of NR2B-containing NMDAR blockade (<15% change). Since previous studies have reported significant enhancement of NR2B-mediated currents following EB replacement in Schaffer collateral-CA1 synapses (Smith and McMahon, 2006; Snyder et al., 2011), we evaluated separately the strong responders to Ro25-6981. In this case, NR2B-containing NMDAR charge transfer represented a significantly larger share of overall NMDAR charge transfer in granule cells from EB-treated rats (n=7 out of 11 cells) compared to oil controls (n=8 out of 11 cells; repeated measures ANOVA F(1,13)=201.126; P<0.0001; Figure 5d), confirming that EB-induced functional up-regulation of NR2B-containing NMDARs occurs in some granule cells.
Discussion
Here we report several novel findings on the mechanisms of EB-induced modulation of synaptic plasticity in the female hippocampal dentate gyrus. We found that, in slices from OVX rats, LTP was not enhanced by strong repeated HFS, while such HFS did enhance LTP in slices from OVX rats after four days of EB replacement (the last injection 24 hour prior to recording), an effect that required mGluR activation that produced a metaplastic modulation of NMDAR synaptic transmission. We found that EB administered to OVX rats increased expression of group I mGluRs which, when activated by HFS, evoked Src kinase-dependent phosphorylation of NR2B-containing NMDARs that primed synapses to express larger NMDAR-dependent LTP in response to subsequent bouts of HFS. On the other hand, we show that EB replacement had no effect on LTP induced by standard HFS, which does not involve activation of mGluRs (Wu et al., 2008; Welsby et al., 2006). These findings are interesting in light of behavioral studies which have reported quite inconsistent effects of EB administration in distinct behavioral learning tasks (Daniel, 2006), inconsistencies that may depend in complex ways on the relative contribution of mGluR and NMDAR plasticity and metaplasticity to a given task.
Snyder et al. (2011) reported in the hippocampal CA1 region that EB did not increase directly either NR2B subunit expression or phosphorylation (Snyder et al., 2011). Similarly, in the dentate gyrus, we did not observe differences in baseline levels of total NR2B protein (containing both synaptic and extrasynaptic pools) between EB-replaced and oil-injected ovariectomized rats. But in contrast to a previous report of EB-induced functional up-regulation of NR2B subunit containing NMDAR-mediated transmission in CA1 pyramidal neurons (Smith and McMahon, 2006), our whole cell recordings showed that the overall percent contribution of NR2B-containing NMDAR to whole-cell NMDAR current did not differ between EB-treated and oil-injected dentate granule neurons prior to HFS. Thus, in the dentate gyrus, EB treatment alone does not result in an enhanced NR2B component of baseline NMDAR transmission. Consistent with this finding, EB replacement had no effect on the magnitude of NMDAR-dependent LTP induced by an HFS, which did not elicit activation of mGluRs. Our data also suggest that dentate granule cells are a non-uniform neuronal population, since we observed neurons with both weak and strong NR2B-mediated synaptic current components. The functional role of such heterogeneity is unknown, but might explain observations of selective vulnerability of some granule cells and resistance of others to damage following seizures or ischemia (Li et al., 1997; Covolan et al., 2000).
Following EB treatment, LTP magnitude was enhanced by activation of a chain of biochemical events initiated by the first HFS train. This effect required sufficient group I mGluR activation by the first HFS train that elicited a “metaplastic” modulation of NMDAR sensitivity to the subsequent HFS, while further activation of mGluRs was not required for this second HFS to evoke LTP. Similar biochemical changes have been reported in dissociated hippocampal neuronal cultures, where NMDAR currents are potentiated by group I mGluRs via a scr tyrosine kinase => protein kinase C (PKC) => calcium activated kinase β (CAKβ) cascade (Kotecha et al., 2003). Our data point to an important role for group I mGluRs in the metaplasticity of NMDAR-dependent LTP mediated by EB. On the other hand, in ovariectomized rats without EB replacement, strong HFS trains appeared to be incapable of sufficient activation of downregulated mGluRs to either phosphorylate NR2B subunits or enhance LTP.
Our data suggest that, if total capacity for LTP is reduced by ovariectomy, HFS should lead to more rapid saturation of LTP. Previous studies have indicated that LTP saturation can be associated with compromised learning and enhanced sensitivity to excitotoxic challenges (Barnes et al., 1994; Abraham, 2008). In fact, we and others have reported that ovariectomy is associated with enhanced sensitivity to neurodegeneration following prolonged seizures, ischemia, or other neurodegenerative disorders in ovariectomized rats and that hippocampal damage is greatly ameliorated by EB replacement [for reviews see (Velíšková, 2007; Pike et al., 2009; Etgen et al., 2011)]. Thus, the absence of EB may place the brain in a state where LTP is saturated more rapidly, and has a greater contribution to neurotoxicity in a variety of disease conditions.
EB administration to OVX rats improves spatial learning and memory formation (Luine et al., 1998; Gibbs and Johnson, 2008). Since it has been shown in the dentate gyrus that regulation of LTP by group I mGluRs is associated with better spatial learning and memory (Naie and Manahan-Vaughan, 2004), we speculate that our findings of mGluR-mediated upregulation of LTP in the dentate gyrus following EB administration in OVX rats could be causally related to improved performance in spatial learning and memory tasks.
Future studies are needed to determine whether enhanced activation of group I mGluR following EB exposure leads to insertion/trafficking of new NR2B NMDAR subunits, and/or lateral movement of existing NR2B subunits between extrasynaptic and synaptic loci (Tovar and Westbrook, 2002; Newpher and Ehlers, 2008; Zhao et al., 2008). A recent study demonstrated a critical role for KIF17, a specific motor transporter for the NR2B subunit, in delivery of NR2B subunits to the synapse (Yin et al., 2011). This raises the interesting possibility that EB-mediated stimulation of KIF17 expression might enhance synaptic insertion of NR2B subunits, and that disruption of this insertion by loss of EB might result in cognitive impairment.
In conclusion, in promoting learning and memory, EB may act by preventing saturation of LTP to improve learning and memory acquisition, particularly in tasks requiring activation of mGluRs. These effects may also apply to antidepressant action of EB (Soares and Frey, 2010), since changes in synaptic plasticity have been reported to play an important role in depression and associated cognitive impairments (Austin et al., 2001). Understanding the mechanisms of action of EB will allow for design of new pharmacological interventions to directly target these mechanisms, instead of using hormone replacement therapy, which combines its benefits with serious risks such as increased occurrence of certain cancers and thrombosis (Chlebowski, 2009; Krieger et al., 2010; Sharpe et al., 2010).
Acknowledgments
We thank to Dr. Regina Hanstein for help with the western blot technique. This work was supported by grants from US National Institutes of Health NS056093 (J.V.), NS072966 (L.V.), NS044421 (P.K.S.) and March of Dimes grant #6-FY-08-214 (L.V.).
Role of funding: This work was supported by grants from US National Institutes of Health NS056093 (J.V.), NS072966 (L.V.), NS044421 (P.K.S.) and March of Dimes grant #6-FY-08-214 (L.V.).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Authors’ disclosure: We report no conflict of interest for all of the authors.
Contributors: Dr. Nino Nebieridze performed the extracellular recordings, western blots, and analyzed data. Drs. Xiao-lei Zhang and Tamar Chachua performed the patch clamp studies and data analysis. Dr. Libor Velíšek participated on designing the experiments, wrote the protocol for extracellular recordings, and helped with statistical analysis and manuscript preparation. Dr. Patric K. Stanton participated on experimental design and preparation of the manuscript. Dr. Jana Velíšková designed the study, performed statistical analysis, and wrote the manuscript. All authors contributed to and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham WC. Metaplasticity: tuning synapses and networks for plasticity. Nat Rev Neurosci. 2008;9:387. doi: 10.1038/nrn2356. [DOI] [PubMed] [Google Scholar]
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- Anwyl R. Metabotropic glutamate receptor-dependent long-term potentiation. Neuropharmacology. 2009;56:735–740. doi: 10.1016/j.neuropharm.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Austin MP, Mitchell P, Goodwin GM. Cognitive deficits in depression: possible implications for functional neuropathology. Br J Psychiatry. 2001;178:200–206. doi: 10.1192/bjp.178.3.200. [DOI] [PubMed] [Google Scholar]
- Barnes CA, Jung MW, McNaughton BL, Korol DL, Andreasson K, Worley PF. LTP saturation and spatial learning disruption: effects of task variables and saturation levels. J Neurosci. 1994;14:5793–5806. doi: 10.1523/JNEUROSCI.14-10-05793.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PE, Errington ML, Kneussel M, Chen G, Annala AJ, Rudhard YH, Rast GF, Specht CG, Tigaret CM, Nassar MA, Morris RG, Bliss TV, Schoepfer R. Behavioral deficits and subregion-specific suppression of LTP in mice expressing a population of mutant NMDA receptors throughout the hippocampus. Learn Mem. 2009;16:635–644. doi: 10.1101/lm.1316909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlebowski RT. Menopausal hormone therapy, hormone receptor status, and lung cancer in women. Semin Oncol. 2009;36:566–571. doi: 10.1053/j.seminoncol.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Colino A, Malenka RC. Mechanisms underlying induction of long-term potentiation in rat medial and lateral perforant paths in vitro. J Neurophysiol. 1993;69:1150–1159. doi: 10.1152/jn.1993.69.4.1150. [DOI] [PubMed] [Google Scholar]
- Covolan L, Ribeiro LT, Longo BM, Mello LE. Cell damage and neurogenesis in the dentate granule cell layer of adult rats after pilocarpine- or kainate-induced status epilepticus. Hippocampus. 2000;10:169–180. doi: 10.1002/(SICI)1098-1063(2000)10:2<169::AID-HIPO6>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Daniel JM. Effects of oestrogen on cognition: what have we learned from basic research? J Neuroendocrinol. 2006;18:787–795. doi: 10.1111/j.1365-2826.2006.01471.x. [DOI] [PubMed] [Google Scholar]
- Dumitriu D, Rapp PR, McEwen BS, Morrison JH. Estrogen and the aging brain: an elixir for the weary cortical network. Ann N Y Acad Sci. 2010;1204:104–112. doi: 10.1111/j.1749-6632.2010.05529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etgen AM, Jover-Mengual T, Suzanne Zukin R. Neuroprotective actions of estradiol and novel estrogen analogs in ischemia: Translational implications. Front Neuroendocrinol. 2011;32:336–352. doi: 10.1016/j.yfrne.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzjohn SM, Bortolotto ZA, Palmer MJ, Doherty AJ, Ornstein PL, Schoepp DD, Kingston AE, Lodge D, Collingridge GL. The potent mGlu receptor antagonist LY341495 identifies roles for both cloned and novel mGlu receptors in hippocampal synaptic plasticity. Neuropharmacology. 1998;37:1445–1458. doi: 10.1016/s0028-3908(98)00145-2. [DOI] [PubMed] [Google Scholar]
- Foster KA, McLaughlin N, Edbauer D, Phillips M, Bolton A, Constantine-Paton M, Sheng M. Distinct roles of NR2A and NR2B cytoplasmic tails in long-term potentiation. J Neurosci. 2010;30:2676–2685. doi: 10.1523/JNEUROSCI.4022-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francesconi A, Kumari R, Zukin RS. Regulation of group I metabotropic glutamate receptor trafficking and signaling by the caveolar/lipid raft pathway. J Neurosci. 2009;29:3590–3602. doi: 10.1523/JNEUROSCI.5824-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, Johnson DA. Sex-specific effects of gonadectomy and hormone treatment on acquisition of a 12-arm radial maze task by Sprague Dawley rats. Endocrinology. 2008;149:3176–3183. doi: 10.1210/en.2007-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Zou S, Guan Y, Ikeda T, Tal M, Dubner R, Ren K. Tyrosine phosphorylation of the NR2B subunit of the NMDA receptor in the spinal cord during the development and maintenance of inflammatory hyperalgesia. J Neurosci. 2002;22:6208–6217. doi: 10.1523/JNEUROSCI.22-14-06208.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Wei F, Zou S, Robbins MT, Sugiyo S, Ikeda T, Tu JC, Worley PF, Dubner R, Ren K. Group I metabotropic glutamate receptor NMDA receptor coupling and signaling cascade mediate spinal dorsal horn NMDA receptor 2B tyrosine phosphorylation associated with inflammatory hyperalgesia. J Neurosci. 2004;24:9161–9173. doi: 10.1523/JNEUROSCI.3422-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP. A behavioral analysis of dentate gyrus function. Prog Brain Res. 2007;163:567–576. doi: 10.1016/S0079-6123(07)63030-1. [DOI] [PubMed] [Google Scholar]
- Kohara A, Takahashi M, Yatsugi S, Tamura S, Shitaka Y, Hayashibe S, Kawabata S, Okada M. Neuroprotective effects of the selective type 1 metabotropic glutamate receptor antagonist YM-202074 in rat stroke models. Brain Res. 2008;1191:168–179. doi: 10.1016/j.brainres.2007.11.035. [DOI] [PubMed] [Google Scholar]
- Kotecha SA, Jackson MF, Al-Mahrouki A, Roder JC, Orser BA, MacDonald JF. Co-stimulation of mGluR5 and N-methyl-D-aspartate receptors is required for potentiation of excitatory synaptic transmission in hippocampal neurons. J Biol Chem. 2003;278:27742–27749. doi: 10.1074/jbc.M301946200. [DOI] [PubMed] [Google Scholar]
- Krieger N, Chen JT, Waterman PD. Decline in US Breast Cancer Rates After the Women’s Health Initiative: Socioeconomic and Racial/Ethnic Differentials. Am J Public Health. 2010 doi: 10.2105/AJPH.2009.181628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni J, de Castella A, Fitzgerald PB, Gurvich CT, Bailey M, Bartholomeusz C, Burger H. Estrogen in severe mental illness: a potential new treatment approach. Arch Gen Psychiatry. 2008;65:955–960. doi: 10.1001/archpsyc.65.8.955. [DOI] [PubMed] [Google Scholar]
- Kurnellas MP, Lee AK, Li H, Deng L, Ehrlich DJ, Elkabes S. Molecular alterations in the cerebellum of the plasma membrane calcium ATPase 2 (PMCA2)-null mouse indicate abnormalities in Purkinje neurons. Mol Cell Neurosci. 2007;34:178–188. doi: 10.1016/j.mcn.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Chopp M, Powers C. Granule cell apoptosis and protein expression in hippocampal dentate gyrus after forebrain ischemia in the rat. J Neurol Sci. 1997;150:93–102. doi: 10.1016/s0022-510x(97)00075-0. [DOI] [PubMed] [Google Scholar]
- Luine VN, Richards ST, Wu VY, Beck KD. Estradiol enhances learning and memory in a spatial memory task and effects levels of monoaminergic neurotransmitters. Horm Behav. 1998;34:149–162. doi: 10.1006/hbeh.1998.1473. [DOI] [PubMed] [Google Scholar]
- Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J Comp Neurol. 2001;429:355–371. [PubMed] [Google Scholar]
- Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, McEwen BS, Warrier S, Alves SE. Ultrastructural localization of estrogen receptor beta immunoreactivity in the rat hippocampal formation. J Comp Neurol. 2005;491:81–95. doi: 10.1002/cne.20724. [DOI] [PubMed] [Google Scholar]
- Naie K, Manahan-Vaughan D. Regulation by metabotropic glutamate receptor 5 of LTP in the dentate gyrus of freely moving rats: relevance for learning and memory formation. Cereb Cortex. 2004;14:189–198. doi: 10.1093/cercor/bhg118. [DOI] [PubMed] [Google Scholar]
- Neal-Perry GS, Zeevalk GD, Santoro NF, Etgen AM. Attenuation of preoptic area glutamate release correlates with reduced luteinizing hormone secretion in middle-aged female rats. Endocrinology. 2005;146:4331–4339. doi: 10.1210/en.2005-0575. [DOI] [PubMed] [Google Scholar]
- Newpher TM, Ehlers MD. Glutamate receptor dynamics in dendritic microdomains. Neuron. 2008;58:472–497. doi: 10.1016/j.neuron.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterlund MK, Witt MR, Gustafsson JA. Estrogen action in mood and neurodegenerative disorders: estrogenic compounds with selective properties-the next generation of therapeutics. Endocrine. 2005;28:235–242. doi: 10.1385/ENDO:28:3:235. [DOI] [PubMed] [Google Scholar]
- Ostlund H, Keller E, Hurd YL. Estrogen receptor gene expression in relation to neuropsychiatric disorders. Ann N Y Acad Sci. 2003;1007:54–63. doi: 10.1196/annals.1286.006. [DOI] [PubMed] [Google Scholar]
- Pike CJ, Carroll JC, Rosario ER, Barron AM. Protective actions of sex steroid hormones in Alzheimer’s disease. Front Neuroendocrinol. 2009;30:239–258. doi: 10.1016/j.yfrne.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca WA, Grossardt BR, Maraganore DM. The long-term effects of oophorectomy on cognitive and motor aging are age dependent. Neurodegener Dis. 2008;5:257–260. doi: 10.1159/000113718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum K, Dudai Y, Richter-Levin G. Long-term potentiation increases tyrosine phosphorylation of the N-methyl-D-aspartate receptor subunit 2B in rat dentate gyrus in vivo. Proc Natl Acad Sci U S A. 1996;93:10457–10460. doi: 10.1073/pnas.93.19.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostas JA, Brent VA, Voss K, Errington ML, Bliss TV, Gurd JW. Enhanced tyrosine phosphorylation of the 2B subunit of the N-methyl-D-aspartate receptor in long-term potentiation. Proc Natl Acad Sci U S A. 1996;93:10452–10456. doi: 10.1073/pnas.93.19.10452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Drew MR, Hen R. Dentate gyrus neurogenesis and depression. Prog Brain Res. 2007;163:697–722. doi: 10.1016/S0079-6123(07)63038-6. [DOI] [PubMed] [Google Scholar]
- Sharpe KH, McClements P, Clark DI, Collins J, Springbett A, Brewster DH. Reduced risk of oestrogen receptor positive breast cancer among peri- and post-menopausal women in Scotland following a striking decrease in use of hormone replacement therapy. Eur J Cancer. 2010 doi: 10.1016/j.ejca.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Surgical menopause, estrogen, and cognitive function in women: what do the findings tell us? Ann N Y Acad Sci. 2005a;1052:3–10. doi: 10.1196/annals.1347.001. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and memory in women: how can we reconcile the findings? Horm Behav. 2005b;47:371–375. doi: 10.1016/j.yhbeh.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Smith CC, McMahon LL. Estradiol-induced increase in the magnitude of long-term potentiation is prevented by blocking NR2B-containing receptors. J Neurosci. 2006;26:8517–8522. doi: 10.1523/JNEUROSCI.5279-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder MA, Cooke BM, Woolley CS. Estradiol potentiation of NR2B-dependent EPSCs is not due to changes in NR2B protein expression or phosphorylation. Hippocampus. 2011;21:398–408. doi: 10.1002/hipo.20756. [DOI] [PubMed] [Google Scholar]
- Soares CN, Frey BN. Challenges and opportunities to manage depression during the menopausal transition and beyond. Psychiatr Clin North Am. 2010;33:295–308. doi: 10.1016/j.psc.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Steele RJ, Morris RG. Delay-dependent impairment of a matching-to-place task with chronic and intrahippocampal infusion of the NMDA-antagonist D-AP5. Hippocampus. 1999;9:118–136. doi: 10.1002/(SICI)1098-1063(1999)9:2<118::AID-HIPO4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. Mobile NMDA receptors at hippocampal synapses. Neuron. 2002;34:255–264. doi: 10.1016/s0896-6273(02)00658-x. [DOI] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Naisbitt S, Yuan JP, Petralia RS, Brakeman P, Doan A, Aakalu VK, Lanahan AA, Sheng M, Worley PF. Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron. 1999;23:583–592. doi: 10.1016/s0896-6273(00)80810-7. [DOI] [PubMed] [Google Scholar]
- Velíšek L, Velíšková J, Giorgi FS, Moshé SL. Sex-specific control of flurothyl-induced tonic-clonic seizures by the substantia nigra pars reticulata during development. Exp Neurol. 2006;201:203–211. doi: 10.1016/j.expneurol.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Velíšková J. Estrogens and epilepsy: why are we so excited? Neuroscientist. 2007;13:77–88. doi: 10.1177/1073858406295827. [DOI] [PubMed] [Google Scholar]
- Velíšková J, Velíšek L. β-Estradiol Increases Dentate Gyrus Inhibition in Female Rats via Augmentation of Hilar Neuropeptide Y. J Neurosci. 2007;27:6054–6063. doi: 10.1523/JNEUROSCI.0366-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsby P, Rowan M, Anwyl R. Nicotinic receptor-mediated enhancement of long-term potentiation involves activation of metabotropic glutamate receptors and ryanodine-sensitive calcium stores in the dentate gyrus. Eur J Neurosci. 2006;24:3109–3118. doi: 10.1111/j.1460-9568.2006.05187.x. [DOI] [PubMed] [Google Scholar]
- Wigstrom H, Gustafsson B. Large long-lasting potentiation in the dentate gyrus in vitro during blockade of inhibition. Brain Res. 1983;275:153–158. doi: 10.1016/0006-8993(83)90428-6. [DOI] [PubMed] [Google Scholar]
- Wu HY, Hsu FC, Gleichman AJ, Baconguis I, Coulter DA, Lynch DR. Fyn-mediated phosphorylation of NR2B Tyr-1336 controls calpain-mediated NR2B cleavage in neurons and heterologous systems. J Biol Chem. 2007;282:20075–20087. doi: 10.1074/jbc.M700624200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Harney S, Rowan MJ, Anwyl R. Involvement of group I mGluRs in LTP induced by strong high frequency stimulation in the dentate gyrus in vitro. Neuroscience Letters. 2008;436:235–238. doi: 10.1016/j.neulet.2008.03.027. [DOI] [PubMed] [Google Scholar]
- Yin X, Takei Y, Kido MA, Hirokawa N. Molecular Motor KIF17 Is Fundamental for Memory and Learning via Differential Support of Synaptic NR2A/2B Levels. Neuron. 2011;70:310–325. doi: 10.1016/j.neuron.2011.02.049. [DOI] [PubMed] [Google Scholar]
- Zhao J, Peng Y, Xu Z, Chen RQ, Gu QH, Chen Z, Lu W. Synaptic metaplasticity through NMDA receptor lateral diffusion. J Neurosci. 2008;28:3060–3070. doi: 10.1523/JNEUROSCI.5450-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]