Abstract
The reduction of neural activity in response to repeated stimuli, repetition suppression, is one of the most robust experience‐related cortical dynamics known to cognitive neuroscience. Functional magnetic resonance imaging (fMRI) studies during episodic memory encoding have demonstrated repetition suppression in the hippocampus and this reduction has been linked to successful memory formation. An emerging body of functional imaging evidence suggests that the posteromedial cortex, in addition to the medial temporal lobes, may have a pivotal role in successful episodic memory. This area typically deactivates during initial memory encoding, but its functional changes in response to repetitive encoding remain poorly specified. Here, we investigate the repetition‐related changes in the posteromedial cortex as well as the hippocampus while the participants underwent an fMRI experiment involving repetitive encoding of face–name pairs. During the first encoding trial of face–name pairs, significant activation in the hippocampus was observed. The second and third encoding trials demonstrated a repetition suppression effect in the hippocampus, indicated by a stepwise decrease of activation. In contrast, the posteromedial cortex demonstrated significant deactivation during the initial encoding trial of face–name pairs. The second and third encoding trials demonstrated a stepwise decrease of deactivation, repetition enhancement, with activity at or above baseline levels in the final encoding trial. These findings demonstrate that hippocampus repetition suppression as well as posteromedial repetition enhancement is related to successful encoding processes and are discussed in relation to the default mode hypothesis as well as potential implications for understanding late‐life amnestic disorders. Hum Brain Mapp, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: episodic memory, functional MRI, hippocampus, posteromedial cortex, repetition
INTRODUCTION
Stimulus repetition induces an attenuated neural response, a phenomenon known as repetition suppression (or habituation). This phenomenon occurs in non‐human primates at the level of single cells [e.g., Desimone, 1996; Miller et al., 1991] and in humans at the cortical level using functional neuroimaging techniques such as functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) [for a review see Grill‐Spector et al., 2006; Henson and Rugg, 2003; Schacter and Buckner, 1998]. In studies of episodic memory, repetition suppression has been observed in the hippocampus and adjacent brain regions in the medial temporal lobe (MTL), suggesting that suppression may reflect successful encoding or consolidation [Gonsalves et al., 2005; Rand‐Giovannetti et al., 2006; Suzuki et al., 2010]. Support for this possibility has been demonstrated with fMRI by examining populations with memory impairment. The common finding in these studies is that MTL activity for repeated stimuli is sustained rather than suppressed in patients with Alzheimer's disease (AD) and individuals with mild cognitive impairment (MCI) compared to healthy older subjects [Golby et al., 2005; Johnson et al., 2004, 2008b; Pihlajamäki et al., 2008]. This failure of normal suppression of the MTL response in AD patients is related to poorer post‐scan memory performance [Pihlajamäki et al., 2008].
An emerging body of functional imaging evidence suggests that a large‐scale neural network interconnected with MTL memory structures is also pivotal for successful episodic memory function [Eichenbaum, 2000]. Decreased activation to repetition is observed in multiple neocortical regions, especially in parietal regions including the lateral posterior parietal cortex and the precuneus extending into the posterior cingulate and retrosplenial cortices [Cabeza et al., 2008; Miller et al., 2008; Rugg et al., 2002; Shannon and Buckner, 2004; Spaniol et al., 2009; Uncapher and Wagner, 2009]. These brain regions, in addition to medial temporal and medial prefrontal regions, together make up what is commonly referred to as the “default mode network,” named in part for its involvement when subjects are at rest and relatively suspended when subjects are engaged in attention‐demanding tasks [Buckner et al., 2008; Greicius and Menon, 2004; Mazoyer et al., 2001; McKiernan et al., 2003; Raichle et al., 2001; Shulman et al., 1997]. With respect to episodic memory, deactivation in the default mode network is thought to reflect the proper reallocation of neuronal resources necessary for an individual to focus on the task at hand, thereby contributing to successful encoding [Buckner et al., 2008]. Failure to exhibits of default mode deactivation has been linked to poor memory performance in patients with AD and MCI [Miller et al., 2008; Pihlajamäki et al., 2009]. Additional evidence for the involvement of the default mode network in task processing is the findings of increased engagement of this network with increasing task demand [McKiernan et al., 2003; Park et al., 2010; Persson et al., 2007], suggesting that as attentional demands increase, reallocation of processing that results in suppression of default network activity occurs. To date, multiple studies have provided support for the view that task‐related deactivation in the default mode network is an indicator of reallocation of information processing that underlies successful task performance [Daselaar et al., 2004; Kao et al., 2005; Miller et al., 2008; Otten and Rugg, 2001; Shrager et al., 2008; Wagner and Davachi, 2001]. However, the role of suppression within the default mode network, particularly the posteromedial cortex, during repetitive encoding remains to be elucidated.
In the current study, we investigate activity in the default mode network and the hippocampus in response to repeated stimuli during encoding. Expecting to observe the robust phenomenon of repetition suppression in the hippocampus [e.g., Rand‐Giovannetti et al., 2006], our analyses focus on the hypothesis that task‐induced deactivation in the posteromedial cortex would be observed during initial encoding and progressively diminish in response to repetition. If, as prior evidence suggests, deactivation in the posteromedial cortices reflects reallocation of neuronal resources for efficient cognitive processing, then the reduced task demands created by repeated exposure to a stimulus should lead to reduced deactivation on subsequent encoding trials. Because of the initial deactivation relative to baseline in these regions, such repetition‐related habituation would result in repetition enhancement of neural activity. Emerging evidence suggests that both the MTL and posteromedial cortices are important brain regions supporting memory formation, and that both these regions exhibit failures in age‐related amnestic dementias such as AD. It is therefore critical to also understand how the activity in these regions contributes to successful encoding. Thus, our study may yield important insights into the contributions of these regions during successful memory formation that may have important implications for understanding late‐life amnestic disorders.
METHODS
Subjects
Participants were twenty‐six (13 female) adults aged between 20 and 29 (M = 23.3, SD = 2.5) with between 12 and 17 years of education (M = 15.6, SD = 1.1). All participants were recruited from advertisements on the Internet and were given compensation for their participation. Data from the first encoding trial for 20 participants have appeared in another publication [Vannini et al., 2011]. All participants had normal or corrected‐to‐normal vision, were right‐handed, and native English speakers. Study procedures were approved by the Human Research Committee at the Brigham and Women's T Hospital and Massachusetts General Hospital (Boston, MA). Informed written consent was obtained before participation.
Face–Name Experimental Task
The fMRI experiment employed a rapid event‐design and consisted of a face–name association encoding phase and retrieval phase described in detail in [Vannini et al., 2011]. Given that this study focuses on encoding, the retrieval phase will only be mentioned briefly (see fMRI statistical analysis section below). Subjects received detailed oral instructions prior to each run, and completed a practice session both inside and outside of the MR‐scanner before the experiment began. During the encoding task, subjects were shown pictures of faces against a black background with a fictional first name printed (Times New Roman 36 point font) in white type underneath. Subjects were explicitly told to try to remember the name associated with each face, and to indicate with a button press whether or not they thought the name was a good “fit” for the face or not. This was a purely subjective decision to enhance successful encoding [Sperling et al., 2003b]. The experiment consisted of four encoding runs alternating with four retrieval runs. During each encoding run, subjects viewed 20 face–name pair stimuli, each shown for 2.75 seconds (s), which were presented in a pseudorandom order in groups of four face–name pairs [see Fig. 1B in Vannini et al., 2011 for an example]. Each of the 20 face–name pairs was repeated three times during one encoding run. Each stimulus was presented twice in the beginning of the run (with a mean delay of 15 s between the first and the second presentation), and at the end of each run all stimuli were repeated a third time (with a mean delay of 2 min from the second presentation). This “delayed‐repetition” paradigm was designed to boost encoding success in a manner similar to what might be done in a clinical setting.
Figure 1.
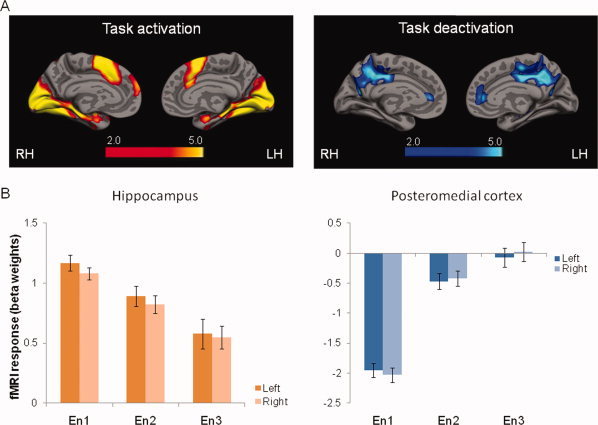
fMRI activation and deactivation patterns and modulation of response during successful repetitive encoding. A: During the first encoding trial of face–name pairs that were subsequently remembered correctly, significant activation in the hippocampus and deactivation in the default network was observed (SPM2, one‐sample t‐test using Fix > RHITenc1, p FDR = 0.05). B: Bar graph of the functional response (mean beta weights) during the three encoding trials (Enc1, En2, and En3) in hippocampus and posteromedial cortex (left and right hemisphere). Error bars denote standard error (S.E.).
Face–name stimuli were randomly intermixed with fixation trials (a white crosshair (+) centred on a black background). During presentation of the fixation cross, subjects were told to focus their attention on the crosshair. The paradigm was designed and generated on an external personal computer using MacStim 2.5 software (WhiteAnt Occasional Publishing, West Melbourne) and projected by means of a magnetic resonance (MR) compatible goggle‐system (VisuaStim XGA, Resonance Technology, Los Angeles, CA). Responses were collected using an MR compatible fiber‐optical key press device with two buttons held in the right hand and responses were recorded by a computer interfaced with the optical switch using MacStim software outside the scanner room.
MR Imaging Acquisition
MRI acquisition was conducted on a General Electric Signa 3.0 Tesla MR system (IGC, Milwaukee, WI) equipped with an eight channel head coil. Changes in blood‐oxygen‐level‐dependent (BOLD) T2*‐weighted MRI signal were measured by using a gradient‐echo EPI sequence: repetition time (TR), 2000 ms; echo time (TE), 30 ms; flip angle (FA), 90° within a field of view (FOV) of 220 cm, and 64 × 64 pixel matrix. Thirty oblique coronal slices, with a slice thickness of 5 mm (interslice distance, 1 mm), perpendicular to the anterior‐posterior commissural (AC‐PC) line, were acquired to cover the whole brain, providing a resolution of 3.44 × 3.44 × 6 mm. Eight functional runs were collected for each subject, consisting of 145 time points per run. The first five (additional) images in each run were discarded to allow the magnetization to reach equilibrium. The total functional scanning time was ∼ 40 min.
High‐resolution T1‐weighted structural images were acquired using a 3D Magnetization Prepared Rapid Acquisition Gradient Echo (MP‐RAGE) sequence: TR = 6.4 ms, TE = 2.8 ms, inversion time (TI) = 900 ms, FA = 8°, FOV = 260 mm, 256 × 256 matrix, 166 sagittal slices, and resolution = 1 mm3.
fMRI Preprocessing
Functional MRI data was preprocessed on a Linux platform running MATLAB version 7.1 (The Mathworks, Sherborn, MA) with Statistical Parametric Mapping (SPM2, Wellcome Department of Cognitive Neurology, London, UK: http://www.fil.ion.ucl.ac.uk). The data were motion corrected using sinc interpolation, by aligning (within‐subject) each time series to the first image volume using a least‐square minimization of a six‐parameter (rigid‐body) spatial transformation. Data were then normalized to the standard SPM2 EPI template and resliced into 3 × 3 × 3‐mm3 resolution in Montreal Neurological Institute (MNI) space. Functional data were smoothed using an isotropic Gaussian kernel of 8 mm full‐width half‐maximum (FWHM). No scaling was implemented for global effects. The coordinates were later converted to Talairach and Tournoux's space [Talairach and Tournoux, 1988] using software available online http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach.
fMRI Statistical Analysis
The face–name stimuli were categorized based on the subjects' responses during the retrieval task administered after completion of the encoding task [for a detailed description see Vannini et al., 2011]. The retrieval task consisted of two phases per stimulus, a cued recall (CR) and a forced choice recognition (FCR) phase. In the CR task, the subject was shown a face and indicated whether he or she remembered or had forgotten the name associated with the face. During the FCR phase, immediately following the CR phase, the face was shown with two names printed underneath; the name originally paired with the face during encoding, and a lure name previously paired with a different face during encoding. Subjects indicated the correct name by pressing one of two buttons. Together, the CR and the FCR phases allowed four possible response conditions for each stimulus: remembered hit (RHIT), forgotten hit (FHIT), remembered miss (RMISS), and forgotten miss (FMISS). Given our goal to investigate successful encoding the current paper focuses on the RHIT variable. For each subject, all runs were concatenated and regressors added, in lieu of global scaling, to account for signal differences between runs.
To provide an overall impression of the regions activated during initial encoding, we computed second level t‐statistics (using random effects) across all subjects, entering the first‐level contrasts for each individual and comparing fMRI activity during the first encoding trial for correctly remembered (RHIT) face–name pairs to a control condition (fixation cross) (RHITen1 > Fix). To investigate regions deactivated during initial encoding the reverse contrast was used (Fix > RHITen1). All maps were thresholded with a false discovery rate (FDR) threshold (p = 0.05) and overlaid on an averaged inflated brain using FreeSurfer software. To investigate the modulation of functional response during successful encoding, we applied two different approaches. First, we performed an individual‐specific region of interest (ROI) analysis, constraining activated voxels to lie within an individually defined anatomical region and selecting above‐threshold voxels from the contrast RHITen1> Fix for activation and Fix > RHITen1 for deactivation using a threshold of p uncorrected < 0.05. Mean beta weights for each of the three encoding trials were extracted within individually defined anatomical regions (using FreeSurfer) in the posteromedial cortex (precuneus) and the hippocampus. These regions were determined on each subject's high resolution MPRAGE image with FreeSurfer (http://surfer.nmr.mgh.harvard.edu/) using a semi‐automated parcellation method based on a probabilistic map [Fischl et al., 2004]. Beta weights were entered into a repeated measure ANOVA with encoding trial (En1, En2, and En3) as the dependent variable. Post hoc analyses using the Bonferroni correction were used to investigate differences in beta weights between trials in case of a significant main effect. Statistical analyses were conducted using STATISTICA 8 (StatSoft Tulsa, OK) software.
Second, to confirm the ROI analysis and to illustrate differences across encoding trials using an exploratory whole brain approach, second level t‐statistics (using random effects) were computed across all subjects; (i) comparing fMRI activity during the first encoding trial with the last encoding trial (RHITen1 > RHITen3), and (ii) comparing fMRI activity during the last encoding trial with the first encoding trial (RHITen3 > RHITen1). Maps were thresholded with p = 0.05, FDR‐corrected for multiple comparisons, and overlaid on an averaged inflated brain using FreeSurfer software.
RESULTS
Task Performance
Participants were generally able to successfully encode the stimuli, correctly reporting face–name pairs as remembered on 77.5% (±3.5 S.E.) of trials, and recording a hit (whether reported as remembered or forgotten) on 92.8% (±1.8 S.E.) of trials. In addition, the low percentage of remembered misses (2.6%) indicates the subjects were able to correctly recognize the right face to the name. See Table I for detailed behavioral results.
Table I.
Behavioral results during the face name association task
| Face name task accuracy (%) | Mean ± S.E |
|---|---|
| Cued recall task | |
| Remember | 77.5 ± 3.5 |
| Forgotten | 20.8 ± 3.6 |
| Forced choice recognition task | |
| Hits | 92.8 ± 1.8 |
| Misses | 5.4 ± 1.1 |
| Combined CR and FCR | |
| Remembered hit | 76.5 ± 3.5 |
| Forgotten hit | 17.9 ± 2.9 |
| Remembered miss | 2.6 ± 0.7 |
| Forgotten miss | 2.9 ± 1.0 |
fMRI Activation Patterns and Modulation of Response During Successful Repetitive Encoding
During the first encoding trial of face–name pairs that were later correctly reported as remembered (contrast used; RHITen1 > Fix), significant activation (p FDR ≤ 0.05) was observed in bilateral visual cortex including inferior occipital gyrus (IOG), frontal cortex including the inferior (IFG) and medial frontal gyri (MeFG) and temporal cortex including the superior temporal gyrus (STG), fusiform gyrus, and hippocampal formation and other subcortical regions including the insula, caudate nucleus and amygdala (Fig. 1A left). Supporting Information Table I lists specific MNI and Talairach coordinates from these activation clusters.
Using the functional ROI approach described in the methods, the mean functional responses (beta weights) from the a priori regions of interest in the (bilateral) hippocampus were extracted for each encoding trial (Fig. 1B left). One subject did not have any activated voxels in this region using the functional threshold set for the analysis and was therefore excluded from the analysis. A significant main effect of encoding trial was observed in both left [F (2,48) = 16.2, p < 0.001] and right [F (2,48) = 18.2, p < 0.001] hemisphere. Post hoc analysis revealed a significant stepwise decrease of activation between the first (M En1right = 1.08, SD = 0.25; M En1left = 1.17, SD = 0.34) and second (M En2right = 0.82, SD = 0.39; M En2left = 0.89, SD = 0.45) encoding trials in left (p = 0.03) and right (p = 0.02) hemispheres, and between the first and third (M En3right = 0.55, SD = 0.49; M En3left = 0.58, SD = 0.64) encoding trials in left (p < 0.001) and right (p < 0.001), and between the second and third encoding trials in left (p = 0.011) and right (p = 0.009) hemispheres.
fMRI Deactivation Patterns and Modulation of Response During Successful Repetitive Encoding
During the first encoding trial of face–name pairs that were later correctly reported as remembered (contrast used; Fix > RHITen1), significant deactivation (p FDR ≤ 0.05) in the default network was observed, particularly in the parietal lobe bilaterally, including inferior parietal lobe (IPL; BA 40) and precuneus (BA 7) extending into posterior cingulate gyrus (BA 31; Fig. 1A right). Significant bilateral clusters of deactivation were also observed in the frontal cortex, including anterior cingulum (BA 32) and middle frontal gyrus (MFG, BA 8 and 31) and temporal cortex (BA 21 and 22). Supporting Information Table II lists specific MNI and Talairach coordinates from these activation clusters.
The mean functional response (beta weights) from the a priori regions of interest in the bilateral posteromedial cortex was extracted for each encoding trial (Fig. 1B right). A significant main effect of encoding trial was observed in both left [F (2,50) = 49.9, p < 0.001] and right [F (2,50) = 56.9, p < 0.001] hemispheres. Similar to the hippocampus, a significant stepwise decrease of deactivation was observed between the first (M En1right = −2.03, SD = 0.62; M En1left = −1.95, SD = 0.61) and second (M En2right = −0.42, SD = 0.65; M En2left = −0.47, SD = 0.67) encoding trials in left (p < 0.001) and right (p < 0.001) hemispheres, and between the first and third (M En3right = 0.03, SD = 0.82; M En3left = −0.08, SD = 0.83) encoding trials in left (p < 0.001) and right (p < 0.001) hemispheres. The difference between the second and third encoding trials did not reach significant in either hemisphere (left: p = 0.15, right: p = 0.10). Notably, activity in the third encoding trial returned to baseline levels (not different from 0, p = 0.65 in left and p = 0.87 in right hemisphere).
Whole Brain Exploratory Effects of Repetition Suppression
To confirm the ROI analysis in the hippocampus and to further explore additional areas that demonstrate repetition related decreases of initial activation we performed a one sample t‐test (p FDR ≤ 0.05) between the first and third encoding trial using the contrast RHITen1 > RHITen3. We again found a significant decrease of activation in the hippocampus (both hemispheres; Fig. 2A). This analysis revealed other regions exhibiting a decrease of activation with repetition (compare to Fig. 1A), including visual cortex and several regions in the frontal cortex. Supporting Information Table III lists specific MNI and Talairach coordinates from these activation clusters.
Figure 2.
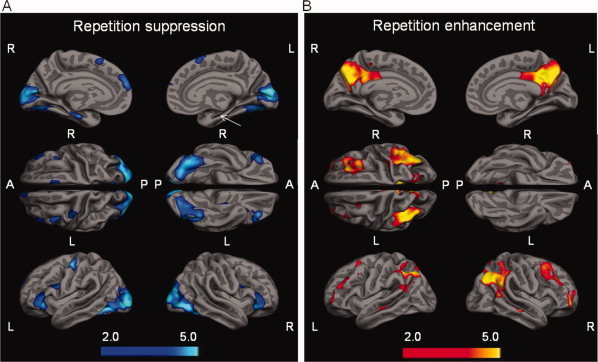
Repetition suppression and repetition enhancement during successful repetitive encoding. A: Displays areas with significantly increased activation in the first encoding trial as compared to the last encoding trial (SPM2, one‐sample t‐test using RHITenc1 > RHITen3, p FDR = 0.05). Right = R, Left = L, Anterior = A, Posterior = P. Lighter colors indicate more significant activation (light blue/dark blue). Arrow depicts cluster of activation in the left hippocampus/parahippocampal gyrus. B: Displays areas with significantly decreased (less) deactivation in the last encoding trial as compared to the first encoding trial (SPM2, one‐sample t‐test using RHITen3 > RHITenc1, p FDR = 0.05). Lighter colors indicate more significant activation (yellow/red).
Whole Brain Exploratory Effects of Repetition Enhancement
To confirm the ROI analysis in the posteromedial cortex and explore other areas that demonstrate a repetition related increase of activation we performed a one sample t‐test (p FDR ≤ 0.05) between the third and first encoding trial using the contrast RHITen3 > RHITen1. Similar to the ROI analysis, we found a significant increase of activation in the functional response within posteromedial cortex (both hemispheres; Fig. 2B). Supporting Information Table IV lists specific MNI and Talairach coordinates from these activation clusters. Notably, many areas demonstrating deactivation (compare to Fig. 1B) during the first encoding trial showed evidence of relatively increased activation during the third encoding trial (see Supporting Information Fig. 1).
DISCUSSION
This study investigated repetition‐related changes in the posteromedial cortex and the hippocampus while participants underwent an event‐related fMRI experiment involving repetitive encoding of face–name pairs. Similar to previous memory studies, the current results demonstrate a robust repetition suppression effect in the hippocampus, further bolstering the hypothesis that an attenuated functional response after repeated presentation in hippocampal areas engaged during initial encoding is associated with subsequent memory performance. In addition, we report repetition related increases of initially deactivated regions in areas associated with the default mode network, particularly the posteromedial cortex. These findings support the idea that less attention was needed to process the task as the stimuli were repeated, (but see alternative explanations discussed below).
The finding of bilateral activation in the hippocampus during the initial encoding of face–name associations that were subsequently remembered correctly is consistent with previous findings in young and healthy elderly individuals [e.g., Chua et al., 2007; Daselaar et al., 2003; Grön et al., 2003; Rand‐Giovannetti et al., 2006; Sperling et al., 2003a]. In addition to increased activation in hippocampus, we also found activation in multiple regions part of a recognized core neural network for face perception [Haxby et al., 2000]. In particular, activation in the inferior occipital gyrus and the frontal gyrus has been demonstrated to mediate face recognition [e.g., Ishai et al., 1999; Kanwisher et al., 1997], whereas increased activation in the insula and amygdala has been shown to be responsive to facial expressions [e.g., Anderson et al., 2003; Whalen et al., 1998]. In addition, activation in the superior temporal gyrus has been proposed to mediate processing of cues for social communication, such as the direction of eye gaze [Puce et al., 1999].
With regard to successful encoding, the current finding of decreased neural responses in regions engaged during initial encoding, particularly the hippocampus, supports previous functional neuroimaging findings demonstrating that intact MTL repetition suppression [but see also Ishai et al., 2004 for a discussion about repetition suppression in other regions related to face processing] is related to successful memory processes and retrieval [Buckner et al., 1995; Gonsalves et al., 2005; Johnson et al., 2004, 2008a; Rand‐Giovannetti et al., 2006; Suzuki et al., 2010]. Although several potential models has been proposed [see Grill‐Spector et al., 2006 for a review; Henson and Rugg, 2003], one of the explanations for the reduced activation associated with repeated presentation of identical stimuli, is that it may reflect a “sharpening” of the cortical representations, whereby neurons coding features unnecessary for processing that stimulus lessen their response [Desimone, 1996; Wiggs and Martin, 1998]. Consequently, this result in a smaller, more selective and specialized population of neurons being active after repeated exposure to a stimulus. Although repetition suppression is observed across a number of brain regions, recent findings indicate that such suppression in most such regions is dependent on the specific stimulus presented to the subject. Another, perhaps more theoretical explanation for the observed repetition suppression effect seen in the hippocampus can be made from the novelty‐encoding hypothesis proposed by Tulving and Kroll [1995]. Originally derived from a study using PET, this hypothesis states that the efficacy of encoding online information into long‐term memory depends on the novelty of the information [Tulving and Kroll, 1995]. Similarly, studies using single‐unit recordings in monkeys have been able to demonstrate that neurons in homologous regions part of the temporal lobe as well as the expanded limbic system respond more vigorously to novel versus familiar or recently seen stimuli [e.g., Fahy et al., 1993; Riches et al., 1991]. Thus, in line with this idea, the repetition suppression effect could then be explained by the fact that the hippocampus responds more actively to novel stimuli, whereas the repeated stimuli could be regarded as more familiar and hence results in less neuronal response. However, while our findings are in accordance with previous studies demonstrating a repetition suppression effect during successful memory formation, the changes in the functional response in the posteromedial cortices in response to repeated stimuli was of particular interest.
The present findings of posteromedial cortical involvement during initial encoding are consistent with a growing body of neuroimaging data demonstrating “beneficial” deactivations during successful encoding [Daselaar et al., 2004; Kao et al., 2005; Miller et al., 2008; Otten and Rugg, 2001; Shrager et al., 2008; Wagner and Davachi, 2001]. The findings of additional deactivation in the medial temporal and medial prefrontal regions as well as the anterior cingulum are also in accordance with the pattern of deactivation subserving the default mode network [Buckner et al., 2008; Greicius and Menon, 2004; Mazoyer et al., 2001; McKiernan et al., 2003; Raichle et al., 2001; Shulman et al., 1997]. Although the exact functional relevance of this deactivation is still a focus of debate, these sets of brain regions has been demonstrated to be maximally engaged during rest and suspended during performance of focused cognitive processing [Buckner et al., 2008; Greicius and Menon, 2004; Mazoyer et al., 2001; McKiernan et al., 2003; Raichle et al., 2001; Shulman et al., 1997]. Consequently, this network has been linked to “mind wandering” or the “stream of consciousness” that is disrupted when the individual performs an attention‐demanding task [Mason et al., 2007; McKiernan et al., 2006]. Thus, until now, the prevailing hypothesis states that the observed deactivation pattern may be the consequence of ongoing attentional demanding processing of information and there is also evidence to suggest that the amount of deactivation is related to task difficulty. This latter idea was originally proposed by McKiernan and colleagues, who by using a parametric auditory target detection task were able to demonstrate that task induced deactivation increased as task demand increased in a subsequently administered cognitive probe task [McKiernan et al., 2003]. This finding can be extended to the interpretation of the observed repetition‐related increase in default mode network activity in the current study. That is, in relation to the findings by McKiernan and colleagues, one can hypothesize that as the task demand or task processing becomes less difficult and more automatic with stimulus repetition, less attention is needed, resulting in less engagement of the default mode network. Notably, the repetition enhancement effect in the current study was generally observed in regions that demonstrated deactivation relative to baseline during initial encoding (see Figs. 1B and 2B). More importantly, in the posteromedial cortex, stepwise decreases of deactivation levels were observed, with activity at or above baseline levels in the last encoding trial (see Fig. 1B). Interestingly, the pattern of repetition enhancement effect observed in this study, especially in the posteromedial cortex, overlaps with the pattern of activation observed during self‐referential and reflective activity [Gusnard and Raichle, 2001; Greicius et al., 2003; Johnson et al., 2006, 2009], including processes such as episodic memory retrieval as well as prospective thinking, autobiographical memory, mental images, emotions, and inner speech [Addis et al., 2004, 2007; Burianova et al., 2010; Greicius et al., 2004; Mazoyer et al., 2001; Svoboda et al., 2006]. Accordingly, we recently demonstrated that the deactivation in the posteromedial cortex during initial successful encoding of the same face–name association task used here overlaps functionally with the activation elicited during successful retrieval of that information [Vannini et al., 2010]. In this region, a significant negative correlation was found, that is, greater deactivation during encoding was related to greater activation during successful retrieval, supporting the hypothesis that the process of retrieving episodic information from memory engages the same network of regions in encoding that information [Vannini et al., 2011]. Similar results between deactivation during encoding and activation during retrieval have also been observed by other groups [Daselaar et al., 2009; Gilbert et al., 2012; Huijbers et al., 2009, 2011; Jaiswal et al., 2010; Kim et al., 2009], and has been referred to as the encoding/retrieval flip or the E/R flip. Although the precise mechanism underlying this toggling phenomenon is still under active investigation, one theory suggests that it may represent functionally collaborating brain systems that work together to increase the likelihood of successful information processing [Buckner et al., 2008]. In line with this, one could speculate that an alternative explanation for the observed increase of activation with repeated stimuli (which notably was above baseline levels in the last repetition encoding trial) might be the results of the activation involved during retrieval processes due to a priming effect from having seen that face in prior encoding trials. Yet, another somewhat related explanation could be the fact that novelty (as presented above) drives the effects seen in the default mode network. Thus, in line with the repetition suppression effect in the hippocampal region the observed reduced deactivation during subsequent encoding stages could be the result of an automatic response that is not reflecting attentional allocation at all.
On a similar topic is the relationship between the repetition enhancement and suppression effect, which supports the hypothesis that these two brain regions may work together for successful execution of the task. The current data revealed a significant relationship (r = 0.53, p = 0.007) in the left hemisphere between the activity in these regions, such that an increased repetition enhancement effect in the posteromedial cortex was related to a decreased repetition suppression effect in the hippocampus. If (as argued above) the repetition enhancement effect represents the decrease in attentional demand and the repetition suppression effect represents neuronal efficacy in the hippocampus, one interpretation of the current results would be that less repetition enhancement in the posteromedial cortices and hence more attentional demand throughout all encoding steps leads to better repetition suppression in the hippocampus. Another explanation for this could be the possibility that different cognitive processes are at work at different repeated steps [Mangels et al., 2009]. By investigating event‐related potentials during three repetitive encoding trials of face–name associations, Mangels et al. [2009] found that associative encoding was strongest in the initial presentation whereas the third presentation benefited later memory processes. The authors concluded that the initial presentation had the greatest impact on memory formation but the third encoding trial provided additional encoding support for the name in each pair, to better differentiate face–name pairs from similar (recombined) pairs [Mangels et al., 2009]. Future research will be needed to disentangle these different possibilities in the current results.
To date, few studies have reported findings of decreased task‐induced deactivation with repetition [Koutstaal et al., 2001; Orfanidou et al., 2006; Simons et al., 2003; Soldan et al., 2008], using perceptual and auditory priming of visual objects and words. Thus, our study adds to these results by demonstrating this effect in a memory task using face–name associations. With regard to the current findings of a repetition suppression effect in the hippocampus and the repetition enhancement effect in the posteromedial cortex, two areas implicated in the default mode network during rest, we believe that the current results are in accordance with previous studies demonstrating a dissociation of the functional activation. More importantly, the present study has provided us with important insights into the differential contributions of these regions during successful memory formation, demonstrating that the effect of learning is distinct in these brain regions. Thus, our findings seem to point to the fact that successful episodic memory requires a coordinated reciprocal pattern in response to repeated stimuli among brain areas that needs to be activated and deactivated. Future studies will investigate the implications of these findings for understanding late‐life amnestic disorders. Our previous work has suggested that failure of deactivation during initial encoding may be associated with paradoxical hyperactivity within the hippocampus in older individuals with mild memory impairment [Miller et al., 2008] and recently we demonstrated that this effect was also evident in cognitively healthy older individuals with evidence of amyloid deposition in the posteromedial cortices [Sperling et al., 2009].
CONCLUSION
In summary, the present study supports the hypothesis that successful episodic memory requires the coordinated patterns of neural activity among brain areas that needs to be activated and deactivated. In particular, the current study demonstrates that hippocampus repetition suppression as well as posteromedial repetition enhancement is related to successful encoding processes.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information Figure 1.
Supporting Information Figure 2.
Supporting Information Table 1.
Supporting Information Table 2.
Supporting Information Table 3.
Supporting Information Table 4.
Acknowledgements
The authors are indebted to the volunteers who participated in this study. They thank Janice Fairhurst, Seung‐Schik Yoo, George Chiuo, and Istvan Akos Morocz for their help with scan acquisition at the Center for Advanced Imaging at Brigham and Women's Hospital. This work was supported by the Swedish Brain foundation (P.V.), The Swedish Society for Medicine (P.V.), and Marie Curie Fellowship: FP7‐PEOPLE‐2007‐4‐1‐IOF from the European Union (P.V.). National Institutes of Health: R01 AG027435‐S1 (R.S.), P01AG036694 (R.S.), P50AG00513421 (R.S.), and the Alzheimer's Association: IIRG‐06‐27374 (R.S.).
REFERENCES
- Addis DR, McIntosh AR, Moscovitch M, Crawley AP, McAndrews MP ( 2004): Characterizing spatial and temporal features of autobiographical memory retrieval networks: A partial least squares approach. Neuroimage 23: 1460–1471. [DOI] [PubMed] [Google Scholar]
- Addis DR, Wong AT, Schacter DL ( 2007): Remembering the past and imagining the future: Common and distinct neural substrates during event construction and elaboration. Neuropsychologia 45: 1363–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AK, Christoff K, Panitz D, De Rosa E, Gabrieli JDE ( 2003): Neural correlates of the automatic processing of threat facial signals. J Neurosci 23: 5627–5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R, Petersen S, Ojemann J, Miezin F, Squire L, Raichle M ( 1995): Functional anatomical studies of explicit and implicit memory retrieval tasks. J Neurosci 15: 12–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R, Andrews‐Hanna JR, Schacter DL ( 2008): The brain's default network: Anatomy, function, and relevance to disease. Ann NY Acad Sci 1124: 1–38. [DOI] [PubMed] [Google Scholar]
- Burianova H, McIntosh AR, Grady CL ( 2010): A common functional brain network for autobiographical, episodic, and semantic memory retrieval. Neuroimage 49: 865–874. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Olson IR, Moscovitch M ( 2008): The parietal cortex and episodic memory: An attentional account. Nat Rev Neurosci 9: 613–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua EF, Schacter DL, Rand‐Giovannetti E, Sperling RA ( 2007): Evidence for a specific role of the anterior hippocampal region in successful associative encoding. Hippocampus 17: 1071–1080. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Veltman DJ, Rombouts SARB, Raaijmakers JGW, Jonker C ( 2003): Neuroanatomical correlates of episodic encoding and retrieval in young and elderly subjects. Brain 126: 43–56. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Prince SE, Cabeza R ( 2004): When less means more: Deactivations during encoding that predict subsequent memory. Neuroimage 23: 921–927. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Prince SE, Dennis NA, Kim H, Cabeza R ( 2009): Posterior midline and ventral parietal acitivity is associated with retrieval success and encoding failure. Front Hum Neurosci 3: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R ( 1996): Neural mechanisms for visual memory and their role in attention. Proc Natl Acad Sci USA 93: 13494–13499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H ( 2000): A cortical‐hippocampal system for declarative memory. Nat Rev Neurosci 1: 41–50. [DOI] [PubMed] [Google Scholar]
- Fahy FL, Riches IP, Brown MW ( 1993): Neuronal activity related to visual recognition memory: Long‐term memory and the encoding of recency and familiarity information in the primate anterior and medial inferior temporal and rhinal cortex. Exp Brain Res 96: 457–472. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM ( 2004): Automatically Parcellating the Human Cerebral Cortex. Cereb Cortex 14: 11–22. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Armbruster DJN, Panagiotidi M ( 2012): Similarity between brain activity at encoding and retrieval predicts successful realization of delayed intentions. J Cogn Neurosci 24: 93–105. [DOI] [PubMed] [Google Scholar]
- Golby A, Silverberg G, Race E, Gabrieli S, O'Shea J, Knierim K, Stebbins G, Gabrieli J ( 2005): Memory encoding in Alzheimer's disease: An fMRI study of explicit and implicit memory. Brain 128: 773–787. [DOI] [PubMed] [Google Scholar]
- Gonsalves BD, Kahn I, Curran T, Norman KA, Wagner AD ( 2005): Memory strength and repetition suppression: multimodal imaging of medial temporal cortical contributions to recognition. Neuron 47: 751–761. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Menon V ( 2004): Default‐mode activity during a passive sensory task: Uncoupled from deactivation but impacting activation. J Cogn Neurosci 16: 1484–1492. [DOI] [PubMed] [Google Scholar]
- Grill‐Spector K, Henson R, Martin A ( 2006): Repetition and the brain: Neural models of stimulus‐specific effects. Trend Cogn Sci 10: 14–23. [DOI] [PubMed] [Google Scholar]
- Grön G, Bittner D, Schmitz B, Wunderlich AP, Tomczak R, Riepe MW ( 2003): Variability in memory performance in aged healthy individuals: An fMRI study. Neurobiol Aging 24: 453–462. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini IM ( 2000): The distributed human neural system for face perception. Trends Cog Sci 4: 223–233. [DOI] [PubMed] [Google Scholar]
- Henson R, Rugg M ( 2003): Neural response suppression, haemodynamic repetition effects, and behavioral priming. Neuropsychologia 41: 263–270. [DOI] [PubMed] [Google Scholar]
- Huijbers W, Pennartz CM, Cabeza R, Daselaar SM ( 2009): When learning and remembering compete: A functional MRI study. PLoS Biol 13: 7:e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbers W, Pennartz CMA, Cabeza R, Daselaar SM ( 2011): The hippocampus is coupled with the default network during memory retrieval but not during memory encoding. PLoS ONE 6: e17463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishai A, Ungerleider LG, Martin A, Schouten JL, Haxby JV ( 1999): Distributed representation of objects in the human ventral visual pathway. PNAS 96: 9379–9384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishai A, Pessoa L, Bikle PC, Ungerleider LG ( 2004): Repetition suppression of faces is modulated by emotion. Proc Natl Acad Sci USA 101: 9827–9832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal N, Ray W, Slobounov S ( 2010): Encoding of visual‐spatial information in working memory requires more cerebral efforts than retrieval: Evidence from an EEG and virtual reality study. Brain Res 1347: 80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, Susskind‐Wilder L, Conngor DJ, Sabbagh MN, Caselli RJ ( 2004): Hippocampal adaptation to face repetition in healthy elderly and mild cognitive impairment. Neuropsychologia 42: 980–989. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Raye CL, Mitchell KJ, Touryan SR, Greene EJ, Nolen‐Hoeksema S ( 2006): Dissociating medial frontal and posterior cingulate activity during self‐reflection. Soc Cogn Affect Neurosci 1: 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JD, Muftuler LT, Rugg MD ( 2008a): Multiple repetitions reveal functionally and anatomically distinct patterns of hippocampal activity during continuous recognition memory. Hippocampus 18: 975–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Schmitz TW, Asthana S, Gluck MA, Myers C ( 2008b): Associative learning over trials activates the hippocampus in healthy elderly but not mild cognitive impairment. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 15: 129–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MK, Nolen‐Hoeksema S, Mitchell KJ, Levin Y ( 2009): Medial cortex activity, self‐reflection and depression. Soc Cogn Affect Neurosci 4: 313–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM ( 1997): The fusiform face area: A module in human extrastriate cortex specialized for face perception. J Neurosci 17: 4302–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao Y‐C, Davis ES, Gabrieli JDE ( 2005): Neural correlates of actual and predicted memory formation. Nat Neurosci 8: 1776–1783. [DOI] [PubMed] [Google Scholar]
- Kim H, Daselaar SM, Cabeza R ( 2009): Overlapping brain activity between episodic memory encoding and retrieval: Roles of the task positive and task negative networks. Neuroimage 49: 1045–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutstaal W, Wagner AD, Rotte M, Maril A, Buckner RL, Schacter DL ( 2001): Perceptual specificity in visual object priming: Functional magnetic resonance imaging evidence for a laterality difference in fusiform cortex. Neuropsychologia 39: 184–199. [DOI] [PubMed] [Google Scholar]
- Mangels JA, Manzi A, Summerfield C ( 2009): The first does the work, but the third time's the charm: The effects of massed repetition on episodic encoding of multimodal face–name associations. J Cogn Neurosci 22: 457–473. [DOI] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN ( 2007): Wandering minds: The default network and stimulus‐independent thought. Science 315: 393–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houde O, Crivello F, Joliot M, Petit L, Tzourio‐Mazoyer N ( 2001): Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull 54: 287–298. [DOI] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera‐Thompson J, Binder JR ( 2003): A parametric manipulation of factors affecting task‐induced deactivation in functional neuroimaging. J Cogn Neurosci 15: 394–408. [DOI] [PubMed] [Google Scholar]
- McKiernan KA, D'Angelo BR, Kaufman JN, Binder JR ( 2006): Interrupting the “stream of consciousness”: An fMRI investigation. Neuroimage 29: 1185–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Li L, Desimone R ( 1991): A neural mechanism for working and recognition memory in inferior temporal cortex. Science 254: 1377–1379. [DOI] [PubMed] [Google Scholar]
- Miller SL, Celone K, DePeau K, Diamond E, Dickerson BC, Rentz D, Pihlajamaki M, Sperling RA ( 2008): Age‐related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proc Natl Acad Sci USA 105: 2181–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orfanidou E, Marslen‐Wilson WD, Davis MH ( 2006): Neural response suppression predicts repetition priming of spoken words, and pseudo words. J Cogn Neurosci 18: 1237–1252. [DOI] [PubMed] [Google Scholar]
- Otten LJ, Rugg MD ( 2001): When more means less: Neural activity related to unsuccessful memory encoding. Curr Biol 11: 1528–1530. [DOI] [PubMed] [Google Scholar]
- Park DC, Polk TA, Hebrank A, Jenkins LJ ( 2010): Age differences in default mode activity on easy and difficult spatial judgement tasks. Front Hum Neurosci 3: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J, Lustig C, Nelson JK, Reuter‐Lorenz PA ( 2007): Age differences in deactivation: A link to cognitive control? J Cogn Neurosci 19: 1021–1032. [DOI] [PubMed] [Google Scholar]
- Pihlajamäki M, DePeau K, Blacker D, Sperling RA ( 2008): Impaired medial temporal repetition suppression is related to failure of parietal deactivation in Alzheimer disease. Am J Geriatr Psychiatry 16: 283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihlajamäki M, O'Keefe K, Bertram L, Tanzi RE, Dickerson BD, Blacker D, Albert MS, Sperling RA ( 2009): Evidence of altered posteromedial cortical fMRI activity in subjects at risk for Alzheimer disease. Alzheimer Dis Assoc Disord 24: 28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puce A, Allison T, McCarthy G ( 1999): Electrophysiological studies of human face perception. III: Effects of top‐down processing on face‐specific potentials. Cereb Cortex 9: 445–458. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL ( 2001): A default mode of brain function. Proc Natl Acad Sci USA 98: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand‐Giovannetti E, Chua EF, Driscoll AE, Schacter DL, Albert MS, Sperling RA ( 2006): Hippocampal and neocortical activation during repetitive encoding in older adults. Neurobiol Aging 27: 173–182. [DOI] [PubMed] [Google Scholar]
- Riches IP, Wilson FA, Brown MW ( 1991): The effects of visual stimulation and memory on the neurons of the hippocampal formation and the neighboring parahippocampal gyurs and the inferior temporal cortex of teh primate. J Neurosci 11: 1763–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg MD, Otten LJ, Henson RNA ( 2002): The neural basis of episodic memory: Evidence from functional neuroimaging. Philos Trans R Soc B 357: 1097–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Buckner RL ( 1998): Priming and the brain. Neuron 20: 185–195. [DOI] [PubMed] [Google Scholar]
- Shannon BJ, Buckner RL ( 2004): Functional‐anatomic correlates of memory retrieval that suggest nontraditional processing roles for multiple distinct regions within posterior parietal cortex. J Neurosci 24: 10084–10092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrager Y, Kirwan CB, Squire LR ( 2008): Activation in both hippocampus and perirhinal cortex predicts the memory strenght of subsequently remembered information. Neuron 59: 547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE ( 1997): Common blood flow changes across visual tasks: II. decreases in cerebral cortex. J Cogn Neurosci 9: 648–663. [DOI] [PubMed] [Google Scholar]
- Simons JS, Koutstaal W, Prince S, Wagner AD, Schacter DL ( 2003): Neural mechanisms of visual object priming: Evidence for perceptual and semantic distinctions in fusiform cortex. Neuroimage 19: 613–626. [DOI] [PubMed] [Google Scholar]
- Soldan A, Gazes Y, Hilton HJ, Stern Y ( 2008): Aging does not affect brain patterns of repetition effects associated with perceptual priming of novel objects. J Cogn Neurosci 20: 1762–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaniol J, Davidson PSR, Kim ASN, Han H, Moscovitch M, Grady CL ( 2009): Event‐related fMRI studies of episodic encoding and retrieval: Meta‐analyses using activation likelihood estimation. Neuropsychologia 47: 1765–1779. [DOI] [PubMed] [Google Scholar]
- Sperling R, Chua E, Cocchiarella A, Rand‐Giovannetti E, Poldrack R, Schacter DL, Albert M ( 2003a): Putting names to faces: Successful encoding of associative memories activates the anterior hippocampal formation. Neuroimage 20: 1400–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Bates JF, Chua EF, Cocchiarella AJ, Rentz DM, Rosen BR, Schacter DL, Albert MS ( 2003b): fMRI studies of associative encoding in young and elderly controls and mild Alzheimer's disease. J Neurol Neurosurg Psychiatry 74: 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, LaViolette PS, O'Keefe K, O'Brien J, Rentz DM, Pihlajamaki M, Marshall G, Hyman BT, Selkoe DJ, Hedden T, Buckner RL, Becker JA, Johnson KA ( 2009): Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron 63: 178–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Johnson JD, Rugg MD ( 2010): Decrements in hippocampal activity with item repetition during continuous recognition: An fMRI study. J Cogn Neurosci 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, Levine B ( 2006): The functional neuroanatomy of autobiographical memory: A meta‐analysis. Neuropsychologia 44: 2189–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P ( 1988): Co‐Planar Stereotaxic Atlas of the Human Brain. Stuttgart: Thieme Medical Publishers. [Google Scholar]
- Tulving E, Kroll NEA ( 1995): Novelty assessment in the brain and long‐term memory encoding. Psychonomic Bull Rev 2: 387–390. [DOI] [PubMed] [Google Scholar]
- Uncapher MR, Wagner AD ( 2009): Posterior parietal cortex and episodic encoding: Insights from fMRI subsequent memory effects and dual‐attention theory. Neurobiol Learn Mem 91: 139–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannini P, O'Brien J, O'Keefe K, Pihlajamaki M, LaViolette PS, Sperling RA ( 2011): What goes down must come up: Role of the posteromedial cortices in encoding and retrieval. Cereb Cortex 21: 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Davachi L ( 2001): Cognitive neuroscience: Forgetting of things past. Curr Biol 11: R964–R967. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA ( 1998): Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci 18: 411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggs CL, Martin A ( 1998): Properties and mechanisms of perceptual priming. Curr Opin Neurobiol 8: 227–233. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information Figure 1.
Supporting Information Figure 2.
Supporting Information Table 1.
Supporting Information Table 2.
Supporting Information Table 3.
Supporting Information Table 4.


