Abstract
Background
Siblings of children with autism (sibs-A) are at increased genetic risk for autism spectrum disorders (ASD) and milder impairments. To elucidate diversity and contour of early developmental trajectories exhibited by sibs-A, regardless of diagnostic classification, latent class modeling was used.
Methods
Sibs-A (n=204) were assessed with the Mullen Scales of Early Learning from age 6–36 months. Mullen T scores served as dependent variables. Outcome classifications at age 36 months included: ASD (n=52); non-ASD social/communication delay (broader autism phenotype; BAP) (n=31); and unaffected (n=121). Child-specific patterns of performance were studied using latent class growth analysis. Latent class membership was then related to diagnostic outcome through estimation of within-class proportions of children assigned to each diagnostic classification.
Results
A 4-class model was favored. Class 1 represented accelerated development and consisted of 25.7% of the sample, primarily unaffected children. Class 2 (40.0% of the sample), was characterized by normative development with above-average nonverbal cognitive outcome. Class 3 (22.3% of the sample) was characterized by receptive language, and gross and fine motor delay. Class 4 (12.0% of the sample), was characterized by widespread delayed skill acquisition, reflected by declining trajectories. Children with an outcome diagnosis of ASD were spread across Classes 2, 3, and 4.
Conclusions
Results support a category of ASD that involves slowing in early non-social development. Receptive language and motor development is vulnerable to early delay in sibs-A with and without ASD outcomes. Non-ASD sibs-A are largely distributed across classes depicting average or accelerated development. Developmental trajectories of motor, language, and cognition appear independent of communication and social delays in non-ASD sibs-A.
Keywords: autism, trajectories, broader autism phenotype
Introduction
Autism spectrum disorders (ASD) are defined by qualitative impairment in social and communication development, often accompanied by repetitive and stereotyped patterns of interests and behaviors. Despite these defining behavioral features, there is heterogeneity in ASD symptom severity, onset pattern, and trajectory of development (Landa & Garrett-Mayer, 2006; Landa, Holman, & Garrett-Mayer, 2007; Ozonoff et al., 2010). In addition, the genetic liability for autism may be expressed in first degree relatives of children with autism as ASD or milder impairments often referred to as the broader autism phenotype (BAP) (Bhat, Galloway, & Landa, 2010; Constantino, Zhang, Frazier, Abbacchi, & Law, 2010; Folstein & Rutter, 1977; Gamliel, Yirmiya, Jaffe, Manor, & Sigman, 2009; Landa & Garrett-Mayer, 2006; Landa et al. 2007; Sullivan et al., 2007). Little is known about the patterns of developmental trajectory in the first three years of life in later-born siblings of children with autism (sibs-A).
On average, 20% of sibs-A have clinically significant social and communication impairment warranting a diagnosis of ASD (Brian et al., 2008; Landa et al., 2007; Ozonoff et al., 2011). Others exhibit an intermediate phenotype (often referred to as Broader Autism Phenotype; BAP) involving social and communication delays that are not sufficiently pervasive or atypical in quality to warrant an ASD diagnosis (Constantino et al., 2010; Landa & Garrett-Mayer, 2006; Landa et al., 2007; Ozonoff et al., 2010). Still other sibs-A have relatively typical development (Landa et al., 2007). In the extant literature, examination of early developmental trajectories of sibs-A have compared groups based on their diagnostic classification at a particular point in time (e.g., 24 months, Landa & Garrett-Mayer, 2006; 36 months, Landa, Holman, & Garrett-Mayer, 2007). Such an analytic approach elucidated trajectories of children with early- or later-identified ASD, those with communication and social delay, and those with no such delays (Landa et al., 2007). However, research is needed to objectively define the diversity of developmental course within this high risk group, without regard to diagnostic status.
In the present study, we employ latent class growth curve analysis (LCGA) to examine patterns of developmental trajectories of language, motor, and nonverbal cognitive functioning from 6 to 36 months of life in children at high risk (siblings of children with autism). LCGA generates classes of growth patterns without a priori information about impairment status. Once the classes of trajectories are defined, the distribution of sibs-A within the three categories of impairment (ASD, non-ASD social and communication impairment [hereafter, BAP], and no such impairments), defined at age 36 months, and across the classes will be examined. This analysis will provide clinically significant information about heterogeneity (or lack thereof) of developmental pathways, involving trajectories, for children with different outcome classifications.
Prospective studies of siblings of children with autism generally report that, among those later diagnosed with autism, development begins to diverge from expected patterns sometime around the first birthday (Landa & Garrett-Mayer, 2006; Landa et al., 2007; Ozonoff et al., 2010). Prior to that age, development appears to be rather typical (Bryson et al., 2007; Landa & Garrett-Mayer, 2006; Ozonoff et al., 2010; Young, Merin, Rogers, & Ozonoff, 2009; Zwaigenbaum et al., 2005), although there is preliminary evidence of motor delay in 6-month-old sibs-A who are later diagnosed with communication and social delays (Bryson, Zwaigenbaum, Brian, Roberts, Szatmari, Rombough, V., et al., 2007; Flanagan, Landa, Bhat, & Bauman, in press). Poor postural control, defined by head lag at mean age 6 months, distinguished infant sibs-A from low risk controls, and was predictive of social and communication delay at 36 months, including ASD (Flanagan et al., in press). By 12–14 months, some sibs-A show social and communication delays (Landa & Garrett-Mayer, 2006; Landa et al., 2007; Ozonoff et al., 2010; Sullivan et al., 2007; Zwaigenbaum et al., 2005).
Examination of heterogeneity in developmental trajectories in sibs-A may reveal patterns of developmental regression, slowing, and/or plateauing in some sibs-A, most likely those with ASD. Retrospective studies indicate that between 10.6% and 38.6% of children with ASD exhibit language regression, depending on how strictly the diagnosis of autism is defined (Baird, Charman, Pickles, Chandler, Loucas, Meldrum, et al., 2008). The timing of the alteration in trajectory reportedly occurs between 16–20 months of age (Davidovitch, Glick, Holtzman, Tirosh, & Safir, 2000; Goldberg, Osann, Filipek, Laulhere, Jarvis, Modahl, et al., 2003; Kurita, 1985; Ozonoff, Williams, & Landa, 2005; Shinnar, Rapin, Arnold, Tuchman, Shulman, Ballaban-Gil et al., 2001), at mean age 19.8 months (Fombonne & Chakrabari, 2001). Prospective studies have also identified developmental slowing, plateauing, and diminishing skills in children with ASD (Landa et al., 2007; Ozonoff et al., 2010). Abnormal development is identifiable in the first 15 months, affecting social, communication, and motor development (Landa & Garrett-Mayer, 2006; Landa et al., 2007; Ozonoff et al., 2010; Werner, Dawson, Munson, & Osterling, 2005), distinguishing autism from typical development and other developmental disorders by age one or two years (Colgan et al., 2006; Landa et al., 2007; Osterling, Dawson, & Munson, 2002; Maestro et al., 2005).
The present study seeks to fill a gap in knowledge about the nature of developmental trajectory in sibs-A and determine whether trajectories are associated with outcome classifications of ASD versus BAP versus no impairment. We focus simultaneously on language, motor, and cognitive development from 6–36 months in infants having an older sibling with autism (sibs-A). At 36 months, children were assigned a diagnostic classification: ASD, BAP, or unaffected (no clinically significant delays). After identifying latent trajectories across multiple developmental systems, we examined relations between diagnostic outcome classification and probability of latent class membership. It was hypothesized that three classes of developmental trajectories would be defined. Specifically, one trajectory class was expected to represent a group with delayed development, and was expected to consist of children from the ASD outcome group, with this group increasingly departing from expected levels of developmental achievement. A second trajectory class was expected to be characterized by mild delay, appearing in the second year of life. This trajectory class was expected to characterize the children with the BAP. Finally, a third trajectory class depicting typical performance (within a standard deviation of the test mean), was expected, characterizing unaffected sibs-A.
Methods
Participants
This study was approved by the Johns Hopkins Medicine and the Lurie Center/LADDERS program at the Massachusetts General Hospital for Children (MGHfC) Institutional Review Boards before data collection; all families gave written informed consent for their child’s participation. Participants included infant siblings of children with autism (sibs-A; n=204; 119 boys). Details about recruitment and diagnosis of probands with autism are described in Landa et al., 2007. Exclusion criteria for sibs-A were: age greater than 14 months at study entry; family’s first language being other than English (language measures are normed on English speakers, disadvantaging non-English speakers); birth weight <1500 grams; severe birth trauma; head injury; prenatal illicit drug or excessive alcohol exposure; and severe birth defects.
One hundred twenty of these children were included in the report by Landa et al. (2007), which examined trajectories of social, communication, and play development (using data from the Communication and Symbolic Behavior Scales Developmental Profile (Wetherby & Prizant, 2001) between 14 and 24 months; data from the Mullen Scales of Early Learning (Mullen, 1995) were not examined in that study. None of the studies from our lab, including the paper we published on Mullen trajectories from 6–24 months on a much smaller sample ranging in age from 6–24 months (Landa & Garrett-Mayer, 2006), have examined trajectories of sibs-A using latent class growth analysis.
Measures
The Mullen Scales of Early Learning (Mullen, 1995) is a standardized developmental test for children aged 3–69 months, with five scales: Gross Motor, Fine Motor, Visual Reception, Receptive Language, and Expressive Language. The Visual Reception scale is a proxy for nonverbal cognitive functioning. Each scale was administered to children at 6, 14, 18, 24, 30, and 36 months of age except Gross Motor, which was administered for the last time at 30 months of age because norms do not extend to 36 months. Dependent variables for this study were age-standardized T scores, enabling us to distinguish between normative and non-normative development and detect departure from normative benchmarks over time.
Outcome diagnostic classification
Confirmatory outcome classification, given by a clinical researcher with a master’s degree or Ph.D. and expertise in early diagnosis of ASD, was made at 30 (n=7) or 36 months (n=197) because an ASD diagnosis at this age is considered reliable (Ventola et al., 2006). One child was diagnosed at age 4 years after missing the 36-month visit. The outcome classifications were:
ASD (n=52)
Clinical judgment of autism or Pervasive Developmental Disorder-Not Otherwise Specified and met Autism Diagnostic Observation Schedule-Generic (ADOS; Lord, Rutter, DiLavore, & Risi, 1999) algorithm criteria for ASD or autism. Beginning at age 14 months, expert clinical researchers documented their clinical impressions about the presence or absence of ASD. Timing of first clinical impression of ASD in children with confirmatory outcome diagnosis of ASD provided the basis stratifying the ASD group into ‘early identified’ (identified by age 14 months) (n=27) versus ‘later identified’ (identified after age 14 months) (n=25) groups.
BAP (n=31)
Clinical judgment of social or language delay, in addition to meeting one of the following criteria: score of at least 1.25 standard deviations below the mean on the Vineland Adaptive Behavior Scales-II Socialization or Communication Domain, Preschool Language Scale (Zimmerman, Steiner, & Pond, 2002) Auditory Comprehension or Expressive Communication subtests; met ADOS Reciprocal Social Interaction algorithm domain criteria for ASD or autism (but did not meet clinical judgment for ASD or autism); met test criterion for delay on the Past Tense or Third Person Singular Present Tense scale of the Test of Early Grammatical Impairment (Rice & Wexler, 2001); and/or qualified for and received speech-language intervention services.
Unaffected (n=121)
Did not meet criteria for ASD or BAP.
Analysis plan
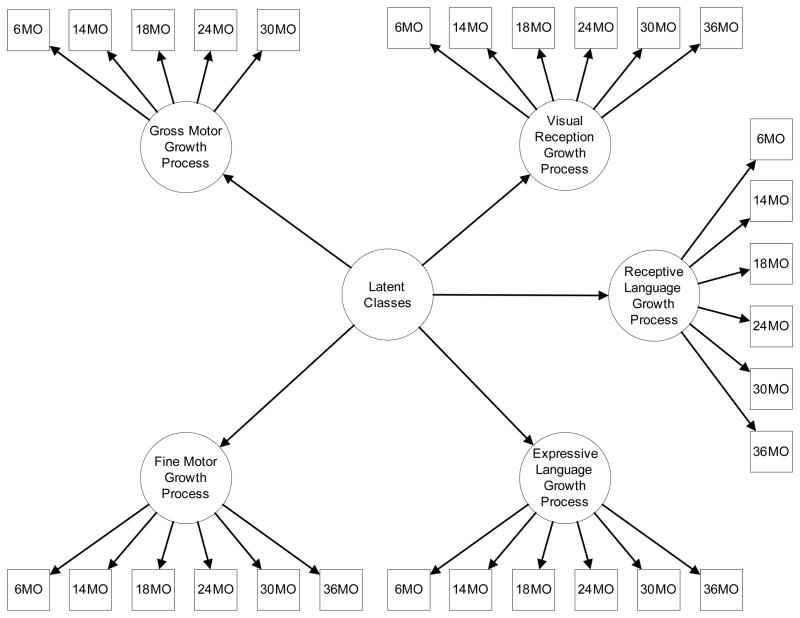
Parallel process latent class growth models (LCGM) were used to study child-specific patterns of trajectory on prospectively measured domains of the Mullen. Latent variables representing intercepts, slopes, and quadratic growth parameters summarize repeatedly measured observed variables (Muthén, 1997; Muthén & Muthén, 2008). In a parallel process LCGM, several growth processes are included in the measurement model. Models with different numbers of latent classes, identified using observed child-specific trajectories, were estimated and interpreted using these latent growth parameters. Latent class membership was then related to clinical diagnoses of ASD or BAP. The general model setup is shown graphically in Figure 1. Trajectories (growth processes) for each of the five Mullen scales are represented by an intercept to denote initial status or baseline score, a slope to represent linear change over time, and a quadratic growth factor (Elliott, Gallo, Ten Have, Bogner, & Katz, 2005). These three components are jointly referred to as the growth parameters (or “growth process”) for each Mullen scale in Figure 1. Means for growth parameters were allowed to vary between classes, and within-class variances of growth parameters were constrained to be 0 in each class. Each latent trajectory class can be interpreted and characterized by the means and variances of its latent growth parameters. Residual variances for repeatedly measured observed outcomes were constrained to be equal within classes.
Figure 1.
Parallel Process Latent Class Growth Curve Model
Parallel process latent class growth curve model of Mullen scale growth processes across six measurement occasions. The model is described notationally in the Methods section. Latent variable intercepts, slopes, and quadratic terms, represented in the graph above as in “growth process” circles, for each score, are used to construct latent classes.
Model development proceeded through three major steps in which components of the model were estimated and checked for model misspecification. This model development procedure follows and expands upon recommended best practices for fitting LCGM (Muthén et al., 2002; Muthén & Shedden, 1999). First, single-class growth models were fit separately for each of the five Mullen scales using data from the six timepoints (6, 14, 18, 24, 30, and 36 months of age, except for the Gross Motor scale, which was administered from 6–30 months). In a second step of model development, linear one-, two-, three-, four, and five-class parallel process LCGM were estimated and compared using model fit criteria and considerations of theoretical utility. The best model was considered to be the most parsimonious model that still provided adequate classification quality of the sample and demonstrated good relative fit using standard fit statistics that are described shortly.
A third step in model development introduced distal clinical outcomes (diagnoses) into the model. Importantly, the identification of classes does not depend on diagnoses. Proportions of diagnostic groups in each class, of each class in diagnostic groups, and tests for significant differences between the proportions are provided. The final model phase is shown graphically in Figure 1 and notationally in equations here. For person-level outcome Y, individual i, time j, Mullen score m, growth parameter q, and class k:
In these equations, Y represents the individual-level outcome for Mullen score m for the ith individual at the jth age. Responses are conditioned on membership in class k. Growth parameters η for each Mullen scale include an initial status factor, a linear slope term, and a quadratic growth factor; subscript q summarizes the growth parameter shapes and β describes random intercepts. Level 1 (ε) and level 2 (ζ) errors are assumed to be normally distributed.
Model parameters were estimated using maximum likelihood estimation using the Mplus version 6.11 software package (Muthén & Muthén, 2010). The number of classes and shapes of trajectories in each class were identified using multiple model fit statistics (Nylund, Asparouhov, & Muthen, 2007). Model fit statistics considered included classification quality (entropy), parsimony indexes including Aikake Information Criterion (AIC; Akaike, 1974) and Bayesian Information Criterion (BIC; Schwartz, 1978), the Lo-Mendel-Rubin likelihood ratio test (LMR-LRT; Lo, Mendell, & Rubin, 2001), and the Bootstrapped likelihood ratio test (BLRT; Feng & McCulloch, 1996). Presence of quadratic growth for the Mullen scales was first tested with likelihood ratio tests comparing the log likelihood of models with quadratic growth in each class with a nested model not allowing for quadratic growth (Satorra & Bentler, 1994). Lower AICs and BICs are used to identify a preferred model that provides adequate fit to the data with the fewest parameters. However, in latent class analysis these statistics do not always increase with additional superfluous parameters (e.g., additional meaningless classes), making them poor indicators of parsimony. Thus, although there is no way to statistically test whether one model’s information criteria is better than another, we choose the k-class model that produced larger drops in information criteria than a k+1 class model. Models with k classes were compared to similar models with k-1 classes using the LMR-LRT and the BLRT. Both provide a p-value testing a null hypothesis that an additional class is unnecessary. The overall entropy statistic, supplemented by class-specific averages of most likely latent class membership, was used to evaluate classification quality of individuals by each model. In addition to these standard fit indices, residual diagnostic procedures were used to assess whether the presented parallel process models adequately reflect observed trajectories and to explore sources of model misspecification (Wang, Brown, & Bandeen-Roche, 2005). Final model choice was guided by these criteria and interpretability of the classes. Only results from the final model are described in this report.
Attrition is common in longitudinal studies (Hansen, Tobler, & Graham, 1990). Parameter estimates from Mplus adjusted for such missing data using a robust full information maximum likelihood (FIML) estimator, which assumes data are missing at random conditional on the variables in the model (Donders, van der Heijden, Stijnen, & Moons, 2006; Little, 1995; Muthén, 2004). FIML is a commonly accepted way to handle missing data (Muthén & Shedden, 1999; Schafer & Graham, 2002). To illustrate the extent of missing data in a dataset, Mplus provides a covariance coverage matrix that shows the proportion of observations available for each pair of variables. The minimum recommended coverage proportion in MPLUS is 10%. The mean coverage proportion among all the variables in analyses was 62.5% and none fell below 35%, so the robust FIML estimator was considered adequate for these data. The estimator used is also robust to non-normally distributed outcomes.
Results
Baseline demographic characteristics are summarized in Table 1. Missing data at six, 18, and 30 months of age was largely due to delays in acquisition of funding to support assessments at these ages. There were significant differences in race between groups (χ2=12.37, df=203, p=.001); the ASD group included significantly more minorities than the Unaffected group (p<.001). No between-group differences were detected in socio-economic status (Hollingshead, 1975). Significant differences in gender were found between groups (χ2=17.04, df=203, p=<.001). The ASD group was comprised of significantly more males than the Unaffected group (p<.001).
Table 1.
Sample characteristics
| Unaffected | Broader Autism Phenotype | Autism Spectrum Disorder
|
|||
|---|---|---|---|---|---|
| Total | Early Identified | Late Identified | |||
| n=121 | n=31 | n=52 | n=27 | n=25 | |
| Sample size at each age | |||||
| 6 months | 91 | 28 | 29 | 13 | 16 |
| 14 months | 115 | 31 | 51 | 26 | 25 |
| 18 months | 63 | 18 | 28 | 15 | 13 |
| 24 months | 117 | 28 | 51 | 26 | 25 |
| 30 months | 56 | 16 | 27 | 14 | 13 |
| 36 months | 114 | 29 | 47 | 23 | 24 |
| Male sex, n (column %) | 53 (43.8%) | 23 (74.2%) | 43 (82.7%) | 21 (77.8%) | 22 (88%) |
| Ethnicity, n (column %) | |||||
| Caucasian | 113 (91.6%) | 34 (89.5%) | 40 (74.1%) | 21 (75%) | 19 (73.1%) |
| African American | 3 (2.1%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Asian | 1 (0.7%) | 1 (2.6%) | 2 (3.7%) | 1 (3.6%) | 1 (3.8%) |
| Multiracial | 3 (2.1%) | 1 (2.6%) | 9 (16.7%) | 5 (17.9%) | 4 (15.4%) |
| Unknown | 2 (3.5%) | 1 (2.6%) | 3 (5.56%) | 1 (3.6%) | 2 (10.5%) |
| SES* | 56.54 | 57.21 | 57.65 | 56.62 | 58.82 |
| ADOS** | |||||
| Communication Total | 1.85 | 3.42 | 4.89 | 4.63 | 5.31 |
| Social Total | 2.06 | 4.63 | 7.96 | 8.85 | 7.23 |
SES refers to the Hollingshead socioeconomic score. This score is derived from both parents’ highest level of education completed and their occupations. A higher score is indicative of higher socioeconomic status. The maximum observed score is 66.
ADOS scores are means from the last visits of children.
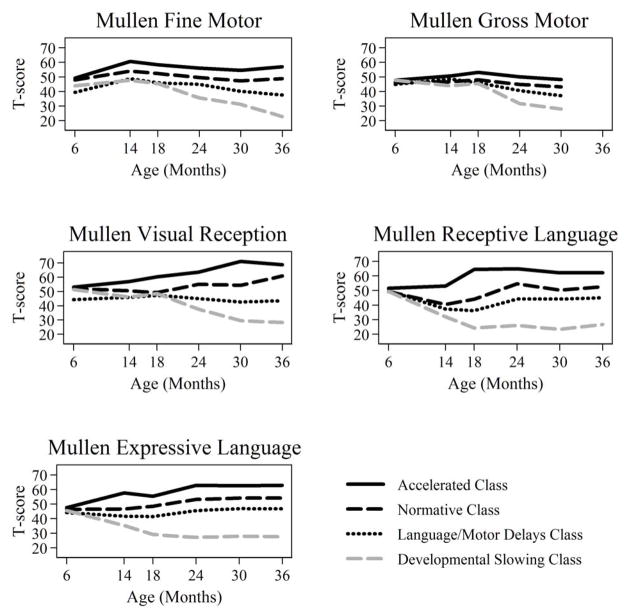
Parallel process latent class growth models without quadratic terms did not fit the observed data as well as models that accommodated quadratic growth. Table 2 shows model fit statistics for one-, two-, three-, four-, and five-class quadratic parallel process LCGM. Model fit statistics provided a mixed picture regarding the optimal number of classes. BIC and AIC parsimony indices never increased with added classes, but the decline plateaued after three or four classes. The BLRT continued to decrease in models up to five classes, but the LMR LRT implied a two-class solution was as good as a three-class solution. The results from the three-and four-class models provided more clarity about developmental trajectories than were represented in the two-class model. Both the three- and four-class models provided excellent classification quality (Entropy: 0.925 and 0.895, respectively), suggesting good separation of individuals into classes. Plots of observed mean trajectories in the four latent classes are shown in Figure 2. Here, we discuss only the results from the four-class model. The rationale is that although Classes 1 and 3 in the three-class model were nearly identical to Classes 1 and 4 in the four-class model, the four-class model provided a more differentiated depiction of trajectories within Class 2 of the three-class model.
Table 2.
Model fit statistics
| Number of classes | Number of Free Parameters | Class proportions based on the estimated model
|
Entropy | BIC | AIC | LMR LRT p-value | BLRT p-value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Class 1 | Class 2 | Class 3 | Class 4 | Class 5 | |||||||
| 1 | 20 | 100.0% | - | - | - | - | - | 35471.8 | 35405.4 | - | - |
| 2 | 36 | 68.6% | 31.4% | - | - | - | 0.955 | 34401.7 | 34282.3 | 0.0004 | <0.0001 |
| 3 | 52 | 33.8% | 50.8% | 15.4% | - | - | 0.925 | 34000.0 | 33827.5 | 0.1023 | <0.0001 |
| 4 | 68 | 25.7% | 40.0% | 22.3% | 12.0% | - | 0.895 | 33928.7 | 33703.1 | 0.194 | <0.0001 |
| 5 | 84 | 18.6% | 22.2% | 18.8% | 12.6% | 27.7% | 0.875 | 33896.7 | 33618.0 | 0.6863 | <0.0001 |
Fit statistics for one, two, and three class parallel process latent growth curve models of Mullen scale scores. Final class proportions for the latent classes are based on the estimated model. Entropy is an index of classification error (values close to 1.0 indicate less error in class assignment). BIC: Bayesian Information Criterion; AIC: Akaike Information Criterion; LMR-LRT: Lo-Mendel-Rubin likelihood ratio test; BLRT: Bootstrap likelihood ratio test.
Figure 2.
Observed Mean Trajectories by Mullen Scale from a Four-Class Parallel Process Latent Class Growth Model (n = 204)
Observed mean trajectories by latent class status from a four-class parallel process latent class growth model (general model setup displayed graphically in Figure 1 and notationally in equations in the Methods when k = 4). In this display, class membership is assigned based on most likely posterior probabilities of class membership.
The first of the four classes, an Accelerated Development class with an expected proportion of 25.7% of the sample, was characterized by initially average levels of functioning based on Mullen norms on all scales at age 6 months. Gross motor development was stable at an average level over time. Through 18 months of age, fine motor and expressive language developmental functioning and trajectories were nearly identical, increasing from average (at the age-normed mean) to about one standard deviation above the mean. From 18 months onward, fine motor development was within the average range but expressive language remained in the above-average range. From 14 to 18 months of age, acceleration in receptive language development was observed, resulting in above-average performance (one standard deviation above the test mean) from 18–36 months (Figure 2). Rate of nonverbal cognitive development steadily accelerated from 6 through 30 months then stabilized at a level that was two standard deviations above the test mean T score.
The second class, exhibiting Normative Development with high nonverbal cognitive outcome, was constituted by 40.0% of the sample. This class was characterized by scores falling at the normative test mean on all Mullen scales at all time points except for nonverbal cognitive functioning, which by age 36 months reached above-average levels (T score of 1 standard deviation above the mean) (Figure 2). Receptive language growth rate in this group dipped between 6 and 14 months, but did not fall into the below-average range.
Class 3 (Language/Motor Delay), consisting of 22.3% of the sample, was best defined by early fine motor (age 6 months) and receptive language (14–18 months) delay followed by normative developmental outcomes in all areas except gross and fine motor, which were delayed. By 24 months, receptive language scores had reached an average level. Gross motor development fell below the average range at age 30 months, the final age at which the Gross Motor scale was administered. From 14–24 months, the Fine Motor T score fell within the average range. However, from 24–36 months, the Fine Motor T score began to drop, indicating a slowing in fine motor development. By 36 months, fine motor performance fell below the Mullen normative mean (Figure 2).
Class 4, consisting of 12.0% of the sample, was characterized by Developmental Slowing, where children increasingly departed from the norm over time. Although the children in this class exhibited average T scores at age 6 months, they performed at least two standard deviations below the normative mean by 36 months. By age 36 months, the Fine Motor T score was at the floor (T=20) for the Mullen. Delays were observed first in receptive and expressive language development, being present by age 14 months. Delays in nonverbal cognitive and motor development manifested between 18 and 24 months. Development increasingly departed from the norm through 30 months for nonverbal cognition and through 36 months for fine motor development. By 30 or 36 months, children in this class showed pervasive delays, affecting language, motor, and cognitive development.
Table 3 shows estimated proportions of latent class in each diagnostic phenotype. Here, the most likely latent class membership for an individual is based on posterior probabilities of being in a certain latent class. Children without ASD or BAP primarily were distributed between the Accelerated and Normative development classes; none of the children in this group were assigned to the Developmental Slowing class (Class 4). Similarly, proportions of children with BAP did not differ between the Accelerated and Normative Development classes, but were less often represented in the Accelerated class than in the class characterized by language and motor delays (Language/Motor Delays class). Proportions of children with BAP were less likely to be in the Developmental Slowing class relative to the Accelerated Development and Language/Motor Delays classes. In contrast, having an ASD diagnosis increased the probability of being assigned to the Developmental Slowing class relative to other classes.
Table 3.
Cross-classification of diagnostic status and likely latent class membership (n = 204)
| N | Proportion of diagnostic phenotypes in each latent class
|
Test of proportions by class (p < 0.05) | ||||
|---|---|---|---|---|---|---|
| Class 1: Accelerated | Class 2: Normative | Class3: Language/Motor Delays | Class 4: Developmental Slowing | |||
| Unaffected | 121 | 38.8 | 49.6 | 11.6 | 0.0 | (1=2)>3>4 |
| Broader Autism Phenotype | 31 | 16.1 | 32.3 | 45.2 | 6.5 | 3>1, 2>4, 3>4 |
| Autism Spectrum Disorder | 52 | 1.9 | 25.0 | 30.8 | 42.3 | 4>2>1, 3>1 |
| Early Identified | 27 | 0.0 | 14.8 | 29.6 | 55.6 | 4>3>2>1 |
| Later Identified | 25 | 4.0 | 36.0 | 32.0 | 28.0 | (2=3=4)>1 |
Within-class proportions of diagnostic categories.
With respect to proportions of diagnostic categories comprising each latent class, the Developmental Slowing class was almost entirely comprised of children with ASD and contained a higher proportion of ASD children than either the Accelerated Development class, the Normative class, or the Language/Motor Delays classes (ps<0.001). A higher proportion of children in the Developmental Slowing class than in any other class were identified as having ASD early (by 14 months of age versus at a later age) (ps<0.001). There were more children from the BAP and ASD groups in the Language/Motor Delays class than in the Accelerated Development class (ps<0.01).
Discussion
This is the first prospective longitudinal study of sibs-A to examine developmental trajectory across multiple developmental domains without a priori classification of participants based on outcome diagnosis. This analytic approach elucidates classes of growth patterns in younger siblings of children with autism, who are at high genetic risk for ASD and milder impairments, without constraining the definition of trajectory patterns to specific categories or degree of outcome impairment as we have done in our prior reports (Landa & Garrett-Mayer, 2006; Landa et al., 2007). The present study uses a large sample and examines trajectory from 6 through 36 months of age. For the most part, results supported our hypotheses. While we had anticipated three classes of trajectories, findings were deemed to best support the presence of four distinct patterns of developmental trajectory within sibs-A. Unexpectedly, we identified an Accelerated Development class. This class was characterized by accelerated cognitive and language development relative to increasing chronological age. The second class of trajectories reflected Normative Development. This class was defined by average, stable development across the Mullen developmental domains, except for nonverbal cognition, for which above-average developmental level was achieved by age 36 months. A third class was characterized by early fine motor and receptive language delay, with later gross motor delay. The receptive language delay resolved by age 24 months, and there was a transient normalization of fine motor development. However, by 30 and 36 months, respectively, gross and fine motor delays were identifiable. A Developmental Slowing class was characterized by typical levels of functioning at 6 months of age followed by clinically significant attenuation in developmental rate relative to increasing chronological age, with severe developmental delay across motor, cognitive, and language domains by the third birthday. These findings reveal distinct developmental trajectories in sibs-A.
Our participants, regardless of trajectory class, performed similarly at six months but subsequently diverged into different patterns of developmental trajectory. The distribution of children across the four classes provides insight into developmental trajectories associated with generally healthy development (Unaffected group), BAP, and ASD. Most children having Unaffected outcome classifications were assigned to the Accelerated or Normative Development classes; none were assigned to the Developmental Slowing class. Most children with 36-month BAP classification were represented in the Normative class or the Language/Motor Delays class. A few children from the BAP group were assigned to the Accelerated class (16.1%) and two were assigned to the Developmental Slowing class (6.5%). About half of the children with ASD (55.6%) were represented in the Developmental Slowing class. No children in the early-identified ASD group, and only one child from the later-identified ASD group, were in the Accelerated Development class. These findings indicate that the Developmental Slowing class is mostly restricted to children with the full ASD phenotype. Children with later-identified ASD were equally distributed across Classes 2–4.
The findings reported herein have considerable clinical significance. First, vulnerability in early language acquisition in sibs-A is indicated by a dip in rate of receptive language growth in Class 2 (which did not fall into the below-average range), the slow launch of fine motor and receptive language development in Class 3, and very delayed receptive and expressive language development in the Developmental Slowing class. Children in the Accelerated Development class exhibited healthy receptive language development, possibly reflecting a neurobiological protective factor in this group that is not present in children within the other classes (Sacco, Papaleo, Hager, Rousseau, Moessner, Militerni, Bravaccio, et al., 2007). In addition, the representation of children with ASD in the Normative Development class confirms that quantitatively typical developmental trajectory in non-social aspects of development is possible in some children with ASD. In such cases, qualifying for intervention may pose a challenge. Yet impairments in social functioning, which are difficult to quantify using standardized measures, may be severe enough to restrict academic (Hoekstra, Happe, Baron-Cohen, & Ronald, 2009) and social-emotional success. Thus, if concerns about social functioning exist, but general developmental measures (including IQ tests) reveal at least average performance, measures that are sensitive to social impairments should be administered (e.g., ADOS). Such measures are not routine in public early intervention settings, so an emphasis on incorporating such measures into assessment protocols is indicated.
These prospectively collected longitudinal data reveal that nearly three quarters of sibs-A with ASD fail to acquire new skills at the normative rate, with language and motor skills being particularly vulnerable to delay. For the most comprehensively affected children with ASD, this developmental pattern continues from 6 through 30 months for nonverbal cognitive and language development, and through 36 months for fine motor development. The attenuation in rate of development is gradual. As this study examined norm-referenced scores, we are not able to determine whether developmental plateau or regression contributed to the declining T scores. Of the three developmental domains assessed using the Mullen, disrupted language development is the first to be apparent in the Developmental Slowing class based on visual inspection of the trajectories. The abnormality is present by 14 months, with development stabilizing well below expected levels in the Developmental Slowing class. This suggests a failure to acquire early-developing language skills at expected ages followed by slow and delayed acquisition of other skills. The clinical significance of this pertains to screening. Early screening for ASD (between 12–18 months) should heavily focus on early-developing communication skills such as comprehension of spoken commands and of familiar objects (outside of highly practiced interaction routines), diversity of communicative gestures and speech sounds, and diversity of words spoken with meaningful communicative intent across a range of contexts (Landa, 2011). Motor development, as measured by the Mullen, is the next to clearly depart from normative development in the Developmental Slowing class. Motor scores being within normal limits at 14 months indicate that early motor milestones, such as walking independently, are generally acquired at expected ages in children with ASD, but more complex and refined motor skills are slow to emerge. This may be an early predictor of impairment in development of skilled movements (Bhat, Galloway, & Landa, 2011), including gesture and skills such as handwriting (Haswell, Izawa, Dowell, Mostofsky, & Shadmehr, 2009). Finally, between 18 and 24 months, children in the Developmental Slowing class depart from the norm in nonverbal cognitive development. Slowing is dramatic between 18–30 months, then stabilizes in the delayed range. The timing of this departure from normative development coincides with typical emergence of symbolic thought, relational thinking, and ability to think about unseen things (object permanence). The scores depicted in Table 3 for the Developmental Slowing class indicate that half of the children with ASD are likely to have had delayed development of such complex cognitive skills. This finding concurs with Minshew and colleagues’ (2002) report that abstract processing skills are impaired in ASD, even individuals with no intellectual impairment.
In the Accelerated and Normative classes, performance on motor scales at 36 months was generally more than a standard deviation below performance on language and nonverbal cognitive scales. This phenomenon suggests that performance on the Mullen motor scales, in the absence of intellectual disability, is independent of cognitive and language functioning. Thus we caution against the standard practice of averaging Mullen Fine Motor and Visual Reception scale scores to generate an estimate of nonverbal cognitive functioning. Another caution is offered. The Mullen motor scales indicate average motor functioning at age 6 months in all but Class 3. Yet a delay in postural control has been identified in sibs-A using the Mullen pull-to-sit task, particularly in those who have outcomes of social and communication delay, including ASD (Flanagan et al., in press). This discrepancy indicates that clinicians should not completely rely on test summary scores to the exclusion of performance on key test items or quality of performance when determining risk for impairment or making clinical recommendations. Finally, delay in semantic and grammatical aspects of language development may not be identified by the Mullen’s non-specific measures of receptive and expressive language.
Our findings and other reports in the literature about atypical developmental trajectories in children with ASD have important implications for developmental surveillance and early intervention. Healthy development at six months of age does not protect against developmental delay, especially for children with ASD who exhibit a prodrome that extends at least through mid-infancy (Landa & Garrett-Mayer, 2006; Ozonoff et al., 2010; Young et al., 2009), even though brain development may be altered at that age (Elsabbagh et al., 2012; Wolff et al., 2012). Thus, children at increased genetic risk for ASD, including sibs-A, should be screened regularly and thoroughly. Given the multiple prospective reports of delay in sibs-A, developmental enrichment programs are recommended for all infant sibs-A, where parents learn to incorporate development-enhancing strategies in their daily routines with the child.
Further research is needed to understand developmental trajectories associated with autism and the BAP. As more is understood about different patterns of trajectory across developmental systems in sibs-A, empirically grounded diagnostic algorithms will emerge. Additionally, information needed to develop targeted early interventions and to refine neurobiological theories of ASD will be gained.
Limitations
Limitations of this study include the absence of social measures and missing data at six, 18, and 30 months of age. However, missing data were handled using appropriate modern statistical methods and estimation procedures. The lack of an assessment point between 6 and 14 months is a limitation, preventing us from understanding the timing of disrupted trajectory in the Language/Motor Delays class and Developmental Slowing class. Our use of standardized Mullen scores enabled us to benchmark trajectories to the norm for children in the first three years of life, yet this metric introduces its own set of limitations. First, the Mullen T scores may exhibit ordinal rather than interval properties, thus may not yield equal spacing across the age intervals examined herein. This limits the precision with which the timing of relative delay across developmental domains can be determined. Secondly, T scores do not allow us to observe whether children in the Developmental Slowing group were actually losing raw score points, which may indicate regression.
Conclusion
In the first three years of life, siblings of children with autism may exhibit accelerated, normative, normative with isolated delay, or widespread attenuation in developmental rate as revealed by analyses conducted without a priori separation of groups based on diagnostic classification. Developmental slowing that becomes more accentuated over time, resulting in declining standardized test scores, is almost exclusively restricted to children having an outcome diagnosis of ASD. In those siblings with the BAP, the most common pattern of trajectory is healthy (accelerated or normative) development as measured by a quantitative tool that does not assess social or emotional development. Future studies of developmental trajectory in children with ASD should examine multiple developmental domains using measures that permit observation of patterns of slowing, plateauing, and actual loss of skills.
Key points.
There is diversity in the patterns of developmental trajectory in siblings of children with autism in the first three years of life.
Four main trajectory phenotypes were identified in young children at high risk for autism (sibs-A): Accelerated Development; Normative Development; Language/Motor Delays; and Developmental Slowing.
Most of the sibs-A with Non-ASD language and/or social delays at age 36 months showed developmental trajectories on a standardized developmental assessment that were accelerated or normative across diverse aspects of development.
About half of children with ASD identified at 14 months exhibited a decline in rate of development from 6 to 36 months.
Acknowledgments
We thank the participants and staff at Kennedy Krieger Institute and the Lurie Center/LADDERS of the Mass General Hospital for Children for their help in data acquisition. All authors had full access to all of the data in the study; Dr. Landa takes responsibility for the integrity of the data; Drs. Gross and Stuart take responsibility for the accuracy of data analysis; Dr. Bauman takes responsibility for the integrity of the data collected at the LurieCenter/LADDERS. Funding from the National Institute of Mental Health, awarded to Rebecca Landa (PI): MH59630 (design; study conduct; data collection, management, analysis, interpretation; preparation, review, approval of manuscript), U54 MH066417-04 and Autism Speaks (data collection).
Footnotes
The authors have no conflicts of interest to report.
Contributor Information
Rebecca J. Landa, Center for Autism and Related Disorders, Kennedy Krieger Institute, Psychiatry and Behavioral Sciences, The Johns Hopkins University School of Medicine
Alden L. Gross, Mental Health, Johns Hopkins Bloomberg School of Public Health
Elizabeth A. Stuart, Mental Health, Johns Hopkins Bloomberg School of Public Health Biostatistics, Johns Hopkins Bloomberg School of Public Health
Margaret Bauman, Lurie Center/LADDERS, Mass General Hospital for Children, Neurology, Harvard Medical School.
References
- Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19:716–723. [Google Scholar]
- Baird G, Charman T, Pickles A, Chandler S, Loucas T, Meldrum D, et al. Regression, developmental trajectory, and associated problems in disorders in the autism spectrum: The SNAP study. Journal of Autism and Developmental Disorders. 2008;38:1827–1836. doi: 10.1007/s10803-008-0571-9. [DOI] [PubMed] [Google Scholar]
- Bhat AN, Galloway JC, Landa RJ. Social and non-social visual attention patterns and associative learning in infants at risk for autism. Journal of Child Psychology and Psychiatry. 2010;51:989–997. doi: 10.1111/j.1469-7610.2010.02262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat A, Landa R, Galloway JC. Perspectives on motor problems in infants, children, and adults with autism spectrum disorders. Physical Therapy. 2011;91:1116–1129. doi: 10.2522/ptj.20100294. [DOI] [PubMed] [Google Scholar]
- Brian J, Bryson SE, Garon N, Roberts W, Smith IM, Szatmari P, et al. Clinical assessment of autism in high-risk 18-month-olds. Autism. 2008;12:433–456. doi: 10.1177/1362361308094500. [DOI] [PubMed] [Google Scholar]
- Bryson SE, Zwaigenbaum L, Brian JA, Roberts SW, Szatmari P, Rombough V, et al. A prospective case series of high-risk infants who developed autism. Journal of Autism and Developmental Disorders. 2007;37:12–24. doi: 10.1007/s10803-006-0328-2. [DOI] [PubMed] [Google Scholar]
- Colgan SE, Lanter E, McComish C, Watson LR, Crais ER, Baranek GT. Analysis of social interaction gestures in infants with autism. Child Neuropsychology. 2006;12:307–319. doi: 10.1080/09297040600701360. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Zhang Y, Frazier T, Abbacchi AM, Law P. Sibling recurrence and the genetic epidemiology of autism. American Journal of Psychiatry. 2010;167:1349–1356. doi: 10.1176/appi.ajp.2010.09101470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidovitch M, Glick L, Holtzman G, Tirosh E, Safir MP. Developmental regression in autism: Maternal perception. Journal of Autism and Developmental Disorders. 2000;30:113–119. doi: 10.1023/a:1005403421141. [DOI] [PubMed] [Google Scholar]
- Donders AR, van der Heijden GJ, Stijnen T, Moons KG. Review: A gentle introduction to imputation of missing values. Journal of Clinical Epidemiology. 2006;59:1087–1091. doi: 10.1016/j.jclinepi.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Elliott MR, Gallo JJ, Ten Have TR, Bogner HR, Katz IR. Using a Bayesian latent growth curve model to identify trajectories of positive affect and negative events following myocardial infarction. Biostatistics. 2005;6:119–143. doi: 10.1093/biostatistics/kxh022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsabbagh M, Mercure E, Hudry K, Chandler S, Pasco G, Charman T, Pickles A. Infant neural sensitivity to dynamic eye gaze is associated with later emerging autism. Current Biology. 2012;22:1–5. doi: 10.1016/j.cub.2011.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z, McCulloch CE. Using bootstrap likelihood ratio in finite mixture models. Journal of the Royal Statistical Society, Series B. 1996;58:609–617. [Google Scholar]
- Flanagan J, Landa R, Bhat A, Bauman M. Head lag in infants at risk for autism: A preliminary study. American Journal of Occupational Therapy. doi: 10.5014/ajot.2012.004192. (in press) [DOI] [PubMed] [Google Scholar]
- Folstein S, Rutter M. Infantile autism: A genetic study of 21 twin pairs. Journal of Child Psychology and Psychiatry. 1977;18:297–321. doi: 10.1111/j.1469-7610.1977.tb00443.x. [DOI] [PubMed] [Google Scholar]
- Fombonne E, Chakrabari S. No evidence for a new variant of measles-mumps- rubella-induced autism. Pediatrics. 2001;108:E58. doi: 10.1542/peds.108.4.e58. [DOI] [PubMed] [Google Scholar]
- Gamliel I, Yirmiya N, Jaffe DH, Manor O, Sigman M. Developmental trajectories in siblings of children with autism: Cognition and language from 4 months to 7 years. Journal of Autism and Developmental Disorders. 2009;39:1131–1144. doi: 10.1007/s10803-009-0727-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godlberg WA, Osann K, Filipek PA, Laulhere T, Jarvis K, Modahl C, et al. Language and other regression: Assessment and timing. Journal of Autism and Developmental Disorders. 2003;33:607–616. doi: 10.1023/b:jadd.0000005998.47370.ef. [DOI] [PubMed] [Google Scholar]
- Hansen WB, Tobler NS, Graham JW. Attrition in substance abuse prevention research: A metaanalysis of 85 longitudinally followed cohorts. Evaluation Review. 1990;14:677–685. [Google Scholar]
- Haswell CC, Izawa J, Dowell LR, Mostofsky SH, Shadmehr R. Representation of internal models of action in the autistic brain. Nature Neuroscience. 2009;12:970–972. doi: 10.1038/nn.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra RA, Happe F, Baron-Cohen S, Ronald A. Association between extreme autistic traits and intellectual disability: insights from a general population twin study. The British Journal of Psychiatry. 2009;195:531–536. doi: 10.1192/bjp.bp.108.060889. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four Factor Index of Social Status. New Haven, CT: Department of Sociology, Yale University; 1975. Unpublished Manuscript. [Google Scholar]
- Kurita H. Infantile autism with speech loss before the age of thirty months. Journal of the American Academy of Child Psychiatry. 1985;24:191–196. doi: 10.1016/s0002-7138(09)60447-7. [DOI] [PubMed] [Google Scholar]
- Landa R, Garrett-Mayer E. Development in infants with autism spectrum disorders: A prospective study. Journal of Child Psychology and Psychiatry. 2006;47:629–638. doi: 10.1111/j.1469-7610.2006.01531.x. [DOI] [PubMed] [Google Scholar]
- Landa RJ, Holman KC, Garrett-Mayer E. Social and communication development in toddlers with early and later diagnosis of autism spectrum disorders. Archives of General Psychiatry. 2007;64:853–864. doi: 10.1001/archpsyc.64.7.853. [DOI] [PubMed] [Google Scholar]
- Landa R. Developmental features and trajectories associated with autism spectrum disorders in infants and toddlers. In: Geschwind DG, Dawson G, Amaral DH, editors. Autism Spectrum Disorders. New York: Oxford Press; 2011. pp. 213–228. [Google Scholar]
- Little RJA. Modeling the drop-out mechanism in repeated-measures studies. Journal of the American Statistical Association. 1995;90:1112–1121. [Google Scholar]
- Lo Y, Mendell NR, Rubin DB. Testing the number of components in a normal mixture. Biometrika. 2001;88:767–778. [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi S. Autism Diagnostic Observation Schedule. Los Angeles, CA: Western Psychological Services; 1999. [Google Scholar]
- Maestro S, Muratori F, Cesari A, Cavallaro MC, Paziente A, Pecini C, et al. Course of autism signs in the first year of life. Psychopathology. 2005;38:26–31. doi: 10.1159/000083967. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Meyer J, Goldstein G. Abstract reasoning in autism: A dissociation between concept formation and concept identification. Neuropsychology. 2002;16:327–334. doi: 10.1037//0894-4105.16.3.327. [DOI] [PubMed] [Google Scholar]
- Mullen EM. Mullen: Scales of Early Learning. Circle Pines, MN: American Guideline Service, Inc; 1995. (AGS edn) [Google Scholar]
- Muthén B. Latent variable modeling with longitudinal and multilevel data. In: Raftery A, editor. Sociological methodology. Boston, MA: Blackwell Publishers; 1997. pp. 453–480. [Google Scholar]
- Muthén B. Latent variable analysis: Growth mixture modeling and related techniques for longitudinal data. In: Kaplan D, editor. Handbook of quantitative methodology for the social sciences. Newbury Park, CA: Sage Publications; 2004. pp. 345–368. [Google Scholar]
- Muthén B, Brown CH, Masyn K, Jo B, Khoo ST, Yang CC, Wang CP, Kellam SG, Carlin JB, Liao J. General growth mixture modeling for randomized preventive interventions. Biostatistics. 2002;3:459–475. doi: 10.1093/biostatistics/3.4.459. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus user’s guide. 6. Los Angeles, CA: Muthén & Muthén; 1998–2010. [Google Scholar]
- Muthén B, Shedden K. Finite mixture modeling with mixture outcomes using the EM algorithm. Biometrics. 1999;55:463–469. doi: 10.1111/j.0006-341x.1999.00463.x. [DOI] [PubMed] [Google Scholar]
- Nylund KL, Asparouhov T, Muthén BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Structural Equation Modeling. 2007;14:535–569. [Google Scholar]
- Osterling JA, Dawson G, Munson JA. Early recognition of 1-year-old infants with autism spectrum disorder versus mental retardation. Developmental Psychopathology. 2002;14:239–251. doi: 10.1017/s0954579402002031. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Williams BJ, Landa R. Parental report of the early development of children with regressive autism: The delays-plus-regression phenotype. Autism. 2005;9:461–486. doi: 10.1177/1362361305057880. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Iosif AM, Baguio F, Cook IC, Moore Hill M, Hutman T, et al. A prospective study of the emergence of early behavioral signs of autism. Journal of the Academy of Child and Adolescent Psychiatry. 2010;49:256–266. [PMC free article] [PubMed] [Google Scholar]
- Rice ML, Wexler K. Rice/Wexler Test of Early Grammatical Impairment. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- Sacco R, Papaleo V, Hager J, Rousseau F, Moessner R, Militerni R, Bravaccio C, et al. Case-control and family-based association studies of candidate genes in autistic disorder and its endophenotypes: TPH2 and GLO1. BMC Medical Genetics. 2007;8:11. doi: 10.1186/1471-2350-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satorra A, Bentler PM. Ensuring positiveness of the scaled difference Chi-Square test statistic. Psychometrika. 1994;75:243–248. doi: 10.1007/s11336-009-9135-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer JL, Graham JW. Missing data: Our view of the state of the art. Psychological Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- Schwartz GE. Estimating the dimension of a model. Annals of Statistics. 1978;6:461–464. [Google Scholar]
- Shinnar S, Rapin I, Arnold S, Tuchman RF, Shulman L, Ballaban-Gil K, et al. Language regression in childhood. Pediatric Neurology. 2001;24:183–189. doi: 10.1016/s0887-8994(00)00266-6. [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Cicchetti DV, Balla DA. Vineland Adaptive Behavior Scales. 2. Circle Pines, MN: American Guidance Services Publishing; 2005. [Google Scholar]
- Sullivan M, Finelli J, Marvin A, Garrett-Mayer E, Bauman M, Landa R. Response to joint attention in toddlers at risk for autism spectrum disorder: A prospective study. Journal of Autism and Developmental Disorders. 2007;37:37–48. doi: 10.1007/s10803-006-0335-3. [DOI] [PubMed] [Google Scholar]
- Ventola P, Kleinman J, Pandey J, Wilson L, Esser E, Boorstein H, et al. Differentiating between autism spectrum disorders and other developmental disabilities in children who failed a screening instrument for ASD. Journal of Autism and Developmental Disorders. 2007;37:425–436. doi: 10.1007/s10803-006-0177-z. [DOI] [PubMed] [Google Scholar]
- Wang CP, Brown CH, Bandeen-Roche K. Residual diagnostics for growth mixture models: Examining the impact of a preventive intervention on multiple trajectories of aggressive behavior. Journal of American Statistical Association. 2005;100:1054–1076. [Google Scholar]
- Werner E, Dawson G, Munson J, Osterling J. Variation in early developmental course in autism and its relation with behavioral outcome at 3–4 years of age. Journal of Autism and Developmental Disorders. 2005;35:337–350. doi: 10.1007/s10803-005-3301-6. [DOI] [PubMed] [Google Scholar]
- Wetherby A, Prizant B. Communication and Symbolic Behavior Scales Developmental Profile. Baltimore, MD: Brookes Publishing; 2001. [Google Scholar]
- Wolff JJ, Gu H, Gerig G, Elison JT, Styner M, Gouttard G, Botteron KN, et al. Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. American Journal of Psychiatry. 2012 doi: 10.1176/appi.ajp.2011.11091447. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young GS, Merin N, Rogers SJ, Ozonoff S. Gaze behavior and affect at 6 months: Predicting clinical outcomes and language development in typically developing infants and infants at risk for autism. Developmental Science. 2009;12:798–814. doi: 10.1111/j.1467-7687.2009.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman IL, Steiner VG, Pond RE. Preschool Language Scale. 4. San Antonio, TX: The Psychological Corporation; 2002. [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. International Journal of Developmental Neuroscience. 2005;23:143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]