Abstract
Previous studies on cellular and molecular mechanisms that regulate vascular development identified key signaling pathways and transcription factors. These findings supported the notion that the formation of vasculature is predominantly regulated by genetic programs, which is generally accepted. However, recent progress in understanding nongenetic factors that can modify the preprogrammed genetic mechanisms added another layer of complexity to our current understanding of vascular development. Here, we briefly summarize historic viewpoints and evolutionary perspectives on vascular development. We also review the cellular and molecular mechanisms that govern the emergence of the endothelial lineage and the subsequent process of vasculogenesis during development, with an emphasis on vascular endothelial growth factor and angiopoietin signaling cascades. Finally, we discuss epigenetic factors such as hemodynamic forces and hypoxic responses that can modulate and override the predetermined genetic mechanisms of vascular development.
Keywords: vasculogenesis, VEGF, angiopoietin, endothelial progenitor, development
The cellular ontogeny and evolutionary origin of the endothelial lineage and the vascular system have been a center of investigation for centuries. Collective efforts so far have determined that the endothelial lineage has a surprisingly heterogeneous origin. Likewise, it is becoming increasingly clear that the molecular mechanisms that govern the initiation of the endothelial lineage are surprisingly intricate.1,2 In recent years, the specification and differentiation of the endothelial lineage have been extensively studied in an effort to delineate genetic mechanisms that modulate this process. These studies have resulted in the identification of numerous diverse signaling pathways associated with the endothelial lineage, including vascular endothelial growth factor (VEGF) and angiopoietin.
Historical Perspective on the Circulatory System
The existence of the circulatory system has been known to many cultures from ancient times. For instance, Yellow Emperor’s Manual of Corporeal Medicine, written in the second century B.C. in China, described the circulation pattern of the blood within the body.3 In ancient Greece, a group of natural philosophers, including the famous Aristotle, recognized that higher animals contain blood.4 The anatomic structure of the circulatory system, however, was not identified until Erasistratus, who discovered membranous structures within the heart that connected to large blood vessels.5 In the second century A.D., Claudius Galenus subsequently demonstrated that blood vessels are used as a conduit for the circulating blood. These observations were rediscovered later during the pinnacle of the Islamic Golden Age. Ibn al-Nafis of Damascus wrote a description about the circulation of blood which was later translated into Latin and served to influence many Renaissance scientists such as Giordano Bruno and Michael Servetus.6,7 However, the actual importance of the circulatory system was not widely recognized until the seminal publication by William Harvey in the seventeenth century, the Exercitatio Anatomica de Motu Cordis et Sanguinis in Animali-bus, (An Anatomic Exercise on the Motion of the Heart and Blood in Living Beings). William Harvey was the first person to suggest the presence of a complete circulatory loop within the body, including the pulmonary circulation, and delineated the function of the heart.8,9 With the advent of the microscope, the Italian scientist Marcello Malpighi discovered that arteries and veins are connected via capillary vessels.9
Although the physiological aspects of the circulatory system were relatively well known by the end of the Renaissance era, its origin during development remained largely unexplored. In the late 1800s, Wilhelm His suggested that the cellular constituents of blood vessels were generated from extraembryonic tissue and that blood vessels themselves were formed by a series of morphogenetic events.10 He used the term “angioblast” to describe the mesenchymal cells that give rise to the cellular constituents of blood vessels. Although this suggested origin of blood vessels by His was proven wrong, he did correctly point out that blood vessels do develop via distinct morphogenetic events. In the late 1910s, Florence Sabin observed that, during development, red blood cells and blood vessels originate concomitantly in adjacent regions of the avian embryo, and postulated that these two lineages might share the same progenitors. She adopted the term “angioblast” from His to describe this hypothetical progenitor,11 which was later renamed “hemangioblast” by Murray to properly reflect that this progenitor is thought to generate both endothelial and hematopoietic lineages.12 Recently, it has been shown that the endothelial lineage shares common progenitors with diverse cell types derived from the mesoderm, including cardiomyocytes, smooth muscle cells, as well as subtypes of blood cells.13,14 Furthermore, in lower vertebrates, it has been reported that mesodermal cells programmed to generate kidney or other mesodermal cell types can differentiate into endothelial progenitors under certain circumstances, 15,16 suggesting that the emergence of the endothelial lineage might be far more diverse than previously thought.
Evolution of the Circulatory System
The essential function of the circulatory system is to provide essential nutrients and oxygen, as well as to remove cellular waste. Various model organisms that are commonly used to study biological sciences reflect distinct evolutionary stages of the circulatory system. In the animal kingdom, some phyla, notably Platyhelminthes, Nematoda, and Porifera, lack any circulatory system. Instead, their body structure allows for all cells to directly absorb nutrients and oxygen. For instance, a well known animal model, Caenorhabditis elegans, has a primitive internal cavity known as the pseudocoelom, which allows every cell in the body to have direct access to nutrients and oxygen.17 In addition, this organism possesses a rudimentary excretory system consisting of two cells which extend a tubular structure along the body axis to regulate osmotic pressure and remove excessive ions and cellular metabolites.17 However, the majority of Bilateria have a functional circulatory system. Depending on whether blood and interstitial fluid mix, the circulatory system in these animals is divided into two major groups: the open circulatory system and the closed circulatory system. In the phyla with the open circulatory system such as Arthropoda, the blood can freely blend with the interstitial fluid to generate hemolymph. The internal organs in these animals are submerged within an internal cavity called the hemocoel and directly receive oxygen and nutrients, and deposit metabolic wastes.18 The hemolymph is drawn back through the ostia (open end pore) when the heart dilates, a process that is often facilitated by muscular movement. Closed circulatory systems can be found in Chordata, Annelida, as well as some species of Mollusca (Cephalopods). In the closed circulatory system, the blood circulates only within the internal lumen of the blood vessels and does not mix with interstitial fluid.
Cellular Origin of Endothelial Cells During Development
During development, endothelial cells appear to emerge from mesodermal tissue residing within diverse regions of the embryo, ranging from blood islands in the yolk sac19,20 and within the embryo proper21,22 to atypical regions such as allantois23,24 and placenta25,26 (Figure). In mice, the first site the endothelial lineage appears is the extraembryonic visceral mesoderm in the yolk sac, where endothelial progenitors contribute to the blood islands.27,28 Endothelial progenitors in the embryo proper subsequently appear in the rostral region of the embryo around embryonic day (E) 8, based on the expression pattern of various endothelial markers. In the avian model, formation of endothelial cells can be observed as early as the gastrulation stage. Similar to the endothelial progenitors in mice, endothelial progenitors in avian embryos are closely associated with the hematopoietic lineage.29–31
Figure.
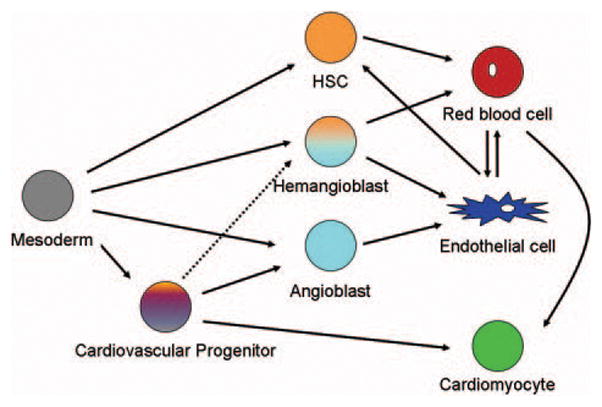
Origin of the endothelial lineage. The endothelial lineage appears to emerge from diverse progenitors and has a close association with other lineages found in the cardiovascular system.
Based on the temporal and spatial proximity of endothelial and hematopoietic lineages during avian development, in the early 1900s Sabin postulated that these two lineages share a common progenitor, which was later named the “hemangioblast” by Murray.11,12 Since then, much indirect evidence supporting this common progenitor notion has been reported, including the phenotype of VEGF receptor2 (Flk1/KDR) knock-out mice27 and the zebrafish mutant, clo.32 Recently, studies using embryonic stem (ES) cell–derived embryoid bodies provided more evidence that supports the existence of the hemangioblast. Using ES cells containing blast colony-forming cells (BL-CFC), Choi and colleagues reported that a single progenitor-derived embryoid body contains both endothelial, hematopoietic, and smooth muscle lineages, implying the existence of the hemangioblast.33 Although these observations do not provide direct evidence of the hemangioblast, they provide invaluable insights into understanding the endothelial or hematopoietic lineage specification process. Despite these findings, providing in vivo evidence that directly supports the existence of the hemangioblast as a bipotential progenitor during development has been extremely difficult because of the technical limitations innate to commonly used model systems. Previously, based on their fate mapping and lineage tracing data, Kinder and colleagues suggested that endothelial and hematopoietic lineages might arise independently, alluding to the fact that the hemangioblast might not exist in vivo.34 However, Huber and colleagues took this one step further and isolated potential hemangioblast cells (identified by the expression of both brachyury and Flk1) from E7.5 mouse embryos and showed that these cells could give rise to both hematopoietic and vascular cells.35 Recently, Vogeli and colleagues demonstrated that developing zebrafish gastrula contain progenitors that generate both endothelial and hematopoietic lineages using single cell resolution fate mapping.36 Surprisingly, they also found that the hemangioblast gives rise to only a small fraction of the endothelial lineage, whereas the majority of the endothelial lineage derives from endothelial-specific progenitors, or angioblasts.36 Similar findings have also been reported in mammalian and avian models.37,38 Although the precise molecular and cellular mechanisms modulating the specification of endothelial progenitors are largely unknown, recent identification of Islet1, Nkx2.5, and Flk1-positive multipotent cardiovascular progenitor cells within the nascent mesodermal cells provide an important insight into the developmental ontogeny of the endothelial lineage13,14 (Figure). It is likely that the hemangioblast is an integral component of early vascular development as well as primitive hematopoiesis. However, whether the hemangioblast exists beyond the early developmental stage to contribute to definitive hematopoiesis, and if so, also plays a critical role in postembryonic stage, still needs to be determined.
The majority of the endothelial lineage does appear to originate from conventional progenitors such as angioblast and hemangioblast, which are predominantly derived from the posterior and lateral mesoderm. However, it is also critical to note that the entire mesoderm (excluding notochord and prechordal mesoderm) can be reprogrammed to generate endothelial cells during development,39 suggesting that the ability to produce the endothelial lineage might be one of the intrinsic properties of the mesoderm. However, this plasticity of mesodermal cells diminishes as embryogenesis progresses, and only a small group of endothelial progenitors retain the ability to produce the endothelial lineage in later development.
Vasculogenesis: De Novo Formation of Vascular Structure
Vascular structures in vertebrates are formed by two distinct mechanisms: vasculogenesis and angiogenesis. Vasculogenesis is a process by which new vessels are generated from endothelial progenitors via differentiation. The subsequent assembly of these differentiated cells leads to the formation of primitive blood vessels. Conversely, angiogenesis describes the process of new vessel formation from the sprouting or splitting of preexisting vessels. Although environmental factors such as hemodynamics40,41 and hypoxic response42,43 appear to modulate the vasculogenesis process, initial development of the vasculature seems to be genetically programmed.44–46 In mice and chick, even before the onset of circulation, endothelial cells that are differentiated from the progenitor population assemble into a capillary network. After the onset of circulation, the capillary network remodels into arteries and veins to generate a functional circulatory loop.
For the functional vertebrate circulatory system, subsequent morphogenesis of a vascular lumen is essential. Previous studies on lumen formation using Mardin-Darby Canine Kidney (MDCK) cell culture provide critical insight on how vascular lumens might be generated.47 Nascent MDCK cells initially establish polarity by actively sorting the apical membrane to the luminal side and subsequently stabilize by deposition of tight junctions. However, because MDCK cells are epithelial in origin, they do not necessarily reflect behaviors of endothelial cells during lumen formation. Furthermore, given that complex interactions between different cell types which might be essential to generate the complicated three-dimensional architecture of a vascular lumen in developing embryos, the precise cellular and molecular mechanisms that modulate vascular lumen formation in vivo still remain elusive. Recently, Kamei and colleagues demonstrated that intracellular vacuoles within endothelial cells, which were first described by Folkman and Haudenschild,48 coalesce to create a functional lumen within the sprouting intersegmental vessels in developing zebrafish embryos.49 This result suggests that vascular lumen formation might recapitulate the similar cellular and molecular processes that have been reported in various cell culture models.49
Many of the signaling pathways responsible for regulating vascular development elicit their function in a noncell autonomous manner,2 suggesting that specific tissue interactions are critical during this process. In mice, it has been proposed that the endoderm is necessary and sufficient for the specification of the endothelial lineage.50,51 Evidence supporting this claim include the fact that the endoderm is juxtaposed to the region of the mesoderm that produces endothelial progenitors and that some of the key signaling molecules that regulate endothelial specification are expressed in the endoderm.51 In particular, Hedgehog has been implicated as a potential signaling molecule secreted by the endoderm because it is expressed in the visceral endoderm starting at E6.5, and loss of Hedgehog signaling causes defects in vasculogenesis as well as hematopoiesis in embryoid bodies.52 However, Vokes and Krieg analyzed this hypothesis and found that Hedgehog signal secreted from the endoderm is dispensable for the specification of the endothelial lineage, although essential for the subsequent morphogenesis of the vascular network in mammalian and avian model systems.51 Likewise, Lawson and colleagues elegantly demonstrated that cell nonautonomous signals, namely Hedgehog from the hypochord and VEGF from the ventral somites, induce arterial differentiation.53 Although the interaction between nascent endothelial progenitors and their surrounding tissue appear to be indispensable for the subsequent differentiation of either arterial or venous endothelial cells, there are always some exceptions. In zebrafish, the function of the endoderm is negligible for vascular development because endoderm-less mutant embryos form a fully lumenized and highly organized vascular network.54
Molecular Pathways That Regulate the Specification of the Endothelial Lineage
The molecular mechanisms that regulate vascular development appear to be highly conserved among vertebrates. Given that endothelial progenitors emerge from the mesoderm, it is expected that several signaling molecules that function during the patterning of the mesoderm would be critical for the specification of the endothelial lineage (Figure). To date, many signaling pathways including the Wnt/Frizzled, Delta/Notch, bone morphogenic protein (BMP), Ephrin/Eph receptor, transforming growth factor beta (TGF-β), platelet-derived growth factor (PDGF), fibroblast growth factor (FGF) signaling pathways, as well as several transcription factors such as Scl, Runx-1, and Ets have been shown to be essential for the specification of the endothelial lineage and subsequent vasculogenesis. The list of factors whose function is essential for vascular development is continuously increasing with the recent addition of microRNA,55,56 BMP antagonist BMPER/Crossveinless,57,58 as well as lysocardiolipin acyltrans-ferase.59,60 However, many of these factors do not function exclusively within the endothelial lineage. For instance, arguably the most critical signaling pathways for the endothelial lineage, the VEGF and the angiopoietin/Tie signaling pathways, appear to be critical for the development of nonendothelial lineages such as hematopoietic stem cells as well as neural stem cells.61–64
Vascular Endothelial Growth Factor and Related Molecules
Although a number of signaling pathways and their effectors have been implicated in vascular development in vertebrates, VEGF is regarded as the most critical factor for the emergence of the endothelial lineage and is also the most extensively studied signaling pathway within the context of vascular development. The function of VEGF is required for all aspects of vascular development—from the earliest event of the endothelial lineage specification to vessel maintenance. During development, activation of the VEGF signaling pathways is the earliest known landmark that defines the endothelial lineage commitment within the nascent mesoderm. The biochemical and molecular properties of VEGF have been extensively studied because of its essential role during vascular development. Biochemically, VEGF is a member of the platelet-derived growth factor (PDGF) family and its function is transduced via an array of tyrosine kinase receptors. In mammals, 5 VEGF molecules, 3 major receptors, VEGFR1/Flt1, VEGFR2/KDR, and VEGFR3/Flt4, and 2 coreceptors, Neuropilin 1 and 2, have been identified. Attenuation of any of these VEGFs or their receptors results in embryonic lethality. Similar phenotypes have been reported in Xenopus and chick embryos with compromised VEGF signaling. In zebrafish, VEGF also appears to be indispensable for the formation of the endothelial lineage, and subsequent differentiation into arterial endothelial cells by inducing arterial specific Notch activation.
It has been proposed that VEGF signaling is transduced via the VEGFR2/KDR receptor whereas VEGFR1/Flt1 modulates this process, functioning as a reservoir for VEGF ligands.65–67 In agreement with its proposed function, the main receptor for VEGF signaling, VEGFR2/KDR, is widely used as a molecular marker that defines the endothelial lineage during development alongside PECAM and the ETS-related transcription factor, Fli. The expression of VEGFR2/KDR can be readily observed in early stages of development in mice,27 chick,22 and zebrafish.67,68 In mammalian and avian model systems, flk1/vegfr2 is the earliest known endothelial-specific marker, being expressed as early as the 5 somite stage (12 hpf) within the presumptive endothelial progenitors alongside progenitors for other mesodermal lineages in the lateral plate mesoderm.22,27 Mice deficient in VEGFR2/KDR die between E8.5 and E9.5 in utero because of defects in endothelial and hematopoietic lineages.27 At E7.5, homozygous null VEGFR2/KDR mice are completely devoid of blood islands, and no organized vasculature can be detected in the embryo proper. In contrast, mice that lack functional Flt1 form both embryonic and extraembryonic endothelial cells, but display grossly dysmorphic vasculature, indicating that the function of Flt1 is dispensable for the specification of the endothelial lineage. VEGF signaling appears to function in a more complex manner in zebrafish partly because of genome duplication.68 There are at least 4 functional VEGF receptors that have been identified in zebrafish to date: VEGFR2/KDR, KDR-related (KDRL), VEGFR1/Flt1, and VEGFR3/Flt4, with KDRL being the main transducer of VEGF signaling.68 Interestingly, unlike in mammalian model systems, mutation in KDRL does not cause any obvious early vascular defects.67,68 Early stages of vascular development, including the specification of the endothelial lineage, occur normally in homozygous KDRL mutant zebrafish embryos that completely lack a functional KDRL receptor. Later vascular development, such as intersegmental vessel formation, is however compromised. Morpholino-mediated knockdown of all VEGF receptors causes significant reduction of the endothelial lineage, suggesting that different VEGF receptors synergistically interact to mediate VEGF signaling in zebrafish.67,68
Despite the extensive studies on VEGF function in vascular development, upstream signaling cascades that regulate the initiation of VEGF and VEGFR2/KDR expression are still largely unknown. However, recent studies show that HoxB5 and FoxH1 can directly bind to the cis-regulatory elements of VEGFR2/KDR in mice and zebrafish, respectively.69,70 Wu and colleagues reported that HoxB5 can bind the regulatory element within the first intron of the Vegfr2/Kdr locus to induce the expression of VEGFR2/KDR.69 Interestingly, overexpression of HoxB5 causes a significant increase in the number of endothelial progenitors, suggesting that HoxB5 might be necessary and sufficient to initiate the specification of endothelial progenitors. In contrast, FoxH1 appears to function as a repressor for vegf2/kdr expression.70 Choi and colleagues demonstrated that FoxH1 can bind the upstream enhancer of vegf2/kdr locus in zebrafish and attenuate its expression.69 Taken together, these data suggest that the expression of VEGF signaling components is highly regulated by complex mechanisms.
Angiopoietins and Related Molecules
The second signaling pathway that predominantly functions within the endothelial lineage is the Angiopoietin (Ang)/Tie-2 pathway. Structural signatures of Angiopoietins are a fibrinogen-like domain which is essential for the binding to the Tie-2 receptor, and a coiled-coil domain which is used for oligomerization.71–73 There are at least 3 members of the Ang family, Ang-1, Ang-2, and Ang-3 (mice)/Ang-4 (human), all of which bind to the receptor tyrosine kinase Tie-2. During development, Ang-1 can be detected as early as E9 in the myocardium and becomes more broadly expressed in later stages extending to the surrounding mesenchyme of developing blood vessels.74 In contrast, the second Angiopoietin gene, Ang-2, is expressed in the smooth muscle cells associated with the major vessels during development.75,76 The expression of Tie-2 can be detected as early as E8 in the endocardium and endothelial cells within the dorsal aorta.77–79 Mice deficient in Ang-1 or its receptor Tie-2 die at E9.5 and 12.5 because of multiple cardiovascular defects. However, vasculogenesis appears to occur normally in these embryos, and the primary reason for their early lethality seems to be the failure of endothelial cells to adhere to the surrounding extracellular matrix.80 This phenotype clearly indicates that Ang-1/Tie-2 signaling is not essential for the specification of the endothelial lineage, but is required for the later aspects of vascular development such as patterning, migration, and maturation of the vascular network. Recent reports suggesting that Ang-1 promotes tight association of adjacent endothelial cells and between endothelial cells and vascular smooth muscle cells further support this conclusion. Interestingly, overexpression of another ligand of Tie-2, Ang-2, causes a phenotype similar to Tie-2–deficient mice,81–83 suggesting that Ang-2 might function as an antagonist of Ang-1 to modulate the activity of Tie-2. Indeed, Scharpfenecker and colleagues found that Ang-2 is expressed before vessel remodeling, possibly functioning as a destabilizing signal, thereby antagonizing the function of Tie-2 in vascular maintenance.84 Taken together, these findings indicate that both Ang-1 and Ang-2 signal through Tie-2 in a distinct manner, and complement VEGF signaling to modulate vascular development. The function of 2 additional Angiopoietins, Ang-3 and Ang-4, during development is largely unknown.85,86
In addition to the Angiopoietins, several molecules that possess a coiled-coil domain and a fibrinogen-like domain have been recently identified. Given their structural resemblance to the Angiopoietins, these molecules were named as angiopoietin-like proteins (Angptls).87,88 Despite their structural similarities, Angptls do not bind to Tie-2 or Tie-1 receptors.89 In humans, Angptl-1 and Angptl-2 have largely overlapping expression patterns, suggesting that these molecules might function redundantly. Recent studies in zebrafish demonstrated that Angptl-1 and Angptl-2 synergistically regulate the patterning of the vascular network. Embryos with attenuated Angptl-1 and Angptl-2 activity display defective intersegmental sprouting and increased level of apoptosis within the dorsal aorta.90 Unlike Angptl-1 and Angptl-2, other Angptls do not seem to have any obvious function related to vascular development.89,91
Local Environmental Factors That Influence Early Vascular Development
As previously mentioned, early vascular development is largely governed by a genetic program. However, increasing evidence suggest that nongenetic components also modulate vascular development. In particular, these nongenetic factors seem to be the major driving force during vascular remodeling when the capillary plexus undergoes morphogenetic changes to create more defined arteries and veins. For instance, hypoxic conditions stimulate vasculogenesis in several model systems. As embryonic development proceeds, cells distantly located from a blood vessel undergo a hypoxic response, which triggers the stabilization of Hypoxia Inducible Factor-1 α (HIF-1 α), which in turn forms a heterodimeric transcription factor complex with constitutively expressed HIF-1β. The activation of HIF-1 induces an array of genes involved in vascular development including the VEGFs and their receptors, and the Angiopoietin receptor Tie-2.92 Mice that lack a functional HIF-1α locus die in utero because of defects in vascular remodeling,93 suggesting an essential role for the HIF pathway in vascular development. However, mouse embryos cultured under hypoxic conditions before the onset of circulation initiate normal vascular remodeling.40 Similar results have been observed in other model systems,94,95 alluding to the possibility that HIF-1α–induced regulation of vascular development might be dispensable for earlier stages of development. Although it is apparent that the HIF pathway does function during early vascular development, analyzing the exclusive function of HIF during this process is problematic because of the fact that the HIF pathway interacts with other signaling pathways involved in vascular development, such as TGF-β and VEGF.
In many vertebrate model systems, the onset of circulation occurs while the vascular network is still forming.96 The blood flow within the developing vessel generates mechanical forces, collectively known as hemodynamic forces, which can be recognized by endothelial cells.97 As a result, endothelial cells within the vessels that are actively experiencing hemodynamic forces turn on the expression of eNOS.98 A series of reports suggest that hemodynamic forces are essential for modulating vascular development. For instance, the secondary defects of the extraembryonic vasculature observed in mice embryos with impaired cardiac contractility support this notion.40 Recent studies by Lucitti and colleagues analyzed this phenotype in detail and successfully demonstrated that the shear stress generated by circulating red blood cells is critical for inducing the expression of eNOS in endothelial cells, which can serve as a driving force for vascular remodeling during embryogenesis.40 Although the exact mechanism is not known yet, it is very likely that mechanical forces created by the circulation can regulate subsequent development of the vascular network during development.
Concluding Remarks
In this review we have discussed how early vascular development proceeds, and we have explored some of the cellular and molecular mechanisms that regulate this process, emphasizing the specification of the endothelial lineage. Although the function of the circulatory system has been conserved throughout different phyla, the endothelial lineage in each species appears to possess diversity in terms of their origin and properties. The endothelial lineage emerges from heterogeneous progenitors residing in different regions within the developing embryo, and diverse signaling pathways and environmental cues regulate the induction of the endothelial lineage. With the help of novel technologies and various animal model systems, the complexity of the endothelial lineage has only just begun to be unveiled.
Footnotes
Disclosures
None.
References
- 1.Roca C, Adams RH. Regulation of vascular morphogenesis by Notch signaling. Genes Dev. 2007;21:2511–24. doi: 10.1101/gad.1589207. [DOI] [PubMed] [Google Scholar]
- 2.Rossant J, Howard L. Signaling pathways in vascular development. Annu Rev Cell Dev Biol. 2002;18:541–73. doi: 10.1146/annurev.cellbio.18.012502.105825. [DOI] [PubMed] [Google Scholar]
- 3.Ilza Veith., translator. The Yellow Emperor’s Classic of Internal Medicine. University of California Press; Berkeley: 2002. [Google Scholar]
- 4.Shoja MM, Tubbs RS, Loukas M, Ardalan MR. The Aristotelian account of “heart and veins”. Int J Cardiol. 2008;125:304–10. doi: 10.1016/j.ijcard.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Lonie I. Erasistratus, The Erasistrateans, and Aristotle. Bull Hist Med. 1964;38:426–443. [PubMed] [Google Scholar]
- 6.Khan IA, Daya SK, Gowda RM. Evolution of the theory of circulation. Int J Cardiol. 2005;98:519–21. doi: 10.1016/j.ijcard.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 7.Khulusi SA. Arab medicine and circulation of the blood. Lancet. 1978;1:1314. doi: 10.1016/s0140-6736(78)91302-8. [DOI] [PubMed] [Google Scholar]
- 8.Harvey W. The Circulation of the Blood and Other Writings. Everyman’s Library; London: 1963. [Google Scholar]
- 9.Brown A, Barnes J. ’William Harvey (1578 –1657) and Marcecllo Malpighi (1628–1694): Linked in blood, paralleled in life. Adler Mus Bull. 1994;20:14–23. [PubMed] [Google Scholar]
- 10.His WS. Die Häute und Höhlen des Körpers. Schwighauser; Basel: 1865. [Google Scholar]
- 11.Sabin F. Preliminary note on the differentiation of angioblasts and the method by which they produce blood-vessels, blood-plasma, and red blod-cells as seen in the living chick. Anat Rec. 1917;13:199–204. doi: 10.1089/152581602753448496. [DOI] [PubMed] [Google Scholar]
- 12.Murray PDF. The development in vitro of the blood of the early chick embryo. Proc Biol Sci. 1932;111:497–521. [Google Scholar]
- 13.Kattman SJ, Huber TL, Keller GM. Multipotent flk-1 + cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev Cell. 2006;11:723–32. doi: 10.1016/j.devcel.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Moretti A, Caron L, Nakano A, Lam JT, Bernshausen A, Chen Y, Qyang Y, Bu L, Sasaki M, Martin-Puig S, Sun Y, Evans SM, Laugwitz KL, Chien KR. Multipotent embryonic isl1 + progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127:1151–65. doi: 10.1016/j.cell.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 15.Mudumana SP, Hentschel D, Liu Y, Vasilyev A, Drummond IA. odd skipped related1 reveals a novel role for endoderm in regulating kidney versus vascular cell fate. Development. 2008;135:3355–67. doi: 10.1242/dev.022830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gering M, Yamada Y, Rabbitts TH, Patient RK. Lmo2 and Scl/Tal1 convert non-axial mesoderm into haemangioblasts which differentiate into endothelial cells in the absence of Gata1. Development. 2003;130:6187–99. doi: 10.1242/dev.00875. [DOI] [PubMed] [Google Scholar]
- 17.Riddle DL, Blumenthal T, Meyer BJ, Priess JR. C elegans II. Cold Spring Harbor Laboratory Press; Cold Spring Harbor: 1997. [PubMed] [Google Scholar]
- 18.Wasserthal LT. Drosophila flies combine periodic heartbeat reversal with a circulation in the anterior body mediated by a newly discovered anterior pair of ostial valves and ‘venous’ channels. J Exp Biol. 2007;210:3707–3719. doi: 10.1242/jeb.007864. [DOI] [PubMed] [Google Scholar]
- 19.Ferkowicz MJ, Yoder MC. Blood island formation: longstanding observations and modern interpretations. Exp Hematol. 2005;33:1041–7. doi: 10.1016/j.exphem.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol. 1995;11:73–91. doi: 10.1146/annurev.cb.11.110195.000445. [DOI] [PubMed] [Google Scholar]
- 21.Quinn TP, Peters KG, De Vries C, Ferrara N, Williams LT. Fetal liver kinase 1 is a receptor for vascular endothelial growth factor and is selectively expressed in vascular endothelium. Proc Natl Acad Sci U S A. 1993;90:7533–7. doi: 10.1073/pnas.90.16.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eichmann A, Marcelle C, Breant C, Le Douarin NM. Two molecules related to the VEGF receptor are expressed in early endothelial cells during avian embryonic development. Mech Dev. 1993;42:33–48. doi: 10.1016/0925-4773(93)90096-g. [DOI] [PubMed] [Google Scholar]
- 23.Caprioli A, Jaffredo T, Gautier R, Dubourg C, Dieterlen-Lievre F. Blood-borne seeding by hematopoietic and endothelial precursors from the allantois. Proc Natl Acad Sci U S A. 1998;95:1641–6. doi: 10.1073/pnas.95.4.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caprioli A, Minko K, Drevon C, Eichmann A, Dieterlen-Lièvre F, Jaffredo T. Hemangioblast commitment in the avian allantois: cellular and molecular aspects. Dev Biol. 2001;238:64–78. doi: 10.1006/dbio.2001.0362. [DOI] [PubMed] [Google Scholar]
- 25.Demir R, Seval Y, Huppertz B. Vasculogenesis and angiogenesis in the early human placenta. Acta Histochem. 2007;109:257–65. doi: 10.1016/j.acthis.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 26.Yamaguchi TP, Dumont DJ, Conlon RA, Breitman ML, Rossant J. flk-1, an flt-related receptor tyrosine kinase is an early marker for endothelial cell precursors. Development. 1993;118:489–98. doi: 10.1242/dev.118.2.489. [DOI] [PubMed] [Google Scholar]
- 27.Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–6. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 28.Risau W, Sariola H, Zerwes HG, Sasse J, Ekblom P, Kemler R, Doetschman T. Vasculogenesis and angiogenesis in embryonic-stem-cell-derived embryoid bodies. Development. 1988;102:471–8. doi: 10.1242/dev.102.3.471. [DOI] [PubMed] [Google Scholar]
- 29.Eichmann A, Corbel C, Pardanaud L, Bréant C, Moyon D, Yuan L. Hemangioblastic precursors in the avian embryo. Curr Top Microbiol Immunol. 2000;251:83–90. doi: 10.1007/978-3-642-57276-0_11. [DOI] [PubMed] [Google Scholar]
- 30.Dieterlen-Lievre F, Pardanaud L, Godin I, Garcia-Porrero J, Cumano A, Marcos M. Developmental relationships between hemopoiesis and vasculogenesis. C R Acad Sci III. 1993;316:892–901. [PubMed] [Google Scholar]
- 31.Dieterlen-Lievre F. Hemopoietic cell progenitors in the avian embryo: origin and migrations. Ann N Y Acad Sci. 1987;511:77–87. doi: 10.1111/j.1749-6632.1987.tb36239.x. [DOI] [PubMed] [Google Scholar]
- 32.Stainier DY, Weinstein BM, Detrich HW, III, Zon LI, Fishman MC. Cloche, an early acting zebrafish gene, is required by both the endothelial and hematopoietic lineages. Development. 1995;121:3141–50. doi: 10.1242/dev.121.10.3141. [DOI] [PubMed] [Google Scholar]
- 33.Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development. 1998;125:725–32. doi: 10.1242/dev.125.4.725. [DOI] [PubMed] [Google Scholar]
- 34.Kinder SJ, Tsang TE, Quinlan GA, Hadjantonakis AK, Nagy A, Tam PP. The orderly allocation of mesodermal cells to the extraembryonic structures and the anteroposterior axis during gastrulation of the mouse embryo. Development. 1999;126:4691–70. doi: 10.1242/dev.126.21.4691. [DOI] [PubMed] [Google Scholar]
- 35.Huber TL, Kouskoff V, Fehling HJ, Palis J, Keller G. Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature. 2004;432:625–30. doi: 10.1038/nature03122. [DOI] [PubMed] [Google Scholar]
- 36.Vogeli KM, Jin SW, Martin GR, Stainier DY. A common progenitor for haematopoietic and endothelial lineages in the zebrafish gastrula. Nature. 2006;443:337–9. doi: 10.1038/nature05045. [DOI] [PubMed] [Google Scholar]
- 37.Ueno H, Weissman IL. Clonal analysis of mouse development reveals a polyclonal origin for yolk sac blood islands. Dev Cell. 2006;11:519–33. doi: 10.1016/j.devcel.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 38.Weng W, Sukowati EW, Sheng G. On hemangioblasts in chicken. PLoS ONE. 2007;2:e1228. doi: 10.1371/journal.pone.0001228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noden DM. Embryonic origins and assembly of blood vessels. Am Rev Respir Dis. 1989;140:1097–103. doi: 10.1164/ajrccm/140.4.1097. [DOI] [PubMed] [Google Scholar]
- 40.Lucitti JL, Jones EA, Huang C, Chen J, Fraser SE, Dickinson ME. Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development. 2007;134:3317–26. doi: 10.1242/dev.02883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones EA, Baron MH, Fraser SE, Dickinson ME. Measuring hemodynamic changes during mammalian development. Am J Physiol Heart Circ Physiol. 2004;287:H1561–H1569. doi: 10.1152/ajpheart.00081.2004. [DOI] [PubMed] [Google Scholar]
- 42.Adelman DM, Gertsenstein M, Nagy A, Simon MC, Maltepe E. Placental cell fates are regulated in vivo by HIF-mediated hypoxia responses. Genes Dev. 2000;14:3191–203. doi: 10.1101/gad.853700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramirez-Bergeron DL, Runge A, Dahl KD, Fehling HJ, Keller G, Simon MC. Hypoxia affects mesoderm and enhances hemangioblast specification during early development. Development. 2004;131:4623–34. doi: 10.1242/dev.01310. [DOI] [PubMed] [Google Scholar]
- 44.Isogai S, Lawson ND, Torrealday S, Horiguchi M, Weinstein BM. Angiogenic network formation in the developing vertebrate trunk. Development. 2003;130:5281–90. doi: 10.1242/dev.00733. [DOI] [PubMed] [Google Scholar]
- 45.Lawson ND, Scheer N, Pham VN, Kim CH, Chitnis AB, Campos-Ortega JA, Weinstein BM. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development. 2001;128:3675–83. doi: 10.1242/dev.128.19.3675. [DOI] [PubMed] [Google Scholar]
- 46.Lawson ND, Weinstein BM. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol. 2002;248:307–18. doi: 10.1006/dbio.2002.0711. [DOI] [PubMed] [Google Scholar]
- 47.Lubarsky B, Krasnow MA. Tube morphogenesis: making and shaping biological tubes. Cell. 2003;112:19–28. doi: 10.1016/s0092-8674(02)01283-7. [DOI] [PubMed] [Google Scholar]
- 48.Folkman J, Haudenschild C. Angiogenesis by capillary endothelial cells in culture. Trans Ophthalmol Soc U K. 1980;100:346–53. [PubMed] [Google Scholar]
- 49.Kamei M, Saunders WB, Bayless KJ, Dye L, Davis GE, Weinstein BM. Endothelial tubes assemble from intracellular vacuoles in vivo. Nature. 2006;442:453–6. doi: 10.1038/nature04923. [DOI] [PubMed] [Google Scholar]
- 50.Belaoussoff M, Farrington SM, Baron MH. Hematopoietic induction and respecification of A-P identity by visceral endoderm signaling in the mouse embryo. Development. 1998;125:5009–18. doi: 10.1242/dev.125.24.5009. [DOI] [PubMed] [Google Scholar]
- 51.Vokes SA, Yatskievych TA, Heimark RL, McMahon J, McMahon AP, Antin PB, Krieg PA. Hedgehog signaling is essential for endothelial tube formation during vasculogenesis. Development. 2004;131:4371–80. doi: 10.1242/dev.01304. [DOI] [PubMed] [Google Scholar]
- 52.Maye P, Becker S, Kasameyer E, Byrd N, Grabel L. Indian hedgehog signaling in extraembryonic endoderm and ectoderm differentiation in ES embryoid bodies. Mech Dev. 2000;94:117–32. doi: 10.1016/s0925-4773(00)00304-x. [DOI] [PubMed] [Google Scholar]
- 53.Lawson ND, Vogel AM, Weinstein BM. sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev Cell. 2002;3:127–36. doi: 10.1016/s1534-5807(02)00198-3. [DOI] [PubMed] [Google Scholar]
- 54.Jin SW, Beis D, Mitchell T, Chen JN, Stainier DY. Cellular and molecular analyses of vascular tube and lumen formation in zebrafish. Development. 2005;132:5199–209. doi: 10.1242/dev.02087. [DOI] [PubMed] [Google Scholar]
- 55.Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, Richardson JA, Bassel-Duby R, Olson EN. The Endothelial-Specific MicroRNA miR-126 Governs Vascular Integrity and Angiogenesis. Dev Cell. 2008;15:261–71. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fish JE, Santoro MM, Morton SU, Yu S, Yeh R, Wythe JD, Ivey KN, Bruneau BG, Stainier DY, Srivastava D. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008;15:272–84. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moser M, Yu Q, Bode C, Xiong JW, Patterson C. BMPER is a conserved regulator of hematopoietic and vascular development in zebrafish. J Mol Cell Cardiol. 2007;43:243–53. doi: 10.1016/j.yjmcc.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moser M, Binder O, Wu Y, Aitsebaomo J, Ren R, Bode C, Bautch VL, Conlon FL, Patterson C. BMPER, a novel endothelial cell precursor-derived protein, antagonizes bone morphogenetic protein signaling and endothelial cell differentiation. Mol Cell Biol. 2003;23:5664–79. doi: 10.1128/MCB.23.16.5664-5679.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang C, Faloon PW, Tan Z, Lv Y, Zhang P, Ge Y, Deng H, Xiong JW. Mouse lysocardiolipin acyltransferase controls the development of hematopoietic and endothelial lineages during in vitro embryonic stem-cell differentiation. Blood. 2007;110:3601–9. doi: 10.1182/blood-2007-04-086827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xiong JW, Yu Q, Zhang J, Mably JD. An acyltransferase controls the generation of hematopoietic and endothelial lineages in zebrafish. Circ Res. 2008;102:1057–64. doi: 10.1161/CIRCRESAHA.107.163907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/Angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 62.Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, During MJ. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet. 2004;36:827–35. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- 63.Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Nat Acad Sci. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tam BY, Wei K, Rudge JS, Hoffman J, Holash J, Park SK, Yuan J, Hefner C, Chartier C, Lee JS, Jiang S, Niyak NR, Kuypers FA, Ma L, Sundram U, Wu G, Garcia JA, Schrier SL, Maher JJ, Johnson RS, Yancopoulos GD, Mulligan RC, Kuo CJ. VEGF modulates erythropoiesis through regulation of adult hepatic erythropoietin synthesis. Nat Med. 2006;12:793–800. doi: 10.1038/nm1428. [DOI] [PubMed] [Google Scholar]
- 65.Fong GH, Zhang L, Bryce DM, Peng J. Increased hemangioblast commitment, not vascular disorganization, is the primary defect in flt-1 knock-out mice. Development. 1999;126:3015–25. doi: 10.1242/dev.126.13.3015. [DOI] [PubMed] [Google Scholar]
- 66.Kearney JB, Ambler CA, Monaco KA, Johnson N, Rapoport RG, Bautch VL. Vascular endothelial growth factor receptor Flt-1 negatively regulates developmental blood vessel formation by modulating endothelial cell division. Blood. 2002;99:2397–407. doi: 10.1182/blood.v99.7.2397. [DOI] [PubMed] [Google Scholar]
- 67.Habeck H, Odenthal J, Walderich B, Maischein H, Schulte-Merker S. Analysis of a zebrafish VEGF receptor mutant reveals specific disruption of angiogenesis. Curr Biol. 2002;12:1405–12. doi: 10.1016/s0960-9822(02)01044-8. [DOI] [PubMed] [Google Scholar]
- 68.Covassin LD, Villefranc JA, Kacergis MC, Weinstein BM, Lawson ND. Distinct genetic interactions between multiple Vegf receptors are required for development of different blood vessel types in zebrafish. Proc Natl Acad Sci U S A. 2006;103:6554–9. doi: 10.1073/pnas.0506886103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Choi J, Dong L, Ahn J, Dao D, Hammerschmidt M, Chen JN. FoxH1 negatively modulates flk1 gene expression and vascular formation in zebrafish. Dev Biol. 2007;304:735–44. doi: 10.1016/j.ydbio.2007.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu Y, Moser M, Bautch VL, Patterson C. HoxB5 is an upstream transcriptional switch for differentiation of the vascular endothelium from precursor cells. Mol Cell Biol. 2003;23:5680–91. doi: 10.1128/MCB.23.16.5680-5691.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barton WA, Tzvetkova D, Nikolov DB. Structure of the angiopoietin-2 receptor binding domain and identification of surfaces involved in Tie2 recognition. Structure. 2005;13:825–32. doi: 10.1016/j.str.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 72.Cho CH, Kammerer RA, Lee HJ, Steinmetz MO, Ryu YS, Lee SH, Yasunaga K, Kim KT, Kim I, Choi HH, Kim W, Kim SH, Park SK, Lee GM, Koh GY. COMP-Ang1: a designed angiopoietin-1 variant with nonleaky angiogenic activity. Proc Natl Acad Sci U S A. 2004;101:5547–52. doi: 10.1073/pnas.0307574101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Davis S, Papadopoulos N, Aldrich TH, Maisonpierre PC, Huang T, Kovac L, Xu A, Leidich R, Radziejewska E, Rafique A, Goldberg J, Jain V, Bailey K, Karow M, Fandl J, Samuelsson SJ, Ioffe E, Rudge JS, Daly TJ, Radziejewski C, Yancopoulos GD. Angiopoietins have distinct modular domains essential for receptor binding, dimerization and super-clustering. Nat Struct Biol. 2003;10:38–44. doi: 10.1038/nsb880. [DOI] [PubMed] [Google Scholar]
- 74.Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC, Yancopoulos GD. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–9. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- 75.Tanaka S, Mori M, Sakamoto Y, Makuuchi M, Sugimachi K, Wands JR. Biologic significance of angiopoietin-2 expression in human hepato-cellular carcinoma. J Clin Invest. 1999;103:341–5. doi: 10.1172/JCI4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yuan HT, Suri C, Yancopoulos GD, Woolf AS. Expression of angiopoietin-1, angiopoietin-2, and the Tie-2 receptor tyrosine kinase during mouse kidney maturation. J Am Soc Nephrol. 1999;10:1722–36. doi: 10.1681/ASN.V1081722. [DOI] [PubMed] [Google Scholar]
- 77.Dumont DJ, Yamaguchi TP, Conlon RA, Rossant J, Breitman ML. tek, a novel tyrosine kinase gene located on mouse chromosome 4, is expressed in endothelial cells and their presumptive precursors. Oncogene. 1992;7:1471–80. [PubMed] [Google Scholar]
- 78.Runting AS, Stacker SA, Wilks AF. tie2, a putative protein tyrosine kinase from a new class of cell surface receptor. Growth Factors. 1993;9:99–105. [PubMed] [Google Scholar]
- 79.Sato TN, Qin Y, Kozak CA, Audus KL. Tie-1 and tie-2 define another class of putative receptor tyrosine kinase genes expressed in early embryonic vascular system. Proc Natl Acad Sci U S A. 1993;90:9355–8. doi: 10.1073/pnas.90.20.9355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schnurch H, Risau W. Expression of tie-2, a member of a novel family of receptor tyrosine kinases, in the endothelial cell lineage. Development. 1993;119:957–68. doi: 10.1242/dev.119.3.957. [DOI] [PubMed] [Google Scholar]
- 81.Dumont DJ, Gradwohl G, Fong GH, Puri MC, Gertsenstein M, Auerbach A, Breitman ML. Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev. 1994;8:1897–909. doi: 10.1101/gad.8.16.1897. [DOI] [PubMed] [Google Scholar]
- 82.Cao Y, Sonveaux P, Liu S, Zhao Y, Mi J, Clary BM, Li CY, Kontos CD, Dewhirst MW. Systemic overexpression of angiopoietin-2 promotes tumor microvessel regression and inhibits angiogenesis and tumor growth. Cancer Res. 2007;67:3835–44. doi: 10.1158/0008-5472.CAN-06-4056. [DOI] [PubMed] [Google Scholar]
- 83.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 84.Scharpfenecker M, Fiedler U, Reiss Y, Augustin HG. The Tie-2 ligand angiopoietin-2 destabilizes quiescent endothelium through an internal autocrine loop mechanism. J Cell Sci. 2005;118:771–80. doi: 10.1242/jcs.01653. [DOI] [PubMed] [Google Scholar]
- 85.Lee HJ, Cho CH, Hwang SJ, Choi HH, Kim KT, Ahn SY, Kim JH, Oh JL, Lee GM, Koh GY. Biological characterization of angiopoietin-3 and angiopoietin-4. Faseb J. 2004;18:1200–8. doi: 10.1096/fj.03-1466com. [DOI] [PubMed] [Google Scholar]
- 86.Nishimura M, Miki T, Yashima R, Yokoi N, Yano H, Sato Y, Seino S. Angiopoietin-3, a novel member of the angiopoietin family. FEBS Lett. 1999;448:254–6. doi: 10.1016/s0014-5793(99)00381-6. [DOI] [PubMed] [Google Scholar]
- 87.Morisada T, Kubota Y, Urano T, Suda T, Oike Y. Angiopoietins and angiopoietin-like proteins in angiogenesis. Endothelium. 2006;13:71–9. doi: 10.1080/10623320600697989. [DOI] [PubMed] [Google Scholar]
- 88.Oike Y, Yasunaga K, Suda T. Angiopoietin-related/angiopoietin-like proteins regulate angiogenesis. Int J Hematol. 2004;80:21–8. doi: 10.1532/ijh97.04034. [DOI] [PubMed] [Google Scholar]
- 89.Hato T, Tabata M, Oike Y. The role of angiopoietin-like proteins in angiogenesis and metabolism. Trends Cardiovasc Med. 2008;18:6–14. doi: 10.1016/j.tcm.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 90.Kubota Y, Oike Y, Satoh S, Tabata Y, Niikura Y, Morisada T, Akao M, Urano T, Ito Y, Miyamoto T, Nagai N, Koh GY, Watanabe S, Suda T. Cooperative interaction of Angiopoietin-like proteins 1 and 2 in zebrafish vascular development. Proc Natl Acad Sci U S A. 2005;102:13502–7. doi: 10.1073/pnas.0501902102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Oike Y, Akao M, Yasunaga K, Yamauchi T, Morisada T, Ito Y, Urano T, Kimura Y, Kubota Y, Maekawa H, Miyamoto T, Miyata K, Matsumoto S, Sakai J, Nakagata N, Takeya M, Koseki H, Ogawa Y, Kadowaki T, Suda T. Angiopoietin-related growth factor antagonizes obesity and insulin resistance. Nat Med. 2005;11:400–8. doi: 10.1038/nm1214. [DOI] [PubMed] [Google Scholar]
- 92.Yamakawa M, Liu LX, Date T, Belanger AJ, Vincent KA, Akita GY, Kuriyama T, Cheng SH, Gregory RJ, Jiang C. Hypoxia-inducible factor-1 mediates activation of cultured vascular endothelial cells by inducing multiple angiogenic factors. Circ Res. 2003;93:664–73. doi: 10.1161/01.RES.0000093984.48643.D7. [DOI] [PubMed] [Google Scholar]
- 93.Kotch LE, Iyer NV, Laughner E, Semenza GL. Defective vascularization of HIF-1alpha-null embryos is not associated with VEGF deficiency but with mesenchymal cell death. Dev Biol. 1999;209:254–67. doi: 10.1006/dbio.1999.9253. [DOI] [PubMed] [Google Scholar]
- 94.Kajimura S, Aida K, Duan C. Understanding hypoxia-induced gene expression in early development: in vitro and in vivo analysis of hypoxia-inducible factor 1-regulated zebra fish insulin-like growth factor binding protein 1 gene expression. Mol Cell Biol. 2006;26:1142–55. doi: 10.1128/MCB.26.3.1142-1155.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Paffett-Lugassy N, Hsia N, Fraenkel PG, Paw B, Leshinsky I, Barut B, Bahary N, Caro J, Handin R, Zon LI. Functional conservation of erythropoietin signaling in zebrafish. Blood. 2007;110:2718–26. doi: 10.1182/blood-2006-04-016535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jones EAV, Le Noble F, Eichmann A. What Determines Blood Vessel Structure? Genetic Prespecification vs. Hemodynamics. Physiology. 2006;21:388–395. doi: 10.1152/physiol.00020.2006. [DOI] [PubMed] [Google Scholar]
- 97.Le Noble F, Moyon D, Pardanaud L, Yuan L, Djonov V, Matthijsen R, Breant C, Fleury V, Eichmann A. Flow regulates arterial-venous differentiation in the chick embryo yolk sac. Development. 2004;131:361–75. doi: 10.1242/dev.00929. [DOI] [PubMed] [Google Scholar]
- 98.Le Cras TD, Tyler RC, Horan MP, Morris KG, Tuder RM, McMurtry IF, Johns RA, Abman SH. Effects of chronic hypoxia and altered hemodynamics on endothelial nitric oxide synthase expression in the adult rat lung. J Clin Invest. 1998;101:795–801. doi: 10.1172/JCI786. [DOI] [PMC free article] [PubMed] [Google Scholar]


