Abstract
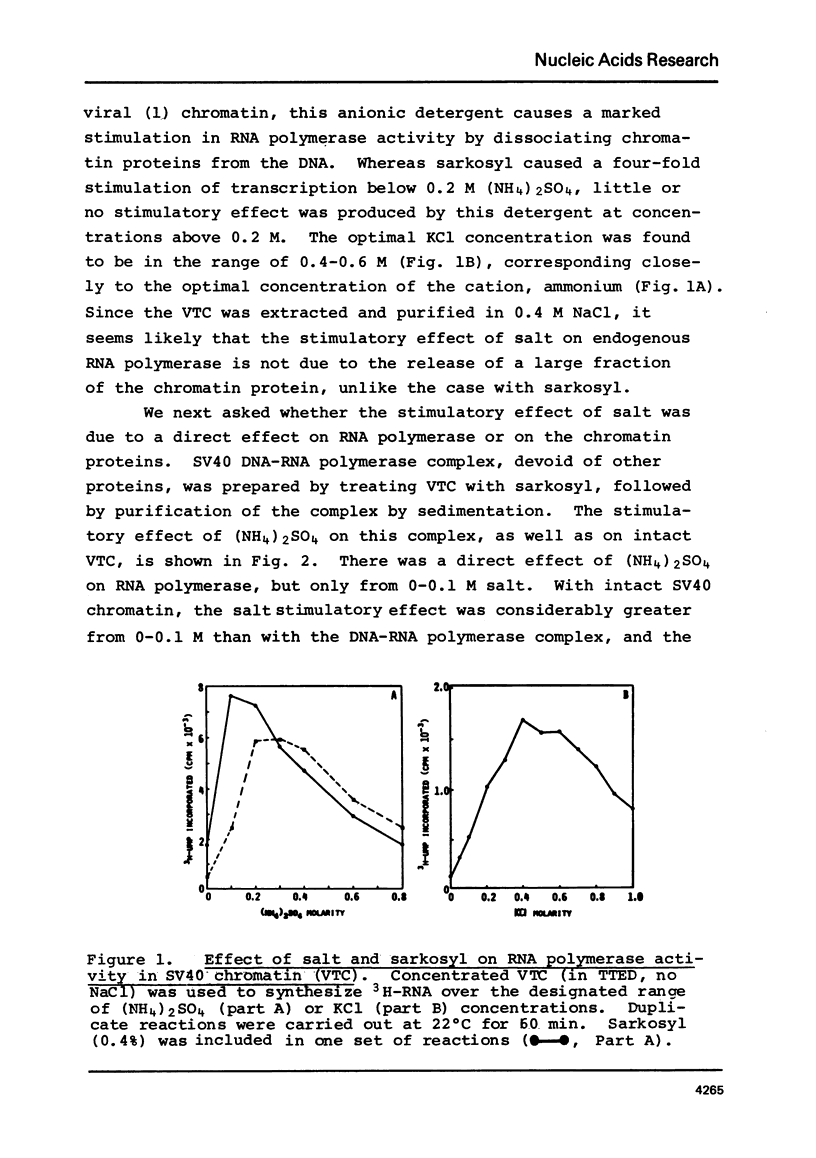
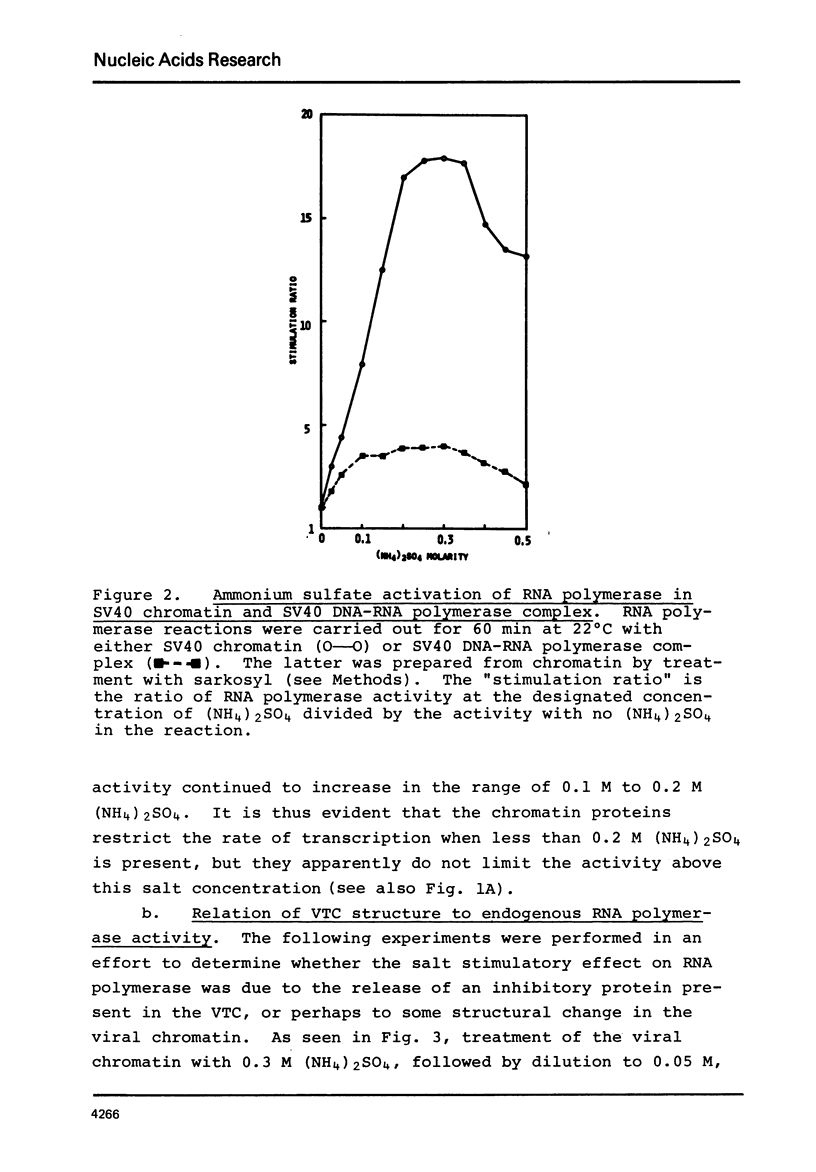
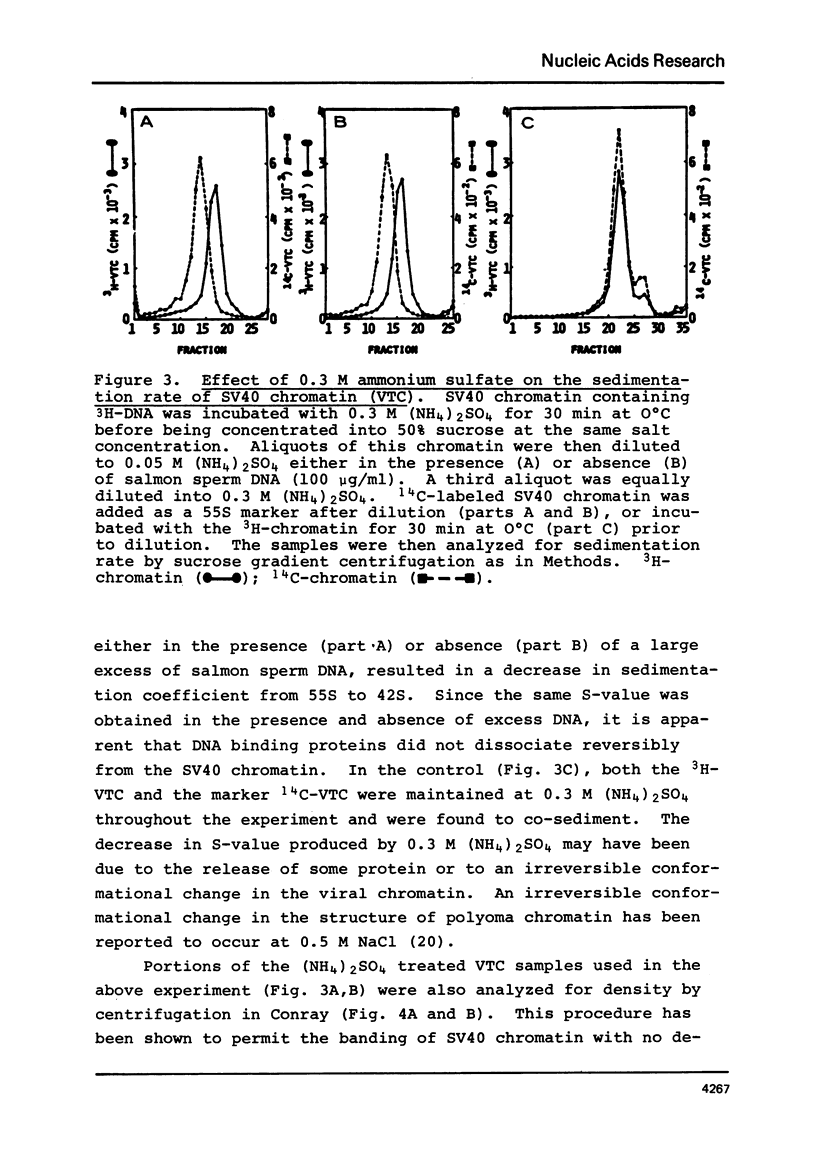
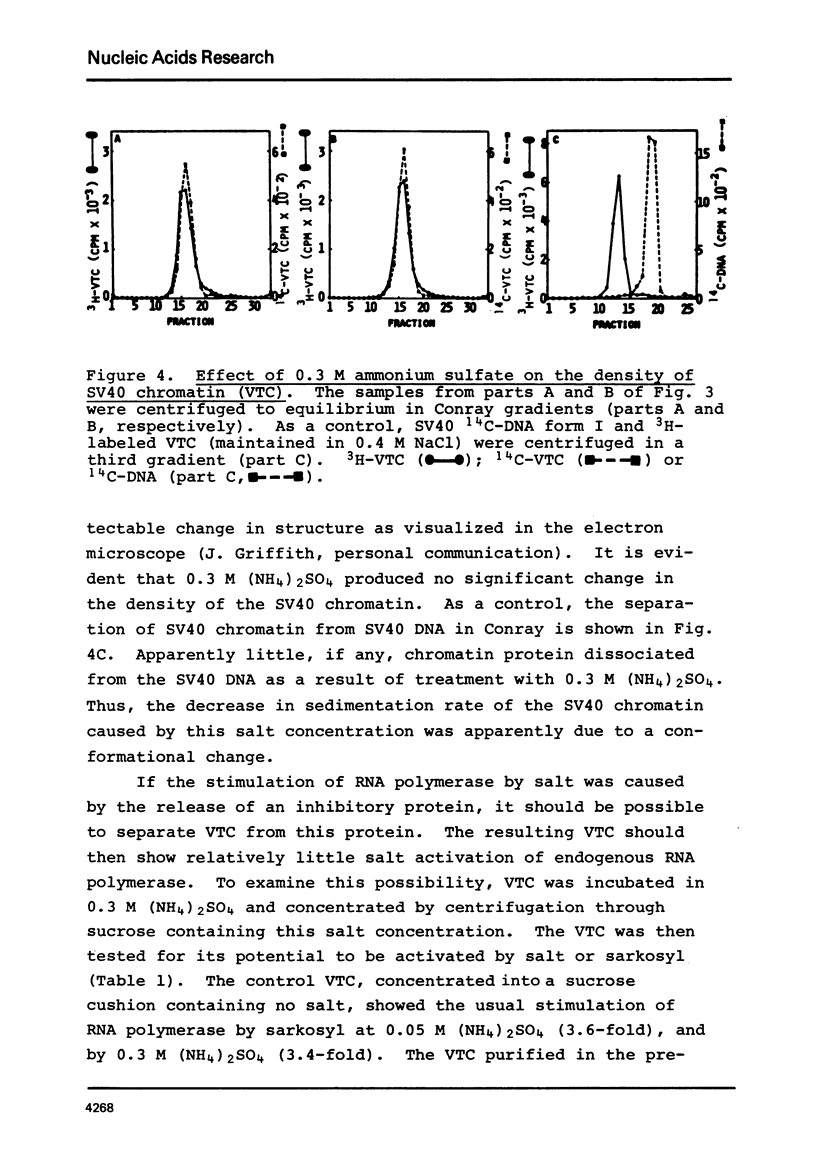
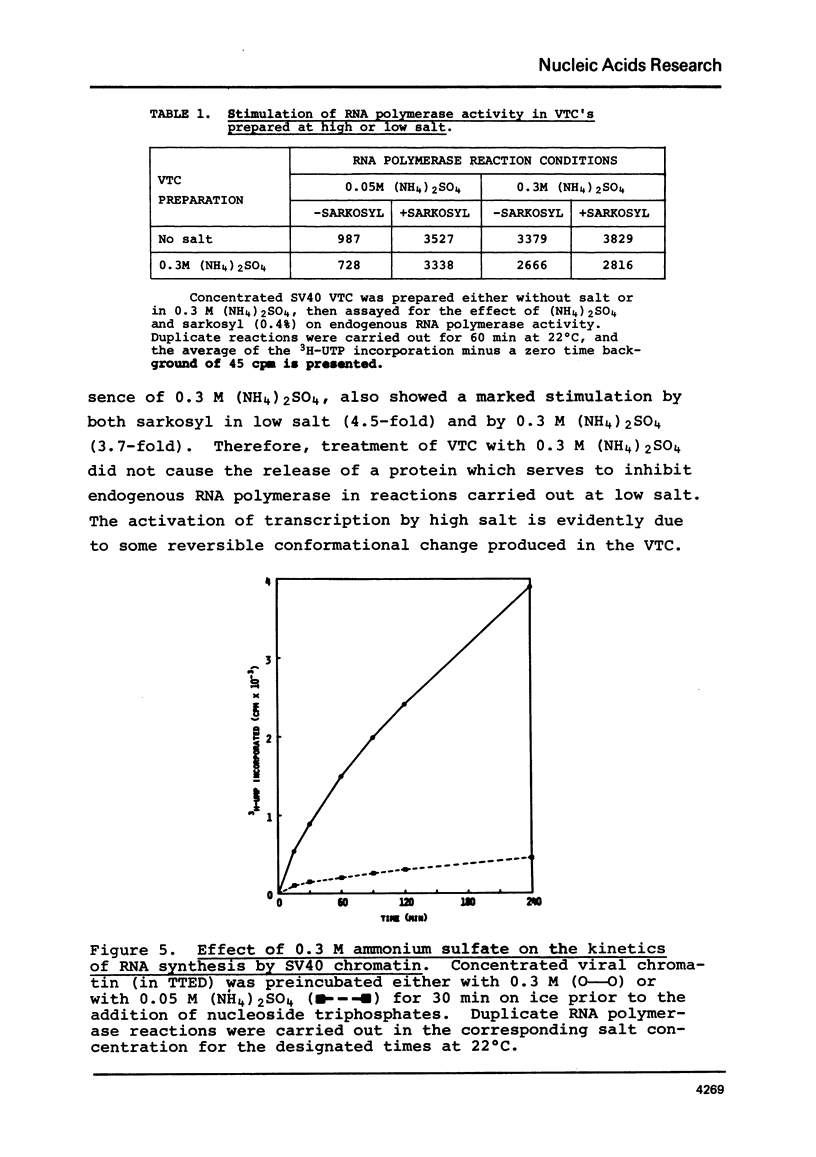
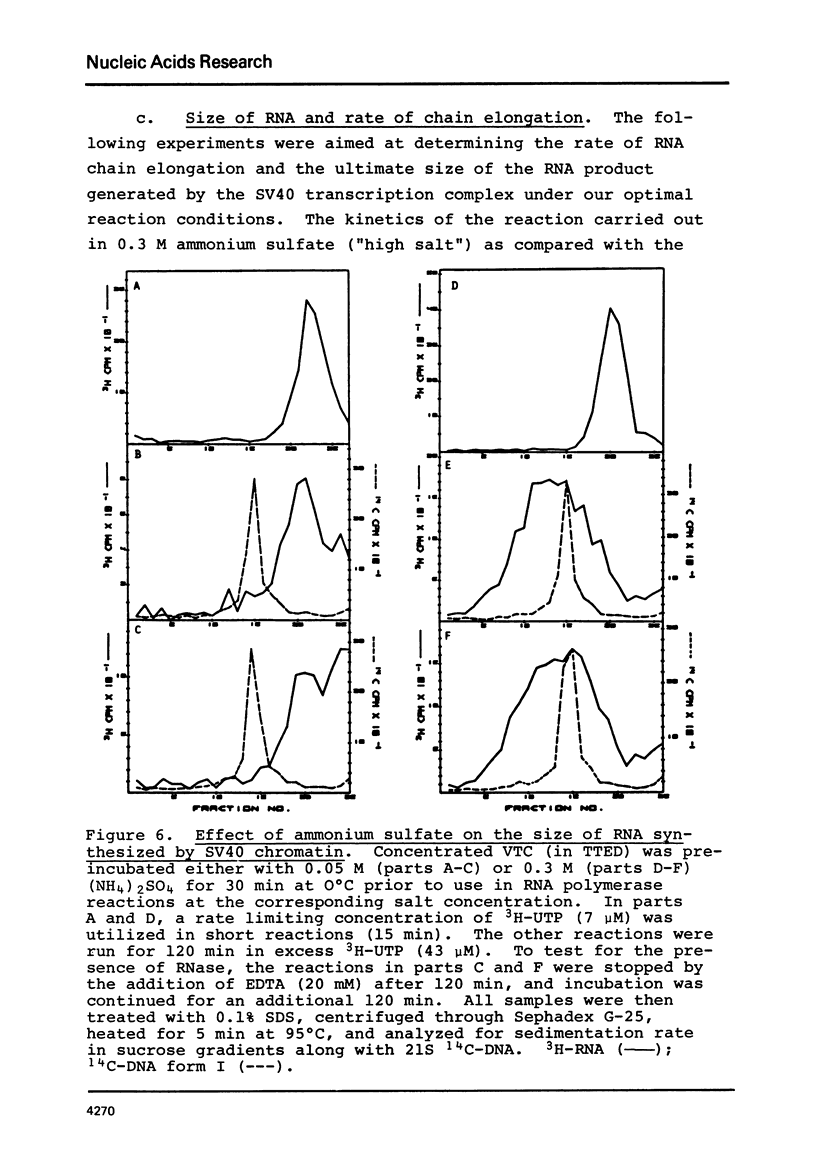
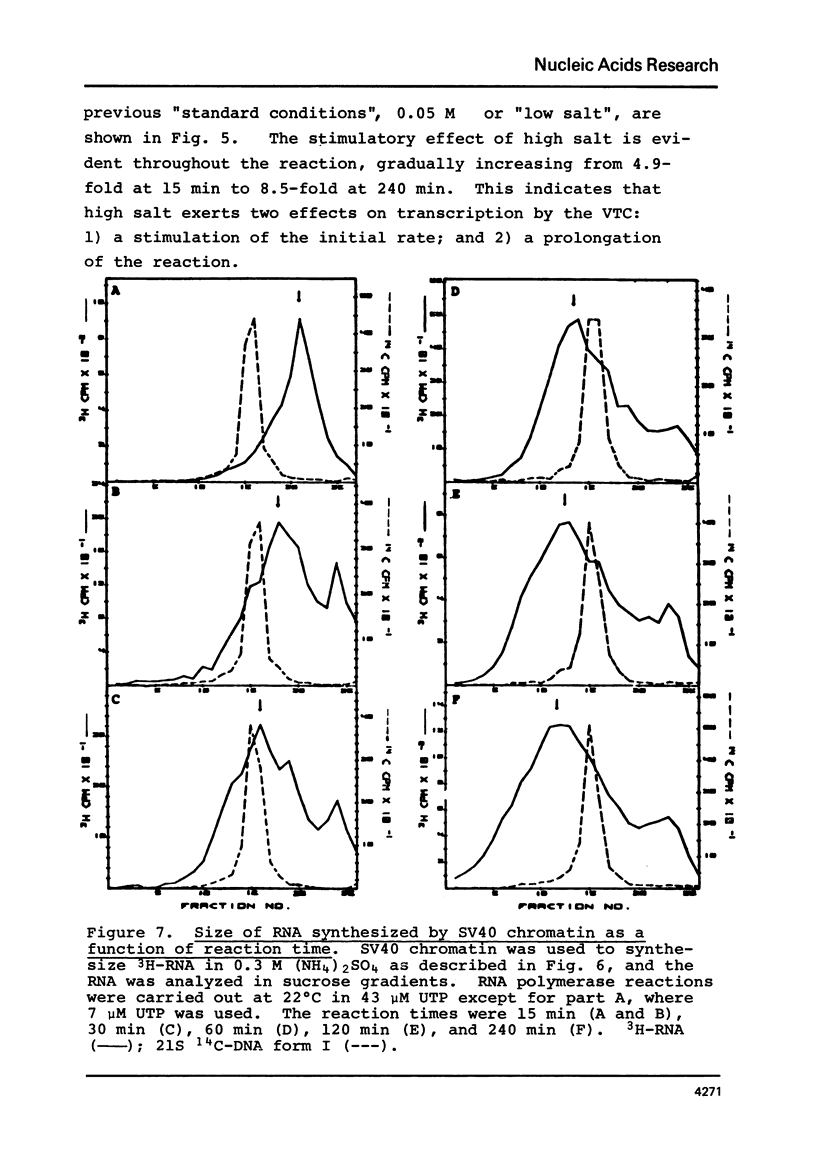
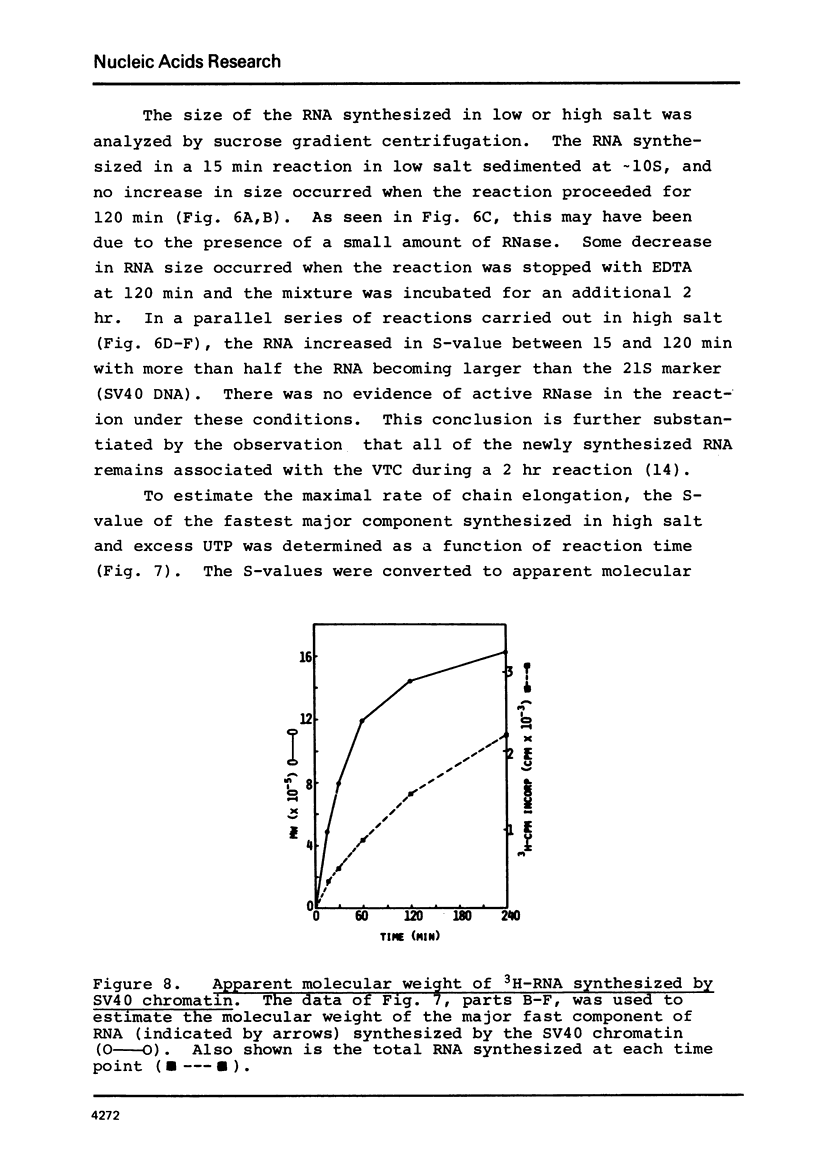
SV40 chromatin obtained from infected monkey cells was used to study the effect of the viral chromatin proteins on endogenous RNA polymerase II. Ammonium sulfate activated the rate of transcription by endogenous RNA polymerase in two ways: 1) by direct action on the enzyme; and 2) by causing a reversible conformational change in the viral chromatin. Under optimal reaction conditions, the viral chromatin proteins did not limit the rate of RNA chain elongation, and high molecular weight RNA (1.6 X 10(6) d) was synthesized by the SV40 chromatin.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloni Y. Biogenesis and characterization of SV40 and polyoma RNAs in productively infected cells. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):165–178. doi: 10.1101/sqb.1974.039.01.023. [DOI] [PubMed] [Google Scholar]
- Aloni Y., Shani M., Reuveni Y. RNAs of simian virus 40 in productively infected monkey cells: kinetics of formation and decay in enucleate cells. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2587–2591. doi: 10.1073/pnas.72.7.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolle A., Epstein R. H., Salser W., Geiduschek E. P. Transcription during bacteriophage T4 development: synthesis and relative stability of early and late RNA. J Mol Biol. 1968 Feb 14;31(3):325–348. doi: 10.1016/0022-2836(68)90413-0. [DOI] [PubMed] [Google Scholar]
- Bonner J., Dahmus M. E., Fambrough D., Huang R. C., Marushige K., Tuan D. Y. The Biology of Isolated Chromatin: Chromosomes, biologically active in the test tube, provide a powerful tool for the study of gene action. Science. 1968 Jan 5;159(3810):47–56. doi: 10.1126/science.159.3810.47. [DOI] [PubMed] [Google Scholar]
- Butterworth P. H., Cox R. F., Chesterton C. J. Transcription of mammalian chromatin by mammalian DNA-dependent RNA polymerases. Eur J Biochem. 1971 Nov 11;23(2):229–241. doi: 10.1111/j.1432-1033.1971.tb01613.x. [DOI] [PubMed] [Google Scholar]
- Cedar H. Transcription of DNA and chromatin with calf thymus RNA polymerase B in vitro. J Mol Biol. 1975 Jun 25;95(2):257–269. doi: 10.1016/0022-2836(75)90394-0. [DOI] [PubMed] [Google Scholar]
- Christiansen G., Griffith J. Salt and divalent cations affect the flexible nature of the natural beaded chromatin structure. Nucleic Acids Res. 1977 Jun;4(6):1837–1851. doi: 10.1093/nar/4.6.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremisi C., Pignatti P. F., Croissant O., Yaniv M. Chromatin-like structures in polyoma virus and simian virus 10 lytic cycle. J Virol. 1975 Jan;17(1):204–211. doi: 10.1128/jvi.17.1.204-211.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fey G., Hirt B. Fingerprints of polyoma virus proteins and mouse histones. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):235–241. doi: 10.1101/sqb.1974.039.01.030. [DOI] [PubMed] [Google Scholar]
- Gariglio P., Mousset S. Isolation and partial characterization of a nuclear RNA polymerase - SV40 DNA complex. FEBS Lett. 1975 Aug 1;56(1):149–155. doi: 10.1016/0014-5793(75)80130-x. [DOI] [PubMed] [Google Scholar]
- Germond J. E., Hirt B., Oudet P., Gross-Bellark M., Chambon P. Folding of the DNA double helix in chromatin-like structures from simian virus 40. Proc Natl Acad Sci U S A. 1975 May;72(5):1843–1847. doi: 10.1073/pnas.72.5.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein D. A., Hall M. R., Meinke W. Properties of nucleoprotein complexes containing replicating polyoma DNA. J Virol. 1973 Oct;12(4):887–900. doi: 10.1128/jvi.12.4.887-900.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. H., Brooks T. L. Isolation of two forms of SV40 nucleoprotein containing RNA polymerase from infected monkey cells. Virology. 1976 Jul 1;72(1):110–120. doi: 10.1016/0042-6822(76)90316-0. [DOI] [PubMed] [Google Scholar]
- Green M. H., Miller H. I., Hendler S. Isolation of a polyoma-nucleoprotein complex from infected mouse-cell cultures. Proc Natl Acad Sci U S A. 1971 May;68(5):1032–1036. doi: 10.1073/pnas.68.5.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith J. D. Chromatin structure: deduced from a minichromosome. Science. 1975 Mar 28;187(4182):1202–1203. doi: 10.1126/science.187.4182.1202. [DOI] [PubMed] [Google Scholar]
- Hall M. R., Meinke W., Goldstein D. A. Nucleoprotein complexes containing replicating Simian virus 40 DNA: comparison with polyoma nucleoprotein complexes. J Virol. 1973 Oct;12(4):901–908. doi: 10.1128/jvi.12.4.901-908.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hossenlopp P., Oudet P., Chambon P. Animal DNA-dependent RNA polymerases. Studies on the binding of mammalian RNA polymerases AI and B to Simian virus 40 DNA. Eur J Biochem. 1974 Jan 16;41(2):397–411. doi: 10.1111/j.1432-1033.1974.tb03281.x. [DOI] [PubMed] [Google Scholar]
- Louie A. J. The organization of proteins in polyoma and cellular chromatin. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):259–266. doi: 10.1101/sqb.1974.039.01.034. [DOI] [PubMed] [Google Scholar]
- Mandel J. L., Chambon P. Animal DNA-dependent RNA polymerases. Analysis of the RNAs synthesized on Simian virus 40 superhelical DNA by mammalian RNA polymerases AI and B. Eur J Biochem. 1974 Jan 16;41(2):379–395. doi: 10.1111/j.1432-1033.1974.tb03280.x. [DOI] [PubMed] [Google Scholar]
- Meinke W., Hall M. R., Goldstein D. A. Proteins in intracellular simian virus 40 nucleoportein complexes: comparison with simian virus 40 core proteins. J Virol. 1975 Mar;15(3):439–448. doi: 10.1128/jvi.15.3.439-448.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkley E. G., Pribnow D. Transcription of the early region of bacteriophage T7: selective initiation with dinucleotides. J Mol Biol. 1973 Jun 25;77(2):255–277. doi: 10.1016/0022-2836(73)90335-5. [DOI] [PubMed] [Google Scholar]
- Olins A. L., Olins D. E. Spheroid chromatin units (v bodies). Science. 1974 Jan 25;183(4122):330–332. doi: 10.1126/science.183.4122.330. [DOI] [PubMed] [Google Scholar]
- Oudet P., Gross-Bellard M., Chambon P. Electron microscopic and biochemical evidence that chromatin structure is a repeating unit. Cell. 1975 Apr;4(4):281–300. doi: 10.1016/0092-8674(75)90149-x. [DOI] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb J. A., Martin R. G. Genetic analysis of simian virus 40. 3. Characterization of a temperature-sensitive mutant blocked at an early stage of productive infection in monkey cells. J Virol. 1972 Jun;9(6):956–968. doi: 10.1128/jvi.9.6.956-968.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahasrabuddhe C. G., Saunders G. F. Salt-induced structural changes in nucleosomes. Nucleic Acids Res. 1977 Apr;4(4):853–866. doi: 10.1093/nar/4.4.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shani M., Birkenmeier E., May E., Salzman N. P. Properties of simian virus 40 transcriptional intermediates isolated from nuclei of permissive cells. J Virol. 1977 Jul;23(1):20–28. doi: 10.1128/jvi.23.1.20-28.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmookler R. J., Buss J., Green M. H. Properties of the polyoma virus transcription complex obtained from mouse nuclei. Virology. 1974 Jan;57(1):122–127. doi: 10.1016/0042-6822(74)90113-5. [DOI] [PubMed] [Google Scholar]
- Solage A., Cedar H. RNA chain elongation on a chromatin template. Nucleic Acids Res. 1976 Sep;3(9):2223–2231. doi: 10.1093/nar/3.9.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshavsky A. J., Bakayev V. V., Chumackov P. M., Georgiev G. P. Minichromosome of simian virus 40: presence of histone HI. Nucleic Acids Res. 1976 Aug;3(8):2101–2113. doi: 10.1093/nar/3.8.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M., Eason R. Nucleoprotein complexes in simian virus 40-infected cells. J Virol. 1971 Oct;8(4):363–371. doi: 10.1128/jvi.8.4.363-371.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F. L., Feigelson P. Studies on the role of ammonium sulfate in nuclear transcription in vitro. Biochim Biophys Acta. 1972 Jun 22;272(1):119–123. doi: 10.1016/0005-2787(72)90039-1. [DOI] [PubMed] [Google Scholar]



