Abstract
Early diagnosis of active tuberculosis (TB) remains an elusive challenge, especially with disseminated TB and HIV co-infection. Recent studies have shown a promise for detection of pathogen biomarkers such as lipoarabinomannan (LAM) in the diagnosis of TB in HIV-positive individuals. Currently, immunoassay platforms that suffer from poor sensitivity and high non-specific interactions are used for biomarker detection. Herein, we demonstrate the development of sandwich immunoassays for the direct detection of three TB-specific biomarkers, namely LAM, early secretory antigenic target 6 (ESAT6) and antigen 85 complex (Ag85), using a waveguide-based optical biosensor. Combining detection within the evanescent field of an optical waveguide with functional surfaces that reduce non-specific interactions allows for the sensitive and quantitative detection of biomarkers in patient samples (urine, serum) within a short time. Using this approach, we demonstrate for the first time, the quantitative detection of LAM in urine from a small cohort of subjects with TB but without HIV co-infection, with excellent sensitivity and specificity. These results suggest that pathogen biomarkers can be applied for the rapid diagnosis of disease. It is likely that detection of a combination of biomarkers offers greater reliability of diagnosis, rather than any single biomarker alone. NCT00341601.
Keywords: Tuberculosis, lipoarabinomannan, Early secretory antigenic target 6, antigen 85 complex, waveguide-based optical biosensor
1. Introduction
Tuberculosis (TB), caused by Mycobacterium tuberculosis, is a major global health threat that was responsible for 9.4 million incident cases worldwide in 2009, resulting in 1.6 million deaths.1 Increased incidence of drug resistant strains of Mycobacterium tuberculosis and HIV co-infection, as well as the continued migration of individuals from areas where TB is endemic to more industrialized countries, have all made TB a global medical emergency.2 Current diagnostic strategies such as sputum smear microscopy are unreliable, especially in individuals with HIV co-infection.3 It is widely accepted that the development of rapid and accurate new diagnostic tools is essential to improve TB control in developing countries, and its consequent dissemination to the industrialized world.
Use of pathogen-specific biomarkers for the diagnosis of TB has been controversial. Several pathogen-specific biomarkers of TB have been identified. Noteworthy among them are the early secretory antigenic target 6 (ESAT6), antigen 85 complex (Ag85) and the cell wall lipoglycan lipoarabinomannan (LAM). ESAT6 is the basis for the diagnosis of TB using the interferon-γrelease assays.4 Talaat et al5 have developed immunoassays for the rapid detection of ESAT6 antibodies. An ELISA-based assay for the detection of ESAT6 antibodies in TB sera was associated with 97.6% sensitivity and 75% specificity in the diagnosis of TB infection. However, discriminating exposed individuals in an endemic background using antibody-detection strategies is difficult. This is because of one of many reasons: an individual may be vaccinated against said disease resulting in a cross-reactive response with antibody-based diagnostics, or may have been exposed to the pathogen or its near neighbors without actually having active infection, resulting in an enhanced antibody response. Antibodies to ESAT6 are also a sensitive strategy for the detection of early bovine TB infection.6 Ag85 has been of interest in the diagnosis of tuberculosis-associated meningitis. Kashyap et al7 have shown that 89% of the population evaluated in their study of tuberculous meningitis (71 of 80 enrolled patients) were positive for Ag85 complex. The protein has also been reported to be present in the blood of patients with extra-central nervous system TB.8, 9
LAM is a prominent lipoglycan component of the cell wall of Mycobacterium tuberculosis. Of late, the direct detection of LAM has garnered interest for the diagnosis of TB, especially in HIV-positive individuals.10, 11 Reither et al evaluated a plate-based sandwich ELISA immunoassay for LAM for the diagnosis of TB in 291 Tanzanian patients with pulmonary tuberculosis.12 They demonstrated that the assay had a high sensitivity (87.8%) for detection of TB and also that the specificity of the test was higher in women (66.7%), and in patients with HIV co-infection (62%) when compared to sputum-smear microscopy. Shah et al10, 13 conducted a nested prospective cohort study in three South African hospitals to evaluate the accuracy of semi-quantitative urinary detection of LAM using the commercially available Clearview TB ELISA (Inverness Medical Innovations, Waltham, MA). The authors concluded that the LAM test detected a subset of HIV-positive individuals with severe TB whose sputum-smear microscopy results were sub-optimal. Further, these studies indicate that urinary LAM correlates with bacillary burden in microbiologically confirmed TB. These studies suggest that urinary detection of biomarkers such as LAM might be of value in the detection of TB, especially in HIV-positive individuals. However, the current assays for LAM detection in urine, such as the Chemogen Inverness Dipstick and ELISA assays, are semi-quantitative. Further, lateral flow immunoassays and plate-based colorimetric assays have poor sensitivity as a consequence of the high non-specific interactions in complex biological samples.14 Indeed, Reither et al reported that although the LAM ELISA was effective in diagnosis of TB in HIV-positive populations, the sensitivity of the assay could be significantly improved.12
Accordingly, the development of ultra-sensitive strategies for the detection of the low concentrations of pathogen-specific biomarkers in patients may permit the sensitive, specific and rapid diagnosis of active TB infection, with low false negatives. As described above, this strategy may be effective in the diagnosis of early TB (ESAT6), disseminated and extra-pulmonary TB (Ag85) and TB in children and HIV-positive individuals (LAM).
We have developed a waveguide-based optical biosensor for the rapid, sensitive and specific detection of biomarkers associated with pathogens and effectively demonstrated it for the detection of biomarkers associated with influenza,15 anthrax,16 breast cancer17 and tuberculosis.18 We have also developed novel surface functionalization chemistry, self-assembled monolayers (SAMs), for the minimization of non-specific interactions associated with complex biological samples, a strategy that effectively enhances the signal over noise ratio, thereby improving assay sensitivity.19, 20 Herein, we demonstrate the application of our sensor platform to the detection of three TB-specific biomarkers, LAM, ESAT6 and Ag85, in a sandwich immunoassay format, and compare them with the sensitivity achieved by traditional plate-based ELISAs. We also demonstrate, in a blinded study, the effectiveness of this strategy for the sensitive and quantitative detection of LAM in urine and ESAT6 in serum, from a small set of human samples collected from a study of TB infected persons.
2. Materials and methods
2.1. Materials
The waveguide-based sensor was developed at the Los Alamos National laboratory.16 Silicon oxynitride (SiONx) planar optical waveguides were fabricated at nGimat (Atlanta) and have been effectively used with the waveguide-based biosensor platform for over a decade.21 SiONx films have a thickness of ~ 120 nm (± 5 nm) and a refractive index of 1.80± 0.06. A thin ~10 nm coating of SiO2 is deposited on the active waveguide surface for functionalization. Materials required for waveguide functionalization with SAMs are described elsewhere.19 LAM (14-19 Kda), ESAT6, and Ag85 complex from Mycobacterium tuberculosis H37Rv culture and, the rabbit polyclonal antibody and monoclonal antibodies and cell lines for the above antigens were procured by a materials contract from the Colorado State University (via BEI Resources). Monoclonal anti-ESAT6 antibodies (HYB 076-08) and EasyLink Streptavidin conjugation kit were purchased from AbCam. EZ-link Sulfo-NHS-LC-LC-Biotin and streptavidin were from Pierce. Alexa Fluor 647 (AF647) labeling kit was from Invitrogen. 1,2-Dioleoyl-sn-glycero-3- phosphocholine (DOPC) and 1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine-N-(cap biotinyl) (cap-biotin-PE) were from Avanti Polar Lipids, Inc. Miniature G-25 sephadex columns were acquired from Harvard Apparatus and bovine serum was purchased from Hyclone Laboratories. Human Serum was obtained from Biomedical Technologies Inc. Nunc Immunosorp plates for use in immunoassays were purchased from Nalgene Nunc Inc., (Milwaukee, WI). Secondary antibodies were purchased from Jackson ImmunoResearch, West Grove, PA and Southern Biotech Ltd, Birmingham, Alabama. All other immunoassay and protein estimation reagents were purchased from Pierce Biologicals Ltd., Rockford, Illinois or Sigma Aldrich.
2.2. Humans Subjects
Korean Ministry of Health Welfare and Family and NIAID co-sponsored a Natural History study conducted at the National Masan Tuberculosis Hospital (NMTH) to investigate occurrence of multidrug resistant tuberculosis (MDR-TB) and the rate of relapse in Korean subjects undergoing treatment for tuberculosis for the first time or those that were being retreated (http://clinicaltrials.gov NCT00341601). Initial blood and periodic sputum samples were collected from all subjects during treatment and subjects who had taken 7 days or less of anti-tubercular therapy were asked to provide a urine sample for analysis. Other objectives of the study included the investigation of possible biomarkers of disease and thus age and gender matched control subjects were recruited from the surrounding province and given a physical exam, chest X-ray, and sputum exam to exclude active TB. The blood of control subjects was also tested with the QuantiFERON-T® Gold assay (QFT-G; Cellestis Limited, Carnegie, Victoria, Australia) to determine their exposure status to M. tuberculosis. The NMTH and NIAID institutional review boards approved the study protocol and all subjects provided written informed consent for the protocol.
2.3. Collection of Urine Samples
Biomarkers of interest (LAM, ESAT6 and Ag85) were spiked into control human urine and subjected to a variety of treatments to design the optimal procedure for their collection. The procedures evaluated were: stability at room temperature (up to 4 hrs), with protease inhibitor treatment, pH correction, upon dialysis, filtration and centrifugal clarification. It was determined that ESAT6 and LAM were stable in spiked urine for 2 hrs, after which their stability deteriorated and that centrifugation did not deter biomarker stability in urine, but it did not contribute to it either. All other methods were, in fact, detrimental, for the stability of LAM in spiked urine. Based on these observations, urine samples were collected from the patients, aliquoted (1 mL each) and snap-frozen in liquid nitrogen within 60 minutes of collection. Urine was collected from HIV-free, TB-infected subjects and control subjects, coded, tested for the absence of culturable M. tuberculosis, and shipped to LANL where they were frozen at −80°C until use.
2.4 Detection of LAM, Ag85 and ESAT6 by Traditional plate-based ELISA
Microtiter plates from Dynatech were used for LAM and Ag85 ELISAs, whereas Nunc MaxiSorp flat-bottom 96 well plates were used for ESAT6. PBS containing 2% BSA was used for blocking (37°C for 2 h). The washing protocol comprised of PBS −0.1% tween 20 (3X), followed by PBS (3X). Horseradish peroxidase (HRP) conjugated reporter antibody was used in all cases (37°C for 1 h). After washing, 100 μl of substrate solution, H2O2–tetramethylbenzidine (TMB), was added to the wells, and incubated for 30 min, RT. The reaction was stopped with 100 μl of 2.5N H2SO4, and the absorbance was read at 450 nm on a Molecular Devices Absorbance Plate Reader. Each test was performed in triplicate, and the mean absorbance of control wells was subtracted from that of wells with LAM before analysis.
For the LAM assay, polyclonal LAM antibody (20mg/ml) was diluted (1:500) in carbonate buffer (pH 9.6) and incubated overnight at 4°C. After washing and blocking, either mannose capped LAM, or an unknown clinical sample was added to the wells (2hrs, 37°C). After washing, HRP-conjugated reporter antibody was added, and the specific reaction measured as described above.
For Ag85 complex, 100 μl of CS90 (150 nM) was coated on the plates (2 hrs at RT, followed by 4°C overnight). After washing and blocking, Ag85 (diluted in serum) was added at varying concentrations. Following incubation (37°C, 2 hrs) and washing, reporter antibody was added and plates developed with TMB substrate.
For ESAT6, wells were coated with anti-ESAT6 polyclonal antibody in PBS (100 μl, 200 nM, overnight at 4°C). After washing and blocking, varying concentrations of ESAT6 in PBS- 0.5% BSA were added, and incubated (37°C for 2 hrs). After washing, 15 nM anti-ESAT6 monoclonal antibody (2 hr, 37°C) was added. After washing, HRP-conjugated goat-anti mouse IgG polyclonal antibody was added (1 hr at 37°C). Plates were washed, and developed with TMB substrate.
2.5 Preparation of Antibodies for Fluorescence Assays on the Waveguide-based Biosensor
The choice of capture and reporter antibodies and their effective concentrations in the assays were determined by conventional plate-based assays, using checkerboard titration. Capture antibodies (CS40 for LAM, HYB 076-08 for ESAT6, CS90 for Ag85) were biotinylated using Sulfo-NHS biotin (20-fold molar excess), as described in detail elsewhere.22 The reporter antibodies (rabbit polyclonal antibodies against LAM, ESAT6 and Ag85) were labeled with AF647 using a fluorescence labeling kit from Invitrogen as per the manufacturer’s instructions. In both cases, labeled protein was separated from unlabeled by gel filtration followed by exhaustive dialysis (if applicable) in PBS. Labeled proteins were tested for binding activity (immunoblot) and the extent of biotinylation (4-hydroxy azo-benzene-2-carboxylic acid analysis) and fluorescence labeling (UV-Vis spectroscopy). Detailed methods for labeling and characterization have been presented elsewhere.22 Only material with optimal protein recovery and degree of labeling was used for the assays described below.
2.6 Functionalization of Waveguides
Single mode planar optical waveguides consisting of a 10 nm coating of SiO2 for the functionalization of the waveguide surface were used. Waveguides and glass coverslips were cleaned by sonication in chloroform and ethanol, and water (5 min each), followed by exposure to UV-light and ozone (40 min) and subsequently functionalized for bioassays with either unilamellar bilayers or SAMs. The preparation of biotinylated (1%) unilamellar vesicles by sonication has been previously described.17, 22 Briefly, thin films of DOPC (5 mM stock in CHCl3, 60 μl) with 1% cap biotinyl (v/v, sodium salt, 5mM stock in CHCl3, 0.6 μl,) were deposited on test tubes by evaporation of chloroform under argon and then, re-hydrated in buffer (PBS, 0.01 M, 60 min). They were then subjected to eight freeze-thaw cycles (frozen in liquid nitrogen and thawed in warm water) and sonicated with a probe tip sonicator (Branson, 50% duty cycle) for 5 min to form uniform vesicles.
Waveguides and coverslips were assembled into a flow cell using a gasket to form a tight seal between them as outlined earlier.17, 21 A supported bilayer was formed on the waveguide surface by injecting the vesicle solution into the flow cell, and allowing it to stabilize (12-24 hrs). The functionalized surface was blocked with PBS containing 2% BSA for 1 hr before use.
Preparation of SAMs has also been described previously.19 Briefly, waveguide and coverslip surfaces were cleaned as described above, and rinsed in water, blown dry, and placed in a vacuum desiccators with a small volume (~50 μl) of 1-(3-aminopropyl)-1-methyldiethoxy silane (APMDES), which was held at static vacuum (200 torr) for 2 h. The coated substrates were annealed (110 °C, 30 min), allowed to cool in a drying desiccator, rinsed with ethanol, blown dry, and analyzed (contact angle). Silanized slides were immersed in a solution containing a mixture of carboxylic acid-terminated polyethylene glycol (PEG) reagents with either a methoxy group (99-99.99% of the total PEG volume) or a 9-fluorenylmethoxycarbonyl (F-moc)-protected amine (0.01-1% of the PEG volume) with standard peptide coupling reagents. After confirmation of coupling by decrease in contact angle, protecting groups were removed (20% piperidine/(N-methylpyrrolidinone) NMP, 2×15 min), and biotin was attached to the resulting free-amine via its commercially available N-hydroxysuccinimide ester. All steps after amino-silanization were carried out in NMP as the solvent. The slides were rinsed thoroughly between steps; ethanol was used after silanization, while NMP, acetone, and ethanol were used subsequently. After biotinylation, the slides were rinsed and dried under a stream of argon.
2.7. Sandwich Immunoassays for Biomarkers on the Waveguide-based Optical Biosensor
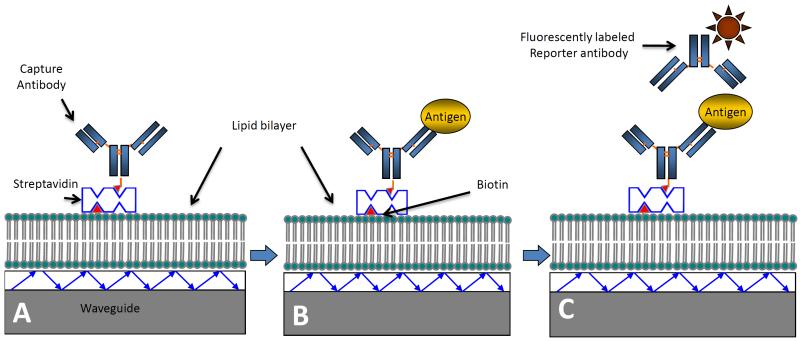
The general scheme for the detection of TB biomarkers on functionalized waveguides by sandwich immunoassay format is described in Scheme 1. This description applies to all three biomarkers for which sandwich immunoassays were developed (LAM, Ag85 and ESAT6). Differences in incubation time and antibody concentrations are specifically mentioned.
Scheme 1.
Illustration of the sandwich immunoassay on a functionalized waveguide. A) Waveguides functionalized with a lipid bilayer are prepared by binding of capture antibody using biotin-streptavidin chemistry. B) Addition of the sample containing the antigen results in specific binding. C) Finally, addition of a fluorescently labeled reporter antibody results in a specific signal measured via a spectrometer interface. Schematic not drawn to scale, and represents process for all three biomarkers investigated in this report.
In all experiments, functionalized waveguides (either SAMs or lipid bilayers, depending on the experiment) were mounted in the flow cell and blocked with 2% BSA in PBS for 60 min at RT, and waveguide-associated background (intrinsic measure of impurities associated with the instrument) measured. After each addition, the flow cell was washed with PBS (~1.5 ml, 60X flow cell volume), unless otherwise specified. Then, fluorescently labeled streptavidin (Strep-AF647, 50 pM) was injected into the flow cell (5 min, RT), and washed and fluorescence due to the binding of streptavidin to the biotinylated functional surface measured to confirm integrity of the functionalization process.
Unlabeled streptavidin (10 nM) was then added (5 min, RT) to saturate the functional surface, followed by addition of the biotinylated capture antibody specific to the biomarker being detected. For ESAT6, streptavidin conjugated capture antibody was added directly to the biotin-functionalized waveguide surface. The concentrations and incubation times were different for the biomarkers (125 nM of anti-LAM CS40 for 15 min, 150 nM of anti-Ag85 CS90 for 30 min, 110 nM of anti-ESAT6 monoclonal for 60 min). Non-specific interactions (NS) were determined in each experiment by the addition of control bovine serum (1:10 dilution, 30-60 min, RT), followed by the reporter antibody (anti-LAM-AF647 (100 nM), anti-ESAT6 (40 nM), anti-Ag85 (75 nM), 15 min, RT). Specific detection of the biomarker was measured by the addition of the antigen (spiked in bovine serum) to the flow cell (30-60 min, RT, depending on the antigen), followed by addition of the reporter antibody (15 min, RT). The fluorescence signal associated with the binding of the biomarker to the reporter was measured using a spectrometer interface.
2.8 Measurement of LAM and ESAT6 in Patient Samples
As indicated in Section 2.3, urine was collected in S. Korea and, evaluated for M. tuberculosis sterility and shipped frozen to LANL. Aliquots were thawed and used in the assay within 30 minutes. In all experiments, a standard measurement of a known concentration of LAM was performed on functionalized waveguides (Section 2.5) to serve as an internal standard for quantitation of the LAM concentration in the unknown sample. The fluorescent signal associated with this measurement was photobleached back to baseline before subsequent measurements. Patient urine, diluted 10X in PBS, was then added to the flow cell and incubated for 60 minutes, RT. After washing with PBS, the reporter antibody was added and the specific signal, indicating LAM in the patient urine, was measured. Extrapolation of the signal against the standard measurement made on the same waveguide provides a quantitative extrapolation of the LAM concentration in urine.
In all experiments, a second standard LAM measurement was made after the measurement of the unknown sample. This measurement confirms the reproducibility of the signal on the same waveguide, the availability of sufficient capture sites to assure reliable multiple measurements on the same waveguide and the stability of optical parameters during the experiment.
3. Results
31. Detection of Biomarkers by Conventional Immunoassays
Biomarker stability and appropriate sample collection protocols are essential to the accurate determination of the success of any diagnostic/detection assay. Therefore, the stability of the spiked biomarkers in serum and/or urine was determined by performing immunoblots (data not shown) under a variety of conditions as described earlier. It was determined that snap freezing urine in liquid nitrogen causes no compromise in sensitivity of detection of LAM whereas treatment of urine with protease inhibitor cocktail, pH correction with PBS tablets (pH 7.4, 1 M final concentration), dialysis, centrifugal clarification and filtration, compromise sensitivity or completely inhibit detection. Similar stability studies were performed for ESAT6 and Ag85 in spiked plasma and urine. It was found that none of the treatments inhibits detection of these antigens nor does it significantly enhance it. Therefore, snap frozen urine samples and frozen serum samples were used for all the patient measurements. Also, stability of LAM in urine decreases after 2 h, RT, and is undetectable by 3 h using immunoblots (data not shown).
We evaluated the sensitivity and specificity of detection of the three biomarkers: LAM, ESAT6 and Ag85, using conventional plate-based ELISAs using the same sample collection protocol and antibodies as used in the waveguide-based assays (Section 3.2) to provides an accurate comparison of the performance of the two assay platforms. Table 1 summarizes the data from standard plate-based ELISAs for the detection of LAM and ESAT6 and Ag85 (average of three measurements) in spiked samples (urine for LAM, serum for ESAT6 and Ag85). The limit of detection (LoD) for LAM using this approach is 78 nM in urine (LoD = average background + 3*(standard deviation), whereas the LoD for ESAT6 is 125 nM and that for Ag85 complex, is 183 nM, both in spiked serum. The table compares the LoD with the measurements made on the waveguide-platform, described in Section 3.2.
Table 1.
Summary of plate-based ELISA measurements for TB biomarkers LAM, ESAT6, and Ag85 complex. The NS binding and the signal measured at the LoD are shown for all three biomarkers, when diluted in serum/urine. The LoD measured on the waveguide-based platform is also indicated.
| Sample | Biomarker | Optical Density |
Limit of Detection (pM) |
||
|---|---|---|---|---|---|
| Control | Positive | ELISA | Waveguide | ||
| Serum | Ag85 | 0.06 ± 0.01 | 0.24 ±0.01 | 183000 | 0.5a |
| Serum | ESAT6 | 0.31 ± 0.1 | 0.9 ± 0.4 | 125000 | 100 |
| Urine | LAM | 0.13 ± 0.005 | 0.18 ± 0.006 | 78000 | 1 |
LoD for Ag85 derived from two measurements. A complete standard curve was not generated because of poor antibody stability
3.2. Detection of Biomarkers in Spiked Samples Using the Waveguide-based Biosensor
To accurately evaluate assay sensitivity and specificity, concentration-dependent measurements of the biomarkers were performed on the waveguide-based platform. Figure 1 demonstrates typical spectra measured with the detection of LAM (A), ESAT6 (B) and Ag85 (C) using the spectrometer interface of the waveguide-based platform. For LAM (Fig. 1A), the waveguide-associated background and NS binding associated are minimal (<150 relative fluorescence units, RFU). The specific signal associated with the detection of 100 pM of LAM spiked in control urine is 1420 RFU. For 200 pM of ESAT6 (Fig. 1B) in 50% human serum (conditions similar to those for patient serum samples), the specific signal (850 RFU) is significantly higher than waveguide-associated background and NS binding (160 RFU). For Ag85 (Fig. 1C), a specific signal of 5020 RFU is shown over a minimal NS binding of 350 RFU, resulting in a phenomenal signal/background of 1673 for 500 pM Ag85 in 50% serum. However, further experiments were not performed for Ag85 owing to the highly unstable nature (< 1 week) of the fluorescently labeled polyclonal reporter antibody, stressing the significance of reliable recognition ligands for immunoassays. We are currently exploring the use of single-chain and aptamer-based antibodies for the detection of Ag85 in waveguide-based sandwich assays. For LAM and ESAT6, measurements were made in triplicate on different waveguides and used for the construction of the standard curve after correction for the waveguide-associated background and NS binding.
Figure 1.
Representative curves for biomarker detection on the waveguide-based platform. A) LAM (100 pM), B) ESAT6 (200 pM) and C) Ag85 complex (500 pM). RFU measured is plotted as function of wavelength. Black lines indicate waveguide-associated background. Gray lines represent NS-binding associated with binding of reporter antibody to a control sample. Black circles indicate specific signal measured for the corresponding biomarker.
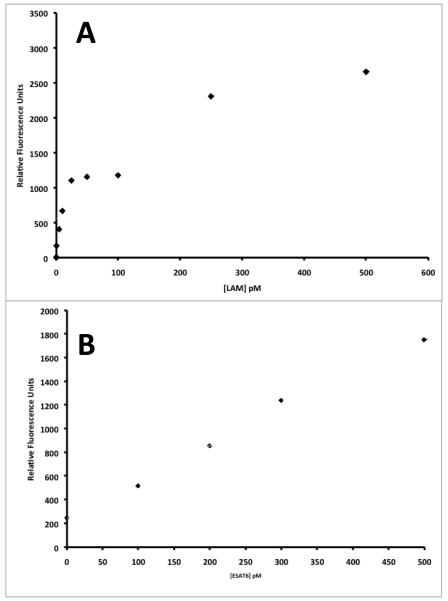
For LAM (Fig. 2A), The NS binding associated with the interaction of the reporter antibody in control urine is 122 RFU, and is considered the baseline value (0 pM). The standard curve for LAM is reasonably linear, with r2 of 0.817, which is reasonable given the intrinsic variability associated with measurements made on different waveguides. The assay has a broad dynamic range and is linear over two orders of magnitude. The standard curve shows linear dependence on concentration in the 1 pM – 25 pM range. The calculated LoD for LAM in urine is 1 pM. For ESAT6 in 50% serum (Fig. 2B), NS binding is 247 RFU. The standard curve for ESAT6 in the concentration range 100 pM to 500 pM is linear, with an r2 of 0.99. The assay has a dynamic range of one order of magnitude, and the LoD for detection of ESAT6 in spiked serum is 100 pM.
Figure 2.
Standard curves constructed for LAM spiked in urine (A) and ESAT6 spiked in serum (B) on the waveguide-based platform. RFU is plotted as a function of concentration for each biomarker. Measurements (n=3/concentration) were made on different waveguides.
3.3. Detection of LAM in Subject Urine
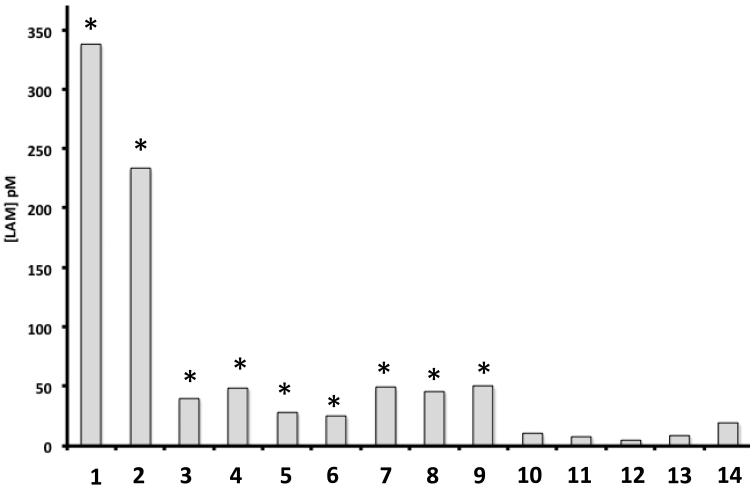
To evaluate the potential use of biomarkers for the accurate detection in clinical samples and possible diagnosis of TB, the LAM assay was performed on 14 blinded urine samples collected from HIV-negative TB patients and healthy controls as per the protocol described in Section 2.2. This small cohort was tested to demonstrate the feasibility of this approach to the sensitive detection of pathogen biomarkers, and not as a clinical trial to establish the diagnostic reliability of said biomarkers. Figure 3 demonstrates that LAM concentrations were significantly higher (student T-test, Fig. 3) in 9 of 14 samples with a concentration range of 24 to 338 pM, based on internal standards. All nine samples were from subjects with a chest X-ray suggestive of active TB, sputum smears positive for acid-fast bacilli, and positive cultures on Lowenstein Jensen medium. Five samples (10-14) gave signals below NS background, and were all determined to be negative for LAM. All these samples were from individuals recruited as controls, with a clean chest X-ray and physical exam and a negative Quantiferon-Gold test, suggesting the subjects did not have active or latent TB.
Figure 3.
Quantitative measurement of urinary LAM in a small cohort of TB patients without HIV co-infection. LAM concentration is plotted as a function of patient ID. LAM was detected only in patients with active TB (1-9) and not in QFG-negative controls (10-14). Each measurement was repeated twice for confirmation.
3.4. Detection of ESAT6 in Serum
The sandwich assay for ESAT6 was performed in six-blinded plasma samples also obtained from NMTH (Fig. 4). ESAT6 was detected in three of the six samples (1-3) but not detected above NS levels in the other three (4-6). Samples 1-3 were collected from individuals with confirmed active TB (see above), and also tested positive for LAM. Samples 4-6 were found to be from the control TB-negative group, with no TB infection. Data is plotted as corrected RFU (measured RFU-NS binding) for each sample. The extrapolated ESAT6 concentration in patients 1-3, based on the standard curve, is between 50-100 pM.
Figure 4.
Quantitative measurement of serum ESAT6 in a small cohort of TB patients without HIV co-infection. ESAT6 concentration is plotted as a function of patient ID. Only samples from patients with active TB were positive for ESAT6 (1-3), whereas it was undetectable in negative controls (4-6). * indicates significant difference from controls.
4. Discussion
Despite being one of the oldest diseases known to mankind, current methods for the accurate diagnosis of active TB are inadequate. Sputum smear microscopy, which is the most common method for diagnosis used in countries with an active vaccination program (where the disease is endemic), is increasingly unreliable especially in individuals with co-infection with HIV.1 There is thus a need for rapid, reliable and sensitive detection of active TB.
Infection in the human host results in the secretion of pathogen-specific biomarkers many of which are immediately recognized by the host innate immune receptors, resulting in an immune response that can culminate in the elimination of the pathogen. Mimicking innate immune recognition of pathogen biomarkers in vitro can allow for a rapid, early, sensitive and reliable method of diagnosis of infectious disease. However, this approach has several limitations: 1) most of the current methods of detection are not capable of quantitative detection of the very low concentrations of the pathogen biomarkers in the human host, 2) sensitive and specific recognition ligands are not available for many pathogen biomarkers, 3) most pathogen-biomarkers are not secreted during the entire course of the disease, and information regarding biomarker expression as a function of disease progression is limited and 4) very little information is available regarding appropriate sample collection and processing strategies to preserve the bio-active properties of pathogen biomarkers.
In this manuscript, we have attempted to evaluate the use of direct detection of M. tuberculosis- specific biomarkers for diagnosis of active infection. The novel findings in this manuscript are:
Lipidated biomarkers like LAM do not tolerate sample processing such as filtration, dialysis and pH correction. Snap freezing urine in liquid nitrogen as soon as it is collected is the most efficient method for preserving urinary LAM. Protein biomarkers like ESAT6 and Ag85 are stable after sample processing methods used here in spiked samples. Improper sample processing may explain some of the discrepancies in existing data regarding the detection of urinary LAM. This is, to our knowledge, the first comprehensive demonstration of the effective of multiple sample processing methods on the stability of LAM in urine. The finding that LAM concentration in urine decreases over time following collection after ~ 2 hours suggests that rigorous sampling protocols must be used to achieve accurate quantitation of urinary LAM.
We have demonstrated sensitive sandwich immunoassays for the detection of urinary LAM (LoD 1 pM), serum ESAT6 (LoD 100 pM) and Ag85 complex (500 fM) on our waveguide-based optical biosensor. Compared to commercial immunoassays such as the Alere® assay or the Inverness® lateral flow urinary LAM assay (LoD 0.1-1 μM),10, 13 or to conventional plate-based ELISAs in our laboratory using the same antibodies (LoD 183,000 pM, Table 1), the waveguide-based LAM assay offers at least a few orders of magnitude greater sensitivity (LoD 1 pM). The assays are rapid (15-60 minutes), quantitative, robust and can be integrated into a multiplex format.23 This is, to our knowledge, the first quantitative estimation of LAM in a sandwich immunoassay format with a LoD of 1 pM.
We have demonstrated the quantitative detection of LAM in a small cohort of TB patients without HIV infection for the first time. This study does not establish the use of LAM as a diagnostic marker, but simply establishes the feasibility of using quantitative measurements of pathogen biomarkers as indicators of infection. We are currently exploring the use of a larger clinical cohort for the determination of diagnostic accuracy of LAM in individuals with and without HIV. Quantitation of LAM may be an indicator of patient bacillary load. However, accurate interpretation of LAM concentration requires a systematic evaluation of stability in urine, and normalization of collection processes to ensure antigenic viability.
We have demonstrated, for the first time, the direct detection of ESAT6 in three plasma samples from patients with active TB.
The current study demonstrates the feasibility of development of sensitive and specific sandwich immunoassays for the direct detection of pathogen biomarkers in patients. LAM, Ag85 and ESAT6 are, by no means, the most suitable biomarkers for the diagnosis of active TB infection. Indeed, no pathogen biomarker is likely expressed during the entire course of the disease. For the effective application of pathogen biomarkers to disease diagnosis, expression profiles of these biomarkers as a course of disease progression should be assessed. We are currently initiating studies to determine expression profiles of LAM in samples collected longitudinally as a function of infection. Similar studies on other biomarkers will help identify a limited suite of these biomolecules that can inform on infection irrespective of the state of disease or treatment.
Lack of effective and stable recognition ligands is another major limitation to the effective use of pathogen biomarkers for disease diagnosis and detection. Whereas we were able to accomplish the sensitive detection of Ag85 complex (LoD, 500 fM) in serum, we were unable to construct an effective standard curve or evaluate expression of the antigen in patients primarily because of the poor stability of the AlexaFluor-647 labeled polyclonal reporter antibody (less than a week at 4°C). The availability of specific, more stable, and high affinity recognition ligands is critical to understanding pathogen biomarker expression in the host and in assessing their potential role in diagnosis. We are currently working on developing better recognition ligands using combinatorial strategies to allow for the reliable detection of Ag85 in future studies.
Acknowledgements
This work was supported in part, by the Intramural Research Program of the NIAID, NIH (CEB) and the South Korean Ministry of Health, Welfare and Family Affairs (CEB and SNC), a WHO/FIND grant (CEB) and a Department of Energy and Los Alamos National Laboratory LDRD Directed Research Award to Drs. B.T. Korber and B.I. Swanson. The authors sincerely thank the many patients that have been willing to sacrifice their time and energy to contribute to this study and the doctors and nurses of the National Masan Tuberculosis Hospital that made this work possible. We also thank Ms. Lisa Goldfelder (NIAID) for assistance with the human research documentation. We thank the Colorado State University Materials Consortium (BEI Resources, NIAID Facility) for all the purified antigens, and some of the antibodies used in this study.
Footnotes
Potential reviewer List
Dr. Susan H Dorman, MD. John’s Hopkins School of Medicine, dsusan1@jhmi.edu Dr. Jerold J. Ellner, MD, Boston Medical College, jerrold.ellner@bmc.org
Dr. R. Waters, Asst Professor, Iowa State University and USDA Scientist, rwaters@nadc.ars.usda.gov
Dr. Mark Chambers, Team Leader and Scientist, Animal Health and Veterinary Laboratories Agency (AHVLA), Department of Bacteriology, mark.chambers@ahvla.gsi.gov.uk
References
- 1.Global tuberculosis control: WHO report. 2011.
- 2.Dye C, Watt CJ, Bleed DM, Hosseini SM, Raviglione MC. Evolution of tuberculosis control and prospects for reducing tuberculosis incidence, prevalence, and deaths globally. JAMA. 2005;293:2767–2775. doi: 10.1001/jama.293.22.2767. [DOI] [PubMed] [Google Scholar]
- 3.Getahun H, Harrington M, O’Brien R, Nunn P. Diagnosis of smear-negative pulmonary tuberculosis in people with HIV infection or AIDS in resource-constrained settings: informing urgent policy changes. Lancet. 2007;369:2042–2049. doi: 10.1016/S0140-6736(07)60284-0. [DOI] [PubMed] [Google Scholar]
- 4.Zwerling A, van den Hof S, Scholten J, Cobelens F, Menzies D, Pai M. Interferon-gamma release assays for tuberculosis screening of healthcare workers: a systematic review. Thorax. 2012;67:62–70. doi: 10.1136/thx.2010.143180. [DOI] [PubMed] [Google Scholar]
- 5.Talaat RM, Radwan GS, Mosaad AA, Saleh AS, Bassiouny K. Rapid immunodiagnostic assays for Mycobacterium Tuberculosis infection. Health. 2010;2:171–176. [Google Scholar]
- 6.Koo HC, Park YH, Ahn J, Waters WR, Palmer MV, Hamilton MJ, Barrington G, Mosaad AA, Park KT, Jung WK, Hwang IY, Cho SN, Shin SJ, Davis WC. Use of rMPB70 protein and ESAT-6 peptide as antigens for comparison of the enzyme-linked immunosorbent, immunochromatographic, and latex bead agglutination assays for serodiagnosis of bovine tuberculosis. J Clin Microbiol. 2005;43:4498–4506. doi: 10.1128/JCM.43.9.4498-4506.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kashyap RS, Dobos KM, Belisle JT, Purohit HJ, Chandak NH, Taori GM, Daginawala HF. Demonstration of components of antigen 85 complex in cerebrospinal fluid of tuberculous meningitis patients. Clin Diagn Lab Immunol. 2005;12:752–758. doi: 10.1128/CDLI.12.6.752-758.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miki K, Nagata T, Tanaka T, Kim YH, Uchijima M, Ohara N, Nakamura S, Okada M, Koide Y. Induction of protective cellular immunity against Mycobacterium tuberculosis by recombinant attenuated self-destructing Listeria monocytogenes strains harboring eukaryotic expression plasmids for antigen 85 complex and MPB/MPT51. Infect Immun. 2004;72:2014–2021. doi: 10.1128/IAI.72.4.2014-2021.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kashyap RS, Biswas SK, Agarwal N, Chandak N, Purohit H, Taori GM, Daginawala HF. Significance of 30 KD protein marker as diagnostic marker in CSF of tuberculous meningitis. Ann. Indian Acad. Neurol. 2001;4:197–201. [Google Scholar]
- 10.Shah M, Variava E, Holmes CB, Coppin A, Golub JE, McCallum J, Wong M, Luke B, Martin DJ, Chaisson RE, Dorman SE, Martinson NA. Diagnostic accuracy of a urine lipoarabinomannan test for tuberculosis in hospitalized patients in a High HIV prevalence setting. J Acquir Immune Defic Syndr. 2009;52:145–151. doi: 10.1097/QAI.0b013e3181b98430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boehme C, Molokova E, Minja F, Geis S, Loscher T, Maboko L, Koulchin V, Hoelscher M. Detection of mycobacterial lipoarabinomannan with an antigen-capture ELISA in unprocessed urine of Tanzanian patients with suspected tuberculosis. Trans R Soc Trop Med Hyg. 2005;99:893–900. doi: 10.1016/j.trstmh.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 12.Reither K, Saathoff E, Jung J, Minja LT, Kroidl I, Saad E, Huggett JF, Ntinginya EN, Maganga L, Maboko L, Hoelscher M. Low sensitivity of a urine LAM-ELISA in the diagnosis of pulmonary tuberculosis. BMC Infect Dis. 2009;9:141. doi: 10.1186/1471-2334-9-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah M, Martinson NA, Chaisson RE, Martin DJ, Variava E, Dorman SE. Quantitative analysis of a urine-based assay for detection of lipoarabinomannan in patients with tuberculosis. J Clin Microbiol. 2010;48:2972–2974. doi: 10.1128/JCM.00363-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hurt AC, Alexander R, Hibbert J, Deed N, Barr IG. Performance of six influenza rapid tests in detecting human influenza in clinical specimens. J Clin Virol. 2007;39:132–135. doi: 10.1016/j.jcv.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Kale RR, Mukundan H, Price DN, Harris JF, Lewallen DM, Swanson BI, Schmidt JG, Iyer SS. Detection of intact influenza viruses using biotinylated biantennary S-sialosides. J Am Chem Soc. 2008;130:8169–8171. doi: 10.1021/ja800842v. [DOI] [PubMed] [Google Scholar]
- 16.Martinez JS, Grace WK, Grace KM, Hartman N, Swanson BI. Pathogen detection using single mode planar optical waveguides. J Mater Chem. 2005;15:4639–4647. [Google Scholar]
- 17.Mukundan H, Kubicek JZ, Holt A, Shively JE, Martinez JS, Grace K, Grace WK, Swanson BI. Planar optical waveguide-based biosensor for the quantitative detection of tumor markers. Sensor Actuat B-Chem. 2009;138:453–460. [Google Scholar]
- 18.Mukundan H, Price DN, Goertz M, Parthasarathi R, Montano GA, Kumar S, Scholfield MR, Anderson AS, Gnanakaran S, Iyer S, Schmidt J, Swanson BI. Understanding the interaction of Lipoarabinomannan with membrane mimetic architectures. Tuberculosis (Edinb) 2012;92:38–47. doi: 10.1016/j.tube.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Anderson AS, Dattelbaum AM, Montano GA, Price DN, Schmidt JG, Martinez JS, Grace WK, Grace KM, Swanson BI. Functional PEG-modified thin films for biological detection. Langmuir. 2008;24:2240–2247. doi: 10.1021/la7033438. [DOI] [PubMed] [Google Scholar]
- 20.Anderson AS, Dattelbaum AM, Mukundan H, Price DN, Grace WK, Swanson BI. Robust Sensing Films for Pathogen Detection and Medical Diagnostics. Proc Spie. 2009;7167 [Google Scholar]
- 21.Mukundan H, Anderson AS, Grace WK, Grace KM, Hartman N, Martinez JS, Swanson BI. Waveguide-Based Biosensors for Pathogen Detection. Sensors-Basel. 2009;9:5783–5809. doi: 10.3390/s90705783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukundan H, Xie H, Anderson AS, Grace WK, Shively JE, Swanson BI. Optimizing a waveguide-based sandwich immunoassay for tumor biomarkers: evaluating fluorescent labels and functional surfaces. Bioconjug Chem. 2009;20:222–230. doi: 10.1021/bc800283e. [DOI] [PubMed] [Google Scholar]
- 23.Mukundan H, Xie HZ, Price D, Kubicek-Sutherland JZ, Grace WK, Anderson AS, Martinez JS, Hartman N, Swanson BI. Quantitative Multiplex Detection of Pathogen Biomarkers on Multichannel Waveguides. Analytical Chemistry. 2010;82:136–144. doi: 10.1021/ac901497g. [DOI] [PubMed] [Google Scholar]