Abstract
The concentration of glutathione (GSH), the most abundant intracellular free thiol and an important antioxidant, is decreased in the lung in both fibrotic diseases and experimental fibrosis models. The underlying mechanisms and biological significance of GSH depletion, however, remain unclear. Transforming growth factor beta (TGF-β) is the most potent and ubiquitous profibrogenic cytokine and its expression is increased in almost all fibrotic diseases. In this study, we show that increasing TGF-β1 expression in mouse lung to a level comparable to those found in lung fibrotic diseases by intranasal instillation of AdTGF-β1223/225, an adenovirus expressing constitutively active TGF-β1, suppressed the expression of both catalytic and modifier subunits of glutamate cysteine ligase (GCL), the rate-limiting enzyme in de novo GSH synthesis, decreased GSH concentration, and increased protein and lipid peroxidation in mouse lung. Furthermore, we show that increasing TGF-β1 expression activated JNK and induced activating transcription factor 3 (ATF3), a transcriptional repressor involved in the regulation of the catalytic subunit of GCL (GCLC), in mouse lung. Control virus (AdDL70-3) had no significant effect on any of these parameters, compared to saline treated control. Concurrent with GSH depletion, TGF-β1 induced lung epithelial apoptosis and robust pulmonary fibrosis. Importantly, lung GSH levels returned to the normal whereas fibrosis persisted at least 21 days after TGF-β1 instillation. Together, the data suggest that increased TGF-β1 expression may contribute to the GSH depletion observed in pulmonary fibrosis diseases and that GSH depletion may be an early event in, rather than a consequence of, fibrosis development.
Keywords: GSH depletion, lung fibrosis, transforming growth factor beta 1, glutamate cysteine ligase, oxidative stress
Introduction
The tripeptide γ-L-glutamyl-L-cysteinyl-glycine, glutathione (GSH), is the most abundant intracellular free thiol and has multiple biological functions [1, 2]. GSH acts as a major cellular storage unit of cysteine and is involved in the regulation of several cellular functions including cell proliferation, differentiation, and apoptosis [3–6]. GSH also provides reducing equivalents for the synthesis of deoxyribonucleotides, dehydroascorbate, and leukotrienes [7, 8]. The most important function of GSH, however, is in antioxidant defense and detoxification of electrophiles [9–11]. GSH can directly scavenge free radicals as well as detoxify hydrogen peroxide and lipid peroxides through GSH peroxidase-catalyzed reactions or electrophiles through glutathione-S-transferase catalyzed reactions [12]. Emerging evidence indicates that GSH is also involved in redox signaling through modification of protein cysteine residues [13–19]. As GSH is involved in many cell processes, maintenance of intracellular GSH homeostasis is critical for cell survival and function.
The lung is exposed constantly to environment pollutants, many of which are oxidants in nature. The epithelial surface of the lower respiratory tract must have mechanisms to protect itself from the damaging effects of oxidants. It has been reported that GSH concentration in the lung lining fluid is higher than that in the plasma [20], suggesting a critical role of GSH in lung antioxidant defense. Importantly, it has been reported that GSH concentration is decreased in the lung lining fluid in patients with fibrotic diseases such as idiopathic pulmonary fibrosis (IPF), cystic fibrosis (CF), acute respiratory distress syndrome (ARDS), and asbestosis [21–26]. A decreased GSH concentration has also been observed in experimental lung fibrosis models [27–30]. The mechanism underlying the GSH depletion in lung fibrotic diseases, however, is unclear. Transforming growth factor beta (TGF-β) is the most potent and ubiquitous profibrogenic cytokine and its expression is increased in almost all fibrotic diseases. Studies from this and other laboratories have shown that TGF-β1 decreases intracellular GSH concentration in various types of cells in vitro [31–35]. Whether increased TGF-β expression is also responsible for the decrease in GSH levels in vivo, however, remains to be determined.
In the present study, we show that increasing TGF-β1 expression in mouse lung to the levels comparable to those found in lung fibrotic diseases via an adenovirus-mediated gene transfer technique down-regulated the expression of GCL, the rata-limiting enzyme in de novo GSH synthesis, and decreased GSH levels in the lung, accompanied by massive fibrotic responses that lasted for at least 21 days although lung GSH levels have returned to the normal by then. These data suggest that increased TGF-β1 expression may contribute to the depletion of GSH in lung fibrotic diseases and that GSH depletion may be an early event in, rather than a consequence of, lung fibrosis development.
Materials and Methods
Overexpression of TGF-β1 in the lung by adenovirus-mediated gene transfer techniques
AdTGF-β1223/225, an adenovirus expressing constitutively active TGF-β1 [36], and AdDL70-3, a control virus constructed based on human adenovirus 5 (Ad5) DNA sequences with expanded deletions in early region 1 (E1) and region 3 (E3) [37] (kindly provided by Dr. Jack Gauldie, Health Sciences Center, McMaster University, Ontario, Canada) were propagated in human embryonic kidney (HEK)-293 cells and purified by double Cesium chloride density gradient centrifugation. Virus counts and activity were determined by UV spectrophotometery and colony formation assay. To study the mechanism underlying GSH depletion in the pulmonary fibrosis, 7–8 week old male C57BL mice were anesthetized and intranasally instilled with 40 µl of saline, AdDL70-3 (109pfu), or AdTGF-β1223/225 (109pfu) as we have described previously [38]. Food and water were allowed ad libitum during the experimental period. Mice were sacrificed one or three weeks after instillation. All animal experimental procedures were approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee and conducted at the UAB Animal Resource Center under specific pathogen-free conditions.
Bronchoalveolar lavage and lung tissue collection
Seven days after saline/AdDL70-3/AdTGF-β1223/225 instillation, mice were anesthetized and bronchoalveolar lavage (BAL) performed with 0.9 ml of saline. BAL solution was centrifuged and the supernatant (BALF) was either stored at −80°C for TGF-β1 or urea analysis or acidified with m-phosphoric acid immediately for the measurement of GSH, glutathione disulfide (GSSG), and ascorbate. After lavage, the pulmonary artery was cannulated and the vascular bed was perfused using 310 mOsm phosphate-buffered saline (PBS). The left main stem bronchus was inflated with 4% paraformaldehyde under constant pressure of 25 cm H2O while the right lobes of the lung were snap-frozen as described previously [38, 39].
Measurement of total and active form of TGF-β in BALF by ELISA
The amounts of total and active TGF-β proteins in the BALF were measured using a ELISA kit from eBioscience (84-7344-88) as we have described previously [40].
Measurement of GSH, glutathione disulfide, and ascorbate concentration in BALF and lung tissue
The concentrations of GSH, glutathione disulfide (GSSG), and reduced ascorbate in BALF were determined using a HPLC method as described previously [41]. Concentrations of urea in BALF and in the plasma were measured using a commercially available kit (Teco Diagnostics, Anaheim, CA) and the dilution factors of epithelial lining fluid (ELF) were calculated based on urea concentrations in the plasma and in BALF, which were used to normalize the antioxidant concentrations in ELF. Total GSH contents in lung tissue were measured using a recycling assay as we have described previously [42]. The protein contents were measured by a BCA kit and GSH concentrations calculated based on standard curve run together with the samples and normalized by protein content.
Real-time PCR analysis of GCL mRNAs in the lung
Total RNA was isolated using Trizol reagent (Invitrogen) according to manufacturer’s instructions. Extracted RNA was then treated with RNA-free (Ambion) DNase and quantitated. One microgram of total RNA was reverse-transcribed using the TaqMan Reverse Transcription Kit (Applied Biosystems) to produce cDNA for use in real-time PCR. Real-time PCR was performed on an Applied Biosystems 7300 using TaqMan Gene Expression Assays. Briefly, each sample was analyzed for a given gene in separate 25 µL reaction mixtures containing 12.5 µL 2× TaqMan Universal PCR Master Mix, 10 pmol of primers and 5 pmol of TaqMan probe, and 100 ng of total RNA for 40 cycles of 95°C for 15 s and 60°C for 1 min. Gene specific TaqMan assays for mouse Gclc (Mm00802658_m1), Gclm (Mm00514996_m1), and Gapdh (Mm99999915_g1) were used. Relative changes in the expression were determined by normalizing the relative amount of gene-specific mRNA comparative threshold (Ct) to the Gapdh (housekeeping gene) Ct using the comparative cycle threshold (ΔΔCT) method.
Western blotting
Lung tissues were homogenized in 0.25 M sucrose buffer containing protease inhibitor (Sigma P8340) and phosphatase inhibitor cocktails (Sigma, P5726) and then centrifuged at 3,000 × g for 10 min at 4°C. The supernatants were centrifuged at 105,000 × g for 30 min at 4°C. The protein concentrations in the supernatants were measured using a BCA protein assay kit (Pierce, Rockford, IL). The amounts of GCLC, GCLM, vimentin, alpha smooth muscle actin (α-SMA), activating transcription factor 3 (ATF3), as well as phosphorylated and non-phosphorylated JNK proteins were determined by Western blotting techniques using specific antibody to GCLC, GCLM, vimentin (SCBT, SC-6260), α-SMA (Biocare Medical LLC), ATF3 (SCBT, sc-188), JNK (SCBT, sc-7345), pJNK (abcam, ab4821), pERK (SCBT, sc-7383), ERK (SCBT, sc-94), pP38 (SCBT, sc-7973), p38 (SCBT, sc-7972), 4HNE (Alpha Diagnostic, HNE-11S), and nitrotyrosine (Alpha Diagnosis, NITT-12A), respectively.
Measurement of GCL activity in lung tissues
GCL activity in lung tissue was measured using a fluorescence-based microliter plate assay as described before [43]. Briefly, 50 µl of GCL reaction cocktail (400 mM Tris, 40 mM ATP, 20 mM l-glutamic acid, 2.0 mM EDTA, 20 mM sodium borate, 2 mM serine, 40 mM MgCl2) were added to each well on a 96-well plate, 6 wells per sample (3 for the activity and 3 for GSH-baseline) and followed by 50 µl of sample. After 5 min pre-incubation, the reaction was initiated by adding 50 µl of 2 mM cysteine to GCL activity wells. The plate was then vortexed, covered, and incubated at 37°C for 30 min. The reaction was stopped by adding 50 µl of 200 mM sulfosalicylic acid and then 50 µl of 2 mM cysteine was then added to GSH-baseline wells. The plate was vortexed and held on ice for 20 min and then centrifuged. Twenty microliters of supernatant from each well were transferred to a separate 96-well plate designed for fluorescence detection and 180 µl of naphthalenedicarboxaldehyde (NDA) derivatization solution (50 mM Tris, pH 10, 0.5 N NaOH, and 10 mM NDA in Me2SO, v/v/v 1.4/0.2/0.2) was added. The plate was covered to protect the wells from light and allowed to incubate at room temperature for 30 min. NDA-glutamylcysteine and NDA–GSH fluorescence intensity were measured on a fluorescence plate reader (472 ex/528 em). The results were calculated based on the standard curve obtained with different concentrations of GSH solutions at the same time.
TUNEL assay
Apoptosis of airway epithelial cells was analyzed using a TUNEL assay kit from Roche (Cat No 11684817001) following the protocol provided by the manufacturer. Multiple digital images [×200] around proximal bronchial structures where fibrosis was identified most in this model from each mouse [4–6 mice/group] were captured. Number of apoptotic events was manually counted from a total of 500 to 1000 cells and the apoptotic index was calculated by dividing the total number of apoptotic cells by total number of cells and multiplying by 100.
Masson’s trichrome staining of collagens
Masson’s Trichrome staining technique was employed to reveal collagen deposition as we have described previously [39]. Briefly, the sections were deparaffinized and hydrated through gradient alcohol, subsequently fixed in Bouin's mordant fluid, and the sections sequentially stained using Weigert's hematoxylin, Biebrich Scarlet-Acid Fuchsin, and Aniline Blue solutions.
Immunohistochemistry staining of α-SMA
Immunohistochemical staining of α-SMA was conducted as we have described previously [38, 39]. Briefly, sections were deparaffinized and hydrated through graded alcohol series. Endogenous peroxidase activity was quenched with 3% hydrogen peroxide. After blocking with 10% normal horse serum, sections were probed with primary antibody (α-SMA 1:200 dilution) at 4°C overnight and then with biotin-conjugated secondary antibody (Vector, Burlingame, CA) for 1 hour at room temperature. After incubation with avidin-biotin enzyme reagent (Vector, Burlingame, CA) the color was developed with DAB chromophoric solution (Scytek Labs, Utah, USA) at room temperature for 5 min and then counterstained with Harris’s hematoxylin (Sigma).
Measurement of hydroxyproline
The hydroxyproline content in mouse lungs was measured as we have described previously [38]. Briefly, lung tissue was homogenized in 300 µl of PBS and hydrolyzed with 12 N HCl at 110°C for 24 hrs. After cooling, samples were mixed with equal amounts of citrate–acetate buffer (5% citric acid, 1.2% glacial acetic acid, 7.25% sodium acetate, and 3.4% sodium hydroxide) and 10 × volume of chloramine-T solution (1.4% chloramine-T, 10% N-propanol, and 80% citrate–acetate buffer) and incubated at room temperature for 30 min. Ehrlich’s solution (2.5 g p-dimethylaminobenzaldehyde added to 9.3 ml of n-propanol and 3.9 ml of 70% perchloric acid) was added and the plate incubated at 65°C for 30 min. After the samples were cooled down on ice, the absorbance was measured at 550 nm. Standard curves were generated for each experiment using hydroxyproline reagent. Results are expressed as micrograms of hydroxyproline per milligram protein.
Data analysis
Data are presented as means ± SEM and evaluated by one-way ANOVA. Statistical significance was determined by Turkey’s test whereby p < 0.05 was considered significant.
Results
Effects of TGF-β1 on GSH concentration in mouse lung
Although it has been reported that TGF-β decreases GSH concentrations in different types of cells in vitro, whether this is the case in vivo is unknown. To elucidate whether increased TGF-β expression could possibly contribute to the decrease in GSH concentration in lung lining fluid observed in patients with lung fibrotic diseases, we utilized a mouse model whereby TGF-β1 expression in the lung was increased by intranasal instillation of AdTGF-β1223/225, an adenovirus expressing constitutively active TGF-β1. Mice instilled with AdDL70-3, a virus vector, or saline were used as controls. The results show that TGF-β protein content in BALF was significantly increased by day 7 in mice intranasally instilled with AdTGF-β1223/225 but not AdDL70-3 as compared to saline instilled controls (the amounts of total TGF-β protein in BALF: saline, 205 ± 18 pg/ml, AdDL70-3, 270 ± 27 pg/ml, AdTGF-β1223/225, 6,900 ± 721 pg/ml; active TGF-β in BALF: saline, 252 ± 11 pg/ml, AdDL70-3, 218 ± 12 pg/ml, AdTGF-β1223/225, 2,041 ± 433 pg/ml). The BALF TGF-β1 levels detected in our animal model are comparable with those found in different lung fibrotic diseases including IPF [44–47], suggesting that our animal model is a clinically relevant model.
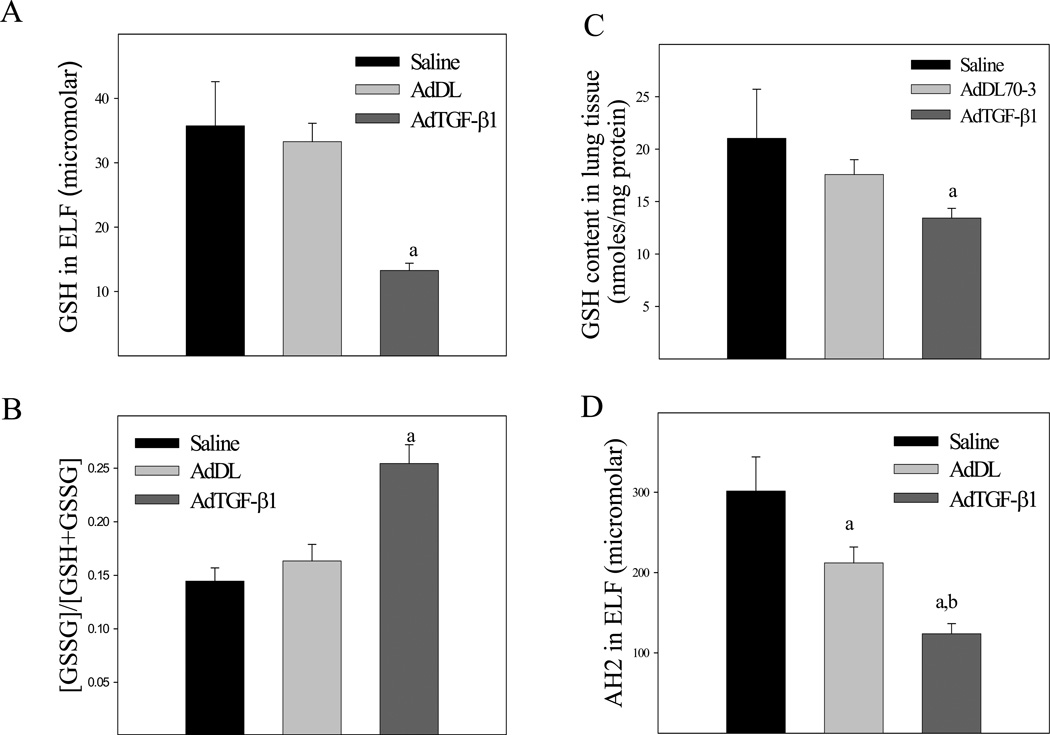
Associated with the increase in lung TGF-β expression, the concentration of GSH in ELF was significantly decreased by day 7 (Fig 1A) although the level of glutathione disulfide (GSSG) was not significantly changed (data not shown), leading to an increase in the ratio of GSSG to GSH (Fig 1B), an indicator of oxidative stress. The total GSH content in lung tissue was also significantly decreased in TGF-β1 treated mice (Fig 1C). However, the GSH concentrations in lung tissue and ELF of TGF-β1 treated mice returned to the normal by day 21 (data not shown). In addition to depleting GSH, increasing TGF-β1 expression in the lung also decreased the concentration of reduced ascorbate (Fig 1D), another small molecule antioxidant present in the lung lining fluid. Control virus had no significant effects on GSH level although it slightly decreased the ascorbate concentration, compared to saline treated mice. These data suggest that increased TGF-β1 expression may contribute to the depletion of GSH observed in fibrotic lung diseases and that GSH depletion may not be a consequence of lung fibrosis.
Fig. 1.
Effects of increasing TGF-β1 expression on GSH concentration in mouse lung. AdTGF-β1223/225, AdDL70-3, or saline was administrated to mouse lung by intranasal instillation. Seven days after instillation, mice were sacrificed. The concentrations of GSH, glutathione disulfide (GSSG), and reduced ascorbate in BALF were determined by HPLC whereas GSH in lung tissue determined by a recycle assay as described in the Materials and Methods section. A) ELF GSH concentration; B) The ratios of GSSG to total GSH in ELF, calculated by the formula of [GSSG]*2/([GSSG]*2+[GSH]); C) Lung tissue GSH content; D) ELF ascorbate (AH2) concentration. a, Significantly different from saline treated mice; b, significantly different from control virus treated mice (p<0.05, n=5–8).
Effects of TGF-β1 on GCLC and GCLM mRNA and protein expression as well as GCL activity in mouse lung
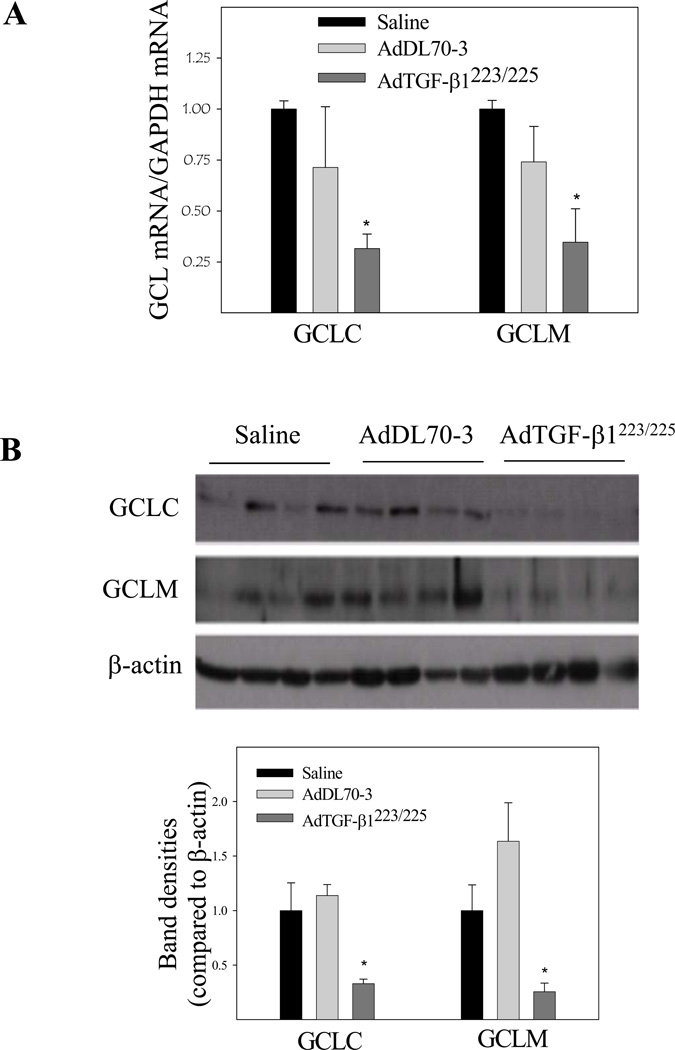
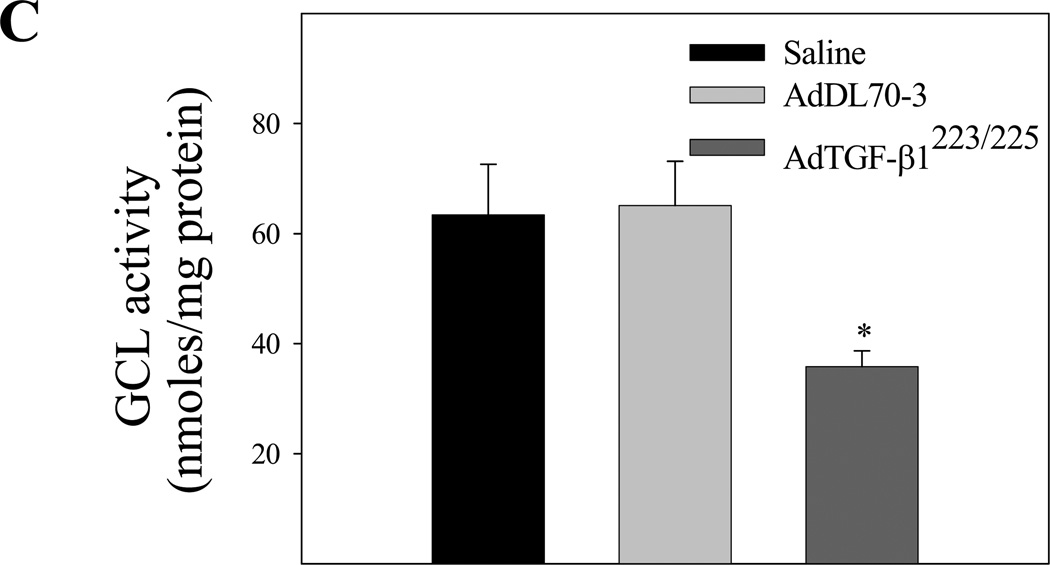
To explore the mechanism whereby TGF-β1 decreases GSH in mouse lung, we further investigated the effects of TGF-β1 on the expression of both catalytic and modifier subunits of GCL, the rate-limiting enzyme in de novo GSH synthesis. The results show that increasing lung TGF-β1 expression down-regulated the expression of both catalytic (GCLC) and modifier (GCLM) subunit mRNAs and proteins (Fig 2A& 2B), which was accompanied by a decrease in GCL activity (Fig 2C) in lung tissue. No significant change in GCL mRNA and protein expression or GCL activity was detected in mice administrated with control virus as compared to saline treated group. These data suggest that TGF-β1 decreases GSH level in vivo at least in part by suppressing the expression and activity of GCL.
Fig. 2.
Effects of increasing lung TGF-β1 expression on GCLC and GCLM mRNA and protein expression as well as GCL activity in mouse lung. AdTGF-β1223/225, AdDL70-3, or saline was administrated to mouse lung by intranasal instillation. Seven days after instillation, mice were sacrificed. A) Real-time PCR analyses of GCLC and GCLM mRNAs. The results were expressed comparative to GAPDH mRNA; B) Western analyses of GCLC and GCLM proteins. Top panel, representative Western blot; bottom panel, semi-quantified data of Western blots (normalized to β-actin protein); C) GCL activity determined by a fluorescence-based microplate assay. *, Significantly different from saline treated controls (p<0.05, n=5–6).
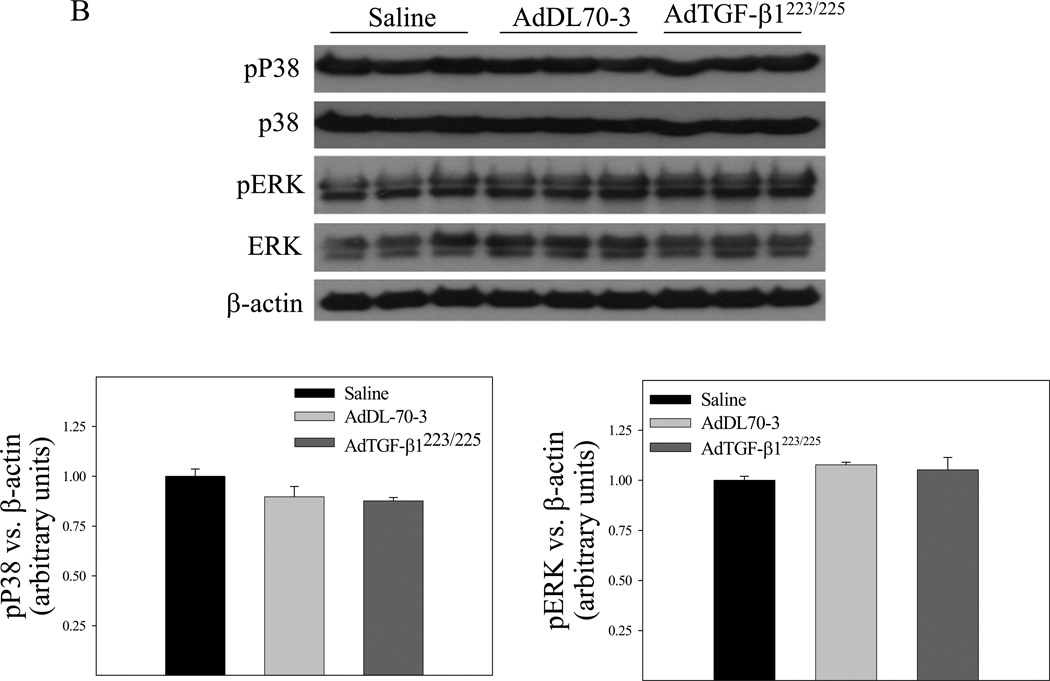
Effects of TGF-β1 on JNK phosphorylation and ATF3 expression in mouse lung
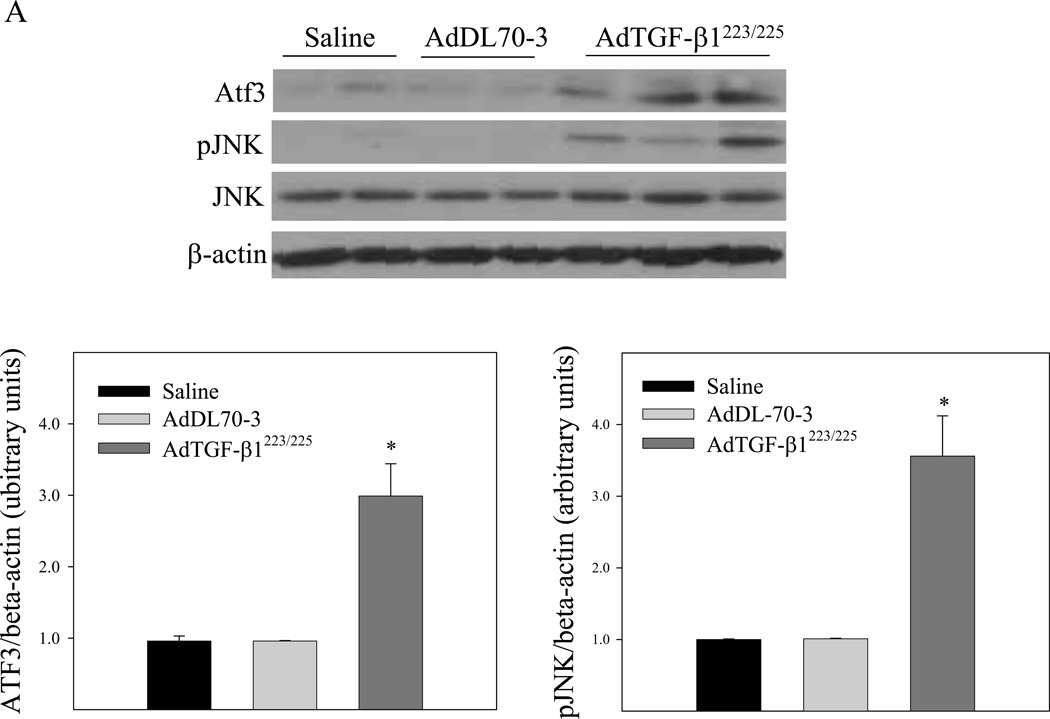
It has been reported that TGF-β suppresses the expression of phase II enzymes including GCLC by inducing the repressor ATF3 in mammary epithelial cells [48] whereas JNK is involved in the induction of ATF3 by different stimuli [49–51]. To further explore the mechanism whereby TGF-β1 suppresses the Gclc gene expression in vivo, we compared the expression levels of ATF3 protein as well as the phosphorylation status of JNK in lung tissue of mice instilled with saline, control virus, or TGF-β1 expressing virus by Western analysis. The results show that instillation of AdTGF-β1223/225, but not AdDL70-3, increased JNK phosphorylation and ATF3 protein level (Fig 3A) in mouse lung tissue. No significant effect of overexpression of TGF-β1 on the phosphorylation of ERK or p38 was detected (Fig 3B). These data suggest that activation of the JNK pathway, which leads to increased expression of the transcriptional repressor AFT3, may be involved in TGF-β1 suppression of Gclc gene expression in mouse lung.
Fig. 3.
Effects of increasing lung TGF-β1 expression on JNK phosphorylation and ATF3 expression in mouse lung. AdTGF-β1223/225, AdDL70-3, or saline was administrated to mouse lung by intranasal instillation. Seven days after instillation, mice were sacrificed. A) Top panel, representative Western blots of Atf3, phosphorylated JNK, total JNK, and β-actin; bottom panel, semi-quantified data of Western blots (normalized to β-actin). *, Significantly different from saline treated controls (p<0.05, n=3–4). B) Top panel, representative Western blots of ERK, pERK, p38, pP38, and β-actin; bottom panel, semi-quantified data of Western blots.
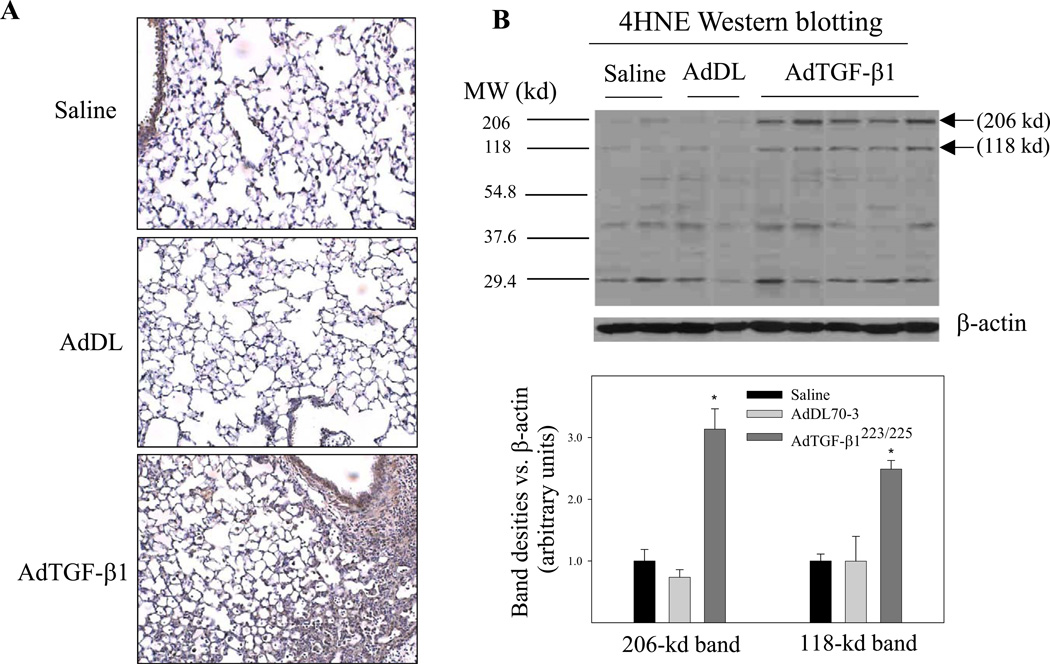
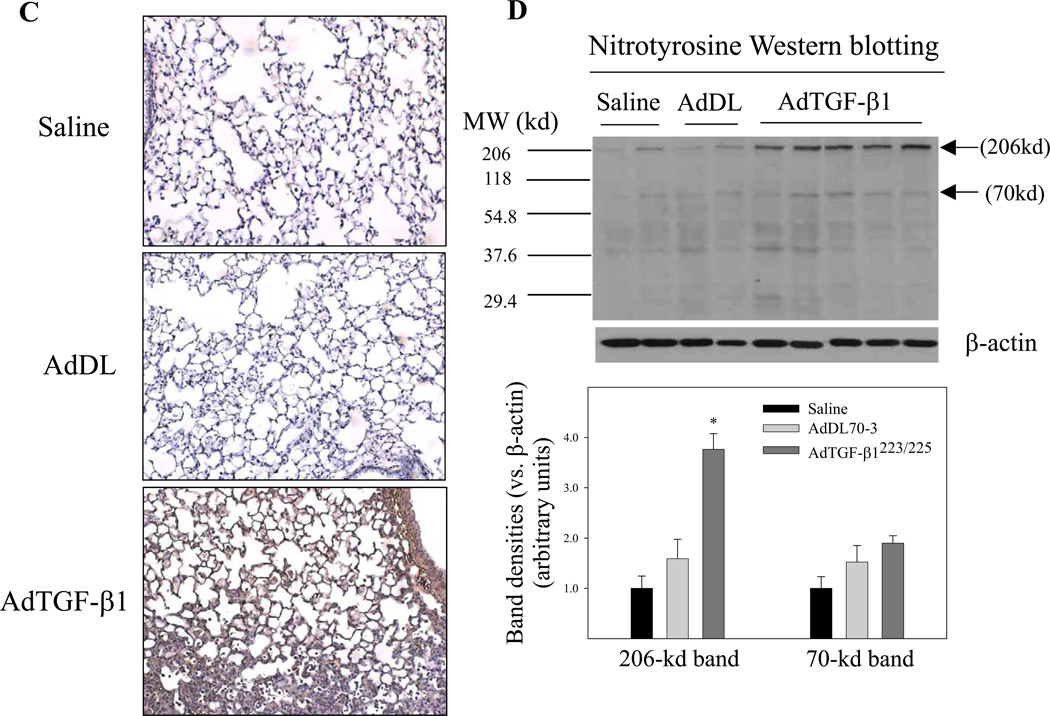
Induction of oxidative modifications of proteins and lipids by TGF-β1 in mouse lung
To determine whether increased TGF-β1 expression, which led to decreases in the concentrations of GSH and ascorbate in the lung, causes oxidative modifications of lipids and proteins, we assessed the levels of 4-hydroxynonenal (4-HNE)-protein adducts, a lipid peroxidation indicator, and protein 3-nitrotyrosine, a protein oxidation product, by immunohistochemistry and Western blotting techniques. The results show that increasing TGF-β1 expression in mouse lung to the level comparable to those found in lung fibrotic diseases was associated with significant increases in the amounts of both 4-HNE-protein adducts (Fig 4A&4B) and protein nitrotyrosine (Fig 4C&4D). Again, control virus administration did not cause any significant increase in either protein oxidation or lipid peroxidation, compared to saline instilled mice. The results suggest that increased TGF-β1 expression may contribute to the increase in oxidative modifications of proteins and lipids observed in these fibrotic lung diseases.
Fig. 4.
Increasing lung TGF-β1 expression induced oxidative modifications of lipids and proteins in mouse lung. AdTGF-β1223/225, AdDL70-3, or saline was administrated to mouse lung by intranasal instillation. Seven days after instillation, mice were sacrificed. A) Immunohistochemical staining of 4HNE-protein adducts; B) Western analyses of 4HNE-protein adducts with β-actin to show equal protein loading. Top panel, selected gel picture; bottom panel, semi-quantified data of the bands indicated by the arrows. C) Immunohistochemical staining of protein nitrotyrosine; D) Western analyses of protein nitrotyrosine comparative to β-actin. Top panel, selected gel picture; bottom panel, semi-quantified data of the bands indicated by the arrows.
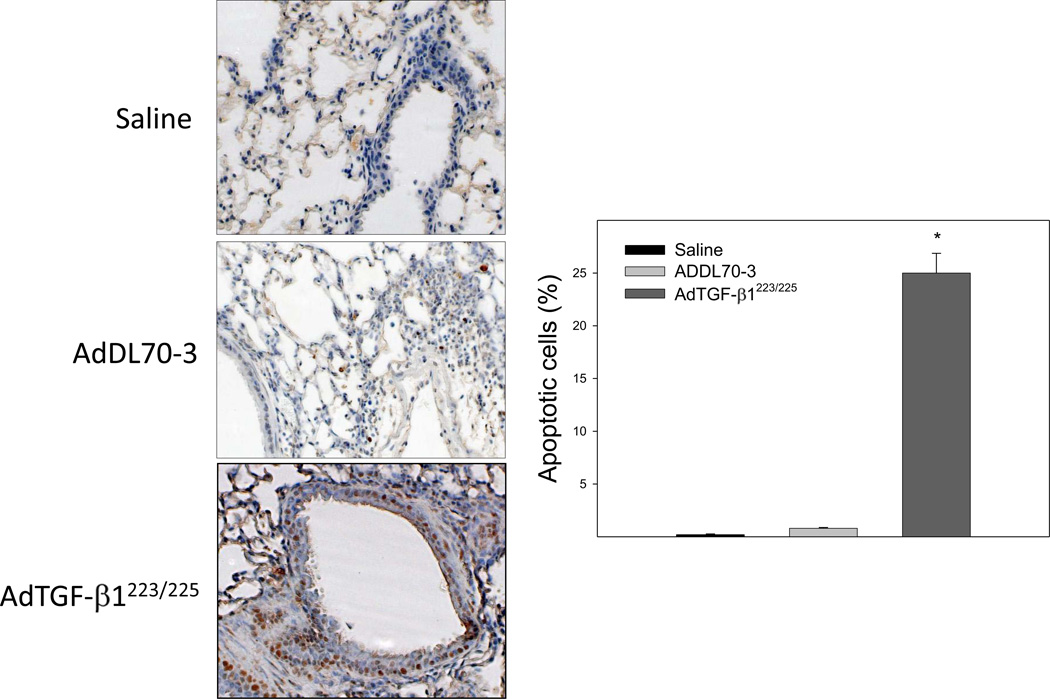
TGF-β1 induced apoptosis in airway epithelial cells associated with massive fibrotic responses in mouse lung
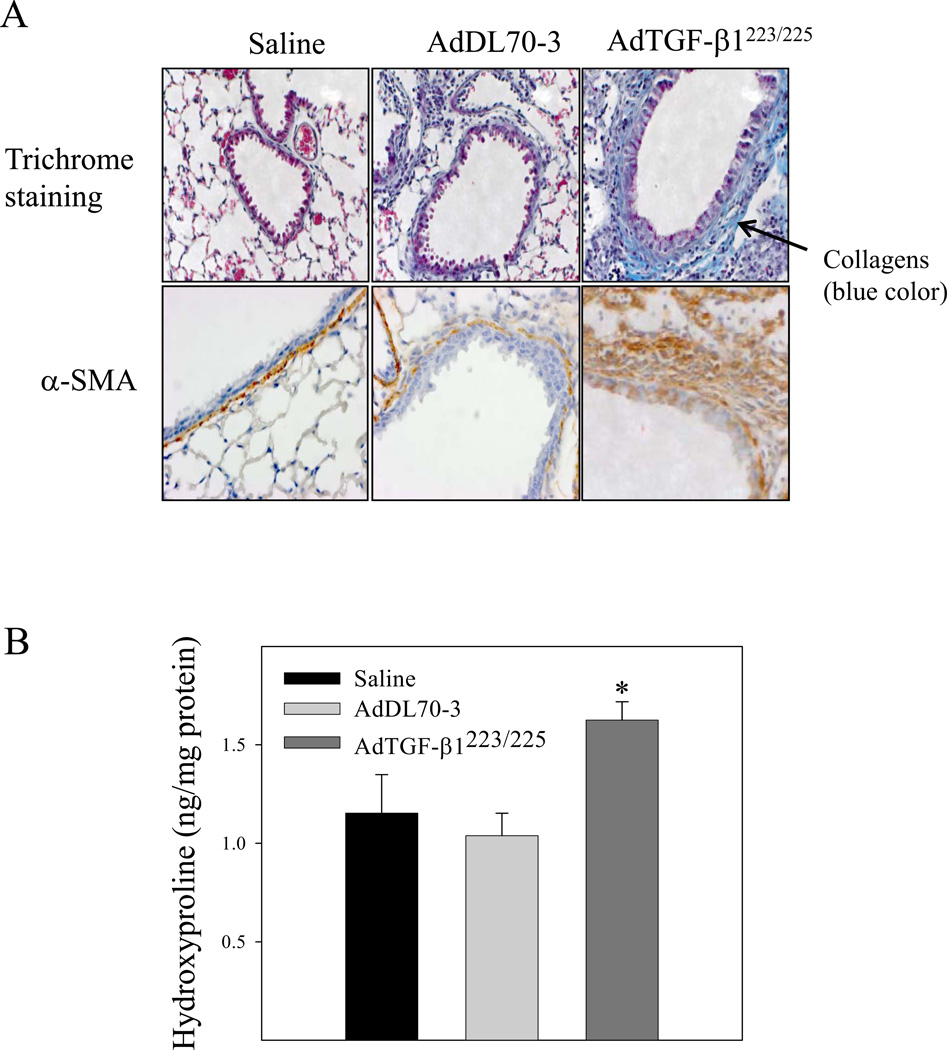
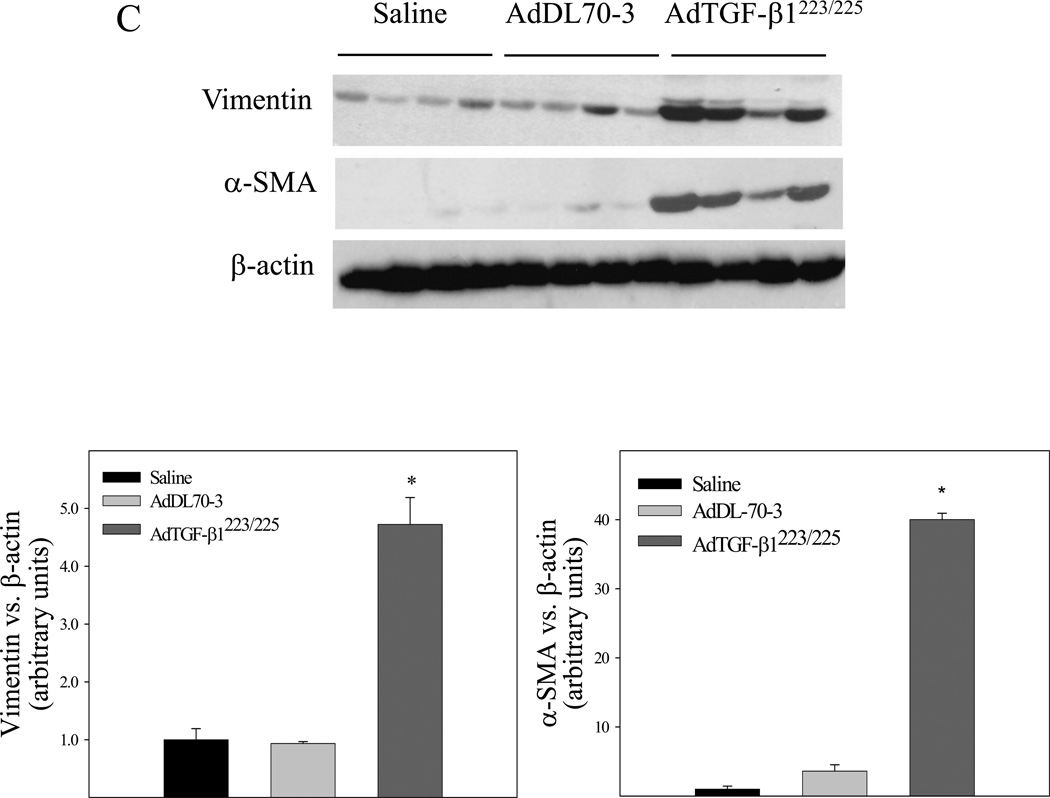
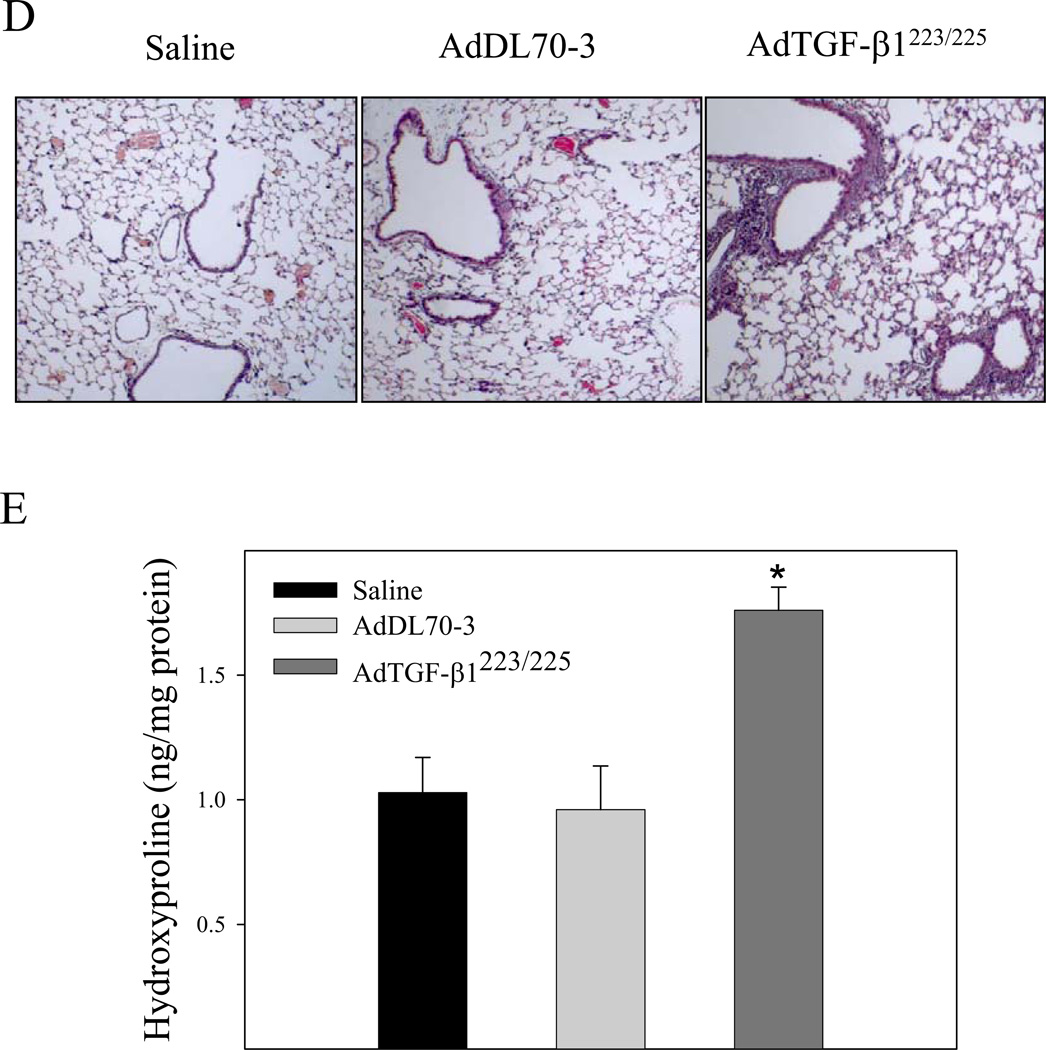
It has been reported that TGF-β1 decreases GSH concentration, which was associated with induction of apoptosis in different types of cells in vitro [31, 33]. To investigate whether increasing TGF-β1 expression in the lung, which led to a decrease in GSH level in BALF and lung tissue as shown in Fig 1 is associated with an increase in apoptotic cell death in mouse lung, TUNEL assay was conducted with lung tissue paraffin sections. The results show that increasing lung TGF-β1 expression dramatically increased the number of apoptotic cells in airway epithelium in mouse lung by day 7 although the virus vector alone had no significant effect, compared to saline instilled control mice (Fig 5). Accompanied with the increase in epithelial apoptosis, TGF-β1 induced massive fibrotic responses in the lung revealed by trichrome staining, α-SMA immunohistochemical staining, hydroxyproline measurement, and Western analyses of vimentin as well as α-SMA (Fig 6A&B). Importantly, the results show that lung fibrosis (collagen deposition and hydroxyproline accumulation) in TGF-β1 treated mice persisted at least 21 days (Fig 6C).
Fig. 5.
Increasing lung TGF-β1 expression induced apoptosis in lung epithelial cells in mice. AdTGF-β1223/225, AdDL70-3, or saline was administrated to mouse lung by intranasal instillation. Seven days after instillation, mice were sacrificed. Apoptotic cells were detected by TUNEL assay as described in the Materials and Methods section. Positive stained cells were quantified and expressed as percentage of total cell number. *, Significantly different from saline treated controls (p<0.05, n=5–6 mice).
Fig. 6.
Increasing lung TGF-β1 expression induced massive long-lasting fibrotic responses in mouse lung. AdTGF-β1223/225, AdDL70-3, or saline was administrated to mouse lung by intranasal instillation. Seven (A&B&C) and twenty-one (D) days after instillation, mice were sacrificed. A) Trichrome staining of collagens and immunohistochemical staining of α-SMA in mouse lung; B) Hydroxyproline content in lung tissue; C) Western analysis of vimentin and α-SMA protein in lung tissue. β-actin was used as a protein loading control. Top panel, representative Western blots; bottom panel, semi-quantified Western data. D) Top panel, representative trichrome staining picture; bottom panel, hydroxyproline content in lung tissue from mice sacrificed 21 days after TGF-β1 challenge. *, Significantly different from saline treated controls (p<0.05, n=3–4).
Discussion
GSH concentration in the lung lining fluid is considerably reduced in fibrotic lung diseases [21–26]; the underlying mechanism and the biological significance of GSH depletion, however, is unclear. In this study, we show that increasing the expression of TGF-β1, the most potent and ubiquitous profibrogenic cytokine whose expression is increased in almost all fibrotic diseases, in mouse lung to the levels comparable to those found in lung fibrotic diseases [44–47] decreased GSH and ascorbate concentrations and increased the amounts of oxidatively modified proteins and lipids in BALF and/or lung tissue by day 7, accompanied by airway epithelial cell apoptosis and lung fibrosis. Importantly, lung GSH levels returned to the normal whereas fibrosis persisted at least 21 days after TGF-β1 challenge. These results suggest that increased TGF-β expression may contribute to the depletion of GSH in the lung lining fluid observed in fibrotic lung diseases and that GSH depletion may be an early event in, rather than a consequence of, lung fibrosis development.
TGF-β is a multi-functional cytokine, which plays a central role in the development of lung fibrosis. It has been reported that TGF-β1 causes time-and dose-dependent depletion of GSH concentration in human alveolar epithelial and pulmonary endothelial cells in vitro [31–34]. Our previous studies have also shown that TGF-β1 decreased the GSH concentration in cultured fibroblasts (NIH3T3 cells) [35]. In this study, we further show that increasing TGF-β1 expression in mouse lung by an adenovirus mediated gene transfer technique led to decreases in GSH concentrations in ELF and lung tissue. As control virus did not have any significant effect on GSH level although it slightly decreased ascorbate concentration in BALF, it is suggested that the decrease in the lung GSH is caused by TGF-β1 not by viral vector. Importantly, it has been reported that TGF-β1 concentrations in BALF of lung fibrotic diseases including IPF, sarcoidosis, bronchopulmonary dysplasia, and acute respiratory distress syndrome (ARDS) range between 1,000 pg/ml to 4 µg/ml [44–47]. Considering that the dilution factors of BALF are normally from 10 to 100 in human and the dilution factors for our BALF vary between 10–20, these data suggest that the TGF-β1 level detected in our animal model is clinical relevant. These data also suggest that increased TGF-β expression may contribute to the depletion of GSH observed in the lung lining fluid of fibrotic lung disease patients.
Although a few types of cells can directly uptake intact GSH from surrounding fluid most cells depend on de novo synthesis to maintain their intracellular GSH homeostasis [10]. De novo GSH synthesis is a two-step process catalyzed by the cytosolic enzymes glutamate cysteine ligase (GCL) and GSH synthase (GS). GCL, composed of a heavy or catalytic subunit (GCLC; Mr ~73 kDa) and a light or modifier subunit (GCLM; Mr ~31 kDa), is the rate-limiting enzyme in de novo GSH synthesis [52]. Studies from this and other laboratories have shown that regulation of Gcl gene expression is critical for maintaining intracellular GSH homeostasis under both physiological and pathological conditions [41, 53–56]. In this study, we show that associated with the depletion of GSH, overexpression of TGF-β1 in mouse lung tissue suppressed the expression of both Gclc and Gclm genes at mRNA and protein levels and decreased GCL activity (Fig 2). These data suggest that TGF-β1 decreased lung GSH in vivo at least in part by suppressing Gcl gene expression and thereby the biosynthesis of GSH.
The catalytic submit of GCL (GCLC) possesses all of the enzymatic activity and is feed-back inhibited by the final product, GSH [57]. Although it has been reported that overexpression of GCLC alone is sufficient to increase the GCL enzyme activity and subsequent GSH synthesis in cells [57–59] both subunits are believed to be required for the optimal enzyme activity in vivo due to a low concentration of glutamate and a high concentration of GSH under physiological conditions [57, 59]. Association of GCLM with the catalytic subunit GCLC lowers the apparent Km for the substrate glutamate and ATP and increases the apparent Ki for GSH [60, 61]. Interestingly, it has been reported that the expressions of GCLC and GCLM are regulated independently under some conditions [53, 54, 62] but coordinately in other situations [62, 63]. The mechanism underlying the coordinate or disassociate regulation of two submits of GCL in these different situation is unknown. In this study, we show that overexpression of TGF-β1 in mouse lung suppressed not only the Gclc but also the Gclm gene expression at both mRNA and protein levels. Our data are consistent with previous studies on GCLC by other laboratories, which show that TGF-β1 suppressed the promoter activity of the Gclc gene and thereby the GCLC mRNA and protein expression in cultured cells [32–34, 48]. As regarding GCLM, one study showed that TGF-β1 had no effect on Gclm gene expression in TGF-α overexpressing hepatocytes [33]. The mechanism underlying such discrepancy between our results and theirs is unclear and probably related to differences in cell types (TGF-α overexpressing hepatocytes vs. various types of lung cells) and the environment (in vitro vs. in vivo). Nonetheless, although the mechanism underlying the inhibition of Gclm gene expression by TGF-β1 is unknown at the moment, the results from this study suggests that increased TGF-β1 expression may contribute importantly to the GSH depletion observed in fibrotic diseases and that TGF-β1 decreases GSH in vivo at least in part by suppressing the expression of GCL
Different mechanisms have been proposed for the suppression of Gclc gene expression by TGF-β1. Franklin et al reported that TGF-β1 suppressed Gclc gene expression in hepatocytes in part by inducing caspase-dependent cleavage of GCLC protein [33]. In this study, we did not detect any cleaved GCLC band in lung tissue in mice instilled with ADTGF-β1223/225, suggesting that TGF-β1 suppressed the Gclc gene expression in vivo probably through other mechanism(s) rather than inducing GCLC protein cleavage. As TGF-β1 reduced the amounts of both mRNA and protein of GCLC in mouse lung (Fig 2), it is suggested that the suppression occurs at the transcription or mRNA level. Arsalane et al. reported that although TGF-β1 had no effect on the half-life of gamma-GCShs (the old term for GCLC) mRNA it suppressed the transcription rate of the gene revealed by nuclear run-on assays [32]. Activating transcription factor 3 (ATF3) is a transcriptional repressor [64]. TGF-β has been shown to induce the expression of ATF3 in mammary epithelial cells, which leads to the suppression of GCLC expression [48]. Interestingly, an increased ATF3 protein level was detected in AdTGF-β1223/225 instilled mouse lung as compared with viral vector or saline instilled mice. These data suggest that induction of the transcriptional repressor ATF3 may be involved in TGF-β1 suppression of GCLC expression in vivo. ATF3 is induced by different stimuli through different signaling pathways. Chen et al reported that hypoxia induced ATF3 expression in endothelial cells through activation of the JNK MAPK pathway [65]. Lu et al showed, on the other hand, that the p38 signaling pathway was involved in the induction of ATF3 by stress signals in HeLa cells [49]. Furthermore, it was reported that TNFα induced ATF3 expression in vascular endothelial cells by activating the JNK pathway whereas the ERK pathway inhibited TNFα-mediated induction of ATF3 mRNA [66]. In this study, we show that an increase in ATF3 expression was accompanied by an increase in the phosphorylation of JNK, but not p38 or ERK, MAPK, suggesting that activation of the JNK pathway may be involved in the induction of ATF3 by TGF-β1 in mouse lung in vivo.
The mechanism underlying the suppression of Gclm gene expression by TGF-β1 is far less clear than that for the Gclc gene. No study had reported a down-regulation of Gclm gene expression by TGF-β1 and little is known about the mechanism leading to a down regulation of Gclm gene expression in other cases either [53, 63]. An interesting finding from our previous studies is that both GCLC and GCLM expression were down regulated in several visceral organs including the lung in old rats [63] whereas only GCLM , not GCLC, expression was decreased in the brain of old rats and in HIV infected hepatocytes [53, 63]. These studies suggest that not only the stimuli but also the environment is important in controlling the expression of two Gcl genes. Nonetheless, more studies are needed to elucidate the mechanisms underlying the down regulation not only Gclm but also Gclc gene expression under these different situations.
The biological significance of GSH depletion in the development of fibrosis remains unclear. It has been reported that GSH depletion mediated TGF-β-induced apoptosis of mammary epithelial cells as well as hepatocytes [31, 33]. In the previous study, we showed that TGF-β1 decreased intracellular GSH levels in NIH3T3 cells, a murine embryo fibroblast cell line, whereas treatment of NIH3T3 cells with exogenous GSH, GSH ester, or the GSH pro-drug N-acetylcysteine (NAC) partially reversed TGF-β1-induced GSH depletion and collagen accumulation [35]. We have also shown that exogenous GSH selectively inhibited TGF-β1-induced expression of plasminogen activator inhibitor 1 (PAI-1), a protease inhibitor that plays a critical role in the development of fibrosis under various pathological conditions, and stimulated collagen degradation in NIH3T3 cells [67, 68]. In this and recent studies, we show that, associated with depletion of lung GSH, TGF-β1 increased PAI-1 expression and induced airway epithelial cell apoptosis, which was accompanied by massive fibrotic responses in mouse lung [38] (Fig 6). Most importantly, we show in this study that GSH level in the lung of TGF-β1 treated mice returned to normal whereas fibrosis persisted 21 days after TGF-β1 instillation, suggesting that GSH depletion may not be a consequence of fibrosis but occurs before or simultaneously with the development of fibrosis.
In conclusion, our data suggest that increased TGF-β expression contributes to GSH depletion observed in fibrotic lung diseases by suppressing the expression of the Gcl genes and that GSH depletion may not be a consequent of the development of lung fibrosis.
Highlights.
-
◆
Clinically relevant level of TGF-β1 decreases GSH in BALF and lung tissue in mice
-
◆
Clinically relevant level of TGF-β1 suppresses GCLC and GCLM expression in mouse lung
-
◆
Clinically relevant level of TGF-β1 induces oxidative stress in mouse lung
-
◆
Clinically relevant level of TGF-β1 activates JNK MAPK and induces ATF3 in mouse lung
-
◆
GSH level returns to the normal whereas fibrosis persists at least 21 days
Acknowledgements
The work was supported by the grants from National Institute of Environmental Health Sciences (NIH ES011831), National Heart, Lung, and Blood Institute (NHLBI HL088141), and the American Lung Association to Rui-Ming Liu; P01 ES and HL54696 to Edward Postlethwait. The authors would also like to thank Dr. Jack Gauldie, Dept. of Pathology and Molecular Medicine, McMaster University, Canada, for providing AdTGF-β1223/225 and AdDL70-3 virus.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Meister A. Selective modification of glutathione metabolism. Science. 1983;220:472–477. doi: 10.1126/science.6836290. [DOI] [PubMed] [Google Scholar]
- 2.Meister A. Biosynthesis and function of glutathione, an essential biofactor. Journal of Nutritional Science and Vitaminology Spec No . 1992:1–6. doi: 10.3177/jnsv.38.special_1. [DOI] [PubMed] [Google Scholar]
- 3.Sies H. Glutathione and its role in cellular functions. Free Radic Biol Med. 1999;27:916–921. doi: 10.1016/s0891-5849(99)00177-x. [DOI] [PubMed] [Google Scholar]
- 4.Kwon YW, Masutani H, Nakamura H, Ishii Y, Yodoi J. Redox Regulation of Cell Growth and Cell Death. Biological Chemistry. 2003;384:991–996. doi: 10.1515/BC.2003.111. [DOI] [PubMed] [Google Scholar]
- 5.Watson WH, Chen Y, Jones DP. Redox state of glutathione and thioredoxin in differentiation and apoptosis. Biofactors. 2003;17:307–314. doi: 10.1002/biof.5520170130. [DOI] [PubMed] [Google Scholar]
- 6.Diaz Vivancos P, Wolff T, Markovic J, Pallardo FV, Foyer CH. A nuclear glutathione cycle within the cell cycle. Biochem J. 2010;431:169–178. doi: 10.1042/BJ20100409. [DOI] [PubMed] [Google Scholar]
- 7.Luthman M, Eriksson S, Holmgren A, Thelander L. Glutathione-dependent hydrogen donor system for calf thymus ribonucleoside-diphosphate reductase. Proc Natl Acad Sci U S A. 1979;76:2158–2162. doi: 10.1073/pnas.76.5.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bing K L. Leukotriene C4 synthase. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2003;69:111–116. doi: 10.1016/s0952-3278(03)00071-1. [DOI] [PubMed] [Google Scholar]
- 9.Meister A. Current studies on glutathione, a major cellular antioxidant. In: Ursini F, Cadenas E, editors. Biological Free Radical Oxidations and Antioxidants. Padova: CLEUP; 1992. pp. 89–93. [Google Scholar]
- 10.Forman HJ, Liu RM, Shi MM. Glutathione synthesis in oxidative stress. In: Packer L, Cadenas E, editors. Biothiols in Health and Disease. 1995. [Google Scholar]
- 11.Forman HJ, Liu R-M, Tian L. Massaro D, Clerch L. Oxygen, Gene Expression and Cellular Function. New York: Marcel Decker, Inc; 1997. Glutathione cycling in oxidative stress. In: Lung Biology in Health and Diseases; p. 99. [Google Scholar]
- 12.Hayes JD, McLellan LI. Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free Radic Res. 1999;31:273–300. doi: 10.1080/10715769900300851. [DOI] [PubMed] [Google Scholar]
- 13.Barrett WC, DeGnore JP, Konig S, Fales HM, Keng YF, Zhang ZY, Yim MB, Chock PB. Regulation of PTP1B via glutathionylation of the active site cysteine 215. Biochemistry. 1999;38:6699–6705. doi: 10.1021/bi990240v. [DOI] [PubMed] [Google Scholar]
- 14.Klatt P, Molina EP, Lamas S. Nitric oxide inhibits c-Jun DNA binding by specifically targeted S-glutathionylation. J Biol Chem. 1999;274:15857–15864. doi: 10.1074/jbc.274.22.15857. [DOI] [PubMed] [Google Scholar]
- 15.Shelton MD, Chock PB, Mieyal JJ. Glutaredoxin: role in reversible protein sglutathionylation and regulation of redox signal transduction and protein translocation. Antioxid Redox Signal. 2005;7:348–366. doi: 10.1089/ars.2005.7.348. [DOI] [PubMed] [Google Scholar]
- 16.Rahman I, Biswas SK, Jimenez LA, Torres M, Forman HJ. Glutathione, stress responses, and redox signaling in lung inflammation. Antioxid Redox Signal. 2005;7:42–59. doi: 10.1089/ars.2005.7.42. [DOI] [PubMed] [Google Scholar]
- 17.Lipton AJ, Johnson MA, Macdonald T, Lieberman MW, Gozal D, Gaston B. S-nitrosothiols signal the ventilatory response to hypoxia. Nature. 2001;413:171–174. doi: 10.1038/35093117. [DOI] [PubMed] [Google Scholar]
- 18.Bindoli A, Fukuto JM, Forman HJ. Thiol chemistry in peroxidase catalysis and redox signaling. Antioxid Redox Signal. 2008;10:1549–1564. doi: 10.1089/ars.2008.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiong Y, Uys JD, Tew KD, Townsend DM. S-glutathionylation: from molecular mechanisms to health outcomes. Antioxid Redox Signal. 2011;15:233–270. doi: 10.1089/ars.2010.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cantin AM, North SL, Hubbard RC, Crystal RG. Normal alveolar epithelial lining fluid contains high levels of glutathione. Journal of Applied Physiology: Respiration, Environmental and Exercise Physiology. 1987;63:152–157. doi: 10.1152/jappl.1987.63.1.152. [DOI] [PubMed] [Google Scholar]
- 21.Cantin AM, Hubbard RC, Crystal RG. Glutathione deficiency in the epithelial lining fluid of the lower respiratory tract in idiopathic pulmonary fibrosis. American Review of Respiratory Disease. 1989;139:370–372. doi: 10.1164/ajrccm/139.2.370. [DOI] [PubMed] [Google Scholar]
- 22.Roum JH, Buhl R, McElvany NG, Borok Z, Crystal RG. Systemic deficiency of glutathione in cystic fibrosis. Journal of Applied Physiology: Respiration, Environmental and Exercise Physiology. 1993;75:2419–2424. doi: 10.1152/jappl.1993.75.6.2419. [DOI] [PubMed] [Google Scholar]
- 23.Pacht ER, Timerman AP, Lykens MG, Merola AJ. Deficiency of alveolar fluid glutathione in patients with sepsis and the adult respiratory distress syndrome. Chest. 1991;100:1397–1403. doi: 10.1378/chest.100.5.1397. [DOI] [PubMed] [Google Scholar]
- 24.Brown DM, Beswick PH, Bell KS, Donaldson K. Depletion of glutathione and ascorbate in lung lining fluid by respirable fibres. Ann Occup Hyg. 2000;44:101–108. [PubMed] [Google Scholar]
- 25.Montaldo C, Cannas E, Ledda M, Rosetti L, Congiu L, Atzori L. Bronchoalveolar glutathione and nitrite/nitrate in idiopathic pulmonary fibrosis and sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2002;19:54–58. [PubMed] [Google Scholar]
- 26.Beeh KM, Beier J, Haas IC, Kornmann O, Micke P, Buhl R. Glutathione deficiency of the lower respiratory tract in patients with idiopathic pulmonary fibrosis. Eur Respir J. 2002;19:1119–1123. doi: 10.1183/09031936.02.00262402. [DOI] [PubMed] [Google Scholar]
- 27.Ma JY, Barger MW, Hubbs AF, Castranova V, Weber SL, Ma JK. Use of tetrandrine to differentiate between mechanisms involved in silica-versus bleomycininduced fibrosis. J Toxicol Environ Health A. 1999;57:247–266. doi: 10.1080/009841099157692. [DOI] [PubMed] [Google Scholar]
- 28.Candan F, Alagozlu H. Captopril inhibits the pulmonary toxicity of paraquat in rats. Hum Exp Toxicol. 2001;20:637–641. doi: 10.1191/096032701718890540. [DOI] [PubMed] [Google Scholar]
- 29.Arslan SO, Zerin M, Vural H, Coskun A. The effect of melatonin on bleomycininduced pulmonary fibrosis in rats. J Pineal Res. 2002;32:21–25. doi: 10.1034/j.1600-079x.2002.10796.x. [DOI] [PubMed] [Google Scholar]
- 30.Nieto N, Cederbaum AI. S-Adenosylmethionine Blocks Collagen I Production by Preventing Transforming Growth Factor-{beta} Induction of the COL1A2 Promoter. J. Biol. Chem. 2005;280:30963–30974. doi: 10.1074/jbc.M503569200. [DOI] [PubMed] [Google Scholar]
- 31.White AC, Das SK, Fanburg BL. Reduction of glutathione is associated with growth restriction and enlargement of bovine pulmonary artery endothelial cells produced by transforming growth factor-beta 1. Am J Respir Cell Mol Biol. 1992;6:364–368. doi: 10.1165/ajrcmb/6.4.364. [DOI] [PubMed] [Google Scholar]
- 32.Arsalane K, Dubois CM, Muanza T, Begin R, Boudreau F, Asselin C, Cantin AM. Transforming growth factor-beta1 is a potent inhibitor of glutathione synthesis in the lung epithelial cell line A549: transcriptional effect on the GSH rate-limiting enzyme gamma-glutamylcysteine synthetase. Am J Respir Cell Mol Biol. 1997;17:599–607. doi: 10.1165/ajrcmb.17.5.2833. [DOI] [PubMed] [Google Scholar]
- 33.Franklin CC, Rosenfeld-Franklin ME, White C, Kavanagh TJ, Fausto N. TGFbeta1-induced suppression of glutathione antioxidant defenses in hepatocytes: caspase-dependent post-translational and caspase-independent transcriptional regulatory mechanisms. Faseb J. 2003;17:1535–1537. doi: 10.1096/fj.02-0867fje. [DOI] [PubMed] [Google Scholar]
- 34.Jardine H, MacNee W, Donaldson K, Rahman I. Molecular mechanism of transforming growth factor (TGF)-beta1-induced glutathione depletion in alveolar epithelial cells. Involvement of AP- 1/ARE and Fra-1. J Biol Chem. 2002;277:21158–21166. doi: 10.1074/jbc.M112145200. [DOI] [PubMed] [Google Scholar]
- 35.Liu R-M, Liu Y, Forman HJ, Olman M, Tarpey MM. Glutathione regulates transforming growth factor-{beta}-stimulated collagen production in fibroblasts. Am J Physiol Lung Cell Mol Physiol. 2004;286:L121–L128. doi: 10.1152/ajplung.00231.2003. [DOI] [PubMed] [Google Scholar]
- 36.Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J Clin Invest. 1997;100:768–776. doi: 10.1172/JCI119590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bett AJ, Haddara W, Prevec L, Graham FL. An efficient and flexible system for construction of adenovirus vectors with insertions or deletions in early regions 1 and 3. Proc Natl Acad Sci U S A. 1994;91:8802–8806. doi: 10.1073/pnas.91.19.8802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang W-T, Vayalil PK, Miyata T, Hagood J, Liu RM. Therapeutic value of small molecule inhibitor to plasminogen activator inhibitor-1 for lung fibrosis. Am. J. Respir. Cell Mol. Biol. doi: 10.1165/rcmb.2011-0139OC. (online publication):2011-0139OC; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katre A, Ballinger C, Akhter H, Fanucchi M, Kim D-K, Postlethwait E, Liu R-M. Increased transforming growth factor beta 1 expression mediates ozone-induced airway fibrosis in mice. Inhalation Toxicology. 2011;23:486–494. doi: 10.3109/08958378.2011.584919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang WT, Vayalil PK, Miyata T, Hagood J, Liu RM. Therapeutic value of small molecule inhibitor to plasminogen activator inhibitor-1 for lung fibrosis. Am J Respir Cell Mol Biol. 2012;46:87–95. doi: 10.1165/rcmb.2011-0139OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu R-M, Choi J. Age-associated decline of gamma-glutamylcysteine synthetase gene expression in rats. Free Radical Biol. Med. 2000;28:566–574. doi: 10.1016/s0891-5849(99)00269-5. [DOI] [PubMed] [Google Scholar]
- 42.Akhter H, Katre A, Li L, Liu X, Liu RM. Therapeutic potential and antiamyloidosis mechanisms of tert-butylhydroquinone for Alzheimer’s disease. J Alzheimers Dis. 2011;26:767–778. doi: 10.3233/JAD-2011-110512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.White CC, Viernes H, Krejsa CM, Botta D, Kavanagh TJ. Fluorescence-based microtiter plate assay for glutamate-cysteine ligase activity. Analytical Biochemistry. 2003;318:175–180. doi: 10.1016/s0003-2697(03)00143-x. [DOI] [PubMed] [Google Scholar]
- 44.Hiwatari N, Shimura S, Yamauchi K, Nara M, Hida W, Shirato K. Significance of elevated procollagen-III-peptide and transforming growth factor-beta levels of bronchoalveolar lavage fluids from idiopathic pulmonary fibrosis patients. Tohoku J Exp Med. 1997;181:285–295. doi: 10.1620/tjem.181.285. [DOI] [PubMed] [Google Scholar]
- 45.Kowalska A, Puscinska E, Czerniawska J, Goljan-Geremek A, Czystowska M, Rozy A, Chorostowska-Wynimko J, Gorecka D. [Markers of fibrosis and inflammation in exhaled breath condensate (EBC) and bronchoalveolar lavage fluid (BALF) of patients with pulmonary sarcoidosis -- a pilot study] Pneumonol Alergol Pol. 2010;78:356–362. [PubMed] [Google Scholar]
- 46.Liu DY, Wu J, Zhang XY, Feng ZC. [Expression of IL-8, SP-A and TGF-beta1 in bronchoalveolar lavage fluid of neonates with bronchopulmonary dysplasia] Zhongguo Dang Dai Er Ke Za Zhi. 2010;12:444–446. [PubMed] [Google Scholar]
- 47.Synenki L, Chandel NS, Budinger GR, Donnelly HK, Topin J, Eisenbart J, Jovanovic B, Jain M. Bronchoalveolar lavage fluid from patients with acute lung injury/acute respiratory distress syndrome induces myofibroblast differentiation. Crit Care Med. 2007;35:842–848. doi: 10.1097/01.CCM.0000257254.87984.69. [DOI] [PubMed] [Google Scholar]
- 48.Bakin AV, Stourman NV, Sekhar KR, Rinehart C, Yan X, Meredith MJ, Arteaga CL, Freeman ML. Smad3-ATF3 signaling mediates TGF-[beta] suppression of genes encoding Phase II detoxifying proteins. Free Radical Biology and Medicine. 2005;38:375–387. doi: 10.1016/j.freeradbiomed.2004.10.033. [DOI] [PubMed] [Google Scholar]
- 49.Lu D, Chen J, Hai T. The regulation of ATF3 gene expression by mitogen-activated protein kinases. Biochem J. 2007;401:559–567. doi: 10.1042/BJ20061081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mei Y, Yuan Z, Song B, Li D, Ma C, Hu C, Ching YP, Li M. Activating transcription factor 3 up-regulated by c-Jun NH(2)-terminal kinase/c-Jun contributes to apoptosis induced by potassium deprivation in cerebellar granule neurons. Neuroscience. 2008;151:771–779. doi: 10.1016/j.neuroscience.2007.10.057. [DOI] [PubMed] [Google Scholar]
- 51.Sandnes D, Muller KM, Akhtar K, Johansen EJ, Christoffersen T, Thoresen GH. Induction of LRF-1/ATF3 by vasopressin in hepatocytes: role of MAP kinases. Cell Physiol Biochem. 2010;25:523–532. doi: 10.1159/000303056. [DOI] [PubMed] [Google Scholar]
- 52.Soltaninassab SR, Sekhar KR, Meredith MJ, Freeman ML. Multi-faceted regulation of gamma-glutamylcysteine synthetase. J Cell Physiol. 2000;182:163–170. doi: 10.1002/(SICI)1097-4652(200002)182:2<163::AID-JCP4>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 53.Choi J, Liu R-M, Kundu RK, Sangiorgi F, Wu W, Maxson R, Forman HJ. Molecular mechanism of decreased glutathione content in Human Immunodeficiency Virus Type 1 Tat-transgenic mice. Journal of Biological Chemistry. 2000;275:3693–3698. doi: 10.1074/jbc.275.5.3693. [DOI] [PubMed] [Google Scholar]
- 54.Liu RM. Down-Regulation of gamma-Glutamylcysteine Synthetase Regulatory Subunit Gene Expression in Rat Brain Tissue During Aging. J. Neuroscience Research. 2002;68:344–351. doi: 10.1002/jnr.10217. [DOI] [PubMed] [Google Scholar]
- 55.Liu RM, Dickinson DA. Decreased synthetic capacity underlies the age-associated decline in glutathione content in Fisher 344 rats. Antioxid Redox Signal. 2003;5:529–536. doi: 10.1089/152308603770310176. [DOI] [PubMed] [Google Scholar]
- 56.Liu H, Wang H, Shenvi S, Hagen TM, Liu R-M. Glutathione Metabolism during Aging and in Alzheimer Disease. Ann NY Acad Sci. 2004;1019:346–349. doi: 10.1196/annals.1297.059. [DOI] [PubMed] [Google Scholar]
- 57.Huang CS, Chang LS, Anderson ME, Meister A. Catalytic and regulatory properties of the heavy subunit of rat kidney gamma-glutamylcysteine synthetase. J Biol Chem. 1993;268:19675–19680. [PubMed] [Google Scholar]
- 58.Yan N, Meister A. Amino acid sequence of rat kidney g-glutamylcysteine synthetase. Journal of Biological Chemistry. 1990;265:1588–1593. [PubMed] [Google Scholar]
- 59.Griffith OW, Mulcahy RT. The enzymes of glutathione synthesis: gammaglutamylcysteine synthetase. Adv Enzymol Relat Areas Mol Biol. 1999;73:209–267. xii. doi: 10.1002/9780470123195.ch7. [DOI] [PubMed] [Google Scholar]
- 60.Huang CS, Anderson ME, Meister A. Amino acid sequence and function of the light subunit of rat kidney gamma-glutamylcysteine synthetase. J Biol Chem. 1993;268:20578–20583. [PubMed] [Google Scholar]
- 61.Chen Y, Shertzer HG, Schneider SN, Nebert DW, Dalton TP. Glutamate Cysteine Ligase Catalysis: DEPENDENCE ON ATP AND MODIFIER SUBUNIT FOR REGULATION OF TISSUE GLUTATHIONE LEVELS. J. Biol. Chem. 2005;280:33766–33774. doi: 10.1074/jbc.M504604200. [DOI] [PubMed] [Google Scholar]
- 62.Liu RM, Borok Z, Forman HJ. 4-Hydroxy-2-nonenal increases gammaglutamylcysteine synthetase gene expression in alveolar epithelial cells. Am J Respir Cell Mol Biol. 2001;24:499–505. doi: 10.1165/ajrcmb.24.4.4307. [DOI] [PubMed] [Google Scholar]
- 63.Liu R, Choi J. Age-associated decline in gamma-glutamylcysteine synthetase gene expression in rats. Free Radic Biol Med. 2000;28:566–574. doi: 10.1016/s0891-5849(99)00269-5. [DOI] [PubMed] [Google Scholar]
- 64.Chen BP, Liang G, Whelan J, Hai T. ATF3 and ATF3 delta Zip. Transcriptional repression versus activation by alternatively spliced isoforms. J Biol Chem. 1994;269:15819–15826. [PubMed] [Google Scholar]
- 65.Chen S-C, Liu Y-C, Shyu K-G, Wang DL. Acute hypoxia to endothelial cells induces activating transcription factor 3 (ATF3) expression that is mediated via nitric oxide. Atherosclerosis. 2008;201:281–288. doi: 10.1016/j.atherosclerosis.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 66.Inoue K, Zama T, Kamimoto T, Aoki R, Ikeda Y, Kimura H, Hagiwara M. TNF_-induced ATF3 expression is bidirectionally regulated by the JNK and ERK pathways in vascular endothelial cells. Genes to Cells. 2004;9:59–70. doi: 10.1111/j.1356-9597.2004.00707.x. [DOI] [PubMed] [Google Scholar]
- 67.Vayalil PK, Olman M, Murphy-Ullrich JE, Postlethwait EM, Liu RM. Glutathione restores collagen degradation in TGF-beta-treated fibroblasts by blocking plasminogen activator inhibitor-1 expression and activating plasminogen. Am J Physiol Lung Cell Mol Physiol. 2005;289:L937–L945. doi: 10.1152/ajplung.00150.2005. [DOI] [PubMed] [Google Scholar]
- 68.Vayalil PK, Iles KE, Choi J, Yi A-K, Postlethwait EM, Liu R-M. Glutathione suppresses TGF-beta-induced PAI-1 expression by inhibiting p38 and JNK MAPK and the binding of AP-1, SP-1, and Smad to the PAI-1 promoter. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1281–L1292. doi: 10.1152/ajplung.00128.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]