Abstract
Polycystin-1, the product of the major gene mutated in autosomal dominant polycystic kidney disease (ADPKD), associates with multiple epithelial cell junctions, including desmosomes. It was our objective to identify the molecular interactions between polycystin-1 and desmosomal components in primary human kidney epithelial cells and to determine if desmosomal adhesion is altered in ADPKD. Using laser scanning confocal microscopy and two models of cell polarization, polycystin-1 and desmosomes were found to colocalize during the initial establishment of cell-cell contact when junctions were forming. However, colocalization was lost in confluent monolayers. Parallel morphological and biochemical evaluations of polycystic kidney tissue and primary epithelial cells from ADPKD cysts revealed a profound mispolarization of desmosomal components to both the apical and basolateral domains of the disease cells. Structural and functional evaluations of the desmosomal assemblies in ADPKD cells provide evidence for impaired cytokeratin expression and increased sensitivity of the monolayers to shear stress. Together, these discoveries suggest a transient role for polycystin-1 activity in the assembly of functional desmosomes at early stages of cell differentiation and polarization, which when disrupted leads to abnormal desmosomal adhesion in ADPKD.
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is a common, life-threatening genetic disease that principally afflicts the kidney, but may also have extrarenal manifestations. ADPKD has a dominant mode of inheritance and afflicts more individuals than cystic fibrosis, sickle cell anemia, hemophilia, muscular dystrophy, Down’s syndrome and Huntington’s disease combined. The disease at a gross anatomical level is characterized by the progressive appearance of multiple fluid filled-cysts in both kidneys which gradually compromise normal function and ultimately result in end stage renal failure (1). Extrarenal pathologies include pancreatic and liver cysts that may cause significant pain and cardiovascular defects that include intracranial aneurysms and heart defects.
At the molecular level the disease has proven to be extraordinarily complex (1, 2). Alterations in growth control and differentiation (3), extracellular matrix deposition (4), gene expression, cell polarity and intracellular signaling have all been closely associated with disease pathophysiology (5). Based on current understanding, cysts develop following inheritance of a germline mutation in one of two genes, PKD1 (80% of all cases) (6–9) or PKD2 (10) and subsequent somatic mutation in the normal PKD1 or PKD2 alleles of a tubular epithelial cell (2, 11). There is no consensus as to whether loss of heterozygosity or dominant negative mutant proteins is the underlying cause of disease pathogenesis. Molecular dissection of the disease is further complicated by the large numbers of disease causing mutations and the dramatic influence of modifying loci on disease severity (12).
A significant breakthrough in the field was realized with the identification and characterization of polycystin-1 and polycystin-2 as the products of the PKD1 and PKD2 genes, respectively. Multiple functions and localizations have been ascribed to the polycystins and they appear to participate in a variety of protein-protein complexes (5, 13, 14). Polycystin-1 is a large transmembrane protein with extracellular domains typical of proteins that mediate cell-cell and cell-extracellular matrix interactions (9, 15) and intracellular domains implicated in the transduction of signals (16). Polycystin-1 interacts via its C-terminus with polycystin-2, a protein that forms a calcium permeable channel and is thought to participate in calcium homeostasis(17–20). Recently it has been reported that polycystin-1 may itself exhibit channel activity even in the absence of polycystin-2 (21). The composite localization and functional studies to date show the polycystins to play an important role in mechanosensory signal transduction, as well as cell adhesion (22–29).
Altered cell-cell adhesion is proving to be a key factor in ADPKD and most likely contributes to both cystogenesis and altered cell growth control. The importance of the polycystins in cell-cell adhesion is in part evidenced by observations that polycystins are associated with key adhesive junctions including adherens junctions and desmosomes (24, 26). A second line of evidence derives from the finding that in primary ADPKD cells there is a dramatic depletion of normal adherens junctions and E-cadherin along with polycystin-1 from the cell surface (25, 30). E-cadherin is replaced by N-cadherin both in cysts in situ as well as in primary cells in culture and β-catenin may be increasingly activated (25, 31). Because different cell surface cadherins display a propensity to self-aggregate and promote segregation of cells during growth and development (32, 33), the expression of N-cadherin may contribute to cystogenesis by inciting the cells expressing mutant polycystin to self-associate, segregate from the normal tubule lumen and invade the parenchyma to form a cyst.
The fact that the disruption of adherens junctions may negatively impact the assembly of desmosomes taken together with the finding that polycystin-1 colocalizes with desmosomes in MDCK cells provide impetus for the present study of the status of desmosomes in primary ADPKD cells. Desmosomes constitute a second key adhesive junction at the basolateral plasma membrane and are critical for stabilizing the epithelial sheet through connections to intermediate filaments. Crosstalk between adherens junction and desmosome formation is thought to be mediated by plakoglobin, a common component of both junctions, as well as through intracellular signaling networks (34). Initial desmosome assembly is calcium dependent, while fully assembled desmosomes lose their calcium dependence and are highly insoluble. Desmosomes may also have growth regulatory roles mediated by associated cytoplasmic plaque proteins (35, 36). The desmosomal cadherins desmoglein (Dsg) and desmocollin (Dsc) constitute the core transmembrane proteins that interact with desmosomal cadherins on adjacent cells via their luminal domains and associate with desmosomal plaque constituents via their cytosolic domains. The desmosomal plaque is constituted of plakoglobin (Pg), plakophilins, and desmoplakin (DP), which bind to the intermediate filament cytoskeleton. In this study, we investigated the status of desmosomal junction assembly and the associated intermediate filament network in primary ADPKD cells in relation to the expression and localization of polycystin-1 in human primary kidney cells.
Methods
Antibodies and Reagents
All reagents were from Sigma (Saint Louis, MO) unless otherwise indicated. The following commercial antibodies against desmosomal components were used in immunofluorescence: mouse mAb directed against desmoglein-2 (1:25, clone 6D8, Zymed), mouse mAb directed against plakoglobin (1:100, clone PG-11E4, Zymed), mouse mAb directed against desmoplakin (1:2, DP 1 and 2 clone 2.15, 2.17, 2.20, Progen). Dr. Kathleen J. Green (Dept. Pathology, Northwestern University, Chicago, IL) kindly provided a rabbit pAb directed against desmoplakin (NW161) and a mouse mAb directed against desmocollin-2 (clone 7G6). Antibodies against cytoskeletal proteins were as follows: mouse mAb directed against vimentin (1:100, clone Vim 3B4, DAKO), mouse mAb directed against cytokeratin 18 (1:100, clone DC10, DAKO). A pAb directed against polycystin-1 (1:100, NM005), was obtained from rabbits injected with the C-terminal region of the protein (exon 46; 4070-4302 aa) and affinity purified in our laboratory. Secondary antibodies were obtained from Jackson ImmunoResearch Laboratories Inc (West Grove, Pennsylvania, USA): FITC-conjugated donkey anti-mouse and rhodamine-conjugated donkey anti-rabbit (used for tissue sections) or Cy5-conjugated donkey anti-rabbit (used for cultured cells). Nuclei were stained with Hoechst 33258 (Eastman Kodak Company, Rochester, NY) or propidium iodide. Cell culture medium was from Gibco Invitrogen Corporation (Grand Island, NY) and serum was from Hyclone (South Logan, UT)
Cell culture
Primary normal epithelial kidney cells were isolated from kidneys of organ donors that were medically unsuitable for transplant and therefore procured for research through the NDRI tissue resource center. Cyst lining epithelial cells were initially derived by Dr. Frank Carone and Ms. Sakie Nakamura and subsequently by dissection in our laboratory of kidneys from patients with autosomal dominant polycystic kidney disease that were removed for clinically indicated reasons. Briefly, upon arrival, normal kidneys were placed on ice; the cortex was excised and minced into fine pieces that were incubated in Enzyme Solution (collagenase, hyaluronidase, DNase l, soybean trypsin inhibitor and DMEM/F12 media) at 37°C with regular vortexing for one hour. The extract was pelleted by centrifugation and the cell pellet resuspended and grown in DMEM/F12 media supplemented with 10% FCS and antibiotics. Polycystic kidney cells were processed as follows: cysts from the kidney surface were individually dissected and put into a solution of 1% trypsin-EDTA at 37°C for one hour. After vortexing, the cells were left to settle and the cyst wall was subjected to a second round of enzyme-vortexing to achieve maximal recovery of the cyst lining epithelial cells. Cells were frozen and stored in liquid nitrogen or expanded. Cells were expanded to passage 4, after which they became retarded in their growth, acquired cytoplasmic vacuoles and were discarded. Cells were phenotypically characterized prior to use by immunofluorescence staining. Their epithelial cell origin was established by staining for tight junctions and adherens junctions. In the case of ADPKD cells, the loss of E-cadherin and expression of N-cadherin was used as a characteristic hallmark (25). At least three normal and three ADPKD samples were used for each experiment. For experiments, 3 × 105 to 1 × 106 cells were seeded on polyethylene terephthalate (PET) (0.4 μm pore size) transparent 6-well or 12-well cell culture inserts (Falcon-BD, New Jersey, USA) and grown for 3–7 days at full confluence to let them achieve maximal polarization.
Immunocytochemistry
Briefly, immunofluorescence of cells grown on filters was conducted as follows. Cells were rinsed with PBS+ (PBS containing 1 mM each MgCl2 and CaCl2), pre-extracted with 0.01–2% saponin (depending on normal/pathologic, passage number, sparse/confluent cells) in PIPES solution (80 mM PIPES, 5mM EGTA and 1mM MgCl2) for 3–5 min. Saponin pre-extraction is a well described method for selectively permeabilizing the plasma membrane and allowing small soluble cytosolic proteins to be extracted, thereby improving membrane protein staining. Following preextraction cells were fixed with 3% paraformaldehyde in PBS+ for 20 min, quenched with NH4Cl for 10 min and permeabilized with 0.1% Triton-X 100 for 5 min after which samples were blocked with 0.4% fish skin for 10 min and washed. Filters were excised from filter support and cut in 2–4 pieces prior incubation with primary antibody. After incubation with secondary antibody plus propidium iodide, cells were post-fixed with 3% paraformaldehyde, quenched and mounted with ProLong or SlowFade (Molecular Probes, Eugene, OR). Nuclei were stained with Hoechst (1:1000) or propidium iodide (1 μg/ml) plus RNAse A (20 μg/ml). Filters were placed on a microscope slide with mounting media cell side up in the center of 4 nail polish posts and covered carefully with a coverslip. For cytoskeleton labeling cells were fixed in methanol at −20°C for 5 min and processed for immunofluorescence staining. All samples were imaged with a Zeiss LSM 510.
Immunohistochemistry
Frozen normal or ADPKD tissue were cryosectioned (4 μm thickness) using a Reichert Ultracut S ultramicrotome with Leica EMFCS cryochamber. Sections were collected on Fisherbrand Superfrost/Plus microscope slides (Fisher Scientific), fixed with 3% paraformaldehyde, blocked/permeabilized with 10% newborn calf serum/0.1% Triton-100 X for 40 min. Tissue samples were stained with primary antibodies for 2 h and secondary antibodies for 30 min, all at 37°C in a humidified chamber. Nuclei were stained with propidium iodide (1μg/ml). Tissue was washed three times for 10 min each with PBS/0.1% TX-100 and mounted in SlowFade mounting media. A Zeiss LSM 510 was used to obtain high resolution images of stained tissue sections. For labeling of cytoskeletal components, tissue sections were fixed in methanol at −20°C for 5 min, after which, they were blocked in 10% newborn calf serum and incubated with the appropriate antibodies.
Calcium switch assay
Cells were seeded on filters at full confluence (5 × 105 cells on 12-well filters) in low calcium (<5 μM Ca2+) DMEM (Gibco Invitrogen Corporation, Grand Island, NY) and incubated overnight at 37°C. The following day, cells were switched to high calcium (>1.0 mM) DMEM/F12 media supplemented with 10% FCS and antibiotics, for different lengths of time ranging from 0 min to 24 h, after which cells were pre-extracted and fixed for immunofluorescence as described above.
Cell surface biotinylation and streptavidin affinity precipitation
Confluent filter-grown cells were washed with ice-cold PBS+ and incubated with ice-cold sulfo-NHS-SS-biotin (Pierce Biotechnology Inc, Rockford, IL) at 0.6 mg/ml prepared in PBS+ pH 8.0. Either the apical (1 ml volume) or basolateral (1.5 ml volume) surface was biotinylated twice in succession for 15 min each, using fresh biotinylation reagent each time. The biotinylation reaction was terminated by replacing the second biotin solution with the same volume of ice-cold 50 mM NH4Cl in PBS+ for 30 min on ice. Following biotinylation, cells were lysed in 100 μl of 1% SDS (w/v), 15 mM Tris-Cl pH 8, 4mM EDTA, 1 μM CLAP (chrimostatin, leupeptin, antipain and pepstain A) and 1 μM AEBSF, boiled for 5 min and diluted in 900 μl of Triton Buffer 1 (0.5% v/v TX-100, 15 mM Tris-Cl pH 8.0, 150 mM NaCl, 4 mM EDTA, CLAP and AEBSF) containing 40 μl of streptavidin-agarose (Pierce Biotechnology Inc, Rockford, IL). The sample was rocked at 4°C for 1 h, and the beads washed and boiled for 5 min in 40 μl of 2X SDS sample buffer containing 50 mM dithiothreitol (DTT). The cell lysates were resolved by SDS-PAGE, the gels blotted onto nitrocellulose membranes (Amersham Biosciences UK limited, UK) and the blots probed with mouse monoclonal antibody directed against Dsg-2 followed by the anti-mouse HRP-conjugated secondary antibody (Amersham Biosciences UK limited, UK). Dsg-2 was detected using ECL (SuperSignal, Pierce).
Coimmunoprecipitation and immunoblot assays
Cells grown to confluence on 6-well Transwell filters were washed 3 times in cold PBS, covered with 250 μl of RIPA buffer without SDS (10 mM Na2HPO4 pH 7.2, 150 mM NaCl, 2mM EDTA, 1% TX-100, 1% sodium deoxycholate and 20 μM AEBSF), left shaking at 4°C for 30 min, after which cells were scraped, lysed in a Dounce homogenizer (pestle “B”), and centrifuged at 40,000 g for 50 min. The supernatant was recovered and a sample was reserved as a total lysate sample for immunoblot analysis. The rest of the sample (4 filter equivalents) was precleared by incubation in 10 μl of packed protein G-agarose (Fast Flow, Upstate, Lake Placid, NY) overnight at 4°C and short, high-speed centrifugation. The resulting supernatant was divided into two aliquots, each incubated for 3 h with an antibody against a desmosomal protein (anti-Dsc, anti-Dsg) to precipitate the protein of interest or an irrelevant Ig of the same isotype (anti-TNP, Pharmingen, San Diego, CA) as a negative control. Subsequently, the samples were incubated with protein G-agarose beads for 2 h, immunoprecipitates recovered by a short, high-speed centrifugation and washed sequentially 3 times in Triton buffer 2 (1% v/v TX-100, 10 mM Tris-Cl pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA), 1 time in high salt buffer (TX-100 buffer with 500 mM NaCl) and 1 time in distilled water. The beads were mixed 1:1 with SDS sample buffer (Bio-Rad Laboratories, Hercules, CA), boiled for 10 min, clarified by centrifugation and the supernatants were loaded on a 7.5% Criterion Bio-Rad gel. After electrophoresis, the proteins were transferred to PVDF membranes, blocked in 5% non-fat milk prepared in PBS and incubated with the desired antibody (anti-Dsc, anti-Dsg and anti-Pg, all 1:500, and anti-PC1, 1:250). Secondary antibodies were mouse or anti-rabbit HRP-peroxidase conjugated that were revealed with ECL.
Dispase assay
The dispase assay described by Calautti et al.(37) was modified for our purposes and performed as follows. Cells grown on collagen lV coated 35 mm dishes were incubated with dispase enzyme (2.4 U/ml; Roche Applied Science) at 34°C for 15–30 min after which they were carefully scraped to separate the monolayer from the dish. The sheets of cells were gently transferred with a wide-tipped transfer pipette into a snap cup tube where they were subjected to shaking on a shaker (model M65125, Integrated Separation Systems Enprotech, MA), at 106 rpm for 3 min. Subsequently, cells were left to settle for 1 minute and 50 μl of the supernatant was taken in duplicate for cell counting in a cell counter (Z2 Coulter Counter, Beckman Inc). The remaining cells were pelleted and incubated with trypsin at 37°C for 10 minutes after which they were counted in the cell counter along with the samples taken just after shaking.
Results
Desmosomal proteins are mislocalized to the apical domain in ADPKD
It has been postulated that adherens junctions are necessary for desmosomal formation and that when E-cadherin is not expressed, desmosome assembly is delayed or blocked (34, 38). In autosomal dominant polycystic kidney disease, E-cadherin is depleted from the lateral membrane of disease cells and found intracelullarly in vesicles, (30). These observations raised questions regarding the status of desmosomal junctions in the disease, which was addressed by examining primary human cells in culture and cystic epithelium in situ.
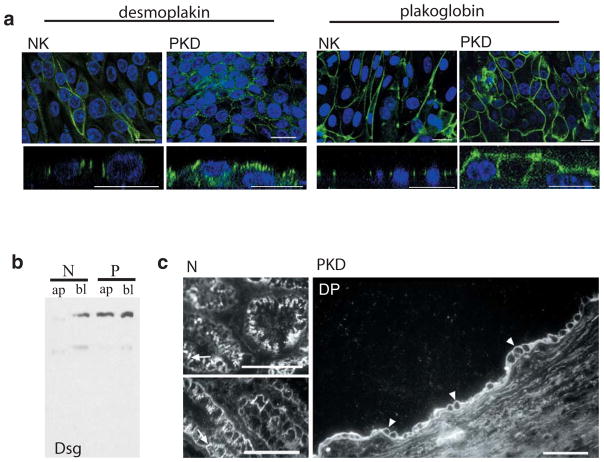
Endogenous desmosomal structures were evaluated by immunolabeling normal and cystic primary human kidney epithelial cells grown on filters (Figure 1a). We discovered that while desmosomal proteins are still present at the lateral plasma membrane (Figure 1a), they are also found at the apical plasma membrane of ADPKD cells (figure 1a, xz view). Labeling for both cytoplasmic desmosomal components, such as desmoplakin and plakoglobin (figure 1a) or for the integral membrane desmosomal cadherins such as desmoglein (data not shown) revealed apical staining in all cases. Desmoplakin and desmoglein were arrayed in a punctate pattern, while plakoglobin exhibited a more homogeneous distribution. The reported distribution was consistently observed in ADPKD cells from three different patients. To further substantiate the extent of desmosomal cadherin mislocalization, a biochemical approach was used. Apical and lateral cell domains were subjected to domain-selective biotinylation and the biotin-labeled proteins were precipitated with streptavidin-agarose beads. The biotinylated proteins were resolved by SDS-PAGE and desmosomal proteins identified by immunoblotting. In fully polarized normal kidney cells desmoglein was exclusively basolateral, while in ADPKD cells desmoglein was equally distributed between the apical and basolateral domains of ADPKD cells (Figure 1b). Moreover, the aberrant polarization of desmosomal components was also confirmed by immunohistochemistry of normal and ADPKD tissue cryosections (Figure 1c), leading us to conclude that desmosomal proteins are mislocalized in situ in ADPKD and that this phenotype is recapitulated by primary ADPKD cells in culture, making it amenable to study the underlying causes.
Figure 1. Desmosomes are mispolarized in ADPKD cells in vitro and in situ.
a. Confocal images of normal and ADPKD cells in culture labeled for desmosomal components (green). Upper panels: xy view to show desmosomal appearance at lateral membrane. Lower panels: xz view to show desmosomal distribution between apical and lateral cell domains. Desmoplakin labeling (two left panels) is seen only at the lateral domain of normal kidney cells (NK) but apical and lateral in ADPKD cells (PKD). Plakoglobin (two right panels) in normal (NK) and ADPKD cells (PKD) reveals same phenomenon. Nuclear staining, used as structural reference is seen in blue. Bars: 20 μm (except first panel: 5μm)
b. Western blot of streptavidin-precipitated, biotinylated proteins, blotted with anti-desmoglein antibody. Only basolateral protein (bl) was recovered in normal kidney cells (N), while basolateral (bl) and apical (ap) desmoglein is seen in ADPKD cells (P).
C. Confocal image of cryosections of normal (two left panels) and polycystic (right panel) tissue. Labeling against desmoplakin shows apical staining of the cyst lining epithelia in polycystic kidney tissue (arrowheads) while only lateral signal can be observed in normal tubules (arrows). Bars: 30 μm
Abnormal intermediate filament protein profile in ADPKD
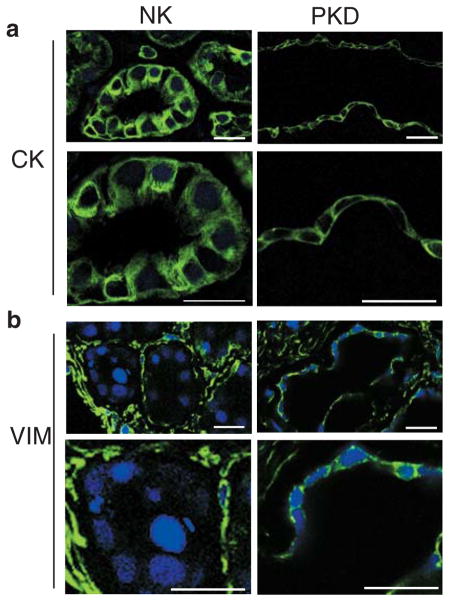
Desmosomal junctions are normally anchored to a keratin intermediate filament network in epithelia that gives strength to intercellular adhesion and protects the monolayer to shear stress. Vimentin is an intermediate filament protein that is characteristic of fibroblasts, endothelial cells and mesenchymal cells. In vivo, there are only few examples of co-expression of desmosomal proteins and intermediate filaments of the vimentin type (39). In order to analyze the whole system of desmosomal anchoring junctions and its attached cytoskeleton, ADPKD tissue sections were immunolabeled for vimentin and cytokeratin, and compared with normal kidney tissue sections. Although cystic cells harbor desmosomal components at the plasma membrane, they abnormally express vimentin in their cytoplasm (Figure 2). ADPKD tissue sections stained with hematoxilin-eosin, showed more interstitial fibrosis in the patients wherein the vimentin labeling was more intense (not shown). Normal kidney tissue sections labeled for comparison show the tubular epithelial cells are completely devoid of vimentin as expected. Although cytokeratin staining could still be seen in the cyst lining epithelia, the signal was rather homogeneous and contrasted with the well-developed, filamentous pattern seen in tubular epithelial cells from normal kidney cryosections. The same profile was observed in three different patients. We conclude that cystic cells have desmosomal junctions, characteristic of epithelia, yet show signs of mesenchymal transformation.
Figure 2. Vimentin is anomalously expressed in cystic epithelia in situ.
a. Cryosection of normal kidney showing cytokeratin (CK) staining in tubule cells (NK) Lower panel is a higher magnification of upper one. ADPKD kidney sectioned at a cyst level (PKD) shows that cells lining the wall cyst conserve cytokeratin expression (magnified in lower panel). Samples were imaged on Zeiss LSM510. Bars, 20 μm.
b. Cryosection of normal tubules (NK) labeled against vimentin protein (VIM), show complete a absence of vimentin from their cytoplasm (magnified in lower panel). Kidney interstitial fibroblasts have a normal vimentin signal. Sections from a cyst of an ADPKD kidney (PKD) show the abnormal presence of vimentin in cyst-lining epithelial cells (detailed in lower panel). Samples were imaged on Zeiss LSM510. Bars, 20 μm.
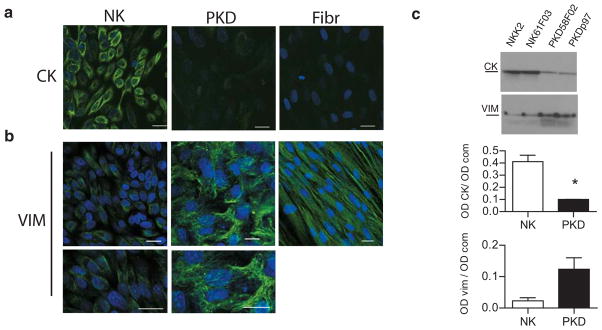
The cytoskeleton abnormalities in ADPKD were further analyzed in cultured primary cells both morphologically and biochemically. Immunofluorescence staining with specific antibodies against vimentin and cytokeratin-18 showed normal human epithelial kidney cells co-expressed vimentin and cytokeratin, when the cells were processed 3 days after reaching full confluence (not shown). The transition to a cytokeratin intermediate filament network is consistent with what has been described for mature epithelial cells (40). Immunofluorescence staining after 7 days at full confluence, revealed a predominant cytokeratin staining and much reduced vimentin expression in normal cells (Figure 3a, NK). In contrast, vimentin remained highly expressed in ADPKD cells even after long-term culture and exhibited a conspicuous filamentous pattern throughout the cytoplasm of ADPKD cells (Figure 3a, PKD). PKD cells also exhibited some cytokeratin staining (Figure 3a), although the levels appeared to be significantly lower than in NK cells. In contrast, fibroblasts (Figure 3a, Fibr), used as a control, completely lacked cytokeratin, while harboring normal vimentin labeling. To quantify vimentin and cytokeratin levels, NK and ADPKD from two different patients each, were grown on filters and analyzed by SDS-PAGE, controlling for equal protein loading, and immunoblotting 7 days after reaching full confluence (Figure 3b, blots). Quantification revealed that PKD cells express 5 times more vimentin and 4 times less cytokeratin than normal cells (Figure 3b, graphs). These results are in agreement with the cytoskeleton alterations found in vivo, and demonstrate that the normal desmosome-intermediate filament system is disrupted in ADPKD.
Figure 3. ADPKD cells express an immature intermediate filament network even when fully confluent.
a. Confocal images of primary normal kidney cells (NK) and polycystic kidney cells (PKD) in culture labeled against cytokeratin intermediate filament (CK). Normal kidney cells show normal cytokeratin labeling, while disease cells show very low signal. Confocal images of normal and polycystic kidney cells in culture, labeled against vimentin intermediate filament (VIM). Normal cells have very little vimentin signal (magnified in bottom panel), but polycystic cells, on the contrary, show a conspicuous vimentin filament network (amplified in lower panel). Normal human fibroblasts (Fibr) express exclusively vimentin. Bars, 20 μm.
b. Immunoblots of lysates from two different samples each of normal (NK) and ADPKD (PKD) kidney cells probed for cytokeratin (CK) or vimentin (VIM). Below, quantification of cytokeratin and vimentin protein expression levels in normal and disease cells grown. The y-axis represents the intensity of the CK or the VIM band (OD CK) normalized according to the total protein loaded per lane as detected by Coomassie blue staining (OD com). CK graph: p=0.01. Vim graph: p=0.057.
Abnormal cell-cell adhesion in polycystic kidney cells
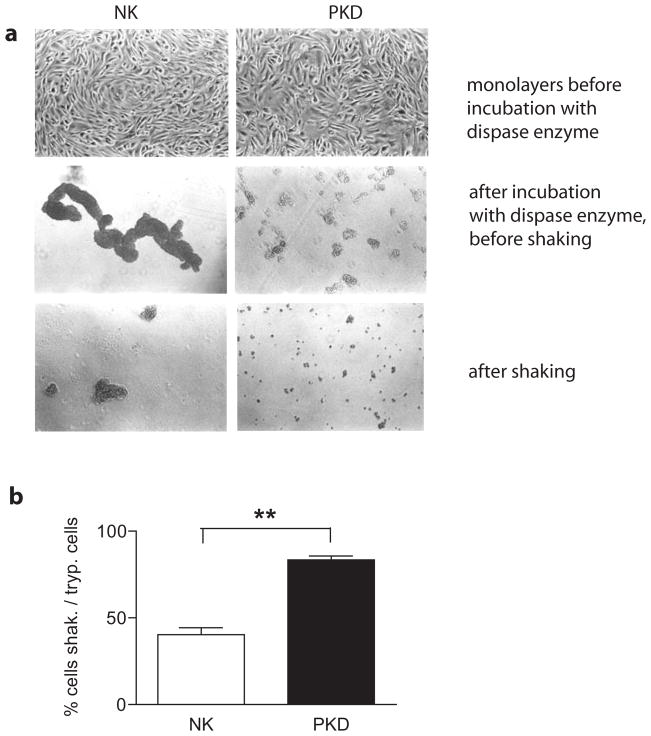
The alterations in adherens junctions shown previously (25, 30) and the structural abnormalities of desmosomal anchoring junctions and cytoskeleton network found in ADPKD in these studies, prompted us to examine impact on cell-cell adhesion at a functional level. Cell-cell adhesion was monitored by releasing monolayers with dispase enzyme, a neutral protease that cleaves fibronectin and type lV collagen, but degrades type I collagen only minimally (41). After dispase treatment, the monolayer was subjected to shear stress using a published protocol modified to suit our kidney primary cells (see methods and (32)). Following shear stress the numbers of single cells in suspension were determined using a Coulter counter. Two different ADPKD and two normal samples were analyzed in triplicate. The cell fraction between 10–20 μm in size was analyzed (single cells or doublets). As shown, the intercellular adhesions between cells from ADPKD patients were significantly more fragile and were largely disaggregated after shaking (Figure 4). The differences in the status of cell-cell adhesion were clearly evident by microscopic inspection after release of the monolayers with dispase and became even more pronounced after shaking (Figure 4a). The numbers of single or doublet cells after shaking are expressed as a percentage of the total number cells obtained after trypsinization. ADPKD cells were 83.4 ± 2.20% single or doublet cells after shaking while only 40.3 ± 3.95 % of NK cells were present as single or doublet cells after the same procedure (Figure 4b). Interestingly, although the ADPKD cells exhibited decreased intercellular adhesion, they remained more firmly attached to the substratum than normal kidney cells during dispase treatment. This was observed irrespective of whether the cells were grown on tissue culture plastic, suspension culture dishes, or collagen IV coated dishes, which is in agreement with the findings of other investigators (22). With this experiment we demonstrate that PKD cells harbor a profound defect in cell-cells adhesion, and that structural alterations in desmosomal junctions have a negative impact on ADPKD epithelia integrity.
Figure 4. Desmosomal mediated cell-cell adhesion is compromised in ADPKD cells.
NK and PKD cell monolayers were incubated with dispase, released from the substrate and briefly shaken for a fixed time.
a. Cells were imaged with a Zeiss, inverted light microscope. Top panels show NK and PKD cells forming confluent monolayers (10X objective). Middle panels show normal kidney cells in large, interconnected sheets (NK cells), while ADPKD cells are in small aggregates (PKD) after incubation with dispase enzyme, but before shaking. Bottom panels show the same samples after shaking, where the difference is further exacerbated.
b. Quantification of cells released after shaking was performed using a Beckman Coulter counter. Plotted are the percentages of single or doublet cells after shaking as a function of the total number of cells assayed by trypsinization. 83.4 ± 2.20 % of PKD cells (black) were present as single or doublet cells after shaking, while only 40.3 ± 3.95 % of NK cells (white) were present as single or doublet cells after the same procedure. P= 0.005.
Polycystin-1 colocalizes with desmosomes transiently during the establishment of cell-cell contacts
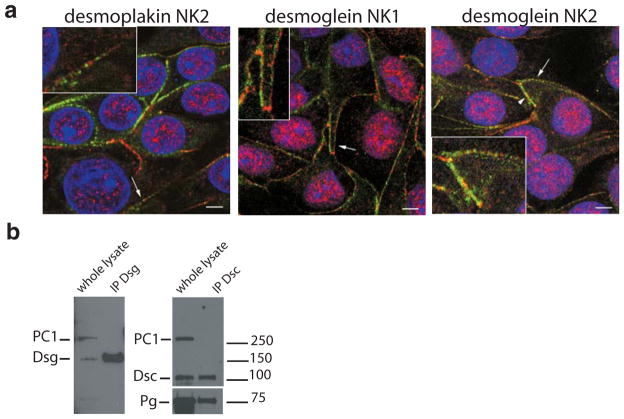
The observed mispolarization of desmosomal components in ADPKD described above and the report by other investigators (26) that polycystin-1 colocalizes with desmoplakin in MDCK cultured on plastic surfaces, prompted us to analyze the relation between polycystin-1 and desmosomal components in our primary human cells, grown on filters. Normal kidney cells from at least three different unrelated people were grown on filters to 3 days of full confluence, after which they were processed for immunofluorescence and co-labeled with antibodies directed against individual desmosomal components and polycystin-1. No colocalization between polycystin-1 and desmosomal proteins could be seen, with the proteins observed in a punctate, alternating pattern, irrespective of whether the desmosomal cadherins or cytosolic desmosomal components were labeled. Representative examples of desmoplakin and desmoglein staining are shown in Figure 5a. In most stretches of the plasma membrane polycystin-1 and desmosomal staining appeared mutually exclusive. Occasional membrane regions, where polycystin-1 and desmosomal protein staining was more homogeneous were also observed (Figure 5, “desmoplakin NK2”, arrowheads). In these areas some overlapping staining was observed and it is thought that this may be due to a different state of cell-cell contact and junctional development, possibly in a cell that has recently undergone cell division.
Figure 5. Polycystin-1 and desmosomal proteins are segregated in fully confluent normal kidney cells.
a. Confocal images of primary normal human kidney cells grown on filters at 100% confluence for three days co-labeled for polycystin-1 (red) and desmosomal components (green). No colocalization between polycystin-1 and desmoplakin (left panel) was seen. Desmoglein staining of normal kidney cells (NK1 and NK2) from two different patients shows no overlap with polycystin-1 signal (arrows). Occasional colocalization could be seen in membrane regions where desmosome and polycystin-1 staining gave a homogeneous pattern (arrowhead, right panel). Insets show a magnification of the regions denoted by arrows. Each image depicts a single confocal section acquired at the level of the nucleus. Bars, 5 μm.
b. Normal kidney cells were lysed and subjected to immunoprecipitation for different desmosomal proteins. Subsequent western blotting was used to probe for the presence of polycystin-1. Polycystin-1 failed to be recovered in immunoprecipitates of desmocollin or desmoglein. A blot for plakoglobin was included as a positive control for the stability of desmosomal protein-protein interactions to the lysis procedure.
A biochemical approach was followed to study the potential interaction between polycystin-1 and desmosomal components. Cells grown on filters were lysed and desmosomes solubilized in RIPA buffer, a buffer used previously to study desmosomal protein associations (42). Immunoprecipitates of desmoglein and desmocollin were analyzed for the presence of polycystin-1 by immunoblotting (Figure 5b), but no polycystin-1 could be detected. The immunoprecipitates were positive when immunoblotted for another desmosomal protein, plakoglobin, serving as evidence that the lysis conditions used were able to preserve protein-protein interactions between known desmosomal components. Together our data show that polycystin-1 is not significantly associated with desmosomes in fully polarized cells that have well developed cell-cell contacts.
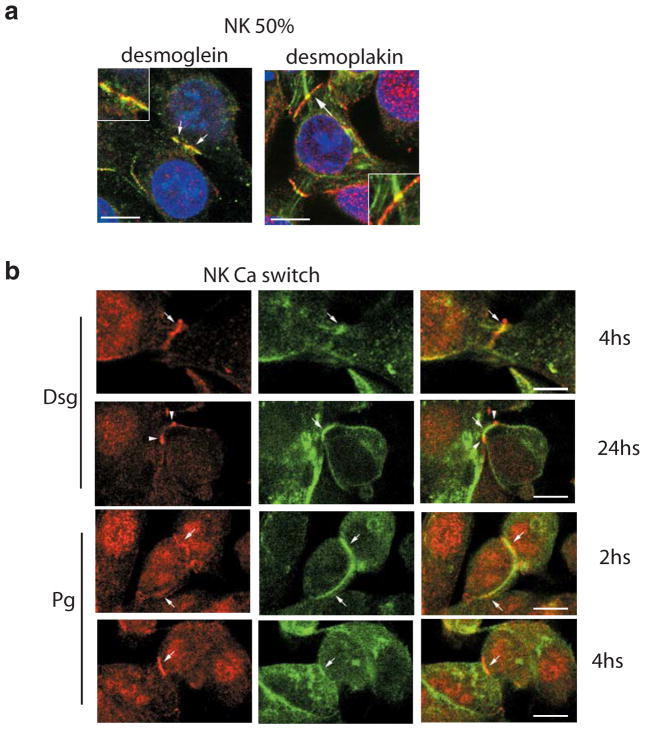
How then could our findings be reconciled with previous ultrastructural studies showing polycystin-1 associated with desmosomes? We noted that the previous studies documenting colocalization between polycystin-1 and desmosomes were performed in MDCK cells grown at subconfluence or before 3 days of full confluence (26, 43). Therefore, we focused on analyzing the dynamics of the possible colocalization between polycystin-1 and desmosomal junctions in our primary normal human kidney epithelial cells. Cells grown on filters to 50% confluence were processed for immunofluorescence and co-labeled for different desmosomal components and polycystin-1. At subconfluence, polycystin-1 indeed colocalizes with desmosomal proteins (Figure 6a). A homogenous, overlapping staining pattern for polycystin-1 and desmosomal proteins was seen at the plasma membrane, at sites of early cell-cell contact. Desmoplakin, but not polycystin-1 staining could be seen lining intermediate filaments that terminate at sites of desmosomal assembly. As an independent measure of the dynamics of polycystin-1/desmosomal colocalization, these proteins were analyzed by immunofluorescence following transfer of cells grown in low calcium to calcium-sufficient medium. The ‘calcium switch’ assay involves seeding cells at full confluence in low calcium medium to prevent cell junction assembly. The calcium-switch assay has been widely used to study cell-cell adhesion since junction assembly in this case occurs synchronously over a short period of time following transfer to normal calcium sufficient medium. Normal primary kidney cells were processed at different time points between 0 minutes and 24 h after raising the media calcium levels and co-labeled with anti-polycystin-1 antibody and antibodies against different desmosomal structures (Figure 6b). At 0 minutes after the calcium switch, the cells were rounded and desmosomal staining predominantly intracellular, while at this stage polycystin-1 was already prominent at the plasma membrane (data not shown). Polcystin-1 could be seen colocalizing with plakoglobin and desmoglein as early as 2 h after the switch to the high calcium media. At 4 h, colocalization between these structures still could be seen. No colocalization was seen after 24 h of the calcium switch, and mutually exclusive, alternating staining was evident analogous to the situation observed in fully confluent filter cultures. The conditions required to solubilize desmosomal cadherins even just 2 hours after calcium switch proved too harsh to preserve the interaction with polycystin-1 in coimmunoprecipitation studies (data not shown), though it is possible to coimmunoprecipitate polycystin-1 and the plaque protein plakoglobin (data not shown and (24)). The data suggest there is a transient, close association between polycystin-1 and desmosomes during the early steps of junctional assembly, which is lost once desmosomal maturity is achieved.
Figure 6. Polycystin-1 and desmosomal proteins transiently colocalize when normal kidney cells are at 50% confluence.
Confocal images of normal primary human kidney cells taken at early stage of cell-cell contact. Co-labeling of polycystin-1 (red) and desmosomal components (green) was carried out.
a. Cells processed at 50% confluence show an overlapping pattern (arrows) between polycystin-1 and desmoglein (left panel) or desmoplakin (right panel). Insets show a magnification of membrane region marked with arrows. Bars, 10 μm.
b. Cells were subjected to a calcium switch assay and processed at different time points after the calcium switch. Desmoglein staining (upper two rows) shows clear colocalization with polycystin-1 at 4 h following the calcium switch. This colocalization is lost after 24 h. Plakoglobin labeling (lower two rows) shows colocalization with polycystin-1 at 2 and 4 h after the switch to normal calcium. Bars, 10 μm.
Discussion
In this study we identify a key cell-cell adhesion defect in ADPKD that makes cystic epithelia increasingly vulnerable to dissociation in response to shear stress. Our data show desmosomal proteins are mispolarized to the apical domain in ADPKD cells in culture and in situ, in tissue sections. The desmosomal protein rearrangements are paralleled by an abnormal intermediate filament network in the disease cells, with decreased cytokeratin expression and abnormal expression of vimentin. Such cytoskeletal alterations are frequently hallmarks of cells undergoing an epithelial to mesenchymal transition. The combination of mispolarized desmosomal proteins and cytoskeletal alterations were found to have functional consequences for the cystic epithelial cells and made them more sensitive to dissociation in a shear stress assay. Several lines of evidence, suggest the observed changes may be coupled to the expression of mutant polycystin-1.
E-cadherin, which is intracellularly located in disease cells (30), has been demonstrated to be necessary for desmosomal assembly (34). In ADPKD cells, N-cadherin has been found to substitute for the loss of E-cadherin (25). N-cadherin, a mesenchymal cadherin, has proven as effective as E-cadherin in its capacity to stimulate desmosome assembly (44) and may account for the continued presence of desmosomal puncta at the lateral membrane of ADPKD cells. For this reason, it is unlikely that alterations in adherens junction assembly alone can account for the observed alterations in desmosome assembly in ADPKD.
Previous research has revealed mislocalization of select cell surface molecules in the disease epithelia. Na+, K+-ATPase and epidermal growth factor receptor (EGFR) have been found apically mislocalized in ADPKD (45, 46). This localization is normal in the developing kidney but not in the adult kidney where these proteins are principally at the basolateral membrane. In the case of the Na+, K+-ATPase, its mispolarization has been attributed to the continued expression of a fetal isoform of the β-subunit, which contains apical targeting information (47). Plakoglobin, a protein shared by adherens junctions and desmosomes, also exhibits an apical localization during kidney development (24). Therefore, the apical mislocalization of desmosomes and expression of an immature intermediate filament cytoskeleton in ADPKD is consistent with the prevalent idea that ADPKD is a disease where the epithelia suffer a regression to a fetal phenotype in response to the expression of mutant polycystins (1).
The abnormalities in desmosome mediated adhesion taken together with previous observations that polycystin-1 associates with desmosomes, raised the question as to how polycystin-1 may be involved in desmosome assembly. The possible colocalization of polycystin-1 with desmosomes was therefore evaluated from a dynamic point of view. Although other investigators have examined desmosome polycystin-1 interactions, the state of cell polarization was not taken in account (26) (43). Both during the transition from subconfluent to fully confluent monolayers and in the synchronous calcium-switch model, polycystin-1 was found to colocalize with desmosomes early during the establishment of cell-cell contacts. This overlap was subsequently lost and polycystin-1 was excluded from mature desmosomes. In previous studies we showed that although expressed, mutant polycystin-1 is not localized to the plasma membrane (25). Owing to the high degree of desmosomal protein insolubility, it was not possible to monitor their association with polycystin-1 by coimmunoprecipitation. Nevertheless our data are in good agreement with those of Scheffers et al (26), who showed by electron microscopy close proximity between polycystin-1 and desmoplakin in subconfluent MDCK cells. Moreover, plakoglobin was found to coimmunoprecipitate with polycystin-1 in a pancreatic cell line (24). Based on the molecular evaluation of the germline mutations harbored by our primary ADPKD cells, various mutations ranging from missense to nonsense mutations cause the loss of plasma membrane polycystin-1 (Harris, Roitbak, Rosetti, Wandinger-Ness, unpublished data) and give rise to the desmosomal phenotype shown here. Based on the published literature and the present findings we conclude that polycystin-1 is probably required at the lateral membrane, where it transiently associates with desmosomal components to promote their proper assembly into mature desmosomal junctions.
With regard to the possible role of polycystins in desmosomal junction assembly, it is interesting to consider that desmosome assembly is initially a calcium dependent process and that polycystin-1 and 2 constitute a calcium permeable channel. Once desmosomes reach maturity they lose their calcium dependence and become highly insoluble. Interestingly, polcystin-1 was present at the plasma membrane even prior to the shift from low to normal calcium (data not shown). Two hours after the calcium-shift, desmosomal components began to appear on the plasma membrane, consistent with previous studies showing that de novo synthesized desmosomal proteins account for the reestablishment of cell junctions upon transfer to normal calcium (48). Hence, polycystin-1 present at the membrane is uniquely poised to promote the assembly of mature desmosomes beginning 2 hours and complete 24 hours after the calcium-switch. It is possible that a complex of polycystin-1 with polycystin-2, disrupted in ADPKD (25), normally promotes desmosome formation and maturation through regulated, localized calcium influx. Alternatively, it has been shown recently that polycystin-1 can produce calcium currents independently of polycystin-2 channel activity (21). The Ig-domains of the extracellular PC1 domain are reported to bind their counterparts on a neighboring cell (49, 50). Therefore polycystin-1 could also be a surface sensor that signals initial cell-cell contact through calcium currents and thereby sets in motion the cell surface recruitment of adhesion molecules. Distinguishing between these postulates will require further testing.
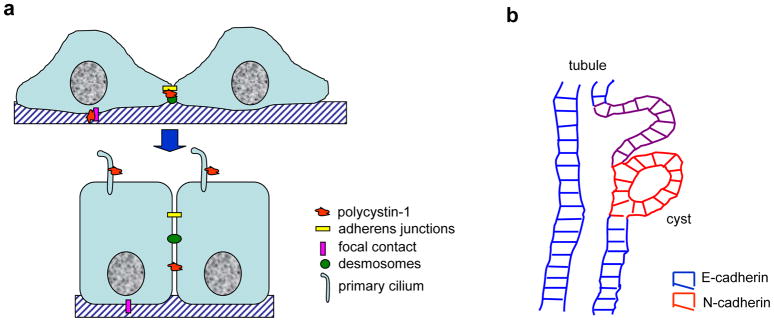
A paradox arises from the facts that ADPKD is an adult onset disease, impacting tubular epithelia after the nephrons are already developed and kidney epithelial cells normally do not divide in the adult kidney. Furthermore, in fully confluent polarized epithelial cells, polycystin-1, although still present at the lateral membrane, no longer colocalizes with desmosomes and makes its presence at the apical primary cilium (Figure 7a). In the cilia polycystin-1 and polycystin-2 serve in mechanosensory transduction and may play a role in the maintenance of cell polarity (28, 51). How then might improper desmosomal assembly contribute to disease pathogenesis in ADPKD? We envisage improper desmosome assembly may contribute at several levels. First, improper desmosome assembly may participate in cystogenesis. As kidney epithelial cells abnormally start proliferating in response to a second site mutation in the PKD1 or PKD2 genes it would dictate the need for new cell-cell junctions. At this moment the requirement for polycystin-1 function to establish normal desmosomal assembly/localization would first become evident. Even though ADPKD cells also have aberrant adherens junctions, in vitro studies suggest that the homotypic and heterotypic binding affinities between N- and E-cadherin are equivalent (33). Hence the alterations in desmosomes may weaken cell-cell contacts with their normal neighbors, so as to gradually allow the increasingly migratory N-cadherin positive cells to be extruded into the parenchyma as the normal cells attempt to reestablish a fully polarized, contiguous tubular epithelium by closing off the cyst (see cartoon, Figure 7b). Second, the increased sensitivity of cystic epithelia to shear stress may also limit tolerance to cyst fluid accumulation and cause breaks in the monolayer that predispose to infection and cyst rupture. Third, improper desmosome assembly may lead to abnormal growth control. Desmosomes, like adherens junctions, serve to anchor cytosolic plaque proteins with dual functions in cytoskeletal attachment and in cellular growth control (52, 53)). It is possible that the derangement of desmosomes interferes with the complete sequestration of desmosomal plaque proteins, leading to an enhanced cytosolic and nuclear pool of plakoglobin. Plakoglobin has the capacity to bind to the TCF/LEF transcription factor and inhibit β-catenin-mediated transcription. Plakoglobin can also compete with β-catenin for the proteasome-mediated degradative APC complex in the cytoplasm. Thus, plakoglobin mislocalization precipitated by incomplete or improper desmosome assembly could impact cell proliferation in ADPKD.
Figure 7. Models.
a. Schematic representation of our hypothesis of changing polycystin-1 distributions with cell polarization. In subconfluent, polarizing monolayers polycystin-1 is expressed at the basal and lateral membranes to facilitate the establishment of focal adhesions, as well as adherens junctions and desmosomes, possibly by mediating localized, regulated influx of calcium. Upon reaching full confluence and establishment of fully mature adhesive junctions, polycystin-1 no longer interacts with those junctions at the basolateral membranes and is relocated to the primary cilium where it is important in mechanosensory signal transduction and may participate in the maintenance of cell polarity.
b. Schematic representation of how altered cell-cell adhesion may contribute to cystogenesis. Cells suffering a second site mutation in polycystin-1 or –2 begin to proliferate, but fail to reestablish a fully polarized epithelium. Weakened cell-cell adhesion caused by improper desmosome assembly coupled with the increased migratory potential of N-cadherin expressing cystic epithelia likely are envisaged to contribute to the extrusion of the cystic cells into the underlying parenchyma. The closure of the cyst may be driven by the normal tubular epithelia attempting to reconnect with their normal counterparts to reestablish a contiguous monolayer.
In summary, polycystin-1 plays a key role in the establishment of mature desmosomal junctions and its mutation leads to defects in cell-cell adhesion that may have significant consequences in disease progression.
Acknowledgments
Studies were supported by NIDDK R01 50141, PKD Foundation 12A2R-12C2R and AHA 0040211N to AWN and subcontracts to RLB. MS is supported by PKDF fellowship 72a2f. We are indebted to the anonymous patients and their families who donated tissue to make this research possible. We gratefully acknowledge Ms. Elsa Romero for expert technical assistance and Ms. Valorie Bivins for administrative support. We thank Michael Grady for invaluable help on the scanning of the film negatives. We acknowledge Dr. Federico Bolognani for helpful discussions of the manuscript. Immunofluorescence images in this paper were generated in the Fluorescence Microscopy Facility, which received support from the NCRR (P20 RR11830; S10 RR14668; S10 RR016918), NSF MCB9982161, NCI R24 CA88339, the University of New Mexico Health Sciences Center, and the University of New Mexico Cancer Center. We thank Dr. Rebecca Lee for expert technical support in the maintenance and operation of the Cancer Center Microscopy Facility.
References
- 1.Arnaout MA. Molecular genetics and pathogenesis of autosomal dominant polycystic kidney disease. Annu Rev Med. 2001;52:93–123. doi: 10.1146/annurev.med.52.1.93. [DOI] [PubMed] [Google Scholar]
- 2.Sutters M, Germino GG. Autosomal dominant polycystic kidney disease: molecular genetics and pathophysiology. J Lab Clin Med. 2003;141:91–101. doi: 10.1067/mlc.2003.13. [DOI] [PubMed] [Google Scholar]
- 3.Grantham JJ, Geiser JL, Evan AP. Cyst formation and growth in autosomal dominant polycystic kidney disease. Kidney Int. 1987;31:1145–1152. doi: 10.1038/ki.1987.121. [DOI] [PubMed] [Google Scholar]
- 4.Carone FA, Makino H, Kanwar YS. Basement membrane antigens in renal polycystic disease. Am J Pathol. 1988;130:466–471. [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson PD. Polycystin: new aspects of structure, function, and regulation. J Am Soc Nephrol. 2001;12:834–845. doi: 10.1681/ASN.V124834. [DOI] [PubMed] [Google Scholar]
- 6.Breuning MH. Gene defect in polycystic kidney disease. Exp Nephrol. 1995;3:267–269. [PubMed] [Google Scholar]
- 7.Breuning MH, Peters DJ. Latest news on the major gene for polycystic kidney disease, PKD1. Nephrol Dial Transplant. 1995;10:1795–1796. [PubMed] [Google Scholar]
- 8.Consortium. Polycystic kidney disease: the complete structure of the PKD1 gene and its protein. The International Polycystic Kidney Disease Consortium. Cell. 1995;81:289–298. doi: 10.1016/0092-8674(95)90339-9. [DOI] [PubMed] [Google Scholar]
- 9.Hughes J, Ward CJ, Peral B, Aspinwall R, Clark K, San Millan JL, Gamble V, Harris PC. The polycystic kidney disease 1 (PKD1) gene encodes a novel protein with multiple cell recognition domains. Nat Genet. 1995;10:151–160. doi: 10.1038/ng0695-151. [DOI] [PubMed] [Google Scholar]
- 10.Kimberling WJ, Kumar S, Gabow PA, Kenyon JB, Connolly CJ, Somlo S. Autosomal dominant polycystic kidney disease: localization of the second gene to chromosome 4q13–q23. Genomics. 1993;18:467–472. doi: 10.1016/s0888-7543(11)80001-7. [DOI] [PubMed] [Google Scholar]
- 11.Wu G, D’Agati V, Cai Y, Markowitz G, Park JH, Reynolds DM, Maeda Y, Le TC, Hou H, Jr, Kucherlapati R, et al. Somatic inactivation of Pkd2 results in polycystic kidney disease. Cell. 1998;93:177–188. doi: 10.1016/s0092-8674(00)81570-6. [DOI] [PubMed] [Google Scholar]
- 12.Brook-Carter PT, Peral B, Ward CJ, Thompson P, Hughes J, Maheshwar MM, Nellist M, Gamble V, Harris PC, Sampson JR. Deletion of the TSC2 and PKD1 genes associated with severe infantile polycystic kidney disease--a contiguous gene syndrome. Nat Genet. 1994;8:328–332. doi: 10.1038/ng1294-328. [DOI] [PubMed] [Google Scholar]
- 13.Boletta A, Germino GG. Role of polycystins in renal tubulogenesis. Trends Cell Biol. 2003;13:484–492. doi: 10.1016/s0962-8924(03)00169-7. [DOI] [PubMed] [Google Scholar]
- 14.Lakkis M, Zhou J. Molecular complexes formed with polycystins. Nephron Exp Nephrol. 2003;93:e3–8. doi: 10.1159/000066648. [DOI] [PubMed] [Google Scholar]
- 15.Malhas AN, Abuknesha RA, Price RG. Interaction of the leucine-rich repeats of polycystin-1 with extracellular matrix proteins: possible role in cell proliferation. J Am Soc Nephrol. 2002;13:19–26. doi: 10.1681/ASN.V13119. [DOI] [PubMed] [Google Scholar]
- 16.Wilson PD. Polycystic kidney disease. N Engl J Med. 2004;350:151–164. doi: 10.1056/NEJMra022161. [DOI] [PubMed] [Google Scholar]
- 17.Hanaoka K, Qian F, Boletta A, Bhunia AK, Piontek K, Tsiokas L, Sukhatme VP, Guggino WB, Germino GG. Co-assembly of polycystin-1 and -2 produces unique cation-permeable currents. Nature. 2000;408:990–994. doi: 10.1038/35050128. [DOI] [PubMed] [Google Scholar]
- 18.Koulen P, Cai Y, Geng L, Maeda Y, Nishimura S, Witzgall R, Ehrlich BE, Somlo S. Polycystin-2 is an intracellular calcium release channel. Nat Cell Biol. 2002;4:191–197. doi: 10.1038/ncb754. [DOI] [PubMed] [Google Scholar]
- 19.Qian F, Germino FJ, Cai Y, Zhang X, Somlo S, Germino GG. PKD1 interacts with PKD2 through a probable coiled-coil domain. Nat Genet. 1997;16:179–183. doi: 10.1038/ng0697-179. [DOI] [PubMed] [Google Scholar]
- 20.Tsiokas L, Kim E, Arnould T, Sukhatme VP, Walz G. Homo- and heterodimeric interactions between the gene products of PKD1 and PKD2. Proc Natl Acad Sci U S A. 1997;94:6965–6970. doi: 10.1073/pnas.94.13.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Babich V, Zeng WZ, Yeh BI, Ibraghimov-Beskrovnaya O, Cai Y, Somlo S, Huang CL. The amino-terminal extracellular domain is required for polycystin-1-dependent channel activity. J Biol Chem. 2004 doi: 10.1074/jbc.M402829200. [DOI] [PubMed] [Google Scholar]
- 22.Wilson PD, Geng L, Li X, Burrow CR. The PKD1 gene product, “polycystin-1,” is a tyrosine-phosphorylated protein that colocalizes with alpha2beta1-integrin in focal clusters in adherent renal epithelia. Lab Invest. 1999;79:1311–1323. [PubMed] [Google Scholar]
- 23.Ibraghimov-Beskrovnaya O, Dackowski WR, Foggensteiner L, Coleman N, Thiru S, Petry LR, Burn TC, Connors TD, Van Raay T, Bradley J, et al. Polycystin: in vitro synthesis, in vivo tissue expression, and subcellular localization identifies a large membrane-associated protein. Proc Natl Acad Sci U S A. 1997;94:6397–6402. doi: 10.1073/pnas.94.12.6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huan Y, van Adelsberg J. Polycystin-1, the PKD1 gene product, is in a complex containing E-cadherin and the catenins. J Clin Invest. 1999;104:1459–1468. doi: 10.1172/JCI5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roitbak T, Ward CJ, Harris PC, Bacallao R, Ness SA, Wandinger-Ness A. A polycystin-1 multiprotein complex is disrupted in polycystic kidney disease cells. Mol Biol Cell. 2004;15:1334–1346. doi: 10.1091/mbc.E03-05-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scheffers MS, van der Bent P, Prins F, Spruit L, Breuning MH, Litvinov SV, de Heer E, Peters DJ. Polycystin-1, the product of the polycystic kidney disease 1 gene, co-localizes with desmosomes in MDCK cells. Hum Mol Genet. 2000;9:2743–2750. doi: 10.1093/hmg/9.18.2743. [DOI] [PubMed] [Google Scholar]
- 27.Cantiello HF. A tale of two tails: ciliary mechanotransduction in ADPKD. Trends Mol Med. 2003;9:234–236. doi: 10.1016/s1471-4914(03)00073-x. [DOI] [PubMed] [Google Scholar]
- 28.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 29.Yoder BK, Hou X, Guay-Woodford LM. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol. 2002;13:2508–2516. doi: 10.1097/01.asn.0000029587.47950.25. [DOI] [PubMed] [Google Scholar]
- 30.Charron AJ, Nakamura S, Bacallao R, Wandinger-Ness A. Compromised cytoarchitecture and polarized trafficking in autosomal dominant polycystic kidney disease cells. J Cell Biol. 2000;149:111–124. doi: 10.1083/jcb.149.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim E, Arnould T, Sellin LK, Benzing T, Fan MJ, Gruning W, Sokol SY, Drummond I, Walz G. The polycystic kidney disease 1 gene product modulates Wnt signaling. J Biol Chem. 1999;274:4947–4953. doi: 10.1074/jbc.274.8.4947. [DOI] [PubMed] [Google Scholar]
- 32.Huen AC, Park JK, Godsel LM, Chen X, Bannon LJ, Amargo EV, Hudson TY, Mongiu AK, Leigh IM, Kelsell DP, et al. Intermediate filament-membrane attachments function synergistically with actin-dependent contacts to regulate intercellular adhesive strength. J Cell Biol. 2002;159:1005–1017. doi: 10.1083/jcb.200206098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niessen CM, Gumbiner BM. Cadherin-mediated cell sorting not determined by binding or adhesion specificity. J Cell Biol. 2002;156:389–399. doi: 10.1083/jcb.200108040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lewis JE, Wahl JK, 3rd, Sass KM, Jensen PJ, Johnson KR, Wheelock MJ. Cross-talk between adherens junctions and desmosomes depends on plakoglobin. J Cell Biol. 1997;136:919–934. doi: 10.1083/jcb.136.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Runswick SK, O’Hare MJ, Jones L, Streuli CH, Garrod DR. Desmosomal adhesion regulates epithelial morphogenesis and cell positioning. Nat Cell Biol. 2001;3:823–830. doi: 10.1038/ncb0901-823. [DOI] [PubMed] [Google Scholar]
- 36.Tselepis C, Chidgey M, North A, Garrod D. Desmosomal adhesion inhibits invasive behavior. Proc Natl Acad Sci U S A. 1998;95:8064–8069. doi: 10.1073/pnas.95.14.8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calautti E, Cabodi S, Stein PL, Hatzfeld M, Kedersha N, Paolo Dotto G. Tyrosine phosphorylation and src family kinases control keratinocyte cell-cell adhesion. J Cell Biol. 1998;141:1449–1465. doi: 10.1083/jcb.141.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amagai M, Fujimori T, Masunaga T, Shimizu H, Nishikawa T, Shimizu N, Takeichi M, Hashimoto T. Delayed assembly of desmosomes in keratinocytes with disrupted classic-cadherin-mediated cell adhesion by a dominant negative mutant. J Invest Dermatol. 1995;104:27–32. doi: 10.1111/1523-1747.ep12613462. [DOI] [PubMed] [Google Scholar]
- 39.Kartenbeck J, Schwechheimer K, Moll R, Franke WW. Attachment of vimentin filaments to desmosomal plaques in human meningiomal cells and arachnoidal tissue. J Cell Biol. 1984;98:1072–1081. doi: 10.1083/jcb.98.3.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Osborn M, Franke W, Weber K. Direct demonstration of the presence of two immunologically distinct intermediate-sized filament systems in the same cell by double immunofluorescence microscopy. Vimentin and cytokeratin fibers in cultured epithelial cells. Exp Cell Res. 1980;125:37–46. doi: 10.1016/0014-4827(80)90186-x. [DOI] [PubMed] [Google Scholar]
- 41.Stenn KS, Link R, Moellmann G, Madri J, Kuklinska E. Dispase, a neutral protease from Bacillus polymyxa, is a powerful fibronectinase and type IV collagenase. J Invest Dermatol. 1989;93:287–290. doi: 10.1111/1523-1747.ep12277593. [DOI] [PubMed] [Google Scholar]
- 42.Windoffer R, Borchert-Stuhltrager M, Leube RE. Desmosomes: interconnected calcium-dependent structures of remarkable stability with significant integral membrane protein turnover. J Cell Sci. 2002;115:1717–1732. doi: 10.1242/jcs.115.8.1717. [DOI] [PubMed] [Google Scholar]
- 43.Bukanov NO, Husson H, Dackowski WR, Lawrence BD, Clow PA, Roberts BL, Klinger KW, Ibraghimov-Beskrovnaya O. Functional polycystin-1 expression is developmentally regulated during epithelial morphogenesis in vitro: downregulation and loss of membrane localization during cystogenesis. Hum Mol Genet. 2002;11:923–936. doi: 10.1093/hmg/11.8.923. [DOI] [PubMed] [Google Scholar]
- 44.Parker HR, Li Z, Sheinin H, Lauzon G, Pasdar M. Plakoglobin induces desmosome formation and epidermoid phenotype in N-cadherin-expressing squamous carcinoma cells deficient in plakoglobin and E-cadherin. Cell Motil Cytoskeleton. 1998;40:87–100. doi: 10.1002/(SICI)1097-0169(1998)40:1<87::AID-CM8>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 45.Wilson PD, Sherwood AC, Palla K, Du J, Watson R, Norman JT. Reversed polarity of Na(+) -K(+) -ATPase: mislocation to apical plasma membranes in polycystic kidney disease epithelia. Am J Physiol. 1991;260:F420–430. doi: 10.1152/ajprenal.1991.260.3.F420. [DOI] [PubMed] [Google Scholar]
- 46.Du J, Wilson PD. Abnormal polarization of EGF receptors and autocrine stimulation of cyst epithelial growth in human ADPKD. Am J Physiol. 1995;269:C487–495. doi: 10.1152/ajpcell.1995.269.2.C487. [DOI] [PubMed] [Google Scholar]
- 47.Wilson PD, Devuyst O, Li X, Gatti L, Falkenstein D, Robinson S, Fambrough D, Burrow CR. Apical plasma membrane mispolarization of NaK-ATPase in polycystic kidney disease epithelia is associated with aberrant expression of the beta2 isoform. Am J Pathol. 2000;156:253–268. doi: 10.1016/s0002-9440(10)64726-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burdett ID, Sullivan KH. Desmosome assembly in MDCK cells: transport of precursors to the cell surface occurs by two phases of vesicular traffic and involves major changes in centrosome and Golgi location during a Ca(2+) shift. Exp Cell Res. 2002;276:296–309. doi: 10.1006/excr.2002.5509. [DOI] [PubMed] [Google Scholar]
- 49.Streets AJ, Newby LJ, O’Hare MJ, Bukanov NO, Ibraghimov-Beskrovnaya O, Ong AC. Functional analysis of PKD1 transgenic lines reveals a direct role for polycystin-1 in mediating cell-cell adhesion. J Am Soc Nephrol. 2003;14:1804–1815. doi: 10.1097/01.asn.0000076075.49819.9b. [DOI] [PubMed] [Google Scholar]
- 50.Ibraghimov-Beskrovnaya O, Bukanov NO, Donohue LC, Dackowski WR, Klinger KW, Landes GM. Strong homophilic interactions of the Ig-like domains of polycystin-1, the protein product of an autosomal dominant polycystic kidney disease gene, PKD1. Hum Mol Genet. 2000;9:1641–1649. doi: 10.1093/hmg/9.11.1641. [DOI] [PubMed] [Google Scholar]
- 51.Ong AC, Wheatley DN. Polycystic kidney disease--the ciliary connection. Lancet. 2003;361:774–776. doi: 10.1016/S0140-6736(03)12662-1. [DOI] [PubMed] [Google Scholar]
- 52.Green KJ, Gaudry CA. Are desmosomes more than tethers for intermediate filaments? Nat Rev Mol Cell Biol. 2000;1:208–216. doi: 10.1038/35043032. [DOI] [PubMed] [Google Scholar]
- 53.Getsios S, Huen AC, Green KJ. Working out the strength and flexibility of desmosomes. Nat Rev Mol Cell Biol. 2004;5:271–281. doi: 10.1038/nrm1356. [DOI] [PubMed] [Google Scholar]