Abstract
Objectives
Tenofovir (TDF) is increasingly used in second-line antiretroviral treatment (ART) in sub-Saharan Africa. We compared outcomes of second-line ART containing and not containing TDF in cohort studies from Zambia and the Republic of South Africa (RSA).
Methods
Patients aged ≥ 16 years starting protease inhibitor-based second-line ART in Zambia (1 cohort) and RSA (5 cohorts) were included. We compared mortality, immunological failure (all cohorts) and virological failure (RSA only) between patients receiving and not receiving TDF. Competing risk models and Cox models adjusted for age, sex, CD4 count, time on first-line ART and calendar year were used to analyse mortality and treatment failure, respectively. Hazard ratios (HRs) were combined in fixed-effects meta-analysis.
Findings
1,687 patients from Zambia and 1,556 patients from RSA, including 1,350 (80.0%) and 206 (13.2%) patients starting TDF, were followed over 4,471 person-years. Patients on TDF were more likely to have started second-line ART in recent years, and had slightly higher baseline CD4 counts than patients not on TDF. Overall 127 patients died, 532 were lost to follow-up and 240 patients developed immunological failure. In RSA 94 patients had virologic failure. Combined HRs comparing tenofovir with other regimens were 0.60 (95% CI 0.41–0.87) for immunologic failure and 0.63 (0.38–1.05) for mortality. The HR for virologic failure in RSA was 0.28 (0.09–0.90).
Conclusions
In this observational study patients on TDF-containing second-line ART were less likely to develop treatment failure than patients on other regimens. TDF seems to be an effective component of second-line ART in southern Africa.
Keywords: Tenofovir, second-line antiretroviral therapy, southern Africa, treatment failure, mortality
INTRODUCTION
Despite the unprecedented scale-up of antiretroviral treatment (ART) in resource-constrained settings, the proportion of patients switching to second-line ART after failing a first-line regimen is low in many resource-limited countries. Earlier detection of treatment failure and switching to second-line protease-inhibitor (PI)-based ART probably reduces mortality 2, but second-line regimens remain considerably more expensive than first line regimens. Only few studies have described clinical outcomes of patients on second-line therapy in sub-Saharan Africa 3–6.
As genotypic drug-resistance testing is not routinely available in the region, the World Health Organization (WHO) recommends the use of standardized second-line ART consisting of a ritonavir-boosted PI plus two nucleoside reverse transcriptase inhibitors (NRTIs). The NRTI backbone should include at least one new agent. Tenofovir (TDF) is increasingly used as a component of second-line ART in patients not previously exposed to this drug. In southern Africa TDF has only recently been introduced for use in first-line ART and the majority of patients failing their first-line regimen are therefore eligible to receive this drug in second-line ART. Although studies from Europe and North America showed favorable clinical outcomes in patients treated with TDF-containing salvage ART8,9, outcomes of second-line regimens containing and not containing TDF have not been compared so far in southern Africa.
HIV-1 subtype C variant represents approximately 50% of global HIV infections and is most prevalent in southern Africa. The K65R mutation, which is associated with TDF resistance, is more frequent in HIV-1 subtype C compared to subtype B viruses, especially when suboptimal first-line regimens including stavudine (D4T) or didanosine (ddI) are used 10–12. A study from Malawi showed that 23% of patients failing first-line ART developed the K65R mutation even without prior exposure to TDF 11. In South Africa, where routine viral load monitoring shortens the time patients spend on failing first-line regimens, the proportion of patients with this mutation was much lower 13–15. In Malawi clinical outcomes after one year were not affected by resistance 6.
We compared outcomes in patients receiving TDF-containing second-line ART with those on other second-line regimens in a collaborative analysis of six cohorts in Zambia and the Republic South Africa (RSA).
METHODS
Antiretroviral treatment programmes
The International epidemiological Databases to Evaluate AIDS in Southern Africa (IeDEA-SA) are a regional collaboration of ART programmes 16. Data are collected at ART initiation (baseline) and each follow-up visit, using standardized instruments, and transferred to data centres at the Universities of Cape Town, Republic of South Africa (RSA) and Bern, Switzerland. All sites have ethical approval to collect data and to participate in IeDEA-SA.
We included all cohorts with more than 50 patients on second-line ART, and 10 or more patients on TDF and not on TDF. Six cohorts met inclusion criteria: the Centre for Infectious Disease Research in Zambia (MoH-CIDRZ) programme in Lusaka, Zambia and five cohorts from RSA: Aurum Institute (community and workplace ART program) and Themba Lethu clinic in Johannesburg, and the Khayelitsha and Tygerberg ART programs in Cape Town. In RSA viral load and CD4 cell counts are monitored every 6 months during the first year of ART and then yearly. In Zambia CD4 counts are monitored every 6 months but viral load measurements are not routinely performed. All treatment programmes trace patients lost to follow-up.
Eligibility criteria
All patients aged 16 years and older who started a second-line ART regimen were included. We defined second-line regimens according to the most recent WHO treatment guidelines as a boosted PI-based regimen, which followed a first-line regimen of one NNRTI and two NRTIs. At least one component of the NRTI backbone had to be replaced by a drug characterised by different resistance mutations pathways. For example, a change from lamivudine (3TC)/D4T to 3TC/zidovudine (AZT) was not considered an eligible backbone change. Patients with ineligible backbone changes and patients treated with a TDF-containing first-line regimen were excluded. The selection of study participants in Zambia and the Republic of South Africa are shown in Webfigures 1 and 2.
Outcomes
We examined time to immunological failure, time to virological failure and time to death, defining treatment failure as proposed by WHO. Briefly, there are 3 possible criteria for immunological failure: (i) a fall of CD4 count to baseline or below, (ii) a 50% fall from on-treatment peak value and (iii) persistent CD4 count levels below 100 cells/μl. Patients were considered to experience immunological failure if at least one of the 3 criteria were fulfilled on two consecutive CD4 cell measurements within 1 year. Virological failure, defined as 2 consecutive viral load measurements above 5,000 copies/ml within a year, was assessed in the South African cohorts.
Statistical analyses
Characteristics at the start of second-line ART were compared between patients on second-line regimens containing and not containing TDF using chi-squared and Mann-Whitney tests. We compared rates of immunological failure and virological failure in Cox regression models, measuring time from 6 months after switching to second-line ART. We used competing risk cumulative incidence curves 17 and competing risk regression models according to Fine and Gray 18 to compare mortality, measuring time from switching to second-line ART. Standard Kaplan-Meier curves ignore the competing risks of death and LTFU and may produce biased results19. All regression models included the variables gender, age (16–29, 30–39 or 40 years and over), CD4 cell count (0–49, 50–99, 100–199, over 200 cells/μl or “not measured”) at the start of second-line ART, time on first-line before switching to second-line ART (less than 18, 18–36 or over 36 months) and calendar year of starting second-line ART (before 2007, 2007, 2008, 2009 and 2010).
All analyses were done separately for Zambia and RSA. Sub-distribution hazard ratios (sHR) and hazard ratios (HR) were then combined in (inverse variance weighted) fixed-effects meta-analysis and shown in a stratified forest plot. Finally, in order to assess the effect of the first-line backbone on second-line outcomes, we examined whether the use of D4T in the first-line ART regimen predicted immunological failure in patients on TDF-containing second-line regimen. All statistical analyses were performed using Stata software version 11 (College Station, Texas, USA).
RESULTS
ART programmes and patients characteristics
Table 1 shows the composition of cohorts. A total of 3,243 patients on second-line ART, including 1,556 (48.0%) on a TDF-containing regimen were included in the analyses. The majority of patients were female in all cohorts except the workplace cohort in South Africa, which was dominated by male miners. The median age ranged from 32 years in Khayelitsha to 45 years in the Aurum workplace cohort. In Zambia, 80% of patients were on TDF-based second-line ART whereas in RSA this percentage ranged from 4% to 25%. Crude mortality rates were similar across South African cohorts except for the Aurum community cohort for which mortality was considerably lower, probably due to under ascertainment of deaths. Such under ascertainment may also explain the lower mortality in Zambia compared to RSA.
Table 1.
Characteristics of participating antiretroviral therapy (ART) programmes.
| Sites | No. of patients | Female (%) | Median age in years (IQR) | No. on second-line ART with TDF (%) | Viral load monitoring | Follow-up time on second-line ART (py) | Mortality (95% CI) (per 1,000 py) | LTFU (95% CI) (per 1,000 py) |
|---|---|---|---|---|---|---|---|---|
| South Africa | ||||||||
| Aurum-C | 323 | 209 (65) | 38 (32–44) | 80 (25) | Yes | 309 | 6.5 (1.6–25.9) | 145.7 (108.8–195.1) |
| Aurum-W | 262 | 15 (6) | 45 (38–51) | 10 (4) | Yes | 331 | 45.2 (27.3–75.0) | 165.8 (127.3–216.0) |
| Khayelitsha | 197 | 144 (73) | 32 (28–40) | 13 (7) | Yes | 227 | 30.8 (14.7–64.7) | 61.7 (36.5–104.1) |
| Themba Lethu | 562 | 341 (61) | 36 (32–43) | 81 (14) | Yes | 766 | 35.3 (24.2–51.4) | 105.8 (85.1–131.5) |
| Tygerberg | 212 | 139 (66) | 35 (31–42) | 22 (10) | Yes | 329 | 39.5 (22.9–68.0) | 54.6 (34.4–86.7) |
| Zambia | ||||||||
| CIDRZ | 1,687 | 954 (57) | 38 (33–45) | 1,350 (80) | No | 2,508 | 25.1 (19.6–32.2) | 127.2 (114.0–141.9) |
| Total | 3,243 | 1,802 (55.6) | 38 (32–45) | 1,556 (48.0) | 4,471 | 28.4 (23.9–33.8) | 119.0 (109.3–129.5) | |
Py, person-years; LTFU, loss to follow-up; Aurum-C, Aurum Community cohort; Aurum-W, Aurum workplace cohort.
Both in Zambia and RSA, the proportion of patients on a TDF containing second-line regimen increased over the years, with the exception of a slight decrease in Zambia in 2010 (Table 2). The median age at start of second-line ART was higher in patients on TDF in Zambia, but identical in both treatment groups in RSA. Conversely, the sex distribution was similar in Zambia whereas in RSA, women were more likely to start a TDF containing regimen than men. In both countries, patients receiving TDF-containing regimens had higher CD4 cell counts and had spent more time on their first-line regimen before switching to second-line ART than those on other regimens. Most patients (3,225 patients; 99.4%) were treated with second-line regimens containing ritonavir-boosted lopinavir (LPV/r) and 1,468 (87.0%) of the patients not on a TDF-containing second-line regimen had a backbone of DDI/AZT or DDI/ABC.
Table 2.
Patient characteristics at start of second-line antiretroviral therapy
| All patients (N=3,243) | Zambia
|
South Africa
|
|||||
|---|---|---|---|---|---|---|---|
| Tenofovir (N=1,350) | No Tenofovir (N=337) | P | Tenofovir (N=206) | No Tenofovir (N=1,350) | P | ||
| Female gender (%) | 1,802 (55.6) | 757 (56.1) | 197 (58.5) | 0.43 | 137 (66.5) | 711 (52.7) | <0.001 |
| Median age at start (IQR) | 38 (32–45) | 39 (33–45) | 37 (32–43) | 0.003 | 37 (32–43) | 38 (32–45) | 0.37 |
| Median CD4 count at start (IQR) | 172 (95–267) | 161 (92–261) | 135 (77–242) | 0.05 | 206 (97–339) | 185 (103–270) | 0.05 |
| missing (%) | 349 (10.8) | 183 (13.6) | 46 (13.6) | 23 (11.1) | 97 (7.2) | ||
| Median time on first-line ART in months (IQR) | 27 (17–38) | 33 (23–43) | 27 (18–35) | <0.001 | 24 (15–34) | 22 (14–32) | 0.04 |
| Calendar year of second-line ART start (%) | <0.001 | <0.001 | |||||
| Before 2007 | 405 (12.5) | 5 (0.4) | 53 (15.7) | 22 (10.7) | 325 (24.1) | ||
| 2007 | 695 (21.4) | 164 (12.2) | 122 (36.2) | 32 (15.5) | 377 (27.9) | ||
| 2008 | 909 (28.0) | 445 (33.0) | 57 (16.9) | 44 (21.4) | 363 (26.9) | ||
| 2009 | 889 (27.4) | 487 (36.1) | 59 (17.5) | 86 (41.8) | 257 (19.0) | ||
| 2010 | 345 (10.6) | 249 (18.4) | 46 (13.7) | 22 (10.7) | 28 (2.1) | ||
| Most common second-line ART backbone (%) | FTC/TDF (37.5) | FTC/TDF (85.8) | DDI/ABC (79.8) | TDF/FTC (28.4) | DDI/AZT (71.1) | ||
| DDI/AZT (29.8) | 3TC/TDF (8.0) | ABC/3TC (16.6) | 3TC/TDF (26.9) | DDI/ABC (17.5) | |||
| DDI/ABC (15.6) | FTC/AZT/TDF (5.9) | DDI/3TC (1.2) | AZT/3TC/TDF (12.2) | DDI/D4T (2.4) | |||
Categorical variables are compared with chi-squared, continuous variables with Mann-Whitney tests. Viral load is routinely monitored in South Africa only. IQR; interquartile range. FTC: emtricitabine; TDF: tenofovir; DDI: didanosine; AZT: zidovudine; ABC: abacavir; 3TC: lamivudine
Descriptive analyses of treatment failure, mortality and LTFU
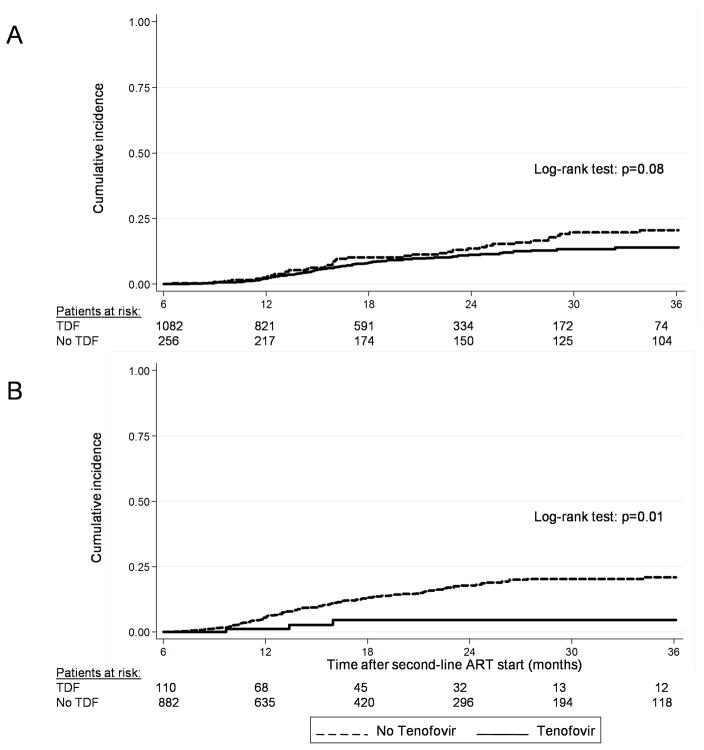
Analyses of immunological treatment failure were based on 2,330 patients (71.8% of total study population) with at least six months of follow-up after starting second-line ART. Virological failure was examined in 992 patients (63.8% of patients treated in RSA). Over 2,782 person-years, 94 patients (7.9%) on TDF and 146 patients (12.8%) on other second-line regimens developed immunological failure. The crude incidence rate of immunological failure was 69.9 (95% CI 57.1–85.6) per 1,000 person-years in the TDF group and 101.6 (86.4–119.4) per 1,000 person-years in the other group. In South Africa, three patients (2.7%) in the TDF group and 107 patients (12.1%) in the group without TDF experienced virological failure. Figure 1 shows the Kaplan-Meier curves of immunological failure in Zambia and virological failure in South Africa, by treatment group.
FIGURE 1.
Cumulative incidence of immunological failure in Zambia (A) and virological failure in the Republic of South Africa (B) during the first three years of second-line ART. TDF, tenofovir.
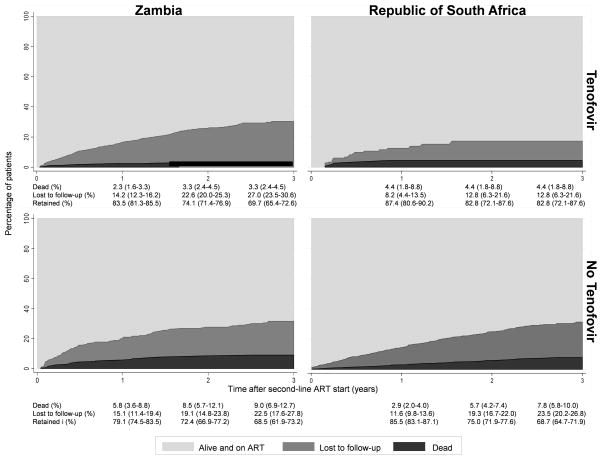
Over 4,471 person-years, 127 patients (3.9%) died and 532 (16.4%) were LTFU (Table 1). Crude rates per 1000 person-years ranged from 6.5 (95% CI 1.6–25.9) to 45.2 (27.3–75.0) for mortality and from 54.6 (34.4–86.7) to 165.8 (127.3–216.0) for LTFU. Figure 2 shows the cumulative incidence of mortality and LTFU by country and type of second-line ART from the competing risk analysis. At 3 years, 3.3% of patients (95% CI 2.3–4.5%) in the TDF group in Zambia and 4.4% (95% CI 1.8–8.8) in South Africa were known to have died. These proportions were higher in the groups treated without TDF: 9.0% (95% CI 6.9–12.7%) in Zambia and 7.8% (95% CI 5.8–10.00) in South Africa. LTFU at 3 years was higher in Zambia than in RSA. In Zambia, LTFU was somewhat higher in patients on TDF compared to patients not on TDF whereas the opposite was observed in RSA: LTFU was lower in patients on TDF compared to other patients (Figure 2). These analyses were not adjusted for differences in patient characteristics at the start of second-line ART and therefore have to be interpreted with caution.
FIGURE 2.
Retention in care by second-line ART category and country.
Regression analyses of treatment failure and mortality
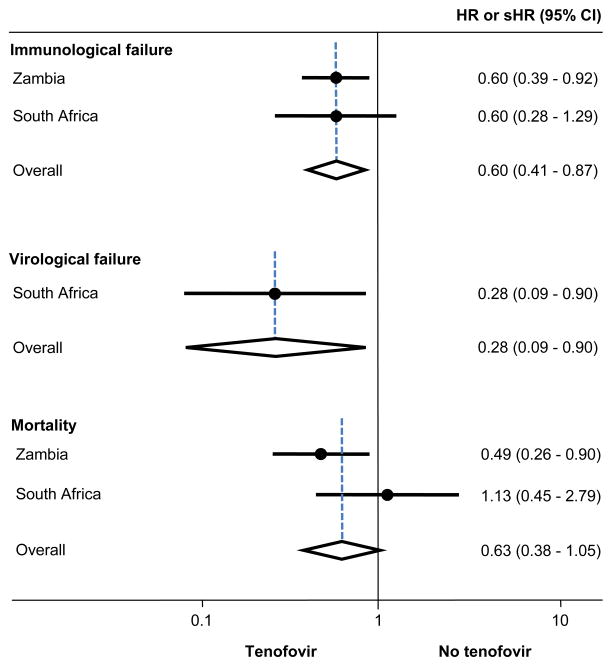
Figure 3 presents the results from the Cox and competing risk regression analyses adjusted for age, sex, CD4 count, time on first-line ART and calendar year, and meta-analyses of these estimates. Results for immunological failure were closely similar in Zambia and RSA (p from test of heterogeneity 0.99), with a combined HR comparing TDF with other regimens of 0.60 (95% CI 0.41–0.87). Similarly, the hazard of virologic failure was reduced with TDF in RSA: HR 0.22 (95% CI 0.07–0.71). Mortality was lower in patients on a TDF-containing regimen compared to those on other regimens in Zambia but not in RSA. However, confidence intervals overlapped and the test of heterogeneity was not statistically significant (p=0.13). The combined subdistribution HR for mortality was 0.63 (95% CI 0.38–1.05).
FIGURE 3.
The risk of treatment failure and death on secondline ART containing and not containing tenofovir in Zambia and the Republic of South Africa. The estimates shown are hazard ratios (HR) for immunological and virological failure and subdistribution hazard ratios (sHR) for mortality.
Results for all variables included in the models are shown in webtable 1 for Zambia and webtable 2 for RSA. In both settings, male patients and those under 30 years of age were more likely to experience treatment failure. Time spent on first-line ART before switching to a second-line regimen did not affect outcomes. Finally, in patients on TDF-containing second-line regimens, the risk of second-line immunological failure in the TDF-group was slightly increased if D4T was used in the first-line backbone, however, confidence intervals around the HR (adjusted for all variables listed above) were wide and included both a decrease and increase of the risk of failure: HR 1.30 (95% CI 0.84–2.02).
DISCUSSION
Even though several countries in southern Africa have introduced TDF in first-line ART, most patients who will be failing their first-line regimen in the coming years will not have been exposed to TDF. As a consequence, these patients might benefit from this drug in their second-line regimen. The comparative effectiveness of second-line regimens including or excluding TDF in southern Africa is therefore of great interest. We compared clinical outcomes between patients receiving TDF-containing second-line ART and patients treated with other second-line regimens in six ART programmes in Zambia and RSA. Overall, mortality and the rate of treatment failure were low in this population, underlining the benefit of PI-based second-line ART in patients failing first-line treatment in the region20. In Zambia, LTFU was similar in patients on second-line ART containing and not containing TDF, but mortality and immunological failure were lower in patients on TDF. In the five South African cohorts with access to routine viral load monitoring the rate of virological failure was also lower in the TDF group.
In contrast to Zambia the use of TDF was not associated with reduced mortality in South Africa. This finding could be the result of differences in the capacity of the health system in South Africa compared to Zambia or reflect differences in ascertainment of deaths and tracing of patients LTFU. Confounding by indication could be another explanation: the relatively few patients who were prescribed TDF in South Africa before 2010 might have been a selected group of sicker patients. Finally, the difference between the two countries could reflect the play of chance: the confidence intervals overlapped widely and a formal test of heterogeneity gave a p value of 0.13. We can thus not exclude a similar reduction in mortality in RSA.
In both groups mortality after one year of second-line ART was somewhat lower than the 5.4% mortality observed after a median of 15.1 months in the Médecins sans Frontiéres (MSF) multi-cohort study of patients on second-line ART 3. In contrast, in a study of patients virologically failing first-line ART in Malawi, 10% of patients on second-line ART died during the first six months 6. The higher mortality in the latter study might be explained by the presence of virological failure in all patients and the very low median CD4 count at the start of second-line ART. Furthermore, patients who are treated in settings without access to routine viral load monitoring are at risk of remaining on failing first-line regimens for long periods before switching to second-line ART2,21, and of accumulating drug resistance mutations which might limit the efficacy of some second line regimens11.
Studies from different regions in sub-Saharan Africa showed a high prevalence of TDF-related resistance mutations in patients failing first-line ART 10,11,22. This raised concerns on the efficacy of TDF in second-line regimens for populations infected with subtype C HIV-1 variants. In high-income countries, the K65R mutation is present only in 2–5% of HIV-1 subtype B infected patients failing first-line ART 23. In contrast, over 20% of patients failing first-line ART in an urban public-sector ART clinic in Malawi had developed this resistance mutation, without prior exposure to TDF 11. Interestingly, in the Malawian study, and the PharmAccess African Studies to Evaluate Resistance (PASER), clinical outcomes one year after initiation of second-line ART were not affected by resistance to TDF 6,24. Another report from PASER nevertheless argued that in light of the high prevalence of the K65R mutation in patients failing a D4T-containing regimen, AZT might be a better option for second-line ART than TDF12. Prolonged treatment with a failing D4T-containing first-line regimen might explain the high levels of TDF resistance mutations in the region 23. We found little evidence for an association of the risk of second-line treatment failure with the presence of D4T in the first-line regimen, however, the power of our study to detect smaller effects was limited.
There are several possible explanations for the superior effect of TDF in second-line ART in Zambia and South Africa. More than 80% of the patients not on TDF were treated with either DDI/AZT or DDI/ABC as the NRTI backbone. Due to its better tolerability and once-daily dosing, treatment adherence might be higher in patients receiving TDF compared to other NRTI combinations, especially those including DDI: the higher toxicity of DDI-based regimens might have led to poorer adherence. Wallis et al. and Van Zyl et al. reported a low prevalence of PI mutations in patients failing second-line ART in the Republic of South Africa 25,26, indicating that failure was due to insufficient drug levels following non-adherence, rather than resistance. Finally, the high potency of LPV/r monotherapy in patients without prior PI exposure could have masked larger differences in treatment outcomes between the two groups 27,28. Patients on TDF-containing second-line regimens might have had favourable outcomes despite potential NRTI mutations, including thymidine-analogue mutations (TAM’s) and K65R. Of note, the difference in treatment failure between the two second-line regimens emerged already after one year in RSA, whereas it was only apparent later during follow-up in Zambia. This could be explained by the earlier diagnosis of treatment failure with virological monitoring in RSA compared to CD4 monitoring in Zambia.
To our knowledge this is the first study to compare second-line regimens in southern Africa. In particular, there are no randomized trials of second-line ART tailored to regions where non-B HIV subtypes dominate. The main limitation of multi-cohort data comparing treatments lies in the lack of randomization and the heterogeneity between the different treatment sites. Confounding by indication and differences between settings in background mortality, monitoring and treatment strategies, and health systems may have biased our results. Of note, the proportion of patients on a TDF-containing second-line regimen varied widely across countries and calendar time, reflecting national treatment guidelines. However, the association of TDF with reduced rates of treatment failure was consistent within countries and cohorts, which adds strength to our findings. Furthermore, over 99% of the patients received the same PI, thus effectively removing one potential source of confounding. We had no data on treatment adherence, which is known to influence ART outcomes29–31. Young age and male gender were risk factors for second-line failure, probably as a consequence of the lower adherence to ART in younger patients and men 32,33. Finally, as no genotypic resistance data is routinely collected in southern Africa, we could not assess the relationship between treatment failure, resistance patterns and clinical outcomes.
We did not evaluate toxicity and side-effects related to the different regimen. Most patients on non-TDF second-line ART had DDI in their backbone. The toxicities of DDI, including lipodystrophy, gastrointestinal intolerance, peripheral neuropathy and pancreatitis, will have influenced our results.34,35 TDF is associated with nephrotoxicity, including an increased risk of loss of kidney function, acute renal failure and tubulopathy36,37. A recent study from South Africa showed that pre-existing renal disease was frequently exacerbated by the use of tenofovir38. Furthermore, patients on PI-based regimens may be at increased risk of renal failure39. Screening for renal dysfunction before the initiation of TDF-containing regimen and close monitoring during treatment is part of treatment guidelines and should be performed routinely.
In conclusion, we found that patients on TDF-containing second-line ART were less likely to develop treatment failure in all cohorts and less likely to die in Zambia than patients on other regimens. Despite the increased prevalence of TDF-related resistance mutations in patients failing first-line ART in southern Africa, TDF seems to be an effective component of second-line ART for many patients who have not been exposed to this drug previously. This finding is of considerable importance, as an increasing number of TDF-unexposed patients failing their first-line treatment will be switched to TDF-containing second-line regimen in the coming years. Second-line ART is becoming more available in sub-Saharan Africa, but most ART programmes in the region do not have access to individual genotypic resistance data. Thus, randomized trials comparing the efficacy and toxicities of different second-line regimens are urgently needed to inform clinical practice and guidelines.
Supplementary Material
Acknowledgments
We thank all study participants and staff of all participating sites. This study was supported by the National Institute of Allergy and Infectious Diseases (grant 1 U01AI069924–01).
Footnotes
Conflicts of Interest: None reported.
Presented in part at the 13th European AIDS Conference, Belgrade, October 12–15, 2011.
Author contributions
G.W, O.K. and M.E. designed the study. G.W. and O.K. performed the statistical analyses. G.W. and M.E. wrote the first draft of the manuscript. All authors contributed to the interpretation of the results and to the final version of the manuscript. G.W. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Financial disclosure: The study was supported by the National Institute of Allergy and Infectious Diseases (NIAID), Grant 5U01-AI069924-05
References
- 1.World Health Organization. Progress Report 2010. Geneva: World Health Organization; 2010. Towards universal access. Scaling up priority HIV/AIDS interventions in the health sector. Available at http://www.who.int/hiv/pub/2010progressreport/en/index.htm. [Google Scholar]
- 2.Keiser O, Chi BH, Gsponer T, et al. Outcomes of antiretroviral treatment in programmes with and without routine viral load monitoring in Southern Africa. AIDS. 2011 Jun 15; doi: 10.1097/QAD.0b013e328349822f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pujades-Rodriguez M, Balkan S, Arnould L, Brinkhof MA, Calmy A. Treatment failure and mortality factors in patients receiving second-line HIV therapy in resource-limited countries. JAMA. 2010 Jul 21;304(3):303–312. doi: 10.1001/jama.2010.980. [DOI] [PubMed] [Google Scholar]
- 4.Murphy RA, Sunpath H, Lu Z, et al. Outcomes after virologic failure of first-line ART in South Africa. AIDS. 2010 Apr 24;24(7):1007–1012. doi: 10.1097/QAD.0b013e3283333639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fox MP, Ive P, Long L, Maskew M, Sanne I. High rates of survival, immune reconstitution, and virologic suppression on second-line antiretroviral therapy in South Africa. J Acquir Immune Defic Syndr. 2010 Apr 1;53(4):500–506. doi: 10.1097/QAI.0b013e3181bcdac1. [DOI] [PubMed] [Google Scholar]
- 6.Hosseinipour MC, Kumwenda JJ, Weigel R, et al. Second-line treatment in the Malawi antiretroviral programme: high early mortality, but good outcomes in survivors, despite extensive drug resistance at baseline. HIV Med. 2010 Sep;11(8):510–518. doi: 10.1111/j.1468-1293.2010.00825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach. Geneva: World Health Organization; 2010. 2010 revision. Available at http://www.who.int/hiv/pub/arv/adult2010/en/index.html. [PubMed] [Google Scholar]
- 8.Piketty C, Gerard L, Chazallon C, et al. Salvage therapy with atazanavir/ritonavir combined to tenofovir in HIV-infected patients with multiple treatment failures: randomized ANRS 107 trial. Antivir Ther. 2006;11(2):213–221. [PubMed] [Google Scholar]
- 9.Johnson M, Grinsztejn B, Rodriguez C, et al. 96-week comparison of once-daily atazanavir/ritonavir and twice-daily lopinavir/ritonavir in patients with multiple virologic failures. AIDS. 2006 Mar 21;20(5):711–718. doi: 10.1097/01.aids.0000216371.76689.63. [DOI] [PubMed] [Google Scholar]
- 10.Doualla-Bell F, Avalos A, Brenner B, et al. High prevalence of the K65R mutation in human immunodeficiency virus type 1 subtype C isolates from infected patients in Botswana treated with didanosine-based regimens. Antimicrob Agents Chemother. 2006 Dec;50(12):4182–4185. doi: 10.1128/AAC.00714-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hosseinipour MC, van Oosterhout JJ, Weigel R, et al. The public health approach to identify antiretroviral therapy failure: high-level nucleoside reverse transcriptase inhibitor resistance among Malawians failing first-line antiretroviral therapy. AIDS. 2009 Jun 1;23(9):1127–1134. doi: 10.1097/QAD.0b013e32832ac34e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamers RL, Sigaloff KC, Wensing AM, et al. Patterns of HIV-1 Drug Resistance After First-Line Antiretroviral Therapy (ART) Failure in 6 Sub-Saharan African Countries: Implications for Second-Line ART Strategies. Clin Infect Dis. 2012 Jun;54(11):1660–1669. doi: 10.1093/cid/cis254. [DOI] [PubMed] [Google Scholar]
- 13.Marconi VC, Sunpath H, Lu Z, et al. Prevalence of HIV-1 drug resistance after failure of a first highly active antiretroviral therapy regimen in KwaZulu Natal, South Africa. Clin Infect Dis. 2008 May 15;46(10):1589–1597. doi: 10.1086/587109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orrell C, Walensky RP, Losina E, Pitt J, Freedberg KA, Wood R. HIV type-1 clade C resistance genotypes in treatment-naive patients and after first virological failure in a large community antiretroviral therapy programme. Antivir Ther. 2009;14(4):523–531. [PMC free article] [PubMed] [Google Scholar]
- 15.van Zyl GU, van der Merwe L, Claassen M, Zeier M, Preiser W. Antiretroviral resistance patterns and factors associated with resistance in adult patients failing NNRTI-based regimens in the Western Cape, South Africa. J Med Virol. 2011 Oct;83(10):1764–1769. doi: 10.1002/jmv.22189. [DOI] [PubMed] [Google Scholar]
- 16.Egger M, Ekouevi DK, Williams C, et al. Cohort Profile: The international epidemiological databases to evaluate AIDS (IeDEA) in sub-Saharan Africa. Int J Epidemiol. 2011 May 18; doi: 10.1093/ije/dyr080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999 Mar 30;18(6):695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 18.Fine JPGR. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94(446):496–509. [Google Scholar]
- 19.Schöni-Affolter FKO, Mwango A, Stringer J, Ledergerber B, Mulenga L, Bucher HC, Westfall AO, Calmy A, Boulle A, Chintu N, Egger M, Chi BH. Estimating loss to follow-up in HIV-infected patients on antiretroviral therapy: The effect of the competing risk of death in Zambia and Switzerland. PLoS ONE. 2011 doi: 10.1371/journal.pone.0027919. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eron JJ, Jr, Murphy RL, Peterson D, et al. A comparison of stavudine, didanosine and indinavir with zidovudine, lamivudine and indinavir for the initial treatment of HIV-1 infected individuals: selection of thymidine analog regimen therapy (START II) AIDS. 2000 Jul 28;14(11):1601–1610. doi: 10.1097/00002030-200007280-00016. [DOI] [PubMed] [Google Scholar]
- 21.Keiser O, Tweya H, Boulle A, et al. Switching to second-line antiretroviral therapy in resource-limited settings: comparison of programmes with and without viral load monitoring. AIDS. 2009 Sep 10;23(14):1867–1874. doi: 10.1097/QAD.0b013e32832e05b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hawkins CA, Chaplin B, Idoko J, et al. Clinical and genotypic findings in HIV-infected patients with the K65R mutation failing first-line antiretroviral therapy in Nigeria. J Acquir Immune Defic Syndr. 2009 Oct 1;52(2):228–234. doi: 10.1097/QAI.0b013e3181b06125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brenner BG, Coutsinos D. The K65R mutation in HIV-1 reverse transcriptase: genetic barriers, resistance profile and clinical implications. HIV Ther. 2009 Nov 1;3(6):583–594. doi: 10.2217/hiv.09.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sigaloff KC, Hamers RL, Wallis CL, et al. Second-Line Antiretroviral Treatment Successfully Resuppresses Drug-Resistant HIV-1 After First-Line Failure: Prospective Cohort in Sub-Saharan Africa. J Infect Dis. 2012 Jun;205(11):1739–1744. doi: 10.1093/infdis/jis261. [DOI] [PubMed] [Google Scholar]
- 25.Wallis CL, Mellors JW, Venter WD, Sanne I, Stevens W. Protease Inhibitor Resistance Is Uncommon in HIV-1 Subtype C Infected Patients on Failing Second-Line Lopinavir/r-Containing Antiretroviral Therapy in South Africa. AIDS Res Treat. 2011;2011:769627. doi: 10.1155/2011/769627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tuboi SH, Pacheco AG, Harrison LH, et al. Mortality associated with discordant responses to antiretroviral therapy in resource-constrained settings. J Acquir Immune Defic Syndr. 2010 Jan;53(1):70–77. doi: 10.1097/QAI.0b013e3181c22d19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arribas JR, Pulido F, Delgado R, et al. Lopinavir/ritonavir as single-drug therapy for maintenance of HIV-1 viral suppression: 48-week results of a randomized, controlled, open-label, proof-of-concept pilot clinical trial (OK Study) J Acquir Immune Defic Syndr. 2005 Nov 1;40(3):280–287. doi: 10.1097/01.qai.0000180077.59159.f4. [DOI] [PubMed] [Google Scholar]
- 28.Pulido F, Delgado R, Perez-Valero I, et al. Long-term (4 years) efficacy of lopinavir/ritonavir monotherapy for maintenance of HIV suppression. J Antimicrob Chemother. 2008 Jun;61(6):1359–1361. doi: 10.1093/jac/dkn103. [DOI] [PubMed] [Google Scholar]
- 29.Mannheimer S, Friedland G, Matts J, Child C, Chesney M. The consistency of adherence to antiretroviral therapy predicts biologic outcomes for human immunodeficiency virus-infected persons in clinical trials. Clin Infect Dis. 2002 Apr 15;34(8):1115–1121. doi: 10.1086/339074. [DOI] [PubMed] [Google Scholar]
- 30.Parruti G, Manzoli L, Toro PM, et al. Long-term adherence to first-line highly active antiretroviral therapy in a hospital-based cohort: predictors and impact on virologic response and relapse. AIDS patient care and STDs. 2006 Jan;20(1):48–56. doi: 10.1089/apc.2006.20.48. [DOI] [PubMed] [Google Scholar]
- 31.Oyugi JH, Byakika-Tusiime J, Ragland K, et al. Treatment interruptions predict resistance in HIV-positive individuals purchasing fixed-dose combination antiretroviral therapy in Kampala, Uganda. AIDS. 2007 May 11;21(8):965–971. doi: 10.1097/QAD.0b013e32802e6bfa. [DOI] [PubMed] [Google Scholar]
- 32.Nachega JB, Hislop M, Nguyen H, et al. Antiretroviral therapy adherence, virologic and immunologic outcomes in adolescents compared with adults in southern Africa. J Acquir Immune Defic Syndr. 2009 May 1;51(1):65–71. doi: 10.1097/QAI.0b013e318199072e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nachega JB, Hislop M, Dowdy DW, et al. Adherence to highly active antiretroviral therapy assessed by pharmacy claims predicts survival in HIV-infected South African adults. J Acquir Immune Defic Syndr. 2006 Sep;43(1):78–84. doi: 10.1097/01.qai.0000225015.43266.46. [DOI] [PubMed] [Google Scholar]
- 34.Bussmann H, Wester CW, Thomas A, et al. Response to zidovudine/didanosine-containing combination antiretroviral therapy among HIV-1 subtype C-infected adults in Botswana: two-year outcomes from a randomized clinical trial. J Acquir Immune Defic Syndr. 2009 May 1;51(1):37–46. doi: 10.1097/QAI.0b013e31819ff102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perry CM, Noble S. Didanosine: an updated review of its use in HIV infection. Drugs. 1999 Dec;58(6):1099–1135. doi: 10.2165/00003495-199958060-00009. [DOI] [PubMed] [Google Scholar]
- 36.Moyle GJ, Schoelles K, Fahrbach K, et al. Efficacy of selected treatments of HIV wasting: a systematic review and meta-analysis. J Acquir Immune Defic Syndr. 2004 Dec 1;37 (Suppl 5):S262–276. doi: 10.1097/01.qai.0000144381.09350.5b. [DOI] [PubMed] [Google Scholar]
- 37.Kuritzkes DR, Bassett RL, Johnson VA, et al. Continued lamivudine versus delavirdine in combination with indinavir and zidovudine or stavudine in lamivudine-experienced patients: results of Adult AIDS Clinical Trials Group protocol 370. AIDS. 2000 Jul 28;14(11):1553–1561. doi: 10.1097/00002030-200007280-00011. [DOI] [PubMed] [Google Scholar]
- 38.Richman DD, Fischl MA, Grieco MH, et al. The toxicity of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N Engl J Med. 1987 Jul 23;317(4):192–197. doi: 10.1056/NEJM198707233170402. [DOI] [PubMed] [Google Scholar]
- 39.Ssali F, Stohr W, Munderi P, et al. Prevalence, incidence and predictors of severe anaemia with zidovudine-containing regimens in African adults with HIV infection within the DART trial. Antivir Ther. 2006;11(6):741–749. doi: 10.1177/135965350601100612. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.