Abstract
Although inactivation of the PTEN gene has been implicated in the development of resistance to the HER2 targeting antibody trastuzumab, the mechanisms mediating this resistance remain elusive. We generated trastuzumab resistant cells by knocking down PTEN expression in HER2 overexpressing breast cancer cell lines and demonstrate that development of trastuzumab resistance in these cells is mediated by activation of an IL-6 inflammatory feedback loop leading to expansion of the cancer stem cell (CSC) population. Long term trastuzumab treatment generates highly enriched CSCs which display an EMT phenotype secreting over 100-fold more IL-6 than parental cells. An IL-6 receptor antibody interrupted this inflammatory feedback loop reducing the cancer stem cell population resulting in decreased tumor growth and metastasis in mouse xenographs. These studies demonstrate that trastuzumab resistance may be mediated by an IL-6 inflammatory loop and suggest that blocking this loop may provide alternative strategy to overcome trastuzumab resistance.
Introduction
The HER2 gene is amplified in approximately 20–25% of human breast cancers which are characterized by an aggressive clinical course (Slamon et al., 1987). The development of HER2 targeted therapeutic agents, such as trastuzumab, has dramatically altered the course of this disease. However, despite the clinical benefits of these HER2 targeted therapies, almost 50% of patients with HER2 amplified cancers fail to respond to trastuzumab and the vast majority of tumors that respond to trastuzumab develop resistance within one to two years of treatment (Lan et al., 2005). Although a number of mechanisms that mediate “de novo” or “acquired” trastuzumab resistance have been proposed, the most common molecular alteration associated with this resistance is inactivation of the tumor suppressor PTEN, found in over 40% of HER2-positive breast cancers (Nagata et al., 2004). The PTEN activity mediates trastuzumab resistance via activation of the downstream signaling molecule Akt, bypassing the requirement for HER2 activation (Berns et al., 2007). In addition, we and others have shown that both HER2 and PTEN are important regulators of subpopulations of breast cancer cells which display stem cell properties (Cicalese et al., 2009; Korkaya et al., 2009). There is increasing evidence that these cancer stem cells mediate tumor growth and metastasis and by virtue of their relative resistance to chemotherapy and radiation therapy may also contribute to tumor recurrence (Eyler and Rich, 2008). However, it is unknown if cancer stem cells play a role in de novo or acquired trastuzumab resistance.
Interleukins 6 (IL-6) and 8 (IL-8) have also been demonstrated to regulate the breast cancer stem cell self-renewal (Ginestier et al., 2010; Iliopoulos et al., 2011). Although these cytokines are regulated by multiple factors, HER2 overexpression in breast cancer stem cells has been shown to increase IL-6 production (Hartman et al., 2011). The IL-6 links inflammation to malignant transformation by activating the NF-κB pathway which, in turn, drives constitutive IL-6 production generating a positive feedback loop. In addition, IL-6 is able to induce epithelialmesenchymal transitions (EMT) which has been implicated in generation of stem cell phenotype (Iliopoulos et al., 2011; Mani et al., 2008; Sullivan et al., 2009). The clinical relevance of these studies is demonstrated by the strong association between serum IL-6 levels and poor clinical outcome in breast cancer patients including those with HER2 amplified breast tumors (Bachelot et al., 2003; Salgado et al., 2003). Together these studies suggest the possibility that the generation of inflammatory feedback loops regulating cancer stem cells may play a role in trastuzumab resistance in HER2 overexpressing breast cancers. To determine whether this is the case, we examined the activation of these pathways and their effects on cancer stem cell populations in genetically engineered breast cancer cell lines and mouse xenograft models. We demonstrate that PTEN deletion in HER2 overexpressing breast cancer cells activates an IL-6 mediated inflammatory feedback loop. This feedback loop expands the cancer stem cell population displaying an EMT phenotype through both autocrine and paracrine mechanisms which confer trastuzumab resistance. In addition, we demonstrate that interfering with this feedback loop utilizing an IL-6 receptor (IL-6R) antibody reduces the cancer stem cell population inhibiting tumor growth and metastasis. These studies define an alternative mechanism of trastuzumab resistance and suggest an effective therapeutic strategy to overcome this resistance.
Results
PTEN down regulation in HER2 overexpressing breast cancer cells increases the proportion of invasive cancer stem cells
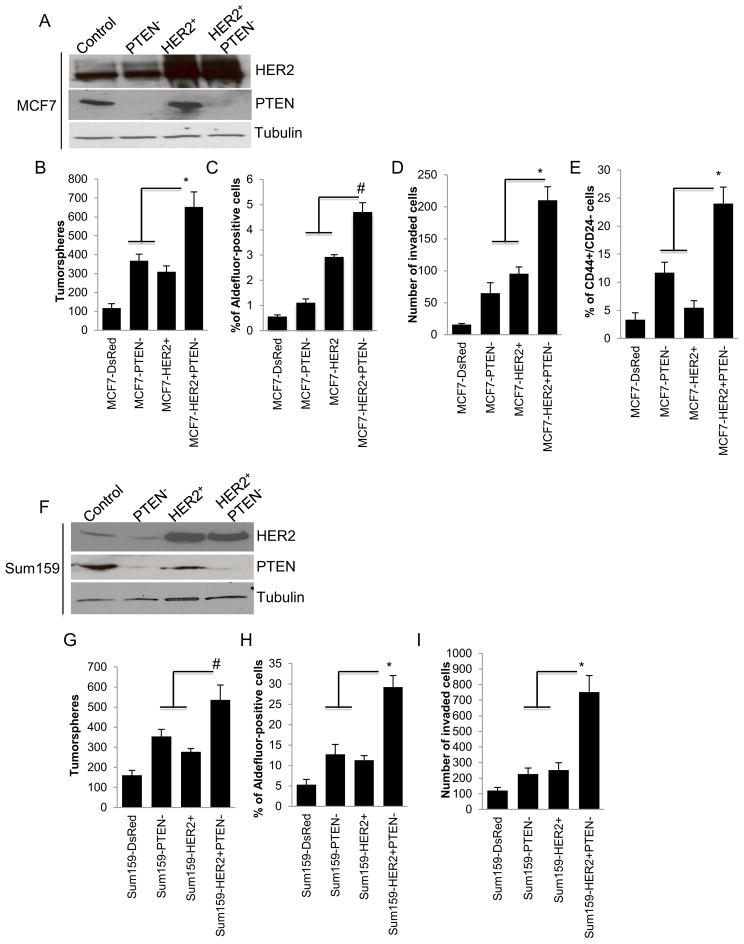
Since PTEN inactivation frequently occurs in the context of the HER2 amplification, a phenotype associated with trastuzumab resistance, we examined the effect of PTEN knockdown on CSC like populations in HER2 overexpressing breast cancer cell lines. The efficiency of HER2 overexpression and PTEN knockdown utilizing lentiviral shRNAs or control vector is demonstrated (Figure 1A and F). We first assessed the effect of these molecular alterations on tumorsphere formation an assay was shown to enrich cancer stem cells (Singh et al., 2003). Both PTEN deletion and HER2 overexpression resulted in significant increase in tumorsphere formation (Figure 1B and G). PTEN knockdown in HER2 overexpressing cells resulted in a 2–3 fold increase in sphere formation and a 6-fold increase over the parental cells. To confirm and extend these observations we examined the effect of PTEN knockdown and HER2 overexpression on the CSC markers, such as the expression of Aldehyde dehydrogenase or the CD44+/CD24− phenotype (Al-Hajj et al., 2003; Ginestier et al., 2007). There was a stepwise increase in the Aldefluor-positive and CD44+/CD24− populations in parental MCF7-DsRed, MCF7-PTEN−, MCF7-HER2+ and MCF7-HER2+PTEN− cells, respectively, when they were analyzed by the Aldefluor assay or CD44+/CD24− expression (Figure 1C and E). SUM159 cells are composed of over 90% CD44+/CD24− cells, precluding the use of these markers to identify CSC in these cell lines. However, there was a two-fold increase in the Aldefluor-positive population in SUM159-HER2+PTEN− cells as compared to SUM159-HER2+ cells (Figure 1H). Together these results demonstrate that the increase in the CSC population induced by HER2 overexpression is further enhanced by PTEN deletion.
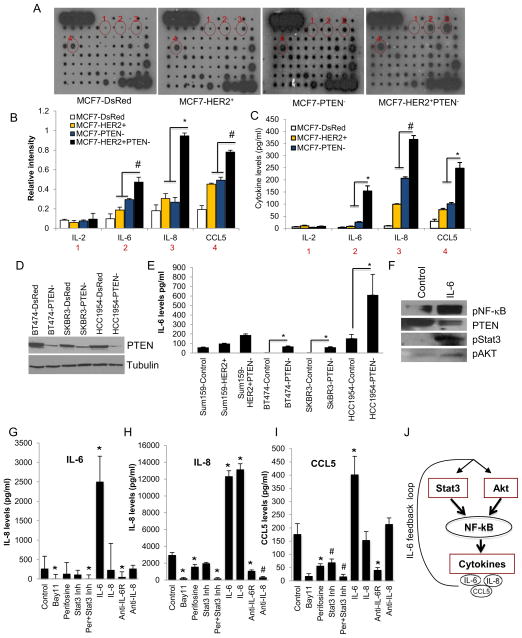
Figure 1. PTEN down regulation and HER2 overexpression synergize to increase the CSC population in vitro.
(A and F) Down regulation of PTEN and/or overexpression of HER2 in MCF7 or Sum159 cells is demonstrated by western blotting. (B and G) PTEN down regulation in HER2 overexpressing cells increased the tumorsphere formation, (C and H) Aldefluor-positive cell populations (D and I) as well as invasion compared to either HER2 overexpression or PTEN down regulation in vitro. (E) MCF7-HER2+PTEN− cells showed a significant increase in the proportion of CD44+/CD24− cells as compared to the MCF7-HER2+ or MCF7PTEN− cells. Error bars represent the mean and standard deviation of two independent experiments performed in duplicate samples. (*p ≤ 0.05, # p ≤ 0.01)
We next assessed the effect of HER2 overexpression and PTEN deletion on invasion of tumor cells through matrigel. As shown in Figure 1D and I, PTEN deletion in HER2 overexpressing MCF7 or SUM159 cells enhanced in vitro invasive capacity as compared to cells with either HER2 overexpression or PTEN deletion alone (Figure 1D and I).
PTEN downregulation generates a trastuzumab resistant CSC population
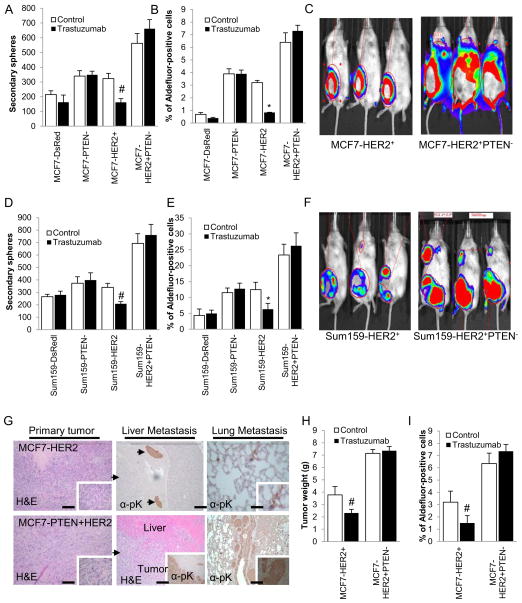
We determined the effect of HER2 blockade using the HER2 targeting antibody trastuzumab on cancer stem cell populations as assessed by tumorsphere formation or by ALDH expression. Primary tumorspheres for each indicated cell line were treated with trastuzumab during the course of 5–7 days and the effects of this treatment on the ability to form secondary tumorspheres was assessed. There was no significant effect of trastuzumab on tumorsphere formation in parental cells. However, HER2 overexpression rendered the tumorsphere forming population sensitive to trastuzumab as reflected by a 50% reduction in tumorsphere formation in MCF7-HER2+ and SUM159-HER2+ cells upon trastuzumab treatment. In contrast, trastuzumab had no significant effect on secondary tumorsphere formation in MCF7-HER2+PTEN− and SUM159-HER2+PTEN− cells (Figure 2A, D). In addition, trastuzumab reduced the Aldefluor-positive population by 75% and 50% in MCF7-HER2+ and SUM159-HER2+ cells, respectively (Figure 2B and E). In contrast, there was a modest non-statistically significant increase in the Aldefluor-positive population in both MCF7-HER+PTEN− and SUM159-HER2+PTEN− cells (Figure 2B and E) upon trastuzumab treatment.
Figure 2. PTEN down regulation and HER2 overexpression generate trastuzumab resistant tumors in NOD/SCID mice.
(A and D) Trastuzumab treatment of primary spheres of MCF7- HER2+ and Sum159-HER2+ reduced the formation of secondary spheres by 50%, while the secondary spheres from MCF7-PTEN−, Sum159-PTEN−, MCF7-HER2+PTEN− and Sum159- HER2+PTEN− cells were not affected by trastuzumab. (B and E) Trastuzumab treatment had no effect on the Aldefluor-positive cell population in MCF7-HER2+PTEN− and Sum159- HER2+PTEN− cells while it reduced this population by more than 50% in MCF7-HER2+ and Sum159-HER2+ cells. (C and F) MCF7-HER2+PTEN− and Sum159-HER2+PTEN− cells compared to MCF7-HER2+ and Sum159-HER2+ cells generated rapidly growing tumors with (G) metastasis to liver and lung. (H and I) While MCF7-HER2+ tumors responded to trastuzumab treatment in mice leading to inhibition of tumor growth and decrease in Aldefluor-positive population in mice, while MCF7-HER2+PTEN− tumors were resistant to trastuzumab. Error bars represent the mean and standard deviation of two independent experiments in panels A, B, D and E, and of the 5 tumor sizes from 5 different mice in panels H and I. (*p ≤ 0.01, #p ≤ 0.05).
PTEN down regulation in HER2 overexpressing cells generates trastuzumab resistant metastatic tumors in NOD/SCID mice
We next examined the biological consequences of increased CSC populations generated by HER2 overexpression and PTEN knockdown by implanting these cells into the mammary fad pads of NOD/SCID mice. Although parental MCF7 and SUM159 xenografts were able to grow in the mammary fat pads of these mice, they failed to generate metastasis in distant organs (data not shown). In contrast, MCF7-HER2+PTEN− and SUM159-HER2+PTEN− cells generated larger, primary tumors, which extensively metastasized to lymph nodes, liver and lung when compared to MCF7-HER2+ and SUM159-HER2+ cells that displayed only occasional metastasis to lung and liver (Figure 2C and F). Furthermore, while MCF7-HER2+ xenografts in mice were responsive to trastuzumab treatment leading to reduced tumor size (Figure 2H), MCF7-HER2+PTEN− xenografts demonstrated de novo resistance to trastuzumab (Figure 2H). The effects of trastuzumab on tumor weight were paralleled by effects on the cancer stem cell populations as assessed by the Aldefluor assay. The percent of Aldefluor-positive tumor cells was reduced by over 50% by trastuzumab treatment in MCF7-HER2+ cells (Figure 2H). In contrast, trastuzumab actually caused a slight increase in the Aldefluor-positive populations in MCF7-HER2+PTEN− cells demonstrating that PTEN deletion in HER2 overexpressing breast cancer cells generates a trastuzumab resistant CSC population (Figure 2I).
PTEN down regulation and HER2 overexpression synergize to increase expression of the cytokines IL-6, IL-8 and CCL5/RANTES
Recent studies demonstrated that a number of cytokines, including IL-6, IL-8 and CCL5/RANTES play a role in CSC regulation as well as in invasion and metastasis (Korkaya et al., 2011). Utilizing an antibody cytokine array, we determined the effects of HER2 overexpression, PTEN deletion or the combination on levels of cytokine expression in MCF7 cells. We detected a stepwise increase in IL-6, IL-8 and CCL5, as well as platelet-derived growth factor B (PDGF-B) secreted from MCF7-DsRed, MCF7-HER2+, MCF7-PTEN− and MCF7-HER2+PTEN− cells (Figure 3A). As assessed by densitometry of the cytokine blots and utilizing an enzyme-linked immunosorbent assay (ELISA), we further confirmed that secretion of these cytokines was increased by 2–3 fold in MCF7-HER2+ or MCF7-PTEN− cells compared to parental cells and by 10–20 fold in MCF7-HER2+PTEN− cells compared to parental MCF7-DsRed cells (Figure 3B and C). This dramatic elevation of cytokines in the MCF7-HER2+PTEN− cells suggests a synergistic effect resulting from PTEN deletion and HER2 overexpression. Although parental MCF7-DsRed cells secreted detectable levels of IL-8, CCL5 and PDGF-B, which were increased in MCF7-HER2+, MCF7-PTEN− and MCF7-HER2+PTEN− cells, there was no detectable expression of IL-6 in parental MCF7 cells. To confirm that PTEN deletion increased cytokine production in HER2 overexpressing cells, we performed PTEN knockdown in BT474, SKBR3 and HCC1954 cells, all of which display endogenous HER2 gene amplification and in SUM159-HER2+ cells. The efficiency of PTEN knockdown is shown in these cells by the western blotting (Figure 3D). The PTEN knockdown in these HER2 amplified cell lines and in SUM159-HER2+PTEN− cells increased IL-6 production (Figure 3E).
Figure 3. PTEN down regulation in HER2 overexpressing cells activates an IL-6/NF-κB mediated inflammatory feedback loop.
(A) MCF7-HER2+PTEN− cells secreted 3–5 fold higher levels of IL-6, IL-8, CCL5 compared to MCF7-HER2+ or MCF7-PTEN− cells as determined by RayBio Human Cytokine Antibody Array 5, (B) the intensity of each blot compared to control was determined by kodak image analyzer and (C) confirmed by ELISA. (D) Down regulation of PTEN in HER2 amplified breast cancer cells, BT474, SKBR3, HCC1954 and Sum159-HER2+ (E) results in increased levels of these cytokines in vitro. (F, G and H) Secretion of all three cytokines in Sum159-HER2+PTEN− cells were completely inhibited by the NF-κB inhibitor, (5μM Bay11-7082), or combined inhibition of Akt and Stat3, while Akt (5μM perfosine) or Stat3 (1μM Stat3 Inhibitor VII) reduced the levels of these cytokines by 50%. (F, G and H) Addition of recombinant IL-6 to Sum159-HER2+PTEN− cells stimulated the levels of all three cytokines while blocking IL-6 using the IL-6R antibody (at 5μg/ml) reduced their levels by more than 50%. (I) IL-6 activated Akt, Stat3 and NF-κB pathways while suppressing PTEN expression. (J) An IL-6 feedback loop is schematically illustrated. (Scale bar 100μm). Error bars represent the mean and standard deviation of two independent experiments performed in duplicate samples. (*p ≤ 0.05, #p ≤ 0.01).
The NF-κB transcription factor is known to transcribe number of cytokine genes including IL-6, IL-8 and CCL5 (Yu et al., 2010). Furthermore, Iliopoulos, et al., recently reported that IL-6 activated NF-κB signaling is mediated by Stat3 and Akt signaling pathways (Iliopoulos et al.). Consistent with these studies, recombinant IL-6 activated Akt, Stat3 and NF-κB pathways while suppressing PTEN expression as shown by western blotting (Figure 3F). We utilized inhibitors of NF-κB, Akt and Stat3 to determine their effects on cytokine production in HER2+PTEN− cells. A Stat3 inhibitor or the Akt inhibitor perifosine only partially inhibited secretion of all three cytokines (Figure 3G, H and I). In contrast, inhibition of NF-κB using Bay11 or combined inhibition of Akt and Stat3 pathways completely suppressed secretion of these cytokines (Figure 3G, H and I). We next determined the effect of recombinant cytokines or cytokine blocking antibodies on cytokine production. IL-6 but not IL-8, increased the production of all three cytokines, an effect that was completely inhibited by anti-IL-6R antibody. In contrast, addition of recombinant IL-8, or an IL-8 blocking antibody, had no significant effect on production of the other cytokines (Figure 3G, H and I). Together, these results suggest that Stat3 and Akt signaling through NF-κB increases the production of cytokines including IL-6, a cytokine whose production is necessary to maintain a positive feedback loop as illustrated in Figure 3J.
Trastuzumab treatment of PTEN deleted cells activates an IL-6 inflammatory loop expanding the CSC population
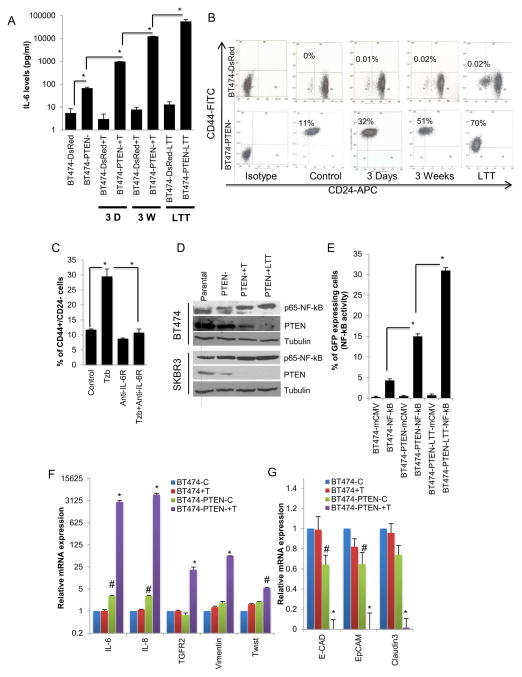
We next examined the effects of trastuzumab treatment on secretion of cytokines in trastuzumab sensitive and trastuzumab resistant cells. Trastuzumab sensitive BT474-DsRed cells showed a modest decrease in IL-6 levels following three days of trastuzumab treatment. However, there was approximately a two-fold increase in IL-6 when cells were treated for three weeks or longer (Figure 4A). In contrast, trastuzumab treatment of resistant BT474-PTEN− cells resulted in greater than 10-fold increase in IL-6 after three days and several hundred-fold after three weeks of trastuzumab treatment (Figure 4A). The effects of trastuzumab-mediated IL-6 production on CSCs was also tested. Parental BT474-DsRed cells contain no detectable CD44+/CD24− cells, a situation that was not significantly altered following trastuzumab treatment. In contrast, down regulation of PTEN in these cells generated a population which contained approximately 10% CD44+/CD24− cells (Figure 4B). Furthermore, culture of BT474-PTEN− cells in the presence of trastuzumab further increased the CD44+/CD24− populations to 32% after 3 days, 51% after 3 weeks and 70% when these cells were cultured for a month in the presence of trastuzumab (long term treatment – LTT) (Figure 4B). Interestingly these BT474-PTEN−LTT cells maintained this phenotype even in the absence of trastuzumab in subsequent passages. To determine whether induction of the CSC phenotype was dependent on IL-6 production, we assessed the effect of addition of IL-6 receptor antibody on induction of the CSC phenotype. Addition of this antibody not only reduced the CD44+/CD24− CSC population in BT474-PTEN− cells, but more importantly completely blocked the increase in this population induced by trastuzumab (Figure 4C). We examined the ability of the anti-IL-6R antibody to effect the CD44+/CD24− population in Sum159 cells which lack the expression of the luminal CD24 marker. Anti-IL-6R antibody treatment of these cells for 5 days resulted in generation of CD24+ cells (11%) and substantial growth arrest (Figure S1A and B). We further confirmed the effect of IL-6 in different cell lines (MCF7, Sum159, BT474 and SKBR3) representing different breast cancer subtypes by analyzing both Aldefluor and CD44+/CD24− phenotypes. Although IL-6 induced CD44+/CD24− phenotype in all cell lines, it only increased the Aldefluor-positive population in MCF7 and Sum159 cells while slightly reducing in HER2 amplified BT474 and SKBR3 cell lines (Figure S1C and D). This discrepancy may be explained by the existence of different stem cell population in different breast cancer subtypes.
Figure 4. An IL-6 mediated inflammatory loop expands the CSC population which displays characteristics of EMT.
(A) Parental BT474-DsRed cells demonstrated a modest decrease in IL-6 levels after 3 days of trastuzumab treatment with a 2 fold increase following long term (more than three weeks) treatment, however treatment of BT474-PTEN− cells resulted in more than 10 fold increase in IL-6 levels after 3 days and more than 100 fold after 3 weeks reaching to several hundred fold increase in LTT cells. (B) Trastuzumab treatment gradually increased the percent of cells expressing the CD44+CD24− markers in BT474-PTEN− cells compared to parental BT474-DsRed cells that are predominantly CD44−CD24+. (C) Blocking IL-6R in early stage inhibited this process. (D) Trastuzumab treatment of BT474-PTEN− but not parental BT474-DsRed cells increased the NF-κB activity as shown by Western blotting for p65-NF-κB or (E) NF-κB reporter activity. (F and G) Increased IL-6 production was accompanied by expression of mesenchymal markers and down regulation of epithelial markers as assessed by qPCR. (See Figure S1). Error bars represent the mean and standard deviation of two independent experiments performed in duplicate samples. (*p ≤ 0.05, #p ≤ 0.01).
To determine whether NF-κB activation was involved in these processes, we assessed the p65 NF-κB phosphorylation. We find an increased NF-κB phosphorylation following the trastuzumab treatment in BT474-PTEN− and SKBR3-PTEN− cells compared to parental and that was further enhanced with long term trastuzumab treatment (Figure 4D). Stepwise activation of NF-κB over time of trastuzumab treatment was also confirmed utilizing an NF-κB reporter assay (Figure 4E).
Since EMT has been linked to the CSC phenotype (Mani et al., 2008), a population known to be regulated by IL-6, we examined whether the dramatic increase in IL-6 production resulted in induction of EMT markers. In addition to several thousand fold increase in the IL-6 and IL-8 transcripts, gene expression of EMT markers, TGFR, Vimentin and Twist were up regulated by 5 to 25 fold in BT474-PTEN− cells upon trastuzumab treatment an effect has not been seen in parental BT474 cells (Figure 4F). Furthermore, trastuzumab treatment of BT474-PTEN− cells resulted in down regulation of gene expressions of epithelial markers, E-cadherin, EpCAM and Claudin (Figure 4G).
Paracrine induction of a trastuzumab resistant CSC phenotype in parental BT474 cells
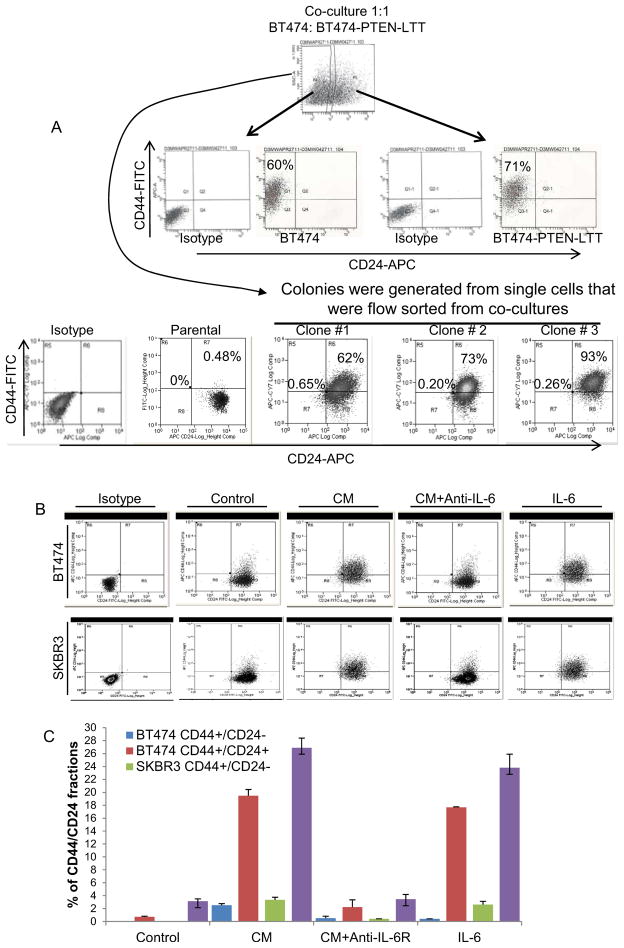
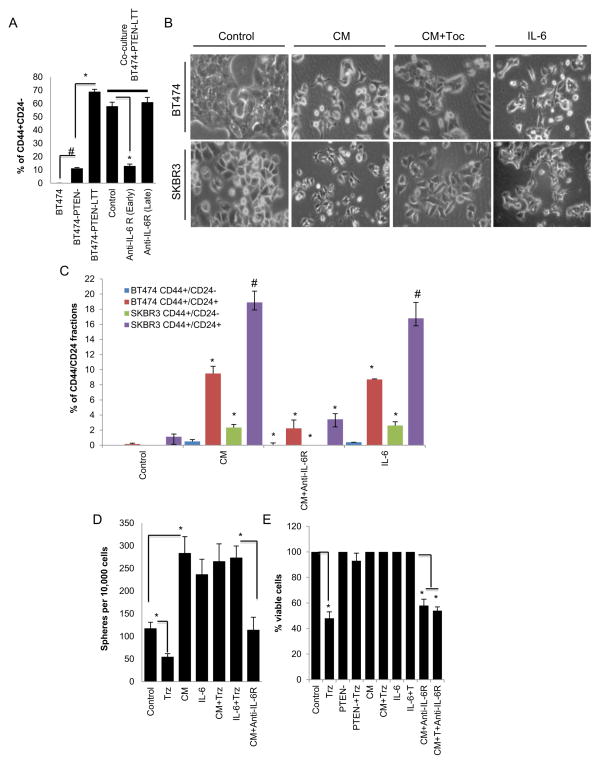
To determine whether paracrine factors could also act upon PTEN wild type (BT474 cells), we co-cultured parental BT474 cells with BT474-PTEN−LTT cells which had been cultured for 4 weeks in the presence of trastuzumab. The GFP label in the parental (PTEN wild type) cells allowed separation of cell populations from co-culture by flow cytometry. Interestingly, co-culture of parental BT474-GFP cells with BT474-PTEN−LTT cells increased the percentage of CD44+/CD24− CSCs from 0.1% to 60% in parental cells (Figure 5A). We next tested whether these parental BT474 cells can maintain this phenotype in the absence of BT474-PTEN−LTT cells. We sorted single GFP expressing parental BT474 cells after three weeks of co-culture with BT474-PTEN−LTT and generated multiple colonies. Although these colonies of parental BT474 cells after sorting did not maintain high CD44+/CD24− phenotype, they were primarily CD44+/CD24+ which is distinctly different from the control BT474 cells (Figure S2A). Furthermore addition of anti-IL-6R antibody at the time of co-culture inhibited the induction of the CD44+/CD24− phenotype while the late treatment with anti-IL-6R antibody had no effect in parental cells (Figure 5A). To examine the direct role of IL-6 in these processes, we stimulated parental BT474 or SKBR cells using recombinant IL-6 or the conditioned medium (CM) from BT474-PTEN-LTT cells in the presence or absence of anti-IL-6R antibody. Either IL-6 or CM treatment of cells for 5 days was able to induce mesenchymal phenotype and 10 fold increase in the expression of the CD44 marker in these cells, while the anti-IL-6R antibody was able to reverse these phenotypic changes (Figure 5B and C). Longer exposure of these cells to CM or IL-6 (10 days) further increased the CD44+/CD24− and CD44+/CD24+ populations (Figure S2B and C) suggesting that over time these populations are enriched.
Figure 5. An IL-6 inflammatory loop mediates trastuzumab resistance through autocrine and paracrine mechanisms.
(A and B) When co-cultured with BT474-PTEN-LTT cells for two weeks, parental BT474-DsRed cells acquired a CD44+CD24− phenotype a transition which was inhibited by addition of anti-IL-6R antibody at the beginning of co-culture. However once cells acquired the CD44+/CD24− phenotype they became resistant to anti-IL-6R antibody (late). (B and C) Conditioned medium (CM) from BT474-PTEN-LTT cells or recombinant IL-6 was able to induce mesenchymal phenotype and CD44 expression in both parental BT474-DsRed and SKBR3-DsRed cells. (D) CM from BT474-PTEN-LTT cells or recombinant human IL-6 increased the sphere formation of BT474-DsRed cells providing resistance to trastuzumab an effect that was reversed by anti-IL-6R antibody. (E) Trastuzumab reduced the number of viable BT474-DsRed cells by 50%, while it had no effect on the viability of BT474-PTEN− cells or BT474-DsRed cells when they were grown in the presence of CM or IL-6, an effect reversed by anti-IL-6R antibody. (See Figure S2). Error bars represent the mean and standard deviation of two independent experiments performed in duplicate samples. (*p ≤ 0.05, #p ≤ 0.01).
We next examined whether the IL-6 renders CSC population resistant to trastuzumab in parental BT474 cells in vitro. While IL-6 or CM significantly increased the number tumorspheres in suspension cultures of BT474 cells, trastuzumab treatment reduced the sphere forming cells by more than 50% (Figure 5D). In contrast, trastuzumab failed to inhibit sphere formation in the presence of IL-6 or CM, while addition of anti-IL-6R antibody reduced the number of tumorspheres induced by CM in BT474 cells (Figure 5D).
BT474 cells were stimulated by CM or IL-6 in the presence or absence of trastuzumab and/or anti-IL-6R antibody. While the parental BT474 cell growth was reduced by 50% following 48 hours of trastuzumab treatment, BT474-PTEN− cells were unaffected (Figure 5E). Addition of CM from BT474-PTEN−LTT cells or recombinant IL-6 rendered parental BT474 cells resistant to trastuzumab, an effect that was blocked by anti-IL-6R antibody (Figure 5E).
Blocking the IL-6 receptor inhibits the CSC population reducing tumor growth and metastasis in trastuzumab resistant mouse xenografts
To demonstrate the in vivo relevance of these findings, we assessed the effects of inhibition of IL-6 signaling utilizing anti-IL-6R antibody, and Akt signaling using perifosine in SUM159- HER2+PTEN− luciferase labeled trastuzumab resistant xenografts (Figure 2E and F). The effects of these treatments on tumor growth were assessed by luminescent imaging or by weighing tumors after mice were sacrificed. Treatments were started on the day of tumor inoculation (early) (Figure 6B) or delayed until palpable tumors were established, at approximately 0.4 cm size (late). The effects of the chemotherapy agent docetaxel, the Akt inhibitor perifosine, anti-IL-6R antibody or perifosine plus anti-IL-6R antibody were assessed after eight weeks of treatment (Figure 6 B, D and E). In contrast to the chemotherapeutic agent docetaxel, perfosine or anti-IL-6R antibody significantly inhibited tumor growth while the combination of perifosine and anti-IL-6R antibody showed greatest inhibition of tumor growth with complete inhibition of tumors in 50% of animals in which treatments were begun early (Figures 6B, C, D and E). Furthermore, while control and docetaxel treated mice lost body weight, those treated with anti-IL-6R antibody alone or in combination with perifosine maintained normal body weight (Figure 6F). The effects of these treatments on the CSC populations were assessed by the Aldefluor assay. Although docetaxel had no significant effect on the percent of Aldefluor-positive cells, both perifosine and anti-IL-6R antibody significantly reduced this population. Furthermore, the combination of perifosine and anti-IL-6R antibody resulted in the greatest reduction in the Aldefluor-positive population with more than 80% reduction compared to control or docetaxel treated tumors (Figure 6G). To determine whether these treatments effected tumor cytokine production, we determined the level of human cytokines in the serum of treated mice utilizing a human specific ELISA assay. Although docetaxel had no significant effect on serum IL-6 or IL-8 levels, perifosine or anti-IL-6R antibody treatment significantly reduced the levels of these cytokines. Furthermore, the combination of perifosine and anti-IL-6R antibody produced greater than an 80% reduction in levels of human serum IL-6 compared to untreated or docetaxel treated mice (Figure 6H).
Figure 6. Targeting of the IL-6 pathway reduces the CSC population inhibiting tumor growth and metastasis in mouse xenografts.
Metastatic Sum159-HER2+PTEN− cells were implanted into the fat pads of NOD-SCID mice (A). In early treatment (started on the day of implantation) settings (B), docetaxel (10mg/kg) once a week, perifosine (20mg/kg) twice a week and anti-IL-6R (10mg/kg) once a week were administered for 8 weeks. (C) Representative pictures of mice for each early treatment group. (D) In late treatment (started after the establishment of tumor ~0.4cm), drugs were administered as in the early treatment. (D and E) Tumors were measured following 8 weeks of treatment. There was a 80% and 50% reduction in tumor size in early and late perifosine+anti-IL-6R antibody combination treatments respectively. (F) Mice treated with perifosine, anti-IL-6R antibody or the combination showed less body weight loss than those treated with docetaxel alone. (G) Tumors from mice treated with perifosine or anti-IL-6R antibody alone or the combination showed substantial reduction in Aldefluor-positive cell population. In contrast, tumors from control or docetaxel treated mice, showed no reduction. (H) Serum levels of human IL-6 and IL-8, measured by ELISA in control and docetaxel treated mice were two fold higher than in the perifosine, anti-IL-6R antibody alone or in combination treated mice. Error bars represent the mean and standard deviation of five independent mice data. (*p ≤ 0.05, #p ≤ 0.01).
Anti-IL-6 receptor antibody overcomes acquired and de novo trastuzumab resistance
We next examined the effect of anti-IL-6R antibody on trastuzumab-sensitive BT474-DsRed and trastuzumab-resistant BT474-PTEN− xenografts. The growth of parental BT474-DsRed xenografts was significantly inhibited by trastuzumab treatment compared to saline treatment over a seven week period (Figure 7A). However, tumors in trastuzumab treated mice began to grow by week 5 demonstrating acquired trastuzumab resistance. Although the anti-IL-6R antibody had little effect on tumor growth on its own, when added to trastuzumab it completely blocked tumor growth up to week 7 of follow up (Figure 7A). In contrast, trastuzumab had no effect on the growth of BT474-PTEN− xenografts while the anti-IL-6R antibody completely blocked tumor growth when given alone or in combination with trastuzumab (Figure 7B). Addition of anti-IL-6R antibody to established tumors (late treatment) completely blocked tumor growth as assessed by tumor weight at sacrifice (Figure 7C). To determine the effects of these treatments on the development of metastasis, we excised primary BT474-PTEN− tumors after eight weeks of treatment and assessed subsequent development of local and distant metastasis by luciferase imaging (Figure 7D). While control or trastuzumab treated mice quickly developed secondary tumors and distant metastasis requiring euthanization, there were no distant metastasis detected in anti-IL-6R antibody treated mice (Figure 7D). The effects of these treatments were also reflected in human cytokine levels in these mice serum as determined by ELISA assay (Figure 7E).
Figure 7. The IL-6 receptor antibody overcomes de novo and acquired trastuzumab resistance in mouse xenografts.
(A) Combining anti-IL-6R antibody with trastuzumab completely suppresses tumor growth in mice bearing trastuzumab sensitive BT474-DsRed tumors, and (B and C) overcomes de novo trastuzumab resistance in BT474-PTEN− xenografts. (D) Anti-IL-6R antibody completely inhibited the development of secondary metastasis in distant organs after the primary tumors were excised in NOD-SCID mice. (E) Serum IL-6 levels were significantly higher in trastuzumab treated mice while anti-IL-6R antibody treated mice showed lowest levels of serum IL-6. (F) Frequency of CSC were calculated in serial reimplantation of residual tumors from treated mice showing significantly lower CSCs in anti-IL-6R antibody alone or combination treatments. Error bars represent the mean and standard deviation of five independent mice data. (*p ≤ 0.05, #p ≤ 0.01).
One of the hallmarks of CSC model is their ability to initiate tumors in secondary reimplantation assays (Clarke et al., 2006). We therefore utilized a re-implantation assay to determine the frequency of breast CSCs (tumor initiating cells) in residual tumors from mice treated with control, trastuzumab, anti-IL-6R alone or in combination. Re-implantation of these residual tumor cells into secondary mice showed that the frequency of breast CSCs was actually increased by trastuzumab treatment alone (Figure 7F). However the frequency of CSCs in anti-IL-6R antibody or anti-IL-6R antibody+trastuzumab treated tumors were reduced by more then 50% and 90% respectively (Figure 7F) suggesting that anti-IL-6R antibody targets the tumorigenic CSC population.
Discussion
There is increasing evidence that many human cancers, including breast cancer, are driven and maintained by a population of cells that display stem cell properties. In addition to mediating tumor invasion and metastasis, the relative resistance of CSCs to cytotoxic chemotherapy and radiation therapy may also contribute to treatment resistance (Morrison et al., 2011). The HER2 gene, which is amplified in approximately 20% of human breast cancers, is an important regulator of breast CSCs. Despite the demonstrated clinical efficacy of HER2 blockade, the existence of de novo and acquired resistance to HER2 targeted therapeutics remains a major therapeutic challenge. Although multiple mechanisms may contribute to this resistant phenotype, inactivation of the PTEN tumor suppressor gene occurs in over 40% of HER2 amplified breast cancers, an alteration associated with trastuzumab resistance (Nagata et al., 2004). We have previously reported that PTEN down regulation increases the breast CSC population by via Akt activation of the Wnt signaling pathway (Korkaya et al., 2009). The Akt inhibitor perifosine was able to partially block this pathway, reducing CSC populations.
Recently Iliopoulos, et al., demonstrated that IL-6 links inflammation to malignant transformation by constitutively activating the NF-κB pathway which in turn drives further IL-6 production creating a positive feedback loop (Iliopoulos et al., 2009). IL-6 also has previously been reported to be capable of expanding the CSC populations, as well as inducing epithelial mesenchymal transition (EMT), both of which are implicated in tumor metastasis and therapeutic resistance (Iliopoulos et al., 2011; Sansone et al., 2007; Sullivan et al., 2009). Resistance to EGFR and Notch targeted therapies in lung cancer may be regulated by IL-6 (He et al., 2011; Yao et al., 2010). Elevated levels of IL-6 have been shown to associate with chronic inflammatory state, obesity and increased risks for developing malignancies (Bromberg and Wang, 2009; Scheller et al., 2006). The clinical relevance of these findings is suggested by the correlation of serum IL-6 levels and poor outcome in breast cancer patients (Yao et al., 2010). Furthermore IL-6 knock-out mouse failed to generate glioma tumors (Weissenberger et al., 2004). In the present studies, we demonstrate that an IL-6 driven inflammatory loop mediates both de novo and acquired trastuzumab resistance. We generated trastuzumab resistant breast cancer cell lines by overexpressing HER2 and/or down regulating PTEN. In addition to inducing trastuzumab resistance, PTEN down regulation in HER2 overexpressing cells expanded the CSC population resulting in cells that were highly metastatic compared to HER2 overexpressing cells with wild type PTEN. We demonstrated that PTEN deletion in multiple HER2 amplified breast cancer cell lines resulted in substantial increases in production of several cytokines, including IL-6. IL-6, in turn, was necessary to maintain a positive feedback loop which also expanded the CSC population as assessed by the Aldefluor assay or by expression of the cancer stem cell marker CD44+/CD24−. Interestingly, when HER2 amplified PTEN deleted cells were cultured in the presence of the HER2 blocking antibody, trastuzumab, they demonstrated progressive increase in cytokine production as well as in the proportion of CSCs. Long term trastuzumab treatment of BT474-PTEN− cells resulted in several 100-fold increase in IL-6 production associated with an increase in the CD44+/CD24− cells from less than 1% to over 70%. It is unclear whether this increase in the CSC population results from expansion of a pre-existing CSC population or induction of this phenotype in non-CSC cells. Further studies will be necessary to demonstrate between these possibilities. However, appearance of a CD44+ cell population in HER2 amplified BT474 and SKBR3 breast cancer lines suggests that IL-6 may induce the CSC phenotype in non-CSC cells. In addition to increasing the CSC population, these cells assumed a mesenchymal appearance with increased expression of EMT markers such as vimentin, TGF-β and Twist and decreased expression of E-cadherin, EpCAM and Claudin. IL-6 has previously been reported to be an inducer of EMT, a state also associated with CSCs (Mani et al., 2008). We demonstrated that an IL-6 receptor blocking antibody was able to prevent the increase in CSCs, EMT and cytokine production demonstrating a critical role for IL-6 in maintaining this feedback loop. Long-term trastuzumab treated cells became resistant to anti-IL-6R antibody suggesting that these cells may have undergone additional epigenetic changes.
We also demonstrated that the IL-6 inflammatory loop is dependent on NF-κB signaling. This transcription factor is known to regulate the production of a number of cytokines including IL-6, IL-8 and CCL5 (Yu et al., 2010). Iliopoulos et al., recently reported that IL-6 activated NF-κB signaling is mediated by both STAT3 and Akt pathways (Iliopoulos et al., 2009; Iliopoulos et al.). Consistent with this, we demonstrate that although combined inhibition of Akt and STAT3 pathways was required to completely inhibit production of these cytokines, blocking IL-6 or inhibiting the NF-κB pathway directly with the inhibitor Bay11 almost completely blocked cytokine production. We also utilized an NF-κB reporter to demonstrate that there was greater than a three-fold increase in the proportion of NF-κB activated cells upon PTEN down regulation. The above experiments suggest that activation of an IL-6 inflammatory loop plays an important role in both de novo and acquired trastuzumab resistance. Activation of this inflammatory loop was dependent upon PTEN down regulation. However, unexpectedly, we found that when parental BT474 cells were co-cultured with BT474/PTEN-/LTT (cultured long term in the presence of trastuzumab), the IL-6 inflammatory loop was activated in parental BT474 cells with wild type PTEN expression via IL-6 dependent paracrine mechanism. These experiments suggest that once the IL-6 inflammatory loop is activated in PTEN deleted cells, paracrine factors, including IL-6 are able to activate similar loops in neighboring cells, even in the absence of genetic alterations. Suppression of PTEN expression by IL-6 has previously been shown to be mediated by the microRNA miR21 (Iliopoulos et al.). Consistent with these, we show here that IL-6 activates Akt, Stat3 and NF-kB pathways while suppressing PTEN. Furthermore, IL-6 has been known to induce epigenetic alterations such as methylation in number of genes including CD44 which is induced by IL-6 mediated hypomethylation resulting in basal/stem cell phenotype (D’Anello et al., 2010). This may explain permanent phenotypic changes in parental BT474 or SKBR3 cells upon IL-6 treatment.
The role of an IL-6 mediated inflammatory loop in trastuzumab resistance was confirmed utilizing NOD/SCID xenograft models, both SUM159/HER2+/PTEN− and HER2 amplified BT474/PTEN− cells generated rapidly growing highly metastatic tumors in NOD/SCID mice that exhibited “de novo” resistance to trastuzumab treatment. Furthermore, the addition of anti-IL-6R antibody to trastuzumab prevented development of “acquired” trastuzumab resistance in mice bearing parental BT474 xenografts. As was the case in vitro, trastuzumab treatment of mice actually accelerated BT474-PTEN− tumor growth and CSC frequency as well as markedly increasing the level of secreted cytokines IL-6 and IL-8 as demonstrated by a human specific ELISA assay. This suggests that the IL-6 inflammatory loop not only mediates de novo trastuzumab resistance, but further amplification of this loop also is involved in acquired trastuzumab resistance.
The functional importance of this inflammatory loop was demonstrated by our studies indicating that the IL-6R antibody alone or in combination with trastuzumab or the Akt inhibitor perifosine not only decreased the population of CSCs in primary tumor, but also completely inhibited development of distant metastasis. Furthermore, primary tumors treated with anti-IL6-R alone or in combination with trastuzumab reduced the frequency of CSCs while trastuzumab treatment alone resulted in enrichment of CSCs in re-implantation assays at serial dilutions. These results are consistent with our findings that IL-6 regulates the CSC population, as well as the process of EMT, both of which have been linked to tumor metastasis.
Anti-IL6R (Tocilizumab) is currently FDA approved for the treatment of rheumatoid arthritis, a condition in which IL-6 plays a role in joint inflammation. Our results suggests that addition of agents targeting the IL-6 pathway such as anti-IL-6R antibody may prove a valuable addition to HER2 targeted agents for treatment of HER2+ breast cancer.
Methods
Cell lines and reagents
MCF7, BT474, SKBR3, HCC1954 cell lines were maintained by ATCC guidelines. The SUM159 cell line was maintained in Ham’s F12 medium supplemented (with 5% FBS, 5 μg/ml insulin, 1μg/ml hydrocortisone and antibiotic/antimycotic 10.000 units/ml penicillin G sodium, 10.000 μg/ml streptomycin sulfate and 25 μg/ml amphotericin B).
Perifosine, Akt inhibitor was obtained from Keryx Biopharmaceuticals Inc., and docetaxel (Taxotere) was from Sanofi Aventis (Bridgewater, NJ). Anti-IL-6R antibody (Tocilizumab) was obtained from Chugai Pharmaceuticals Co. Ltd. (Shizuoka, Japan). Trastuzumab was purchased from University of Michigan Cancer Center Pharmacy. NF-κB inhibitor, Bay11-7082 and Stat3 Inhibitor VII were purchased from EMD Chemicals (NJ, USA).
The PTEN antibody was purchased from Cell Signaling Technology Inc., the α-Tubulin antibody was from Santa Cruz Biotechnology Inc., Phospho-NF-κB (p65) antibody was from Cell Applications. Fluorescent-conjugated antibodies to CD44, CD24, CD49f and EpCAM are from BD Biosciences (San Jose, CA).
Cytokine Antibody Array and ELISA
The equal numbers of cells were plated and cultured for three days. Subsequently conditioned medium from these cell cultures were collected and analyzed by the RayBio Human Cytokine Antibody Array 5 (RayBiotech, Inc. Norcross, GA). ELISA assay was performed using the conditioned medium collected from two day cultures of cells seeded at 200,000 cells/plate. Blood samples were drawn through orbital vein just before sacrificing the mice. Plasma separated from whole blood by centrifugation at 14,000 rpm at 4 °C. Plasma from tumor bearing mice and the conditioned medium from in vitro cultures were then analyzed for the indicated cytokines by UM Cytokine Core facility.
Tumorsphere assay
Single cells were plated on ultra-low attachment plates at a density of 1×105/ml and grown for 7 days in a mammocult medium (Stem Cell Technologies). Following the treatment of primary spheres, they were dissociated into single cell suspension and plated at a density of 5×103–1×104/ml for the subsequent passages. Secondary spheres were counted after 5 to 7 days in culture.
Lentiviral constructs and infection of NMECs and breast cancer cell lines
The lentiviral shRNA, pLL3.7-shPTEN targets the human PTEN gene and pLenti-RSV-HER2 constructs overexpress HER2 were previously described (Korkaya et al., 2009; Korkaya et al., 2008). Using both pLL3.7-shPTEN and pLenti-RSV-HER2, we co-infected SUM159 and MCF7 cell lines to generate MCF7-HER2+PTEN− and Sum159-HER2+PTEN− cells. Stables clones of MCF7-HER2+, Sum159-HER2+, MCF7-PTEN− and Sum159-PTEN− cells were previously generated (Korkaya et al., 2009; Korkaya et al., 2008).
Aldefluor assay and Flow cytometry
To measure ALDH activity, the Aldefluor assay was carried out according to manufacturer’s (Stemcell Technologies, Durham, NC) guidelines. Indicated cells were incubated with fluorophore conjugated CD44 or CD24 antibodies alone or in combination on ice for 30 minutes, washed with HBSS and re-suspended in DAPI containing HBSS buffer for flow cytometry analyses.
Implantation of cells in NOD/SCID mice and drug treatments
In mouse xenografts, we utilized the luciferase expressing breast cancer cell lines for in vivo bioluminescence imaging using the Caliper IVIS imaging systems. Breast cancer cells expressing the luciferase gene implanted into the fat pads of five week old NOD/SCID mice. These mice were imaged the following day to ensure the implantation of tumor cells.
Early drug treatments were started right after the implantation of cells in mice, trastuzumab was given by IP at 20mg/kg dose once per week, docetaxel was given by IP at 10mg/kg dose once per week, anti-IL-6R antibody was given at 10mg/kg once a week and perifosine was given at 20mg/kg twice per week. Treatments for all early settings were 8 weeks long. Late treatments were started after the establishment of primary tumors (roughly when they reached 0.4 cm size). Drug doses were as described in early setting.
Immunoblotting and Immunostaining assays were performed as previously described (Korkaya et al., 2009).
Statistical analyses
Statistical differences for the number of spheres, GFP positive cells, Aldefluor assays and tumor growths were determined using students t test.
Supplementary Material
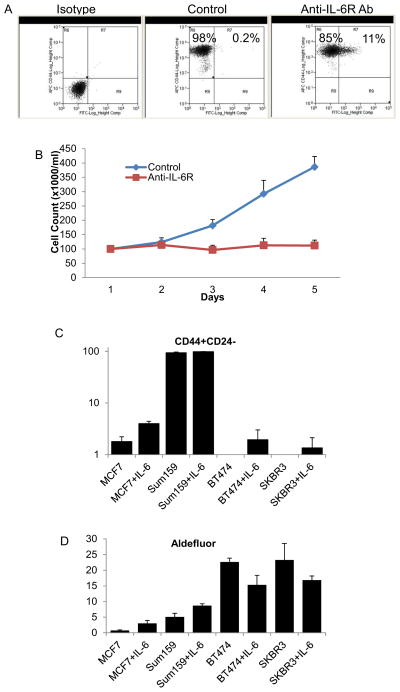
Figure S1, related to Figure 4. (A) Treatment of Sum159 cells with anti-IL-6R antibody for 5 days reduced the CD44+CD24− population and (B) there was a significant growth arrest following 5 days of anti-IL-6R ab treatment despite no significant apoptosis induced.
IL-6 induced CD44+/CD24− and Aldefluor-positive population in MCF7, Sum159, BT474 and SKBR3. (C) IL-6 induces CD44+/CD24− populations in all four breast cancer cell lines. (D) However IL-6 was only able to increase Aldefluor-positive cell population in MCF7 and Sum159 cells, while slightly reducing this population in HER2 amplified BT474 and SKBR3 cell lines. Error bars represent the mean and standard deviation of two independent experiments performed in duplicate samples. (*p ≤ 0.05, #p ≤ 0.01).
Figure S2, related to Figure 5. (A) Co-culture of parental BT474 (GFP) cells with BT474-PTEN-LTT (DsRed) cells for three weeks resulted in induction of CD44+CD24− population in parental cells. To eliminate the cross contamination of parental cells and BT474-PTEN-LTT cells, single GFP expressing cells were flow sorted from these co-cultures and multiple colonies of parental BT474 cells were generated. Three representative colonies show that these cells even in the absence of BT474-PTEN-LTT cells can maintain high CD44+ cell population despite regaining the CD24 expression.
(B and C) Long term treatment (10 days) of parental BT474-DsRed and SKBR3-DsRed cells with conditioned medium (CM) from BT474-PTEN-LTT cells or recombinant IL-6 further increased CD44+ expression which is more than the 5 days treatment as shown in Figure 5C.
Error bars represent the mean and standard deviation of two independent experiments performed in duplicate samples.
Highlights.
PTEN deletion in HER2+ breast tumors activates IL-6 feedback loop.
IL-6/NFκB signaling induces breast cancer stem cell population with EMT phenotype.
IL-6 induced breast cancer stem cells are resistant to anti-HER2 therapy.
Anti-IL-6R antibody overcomes trastuzumab resistance in mouse xenograft models.
Acknowledgments
We would like to thank the UMCCC Core facilities (vector, flow cytometry and animal husbandry) and the pathology team for their technical help. We are also grateful to Denise Poirier for extraordinary typing assistance. This work was supported by NIH grants, CA129765 and CA101860 to MSW and Komen for the cure, KG110230 to HK. Indo-US fellowship to FM
Footnotes
Disclosure of potential conflicts of interest
M. S. Wicha has financial holdings in OncoMed Pharmaceuticals and receives research support from Dompe.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachelot T, Ray-Coquard I, Menetrier-Caux C, Rastkha M, Duc A, Blay JY. Prognostic value of serum levels of interleukin 6 and of serum and plasma levels of vascular endothelial growth factor in hormone-refractory metastatic breast cancer patients. Br J Cancer. 2003;88:1721–1726. doi: 10.1038/sj.bjc.6600956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns K, Horlings HM, Hennessy BT, Madiredjo M, Hijmans EM, Beelen K, Linn SC, Gonzalez-Angulo AM, Stemke-Hale K, Hauptmann M, et al. A Functional Genetic Approach Identifies the PI3K Pathway as a Major Determinant of Trastuzumab Resistance in Breast Cancer. Cancer Cell. 2007;12:395–402. doi: 10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- Bromberg J, Wang TC. Inflammation and cancer: IL-6 and STAT3 complete the link. Cancer Cell. 2009;15:79–80. doi: 10.1016/j.ccr.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicalese A, Bonizzi G, Pasi CE, Faretta M, Ronzoni S, Giulini B, Brisken C, Minucci S, Di Fiore PP, Pelicci PG. The tumor suppressor p53 regulates polarity of self-renewing divisions in mammary stem cells. Cell. 2009;138:1083–1095. doi: 10.1016/j.cell.2009.06.048. [DOI] [PubMed] [Google Scholar]
- Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, Visvader J, Weissman IL, Wahl GM. Cancer Stem Cells--Perspectives on Current Status and Future Directions: AACR Workshop on Cancer Stem Cells. Cancer Res. 2006 doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- D’Anello L, Sansone P, Storci G, Mitrugno V, D’Uva G, Chieco P, Bonafe M. Epigenetic control of the basal-like gene expression profile via Interleukin-6 in breast cancer cells. Mol Cancer. 2010;9:300. doi: 10.1186/1476-4598-9-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyler CE, Rich JN. Survival of the fittest: cancer stem cells in therapeutic resistance and angiogenesis. J Clin Oncol. 2008;26:2839–2845. doi: 10.1200/JCO.2007.15.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginestier C, Liu S, Diebel ME, Korkaya H, Luo M, Brown M, Wicinski J, Cabaud O, Charafe-Jauffret E, Birnbaum D, et al. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J Clin Invest. 2010;120:485–497. doi: 10.1172/JCI39397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman ZC, Yang XY, Glass O, Lei G, Osada T, Dave SS, Morse MA, Clay TM, Lyerly HK. HER2 Overexpression Elicits a Proinflammatory IL-6 Autocrine Signaling Loop That Is Critical for Tumorigenesis. Cancer Res. 2011;71:4380–4391. doi: 10.1158/0008-5472.CAN-11-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Luistro L, Carvajal D, Smith M, Nevins T, Yin X, Cai J, Higgins B, Kolinsky K, Rizzo C, et al. High tumor levels of IL6 and IL8 abrogate preclinical efficacy of the gamma-secretase inhibitor, RO4929097. Mol Oncol. 2011;5:292–301. doi: 10.1016/j.molonc.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139:693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliopoulos D, Hirsch HA, Wang G, Struhl K. Inducible formation of breast cancer stem cells and their dynamic equilibrium with non-stem cancer cells via IL6 secretion. Proc Natl Acad Sci U S A. 2011;108:1397–1402. doi: 10.1073/pnas.1018898108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliopoulos D, Jaeger SA, Hirsch HA, Bulyk ML, Struhl K. STAT3 activation of miR-21 and miR-181b-1 via PTEN and CYLD are part of the epigenetic switch linking inflammation to cancer. Mol Cell. 39:493–506. doi: 10.1016/j.molcel.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkaya H, Liu S, Wicha MS. Regulation of Cancer Stem Cells by Cytokine Networks: Attacking Cancers Inflammatory Roots. Clin Cancer Res. 2011 doi: 10.1158/1078-0432.CCR-10-2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkaya H, Paulson A, Charafe-Jauffret E, Ginestier C, Brown M, Dutcher J, Clouthier SG, Wicha MS. Regulation of mammary stem/progenitor cells by PTEN/Akt/beta-catenin signaling. PLoS Biol. 2009;7:e1000121. doi: 10.1371/journal.pbio.1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkaya H, Paulson A, Iovino F, Wicha MS. HER2 regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion. Oncogene. 2008;27:6120–6130. doi: 10.1038/onc.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan KH, Lu CH, Yu D. Mechanisms of trastuzumab resistance and their clinical implications. Ann N Y Acad Sci. 2005;1059:70–75. doi: 10.1196/annals.1339.026. [DOI] [PubMed] [Google Scholar]
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison R, Schleicher SM, Sun Y, Niermann KJ, Kim S, Spratt DE, Chung CH, Lu B. Targeting the mechanisms of resistance to chemotherapy and radiotherapy with the cancer stem cell hypothesis. J Oncol. 2011;2011:941876. doi: 10.1155/2011/941876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata Y, Lan KH, Zhou X, Tan M, Esteva FJ, Sahin AA, Klos KS, Li P, Monia BP, Nguyen NT, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6:117–127. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- Salgado R, Junius S, Benoy I, Van Dam P, Vermeulen P, Van Marck E, Huget P, Dirix LY. Circulating interleukin-6 predicts survival in patients with metastatic breast cancer. Int J Cancer. 2003;103:642–646. doi: 10.1002/ijc.10833. [DOI] [PubMed] [Google Scholar]
- Sansone P, Storci G, Tavolari S, Guarnieri T, Giovannini C, Taffurelli M, Ceccarelli C, Santini D, Paterini P, Marcu KB, et al. IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J Clin Invest. 2007;117:3988–4002. doi: 10.1172/JCI32533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller J, Ohnesorge N, Rose-John S. Interleukin-6 trans-signalling in chronic inflammation and cancer. Scand J Immunol. 2006;63:321–329. doi: 10.1111/j.1365-3083.2006.01750.x. [DOI] [PubMed] [Google Scholar]
- Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- Sullivan NJ, Sasser AK, Axel AE, Vesuna F, Raman V, Ramirez N, Oberyszyn TM, Hall BM. Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human breast cancer cells. Oncogene. 2009;28:2940–2947. doi: 10.1038/onc.2009.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenberger J, Loeffler S, Kappeler A, Kopf M, Lukes A, Afanasieva TA, Aguzzi A, Weis J. IL-6 is required for glioma development in a mouse model. Oncogene. 2004;23:3308–3316. doi: 10.1038/sj.onc.1207455. [DOI] [PubMed] [Google Scholar]
- Yao Z, Fenoglio S, Gao DC, Camiolo M, Stiles B, Lindsted T, Schlederer M, Johns C, Altorki N, Mittal V, et al. TGF-beta IL-6 axis mediates selective and adaptive mechanisms of resistance to molecular targeted therapy in lung cancer. Proc Natl Acad Sci U S A. 2010;107:15535–15540. doi: 10.1073/pnas.1009472107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Baxter PA, Voicu H, Gurusiddappa S, Zhao Y, Adesina A, Man TK, Shu Q, Zhang YJ, Zhao XM, et al. A clinically relevant orthotopic xenograft model of ependymoma that maintains the genomic signature of the primary tumor and preserves cancer stem cells in vivo. Neuro Oncol. 2010;12:580–594. doi: 10.1093/neuonc/nop056. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1, related to Figure 4. (A) Treatment of Sum159 cells with anti-IL-6R antibody for 5 days reduced the CD44+CD24− population and (B) there was a significant growth arrest following 5 days of anti-IL-6R ab treatment despite no significant apoptosis induced.
IL-6 induced CD44+/CD24− and Aldefluor-positive population in MCF7, Sum159, BT474 and SKBR3. (C) IL-6 induces CD44+/CD24− populations in all four breast cancer cell lines. (D) However IL-6 was only able to increase Aldefluor-positive cell population in MCF7 and Sum159 cells, while slightly reducing this population in HER2 amplified BT474 and SKBR3 cell lines. Error bars represent the mean and standard deviation of two independent experiments performed in duplicate samples. (*p ≤ 0.05, #p ≤ 0.01).
Figure S2, related to Figure 5. (A) Co-culture of parental BT474 (GFP) cells with BT474-PTEN-LTT (DsRed) cells for three weeks resulted in induction of CD44+CD24− population in parental cells. To eliminate the cross contamination of parental cells and BT474-PTEN-LTT cells, single GFP expressing cells were flow sorted from these co-cultures and multiple colonies of parental BT474 cells were generated. Three representative colonies show that these cells even in the absence of BT474-PTEN-LTT cells can maintain high CD44+ cell population despite regaining the CD24 expression.
(B and C) Long term treatment (10 days) of parental BT474-DsRed and SKBR3-DsRed cells with conditioned medium (CM) from BT474-PTEN-LTT cells or recombinant IL-6 further increased CD44+ expression which is more than the 5 days treatment as shown in Figure 5C.
Error bars represent the mean and standard deviation of two independent experiments performed in duplicate samples.