Abstract
Lymphocytic infiltration of the lacrimal gland and ocular surface in autoimmune diseases such as Sjögren's syndrome (SS) causes an aqueous-deficient dry eye that is associated with significant morbidity. Previous studies from our laboratory and others have established autoimmune regulator (Aire)–deficient mice as a useful model to examine exocrinopathy and ocular surface disease associated with SS. Consistent with human SS, autoreactive CD4+ T cells play an indispensible role in the development of exocrine and ocular surface disease in Aire knockout mice. We report that in addition to CD4+ T cells, a large number of macrophages infiltrate the corneal stroma, limbus, and lacrimal glands of diseased mice. Adoptive transfer of autoreactive CD4+ T cells from Aire knockout mice led to local infiltration of macrophages and ocular surface damage in immunodeficient recipients. Depletion of local macrophages, through subconjunctival injection of clodronate liposome, attenuated lissamine green staining and improved ocular phenotype. Alternatively, systemic depletion of macrophages had no effect on ocular phenotype but led to significant improvements in lacrimal gland exocrinopathy and tear secretion. Our results suggested that autoreactive CD4+ T cells provoked macrophage infiltration to the eye and lacrimal gland, where they played a functional role in directing the development of autoimmune dry eye.
Sjögren's syndrome (SS) is a disease characterized by autoimmune destruction of the salivary and lacrimal glands that can progress to severe xerostomia (dry mouth) and keratoconjunctivitis sicca (dry eye). Loss of aqueous tears sets off a proinflammatory stress response at the ocular surface that leads to immune cell infiltration and loss of epithelial integrity with increased lissamine green staining. With persistent inflammation, the ocular mucosa transitions from a nonkeratinized, mucous-secreting epithelium to one that is pathologically keratinized and skinlike. This process, known as squamous metaplasia (SQM), represents a devastating end-stage consequence of autoimmune dry eye with clinical manifestations of corneal opacification and surface keratinization.1,2 As of today, there is no efficient clinical approach to prevent or reverse aqueous-deficient dry eye and associated SQM. A better understanding of the mechanisms of disease development in the context of autoimmunity is needed.
Previously, we described the use of autoimmune regulator (Aire)–deficient mice as an animal model of aqueous-deficient dry eye in autoimmune diseases such as SS.3,4 Aire, a transcription factor expressed in a subset of medullary thymic epithelial cells, regulates expression of numerous tissue-specific self-antigens essential for the removal of autoreactive lymphocytes.5 Loss of functional Aire leads to multiorgan autoimmune disease, including an exocrinopathy that affects the salivary and lacrimal glands.5,6 Adult Aire knockout (KO) mice show decreased production of saliva and tears and lissamine green staining that mimics the clinical characteristics of SS dry eye.
Similar to human patients with SS, lymphocytes infiltrate the tissues of Aire KO mice,7 and autoreactive CD4+ T cells play a critical role in the development of dry eye and SQM.4,7 Severity of ocular surface disease was correlated with proinflammatory activity mediated via IL-1, and IL-1 signaling proved to be a critical component initiating and perpetuating CD4+ T cells to provoke ocular disease development.3,8 To further decipher immune events that promote autoimmune dry eye and SQM in SS-associated autoimmunity, we sought to identify cellular intermediates that coordinate the local inflammatory response of resident cells with infiltrating CD4+ T cells.
Macrophages interact intimately with CD4+ T cells, serving as both T cell–directed phagocytes and T cell–activating antigen-presenting cells (APCs). Macrophages present antigenic peptides complexed with major histocompatibility complex class II to antigen-specific CD4+ T cells and reciprocally are activated by CD4+ T cells. As we have previously demonstrated, macrophages secrete a variety of proinflammatory cytokines, including IL-1, that play a critical role in promoting the development of SQM.3 Infiltration of CD68+ macrophages has been noted in the salivary gland of SS patients, where interferon-γ secreted by TH1 and IL-17 secreted by TH17 cells can directly activate macrophage infiltration and exocrinopathy.9,10 The functional involvement of macrophages in autoimmune disease has been gaining more attention and is greatly facilitated by the use of clodronate liposome to effectively deplete macrophage in vivo.11 With this approach, clodronate depletion has attenuated disease progression in autoimmune disorders, such as psoriasis and experimental uveitis.12,13
In Aire KO mice, we observed a large number of infiltrating macrophages adjacent to CD4+ T cells throughout the ocular surface and lacrimal glands. We hypothesized that these macrophages were recruited and activated after CD4+ T-cell infiltration, where they amplify and sustain ocular inflammation in autoimmune dry eye. Our data suggested that macrophage infiltration in Aire KO mice was CD4+ T-cell dependent. Ocular manifestations of disease were alleviated after local macrophage depletion via subconjunctival clodronate liposome injection and lacrimal gland disease improved after systemic depletion. Protection occurred without a significant reduction in local CD11c+ dendritic cell or CD4+ T-cell infiltration. Thus, we believe infiltrating macrophages play an essential role in the pathogenesis of autoimmune dry eye by acting downstream of autoreactive CD4+ T cells to mediated local tissue damage.
Materials and Methods
Mice and Reagents
Mouse strains with targeted mutation in the Aire gene [BALB/c and nonobese diabetic (NOD) Lt/J background] were gifts from Dr. Mark Anderson, University of California, San Francisco. Mice were housed in a pathogen-free barrier facility at University of California, San Francisco. Offspring were genotyped for the Aire mutation by PCR of genomic DNA as previously described.5 All experimental procedures adhered to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
Lissamine green dye was purchased from Leiter's Pharmacy and Compounding Center (San Jose, CA). Pilocarpine was from Sigma-Aldrich (St. Louis, MO), optimal cutting temperature compound was from Sakura (Tokyo, Japan), and DAPI was purchased from Molecular Probes (Eugene, OR). Rat IgG, F4/80, and MOMA-2 antibodies were from AbD Serotec (Raleigh, NC). CD11c and CD4 antibodies were from BD Pharmingen (San Diego, CA). Alexa Fluor 488–conjugated donkey–anti-rat IgG and goat–anti-mouse IgG were from Molecular Probes. Cyanine cy3-conjugated goat–anti-mouse–horseradish peroxidase and goat–anti-Armenian hamster IgG were from Jackson Immunoresearch Lab (West Grove, PA). Criterion precast gel (4% to 20%; Tris-Hcl) was from Bio-Rad (Hercules, CA). ECL Western Blotting Analysis System was from Amersham (Piscataway, NJ).
Adoptive Transfer of CD4+ T Cells
Adoptive transfer of CD4+ T cells was done as previously described.7 Briefly, lymphocytes were isolated from the regional lymph node and spleen of 7-week-old Aire KO or Aire wild-type (WT) NOD mice. The CD4+ T-cell population was enriched using magnetic beads sorting, and 5 × 106 cells were injected through tail veins to 6- to 8-week-old NOD, severe combined immunodeficiency (SCID) recipients. Disease development was monitored and mice were sacrificed 6 weeks after the adoptive transfer.
Macrophage Depletion
Clodronate was a gift from Roche.11 To deplete local macrophages, 15 μL (∼100 μg) of clodronate or PBS liposomes was injected into the subconjunctival space of Aire KO mice (7 to 10 weeks of age) at a frequency of twice per week for 2 weeks. To deplete systemic macrophages, 100 to 400 μL (∼0.7 to 2.8 mg) of clodronate or PBS liposomes was injected i.p. three times per week for 3 weeks to Aire KO mice (6 to 9 weeks of age). Local depletion of macrophages after subconjunctival injection was confirmed by monitoring F4/80+cells in the ocular tissues, whereas systemic depletion after i.p. injection was monitored by F4/80+ immunostaining of the spleen in Aire KO mice.
Lissamine Green Staining of the Ocular Surface
Mice were anesthetized with isoflurane, and 5 μL of lissamine green dye (1%) was applied to the ocular surface. Images were taken using an Olympus Zoom Stereo Microscope (Olympus, Center Valley, PA). Quantification was performed using NIS Elements Advanced Research 3.10 (Nikon Instruments Inc., Melville, NY). A region of interest, encompassing the entire ocular surface, was selected, and the total area of positive lissamine green staining was calculated using the binary-threshold tool of NIS Elements. The extent of lissamine green staining was expressed as the ratio of total lissamine green positive signal area to the total region of interest area.
Quantification of Epithelial Hyperplasia
Ocular surface tissue sections photographed at ×20 were appropriately calibrated to measure the actual distance between any two points in the image using NIS Elements Advanced Research 3.10. The corneal epithelium was measured in micrometers at three different cross-sectional positions in the central cornea, and mean thickness was calculated for each animal.
Tear Secretion Measurement
Mice were anesthetized with isoflurane. Pilocarpine diluted in saline was injected i.p. at a dosage of 4.5 mg/kg. Ten minutes later, mice were anesthetized with isoflurane and tear secretion (as indicated by the length of the tear-absorbed region) was measured using Zone-Quick phenol red thread (Showa Yakuhin Kako Co. Ltd., Tokyo, Japan). Aire KO mice demonstrating a phenotypic decrease in tear secretion, defined as a measurement of ≤10 mm on the phenol red thread test, were included in our macrophage depletion studies.
Immunofluorescence and H&E Staining
For immunofluorescence detection of cells expressing F4/80, MOMA-2, CD4, or CD11c, frozen tissue sections were fixed in acetone followed by washing with PBS Tween. After blocking for 1 hour, sections were incubated with primary antibodies or isotype controls at 1:25 dilution at 4°C overnight. After washing, appropriate secondary antibody was added at a 1:200 dilution and nuclei were stained with DAPI. Immunofluorescent staining of cells was photographed with a Nikon Eclipse Ti-E microscope. Photographed images were analyzed with NIS Elements Advanced Research 3.10 to estimate the number of nucleus-associated F4/80+ and MOMA-2+ cells across the entire ocular surface, including the limbus and cornea. F4/80+ and MOMA-2+ cells (stained green with Alexa Fluor 488) with associated nuclei (stained blue with DAPI) were manually counted after merging the signals from the two fluorophores. Similarly, CD4+ and CD11c+ cells labeled with Cy3 were manually counted with their associated nuclei. Data have been reported as the total number of nucleus-associated F4/80+, MOMA-2+, CD4+, or CD11c+ cells.
For H&E staining, slides were incubated in Gill's hematoxylin for 3 minutes followed by destaining with 4% acetic acid. Staining nuclei in Scott's water was followed by counterstaining with Eosin Y. Sections were covered with Cytoseal 60 and coverslips after dehydration. Immune cell infiltration of the lacrimal glands was estimated by analyzing H&E-stained images of gland sections using NIS Elements. For quantitative analysis representative of the entire gland, we selected three sections from three different planar regions of the lacrimal gland, and all three sections were analyzed. The total area of the lacrimal gland section was selected as the region of interest, and the total area of signals from immune cells was calculated and expressed as a percentage of the region of interest area using the binary-threshold tool of NIS Elements. The data were reported as the mean immune cell infiltration of three sections.
Detection of Lacrimal Gland-Specific Autoantibodies in Serum
The method was modified from a previous publication.6 Briefly, two lacrimal glands from a WT mouse were homogenized in 250 μL of 2× Laemmli buffer and boiled for 10 minutes. After spinning down debris, supernatant samples were loaded on a 4% to 20% gradient Criterion gel in multiple wells (25 μL per well) and transferred to a nitrocellulose membrane after SDS-PAGE separation. After overnight blocking with 5% milk, individual lanes on membrane were incubated with diluted serum samples (1:2000) at room temperature for 90 minutes. Antibodies bound to the membrane were detected by incubation with horseradish peroxidase goat–anti-mouse (1:10,000 dilution) for 1 hour followed by chemiluminescence reagent treatment and autoradiography.
Statistical Analysis
We used unpaired t-test (two-tailed) or nonparametric Kruskal-Wallis test, depending on data distribution, to compare macrophage infiltration in i) Aire WT versus Aire KO mice, ii)Aire WT → SCID versus Aire KO → SCID adoptive transfer recipients, and iii)clodronate versus PBS liposome-injected Aire KO mice. Differences in lissamine green staining, epithelial thickness, tear secretion, and immune cell infiltration were compared after clodronate versus PBS liposome injection using the unpaired t-test. All analyses were performed using Stata 9.0 (Stata Corp., College Station, TX) for MacIntosh. P ≤ 0.05 was considered statistically significant.
Results
Macrophages Infiltrate the Corneal Stroma and Limbus in Aire-Deficient Mice
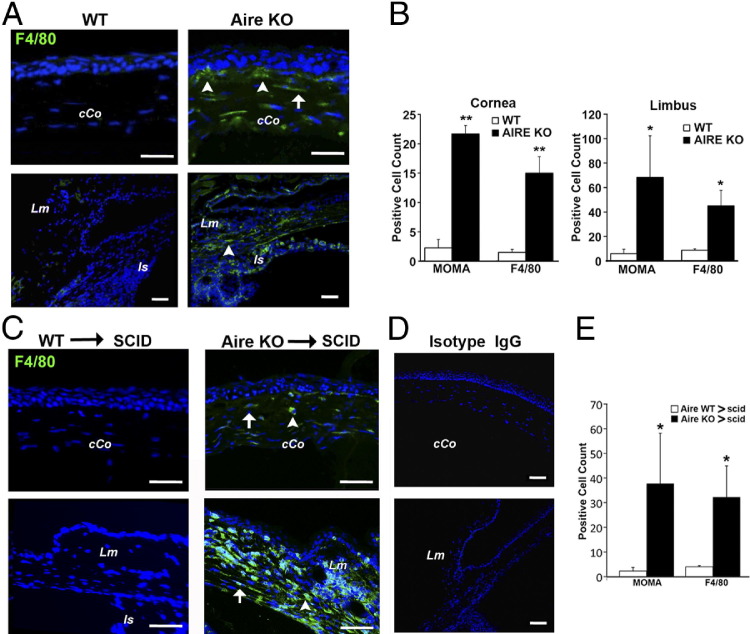
Aire KO mice spontaneously develop SS-like disease with associated exocrinopathy and corneal epitheliopathy as indicated by decreased tear secretion, severe lissamine green staining, and decreased conjunctival goblet cell density.4,6 Using extracellular and intracellular macrophage markers, F4/80 and MOMA-2, respectively, we revealed an accumulation of macrophages in the limbus and central corneal of Aire KO mice (Figure 1A), whereas few macrophages were noted in the eyes of Aire-sufficient, WT mice (Figure 1A). Although F4/80 and MOMA-2 have been reported to detect different macrophage subpopulations,14 similar staining patterns were observed with F4/80 and MOMA-2 antibodies in Aire KO mice. Positively stained cells were enumerated and cell counts are provided in Figure 1B. Both MOMA-2+ and F4/80+ cells were significantly increased in the central cornea (MOMA-2: 22 ± 1.45 versus 2 ± 1.44, P < 0.01; and F4/80: 15 ± 2.80 versus 1.5 ± 0.5, P < 0.01) and limbus (MOMA-2: 68 ± 34.03 versus 6 ± 3.51, P < 0.05; and F4/80: 45 ± 12.77 versus 9 ± 1.11, P < 0.05) of Aire KO versus Aire WT mice.
Figure 1.
Macrophage infiltration of the central corneal (cCo) and limbus (Lm) in Aire KO mice. A: Eye tissue sections from Aire KO or WT mice were stained with antibodies directed against F4/80, MOMA-2, or IgG isotype control. F4/80+ cells infiltrating the subepithelial (arrowheads) and stromal (arrow) compartments of the cCo are indicated. The limbal region was identified using the iris insertion (Is) as an anatomical landmark. Results shown are representative of at least four independent tests. B: Quantitative analysis of MOMA-2+ and F4/80+ macrophages in the cornea and limbus of Aire KO and WT mice, shown as mean ± SE cell count. *P < 0.05 and **P < 0.01. C: CD4+ T cells from Aire KO or WT mice were adoptively transferred to immunodeficient SCID recipients. Results shown are representative of four independent tests. Mice were sacrificed at 40 days and eye sections assessed by immunofluorescence using antibodies directed against F4/80, MOMA-2, or isotype control (D). E: Quantitative analysis of corneal MOMA-2+ and F4/80+ macrophages in recipients of autoreactive CD4+ T cells (Aire KO → SCID) or WT CD4+ T cells (Aire WT → SCID), shown as mean ± SE cell number. *P < 0.05.
Adoptive Transfer of Autoreactive CD4+ T Cells Promotes Ocular Infiltration of Macrophages
CD4+ T cells are the essential mediators of autoimmune dry eye and SQM in Aire KO mice.6,15 Previously, we reported that adoptive transfer of autoreactive CD4+ T cells from Aire KO mice to SCID recipients resulted in autoimmune dry eye and SQM in <6 weeks.3 Consistent with dry eye development, we observed limbal and stromal infiltration of F4/80+ macrophages after adoptive transfer of autoreactive CD4+ T cells (Figure 1C). Staining with isotype control antibody produced negative results (Figure 1D). Quantification of MOMA-2– and F4/80-stained cells revealed that the overall infiltration of macrophages was enhanced after adoptive transfer of autoreactive CD4+ T cells (Figure 1E). MOMA-2+ (38 ± 20.41 versus 2 ± 1.44, P < 0.05) and F4/80+ (32 ± 12.66 versus 4 ± 0.45, P ≤ 0.05) cells in the cornea were significantly increased in Aire KO → SCID versus Aire WT → SCID recipients. Infiltration of the limbus was especially intense, making it difficult to enumerate. These results suggested that macrophage infiltration of the ocular surface was autoreactive CD4+ T-cell dependent. We hypothesized that CD4+ T cells infiltrating the ocular tissues promoted the local production of chemokines to elicit macrophage recruitment.
Local Depletion of Macrophages Improves Ocular Phenotype
To test the hypothesis that CD4+ T cells infiltrating the ocular tissues promoted the local production of chemokines to elicit macrophage recruitment, we used an approach previously shown to efficiently deplete local macrophages through subconjunctival injection of clodronate liposome.16,17 Lissamine green staining measured before versus after biweekly injections for 2 weeks (total of four injections) revealed remarkably reduced lissamine green staining in mice treated with clodronate (40.0% ± 4.5% versus 5.9% ± 1.8%, P < 0.01) compared with PBS liposome control (28.7% ± 5.4% versus 25.7% ± 7.9%, P > 0.05) (Figure 2, A and B). F4/80+ cells were decreased throughout the ocular surface (counting limbus to limbus) in clodronate versus PBS-treated Aire KO mice (32 ± 4.53 versus 104 ± 20.10, P < 0.01) (Figure 2C). In contrast, CD4+ T-cell infiltration (Figure 2C) was unaltered by clodronate treatment (122 ± 17.08 versus 101 ± 20.69, P > 0.05). Similarly, the intensity and localization of CD11c+ dendritic cells (Figure 2C) were unchanged in clodronate versus PBS-treated mice (63 ± 15 versus 72 ± 15, P > 0.05), suggesting that clodronate depletion was largely specific to local macrophages. Also, focal lymphocyte infiltration of the lacrimal gland and pilocarpine-induced tear secretion were similar between the PBS and clodronate liposome groups (data not shown).
Figure 2.
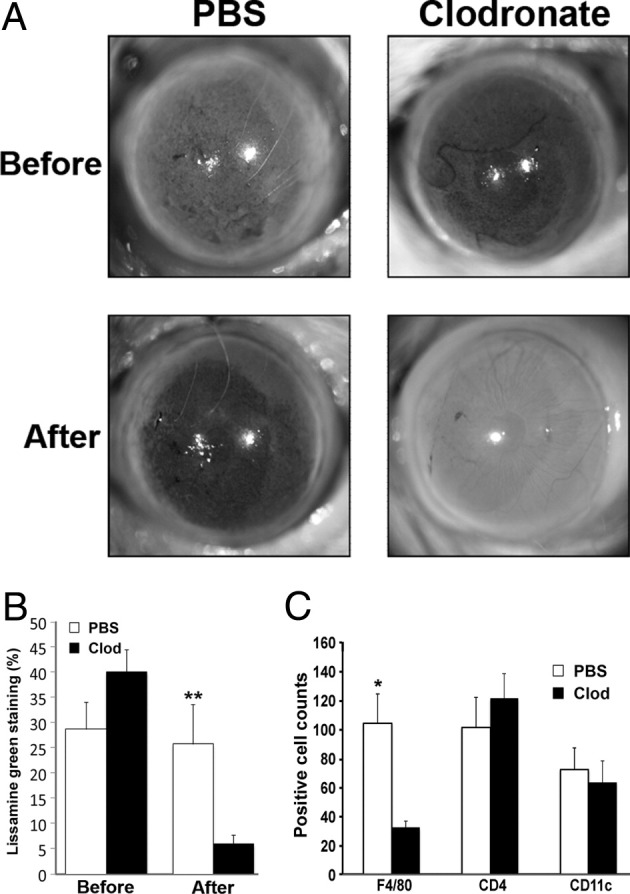
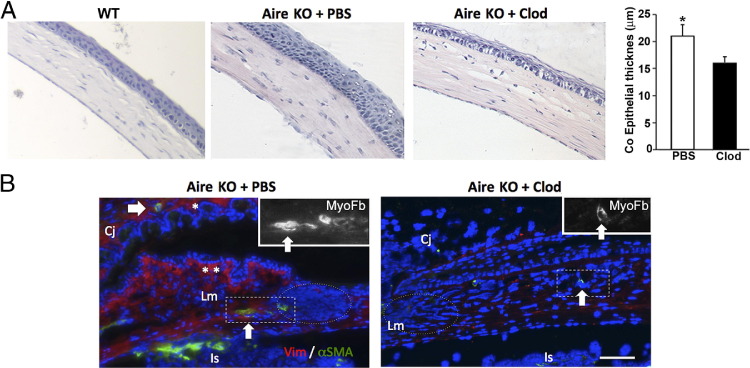
Effect of local macrophage depletion on ocular surface integrity and immune cell infiltration. A: Aire KO mice receiving clodronate liposome via subconjunctival injection twice a week for 2 weeks demonstrated significant improvement of ocular surface integrity as assessed by lissamine green staining. Staining was performed before and after the 2-week treatment with clodronate liposome (Clod) or PBS liposome control (PBS). B: Percentage of ocular surface staining with lissamine green is shown as mean ± SE (n = 5 to 7 per group). C: Quantification of F4/80+, CD4+, and CD11c+ cells across the ocular surface of Aire KO mice treated with Clod or PBS liposome. Data shown as mean ± SE cell number (n = 5 to 7 mice per group), *P < 0.05 and **P < 0.01.
Chronic aqueous tear deficiency is accompanied by phenotypic alterations of the ocular surface, which can include corneal epithelial cell hyperplasia and stromal fibrosis. In previous reports, we found that corneal epithelial hyperplasia in Aire KO mice was associated with CD4+ T-cell–dependent, local IL-1R1–mediated inflammation of the ocular surface.3 We observed and quantified (Figure 3A) epithelial hyperplasia in the presence and absence of macrophages. We observed a substantial decrease in corneal epithelial thickness in clodronate versus PBS liposome–treated mice (16 ± 1.1 versus 21 ± 2.1 μm, P < 0.05), suggesting the potential role of macrophages in promoting structural changes of the ocular epithelium in autoimmune keratoconjunctivitis sicca. Moreover, immunofluorescent staining with stromal fibroblast and myofibroblast markers, vimentin, and α-smooth muscle actin, respectively, revealed alterations in stromal architecture and cell types in Aire KO mice. Fibrotic changes were indicated by subepithelial, hypertrophic vimentin-positive (asterisk), and α-smooth muscle actin–positive fibroblasts (white arrow) in the peripheral cornea, limbus, and conjunctiva (Figure 3B). These results suggest that the development of corneal epitheliopathy, epithelial hyperplasia, and fibrotic changes in the corneal stroma of Aire KO mice were dependent, in part, on macrophage recruitment.
Figure 3.
Histologic analysis of ocular phenotype after local macrophage depletion with clodronate liposome. A: H&E staining reveals ocular surface hyperplasia as evidenced by significant thickening of the corneal (Co) epithelium, plotted as mean ± SE micrometers (n = 5 per group), P < 0.05. B: Pronounced fibrotic changes in Aire KO mice treated with PBS liposome were suggested by the increased number of subepithelial fibroblasts staining positive with vimentin (Vim+ red cells indicated by asterisks) and deeper stromal myofibroblast stained with α-smooth muscle actin (α-SMA+ green cells indicated by an arrow) adjacent to dense cellular infiltration (nuclei stained blue with DAPI, dotted circle). Aire KO mice treated with clodronate (Clod) revealed less infiltrate and lower density of fibroblasts and myofibroblast (right). Insets are high-power views of the myofibroblasts in the boxed areas. Cj, conjunctiva; Is, iris insertion; Lm, limbus. Scale bar = 50 μm. Data are representative of n = 5 per group.
Systemic Depletion of Macrophage Improves Exocrinopathy and Tear Secretion
Lymphocytic infiltration and destruction of acinar units in the lacrimal gland by autoreactive T cells is an essential component of SS-associated dry eye. Although local depletion of macrophages using subconjunctival clodronate liposome did not diminish macrophage infiltration of the lacrimal gland, we speculated that systemic depletion of macrophages would alleviate lacrimal gland inflammation and improve tear secretion. We administered clodronate liposome i.p. three times weekly for 3 weeks to deplete systemic macrophages. Initially, we used 400 μL of liposome but observed a high mortality rate (84.6%) in both Aire KO and WT mice backcrossed to the BALB/c background. The high mortality rate was substantially reduced after decreasing the clodronate liposome dosage to 100 μL (61.1%). Although the cause of death was not clear, systemic infection after macrophage depletion was a plausible explanation. Environmental factors and genetic background may also have contributed. Animals receiving clodronate consistently experienced weight loss without diarrhea. For the purposes of our study, mice experiencing ≥20% loss of body weight during the 3-week treatment period were excluded from analysis. Accordingly, the mean weight loss of clodronate-treated mice that met the inclusion criteria of our study was <1.0 g. Efficient systemic depletion was confirmed at sublethal doses by monitoring the presence of F4/80+ macrophages in the spleen of Aire KO mice (Figure 4A). Diffuse infiltration of the Aire KO lacrimal glands with F4/80+ macrophages was effectively depleted through the systemic administration of clodronate liposome (Figure 4B). Despite efficient systemic depletion, there was no change in serum autoantibody levels in Aire KO mice (Figure 4C), and ocular surface staining with lissamine green did not improve (data not shown). Alternatively, immune cell infiltration throughout the lacrimal gland was significantly reduced (36.8% ± 4.52% versus 16.6% ± 2.89%, P < 0.01) (Figure 4, D and E) and tear secretion significantly increased (6.25 ± 0.9 mm versus 12.1 ± 1.6 mm, P < 0.05) (Figure 4F) after clodronate injection. These results suggested that systemic depletion of macrophages reduced immune cell infiltration and restored lacrimal gland function.
Figure 4.
Systemic depletion of macrophages increased tear secretion in Aire KO mice by preserving acinar tissues. Immunofluorescent staining of F4/80+ macrophages in the spleen (A) and lacrimal gland (B) (asterisks) are depleted after i.p. injection of clodronate liposome (Clod). F4/80+ cells appear to surround dense cellular aggregates (nuclei stained blue with DAPI, dotted circle), and bordering F4/80+ cells are depleted after i.p. clodronate (yellow box). C: Tissue lysate from WT lacrimal gland were resolved on SDS-PAGE and transferred to nitrocellulose membrane. Western blot was performed to assess the presence of lacrimal gland specific autoantibodies against an 18-kDa self-antigen, oderant binding protein, in serum samples from WT and Aire KO mice treated with Clod or PBS liposome. D: Histologic findings of lacrimal gland tissues from WT or Aire KO mice treated with Clod or PBS liposome (H&E staining, original magnification ×4). E: Percentage of immune cell infiltration in the lacrimal parenchyma. Data are presented as mean ± SE (n = 5 per group). F: Tear secretion was measured by Zone-Quick thread before and after systemic treatment with Clod or PBS. Reported as mean ± SE millimeters of wetting (n = 5 per group). *P < 0.05 and **P < 0.01.
Discussion
CD4+ T cells are the essential driving force promoting autoimmune dry eye in Aire KO mice and patients with SS.18 The current literature supports the idea that the combined effects of TH1 and TH17 CD4+ T-cell subsets dictate disease development in patients with SS. In the current study, we aimed to reveal the link between autoreactive CD4+ T cells and the development of ocular surface disease. In addition to providing a major source of proinflammatory cytokines, including IL-1, macrophages play many roles in both innate and adaptive immunity. As multitasking inflammatory cells, they are activated by TH1 and TH17 CD4+ T cells and also interact with them through antigenic epitopes presented by major histocompatibility complex class II molecules. The activation of macrophages by T cells is critical in the control of intracellular pathogens, and, on activation, macrophages are essential for coordinating T-cell–mediated autoimmune diseases, such as psoriasis and experimental uveitis.12,19,20
Although macrophages express major histocompatibility complex class II and are classified as professional APCs, unlike dendritic cells, they are not considered to be the primary APC responsible for activating naive CD4+ T cells. Instead, they interact with primed CD4+ T cells and are activated by T cells through T cell receptor and major histocompatibility complex class II/peptide complex engagement. In this study, we observed significant macrophage infiltration of the cornea and limbus in Aire KO mice, with the presence of F4/80+ and MOMA-2+ macrophages outnumbering that of CD11c+ dendritic cells. Using adoptive transfer, we provided direct evidence that autoreactive CD4+ T cells function independent of other immune cells to promote macrophage infiltration and accumulation. Thus, we hypothesized that autoreactive CD4+ T cells governed macrophage infiltration through the local release of chemokines. Indeed, it has been shown that infiltrating CD4+ T cells can lead to increased levels of chemokines, including monocyte chemoattractant proteins responsible for monocyte/macrophage recruitment. For example, TH1 cytokine interferon-γ stimulates the production of monocyte chemoattractant protein 1 in human endothelial cells,21 peripheral blood mononuclear leukocytes,22 and mesangial cells.23 IL-17 secreted by TH17 CD4+ T cells stimulates epithelial and fibroblastic cells to produce proinflammatory chemokines, including IL-8 and monocyte chemoattractant protein 1.24 Thus, the recruitment of macrophages by autoreactive CD4+ T cells is likely one mechanism whereby chronic inflammation is established and local tissues are ultimately destroyed.
To address the involvement of macrophages in Aire-mediated autoimmunity, we treated mice both locally and systemically with clodronate liposomes. Clodronate effectively depleted local macrophages without altering the levels of CD11c+ dendritic cells. The beneficial effect of clodronate in Aire KO mice was in line with clodronate's effects in other autoimmune disease models. For example, depletion of skin macrophages by subcutaneous injection of clodronate liposomes resulted in significant attenuation of psoriasis-like skin changes in mice.13,19 Subconjunctival injection of clodronate liposomes prevented corneal graft rejection in rats,17 whereas intraperitoneal administration of clodronate attenuated retention of lymphocytes in insulitis of NOD mice.25 In Aire KO mice, subconjunctival injection proved efficient in attenuating corneal epitheliopathy, hyperplasia, and stromal fibrosis, suggesting both growth and immune regulatory roles for macrophages in the development of autoimmune keratoconjunctivitis sicca.
Lymphocytic infiltration of the limbus and stroma in Aire KO mice was unaltered by clodronate treatment. Improved ocular surface phenotype in the presence of autoreactive CD4+ T cells mimicked earlier studies in which Aire KO mice deficient in IL-1/IL-1R1 signaling showed significant improvement in ocular phenotype without significant reduction of infiltrating CD4+ T cells. Thus, both macrophages and IL-1/IL-1R1 appear to function locally, downstream of CD4+ T-cell activation and infiltration, to mediate the immunopathologic features of autoimmune dry eye. This finding is in contrast to a recent study using a mouse model of dry eye induced by desiccating stress where subconjunctival administration of clodronate liposomes efficiently inhibited CD4+ T-cell accumulation within the ocular surface and the generation of autoreactive CD4+ T cells.26 The difference between these studies may be attributed to the unique nature of Aire KO mice as a model of spontaneous autoimmunity, where centrally primed autoreactive CD4+ T cells do not need to reencounter their cognate ocular APCs for peripheral maintenance. Rather, after their recruitment and activation by autoreactive CD4+ T cells, infiltrating macrophages function locally to amplify inflammation and upset tissue homeostasis to promote autoimmune dry eye. Thus, in Aire KO mice, infiltrating macrophages, and perhaps some monocyte-derived dendritic cells, function as the effector cells of preexisting autoimmune CD4+ T cells with ocular surface antigen specificity.
Given the functional significance of infiltrating macrophages in provoking ocular manifestations associated with autoimmune dry eye, we considered the extent to which they contributed to lacrimal gland exocrinopathy. Although repetitive i.p. injection of clodronate did not improve ocular surface epitheliopathy, it promoted a striking restoration of tear secretion and attenuated the destruction of lacrimal gland acinar units. Although it is not clear why i.p. clodronate removed macrophages from the lacrimal gland and not the conjunctiva, we believe the local density of clodronate liposome delivered via i.p. injection to the immune privilege eye may have been below that required for macrophage depletion, whereas that reaching the lacrimal gland was sufficient. Given the relatively low concentration of i.p. clodronate necessary to achieve a sublethal dose, we determined local administration as the most efficient way to selectively deplete ocular resident macrophages. Although ocular surface epitheliopathy is traditionally believed to occur as a direct consequence of lacrimal gland destruction and aqueous tear deficiency, we found that reducing local inflammation through subconjunctival clodronate injection significantly improved ocular surface integrity even in the absence of improved lacrimal gland function. Thus, blocking the local effector function of ocular macrophages provides promise as a treatment for autoimmune dry eye even in the setting of persistent aqueous tear deficiency.
Footnotes
Supported by an NIH National Eye Institute grant (EY016203 to N.A.M.) and a University of California, San Francisco, core grant (EY02162).
References
- 1.Kinoshita S., Nakamura T., Nishida K. Pathological keratinization of ocular surface epithelium. Adv Exp Med Biol. 2002;506:641–646. doi: 10.1007/978-1-4615-0717-8_90. [DOI] [PubMed] [Google Scholar]
- 2.McNamara N.A. Molecular mechanisms of keratinizing ocular surface disease. Optom Vis Sci. 2010;87:233–238. doi: 10.1097/OPX.0b013e3181c914ed. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y.T., Nikulina K., Lazarev S., Bahrami A.F., Noble L.B., Gallup M., McNamara N.A. Interleukin-1 as a phenotypic immunomodulator in keratinizing squamous metaplasia of the ocular surface in Sjögren's syndrome. Am J Pathol. 2010;177:1333–1343. doi: 10.2353/ajpath.2010.100227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li S., Nikulina K., DeVoss J., Wu A.J., Strauss E.C., Anderson M.S., McNamara N.A. Small proline-rich protein 1B (SPRR1B) is a biomarker for squamous metaplasia in dry eye disease. Invest Ophthalmol Vis Sci. 2008;49:34–41. doi: 10.1167/iovs.07-0685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson M.S., Venanzi E.S., Klein L., Chen Z., Berzins S.P., Turley S.J., von Boehmer H., Bronson R., Dierich A., Benoist C., Mathis D. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 6.DeVoss J.J., LeClair N.P., Hou Y., Grewal N.K., Johannes K.P., Lu W., Yang T., Meagher C., Fong L., Strauss E.C., Anderson M.S. An autoimmune response to odorant binding protein 1a is associated with dry eye in the aire-deficient mouse. J Immunol. 2010;184:4236–4246. doi: 10.4049/jimmunol.0902434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devoss J.J., Shum A.K., Johannes K.P., Lu W., Krawisz A.K., Wang P., Yang T., Leclair N.P., Austin C., Strauss E.C., Anderson M.S. Effector mechanisms of the autoimmune syndrome in the murine model of autoimmune polyglandular syndrome type 1. J Immunol. 2008;181:4072–4079. doi: 10.4049/jimmunol.181.6.4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y.T., Lazarev S., Bahrami A.F., Noble L.B., Chen F.Y., Zhou D., Gallup M., Yadav M., McNamara N.A. Interleukin-1 receptor mediates the interplay between CD4(+) T cells and ocular resident cells to promote keratinizing squamous metaplasia in Sjogren's syndrome. Lab Invest. 2012;92:556–570. doi: 10.1038/labinvest.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christodoulou M.I., Kapsogeorgou E.K., Moutsopoulos H.M. Characteristics of the minor salivary gland infiltrates in Sjögren's syndrome. J Autoimmun. 2010;34:400–407. doi: 10.1016/j.jaut.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Mills K.H. Induction, function and regulation of IL-17-producing T cells. Eur J Immunol. 2008;38:2636–2649. doi: 10.1002/eji.200838535. [DOI] [PubMed] [Google Scholar]
- 11.van Rooijen N., van Kesteren-Hendrikx E. Clodronate liposomes: perspectives in research and therapeutics. J Liposome Res. 2002;12:81–94. doi: 10.1081/lpr-120004780. [DOI] [PubMed] [Google Scholar]
- 12.Broekhuyse R.M., Huitinga I., Kuhlmann E.D., Rooijen N.V., Winkens H.J. Differential effect of macrophage depletion on two forms of experimental uveitis evoked by pigment epithelial membrane protein (EAPU), and by melanin-protein (EMIU) Exp Eye Res. 1997;65:841–848. doi: 10.1006/exer.1997.0396. [DOI] [PubMed] [Google Scholar]
- 13.Wang H., Peters T., Kess D., Sindrilaru A., Oreshkova T., Van Rooijen N., Stratis A., Renkl A.C., Sunderkotter C., Wlaschek M., Haase I., Scharffetter-Kochanek K. Activated macrophages are essential in a murine model for T cell-mediated chronic psoriasiform skin inflammation. J Clin Invest. 2006;116:2105–2114. doi: 10.1172/JCI27180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leenen P.J., de Bruijn M.F., Voerman J.S., Campbell P.A., van Ewijk W. Markers of mouse macrophage development detected by monoclonal antibodies. J Immunol Methods. 1994;174:5–19. doi: 10.1016/0022-1759(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y.T., Li S., Nikulina K., Porco T., Gallup M., McNamara N. Immune profile of squamous metaplasia development in autoimmune regulator-deficient dry eye. Mol Vis. 2009;15:563–576. [PMC free article] [PubMed] [Google Scholar]
- 16.Bauer D., Wasmuth S., Hermans P., Hennig M., Meller K., Meller D., van Rooijen N., Tseng S.C., Steuhl K.P., Heiligenhaus A. On the influence of neutrophils in corneas with necrotizing HSV-1 keratitis following amniotic membrane transplantation. Exp Eye Res. 2007;85:335–345. doi: 10.1016/j.exer.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slegers T.P., van der Gaag R., van Rooijen N., van Rij G., Streilein J.W. Effect of local macrophage depletion on cellular immunity and tolerance evoked by corneal allografts. Curr Eye Res. 2003;26:73–79. doi: 10.1076/ceyr.26.2.73.14510. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto K. Pathogenesis of Sjogren's syndrome. Autoimmun Rev. 2003;2:13–18. doi: 10.1016/s1568-9972(02)00121-0. [DOI] [PubMed] [Google Scholar]
- 19.Stratis A., Pasparakis M., Rupec R.A., Markur D., Hartmann K., Scharffetter-Kochanek K., Peters T., van Rooijen N., Krieg T., Haase I. Pathogenic role for skin macrophages in a mouse model of keratinocyte-induced psoriasis-like skin inflammation. J Clin Invest. 2006;116:2094–2104. doi: 10.1172/JCI27179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ward N.L., Loyd C.M., Wolfram J.A., Diaconu D., Michaels C.M., McCormick T.S. Depletion of antigen-presenting cells by clodronate liposomes reverses the psoriatic skin phenotype in KC-Tie2 mice. Br J Dermatol. 2011;164:750–758. doi: 10.1111/j.1365-2133.2010.10129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rollins B.J., Yoshimura T., Leonard E.J., Pober J.S. Cytokine-activated human endothelial cells synthesize and secrete a monocyte chemoattractant, MCP-1/JE. Am J Pathol. 1990;136:1229–1233. [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshimura T., Yuhki N., Moore S.K., Appella E., Lerman M.I., Leonard E.J. Human monocyte chemoattractant protein-1 (MCP-1): full-length cDNA cloning, expression in mitogen-stimulated blood mononuclear leukocytes, and sequence similarity to mouse competence gene JE. FEBS Lett. 1989;244:487–493. doi: 10.1016/0014-5793(89)80590-3. [DOI] [PubMed] [Google Scholar]
- 23.Grandaliano G., Valente A.J., Rozek M.M., Abboud H.E. Gamma interferon stimulates monocyte chemotactic protein (MCP-1) in human mesangial cells. J Lab Clin Med. 1994;123:282–289. [PubMed] [Google Scholar]
- 24.Aggarwal S., Ghilardi N., Xie M.H., de Sauvage F.J., Gurney A.L. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278:1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 25.Nikolic T., Geutskens S.B., van Rooijen N., Drexhage H.A., Leenen P.J. Dendritic cells and macrophages are essential for the retention of lymphocytes in (peri)-insulitis of the nonobese diabetic mouse: a phagocyte depletion study. Lab Invest. 2005;85:487–501. doi: 10.1038/labinvest.3700238. [DOI] [PubMed] [Google Scholar]
- 26.Schaumburg C.S., Siemasko K.F., De Paiva C.S., Wheeler L.A., Niederkorn J.Y., Pflugfelder S.C., Stern M.E. Ocular surface APCs are necessary for autoreactive T cell-mediated experimental autoimmune lacrimal keratoconjunctivitis. J Immunol. 2011;187:3653–3662. doi: 10.4049/jimmunol.1101442. [DOI] [PubMed] [Google Scholar]