Abstract
Mastitis is a substantial clinical problem in lactating women that may result in severe pain and abrupt termination of breastfeeding, thereby predisposing infants to long-term health risks. Many cases of mastitis involve no known infectious agent and may fundamentally be due to autoimmune-mediated inflammation of the breast. Herein, we develop a murine model of autoimmune mastitis and provide a detailed characterization of its resulting phenotype of breast failure and lactation insufficiency. To generate breast-specific autoimmunity, we immunized SWXJ mice with recombinant mouse α-lactalbumin, a lactation-dependent, breast-specific differentiation protein critical for production of lactose. Mice immunized with α-lactalbumin showed extensive T-cell–mediated inflammation in lactating normal breast parenchyma but none in nonlactating normal breast parenchyma. This targeted autoimmune attack resulted in breast failure characterized by lactation insufficiency and decreased ability to nurture offspring. Although immunization with α-lactalbumin had no effect on fertility and birth numbers, pups nursed by α-lactalbumin–immunized mice showed significantly disrupted growth often accompanied by kwashiorkor-like nutritional abnormalities, including alopecia, liver toxicity, and runting. This experimental model of autoimmune breast failure has useful applications for prophylactic breast cancer vaccination and for addressing inflammatory complications during breastfeeding. In addition, this model is suited for investigating nutritionally based “failure-to-thrive” issues, particularly regarding the long-term implications of postnatal nutritional deprivation.
Mastitis is an inflammation of the breast that often causes women to stop breastfeeding. It is clinically characterized by breast engorgement, formation of noncaseating granulomatous inflammation with leukocytic infiltrates, and blocked milk ducts. If left untreated, mastitis can lead to lactation failure, recurrent inflammation, or breast abscess.1 Mastitis has been traditionally viewed to affect less than 3% of American women in their childbearing years.2 However, several studies indicate that mastitis may be much more prevalent, perhaps affecting up to 33% of lactating women.3–6
Mastitis has been predominantly associated with Staphylococcus aureus infections.7,8 Several other etiopathogenic factors have also been implicated, including the use of illicit drugs, stress during labor, and trauma to the breast, all of which may cause or predispose to inflammation and related abnormalities.9 Also, some cases of mastitis seem to involve no known infectious or other causative agent and, as such, suggest that breast-specific autoimmunity may be implicated. Indeed, idiopathic granulomatous mastitis is one form of this disorder that is often responsive to glucocorticoid treatment10–13 and has been attributed to a localized immune response to an unidentified breast-specific antigen.14
Inflamed breast tissues typically show substantial neutrophilic and lymphocytic infiltration involving T and B cells.15,16 Immunohistochemical analyses of breast infiltrates often show a predominance of T cells17,18 but may also show dense intralobular, perilobular, and perivascular B-cell infiltrates with lobular atrophy and sclerosis.19 However, breast inflammation in the absence of any infectious pathogens or other contributing factors distinguishes idiopathic mastitis and a putative autoimmune etiology.
In the present study, we define the detailed phenotype of breast failure by creating a murine experimental autoimmune mastitis. We selected mouse α-lactalbumin as the target antigen to induce autoimmune disruption of breast function because it is a differentiation protein involved in regulation of lactose biosynthesis and because its expression is breast specific and lactation dependent.20–23 We found that immunization of SWXJ mice with recombinant mouse α-lactalbumin causes extensive T-cell–mediated inflammation in lactating normal breast parenchyma and none in nonlactating normal breast parenchyma. This targeted autoimmune attack results in breast failure characterized by lactation insufficiency and decreased ability to nurture offspring. Although we observed no effect on fertility, pups nursed by α-lactalbumin–immunized mice consistently showed significant growth inhibition, often complicated by kwashiorkor-like nutritional abnormalities and runting. This experimental model has useful applications for prophylactic breast cancer vaccination, for addressing inflammatory complications during breastfeeding, and for investigating nutritionally based “failure-to-thrive” issues, particularly regarding the long-term implications of nutritional deprivation during the early stages of development.
Materials and Methods
Production of Recombinant Mouse α-Lactalbumin
mRNA was extracted (Qiagen Inc., Valencia, CA) from lactating mouse mammary tissue taken from 8-week-old female SWXJ mice, and α-lactalbumin cDNA was generated by RT-PCR using α-lactalbumin–specific primers (Table 1). The cDNA for mouse α-lactalbumin was inserted into the pQE-82L expression vector (Qiagen Inc.) for producing a 6X-His–tagged fusion protein. SG13009 Escherichia coli (Qiagen Inc.) was transformed and screened for expression with horseradish peroxidase–conjugated His antibody (Qiagen Inc.). High-level expression colonies were selected after induction with isopropyl thiogalactopyronidase and treatment with His antibody. Plasmids from selected colonies were purified using the Plasmid Maxi Kit (Qiagen Inc.) and sequenced for verifying proper orientation and alignment. His-tagged α-lactalbumin was purified under denaturing conditions using a nickel-nitrilo triacetic acid affinity chromatography column (Qiagen Inc.). The column was washed, and multiple elution fractions were collected and electrophoresed on 15% Tris-HCl SDS-PAGE denaturing gels with Kaleidoscope prestained molecular weight markers (Bio-Rad Laboratories, Hercules, CA). The gels were blotted onto Immobilon-P polyvinylidene difluoride membrane (Millipore, Bedford, MA), blocked with 3% bovine serum albumin, and stained using polyclonal goat anti-mouse α-lactalbumin primary antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and horseradish peroxidase–labeled donkey anti-goat IgG secondary antibody (Santa Cruz Biotechnology). Detection was performed using ECL chemiluminescence reagents (GE Healthcare, Buckinghamshire, UK) and HyBlot autoradiography film (Denville Scientific, Metuchen, NJ). Before immunization or use in cultures, His-tagged α-lactalbumin was further purified by reverse-phase high-performance liquid chromatography to yield endotoxin-free protein.24
Table 1.
Primer Pairs for Quantitative RT-PCR, Cloning of α-Lactalbumin, and Conventional RT-PCR
| Protein | Sequence | Amplicon length (bp) |
|---|---|---|
| Quantitative RT-PCR | ||
| IFN-γ | ||
| Forward | 5′-TCAAGTGGCATAGATGTGGAAGAA-3′ | 192 |
| Reverse | 5′-TGGCTCTGCAGGATTTTCATG-3′ | |
| IL-10 | ||
| Forward | 5′-GGTTGCCAAGCCTTATCGGA-3′ | 190 |
| Reverse | 5′-ACCTGCTCCACTGCCTTGCT-3′ | |
| α-Casein | ||
| Forward | 5′-AAGTTTCCCCAGCACAGCAATCT-3′ | 248 |
| Reverse | 5′-CCAAAGGGGAAAGGCATCATACT-3′ | |
| α-Lactalbumin | ||
| Forward | 5′-TCAACGACAACGGCAGCACAGAGT-3′ | 292 |
| Reverse | 5′-CACAGGGGCAGGAGCAGCAAGGAC-3′ | |
| GAPDH | ||
| Forward | 5′-TTCACCACCATGGAGAAGGGC-3′ | 236 |
| Reverse | 5′-GGCATCGACTGTCATGA-3′ | |
| Cloning of α-Lactalbumin | ||
| α-Lactalbumin | ||
| Forward | 5′-TTCGTTCCTTTGTTCCTGGTGTG-3′ | 581 |
| Reverse | 5′-GTCCTCTAGAGTCCGGTGGTGTC-3′ | |
| Conventional RT-PCR | ||
| IFN-γ | ||
| Forward | 5′-TGATGGCCTGATTGTCTTTCAA-3′ | 110 |
| Reverse | 5′-GGATATCTGGAGGAACTGGCAA-3′ | |
| β-Actin | ||
| Forward | 5′-CAGGGTGTGATGGTGGGAATGG-3′ | 409 |
| Reverse | 5′-GCAGGATGGCGTGAGGGAGAGC-3′ |
GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Mice and Immunization
SWXJ (H-2q,s) mice were generated by mating SJL/J (H-2s) males with SWR/J (H-2q) females (The Jackson Laboratory, Bar Harbor, ME). At 8 to 10 weeks of age, mice were injected s.c. in the abdominal flank with 150 μg of recombinant mouse α-lactalbumin in 200 μL of emulsion of equal volumes of water and complete Freund's adjuvant (CFA) containing 400 μg of Mycobacteria tuberculosis H37RA (Difco, Detroit, MI). Control mice were immunized with CFA alone. All the protocols were approved by the Institutional Animal Care and Use Committee of the Cleveland Clinic and were performed in compliance with the Public Health Service policy on humane care and use of laboratory animals.
Phenotype of Autoimmune-Induced Breast Failure
To determine the phenotype of autoimmune-induced breast failure, α-lactalbumin–immunized female SWXJ mice and control female mice immunized with CFA alone were mated serially with the same SWXJ male partners over several mating periods. All the litters were weighed daily until weaning at 3 weeks of age.
Proliferation Assays
To determine the immunogenicity of recombinant mouse α-lactalbumin, female SWXJ mice were immunized with 150 μg of protein, and 10-day primed inguinal and axillary lymph node cells (LNCs) were teased into single cell suspensions, washed thoroughly in HBSS (Life Technologies, Grand Island, NY), and plated at 3 × 105 cells per microititer well in 96-well flat-bottomed Falcon plates (BD, Franklin Lakes, NJ) in Dulbecco's modified Eagle's medium (Mediatech Cellgro, Herndon, VA) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT), 2 mmol/L fresh l-glutamine, 100 U/mL of penicillin, 100 μg/mL of streptomycin, and 30 mmol/L HEPES buffer (Invitrogen, Carlsbad, CA) at a final volume of 200 μL per well. Recombinant α-lactalbumin was added in serial 10-fold dilutions to triplicate wells, with positive control wells containing 2 μg/mL of anti-mouse CD3 (BD Biosciences, San Jose, CA) and negative control wells containing no antigen. After 72 hours of culture, each well was pulsed with [methyl-3H]thymidine (l.0 μCi per well; specific activity, 6.7 Ci/mmol; New England Nuclear, Boston, MA) and harvested 16 hours later by aspiration onto glass fiber filters (Tomtec Inc., Hamden, CT). Levels of incorporated radioactivity were determined by scintillation spectrometry. Results are expressed as mean counts per minute of experimental cultures with antigen divided by mean counts per minute of cultures without antigen (stimulation index).
ELISA
Cytokine concentrations were determined by enzyme-linked immunosorbent assay (ELISA) measurement of supernatants of 10-day primed lymphocytes cultured in supplemented Dulbecco's modified Eagle's medium for 48 hours at a concentration of 5 × 106 cells per well in 24-well flat-bottomed Falcon plates (BD) in the presence of 20 μg/mL of antigen in a final volume of 2.0 mL per well. Purified capture/detection antibody pairs and recombinant cytokines were obtained commercially (BD Biosciences) and included anti-mouse interferon-gamma (IFN-γ; R4-6A2 and biotin XMG 1.2), anti-mouse IL-2 (JES6-1A12 and biotin JES6-5H4), anti-mouse IL-5 (TRFK5 and biotin TRFK4), and anti-mouse IL-10 (JES5-2A5 and biotin SXC-1). Absorbance was measured at 405 nm using a model 550 enzyme-linked immunosorbent assay microplate reader (Bio-Rad Laboratories). Standard values were plotted as absorbance versus cytokine concentration, and sample cytokine concentrations were determined as values in the linear part of the standard curve established using known concentrations of each cytokine.
Real-Time qRT-PCR
Eight weeks after immunization with α-lactalbumin or CFA alone, total RNA was extracted from lactating mammary tissue using the RNeasy mini kit (Qiagen Inc.). cDNA was synthesized using reverse transcription mix (RETROscript for RT-PCR; Ambion, Austin, TX) containing 2 μL of oligo dT primers, 1 mmol/L dNTPs, 100 IU of Moloney murine leukemia virus reverse transcriptase, and 10 IU of RNase inhibitor. The reaction mixture was heated to 42°C for 60 minutes followed by heat inactivation at 95°C for 5 minutes. Quantitative real-time RT-PCR (qRT-PCR) was performed using primer pairs for murine IFN-γ, IL-10, α-casein, α-lactalbumin, and glyceraldehyde-3-phosphate dehydrogenase (Table 1). cDNAs were assayed separately in 96-well plates (Applied Biosystems, Foster City, CA) in 25 μL of reaction volume containing 1XSYBR Green PCR buffer, 3 mmol/L MgCl2, 1 mmol/L dNTPs, 0.01 IU/μL of AmpErase uracil N-glycosylase, and 0.025 IU/μL of AmpliTaq Gold DNA polymerase (Applied Biosystems). Samples were amplified in an automated fluorometer (ABI Prism 7700 sequence detection system; Applied Biosystems), and transcripts were calculated according to the comparative threshold cycle method using glyceraldehyde-3-phosphate dehydrogenase transcripts as endogenous controls. The data are represented as relative gene expression, defined as the ratio of test gene expression to glyceraldehyde-3-phosphate dehydrogenase gene expression for each tissue analyzed. Conventional RT-PCR was performed using IFN-γ–specific primers, with β-actin primers as amplification controls (Table 1). All the RT-PCR reactions were performed in 25 μL of volume using 1X PCR buffer, 3 mmol/L MgCl2, 1 mmol/L dNTPs, and 0.025 IU/μL of AmpliTaq DNA polymerase. Amplified products were electrophoresed on a 1.5% agarose gel and were stained with ethidium bromide, and amplicons were recorded on a UV transilluminator using a gel documentation system (Bio-Rad Laboratories).
Liver Enzyme Analysis
Concentrations of serum glutamic oxaloacetic transaminase (aka aspartate aminotransferase) and serum glutamic pyruvic transaminase (aka alanine aminotransferase) were determined by the UV-kinetic spectrometry method using alanine aminotransferase/glutamic pyruvic transaminase and aspartate aminotransferase/glutamic oxaloacetic transaminaseassay kits (Teco Diagnostics, Anaheim, CA).
T-Cell Purification
Ten days after immunization with recombinant mouse α-lactalbumin, CD4+ and CD8+ T cells were positively purified from whole LNCs by magnetic bead separation using a MidiMACS cell separator (Miltenyi Biotec Inc., Auburn, CA). Purities of enriched fractions were determined by flow cytometry analysis using CellQuest software version 3.3 (BD Biosciences) and were consistently found to be >90%.
Immunohistochemical Analysis
Mouse mammary glands were fixed in 10% phosphate-buffered formalin (Fisher Scientific, Fair Lawn, NJ) and embedded in paraffin. Sections (10 μm) were sequentially unmasked in 1 mmol/L EDTA (pH 8.0), blocked with 5% normal goat serum (Vector Laboratories, Burlingame, CA), incubated with a 1:50 dilution of rat anti-mouse CD3 (Novacastra, Newcastle, UK), incubated with 1:100 dilution of mouse-adsorbed biotinylated goat anti-rat IgG (BD Biosciences), treated with 1.5% H2O2 in methanol, and developed by sequential treatment with streptavidin–horseradish peroxidase complex (ABC kit; Vector Laboratories), diaminobenzidine, and H2O2 substrate (BioGenex, San Ramon, CA). Slides were counterstained with hematoxylin 2 (Richard-Allan Scientific, Kalamazoo, MI), dehydrated in an ascending gradient of ethanol followed by xylene, and mounted in Cytoseal 60 (Stephens Scientific, Riverdale, NJ) for examination under light microscopy.
Passive Transfer of Autoimmune-Induced Breast Failure
SWXJ female mice were immunized with either 150 μg of α-lactalbumin in CFA or 150 μg of recombinant human cochlin as an irrelevant control antigen. Ten days after immunization, LNCs were activated for 4 days with 20 μg/mL of recombinant mouse α-lactalbumin, and 2 to 3 × 107 cells were injected i.p. into naive lactating female SWXJ recipients. In addition, CD4+ T cells and CD8+ T cells were positively selected from antigen-activated LNC cultures by magnetic bead separation as described previously herein and injected i.p. into naive lactating female recipients at 2 to 3 × 107 cells per mouse. Four weeks after each transfer, RNA was isolated from lactating mammary glands and was analyzed by qRT-PCR for gene expression as described previously herein using primer pairs for murine α-casein and glyceraldehyde-3-phosphate dehydrogenase (Table 1).
Biostatistical Analysis
Significance between levels of mRNA expression and serum liver enzymes was determined by the t-test. Significance between pup growth curves was determined by one-way analysis of variance.
Results
α-Lactalbumin Immunization Induces a Proinflammatory Type-1 Immune Response
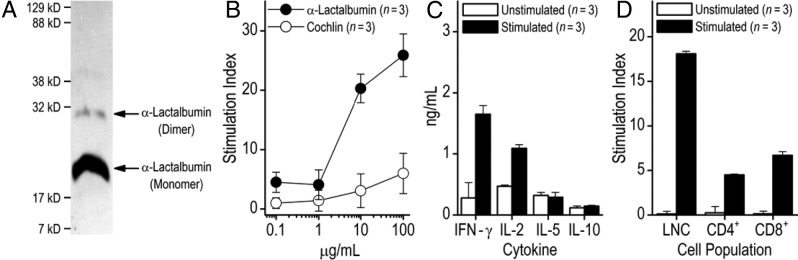
We selected α-lactalbumin as the target autoantigen because it is a lactation-dependent differentiation protein expressed exclusively and at high levels in lactating mammary epithelial cells but not in nonlactating breast tissues.20–23 α-Lactalbumin cDNA was generated by reverse transcription of RNA extracted from lactating mouse breast tissue and inserted into an E. coli expression vector for producing recombinant fusion proteins with N-terminal 6X-His tag. Production of 6X-His–tagged α-lactalbumin in E. coli was induced by adding 1 mmol/L isopropyl thiogalactopyronidase to cultures in log phase and purified by nickel-nitrilo triacetic acid affinity chromatography involving serial acidic elutions (Figure 1A). The molecular weight of α-lactalbumin was determined by gel electrophoresis using Kaleidoscope prestained protein standards, and the protein was further purified before use by reverse-phase high-performance liquid chromatography to yield an endotoxin-free product.24
Figure 1.
α-Lactalbumin immunization induces a proinflammatory type-1 immune response. A: Western blot analysis to detect His-tagged mouse α-lactalbumin. Arrows show the positions of the α-lactalbumin monomer and dimer. Positions of molecular weight markers are indicated on the left side of the gel. B: SWXJ female mice were immunized with 150 μg of recombinant mouse α-lactalbumin in CFA. Ten days later, LNCs showed antigen-specific proliferation in recall responses over a broad dose range to α-lactalbumin but not to recombinant human cochlin, an irrelevant control antigen cloned and purified in an identical manner. C: ELISA of culture supernatants from 10-day LNCs stimulated with 20 μg/mL of α-lactalbumin showed production of the type 1 proinflammatory cytokines IFN-γ and IL-2 with minimal production of the type 2 regulatory cytokines IL-5 and IL-10. D: Recall responses to 20 μg/mL of α-lactalbumin were elicited from CD4+ and CD8+ T cells purified by magnetic bead separation from LNCs 10 days after immunization with α-lactalbumin. Data are given as mean ± SE.
Ten days after immunization of female SWXJ mice with recombinant mouse α-lactalbumin, LNCs showed dose-dependent proliferation in recall responses to α-lactalbumin and were unresponsive to recombinant human cochlin generated in E. coli in a virtually identical manner (Figure 1B).25 Responsiveness to α-lactalbumin involved cytokine production consistent with a proinflammatory type-1 response with high production of IFN-γ and IL-2 and low production of IL-5 and IL-10 (Figure 1C). Responsiveness to α-lactalbumin involved CD4+ and CD8+ T cells, as shown by magnetic bead separation of purified populations from LNCs 10 days after immunization (Figure 1D).
α-Lactalbumin Immunization Causes Infiltration of CD3+ T Cells in Lactating Breast Tissue Only
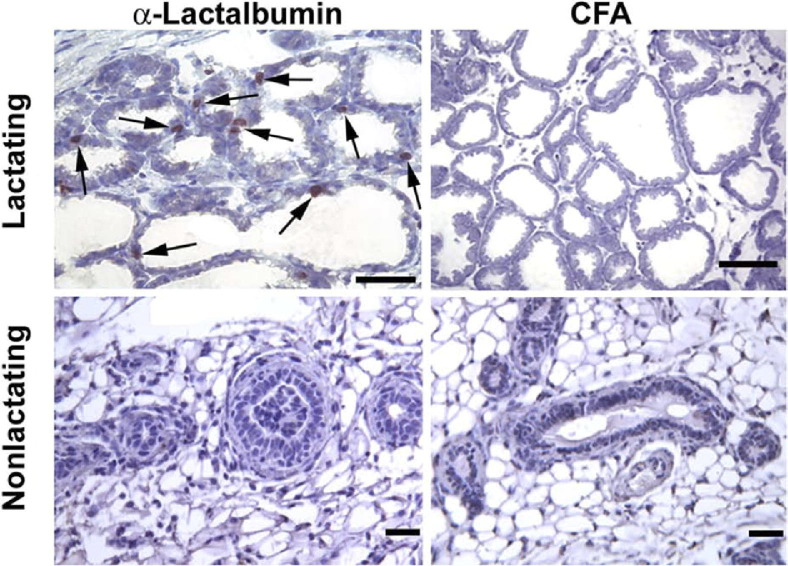
We next examined histologic changes associated with α-lactalbumin immunization. Lactating and nonlactating SWXJ female mice were immunized with α-lactalbumin in CFA or with CFA alone, and breast tissues were examined 6 to 7 weeks after immunization. Immunohistochemical analysis using CD3 antibody showed numerous CD3+ T cells in breast tissues from lactating mice immunized with α-lactalbumin but not from control lactating mice immunized with CFA alone or from nonlactating mice immunized with either α-lactalbumin or CFA (Figure 2).
Figure 2.
α-Lactalbumin immunization causes infiltration of CD3+ T cells in lactating breast tissue only. Immunohistochemical analysis shows numerous CD3+ T cells clustered in periductal areas (arrows) of breast tissues from lactating mice immunized with α-lactalbumin 6 to 7 weeks before initiation of lactation (top left panel). Clusters of CD3+ T cells were not observed in breast tissues from lactating mice immunized with CFA (top right panel) or in nonlactating mice immunized with either α-lactalbumin (bottom left panel) or CFA (bottom right panel). Scale bars: 50 μm.
Altered Gene Expression in Lactating Breast Tissues From α-Lactalbumin–Immunized Mice
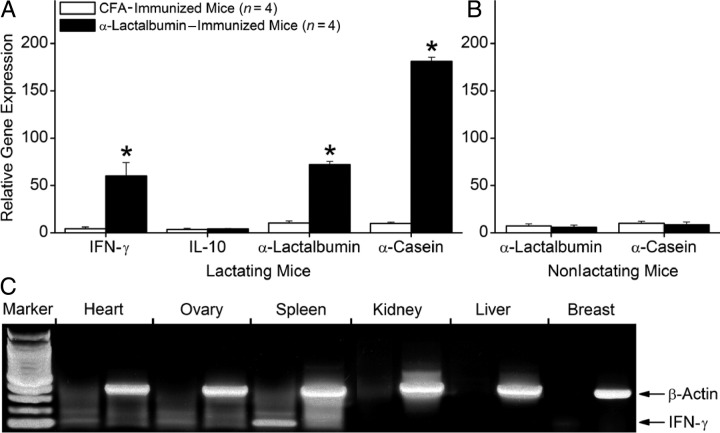
We next assessed molecular changes occurring in lactating breast tissues from mice immunized with α-lactalbumin. Six weeks after immunization with either α-lactalbumin or CFA alone, RNA from lactating mammary tissue was analyzed for gene expression by real-time qRT-PCR. We found that lactating breast tissue from α-lactalbumin–immunized mice showed significantly elevated gene expression levels of IFN-γ (P = 0.001) but not IL-10 (P > 0.10), indicating the induction of a type 1 proinflammatory T-cell response (Figure 3A). In addition, we found significantly increased gene expression of the breast-specific, lactation-dependent differentiation proteins α-lactalbumin (P < 0.002) and α-casein (P = 0.0008), indicating a compensatory homeostatic response of the breast tissue to produce more milk in the presence of an autoimmune attack. Enhanced expression of these lactation genes did not occur in nonlactating breast tissue from mice immunized with either α-lactalbumin or CFA (Figure 3B). Thus, enhanced expression of these lactation proteins serves as a surrogate marker for detecting breast failure. It is important to note that 6 weeks after immunization of nonlactating female mice with α-lactalbumin, we did not observe any measurable increase in gene expression levels of IFN-γ, a cytokine whose expression is closely linked to the presence of activated proinflammatory type-1 T cells (Figure 3C). Thus, α-lactalbumin autoimmune inflammation is normally confined to the lactating breast and spares all other normal tissues.
Figure 3.
Altered gene expression in lactating breast tissues from α-lactalbumin–immunized mice. Six weeks after immunization with either α-lactalbumin or CFA alone, total RNA from lactating mammary tissue was analyzed for gene expression by real-time qRT-PCR. A: Lactating breast tissue from α-lactalbumin–immunized mice showed significantly elevated gene expression levels of IFN-γ (P = 0.001) but not IL-10 (P > 0.10) and significantly increased gene expression of α-lactalbumin (P < 0.002) and α-casein (P = 0.0008). Asterisks indicate significance. B: α-Lactalbumin and α-casein gene expression levels were not elevated in nonlactating breast tissue from α-lactalbumin–immunized mice. C: Except for the spleen, IFN-γ gene expression levels were not elevated in any tissues examined from normal nonlactating α-lactalbumin–immunized mice. Data are given as mean ± SE.
Functional Phenotype of Autoimmune Breast Failure
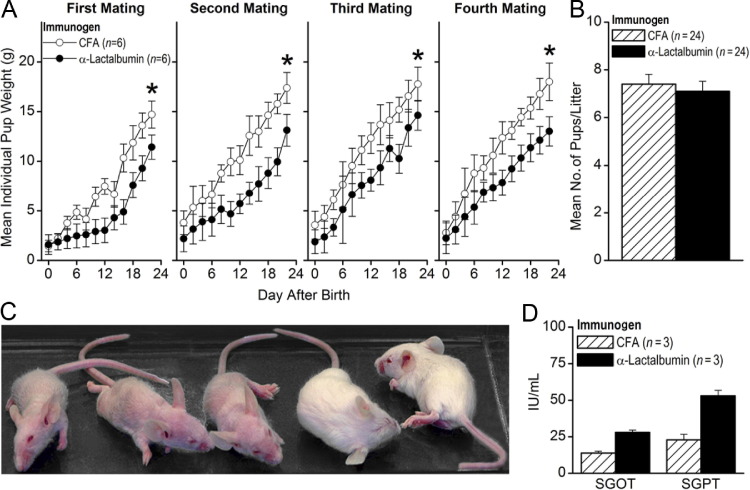
Since we observed inflammation in lactating breast tissue only, we hypothesized that α-lactalbumin–immunized mice may manifest lactation abnormalities that affect pup growth. Thus, 4 weeks after immunization with either α-lactalbumin or CFA alone, females were mated with the same males for the same period, and litters were examined daily for 3 weeks after birth over several matings for differences in mean pup weights. Pups from α-lactalbumin–immunized mothers consistently showed a failure to thrive as measured by significantly decreased mean pup weights over several mating cycles (P < 0.04 for every individual mating; Figure 4A). The decreased mean individual pup weights were not accompanied by decreased fertility since there were no significant differences (P > 0.60) in the mean number of mice per litter between females immunized with α-lactalbumin and those immunized with CFA alone (Figure 4B). The observed nourishment failure often presented with pups showing alopecia (Figure 4C) and liver toxicity (Figure 4D) consistent with kwashiorkor, a severe nutritional deficiency often observed in children during famine.26 Severe runting occurred only in pups from mothers immunized with α-lactalbumin and never occurred in pups from control-immunized mice. In addition, severe irritation of the nipples occurred only in α-lactalbumin–immunized nursing mice, indicating determined efforts by the pups to obtain needed nourishment (data not shown). This breast failure phenotype mimics that observed in α-lactalbumin–deficient transgenic mice27 and in epidermal growth factor receptor 2 (HER2)–deficient transgenic mice immunized with xenogeneic HER2 DNA.28
Figure 4.
Functional phenotype of autoimmune breast failure. Four weeks after immunization with either α-lactalbumin or CFA alone, females were mated with identical males, and the resultant litters were examined daily for differences in mean pup weight. A: Pups from α-lactalbumin–immunized mothers consistently showed a failure to thrive as measured by significantly decreased mean pup weights over several mating cycles (P < 0.04 for every individual mating). Asterisks indicate significance. B: There were no significant differences (P > 0.60) in mean number of mice per litter between females immunized with α-lactalbumin or CFA alone. Nourishment failure often presented with kwashiorkor-like signs, including alopecia (three pups on the left) (C) and liver toxicity as indicated by increased serum glutamic oxaloacetic transaminase (SGOT) and serum glutamic pyruvic transaminase (SGPT) levels (D). Data are given as mean ± SE.
Passive Transfer of Autoimmune Breast Failure with Primed T Cells
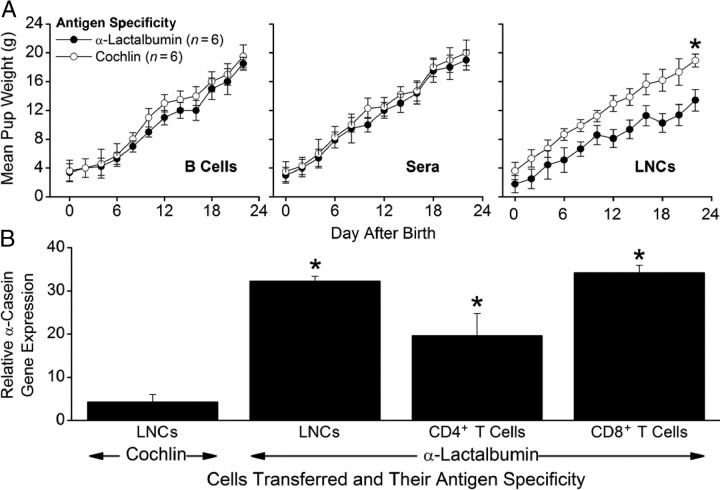
We next determined whether autoimmune-induced breast failure was mediated by T or B cells. To this end, we transferred defined lymphocyte populations and sera from α-lactalbumin–immunized mice into naive recipients and found that the pup growth inhibition phenotype could be transferred into lactating recipients with α-lactalbumin–activated LNCs (P < 0.04) but not with α-lactalbumin–primed B cells (P > 0.50) or sera (P > 0.60) (Figure 5A). Moreover, we found that significantly elevated α-casein gene expression, a surrogate marker for autoimmune breast failure (Figure 3A), did not occur in lactating breast tissues from control females receiving cochlin-primed and activated LNCs (P > 0.10) but did occur in lactating breast tissues from females receiving α-lactalbumin–primed LNCs (P = 0.02), CD4+ T cells (P = 0.03), or CD8+ T cells (P = 0.02) (Figure 5B). Taken together, these results indicate that autoimmune-induced breast failure is mediated by α-lactalbumin–specific CD4+ or CD8+ T cells and not by B cells or antibody.
Figure 5.
Passive transfer of autoimmune breast failure with primed T cells. Lactating female SWXJ mice were injected i.p. with LNCs, CD4+ T cells, CD8+ T cells, B cells, or sera from mice immunized previously with either recombinant α-lactalbumin or recombinant cochlin in CFA. After weaning (approximately 3 to 4 weeks after transfer), mice were euthanized for molecular analysis of tissues. A: Inhibition of growth did not occur in pups from mothers that received B cells (P > 0.50; left panel) or sera (P > 0.60; middle panel) from α-lactalbumin–immunized mice but did occur in pups from mothers that received α-lactalbumin–activated LNCs (P < 0.04; right panel). B: Significantly elevated α-casein gene expression, a surrogate marker for breast failure, did not occur in lactating breast tissues from control females receiving cochlin-activated LNCs (P > 0.10) but did occur in lactating breast tissues from females receiving α-lactalbumin–activated LNCs (P = 0.02), CD4+ T cells (P = 0.03), or CD8+ T cells (P = 0.02). Data are given as mean ± SE. Asterisks indicate significance. In all experiments, n = 6.
Discussion
In the present study, we developed and characterized a novel mouse model for autoimmune breast failure. Immunization against the breast-specific lactation protein α-lactalbumin causes T-cell–mediated mastitis in lactating mice. The targeted breast-specific inflammation causes functional failure of the breast characterized by lactation insufficiency, resulting in decreased ability to nurture offspring. Pups nursed by α-lactalbumin–immunized mice show significantly delayed growth often accompanied by kwashiorkor-like nutritional abnormalities, including alopecia, liver toxicity, and runting.
It is important to note that nonlactating mice immunized with α-lactalbumin were completely unaffected by inflammatory signs associated with the breast failure phenotype. They showed no histologic signs of T-cell infiltrates in breast tissues and no local or systemic molecular indications of any inflammatory mediators in breast and other tissues. Indeed, the benign nature of α-lactalbumin immunization in nonlactating mice has formed the basis for designing a prophylactic breast cancer vaccine.29 Vaccination against α-lactalbumin provides immune protection against the development of transplantable and autochthonous breast tumors in mice without causing inflammatory complications. Thus, conditionally expressed self-proteins or self-proteins that are “retired” and are no longer expressed may substitute for unavailable viral targets in the development of a prophylactic cancer vaccination.30 The present results reevaluate and confirm the safety of immunization against breast-specific, conditionally expressed lactation proteins as prophylaxis against breast cancer provided that lactation is avoided after immunization.
This experimental model of autoimmune breast failure may have useful applications for addressing inflammatory complications during lactation and the associated inability to continue breastfeeding. This inflammatory mastitis manifests in a substantial population of lactating women usually within the first few weeks of delivery and often leads to cessation of breastfeeding. It is well established that early postnatal nutrition plays an important role in the long-term health of offspring.31,32 The benefits of breastfeeding for infants include optimal growth and nutrition, enhanced ability to fight infections and avoid allergic disorders, and enhancement of maternal-infant bonding.33 Thus, it is important to encourage and facilitate breastfeeding, and this mouse model may be useful in finding ways to improve the quality and effectiveness of lactation and breastfeeding.
This experimental model of autoimmune breast failure also has useful applications for addressing nutritionally based failure-to-thrive issues, particularly regarding the long-term implications of postnatal nutritional deprivation. Nutritional deficiencies at critical periods of infant growth may induce permanent changes in physiologic function. Breastfeeding is associated with a reduced incidence of gastroenteritis and respiratory disorders during infancy33–36 and leads to benefits during adulthood that include reduced serum cholesterol concentrations,37–39 reduced mortality from ischemic heart disease,40 reduced incidence of type 2 diabetes mellitus,41 and Helicobacter pylori infection associated with gastric ulcer formation.42 Other studies indicate that breastfeeding is also associated with a reduced prevalence of childhood obesity.43 Malnutrition in childhood has long-term implications, including the tendency to develop impaired glucose tolerance and pancreatic damage, leading to malnutrition diabetes.44 However, there is still much more to learn about the long-term effects of early childhood nutritional deficiency, particularly regarding long-term defined cognitive impairment, adult immune dysfunction, and overall longevity, and this autoimmune breast failure model may facilitate such investigations.45
It is important to note the key observation that immunization with α-lactalbumin inhibits mean pup growth without any significant differences in mean birth weight or in fertility as measured by the mean number of mice per litter. This indicates that the observed pathologic abnormalities associated with autoimmune breast failure are exclusively due to postnatal lactation insufficiency, with no measurable prenatal effect on fertility. Note that during the four mating cycles examined, none of the female mice immunized with α-lactalbumin showed any clinical signs other than irritated nipples consistent with determined attempts by the pups to gain nourishment and determined efforts by the nursing moms to provide nourishment. None of the α-lactalbumin–immunized lactating mice showed any signs of morbidity, and they seemed relatively normal in behavior, particularly in terms of their social interactions and grooming behavior when they stopped lactating.
Notably, gene expression for α-casein and α-lactalbumin was greatly enhanced in breasts undergoing autoimmune attack. Similar examples of up-regulated gene expression of tissue-specific differentiation proteins include enhanced acetylcholine receptor gene expression in patients with myasthenia gravis,46 enhanced ghrelin gene expression in joint tissues of patients with rheumatoid arthritis,47 and up-regulation of developmental isoforms of myelin proteins in central nervous system tissues during inflammatory stages of autoimmune demyelinating disease.48 This up-regulated gene expression of tissue-specific differentiation proteins seems to represent a compensatory response by the damaged tissue to achieve functional homeostasis. In the case of autoimmune mastitis, the enhanced gene expression for lactation proteins likely represents remuneration for the lactation inadequacy in the autoimmune targeted breast and may have biomarker potential for determining breast failure.
Finally, this autoimmune mastitis model may serve as a basis for understanding bovine mastitis, a most prevalent and costly disease in dairy cows resulting in considerable economic loss.49 Although bovine mastitis is often associated with bacterial infections, antibiotics are not always successful in alleviating clinical signs in bovine mastitis, and a substantial number of cases are not associated with any known pathogen and are diagnosed as sterile mastitis.50 In addition, the relapsing nature of bovine mastitis mimics the typical clinical presentation of autoimmune disease, and the high frequency of antibiotic-resistant cases of bovine mastitis suggests that microbial infection may not account for all cases of bovine mastitis. We hypothesize that a cohort of dairy cows with bovine mastitis may actually have an underlying autoimmune etiopathogenic process accounting for inflammatory signs that may be indistinguishable from those produced by infection. This hypothesis may be tested rather easily by examining autoantibody responses to a broad spectrum of primary milk proteins, including α-lactalbumin and α-casein.
In summary, this experimental autoimmune breast failure model has useful applications for prophylactic breast cancer vaccination, for addressing inflammatory complications that prevent breastfeeding, and for investigating long-term implications of postnatal nutritional deprivation, and it may be relevant in cases of sterile mastitis in the dairy industry.
Footnotes
Supported by NIH grant R01CA-140350 (V.K.T.).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Current address of C.Z.A., Department of Urology, Case Western Reserve University, Cleveland, Ohio.
References
- 1.Lawrence R.A., Lawrence R.M. Medical complications of the mother. In: Lawrence R.A., Lawrence R.H., editors. Breastfeeding: A Guide for the Medical Profession. ed 7. Elsevier Mosby; Maryland Heights, MO: 2011. pp. 550–613. [Google Scholar]
- 2.Foxman B., D'Arcy H., Gillespie B., Bobo J.K., Schwartz K. Lactation mastitis: occurrence and medical management among 946 breastfeeding women in the United States. Am J Epidemiol. 2002;155:103–114. doi: 10.1093/aje/155.2.103. [DOI] [PubMed] [Google Scholar]
- 3.Riordan J.M., Nichols F.H. A descriptive study of lactation mastitis in long-term breastfeeding women. J Hum Lact. 1990;6:53–58. doi: 10.1177/089033449000600213. [DOI] [PubMed] [Google Scholar]
- 4.Jonsson S., Pulkkinen M.O. Mastitis today: incidence, prevention and treatment. Ann Chir Gynaecol Suppl. 1994;208:84–87. [PubMed] [Google Scholar]
- 5.Fetherston C. Characteristics of lactation mastitis in a Western Australian cohort. Breastfeed Rev. 1997;5:5–11. [PubMed] [Google Scholar]
- 6.Kinlay J.R., O'Connell D.L., Kinlay S. Incidence of mastitis in breastfeeding women during the six months after delivery: a prospective cohort study. Med J Aust. 1998;169:310–312. doi: 10.5694/j.1326-5377.1998.tb140282.x. [DOI] [PubMed] [Google Scholar]
- 7.Melish M.E., Campbell K.A. Coagulase-positive staphylococcal infections: breast abcesses. In: Feigin R.D., Cherry J.D., editors. Textbook of Pediatric Infectious Diseases. ed 4. WB Saunders; Philadelphia: 1998. pp. 1039–1066. [Google Scholar]
- 8.Amir L.H., Harris H., Andriske L. An audit of mastitis in the emergency department. J Hum Lact. 1999;15:221–224. doi: 10.1177/089033449901500312. [DOI] [PubMed] [Google Scholar]
- 9.Bair-Merritt M.H., Blackstone M., Feudtner C. Physical health outcomes of childhood exposure to intimate partner violence: a systematic review. Pediatrics. 2006;117:e278–e290. doi: 10.1542/peds.2005-1473. [DOI] [PubMed] [Google Scholar]
- 10.Azlina A.F., Ariza Z., Arni T., Hisham A.N. Chronic granulomatous mastitis: diagnostic and therapeutic considerations. World J Surg. 2003;27:515–518. doi: 10.1007/s00268-003-6806-1. [DOI] [PubMed] [Google Scholar]
- 11.Kim J., Tymms K.E., Buckingham J.M. Methotrexate in the management of granulomatous mastitis. ANZ J Surg. 2003;73:247–249. doi: 10.1046/j.1445-1433.2002.02564.x. [DOI] [PubMed] [Google Scholar]
- 12.Katz U., Molad Y., Ablin J., Ben-David D., Paran D., Gutman M., Langevitz P. Chronic idiopathic granulomatous mastitis. Ann NY Acad Sci. 2007;1108:603–608. doi: 10.1196/annals.1422.063. [DOI] [PubMed] [Google Scholar]
- 13.Patel R.A., Strickland P., Sankara I.R., Pinkston G., Many W., Jr, Rodriguez M. Idiopathic granulomatous mastitis: case reports and review of literature. J Gen Intern Med. 2010;25:270–273. doi: 10.1007/s11606-009-1207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown K.L., Tang P.H. Postlactational tumoral granulomatous mastitis: a localized immune phenomenon. Am J Surg. 1979;138:326–329. doi: 10.1016/0002-9610(79)90397-0. [DOI] [PubMed] [Google Scholar]
- 15.Milward T.M., Gough M.H. Granulomatous lesions in the breast presenting as carcinoma. Surg Gynecol Obstet. 1970;130:478–482. [PubMed] [Google Scholar]
- 16.Tamiolakis D., Lambropoulou M., Koutlaki N., Manavis J., Alexiadis G., Tolparidou I., Papadopoulos N., Sivridis E., Anastasiadis P. Imprint cytology of non-specific granulomatous mastitis. Clin Exp Obstet Gynecol. 2001;28:176–178. [PubMed] [Google Scholar]
- 17.Carmalt H.L., Ramsey-Stewart G. Granulomatous mastitis. Med J Aust. 1981;1:356–359. doi: 10.5694/j.1326-5377.1981.tb135631.x. [DOI] [PubMed] [Google Scholar]
- 18.Galea M.H., Robertson J.F., Ellis I.O., Elston C.W., Blamey R.W. Granulomatous lobular mastitis. Aust N Z J Surg. 1989;59:547–550. doi: 10.1111/j.1445-2197.1989.tb01629.x. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz I.S., Strauchen J.A. Lymphocytic mastopathy: an autoimmune disease of the breast? Am J Clin Pathol. 1990;93:725–730. doi: 10.1093/ajcp/93.6.725. [DOI] [PubMed] [Google Scholar]
- 20.Nagamatsu Y., Oka T. Purification and characterization of mouse α-lactalbumin and preparation of its antibody. Biochem J. 1980;185:227–237. doi: 10.1042/bj1850227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vilotte J.L., Soulier S., Mercier J.C. Sequence of the murine α-lactalbumin-encoding cDNA: interspecies comparison of the coding frame and deduced pre-protein. Gene. 1992;112:251–255. doi: 10.1016/0378-1119(92)90385-3. [DOI] [PubMed] [Google Scholar]
- 22.Vilotte J.L., Soulier S. Isolation and characterization of the mouse alpha-lactalbumin-encoding gene: interspecies comparison, tissue- and stage-specific expression. Gene. 1992;119:287–292. doi: 10.1016/0378-1119(92)90285-w. [DOI] [PubMed] [Google Scholar]
- 23.Ren J., Stuart D.I., Acharya K.R. α-Lactalbumin possesses a distinct zinc binding site. J Biol Chem. 1993;268:19292–19298. [PubMed] [Google Scholar]
- 24.Dudley A., McKinstry W., Thomas D., Best J., Jenkins A. Removal of endotoxin by reverse phase HPLC abolishes anti-endothelial cell activity of bacterially expressed plasminogen kringle 5. Biotechniques. 2003;35:724–726. doi: 10.2144/03354st02. [DOI] [PubMed] [Google Scholar]
- 25.Baek M.J., Park H.M., Johnson J.M., Altuntas C.Z., Jane-Wit D., Jaini R., Solares C.A., Thomas D.M., Ball E.J., Robertson N.G., Morton C.C., Hughes G.B., Tuohy V.K. Increased frequencies of cochlin-specific T cells in patients with autoimmune sensorineural hearing loss. J Immunol. 2006;177:4203–4210. doi: 10.4049/jimmunol.177.6.4203. [DOI] [PubMed] [Google Scholar]
- 26.Scrimshaw N.S. Fifty-five-year personal experience with human nutrition worldwide. Annu Rev Nutr. 2007;27:1–18. doi: 10.1146/annurev.nutr.27.061406.093746. [DOI] [PubMed] [Google Scholar]
- 27.Stinnakre M.G., Vilotte J.L., Soulier S., Mercier J.C. Creation and phenotypic analysis of α-lactalbumin-deficient mice. Proc Natl Acad Sci U S A. 1994;5:6544–6548. doi: 10.1073/pnas.91.14.6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pupa S.M., Iezzi M., Di Carlo E., Invernizzi A., Cavallo F., Meazza R., Comes A., Ferrini S., Musiani P., Ménard S. Inhibition of mammary carcinoma development in HER-2/neu transgenic mice through induction of autoimmunity by xenogeneic DNA vaccination. Cancer Res. 2005;65:1071–1078. [PubMed] [Google Scholar]
- 29.Jaini R., Kesaraju P., Johnson J.M., Altuntas C.Z., Jane-Wit D., Tuohy V.K. An autoimmune-mediated strategy for prophylactic breast cancer vaccination. Nat Med. 2010;16:799–803. doi: 10.1038/nm.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tuohy V.K., Jaini R. Prophylactic cancer vaccination by targeting functional non-self. Ann Med. 2011;43:356–365. doi: 10.3109/07853890.2011.565065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Businco L., Marchetti F., Pellegrini G., Cantani A., Perlini R. Prevention of atopic disease in “at-risk newborns” by prolonged breast-feeding. Ann Allergy. 1983;51:296–299. [PubMed] [Google Scholar]
- 32.Barker D.J.P. Childhood infections and disease in later life. In: Barker D.J.P., editor. Mothers, Babies and Disease in Later Life. ed 2. Churchill Livingstone-Robert Stevenson House; Edinburgh: 1998. pp. 151–166. [Google Scholar]
- 33.Cunningham A.S. Morbidity in breast-fed and artificially fed infants. J Pediatr. 1977;90:726–729. doi: 10.1016/s0022-3476(77)81236-5. [DOI] [PubMed] [Google Scholar]
- 34.Feachem R.G., Koblinsky M.A. Interventions for the control of diarrhoeal diseases among young children: promotion of breast-feeding. Bull World Health Organ. 1984;62:271–291. [PMC free article] [PubMed] [Google Scholar]
- 35.Jason J.M., Nieburg P., Marks J.S. Mortality and infectious disease associated with infant-feeding practices in developing countries. Pediatrics. 1984;74:702–727. [PubMed] [Google Scholar]
- 36.Victora C.G. Infection and disease: the impact of early weaning. Food Nutr Bull. 1996;17:390–396. [Google Scholar]
- 37.Marmot M.G., Page C.M., Atkins E., Douglas J.W. Effect of breast-feeding on plasma cholesterol and weight in young adults. J Epidemiol Commun Health. 1980;34:164–167. doi: 10.1136/jech.34.3.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mott G.E., McMahan C.A., Kelley J.L., Farley C.M., McGill H.C., Jr Influence of infant and juvenile diets on serum cholesterol, lipoprotein cholesterol, and apolipoprotein concentrations in juvenile baboons (Papio sp.) Atherosclerosis. 1982;45:191–202. doi: 10.1016/0021-9150(82)90138-1. [DOI] [PubMed] [Google Scholar]
- 39.Ravelli A.C., van der Meulen J.H., Osmond C., Barker D.J., Bleker O.P. Infant feeding and adult glucose tolerance, lipid profile, blood pressure, and obesity. Arch Dis Child. 2000;82:248–252. doi: 10.1136/adc.82.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fall C.H., Barker D.J., Osmond C., Winter P.D., Clark P.M., Hales C.N. Relation of infant feeding to adult serum cholesterol concentration and death from ischaemic heart disease. BMJ. 1992;304:801–805. doi: 10.1136/bmj.304.6830.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pettitt D.J., Forman M.R., Hanson R.L., Knowler W.C., Bennett P.H. Breastfeeding and incidence of non-insulin-dependent diabetes mellitus in Pima Indians. Lancet. 1997;350:166–168. doi: 10.1016/S0140-6736(96)12103-6. [DOI] [PubMed] [Google Scholar]
- 42.Fall C.H., Goggin P.M., Hawtin P., Fine D., Duggleby S. Growth in infancy, infant feeding, childhood living conditions, and Helicobacter pylori infection at age 70. Arch Dis Child. 1997;77:310–314. doi: 10.1136/adc.77.4.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ryan A.S. Breastfeeding and the risk of childhood obesity. Coll Anthropol. 2007;31:19–28. [PubMed] [Google Scholar]
- 44.Abu-Bakare A., Taylor R., Gill G.V., Alberti K.G. Tropical or malnutrition-related diabetes: a real syndrome? Lancet. 1986;1:1135–1138. doi: 10.1016/s0140-6736(86)91846-5. [DOI] [PubMed] [Google Scholar]
- 45.Lucas A. Programming by early nutrition: an experimental approach. J Nutr. 1998;128:401S–406S. doi: 10.1093/jn/128.2.401S. [DOI] [PubMed] [Google Scholar]
- 46.Moulian N., Wakkach A., Guyon T., Poea S., Aissaoui A., Levasseur P., Cohen-Kaminsky S., Berrih-Aknin S. Respective role of thymus and muscle in autoimmune myasthenia gravis. Ann N Y Acad Sci. 1998;841:397–406. doi: 10.1111/j.1749-6632.1998.tb10953.x. [DOI] [PubMed] [Google Scholar]
- 47.Otero M., Nogueiras R., Lago F., Dieguez C., Gomez-Reino J.J., Gualillo O. Chronic inflammation modulates ghrelin levels in humans and rats. Rheumatology. 2004;43:306–310. doi: 10.1093/rheumatology/keh055. [DOI] [PubMed] [Google Scholar]
- 48.Mathisen P.M., Kawczak J.A., Yu M., Johnson J.M., Tuohy V.K. Differential DM20 mRNA expression distinguishes two distinct patterns of spontaneous recovery from murine autoimmune encephalomyelitis. J Neurosci Res. 2001;64:542–551. doi: 10.1002/jnr.1106. [DOI] [PubMed] [Google Scholar]
- 49.Seegers H., Fourichon C., Beaudeau F. Production effects related to mastitis and mastitis economics in dairy cattle herds. Vet Res. 2003;34:475–491. doi: 10.1051/vetres:2003027. [DOI] [PubMed] [Google Scholar]
- 50.Schukken Y.H., Wilson D.J., Welcome F., Garrison-Tikofsky L., Gonzalez R.N. Monitoring udder health and milk quality using somatic cell counts. Vet Res. 2003;34:579–596. doi: 10.1051/vetres:2003028. [DOI] [PubMed] [Google Scholar]