Abstract
Dysregulation of the WNT and insulin-like growth factor 2 (IGF2) signaling pathways has been implicated in sporadic and syndromic forms of adrenocortical carcinoma (ACC). Abnormal β-catenin staining and CTNNB1 mutations are reported to be common in both adrenocortical adenoma and ACC, whereas elevated IGF2 expression is associated primarily with ACC. To better understand the contribution of these pathways in the tumorigenesis of ACC, we examined clinicopathological and molecular data and used mouse models. Evaluation of adrenal tumors from 118 adult patients demonstrated an increase in CTNNB1 mutations and abnormal β-catenin accumulation in both adrenocortical adenoma and ACC. In ACC, these features were adversely associated with survival. Mice with stabilized β-catenin exhibited a temporal progression of increased adrenocortical hyperplasia, with subsequent microscopic and macroscopic adenoma formation. Elevated Igf2 expression alone did not cause hyperplasia. With the combination of stabilized β-catenin and elevated Igf2 expression, adrenal glands were larger, displayed earlier onset of hyperplasia, and developed more frequent macroscopic adenomas (as well as one carcinoma). Our results are consistent with a model in which dysregulation of one pathway may result in adrenal hyperplasia, but accumulation of a second or multiple alterations is necessary for tumorigenesis.
Adrenocortical tumors are fairly common, occurring in approximately 3% to 10% of the population.1 Frequently, adrenocortical tumors are discovered as part of an unrelated imaging examination, and most are benign adrenocortical adenomas (ACAs). In contrast, adrenocortical carcinomas (ACCs) are rare, with an annual incidence of 1 to 2 per million of the U.S. population.1,2 Unfortunately, a substantial number of ACC patients present with advanced disease, including 30% to 40% with clear evidence of metastatic disease, and the available treatments for advanced disease are rarely curative. The overall 5-year survival rate has been reported to range from 10% to 40%.3–5
Although most ACCs arise sporadically, certain genetic syndromes have been associated with an increased incidence of ACC. Familial adenomatous polyposis (FAP) patients have a mutated adenomatous polyposis coli gene (APC) and higher rates of a variety of cancers, most notably colorectal cancer. ACC is also thought to occur at a higher rate in these patients, although the statistical significance of this correlation has been called into question.6 APC is a component of the cadherin-associated protein β 1 (CTNNB1; commonly known as β-catenin) destruction complex, which, in the absence of WNT (wingless-type MMTV integration) signaling, helps facilitate the phosphorylation of β-catenin, targeting it for degradation. The presence of WNT signaling or decreased expression/mutation of APC results in stabilization and nuclear translocation of active β-catenin. In the nucleus, β-catenin acts as a transcriptional cofactor with members of the T-cell factor/lymphoid enhancer factor (TCF/LEF) family to regulate target gene expression.7
WNT signaling has been shown to play an important role in organ development and has been implicated as a causative factor in a variety of cancers.7 In the adrenal gland, the Wnt pathway is required for normal gland formation, and adrenocortical cell-specific knock out of Ctnnb1 in mice results in adrenal gland aplasia.8 A number of tumor profiling studies have revealed abnormalities in β-catenin in both ACA and ACC.9–12 Here, we present data from a cohort of 118 adult patients with ACA or ACC, of whom 24% and 30%, respectively, displayed cytoplasmic and/or nuclear β-catenin staining, compared with the membranous staining found in the normal adrenal cortex. Approximately 20% of the patients in the cohort had exon 3 CTNNB1 mutations, which alter the consensus site for glycogen synthase kinase 3β (GSK3β)-mediated phosphorylation and thus abrogate β-catenin degradation.13–15
Insulin-like growth factor 2 (IGF2) is a critical growth factor in the development of many organ systems, including the adrenal cortex. Reciprocal imprinting defects of the 11p15.5 locus result in either Silver-Russell syndrome or Beckwith-Wiedemann syndrome (BWS). Silver-Russell syndrome is characterized by elevated expression of H19, decreased expression of IGF2, hypomethylation of the imprinting control region, and growth retardation.16 BWS is characterized by elevated expression of IGF2, hypermethylation of the imprinting control region, and organ overgrowth.17 BWS patients have, among other manifestations, an increased rate of ACC.17 IGF2 is a mitogen that acts by binding the cell surface receptor, IGF-1R, resulting in its autophosphorylation and a downstream signaling cascade that engages a number of cellular processes, including cell-cycle progression and proliferation.18 Large-scale gene expression studies from our research group and from others have revealed that IGF2 expression is dramatically up-regulated in at least 80% of sporadic ACCs, compared with either ACA or normal adrenal tissue.19–23
To examine the potential interaction of Wnt and Igf2 signaling pathways in ACC, we developed mutant mouse models in which these pathways are perturbed. Mice lacking Apc specifically in the adrenal cortex displayed age-dependent adrenocortical hyperplasia as microscopic to macroscopic adenomas. Adrenal cortex-specific loss of imprinting at the Igf2/H19 region in mice results in elevated Igf2 expression and minor adrenocortical dysplasia, but no actual hyperplasia. When the Apc and Igf2/H19 alterations are combined in the adrenal cortex, mice exhibited earlier signs of adrenocortical hyperplasia, earlier and more severe microscopic and macroscopic adenomas, and cancer formation. Our present results, along with data from other genetic profiling studies, as discussed below, suggest that multiple genetic aberrations are required for ACC development.
Materials and Methods
Frozen Human Tissues, Histopathology, and RNA isolation
Frozen tumor samples from the 55 patients in the University of Michigan Health System (UMHS) cohort were either obtained from the Cooperative Human Tissue Network (CHTN) or derived from UMHS surgical specimens procured by the Tissue Procurement Service of the University of Michigan Comprehensive Cancer Center. Histopathology and clinical and pathological features of the tissues (including stage, grade, and mitotic rate) were as described previously.19 Samples from the University of São Paulo (USP) cohort included frozen tissues from 63 adrenocortical tumors that were used for mRNA isolation. In the USP cohort, 27 of the 63 tumors were classified as carcinomas according to Weiss scoring criteria24–26 (score >2). All human data that contributed to analysis of patient prognosis (including mitotic rate, stage, grade, hormone secretion, and β-catenin status) are available as part of our Gene Expression Omnibus (GEO) series deposited with the National Center for Biotechnology Information (accession no. GSE33371 at http://www.ncbi.nlm.nih.gov).
Analysis of Human RNA Samples
Microarray data have been described previously.19 The raw array data (.CEL files) and the data set with the statistical tests used to select for differentially expressed genes have been deposited in the GEO database (GSE33371).27 Probe-set annotations were updated from the Affymetrix website (version na32, June 2011 edition). Quantitative real-time PCR (qPCR) of samples from the USP cohort was performed as described previously.28 Gene expression was analyzed using TaqMan expression arrays: Hs01005963_m1 for detection of IGF2 and 4326315E for ACTB (β-actin) as a control.
Tissue Microarray of Human Samples and Analysis
UMHS Cohort
An adrenal tissue array of samples, designated AdrenalTMA3, was constructed as described previously.19 Immunohistochemistry for β-catenin was performed using this AdrenalTMA3 as described previously.29 Because staining patterns were consistent throughout the ACC tissue sections, β-catenin immunoreactivity was scored in a simple dichotomous manner by evaluating the tumor cells for membranous staining versus cytoplasmic and/or nuclear immunolocalization.
USP Cohort
TMA blocks were prepared from representative tumor areas as described previously,28 and were evaluated by IHC with mouse anti-human β-catenin antibody (1:200; M3539; DakoCytomation, Carpinteria, CA). Two investigators without knowledge of the clinical data independently evaluated β-catenin staining. Immunoreactivity for β-catenin was evaluated in both nucleus and cytoplasm. Each sample was scored as described previously.10,11
Genomic Mutational Analysis of the CTNNB1 Gene
Somatic mutations of exon 3 of the CTNNB1 gene were screened in UMHS tumor samples by DNA sequencing of exon 3, as described previously.29 USP tumor samples of from adult patients (24 adenomas and 18 carcinomas) were also screened for somatic mutations of exon 3 of the CTNNB1 gene by automatic sequencing. Briefly, primers were designed to amplify exon 3 and the flanking intronic sequences of the β-catenin gene (CTNNB1): 5′-TGGGTCATATCACAGATTCTTTTTTT-3′ and 5′-TCAAAACTGCATTCTGACTTTCA-3′. PCR amplification used Taq DNA polymerase (Promega, Madison, WI). The amplified products were directly sequenced on an ABI 7000 sequence detection system (Life Technologies-Applied Biosystems, Foster City, CA). Mutations were verified in both sense and anti-sense directions.
Mouse Models
Steroidogenic factor 1 (Sf1)-Cre transgenic mice were obtained from Keith Parker (University of Texas-Southwestern, Dallas, TX).30 Sf1 is also known as nuclear receptor subfamily 5, group A member 1 (encoded by Nr5a1). The Sf1-Cre single-copy transgene targets low levels of expression of Cre recombinase to the urogenital ridge by E10.0 and activates a Cre-dependent reporter gene stochastically throughout the adrenal cortex.8,30 Mice carrying floxed exon 14 of the Apc allele31 (ApcloxP/loxP) were obtained from Bart Williams (Van Andel Institute, Grand Rapids, MI). In mice harboring two floxed Apc alleles and the Sf1-Cre transgenes, Cre recombination results in a frameshift mutation generating a null product in Sf1-expressing tissues, including the adrenal cortex [APC knock-out (KO)] (see Supplemental Figure S1A at http://ajp.amjpathol.org). Mice carrying the floxed Ctnnb1 gene (Ctnnb1tm2kem) were purchased from the Jackson Laboratory (Bar Harbor, ME). This cistron contains loxP sites flanking exons 2 to 6 and produces an inactive β-catenin product on Cre-mediated excision32 (BCAT KO) (see Supplemental Figure S1B at http://ajp.amjpathol.org). Some studies involved mice that were the result of crossing APC KO mice with BCAT KO mice (APC-BCAT KO) (see Supplemental Figure S1C at http://ajp.amjpathol.org). Mice with loxP sites flanking the Igf2/H19 imprinting control region (differentially methylated domain, or DMD; H19lxDMD/lxDMD) were a kind gift from Marisa Bartolomei (University of Pennsylvania, Philadelphia, PA).33 On crossing a female H19lxDMD/lxDMD mouse with a male Sf1-Cre mouse, Cre recombination in Sf1-expressing tissues results in loss of imprinting at the maternal Igf2/H19 locus and expression of Igf2 from both maternal and paternal alleles (H19ΔDMD) (see Supplemental Figure S2A at http://ajp.amjpathol.org). Combined loss of Apc and Igf2/H19 imprinting was achieved by breeding Apcfloxed/floxed mice with H19lxDMD/lxDMD mice (APC KO-H19ΔDMD) (see Supplemental Figure S2B at http://ajp.amjpathol.org). Genotyping protocols for the mouse models are given in Table 1. After harvest of tissues from the animals, both adrenals were weighed; the left adrenal was processed for immunohistochemistry and the right adrenal was flash-frozen for biochemical analysis. In some instances in which macroadenomas were present, tumors were cut in half to allow for immunohistochemical and biochemical analyses of the same tissue.
Table 1.
Genotyping of Mouse Models
| Transgene | Primer | TA (°C) | Product size (bp) |
|---|---|---|---|
| Sf1-Cre | Fwd 5′-CAATTTACTGACCGTACAC-3′ | 61 | WT: no band |
| Rev 5′-AGCTGGCCCAAATGTTGCTG-3′ | Cre: 280 | ||
| ApcloxP/loxP | Fwd 5′-GTTCTGTATCATGGAAAGATAGGTGG-3′ | 55 | WT: 320 |
| Rev 5′-CACTCAAAACGCTTTTGAGGGTTGAT-3′ | floxed: 370 | ||
| Ctnnb1tm2kem | Fwd 5′-AAGGTAGAGTGATGAAAGTTGTT-3′ | 60 | WT: 300 |
| Rev 5′-CACCATGTCCTCTGTCTATTC-3′ | floxed: 400 | ||
| H19lxDMD/lxDMD | Fwd 5′-CACACAAAGGATTCTTTGCAGAGAG-3′ | 58 | WT: 297 |
| Rev 5′-TGCAAGGAGACCATGCCTATTCTTG-3′ | floxed: 347 |
TA, annealing temperature; WT, wild type.
Immunoblot Analysis of Mouse Adrenal Lysates
Protein lysates were prepared from mouse adrenal glands and analyzed by immunoblotting as described previously.34 We used the following primary antibodies directed against specific proteins: total β-catenin (610154; BD Biosciences, San Jose, CA), proliferating cellular nuclear antigen (PCNA; sc56, Santa Cruz Biotechnology, Santa Cruz, CA), Dax1 [dosage-sensitive sex reversal, adrenal hypoplasia critical region on chromosome X, gene 1; also known as nuclear receptor subfamily 0 group B member 1; encoded by Nr0b1); kindly provided by Enzo Lalli, Institute of Molecular and Cellular Pharmacology, Valbonne, France], total Akt (thymoma viral proto-oncogene 1; 9272, Cell Signaling Technology, Danvers, MA), phosphorylated Akt at Ser473 (4051S; Cell Signaling Technology), and β-actin (A5441; Sigma-Aldrich, St. Louis, MO). Secondary antibodies used were horseradish peroxidase-conjugated (Roche, Indianapolis, IN) or IRdye conjugated (LI-COR Biosciences, Lincoln, NE); for detection, we used West Dura enhanced chemiluminescence reagent (Pierce; Thermo Fisher Scientific, Rockford, IL) and autoradiography or a LI-COR Odyssey infrared system.
Analysis of Mouse Adrenal Gland Histology and Immunohistochemistry
Adrenal glands were collected at 6, 15, 30, 45, and >45 weeks of age and were fixed, processed, and sectioned as described previously.8 The following antibodies were used: Dax1 (generously provided by Ken Morohashi, Kyushu University, Japan35), 20α-Hsd (20α-hydroxysteroid dehydrogenase; generously provided by Yacob Weinstein, Ben Gurion University, Israel), β-catenin (610154 from BD Biosciences, or sc7199 from Santa Cruz Biotechnology, Santa Cruz, CA), tyrosine hydroxylase (TH; MAB318; Millipore, Billerica, MA) and Sf1 (custom anti-Sf1 prepared by Proteintech Group, Inc, Chicago, IL). IHC was performed using a Vectastain ABC (avidin/biotinylated enzyme complex) kit or a Mouse on Mouse (M.O.M.) kit according to the manufacturer's protocol (Vector Laboratories, Burlingame, CA). Tissue sections were incubated at 4°C overnight with primary antibody, washed, and incubated with secondary antibodies provided with the Vectastain ABC kit or M.O.M kit. Antigen was detected through the use of SIGMAFAST 3,3′-diaminobenzidine tablets (D4168, Sigma-Aldrich). Secondary antibodies used for immunofluorescence were DyLight dyes 488 and 549, goat anti-mouse or goat anti-rabbit from Jackson ImmunoResearch (West Grove, PA). Sections were analyzed by normal light or fluorescent microscopy. Ki-67 detection (ab16667; Abcam) in mouse adrenals was conducted by the Pathology Cores for Animal Research at the University of Michigan, Unit for Laboratory Animal Medicine.
Analysis of Mouse RNA Samples
Total adrenal gland mRNA was prepared and analyzed by qPCR as described previously.8 Primer sequences were as follows: Axin2: 5′-GCAGGAGCCTCACCCTTC-3′to 5′-TGCCAGTTTCTTTGGCTCTT-3′; β-actin: 5′-ACCCGCCACCAGTTCGCCAT-3′ to 5′-TACAGCCCGGGAGCATCGT-3′; Igf2: 5′-CGCTTCAGTTTGTCTGTTCG-3′ to 5′-GCAGCACTCTTCCACGATG-3′; and Lef1 (lymphoid enhancer binding factor 1): 5′-CTGAAATCCCCACCTTCTACC-3′ to 5′-TGGGATAAACAGGCTGACCT-3′. All primers were validated.36
Human and Animal Research Statements
The Endocrine Database of the University of Michigan Endocrine Bank securely joins clinical data about a patient's course of disease, pathology data, and outcome of treatment. The database is Institutional Review Board-approved as a repository of retrospectively gathered patient data behind the UMHS firewall and under control of a small research team. Each research project that intends to query these data to address a research hypothesis undergoes a separate Institutional Review Board approval process based on its own merits and risks, as did the present study. Studies from the University of São Paulo were approved by the ethics committee of Clinical Hospital, São Paulo, Brazil, and informed consent was obtained from all patients and/or responsible representatives.
All experiments involving mice were performed in accordance with an institutionally approved protocol under the auspices of the University of Michigan Committee on Use and Care of Animals. Veterinary care was provided by the Unit for Laboratory Animal Medicine staff at the University of Michigan based on standards in the current Guide for Care and Use of Laboratory Animals, the Animal Welfare Act Regulations, and the Public Health Service Policy on Humane Care and Use of Laboratory Animals.
Results
Abnormal β-Catenin Staining and CTNNB1 Mutations Correlate with Poor Prognosis in Human ACCs
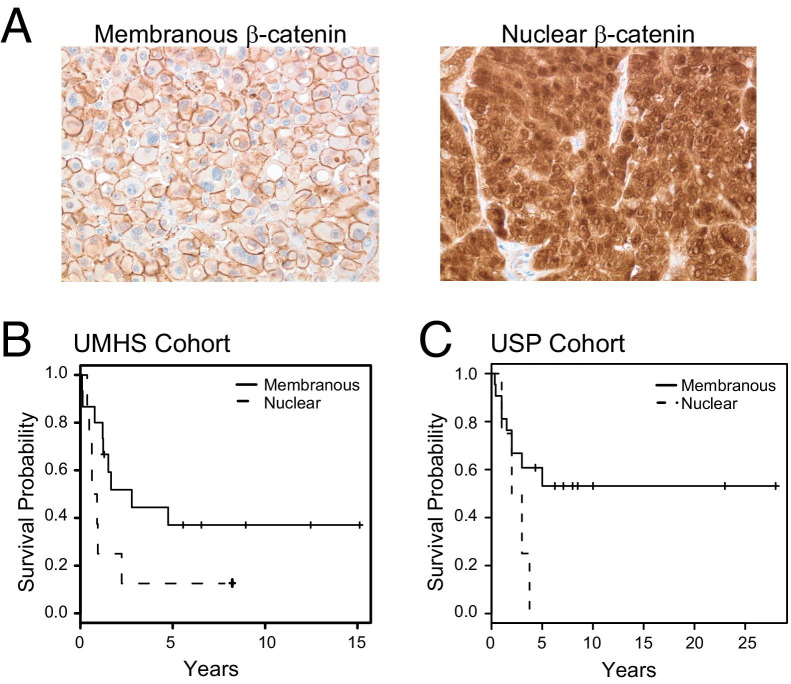
The present study population comprises a combined cohort of 118 adult adrenal tumor samples from the UMHS and the USP. Of these samples, 58 were classified as adenomas (22 UMHS and 36 USP; Weiss score ≤ ū2) and 60 as carcinomas (33 UMHS and 27 USP; Weiss score > 2). Previous studies have demonstrated abnormal cytoplasmic and nuclear accumulation of β-catenin in ACAs and ACCs.9,12,37 We therefore evaluated the status of WNT pathway activation by IHC for β-catenin in tumors from this cohort, using tissue arrays. Representative images from IHC analysis for membranous β-catenin and abnormal nuclear β-catenin staining are presented in Figure 1A. Abnormal cytoplasmic and/or nuclear β-catenin immunoreactivity was observed in 24.1% (14/58) of the ACAs and 30% (18/60) of the ACCs.
Figure 1.
Abnormal β-catenin localization correlates with poor prognosis in humans. A: Immunohistochemistry in ACC showing typical membranous β-catenin or nuclear β-catenin. B: Kaplan-Meier analysis of the UMHS cohort indicated that patients with membranous β-catenin staining (n = 15) had a better survival rate than those with nuclear β-catenin staining (n = 8; P = 0.06 log-rank test). C: Kaplan-Meier analysis of the USP cohort indicated that patients with membranous β-catenin staining (n = 22) had a better survival rate than those with nuclear β-catenin staining (n = 5; P = 0.09 log-rank test).
DNA sequencing of exon 3 of the CTNNB1 gene was performed for 46 ACAs and 31 ACCs, revealing mutations in 21.7% and 22.6% of the cases, respectively. Mutations observed included those previously found in ACC, which result in serine to proline (S45P) and proline to alanine (P44A), and a change at aspartic acid 32.9,12,38 In addition, we found missense mutations responsible for amino acid changes, including serine to cysteine (S37C), glycine to glutamine (G34E), leucine to proline (L46P), or aspartic acid to glycine (D32G). Deletions within exon 3 were also observed (S45del, 41del 5, 43del 8, and 44del1).
In a recent study of both French and German patients, Gaujoux et al12 found that WNT pathway activation as assessed by β-catenin immunohistochemistry was associated with survival. To confirm and extend those results, we performed Kaplan-Meier analysis using data from a total of 50 patients with follow-up data available (23 UMHS and 27 USP), and found that ACC patients with tumors displaying cytoplasmic/nuclear β-catenin had poorer survival than patients with tumors displaying membranous β-catenin (P = 0.006 log-rank test, UMHS and USP combined; Figure 1, B and C). Moreover, although β-catenin status in ACCs was not associated with McFarlane stage, it was associated with high-grade disease as assessed by mitotic rate >20 and analyzed as log2-transformed data (Table 2). In the UMHS cohort, 8/8 tumors with nuclear β-catenin staining were high grade, compared with only 9/15 hours without abnormal β-catenin (P = 0.058 two-sided Fisher's exact test); in the USP cohort, only 1/5 tumors with nuclear β-catenin staining were high grade, whereas none of the 22 tumors without abnormal β-catenin were high grade (Table 3). The β-catenin status of the tumors was significantly associated with prognosis in ACC in univariable models, as others have reported.12 In multivariate Cox models, however, using stage and grade (mitotic rate), we found including β-catenin status did not significantly improve survival prediction (Table 2). The Gaujoux et al12 group that reported independent predictive power for β-catenin did examine mitotic rate, but did not incorporate it as one of the clinical measures in their models. Because abnormal β-catenin status is preferentially present in ACC with high mitotic rates (high-grade ACC), it is not surprising that the predictive power of β-catenin is diminished when tumor grade is considered.39 Thus, our results confirm that CTNNB1 mutations and abnormal β-catenin localization are found with similar frequency in ACA and ACCs. Furthermore in ACC, abnormal β-catenin is associated with increased mitotic rates and poor prognosis.
Table 2.
Multivariable Cox Proportional Hazard Models for Patient Survival
| Cohort and effect | P value (Wald test) | Relative Risk (95% CI) |
|---|---|---|
| UMHS cohort | ||
| β-catenin, nuclear vs membranous | 0.220 | 1.89 (0.69–5.21) |
| Mitotic rate, log2-transformed⁎ | 0.020 | 1.84 (1.10–3.07) |
| Stage III+IV vs I+II | 0.049 | 2.96 (1.00–8.70) |
| USP cohort | ||
| β-catenin, nuclear vs membranous | 0.92 | 1.07 (0.30–3.79) |
| Mitotic rate, log2-transformed⁎ | 0.21 | 1.36 (0.84–2.20) |
| Stage III+IV vs I+II | 0.0033 | 8.06 (2.00–32.36) |
| Combined | ||
| β-catenin, nuclear vs membranous | 0.29 | 1.54 (0.70–3.41) |
| Mitotic rate, log2-transformed⁎ | 0.0016 | 1.55 (1.18–2.03) |
| Stage III+IV vs I+II | 0.00085 | 4.14 (1.80–9.52) |
Mitotic rates of <5 set equal to 5.
Table 3.
Association of β-Catenin status with Stage and Grade
| Cohort | Nuclear β-catenin with high stage or grade (n/N) | Membranous β-catenin with high stage or grade (n/N) | P value⁎ |
|---|---|---|---|
| Association of β-catenin and stage | |||
| UMHS cohort | 4/8 | 7/15 | 1.0 |
| USP cohort | 3/5 | 8/22 | 0.37 |
| Combined | 7/13 | 15/37 | 0.52 |
| Association of β-catenin and grade | |||
| UMHS cohort | 8/8 | 9/15 | 0.058 |
| USP cohort | 1/5 | 0/22 | 0.19 |
| Combined | 9/13 | 9/37 | 0.007 |
Two-sided Fisher's exact test.
Studies in mice and humans have suggested an association between abnormal β-catenin expression and hyperaldosteronism.40,41 Other studies, however, have not found a clear correlation between tumor hormone production and β-catenin status.12,37 In the UMHS cohort, of the 22 ACAs with hormone data available, 8 tumors displayed abnormal β-catenin staining, and 5 of these were found to be non-hormone secreting (P = 0.01 Fisher's exact test; Table 4). In contrast, 12 of 29 ACCs in the UMHS cohort displayed abnormal β-catenin staining, and 11 of these were hormone-producing (P = 0.02). These associations were not found to be significant in the USP cohort. Of 36 ACAs in the USP cohort with hormone data available, 10 had abnormal β-catenin, and 6 of these 10 were hormone-producing (P = 0.23; Table 4). Of the 27 ACCs in the USP cohort, 4 of 5 tumors with abnormal β-catenin were hormone-producing (P = 0.47). These data suggest variability in the UMHS versus USP patient cohorts or in the clinical diagnosis of hormone excess in each population. It is intriguing that β-catenin mutations and hormone production have been shown independently to predict worse prognosis in ACC.42
Table 4.
Correlation of β-catenin status to Hormone Secretion in ACAs and ACCs
| UMHS cohort (no.) |
USP cohort (no.) |
|||
|---|---|---|---|---|
| Membranous β-catenin | Nuclear/cytoplasmic β-catenin | Membranous β-catenin | Nuclear/cytoplasmic β-catenin | |
| Adrenocortical adenomas | ||||
| Nonfunctional | 1 | 5⁎ | 5 | 4 |
| Functional | 13 | 3 | 21 | 6† |
| Total | 14 | 8 | 26 | 10 |
| Adrenocortical carcinomas | ||||
| Nonfunctional | 9 | 1 | 2 | 1 |
| Functional | 8 | 11‡ | 20 | 4§ |
| Total | 17 | 12 | 22 | 5 |
Abnormal β-catenin status is correlated with nonsecretion; P = 0.01, Fisher's exact test.
Abnormal β-catenin status is not correlated with secretion; P = 0.23, Fisher's exact test.
Abnormal β-catenin status is correlated with secretion; P = 0.02, Fisher's exact test.
Abnormal β-catenin status is not correlated with secretion; P = 0.47, Fisher's exact test.
Abnormal β-Catenin Status Correlates with Changes in Gene Expression and an Enrichment of Up-Regulated LEF1 Target Genes
To examine global changes in gene expression, we used our previous array data from the GEO series (GSE33371; formerly GSE10927) consisting of mRNA abundance assays of the 33 ACCs, 22 ACAs, and 10 normal adrenal cortex samples from the UMHS cohort run on HG-U133_Plus_2 arrays, which hold 54,675 probe sets.19 Both ACA and ACC samples were divided into two groups, based on β-catenin status: one group with cytoplasmic/nuclear staining or CTNNB1 mutations (Bcat+) and the other with normal membranous staining and no CTNNB1 mutations (Bcat−). Thus, including normal adrenal cortex samples, we had five groups (normal, n = 10; ACC Bcat+, n = 13; ACC Bcat− n = 19; ACA Bcat+, n = 8; and ACA Bcat− n = 14) to which we fit analysis of variance models with separate means for each group. One ACC, for which no β-catenin analysis was performed, was excluded. Using pairwise contrasts between Bcat+ versus Bcat− ACCs, we obtained 4559 probe sets corresponding to transcripts that differed significantly (P < 0.01). A similar comparison in ACAs revealed that signal from only 763 probe sets differed significantly (P < 0.01). In both cases, we expected approximately 547 (ie, 54,675 × 0.01) P values this small by chance, indicating that only approximately 12% of the probe sets representing transcripts whose expression levels appear significantly up or down in ACC Bcat+ are false positives. Of the probe sets with P < 0.01, we further selected only those in the ACC comparison with average differences of at least 1.5-fold and thereby obtained a list of probe sets representing 1230 increased and 1087 decreased transcripts (see Supplemental Table S1 at http://ajp.amjpathol.org). Compared with ACC, β-catenin status in our ACA samples corresponded to fewer transcripts that were changed more that 1.5-fold and with P < 0.01 (278 increased and 211 decreased). In addition, there was minimal overlap in similarly affected transcripts in ACC and ACA (a total of 14 probe sets). In fact, the probe sets were significantly negatively associated; there were more transcripts for which abnormal β-catenin correlated with an increase in ACC and a decrease in ACA and vice versa (a total of 16 and 21 probe sets, respectively), compared with transcripts that were regulated in the same direction in both ACC and ACA (see Supplemental Table S1 at http://ajp.amjpathol.org). The full array data and results for this and other statistical tests are available in the GEO database (GSE33371).
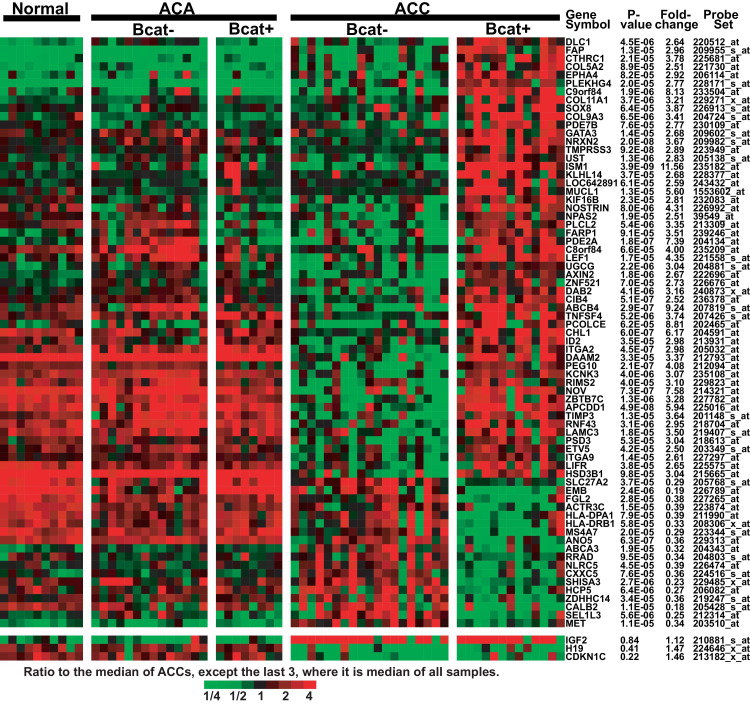
A subset of the most profound differences (fold change > 2.5, P < 0.0001) is presented in Figure 2. Transcripts for the β-catenin target genes LEF1, axin 2 (AXIN2), and isthmin 1 homolog (ISM1) are increased in ACC with abnormal β-catenin status. Surprisingly, these targets are not increased in Bcat+ ACA. Certain other transcripts are decreased in Bcat+ ACC, including the met proto-oncogene (MET), which has been reported to be associated with β-catenin in a variety of cancers.43–45 This evaluation also revealed genes whose expression levels differ (generally as a decrease) specifically in ACC with normal β-catenin status (Bcat−). This group includes nephroblastoma overexpressed (NOV), also known as connective tissue growth factor 3 (CCN3), an apoptotic factor that is also decreased in pediatric ACC.46,47
Figure 2.
Gene expression differences between ACCs with normal membranous β-catenin (Bcat−) versus cytoplasmic/nuclear β-catenin staining (Bcat+). An analysis of variance model fit to the five groups (normal, ACC Bcat+, ACC Bcat−, ACA Bcat+, and ACA Bcat−) was used to compare Bcat+ versus Bcat− ACCs. Probe sets that yielded P < 0.0001 and an average fold change of at least 2.5 were selected, and reduced to those annotated with Entrez gene identifiers. Only the probe set with the smallest P value for a gene is shown. Bcat+ ACAs were those with either β-catenin nuclear staining or mutation of β-catenin exon 3. Data are expressed as the ratio to the median of ACCs, except that in the last three rows the median is for all samples. Red indicates the highest expression (ratio 4) and green indicates the lowest expression (ratio 1/4).
Altered gene expression in Bcat+ ACC may be expected to reflect changes in specific cellular processes. To examine this possibility, we performed enrichment testing using several publicly available sets of gene lists. After removing redundancy in the 1230 up and 1087 down probe sets, we obtained lists of 678 up and 742 down distinct transcripts (see Supplemental Table S2 at http://ajp.amjpathol.org). Genes with Gene Ontology (GO) terms for cell division and cell adhesion were significantly over-represented in the list of genes whose expression was elevated in Bcat+ ACCs, compared with Bcat−, whereas genes involved in antigen processing and immune response were under-represented. Transcripts increased in Bcat+ ACC were enriched for Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways relating to the cell cycle, focal adhesion, and p53, WNT, and TGF-β signaling, whereas transcripts decreased in Bcat+ ACC were enriched for pathways involving the immune system. Over-representation of genes involved in cell division and cell cycle in both enrichment tests suggests an increased proliferative capacity in Bcat+ ACC, compared with Bcat− ACC. We also tested lists of genes in the Molecular Signatures Database (MSigDB; http://w/broadinstitute.org/gsea/msigdb/index.jsp) curating cis-regulatory DNA motifs and found up-regulated genes most enriched for LEF1 and NFAT (nuclear factor of activated T cells) binding sites.48 Two DNA motifs for the LEF1 transcription factor were particularly significant (P < 10−10; see Supplemental Table S2 at http://ajp.amjpathol.org). From these lists, selected genes were identified (those that yielded P < 0.01 and an average expression increase of at least 1.5-fold in a comparison of Bcat+ ACCs with Bcat− ACCs), representing transcripts up-regulated in Bcat+ ACC (see Supplemental Figure S3 at http://ajp.amjpathol.org).
These findings demonstrate that abnormal accumulation of β-catenin results in relatively few changes in the transcriptome of ACAs. In contrast, β-catenin status in ACC correlates with enhanced proliferative potential and an altered gene signature rich in TCF/LEF target genes, suggesting that nuclear β-catenin is transcriptionally active and potentially contributing to the markedly worse prognosis.
Conditional Knock-Out of Apc Causes Stabilization of β-Catenin and Aberrant Expression of Adrenocortical Markers in Mice
To achieve β-catenin stabilization in the adrenal cortex, we modeled adrenocortical tumors that occur in FAP patients and in some sporadic ACCs through the use of conditional knock out of the Apc gene.12,31 Previous studies have evaluated mouse models with mutations in exon 3 of Ctnnb1 in the adrenal cortex.40 Both approaches result in stabilization of β-catenin and mimic overactivation of Wnt signaling. To knock out Apc in only the steroidogenic tissues (adrenal cortex and gonads), our studies used the Sf1-Cre stochastic driver.8,30 This Cre driver, which has only one copy of the Cre transgene, has been shown to lead to incomplete recombination in the adrenal cortex; in contrast, the Sf1-Cre complete driver has five copies of the Cre transgene and leads to recombination throughout the entire adrenal cortex.8,30 We opted to use the stochastic driver, because it more closely resembles the sporadic nature of ACC formation in humans. Indeed, use of the Sf1-Cre complete driver to eliminate Apc expression leads to improper development of the adrenal gland, resulting in severely hypoplastic adrenals, which would confound any studies looking at tumor formation (see Supplemental Figure S4 at http://ajp.amjpathol.org).
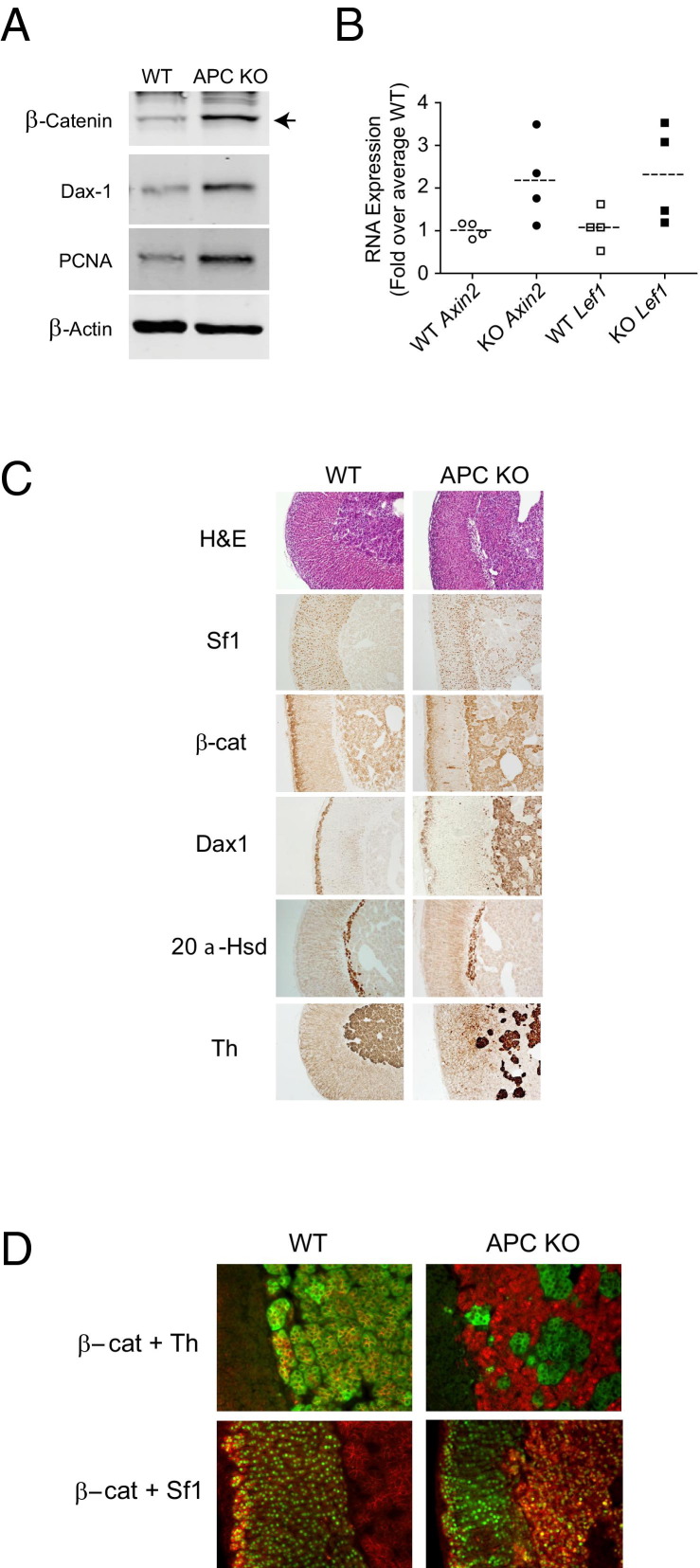
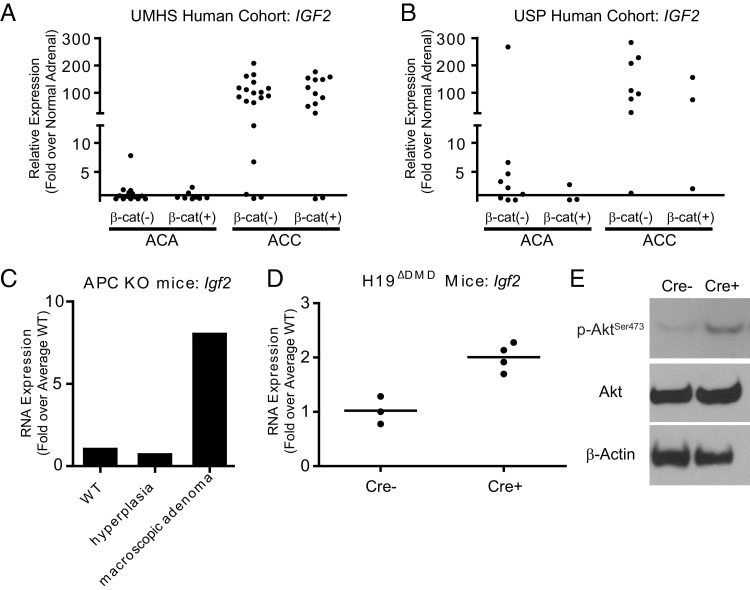
Conditional knock out of exon 14 of the Apc gene was achieved by crossing Sf1-Cre stochastic transgenic mice with mice harboring floxed Apc to produce progeny with the Sf1-Cre/ApcloxP/loxP genotype,30,31 here termed APC KO (see Supplemental Figure S1A at http://ajp.amjpathol.org). APC KO mice have elevated levels of β-catenin, as confirmed by immunoblot analysis (Figure 3A). In addition, examination of mRNA levels revealed an approximately twofold increase in the β-catenin target genes Axin2 and Lef1 (P = 0.018 and P = 0.042, respectively) in APC KO adrenal glands (n = 4), compared with the mean of Cre− wild-type (WT) controls (n = 4) (Figure 3B). The APC KO adrenal glands also exhibited increased levels of PCNA, suggesting that there are more cells undergoing S-phase (Figure 3A).
Figure 3.
Conditional knock out of Apc in mice results in an increase in activated β-catenin and aberrant expression of cortical markers. A: Immunoblot analysis of whole adrenal protein lysates from 15-week-old WT (Cre− controls) or APC KO mice. Whole adrenal lysates were prepared from individual mice, and immunoblot analysis was performed using 10 μg protein. Membranes were probed with antibodies against β-catenin, PCNA, and Dax1, with β-actin as a loading control. Blots shown are representative of four replicates (four different mice of each genotype) with similar results. B: Analysis of mRNA expression from 15-week-old WT and APC KO mice. Total RNA from whole adrenal glands was evaluated by qPCR for expression of the β-catenin downstream targets Axin2 and Lef1 in both WT (n = 4) and APC KO mice (n = 4). Individual data points represent expression in individual adrenal glands; dashed lines represent the mean of all adrenal glands evaluated for a given genotype. The mean WT levels were set equal to 1, and expression was normalized to β-actin. Levels of both Axin2 and Lef1 were elevated approximately twofold in APC KO mice (P = 0.018 and P = 0.042, respectively, one-tailed t-test on ΔΔCT). C: Histological comparison of adrenal glands from 15-week-old WT or APC KO mice. Tissue sections were subjected to H&E staining or to IHC. Representative sections are shown. D: Immunofluorescent costaining with β-catenin (red) and TH (green) or β-catenin (red) and Sf1 (green). Cells staining red with green nuclei are Sf1-expressing cells with cytoplasmic β-catenin, and those with yellow nuclei are Sf1-expressing cells with nuclear β-catenin. Original magnification: ×100 (C); ×200 (D).
Histological analysis of adrenal glands from 15-week-old APC KO mice was compared with Cre− WT adrenal glands of the same age (Figure 3C). Organization of the cortex and the medulla is preserved, but aberrant cortical cells are found intermingled with cells of the adrenal medulla in the inner region of the gland (H&E staining in Figure 3C). These aberrant cells in the inner region express the essential nuclear receptor Sf1 and lack expression of TH. Rather, the TH-positive medullary cells, which normally fill the entire central portion of the adrenal gland, appear as patches surrounded by Sf1-positive cells (Figure 3C). At higher magnification, the immunofluorescent costaining clearly distinguishes the aberrant cells from the medullary cells (Figure 3D). In the WT adrenal, TH-expressing cells with membranous β-catenin fill the medullary space. In stark contrast, the APC KO adrenal displays an inner region that is invaded with cells expressing cytoplasmic/nuclear β-catenin but not TH. TH-expressing cells of the medulla are distinct and clustered in small islands. The β-catenin-expressing aberrant cells also expressed Sf1, in the presence of either nuclear or cytoplasmic β-catenin (Figure 3D).
Both β-catenin and Dax1 have been implicated in adrenocortical progenitor cell maintenance and are normally expressed only in cells of the subcapsular zone.8,49,50 In the APC KO mouse adrenal, however, both β-catenin and Dax1 are expressed in the aberrant cells (Figure 3C), suggesting that these cells have a phenotype similar to subcapsular cells.51–54 Additionally, cells positive for β-catenin immunoreactivity are found sporadically and centripetally throughout the cortex. The X/fetal zone, marked by the expression of 20α-hydroxysteroid dehydrogenase (20α-Hsd), lies at the corticomedullary boundary,55 In female mice, IHC revealed no difference in the expression pattern of 20α-Hsd, suggesting that the X-zone is not affected (Figure 3C). These data show that knock out of Apc in mouse adrenocortical cells results in stabilized active β-catenin and the appearance of aberrant cells primarily in the inner region of the gland.
Conditional Knock-Out of Apc Leads to Adrenal Hyperplasia and Adenomas
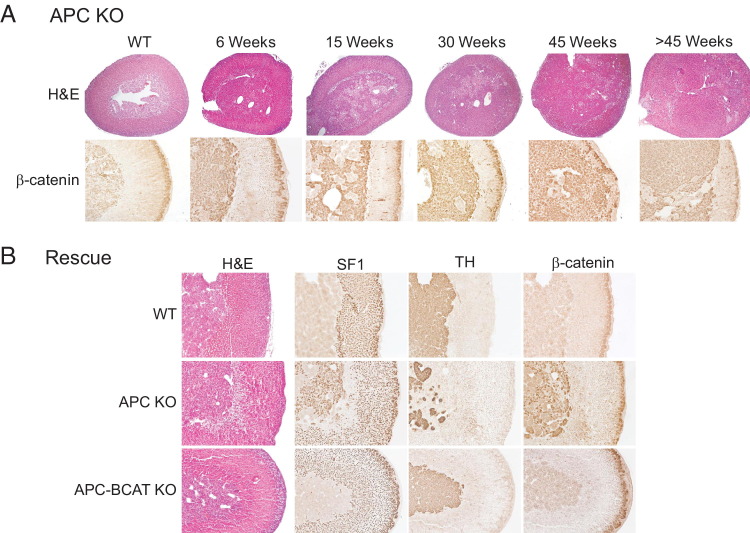
Because stabilized β-catenin correlates with a poor clinical outcome in ACC patients and because data from APC KO mice reveal that increased β-catenin activity leads to aberrant adrenal cells as early as 15 weeks after birth, it was important to determine whether these APC KO mice would also develop adrenal tumors. To this end, we analyzed adrenal glands from APC KO mice at 6, 15, 30, 45, and >45 weeks of age. As early as 6 weeks, the adrenal glands from APC KO mice began to display aberrant β-catenin staining and cortical hyperplasia identifiable on histological analysis (Figure 4A); however, no adrenocortical tumors were found in mice younger than 30 weeks of age. In some mice, by 45 weeks and later hyperplasia had progressed to the formation of microscopic adenomas, visible only on histological examination (Figure 4A), and macroscopic adenomas visible to the naked eye. Quantitation of this progression is discussed in more detail below.
Figure 4.
Conditional knock out of Apc in mice leads to β-catenin-dependent adrenal hyperplasia and adenomas. A: Histological analysis and immunohistochemical staining for β-catenin in adrenal glands from a 30-week-old WT mouse and from APC KO mice at 6, 15, 30, 45, and >45 weeks of age. APC KO mice exhibit adrenal hyperplasia that increases with age and some develop adenomas at later ages. Abnormal β-catenin expression is evident primarily in the inner region of the gland, although a few aberrant cells appear throughout the cortex. B: Rescue of APC KO phenotype. Adrenal glands were harvested from 6-week-old WT, APC KO, and APC-BCAT KO mice. H&E staining and IHC for Sf1, TH, and β-catenin were performed. Adrenals from APC-BCAT KO mice display staining characteristics similar to those of WT mice and unlike those of APC KO mice. Original magnification: ×40 (A, H&E); ×100 (A, β-catenin; B).
Adrenocortical Dysplasia in APC KO Mice Is Dependent on β-Catenin Expression
Apc has biological functions independent of Wnt-mediated β-catenin degradation.56 To confirm that the phenotype we observed in our mouse model is mediated by activated β-catenin, we generated mice harboring stochastic mutations of both Apc and Ctnnb1 in Sf1-expressing adrenocortical cells (APC-BCAT KO). The breeding scheme is presented in Supplemental Figure S1C (available at http://ajp.amjpathol.org). If adrenocortical dysplasia is β-catenin-dependent, loss of Ctnnb1 would be expected to lead to genetic rescue of the phenotype described in the APC KO mouse. When we examined the adrenal glands from the adult APC-BCAT KO mice, we observed a complete absence of the aberrant cells in the inner space (Figure 4B). Evaluation of Sf1 and TH expression by IHC revealed a clear delineation between the adrenal cortex and the medulla in the APC-BCAT KO mice (Figure 4B). Moreover, cytoplasmic/nuclear β-catenin accumulation was not detected in either the adrenal cortex or the medulla (Figure 4B). These results confirm that loss of β-catenin prevents the formation of the aberrant cells and adrenocortical dysplasia seen in APC KO mice, demonstrating that the phenotype of APC KO mice is mediated by activated β-catenin.
Elevated IGF2 Expression in Human ACCs and Mouse Tumors
Alterations in the 11p15.5 locus have been reported in many studies of ACC and, as we and others have reported, IGF2 is the most highly up-regulated gene observed in human ACC samples.19,21,26,57,58 Quantitation of the microarray data from the UMHS cohort demonstrated that IGF2 levels were elevated 7- to 200-fold, relative to normal adrenal gland IGF2 levels, in 85% (28/33) of the ACCs (Figures 2 and 5A). In that cohort, only 1 of 22 ACAs (4.5%) exhibited similarly elevated IGF2 levels (7.8-fold greater than normal). Comparison of IGF2 mRNA levels between groups defined by β-catenin status (Bcat+ versus Bcat−) failed to demonstrate an association between IGF2 expression and β-catenin staining in either ACA or ACC. The USP cohort showed a similar pattern; qPCR analysis revealed 9/11 (82%) ACCs tested have IGF2 levels 25- to 300-fold above normal adrenal gland IGF2 levels. Interestingly, 1/12 (8%) ACAs exhibited an IGF2 level 200-fold greater than normal (Figure 5B). Similar to the UMHS cohort, β-catenin status was independent of IGF2 levels. In light of these data, we examined Igf2 mRNA levels in the largest macroscopic adrenal adenoma observed in the APC KO mice (Weiss score = 2). This macroscopic adenoma had approximately 10-fold higher Igf2 expression, compared with a normal (Cre−/ApcloxP/+) or with an APC KO (Cre+/ApcloxP/loxP) that displayed hyperplasia but no gross tumor formation (Figure 5C).
Figure 5.
IGF2 and Igf2 expression is elevated in human ACCs and in mice with conditional knock out of the Igf2/H19 DMD. IGF2 levels were evaluated in human samples from ACA and ACC using microarray data from the UMHS cohort (GEO series GSE33371; individual tumor samples assessed relative to an average of 10 normal samples) (A) and by qPCR of individual human samples from the USP cohort (individual tumor samples assessed relative to 61 normal samples) (B). IGF2 levels were dramatically elevated in ACCs in both cohorts. No association of β-catenin status with IGF2 levels was detected in either ACAs or ACCs. C: qPCR determination of Igf2 levels in adrenals from one Cre− control mouse (WT), one APC KO with hyperplasia, and one APC KO with a large macroscopic adenoma. Igf2 mRNA was elevated dramatically in the macroscopic adenoma. D: qPCR determination of Igf2 levels in Cre− control mice (n = 3) and in H19ΔDMD mice (Cre+; n = 4). Igf2 levels were elevated approximately twofold in H19ΔDMD mice (P = 0.005, one-tailed t-test on ΔΔCT). Horizontal bars represent the mean value of each group. E: Whole adrenal gland lysates were analyzed by immunoblotting for phosphorylated Akt at Ser473 and total Akt, with β-actin as a loading control. Elevated levels of p-Akt in H19ΔDMD (Cre+) mice, relative to that in Cre− mice, indicate activation of Igf2 signaling. Blots shown are representative of four replicates (four different mice of each genotype) with similar results.
Loss of Imprinting at the IGF2/H19 Differentially Methylated Domain by Itself Does Not Induce Tumor Formation in Mice
Transactivation of endogenous mouse Igf2 results in a BWS-like syndrome, and postnatal overexpression of mouse Igf2 results in adrenocortical hyperplasia.59–61 In addition, given that most human ACCs and the large macroscopic adenoma from the APC KO mouse displayed elevated IGF2 and Igf2 expression, we set out to determine whether Igf2 overexpression alone could induce adrenal tumor formation, as occasionally occurs in BWS. To test this hypothesis, we used the conditional Igf2/H19 loss-of-imprinting mouse strain.33 The original characterization of this mutant strain demonstrated that the DMD is necessary for maternal allele silencing, allowing Igf2 expression only from the paternal allele.33 Maternally inherited deletion of the DMD leads to additional expression of Igf2 from the maternal allele, resulting in approximately twofold greater expression.33 To characterize the effects of loss of imprinting of the Igf2/H19 region in the adrenal cortex, we bred female H19lxDMD/lxDMD mice with male Sf1-Cre mice to obtain progeny with excision of the maternally inherited DMD stochastically in Sf1-expressing cells (H19ΔDMD). A breeding scheme is presented in Supplemental Figure S2A (available at http://ajp.amjpathol.org). Analysis by qPCR of mRNA from WT (n = 3) and H19ΔDMD (n = 4) mouse adrenal glands confirmed Igf2 expression to be twofold greater in H19ΔDMD mice than in control animals (P = 0.005; Figure 5D). Active Igf2 signaling through its membrane-bound receptor (Igf1r) can lead to phosphorylation and thereby activation of Akt, a kinase involved in cell growth and proliferation (among other downstream effects).18 Immunoblotting of whole adrenal gland lysates from H19ΔDMD mice (n = 5, WT; n = 4, H19ΔDMD) demonstrated the expected increase in p-AktSer473 (Figure 5E). This result, in the absence of an increase in overall Akt levels, confirms elevated Igf2 signaling.
Having established that we could achieve the loss of genetic imprinting, we set out to characterize the effects of Igf2 overexpression in the adrenal cortex. The histological architecture of adrenal glands from 15-week-old H19ΔDMD mice shows minor differences from littermate controls, as determined by H&E staining (see Supplemental Figure S5 at http://ajp.amjpathol.org). We allowed mice to age, and evaluated adrenal gland histology at 30, 45, and >45 weeks with similar results. Furthermore, even at >45 weeks of age the H19ΔDMD mice exhibited no evidence of hyperplasia or tumor formation. These data suggest that loss of imprinting through the Igf2/H19 DMD is not sufficient for tumor formation.
Loss of Apc and Overexpression of Igf2 in Combination Accelerate Tumor Formation in Mice
To model the dual-hit phenomenon of Apc loss and elevated Igf2 expression, we crossed the APC KO mice with the H19ΔDMD mice to obtain APC KO-H19ΔDMD. The breeding schemes were as detailed under Materials and Methods and presented in Supplemental Figure S2B (available at http://ajp.amjpathol.org). APC KO-H19ΔDMD mice were evaluated on the basis of total adrenal mass, histology, and tumor formation at 15, 30, 45, and >45 weeks of age and were compared with the APC KO and H19ΔDMD single-mutation mouse models.
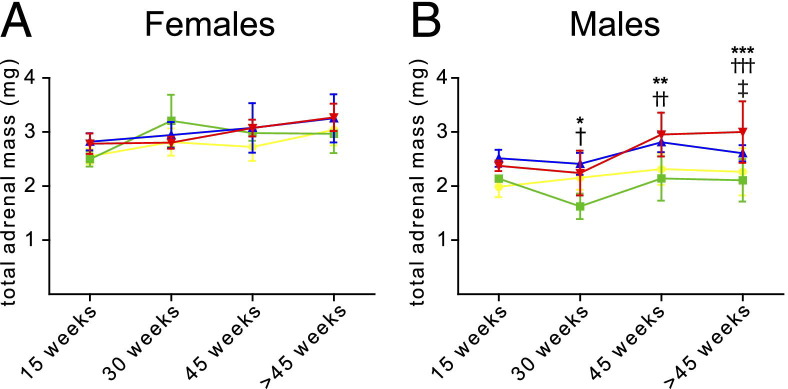
For each mouse, the sum of the mass of both adrenal glands was measured, and fit to an analysis of variance model with separate means for 32 groups of mice defined by four age groups (15, 30, 45, and >45 weeks, respectively), the two sexes, and four genetic classes (APC KO, H19ΔDMD, APC KO-H19ΔDMD, and Cre− littermates), and pairwise contrasts were performed. Two adrenal glands displayed extraordinarily large tumors (one male APC KO and one female APC KO-H19ΔDMD mouse). Adrenal masses of each mouse, including these two outliers, are presented in Supplemental Figure S6 (available at http://ajp.amjpathol.org). While keeping these exceptional tumors in mind, we examined differences in the adrenal mass data even when these samples were excluded. In female mice, adrenal mass did not significantly increase between 15 and >45 weeks of age and did not differ between mouse models (Figure 6A). In males, H19ΔDMD mice did not differ significantly from Cre− controls; however, in male APC KO and APC KO-H19ΔDMD mice the adrenal masses increased between 15 and >45 weeks of age (Figure 6B). Total adrenal masses of male APC KO mice were larger, relative to those of H19ΔDMD male mice, by 30 weeks (P = 5.7 × 10−5) and remained larger at 45 weeks (P = 7.8 × 10−5) and beyond 45 weeks (P = 0.012). In male APC KO-H19ΔDMD mice, total adrenal mass was significantly greater than for H19ΔDMD mice by 30 weeks (P = 0.002) and remained greater at 45 weeks (P = 4.45 × 10−7) and beyond 45 weeks (P = 6.7 × 10−8). In addition, the male APC KO-H19ΔDMD mice exhibited significantly larger total adrenal masses than the APC KO male mice at >45 weeks of age (P = 0.04; Figure 6B). In summary, statistically significant increases in total adrenal masses were observed in male mice with the genetic loss of both Apc and the Igf2/H19 DMD region.
Figure 6.
Total adrenal mass increases more rapidly in APC KO-H19ΔDMD mice, compared with APC KO mice. Adrenal glands were harvested from mice of the indicated ages and weighed individually. The sum of the two adrenal gland masses for each mouse was measured and fit to an analysis of variance model with separate means for 32 groups of mice, defined by four age groups (15, 30, 45, and >45 weeks), the two sexes, and four genetic classes: H19DDMD (green), APC KO (blue), APC KO-H19DDMD (red), and control (yellow). Pairs of groups were compared with F tests for pairwise contrasts. Female mice (A) exhibited no significant differences; in male mice (B), significant differences appeared from 30 weeks of age. Data are expressed as means ± SD. *P = 5.7 × 10−5, **P = 7.9 × 10−5, and ***P = 0.012 APC KO versus H19ΔDMD; †P = 0.0023, ††P = 4.5 × 10−7, and †††P = 6.7 × 10−8 APC KO-H19ΔDMD versus H19ΔDMD; and ‡P = 0.040 APC KO versus APC KO-H19ΔDMD.
To supplement the adrenal mass data, we measured cell proliferation in adrenals from >45-week-old mice. Most adrenals from H19ΔDMD and control mice displayed a basal level of proliferation (<1% of cells per adrenal) (Table 5). All adrenals from APC KO-H19ΔDMD mice displayed an increased number of proliferating cells, compared with control adrenals, whereas 67% of APC KO mice had adrenals with Ki-67 staining in >1% of cells. Furthermore, adrenals from multiple APC KO and APC KO-H19ΔDMD mice presented with accelerated organ growth (>5% of Ki-67-positive cells per adrenal; P = 0.0009 Mantel-Haenszel χ2 test of linear association between all models; Table 5). However, the difference in Ki-67 staining between APC KO and APC KO-H19ΔDMD mice did not reach statistical significance (P = 0.18).
Table 5.
Ki-67 Staining in Mouse Models of Adrenal Tumors
| Mouse model⁎ | Tumors with given percentage of Ki-67+ cells (no.) |
Total adrenals evaluated | |||
|---|---|---|---|---|---|
| <1% | 1%–5% | 5%–10% | 10–20% | ||
| Control (Cre−) | 4 | 2 | 0 | 0 | 6 |
| H19ΔDMD | 6 | 2 | 0 | 0 | 8 |
| APC KO | 5 | 6 | 3 | 1 | 15 |
| APC KO-H19ΔDMD† | 0 | 10 | 2 | 2 | 14 |
Mantel-Haenszel chi-square analysis of linear association between all models: P = 0.0009.
Comparison of APC KO and APC KO-H19ΔDMD by Mantel-Haenszel chi-square: P = 0.18.
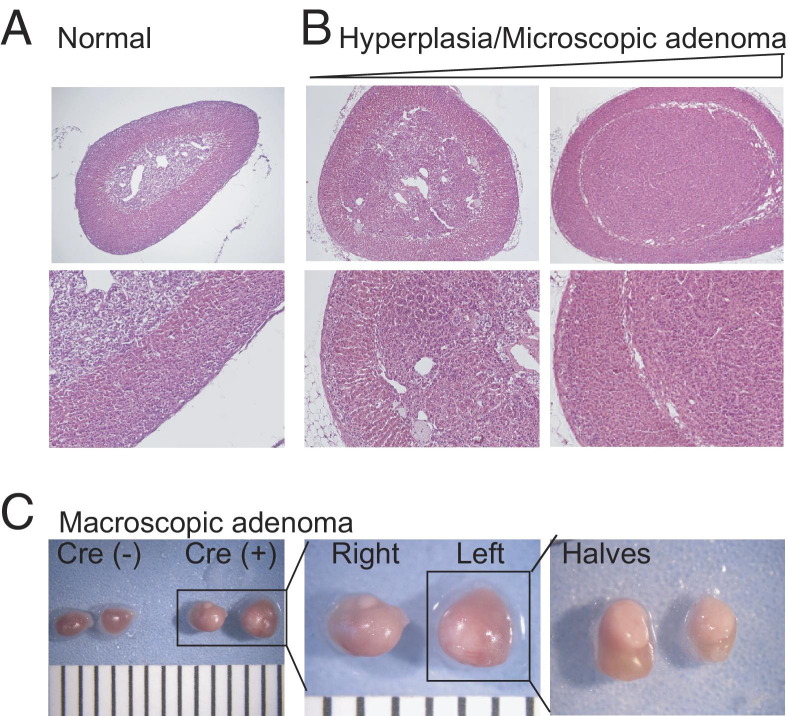
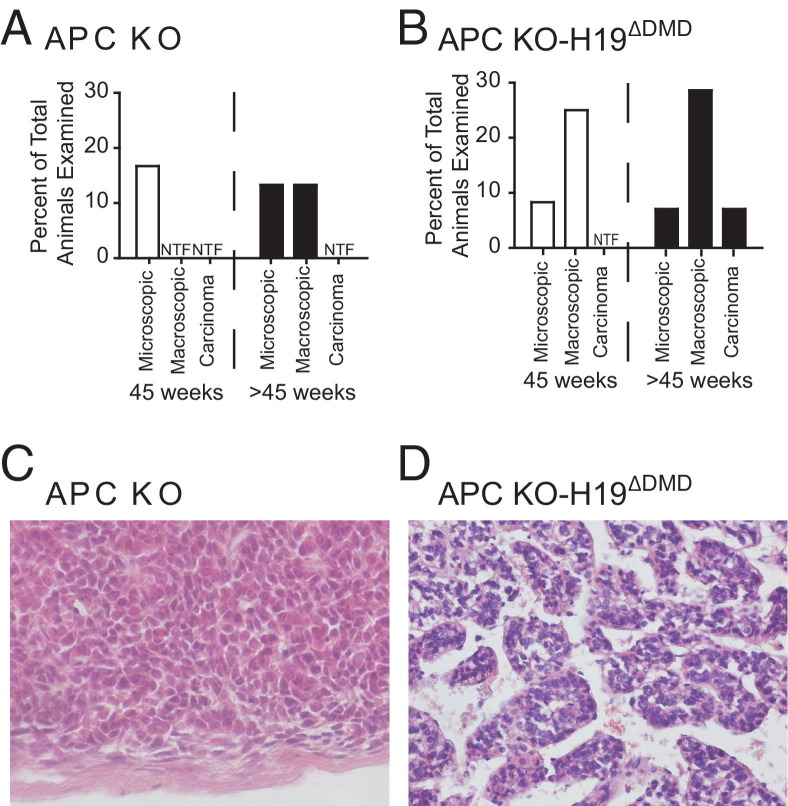
As adrenal glands were harvested, it became apparent that adrenal tumors were present in the older APC KO and APC KO-H19ΔDMD mice. Furthermore, in approximately 40% (4 of 10) of mice with tumors, both adrenals were visibly enlarged. Therefore, a single pathologist not directly involved in the animal studies (T.J.G.) did a blind-study of sections from each of these adrenal glands and classified them according to guidelines similar to those used for humans (Figure 7, A–C). The histology of the adrenal gland was classified as normal, hyperplastic, microscopic adenoma, macroscopic adenoma, or carcinoma (tumors with mitotic figures). By these criteria, H19ΔDMD mice displayed normal adrenal glands at all ages examined (Table 6), as did Cre− controls (data not shown). All adrenal glands from APC KO mice evaluated at 15 weeks were hyperplastic, and a shift toward microscopic adenomas was detected by 45 weeks, although adrenals lacking histological changes were still found (Figure 8A and Table 6). Macroscopic adenomas were not detected in APC KO mice until after 45 weeks of age, and no carcinomas were detected. Using the Mantel-Haenszel χ2 test of linear association, we found significantly worse histological findings for APC KO mice, compared with H19ΔDMD mice (P = 0.004, two-sided test). Like the APC KO mice, all APC KO-H19ΔDMD mice displayed adrenocortical hyperplasia by 15 weeks of age. In contrast, at 45 weeks of age, microscopic and/or macroscopic adenomas were present in adrenal glands from 33.3% (4 of 12) of APC KO-H19ΔDMD mice, with a single carcinoma evident after 45 weeks (Figure 8B and Table 6). APC KO-H19ΔDMD mice also fared worse, compared with H19ΔDMD mice (P = 0.0005, χ2 test of linear association); however, statistical significance between tumor formation in APC KO and APC KO-H19ΔDMD mice reached only a value of P = 0.047, which may reflect too small a sample.
Figure 7.
Combined loss of Apc and loss of imprinting at the DMD of the Igf2/H19 region results in tumor formation in mice. Images (H&E) shown are representative of normal adrenal glands from a 45-week-old normal mouse (A) and a 60-week-old APC KO mouse demonstrating hyperplasia to adenoma formation (B). C: Macroscopic adenomas were also found in APC KO and APC KO-H19ΔDMD mice. Gross anatomy of adrenal glands from 45-week-old WT (Cre−) and APC KO-H19ΔDMD (Cre+) mice are shown. For scale (C), black lines are spaced 1 mm apart. Original magnification: ×40 (A, upper row); ×100 (A, lower row).
Table 6.
Prevalence of Hyperplasia and Tumors in Mouse Models
| Age, sample size, and findings | H19ΔDMD [no. (%)] | APC KO [no. (%)] | APC KO-H19ΔDMD [no. (%)] |
|---|---|---|---|
| 15 weeks | |||
| Total examined | 3 | 10 | 7 |
| Normal | 3 (100) | 0 | 0 |
| Hyperplasia | 0 | 10 (100) | 7 (100) |
| Microscopic adenoma | 0 | 0 | 0 |
| Macroscopic adenoma | 0 | 0 | 0 |
| Carcinoma | 0 | 0 | 0 |
| 30 weeks | |||
| Total examined | 3 | 8 | 9 |
| Normal | 3 (100) | 0 | 0 |
| Hyperplasia | 0 | 8 (100) | 9 (100) |
| Microscopic adenoma | 0 | 0 | 0 |
| Macroscopic adenoma | 0 | 0 | 0 |
| Carcinoma | 0 | 0 | 0 |
| 45 weeks | |||
| Total examined | 6 | 6 | 12 |
| Normal | 6 (100) | 0 | 1 (8.3) |
| Hyperplasia | 0 | 5 (83.3) | 7 (58.3) |
| Microscopic adenoma | 0 | 1 (16.7) | 1 (8.3) |
| Macroscopic adenoma | 0 | 0 | 3 (25) |
| Carcinoma | 0 | 0 | 0 |
| >45 Weeks | |||
| Total examined | 8 | 15 | 14 |
| Normal | 8 (100) | 3 (20) | 0 |
| Hyperplasia | 0 | 8 (53.3) | 8 (57.1) |
| Microscopic adenoma | 0 | 2 (13.3) | 1 (7.1) |
| Macroscopic adenoma | 0 | 2 (13.3) | 4 (28.6) |
| Carcinoma | 0 | 0 | 1 (7.1) |
Figure 8.
APC KO and APC KO-H19ΔDMD mice develop tumors of increased severity. A and B: Tumor types present in APC KO mice and APC KO-H19ΔDMD mice at 45 and >45 weeks of age. C: Adrenal (H&E) of a 48-week-old APC KO mouse. The APC KO mice exhibit some microscopic and macroscopic adenomas but no tumors with a Weiss score of >2. D: Adrenal (H&E) of a 55-week-old APC KO-H19ΔDMD mouse with adrenocortical carcinoma. H&E staining was performed on adrenal glands harvested from mice at 15, 30, 45, and >45 weeks of age. H&E-stained sections of all available adrenal glands from WT (Cre− controls), H19ΔDMD, APC KO, and APC KO-H19ΔDMD mice were evaluated by a single pathologist (T.J.G.) and categorized as normal or as having hyperplasia, microscopic adenoma, macroscopic adenoma, or carcinoma. Representative images are shown in Figure 7, and the data are summarized in Table 6. Original magnification, ×200. NTF, no tumors found.
Under Weiss parameters, a single macroscopic adenoma from an APC KO mouse was scored as high as 2, consistent with a benign lesion, whereas a tumor from one APC KO-H19ΔDMD mouse received a score of 3, indicative of carcinoma (Figure 8, C and D). Given our human data and the qPCR data from APC KO mice, we measured the β-catenin activity and Igf2 mRNA expression levels in these two tumors. Both tumors displayed elevated β-catenin signaling, including up-regulation of the downstream target gene Axin2 (Figure 3B). Compared with adrenals from control (Cre−) mice, the Weiss 2 tumor from an APC KO mouse showed dramatically increased Igf2 mRNA levels (Figure 5C). Surprisingly, Igf2 mRNA expression in the Weiss 3 tumor harvested from an APC KO-H19ΔDMD was actually decreased, compared with Cre− controls (data not shown). This unexplained result may indicate that as yet unidentified additional mutations or alterations in gene regulation are involved in this unique tumor. Nevertheless, in total, our data indicate that the combination of activated β-catenin and loss of imprinting of the Igf2/H19 locus (loss of Apc and the Igf2/H19 DMD) shifts the tumor spectrum in the adrenal cortex to earlier and more aggressive neoplasia.
Discussion
Adrenocortical carcinoma is an aggressive and often lethal disease, largely because it typically escapes diagnosis until it has already progressed to a late stage and because its inherent chemoresistance renders most cytotoxic therapies ineffective. The relative rarity of ACC hinders efforts to understand its etiology; few clinical samples are available for laboratory studies, and ACC cell lines are notoriously difficult to establish. These facts provide impetus for the generation of better mouse models of ACC, to further understanding of underlying mechanisms and to develop effective targeted therapies. In the present study, we modeled adrenocortical carcinoma through stabilization of β-catenin (via loss of Apc) and elevation of Igf2 levels (via loss of Igf2/H19 DMD) in mice, providing a tool to study the contribution of multiple oncogenic mutations to ACC development.
Although adrenocortical tumors may manifest in the context of a familial cancer syndrome, such as FAP or BWS, the majority of ACCs arise sporadically.62,63 Approximately 13% of FAP patients, characterized by mutations in APC and elevated Wnt/β-catenin signaling, develop adrenocortical tumors; however, these tumors rarely progress to ACC.64–66 Similarly, BWS patients, with genetic mutations in the 11p15.5 locus and a resultant increase in IGF2 expression, develop adrenocortical hyperplasia but rarely ACC, which accounts for only 7% of the malignancies found in this syndrome.6 In contrast, in sporadic ACC, IGF2 is up-regulated in 80% to 90% of cases but is increased in only approximately 5% of ACAs.19–21,23,47,67 Despite anecdotal clinical reports, it remains unclear how frequently carcinomas of the adrenal cortex arise from pre-existing benign neoplasms.40 Recent molecular and clinical studies, however, increasingly support a multistep model whereby normal adrenal glands become hyperplastic and progress to ACC.68,69 Differences in size between ACA and ACC tumors and the presence of aggressive ACCs embedded within ACA tissue also support a multistep progression,70–72 consistent with our understanding of most carcinoma types.
Previous studies of large human cohorts have revealed that the WNT/β-catenin signaling pathway is up-regulated in many adrenocortical tumors.9,12,38 The combined UMHS and USP data in the present study revealed exon 3 mutations in 21.7% (10 of 46 tested) of ACAs and 22.6% (7 of 31 tested) of ACCs, similar to previously reported frequency of CTNNB1 mutations in ACAs (27%) and ACCs (16% to 31%).9,12 A missense mutation in one of the phosphorylation sites, codon 45 of exon 3, was the most frequent genetic alteration of CTNNB1. CTNNB1 mutations have been correlated with abnormal β-catenin localization by classical IHC, and as many as 39% of ACAs and 77% of ACCs have abnormal cytoplasmic and nuclear accumulation of β-catenin.9–12,37 The present study revealed that abnormal cytoplasmic or nuclear β-catenin staining was observed in 24.1% of the adenomas and 30% of the carcinomas. As expected, most tumors with CTNNB1 mutations also had abnormal β-catenin staining. Interestingly, four ACA samples with mutations did not exhibit obvious abnormal staining, an observation consistent with the array results discussed below. Nuclear staining is not always accompanied by CTNNB1 mutations, indicating that other mutations not examined here may also contribute to tumorigenesis.
Consistent with previous reports, we observed that abnormal or nuclear β-catenin accumulation in ACC is associated with a dismal prognosis.12,37 In the present study, however, when we combined stage and grade with β-catenin status to evaluate prognosis, we found that β-catenin status does not improve predictive power. This is not surprising, given that abnormal β-catenin is prevalent in high-grade ACC. Thus, measuring cell proliferation directly can capture much of the influence of β-catenin on survival, as well as increases in proliferation due to some other mutations, and might therefore be a better predictor of outcome than β-catenin status alone.39 In addition, we attempted to correlate β-catenin status with tumor functionality. Significantly, in the UMHS cohort, ACAs with abnormal β-catenin are less likely to be hormone secreting (37.5%; 3 of 8 tested), whereas in ACC with abnormal β-catenin status are more likely to be hormone secreting (92% or 11 of 12 samples). Similar results were not found for the USP cohort. More studies are required to determine the relationships among β-catenin, functionality, and malignancy.
Using our published array data, we compared gene expression in adrenocortical tumors with membranous β-catenin staining with the gene expression of adrenocortical tumors with cytoplasmic/nuclear β-catenin staining. This approach revealed that the presence of abnormal β-catenin staining in ACC (and not so evident in ACA) corresponded to differential expression of a significant number of genes. ACCs with nuclear or cytoplasmic β-catenin staining had increased transcripts for genes involved in cell division, p53 function, and Wnt signaling, consistent with the finding here and elsewhere12,39 that Bcat+ ACCs have a higher mitotic rate and may confer an advantage for growth in these tumors. The genes that had increased expression in Bcat+ ACC contained an abundance of TCF/LEF transcription factor binding sites in their promoters, suggesting that nuclear β-catenin is stabilized, transcriptionally active, and contributing to tumorigenesis. However, our array data demonstrate that WNT pathway activation has transcriptionally distinct consequences in ACA and ACC, suggesting that WNT activation in benign and malignant settings is not functionally equivalent. It is possible that this reflects differences in their genetic milieu, such as the dramatically up-regulated expression of IGF2 observed in most human ACCs but not ACAs. Alternatively, it is possible that β-catenin mutation in ACA is subclonal, manifesting as nuclear β-catenin in only a small percentage of cells, which is consistent with our observation that nuclear β-catenin immunoreactivity is less intense in these tumors and present in scattered isolated cells (personal observation, T.J.G.).
In a separate study, Berthon et al40 found that constitutively active β-catenin can act as an adrenocortical oncogene to induce adrenal hyperplasia and tumor formation. In their mouse model, exon 3 was mutated in cells in which Ark1b7 is expressed (in the zona fasciculata) but not in cells in which β-catenin is normally expressed (in the subcapsular region, including the adrenocortical stem/progenitor cells and zona glomerulosa), making the physiological interpretation difficult. In the Berthon et al40 study, although samples from mice up to 10 months of age displayed abnormalities in adrenal gland architecture, the adenomas present were graded with a Weiss score of ≤2. However, after 17 months of age (∼68 weeks), adrenal glands from 2 of 17 animals analyzed were thought to have carcinoma-like characteristics.40 In the present study, we have attempted to demonstrate the importance of β-catenin stabilization in adrenal tumorigenesis through the loss of Apc in Sf1-expressing cells, including those cells in which β-catenin is normally expressed. In our model, we detected hyperplasia that progressed to microscopic adenomas and macroscopic adenomas as early as 15 weeks of age. Loss of Apc led to the biochemical activation of β-catenin and the presence of Sf1-positive, β-catenin-positive cells primarily in the inner region of the gland. Because loss of Apc might be predicted to lead to other effects unrelated to β-catenin, we attempted to rescue the APC KO phenotype by deleting Ctnnb1. Loss of both Ctnnb1 and Apc led to formation of adrenal glands that resembled WT glands, confirming that tumor formation was dependent on stabilization of β-catenin.
Having established a functional model of adrenocortical tumorigenesis, we wanted to explore the combinatorial effect of two mutations known to participate in this process. IGF2 expression is dramatically elevated in most human ACCs. We therefore chose to model the combined loss of Apc and up-regulation of Igf2. The H19ΔDMD model led to an approximate twofold increase in Igf2 levels and activation of Igf signaling. This is in accord with expression of Igf2 from both maternal and paternal alleles and the modest two- to threefold increase in most human tumors displaying loss of imprinting, but less than seen in most patients with ACC.73 Consistent with reports from previous models of Igf2 overexpression,60,61,74 we did not observe tumors in aged H19ΔDMD mice.
Our main objective was to determine how this loss of imprinting could cooperate with loss of Apc to contribute to carcinogenesis. Interaction between Apc and Igf2 in tumor formation is supported by mouse modeling studies of intestinal tumorigenesis in which Min mice (harboring a mutation in Apc and a mouse model for FAP) were crossed with mice exhibiting a loss of imprinting at the Igf2/H19 DMD. Compared with Min mice, mice harboring both mutations exhibited more intestinal adenomas (2.2-fold increase in tumor number) of larger size (2.5-fold increase in tumor surface area).75 In our mouse models, combined loss of Apc and loss of imprinting at the Igf2 locus resulted in increased adrenal masses and earlier onset of tumor formation. The explanation for the difference in effects of these mutations on adrenal masses of males versus females is unclear, although sex differences in adrenal development as well as hormonal milieu may contribute. As in APC KO mice, hyperplasia in APC KO-H19ΔDMD mice was detected as early as 15 weeks of age. In contrast to APC KO mice, however, both microscopic and macroscopic adenomas were present in APC KO-H19ΔDMD by 45 weeks, and one carcinoma was found after 45 weeks (at 56 weeks of age).
We were somewhat surprised that the percentage of carcinomas in our model was not higher. We chose the model of sporadic loss of Apc and the Igf2/H19 DMD region in adrenocortical cells, because it is a more accurate representation of tumor progression in humans. Our results could be due in part to the use of the Sf1-Cre stochastic driver, which results in recombination in only a subset of adrenocortical cells and potentially at different times in Sf1-expressing cells. In addition, the low penetrance of ACC in patients with germline mutations in the APC gene in FAP and loss of imprinting in the IGF2 locus in BWS indicates that additional mutations contribute to the ultimate manifestation of cancer.6 Indeed, it has been proposed that human ACCs may harbor as many as 30 mutations or genetic aberrations.6 The largest tumor found in the APC KO mice was graded Weiss 2 and had elevated β-catenin signaling and distinctly elevated Igf2 expression, whereas the unique Weiss 3 tumor from the APC KO-H19ΔDMD mouse had elevated β-catenin and Wnt signaling but decreased Igf2 expression. This could suggest that the enhanced stability of β-catenin (or some other mutated gene product) may preclude expression from the floxed maternal Igf2/H19 gene locus.
Elimination of the DMD region in the presence of stabilized β-catenin may have unintended consequences on the expression of other transcripts in this complex locus, including H19, Cdkn1c, and miR-483. Of these transcripts, miR-483 has been shown to be highly expressed in ACC.76 Indeed, recent studies have shown that β-catenin can transcriptionally activate miR-483 expression separate from Igf277,78. A few human hepatocarcinomas and colorectal cancer samples have displayed elevated miR-483 levels associated with decreased Igf2 expression.77 Nevertheless, it remains clear that mice with stabilized β-catenin and loss of imprinting at the Igf2/H19 locus develop more tumors at an earlier age. Additional studies are required to determine the precise mechanism by which transcripts from the Igf2/H19 locus, the expression of Igf2, and stabilization of β-catenin interact to accelerate tumor formation.
In summary, we have integrated human clinicopathologic and DNA microarray data with mouse models to show that, although abnormal activation of either the WNT or the IGF2 pathway is not sufficient to drive the development of carcinomas, the combined dysregulation of both is a major contributor to ACC. These results provide a model in which dysregulation of one pathway may result in adrenal hyperplasia, but accumulation of a second or multiple alterations is necessary for tumorigenesis. Further studies will be required to uncover the additional genetic and epigenetic events that contribute to this highly aggressive and deadly disease.
Acknowledgments
We thank Drs. Keith Parker for the Sf1-Cre mice, Bart Williams for the Apcfl/fl mice, Marisa Bartolomei for the H19lx/DMDlxDMD mice, Yacob Weinstein for the 20a-Hsd antibody, and Enzo Lalli and Ken Morohashi for the Dax1 antibody.
Footnotes
Supported in part by the University of Michigan's Millie Schembechler Adrenal Cancer Research Fund, NIHDK062027 and CA134606 (G.D.H.), NIHT32 DK07245 (M.A.W.), NIHT32 HD007505 (F.M.B), NIHT32-CA009676 (E.S.), NIHT32-CA009676 (R.K., T.J.G., and D.G.T), the National Council for Scientific and Technological Development of Brazil (CNPq 300209/2008-8 to A.C.L.), and the Coordination for Improvement of Higher Education (CAPES), Brazil (L.O.L).
J.H.H. and M.A.W. contributed equally to this work.
Supplemental material for this article can be found at http://ajp.amjpathol.org or at http://dx.doi.org/10.1016/j.ajpath.2012.05.026.
Supplementary data
Breeding schemes for APC KO, BCAT KO, and APC-BCAT KO mice. All mice were developed using the Sf1-Cre stochastic driver. A: APC KO mice were created by elimination of exon 14, resulting in a frameshift mutation that generates a null product. B: BCAT KO mice were created by elimination of exons 2 through 6, resulting in complete inactivation. C: APC-BCAT KO mice were created by crossing APC KO mice with BCAT KO mice.
Breeding schemes for H19ΔDMD and APC KO-H19ΔDMD mice. All mice were developed using the Sf1-Cre stochastic driver. A:H19ΔDMD mice are a result of loss of the differentially methylated region of the Igf2/H19 cistron, which causes Igf2 expression from both the maternal and paternal alleles. Mice with normal imprinting at this locus express Igf2 only from the paternal allele. B: APC KO-H19ΔDMD mice were bred to generate females with loxP sites surrounding both Apc exon 14 and the Igf2/H19 DMD. Mating with male mice expressing the Cre transgene that are heterozygous for Apc loxP sites results in both Cre− littermates and Cre+ mice with excision of Apc exon 14 and the imprinting control region of the Igf2/H19 region.
Genes up-regulated in ACCs with nuclear β-catenin staining annotated as having either of two DNA motifs for LEF1 binding. The genes selected were those that yielded P < 0.01 and an average expression increase of at least 1.5-fold in comparison of Bcat+ ACCs with Bcat− ACCs. Listed are genes with motifs CTTTGT_V$LEF1_Q2 and CTTTGA_V$LEF1_Q2, as given by version 3 of MSigDB. The MSigDB lists are for the region within 2 kb from the transcription start sites, and demand that the motif be conserved in mouse, rat, and dog. Data are expressed as the ratio to the median of ACCs. Red indicates the highest expression (ratio 4) and green indicates the lowest expression (ratio 1/4). All values are normalized to the median of ACCs.
Knock out of Apc in mice with Sf1-Cre complete driver results in a developmental defect. Embryos (embryonic day E16.5) were harvested and genotyped. Embryos of the WT, APC KO (stochastic driver), and APC-KO (complete driver) genotypes were processed, sectioned, and stained with H&E. Excision of Apc exon 14 with the Sf1-Cre stochastic driver resulted in apparently normal adrenal gland formation, as observed by H&E staining. When Apc was conditionally knocked out with the Sf1-Cre complete driver, a developmental defect was detected. Original magnification, ×100.
Adrenal gland histology of H19ΔDMD mice does not reveal a significantly altered phenotype. H&E analysis of adrenal glands from 15, 30, 45, or >45-week-old H19ΔDMD mice (A) and Cre− control mice (B) revealed no significant changes in morphology of the adrenal gland. Representative images are shown. Original magnification: ×40 (top row); ×100 (bottom row). Scale bars: 2 mm (top row); 50 μm (bottom row).
Adrenal gland mass of individual mice. Adrenal glands were harvested from mice at age 15, 30, 45, or >45 weeks and were weighed. The sum of the two adrenal gland masses for each mouse was measured, and these data were used to generate Figure 6. Here, the total adrenal mass of an individual mouse is plotted separately for female and male samples. Data points represent the total adrenal mass of one animal; horizontal lines represent the mean of each group of animals. Two outlier values (boxed) were eliminated from the analysis presented in Figure 6.
References
- 1.Fassnacht M., Allolio B. Epidemiology of adrenocortical carcinoma. In: Hammer G.D., Else T., editors. Adrenocortical Carcinoma: Basic Science and Clinical Concepts. Springer; New York: 2011. pp. 23–30. [Google Scholar]
- 2.Bilimoria K.Y., Shen W.T., Elaraj D., Bentrem D.J., Winchester D.J., Kebebew E., Sturgeon C. Adrenocortical carcinoma in the United States: treatment utilization and prognostic factors. Cancer. 2008;113:3130–3136. doi: 10.1002/cncr.23886. [DOI] [PubMed] [Google Scholar]
- 3.Kirschner L.S. Emerging treatment strategies for adrenocortical carcinoma: a new hope. J Clin Endocrinol Metab. 2006;91:14–21. doi: 10.1210/jc.2005-1739. [DOI] [PubMed] [Google Scholar]
- 4.Libé R., Fratticci A., Bertherat J. Adrenocortical cancer: pathophysiology and clinical management. Endocr Relat Cancer. 2007;14:13–28. doi: 10.1677/erc.1.01130. [DOI] [PubMed] [Google Scholar]
- 5.Bertherat J., Bertagna X. Pathogenesis of adrenocortical cancer. Best Pract Res Clin Endocrinol Metab. 2009;23:261–271. doi: 10.1016/j.beem.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Else T. Overview of genetic syndromes associated with adrenocortical cancer. In: Hammer G.D., Else T., editors. Adrenocortical Carcinoma: Basic Science and Clinical Concepts. Springer; New York: 2011. pp. 153–172. [Google Scholar]
- 7.Gaujoux S., Tissier F., Bertherat J. WNT/β-catenin signaling in adrenocortical carcinoma. In: Hammer G.D., Else T., editors. Adrenocortical Carcinoma: Basic Science and Clinical Concepts. Springer; New York: 2011. pp. 263–282. [Google Scholar]
- 8.Kim A.C., Reuter A.L., Zubair M., Else T., Serecky K., Bingham N.C., Lavery G.G., Parker K.L., Hammer G.D. Targeted disruption of beta-catenin in Sf1-expressing cells impairs development and maintenance of the adrenal cortex. Development. 2008;135:2593–2602. doi: 10.1242/dev.021493. [DOI] [PubMed] [Google Scholar]
- 9.Tissier F., Cavard C., Groussin L., Perlemoine K., Fumey G., Hagneré A.M., René-Corail F., Jullian E., Gicquel C., Bertagna X., Vacher-Lavenu M.C., Perret C., Bertherat J. Mutations of beta-catenin in adrenocortical tumors: activation of the Wnt signaling pathway is a frequent event in both benign and malignant adrenocortical tumors. Cancer Res. 2005;65:7622–7627. doi: 10.1158/0008-5472.CAN-05-0593. [DOI] [PubMed] [Google Scholar]
- 10.Tadjine M., Lampron A., Ouadi L., Horvath A., Stratakis C.A., Bourdeau I. Detection of somatic beta-catenin mutations in primary pigmented nodular adrenocortical disease (PPNAD) Clin Endocrinol (Oxf) 2008;69:367–373. doi: 10.1111/j.1365-2265.2008.03273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tadjine M., Lampron A., Ouadi L., Bourdeau I. Frequent mutations of beta-catenin gene in sporadic secreting adrenocortical adenomas. Clin Endocrinol (Oxf) 2008;68:264–270. doi: 10.1111/j.1365-2265.2007.03033.x. [DOI] [PubMed] [Google Scholar]
- 12.Gaujoux S., Grabar S., Fassnacht M., Ragazzon B., Launay P., Libé R., Chokri I., Audebourg A., Royer B., Sbiera S., Vacher-Lavenu M.C., Dousset B., Bertagna X., Allolio B., Bertherat J., Tissier F. beta-catenin activation is associated with specific clinical and pathologic characteristics and a poor outcome in adrenocortical carcinoma. Clin Cancer Res. 2011;17:328–336. doi: 10.1158/1078-0432.CCR-10-2006. [DOI] [PubMed] [Google Scholar]
- 13.Yost C., Torres M., Miller J.R., Huang E., Kimelman D., Moon R.T. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996;10:1443–1454. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]
- 14.Behrens J., Lustig B. The Wnt connection to tumorigenesis. Int J Dev Biol. 2004;48:477–487. doi: 10.1387/ijdb.041815jb. [DOI] [PubMed] [Google Scholar]
- 15.Klaus A., Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8:387–398. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- 16.Bartholdi D., Krajewska-Walasek M., Ounap K., Gaspar H., Chrzanowska K.H., Ilyana H., Kayserili H., Lurie I.W., Schinzel A., Baumer A. Epigenetic mutations of the imprinted IGF2-H19 domain in Silver-Russell syndrome (SRS): results from a large cohort of patients with SRS and SRS-like phenotypes. J Med Genet. 2009;46:192–197. doi: 10.1136/jmg.2008.061820. [DOI] [PubMed] [Google Scholar]
- 17.DeBaun M., Horst J. Beckwith-Wiedemann syndrome. In: Hammer G.D., Else T., editors. Adrenocortical Carcinoma: Basic Science and Clinical Concepts. Springer; New York: 2011. pp. 227–234. [Google Scholar]
- 18.Fottner C., Niederle I., Weber M.M. The insulin-like growth factor system in adrenocortical growth control and carcinogenesis. In: Hammer G.D., Else T., editors. Adrenocortical Carcinoma: Basic Science and Clinical Concepts. Springer; New York: 2011. pp. 235–262. [Google Scholar]
- 19.Giordano T.J., Kuick R., Else T., Gauger P.G., Vinco M., Bauersfeld J., Sanders D., Thomas D.G., Doherty G., Hammer G. Molecular classification and prognostication of adrenocortical tumors by transcriptome profiling. Clin Cancer Res. 2009;15:668–676. doi: 10.1158/1078-0432.CCR-08-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Fraipont F., El Atifi M., Cherradi N., Le Moigne G., Defaye G., Houlgatte R., Bertherat J., Bertagna X., Plouin P.F., Baudin E., Berger F., Gicquel C., Chabre O., Feige J.J. Gene expression profiling of human adrenocortical tumors using complementary deoxyribonucleic acid microarrays identifies several candidate genes as markers of malignancy. J Clin Endocrinol Metab. 2005;90:1819–1829. doi: 10.1210/jc.2004-1075. [DOI] [PubMed] [Google Scholar]
- 21.Gicquel C., Bertagna X., Gaston V., Coste J., Louvel A., Baudin E., Bertherat J., Chapuis Y., Duclos J.M., Schlumberger M., Plouin P.F., Luton J.P., Le Bouc Y. Molecular markers and long-term recurrences in a large cohort of patients with sporadic adrenocortical tumors. Cancer Res. 2001;61:6762–6767. [PubMed] [Google Scholar]
- 22.Velázquez-Fernández D., Laurell C., Geli J., Höög A., Odeberg J., Kjellman M., Lundeberg J., Hamberger B., Nilsson P., Bäckdahl M. Expression profiling of adrenocortical neoplasms suggests a molecular signature of malignancy. Surgery. 2005;138:1087–1094. doi: 10.1016/j.surg.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 23.Giordano T.J., Thomas D.G., Kuick R., Lizyness M., Misek D.E., Smith A.L., Sanders D., Aljundi R.T., Gauger P.G., Thompson N.W., Taylor J.M., Hanash S.M. Distinct transcriptional profiles of adrenocortical tumors uncovered by DNA microarray analysis. Am J Pathol. 2003;162:521–531. doi: 10.1016/S0002-9440(10)63846-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiss D.W. Tumor origin, progression, immunogenicity, and immunotherapy. Transplant Proc. 1984;16:528–533. [PubMed] [Google Scholar]
- 25.Weiss D.W. Reflections on tumor origin, immunogenicity, and immunotherapy. Cancer Immunol Immunother. 1984;18:1–4. doi: 10.1007/BF00205391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Almeida M.Q., Fragoso M.C., Lotfi C.F., Santos M.G., Nishi M.Y., Costa M.H., Lerario A.M., Maciel C.C., Mattos G.E., Jorge A.A., Mendonca B.B., Latronico A.C. Expression of insulin-like growth factor-II and its receptor in pediatric and adult adrenocortical tumors. J Clin Endocrinol Metab. 2008;93:3524–3531. doi: 10.1210/jc.2008-0065. [DOI] [PubMed] [Google Scholar]
- 27.Barrett T., Troup D.B., Wilhite S.E., Ledoux P., Rudnev D., Evangelista C., Kim I.F., Soboleva A., Tomashevsky M., Edgar R. NCBI GEO: mining tens of millions of expression profiles–database and tools update. Nucleic Acids Res. 2007;35:D760–D765. doi: 10.1093/nar/gkl887. (Database issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Almeida M.Q., Soares I.C., Ribeiro T.C., Fragoso M.C., Marins L.V., Wakamatsu A., Ressio R.A., Nishi M.Y., Jorge A.A., Lerario A.M., Alves V.A., Mendonca B.B., Latronico A.C. Steroidogenic factor 1 overexpression and gene amplification are more frequent in adrenocortical tumors from children than from adults. J Clin Endocrinol Metab. 2010;95:1458–1462. doi: 10.1210/jc.2009-2040. [DOI] [PubMed] [Google Scholar]
- 29.Hayes M.J., Thomas D., Emmons A., Giordano T.J., Kleer C.G. Genetic changes of Wnt pathway genes are common events in metaplastic carcinomas of the breast. Clin Cancer Res. 2008;14:4038–4044. doi: 10.1158/1078-0432.CCR-07-4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bingham N.C., Verma-Kurvari S., Parada L.F., Parker K.L. Development of a steroidogenic factor 1/Cre transgenic mouse line. Genesis. 2006;44:419–424. doi: 10.1002/dvg.20231. [DOI] [PubMed] [Google Scholar]
- 31.Shibata H., Toyama K., Shioya H., Ito M., Hirota M., Hasegawa S., Matsumoto H., Takano H., Akiyama T., Toyoshima K., Kanamaru R., Kanegae Y., Saito I., Nakamura Y., Shiba K., Noda T. Rapid colorectal adenoma formation initiated by conditional targeting of the Apc gene. Science. 1997;278:120–123. doi: 10.1126/science.278.5335.120. [DOI] [PubMed] [Google Scholar]
- 32.Brault V., Moore R., Kutsch S., Ishibashi M., Rowitch D.H., McMahon A.P., Sommer L., Boussadia O., Kemler R. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- 33.Thorvaldsen J.L., Fedoriw A.M., Nguyen S., Bartolomei M.S. Developmental profile of H19 differentially methylated domain (DMD) deletion alleles reveals multiple roles of the DMD in regulating allelic expression and DNA methylation at the imprinted H19/Igf2 locus. Mol Cell Biol. 2006;26:1245–1258. doi: 10.1128/MCB.26.4.1245-1258.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Looyenga B.D., Hammer G.D. Genetic removal of Smad3 from inhibin-null mice attenuates tumor progression by uncoupling extracellular mitogenic signals from the cell cycle machinery. Mol Endocrinol. 2007;21:2440–2457. doi: 10.1210/me.2006-0402. [DOI] [PubMed] [Google Scholar]
- 35.Kawabe K., Shikayama T., Tsuboi H., Oka S., Oba K., Yanase T., Nawata H., Morohashi K. Dax-1 as one of the target genes of Ad4BP/SF-1. Mol Endocrinol. 1999;13:1267–1284. doi: 10.1210/mend.13.8.0325. [DOI] [PubMed] [Google Scholar]
- 36.Bookout A.L., Cummins C.L., Mangelsdorf D.J., Pesola J.M., Kramer M.F. High-throughput real-time quantitative reverse transcription PCR. Curr Protoc Mol Biol. 2006 doi: 10.1002/0471142727.mb1508s73. Chapter 15:Unit 15.8. [DOI] [PubMed] [Google Scholar]
- 37.Bonnet S., Gaujoux S., Launay P., Baudry C., Chokri I., Ragazzon B., Libé R., René-Corail F., Audebourg A., Vacher-Lavenu M.C., Groussin L., Bertagna X., Dousset B., Bertherat J., Tissier F. Wnt/beta-catenin pathway activation in adrenocortical adenomas is frequently due to somatic CTNNB1-activating mutations, which are associated with larger and nonsecreting tumors: a study in cortisol-secreting and -nonsecreting tumors. J Clin Endocrinol Metab. 2011;96:E419–E426. doi: 10.1210/jc.2010-1885. [DOI] [PubMed] [Google Scholar]
- 38.Masi G., Lavezzo E., Iacobone M., Favia G., Palù G., Barzon L. Investigation of BRAF and CTNNB1 activating mutations in adrenocortical tumors. J Endocrinol Invest. 2009;32:597–600. doi: 10.1007/BF03346515. [DOI] [PubMed] [Google Scholar]
- 39.Giordano T.J. The argument for mitotic rate-based grading for the prognostication of adrenocortical carcinoma. Am J Surg Pathol. 2011;35:471–473. doi: 10.1097/PAS.0b013e31820bcf21. [DOI] [PubMed] [Google Scholar]
- 40.Berthon A., Sahut-Barnola I., Lambert-Langlais S., de Joussineau C., Damon-Soubeyrand C., Louiset E., Taketo M.M., Tissier F., Bertherat J., Lefrançois-Martinez A.M., Martinez A., Val P. Constitutive beta-catenin activation induces adrenal hyperplasia and promotes adrenal cancer development. Hum Mol Genet. 2010;19:1561–1576. doi: 10.1093/hmg/ddq029. [DOI] [PubMed] [Google Scholar]
- 41.Boulkroun S., Samson-Couterie B., Golib-Dzib J.F., Amar L., Plouin P.F., Sibony M., Lefebvre H., Louiset E., Jeunemaitre X., Meatchi T., Benecke A., Lalli E., Zennaro M.C. Aldosterone-producing adenoma formation in the adrenal cortex involves expression of stem/progenitor cell markers. Endocrinology. 2011;152:4753–4763. doi: 10.1210/en.2011-1205. [DOI] [PubMed] [Google Scholar]
- 42.Allolio B., Fassnacht M. Clinical presentation and initial diagnosis. In: Hammer G.D., Else T., editors. Adrenocortical Carcinoma: Basic Science and Clinical Concepts. Springer; New York: 2011. pp. 31–48. [Google Scholar]
- 43.Purcell R., Childs M., Maibach R., Miles C., Turner C., Zimmermann A., Sullivan M. HGF/c-Met related activation of beta-catenin in hepatoblastoma. J Exp Clin Cancer Res. 2011;30:96. doi: 10.1186/1756-9966-30-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Previdi S., Maroni P., Matteucci E., Broggini M., Bendinelli P., Desiderio M.A. Interaction between human-breast cancer metastasis and bone microenvironment through activated hepatocyte growth factor/Met and beta-catenin/Wnt pathways. Eur J Cancer. 2010;46:1679–1691. doi: 10.1016/j.ejca.2010.02.036. [DOI] [PubMed] [Google Scholar]
- 45.Anderson M.R., Harrison R., Atherfold P.A., Campbell M.J., Darnton S.J., Obszynska J., Jankowski J.A. Met receptor signaling: a key effector in esophageal adenocarcinoma. Clin Cancer Res. 2006;12:5936–5943. doi: 10.1158/1078-0432.CCR-06-1208. [DOI] [PubMed] [Google Scholar]
- 46.Doghman M., Arhatte M., Thibout H., Rodrigues G., De Moura J., Grosso S., West A.N., Laurent M., Mas J.C., Bongain A., Zambetti G.P., Figueiredo B.C., Auberger P., Martinerie C., Lalli E. Nephroblastoma overexpressed/cysteine-rich protein 61/connective tissue growth factor/nephroblastoma overexpressed gene-3 (NOV/CCN3), a selective adrenocortical cell proapoptotic factor, is down-regulated in childhood adrenocortical tumors. J Clin Endocrinol Metab. 2007;92:3253–3260. doi: 10.1210/jc.2007-0342. [DOI] [PubMed] [Google Scholar]
- 47.West A.N., Neale G.A., Pounds S., Figueredo B.C., Rodriguez Galindo C., Pianovski M.A., Oliveira Filho A.G., Malkin D., Lalli E., Ribeiro R., Zambetti G.P. Gene expression profiling of childhood adrenocortical tumors. Cancer Res. 2007;67:600–608. doi: 10.1158/0008-5472.CAN-06-3767. [DOI] [PubMed] [Google Scholar]
- 48.Subramanian A., Tamayo P., Mootha V.K., Mukherjee S., Ebert B.L., Gillette M.A., Paulovich A., Pomeroy S.L., Golub T.R., Lander E.S., Mesirov J.P. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scheys J.O., Heaton J.H., Hammer G.D. Evidence of adrenal failure in aging Dax1-deficient mice. Endocrinology. 2011;152:3430–3439. doi: 10.1210/en.2010-0986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mizusaki H., Kawabe K., Mukai T., Ariyoshi E., Kasahara M., Yoshioka H., Swain A., Morohashi K. Dax-1 (dosage-sensitive sex reversal-adrenal hypoplasia congenita critical region on the X chromosome, gene 1) gene transcription is regulated by wnt4 in the female developing gonad. Mol Endocrinol. 2003;17:507–519. doi: 10.1210/me.2002-0362. [DOI] [PubMed] [Google Scholar]
- 51.Wood M.A., Hammer G.D. Adrenocortical stem and progenitor cells: unifying model of two proposed origins. Mol Cell Endocrinol. 2011;336:206–212. doi: 10.1016/j.mce.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim A.C., Barlaskar F.M., Heaton J.H., Else T., Kelly V.R., Krill K.T., Scheys J.O., Simon D.P., Trovato A., Yang W.H., Hammer G.D. In search of adrenocortical stem and progenitor cells. Endocr Rev. 2009;30:241–263. doi: 10.1210/er.2008-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang C.C., Miyagawa S., Matsumaru D., Parker K.L., Yao H.H. Progenitor cell expansion and organ size of mouse adrenal is regulated by sonic hedgehog. Endocrinology. 2010;151:1119–1128. doi: 10.1210/en.2009-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.King P., Paul A., Laufer E. Shh signaling regulates adrenocortical development and identifies progenitors of steroidogenic lineages. Proc Natl Acad Sci USA. 2009;106:21185–21190. doi: 10.1073/pnas.0909471106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hershkovitz L., Beuschlein F., Klammer S., Krup M., Weinstein Y. Adrenal 20alpha-hydroxysteroid dehydrogenase in the mouse catabolizes progesterone and 11-deoxycorticosterone and is restricted to the X-zone. Endocrinology. 2007;148:976–988. doi: 10.1210/en.2006-1100. [DOI] [PubMed] [Google Scholar]
- 56.Fodde R. The multiple functions of tumour suppressors: it's all in APC. Nat Cell Biol. 2003;5:190–192. doi: 10.1038/ncb0303-190. [DOI] [PubMed] [Google Scholar]
- 57.Libé R., Groussin L., Tissier F., Elie C., René-Corail F., Fratticci A., Jullian E., Beck-Peccoz P., Bertagna X., Gicquel C., Bertherat J. Somatic TP53 mutations are relatively rare among adrenocortical cancers with the frequent 17p13 loss of heterozygosity. Clin Cancer Res. 2007;13:844–850. doi: 10.1158/1078-0432.CCR-06-2085. [DOI] [PubMed] [Google Scholar]
- 58.Bertherat J., Groussin L., Bertagna X. Mechanisms of disease: adrenocortical tumors–molecular advances and clinical perspectives. Nat Clin Pract Endocrinol Metab. 2006;2:632–641. doi: 10.1038/ncpendmet0321. [DOI] [PubMed] [Google Scholar]
- 59.Sun F.L., Dean W.L., Kelsey G., Allen N.D., Reik W. Transactivation of Igf2 in a mouse model of Beckwith-Wiedemann syndrome. Nature. 1997;389:809–815. doi: 10.1038/39797. [DOI] [PubMed] [Google Scholar]
- 60.Weber M.M., Fottner C., Schmidt P., Brodowski K.M., Gittner K., Lahm H., Engelhardt D., Wolf E. Postnatal overexpression of insulin-like growth factor II in transgenic mice is associated with adrenocortical hyperplasia and enhanced steroidogenesis. Endocrinology. 1999;140:1537–1543. doi: 10.1210/endo.140.4.6660. [DOI] [PubMed] [Google Scholar]
- 61.Caspary T., Cleary M.A., Perlman E.J., Zhang P., Elledge S.J., Tilghman S.M. Oppositely imprinted genes p57(Kip2) and Igf2 interact in a mouse model for Beckwith-Wiedemann syndrome. Genes Dev. 1999;13:3115–3124. doi: 10.1101/gad.13.23.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kirschner L.S. Signaling pathways in adrenocortical cancer. Ann N Y Acad Sci. 2002;968:222–239. doi: 10.1111/j.1749-6632.2002.tb04338.x. [DOI] [PubMed] [Google Scholar]
- 63.Soon P.S., McDonald K.L., Robinson B.G., Sidhu S.B. Molecular markers and the pathogenesis of adrenocortical cancer. Oncologist. 2008;13:548–561. doi: 10.1634/theoncologist.2007-0243. [DOI] [PubMed] [Google Scholar]
- 64.Smith T.G., Clark S.K., Katz D.E., Reznek R.H., Phillips R.K. Adrenal masses are associated with familial adenomatous polyposis. Dis Colon Rectum. 2000;43:1739–1742. doi: 10.1007/BF02236860. [DOI] [PubMed] [Google Scholar]
- 65.Marchesa P., Fazio V.W., Church J.M., McGannon E. Adrenal masses in patients with familial adenomatous polyposis. Dis Colon Rectum. 1997;40:1023–1028. doi: 10.1007/BF02050923. [DOI] [PubMed] [Google Scholar]
- 66.Ono C., Iwama T., Mishima Y. A case of familial adenomatous polyposis complicated by thyroid carcinoma, carcinoma of the ampulla of vater and adrenocortical adenoma. Jpn J Surg. 1991;21:234–240. doi: 10.1007/BF02470915. [DOI] [PubMed] [Google Scholar]
- 67.Gicquel C., Raffin-Sanson M.L., Gaston V., Bertagna X., Plouin P.F., Schlumberger M., Louvel A., Luton J.P., Le Bouc Y. Structural and functional abnormalities at 11p15 are associated with the malignant phenotype in sporadic adrenocortical tumors: study on a series of 82 tumors. J Clin Endocrinol Metab. 1997;82:2559–2565. doi: 10.1210/jcem.82.8.4170. [DOI] [PubMed] [Google Scholar]
- 68.Libé R., Tissier F., Bienvenu M., Groussin L., Joannidis S., Hignette C., Dousset B., Legmann P., Faraggi M., Richard B., Bertagna X., Tenenbaum F. Adrenocortical tumor with two distinct elements revealed by combined (18)F-fluorodeoxyglucose positron emission tomography and (131)I nor-cholesterol scintigraphy. J Clin Endocrinol Metab. 2009;94:3631–3632. doi: 10.1210/jc.2009-1025. [DOI] [PubMed] [Google Scholar]
- 69.Heaton J.H., Hammer G.D. Adrenocortical stem and progenitor cells: implications for cancer. In: Hammer G.D., Else T., editors. Adrenocortical Carcinoma: Basic Science and Clinical Concepts. Springer; New York: 2011. pp. 285–304. [Google Scholar]
- 70.Nieman L.K. Approach to the patient with an adrenal incidentaloma. J Clin Endocrinol Metab. 2010;95:4106–4113. doi: 10.1210/jc.2010-0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Trezzi R., Poli F., Fellegara G. “Dedifferentiated” adrenal cortical neoplasm. Int J Surg Pathol. 2009;17:343–344. doi: 10.1177/1066896909335155. [DOI] [PubMed] [Google Scholar]
- 72.Bernard M.H., Sidhu S., Berger N., Peix J.L., Marsh D.J., Robinson B.G., Gaston V., Le Bouc Y., Gicquel C. A case report in favor of a multistep adrenocortical tumorigenesis. J Clin Endocrinol Metab. 2003;88:998–1001. doi: 10.1210/jc.2002-021117. [DOI] [PubMed] [Google Scholar]
- 73.Woodson K., Flood A., Green L., Tangrea J.A., Hanson J., Cash B., Schatzkin A., Schoenfeld P. Loss of insulin-like growth factor-II imprinting and the presence of screen-detected colorectal adenomas in women. J Natl Cancer Inst. 2004;96:407–410. doi: 10.1093/jnci/djh042. [DOI] [PubMed] [Google Scholar]
- 74.Wolf E., Kramer R., Blum W.F., Föll J., Brem G. Consequences of postnatally elevated insulin-like growth factor-II in transgenic mice: endocrine changes and effects on body and organ growth. Endocrinology. 1994;135:1877–1886. doi: 10.1210/endo.135.5.7525257. [DOI] [PubMed] [Google Scholar]
- 75.Sakatani T., Kaneda A., Iacobuzio-Donahue C.A., Carter M.G., de Boom Witzel S., Okano H., Ko M.S., Ohlsson R., Longo D.L., Feinberg A.P. Loss of imprinting of Igf2 alters intestinal maturation and tumorigenesis in mice. Science. 2005;307:1976–1978. doi: 10.1126/science.1108080. [DOI] [PubMed] [Google Scholar]
- 76.Patterson E.E., Holloway A.K., Weng J., Fojo T., Kebebew E. MicroRNA profiling of adrenocortical tumors reveals miR-483 as a marker of malignancy. Cancer. 2011;117:1630–1639. doi: 10.1002/cncr.25724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Veronese A., Lupini L., Consiglio J., Visone R., Ferracin M., Fornari F., Zanesi N., Alder H., D'Elia G., Gramantieri L., Bolondi L., Lanza G., Querzoli P., Angioni A., Croce C.M., Negrini M. Oncogenic role of miR-483-3p at the IGF2/483 locus. Cancer Res. 2010;70:3140–3149. doi: 10.1158/0008-5472.CAN-09-4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Veronese A., Visone R., Consiglio J., Acunzo M., Lupini L., Kim T., Ferracin M., Lovat F., Miotto E., Balatti V., D'Abundo L., Gramantieri L., Bolondi L., Pekarsky Y., Perrotti D., Negrini M., Croce C.M. Mutated beta-catenin evades a microRNA-dependent regulatory loop. Proc Natl Acad Sci USA. 2011;108:4840–4845. doi: 10.1073/pnas.1101734108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Breeding schemes for APC KO, BCAT KO, and APC-BCAT KO mice. All mice were developed using the Sf1-Cre stochastic driver. A: APC KO mice were created by elimination of exon 14, resulting in a frameshift mutation that generates a null product. B: BCAT KO mice were created by elimination of exons 2 through 6, resulting in complete inactivation. C: APC-BCAT KO mice were created by crossing APC KO mice with BCAT KO mice.
Breeding schemes for H19ΔDMD and APC KO-H19ΔDMD mice. All mice were developed using the Sf1-Cre stochastic driver. A:H19ΔDMD mice are a result of loss of the differentially methylated region of the Igf2/H19 cistron, which causes Igf2 expression from both the maternal and paternal alleles. Mice with normal imprinting at this locus express Igf2 only from the paternal allele. B: APC KO-H19ΔDMD mice were bred to generate females with loxP sites surrounding both Apc exon 14 and the Igf2/H19 DMD. Mating with male mice expressing the Cre transgene that are heterozygous for Apc loxP sites results in both Cre− littermates and Cre+ mice with excision of Apc exon 14 and the imprinting control region of the Igf2/H19 region.
Genes up-regulated in ACCs with nuclear β-catenin staining annotated as having either of two DNA motifs for LEF1 binding. The genes selected were those that yielded P < 0.01 and an average expression increase of at least 1.5-fold in comparison of Bcat+ ACCs with Bcat− ACCs. Listed are genes with motifs CTTTGT_V$LEF1_Q2 and CTTTGA_V$LEF1_Q2, as given by version 3 of MSigDB. The MSigDB lists are for the region within 2 kb from the transcription start sites, and demand that the motif be conserved in mouse, rat, and dog. Data are expressed as the ratio to the median of ACCs. Red indicates the highest expression (ratio 4) and green indicates the lowest expression (ratio 1/4). All values are normalized to the median of ACCs.
Knock out of Apc in mice with Sf1-Cre complete driver results in a developmental defect. Embryos (embryonic day E16.5) were harvested and genotyped. Embryos of the WT, APC KO (stochastic driver), and APC-KO (complete driver) genotypes were processed, sectioned, and stained with H&E. Excision of Apc exon 14 with the Sf1-Cre stochastic driver resulted in apparently normal adrenal gland formation, as observed by H&E staining. When Apc was conditionally knocked out with the Sf1-Cre complete driver, a developmental defect was detected. Original magnification, ×100.
Adrenal gland histology of H19ΔDMD mice does not reveal a significantly altered phenotype. H&E analysis of adrenal glands from 15, 30, 45, or >45-week-old H19ΔDMD mice (A) and Cre− control mice (B) revealed no significant changes in morphology of the adrenal gland. Representative images are shown. Original magnification: ×40 (top row); ×100 (bottom row). Scale bars: 2 mm (top row); 50 μm (bottom row).
Adrenal gland mass of individual mice. Adrenal glands were harvested from mice at age 15, 30, 45, or >45 weeks and were weighed. The sum of the two adrenal gland masses for each mouse was measured, and these data were used to generate Figure 6. Here, the total adrenal mass of an individual mouse is plotted separately for female and male samples. Data points represent the total adrenal mass of one animal; horizontal lines represent the mean of each group of animals. Two outlier values (boxed) were eliminated from the analysis presented in Figure 6.