Summary
In Drosophila, the body axes are specified during oogenesis through interactions between the germline and the overlying somatic follicle cells [1–5]. A Gurken/TGF-alpha signal from the oocyte to the adjacent follicle cells assigns them a posterior identity [6, 7]. These posterior cells then signal back to the oocyte, thereby inducing the repolarization of the microtubule cytoskeleton, the migration of the oocyte nucleus, and the localization of the axis specifying mRNAs [8–10]. However, little is known about the signaling pathways within or from the follicle cells responsible for these patterning events. We show that the Salvador Warts Hippo (SWH) tumor-suppressor pathway is required in the follicle cells in order to induce their Gurken-and Notch-dependent differentiation and to limit their proliferation. The SWH pathway is also required in the follicle cells to induce axis specification in the oocyte, by inducing the migration of the oocyte nucleus, the reorganization of the cytoskeleton, and the localization of the mRNAs that specify the anterior-posterior and dorsal-ventral axes of the embryo. This work highlights a novel connection between cell proliferation, cell growth, and axis specification in egg chambers.
Results and Discussion
Multicellular organisms develop through an orchestrated temporal and spatial pattern of cell behavior, which is controlled by cell-to-cell signaling. In Drosophila melanogaster, the establishment of the embryonic axes occurs in the oocyte and depends on a sequence of signals between the germline and the somatic cells. First, Gurken (Grk) signals from the oocyte to the adjacent follicle cells (FCs), in which Torpedo (Top, EGFR) is activated, and this signal instructs them to adopt a posterior identity [6, 7]. The posterior FCs (PFCs) then send an unidentified signal back to the oocyte, leading to the movement of the nucleus from the posterior to the dorsoanterior (DA) corner and the repolarization of the microtubule (MT) cytoskeleton, with the minus ends at the anterior and lateral cortex and the plus ends at the posterior [8–10]. This repolarization results in the localization of the mRNAs that encode key patterning factors. grk mRNA is next to the nucleus at the DA corner of the oocyte (Figure 1A). At this corner, Grk instructs the overlying FCs to adopt dorsal fates. In contrast, oskar (osk) and bicoid (bcd) mRNAs are localized at the posterior and anterior pole, respectively (Figures 1B and 1C), thus defining the anterior posterior (AP) embryonic axis and the germ cells. Although several genes are required in the FCs to control these events, little is known about the signaling pathways within and from the FCs.
Figure 1.
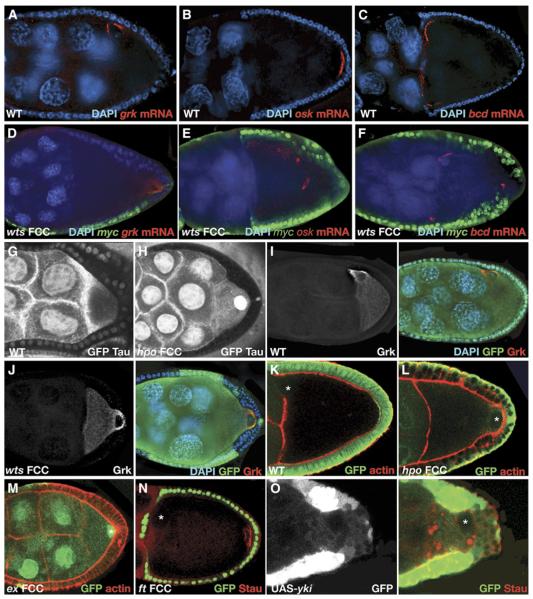
The SWH Pathway, except Ft, Is Required for Germline Axis Specification
(A–F) warts (wts) is required in the follicle cells (FCs) to localize gurken (grk), oskar (osk), and bicoid (bcd) mRNAs in the oocyte. (A)–(C) show that in wild-type stage 9–10 oocytes, grk mRNA is localized to the dorso-anterior corner (A), whereas osk (B) and bcd (C) mRNAs localize to the posterior and anterior pole, respectively. (D)–(F) show that in egg chambers with wts mutant FC clones, the mRNAs are mislocalized: grk mRNA is found at the posterior (D); osk mRNA is found at the center (E), and bcd mRNA is found at the anterior and posterior poles (F). Mutant clones are scored by the lack of Myc staining (green), mRNAs are shown in red, and nuclei are stained with DAPI in blue. In (A)–(M), mutant clones are indicated by the absence of GFP (green), unless stated otherwise.
(G and H) hippo (hpo) is required in the FCs to organize the microtubule cytoskeleton in the oocyte. (G) shows that in wild-type oocytes, the microtubules (MTs) are organized in an anterior-to-posterior gradient, as can been seen by TauGFP (white). (H) shows that in egg chambers with hpo mutant FCs, this gradient is lost and the MTs are distributed diffusely all over the oocyte cytoplasm. Mutant cells are marked by the absence of GFP (white).
(I and J) wts is not required to translate grk mRNA. (I) shows that in wild-type stage 9 oocytes, grk mRNA is localized to and translated at the dorsoanterior corner of the oocyte. (J) shows that in egg chambers with wts mutant FC clones, grk mRNA is mislocalized to the posterior pole (as shown in [D]), but the RNA is translated, and Grk protein is detected at that pole (J). Grk protein is labeled in white (left panel) and red (right panel), and nuclei are stained with DAPI in blue.
(K–N) hippo and expanded (ex), but not fat (ft), are required in the follicle cells to induce polarization of the oocyte. (K) shows that in wild-type stage 9 oocytes, the oocyte nucleus is positioned at the dorsoanterior corner. (L) and (M) show that in egg chambers with hpo (L) and ex (M) mutant FC clones, the oocyte nucleus does not migrate to the dorsoanterior corner and is found instead at the posterior pole. (N) shows that in egg chambers with ft FC clones, both the oocyte nucleus and Staufen (red) are properly localized at the dorsoanterior and posterior pole, respectively. ft mutant cells are marked by the presence of GFP. As shown in (K)–(M), actin is stained with phalloidin (Red). White asterisks mark the oocyte nucleus.
(O) Overexpression of yki in the posterior FCs causes the mislocalization of Staufen (red) and the nucleus in the oocyte. UASyki overexpressing cells are marked by the presence of GFP. The image is overexposed so that all the cells expressing GFP (white and green in left and right panels, respectively) could be visualized. The white asterisk marks the oocyte nucleus.
The SWH Pathway Is Required in the Posterior Follicle Cells for Oocyte Polarity
One of the genes required for axis formation during oogenesis is the tumor suppressor merlin (mer) [10]. However, it is not known whether Mer influences axis specification directly or what signaling pathways lie downstream of Mer. In other tissues, Mer is known to activate the Salvador Warts Hippo (SWH) pathway, which is a tumor-suppressor pathway [11]. Inhibition of the SWH pathway leads to a characteristic overgrowth phenotype in adult organs because of an overproliferation of cells, increased cell growth, and defects in apoptosis [12–14]. To test whether the SWH pathway is required in the function of Mer in axis formation, we examined the localization of grk, bcd, and osk mRNA in egg chambers with warts (wts) and hippo (hpo) mutant FCs, two serine/threonine kinases that are core components of this pathway (Figure 1 and data not shown). In both cases, grk mRNA is mislocalized at the posterior (Figure 1D), osk mRNA is mislocalized at the center (Figure 1E), and bcd mRNA is mislocalized at the posterior and anterior poles (Figure 1F). The mislocalization of these mRNAs could be due to failure of the MTs to repolarize, as has been previously shown in grk/EGFR and mer mutants [6, 7, 10]. In wild-type oocytes, the MTs are organized in an AP gradient (Figure 1G). In contrast, in egg chambers with hpo mutant FCs, the MTs are distributed diffusely all over the oocyte cytoplasm (Figure 1H). Considering these results, together with previous characterizations of similar phenotypes, we conclude that the oocyte cytoskeleton in mutant egg chambers for the SWH pathway is disorganized with the MT plus ends at the center and the minus ends at the anterior and posterior poles. These defects resemble those described in oocytes lacking the Grk signal [6, 7]. In wts mutants, however, Grk protein is detected at the posterior pole, where grk mRNA is mislocalized (Figure 1J). This demonstrates that the axis-specification defects in wts mutant egg chambers are not a consequence of the absence of Grk protein.
It was shown that mer is required in the FCs for the repolarizing signal back to the germline and consequently for the migration of the oocyte nucleus from the posterior to the DA corner [10]. Similarly, when we generated mutant FC clones for wts, hpo, and expanded (ex), an activator of the SWH pathway, the oocyte nucleus fails to migrate to the anterior (Figures 1J, 1L, and 1M and Table 1). Another protein that is upstream of the SWH pathway is the giant atypical cadherin fat (ft) (reviewed in [15]). However, egg chambers with ft mutant FCs show no defects in oocyte polarity, and both the nucleus and Staufen (Stau)—a marker for osk mRNA—are always properly localized (Figure 1N, n = 70). In other epithelia, hpo and wts are required to repress the activity of Yorkie (Yki) and overexpression of yki phenocopies loss-of-function mutations of hpo and wts. Similarly, we found that overexpression of yki in the FCs also causes the mislocalization of Stau and the oocyte nucleus (Figure 1O). These results indicate that the SWH pathway, with the exception of Ft, might be required for the repolarizing signal back from the FCs to the oocyte.
Table 1.
The SWH Pathway Is Required in the Posterior Follicle Cells for Differentiation and Oocyte Polarity, and in the Anterior and Posterior FCs to Control Proliferation
| Defective in | Mutation | Full A Clone (and/or L) |
A and/or L Clone (P Wild-Type) |
Partial P Clone (and/or A and/or L) |
Full P Clone (and/or A and/or L) |
|---|---|---|---|---|---|
| Oocyte Nucleus Migration | |||||
| hpo | 0% (0/2) | 0% (0/16) | 0% (0/13) | 96.1% (50/52) | |
| wts | ND | 0% (0/11) | 35.4% (17/48) | 94.2% (33/35) | |
| ex | 0% (0/1) | 0% (0/1) | 16.6% (1/6) | 34.7% (8/23) | |
|
| |||||
| Epithelium with Proliferation | |||||
| hpo, 2 layers | 48% (12/25) | 0% (0/31) | 80% (4/5) | 88.9% (24/27) | |
| hpo, >2 layers | 20% (5/25) | 0% (0/31) | 0% (0/5) | 11.1% (3/27) | |
| wts, 2 layers | 75% (15/20) | 0% (0/22) | 64.6% (31/48) | 28.6% (10/35) | |
| wts, >2 layers | 15% (3/20) | 0% (0/22) | 33.3% (16/48) | 71.4% (25/35) | |
| ex, 2 layers | 16.6% (1/6) | 0% (0/2) | 33.3% (1/3) | 34.3% (12/35) | |
| ex, >2 layers | 0% (0/6) | 0% (0/6) | 0% (0/3) | 0% (0/5) | |
|
| |||||
|
grk mRNA | |||||
| wts | 0% (0/1) | 0% (0/6) | 84.2% (16/19) | 100% (11/11) | |
|
| |||||
| Grk | |||||
| wts | 0% (0/5) | 0% (0/12) | 56.2% (9/16) | 100% (12/12) | |
|
| |||||
| PH3 | |||||
| hpo | 33.3% (3/9) | 0% (0/6) | 84.2% (16/19) | 100% (11/11) | |
|
| |||||
| FasIII | |||||
| hpo | 0% (0/1) | 0% (0/3) | 0% (0/4) | 68.7% (11/16) | |
|
| |||||
| Hnt | |||||
| hpo | 0% (0/10) | 0% (0/9) | ND | 100% (10/10) | |
|
| |||||
| DG | |||||
| hpo | 0% (0/9) | 0% (0/7) | 0% (0/9) | 0% (0/10) | |
|
| |||||
| Pnt LacZ 998/12 | |||||
| hpo | 0% (0/1) | 0% (0/3) | 60% (3/5) | 100% (8/8) | |
Because this signal is sent by the PFCs, we analyzed whether the SWH pathway is only required in these cells. In egg chambers with wild-type PFCs within an otherwise hpo or wts mutant epithelium (Figure 2A and Table 1), as well as in hpo, wts, and ex germline clones (Figure 2B and data not shown), the oocyte polarity is unaffected. However, in egg chambers with hpo mutant PFCs in an otherwise wild-type epithelium, the oocyte nucleus is mislocalized (Figure 2C and Table 1). We also observed that when only a few cells at the posterior are mutant, Stau localizes in the region of the oocyte that faces the posterior wild-type cells (Figure 2D). The SWH pathway is not required in the polar cells for axis determination because egg chambers with hpo or wts mutant PFCs and wild-type polar cells show oocyte polarity defects (Figure 2C and data not shown). We conclude that the SWH pathway is required only in the PFCs to induce axis specification in the oocyte.
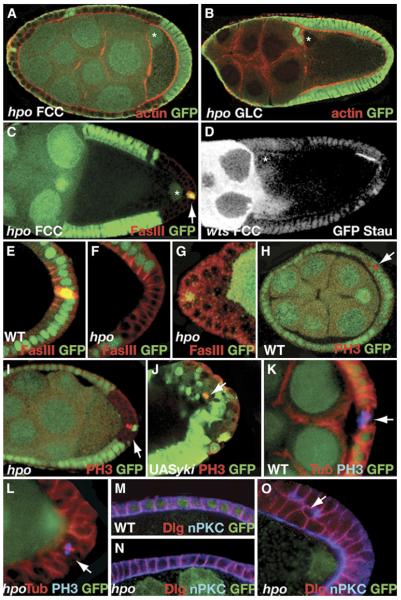
Figure 2.
The SWH Pathway Is Required in the Posterior FCs to Induce Oocyte Polarity and in the Anterior and Posterior FCs to Control Proliferation
(A–D) The SWH pathway is required in the posterior FCs to induce oocyte polarity. As shown in (A), wild-type posterior FCs in an otherwise hpo mutant epithelium induce the migration of the oocyte nucleus to the dorsoanterior (DA) corner. In (B), hippo (hpo) germline clones show no defects in the localization of the oocyte nucleus, which is found at the DA corner of the oocyte. In (C), a hpo mutant clone at the posterior in an otherwise wild-type epithelium shows defects in the positioning of the oocyte nucleus. The polar cells (stained by Fasciclin III in red, arrow) are wild-type. (D) shows the localization of the oocyte nucleus and Staufen (GFPStau, white) in an egg chamber with a partial warts (wts) posterior FC clone. In this case, Stau is localized in the oocyte region that faces the wild-type FCs. Clones are visualized by the absence of GFP (white). The oocyte nuclei are marked with an asterisk, and actin is stained by phalloidin (red) in (A), (B), and (D). In (A)–(O), clones are marked by the absence of GFP (green), unless stated otherwise.
(E–J) The SWH pathway is required in the anterior and posterior FCs to control proliferation. As shown in (E)–(G), wild-type FCs form a cuboidal monolayered epithelium (E), whereas hpo mutant anterior and posterior FCs form an epithelium with two or more layers (F and G). Cells are stained with FasIII (red), which accumulates at the apical lateral side of the membranes in wild-type (E) but not in hpo FCs (F). (H) and (I) show that in wild-type egg chambers, mitotic cells (labeled by phosphohistone 3 [PH3] in red) are detected at early stages ([H], arrow), but never at stage 9, whereas hpo FC clones show cells in mitosis at the posterior of stage 9 egg chambers ([I], arrow). As shown in (J), mitotic cells are also detected in FC clones overexpressing yki (PH3 in red). Clones are marked by the presence of GFP. The image is overexposed so that all the cells expressing GFP (green) could be visualized.
(K–L) hpo controls mitotic-spindle orientation in the follicle cells. (K) shows that in wild-type FCs, the mitotic spindle orientates parallel to the surface of the cells. However, in hpo mutant FCs the mitotic spindle is orientated perpendicular to the surface of the cells, as shown in (L). Microtubules are in red, and phosphohistone 3, PH3, are in blue.
(M–O) Localization of apical and basolateral markers in hippo and warts mutant cells. (M) shows wild-type localization of nPKC at the apical side (blue) and Dlg at the lateral side (red) of the follicle cells. (N) shows that in hpo FC clones that form a monolayer, nPKC and Dlg are properly localized. However, nPKC and Dlg are often mislocalized in mutant cells that form extra layers, as shown in (O). In these cases, nPKC is always apical in the cells that are in contact with the oocyte but never in the cells that do not contact the germline or the basement membrane ([O], arrow).
The SWH Pathway Is Required in the Follicle Cells to Control Proliferation
In contrast to the monolayered wild-type epithelium (Figure 2E), anterior and posterior, but not lateral, hpo and wts mutant cells form a bilayered, and occasionally a multilayered, epithelium (Figures 2F and 2G and Table 1). Given that the SWH pathway is required to control proliferation in epithelia of imaginal discs, we analyzed whether the bilayered epithelium is a result of overproliferation [16–18]. At stage 6 of oogenesis, wild-type FCs undergo a Notch-dependent switch from a mitotic cell cycle to an endocycle. For this reason, phosphohistone 3 (PH3), a marker for mitotic cells, is only detected until that stage and never later (Figure 2H, Table 1, and Figure S1 in the Supplemental Data available online) [19, 20]. In contrast, hpo mutant anterior and posterior FCs are often positive for PH3 at stage 7–10B, indicating that these cells are still dividing (Figure 2I, Table 1, and Figure S1). Similar results are obtained in yki overexpressing FCs (Figure 1O and Figure 2J). Taken together, our findings show that the SWH pathway is required for the control of proliferation at the anterior and posterior FCs.
We also observed the formation of a multilayered epithelium in stage 3–5 mutant FCs (data not shown), although the number of dividing cells is similar to that of the wild-type (Figure S1). It has been recently shown that the aberrant orientation of the mitotic spindle in the FCs results in the formation of a multilayered epithelium [21]. We therefore analyzed the orientation of the mitotic spindle in wild-type and hpo mutant cells. We observed that, contrary to wild-type cells (Figure 2K), the mitotic spindle in mutant FCs is often at an angle or perpendicular to the membrane (Figure 2L). This aberrant orientation disrupts the remaining daughter cells within the same plane, thereby resulting in a bilayered epithelium.
Often, tumor suppressors are important for the polarity of the epithelia. To determine whether this is the case for the SWH pathway, we examined the atypical (novel) Protein Kinase C (nPKC), an apical marker, and Disc large (Dlg), a lateral marker, in the FCs. In wild-type cells (Figure 2M), as well as in hpo mutant FCs that maintain a monolayer epithelium (Figure 2N), nPKC and Dlg localize at the apical and lateral membrane, respectively. However, when the mutant epithelium forms several layers of cells, nPKC and Dlg are often mislocalized, with a reduction of the nPKC staining and an expansion of the Dlg-positive membrane (Figure 2O). Nevertheless, a certain degree of the polarity in these cells is maintained because nPKC is always apical in the cells that are in contact with the oocyte (Figure 2O).
The SWH Pathway Is Essential for Maturation of the Follicle Cells
Because SWH pathway mutant cells do not exit mitosis and keep dividing, it is possible that their differentiation is impaired. To address this question, we analyzed the expression of Fasciclin III (FasIII) and eyes absent (eya) in wild-type and wts and hpo mutant FCs. FasIII and Eya are downregulated in a Notch-dependent manner in the main-body FCs after stage 6 of oogenesis (Figures 3A and 3C) [22–24]. However, the levels of FasIII in hpo mutant PFCs and Eya in wts mutant PFCs remain high after stage 6 (Figures 3B and 3D and Table 1). To further assess the effect of the SWH pathway on the Notch-dependent maturation of the FCs, we examined the expression of Hindsight (Hnt), a transcription factor that is upregulated by Notch signaling in all FCs (Figure 3E) [25]. In hpo posterior FC clones, this Hnt upregulation is blocked (Figure 3F). Contrary to notch clones, however, hpo lateral and anterior clones do not show defects in FasIII, Eya, or Hnt expression (Figures 3B and 3F, Table 1, and data not shown). Furthermore, border, centripetal, and stretched cells that are mutant for hpo migrate normally (Figures S2A–S2D). Considering all these results together, we conclude that the SWH pathway is essential for the PFCs to fully differentiate.
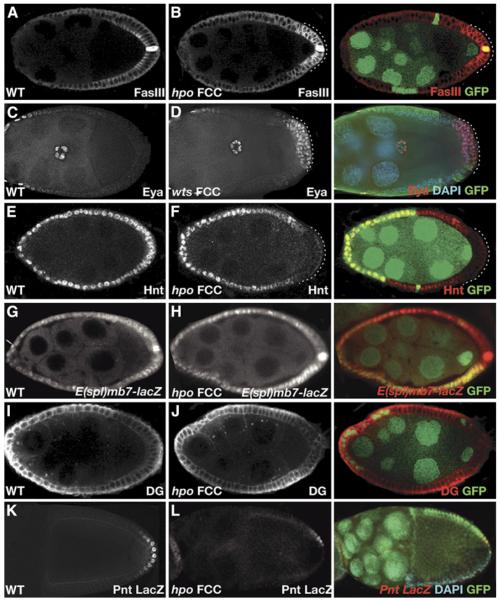
Figure 3.
hippo and warts Regulate Posterior Follicle Cell Differentiation
(A and B) hippo (hpo) is required for the downregulation of Fasciclin III (FasIII). (A) shows that in stage 9 wild-type egg chambers, FasIII is strongly expressed in the polar cells and weakly localizes to the apical lateral membrane in the posterior FCs. In (B), hpo FC clones show both overexpression of FasIII at the posterior follicle cells and redistribution of the protein to the basolateral membrane. In (A)–(F), cells with aberrant expression of FasIII, Eya, and Hnt are marked with a dotted line. In all panels, mutant clones are marked by the absence of GFP (green); expression of FasIII, Eya, Hnt, E(spl)mb7-lacZ, DG and pntLacZ are shown in white or red, and nuclei are stained with DAPI in blue.
(C and D) warts (wts) is required for the down-regulation of Eyes absent (Eya). Staining of Eya in stage 9 wild-type (C) and wts (D) egg chambers is shown. wts FC clones show overexpression of Eya in the posterior follicle cells.
(E and F) hpo is required for the Notch-dependent expression of Hindsight (Hnt) in the posterior FCs. (E) shows that in wild-type egg chambers, Hnt is expressed in all FCs upon activation of Notch at stage 6 of oogenesis. However, Hnt expression is not activated in hpo posterior FCs, as shown in (F).
(G and H) hpo is required for the expression of Enhancer of split [E(spl)mb7-lacZ] in the posterior FCs. As shown in (G), wild-type egg chambers express E(spl)mb7-lacZ in all FCs. (H) shows that in hpo FC clones, the expression is weakly reduced. The hpoJM1 allele was used in this experiment.
(I and J) hpo is not required for the Gurken-dependent expression of Dystroglycan (DG). (I) shows that in wild-type egg chambers, DG is expressed in an anterior-to-posterior gradient. (J) shows that this gradient of DG is also present in hpo FC clones.
(K and L) hpo is required for the Gurken-dependent expression of pointed (pnt). (K) shows wild-type expression of pointed-LacZ line pnt99812. (L) shows that in hpo FC clones, the expression of the pointed-LacZ line pnt99812 is affected, and cannot be detected in posterior mutant cells. Nuclei are stained with DAPI in blue.
The findings described above, together with the proliferation defects in hpo and wts mutant cells, suggest that the SWH pathway is required for Notch signaling. To test whether this is the case, we analyzed the expression of universal Notch transcriptional reporters in wild-type and hpo mutant FCs. In wild-type egg chambers, the Notch reporter E(spl)mß7-lacZ is expressed in all FCs upon Notch activation at stage 6 of oogenesis (Figure 3G). In contrast, we found that in hpo mutant cells, the levels of E(spl)mß7-lacZ are weakly reduced in 53% of the clones and normally expressed in the rest (n = 17, Figure 3H and data not shown). It has been shown that in wing imaginal discs, mer and ex are required to control Notch localization in the cell and consequently its activity [26]. Similarly, the subcellular distribution of Notch is affected in hpo mutant FCs. Contrary to the wild-type, in which Notch accumulates in the apical membrane (Figure S2E), Notch expands to other membranes and is often detected in clusters in hpo clones (Figure S2F). Our results point out that hpo is essential in the PFCs for the Notch-dependent expression of several differentiation markers, such as FasIII, Eya, and Hnt, and for Notch subcellular localization. These observations and the weak defects on the Notch reporters support a function of the SWH pathway in modulating Notch signaling.
Because the SWH pathway is required for the polarization of the oocyte, as well as for the differentiation of the PFCs, we analyzed whether the mutant cells are competent to respond to Grk and indeed adopt a posterior fate. Dystroglycan (DG) is expressed in all FCs at early stages of oogenesis, but upon Grk signaling, DG forms an AP gradient with lower levels at the PFCs (Figure 3I) [27]. The fact that this Grk-dependent gradient of DG is also observed in the hpo mutant epithelia (Figure 3J and Table 1) suggests that the mutant cells are responsive to the Grk signaling. Similarly, when hpo clones affect only a portion of the PFCs, the posterior fate marker pointed is expressed as in the wild-type in 40% of the cases (Figure 3K and Table 1) [3, 28]. However, in 60% of the egg chambers with partial hpo posterior clones, and in all cases when all the PFCs are mutant, the expression of pointed is abolished (Figure 3L and Table 1). These results illustrate that hpo is required to fully process the Grk/EGFR signal in the PFCs. Conversely, in grk mutant egg chambers, the Hpo-dependent expression of Hnt is not affected, suggesting that the EGFR pathway is not required for the activation of the SWH pathway in the PFCs (data not shown).
Considering all these results together, we conclude that the SWH pathway is involved in the Notch- and Gurken-dependent maturation of the PFCs. Whether the SWH pathway modulates this maturation directly or indirectly, for example by affecting membrane properties (reviewed in [26]), needs to be further investigated.
Is the SWH Pathway Directly Required for the Repolarizing Signal Back to the Oocyte?
To study whether the oocyte polarity defects in egg chambers with FCs mutants for the SWH pathway are a consequence of the FCs proliferation and differentiation defects, we analyzed egg chambers with ex and ft mutant PFCs. Egg chambers with ft PFCs occasionally form a bilayer (7% of the clones; Figures S3D and S3D’), although they never have defects in oocyte polarity (Figure 1N, n = 70), suggesting that the morphological disruption of the epithelia in itself doesnot block the repolarizing signal. Egg chambers with ex PFCs show weak defects in the epithelium, with a bilayer rarely formed and restricted to only a few mutant cells (Figure 1M), but Stau is never properly localized (Figures S3A, S3A’ and S3C, Table 1, and Table S1). However, Hnt is not properly expressed in stage 7 ex mutant FCs, suggesting that the mislocalization of Stau is a consequence of the ex mutant cells being undifferentiated at the stage when the repolarizing signal is sent to the oocyte (Figure S3B and Table S1). These results suggest that the defects in oocyte polarity are probably due to a lack of proper differentiation of FCs in SWH mutant egg chambers.
In this study, we have analyzed the requirement of the SWH pathway during oogenesis. We have shown that several of the components of this pathway, but not ft, are required in the PFCs to induce the axis specification in the germline. The defects in oocyte polarity, however, are probably due to a lack of proper differentiation of the PFCs in SWH mutant egg chambers. In addition, the pathway is required in the terminal cells to control their proliferation. It has already been shown that terminal follicle cells are different from lateral follicle cells. The distinct spatial requirement of the SWH pathway for differentiation and proliferation is another feature that distinguishes the terminal from the lateral FCs, and the posterior from the anterior FCs. Our results point out that this dual function of the SWH pathway might be achieved by modulation of the Notch and EGFR signals. In conclusion, the SWH pathway lies at the intersection of two signaling pathways and is permissive for the signal that is sent from the follicle cells to repolarize the oocyte.
Experimental Procedures
Fly Stocks and Induction of Mutant Clones
The following stocks were used: w;FRT42D hpo42-47/CyO [17], w;FRT42D hpoJM1/CyO [29], yw;Sp/CyO;FRT82B wtsx1/TM6B [30], ywhs-FLP122;Sp/CyO;UASyki [18], yw;FRT40A exe1/CyO [31], ywhs-FLP122;FRT39 ft15 [32], whs-FLP,GFP-Staufen;FRT82B-nlsGFP [33], Tau-GFP [28], and ywhs-FLP122 tub-Gal4 UAS-nlsGFP/FM7; FRT42D tub-Gal80/CyO for MARCM clones [34]. The reporter lines used were yw; 998/12/TM6B for pointed (P. Deak, M. Bownes, and D. Glover) and E(spl)mß7-lacZ (an enhancer trap in the E(spl)mβ7 gene; gift of S. Bray, University of Cambridge, Cambridge, UK). All experiments with hpo were done with the hpo42-47 allele unless stated otherwise.
All follicle cell and germline clones were generated by the FRT/FLP recombinase with either the lack or the presence of GFP and the lack of π-Myc as a marker for homozygous clones [34–37]. The heat shock was performed either in larvae for 2 hr at 37°C during 2 consecutive days or adult flies for 1 hr at 37°C during 2 consecutive days (for wts and yki). The expression of Myc is induced for 1 hr at 37°C 4 hr before dissection. For the MARCM clones, the heat shock was performed 5 min at 37°C.
Whole-Mount In Situ Hybridizations and Antibody Stainings
Females were fattened for 20 hr, and the ovaries were dissected in PBT (PBS + 0.2% Tween), fixed for 20 min in 4% paraformaldehyde/PBT, washed with PBT, and kept in methanol at –20°C.
In situ hybridization was performed as previously described [38] with fluorescent tyramide detection (NEN LifeSciences). Digoxigenin antisense probes were prepared with full-length grk (G. Schüpbach), osk (A. Ephrussi), and bcd (C. Nüsslein-Volhard) cDNA.
For antibody stainings, the ovaries were then washed with PBT, blocked with PBT + 10% BSA, NGS (Normal Goat Serum) or NDS (Normal Donkey Serum) for 1 hr, and incubated with the antibody in PBS + 2% Tween + 1% BSA for 12–16 hr. After washing the ovaries with PBS + 2% Tween + 1% BSA several times for 30 min, we incubated them with the secondary antibody for at least 3 hr. They were finally washed three times with PBS for 15 min and mounted in Vectashield (Vector). All steps were performed at room temperature. Flies with hsmyc are heat shocked 1 hr at 37°C 4 hr before dissecting. The following antibodies were used: rat anti-Tubulin (1:500) (Chemicon), rabbit anti-Staufen (1:3000) [39], rabbit anti-nPKC (1:1000) (C-20, Santa Cruz Biotechnology), mouse anti-Dlg (1:500) (DSHB), mouse anti-Grk concentrate (1:200) (DSHB), mouse anti-Eya (1:20) (DSHB), mouse anti-Notch (1:10) (DSHB), mouse anti-Fasciclin III (7G10) (1:100) (DSHB), mouse anti-Hnt (1:15) (DSHB), rabbit anti-DG (1:3000) (Deng et al., 2003), rabbit anti-α-phopho-histone 3 (PH3) (1:500) (Upstate Biotechnology), rabbit anti-LacZ (1:1000) (Cappel), goat anti-LacZ (1:1000) (Biogenesis), mouse anti-GFP (1:1000) (Sigma), rabbit anti-GFP (Invitrogen), goat anti-GFP (1:1000) (Abcam), rabbit anti-Myc (1:1000) (Santa Cruz), and sheep anti-Digoxigenin-POD (1:1000) (Roche). Secondary antibodies were Alexa Fluor 488, Alexa Fluor 568, and Alexa Fluor 647 coupled (Molecular Probes). DAPI and Alexa-coupled Phalloidin (1:200, Invitrogen) were used for visualization of DNA and actin.
Imaging and Deconvolution
Immunofluorescence was visualized with a Leica SP confocal microscope (Leica Microsystems, Wetzlar, Germany) or a wide-field DeltaVision microscope (Applied Precision, Olympus IX70, and Roper Coolsnap HQ). Images were acquired with 20×/0.75 NA or 40×/1.5NA and then deconvolved [40].
Supplementary Material
Acknowledgments
We would like to thank Peter Lawrence for critical reading of the manuscript; J. Casal and H. López-Shier for fruitful discussions; D. St Johnston, J. Casal, G. Halder, R. Fehon, G. Struhl, and the Bloomington Drosophila Stock Center for fly stocks; and the Developmental Studies Hybridoma Bank (DSHB), K. Campbel, and D. St Johnston’s lab for providing antibodies. We are very grateful to C. Polesello and N. Tapon for fruitful discussions and the sharing of material and unpublished results. I.P. is funded by the Royal Society, I.D. is funded by a Senior Fellowship from the Wellcome Trust, C.M. is funded by the Wellcome Trust and I.A.G. is funded by the Biotechnology and Biological Sciences Research Council.
Footnotes
Supplemental Data Three figures and one table are available at http://www.current-biology.com/cgi/content/full/17/21/1871/DC1/.
References
- 1.Lopez-Schier H. The polarisation of the anteroposterior axis in Drosophila. Bioessays. 2003;25:781–791. doi: 10.1002/bies.10309. [DOI] [PubMed] [Google Scholar]
- 2.Ruohola H, Bremer KA, Baker D, Swedlow JR, Jan LY, Jan YN. Role of neurogenic genes in establishment of follicle cell fate and oocyte polarity during oogenesis in Drosophila. Cell. 1991;66:433–449. doi: 10.1016/0092-8674(81)90008-8. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez-Reyes A, St Johnston D. Patterning of the follicle cell epithelium along the anterior-posterior axis during Drosophila oogenesis. Development. 1998;125:2837–2846. doi: 10.1242/dev.125.15.2837. [DOI] [PubMed] [Google Scholar]
- 4.Deng WM, Bownes M. Patterning and morphogenesis of the follicle cell epithelium during Drosophila oogenesis. Int. J. Dev. Biol. 1998;42:541–552. [PubMed] [Google Scholar]
- 5.Sapir A, Schweitzer R, Shilo BZ. Sequential activation of the EGF receptor pathway during Drosophila oogenesis establishes the dorsoventral axis. Development. 1998;125:191–200. doi: 10.1242/dev.125.2.191. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez-Reyes A, Elliott H, St Johnston D. Polarization of both major body axes in Drosophila by gurken-torpedo signalling. Nature. 1995;375:654–658. doi: 10.1038/375654a0. [DOI] [PubMed] [Google Scholar]
- 7.Roth S, Neuman-Silberberg FS, Barcelo G, Schüpbach T. Cornichon and the EGF receptor signalling process are necessary for both anterior-posterior and dorsal-ventral pattern formation in Drosophila. Cell. 1995;81:967–978. doi: 10.1016/0092-8674(95)90016-0. [DOI] [PubMed] [Google Scholar]
- 8.Deng WM, Ruohola-Baker H. Laminin A is required for follicle cell-oocyte signaling that leads to establishment of the anterior-posterior axis in Drosophila. Curr. Biol. 2000;10:683–686. doi: 10.1016/s0960-9822(00)00514-5. [DOI] [PubMed] [Google Scholar]
- 9.Frydman HM, Spradling AC. The receptor-like tyrosine phosphatase lar is required for epithelial planar polarity and for axis determination within drosophila ovarian follicles. Development. 2001;128:3209–3220. doi: 10.1242/dev.128.16.3209. [DOI] [PubMed] [Google Scholar]
- 10.MacDougall N, Lad Y, Wilkie GS, Francis-Lang H, Sullivan W, Davis I. Merlin, the Drosophila homologue of neurofibromatosis-2, is specifically required in posterior follicle cells for axis formation in the oocyte. Development. 2001;128:665–673. doi: 10.1242/dev.128.5.665. [DOI] [PubMed] [Google Scholar]
- 11.Hamaratoglu F, Willecke M, Kango-Singh M, Nolo R, Hyun E, Tao C, Jafar-Nejad H, Halder G. The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat. Cell Biol. 2006;8:27–36. doi: 10.1038/ncb1339. [DOI] [PubMed] [Google Scholar]
- 12.Edgar BA. From cell structure to transcription: Hippo forges a new path. Cell. 2006;124:267–273. doi: 10.1016/j.cell.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Hariharan IK. Growth regulation: A beginning for the hippo pathway. Curr. Biol. 2006;16:R1037–R1039. doi: 10.1016/j.cub.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 14.Hipfner DR, Cohen SM. Connecting proliferation and apoptosis in development and disease. Nat. Rev. Mol. Cell Biol. 2004;5:805–815. doi: 10.1038/nrm1491. [DOI] [PubMed] [Google Scholar]
- 15.Harvey K, Tapon N. The Salvador-Warts-Hippo pathway - an emerging tumour-suppressor network. Nat. Rev. Cancer. 2007;7:182–191. doi: 10.1038/nrc2070. [DOI] [PubMed] [Google Scholar]
- 16.Harvey KF, Pfleger CM, Hariharan IK. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell. 2003;114:457–467. doi: 10.1016/s0092-8674(03)00557-9. [DOI] [PubMed] [Google Scholar]
- 17.Wu S, Huang J, Dong J, Pan D. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell. 2003;114:445–456. doi: 10.1016/s0092-8674(03)00549-x. [DOI] [PubMed] [Google Scholar]
- 18.Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122:421–434. doi: 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 19.Deng WM, Althauser C, Ruohola-Baker H. Notch-Delta signaling induces a transition from mitotic cell cycle to endocycle in Drosophila follicle cells. Development. 2001;128:4737–4746. doi: 10.1242/dev.128.23.4737. [DOI] [PubMed] [Google Scholar]
- 20.Schaeffer V, Althauser C, Shcherbata HR, Deng WM, Ruohola-Baker H. Notch-dependent Fizzy-related/Hec1/Cdh1 expression is required for the mitotic-to-endocycle transition in Drosophila follicle cells. Curr. Biol. 2004;14:630–636. doi: 10.1016/j.cub.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez-Minan A, Martin-Bermudo MD, Gonzalez-Reyes A. Integrin signaling regulates spindle orientation in Drosophila to preserve the follicular-epithelium monolayer. Curr. Biol. 2007;17:683–688. doi: 10.1016/j.cub.2007.02.052. [DOI] [PubMed] [Google Scholar]
- 22.Edgar BA, Orr-Weaver TL. Endoreplication cell cycles: More for less. Cell. 2001;105:297–306. doi: 10.1016/s0092-8674(01)00334-8. [DOI] [PubMed] [Google Scholar]
- 23.Lopez-Schier H, St Johnston D. Delta signaling from the germ line controls the proliferation and differentiation of the somatic follicle cells during Drosophila oogenesis. Genes Dev. 2001;15:1393–1405. doi: 10.1101/gad.200901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bai J, Montell D. Eyes absent, a key repressor of polar cell fate during Drosophila oogenesis. Development. 2002;129:5377–5388. doi: 10.1242/dev.00115. [DOI] [PubMed] [Google Scholar]
- 25.Sun J, Deng WM. Hindsight mediates the role of notch in suppressing hedgehog signaling and cell proliferation. Dev. Cell. 2007;12:431–442. doi: 10.1016/j.devcel.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maitra S, Kulikauskas RM, Gavilan H, Fehon RG. The tumor suppressors Merlin and Expanded function cooperatively to modulate receptor endocytosis and signaling. Curr. Biol. 2006;16:702–709. doi: 10.1016/j.cub.2006.02.063. [DOI] [PubMed] [Google Scholar]
- 27.Poulton JS, Deng WM. Dystroglycan down-regulation links EGF receptor signaling and anterior-posterior polarity formation in the Drosophila oocyte. Proc. Natl. Acad. Sci. USA. 2006;103:12775–12780. doi: 10.1073/pnas.0603817103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Micklem DR, Dasgupta R, Elliott H, Gergely F, Davidson C, Brand A, Gonzalez-Reyes A, St Johnston D. The mago nashi gene is required for the polarisation of the oocyte and the formation of perpendicular axes in Drosophila. Curr. Biol. 1997;7:468–478. doi: 10.1016/s0960-9822(06)00218-1. [DOI] [PubMed] [Google Scholar]
- 29.Jia J, Zhang W, Wang B, Trinko R, Jiang J. The Drosophila Ste20 family kinase dMST functions as a tumor suppressor by restricting cell proliferation and promoting apoptosis. Genes Dev. 2003;17:2514–2519. doi: 10.1101/gad.1134003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu T, Wang W, Zhang S, Stewart RA, Yu W. Identifying tumor suppressors in genetic mosaics: The Drosophila lats gene encodes a putative protein kinase. Development. 1995;121:1053–1063. doi: 10.1242/dev.121.4.1053. [DOI] [PubMed] [Google Scholar]
- 31.Boedigheimer M, Laughon A. Expanded: A gene involved in the control of cell proliferation in imaginal discs. Development. 1993;118:1291–1301. doi: 10.1242/dev.118.4.1291. [DOI] [PubMed] [Google Scholar]
- 32.Bryant PJ, Huettner B, Held LI, Jr., Ryerse J, Szidonya J. Mutations at the fat locus interfere with cell proliferation control and epithelial morphogenesis in Drosophila. Dev. Biol. 1988;129:541–554. doi: 10.1016/0012-1606(88)90399-5. [DOI] [PubMed] [Google Scholar]
- 33.Palacios IM, St Johnston D. Kinesin light chain-independent function of the Kinesin heavy chain in cytoplasmic streaming and posterior localisation in the Drosophila oocyte. Development. 2002;129:5473–5485. doi: 10.1242/dev.00119. [DOI] [PubMed] [Google Scholar]
- 34.Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- 35.Chou T-B, Noll E, Perrimon N. Autosomal P[ovoD1] dominant female-sterile insertions in Drosophila and their use in generating germ-line chimeras. Development. 1993;119:1359–1369. doi: 10.1242/dev.119.4.1359. [DOI] [PubMed] [Google Scholar]
- 36.Chou TB, Perrimon N. The autosomal FLP-DFS technique for generating germline mosaics in Drosophila melanogaster. Genetics. 1996;144:1673–1679. doi: 10.1093/genetics/144.4.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117:1223–1237. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
- 38.Wilkie GS, Shermoen AW, O’Farrell PH, Davis I. Transcribed genes are localized according to chromosomal position within polarized Drosophila embryonic nuclei. Curr. Biol. 1999;9:1263–1266. doi: 10.1016/s0960-9822(99)80509-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.St Johnston D, Beuchle D, Nusslein-Volhard C. Staufen, a gene required to localize maternal RNAs in the Drosophila egg. Cell. 1991;66:51–63. doi: 10.1016/0092-8674(91)90138-o. [DOI] [PubMed] [Google Scholar]
- 40.Davis I. Visualising fluorescence in Drosophila—optimal detection in thick specimens. In: Allan VJ, editor. Protein Localisation by Fluorescence Microscopy: A Practical Approach. Oxford University Press; Oxford: 2000. pp. 131–162. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.