Abstract
Purpose
In performing search tasks, the visual system encodes information across the visual field at a resolution inversely related to eccentricity and deploys saccades to place visually interesting targets upon the fovea where resolution is highest. The serial process of fixation, punctuated by saccadic eye movements, continues until the desired target has been located. Loss of central vision restricts the ability to resolve the high spatial information of a target, interfering with this visual search process. We investigate oculomotor adaptations to central visual field loss with gaze-contingent artificial scotomas.
Methods
Spatial distortions were placed at random locations in 25° square natural scenes. Gaze-contingent artificial central scotomas were updated at the screen rate (75Hz) based on a 250Hz eyetracker. Eight subjects searched the natural scene for the spatial distortion and indicated its location using a mouse-controlled cursor.
Results
As the central scotoma size increased, the mean search time increased [F(3,28)= 5.27, p= .05] and the spatial distribution of gaze points during fixation increased significantly along the x [F(3,28)= 6.33, p= .002] and y [F(3,28)= 3.32, p= .034] axes. Oculomotor patterns of fixation duration, saccade size and saccade duration did not change significantly, regardless of scotoma size.
Conclusions
There is limited automatic adaptation of the oculomotor system following simulated central vision loss.
Keywords: scotoma, saccade, fixation, eyetracking, visual search
Saccades deliver the retinal image of an object to a specific retinal locus for detailed inspection during a period of fixation. The retinal locus for fixation in normally-sighted subjects remains within 160 arcmin2 1 corresponding to a retinal diameter of approximately 35 foveal cone cells. Macular diseases, including age-related macular degeneration (AMD), may damage the fovea through various pathologic processes resulting in a discrete region of blindness called a scotoma. Scotomas arising from macular disease are the leading cause of visual impairment in North America and Europe 2. Attempting to fixate the former location of the fovea in an eye with central scotoma will image objects within the scotoma. Consequently subjects may perceive objects as blurred, distorted, indistinct or having vanished entirely from view 3. To fixate without interference from foveal scotomas, many patients position their eyes so as to image objects upon a locus of relatively unimpaired eccentric retina 4, 5 called a preferred retinal locus (PRL). Failure to efficiently use existing unimpaired retina may preclude patients from achieving optimal performance in everyday visual tasks.
Bertera 6 highlighted that studying central retinal scotomas in patient populations is complicated by concurrent ocular disease as well as variation in the characteristics of scotomas between individuals. Artificial central scotomas provide a practical alternative since the experimenter can systematically manipulate individual scotoma characteristics in the same subject thus eliminating confounding influences and greatly increasing statistical power.
Many techniques have been used to generate artificial central scotomas, including modified scleral contact lenses 7. Currently, the preferred technique employs eye tracking 6, 8–10 to achieve selective image stabilisation. In brief, this involves producing an arbitrarily-shaped mask that obscures part of a stimulus background or scene. The mask is moveable, controlled by means of signals from the eye tracker. When a subject moves their eyes, the obscuring mask is displaced in such a manner to remain fixed with respect to the subject’s fovea, simulating the effects of central visual loss.
In the last few decades researchers have investigated the effect of artificial central scotomas on the oculomotor behaviour of normal subjects. To elicit eye movements, these studies employed visual search tasks 6, 10. However, the visual search stimuli have been presented in highly regular matrices and lacked features and contrast variation normally present in natural scenes. Consequently, the location of candidate targets is highly predictable and the oculomotor behaviour for such search tasks may not correspond to that under more natural viewing conditions.
By introducing natural variation in luminance, contrast, textures, edge density and object sizes into a visual search task we aim to study the impact of artificial central vision loss under more realistic conditions than have been employed previously. Natural variation in spatial structure can be implemented by requiring subjects to search for targets embedded within calibrated natural images. If the location of the target and non-target features were entirely random prior knowledge of the stimulus layout could not influence oculomotor behavior 11. Alternatively, replacing the search target with that of a random spatial distortion does not change natural image statistics (local spatial structure, luminance, contrast, edge density or amplitude spectrum) in a predictable way 12. This avoids the introduction of abrupt changes in luminance, chrominance or contrast and prevents the use of a single filter to make the task unnaturally simple– e.g. the task of finding a ‘C’ target among ’O’ distractors 10 or finding a Gabor target in pink noise 13. Furthermore, distortion targets can be introduced anywhere in the natural scene, which prevents subjects from searching in expected locations (e.g. table tops only for cups) and avoids the introduction of obvious contextual anomalies (e.g. a cup in a tree).
Recent work suggests that training on a visual search task can improve mobility performance under low light conditions for people with visual impairments including AMD 14, 15. To develop novel rehabilitation interventions, a better understanding of the short-term oculomotor adaptations of the visual system following scotoma onset is needed. We therefore investigate short term oculomotor adaptations to artificial central visual loss while normally-sighted subjects conduct a visual search task within natural scenes.
METHODS
The research was conducted at the Schepens Eye Research Institute and followed the tenets of the Declaration of Helsinki. The research was approved by the departmental Institutional Review Board. Subjects gave their informed consent prior to commencing the study.
Subjects
One of the authors (LM) and 7 naïve volunteers participated in the study (mean age 23.1 years; range 21 – 25 years). All subjects had normal or corrected-to-normal visual acuity of 0.0 logMAR or better, a Pelli-Robson contrast sensitivity of 1.80 log units or better and no known ophthalmic disease. Subjects performed all trials under monocular conditions using their preferred eye, with their contra-lateral eye occluded with an eye patch. Other than one author (LM), no subject had previously participated in artificial scotoma or eye movement experiments.
Stimuli were generated on a PC computer using MATLAB (Mathworks, Natick, MA) and employed routines from the PsychToolbox 16, 17. Stimuli were displayed on a gamma-corrected LaCie Electon 22” CRT monitor (LaCie USA, OR) with a mean luminance of 50 cdm−2 at a frame rate of 75 Hz calibrated with a Minolta LS100 photometer. The display measured 36° horizontally (1,152 pixels), 27° vertically (864 pixels) and was positioned 57 cm from the observer in an otherwise dark room.
Stimuli
A set of 4,165 calibrated 16-bit greyscale natural images was downloaded from an online database (http://hlab.phys.rug.nl/archive.html) whose characteristics have been described in detail elsewhere 18. On each trial, a 1,536 × 1,024 pixel source image was selected at random from all 4,165 images and an 800 × 800 pixel area (25° × 25° square) was then sampled at random from the source image and became the experimental image. No attempt was made to select images for particular content. The global RMS contrast (the standard deviation of pixel values divided by the mean) of the experimental images was fixed at 0.2.
An area within the central 23° × 23°, thus avoiding the edge of the test image, was selected at random to contain the distortion. Figure 1 shows a representative image in which a distortion has been introduced at a random location indicated by the red dashed circle (that was not present in the experiment). Spatial distortions were introduced by remapping the pixels from the source image to the distorted image. The remapping was controlled with band-pass filtered noise, newly randomly generated each trial, using log exponential filters:
where ω is spatial frequency, ωpeak specifies the peak frequency, which was fixed at 0.5 cycles per degree, and b0.5 the half bandwidth of the filter in octaves, which was fixed at 0.5 octaves (full width = 1 octave). The mean value of the band-pass filtered noise was fixed at 0 and its amplitude (−α to +α), which controlled the magnitude of distortion, was fixed at 0.5°. One band-pass filtered random noise sample controlled the horizontal displacement of each pixel and a separate band-pass filtered noise sample (with the same peak spatial frequency and amplitude) controlled the vertical displacement of each pixel. Bilinear interpolation was employed to support sub-pixel precision. Spatial distortions were smoothly blended into the image with a Gaussian window with a standard deviation of 1°, thus the magnitude of distortion beyond a 2° radius (±2σ) approached 0, which ensured that there were no abrupt transitions between distorted and undistorted areas of the image. The magnitude of the spatial distortion and the size of the Gaussian window were based on data from a recent study 12 in which we measured detection thresholds for spatial distortions in natural scenes across the visual field. The magnitude (0.5°) and spatial period (2°) of spatial distortion employed in the present study were based on those data were above detection threshold at all eccentricities and should therefore be detectable by peripheral vision with artificial central vision loss.
Figure 1.
Illustration of spatial distortion in natural image. Distortions were smoothly blended into a random location with a Gaussian (σx,y = 1°). Even though these images are probably unfamiliar and the first order statistics of the distorted region do not differ from the rest of the image, the distortion (ringed with a red dashed circle that was not present in the experiments) can be detected with minimum effort.
Central field loss was simulated with a circular Gaussian mask that occluded the background natural image on the CRT display. The Gaussian mask was continuously centered on fixation and progressively reduced the contrast to zero at the center of fixation. Three Gaussian central scotoma sizes were used: σx,y = 1°, 2°, or 4°. The x and y coordinates of the artificial visual loss were defined by the gaze position data of the subject’s preferred eye, sampled at 250 Hz with a video eye tracker (High Speed Video Eyetracker Toolbox; Cambridge Research Systems, Cambridge, UK). To determine the latency of the system, the delay between a drawing command to the graphics card and its corresponding update on the screen was measured with a photodiode and a USB-attached digital I/O acquisition board. The mean latency was 20.35 ms (σlatency = 3.75 ms) at the top of the screen. At 75 Hz, one refresh takes 13.33 ms so the gaze contingent updates nearly always occurred on the next video frame, but the gaze-contingent scotoma was potentially misaligned for 20 ms. The results are unlikely to be compromised by such a small temporal lag as any information present for 13 ms or less will be unlikely to reach detection threshold 19. To maintain the correct viewing distance and accurate eye tracking, a chin and forehead rest were used to restrict head movements. Many patients with central scotomas less than 6° do not readily perceive their scotoma on clinical testing 20 and the scotoma can appear filled-in with the surrounding spatial structure 3, 21–24. The reported time course for the filling-in of scotomas depends on whether it is real or simulated, and if simulated the method employed to generate them. For real scotomas perceptual completion took place instantaneously 22. Scotomas simulated as an eccentrically viewed homogenous grey square filled in within 2–3 seconds 25 while those simulated by retinal stabilization filled in after several seconds 26. Subjective reports from all subjects in this current study indicated that filling-in of artificial scotomas did not occur. Nevertheless, whether the observer experienced filling in over the artificial scotoma or not is irrelevant for the present task. The central gaze point of the search image was occluded by the simulated scotoma and whether it appeared filled in or randomly textured did not alter the absence of a foveal view of the stimulus.
Procedure
A minimum of two runs of visual search trials were performed in succession. Each run consisted of 70 search trials, 10 trials for each of the six artificial visual loss conditions: 3 central simulated scotomas, 3 peripheral simulated scotomas (not discussed here) and a control condition with no simulated visual field loss. Prior to each run, subjects performed an eye tracker calibration for a set of 20 known target positions, evenly spaced over an area subtending 20° × 16°, using the calibration routines supplied with the eye tracker, accessed from MATLAB. Each run was relatively short and it was not necessary to recalibrate the eye tracker between trials. Furthermore, the gaze-contingent scotoma was continuously visible during the experiment and observers were instructed to inform the experimenter if the center of the scotoma drifted from the center of fixation. No subject reported any drift.
The search trials commenced with subjects fixating an isoluminant green central dot subtending 11 arc min in the center of an otherwise uniform field of mean luminance. To initiate a trial, the subject pressed a response button on a computer mouse, which caused the uniform background and fixation target to be immediately replaced with the experimental image and gaze-contingent artificial visual field loss superimposed. It was then the task of the subject to search the natural scene for the spatial distortion and once located, to indicate its position by moving a mouse controlled cursor to the center of the distortion and pressing a response button on the mouse. The standard Microsoft Windows XP operating system arrow was used in this experiment. It was composed of a white center (100cd/m2) and a black outline (0.1cd/m2) and was easily visible to subjects due to its relatively high contrast against 0.2 RMS contrast natural images. A correct feedback signal was provided if the cursor fell within a radius of 2° from the center of the distortion, outside which an incorrect response was recorded. Visual feedback was provided at the fixation dot, which was isoluminant green following a correct response or isoluminant red following an incorrect response. Finally, the subject returned their fixation to the central dot ready to begin the next trial. At the commencement of each trial the cursor remained at, and was continuous with, the last cursor position. The order of the ten trials for each of seven conditions was randomly interleaved.
No time limit was imposed on any trial. Subjects were not provided with any instruction on adopting a specific search strategy but were encouraged to search freely within the experimental image. Subjects were instructed not to guess but to be confident of the location of the spatial distortion before responding, if possible. Subjects were given sufficient practice trials to demonstrate all conditions of artificial visual loss and the spatial distortion target.
Data Analysis
Eye movement data were analysed offline using MATLAB software with routines written specifically for this purpose. A time-stamped data file of the x and y coordinates of gaze position, in millimetres from the screen center, was recorded at 250 Hz during all search trials. Gaze positions were not tracked between search trials. Prior to the statistical analysis of eye movement data, lost data samples arising from blink movements and eye tracking failures were identified. Such lost gaze points amounted to less than 9.5% of the total data recorded. Gaze positions during lost intervals were linearly interpolated between the adjacent gaze positions, evenly distributing missing data points between recorded data points. Similar results were obtained when lost data points were removed or substituted with the last successfully recorded gaze position, which were alternative methods we considered. Once the interpolated data was substituted, all eye position data were analysed in the same way. A saccade was defined statistically as any change in eye position greater than 1.96σ above the mean of all changes in eye position made within a particular trial. For a comprehensive discussion on the use of our fixation-saccade classification technique, the reader is directed to Appendix 1 (available at [LWW insert link]).
Saccade duration was defined as the time elapsed between saccade onset and saccade offset and saccade amplitude was defined as the absolute distance between the last eye position of one fixation epoch and the first eye position of the next fixation epoch. Saccade frequency was expressed as the number of saccades per second. Fixations were defined as the collection of gaze points that were separated by eye movements less than 1.96σ above the mean made in a particular trial. The small eye movements that occur during fixation were defined as micro-saccades. Fixation duration was defined as the time elapsed between two successive saccades. Fixation frequency was expressed as the number of fixations per second. Fixation stability was defined as the spatial distribution of eye positions during a period of fixation. We calculated the horizontal and vertical standard deviation of fixation stability and the bivariate contour ellipse area (BCEA), with a probability area of 68% 27, as an estimate of the area of a given fixation.
Search time was defined as the time elapsed between start of a trial and the mouse button press ending the trial. Prior to statistical analysis of search times, data were examined to determine the proportion of trials in which subjects had failed to locate the spatial distortion i.e. the position of the mouse controlled cursor exceeded a 2° radius from the center of the target spatial distortion at the time of the mouse button press. Unfortunately data on the accuracy of mouse placement was collected for only two of the eight subjects tested. Each of the two subjects completed 80 trials each, 20 trials for each of the 4 conditions. The combined data for both subjects is presented below.
Given that subjects were permitted unlimited search time, no significant correlation was found between search time and failure to identify the spatial distortion. This suggests that despite the difficulty posed in searching with artificial visual field loss, subjects did not forfeit trials but rather made a persistent attempt to locate the spatial distortion. In addition, search time data exceeded 1 second in duration in more than 99% of search trials, indicating that subjects made a reasonable attempt to locate the spatial distortion each trial.
To determine whether search time, fixation duration, number of saccades, saccade duration and saccade size were Normally-distributed, the raw data were analyzed with a two-sample Kolmogorov-Smirnov test, accessed with MATLAB’s ‘ktest2’ function. This analysis confirmed that the data were not significantly different from normally distributed and parametric statistics could be applied. Uncorrected raw data was then analysed by within subjects ANOVA statistical analysis using SPSS (SPSS, IBM Corporation). For group analyses the data for each subject were normalized to their performance in the control condition (no artificial visual field loss). This corrected for variations in baseline performance between subjects and for differences in the total number of trials completed by each subject. Search times and eye movement parameters for each condition reported are the normalized mean and standard deviations of a minimum of twenty searches per condition per subject. Pearson’s linear correlation coefficient, r, was computed using the ‘corr’ function accessed through MATLAB. The corresponding p-value, an output of the ‘corr’ function, indicates if the correlation is significantly different from zero based on a Student’s t test.
RESULTS
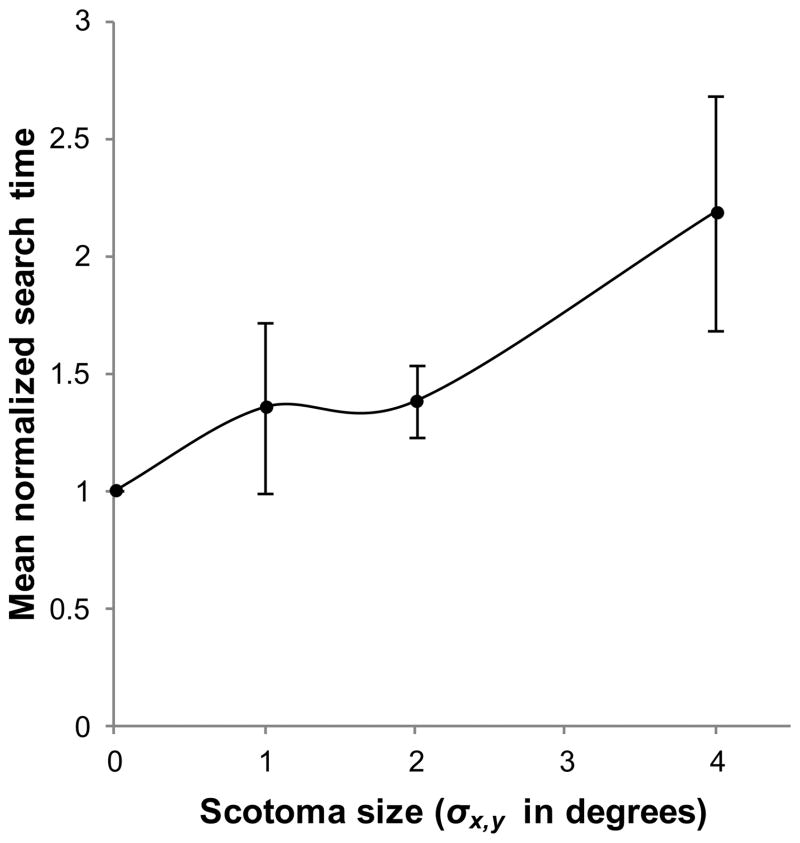
A summary of the raw search time data for each condition is provided in table 1. Figure 2 illustrates mean normalized search time as a function of scotoma size. Mean normalized search time increased significantly with scotoma size [F(3,28)= 5.27, p= .05] and the two factors were significantly correlated [r(30)= .569, p= < .001, with degrees of freedom based on 8 subjects and 4 conditions]. The largest central scotoma condition, σx,y= 4°, resulted in a doubling of the mean search time. Tukey post hoc comparisons show that only this scotoma condition differed significantly from the control condition (p= .003).
Table 1.
Summary of data for two subjects on the proportion incorrect trials.
| Scotoma size (σx,y) | μ (± σ) search time (seconds) | μ (± σ) error distance (degrees) | μ (± σ) proportion of incorrect trials (%) |
|---|---|---|---|
| Control | 4.54 (± 4.69) | 1.85 (± 2.52) | 25.0 (± 10) |
| 1° | 4.57 (± 3.07) | 2.46 (± 3.81) | 32.5 (± 13) |
| 2° | 6.32 (± 5.74) | 3.21 (± 4.60) | 40.0 (± 16) |
| 4° | 8.92 (± 8.84) | 4.32 (± 5.07) | 42.5 (± 17) |
Figure 2.
Mean normalized search time as a function of scotoma size. Search time was defined as the time elapsed between the start of a trial and the mouse button press ending the trial. Error bars show ±95% confidence intervals.
There were strong correlations of the mean normalized number of saccades with both search time [r(30)= .8337, p= < .001] and scotoma size [r(30) = .538, p= .002]. Saccade frequency remained constant across the control and artificial scotoma conditions (μfreq = 2.57 saccades per second, σfreq = 0.11 saccades per second). No statistical difference was found in saccade duration between the control condition and the scotoma condition.
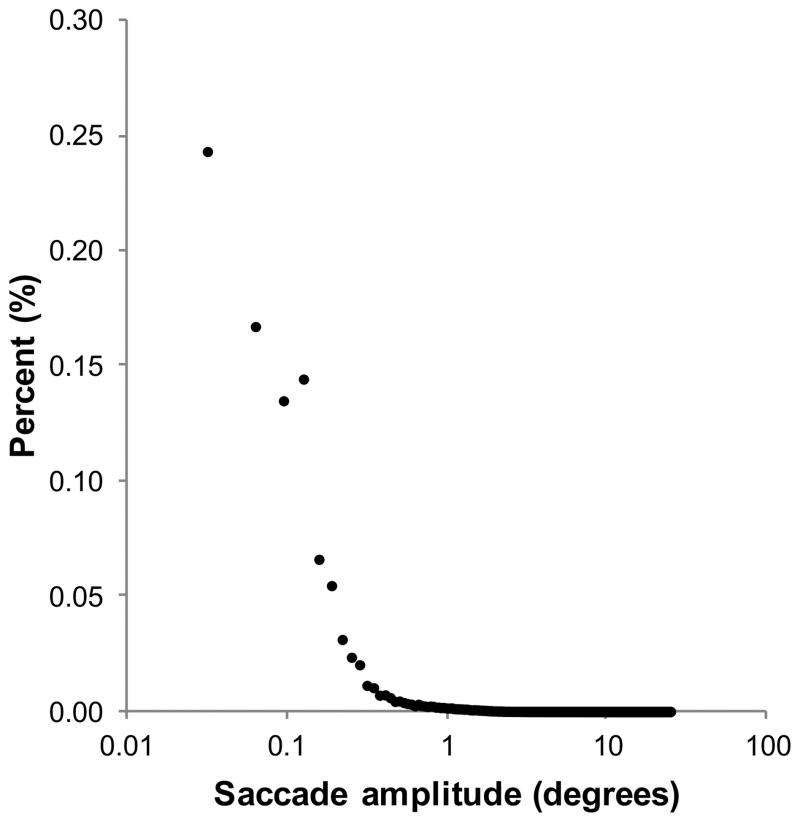
Of the saccades made in the control condition and in each of the scotoma conditions, 99% were 15° or less in amplitude. Figure 3 shows the frequency distribution of saccade amplitude for 24010 saccades made in the control condition. This distribution was fitted with an exponential curve, of the form y=a*exp(−1/b*x), using the curve fitting toolbox within MATLAB. The decay constant for the control condition exponential was 0.108 degree−1 (R2 = .982). Similar decay constants of, 0.100 degree−1 (R2 = .984), 0.101 degree−1 (R2 = .982) and 0.115 degree−1 (R2 = .977) were obtained for central scotoma conditions of 1°, 2° and 4° respectively. This suggests no systematic relationship between saccade amplitude and scotoma size.
Figure 3.
Frequency distribution of saccade amplitude (degrees) for the control condition.
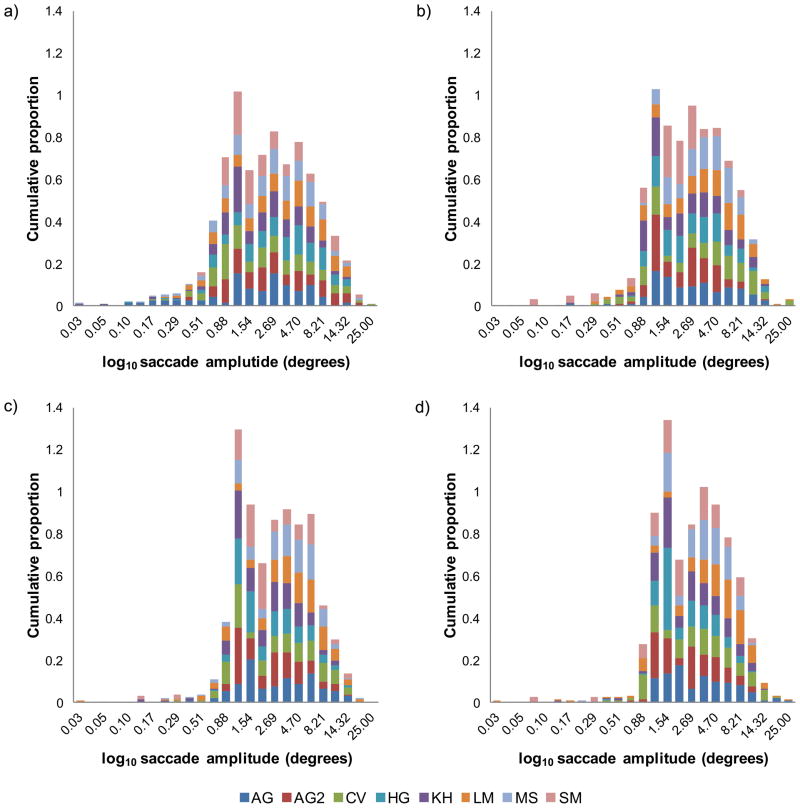
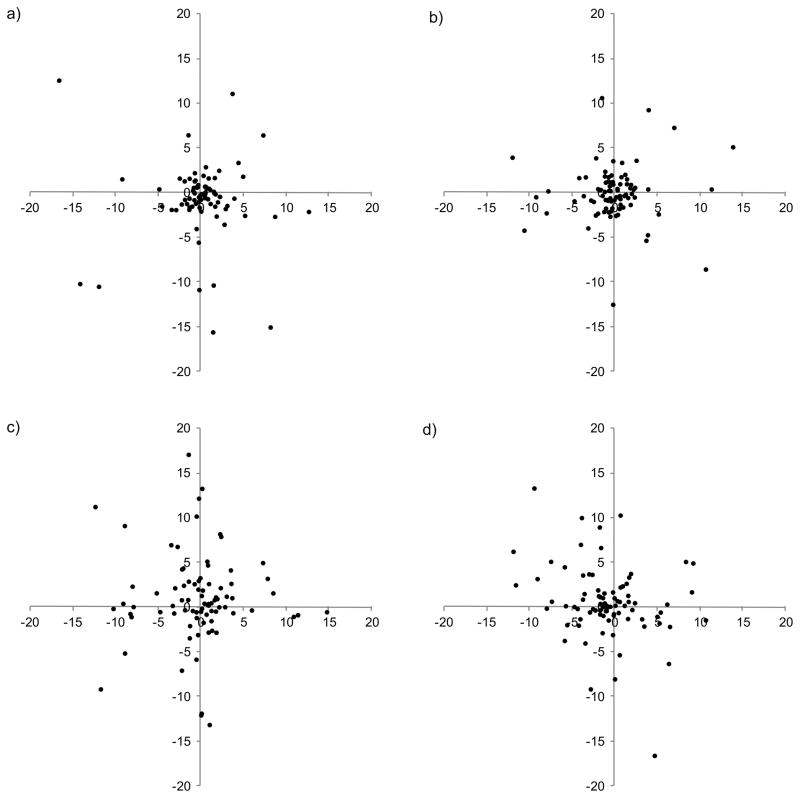
Figures 4(a) – (d) illustrate the distributions of saccade amplitudes, for each subject plotted as histograms, for the control condition and scotoma conditions. In each case saccade amplitudes have been log10 scaled to reveal the underlying distribution because the linear distributions were excessively skewed. The general distribution of saccade amplitudes in the control condition, figure 4(a) are negatively skewed. While none of the mean normalized saccade amplitudes for the scotoma conditions differs significantly from the control condition, figures 4(b), (c) and (d) show that as scotoma size is increased, the distribution becomes less skewed and appears more bimodal, with a first peak for saccades of 1–1.5°and a second peak at approximately 3.5°. The decrease in negative skew indicates that subjects made fewer very small saccades when the scotoma was present (see discussion of fixation stability below).
Figure 4.
Distribution of log10 mean saccade amplitudes for eight subjects, indicated by the caption. Cumulative proportion of saccade amplitudes for (a) control no scotoma condition, (b) scotoma σx,y = 1°, (c) scotoma σx,y = 2° and (d) scotoma σx,y = 4°. It is important to note that the cumulative proportions for each subject, represented by a different color, sum to unity.
The statistical significance of bimodality was tested using the Hartigan dip test of unimodality. However, while there was a trend towards bimodality, none of the distributions in figure 4(a) to (d) were significantly different from unimodal, 4(a) [dip= .08, p= .196], (b) [dip= .0875, p= .094], (c) [dip= .0626, p= .607] and (d) [dip= .081, p= .189].
Given that the artificial scotoma was centerd on fixation, if oculomotor behaviour remained referenced on the fovea, then there should be no saccades smaller than the radius of the scotoma because any peripheral target would therefore remain within the scotoma. We therefore determined the proportions of saccades that were smaller than the scotoma (i.e. saccades ≤ σx,y). These were .119, .390 and .630 for σx,y of 1°, 2°, and 4° respectively. This means that for the smallest scotoma (σx,y= 1°), approximately 12% of eye movements failed to move the (masked) fovea out of the scotoma, this number rose to 63% for the largest scotoma (σx,y= 4°).
Since each saccade is followed by a fixation and vice versa, the data for fixation number and frequency is identical to that for saccades. As with saccade frequency, since the fixation frequency remained constant across the control and scotoma conditions it must follow that fixation duration remained constant across the control and scotoma conditions. No statistical difference was found in fixation duration between the control condition and the scotoma conditions, however it did vary a mean factor of 1.25 (σ=0.28), 1.14 (σ=0.22) and 1.23 (σ=0.21) from the control condition for scotomas of σx,y = 1, 2, and 4° respectively.
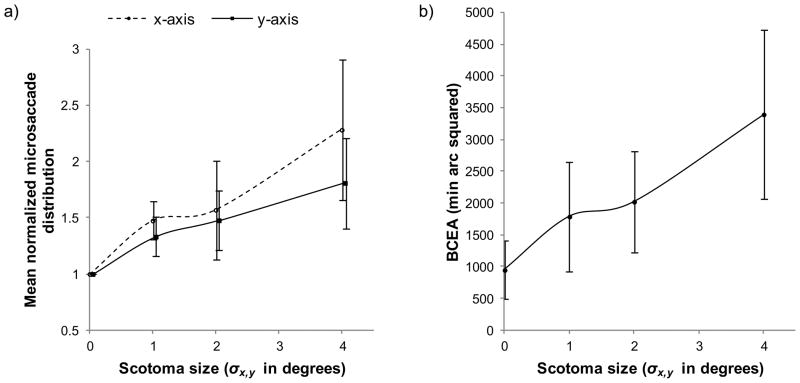
Figure 5(a) plots the standard deviation of micro-saccades during periods of fixation as a function of scotoma size and figure 5(b) plots the BCEA as a function of scotoma size. The spatial distribution of micro-saccades during fixation and the BCEA are both indications of fixation instability i.e. when eye position is distributed over a larger area during a fixation, then fixation is more unstable. The mean BCEA was 947 min arc 2 for the control condition, which increased to 3398 min arc 2 for the 4° scotoma. We next examined horizontal and vertical standard deviations in order to determine the principal source of this additional variation. The standard deviation of x-axis [F(3,28)= 6.33, p= .002] and y-axis [F(3,28)= 3.32, p= .034] micro-saccades during fixation increased significantly with scotoma size. The x and y-axis standard deviations were significantly correlated with scotoma size, [r(30)= .600, p= < .001] and [r(30)= .493, p= .004] respectively. Tukey post hoc comparisons showed that only the largest scotoma condition (σx,y= 4°) differed significantly from the control condition on both the x-axis (p= .001) and the y-axis (p= .021) micro-saccade distributions. These data show that fixation became progressively more unstable as the size of the artificial scotoma increased, with slightly larger increases in horizontal micro-saccadic eye movements.
Figure 5.
(a) Mean normalized micro-saccade standard deviation as a function of scotoma size for x-axis (dashed line) and y-axis (solid line). (b) Mean bivariate contour ellipse area (BCEA) as a function of scotoma size.
While the magnitude of the horizontal fixation distribution exceeds that of the vertical (μdifference = 1.426 pixels/.0445°, σdifference = 1.983 pixels/.062°), there was no significant difference between the x and y axis distributions for the control condition [F(1,14)= .018, p= .896], σx,y= 1° [F(1,14)= .399, p= .538], σx,y= 2° [F(1,14)= .005, p=.946] or for σx,y= 4° [F(1,14)= 1.281, p= .227].
DISCUSSION
Search time increased with scotoma size. Individual variation in search time was observed, suggesting that some subjects were more impaired than others by the presence of a central scotoma. While there was a trend at all sizes, only the largest scotoma condition (σx,y = 4°), was significantly different from the control condition. From this observation, we can conclude either that the visual system is either able to immediately compensate for small to moderate amounts of central visual deprivation or that the resolution of parafoveal vision with the two smaller scotomas (σx,y ≤ 2°) was adequate for the present task. The latter suggests that the parafoveal vision can be sufficient for visual search within natural scenes, as long as the target object subtends an area that is larger than the scotoma. If so, the expected impairment in visual search following central vision loss will be highly dependent on the extent of loss and the size of the target to be detected.
No relationship was found between saccade frequency and scotoma size; trials in which search times were longer simply had a greater number of saccades. The amplitude of most saccades does not exceed 15° when viewing images or walking outdoors 28, consistent with 99% of saccades in all conditions of the present study. However, the decay constants reported for the saccade frequency distributions are, in general, half of that measured in normal subjects for an outdoor walking task 28. This indicates that saccades made during our visual search task were smaller than those made in full field, outdoor natural scenes.
No relationship was found between the mean saccade amplitude and scotoma size. In the present task, subjects were required to search within a natural scene and to identify and locate a spatial distortion. The position of the distortion was completely random, thereby precluding knowledge or priors about the likely location of particular objects in natural scenes 11 and thereby forcing the task to be entirely visually-based. The magnitude and period of the spatial distortion was chosen to be identifiable across the retina so that any retinal location could in principle be used to complete the task. Therefore, the task does not provide any implicit stimulus for PRL development, and choosing a PRL closest to the scotoma boundary will improve the resolution of local spatial information. Although subjects were naive to this fact, the results seem to suggest that in the presence of a central scotoma, subjects continued to make saccades as normal, possibly using a temporary PRL.
The observation that many saccades (63% for the 4° scotoma) were smaller than the radius of the scotoma indicates that a non-foveal retinal locus drove eye movements. This is because if eye movements remained referenced on the former fovea, then all saccades smaller than the radius of the scotoma would retain the point of regard within the scotoma. This suggests that a temporary non-foveal location may have been referenced for eye movements. We attempted to identify whether subject had spontaneously developed a PRL in two ways. Firstly, we examined the direction of the center of fixation relative to the end point of each preceding saccade. We hypothesised that each saccade would initially bring the target into the location of the masked fovea. Next, a small corrective saccade would be deployed to bring the target out of the scotoma and into a PRL if one existed. According to this hypothesis, the direction of such a corrective saccade should be relatively constant. The presence of a second peak in the distribution of saccade sizes in scotoma conditions (Figure 4) was consistent with this hypothesis. However, there was no consistent relationship between the end point of the saccade and the center of the following fixation. Secondly, we examined the relative location of gaze with respect to the target location at the end of the trial. At this point, we hypothesised that subjects would attend to the target with any PRL, had they developed one, and this would produce a fixed directional or angular relationship with the target and the calibrated foveal location. However, there was no consistent relationship between the final gaze point and the location of the target. Therefore, the non-foveal reference varied across saccades and therefore failed to meet the definition of a PRL. Despite this finding, we attempted to determine if subjects maximised the resolution of visual information, based on distance from former fovea. This is possible only by analysing the final fixation relative to the spatial distortion as it is impossible to know what subjects were attending to as they searched within the natural scenes. Since the spatial distortion was smoothly blended into the image with a Gaussian window of σx,y=1°, the magnitude of distortion beyond a 2° radius (representing ±2σx,y) approached 0. To maximise the resolution of visual information in the control condition, subjects would have to have placed their fovea as close as possible to the spatial distortion. Thus the distance between the geometric center of the distortion and the fovea would be less than the radius of the distortion (2°). In scotoma conditions, subjects would have to place the edge of their scotoma (defined as 1σx,y = 61% amplitude) within the radius of the spatial distortion (corresponding to a former foveal distance of less than 2°+ σx,y). Using these definitions, subjects maximised the resolution of visual information of the target on 48.2, 67.1, 55.3 and 71.7% of trials for the control, and σx,y=1,2,4° scotoma conditions respectively.
Is it plausible for subjects to intuitively search natural scenes without the need for a PRL or to maximise the resolution of potential targets? The fovea is typically considered to subtend the central 5° of a monocular visual field that subtends approximately 130° vertically and 150° horizontally. At any one time, most of the visual field is imaged not on the high-resolution fovea but on lower resolution non-foveal regions. In addition, the world we inhabit is not one composed solely of high contrast or high spatial frequency. The benefit expected to be gained through the use of a PRL for visual search tasks is questionable. This idea is supported in a case report by 29 detailing “An unusual strategy for fixation” in a patient with advanced bilateral AMD. The patient’s fixation behaviour suggested a preference for a larger area of lower resolution retina for distance tasks and navigation but a small smaller area of higher resolution retina for reading.
We observed no significant relationship between fixation duration and scotoma size. Geisler 13 found that fixation duration increased, by a factor of up to 1.5, when subjects took longer to search for a Gabor in pink noise. In this study we found a comparable increase in fixation duration, but this failed to reach significance. Our results for fixation duration conflict with other studies that have used artificial scotomas with different tasks, including reading 30 and visual search tasks in highly regular search arrays 6, 10. These tasks did not use a natural images but instead resolution-dependent stimuli. For the highly regular search arrays this took the form of a gap subtending either 0.05° or 0.10° 6 or 0.06° to 0.93° 10, while for reading, text subtending 0.52° to 2°. The variation in spatial structure within our experimental images did not require an explicit resolution threshold to achieve the task - we intentionally selected a distortion that could be detected equally well at all eccentricities. Given that the temporal integration period increases 31 with retinal eccentricity, if subjects had of attempted to use non foveal vision to maximise resolution, we would have expected an increase in fixation duration with scotoma size. However, subjects did not spontaneously make this adaptation and this suggests that rehabilitation efforts might concentrate on training longer, more efficient fixations.
A strong relationship was found between fixation stability and scotoma size. The BCEA for normal subjects without macular disease who fixate a single, isolated target for several seconds, often within a micro-perimetry system is generally less than 1200 min arc 2 27, 32–35. The present results show comparable BCEA values for brief periods of fixation during visual search within a natural scene. Previous studies failed to find a clear relationship between scotoma size and BCEA in patients with macular disease, both longstanding 33, 36 and of recent onset 27. While a larger scotoma may be expected to force a subject fixate with more eccentric retina, this is based on the assumption of a symmetrical and foveally-centerd scotoma, as in the present study. Unlike our simulation, this is rarely, if ever the case for pathological central scotomas. A more accurate prediction of fixation impairment in pathological vision loss may be achieved by considering the minimum distance between the scotoma boundary and the former fovea, i.e. the minimum eccentricity of the scotoma boundary. We found an increase in fixation instability with scotoma size, but this reached statistical significance only for the largest scotoma. This implies a critical scotoma eccentricity, beyond which fixation becomes significantly unstable. This idea is consistent with previous research that found unstable fixation for artificial scotomas greater than 10° diameter (for comparison, a σx,y of approximately 5°), but found little reduction in fixation stability with scotomas of 5° diameter (for comparison a σx,y of approximately 2.5°) 37. It is likely that this impairment in fixation stability contributes to the impairment of visual search performance observed by reducing identification performance during periods of fixation. Although fixation instability is likely to reduce contrast sensitivity at a given eccentricity relative to an eye with stable fixation, it has been previously shown that acuity for high contrast targets is relatively unaffected unless the fixation instability moves the target to more eccentric locations 38. Crowding of features within the natural scenes is also likely to have occurred as subjects viewed eccentrically 39. This may have led to difficulty in disambiguating features when viewing with peripheral retina. Note that in previous studies, targets were presented at relatively isolated locations, surrounded by blank backgrounds of local mean luminance, thus minimising the effects of crowding, but limiting the relevance to visual search in natural scenes.
Both horizontal and vertical components of fixation became increasingly unstable with an increase in scotoma size. Despite a symmetrical central scotoma, this was more pronounced along the horizontal axis than vertical axis. Given the asymmetry between the two meridia, tasks requiring eye movements parallel to the meridian of greatest instability (eg horizontal for reading) are likely to be more detrimentally affected than tasks requiring perpendicular eye movements.
Several recent studies have reported benefits of visual search training for mobility in patients with low vision, mostly under conditions of low luminance or contrast 14, 15. The present results suggest that the main oculomotor change with the use of peripheral vision is an increase in fixation instability. It is therefore possible that visual search training in these studies aided either the stability or the duration of fixation. Measures to counteract fixation instability, either by means of meridian specific training or manipulation of stimuli along the axis of greatest fixation instability (magnification, contrast enhancement etc), may facilitate the development of viewing strategies beneficial to patients with central vision loss.
We acknowledge that a temporary simulated scotoma may only partially reproduce the effects of a chronic pathological scotoma, which cannot be simulated reliably with current technology. We speculate that for stable pathological scotomas, search efficiency and eye movement accuracy may improve with a trained PRL. However, owing to the progressive nature of central vision loss from AMD, it is likely that this performance gain may require ongoing rehabilitation. A limitation of this study is that a spatial distortion is an ideal search target only in subjects with normal vision, no ophthalmic disease and no metamorphopsia. We have devised alternative visual search tasks for ongoing work in clinical subjects with ophthalmic disease with and without metamorphopsia.40
CONCLUSIONS
This study found impairment in visual search of natural scenes in the presence of central scotomas. This impairment is primarily due to a reduction in fixation stability associated with the use of peripheral vision for fixation in the presence of a central scotoma. The results indicate a minimum scotoma extent beyond which fixation becomes significantly unstable. For absolute central scotomas the eccentricity of the scotoma boundary from the former fovea is a better predictor than scotoma size for fixation impairment. No difference was found between the oculomotor system between normal subjects and those viewing with artificial central scotomas. This result suggests that subjects intuitively conduct visual search without any attempt to maximise resolution. This failure may be particularly problematic for reading, which is limited by high spatial frequency resolution and may critically depend on PRL development. Therefore the requirement for the detection of high spatial frequency targets may facilitate PRL training and rehabilitation. While the use of a PRL may facilitate detailed tasks it remains unclear whether a non-central focus of optic flow impacts on navigation and mobility. Spatial resolution does not appear to be a factor in the present visual search task, in which targets were designed to be visible at all eccentricities. This property may be desirable for overcoming the fixation instability but it may not promote the development of a stable PRL.
Supplementary Material
Figure 6.
Absolute distance, in degrees, of final fixation locations relative to the geometric center of the spatial distortion. Each plot is a representative sample for (a) control condition for subject LM, (b) σx,y=1° for subject CV, (c) σx,y= 2° for subject SM, (d) σx,y=4° for subject MS.
Table 2.
Summary of raw search time data for the control and each of the scotoma conditions.
| Scotoma size (σx,y) | μ (±σ) raw search time (seconds) | Range (seconds) | |
|---|---|---|---|
| Min | Max | ||
| Control | 3.41 (± 1.24) | 1.46 | 5.30 |
| 1 | 4.41 (± 2.27) | 2.81 | 9.76 |
| 2 | 4.70 (± 1.77) | 1.89 | 7.31 |
| 4 | 6.84 (± 1.68) | 4.82 | 9.94 |
Acknowledgments
Supported by R01 EY 018664, R01 EY 019281 and R01 EY 018196
APPENDIX
The Appendix is available at [LWW insert link].
References
- 1.Kosnik W, Kline D, Fikre J, Sekuler R. Ocular fixation control as a function of age and exposure duration. Psychol Aging. 1987;2:302–5. doi: 10.1037//0882-7974.2.3.302. [DOI] [PubMed] [Google Scholar]
- 2.Klein R, Klein BE, Tomany SC, Meuer SM, Huang GH. Ten-year incidence and progression of age-related maculopathy: The Beaver Dam eye study. Ophthalmology. 2002;109:1767–79. doi: 10.1016/s0161-6420(02)01146-6. [DOI] [PubMed] [Google Scholar]
- 3.Burke W. Psychophysical observations concerned with a foveal lesion (macular hole) Vision Res. 1999;39:2421–7. doi: 10.1016/s0042-6989(98)00323-x. [DOI] [PubMed] [Google Scholar]
- 4.Von Noorden GK, Mackensen G. Phenomenology of eccentric fixation. Am J Ophthalmol. 1962;53:642–60. doi: 10.1016/0002-9394(62)91987-6. [DOI] [PubMed] [Google Scholar]
- 5.Whittaker SG, Cummings RW, Swieson LR. Saccade control without a fovea. Vision Res. 1991;31:2209–18. doi: 10.1016/0042-6989(91)90173-3. [DOI] [PubMed] [Google Scholar]
- 6.Bertera JH. The effect of simulated scotomas on visual search in normal subjects. Invest Ophthalmol Vis Sci. 1988;29:470–5. [PubMed] [Google Scholar]
- 7.Yarbus AL. Eye movements during the examination of complicated objects. Biofizika. 1961;6(2):52–6. [PubMed] [Google Scholar]
- 8.Crane HD, Kelly DH. Accurate simulation of visual scotomas in normal subjects. Appl Opt. 1983;22:1802. doi: 10.1364/ao.22.001802. [DOI] [PubMed] [Google Scholar]
- 9.Crane HD, Steele CM. Generation-V dual-Purkinje-image eyetracker. Appl Opt. 1985;24:527. doi: 10.1364/ao.24.000527. [DOI] [PubMed] [Google Scholar]
- 10.Cornelissen FW, Bruin KJ, Kooijman AC. The influence of artificial scotomas on eye movements during visual search. Optom Vis Sci. 2005;82:27–35. [PubMed] [Google Scholar]
- 11.Ehinger KA, Hidalgo-Sotelo B, Torralba A, Oliva A. Modeling search for people in 900 Scenes: a combined source model of eye guidance. Vis Cogn. 2009;17:945–78. doi: 10.1080/13506280902834720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bex PJ. Sensitivity to spatial distortion in natural scenes. J Vis. 2010;10:23, 1–15. doi: 10.1167/10.2.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geisler WS, Perry JS, Najemnik J. Visual search: the role of peripheral information measured using gaze-contingent displays. J Vis. 2006;6:858–73. doi: 10.1167/6.9.1. [DOI] [PubMed] [Google Scholar]
- 14.Fuhr PS, Liu L, Kuyk TK. Relationships between feature search and mobility performance in persons with severe visual impairment. Optom Vis Sci. 2007;84:393–400. doi: 10.1097/OPX.0b013e31804f5afb. [DOI] [PubMed] [Google Scholar]
- 15.Kuyk T, Liu L, Elliott J, Fuhr P. Visual search training and obstacle avoidance in adults with visual impairments. J Visual Impair Blind. 2010;104:215–27. [Google Scholar]
- 16.Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10:433–6. [PubMed] [Google Scholar]
- 17.Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis. 1997;10:437–42. [PubMed] [Google Scholar]
- 18.van Hateren JH, van der Schaaf A. Independent component filters of natural images compared with simple cells in primary visual cortex. Proc Biol Sci. 1998;265:359–66. doi: 10.1098/rspb.1998.0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nachmias J. Effect of exposure duration on visual contrast sensitivity with square-wave gratings. J Opt Soc Am. 1967;57:421–7. [Google Scholar]
- 20.Schuchard RA. Validity and interpretation of Amsler grid reports. Arch Ophthalmol. 1993;111:776–80. doi: 10.1001/archopht.1993.01090060064024. [DOI] [PubMed] [Google Scholar]
- 21.Cohen SY, Lamarque F, Saucet JC, Provent P, Langram C, LeGargasson JF. Filling-in phenomenon in patients with age-related macular degeneration: differences regarding uni-or bilaterality of central scotoma. Graefes Arch Clin Exp Ophthalmol. 2003;241:785–91. doi: 10.1007/s00417-003-0744-3. [DOI] [PubMed] [Google Scholar]
- 22.Gerrits HJ, Timmerman GJ. The filling-in process in patients with retinal scotomata. Vision Res. 1969;9:439–42. doi: 10.1016/0042-6989(69)90092-3. [DOI] [PubMed] [Google Scholar]
- 23.Sergent J. An investigation into perceptual completion in blind areas of the visual field. Brain. 1988;111(Pt. 2):347–73. doi: 10.1093/brain/111.2.347. [DOI] [PubMed] [Google Scholar]
- 24.Zur D, Ullman S. Filling-in of retinal scotomas. Vision Res. 2003;43:971–82. doi: 10.1016/s0042-6989(03)00038-5. [DOI] [PubMed] [Google Scholar]
- 25.Ramachandran VS, Gregory RL. Perceptual filling in of artificially induced scotomas in human vision. Nature. 1991;350:699–702. doi: 10.1038/350699a0. [DOI] [PubMed] [Google Scholar]
- 26.Gerrits HJ, De Haan B, Vendrik AJ. Experiments with retinal stabilized images. Relations between the observations and neural data. Vision Res. 1966;6:427–40. doi: 10.1016/0042-6989(66)90051-4. [DOI] [PubMed] [Google Scholar]
- 27.Crossland MD, Culham LE, Rubin GS. Fixation stability and reading speed in patients with newly developed macular disease. Ophthalmic Physiol Opt. 2004;24:327–33. doi: 10.1111/j.1475-1313.2004.00213.x. [DOI] [PubMed] [Google Scholar]
- 28.Bahill AT, Adler D, Stark L. Most naturally occurring human saccades have magnitudes of 15 degrees or less. Invest Ophthalmol. 1975;14:468–9. [PubMed] [Google Scholar]
- 29.Crossland MD, Kabanarou SA, Rubin GS. An unusual strategy for fixation in a patient with bilateral advanced age related macular disease. Br J Ophthalmol. 2004;88:1479–80. doi: 10.1136/bjo.2004.046573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scherlen AC, Bernard JB, Calabrese A, Castet E. Page mode reading with simulated scotomas: oculo-motor patterns. Vision Res. 2008;48:1870–8. doi: 10.1016/j.visres.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Cheong AM, Legge GE, Lawrence MG, Cheung SH, Ruff MA. Relationship between slow visual processing and reading speed in people with macular degeneration. Vision Res. 2007;47:2943–55. doi: 10.1016/j.visres.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rohrschneider K, Becker M, Kruse FE, Fendrich T, Volcker HE. Stability of fixation: results of fundus-controlled examination using the scanning laser ophthalmoscope. Ger J Ophthalmol. 1995;4:197–202. [PubMed] [Google Scholar]
- 33.Timberlake GT, Mainster MA, Peli E, Augliere RA, Essock EA, Arend LE. Reading with a macular scotoma. I. Retinal location of scotoma and fixation area. Invest Ophthalmol Vis Sci. 1986;27:1137–47. [PubMed] [Google Scholar]
- 34.Kosnik W, Fikre J, Sekuler R. Visual fixation stability in older adults. Invest Ophthalmol Vis Sci. 1986;27:1720–5. [PubMed] [Google Scholar]
- 35.Steinman RM. Effect of target size, luminance, and color on monocular xation. J Opt Soc Am. 1965;55:1158–65. [Google Scholar]
- 36.White JM, Bedell HE. The oculomotor reference in humans with bilateral macular disease. Invest Ophthalmol Vis Sci. 1990;31:1149–61. [PubMed] [Google Scholar]
- 37.Boman DK, Kertesz AE. Horizontal fusional responses to stimuli containing artificial scotomas. Invest Ophthalmol Vis Sci. 1985;26:1051–6. [PubMed] [Google Scholar]
- 38.Falkenberg HK, Rubin GS, Bex PJ. Acuity, crowding, reading and fixation stability. Vision Res. 2007;47:126–35. doi: 10.1016/j.visres.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 39.Bouma H. Interaction effects in parafoveal letter recognition. Nature. 1970;226:177–8. doi: 10.1038/226177a0. [DOI] [PubMed] [Google Scholar]
- 40.Wiecek EK, Jackson ML, Dakin S, Bex PJ. Visual search with image enhancements in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2012;53:E-Abstract 4392. doi: 10.1167/iovs.12-10012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.