Abstract
Sox2 (sex-determining region Y-Box) is one of the master transcriptional factors that are important in maintaining the pluripotency of embryonic stem cells (ESCs). In line with this function, Sox2 expression is largely restricted to ESCs and somatic stem cells. We report that Sox2 is expressed in cell lines and tumor samples derived from ALK-positive anaplastic large cell lymphoma (ALK+ALCL), for which the normal cellular counterpart is believed to be mature T-cells. The expression of Sox2 in ALK+ALCL can be attributed to nucleophosmin-anaplastic lymphoma kinase (NPM-ALK), the oncogenic fusion protein carrying a central pathogenetic role in these tumors. By confocal microscopy, Sox2 protein was detectable in virtually all cells in ALK+ALCL cell lines. However, the transcriptional activity of Sox2, as assessed using a Sox2-responsive reporter construct, was detectable only in a small proportion of cells. Importantly, downregulation of Sox2 using short interfering RNA in isolated Sox2active cells, but not Sox2inactive cells, resulted in a significant decrease in cell growth, invasiveness and tumorigenicity. To conclude, ALK+ALCL represents the first example of a hematologic malignancy that aberrantly expresses Sox2, which represents a novel mechanism by which NPM-ALK mediates tumorigenesis. We also found that the transcriptional activity and oncogenic effects of Sox2 can be heterogeneous in cancer cells.
Keywords: Sox2, transcriptional activity, NPM-ALK, STAT3, tumorigenicity
Introduction
The Sox (sex-determining region Y-Box) family of proteins includes a host of transcriptional factors that are known to have crucial roles in embryogenesis and development.1, 2 Members of the Sox family have been reported to regulate a diversity of developmental processes, including the maintenance of pluripotency of embryonic stem cells (ESCs; Sox2), testis determination (Sry), chondrogenesis (Sox5, Sox6, Sox9), as well as the development of the cardiac and lymphoid systems (Sox4), lens (Sox1 and Sox2), neural tissues and the brain (Sox1, Sox3, Sox11, Sox14, Sox21).3, 4 The biological importance of Sox2 is highlighted by the observations that Sox2 homozygous-null mouse embryos die soon after implantation,5 and mutations of the Sox2 gene have been linked to optic nerve hypoplasia and syndromic microphthalmia in humans.6 Sox2 is believed to work in concert with other ESC proteins, particularly Oct4, to maintain self-renewal and the pluripotency of ESCs.5 Similar to the other Sox family members, Sox2 binds to DNA in a highly sequence-specific manner.3 Genes that are transcriptionally regulated by Sox2 often contain a contiguous composite Sox-Oct cis-regulatory element to which Sox2 and Oct4 bind synergistically.7, 8 On the basis of results of chromatin immunoprecipitation-on-chip studies, it appears that the Sox2–Oct4 regulatory complex upregulates a large number of genes important for the maintenance of the pluripotency of ESCs and downregulates genes responsible for the initiation of differentiation.9, 10 Recent studies have implicated Sox2 in cancer biology. Sox2 has been reported to be highly expressed in a number of solid tumors, including cancers of the prostate,11 stomach,12, 13 breast,14 colorectum,15 brain16, 17 and testicles,18 and most of these reports focus on the correlation between Sox2 expression and various clinicopathological parameters. Mechanistic studies investigating the role of Sox2 in cancer cells are relatively scarce, but a few recent publications have provided evidence that Sox2 indeed contributes to tumorigenesis and invasiveness. For instance, Sox2 was found to enhance the migration and proliferation of lung cancer cell lines.19, 20 In another study, inhibition of Sox2 expression using short interfering RNA (siRNA) was shown to decrease cell proliferation and tumorigenicity in glioblastoma cell lines.16 To our knowledge, no study has specifically examined whether Sox2 is expressed in hematological malignancies. Of note, a previously published gene array study of a variety of hematological malignancies did not identify Sox2 expression.21
ALK-positive anaplastic large cell lymphoma (ALK+ALCL), a distinct type of non-Hodgkin's lymphoma of T/null-cell immunophenotype, primarily affects children and young adults, and constitutes 10–30% of all pediatric lymphomas.22 The normal cellular counterpart of ALK+ALCL is believed to be cytotoxic T cells.22 Most of these tumors carry the t(2;5)(p23;q35) cytogenetic abnormality, which places the ALK (anaplastic lymphoma kinase) gene under the regulation of the NPM (nucleophosmin) gene promoter. The resulting fusion protein (NPM-ALK) has constitutively active tyrosine kinase activity, which has been shown to be critically important for its transformation property.23 NPM-ALK is known to activate a host of cell signaling pathways, including those of Janus-family tyrosine kinase/signal transducer and activator of transcription 3 (STAT3),24, 25 Ras/extracellular signal-regulated kinase (ERK)26 and phosphoinositide kinase-3/AKT,27 so as to deregulate various cellular functions such as cell cycle progression and cell survival. Of these pathways, the STAT3 pathway is the best characterized and the NPM-ALK/STAT3 signaling axis is believed to be central to the pathogenesis of ALK+ALCL.28, 29 As STAT3 has been shown to contribute to Sox2 expression in neural precursor cells,30 we hypothesized that Sox2 expression may be induced by the NPM-ALK/STAT3 axis in ALK+ALCL cells. In this study, we demonstrate that this oncogenic signaling axis indeed promotes the expression of Sox2 in ALK+ALCL, and that Sox2 activity exerts important biological effects in this lymphoma.
Materials and methods
ALK+ALCL cell lines and patient samples
Three ALK+ALCL cell lines were used in this study (Karpas 299, SUP-M2 and UCONN-L2), and their characteristics have been described previously.31 Ntera-2, a human teratocarcinoma cell line, and Hela, a human cervical cancer cell line, were purchased from American Type Culture Collection (Manassas, VA, USA) and cultured in Dulbecco's modified Eagle's medium (Gibco, Carlsbad, CA, USA) containing 2 mℳ of ℒ-glutamine supplemented with 10% fetal bovine serum (Gibco). All ALK+ALCL tumors and non-ALK T-cell neoplasms used in this study were diagnosed at the Cross Cancer Institute, and the diagnostic criteria were based on those described in the World Health Organization Classification Scheme.22 The use of these human tissue samples has been approved by our Institutional Ethics Committee. Peripheral blood mononuclear cells were obtained from healthy individuals and isolated using Ficoll-paque (GE Healthcare Bio-Sciences Corp., Piscataway, NJ, USA). T lymphocytes were then enriched using the EasySep human T-cell enrichment kit (catalog no. 19051) from Stem Cell Technology (Vancouver, BC, Canada) following the manufacturer's recommendation. The quality of purification, as evaluated by CD3 immunostaining and flow cytometry, was >99.5%.
Antibodies and drugs
Anti-ALK was purchased from Dako (1:500, Glostrup, Denmark) and anti-Sox2 was purchased from R&D Systems Inc. (1:1000, Minneapolis, MN, USA). Anti-c-myc (1:500) and anti-β-actin (1:1000) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). All of the following antibodies were purchased from Cell Signaling (Danvers, MA, USA): anti-ERK (1:1000), anti-pERK (1:1000), anti-STAT3 (1:1000), anti-pSTAT3 (1:1000) and anti-cyclin D3 (1:1000), anti-AKT (1:1000), anti-pAKT (1:1000), anti-p38α (1:1000) and anti-p38β (1:1000). Doxorubicin was purchased from LC Laboratories (Woburn, MA, USA).
Subcellular protein fractionation, western blots and immunohistochemistry
For subcellular protein fractionation, we employed a kit purchased from Active Motif (Carlsbad, CA, USA) and followed the manufacturer's instructions. Western blots and immunohistochemistry were performed using standard techniques as described in our previous publications.25, 32, 33
Immunofluorescence and confocal microscopy
Cells were grown on cover slips coated with poly-ℒ-lysine (Sigma-Aldrich, St Louis, MO, USA) placed in a six-well plate. Before staining, cells were fixed with 3% paraformaldehyde in phosphate-buffered saline (PBS; pH 7.4). Subsequently, cells were rinsed three times with PBS, permeabilized with triton, washed again with PBS and incubated with 200 μl of anti-Sox2 antibody (1:50, R&D Systems Inc.) overnight at room temperature in a humidified chamber. After washing in PBS, cells were incubated with a secondary antibody conjugated with Alexa Fluor 488 (Invitrogen, Burlington, ON, Canada, 1:250) for 1 h at room temperature. After washing in PBS, cover slips were mounted on slides using the mounting media (Dako). Cells were visualized with a Zeiss LSM 510 confocal microscope (Oberkochen, Germany) at the Core Cell Imaging Facility, Cross Cancer Institute.
Short interfering RNA and gene transfection
To downregulate the expression of Sox2, Karpas 299 and SUP-M2, cells (5 × 106 cells in 0.5 ml of culture medium) were transfected with 100 pmol of SMARTpool-designed siRNA against Sox2. Cells transfected with scrambled siRNA (Dharmacon, Lafayette, CO, USA) were used as negative controls. To downregulate the expression of NPM-ALK, we transiently transfected ALK+ALCL cells with SMARTpool-designed siRNA specific for NPM-ALK. Cells transfected with scrambled siRNA (Dharmacon) were used as negative controls. To downregulate STAT3 expression, specific siRNA against STAT3 and scrambled siRNA were purchased from Qiagen (Mississauga, ON, Canada). All siRNA transfection experiments were performed using the BTX ECM 800 square wave electroporator (BTX, Holliston, MA, USA) at 225 V (8.0 ms pulse length, 3 pulses, 1 s between pulses).
Generation of ALK+ALCL cells stably transduced with the Sox2 reporter construct
Lentiviral particles were generated by transfecting the 293T packaging cell line with the pGreenFire1-mCMV-EF1-Puro lentiviral vector (SBI System Biosciences, Mountain View, CA, USA) or the pGreenFire1-Sox2SRR2-mCMV-EF1-Puro lentiviral vector (SBI System Biosciences). Characterization of the transcriptional response element in the Sox2 reporter (labeled as Sox2SRR2 in the vector) has been previously characterized and published.34, 35 Briefly, as illustrated in Supplementary Figure 1, the Sox2 reporter vector contains three tandem transcriptional response elements, each of which contains a Sox2 consensus binding sequence 5′-CATTGTG-3′. Cells with Sox2 transcriptional activity express the green fluorescence protein (GFP), detectable by flow cytometry. The reporter vector containing mouse cytomegalovirus without the Sox2SRRS segment served as the negative control; cells transfected with this negative control vector did not show any GFP expression detectable by flow cytometry (Supplementary Figure 2). To generate the viral particles required for the experiments, 293T cells were cultured at 37 °C, in the presence of 5% CO2, in 100 mm tissue culture dishes (Corning Life Sciences, Lowell, MA, USA) containing Dulbecco's modified Eagle's medium (Gibco), 10% fetal bovine serum (Sigma- Aldrich, Oakville, ON, Canada), 2 mℳ glutamine (Gibco) and 100 units/ml penicillin with 100 g/ml streptomycin (Gibco). Gene transfection was performed using 10 μg per dish of lentiviral vectors diluted in Opti-MEM (Gibco) and the lipofectamine 2000 reagent (Invitrogen). After 16 h, 293T cells were placed in the regular culture medium. The viral supernatant was harvested at 48 h post-transfection, centrifuged at 2000 g for 5 min and filtered through a 0.45 μm acetate filter (Millipore, Billerica, MA, USA). Two ALK+ALCL cell lines, Karpas 299 and SUP-M2, were infected with the generated viral supernatant in the presence of polybrene (8 μg/ml; Sigma-Aldrich). At 24 h post-infection, cells were washed and cultured in the presence of puromycin selection at all times (2 μg/ml). Immediately before each experiment, ALK+ALCL cells were placed in puromycin-free culture media.
Flow cytometry and cell sorting
To obtain isolated Sox2active and Sox2inactive cell subsets derived from Karpas 299 or SUP-M2 cells, cells stably transfected with the Sox2 reporter were subjected to flow cytometric cell sorting (Aria Cell Sorter, Becton Dickinson Biosciences, Franklin Lakes, NJ, USA). The purity of the resulted Sox2active and Sox2inactive cell subsets derived from Karpas 299 or SUP-M2 cells was >98%.
Assessment of cell growth
To assess if the Sox2active and Sox2inactive cell subsets have a different growth rate, cells were plated at a density of 50 000/ml, and cell count was performed using trypan blue staining (Sigma-Aldrich) and followed for 4 days. Triplicate experiments were performed. To assess if Sox2 contributes to the growth of ALK+ALCL cells, Karpas 299 and SUP-M2 cells were transfected with Sox2-specific siRNA or scrambled siRNA (negative control) as described above. Cells were then plated at a density of 20 000/ml. Cell count was done after 48 h using trypan blue staining (Sigma-Aldrich) and results are expressed as the percentage of the results obtained from the negative controls. Triplicate experiments were performed.
Cell invasiveness assay
Assessment of cell invasiveness was performed using the CytoSelect 96-well cell invasion assay, basement membrane (Cell Biolabs Inc., San Diego, CA, USA), and the procedures were carried out following the manufacturer's suggested protocol. Briefly, 1 × 105 of isolated Sox2active or Sox2inactive cells were plated onto the 96 invasive well plates. After 24 h, quantification of fluorescence signals was performed using the Fluostar Optima fluorometer (BMG Labtech, Cary, NC, USA) at excitation 480 nm/emission 520 nm. Results are expressed in RFU (relative factor unit), which is a measure of the cell number. Triplicate experiments were performed.
Methylcellulose colony formation assay
Methylcellulose-based media was purchased from R&D Systems Inc. Briefly, isolated Sox2active or Sox2inactive cells were plated into a six-well tissue culture plate at 100 or 500 cells per 1 ml of 1.2% methylcellulose, 30% fetal bovine serum, 1% bovine serum albumin, 10−4 ℳ 2-mercaptoethanol and 2 mℳℒ-glutamine. Cells were incubated for 7 days at 37 °C in the presence of 5% CO2. The number of colonies containing more than 30 cells were counted using an inverted phase-contrast microscope. Triplicate experiments were performed.
SCID mouse xenograft studies
CB-17 strain SCID mice were purchased from Taconic (Hudson, NY, USA). These animals were kept under sterile conditions. Briefly, 2 × 107 isolated Sox2active or Sox2inactive cells growing exponentially were injected into the right flank of 4-week-old male mice. These animals were killed when a tumor of >10 mm in the greatest dimension became palpable.
Statistical analysis
Data is expressed as mean±s.d. Statistical significance is determined using Student's t-test. A P-value of less than 0.05 is considered to be statistically significant.
Results
Aberrant expression of Sox2 in ALK+ALCL cell lines
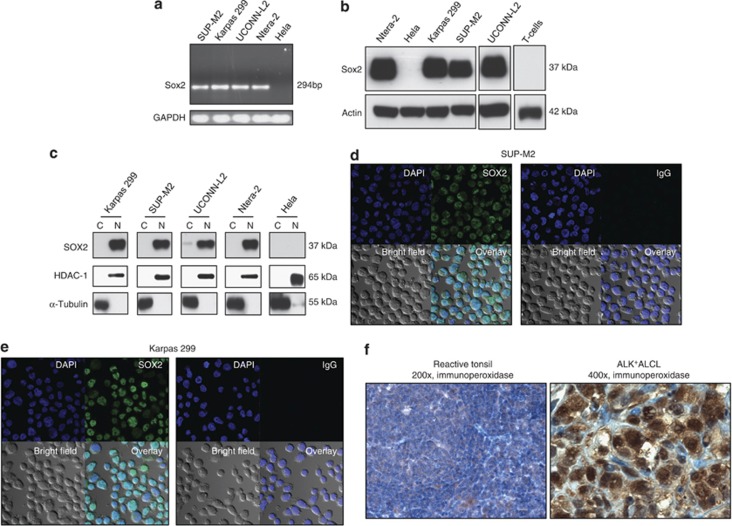
As shown in Figure 1a, all 3 ALK+ALCL cell lines examined expressed the Sox2 transcript of 294 bp detectable by reverse-transcription PCR. Using western blotting, we were able to detect the protein expression of Sox2 at 37 kDa in all three cell lines (Figure 1b). Ntera-2 (derived from teratocarcinoma) and Hela (derived from cervical carcinoma) cells were used as a positive and negative control, respectively, for Sox2 expression.36, 37 Normal peripheral blood T cells had no detectable Sox2 by western blotting (Figure 1b). Using subcellular fractionation, we found that the Sox2 protein was largely restricted to the nuclear fraction of the lysates of ALK+ALCL cells (Figure 1c), and this finding was consistent with the confocal microscopy results showing that Sox2 was localized to the nuclei of ALK+ALCL cells (Figures 1d and e). Of note, confocal microscopy showed that the Sox2 protein expression was detectable in virtually all cells in both cell lines (Figures 1d and e).
Figure 1.
Aberrant expression of Sox2 in ALK+ALCL cell lines and patient samples. (a) Reverse-transcription PCR studies demonstrated the expression of Sox2 mRNA (at 294 bp) in three ALK+ALCL cell lines. Ntera-2 (a teratocarcinoma cell line) and Hela (a cervical cancer cell line) were used as the positive and negative control, respectively. (b) The Sox2 protein in ALK+ALCL cell lines was detectable by western blots. Again, Ntera-2 and Hela cells were used as a positive and negative control, respectively. T-cells isolated from the peripheral blood of healthy donors were negative for Sox2 protein. (c) Nuclear cytoplasmic fractionation experiments showed the nuclear localization of Sox2 in ALK+ALCL cells. Ntera-2 and Hela cells were used as a positive and negative control, respectively. (d, e) Confocal immunofluorescence microscopy studies showed that virtually all Karpas 299 and SUP-M2 cells had Sox2 expression, which was localized to the nuclei. (f) Immunohistochemical staining of paraffin-embedded tissue sections revealed that ALK+ALCL tumor cells expressed Sox2, which had a predominantly nuclear staining pattern. This was in contrast with the lack of definitive Sox2 staining in benign tonsillar lymphocytes.
Nuclear expression of Sox2 is found in ALK+ALCL tumor patient cells
To show that the aberrant expression of Sox2 is not restricted to the ALK+ALCL cell lines, we assessed Sox2 expression using immunohistochemistry applied to archival paraffin-embedded patient samples. As illustrated in Figure 1f, in 8 of 10 cases, the tumor cells examined had readily detectable nuclear staining. In contrast, benign lymphocytes in reactive tonsils showed no definitive nuclear or cytoplasmic staining. Interestingly, in our survey of 10 cases of T-cell lymphomas that were ALK-negative, we found strong Sox2 nuclear staining in two of two transformed mycosis fungoides, whereas all five cases classified as peripheral T-cell lymphoma, not further classified, showed no definitive nuclear staining in the cells (Supplementary Figure 3).
Aberrant Sox2 expression in ALK+ALCL cells can be attributed to NPM-ALK and STAT3 signaling
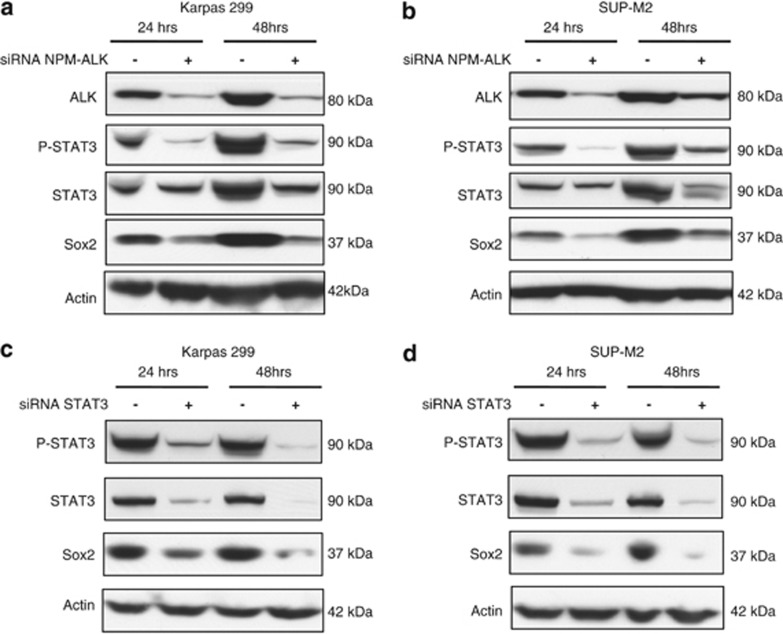
The NPM-ALK/STAT3 signaling axis is the central pathogenetic factor for ALK+ALCL. Thus, we asked if NPM-ALK and STAT3 are responsible for the aberrant expression of Sox2 in these cells. As illustrated in Figures 2a and b, siRNA-induced downregulation of NPM-ALK in Karpas 299 and SUP-M2 cells resulted in a dramatic downregulation of pSTAT3, a surrogate marker of the NPM-ALK oncogenic activity.38, 39 In the same experiment, the protein level of Sox2 was also dramatically decreased. As shown in Figures 2c and d, we found evidence that the expression of Sox2 was also dependent on STAT3, as knockdown of STAT3 using siRNA resulted in a dramatic downregulation of Sox2. To investigate if the regulation of Sox2 by the NPM-ALK/STAT3 axis occurs at the transcriptional level, we compared the mRNA levels of Sox2 before and after the knockdown of NPM-ALK or STAT3. We found that downregulation of STAT3 or NPM-ALK expression was followed by a significant decrease in the Sox2 mRNA level (Supplementary Figures 4A and B). We also asked the question as to whether the presence of NPM-ALK is sufficient to induce Sox2 expression. Thus, we transfected NPM-ALK into two ALK-negative lymphoma cell lines that do not express Sox2, namely Jurkat (a T-cell lymphoblastic lymphoma) and DG75 (a Burkitt's lymphoma cell line). Despite the expression of NPM-ALK and the activation of STAT3, no Sox2 expression was detectable (Supplementary Figures 4C and D). These results suggest that Sox2 expression in ALK+ALCL cells is cell-type-specific.
Figure 2.
Sox2 expression in ALK+ALCL can be attributed to NPM-ALK and STAT3. Downregulation of NPM-ALK using specific siRNA decreased the Sox2 protein levels in Karpas 299 (a) and SUP-M2 (b) cells. Similar effects were observed with siRNA downregulation of STAT3 in Karpas 299 (c) and SUP-M2 (d) cells. Results shown are representative of three independent experiments. Of note, cell lysates for this experiment were obtained at 24 and 48 h after the siRNA treatment, and there was no significant cell death observed within this time frame.
Sox2 is transcriptionally active in subsets of ALK+ALCL cell lines
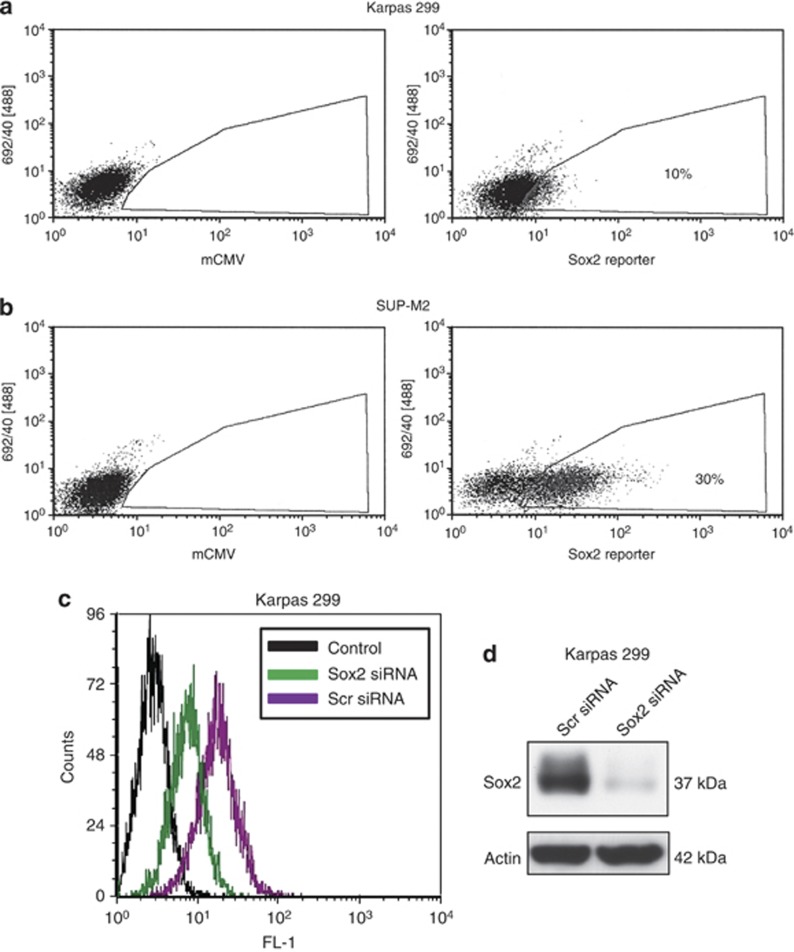
We then assessed if Sox2 is transcriptionally active in ALK+ALCL cells. Two ALK+ALCL cell lines (Karpas 299 and SUP-M2) were infected with lentiviral vectors containing either the negative control reporter construct (that is, mouse cytomegalovirus) or the Sox2 reporter construct (that is, contains three tandem Sox2 transcriptional response elements). To facilitate our studies, we generated stable cell clones for Karpas 299 and SUP-M2. Assessment of GFP expression using flow cytometry revealed that approximately 10% of the cells in Karpas 299 stably transduced with the reporter construct were GFP-positive, whereas 30% of the SUP-M2 cells stably transduced with the reporter construct were GFP-positive (Figures 3a and b). The transcriptional activity of Sox2 was further confirmed using the luciferase assay, as the Sox2 reporter construct contains the luciferase gene (Supplementary Figure 5). In support that the expression of GFP is specific for Sox2, we found that the GFP expression in isolated Sox2active cells decreased dramatically when Sox2 was downregulated using siRNA (Figures 3c and d). In long-term culture, the percentage of GFP-positive cells in isolated Sox2active cells gradually decreased, whereas no appreciable gain of GFP-positive cells was found in the long-term culture of Sox2inactive cells (Supplementary Figure 6).
Figure 3.
Sox2 is transcriptionally active in relatively small subsets of ALK+ALCL cell. The percentage of GFP-positive cells in Karpas 299 cells stably transduced with the Sox2 reporter was approximately 10% (a), whereas the percentage of GFP-positive cells in SUP-M2 cells stably transduced with the Sox2 reporter was approximately 30% (b). The results are representative of two different clones for each cell line. Results shown are representative of three independent experiments. (c) To confirm the validity of Sox2 reporter assay, Karpas 299 Sox2active cells (that is, GFP-positive) were transfected with siRNA targeting Sox2, and we found a substantial decrease in the level of green fluorescence as assessed by flow cytometry. (d) Sox2 expression was confirmed to be dramatically decreased after treatment with siRNA Sox2 by western blotting. Results shown are representative of three independent experiments.
Sox2active and Sox2inactive subsets are biologically distinct
To determine the biological significance of the transcriptional activity of Sox2 in ALK+ALCL cells, we performed a number of assays comparing the biological properties of purified Sox2active cells with those of Sox2inactive cells. Before these experiments, the purities of the two cell subsets were confirmed to be >98% as assessed by flow cytometry.
Biochemical analysis of the Sox2active and Sox2inactive cells
Our first task was to ensure that the lack of GFP expression in Sox2inactive cells was not due to the absence or loss of the Sox2 reporter in these cells. We considered this scenario to be highly unlikely, as these cells grew in the presence of the selection antibiotics. Two additional pieces of evidence also argue against this scenario. First, we re-infected the Sox2inactive cells with the lentiviral vector carrying the Sox2 reporter construct, and no GFP-expressing cells were detected (data not shown). Second, by PCR amplification of genomic DNA, we were able to detect the presence of the GFP and luciferase genes in both the Sox2active and Sox2inactive subsets (Supplementary Figure 7 and Supplementary Table 1).
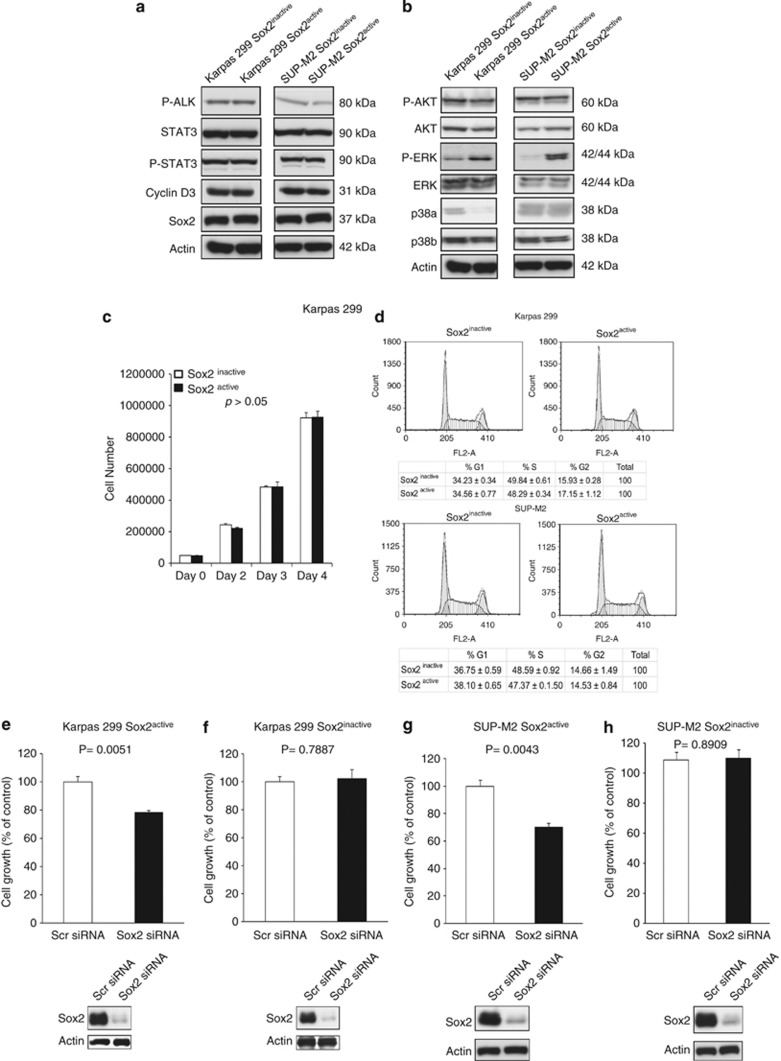
We then asked if the lack of the Sox2 activity in Sox2inactive cells is due to a lower protein expression level of Sox2 and NPM-ALK, and/or the downstream targets of NPM-ALK. By western blotting, the only consistent difference we identified was an upregulation of phospho-ERK (p-ERK) in the Sox2active cells (Figures 4a and b). However, the increased ERK signaling is not a direct result of the higher Sox2 activity in these cells, as downregulation of Sox2 with siRNA in the Sox2active subset did not result in an appreciable difference in the amount of p-ERK (data not shown). Subcellular fractionation experiments were also performed and we found no difference in the nuclear localization of Sox2 in these two cell subsets (Supplementary Figure 8). Using electrophoretic mobility shift assay (EMSA), we found no detectable difference in the probe/protein complexes formed when nuclear extracts from Sox2active or Sox2inactive nuclear extracts were incubated with a biotinylated probe based on the Sox2 reporter construct (Supplementary Figure 8E).
Figure 4.
Biochemical comparison of Sox2active and Sox2inactive cells. (a, b) By western blots, there was no substantial difference in the total protein levels and the activation/phosphorylation levels of NPM-ALK, STAT3, Akt and p38. However, a slightly higher level of phospho-ERK was found in the Sox2active cell subset. (c, d) There was no significant difference in cell growth and cell cycle analyses between the Sox2active and Sox2inactive subsets derived from Karpas 299 cells. Downregulation of Sox2 expression in ALK+ALCL cell lines using siRNA in the Sox2active cell subset resulted in a significant decrease in cell growth in both Karpas 299 (e) and SUP-M2 (g). Downregulation of Sox2 expression in the Sox2inactive cell subset using siRNA resulted in no significant change in cell growth in both Karpas 299 (f) and SUP-M2 (h). Results shown are representative of three independent experiments.
To assess if the Sox2 transcriptional activity in ALK+ALCL cells is associated with any substantial differences in the expression of potential Sox2 targets, we performed quantitative PCR and western blotting, and found that Notch1 and platelet-derived growth factor receptor-α were expressed at higher levels in Sox2active cells, whereas BCL2 was expressed at a higher level in Sox2inactive cells; these findings were confirmed by western blots (Supplementary Figure 9). Lastly, we assessed if Sox2 regulates the NPM-ALK/STAT3 signaling axis. Downregulation of Sox2 using siRNA did not result in any appreciable change to the levels of ALK, p-ALK, STAT3 and pSTAT3 (data not shown).
Cell growth
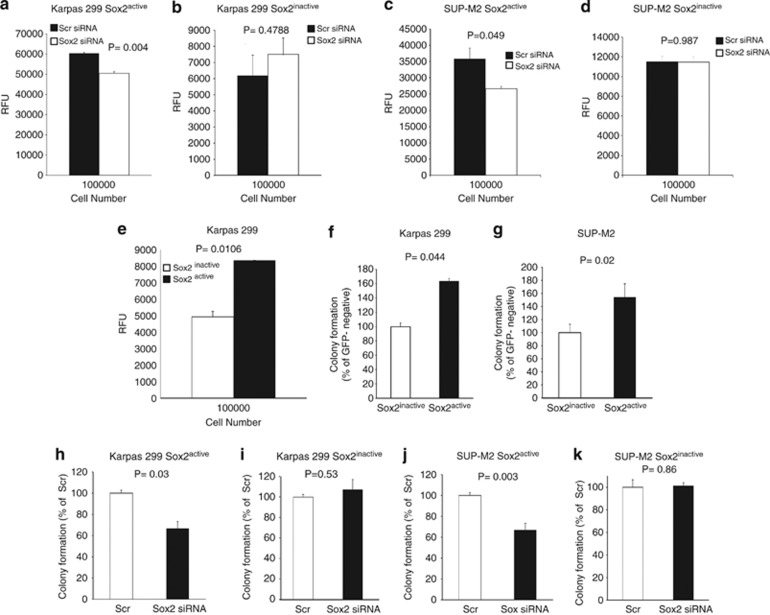
To investigate if the Sox2 transcription activity promotes cell growth in ALK+ALCL, we assessed the growth rate between the Sox2active and Sox2inactive cells. No significant difference was found for Karpas 299 (Figure 4c). We also performed cell cycle analysis and no significant differences were seen (Figure 4d). Similar results were obtained for SUP-M2. Nevertheless, siRNA knockdown of Sox2 expression in the Sox2active subset resulted in a significant decrease in cell growth in both Karpas 299 and SUP-M2, as assessed by the trypan blue cell-count assay (Figures 4e and g). No significant difference was observed for the Sox2inactive subset (Figures 4f and h).
Sox2active cells are more resistant to doxorubicin-induced apoptosis
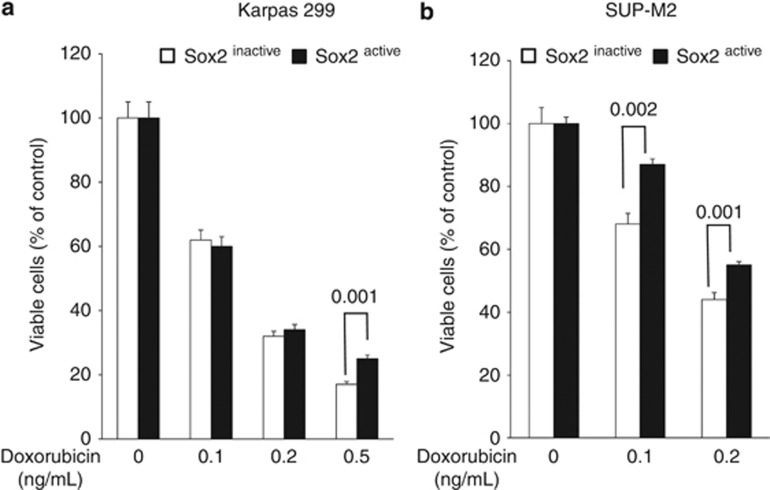
To further assess the biological significance of Sox2, we tested if Sox2 contributes to the resistance to chemotherapeutic agents. We subjected the Sox2active and Sox2inactive subsets to varying concentrations of doxorubicin. We found that Sox2active cells were significantly more resistant to doxorubicin than Sox2inactive cells in both ALK+ALCL cell lines (Figures 5a and b).
Figure 5.
Sox2 transcriptional activity correlates with the sensitivity to doxorubicin. Sox2active subset of cells in both Karpas 299 (a) and SUP-M2 (b) were more resistant to doxorubicin as compared with the Sox2inactive subset. Results shown are representative of three independent experiments.
The Sox2 transcriptional activity is associated with increased invasiveness of ALK+ALCL cells
We then assessed if the transcriptional activity of Sox2 in ALK+ALCL cells contributes to invasiveness. As illustrated in Figures 6a and c, downregulation of Sox2 expression using siRNA in the Sox2active cell subset led to a significant decrease in cell invasiveness in both Karpas 299 and SUP-M2 cells. However, no significant difference was observed when the same experiment was repeated using Sox2inactive cells (Figures 6b and d). As shown in Figure 6e, Sox2active cells were significantly more invasive than Sox2inactive cells.
Figure 6.
Sox2 transcriptional activity correlates with increased invasiveness and contributes to tumorigenicity of ALK+ALCL cell lines. Downregulation of Sox2 expression in the Sox2active subset of Karpas 299 and SUP-M2 cells led to a significant decrease in cell invasiveness (a, c). However, downregulation of Sox2 expression in the Sox2inactive subset resulted in no significant change (b, d). Furthermore, isolated Karpas 299 Sox2active cells were significantly more invasive than Sox2inactive cells (e). Results are expressed in RFU (relative factor unit), which is a measure of cells that have invaded the membrane. The Sox2active subsets of both Karpas 299 (f) and SUP-M2 (g) showed a significantly higher number of colonies in methylcellulose colony formation assay, as compared with the Sox2inactive subsets. Data is presented in such a way that the colony numbers of Sox2inactive subsets were set as 100%. Downregulation of Sox2 expression in the Sox2active subset of both Karpas 299 (h) and SUP-M2 (j) led to a significant decrease in colony formation. However, downregulation of Sox2 expression in the Sox2inactive subset resulted in no significant change in both Karpas 299 (i) and SUP-M2 (k). Results shown are representative of three independent experiments.
The Sox2 transcriptional activity increases tumorigenecity in ALK+ALCL cells
Using methylcellulose colony formation assay, we tested if Sox2active cells differ from Sox2inactive cells in promoting tumorigenecity. We found a significant difference in the number of colonies between Sox2active and Sox2inactive cells for Karpas 299 and SUP-M2 (Figures 6f and g). Downregulation of Sox2 using siRNA in the Sox2active subset led to a significant decrease in the number of colonies in both Karpas 299 and SUP-M2 cells (Figures 6h and j). However, no significant difference was observed when the same experiment was repeated using Sox2inactive cells (Figures 6i and k).
Sox2active cells are more tumorigenic than Sox2inactive cells in the SCID mouse xenograft model
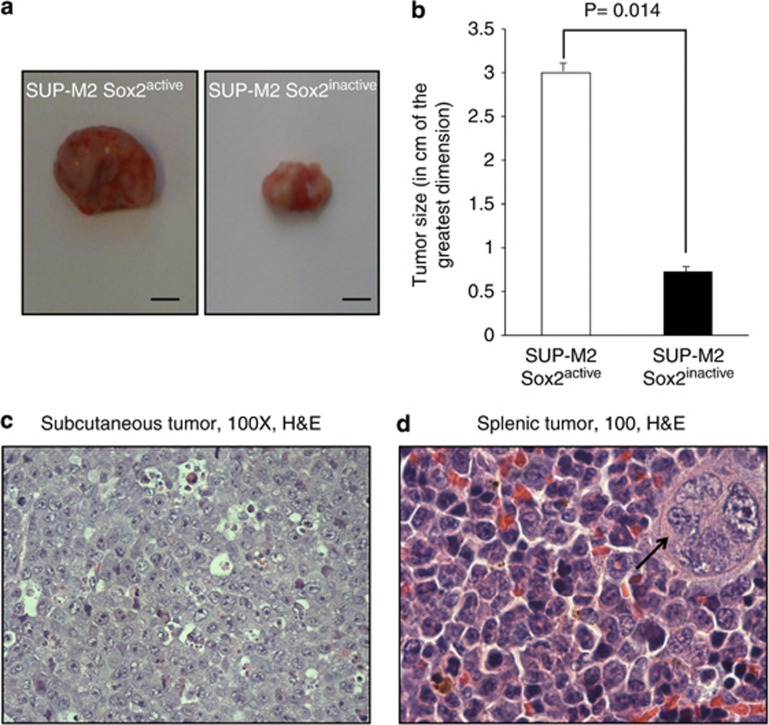
Sox2active or Sox2inactive cells derived from SUP-M2 were injected intraperitoneally in SCID mice of 4 weeks of age. Mice were killed when a sizable (estimated to be at least 1 cm in greatest dimension) tumor became palpable. Of the three SCID mice xenografted with Sox2active cells, all three developed palpable tumors on day 28. The necropsy findings were similar among the three animals; there was a large subcutaneous tumor in the abdominal region, with an average tumor size of 3.0 cm in the greatest dimension. The gross appearance of the tumor is illustrated in Figure 7a. The spleen was enlarged in all three animals xenografted with Sox2active cells and confirmed to contain ALK+ALCL tumor cells microscopically. Of the three SCID mice xenografted with Sox2inactive cells, two developed palpable tumors on day 35. On necropsy, both of these two animals were found to have a subcutaneous tumor in the abdominal region measuring 1.0 cm in the greatest dimension. The remaining animal xenografted with Sox2inactive cells was found on necropsy (day 35) to have a minute tumor in the abdominal subcutaneous region measuring up to 0.2 cm in the greatest dimension. Overall, the difference in the tumor size between the two groups is statistically significant (P=0.014, Student t-test) (Figure 7b). Histologically, tumor cells from all xenografts appeared to be similar (illustrated in Figures 7c and d).
Figure 7.
Sox2 transcriptional activity correlates with tumorogenicity in the SCID xenograft mouse model. Xenografts derived from the Sox2active subset of SUP-M2 cells were significantly larger than those derived from the Sox2inactive subset (a, b). A hematoxylin and eosin section of the subcutaneous tumor harvested from an animal xenografted with Sox2active cells reveals sheets of anaplastic lymphoma cells (c). A high magnification of the splenic tumor from the same animal is illustrated; a megakaryocyte is highlighted by a black arrow (d).
Discussion
In this study, we have shown that Sox2 expression is a consistent feature of ALK+ALCL, being detectable in all three cell lines and most of the primary tumors tested. To our knowledge, ALK+ALCL represents the first example of a hematological malignancy that manifests this feature. In contrast to ALK+ALCL cells, we did not find Sox2 expression in benign lymphocytes, including those present in peripheral blood and tonsils. Corroborating our findings, a recent study performed by Cimpean et al.40 also found no evidence of Sox2 expression in normal lymphocytes. Nevertheless, it has been reported that Sox2 is expressed in CD34+ hematopoietic stem cells.41, 42 Taken together, these observations suggest that the expression of Sox2 in ALK+ALCL cells represents an aberrant event. Of note, our immunohistochemical studies demonstrate that Sox2 expression is not limited to ALK+ALCL in the spectrum of hematological malignancies, as cases of transformed mycosis fungoides and one case of T-cell lymphoma arising in the setting of post-transplantation manifested this phenotype. Further studies are required to confirm whether Sox2 in these T-cell neoplasms without ALK expression is indeed transcriptionally active.
The link between Sox2 and STAT3 has been previously suggested in a study of neural precursor cells.30 Sox2 expression was shown to be upregulated by STAT3, and the promoter region of the Sox2 gene was found to carry multiple STAT3-binding consensus sequences.30 As one of the key characteristics of ALK+ALCL cells is the constitutive STAT3 activation,23 we hypothesized that the aberrant expression of Sox2 in this cell type is attributed to STAT3. Our data supports this assertion. As the oncogenic tyrosine kinase NPM-ALK is known to be the major activator of STAT3 in ALK+ALCL, it is not surprising to observe that siRNA downregulation of NPM-ALK also substantially decreased Sox2 expression. In other words, the NPM-ALK/STAT3 signaling axis, which is considered to be the key oncogenic driving force in ALK+ALCL, is primarily responsible for the aberrant expression of Sox2 in this cancer cell type.
We were rather surprised with the finding that the transcriptional activity of Sox2 is heterogeneous in ALK+ALCL cell lines, as Sox2 protein was expressed in virtually all cells as evidenced by our confocal microscopy experiments. To our knowledge, the observation that the transcriptional activity of Sox2 is heterogeneous in cancer cells has never been previously described. The validity of this novel finding is supported by the following observations. First, ALK+ALCL cell lines stably transduced with the Sox2 reporter construct were cultured in the presence of puromycin selection at all times; thus, it is highly unlikely that the lack of the Sox2 activity in the Sox2inactive subset is due to a loss of the reporter construct. Second, to confirm that the Sox2inactive cells were truly Sox2 inactive, we re-infected these cells with the Sox2 reporter construct, and no increase in GFP expression in these cells was found. Third, using PCR, we were able to detect the presence of the GFP and luciferase genes in the Sox2inactive cells, and thus, the absence of GFP and luciferase expression in these cells is not due to a loss of the reporter. Fourth, the transcriptional activity of Sox2 is associated with different biological characteristics, including cell growth, invasiveness and tumorigenesis. On the basis of our finding that Sox2 transcriptional activity, rather than protein expression, dictates its oncogenic potential, we suggest that future studies investigating Sox2 in cancer should include the transcriptional activity of Sox2 as a major parameter.
An obvious question emerging from our observations is related to how the Sox2 transcriptional activity is regulated. As mentioned above, the differential activity of Sox2 is not due to a difference in Sox2 protein expression, as our confocal microscopy results showed that virtually all cells express this protein. Recently, it was demonstrated that the nuclear localization of Sox2 is regulated by phosphorylation through the AKT signaling.43 Thus, we speculated that the activity of Sox2 is dependent on whether Sox2 is localized to the nuclei where it functions as a transcriptional factor. However, we did not observe any difference in the subcellular localization of Sox2 between the Sox2active and Sox2inactive subsets. In another recent study, Van Hoof et al.44 identified that serine phosphorylation of Sox2 can modulate its activity. Again, we did not observe any difference in the serine phosphorylation of Sox2 between the two subsets (Supplementary Figure 10). Lastly, based on our EMSA results, we observed no obvious difference in the formation of the probe/protein complexes between the two cell subsets. Considering the fact that the transcriptional activity of Sox2 is tightly regulated by a number of co-factors,4, 45 it is likely that one or more of these cofactors are important in regulating the transcriptional activity of Sox2 in ALK+ALCL cells. In this regard, we investigated the expression of Oct4a, the most studied Sox2 cofactor.5 However, by western blotting, we did not detect any Oct4a expression in ALK+ALCL cell lines (data not shown). Overall, further studies are needed to delineate how the transcriptional activity of Sox2 is regulated in cancer cells.
In this study, we found that the transcriptional activity of Sox2 correlated with sensitivity to doxorubicin, a conventional chemotherapeutic agent used to treat ALK+ALCL in the clinic. Interestingly, Mallanna et al.46 have previously reported that Sox2 interacts with DNA repair proteins, such as members of the replication protein A family. Thus, it is possible that the mechanisms that regulate the transcriptional activity of Sox2 may also regulate how Sox2 interacts with DNA repair proteins, thereby modulating the efficiency of DNA repair. Alternatively, some of the downstream targets of Sox2 might be responsible for DNA repair; thus, upregulation of these genes in the Sox2active subset contributes to enhanced DNA repair and a resistance to doxorubicin.
We have examined the possible mechanisms by which Sox2 exerts its oncogenic effects. We first asked if Sox2 regulates the expression and activation of the NPM-ALK/STAT3 axis. As described, siRNA knockdown of Sox2 in Sox2active cells did not result in any detectable change to the levels of NPM-ALK, pALK, STAT3 and pSTAT3. The other approach used was to compare the expression and activation status of NPM-ALK and its downstream targets between the Sox2active and Sox2inactive cells. A comparison of these two cell subsets showed no substantial difference in the expression/activation status of NPM-ALK, STAT3 and a host of other cellular signaling proteins known to be activated by NPM-ALK. The only exception was p-ERK, which was expressed at a slightly higher level in Sox2active subset. As described in the results, this increase in p-ERK is not apparently a direct result of Sox2, as siRNA downregulation of Sox2 in the Sox2active subset did not result in any appreciable change in p-ERK. We also asked if the Sox2 downstream targets in ESCs are differentially expressed between the two cell subsets. As shown in Supplementary Figure 9, we confirmed the differential expression of the three known Sox2 downstream targets, including notch1, BCL2 and platelet-derived growth factor receptor-α.47, 48 These findings further support our findings of two cell subsets in ALK+ALCL cells based on their differential Sox2 transcriptional activity. Of interest, both notch1 and platelet-derived growth factor receptor-α have been previously implicated in the pathobiology of ALK+ALCL.49, 50
To conclude, we have demonstrated that ALK+ALCL aberrantly expresses Sox2, one of the master transcriptional factors in ESCs. We have also shown that Sox2 protein expression is dependent on NPM-ALK/STAT3 activation. Interestingly, we have observed that the transcriptional activity of Sox2 is restricted to a relatively small subset of cells, despite the fact that Sox2 protein is expressed in all cells. In view of the fact that the transcriptional activity of Sox2 is critical in mediating tumorigenesis, we believe that further studies investigating how Sox2 activity is regulated will be highly worthwhile.
Acknowledgments
SH is a graduate student supported by an Egyptian governmental doctoral scholarship and the Queen Elisabeth II graduate scholarship administered by the University of Alberta. KB is a recipient of the Izaak Walton Killam Memorial Scholarship (University of Alberta). This study is supported by operating research grants from the Canadian Institute of Health Research, Alberta Cancer Foundation and the Canadian Cancer Society Research Institute awarded to RL. JDP is a recipient of an Alberta Cancer Foundation studentship.
Author contributions
PG, SH, YM and RL designed the experiments. PG, SH, PW, KB, JDP and MA performed the experimental procedures. PW, DS and MH generated the stable ALK+ALCL cell clones. PG, SH, RJI and RL analyzed the data. SH and RL reviewed the immunohistochemical study results. PG, SH and RL wrote the manuscript.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Blood Cancer Journal website (http://www.nature.com/bcj)
Supplementary Material
References
- Rizzino A. Sox2 and Oct-3/4: a versatile pair of master regulators that orchestrate the self-renewal and pluripotency of embryonic stem cells. Wiley Interdiscip Rev Syst Biol Med. 2009;1:228–236. doi: 10.1002/wsbm.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keramari M, Razavi J, Ingman KA, Patsch C, Edenhofer F, Ward CM, et al. Sox2 is essential for formation of trophectoderm in the preimplantation embryo. PLoS One. 2010;5:e13952. doi: 10.1371/journal.pone.0013952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles J, Schepers G, Koopman P. Phylogeny of the SOX family of developmental transcription factors based on sequence and structural indicators. Dev Biol. 2000;227:239–255. doi: 10.1006/dbio.2000.9883. [DOI] [PubMed] [Google Scholar]
- Wegner M. From head to toes: the multiple facets of Sox proteins. Nucleic Acids Res. 1999;27:1409–1420. doi: 10.1093/nar/27.6.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avilion AA, Nicolis SK, Pevny LH, Perez L, Vivian N, Lovell-Badge R. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 2003;17:126–140. doi: 10.1101/gad.224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Bardakjian T, Reis LM, Tyler RC, Semina EV. Novel SOX2 mutations and genotype-phenotype correlation in anophthalmia and microphthalmia. Am J Med Genet A. 2009;149A:2706–2715. doi: 10.1002/ajmg.a.33098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda T, Tada M, Kubota H, Kimura H, Hatano SY, Suemori H, et al. Octamer and Sox elements are required for transcriptional cis regulation of Nanog gene expression. Mol Cell Biol. 2005;25:2475–2485. doi: 10.1128/MCB.25.6.2475-2485.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew JL, Loh YH, Zhang W, Chen X, Tam WL, Yeap LS, et al. Reciprocal transcriptional regulation of Pou5f1 and Sox2 via the Oct4/Sox2 complex in embryonic stem cells. Mol Cell Biol. 2005;25:6031–6046. doi: 10.1128/MCB.25.14.6031-6046.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura-Nakanishi S, Saito M, Niwa H, Ishikawa F. Oct-3/4 and Sox2 regulate Oct-3/4 gene in embryonic stem cells. J Biol Chem. 2005;280:5307–5317. doi: 10.1074/jbc.M410015200. [DOI] [PubMed] [Google Scholar]
- Sun Y, Li H, Yang H, Rao MS, Zhan M. Mechanisms controlling embryonic stem cell self-renewal and differentiation. Crit Rev Eukaryot Gene Expr. 2006;16:211–231. doi: 10.1615/critreveukargeneexpr.v16.i3.20. [DOI] [PubMed] [Google Scholar]
- Sattler HP, Lensch R, Rohde V, Zimmer E, Meese E, Bonkhoff H, et al. Novel amplification unit at chromosome 3q25-q27 in human prostate cancer. Prostate. 2000;45:207–215. doi: 10.1002/1097-0045(20001101)45:3<207::aid-pros2>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Li XL, Eishi Y, Bai YQ, Sakai H, Akiyama Y, Tani M, et al. Expression of the SRY-related HMG box protein SOX2 in human gastric carcinoma. Int J Oncol. 2004;24:257–263. [PubMed] [Google Scholar]
- Tsukamoto T, Mizoshita T, Tatematsu M. Gastric-and-intestinal mixed-type intestinal metaplasia: aberrant expression of transcription factors and stem cell intestinalization. Gastric Cancer. 2006;9:156–166. doi: 10.1007/s10120-006-0375-6. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Pinilla SM, Sarrio D, Moreno-Bueno G, Rodriguez-Gil Y, Martinez MA, Hernandez L, et al. Sox2: a possible driver of the basal-like phenotype in sporadic breast cancer. Mod Pathol. 2007;20:474–481. doi: 10.1038/modpathol.3800760. [DOI] [PubMed] [Google Scholar]
- Park ET, Gum JR, Kakar S, Kwon SW, Deng G, Kim YS. Aberrant expression of SOX2 upregulates MUC5AC gastric foveolar mucin in mucinous cancers of the colorectum and related lesions. Int J Cancer. 2008;122:1253–1260. doi: 10.1002/ijc.23225. [DOI] [PubMed] [Google Scholar]
- Gangemi RM, Griffero F, Marubbi D, Perera M, Capra MC, Malatesta P, et al. SOX2 silencing in glioblastoma tumor-initiating cells causes stop of proliferation and loss of tumorigenicity. Stem Cells. 2009;27:40–48. doi: 10.1634/stemcells.2008-0493. [DOI] [PubMed] [Google Scholar]
- Phi JH, Park SH, Kim SK, Paek SH, Kim JH, Lee YJ, et al. Sox2 expression in brain tumors: a reflection of the neuroglial differentiation pathway. Am J Surg Pathol. 2008;32:103–112. doi: 10.1097/PAS.0b013e31812f6ba6. [DOI] [PubMed] [Google Scholar]
- Nonaka D. Differential expression of SOX2 and SOX17 in testicular germ cell tumors. Am J Clin Pathol. 2009;131:731–736. doi: 10.1309/AJCP7MNCNBCRN8NO. [DOI] [PubMed] [Google Scholar]
- Hussenet T, Dali S, Exinger J, Monga B, Jost B, Dembele D, et al. SOX2 is an oncogene activated by recurrent 3q26.3 amplifications in human lung squamous cell carcinomas. PLoS One. 2010;5:e8960. doi: 10.1371/journal.pone.0008960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Futtner C, Rock JR, Xu X, Whitworth W, Hogan BL, et al. Evidence that SOX2 overexpression is oncogenic in the lung. PLoS One. 2010;5:e11022. doi: 10.1371/journal.pone.0011022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenhals M, Kassambara A, De Vos J, Hose D, Moreaux J, Klein B. Embryonic stem cell markers expression in cancers. Biochem Biophys Res Commun. 2009;383:157–162. doi: 10.1016/j.bbrc.2009.02.156. [DOI] [PubMed] [Google Scholar]
- Delsol GFB, Muller-Hermelink HK, Campo E, Jaffe ES, Gascoyne RD, Stein H, et al. Anaplastic Large Cell Lymphoma (ALCL), ALK-Positive. Vol. 2. IARC: Lyon; 2008. [Google Scholar]
- Amin HM, Lai R. Pathobiology of ALK+ anaplastic large-cell lymphoma. Blood. 2007;110:2259–2267. doi: 10.1182/blood-2007-04-060715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamo A, Chiarle R, Piva R, Howes J, Fan Y, Chilosi M, et al. Anaplastic lymphoma kinase (ALK) activates Stat3 and protects hematopoietic cells from cell death. Oncogene. 2002;21:1038–1047. doi: 10.1038/sj.onc.1205152. [DOI] [PubMed] [Google Scholar]
- Dien Bard J, Gelebart P, Anand M, Zak Z, Hegazy SA, Amin HM, et al. IL-21 contributes to JAK3/STAT3 activation and promotes cell growth in ALK-positive anaplastic large cell lymphoma. Am J Pathol. 2009;175:825–834. doi: 10.2353/ajpath.2009.080982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner SD, Yeung D, Hadfield K, Cook SJ, Alexander DR. The NPM-ALK tyrosine kinase mimics TCR signalling pathways, inducing NFAT and AP-1 by RAS-dependent mechanisms. Cell Signal. 2007;19:740–747. doi: 10.1016/j.cellsig.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Bai RY, Ouyang T, Miething C, Morris SW, Peschel C, Duyster J. Nucleophosmin-anaplastic lymphoma kinase associated with anaplastic large-cell lymphoma activates the phosphatidylinositol 3-kinase/Akt antiapoptotic signaling pathway. Blood. 2000;96:4319–4327. [PubMed] [Google Scholar]
- Amin HM, McDonnell TJ, Ma Y, Lin Q, Fujio Y, Kunisada K, et al. Selective inhibition of STAT3 induces apoptosis and G(1) cell cycle arrest in ALK-positive anaplastic large cell lymphoma. Oncogene. 2004;23:5426–5434. doi: 10.1038/sj.onc.1207703. [DOI] [PubMed] [Google Scholar]
- Chiarle R, Simmons WJ, Cai H, Dhall G, Zamo A, Raz R, et al. Stat3 is required for ALK-mediated lymphomagenesis and provides a possible therapeutic target. Nat Med. 2005;11:623–629. doi: 10.1038/nm1249. [DOI] [PubMed] [Google Scholar]
- Foshay KM, Gallicano GI. Regulation of Sox2 by STAT3 initiates commitment to the neural precursor cell fate. Stem Cells Dev. 2008;17:269–278. doi: 10.1089/scd.2007.0098. [DOI] [PubMed] [Google Scholar]
- Pulford K, Lamant L, Morris SW, Butler LH, Wood KM, Stroud D, et al. Detection of anaplastic lymphoma kinase (ALK) and nucleolar protein nucleophosmin (NPM)-ALK proteins in normal and neoplastic cells with the monoclonal antibody ALK1. Blood. 1997;89:1394–1404. [PubMed] [Google Scholar]
- Anand M, Lai R, Gelebart P. Beta-catenin is constitutively active and increases STAT3 expression/activation in anaplastic lymphoma kinase-positive anaplastic large cell lymphoma. Haematologica. 2011;96:253–261. doi: 10.3324/haematol.2010.027086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelebart P, Anand M, Armanious H, Peters AC, Dien Bard J, Amin HM, et al. Constitutive activation of the Wnt canonical pathway in mantle cell lymphoma. Blood. 2008;112:5171–5179. doi: 10.1182/blood-2008-02-139212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagi S, Saito T, Mizutani K, Masuyama N, Gotoh Y, Iwama A, et al. The Sox-2 regulatory regions display their activities in two distinct types of multipotent stem cells. Mol Cell Biol. 2004;24:4207–4220. doi: 10.1128/MCB.24.10.4207-4220.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomioka M, Nishimoto M, Miyagi S, Katayanagi T, Fukui N, Niwa H, et al. Identification of Sox-2 regulatory region which is under the control of Oct-3/4-Sox-2 complex. Nucleic Acids Res. 2002;30:3202–3213. doi: 10.1093/nar/gkf435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevanovic M. Modulation of SOX2 and SOX3 gene expression during differentiation of human neuronal precursor cell line NTERA2. Mol Biol Rep. 2003;30:127–132. doi: 10.1023/a:1023961009869. [DOI] [PubMed] [Google Scholar]
- Ji J, Zheng PS. Expression of Sox2 in human cervical carcinogenesis. Hum Pathol. 2010;41:1438–1447. doi: 10.1016/j.humpath.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Raghunath PN, Xue L, Majewski M, Carpentieri DF, Odum N, et al. Multilevel dysregulation of STAT3 activation in anaplastic lymphoma kinase-positive T/null-cell lymphoma. J Immunol. 2002;168:466–474. doi: 10.4049/jimmunol.168.1.466. [DOI] [PubMed] [Google Scholar]
- Han Y, Amin HM, Franko B, Frantz C, Shi X, Lai R. Loss of SHP1 enhances JAK3/STAT3 signaling and decreases proteosome degradation of JAK3 and NPM-ALK in ALK+ anaplastic large-cell lymphoma. Blood. 2006;108:2796–2803. doi: 10.1182/blood-2006-04-017434. [DOI] [PubMed] [Google Scholar]
- Cimpean AM, Encica S, Raica M, Ribatti D. SOX2 gene expression in normal human thymus and thymoma. Clin Exp Med. 2011;11:251–254. doi: 10.1007/s10238-010-0127-0. [DOI] [PubMed] [Google Scholar]
- Loh YH, Agarwal S, Park IH, Urbach A, Huo H, Heffner GC, et al. Generation of induced pluripotent stem cells from human blood. Blood. 2009;113:5476–5479. doi: 10.1182/blood-2009-02-204800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eminli S, Foudi A, Stadtfeld M, Maherali N, Ahfeldt T, Mostoslavsky G, et al. Differentiation stage determines potential of hematopoietic cells for reprogramming into induced pluripotent stem cells. Nat Genet. 2009;41:968–976. doi: 10.1038/ng.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong CH, Cho YY, Kim MO, Kim SH, Cho EJ, Lee SY, et al. Phosphorylation of Sox2 cooperates in reprogramming to pluripotent stem cells. Stem Cells. 2010;28:2141–2150. doi: 10.1002/stem.540. [DOI] [PubMed] [Google Scholar]
- Van Hoof D, Munoz J, Braam SR, Pinkse MW, Linding R, Heck AJ, et al. Phosphorylation dynamics during early differentiation of human embryonic stem cells. Cell Stem Cell. 2009;5:214–226. doi: 10.1016/j.stem.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Kamachi Y, Uchikawa M, Kondoh H. Pairing SOX off: with partners in the regulation of embryonic development. Trends Genet. 2000;16:182–187. doi: 10.1016/s0168-9525(99)01955-1. [DOI] [PubMed] [Google Scholar]
- Mallanna SK, Ormsbee BD, Iacovino M, Gilmore JM, Cox JL, Kyba M, et al. Proteomic analysis of Sox2-associated proteins during early stages of mouse embryonic stem cell differentiation identifies Sox21 as a novel regulator of stem cell fate. Stem Cells. 2010;28:1715–1727. doi: 10.1002/stem.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Yoon JG, Li L, Yu W, Shao J, Hua D, et al. The SOX2 response program in glioblastoma multiforme: an integrated ChIP-seq, expression microarray, and microRNA analysis. BMC Genomics. 2011;12:11. doi: 10.1186/1471-2164-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharov AA, Masui S, Sharova LV, Piao Y, Aiba K, Matoba R, et al. Identification of Pou5f1, Sox2, and Nanog downstream target genes with statistical confidence by applying a novel algorithm to time course microarray and genome-wide chromatin immunoprecipitation data. BMC Genomics. 2008;9:269. doi: 10.1186/1471-2164-9-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YP, Chang KC, Su WC, Chen TY. The expression and prognostic significance of platelet-derived growth factor receptor alpha in mature T- and natural killer-cell lymphomas. Ann Hematol. 2008;87:985–990. doi: 10.1007/s00277-008-0539-z. [DOI] [PubMed] [Google Scholar]
- Jundt F, Anagnostopoulos I, Forster R, Mathas S, Stein H, Dorken B. Activated Notch1 signaling promotes tumor cell proliferation and survival in Hodgkin and anaplastic large cell lymphoma. Blood. 2002;99:3398–3403. doi: 10.1182/blood.v99.9.3398. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.