Abstract
Aberrantly expressed tyrosine kinases have emerged as promising targets for drug development in acute myeloid leukemia (AML). We report that AKN-028, a novel tyrosine kinase inhibitor (TKI), is a potent FMS-like receptor tyrosine kinase 3 (FLT3) inhibitor (IC50=6 nℳ), causing dose-dependent inhibition of FLT3 autophosphorylation. Inhibition of KIT autophosphorylation was shown in a human megakaryoblastic leukemia cell line overexpressing KIT. In a panel of 17 cell lines, AKN-028 showed cytotoxic activity in all five AML cell lines included. AKN-028 triggered apoptosis in MV4-11 by activation of caspase 3. In primary AML samples (n=15), AKN-028 induced a clear dose-dependent cytotoxic response (mean IC50 1 μℳ). However, no correlation between antileukemic activity and FLT3 mutation status, or to the quantitative expression of FLT3, was observed. Combination studies showed synergistic activity when cytarabine or daunorubicin was added simultaneously or 24 h before AKN-028. In mice, AKN-028 demonstrated high oral bioavailability and antileukemic effect in primary AML and MV4-11 cells, with no major toxicity observed in the experiment. In conclusion, AKN-028 is a novel TKI with significant preclinical antileukemic activity in AML. Possible sequence-dependent synergy with standard AML drugs and good oral bioavailability has made it a candidate drug for clinical trials (ongoing).
Keywords: acute myeloid leukemia, drug development, tyrosine kinase inhibitor, signal transduction, FLT3
Introduction
Despite recent progress in understanding the pathogenesis and molecular genetics of acute myeloid leukemia (AML), the prognosis for most patients is still dismal. Following the standard treatment with intense combination chemotherapy, 50–80% of adult patients obtain remission, but the majority ultimately relapses and only 20–30% are cured.1 Patients with high age, secondary AML or unfavorable cytogenetics, as well as those unfit for intense chemotherapy, rarely obtain long-standing remission,2 further underscoring the need for new, improved therapies.
In hematopoietic cells, genetic changes involving protein tyrosine kinases leading to deregulation of intracellular signaling pathways, have been linked to leukemogenesis as well as to progression of the leukemic disease.3 Activation of the FMS-like receptor tyrosine kinase 3 (FLT3), expressed on early multipotent hematopoietic cells, promotes cell survival and proliferation by interacting with multiple downstream targets including the AKT, STAT and MAP-kinase pathways.4, 5 In the majority of patients with AML, FLT3 is overexpressed.6, 7 Activating FLT3 mutations occur in up to 30% of patients, out of which three-fourth consists of FLT3-internal tandem duplications (FLT3-ITD) located in the juxtamembrane domain, and approximately one-fourth of point mutations in the FLT3-tyrosine kinase domain (FLT3-TKD),8 the former being associated with increased risk of relapse and poor overall survival.9, 10, 11 Moreover, a high frequency of mutations in the tyrosine kinase KIT has been reported in core binding factor AML, with an adverse impact on prognosis.12, 13
Thus, aberrantly expressed receptor tyrosine kinases have emerged as promising targets for drug development in AML, as well as in other hematological malignancies. During the last decade, several FLT3-inhibitors, ranging from relatively FLT3-selective to broad multikinase inhibitors, have been introduced and subsequently tested in clinical trials in patients with AML, either as single agents or in combination with chemotherapy.14, 15, 16, 17 So far, only a minority of patients, mainly those with FLT3-mutated leukemia, have shown some degree of clinical response although most often of limited duration.18 Notably, some FLT3-ITD patients do not respond to FLT3 inhibition treatment, despite almost complete inhibition of FLT3 autophosphorylation.19
We have previously shown that 2-aminopyrazine tyrosine kinase inhibitors (TKIs) can induce significant in vitro activity in AML, seemingly irrespective of FLT3 mutation status.20 We now present a novel compound from this group, AKN-028, which has been investigated with respect to kinase inhibition profile, pharmacokinetics and cytotoxic activity in cell lines, primary tumor cells and the hollow-fiber mouse model. In addition, we have studied the antileukemic activity of AKN-028 in combination with cytarabine or daunorubicin, as well as the importance of FLT3 mutation-status and quantitative FLT3 expression for the cytotoxic response.
Materials and methods
Reagents
AKN-028 (N-3-(1H-indol-5-yl)-5-pyridin-4-yl-pyrazine-2,3-diamine, for molecular structure, see Figure 1a), multikinase inhibitor sunitinib, kindly provided by Biovitrum AB (Stockholm, Sweden) and Akinion Pharmaceuticals (Stockholm, Sweden), and selective FLT3 inhibitor AC220 (provided by Fredrik Lehmann) were stored at −70 °C, dissolved as a 10-mℳ stock in dimethylsulphoxide and diluted with culture medium (Sigma-Aldrich Co, St Louis, MO, USA), as needed. Etoposide, daunorubicin and cytarabine were purchased from Apoteket AB (Stockholm, Sweden), and staurosporine was provided by ProQinase GmbH (Freiburg, Germany).
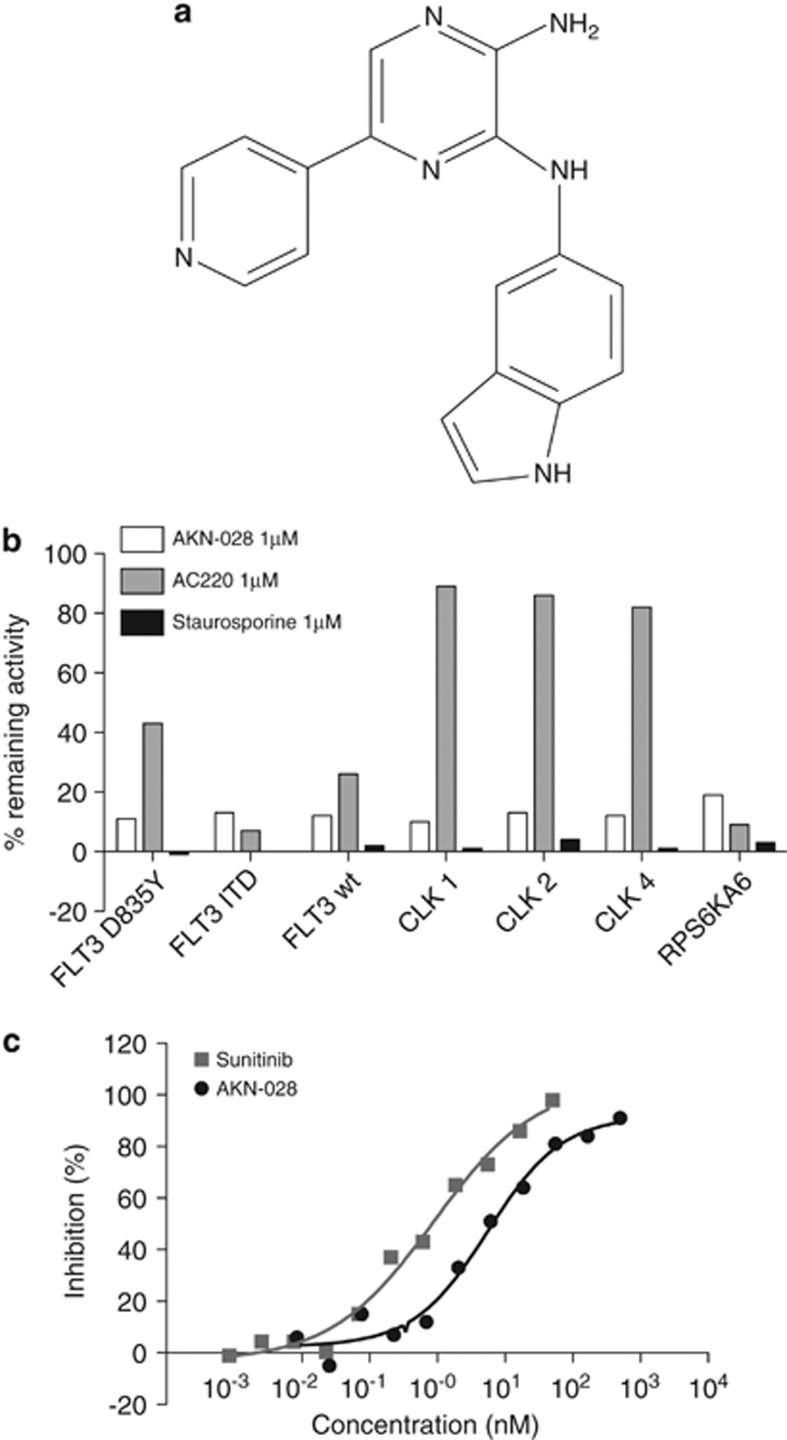
Figure 1.
(a–c) Molecular structure of AKN-028 (a). Inhibition of FLT3, CLK and RPS6 kinases in the kinase-panel screen after exposure to 1 μℳ of AKN-028, AC220 and staurosporine (b). Figure 1c displays the inhibitory activity on FLT3 enzyme of AKN-028 and sunitinib outlined as dose–response curves from a typical experiment in the FLT3 enzyme-inhibition assay.
Cell lines and primary patient samples
AKN-028 was tested against a cell line panel, described in detail previously.20 The panel was expanded to a total of 17 cell lines (Supplementary I), whereof five AML cell lines: MV4-11 (naturally occurring FLT3 ITD mutation),21 Kasumi-1 (t(8;21), activating KIT mutation),22, 23 HL-60 (capability to differentiate),24 KG1a (high content of immature CD34-expressing cells)25 (obtained from American Type Culture Collection; ATCC, Rockville, MD, USA) and MOLM-13 (heterozygote FLT3-ITD mutation, provided by ProQinase).26 Cells were kept in culture medium appropriate to cell type supplemented with fetal calf serum, glutamine and antibiotics (Sigma-Aldrich Co). Mouse embryonal fibroblasts transfected to overexpress FLT3 wild type (FLT3-wt), D835Y point-mutated FLT3 (FLT3-TKD) or FLT3-ITD, as well as human acute megakaryoblastic leukemia M07 cells overexpressing KIT, were used to assess inhibition of FLT3 or KIT autophosphorylation (cells provided by ProQinase).
The cytotoxic effect of AKN-028 was evaluated in an initial screen in tumor cells from adult patients with different hematological malignancies: AML (n=10), acute lymphocytic leukemia (n=10) and chronic lymphocytic leukemia (n=9). Further characterization was performed in tumor cells from adult AML patients (n=26, clinical information in Table 1). Selection of patient samples was based on sample availability and drugs were run in parallel. Leukemic cells were isolated, as previously described;20 a proportion of at least 70% viable tumor cells after thawing was required. The sampling was approved by the Ethics Committee of Uppsala University (No 21/93 and 2007/237).
Table 1. Acute myeloid leukemia patients (n=26).
| Patient (no.) | Age (years) | Sex | Subclass (FAB) | Ratio (FLT3/GUS × 100) | Allelic burden (ITD/total FLT3) | FLT3 status ITD/TKD/wt | Karyotype | Status | IC50 AKN-028 (μℳ) | IC50 cytarabine (μℳ) |
|---|---|---|---|---|---|---|---|---|---|---|
| UPN1 | 63 | M | M5 | 51.2 | NA | wt | 46,XY | Newly diagnosed | 1.22 | 2.04 |
| UPN2 | 38 | M | M2 | 99.4 | NA | wt | Complex | Relapse | 0.265 | 0.347 |
| UPN3 | 64 | F | M1 | 34.1 | NA | wt | Complex | Relapse | 1.82 | 15.5 |
| UPN4 | 46 | M | ND | 0.340 | NA | wt | ND | Relapse | 3.41 | 7.39 |
| UPN5 | 24 | M | ND | 24.4 | NA | wt | 46,XY, t(8;21) | Newly diagnosed | 2.88 | 3.08 |
| UPN6 | 76 | M | M5 | 75.6 | NA | wt | 45,XY,+8,-10,der(17)t(17;21)(q;10;q10)/46,XY | Newly diagnosed | 3.47 | 22.6 |
| UPN7 | 47 | M | M2 | 26.4 | NA | wt | 46,XY | Newly diagnosed | 0.928 | 0.241 |
| UPN8 | 58 | F | M2 | 108 | 0.49 | ITD | 46,XX | Relapse | 0.890 | ND |
| UPN9 | 59 | M | ND | 23.3 | 0.48 | ITD | ND | Relapse | 1.06 | >30 |
| UPN10 | 54 | M | ND | 22.9 | 0.49 | ITD | ND | Relapse | 0.306 | 3.24 |
| UPN11 | 65 | M | M2 | 42.9 | 0.50 | ITD | Complex | Relapse | 5.29 | ND |
| UPN12 | 79 | M | M2 | 70.9 | 0.48 | ITD | 46,XY | Relapse | 1.36 | 3.19 |
| UPN13 | 26 | F | M1 | 85.6 | 0.74 | ITD | 46,XX | Relapse | 0.0465 | 0.167 |
| UPN14 | 68 | M | M1 | 128 | 0.22 | ITD | 46,XY | Relapse | 0.738–1.27 | 0.309–0.450 |
| UPN15 | 79 | M | M0 | 94.6 | NA | wt | 46,XY,t(11;17)(q23?4)/46,XY | Newly diagnosed | 0.622–4.02 | 0.812–5.79 |
| UPN16 | 21 | F | M5 | ND | NA | wt | 47,XX+8, (t(10;11)(p11;q23) | Relapse | 0.00436 | 1.23 |
| UPN17 | 57 | F | M0 | ND | 0.48 | ITD | 46,XX | Relapse | 0.304 | 1.13 |
| UPN18 | 51 | F | M2 | ND | 0.22 | ITD | 46,XX | Relapse | 0.140 | 0.777 |
| UPN19 | 68 | M | M4 | ND | 0.21 | ITD | 46,XY | Newly diagnosed | 0.420 | 2.43 |
| UPN20 | 61 | F | M1 | ND | NA | wt | 46,XX | Relapse | 1.32 | 6.95 |
| UPN21 | 32 | F | M1 | ND | 0.59 | ITD | 46,XX | Relapse | 0.965 | 1.18 |
| UPN22 | 54 | M | M1 | ND | NA | wt | 46,XY | Newly diagnosed | 1.92 | 0.718 |
| UPN23 | 41 | M | M4 | ND | 0.48 | ITD | 46,XY, del (7)(q22) | Relapse | 2.55 | 1.42 |
| UPN24 | 81 | F | M2 | ND | 0.03 | ITD | 46,XX | Newly diagnosed | 11.3 | 9.81 |
| UPN25 | 53 | M | M0 | ND | NA | wt | Complex | Relapse | 5.58 | n.d |
| UPN26 | 47 | M | M1 | ND | NA | wt | 46XY, inv(16)(p13q22)(25) | Newly diagnosed | 3.89 | n.d |
Abbreviations: FAB, French–American–British; FLT3, FMS-like receptor tyrosine kinase 3; FMCA, fluorometric microculture cytotoxicity assay; ITD, internal tandem duplications; ND, not done; NA, not applicable; wt, wild type.
Complex karyotype indicates karyotype with three or more abberations. Clinical characteristics, FLT3 mutation status and quantitative FLT3 expression (displayed as a ratio of mean copy number FLT3/mean copy number of reference gene GUSB × 100), allelic burden for FLT3 ITD-mutated patients (ITD/total FLT3) and corresponding IC50 values from the FMCA for AKN-028 and cytarabine.
Kinase inhibition assessment
The inhibitory effect of AKN-028 on the FLT3 enzyme was evaluated in an enzyme inhibition assay for the tyrosine kinase domain of FLT3 using the immobilized metal ion affinity-based fluorescence polarization technique (IMAP, Molecular Devices, CA, USA), as described previously.20
The kinase inhibitory profile of AKN-028 at 1 μℳ was evaluated over a 320-kinase panel at ProQinase. The profiling was performed by a radiometric protein kinase assay, using FlashPlates (Perkin-Elmer, Boston, MA, USA) in a 50-μl reaction volume, according to the protocol of ProQinase (33PanQinase1 Activity Assay). Grade of remaining kinase activity (%) was determined after 60 min of incubation with AKN-028 or the reference compounds AC220 and staurosporine. The inhibitory effects were verified by full dose–response curves on the following kinases: FLT3 (IMAP), CDC-like kinase 1, Ribosomal Protein S6 (RPS6) (ProQinase FlashPlates), vascular endothelial growth factor receptor 2 and fibroblast growth factor 2 (both performed at CEREP Laboratories (Celle l'Evescault, France) according to manufacturers standards)
Phospho-ELISA, western blot and AKT and ERK protein kinase assay
Inhibition of FLT3 and KIT autophosphorylation was assessed by phospho-ELISA at ProQinase. Mouse embryonal fibroblasts either overexpressing FLT-wt, FLT3-TKD or FLT3-ITD and human acute megakaryoblastic leukemia M07 cells overexpressing KIT, were incubated for 5 min with eight concentrations of AKN-028 before cell lysis and determination of phosphorylation levels by a sandwich ELISA according to ProQinase standards. Sunitinib and AC220 were used for comparison.
Inhibition of FLT3 autophosphorylation in MV4-11 cells after exposure to AKN-028 for 15 h was evaluated by western blot analysis, as described previously.20 To further explore the potential effects on downstream targets of FLT3, the inhibition of AKT 1, 2, 3 and ERK 1, 2 was measured by use of the radiometric protein kinase assay, as described above. Grade of inhibition of kinase activity (%) was determined after 60 min of incubation with 1 μℳ of AKN-028 or reference compounds AC220 and staurosporine.
Cytotoxicity assays
For cytotoxic evaluation, the fluorometric microculture cytotoxicity assay (FMCA), as described previously,27 was used. Cells were seeded into drug-prepared microplates at varying density per well, depending on cell type and plate size. After 72 h of incubation, the living-cell density was assessed using the FMCA; results presented as survival index (%), defined as fluorescence in test wells in percent of control cultures, blank values subtracted. IC50 was determined from log concentration–effect curves in Graph Pad Prism (GraphPad software Inc., CA, USA) using nonlinear regression analysis. Cytotoxic activity in MOLM-13 cells was analyzed by Alamar Blue assay28 at ProQinase.
Combination studies
AKN-028 was studied in combination with cytarabine or daunorubicin with a fixed molar ratio between the agents (cytarabine: AKN-028 2.5:1; daunorubicin: AKN-028 1:20), intended to be equipotent. Drug addition to 96-well plates containing MV4-11 cells was performed directly after cell seeding and again after 24 h. Combinations were tested in three sequences: (I): pretreatment with chemotherapy (24 h), followed by AKN-028; (II): simultaneous treatment with both agents; (III): pretreatment with AKN-028 (24 h), followed by chemotherapy. Cytotoxic activity was assessed by the FMCA after an incubation time of 72 h.
Drug combination analysis
Possible interactions between the drugs in the combination studies was analyzed as proposed by Chou and Talalay29 by median-effect analysis using CalcuSyn software (Biosoft, Cambridge, UK). Each dose–response curve was fit to a linear model using the median effect equation, allowing calculation of a median effect value Dm (corresponding to the IC50). The extent of drug interaction between the two drugs was presented using the combination index (CI) for mutually exclusive drugs (assumption of mutual exclusivity proposed as a universal standard for the analysis of synergism and antagonism30): CI=d1/D1+d2/D2, where D1 and D2 represent the concentration of drugs 1 and 2 alone, required to produce a certain effect, and d1 and d2 represent the concentrations of drugs 1 and 2 in combination, required to produce the same effect. Different CI values are obtained when solving the equation for different effect levels. A CI close to 1 indicates additivity, a CI <0.7 indicates synergy and >1.45 indicates antagonism (arbitrary levels suggested in the software manual).
Apoptosis assay
The cell-death characteristics of AKN-028 were studied with regard to caspase-3 activation. In all, 20 000 MV4-11 cells per well were seeded into 96-well optic plates and subjected to 10 μℳ of either AKN-028 or the positive control etoposide. A probe staining cells with active caspase 3 (DEVD-NucView 488 caspase-3 substrate, Essen BioScience Ltd, Welwyn Garden City, UK) was added to each well at a final concentration of 2–2.2 μℳ, a caspase-3 inhibitor (N-acetyl-Asp-Glu-Val-Asp-al, Sigma-Aldrich Co) at a concentration of 16.4 μℳ was used as a control. The total amount of dimethylsulphoxide in each well was 0.21%. The cells were analyzed in the live-cell imaging instrument IncuCyte (Essen BioScience Ltd); the total duration of the experiment was 48 h.
FLT3 mutation detection
FLT3-ITD length mutation analysis was performed, as described previously.10, 31 Briefly, following PCR amplification, PCR products were run on the ABI 3130XL genetic analyzer (Applied Biosystems, Foster City, CA, USA) and the chromatograms analyzed using GeneMapper 4.0 software (Applied Biosystems). The proportion of FLT3-mutated alleles (allelic burden) was calculated as area under the curve (AUC) for FLT3-ITD divided by (AUC for FLT3-ITD+AUC for FLT3-wt). For FLT3-TKD point mutations in codon D835, genomic DNA was analyzed by PCR, as previously described.31
Quantitative FLT3 expression analysis
Total RNA was isolated from primary AML cells using Trizol reagent (Invitrogen, Carlsbad, CA, USA). One microgram of RNA was reverse-transcribed into complementary DNA (cDNA) using pd(N)6 random hexamer (GE Healthcare, Little Chalfont, UK) and M-MLV enzyme (Invitrogen). The FLT3 mRNA transcripts were quantified by quantitative real-time reverse transcriptase-PCR, using forward primer 5′-CAG GAC TTG GAC AGA GAT TTT CAA-3′, reverse primer 5′-TCC GGG TGT ATC TGA′ACT TCT CTT-3′ and probe 5′-FAM-CCC ACT TTC CAA TCA CAT CCA AAT TCC A-TAMRA-3′. The analysis measured the overall FLT3 expression. Primer and probe sequences for FLT3 were chosen using Primer Express software (Applied Biosystems). A standard curve for FLT3 was created using 10-fold dilutions of the plasmid pCDHF3 containing wild-type FLT3.32 GUSB was used as reference gene and the GUSB mRNA transcripts were quantified by real-time reverse transcriptase-PCR, as previously described.33 The amount of FLT3 transcripts was expressed as a ratio of FLT3 copy number relative to 100 GUS copies (mean copy number FLT3/mean copy number GUSB × 100). Correlations were assessed using Spearman's correlation test.
In vivo studies
The pharmacokinetic properties of AKN-028 were evaluated in male C57 black mice (Supplementary II) and the in vivo activity of AKN-028 further assessed in the AML cell line MV4-11 and two individual AML patients (UPN 25 and 26, Table 1) using the hollow-fiber mouse model, as described previously.34 NMRI male mice (Scanbur), were injected subcutaneously twice daily with 15 mg/kg of AKN-028 or vehicle only, eight animals per group, three fibers per animal. After 6 days of repeated drug administration, the fibers were extracted and the living cell mass assessed by use of the MTT-assay.20, 35 Student's t-test was used for comparison between groups, P<0.05 was considered significant. The study was approved by the Animal Ethics Committee in Uppsala (No. C243/6).
Results
AKN-028 inhibits FLT3 kinase
AKN-028 was identified in a kinase-inhibition screen including ∼400 analogs of AKN-032, a previously reported small molecular 2-aminopyrazine TKI.20 Using a radiometric protein kinase assay, the inhibitory effect of AKN-028 at 1 μℳ was investigated in a panel screen of 320 kinases with AC220 and staurosporine used as reference compounds. Less than 20% remaining enzyme activity after exposure to AKN-028 was observed only for FLT3 kinase, CLK kinases and RPS6K (Figure 1b). AKN-028 induced a dose-dependent inhibition of the FLT3 kinase with an IC50 value of 6 nℳ (n=20), as determined by a kinase inhibition assay; sunitinib was tested for comparison (Figure 1c). Further characterization with full dose–response experiments were made for FLT3, CDC-like kinase 1, RPS6KA, fibroblast growth factor 2 and vascular endothelial growth factor receptor 2; IC50 values are presented in Table 2.
Table 2. IC50 values for AKN-028 and positive-control Staurosporine for kinases FLT3, CLK1, RPS6KA, FGFR2 and VEGFR2.
| FLT3 | CLK1 | RPS6KA | FGFR2 | VEGFR2 | |
|---|---|---|---|---|---|
| AKN-028 | 6 nℳ | 140 nℳ | 220 nℳ | 1200 nℳ | 520 nℳ |
| Staurosporine | <1 nℳ | 66 nℳ | 3 nℳ | 2 nℳ | 4 nℳ |
Abbreviations: FLT3, FMS-like receptor tyrosine kinase 3; CLK1, CDC-like kinase 1; RPS6K, ribosomal protein S6 kinase; FGFR2, fibroblast growth factor 2; VEGFR2, vascular endothelial growth factor receptor 2.
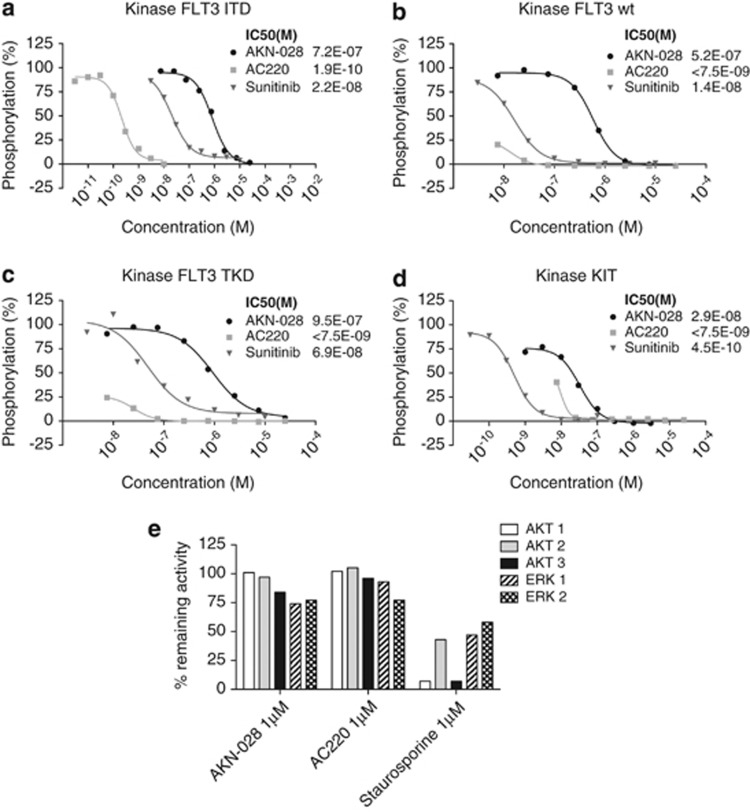
Inhibition of FLT3 autophosphorylation in a cellular assay was verified by phospho-ELISA and western blot analysis (Figures 2a–c and Supplementary III). Surprisingly, inhibition of KIT was also demonstrated by the phospho-ELISA (Figure 2d). To further evaluate the effect of AKN-028 on targets downstream of FLT3, the compound was evaluated in AKT and ERK protein kinase assays. No direct inhibition of AKT 1, 2, 3 or ERK 1, 2 was seen at 1 μℳ; AC220 and staurosporine used as reference compounds (Figure 2e).
Figure 2.
(a–e) Inhibition of kinase autophosphorylation by AKN-028, AC220 and sunitinib shown by phospho-ELISA on cells transfected with FLT3-wt (a), FLT3-ITD (b), FLT3-TKD (c) and KIT (d) outlined as dose–response curves, results presented as grade of phosphorylation (%). Figure 2e displays inhibitory activity (% remaining activity) of AKN-028, AC220 and positive-control staurosporine on AKT 1,2,3 and ERK 1 and 2 at 1 μℳ in the radiometric protein kinase assay.
AKN-028 is cytotoxic to AML cell lines and induces apoptosis in the AML cell line MV4-11
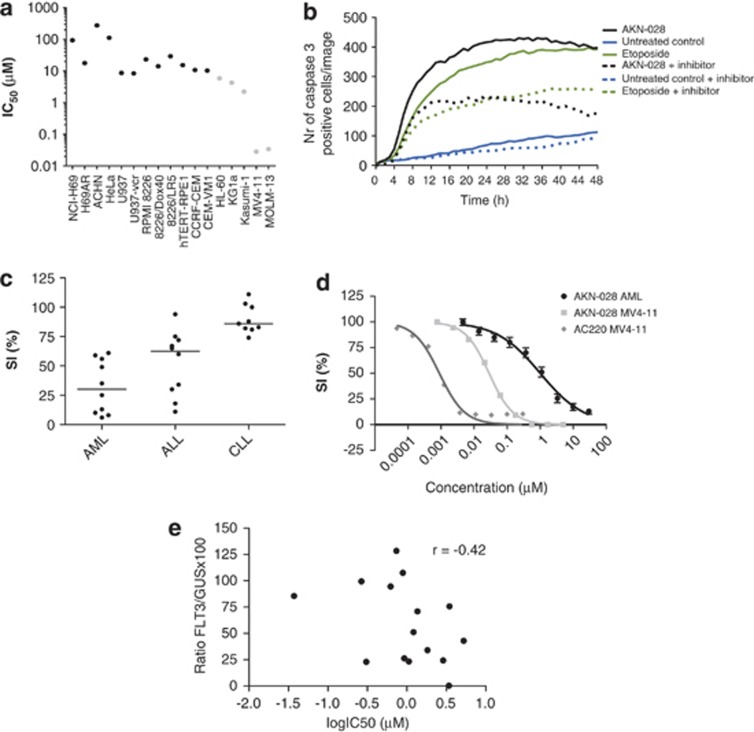
The cytotoxic activity of AKN-028 was investigated in a panel of 17 cell lines, including five AML cell lines. The cytotoxic activity was highest in MV4-11 and MOLM-13 (IC50 <50 nℳ), followed by the three other AML cell lines (IC50 0.5–6 μℳ). Almost no effect was observed in the solid tumor cell lines HeLa, ACHN and NCI-H69 (Figure 3a).
Figure 3.
(a–e) In vitro cytotoxic response to AKN-028 in 17 cell lines displayed as mean IC50 values (μℳ) on a log axis (a). The number of caspase-3 positive MV4-11 cells per image after exposure to AKN-028 positive-control etoposide or untreated control. The experiments were performed with or without the addition of a caspase inhibitor as indicated. One typical experiment from the apoptosis assay is shown (b). In vitro cytotoxic response to 10 μℳ AKN-028 in primary samples from patients with different hematological malignancies (c) and as mean dose–response curves in AML (n=15, ±s.e.m.) compared with MV4-11 cell line, AC220 used as reference compound (d), and as IC50 values (μℳ) plotted against quantitative expression of FLT3 (ratio FLT3/GUSB × 100) (e). Results in the dose–response curves are presented as survival index %, defined as fluorescence in test wells in percent of control cultures, blank values subtracted. ALL, acute lymphocytic leukemia; CLL, chronic lymphocytic leukemia; SI, survival index.
A probe staining activated caspase 3 was used to study the cell death characteristics of AKN-028 in the MV4-11 cell line. Incubation with 10 μℳ AKN-028 induced apoptosis by activation of caspase 3; etoposide was used as a positive control (Figure 3b).
AKN-028 is cytotoxic to primary AML cells, irrespective of FLT3 mutation status and quantitative FLT3 expression
In an initial screen, studying the cytotoxic effect of AKN-028 at 10 μℳ in primary tumor cells from 29 patients with three hematological malignancies (acute myeloid leukemia, n=10, acute lymphocytic leukemia, n=10, chronic lymphocytic leukemia, n=9), AML cells tended to be the most sensitive (Figure 3c). The cytotoxic activity of AKN-028, and of reference compound cytarabine, was further evaluated in leukemic blast cells from patients with newly diagnosed or relapsed AML (n=15: Table 1, Figure 3d), showing a dose-dependent response to AKN-028 in all samples tested. No significant difference was seen between newly diagnosed and relapse patients (P=0.10: Student's t-test). Notably, the sensitivity to AKN-028 did not differ between samples from FLT3-mutated and FLT3-wt patients (P=0.79: Student's t-test). As for FLT3-mutated patients, no correlation between allelic burden (see Table 1) and cytotoxic response to AKN-028 was observed (Spearman's correlation test r=−0.15). As no correlation between FLT3 mutation status and cytotoxic response was seen, the samples were further examined regarding overall quantitative FLT3 expression, which were plotted against corresponding IC50 values for AKN-028 (Figure 3e). No apparent correlation between the level of FLT3 RNA expression and cytotoxic effect was observed (Spearman's correlation test, r=−0.42).
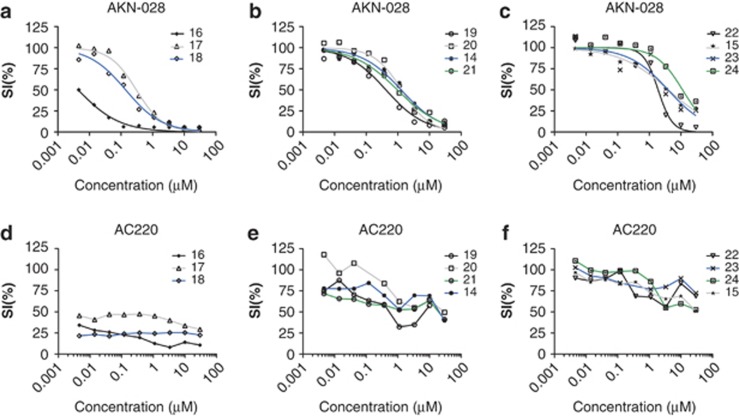
To further explore the cytotoxic properties of AKN-028, and to compare it with the specific FLT3-inhibitor AC220, 11 additional AML patient samples were analyzed by the FMCA (Table 1, Figures 4a–f). Again, AKN-028 presented a dose-dependent response, reaching IC50 in all samples tested. By contrast, the response to AC220 varied considerably, displaying flat curves in general, with a degree of dose-dependant response before reaching a plateau, sometimes with a cell survival of >50%, despite the full efficacy obtained in MV4-11 (Figures 3d and 4d–f).
Figure 4.
(a–f) In vitro cytotoxic response to AKN-028 and AC220 in 11 primary AML samples (UPN 14-24—see Table 1), outlined as individual dose–response curves from the FMCA (a–f). Samples are grouped according to in vitro response to AC220. Because of the configuration of data for AC220, a sigmoid curve fitting was not possible. SI, survival index.
Improved antileukemic activity when cytarabine or daunorubicin is administered simultaneously or 24h before AKN-028
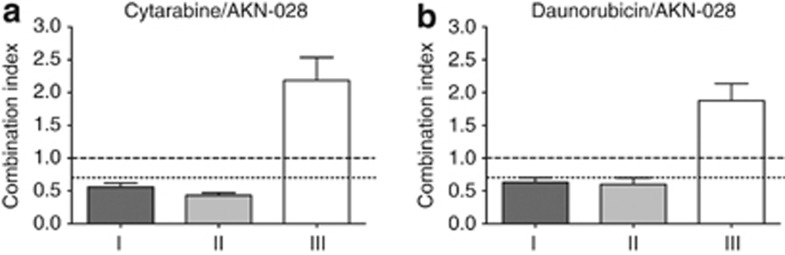
AKN-028 was tested in combination with antileukemic agents cytarabine or daunorubicin in MV4-11 cells and evaluated by the FMCA. Median-effect analysis generated 80 combination indices when data points with drug effect between 10 and 90% were pooled. The mean CI for each sequence regimen is presented in Figures 5a and b, showing a sequence-dependent synergy, with better antileukemic activity when the cells were exposed to cytarabine or daunorubicin simultaneously or for 24 h before adding AKN-028, whereas antagonism was observed when cells were pre-treated with AKN-028.
Figure 5.
(a–b) Cytotoxic response to AKN-028 in combination with standard cytotoxic agents cytarabine (a) and daunorubicin (b) in MV4-11 cell line in three different sequences; (I): pretreatment with chemotherapy (24 h), followed by AKN-028; (II): simultaneous treatment with both agents; (III): pretreatment with AKN-028 (24 h). Results are presented as mean CI, (n=11–16) of all experimental combination data points with effect levels between 10–90%, error bars representing s.e.m. CI <0.7 indicate synergy and CI of 1 additive interactions.
AKN-028 inhibits growth of primary AML and MV4-11 cells in mice
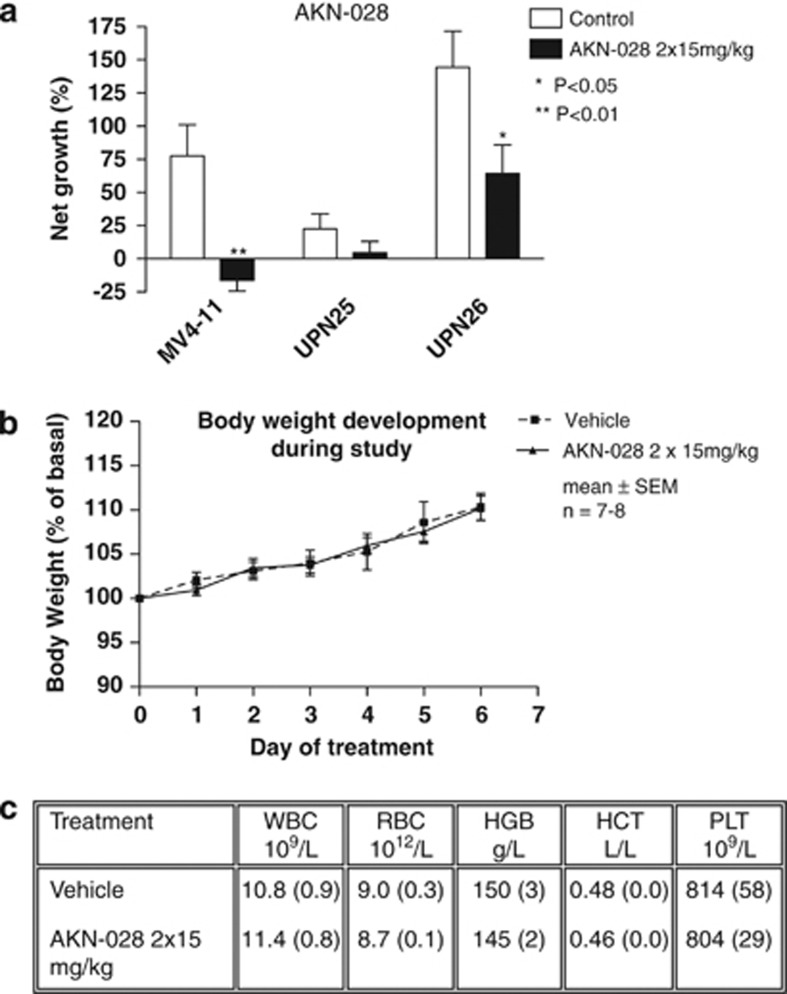
The cytotoxic effect of AKN-028 in vivo was examined in the mouse hollow-fiber model. Based on the pharmacokinetic studies (supplementary), the predicted area under the curve for this experiment was 6 μℳ·h, given no strain difference and linear pharmacokinetics. Twice daily subcutaneous administration with 15 mg/kg of AKN-028 in NMRI male mice inhibited net growth of one of the primary AML samples (UPN26) in vivo and furthermore reduced the tumor mass of MV4-11 cell line (Figure 6a), the total cell growth in the other AML sample (UPN25) was very modest, with no significant inhibition noted. No major toxicity was observed (Figures 6b and c).
Figure 6.
(a–c) In vivo activity of subcutaneously administrated 2 × 15 mg/kg of AKN-028 in MV4-11 cell line and primary AML cells from two individual patients (UPN 25 and 26 in Table 1) in the mouse hollow-fiber assay (n=7–8) (a). Results are presented as net growth, defined as the percent change in cell density in the fibers during the 6 days of in vivo experiment (mean+s.e.m.). Body weight development of the animals over time during the mouse hollow-fiber assay (n=7–8, mean±s.e.m.) (b) and hematological profile in the animals at the end of the study (c). Blood samples were analyzed on day 6 for the following parameters: white blood cell count (WBC), red blood cell count (RBC), hemoglobin (HGB), hematocrite (HCT) and platelet count (PLT). Results are presented as mean±s.e.m.
Discussion
In this study, we report that AKN-028, a novel small molecular TKI identified in an extensive kinase screen as active against FLT3, inhibits the FLT3 enzyme in a dose-dependent manner at very low concentrations. Inhibition of wild-type as well as FLT3-mutated autophosphorylation was confirmed in cellular assays. In vitro cytotoxic activity was further investigated in a cell line panel representing different malignancies and their drug-resistant sublines, as well as immortalized non-tumor cells. Notably, AKN-028 had a significant activity in all the five AML cell lines tested, whereas no or only marginal effect was observed in the other cell lines, suggesting a specific effect in AML. Interestingly, the FLT3-ITD-mutated cell lines, MV4-11 and MOLM-13, was by far the most sensitive cell lines with IC50 <50 nℳ. Moreover, AKN-028 induced apoptosis in AML cell line MV4-11, at least partly mediated by activation of caspase 3.
However, cell lines may have limitations in predicting clinical activity of cytotoxic drugs, possibly owing to their acquisition of additional genetic changes in becoming immortalized and adapted to continuous growth.36 As primary cultures of patient tumor cells may be a model that is better suited for predicting clinical activity,37 we evaluated the cytotoxic effect of AKN-028 in leukemic blasts from AML patients, among those several cases with complex karyotype and chemotherapy-resistant disease. Importantly, AKN-028 showed a dose-dependent cytotoxic response in all patient samples tested, including those with no or only minor in vitro response to AC220, a potent, selective FLT3 TKI having recently shown clinical efficacy in phase-II trials.38 The antileukemic effect was confirmed in vivo using the hollow-fiber mouse model, where AKN-028 had significant effect in MV4-11, as well as in one of the primary AML samples. The hollow-fiber model was developed as a tool for anticancer drug screening. The method enables studies of hematological toxicity, pharmacokinetics and tumor effect in cell lines and primary patient samples within the same immunocompetent animal, which in turn reduces the number of animals needed. The hollow-fiber method is relatively resistant, with a risk of underestimating drug effect owing to low efficacy of drug delivery to the subcutaneously implanted fibers.39
Unexpectedly, we observed no difference in sensitivity to AKN-028 between FLT3-mutated and wild-type AML cases. As a pathogenetic role of the wild-type FLT3-receptor in AML has been proposed, we investigated whether a variance in quantitative FLT3 expression, regardless of mutation status, might explain a different response to AKN-028.6, 40 However, there was no clear correlation between quantitative FLT3 expression and cytotoxic activity of AKN-028. Although, the four patients (two FLT3-ITD, two FLT3-wt) with the highest FLT3/GUS × 100 ratio had all relatively low IC50 values, several cases with relatively low quantitative expression of FLT3 but with corresponding low IC50 values were present in our material.
Interestingly, AKN-028 showed ex vivo efficacy in primary AML tumor cells that were resistant to treatment with the potent FLT3- and KIT-inhibitor AC220. This finding, together with the lack of correlation between FLT3 mutation status or quantitative expression of FLT3 and in vitro response to AKN-028 in AML, indicates that the compound is also targeting other pathways than those directly associated with FLT3 inhibition. Using a radiometric protein kinase assay, we could exclude that AKN-028 inhibited the FLT3 downstream targets AKT 1-3 and ERK 1-2. Aberrant expression of receptor tyrosine kinases such as KIT, PDGF and JAK may have a role in leukemogenesis 41, 42, 43 and share in part the same downstream targets as FLT3.44 As many FLT3-inhibitors also target KIT, AKN-028 was tested in a human megakaryoblastic leukemia cell line overexpressing KIT, in which the substance induced a significant inhibition of KIT autophosphorylation. The inhibition was higher in this cell assay than predicted by the results from our kinase screen. A possible explanation for this discrepancy may be that only the active form of KIT is present in the kinase panel, whereas the cell assay might reflect effects on both the active and the inactive form of the kinase.
Our kinase-panel screen also revealed inhibition of RPS6- and CLK kinases. Recently, it has been shown that RPS6K, a downstream effector in the mammalian target of rapamycin (mTOR) signaling pathway, is activated in primary FLT3-mutated AML-cells.45 The mTOR pathway is involved in cellular growth and survival, and mTOR dysregulation has been implicated in several malignancies. Furthermore, mTOR inhibitors have been developed as anticancer treatment.46 AC220 did not inhibit RPS6K, in accordance with the published kinase-inhibition profile of this compound. Thus, inhibition of RPS6K could possibly be part of the differentiating effect between AKN-028 and AC220 on the cell kill induced in primary cells. CLK kinases have been reported to play a role in the AKT pathway,47 but their role (if any) in leukemogenesis is presently unknown. Further studies of AKN-028-mediated inhibition of KIT, and of other possible targets, are underway.
AML is biologically a heterogeneous disease. Hitherto, patients treated with FLT3 inhibitors have, with the possible exception of AC220, shown only modest and transient clinical response.48 Indeed, inhibition of FLT3 autophosphorylation does not always lead to cell death, and some leukemic cells may not be dependent on FLT3 signaling.18 In addition, monotherapy with TKIs is associated with the risk of developing resistance.49, 50 Thus, a TKI such as AKN-028, targeting other pathways apart from FLT3, may be potentially advantageous. Nevertheless, combinations with other agents including conventional chemotherapeutics are probably necessary to optimize clinical efficacy. Results from our combination study suggest a sequence-dependent synergistic effect between AKN-028 and cytarabine or daunorubicin, with better antileukemic activity when cells were exposed to chemotherapy simultaneously or 24 h before adding AKN-028, whereas antagonism was observed when cells were pre-treated with AKN-028. The finding of possible synergy between cytotoxic drugs and targeted therapy is in line with the results reported from other TKIs.51, 52 Sequence-dependent antagonism after pre-treatment with TKIs has also been reported, possibly because of the fact that (some) TKIs may cause cell cycle arrest.52 Further studies of the cell cycle effect of AKN-028 are underway.
Pharmacokinetic studies show that anticipated effective plasma exposure can be obtained in mice after oral administration. If the IC90 value is divided with fraction unbound of the compound, taking into account the plasma protein binding of 96,5%, the plasma target concentration should be in the range of 1–3 μℳ. Given no strain difference and linear pharmacokinetics, a similar exposure in NMRI mice as in the C57 black mice would be expected. The predicted plasma profile in the hollow-fiber experiment corresponds to 1 h of exposure above the plasma target concentration of 1–3 μℳ twice daily. Thus, continuous exposure was not necessary to obtain an antitumoral effect, which is in accordance with some other TKIs, for example, dasatinib, being clinically effective despite a relatively short plasma half-life.53
In conclusion, AKN-028 is a novel TKI with potent in vitro and in vivo activity in AML, irrespective of FLT3 status. In vitro synergy with the standard antileukemic agents cytarabine and daunorubicin, along with good oral bioavailability, make AKN-028 a candidate drug for clinical trials. An international two-part multi-center phase-I study of AKN-028 in patients with AML started in January 2012 (ClinicalTrials.gov NCT01573247). Additional studies on its mechanism of action are underway.
Acknowledgments
We thank Dr Saadia Bashir Hassan, Mrs Lena Lenhammar and Ms Christina Leek for skillful technical assistance. We also thank Dr Randi Hovland, Center for Medical Genetics and Molecular Medicine, Haukeland Hospital, Bergen, Norway, for kindly providing the pCDHF3 plasmid. This study was supported by Biovitrum AB, Akinion Pharmaceuticals AB and Erik, Karin and Gösta Selanders Research Foundation.
The following authors were employees of Biovitrum AB when the experiments were performed or when this manuscript was written and revised: C Ekholm, A Jenmalm Jensen, A Löthgren, F Lehmann and V Parrow. V Parrow is now primary affiliated to and part owner of Akinion Pharmaceuticals AB. A Eriksson has received an unrestricted research grant from Akinion Pharmaceuticals AB. C Ekholm is a minor stockholder of Biovitrum AB. The remaining authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Blood Cancer Journal website (http://www.nature.com/bcj)
Supplementary Material
References
- Juliusson G, Antunovic P, Derolf A, Lehmann S, Mollgard L, Stockelberg D, et al. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood. 2009;113:4179–4187. doi: 10.1182/blood-2008-07-172007. [DOI] [PubMed] [Google Scholar]
- Burnett A, Wetzler M, Lowenberg B. Therapeutic advances in acute myeloid leukemia. J Clin Oncol. 2011;29:487–494. doi: 10.1200/JCO.2010.30.1820. [DOI] [PubMed] [Google Scholar]
- Stapnes C, Gjertsen BT, Reikvam H, Bruserud O. Targeted therapy in acute myeloid leukaemia: current status and future directions. Expert Opin Investig Drugs. 2009;18:433–455. doi: 10.1517/14728220902787628. [DOI] [PubMed] [Google Scholar]
- Mizuki M, Fenski R, Halfter H, Matsumura I, Schmidt R, Muller C, et al. Flt3 mutations from patients with acute myeloid leukemia induce transformation of 32D cells mediated by the Ras and STAT5 pathways. Blood. 2000;96:3907–3914. [PubMed] [Google Scholar]
- Brandts CH, Sargin B, Rode M, Biermann C, Lindtner B, Schwable J, et al. Constitutive activation of Akt by Flt3 internal tandem duplications is necessary for increased survival, proliferation, and myeloid transformation. Cancer Res. 2005;65:9643–9650. doi: 10.1158/0008-5472.CAN-05-0422. [DOI] [PubMed] [Google Scholar]
- Ozeki K, Kiyoi H, Hirose Y, Iwai M, Ninomiya M, Kodera Y, et al. Biologic and clinical significance of the FLT3 transcript level in acute myeloid leukemia. Blood. 2004;103:1901–1908. doi: 10.1182/blood-2003-06-1845. [DOI] [PubMed] [Google Scholar]
- Small D. FLT3 mutations: biology and treatment. Hematol Am Soc Hematol Educ Program. 2006. pp. 178–184. [DOI] [PubMed]
- Mead AJ, Linch DC, Hills RK, Wheatley K, Burnett AK, Gale RE. FLT3 tyrosine kinase domain mutations are biologically distinct from and have a significantly more favorable prognosis than FLT3 internal tandem duplications in patients with acute myeloid leukemia. Blood. 2007;110:1262–1270. doi: 10.1182/blood-2006-04-015826. [DOI] [PubMed] [Google Scholar]
- Kottaridis PD, Gale RE, Frew ME, Harrison G, Langabeer SE, Belton AA, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98:1752–1759. doi: 10.1182/blood.v98.6.1752. [DOI] [PubMed] [Google Scholar]
- Gale RE, Green C, Allen C, Mead AJ, Burnett AK, Hills RK, et al. The impact of FLT3 internal tandem duplication mutant level, number, size, and interaction with NPM1 mutations in a large cohort of young adult patients with acute myeloid leukemia. Blood. 2008;111:2776–2784. doi: 10.1182/blood-2007-08-109090. [DOI] [PubMed] [Google Scholar]
- Patel JP, Gonen M, Figueroa ME, Fernandez H, Sun Z, Racevskis J, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366:1079–1089. doi: 10.1056/NEJMoa1112304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaise M, Steinbach D, Corbacioglu S. Clinical implications of c-Kit mutations in acute myelogenous leukemia. Curr Hematol Malig Rep. 2009;4:77–82. doi: 10.1007/s11899-009-0011-8. [DOI] [PubMed] [Google Scholar]
- Stone RM. Prognostic factors in AML in relation to (ab)normal karyotype. Best Pract Res Clin Haematol. 2009;22:523–528. doi: 10.1016/j.beha.2009.07.003. [DOI] [PubMed] [Google Scholar]
- DeAngelo DJ, Stone RM, Heaney ML, Nimer SD, Paquette RL, Klisovic RB, et al. Phase 1 clinical results with tandutinib (MLN518), a novel FLT3 antagonist, in patients with acute myelogenous leukemia or high-risk myelodysplastic syndrome: safety, pharmacokinetics, and pharmacodynamics. Blood. 2006;108:3674–3681. doi: 10.1182/blood-2006-02-005702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravandi F, Cortes JE, Jones D, Faderl S, Garcia-Manero G, Konopleva MY, et al. Phase I/II study of combination therapy with sorafenib, idarubicin, and cytarabine in younger patients with acute myeloid leukemia. J Clin Oncol. 2010;28:1856–1862. doi: 10.1200/JCO.2009.25.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levis M, Ravandi F, Wang ES, Baer MR, Perl A, Coutre S, et al. Results from a randomized trial of salvage chemotherapy followed by lestaurtinib for patients with FLT3 mutant AML in first relapse. Blood. 2011;117:3294–3301. doi: 10.1182/blood-2010-08-301796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone R, Fischer T, Paquette R, Schiller G, Schiffer C, Ehninger G, et al. A Phase 1b study of midostaurin (PKC412) in combination with daunorubicin and cytarabine induction and high-dose cytarabine consolidation in patients under age 61 with newly diagnosed de novo acute myeloid leukemia: overall survival of patients whose blasts have FLT3 mutations is similar to those with wild-type FLT3 Blood (ASH Annual Meeting Abstracts) [Abstract] 2009114(Abstract 634.). [Google Scholar]
- Pratz KW, Sato T, Murphy KM, Stine A, Rajkhowa T, Levis M. FLT3-mutant allelic burden and clinical status are predictive of response to FLT3 inhibitors in AML. Blood. 2010;115:1425–1432. doi: 10.1182/blood-2009-09-242859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindler T, Lipka DB, Fischer T. FLT3 as a therapeutic target in AML: still challenging after all these years. Blood. 2010;116:5089–5102. doi: 10.1182/blood-2010-04-261867. [DOI] [PubMed] [Google Scholar]
- Eriksson A, Hoglund M, Lindhagen E, Aleskog A, Hassan SB, Ekholm C, et al. Identification of AKN-032, a novel 2-aminopyrazine tyrosine kinase inhibitor, with significant preclinical activity in acute myeloid leukemia. Biochem Pharmacol. 2010;80:1507–1516. doi: 10.1016/j.bcp.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Lange B, Valtieri M, Santoli D, Caracciolo D, Mavilio F, Gemperlein I, et al. Growth factor requirements of childhood acute leukemia: establishment of GM-CSF-dependent cell lines. Blood. 1987;70:192–199. [PubMed] [Google Scholar]
- Asou H, Tashiro S, Hamamoto K, Otsuji A, Kita K, Kamada N. Establishment of a human acute myeloid leukemia cell line (Kasumi-1) with 8;21 chromosome translocation. Blood. 1991;77:2031–2036. [PubMed] [Google Scholar]
- Beghini A, Magnani I, Ripamonti CB, Larizza L. Amplification of a novel c-Kit activating mutation Asn(822)-Lys in the Kasumi-1 cell line: a t(8;21)-Kit mutant model for acute myeloid leukemia. Hematol J. 2002;3:157–163. doi: 10.1038/sj.thj.6200168. [DOI] [PubMed] [Google Scholar]
- Gallagher R, Collins S, Trujillo J, McCredie K, Ahearn M, Tsai S, et al. Characterization of the continuous, differentiating myeloid cell line (HL-60) from a patient with acute promyelocytic leukemia. Blood. 1979;54:713–733. [PubMed] [Google Scholar]
- Koeffler HP. Induction of differentiation of human acute myelogenous leukemia cells: therapeutic implications. Blood. 1983;62:709–721. [PubMed] [Google Scholar]
- Quentmeier H, Reinhardt J, Zaborski M, Drexler HG. FLT3 mutations in acute myeloid leukemia cell lines. Leukemia. 2003;17:120–124. doi: 10.1038/sj.leu.2402740. [DOI] [PubMed] [Google Scholar]
- Lindhagen E, Nygren P, Larsson R. The fluorometric microculture cytotoxicity assay. Nat Protoc. 2008;3:1364–1369. doi: 10.1038/nprot.2008.114. [DOI] [PubMed] [Google Scholar]
- Ahmed SA, Gogal RM, Walsh JE. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: an alternative to [3H]thymidine incorporation assay. J Immunol Methods. 1994;170:211–224. doi: 10.1016/0022-1759(94)90396-4. [DOI] [PubMed] [Google Scholar]
- Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- Thiede C, Steudel C, Mohr B, Schaich M, Schakel U, Platzbecker U, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99:4326–4335. doi: 10.1182/blood.v99.12.4326. [DOI] [PubMed] [Google Scholar]
- Rosnet O, Buhring HJ, Marchetto S, Rappold I, Lavagna C, Sainty D, et al. Human FLT3/FLK2 receptor tyrosine kinase is expressed at the surface of normal and malignant hematopoietic cells. Leukemia. 1996;10:238–248. [PubMed] [Google Scholar]
- Gabert J, Beillard E, van der Velden VH, Bi W, Grimwade D, Pallisgaard N, et al. Standardization and quality control studies of ‘real-time' quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia - a Europe Against Cancer program. Leukemia. 2003;17:2318–2357. doi: 10.1038/sj.leu.2403135. [DOI] [PubMed] [Google Scholar]
- Hollingshead MG, Alley MC, Camalier RF, Abbott BJ, Mayo JG, Malspeis L, et al. In vivo cultivation of tumor cells in hollow fibers. Life Sci. 1995;57:131–141. doi: 10.1016/0024-3205(95)00254-4. [DOI] [PubMed] [Google Scholar]
- Alley MC, Scudiero DA, Monks A, Hursey ML, Czerwinski MJ, Fine DL, et al. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988;48:589–601. [PubMed] [Google Scholar]
- Larsson R.Prediction of Cytotoxic Drug Resistance in Acute LeukemiaBernal S (ed).Marcel Dekker Inc: New York; 1998 [Google Scholar]
- Nygren P, Fridborg H, Csoka K, Sundstrom C, de la Torre M, Kristensen J, et al. Detection of tumor-specific cytotoxic drug activity in vitro using the fluorometric microculture cytotoxicity assay and primary cultures of tumor cells from patients. Int J Cancer. 1994;56:715–720. doi: 10.1002/ijc.2910560517. [DOI] [PubMed] [Google Scholar]
- Cortes J, Perl A, Smith C, Kovacsovics T, Dombret H, Döhner H, et al. A phase II open-label, AC220 monotherapy efficacy (ACE) study in patients with acute myeloid leukemia (AML) with FLT3-ITD activating mutations: interim results European Hematology Association MeetingAbstract No 10192011
- Mi Q, Pezzuto JM, Farnsworth NR, Wani MC, Kinghorn AD, Swanson SM. Use of the in vivo hollow fiber assay in natural products anticancer drug discovery. J Nat Prod. 2009;72:573–580. doi: 10.1021/np800767a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapper S, Mills KI, Gilkes AF, Austin SJ, Walsh V, Burnett AK. The effects of lestaurtinib (CEP701) and PKC412 on primary AML blasts: the induction of cytotoxicity varies with dependence on FLT3 signaling in both FLT3-mutated and wild-type cases. Blood. 2006;108:3494–3503. doi: 10.1182/blood-2006-04-015487. [DOI] [PubMed] [Google Scholar]
- Frohling S, Scholl C, Gilliland DG, Levine RL. Genetics of myeloid malignancies: pathogenetic and clinical implications. J Clin Oncol. 2005;23:6285–6295. doi: 10.1200/JCO.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Vainchenker W, Dusa A, Constantinescu SN. JAKs in pathology: role of Janus kinases in hematopoietic malignancies and immunodeficiencies. Semin Cell Dev Biol. 2008;19:385–393. doi: 10.1016/j.semcdb.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Foss B, Ulvestad E, Bruserud O. Platelet-derived growth factor (PDGF) in human acute myelogenous leukemia: PDGF receptor expression, endogenous PDGF release and responsiveness to exogenous PDGF isoforms by in vitro cultured acute myelogenous leukemia blasts. Eur J Haematol. 2001;67:267–278. doi: 10.1034/j.1600-0609.2001.0430a.x. [DOI] [PubMed] [Google Scholar]
- Parcells BW, Ikeda AK, Simms-Waldrip T, Moore TB, Sakamoto KM. FMS-like tyrosine kinase 3 in normal hematopoiesis and acute myeloid leukemia. Stem Cells. 2006;24:1174–1184. doi: 10.1634/stemcells.2005-0519. [DOI] [PubMed] [Google Scholar]
- Chen W, Drakos E, Grammatikakis I, Schlette EJ, Li J, Leventaki V, et al. mTOR signaling is activated by FLT3 kinase and promotes survival of FLT3-mutated acute myeloid leukemia cells. Mol Cancer. 2010;9:292. doi: 10.1186/1476-4598-9-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling RJ, Topisirovic I, Fonseca BD, Sonenberg N. Dissecting the role of mTOR: lessons from mTOR inhibitors. Biochim Biophys Acta. 2010;1804:433–439. doi: 10.1016/j.bbapap.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Rodgers JT, Haas W, Gygi SP, Puigserver P. Cdc2-like kinase 2 is an insulin-regulated suppressor of hepatic gluconeogenesis. Cell Metab. 2010;11:23–34. doi: 10.1016/j.cmet.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu SH, Small D. Mechanisms of resistance to FLT3 inhibitors. Drug Resist Updat. 2009;12:8–16. doi: 10.1016/j.drup.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools J, Mentens N, Furet P, Fabbro D, Clark JJ, Griffin JD, et al. Prediction of resistance to small molecule FLT3 inhibitors: implications for molecularly targeted therapy of acute leukemia. Cancer Res. 2004;64:6385–6389. doi: 10.1158/0008-5472.CAN-04-2148. [DOI] [PubMed] [Google Scholar]
- Kindler T, Lipka DB, Fischer T. FLT3 as a therapeutic target in AML: still challenging after all these years. Blood. 2010;116:5089–5102. doi: 10.1182/blood-2010-04-261867. [DOI] [PubMed] [Google Scholar]
- Furukawa Y, Vu HA, Akutsu M, Odgerel T, Izumi T, Tsunoda S, et al. Divergent cytotoxic effects of PKC412 in combination with conventional antileukemic agents in FLT3 mutation-positive versus -negative leukemia cell lines. Leukemia. 2007;21:1005–1014. doi: 10.1038/sj.leu.2404593. [DOI] [PubMed] [Google Scholar]
- Levis M, Pham R, Smith BD, Small D. In vitro studies of a FLT3 inhibitor combined with chemotherapy: sequence of administration is important to achieve synergistic cytotoxic effects. Blood. 2004;104:1145–1150. doi: 10.1182/blood-2004-01-0388. [DOI] [PubMed] [Google Scholar]
- Snead JL, O'Hare T, Adrian LT, Eide CA, Lange T, Druker BJ, et al. Acute dasatinib exposure commits Bcr-Abl-dependent cells to apoptosis. Blood. 2009;114:3459–3463. doi: 10.1182/blood-2007-10-113969. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.