Abstract
Epstein-Barr Virus (EBV) is a γ-herpesvirus that infects >90% of the human population. Although EBV persists in its latent form in healthy carriers, the virus is also associated with several human cancers. EBV is strongly associated with Burkitt lymphoma (BL), even though there is still no satisfactory explanation of how EBV participates in BL pathogenesis. However, new insights into the interplay between viruses and microRNAs (miRNAs) have recently been proposed. In particular, it has been shown that B-cell differentiation in EBV-positive BL is impaired at the post-transcriptional level by altered expression of hsa-miR-127. Here, we show that the overexpression of hsa-miR-127 is due to the presence of the EBV-encoded nuclear antigen 1 (EBNA1) and give evidence of a novel mechanism of direct regulation of the human miRNA by this viral product. Finally, we show that the combinatorial expression of EBNA1 and hsa-miR-127 affects the expression of master B-cell regulators in human memory B cells, confirming the scenario previously observed in EBV-positive BL primary tumors and cell lines. A good understanding of these mechanisms will help to clarify the complex regulatory networks between host and pathogen, and favor the design of more specific treatments for EBV-associated malignancies.
Keywords: Epstein-Barr virus, microRNAs, Burkitt lymphoma
Introduction
The herpesviruses represent a very large, clearly defined group of viruses of considerable medical importance and uniqueness. Epstein-Barr virus (EBV) is the best-known and most widely studied member, due to its clinical and oncogenic importance.1 Following primary infection, EBV preferentially infects B-lymphocytes and establishes a persistent infection, which is maintained throughout the host's lifetime. Infection of other cell types occurs (principally epithelial cells) but is much less efficient.2 Primary EBV infection in vivo generally arises at an early age and is usually asymptomatic. However, if the infection is acquired during adolescence or later, it can result in infectious mononucleosis.3 Of note, EBV has been implicated in the pathogenesis of an increasing number of human malignancies, including a strong association with Burkitt lymphoma (BL).4, 5, 6 The World Health Organization classification recognizes three subsets of BL: endemic (eBL), sporadic (sBL) and immunodeficiency-associated (ID-BL).7 Each affects different populations and can present in different forms.8 More than 90% of eBL carry latent EBV in the form of nuclear extra-chromosomal episomes, whereas only about 20% of sBL are associated with EBV.8 The common characteristic of all BL virtually is the translocation of the MYC proto-oncogene to an immunoglobulin (Ig) locus, determining the overexpression of c-MYC.7
Although EBV is not generally regarded as a driving force of BL cell proliferation, it has an important role in its pathogenesis.8 One striking feature of EBV-positive BLs is their unique pattern of viral latent protein expression, which is restricted to EBV-encoded nuclear antigen 1 (EBNA1): the same pattern of viral latency is found in latently infected memory B cells when they divide to maintain normal homeostasis in healthy carriers.9, 10 Only EBNA1 can allow the viral genome to be transferred to daughter cells.9 Similar to memory B cells, EBV-positive BL B cells (mostly eBL and ID-BL) carry high numbers of Ig heavy chain somatic mutations and signs of antigen selection.11
Taken together, these data might suggest that memory B cells are the normal counterpart to EBV-positive BL. However, latter findings are in contrast with the germinal center (GC) phenotype shared by all of the BL variants.11 Recently, this discrepancy has been unraveled by investigation of microRNA (miRNA) dysregulation.12 In fact, we have previously demonstrated a strong upregulation of the cellular miRNA hsa-miR-127 in EBV-positive BL primary tumors, which results in the impairment of B-cell differentiation by modulation of the master regulators of GC B cells in a B cell that is already differentiated.12
Although the mechanism of action of miRNAs is reasonably well known, the different mechanisms by which miRNAs are regulated are continuously emerging.13, 14, 15, 16 The interplay between miRNAs and viruses, for example, has recently come to light.17, 18, 19 Viruses can adopt various strategies to regulate miRNA expression of host and viral miRNAs targeting either viral transcripts or cellular transcripts.20 Interestingly, it is ultimately emerging that viral factors can regulate host miRNAs. In particular, the EBV-encoded latent membrane protein 1 induces the expression of hsa-miR-146a via nuclear factor kappa-light-chain-enhancer of activated B cells.21, 22
This study was designed to ascertain whether hsa-miR-127 overexpression in BL is really related to the presence of the virus. We have focused on EBNA1 as the only viral product present in EBV-positive BL. Our results show that the upregulation of hsa-miR-127 is mediated by EBNA1, shedding new light on the function of miRNAs and their regulation by viral products.
Materials and methods
Cell culture
Raji (EBV-positive BL-derived cell line), Ramos (EBV-negative BL-derived cell line), 293T (human embryonic kidney cell line) and HeLa (cervical cancer cell line) cells were obtained from the American Type Culture Collection (ATCC, Milan, Italy) (references: #CCL-86, #CRL-1596, #CCL-2, #CRL-11268). Raji, Ramos and 293T cells were cultured in RPMI 1640 medium (Invitrogen, Milan, Italy) supplemented with 10% fetal bovine serum (FBS) (Lonza, Basel, Switzerland), 2 mℳ glutamine (Lonza), 100 U/ml penicillin (Lonza) and 100 μg/ml streptomycin (Lonza). HeLa cells were cultured in Dulbecco's modified Eagle medium (Invitrogen) supplemented with 10% FBS, 2 mℳ glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin.
B-cell isolation
For the preparation of peripheral blood mononuclear cells (PBMC), buffy coats (about 50–70 ml) from normal healthy donors were obtained from the Immunohaematology and Transfusion Medicine Service (Azienda Ospedaliera Senese, Siena, Italy) and processed within 6 h. All donors were informed about the objectives of the study and gave their consent. The study was approved by the ethical committee of the University of Siena, Italy. PBMC were prepared using lympholyte gradient medium (Cedarlane Laboratories, Celbio, Pero, Italy). Fifteen milli litre of lympholyte medium were pipetted onto the filtering disk of a 50 ml tube. After 30 s of centrifugation at 300 g the lympholyte was beneath the disk and 35 ml of blood were poured on top of the disc. After centrifugation for 10 min at 800 g, the PBMC and serum were collected separately from above the disk. The PBMC were washed twice with phosphate-buffered saline (PBS) and immediately subjected to magnetic separation. Purified memory B cells (CD27+CD19+) were isolated from the human PBMC of normal donors using the memory B isolation kit (#130-093-546, Miltenyi Biotec, Cologne, Germany) according to the manufacturer's instructions. The purity of the enriched cell populations was always checked by flow cytometry. Memory B cells were cultured in RPMI 1640 medium (Invitrogen) supplemented with 10% FBS (Lonza), 2 mℳ glutamine (Lonza), 100 U/ml penicillin (Lonza) and 100 μg/ml streptomycin (Lonza).
Nucleofection
Transient transfections of the Ramos cell line were performed by nucleofection using an Amaxa nucleofector apparatus (Amaxa, Cologne, Germany), programme T16 and solution T (Lonza) as a nucleofector buffer solution, according to the manufacturer's instructions. Ramos cells (5 × 106) were transfected with PBS as mock, 5 μg of pcDNA3 and pcDNA3-EBNA1, kindly provided by Professor Ming-Ching Kao (Department of Biochemistry, National Defense Medical Center, Taipei, Taiwan). Transfection efficiency was assessed using the green fluorescent protein (GFP) reporter gene, after transfection of Ramos cells with 2 μg of pmaxGFP Vector (Amaxa). For flow cytometry analyses, performed 24 h post transfection, cells were washed three times in PBS and analyzed by FACScan flow cytometer (BD Biosciences, San Jose, CA, USA). RNA and proteins were extracted 24 h after nucleofection.
Transient transfection of human memory B cells was performed by nucleofection using an Amaxa apparatus, programme U15 and solution for human memory B cells (Lonza) as a nucleofector buffer solution, following the manufacturer's instructions. Human memory B cells (1 × 106) were transfected with PBS as mock, pcDNA3-EBNA1 (1 μg) or cotransfected with pcDNA3-EBNA1 (1 μg) plus 100 nℳ of hsa-miR-127 mimic (mature sequence 5′-UCGGAUCCGUCUGAGCUUGGCU-3′, MIMAT0000446, Dharmacon, Thermo Scientific, Bonn, Germany), and their corresponding controls: pcDNA3 (1 μg) or pcDNA3 (1 μg) plus 100 nℳ of negative control (NC) of the hsa-miR-127 mimic (CN-001000-01 Dharmacon, Thermo Scientific). Transfection efficiency was assessed using the GFP reporter gene, after transfection of memory B cells with 2 μg of pmaxGFP Vector (Amaxa). For flow cytometry analyses, performed 48 h post transfection, cells were washed three times in PBS and analyzed by FACScan flow cytometer (BD Biosciences). RNA and protein were extracted 48 h after nucleofection.
Retroviral infection
Drug selection of the 293T cell line was carried out using 0.3 mg/ml Geneticin (G418, #G9516, Sigma-Aldrich, Saint Louis, MI, USA). 293T cells were seeded on 100 mm dishes to give a maximum of 70% confluence/plate on the day of transfection. The 293T cells were cotransfected with 10 μg of the retroviral plasmids pMSCV and pMSCV-EBNA1 silencer (siEBNA1, a generous gift from Professor Erik K Flemington, Department of Pathology and Laboratory Medicine, Tulane University School of Medicine, New Orleans, LA, USA), and 10 μg each of the packaging plasmids (p60 gag-pol and p456 env),23 using calcium phosphate (Invitrogen). The medium was replaced the following day. Forty-eight hours later, viral supernatants were collected and used for infection. A total of 5 × 105 Raji cells were plated in 100-mm dishes. Twenty-four hours later cells were spun down and suspended in 2 ml of viral supernatant and 16 μg/ml of polybrene (Sigma-Aldrich). After a further 6 h, 8 ml of medium were added to the plates. Infected Raji cells were collected at day 0 and after 7, 14 and 21 days for further analyses.
Immunization and cell sorting
Five C57BL/6 mice were immunized with sheep red blood cells (200 μl, 10%v/v) to induce GC B cells. At day 7, splenic naïve B cells (B220+Fas−) and antigen-experienced B cells (FashighB220+) were sorted using a FACSAria (BD Biosciences).
Quantitative reverse transcription-PCR (qRT-PCR)
RNA was extracted with TRIzol (Invitrogen) and converted into cDNA using random primers (Roche, Milan, Italy) and SuperScriptII reverse transcriptase (Invitrogen). The amount and quality of RNA were evaluated by measuring the optical density at 260 nm, the 260/230 and the 260/280 ratios using a Nanodrop spectrophotometer (Celbio). Target mRNA was quantified using a SYBR green PCR assay (Qiagen). Hypoxanthine phosphoribosyltransferase was used as the normalization control. The primers used are listed in Table 1.
Table 1. Primer sequences used for quantitative real-time PCR.
| Gene symbol | Forward primer 5′–3′ | Reverse primer 5′–3′ |
|---|---|---|
| EBNA1 | GGATCCATGGGTGATGGAGGCAGG | AAGATATCACTCCTGCCCTTC |
| PRDM1/BLIMP-1 | AGACTTGCAACAAGGGCTTT | CCCGTGTGTACCAGGTAGTG |
| XBP-1 | AGTCCGCAGCACTCAGACTA | GGGTCCAAGTTGTCCAGAAT |
| BCL-6 | GAAGCCCTATCCCTGTGAAA | TTCTCTCCTGTGTGGATTCG |
| IRF-4 | ACCCGCAGATGTCCATGAG | GTGGCATCATGTAGTTGTGAACCT |
| CALLA/CD10 | CTGGAGATTCATAATGGATCTTGTAAGCAGC | CCATCCAAGTGAGGTCATCTAAAGTCTG |
| HPRT | AGCCAGACTTTGTTGGATTTG | TTTACTGGCGATGTCAATAAG |
Abbreviations: BCL-6, B-cell lymphoma 6; CALLA/CD10, common acute lymphocytic leukemia antigen/CD10; EBNA1, Epstein-Barr nuclear antigen 1; HPRT, hypoxanthine phosphoribosyltransferase; IRF-4, interferon regulatory factor 4; PRDM1/BLIMP-1, PR domain zinc-finger protein 1/B lymphocyte-induced maturation protein 1; XBP-1, X-box-binding protein 1.
Hsa-miR-127 expression was assessed by qRT-PCR after RNA extraction with TRIzol (Invitrogen), reverse-transcribed with hsa-miR-127-3p-specific primers ( Cat#000452, Applied Biosystems, Applera, Carlsbad, CA, USA) and amplified using an hsa-miR-127-3p-specific primer and TaqMan probe (Cat#4427975, Applied Biosystems, Applera). RNU6B, RNU43 and hsa-miR-191 (Cat#001093, Cat#001095, Cat#002299, Applied Biosystems, Applera), which were stably expressed among the samples, were used for normalization. To check the expression of mmu-miR-127 in the murine cells, we used the same primer set and Taqman probe (Cat#000452, Applied Biosystems, Applera) used for human cells, as the sequence homology was 100%.
qRT-PCR was performed using an Opticon 2 system (Bio-Rad, MJ Research, Hercules, CA, USA). Differences in gene and miRNA expression were calculated using the ΔΔCt method.24
Indirect immunofluorescence
For EBNA1 detection, human memory B cells were smeared on positively charged slides and fixed in cold acetone for 8 min at 4 °C. Permeabilization was achieved by washing cells in PBS, 0.2% Triton X-100 and 1% bovine serum albumin. Saturation was performed for 1 h at room temperature in goat serum (Zymed laboratories, Milan, Italy). The primary antibody anti-EBNA1 (ab20777, Abcam, Cambridge, UK) was diluted in goat serum at a dilution of 1:100. Primary antibody incubation was carried out at room temperature for 1 h. Secondary goat anti-mouse antibody, conjugated with Alexa Fluor568 (Molecular Probes, Invitrogen, Milan, Italy), was diluted 1:100 in goat serum and incubated at room temperature for 45 min. The slides were examined under an Axiovert 200 microscope (Carl Zeiss, Oberkochen, Germany) and processed with Zeiss software (Carl Zeiss).
Western blotting (WB)
Raji and Ramos cell pellets were lysed in EBC buffer (50 mℳ Tris-HCl pH 8.0, 120 mℳ NaCl, 0.5% NP40 and fresh protease inhibitors). WB was performed with anti-EBNA1 ((1:100), 1EB12, sc-81581, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and anti-ACTIN ((1:1000), mAbcam 8224, AbCam) using the enhanced chemiluminiscence system (ECL, Pierce, Rockford, IL, USA), according to the manufacturer's instructions.
Luciferase assay
A luciferase construct containing the hsa-miR-127 promoter (pGL3-miR127) was kindly donated by Professor Li Wang of the University of Utah.25 Briefly, 1.5 × 105 HeLa cells were seeded in a 6-well plate and transfected with different combinations of pRL Renilla luciferase reporter vector (1 μg), pGL3 basic (2 μg), pGL3-miR127 (2 μg) and pcDNA3-EBNA1 (2 μg), using calcium phosphate (Invitrogen). Cells were harvested 48 h after transfection and subjected to luciferase assay. The reaction was normalized with Renilla luciferase activity. A dual-luciferase assay was performed in triplicate, according to the manufacturer's instructions (Promega, Milan, Italy).
Results
Mmu-miR-127 is downregulated in the course of B-cell differentiation
We previously reported that hsa-miR-127 is differentially expressed in B-cell subsets isolated from peripheral blood, being downregulated in both human plasma cells (CD138+) and memory B cells (CD27+), compared with naïve B cells (CD27−).12 Furthermore, after induction of the in vitro differentiation of resting B cells into plasmablasts, the downregulation of hsa-miR-127 was only visible in fully stimulated samples with interleukin-2, cytosine–phosphate–guanine and two fragments antigen-binding (F(ab')2), whereas no changes were detectable in cells stimulated with cytosine–phosphate–guanine alone. The differential expression of this miRNA in distinct B-cell subsets strongly suggests its significant role in the B-cell differentiation program, and that the physiological regulation of hsa-miR-127 in B cells might pass through B-cell receptor (BCR) signaling, as only full induction with F(ab')2 directed against the BCR is able to downregulate its expression.12
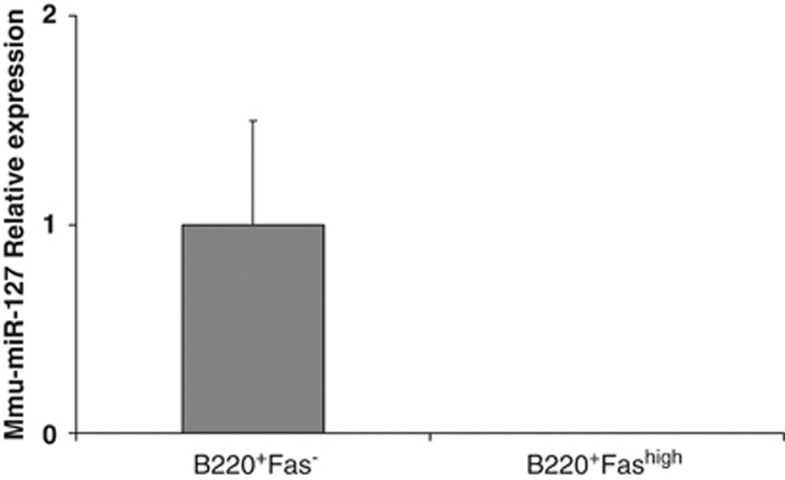
To confirm that the downregulation of hsa-miR-127 is due to the activation of BCR signaling, we extended our previous findings to an in vivo murine system. To this end, we sorted murine splenic naïve B cells (B220+Fas−) and antigen-experienced B cells (B220+Fashigh) 7 days after sheep red blood cell immunization and measured the expression of mmu-miR-127 by qRT-PCR. Similar to human cells, mmu-miR-127 expression was low or undetected in B cells that had experienced the GC reaction compared with their naïve counterparts (Figure 1), suggesting that mmu-miR-127 needs to be downregulated during B-cell differentiation and exit from the GC.
Figure 1.
Expression of mmu-miR-127 in a murine in vivo model: Expression of mmu-miR-127 was evaluated by qRT-PCR in naïve (B220+Fas−) and antigen-experienced (B220+Fashigh) sorted B cells, after sheep red blood cell immunization of C57BL/6 mice. A strong downregulation of mmu-miR-127 was observed in the course of an immune response. The graph shows the results of five independent experiments. Error bars represent s.d. between replicates.
Hsa-miR-127 is upregulated after ectopic expression of EBNA1
To ascertain whether hsa-miR-127 overexpression in BL is related to the presence of the virus, we focused on the EBV-encoded EBNA1 protein, as this is the only viral product expressed in EBV-positive BL cells.
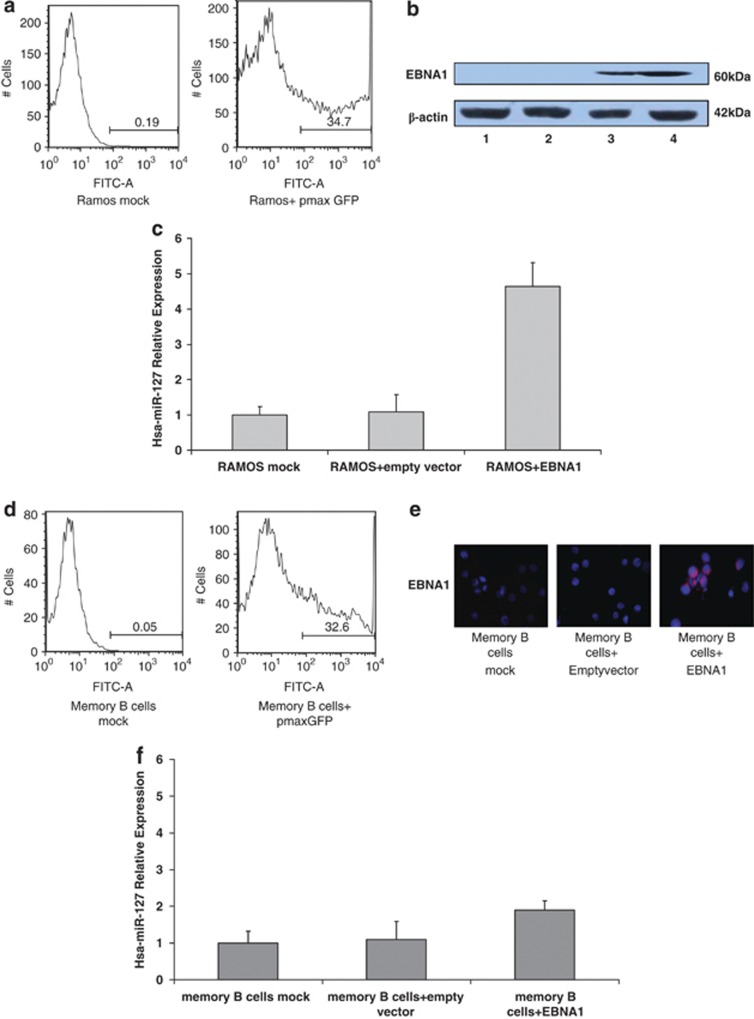
We transiently transfected EBNA1 in the EBV-negative BL Ramos cell line, in which we previously reported low expression of hsa-miR-127.12 A transfection efficiency of 35% was determined by FACS analysis (Figure 2a) and EBNA1 protein was assessed by western blot (Figure 2b). We found an increase in endogenous hsa-miR-127 expression 24 h after transfection in the Ramos cells transfected with EBNA1, compared with the controls (Figure 2c).
Figure 2.
Expression of hsa-miR-127 after transient transfection with EBNA1 in Ramos and human memory B cells. (a) The percentage of cells expressing GFP was determined by FACS analysis in Ramos cells 48 h post transfection (Ramos+pmax GFP) and compared with the control (Ramos mock). A transfection efficiency of 35% was achieved. (b) EBNA1 protein levels were evaluated by WB in the EBV-negative BL cell line (Ramos) after transient transfection of EBNA1. EBNA1 was upregulated 48 h after transfection. Lane 1—Ramos mock; lane 2—Ramos transfected with the empty vector pcDNA3; lane 3—Ramos transfected with pcDNA3-EBNA1; lane 4—positive control (Raji). (c) Expression of hsa-miR-127 was evaluated by qRT-PCR in the EBV-negative BL cell line (Ramos) after transient transfection of EBNA1. Hsa-miR-127 was upregulated 48 h after transfection in Ramos cells transfected with EBNA1, in comparison with the controls (Ramos mock and Ramos+ empty vector). The graph shows the results of three independent experiments. Error bars represent s.d. between replicates. (d) The percentage of cells expressing GFP was determined by FACS analysis in memory B cells 24 h post transfection (memory B cells+pmax GFP) and compared with the control (memory B cells mock). A transfection efficiency of 33% was achieved. (e) EBNA1 protein levels in memory B cells were evaluated by indirect immunofluorescence after transient transfection of EBNA1. EBNA1 was upregulated 24 h after transfection. (f) Expression of hsa-miR-127 was evaluated by qRT-PCR in human memory B cells purified from the peripheral blood of normal donors after transient transfection of EBNA1. Hsa-miR-127 was upregulated 24 h after transfection in memory B cells transfected with EBNA1, in comparison with the controls (memory B cells mock and memory B cells+ empty vector). The graph shows the results of three independent experiments. Error bars represent s.d. between replicates.
Furthermore, we performed ectopic expression of EBNA1 in memory B cells (CD27+, CD19+) purified from the peripheral blood of normal human donors. A transfection efficiency of 33% was determined by 5 fluorescence-activated cell sorting (FACS) analysis (Figure 2d) and EBNA1 protein was assessed by immunofluorescence (Figure 2e). An increase in hsa-miR-127 levels was observed 48 h post transfection in memory B cells transfected with EBNA1, compared with the controls (Figure 2f).
Hsa-miR-127 and EBNA1 ectopic expression modulate B-cell differentiation markers
In a previous study, functional in vitro analyses performed in lymphoblastoid cell lines provided evidence that hsa-miR-127 was able to decrease the expression of BLIMP-1 and XBP-1 in a dose-dependent fashion, resulting in a consequent overexpression of BCL-6. These data were also confirmed in an EBV-positive BL (Raji) cell line after transfection of hsa-miR-127 inhibitor: upregulation of IRF-4 expression was also observed.12
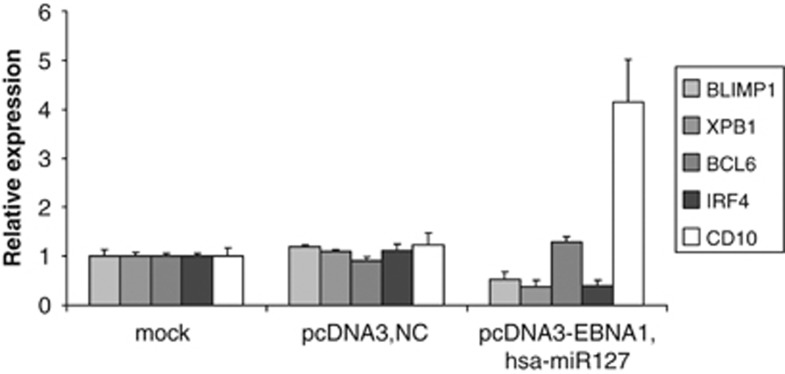
To test the effect of hsa-miR-127 on B-cell differentiation, we purified human memory B cells—the proposed normal counterpart of EBV-positive BL cells—and we ectopically expressed both EBNA1 and hsa-miR-127, in order to closely imitate a possible molecular pathway occurring during the pathogenesis of EBV-positive BL. As shown in Figure 3, the synergistic upregulation of EBNA1 and hsa-miR-127 in memory B cells determines a downregulation of BLIMP-1, XBP-1 and IRF-4 mRNA and an increase in BCL-6 and CD10 expression, compared with the control sample, as previously observed in BL cell lines and primary tumors.
Figure 3.
Effects of cotransfection with hsa-miR-127 and EBNA1 on B-cell markers in human memory B cells. Expression of BLIMP-1, XBP-1, BCL-6, IRF-4 and CD10 was evaluated by qRT-PCR in human memory B cells purified from peripheral blood of normal donors after cotransfection with both hsa-miR-127 mimic and EBNA1 vector. A downregulation of BLIMP-1, XBP-1 and IRF-4 was observed, whereas the expression of BCL-6 and CD10 was increased in memory B cells+ pcDNA3-EBNA1+ hsa-miR-127 mimic compared with the control (memory B cells mock and memory B cells+ pcDNA3+ NC). The graph shows the results of three independent experiments. Error bars represent s.d. between replicates.
EBNA1 activates a luciferase construct driven by hsa-miR-127
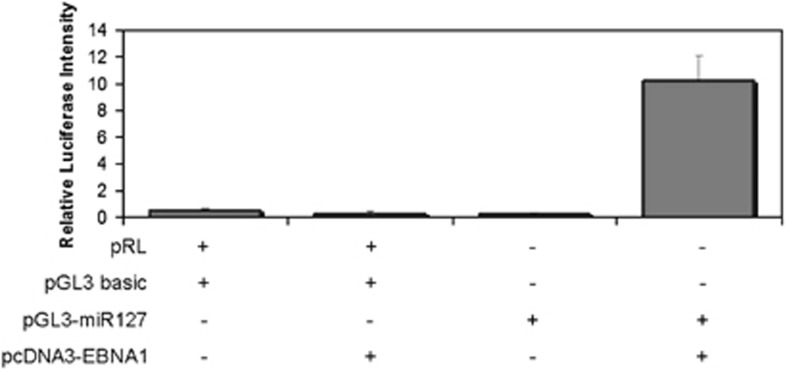
To test whether EBNA1 can modulate the promoter activity of hsa-miR-127, we transfected HeLa cells with a luciferase reporter construct containing the promoter region of hsa-miR-127 in combination with a vector expressing EBNA1. Ectopic expression of EBNA1 led to increased activity of the hsa-miR-127 promoter, suggesting that EBV may directly control the levels of hsa-miR-127 (Figure 4).
Figure 4.
Relative luciferase activity of the hsa-miR-127 promoter after ectopic expression of EBNA1. The promoter region of hsa-miR-127, linked to a luciferase reporter gene, was cotransfected with a vector coding for EBNA1 in a HeLa cell line, and relative luciferase activity was measured. The increase in luciferase activity following ectopic expression of EBNA1 indicates regulation of hsa-miR-127 expression by this viral product. Different combinations of pRL, pGL3 basic, pGL3-miR127 and pcDNA3-EBNA1 were transfected in HeLa cells: (+) and (−) indicate the presence or absence of the corresponding plasmids, respectively. The graph shows the results of three independent experiments. Error bars represent s.d. between replicates.
Hsa-miR-127 is downregulated after EBNA1 silencing
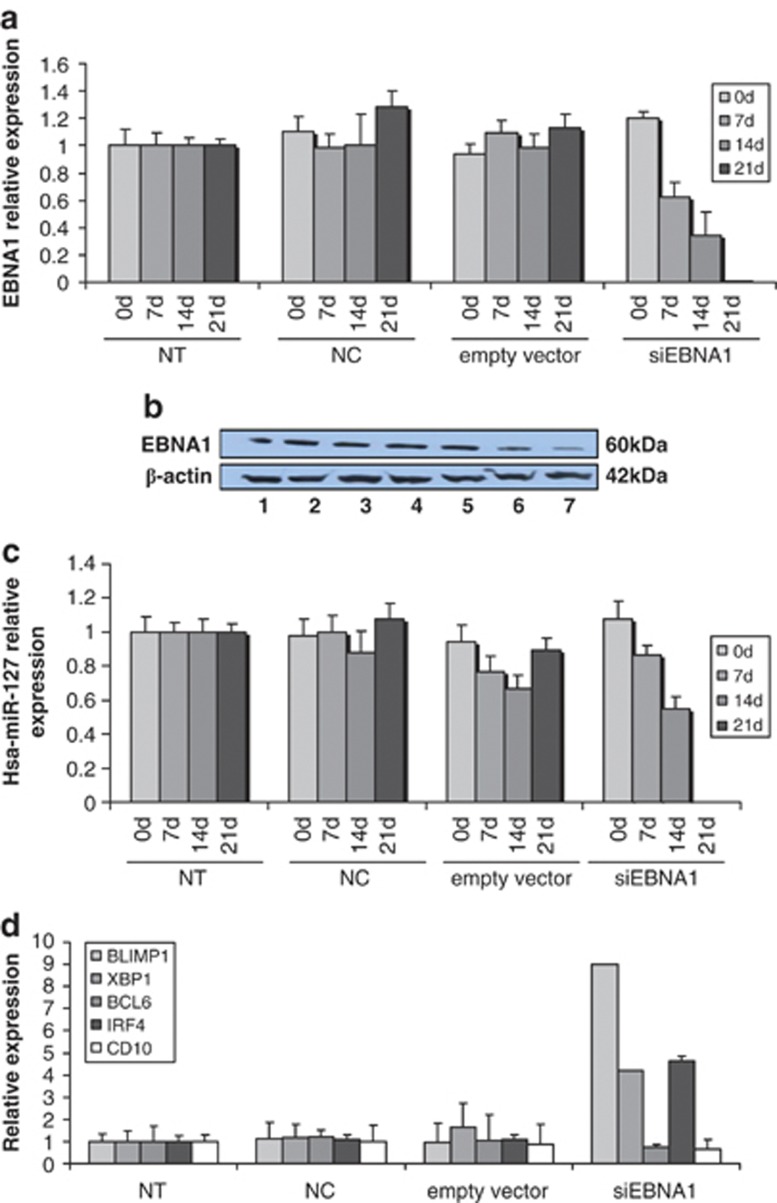
To confirm whether or not hsa-miR-127 regulation is mediated by EBNA1, we performed a retroviral infection using a siEBNA1 vector in EBV-positive Raji cells. EBNA1 mRNA and protein levels were checked by qRT-PCR and WB, respectively (Figures 5a and b).
Figure 5.
Expression of hsa-miR-127 and B-cell markers after EBNA1 silencing. (a) The expression of EBNA1 mRNA was evaluated by qRT-PCR at day 0 and 7, 14 and 21 days after infection of Raji cells with viral supernatants expressing p60 gag-pol and p456 env only (NC), pMSCV (empty vector) and pMSCV-EBNA1 (siEBNA1), compared with untreated (NT) Raji cells. EBNA1 was silenced throughout the experiment. The graph shows the results of three independent experiments. Error bars represent s.d. between replicates. (b) The expression of EBNA1 protein was evaluated by WB at day 0 and 7, 14 and 21 days after infection of Raji cells with viral supernatants expressing p60 gag-pol and p456 env only (NC), pMSCV (empty vector) and pMSCV-EBNA1 (siEBNA1), compared with untreated (NT) Raji cells. EBNA1 was silenced throughout the experiment. Line 1—NT Raji; line 2—Raji+ NC; line 3—Raji+ empty vector; line 4—Raji+ siEBNA1 on day 0; line 5—Raji+ siEBNA1 after 7 days; line 6—Raji+ siEBNA1 after 14 days; line 7—Raji+ siEBNA1 after 21 days. (c) The expression of hsa-miR-127 was evaluated by qRT-PCR at day 0 and 7, 14 and 21 days after infection of Raji cells with viral supernatants expressing p60 gag-pol and p456 env only (NC), pMSCV (empty vector) and pMSCV-EBNA1 (siEBNA1), compared with untreated (NT) Raji cells. Hsa-miR-127 was strongly downregulated throughout the experiment. The graph shows the results of three independent experiments. Error bars represent s.d. between replicates. (d) Expression of BLIMP-1, XBP-1, BCL-6, IRF-4 and CD10 was evaluated by qRT-PCR at day 21 after infection of Raji cells with viral supernatants expressing p60 gag-pol and p456 env only (NC), pMSCV (empty vector) and pMSCV-EBNA1 (siEBNA1), compared with untreated (NT) Raji cells. An upregulation of BLIMP-1, XBP-1 and IRF-4 was observed, whereas the expression of BCL-6 and CD10 was decreased in Raji+ siEBNA1 compared with the controls. The graph shows the results of three independent experiments. Error bars represent s.d. between replicates.
As expected, silencing of EBNA1 determined a decrease in hsa-miR-127 levels starting 7 days post infection (Figure 5c), as well as consequently high levels of BLIMP-1, XBP-1 and IRF-4 mRNAs, in contrast to BCL-6 and CD10 mRNA (Figure 5d).
Discussion
EBV infects humans and persists for life in about 90% of adults without causing disease.5 However, even in its latent form, EBV is implicated in the pathogenesis of an increasing number of human malignancies, including nasopharyngeal carcinoma, 30–50% of Hodgkin's lymphomas and approximately 50% of lymphomas arising in immunosuppressed patients.4 It is with the eBL that the virus has a very close association, being present in more than 90% of cases.26
EBV-positive BL is often spoken of as a tumor of GC origin because it bears phenotypic markers of GC B cells.5 However, this view has recently been challenged by the finding that the pattern and rate of somatic hypermutations in EBV-positive BLs are identical to those found in EBV-positive memory B-cells from peripheral blood.11, 27 Furthermore, BL tumor cells share the same pattern of viral latency as found in latently EBV-infected memory B cells.28 Recent findings suggest that this discrepancy in BL might be due to an altered expression of hsa-miR-127 in EBV-positive BL cases, compared with EBV-negative cases and normal controls.19, 29 Our previous findings suggested that upregulation of hsa-miR-127 in EBV-positive BL may determine the downregulation of BLIMP-1 and XBP-1 and the consequent persistence of BCL-6 expression, as well as the GC phenotype in B cells that have already differentiated towards memory B cells in terms of Ig mutation pattern.12 Furthermore, the expression of putative targets of hsa-miR-127 was tested by Gene Set Enrichment Analysis on a recent eBL signature.30 Interestingly, 10 out of 18 of supposed targets of hsa-miR-127 were found to be enriched in the eBL signature.30 Although studies are providing increasing evidence of miRNA deregulation in this tumor, the data currently available remains limited. The most recent cellular miRNA profiling data suggest that a few miRNAs are differentially expressed between eBL and sBL primary tumors.30, 31 Hsa-miR-127 did not emerge from human miRNA profiling of eBL and sBL, probably due to the different degrees of sensitivity of microarray and qRT-PCR techniques. For this reason, the expression of hsa-miR-127, which was identified by qRT-PCR, might remain undetected by microarray, whereas qRT-PCR still represents the proof-of-principle for gene quantification. Furthermore, viral miRNAs and other viral products in BL might interfere in the regulation of human mRNAs and miRNAs.31
One striking feature of EBV-positive BL is the unique pattern of viral latent protein expression, which is restricted to EBNA1.10 The question of how EBNA1 promotes lymphomagenesis is still the subject of debate. This antigen's role as a transcription factor for cellular genes potentially involved in oncogenesis has recently found support in the notion that EBNA1 may contribute to DNA damage and genomic instability.32, 33 A direct antiapoptotic function of EBNA1, which is able to antagonize p53 function, has also been reported.34, 35 Accordingly, it has been hypothesized that the real contribution of the virus is to make tumor cells resistant to the apoptosis induced by c-MYC.5
In this study, we sought to understand the role of EBNA1 in the regulation of hsa-miR-127 and to clarify its involvement in the pathogenesis of BL. Our previous findings suggested that the physiological regulation of hsa-miR-127 in B cells might pass through BCR signaling.12 Here, we confirmed the regulation of the cellular miRNA-127 during normal immune function in an in vivo murine model. Furthermore, we showed that the increase of hsa-miR-127 levels previously observed in both EBV-positive primary tumors and cell lines is due to regulation mediated by the viral product EBNA1. In fact, ectopic expression of EBNA1 results in hsa-miR-127 upregulation in Ramos cells and human memory B cells. Furthermore, the combinatorial expression of hsa-miR-127 and EBNA1 provides evidence of a modulation of B-cell markers in human memory B cells, resulting in a decrease of BLIMP-1, XBP-1 and IRF-4, and an increase in BCL-6 and CD10 mRNA, thus confirming the scenario previously observed in EBV-positive BL primary tumors and cell lines. On the other hand, EBNA1 silencing in an EBV-positive cell line reduces the endogenous level of hsa-miR-127 and affects the expression of the B-cell markers. Finally, the increased luciferase activity of the hsa-miR-127 promoter in the presence of EBNA1 prompted us to suggest a novel mechanism of direct regulation of the cellular miRNA-127 by the viral product EBNA1, which may result in the modulation of genes involved in B-cell differentiation.
On the basis of our results, we can conclude that EBNA1 is involved in the activation of hsa-miR-127, determining transcriptional reprogramming in EBV-infected memory B cells. The generation of GC lymphocytes and their subsequent differentiation to memory and plasma cells is characterized by the strict regulation of specific genes. Given that tumorigenesis is a multistep process that occurs over long periods of time, it is virtually impossible to know how directly the final cellular or viral phenotype of BL relates to the original infected precursor.
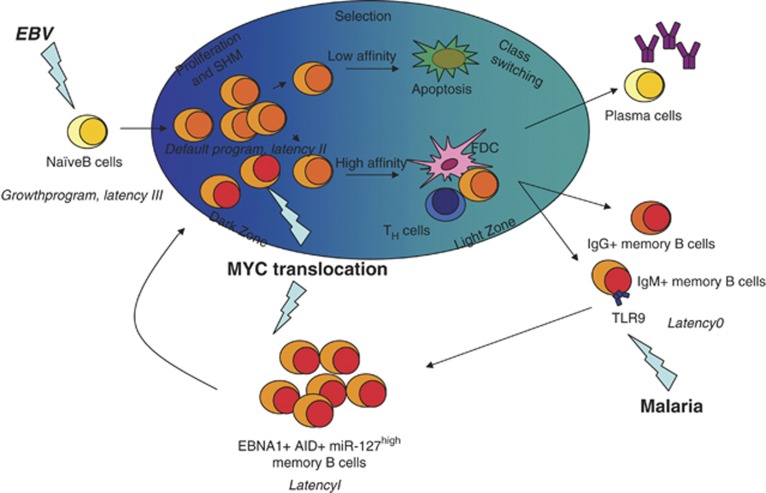
It has recently been shown that memory B cells can be composed of antigen-dependent as well as antigen-independent subsets.36, 37 Both of these subsets are enriched by an IgM component, in addition to an IgG one. In particular, IgM memory B cells ensure the replenishment of the memory pool from antigen-experienced precursors by their rapid mobilization in GCs.38 In holoendemic malaria areas, where EBV-positive BL is endemic, Plasmodium falciparum infection induces the clonal expansion of infected memory B cells, through interaction with Toll-like receptor 9.39, 40, 41 When this subset of cells divide they express EBNA1, which in turn determines the upregulation of hsa-miR-127, and the shift to the characteristic GC phenotype, as shown in this study. However, it should be taken into account that the Toll-like receptor 9 ligand binding also results in the induction of activation-induced cytidine deaminase.42, 43 In particular, the active form of activation-induced cytidine deaminase causes DNA breaks in the heavy chain (IgH) regions, regardless of the stage of B-cell differentiation. This may predispose the cell to chromosomal abnormalities such as MYC/IgH translocations.39 However, it remains to be determined when MYC translocation occurs: in an EBV-infected memory B cell during clonal expansion or in a memory B cell that has re-entered the GC reaction (Figure 6). Intriguingly, this scenario is in accordance with a recent gene expression profiling study, which showed an enrichment of the BCR signaling pathway in EBV-positive BL, suggesting a consistent role for chronic antigenic stimulation in the pathogenesis of BL.30
Figure 6.
Model of EBV-associated BL pathogenesis. EBV-infected naïve B cells undergo a GC reaction to gain access to the memory B-cell pool. In infected naïve B cells, EBV activates a growth programme (also termed ‘latency III'), which is characterized by their expression of nine latent viral genes. Although these cells will be recognized and targeted by T-cell-mediated immune response, a fraction of them will enter the GC, where they will express only three latent viral genes (default programme or ‘latency II'). In the GC reaction, somatic mutations are introduced into variable (V) genes of antigen-activated B cells during proliferation. GC B cells are subsequently selected for high-affinity binding to the respective antigen, and finally differentiate into memory B cells or plasma cells. Thus, the virus gains access to the memory B-cell compartment, its main reservoir during persistence, where no latent viral genes are expressed (‘latency 0'). Stimulation of the Toll-like receptor 9 (TLR9) by malaria infection induces the clonal expansion of memory B cells, which express EBNA1 (‘latency I') and activation-induced cytidine deaminase (AID) when they divide. EBNA1 expression in this subset of cells allows viral DNA to replicate and may thus result in the upregulation of hsa-miR-127 and in the shift to the characteristic GC phenotype and re-entry into the GC reaction. The active form of AID causes DNA breaks in the heavy chain (IgH) regions, regardless of the stage of B-cell differentiation. This may predispose to chromosomal abnormalities such as MYC/IgH translocations. However, it remains to be determined when MYC translocation occurs: in an EBV-infected memory B cell during clonal expansion or in a memory B cell that has re-entered the GC reaction.
Collectively, these findings suggest a novel mechanism of interaction between viral products and cellular miRNAs. Notwithstanding the encouraging results of studies on miRNAs in this regard, many challenges remain and further studies will be necessary to better elucidate the complexity of the viral mechanisms involved in BL lymphomagenesis.
Acknowledgments
This work was funded by the ‘Regional Health Research Programme 2009' grant from the Region of Tuscany. We thank Emma Thorley for proofreading the manuscript.
Author contributions
OA and LL conceived and designed the experiments; OA, VE, NM, AG, MF, MS performed the experiments; OA, VE, DG and LL analyzed the data; OA and LL wrote the paper.
The authors declare no conflict of interest.
References
- Khanna R, Burrows SR, Moss DJ. Immune regulation in Epstein-Barr virus-associated diseases. Microbiol Rev. 1995;59:387–405. doi: 10.1128/mr.59.3.387-405.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LS, Rickinson A. Epstein-Barr virus: 40 years on. Nat Rev Cancer. 2004;4:757–768. doi: 10.1038/nrc1452. [DOI] [PubMed] [Google Scholar]
- Joncas J, Boucher J, Granger-Julien M, Filion C. Epstein-Barr virus infection in the neonatal period and in childhood. Can Med Assoc J. 1974;110:33–37. [PMC free article] [PubMed] [Google Scholar]
- Bakaletz LO. Developing animal models for polymicrobial diseases. Nat Rev Microbiol. 2004;2:552–568. doi: 10.1038/nrmicro928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorley-Lawson DA, Allday MJ. The curious case of the tumour virus: 50 years of Burkitt's lymphoma. Nat Rev Microbiol. 2008;6:913–924. doi: 10.1038/nrmicro2015. [DOI] [PubMed] [Google Scholar]
- Bornkamm GW. Epstein-Barr virus and its role in the pathogenesis of Burkitt's lymphoma: an unresolved issue. Semin Cancer Biol. 2009;19:351–365. doi: 10.1016/j.semcancer.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Swerdlow SH, Campo E, Harris NL, Jaffe E, Pileri S, Stein H.WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues(ed) IARC: Lyon, France, 2008
- Allday MJ. How does Epstein-Barr virus (EBV) complement the activation of Myc in the pathogenesis of Burkitt's lymphoma. Semin Cancer Biol. 2009;19:366–376. doi: 10.1016/j.semcancer.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg D, Middeldorp JM, Catalina M, Sullivan JL, Luzuriaga K, Thorley-Lawson DA. Demonstration of the Burkitt's lymphoma Epstein-Barr virus phenotype in dividing latently infected memory cells in vivo. Proc Natl Acad Sci USA. 2004;101:239–244. doi: 10.1073/pnas.2237267100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy G, Komano J, Sugden B. Epstein-Barr virus provides a survival factor to Burkitt's lymphomas. Proc Natl Acad Sci USA. 2003;100:14269–14274. doi: 10.1073/pnas.2336099100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellan C, Lazzi S, Hummel M, Palummo N, De Santi M, Amato T, et al. Immunoglobulin gene analysis reveals 2 distinct cells of origin for EBV-positive and EBV-negative Burkitt lymphomas. Blood. 2005;106:1031–1036. doi: 10.1182/blood-2005-01-0168. [DOI] [PubMed] [Google Scholar]
- Leucci E, Onnis A, Cocco M, De Falco G, Imperatore F, Antonicelli G, et al. B-cell differentiation in EBV-positive Burkitt lymphoma is impaired at posttranscriptional level by miRNA-altered expression. Int J Cancer. 2009;126:1316–1326. doi: 10.1002/ijc.24655. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115:787–798. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- Lee YS, Dutta A. MicroRNAs in cancer. Annu Rev Pathol Mech Dis. 2009;4:199–227. doi: 10.1146/annurev.pathol.4.110807.092222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottwein E, Cullen BR. Viral and cellular microRNAs as determinants of viral pathogenesis and immunity. Cell Host Microbe. 2008;3:375–387. doi: 10.1016/j.chom.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecellier CH, Dunoyer P, Arar K, Lehmann-Che J, Eyquem S, Himber C, et al. A cellular microRNA mediates antiviral defense in human cells. Science. 2005;308:557–560. doi: 10.1126/science.1108784. [DOI] [PubMed] [Google Scholar]
- Scaria V, Hariharan M, Maiti S, Pillai B, Brahmachari SK. Host-virus interaction: a new role for microRNAs. Retrovirology. 2006;3:68. doi: 10.1186/1742-4690-3-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaria V, Hariharan M, Pillai B, Maiti S, Brahmachari SK. Host-virus genome interactions: macro roles for microRNAs. Cell Microbiol. 2007;9:2784–2794. doi: 10.1111/j.1462-5822.2007.01050.x. [DOI] [PubMed] [Google Scholar]
- Motsch N, Pfuhl T, Mrazek J, Barth S, Grasser FA. Epstein-Barr virus-encoded latent membrane protein 1 (LMP1) induces the expression of the cellular microRNA miR-146a. RNA Biol. 2007;4:131–137. doi: 10.4161/rna.4.3.5206. [DOI] [PubMed] [Google Scholar]
- Cameron JE, Yin Q, Fewell C, Lacey M, McBride J, Wang X, et al. Epstein-Barr virus latent membrane protein 1 induces cellular MicroRNA miR-146a, a modulator of lymphocyte signaling pathways. J Virol. 2008;82:1946–1958. doi: 10.1128/JVI.02136-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claudio PP, Howard CM, Pacilio C, Cinti C, Romano G, Minimo C, et al. Mutations in the retinoblastoma-related gene RB2/p130 in lung tumors and suppression of tumor growth in vivo by retrovirus-mediated gene transfer. Cancer Res. 2000;60:372–382. [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Song G, Wang L. Transcriptional mechanism for the paired miR-433 and miR-127 genes by nuclear receptors SHP and ERRgamma. Nucleic Acids Res. 2008;36:5727–5735. doi: 10.1093/nar/gkn567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornkamm GW. Epstein-Barr virus and the pathogenesis of Burkitt's lymphoma: more questions than answers. Int J Cancer. 2009;124:1745–1755. doi: 10.1002/ijc.24223. [DOI] [PubMed] [Google Scholar]
- Thorley-Lawson DA, Gross A. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N Eng J Med. 2004;350:1328–1337. doi: 10.1056/NEJMra032015. [DOI] [PubMed] [Google Scholar]
- Souza TA, Stollar BD, Sullivan JL, Luzuriaga K, Thorley-Lawson DA. Influence of EBV on the peripheral blood memory B cell compartment. J Immunol. 2007;179:3153–3160. doi: 10.4049/jimmunol.179.5.3153. [DOI] [PubMed] [Google Scholar]
- De Falco G, Antonicelli G, Onnis A, Lazzi S, Bellan C, Leoncini L. Role of EBV in microRNA dysregulation in Burkitt lymphoma. Semin Cancer Biol. 2009;19:401–406. doi: 10.1016/j.semcancer.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Piccaluga PP, De Falco G, Kustagi M, Gazzola A, Agostinelli C, Tripodo C, et al. Gene expression analysis uncovers similarity and differences among Burkitt lymphoma subtypes. Blood. 2011;117:3596–3608. doi: 10.1182/blood-2010-08-301556. [DOI] [PubMed] [Google Scholar]
- Lenze D, Leoncini L, Hummel M, Volinia S, Liu CG, Amato T, et al. The different epidemiologic subtypes of Burkitt lymphoma share a homogenous micro RNA profile distinct from diffuse large B-cell lymphoma. Leukemia. 2011;25:1869–1876. doi: 10.1038/leu.2011.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamranvar SA, Gastaldello S, Masucci MG. The Epstein-Barr virus nuclear antigen-1 promotes genomic instability via induction of reactive oxygen species. Proc Natl Acad Sci USA. 2008;106:2313–2318. doi: 10.1073/pnas.0810619106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamranvar SA, Masucci MG. The Epstein-Barr virus nuclear antigen-1 promotes telomere dysfunction via induction of oxidative stress. Leukemia. 2011;25:1017–1025. doi: 10.1038/leu.2011.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saridakis V, Sheng Y, Sarkari F, Holowaty MN, Shire K, Nguyen T, et al. Structure of the p53 binding domain of HAUSP/USP7 bound to Epstein-Barr nuclear antigen 1 implications for EBV-mediated immortalization. Mol Cell. 2005;18:25–36. doi: 10.1016/j.molcel.2005.02.029. [DOI] [PubMed] [Google Scholar]
- Renouf B, Hollville E, Pujals A, Tetaud C, Garibal J, Wiels J. Activation of p53 by MDM2 antagonists has differential apoptotic effects on Epstein-Barr virus (EBV)-positive and EBV-negative Burkitt's lymphoma cells. Leukemia. 2009;23:1557–1563. doi: 10.1038/leu.2009.92. [DOI] [PubMed] [Google Scholar]
- Dogan I, Bertocci B, Vilmont V, Delbos F, Megret J, Storck S, et al. Multiple layers of B cell memory with different effector functions. Nat Immunol. 2009;10:1292–1299. doi: 10.1038/ni.1814. [DOI] [PubMed] [Google Scholar]
- Lanzavecchia A, Bernasconi N, Traggiai E, Ruprecht CR, Corti D, Sallusto F. Understanding and making use of human memory B cells. Immunol Rev. 2006;211:303–309. doi: 10.1111/j.0105-2896.2006.00403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bende RJ, van Maldegem F, Triesscheijn M, Wormhoudt TA, Guijt R, van Noesel CJ. Germinal centers in human lymph nodes contain reactivated memory B cells. J Exp Med. 2007;204:2655–2665. doi: 10.1084/jem.20071006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moormann AM, Snider CJ, Chelimo K. The company malaria keeps: how co-infection with Epstein-Barr virus leads to endemic Burkitt lymphoma. Curr Opin Infect Dis. 2011;24:435–441. doi: 10.1097/QCO.0b013e328349ac4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrath I. Epidemiology: clues to the pathogenesis of Burkitt lymphoma. Br J Haematol. 2012;156:744–756. doi: 10.1111/j.1365-2141.2011.09013.x. [DOI] [PubMed] [Google Scholar]
- Peng S. Signaling in B cells via Toll-like receptors. Curr Opin Immunol. 2005;17:230–236. doi: 10.1016/j.coi.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Ramiro AR, Jankovic M, Callen E, Difilippantonio S, Chen HT, McBride KM, et al. Role of genomic instability and p53 in AID-induced c-myc-Igh translocations. Nature. 2006;440:105–109. doi: 10.1038/nature04495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709–760. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]