Abstract
Despite the increased prevalence of cocaine use and abuse in males as compared to females, possible effects of paternal cocaine exposure on biobehavioral development has received little attention. We therefore exposed male mice to cocaine (20 mg/kg, i.p.) or vehicle for 10 weeks, and then used those mice as sires. We then behaviorally phenotyped the F1 offspring in order to assess the consequences of paternal cocaine exposure on brain function. We report the presence of a subtle but significant increase in immobility in the tail suspension test, a measure of behavioral depression, following paternal cocaine. Body weight was also significantly decreased in paternal cocaine-exposed offspring. Other aspects of neurobehavioral function, including locomotor activity, anxiety, and learning and memory, were not affected by paternal cocaine history. These data suggest alterations in brain systems and/or circuitry underlying mood regulation in the offspring of cocaine-using fathers.
Keywords: mouse, anxiety, memory, depression, tail suspension, elevated maze, activity
INTRODUCTION
The psychostimulant drug cocaine remains highly abused with recent prevalence rates approaching 15% for lifetime and 1% for use within the immediately prior month (Office of Applied Studies, 2003). These numbers are especially alarming as more research becomes available on long lasting effects of cocaine on the brain across development. The rewarding effects of cocaine are largely mediated by its actions in blocking brain dopamine transporters to increase extracellular levels of the neurotransmitter in the brain’s reward circuits (Kuhar et al., 1991; Withers et al., 1995).
Numerous clinical and animal studies have pointed to significant long-lasting effects of maternal cocaine exposure on biobehavioral development and the development of normal brain architecture (Bandstra et al., 2001; Bhide, 2009; Chelonis et al., 2003; Dow-Edwards, 2011; Lester et al., 2003; Lidow, 1998; Mactutus, 1999; Malanga et al., 2008; Mayes, 1999; Ren et al., 2004; Richardson et al., 1996; Stanwood and Levitt, 2007a; Stanwood et al., 2001; Thompson et al., 2009). Possible effects of paternal exposure, in contrast, have received little attention, despite the fact that males are more than twice as likely as females to have used cocaine in the past year and to have met the criteria for abuse of or dependence upon cocaine in the past year (Office of Applied Studies, 2003). Cocaine can accumulate within testicular tissues and semen (Cone et al., 1996; Yazigi et al., 1991; Yazigi and Polakoski, 1992). Specific cocaine binding sites have been described within sperm cells (Yazigi and Polakoski, 1992). Moreover, a handful of previous studies have associated paternal cocaine exposure prior to mating with a reduction in birth weight (George et al., 1996) and behavioral abnormalities (Abel et al., 1989a; Abel et al., 1989b; He et al., 2006), effects which may be mediated through epigenetic alterations within male gametes (He et al., 2006).
The present study was therefore undertaken to explore the possibility that chronically exposing future sires to cocaine (20 mg/kg, i.p.) might alter behavioral development of their offspring. We report the presence of a subtle but significant decrease in body weight and an increase in a measure of behavioral depression following paternal cocaine. Other aspects of neurobehavioral function, including locomotor activity, anxiety, and learning and memory, did not appear to be affected by paternal cocaine history.
MATERIALS AND METHODS
Animals
Twelve male C57Bl/6J mice (10–12 weeks of age) were acquired from the Jackson Laboratory (Bar Harbor, ME). Mice were then acclimated to a Vanderbilt University School of Medicine vivarium for one week, during which time they were ear tagged for identification and handled by investigators. Mice were housed 4 per cage, maintained on a 12/12-h light/dark cycle, and provided ad libitum access to food and water. Female mice (also 10–12 weeks of age at delivery; Jackson Laboratory) arrived 2–4 weeks prior to breeding and were initially housed 4–5 per cage until the mating procedure began. All procedures were approved by the Animal Care and Use Committee at Vanderbilt University.
Injections
Male mice were divided into 2 groups of 6 mice each that received either cocaine (20 mg/kg, 4mg/cc, i.p., considered equivalent to a moderate dosing regimen) or 0.9% saline control solution. The mice were injected daily between the hours of 1–4 pm for 10 weeks. Body weights were obtained and recorded several times per week to calculate injection volumes and confirm that the treatment did not produce any overt physiological or anorectic effects.
Breeding
Females were exposed to lightly soiled male bedding in order to synchronize estrous cycles and increase breeding success as previously described (Marsden and Bronson, 1964). Sires were added to the cages in the second day after the female was exposed to soiled bedding (of that same future sire). Daily injections of the sire continued during this time. Females were examined for vaginal plugs the following morning and pregnancies were confirmed by palpation and weight gain at days E14-E15. If plugs were not observed or successful pregnancy was not observed within 3 days of pairing, the female was repaired with a male mouse still receiving the same prescribed treatment. The sire was then removed from the cage so as not to modulate the dam’s behavior or interact with the offspring. Each saline- or cocaine-injected male was used to produce one litter from a single dam. Pups were reared by their birth mothers and weighed weekly until weaning. The pups were weaned at P21, and male and female offspring were then housed in same-sex groups (3–5 per cage).
Behavioral Testing
Mice were extensively handled for at least one week prior to the beginning of experiments, were at least P60 prior to testing, and were habituated to the testing rooms for ~30 min prior to testing. Testing apparati were cleaned with Vimoba spray (Quip Labs, Wilmington, DE) between animals and wiped with paper towels to clear the solution and any debris.
Elevated Plus Maze (EPM)
Anxiety responses were examined using a custom EPM (White, 67 cm length × 6.5 cm width × 15 cm height in the closed arms). The entire apparatus was elevated 40 cm from the ground, and testing was conducted under normal lighting (250–300 lux). At the start of the 5 min trial, each mouse was lowered by its tail onto an open section of the maze, directly next to and facing a closed section. Sessions were recorded by a ceiling-mounted video camera connected to a computer using video acquisition and analysis software (ANY-maze, Stoelting, Wood Dale, IL). Data analyzed included the amount of time in and number of entries into each zone of the maze (Carpenter et al., 2012; McLaughlin et al., 2012).
Open Field Activity
Locomotor activity in a novel open field was measured using commercial open field activity chambers (Med Associates, 27 × 27 × 20.5 cm) that were contained within light- and air-controlled environmental chambers (Med Associates, 64 × 45 × 42 cm). Location and movement were detected by the interruption of infrared beams by the body of the mouse (16 photocells in each horizontal direction, as well as 16 photocells elevated 4 cm to measure rearing) and were measured by the Med Associates Activity Monitor program (McLaughlin et al., 2012; Stanwood and Levitt, 2007b). Activity was measured for a 60 min session.
Tail Suspension Test (TST)
Behavioral despair was measured by suspending mice for 7 min by the tail from a vertical aluminum bar attached to the top of a box-like enclosure (Med Associates, 33 × 33 × 32 cm) that is open in the front (McLaughlin et al., 2012). Mice were attached to the bar by tape placed ~1.5 cm from the tip of the tail. Force transducers and automated software (Med Associates) were used to measure immobility. Settings utilized were a lower threshold of 7, upper threshold of 20, gain of 8, and resolution of 220 ms.
Novel Object Recognition
Nonspatial working memory was assessed in a two object recognition task (Gustin et al., 2011; McLaughlin et al., 2012; Thompson et al., 2005). The day before testing, mice were habituated to the novel object arena (39.5 ×28.5 × 19.5 cm, Allentown, Inc., Allentown, NJ) for 20 min. On the test day, mice were placed into the cage with two identical objects (either 50 ml conical tubes weighted with sand or small blue microscope slide boxes on their side) placed on the two sides of the cage for 5 min. After a 10 min interlude in their home cage, they were returned to the arena and allowed to explore two objects, one identical to the original objects and the other different, for an additional 5 min. Time spent exploring novel versus familiar objects was recorded by post-hoc video analysis. A counter-balanced design was used to control for side of presentation and which of the pair of objects was novel. Exploration of the two identical objects was compared to verify that there was not a measurable object or side bias despite the experimental design.
Morris Water Maze
Spatial learning and memory was assessed using the Morris water maze task. Hidden-platform testing was conducted in a 107-cm diameter pool with a circular acrylic platform (10 cm diameter) submerged 1 cm below the surface of the water, as previously described (Harrison et al., 2009a; Harrison et al., 2009b). Mice were given four acquisition trials per day for 10 days in a massed fashion, i.e., each mouse completed all four trials before the next mouse began its trials. The water maze was located in the center of a room with distinct, visual cues fixed to the walls that were clearly visible from the pool. These extra-maze cues remained stationary throughout acquisition and probe test sessions. Sessions were captured by an overhead camera and analyzed using video acquisition and analysis software (ANY-maze, Stoelting, Wood Dale, IL). Escape latency and path length to reach the hidden platform were recorded during acquisition. Twenty-four hours following target acquisition a 60-s probe trial was conducted. The time spent in the target quadrant and swim speed were the primary dependent measures derived from the probe trial.
Statistics
Differences between treatment groups were assessed by unpaired Student’s t-test or ANOVA as appropriate with significance defined as two-tailed p < 0.05. Data were first analyzed to determine if any potential phenotypes were sex-dependent. No significant differences of paternal treatment differed between male and female offspring, so data from both sexes were combined for the presented analyses. Data from offspring from the same litter were then merged prior to statistical comparisons, to eliminate litter as a potential confound. Ns therefore are six per group.
RESULTS
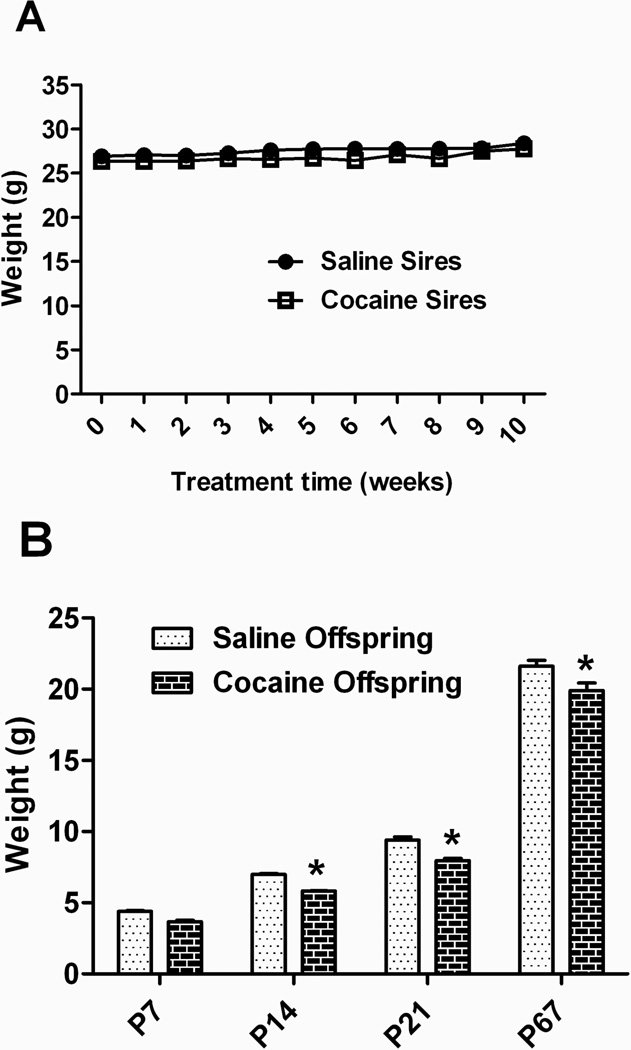
Daily cocaine injections (20 mg/kg) for ten weeks produced noticeable locomotor activation in the future sires, as expected. Body weight in this group was not significantly affected by the chronic treatment (Figure 1A). Both saline- and cocaine-treated males bred successfully and resultant litter sizes were not different from one another (6.0 ± 1.1 pups for saline, 6.0 ± 2.0 for cocaine). All deliveries were at term (~E20). Offspring of cocaine-exposed sires had significantly smaller body weights by P14 (Figure 1B), an effect that was maintained into adulthood. No other overt behavioral or physiological differences were noted.
Figure 1. Body weights of sires and offspring following paternal cocaine.
A) Chronic cocaine exposure did not significantly affect body weight in adult males, eventually used as sires in the study (n = 6 per group). B) Offspring of sires chronically treated with cocaine (10+ weeks) have significantly decreased body weight as compared to controls, beginning by postnatal day (P) 14 and lasting to adulthood (data for littermates are averaged together as specified in Materials and Methods, thus n=6 per group). *p < 0.01
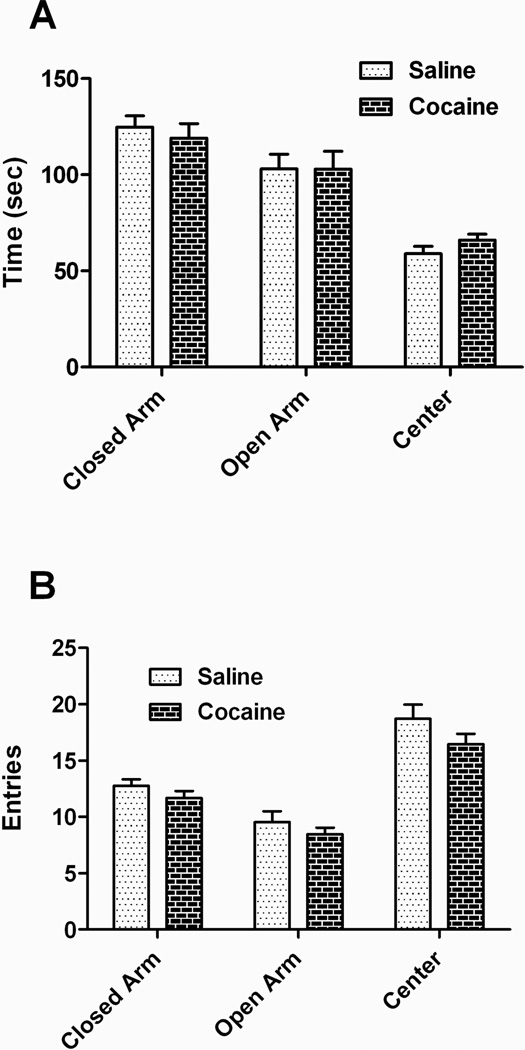
Paternal saline- and cocaine-exposed offspring were tested behaviorally as young adults (starting at P60 and continuing until ~P90). Anxiety behavior was assessed using the EPM (Figure 2). No differences in the times spent in different areas of the maze (Figure 2A) or entries (Figure 2B) were observed. Total number of entries also did not differ significantly (paternal saline = 41.01 ± 2.73; paternal cocaine = 36.61 ± 1.21, p = 0.17).
Figure 2. Anxiety-related behaviors are not altered following paternal cocaine.
Neither the time spent in each type of arm (A) nor number of arm entries (B) in an elevated plus maze differed between the treatment groups, suggesting that paternal cocaine exposure does not alter anxiety responses.
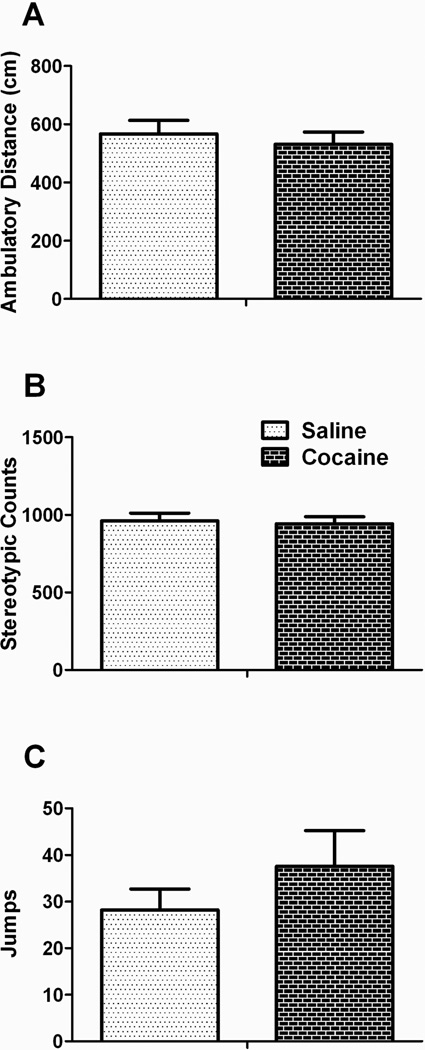
Locomotor activity patterns in open field chambers also did not significantly differ across paternal treatments (Figure 3). Analysis of center-surround activity (thigmotaxis) was also not different, confirming a lack of effect of paternal treatment on anxiety (percentage time in periphery of chamber for paternal saline = 77 ± 4.4%; paternal cocaine = 82 ± 2.2%, p = 0.39). Cocaine-induced (10 mg/kg, ip) locomotor activity was also identical in offspring from control and cocaine-exposed sires (data not shown).
Figure 3. Locomotor activity in an open field is not altered following paternal cocaine.
Paternal cocaine did not induce changes in spontaneous motor activity in a novel open field. Data presented are A) ambulatory distance, B) stereotypic counts, and C) jumping.
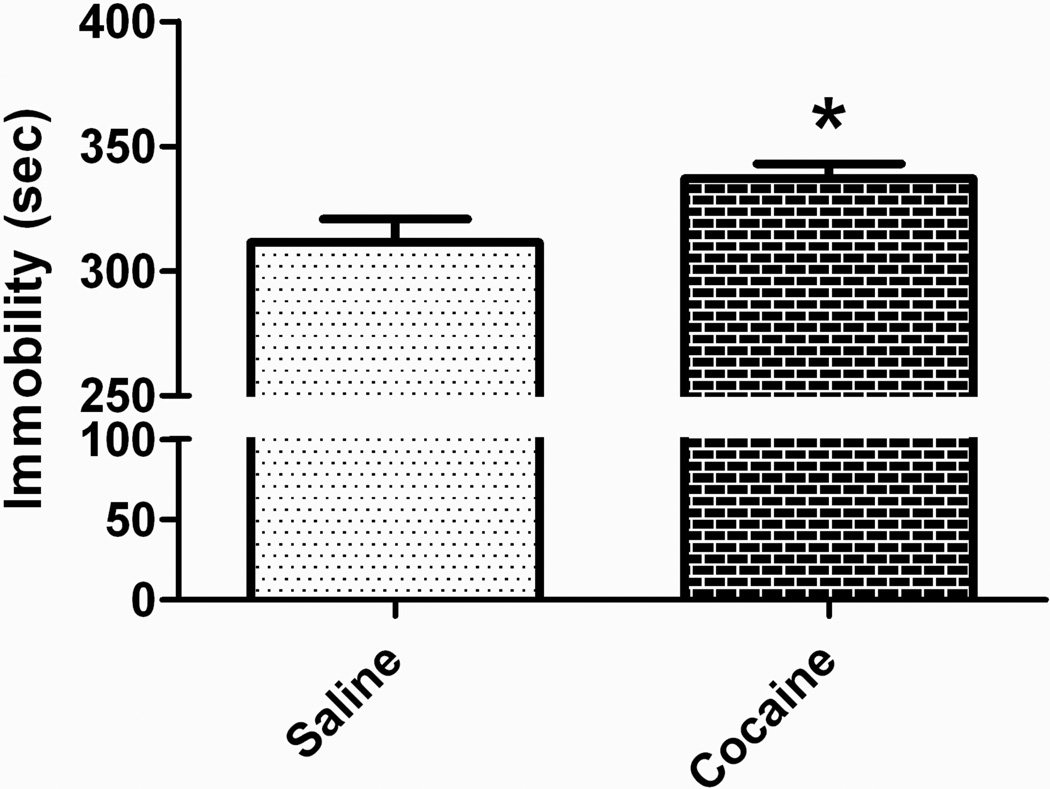
The tail suspension test revealed a significant increase in immobility in the offspring of cocaine-exposed sires (Figure 4). These data suggest the presence of increased behavioral depression following paternal cocaine.
Figure 4. Increased depression-like behavior following paternal cocaine.
Paternal cocaine resulted in increased immobility in the tail suspension test, suggestive of an increase in behavior despair. *p < 0.05
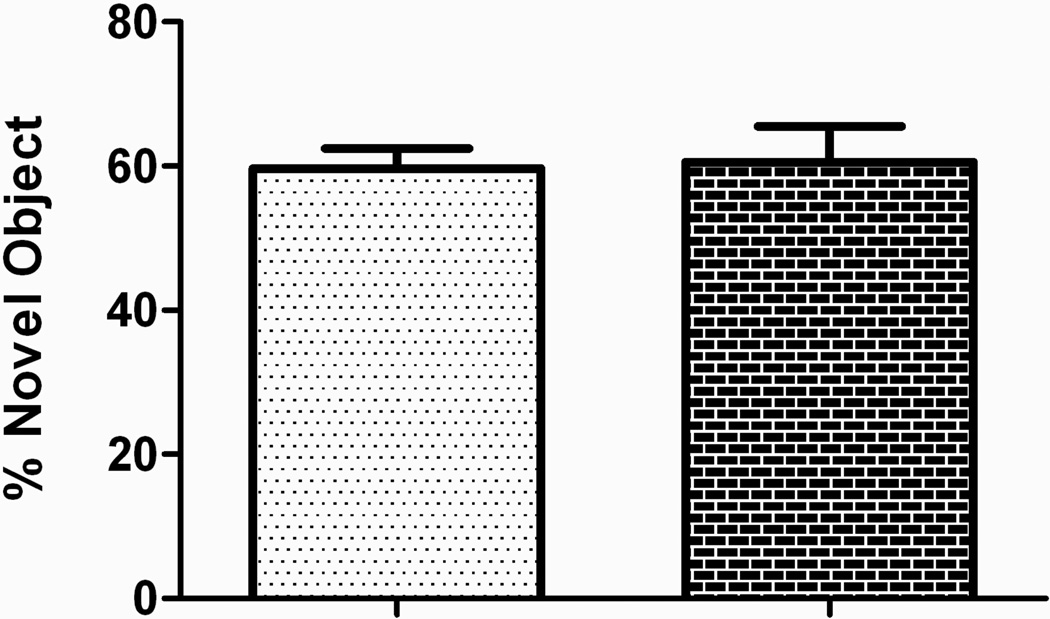
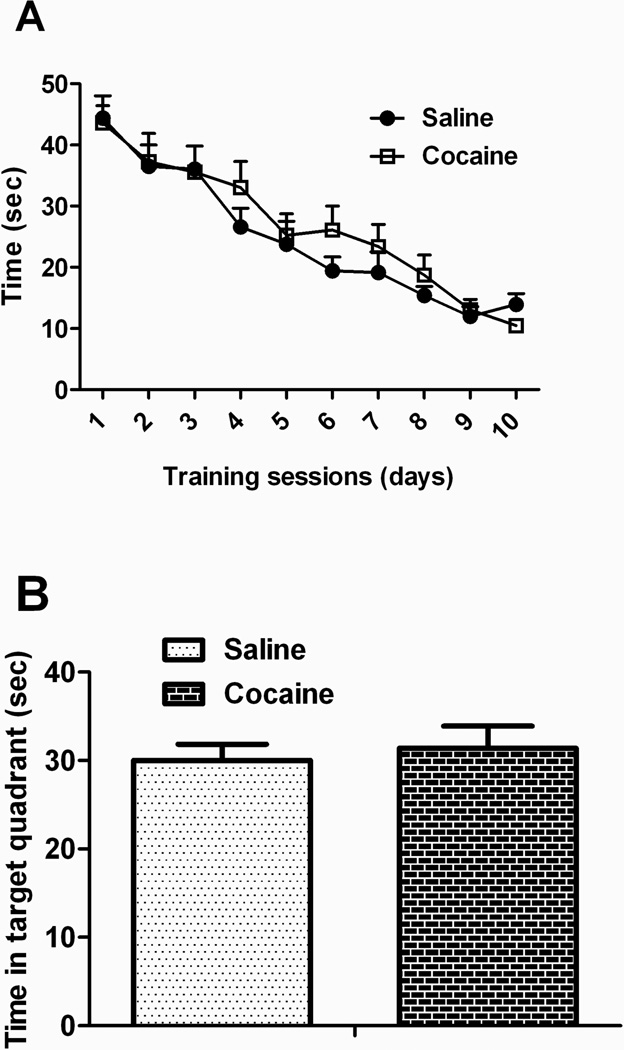
Nonspatial working memory was assessed in a 2-object novel object discrimination paradigm which revealed no differences between the groups in either their initial exploration of the objects during the familiarization trial (data not shown) nor in the novelty-test trial given 10 min later (Figure 5). Spatial learning in a Morris water maze was also intact (Figure 6). There was a significant effect of trial, but not treatment condition, during acquisition. There was also no effect of treatment in target quadrant exploration time during the probe trial (Figure 6B). Swim speed did not differ between treatment groups (data not shown).
Figure 5. Paternal cocaine does not appear to alter nonspatial working memory.
There were no differences of paternal treatment observed within the discrimination index of a novel object discrimination task.
Figure 6. Paternal cocaine does not appear to alter spatial learning or memory.
There were no differences of paternal treatment on the acquisition or probe trial of the Morris water maze, using a hidden platform design.
DISCUSSION
Paternal Cocaine Exposure
While there are vast animal and clinical literatures on the effects of maternal cocaine exposure (Bandstra et al., 2001; Bhide, 2009; Chelonis et al., 2003; Dow-Edwards, 2011; Lester et al., 2003; Lidow, 1998; Mactutus, 1999; Malanga et al., 2008; Mayes, 1999; Ren et al., 2004; Richardson et al., 1996; Stanwood and Levitt, 2007a; Stanwood et al., 2001; Thompson et al., 2009), only a handful of studies have addressed potential effects of paternal cocaine abuse (Abel et al., 1989a; Abel et al., 1989b; He et al., 2006). It is inherently difficult to track paternal data in clinical studies, and many teratological/toxicological studies in animals have been limited to examination of male fertility and measures of reproductive success.
The results of the current study suggest that long-term paternal cocaine exposure prior to conception can produce significant physiological and behavioral alterations in offspring, but that these changes are quite subtle. The developing nervous system in the offspring may be able to compensate and overcome any maladaptive consequences induced within the paternal germ cell.
First, we observed modest (but statistically significant) growth restriction (~15%). Offspring of cocaine-exposed sires were significantly smaller than controls. The difference was statistically significant at P14, but a trend was present even at P7. This effect was found in both male and female offspring, and lasted for the duration of the study; that is, the offspring of the cocaine-treated fathers never caught up, suggesting some type of developmental reprogramming of body weight. Future studies should seek to confirm this phenotype, and further assess fat versus lean muscle mass and food intake in offspring.
Secondly, we noted a significant increase in behavioral despair/depression in the paternal cocaine group, as indicated by increased immobility in a tail suspension test. This effect was relatively selective, as no changes in anxiety, motor activity, or learning and memory were apparent. It remains to be seen whether this phenotype may translate to the human condition, but our data suggests that clinicians should be alert for mood disorders in children and adults born to cocaine-abusing fathers. Studies of neurodevelopmental phenotypes following maternal cocaine exposure may also be affected by non-controlled differences in paternal exposure, since the fathers of these children also are more likely than controls to be chronic drug users (Frank et al., 2002).
Our observed effects were more subtle than those reported previously by Lidow and colleagues (He et al., 2006). There are several possible explanations for this. First, we used passive, experimenter-administered exposure of cocaine rather than active self-administration. Second, the intraperitoneal administration to sires that we utilized will have produced fairly prolonged cocaine and metabolite exposure, but the inhalational route utilized by the previous study from the Lidow lab (He et al., 2006) would have produced much higher peak plasma and tissue levels of cocaine. Background strain also varied between the studies as we used the inbred C57Bl/6J line and the previous study used outbred CD-1 mice. Lastly, the male mice in the previous study began cocaine exposure during adolescence (P28) while we waited until adulthood to begin exposure.
An even earlier study also examined the effects of cocaine on offspring behavior (Abel et al., 1989a). In this experiment, male rats were injected with vehicle, 15mg/kg, or 30 mg/kg cocaine subcutaneously for 72 days. There were significantly greater spermatozoa abnormalities in chronic cocaine-treated males, and subtle but significant changes in activity levels and learning in offspring. Importantly, the offspring were assessed for these behaviors developmentally, from P16-P35, rather than as adults (Abel et al., 1989a). Perhaps performance in those tasks within that model would also have normalized by adulthood, when our current measurements occurred.
Paternal Exposures to other Drugs and Toxins
Perhaps the largest literature regarding paternal drug effects on offspring come from the alcohol field (Friedler, 1996; Warner and Rosett, 1975). A previous history of alcohol exposure in rodent sires has been associated with growth retardation, altered locomotor activity patterns, altered reflex development, reduced grooming, learning impairments, and changes in cerebral cortical thickness (Abel, 1989; Abel, 1991; Abel and Lee, 1988; Jamerson et al., 2004; Ledig et al., 1998; Wozniak et al., 1991). At least some of these phenotypes can be ameliorated by treatment of offspring with physostigmine, suggesting cholinergic mediation of these “neuromaladaptations” (Abel, 1994).
Paternal opiate exposure has also been linked to alterations in offspring outcomes, including behavior and drug responsiveness (Cicero et al., 1995; Friedler, 1985; Friedler, 1996; Friedler and Wheeling, 1979). Similarly, paternal exposures to potential neurotoxins such as lead, solvents, and pesticides have also been linked to behavioral deficits in offspring (Brady et al., 1975; El-Helaly et al., 2011; Lowery et al., 1990).
Mechanisms of Paternal Transmission of Phenotypes
Even in the absence of drug or environmental exposures, genetically identical inbred mice can have phenotypic traits transmitted paternally. A recent compelling paper by Hen and colleagues controlled even for paternal rearing factors and demonstrated that Balb c/J offspring open field activity was strongly associated with paternal open field activity (Alter et al., 2009). Moreover, the offspring of male C57Bl/6J mice who have experienced social defeat stress exhibit increased anxiety- and depressive-like behaviors (Dietz et al., 2011). These effects are almost certainly based in epigenetics, although this has not yet been confirmed. Epigenetic regulation and multigenerational inheritance of stress responses has recently been described in another system (Crews et al., 2012). Similar mechanisms may contribute to the links between increased paternal age and increased risk of autism and other neurodevelopmental disorders (Alter et al., 2011; Malaspina et al., 2002; Malaspina et al., 2005; Reichenberg et al., 2006).
Conclusions
In conclusion, we report the presence of significant alterations in body weight and behavioral depression following paternal cocaine exposure. In one sense, our study may represent a “best possible outcome” for these effects in that the dam was not exposed to cocaine or other drugs of abuse and the same non-exposed mother raised the offspring in a typical environment. In the human condition, neither of these variables can typically be well-controlled. It is also worth noting that our current work represents a “pilot” study with only behavioral endpoints in the mouse. Future work needs to address these issues in larger cohorts, include developmental endpoints, concomitantly examine cellular and neuroanatomical measures, and examine the likely molecular mechanism, epigenetic modulation of spermatoza.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge Heather Durai, Rebecca Bluett and Dr. John Allison for excellent technical assistance and Dr. Devon Graham for constructive criticism of the manuscript. Studies were conducted using the Mouse Neurobehavioral Laboratory at Vanderbilt University and were supported in part by NICHD P30HD15052 and the Vanderbilt Kennedy Center. Work of the late Dr. Michael Lidow inspired this study.
REFERENCES
- Abel EL. Paternal behavioral mutagenesis. Neurotoxicology. 1989;10(3):335–345. [PubMed] [Google Scholar]
- Abel EL. Paternal alcohol consumption affects grooming response in rat offspring. Alcohol. 1991;8(1):21–23. doi: 10.1016/0741-8329(91)91168-2. [DOI] [PubMed] [Google Scholar]
- Abel EL. Effects of physostigmine on male offspring sired by alcohol-treated fathers. Alcohol Clin Exp Res. 1994;18(3):648–652. doi: 10.1111/j.1530-0277.1994.tb00925.x. [DOI] [PubMed] [Google Scholar]
- Abel EL, Lee JA. Paternal alcohol exposure affects offspring behavior but not body or organ weights in mice. Alcohol Clin Exp Res. 1988;12(3):349–355. doi: 10.1111/j.1530-0277.1988.tb00205.x. [DOI] [PubMed] [Google Scholar]
- Abel EL, Moore C, Waselewsky D, Zajac C, Russell LD. Effects of cocaine hydrochloride on reproductive function and sexual behavior of male rats and on the behavior of their offspring. J Androl. 1989a;10(1):17–27. doi: 10.1002/j.1939-4640.1989.tb00051.x. [DOI] [PubMed] [Google Scholar]
- Abel FL, Wilson SP, Zhao RR, Fennell WH. Cocaine depresses the canine myocardium. Circ Shock. 1989b;28(4):309–319. [PubMed] [Google Scholar]
- Alter MD, Gilani AI, Champagne FA, Curley JP, Turner JB, Hen R. Paternal transmission of complex phenotypes in inbred mice. Biol Psychiatry. 2009;66(11):1061–1066. doi: 10.1016/j.biopsych.2009.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter MD, Kharkar R, Ramsey KE, Craig DW, Melmed RD, Grebe TA, Bay RC, Ober-Reynolds S, Kirwan J, Jones JJ, Turner JB, Hen R, Stephan DA. Autism and increased paternal age related changes in global levels of gene expression regulation. PLoS One. 2011;6(2):e16715. doi: 10.1371/journal.pone.0016715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandstra ES, Morrow CE, Anthony JC, Accornero VH, Fried PA. Longitudinal investigation of task persistence and sustained attention in children with prenatal cocaine exposure. Neurotoxicol Teratol. 2001;23(6):545–559. doi: 10.1016/s0892-0362(01)00181-7. [DOI] [PubMed] [Google Scholar]
- Bhide PG. Dopamine, cocaine and the development of cerebral cortical cytoarchitecture: a review of current concepts. Semin Cell Dev Biol. 2009;20(4):395–402. doi: 10.1016/j.semcdb.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Brady K, Herrera Y, Zenick H. Influence of parental lead exposure on subsequent learning ability of offspring. Pharmacol Biochem Behav. 1975;3(4):561–565. doi: 10.1016/0091-3057(75)90173-2. [DOI] [PubMed] [Google Scholar]
- Carpenter AC, Saborido TP, Stanwood GD. Development of Hyperactivity and Anxiety Responses in Dopamine Transporter-Deficient Mice. Dev Neurosci. 2012 doi: 10.1159/000336824. [DOI] [PubMed] [Google Scholar]
- Chelonis JJ, Gillam MP, Paule MG. The effects of prenatal cocaine exposure on reversal learning using a simple visual discrimination task in rhesus monkeys. Neurotoxicol Teratol. 2003;25(4):437–446. doi: 10.1016/s0892-0362(03)00017-5. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Nock B, O'Connor L, Adams M, Meyer ER. Adverse effects of paternal opiate exposure on offspring development and sensitivity to morphine-induced analgesia. J Pharmacol Exp Ther. 1995;273(1):386–392. [PubMed] [Google Scholar]
- Cone EJ, Kato K, Hillsgrove M. Cocaine excretion in the semen of drug users. J Anal Toxicol. 1996;20(2):139–140. doi: 10.1093/jat/20.2.139. [DOI] [PubMed] [Google Scholar]
- Crews D, Gillette R, Scarpino SV, Manikkam M, Savenkova MI, Skinner MK. Epigenetic transgenerational inheritance of altered stress responses. Proc Natl Acad Sci U S A. 2012 doi: 10.1073/pnas.1118514109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz DM, Laplant Q, Watts EL, Hodes GE, Russo SJ, Feng J, Oosting RS, Vialou V, Nestler EJ. Paternal transmission of stress-induced pathologies. Biol Psychiatry. 2011;70(5):408–414. doi: 10.1016/j.biopsych.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow-Edwards D. Translational issues for prenatal cocaine studies and the role of environment. Neurotoxicol Teratol. 2011;33(1):9–16. doi: 10.1016/j.ntt.2010.06.007. [DOI] [PubMed] [Google Scholar]
- El-Helaly M, Abdel-Elah K, Haussein A, Shalaby H. Paternal occupational exposures and the risk of congenital malformations--a case-control study. International journal of occupational medicine and environmental health. 2011;24(2):218–227. doi: 10.2478/s13382-011-0019-x. [DOI] [PubMed] [Google Scholar]
- Frank DA, Brown J, Johnson S, Cabral H. Forgotten fathers: an exploratory study of mothers' report of drug and alcohol problems among fathers of urban newborns. Neurotoxicol Teratol. 2002;24(3):339–347. doi: 10.1016/s0892-0362(02)00196-4. [DOI] [PubMed] [Google Scholar]
- Friedler G. Effects of limited paternal exposure to xenobiotic agents on the development of progeny. Neurobehavioral toxicology and teratology. 1985;7(6):739–743. [PubMed] [Google Scholar]
- Friedler G. Paternal exposures: impact on reproductive and developmental outcome. An overview. Pharmacol Biochem Behav. 1996;55(4):691–700. doi: 10.1016/s0091-3057(96)00286-9. [DOI] [PubMed] [Google Scholar]
- Friedler G, Wheeling HS. Behavioral effects in offspring of male mice injected with opioids prior to mating. Pharmacol Biochem Behav. 1979;11(Suppl):23–28. [PubMed] [Google Scholar]
- George VK, Li H, Teloken C, Grignon DJ, Lawrence WD, Dhabuwala CB. Effects of long-term cocaine exposure on spermatogenesis and fertility in peripubertal male rats. J Urol. 1996;155(1):327–331. [PubMed] [Google Scholar]
- Gustin RM, Shonesy BC, Robinson SL, Rentz TJ, Baucum AJ, 2nd, Jalan-Sakrikar N, Winder DG, Stanwood GD, Colbran RJ. Loss of Thr286 phosphorylation disrupts synaptic CaMKIIalpha targeting, NMDAR activity and behavior in pre-adolescent mice. Mol Cell Neurosci. 2011;47(4):286–292. doi: 10.1016/j.mcn.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison FE, Hosseini AH, Dawes SM, Weaver S, May JM. Ascorbic acid attenuates scopolamine-induced spatial learning deficits in the water maze. Behav Brain Res. 2009a;205(2):550–558. doi: 10.1016/j.bbr.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison FE, Hosseini AH, McDonald MP, May JM. Vitamin C reduces spatial learning deficits in middle-aged and very old APP/PSEN1 transgenic and wild-type mice. Pharmacol Biochem Behav. 2009b;93(4):443–450. doi: 10.1016/j.pbb.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Lidow IA, Lidow MS. Consequences of paternal cocaine exposure in mice. Neurotoxicol Teratol. 2006;28(2):198–209. doi: 10.1016/j.ntt.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Jamerson PA, Wulser MJ, Kimler BF. Neurobehavioral effects in rat pups whose sires were exposed to alcohol. Brain Res Dev Brain Res. 2004;149(2):103–111. doi: 10.1016/j.devbrainres.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Kuhar MJ, Ritz MC, Boja JW. The dopamine hypothesis of the reinforcing properties of cocaine. Trends in Neurosciences. 1991;14(7):299–302. doi: 10.1016/0166-2236(91)90141-g. [DOI] [PubMed] [Google Scholar]
- Ledig M, Misslin R, Vogel E, Holownia A, Copin JC, Tholey G. Paternal alcohol exposure: developmental and behavioral effects on the offspring of rats. Neuropharmacology. 1998;37(1):57–66. doi: 10.1016/s0028-3908(97)00185-8. [DOI] [PubMed] [Google Scholar]
- Lester BM, Lagasse L, Seifer R, Tronick EZ, Bauer CR, Shankaran S, Bada HS, Wright LL, Smeriglio VL, Liu J, Finnegan LP, Maza PL. The Maternal Lifestyle Study (MLS): effects of prenatal cocaine and/or opiate exposure on auditory brain response at one month. J Pediatr. 2003;142(3):279–285. doi: 10.1067/mpd.2003.112. [DOI] [PubMed] [Google Scholar]
- Lidow MS. Nonhuman primate model of the effect of prenatal cocaine exposure on cerebral cortical development. Annals of the New York Academy of Sciences. 1998;846:182–193. [PubMed] [Google Scholar]
- Lowery MC, Au WW, Adams PM, Whorton EB, Jr, Legator MS. Male-mediated behavioral abnormalities. Mutat Res. 1990;229(2):213–229. doi: 10.1016/0027-5107(90)90095-l. [DOI] [PubMed] [Google Scholar]
- Mactutus CF. Prenatal intravenous cocaine adversely affects attentional processing in preweanling rats. Neurotoxicol Teratol. 1999;21(5):539–550. doi: 10.1016/s0892-0362(99)00024-0. [DOI] [PubMed] [Google Scholar]
- Malanga CJ, Riday TT, Carlezon WA, Jr, Kosofsky BE. Prenatal exposure to cocaine increases the rewarding potency of cocaine and selective dopaminergic agonists in adult mice. Biol Psychiatry. 2008;63(2):214–221. doi: 10.1016/j.biopsych.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaspina D, Corcoran C, Fahim C, Berman A, Harkavy-Friedman J, Yale S, Goetz D, Goetz R, Harlap S, Gorman J. Paternal age and sporadic schizophrenia: evidence for de novo mutations. Am J Med Genet. 2002;114(3):299–303. doi: 10.1002/ajmg.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaspina D, Reichenberg A, Weiser M, Fennig S, Davidson M, Harlap S, Wolitzky R, Rabinowitz J, Susser E, Knobler HY. Paternal age and intelligence: implications for age-related genomic changes in male germ cells. Psychiatric genetics. 2005;15(2):117–125. doi: 10.1097/00041444-200506000-00008. [DOI] [PubMed] [Google Scholar]
- Marsden HM, Bronson FH. Estrous Synchrony In Mice: Alteration By Exposure To Male Urine. Science. 1964;144:1469. doi: 10.1126/science.144.3625.1469. [DOI] [PubMed] [Google Scholar]
- Mayes LC. Developing brain and in utero cocaine exposure: effects on neural ontogeny. Development & Psychopathology. 1999;11(4):685–714. doi: 10.1017/s0954579499002278. [DOI] [PubMed] [Google Scholar]
- McLaughlin B, Buendia MA, Saborido TP, Palubinsky AM, Stankowski JN, Stanwood GD. Haploinsufficiency of the E3 Ubiquitin Ligase C-Terminus of Heat Shock Cognate 70 Interacting Protein (CHIP) Produces Specific Behavioral Impairments. PLoS One. 2012;7(5):e36340. doi: 10.1371/journal.pone.0036340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office of Applied Studies. Results from the 2002 National Survey on Drug Use and Health: National findings. Rockville, MD: SDUH Series H-22; 2003. [Google Scholar]
- Reichenberg A, Gross R, Weiser M, Bresnahan M, Silverman J, Harlap S, Rabinowitz J, Shulman C, Malaspina D, Lubin G, Knobler HY, Davidson M, Susser E. Advancing paternal age and autism. Arch Gen Psychiatry. 2006;63(9):1026–1032. doi: 10.1001/archpsyc.63.9.1026. [DOI] [PubMed] [Google Scholar]
- Ren JQ, Malanga CJ, Tabit E, Kosofsky BE. Neuropathological consequences of prenatal cocaine exposure in the mouse. Int J Dev Neurosci. 2004;22(5–6):309–320. doi: 10.1016/j.ijdevneu.2004.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson GA, Conroy ML, Day NL. Prenatal cocaine exposure: effects on the development of school-age children. Neurotoxicology & Teratology. 1996;18(6):627–634. doi: 10.1016/s0892-0362(96)00121-3. [DOI] [PubMed] [Google Scholar]
- Stanwood GD, Levitt P. Prenatal exposure to cocaine produces unique developmental and long-term adaptive changes in dopamine D1 receptor activity and subcellular distribution. J Neurosci. 2007a;27(1):152–157. doi: 10.1523/JNEUROSCI.4591-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanwood GD, Levitt P. Waved-1 mutant mice are hypersensitive to the locomotor actions of cocaine. Synapse. 2007b;61(4):259–262. doi: 10.1002/syn.20364. [DOI] [PubMed] [Google Scholar]
- Stanwood GD, Washington RA, Levitt P. Identification of a sensitive period of prenatal cocaine exposure that alters the development of the anterior cingulate cortex. Cereb Cortex. 2001;11(5):430–440. doi: 10.1093/cercor/11.5.430. [DOI] [PubMed] [Google Scholar]
- Thompson BL, Levitt P, Stanwood GD. Prenatal cocaine exposure specifically alters spontaneous alternation behavior. Behav Brain Res. 2005;164(1):107–116. doi: 10.1016/j.bbr.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Thompson BL, Levitt P, Stanwood GD. Prenatal exposure to drugs: effects on brain development and implications for policy and education. Nat Rev Neurosci. 2009;10(4):303–312. doi: 10.1038/nrn2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner RH, Rosett HL. The effects of drinking on offspring: an historical survey of the American and British literature. Journal of studies on alcohol. 1975;36(11):1395–1420. doi: 10.15288/jsa.1975.36.1395. [DOI] [PubMed] [Google Scholar]
- Withers NW, Pulvirenti L, Koob GF, Gillin JC. Cocaine abuse and dependence. J Clin Psychopharmacol. 1995;15(1):63–78. doi: 10.1097/00004714-199502000-00010. [DOI] [PubMed] [Google Scholar]
- Wozniak DF, Cicero TJ, Kettinger L, 3rd, Meyer ER. Paternal alcohol consumption in the rat impairs spatial learning performance in male offspring. Psychopharmacology (Berl) 1991;105(2):289–302. doi: 10.1007/BF02244324. [DOI] [PubMed] [Google Scholar]
- Yazigi RA, Odem RR, Polakoski KL. Demonstration of specific binding of cocaine to human spermatozoa. JAMA. 1991;266(14):1956–1959. [PubMed] [Google Scholar]
- Yazigi RA, Polakoski KL. Distribution of tritiated cocaine in selected genital and nongenital organs following administration to male mice. Arch Pathol Lab Med. 1992;116(10):1036–1039. [PubMed] [Google Scholar]