Abstract
Mesenchymal stem cells (MSCs) are multipotent, can be easily expanded in culture and hence are an attractive therapeutic tool for cardiac repair. MSCs have tremendous potential to transdifferentiate to cardiac lineage both in vitro and in vivo. The present study examined the differentiation capacity of conditioned media derived from ischemic cardiac tissue on human MSCs. Human Bone marrow-derived MSCs after due characterization by immunocytochemistry and flow cytometry for MSC specific markers were induced by culture media derived from ischemic (n = 13) and non-ischemic (n = 18) human cardiac tissue. Parallel cultures were treated with 5-azacytidine (5-azaC), a potent cardiomyogen. MSCs induced with ischemic conditioned media formed myotube like structures, expressed sarcomeric Troponin I, alpha myosin heavy chain proteins and were positive for cardiac specific markers (Nkx2.5, human atrial natriuretic peptide, myosin light chain-2a, GATA-4) as was observed in 5-azaC treated cells. However, uninduced MSCs as well as those induced with non-ischemic cardiac conditioned media still maintained the fibroblast morphology even after 3 weeks post-induction. Transmission electron microscopic studies of cardiomyocyte-like cells derived from MSCs revealed presence of sarcomeric bands but failed to show gap junctions and intercalated discs as of adult cardiomyocytes. These findings demonstrate that ischemic cardiac conditioned media induces morphological and molecular changes in MSCs with cardiac features, but at a primitive stage. Proteomics analysis of the ischemic conditioned media revealed differential expression of three relevant proteins (C-type lectin superfamily member 13, Testis-specific chromodomain protein Y2 and ADP/ATP translocase 1), whose exact role in cardiac regeneration needs further analysis.
Keywords: Bone marrow, Stem cell, Cardiomyocyte, Differentiation, Conditioned media
Introduction
Bone marrow-derived mesenchymal stem cells (BMSCs) are capable of differentiating along multiple lineages into bone, cartilage, skeletal muscle, adipose tissue and are therefore a potential cell source for therapeutic applications (Jiang et al. 2002; Pittenger et al. 1999). MSCs are therapeutically effective in transplantation because of their unique immunomodulatory properties (Tyndall et al. 2007). Cellular therapy for heart failure and allied diseases by bone marrow stem cells has the potential to restore cardiac function by inducing neovascularization, and regenerating cardiomyocytes (Fraser et al. 2004). Cardiomyogenic differentiation of BMSCs has been achieved by culturing stem cells in vitro using culture medium supplemented with Hyaluronan mixed esters of butyric acid and retinoic acid (HBR), Dimethyl sulphoxide (DMSO), oxytocin, Ascorbic acid, 5-azaC, Bone morphogenetic protein (BMP4), Activin A, and also by co-culturing with adult cardiomyocytes (Ventura et al. 2007; Paquin et al. 2002; Zhang et al. 2005; Heng et al. 2004).
Myocardial regeneration with stem cell therapy in patients with heart failure and post-myocardial infarction is receiving remarkable importance despite various challenges. Bone marrow-derived mononuclear cells (BMMNC) and endothelial progenitor cells (EPC) have been applied for cardiovascular disease in human studies (Sharpe 2004). MSCs not only differentiate into specific cell types such as cardiomyocytes and vascular endothelial cells, but also secrete a variety of paracrine angiogenic and cytoprotective factors. It has also been suggested that endogenous MSCs as well as exogenously transplanted MSCs migrate and participate in cardiac repair (Kamihata et al. 2001).
In vitro differentiation of MSCs to progenitor cardiomyocytes prior to stem cell transplantation is the potential requirement for treating heart failure (Hassink et al. 2003). Although a small percentage of adult cardiac muscle cells are capable of proliferation, they lack the ability to regenerate the whole infarcted region and hence the heart gets compromised, leading to heart failure (Mariani et al. 2004). Stem cell therapy for myocardial damage appears promising as it can replace damaged myocytes and help restoration of blood flow (Weissberg and Qasim 2005). In case of coronary artery disease (CAD) and related disorders, cardiac tissue receives insufficient blood supply leading to ischemia and scar formation. To circumvent this problem, the ischemic myocardium produces specific angiogenic and cardio-regenerative factors to maintain cardiac homeostasis (Lee et al. 2000). The impact of acute myocardial infarction (AMI) on MSCs differentiation into cardiomyocyte lineage suggests that infarct-related biological factors in AMI induce commitment of MSCs to cardiomyocyte-like cells through TGF-β/BMP-2 pathways (Chang et al. 2008). The fact that these pathways are fundamental to cardiac remodeling lends support to the hypothesis that in vitro conditions simulating in vivo environments may reinforce the use of well-defined MSCs preparations for cardiomyogenic differentiation. Therefore, in the present study, we attempted to elucidate the cardiomyogenic potential of ischemic cardiac tissue conditioned media upon bone marrow-derived mesenchymal stem cells.
Materials and methods
Isolation and culture of MSCs
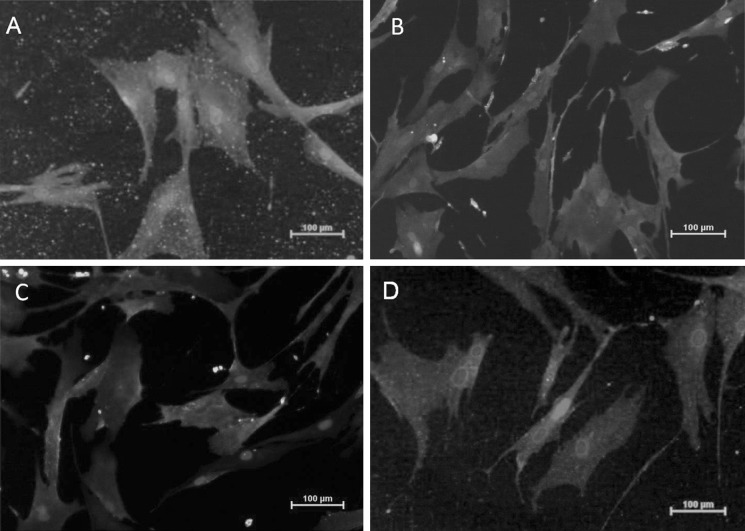
The present study was conducted after due approval by the institutional research and ethical committees of the Frontier Lifeline Hospital. Human bone marrow from sternum was collected from patients undergoing cardiac surgery after pre-informed consent. The bone marrow was diluted at 1:1 concentration with Dulbecco’s modified Eagles media (DMEM), and separated on Ficoll gradient (ACCUSPIN™ System-Histopaque®-1077, Sigma) as per manufacturer’s instructions. The mononuclear enriched fraction was collected and washed twice with Dulbecco’s phosphate buffered saline (DPBS). The cells were resuspended at 3 × 106 cells in 90 mm cell culture plates in DMEM with low glucose, 10% Fetal bovine serum (FBS), l-glutamine and antibiotics. The plates were maintained at 37 °C in a humidified atmosphere of 95% air and 5% CO2 and subcultured prior to confluency. The MSCs were tested positive for CD90, CD105, CD73, and CD44 as confirmed by Flow cytometry and immunocytochemistry (Figs. 1, 2).
Fig. 1.
Immunostained human bone marrow derived mesenchymal stem cells a CD90, b CD44, c CD105, d CD73 [Phycoerythrin (PE) stain - Red color, Fluorescein isothiocyanate (FITC) stain - Green color, and DAPI (4’,6-diamidino-2-phenylindole) nuclear stain - Blue color]. (Color figure online)
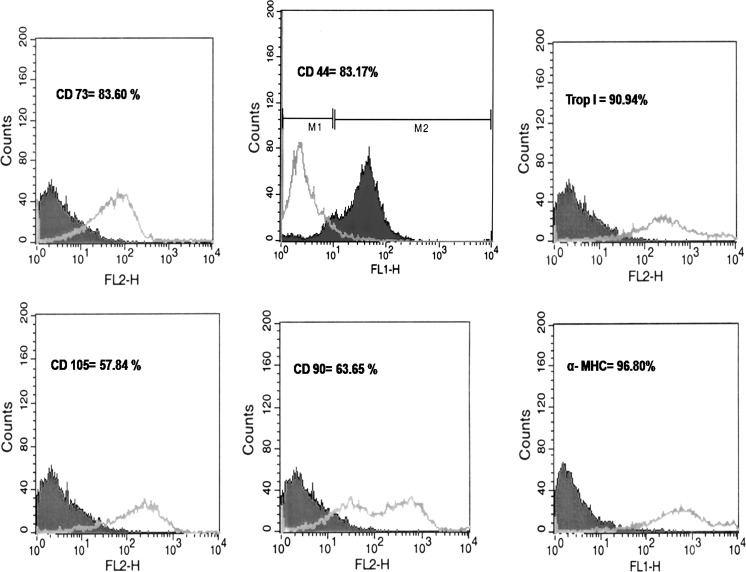
Fig. 2.
Flowcytometry anlaysis for MSCs expressing markers (CD44, CD105, CD90, and CD73) and differentiated MSCs with ischemic conditioned media expressing cardiac specific markers (Troponin I and alpha myosin heavy chain)
Preparation of cardiac conditioned media
Right atrial cardiac tissues were collected from patients while decannulation with pre-informed consent. Tissues collected from patients with CAD and related disorders were considered as Ischemic group (N = 13) (Table 1) and tissues collected from patients with acyanotic congential heart disease (ACHD), complex congenital heart disease (CCHD) and valvular replacement surgery were considered as Non-ischemic group (N = 18) (Table 2). Fresh cardiac tissues were processed for conditioned media preparation within 6 h of sample collection. Cardiac tissue collected from different patients were chopped into small pieces (not exceeding 1 mm in size), incubated along with 10 mL DMEM, 10% FBS and incubated for 7 days under 5% CO2 and 37 °C. The media incubated with each chopped tissues were centrifuged and filtered using 0.2 μm syringe filter to remove the cardiac bits and the myocytes. The cell free media from different patients within the same group (either ischemic/non-ischemic) were pooled and stored in −80 °C freezer.
Table 1.
Ischemic tissue details
| S. no | Age/sex | Cardiac disease |
|---|---|---|
| 1 | 63/M | CAD, TVD, OLD AWMI SEVERE.LVD, SEV.PAH, GRADE II MR |
| 2 | 61/M | CAD, TVD, AF, DM II, SHTN |
| 3 | 42/M | CAD, TVD, CHRONIC STABLE ANGINA, MILD MR, NIDDM |
| 4 | 60/M | CAD, TVD, SHTN |
| 5 | 59/M | CAD, TVD, CHRONIC STABLE ANGINA, DM II |
| 6 | 65/M | CAD, TVD, UNSTABLE ANGINA, MOD.LVD, NIDDM |
| 7 | 57/M | CAD, IWMI (1998), UAI b (recent) TVD, MILD LVD, SHTN |
| 8 | 57/M | CAD, TVD, SEV.LVD, GRADE II MR, SHTN |
| 9 | 65/M | CAD, DVD, SHTN |
| 10 | 63/M | CAD, TVD, CRITICAL LMCAD, 100% RCA, 100% LAD, SHTN |
| 11 | 65/M | CAD, TVD, UNSTABLE ANGINA, MILD MR, DM II, SHTN |
| 12 | 62/M | CAD, DVD, TRIVAL MR, NIDDM |
| 13 | 51/M | CAD, TVD, TRIVAL MR |
CAD coronary artery disease, TVD triple vessel disease, DVD double vessel disease, MR mitral regurgitation, NIDDM non insulin dependent diabetis mellitus, RCA right coronary artery, LMCAD left main coronary artery disease, SHTN systemic hypertension, LVD left ventricular dysfunction, AF atrial fibrillation, DMII type II diabetes, IWMI inferior wall myocardial infarction
Table 2.
Non-ischemic tissue details
| S.no | Age/sex | Cardiac disease |
|---|---|---|
| 1 | 2.5/M | ACHD, LARGE ASD, RIGHT AORTIC ARCH, SUB GLOTTIC STENOSIS. |
| 2 | 2/F | ACHD, VSD |
| 3 | 5/F | CCHD, EBSTEINS ANOMALY OF TRICUSPID VALVE MODERATE SIZE ASD, RV DYSFUNCTION |
| 4 | 12/M | ACHD, LARGE SUB AORTIC MALALIGNED VSD |
| 5 | 6/F | ACHD, LARGE OS ASD |
| 6 | 6/M | ACHD, LARGE MALALIGNED SUB AORTIC VSD |
| 7 | 07/M | ACHD, VSD |
| 8 | 2.4//M | ACHD, LARGE PERIMEMBRANOUS VSD |
| 9 | 43/F | MVR |
| 10 | 5.6/M | ACHD, AORTA PULMONARY WINDOW, SEV.PAH, ABSENT LPA, RETRO AORTIC INNOMINATE VEIN |
| 11 | 3.11/F | ACHD, DORV, LARGE SUBAORTIC VSD |
| 12 | 12/M | ACHD, MOD. PERIMEM.VSD |
| 13 | 6/F | CCHD, DORV, D MALPOSED ARTERIES, LARGE SUB AORTIC VSD, LARGE VERTICAL PDA |
| 14 | 12/M | ACHD, SUB AORTIC VSD, RCC CUSP PROLAPSE. |
| 15 | 18/M | ACHD, TOF, BICUSPID PULMONARY VALVE |
| 16 | 8/M | ACHD, BICUSPID AORTIC VALVE, |
| 17 | 1.5/M | ACHD, SUB AORTIC VSD |
| 18 | 13/M | ACHD, VSD |
ACHD acyanotic congential heart disease, CCHD complex congenital heart disease, VSD ventricular septal defect, MVR mitral valve replacement, PDA patent ductus arteriosus, ASD atrial septal defect, RV right ventricle, RCC right coronary cusp, LPA left pulmonary artery, PAH pulmonary artery hypertension, TOF tetrology of fallot
The expanded and purified MSCs after second passage were induced for 4 weeks in (a) ischemic cardiac tissue conditioned media) (b) non-ischemic cardiac tissue conditioned media and (c) DMEM with 10% FBS, and antibiotics supplemented with 5-azaC (10 μmol/L). The 5-azaC supplemented media were withdrawn after 48 h and cells were nourished with DMEM, 10% FBS, and antibiotics. Cells grown in DMEM with 10%FBS and antibiotics were treated as control. The media were changed twice per week and the cells were maintained for 4 weeks in 5% CO2 and 37 °C humidified atmosphere. The experiments were repeated in triplicates.
Immunocytochemistry and flow cytometry
Immunocytostaining was performed for cardiac specific markers, anti-human Troponin I (Trop I) and anti-human alpha myosin heavy chain (α MHC) (Abcam). The cells were fixed with 4% paraformaldehyde, followed by permeabilization with 0.25% Triton X and were incubated for 1 h with primary antibody at room temperature (1/100 dilution in 5% BSA). Thereafter, the cells were washed with DPBS and incubated with Fluorescein isothiocyanate (FITC) or Phycoerythrin (PE) conjugated secondary antibody at room temperature for 1 h. The immunostained cells were observed under fluorescent microscope (Olympus).
For Flow cytometric analysis, cells were detached with 0.25% trypsin and 0.2% EDTA, blocked with 1% bovine serum albumin in Dulbecco’s phosphate buffered saline (DPBS) and incubated with primary antibody (20 μg/mL in 1%BSA) at 4 °C overnight. Cells were washed twice with DPBS and incubated with fluophore (FITC or PE) conjugated secondary antibody at room temperature for 30 min. The cells were fixed with 2% paraformaldehyde and approximately 106 cells were subjected for flow cytometry by FACS Calibur (Becton–Dickinson).
Reverse transcription PCR
Total RNA was extracted using TRIZOL (Sigma). cDNA was synthesized from 1 μg total RNA using Super Script II reverse transcriptase (New England Bio labs) following the manufacturer’s protocol. The concentration and the purity of RNA were determined spectrophotometricaly. After the reverse transcription step, cDNA samples were subjected to PCR amplification with primers selective for human cardiac genes Nkx2.5, human atrial natriuretic peptide (hANP) and Myosin light chain (MLC-2a, MLC-2v) (Table 3). After an initial denaturation step for 5 min at 95 °C, 35 cycles of amplification were performed as follows: denaturation at 94 °C for 30 s, annealing at 57 °C for 30 s and DNA extension at 72 °C for 60 s, followed by an additional cycle of 5 min at 72 °C to complete partial polymerizations. Amplified products were analyzed using horizontal 1.5% agarose gel electrophoresis.
Table 3.
Primers for RT-PCR
| Gene | Sequence | Product size (bp) |
|---|---|---|
| NKx-2.5 | 5′-GGTGGAGCTGGAGAAGACAG-3′ 5′-AGATCTTGACCTGCGTGGAC-3′ |
197 |
| hANP | 5′-GCTGGACCATTTGGAAGAAA-3′ 5′-TTGCTTTTTAGGAGGGCAGA-3′ |
213 |
| MLC-2a | 5′-GTCTTCCTCACGCTCTTTGG-3′ 5′-CCACCTCAGCTGGAGAGAAC-3′ |
166 |
| GATA-4 | 5′-TCCAAACCAGAAAACGGAAG-3′ 5′-CTGTGCCCGTAGTGAGATGA-3′ |
187 |
| Beta-actin | 5′-AGAGCTACGAGCTGCCTGAC-3′ 5′-AGCACTGTGTTGGCGTACAG-3′ |
194 |
Transmission electron microscopy
Ultrastructural analysis with transmission electron microscopy (TEM) was performed on induced and uninduced cells. Cultured cells from each experimental condition were detached with trypsin EDTA and collected by rinse in PBS. The cells were fixed initially with 2.5% glutaraldehyde and post-fixation was performed in 2% osmium tetroxide, followed by dehydration with ethanol and strained in uranyl acetate solution. The cells were embedded in epoxy resin, subjected to ultra thin sections and subsequently examined in electron microscope.
Proteomic analysis of cardiac conditioned media
The ischemic and non-ischemic cardiac tissue conditioned media were subjected to proteomic analysis to check for differentially expressed proteins.
Sample preparation and SDS-PAGE analysis
Proteins present in the conditioned media were precipitated by methanol/chloroform method. After precipitation, total proteins were dialysed overnight in cold environment. During dialysis, phenylmethanesulfonylfluoride (PMSF) (1 mmol) was added to prevent protease activity, if any. Finally, it was desalted using vivaspin column. Total protein concentration was measured by Bradford reagent and 80 µg protein was subjected to SDS-PAGE with 10% resolving gel and 5% staking gel at 100 V. The Coomassie brilliant blue dye (R250) stained gel was viewed under gel documentation system. The experiment was repeated twice for reproducibility and finally the differentially expressed bands were excised and stored in 0.5% acetic acid for further Mass spectrometry analysis.
Matrix-assisted laser desorption/ionization analysis (MALDI)
Coomassie stained bands excised from gel were washed twice with 100 mM ammonium bicarbonate (ABC)/50% acetonitrile for 30 min each. Gel pieces were dehydrated by 100% acetonitrile and dried in vacuum concentrator, reduced and alkylated using 25 mM dithiothreitol (DTT) and 55 mM iodoacetamide and incubated with 200 ng Trypsin Gold (Promega) in 25 mM ABC for 3 h at 37 °C. After the digestion samples were aspirated and eluted once with 50% acetonitrile, 2.5% TFA (stop solution for digestion). Extracts were combined and evaporated to 5 μL of final volume. Samples were spotted onto a MALDI target and overlaid with 0.5 μL of 5 mg/mL MALDI matrix (α-cyano-4-hydroxycinnamic acid) and 10 mM ammonium mono basic phosphate.
Mass spectrometric data were collected using an ABI 4800 MALDI TOF/TOF (Applied Biosystems, Foster City/CA). The mass data were acquired in reflector mode from a mass range of 600–4,000 Daltons. Five most intense ions from the mass spectrometry (MS) analysis, which are not on the exclusion list, were subjected to MS/MS with appropriate precursor mass range between 60 Dalton to initial mass of precursor ion. GPS software (Applied Biosystems, Foster City/CA) was used and the peak list was generated from the raw data generated from the ABI 4800. The peak list generated based on signal to noise filtering, exclusion list and de-isotoping parameters. The resulting peak list file was then searched and compared against existing data bases like Swissprot and NCBI using Mascot (Matrix Science, Boston; MA). For analysis in GPS explorer software with a tolerance of 20 ppm was used if the sample was internally calibrated and 100 ppm tolerance if the default calibration was applied. Database search parameters include one missed cleavage, oxidation of methionines and carbamidomethylation of cysteines.
Results and discussion
MSCs after 3 days in primary culture adhered to the plastic surface, presenting a small population of cells. The colonies reached confluency at 21–28 days after initial plating. Cytometric analysis revealed that MSCs were positive for CD44, CD90, CD105, CD73 (Figs. 1, 2) and negative for hematopoietic lineage markers CD34 and CD45 (data not shown), Uninduced MSCs maintained the fibroblast morphology while the cells incubated with conditioned media or 5-azaC formed myotube-like structures by 4th week of incubation (Fig. 3). In general, a shift from an immature phenotype in early stage to a more organized sarcomeric structure in later stage was noted in differentiated cells. The cells were fixed and immunostained for early markers of cardiomyogenic differentiation. Figures 2 and 4 show positive immunostaining of myotube like cells with anti-cardiac myosin heavy chain and anti-Troponin I monoclonal antibodies. Varying degrees of myofibrillar organization were noted among the cells. The staining patterns ranged from clumps in some cells to bundles of elongated fibrillar structures in others. Morphologically, in vitro differentiation of MSCs with conditioned media or 5-azaC appears to follow similar pathways. The presence of sarcomeric proteins, differentiation markers of cardiomyocyte phenotype and varying degrees of myofibrillar organization (from disorganized myofibrils to the more organized sarcomeric pattern) described here have also been noted in 5-azaC induced MSCs.
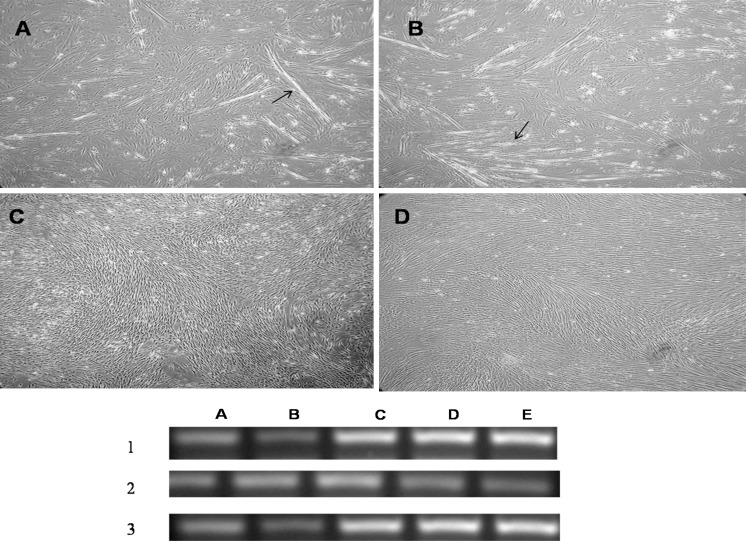
Fig. 3.
Upper panela MSCs under the influence of 5-AzaC at the end of 3rd week, b MSCs under the influence of ischemic conditioned medium at 3rd week, c MSC under the influence of non-ischemic conditioned medium at 3rd week, d negative control of mesenchymal stem cells without any induction. (All magnifications ×40). Lower panel RT-PCR analysis of cardiac specific markers in 1 5-azaC treated MSCs, 2 non ischemic conditioned medium treated MSCs, 3 ischemic conditioned medium treated MSCs. (Lane A GATA4, lane B: Nkx 2.5, lane C myosin light chain 2 a, lane D human atrial natriuretitic peptide (hANP), lane E actin) [arrows ina and b indicates the myotube formation]
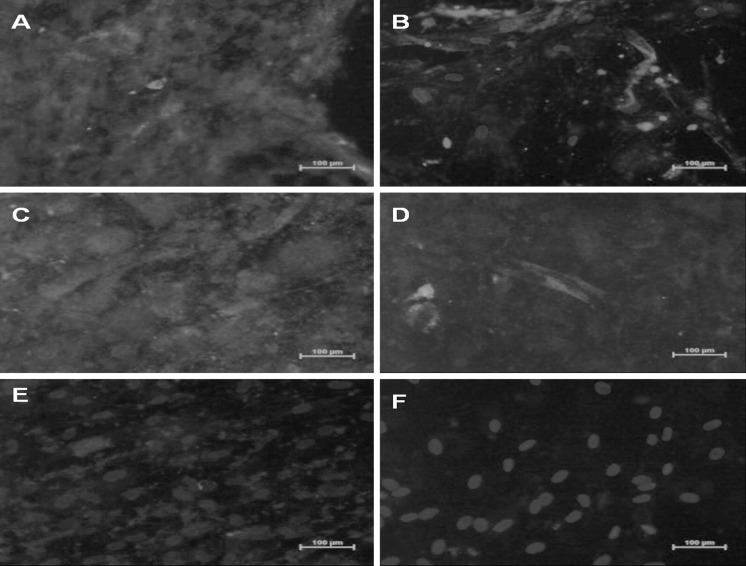
Fig. 4.
Immunocytochemistry studies for Trop I expression in a 5-Aza-C treated cells, c ischemic conditioned medium treated MSCs and e non-ischemic conditioned medium treated MSCs & Myosin heavy chain expression in b 5-aza C treated MSCs, d ischemic conditioned medium treated MSCs and f non-ischemic conditioned medium [Protein expression was confirmed by Phycoerythrin (PE) stain - Red color and DAPI (4’,6-diamidino-2-phenylindole) nuclear stain - Blue color]. (Color figure online)
MSCs under the influence of conditioned media were positive for differentiation markers of cardiomyocyte phenotype Nkx 2.5, hANP and MLC-2a, GATA 4 (Fig. 3 lower panel). However, MSCs treated with factors derived from non-ischemic cardiac tissue showed negligible differentiation and untreated cells showed none of the cardiac features. These data suggest that ischemic cardiac tissue promotes cardiomyogenic differentiation, whereas a non-ischemic environment exerts a partial effect on the differentiation capacity of MSCs.
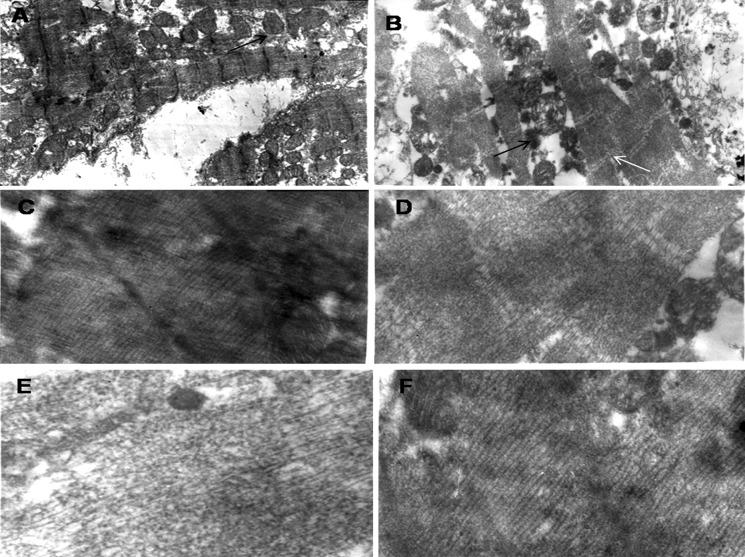
To further assess we performed TEM analysis which revealed that differentiated MSCs expressed sarcomeric proteins without gap junctions and intercalating disks between adjacent cells. This phenotype is most likely related to a cardiomyocyte-like precursor lineage (Fig. 5). The ultrastructural maturation in differentiated cardiomyocytes seemed more heterogeneous, and did not reach the fully mature adult phenotype during the observation period. Recently published data reported that murine MSCs inspite of expressing cardiac-specific markers do not become functional cardiomyocytes in vitro (Rose et al. 2008). Our result also suggests that human bone marrow-derived MSCs follow a similar pattern in vitro and differentiate into cardiomyocytes with limited functional ability.
Fig. 5.
Transmission electron microscopy studies a human cardiomyocytes expressing mitochondria and sacromere (black arrow labeling for mitochondria under magnification ×8,000). b ischemic conditioned media treated MSC expressing abundant mitochondria and sacromere (black arrow for mitochondria and white for sacromere under magnification ×8,000). c sacromere in human cardiomyocytes. d sacromere in ischemic conditioned media treated MSCs. e sacromere in non-ischemic conditioned media treated MSCs. f sacromere in 5-azaC treated MSCs (c, d, e, f; magnification ×40,000)
There are many types of cells considered to be safe and feasible for cardiomyoplasty based on murine and human experiments, e.g. C kit positive cardiac residing stem cells, endothelial progenitor cells, Satellite progenitors of cardiac cells (SPOC) and embryonic stem cells (Ohnishi and Nagaya 2007; Beltrami et al. 2001). In this regard, differentiating MSCs towards a particular lineage prior to transplantation could make the cellular therapy highly effective as they will be able to home into the target site of tissue damage (Fukuda and Yuasa 2003). When treated with 5-azaC, MSCs are capable of transdifferentiating into functional cardiomyocytes. However, the significance of this approach for treating ischemic heart disease is unclear, as 5-azaC at high doses induces DNA damage and is considered carcinogenic (Juttermann et al. 1994; Antonitsis et al. 2007). In vivo, it has been revealed that in an acute ischemia model, MSCs differentiate into cardiomyocyte-like cells that express Troponin I and sarcomeric MHC (Toma et al. 2002). Ischemic myocardium produces several stem cell-attracting and survival-promoting cytokines or transcription factors, such as vascular endothelial growth factor and stromal cell derived factor 1, which augment survival of stem cells (Lee et al. 2000).
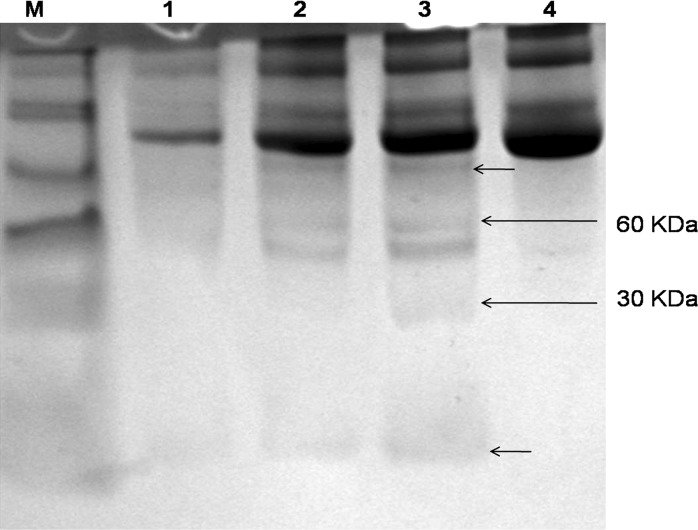
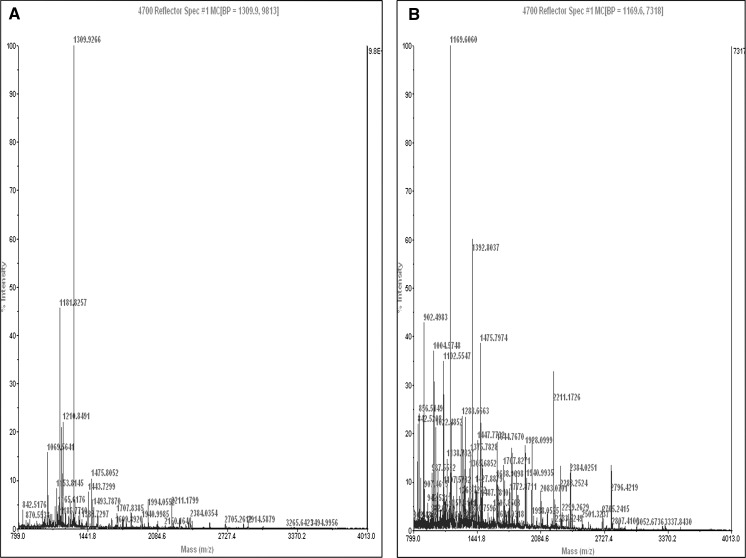
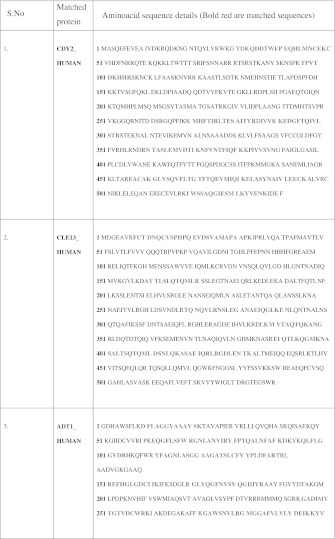
To explore the presence of differentially expressed proteins in the ischemic cardiac tissue conditioned media, we subjected it to SDS-PAGE analysis followed by MALDI-TOF. SDS-PAGE revealed differential expression of four different protein bands (Fig. 6). Two smaller kDa proteins were manually excised and subjected to MALTI-TOF analysis (Fig. 7). The 60 kDa protein band was a mixture of two different proteins. One of them matching 24% with the aminoacid sequence of CDY2_HUMAN (Testis-specific chromodomain protein Y2) and the other matching 21% with C-Type Lectin superfamily member 13. The low molecular weight differentially expressed protein matched 48% aminoacid sequence with ADT1_HUMAN (ADP, ATP carrier protein, heart/skeletal muscle isoforms characterized as T1 or ATP translocase 1) (Table 4).
Fig. 6.
SDS-PAGE analysis of ischemic and non ischemic conditioned media. (Lane M Spectra™ Multicolor Broad Range Protein Ladder (10–260 kDa); Lane 1 7 days incubated control media; Lane 2 and 3 ischemic conditioned media and Lane 4 non-ischemic conditioned media. [Two small arrows indicates differentially expressed unidentified proteins]
Fig. 7.
In-gel digestion studies for differentially expressed proteins and their MALDI-TOF mass spectrometry data for a 60 kDa protein band and b 30 KDa protein band in ischemic cardiac conditioned media
Table 4.
Mass spectrometry—Aminoacid sequence details for the differentially expressed proteins. (Colour table online)
CDY2_HUMAN: Testis-specific chromodomain protein Y 2 Nominal mass (Mr): 61113; Calculated pI value: 9.14. CLE13_HUMAN: C-type lectin superfamily member 13 (C-type lectin 13) Nominal mass (Mr): 60399; Calculated pI value: 6.04. ADT1_HUMAN: ADP, ATP carrier protein, heart/skeletal muscle isoform T1 (ADP/ATP translocase 1) Nominal mass (Mr): 33140; Calculated pI value: 9.78
The ATP translocase, a mitochondrial membrane protein, plays a key role in cellular energy metabolism. There are three isoforms in humans as T1, T2 and T3. The multiple genes encoding for Adenine nucleotide carrier protein are differentially expressed at mRNA level in various stages of myogenic differentiation (Lunardi et al. 1992). Therefore, the overexpression of this protein in ischemic myocardial tissue might be relevant to myocardial regeneration process. C-Type lectin, another protein differentially expressed in ischemic conditioned media, is a carbohydrate binding protein that requires calcium for binding. It has various functional roles such as cell-to-cell adhesion, migration, apoptosis and immune response to pathogens (Kato et al. 2007). Such a feature of C-type lectin might be relevant in terms of early cardiac differentiation of human MSCs in vitro and in vivo. The human chromodomain Y-related (CDY) protein family contains six proteins (CDY1, CDY1B, CDY2A, CDY2B, CDYL, and CDYL2), with two functional domains: a chromo domain involved in chromatin binding and a catalytic domain found in many Coenzymes A (CoA)-dependent acylation enzymes. The presence of these two domains indicates a role for this protein family involved in histone modification and recognition (Wu et al. 2009). The role of CDY2A may also promote epigenetic changes by histone modification in early cardiac differentiation of MSCs. However, a clear role of these three proteins needs to be elucidated in details prior to affirm their specific role in myocardial regeneration.
Conclusion
Conclusively, MSCs when supplemented with factors derived from ischemic cardiac tissue, generated myocytes that were shown to display structural properties consistent with early-stage cardiomyocytes. Based on these data, it is conceivable that some of the improvements in cardiac function that have been described following MSCs injection in the setting of heart disease are related to the generation of new cardiomyocytes. These findings suggest that pre-treatment of MSCs with ischemic tissue derived factors may be a feasible strategy to improve the efficacy of stem cell therapy in patients with heart failure and post-myocardial infarction. Moreover, these data add to recent findings that infarct-related biological factors in AMI induce commitment of MSCs to cardiomyocyte-like cells. Further studies are required to dissect the infarcted tissue specific factors and to determine whether the paradigm applies to the complex in vivo environment.
Acknowledgments
Our thanks are due to the Indian council of medical research (ICMR) for partial funding this project and our team members Dr. Santhosh Mathapati, Mr. Satish Galla, Ms. Sheerin Begam Naser and Mr. A. Pandian for their kind help rendered.
Conflict of interest
The authors have no conflict of interest to disclose with regard to the subject matter of this present manuscript.
References
- Antonitsis P, Ioannidou-Papagiannaki E, Kaidoglou A, Papakonstantinou C. In vitro cardiomyogenic differentiation of adult human bone marrow mesenchymal stem cells. The role of 5-azacytidine. Interact Cardiovasc Thorac Surg. 2007;6:593–597. doi: 10.1510/icvts.2007.157875. [DOI] [PubMed] [Google Scholar]
- Beltrami AP, Urbanek K, Kajustura J, Yan SM, Finato N, Bussani R, Nadal-Ginard B, Silvestri F, Leri A, Beltrami A, Anversa P. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001;344:1750–1757. doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
- Chang SA, Lee EJ, Kang HJ, Zhang SY, Kim JH, Li L, Youn SW, Lee CS, Kim KH, Won JY, Sohn JW, Park KW, Cho HJ, Yang SE, Oh WI, Yang YS, Ho WK, Park YB, Kim HS (2008) Impact of myocardial infarct proteins and oscillating pressure on the differentiation of mesenchymal stem cells: effect of acute myocardial infarction on stem cell differentiation. Stem Cells 26:1901–1912 [DOI] [PubMed]
- Fraser JK, Schreiber RE, Zuk PA, Hedrick MH. Adult stem cell therapy for the heart. Int J Biochem Cell Biol. 2004;36:658–666. doi: 10.1016/j.biocel.2003.10.018. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Yuasa S. Stem cells as a source of regenerative cardiomyocytes use of adult marrow mesenchymal stem cells for regeneration of cardiomyocytes. Bone Marrow Transplant. 2003;32:S25–S27. doi: 10.1038/sj.bmt.1703940. [DOI] [PubMed] [Google Scholar]
- Hassink RJ, Brutel de la Rivière A, Mummery CL, Doevendans PA. Transplantation of cells for cardiac repair. J Am Coll Cardiol. 2003;41:711–717. doi: 10.1016/S0735-1097(02)02933-9. [DOI] [PubMed] [Google Scholar]
- Heng BC, Haider HK, Sim EK, Cao T, Ng SC. Strategies for directing the differentiation of stem cells into the cardiomyogenic lineage in vitro. Cardiovasc Res. 2004;62:34–42. doi: 10.1016/j.cardiores.2003.12.022. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM (2002) Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 418:41–49 [DOI] [PubMed]
- Juttermann R, Li E, Jaenisch R. Toxicity of 5-axa-2-deoxycytidine to mammalian cells is mediated primarily by co-valent trapping of DNA methyl transferase rather than DNA demethylation. Proc Natl Acad Sci USA. 1994;19:11797–11801. doi: 10.1073/pnas.91.25.11797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamihata H, Matsubara H, Nishiue T, Fujiyama S, Tsutsumi Y, Ozono R, Masaki H, Mori Y, Iba O, Tateishi E, Kosaki A, Shintani S, Murohara T, Imaizumi T, Iwasaka T (2001) Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation 104:1046–1052 [DOI] [PubMed]
- Kato M, Khan S, d’Aniello E, McDonald KJ, Hart DN (2007) The novel endocytic and phagocytic D-type lectin receptor DCL-1/CD302 on macrophages is colacalized with F-Actin, sugessing a role in cell adhesion and migration. J Immunol 179:6052–6063 [DOI] [PubMed]
- Lee SH, Wolf PL, Escudero R, Deutsch R, Jamieson SW, Thistlethwaite PA. Early expression of angiogenesis factors in acute myocardial ischemia and infarction. N Engl J Med. 2000;342:626–633. doi: 10.1056/NEJM200003023420904. [DOI] [PubMed] [Google Scholar]
- Lunardi J, Hurko O, Engel WK, Attardi G. The multiple ADP/ATP translocase genes are differentially expressed during human muscle development. J Biol Chem. 1992;267:15267–15270. [PubMed] [Google Scholar]
- Mariani MA, Alfonso AD, Croccia M, Limbruno U, Stefano RD, Grandjean JG. Stem cell transplantation for ischemic myocardium. Ital Heart J. 2004;5:340–342. [PubMed] [Google Scholar]
- Ohnishi S, Nagaya N. Prepare cells to repair heart: mesenchymal stem cells for treatment of heart failure. Am J Nephrol. 2007;27:301–307. doi: 10.1159/000102000. [DOI] [PubMed] [Google Scholar]
- Paquin J, Danalache BA, Jankowski M, Mc Cann SM, Gutkowska J. Oxytocin induces differentiation of P19 embryonic stem cells to cardiomyocytes. Proc Natl Acad Sci USA. 2002;99:9550–9555. doi: 10.1073/pnas.152302499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147 [DOI] [PubMed]
- Rose RA, Jiang H, Wang X, Helke S, Tsoporis JN, Gong N, Keating SC, Parker TG, Backx PH, Keating A (2008) Bone marrow-derived mesenchymal stromal cells express cardiac-specific markers retain the stromal phenotype and do not become functional cardiomyocytes invitro. Stem Cells 26:2884–2892 [DOI] [PubMed]
- Sharpe N. Cardiac remodeling in coronary artery disease. Am J Cardiol. 2004;93:17B–20B. doi: 10.1016/j.amjcard.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- Tyndall A, Walker UA, Cope A, Dazzi F, De Bari C, Fibbe W, Guiducci S, Jones S, Jorgensen C, Le Blanc K, Luyten F, McGonagle D, Martin I, Bocelli-Tyndall C, Pennesi G, Pistoia V, Pitzalis C, Uccelli A, Wulffraat N, Feldmann M (2007) Immunomodulatory properties of mesenchymal stem cells: a review based on an interdisciplinary meeting held at the Kennedy institute of rheumatology division London UK. 31 October 2005. Arthritis Res Ther 9:301 [DOI] [PMC free article] [PubMed]
- Ventura C, Cantoni S, Bianchi F. Hyaluronan mixed esters of butyric and retinoic acid drive cardiac and endothelial fate in term placenta human mesenchymal stem cells and enhance cardiac repair in infracted rat hearts. J Biol Chem. 2007;282:14243–14252. doi: 10.1074/jbc.M609350200. [DOI] [PubMed] [Google Scholar]
- Weissberg PL, Qasim A. Stem cell therapy for myocardial repair. Heart. 2005;91:696–702. doi: 10.1136/hrt.2004.038224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Min J, Antoshenko T, Plotnikov AN. Crystal structures of human CDY proteins reveal a crotonase-like fold proteins. Proteins. 2009;76:1054–1061. doi: 10.1002/prot.22472. [DOI] [PubMed] [Google Scholar]
- Zhang SX, Garcia-Gras E, Wycuff DR, Marriot SJ, Kadeer N, Yu W, Olson EN, Garry DJ, Parmacek MS, Schwartz RJ (2005) Identification of direct serum-response factor gene targets during Me2SO-induced P19 cardiac cell differentiation. J Biol Chem 280:19115–19126 [DOI] [PubMed]