Abstract
Omentum fat derived stem cells have emerged as an alternative and accessible therapeutic tool in recent years in contrast to the existing persuasive sources of stem cells, bone marrow and subcutaneous adipose tissue. However, there has been a scanty citation on human omentum fat derived stem cells. Furthermore, identification of specific cell surface markers among aforesaid sources is still controversial. In lieu of this existing perplexity, the current research work aims at signifying omentum fat as a ground-breaking source of stem cells by surface antigenic profiling of stem cell population. In this study, we examined and compared the profiling of cell surface antigenic expressions of hematopoietic stem cells, mesenchymal stem cells, cell adhesion molecules and other unique markers such as ABCG2, ALDH and CD 117 in whole cell population of human omentum fat, subcutaneous fat and bone marrow. The phenotypic characterization through flowcytometry revealed the positive expressions of CD 34, CD 45, CD 133, HLADR, CD 90, CD 105, CD 73, CD 29, CD 13, CD 44, CD 54, CD 31, ALDH and CD 117 in all sources. The similarities between the phenotypic expressions of omentum fat derived stem cells to that of subcutaneous fat and bone marrow substantiates that identification of ultimate source for curative therapeutics is arduous to assess. Nevertheless, these results support the potential therapeutic application of omentum fat derived stem cells.
Electronic supplementary material
The online version of this article (doi:10.1007/s10616-012-9427-4) contains supplementary material, which is available to authorized users.
Keywords: Omentum fat derived stem cells, Cell surface marker, Mesenchymal stem cells, Cell adhesion molecules, Flowcytometry
Introduction
Adult stem cells comprise the first line repair mechanism, called into action by normal wear and tear of the body as well as by any serious damage or attack caused by disease or infection. Conceptually and from a practical standpoint, bone marrow (BM) was considered a persuasive source of stem cell for regenerative medicine from ancient days (Horwitz et al. 1999; Kuethe et al. 2004a, b; Wilson and Trumpp 2006; Kopp et al. 2005; Tsiftsoglou et al. 2009). Irrespective of it being a prehistoric source, bone marrow was not considered promising in attempting curative therapies for all diseases (Hallam and Gribben 2010). It was postulated that bone marrow yields approximately lesser nucleated cells (De Ugarte et al. 2003a, b; Pountos et al. 2007) and frequency of mesenchymal stem cell (MSC) is seemingly low (De Ugarte et al. 2003a, b; Zhu et al. 2008; Pountos et al. 2007). It was also reported that the frequency and differentiation capacity of Bone marrow derived MSC decline with age, body mass index and tissue harvest site, again resulting in producing low stem cell number (Stolzing et al. 2008).
Hence, search for an ideal alternative source of autologous stem cells was considered utmost important. Subcutaneous adipose tissue exhibited features corresponding to such an alternative contemporary source (Zuk et al. 2002; Gimble et al. 2007, 2010). The sheer number of stem cells that can be harvested at once from fat makes this best in human body (Zhu et al. 2008; Jurgens et al. 2008; Kotaro et al. 2006; Aust et al. 2004; Varma et al. 2007). Additionally, the proliferative capacity and plasticity of subcutaneous adipose derived stem cells was found to be much more effective than of bone marrow derived stem cells (Wagner et al. 2005; Gimble and Guilak 2003; Mesimaki et al. 2009; Garcia-Olmo et al. 2008; Bai et al. 2010).
Omentum fat is yet another potent source of stem cells along with bone marrow and subcutaneous adipose tissue, and provide wide spectrum towards cell based therapy. Scientists isolated, cultured and characterised these omentum fat derived cells from rat and asserted that these cells exhibited multilineage properties of stem cells which are capable of finding its use in repair and regenerative applications (Tholpady et al. 2003; Singh et al. 2008). Subsequently, there has been a very scanty citation of similar research with human omentum fat derived stem cells (Baglioni et al. 2009; Toyoda et al. 2009). Therefore omentum fat raises the issue of rivalry in choosing the best possible source for cell based therapeutics along with bone marrow and subcutaneous fat.
Whatsoever, be it omentum fat, subcutaneous fat or bone marrow, there is no specific marker that can reliably identify stem cells (Mitchell et al. 2006; Pountos et al. 2007; Wagner et al. 2005; Katz et al. 2005; Varma et al. 2007; Vater et al. 2011). Moreover, the ultimate source of stem cell for curative therapeutics is not yet defined. Thus, this study focused on the precise characterization of human omentum fat (OF) in comparison with subcutaneous fat (SF) and bone marrow (BM) in facets of complete profiling of various cell surface markers using flowcytometry in order to identify the specific cell surface markers of each source. Additionally, we aimed at obviating the controversies existing upon the ideal source for stem cell therapy.
Materials and methods
Collection of human bone marrow
Human bone marrow samples were obtained from the iliac crest region of 5 patients with spinal cord injury (Paraplegia), who were aged between 32 and 50 (Mean age being 41) and were applied for stem cell transplantation procedure, after the approval of institutional ethical committee. Formal written consent from the donors was obtained before collection.
Adipose tissue collection
Abdominal subcutaneous fat and omentum fat was obtained from five obese male and female patients undergoing a Bariatric surgical procedure of either sleeve gastrectomy or abdominoplasty at Lifeline Multispecialty Hospital, Chennai, Tamilnadu, India, who were aged between 35 and 60 (Mean age being 48). The tissue was collected after obtaining the informed consent of the patient and abiding by the institutional ethical committee approval.
Isolation of whole cell population
Isolation of mononuclear cells from bone marrow
The mononuclear cells from the bone marrow sample were isolated using density gradient centrifugation method. Briefly, the blood is diluted with PBS (Invitrogen) and layered on to Ficoll Paque (Stemcell Technologies). The mononuclear cell layer was collected after centrifugation. The cells were further washed with PBS to remove residual Ficoll and other contaminants. Contaminating erythrocytes were eliminated by treatment with osmotic buffer. Yield of whole cell population was determined using Trypan blue dye exclusion method and characterised using flow cytometry.
Isolation of stromal vascular fraction from subcutaneous and omentum fat tissue
Stromal vascular fraction was obtained from adipose tissue using a previously standardised protocol (Zuk et al. 2002) with modifications. The harvested tissues were washed several times with PBS, minced with scalpels into small pieces with simultaneous removal of visible blood vessels to maximum possible extent. The minced tissues were enzymatically dissociated with collagenase type I (Himedia Laboratories Pvt. Ltd) and centrifuged. The upper aqueous layer containing lipocytes was discarded and the pelleted stromal vascular fraction was washed twice with Phosphate Buffered Saline. The cells were then filtered and the contaminating erythrocytes lysed with an osmotic buffer. The resulting cells were counted and viability assay performed and characterized using flowcytometry.
Flowcytometric characterization
Flow cytometry was performed on a Becton, Dickinson FACS Aria (http://www.bd.com/) using a 488-nm argon-ion LASER and 632 nm red LASER for excitation; fluorescence emission was collected using its corresponding detectors. The antibodies used for enumeration are listed (Table 1) along with its fluorochrome, cat no and the manufacturer.
Table 1.
Facets of an assortment of cell surface marker
| Sl. No | CD marker | Alternate name | Fluorochrome | Cat No | Company |
|---|---|---|---|---|---|
| Hematopoietic stem cell markers | |||||
| 1 | CD 34 | Sialomucin | PE | 348057 | BD Bioscience |
| 2 | CD 45 | PTPRC | APC CY 7 | 348795 | BD Bioscience |
| 3 | CD 133 | Prominin 1 | APC | 17-1338-42 | eBioscience |
| 4 | HLADR | MHC-II | PER CP | 347364 | BD Bioscience |
| Mesenchymal stem cell marker | |||||
| 5 | CD 90 | Thy 1 | PE CY 5 | 15-0909-73 | eBioscience |
| 6 | CD 105 | Endoglin | APC | 17-1057-73 | eBioscience |
| 7 | CD 73 | NT5E | PE | 550257 | BD Bioscience |
| Cell adhesion molecules | |||||
| 8 | CD 29 | Integrin Beta 1 | PE | 555443 | BD Bioscience |
| 9 | CD 49d | Integrin Alpha 4 | PE | 12-0499-73 | eBioscience |
| 10 | CD 44 | HCELL | FITC | 555478 | BD Bioscience |
| 11 | CD 166 | ALCAM | PE | 559263 | BD Bioscience |
| 12 | CD 13 | ANPEP | APC | 557454 | BD Bioscience |
| 13 | CD 106 | VCAM-1 | FITC | 551146 | BD Bioscience |
| 14 | CD 54 | ICAM-1 | PER CP | 555512 | BD Bioscience |
| 15 | CD 31 | PECAM-1 | FITC | 555445 | BD Bioscience |
| Unique MSC marker | |||||
| 16 | CD 117 | C-Kit/SCF | APC | 17-1179-73 | eBioscience |
| 17 | ABCG2 | CDw338 | PE | 12-8888-73 | eBioscience |
| 18 | ALDH | – | – | 01700 | Stemcell Technologies |
About 1 × 106 cells were stained with saturating concentrations of fluorochrome conjugated antibodies. The cells were incubated in the dark for 20 min at RT. After incubation, cells were washed three times with wash flow buffer and resuspended in 500 μl of Phosphate-buffered solution (PBS). Wash flow buffer consisted of phosphate buffer supplemented with 2% (v/v) FBS (Sigma Aldrich) and 0.1% (w/v) sodium azide, NaN3 (Sigma Aldrich). Data analysis and acquisition was then performed using DIVA Software, Becton–Dickinson. A minimum of 10,000 events were characterized and recorded.
Statistical analysis
Data from omentum fat, subcutaneous fat and bone marrow (N = 5) were shown in Mean ± SEM, as individual variables with respect to n = 5 and p value was analyzed using two-tailed student’s t test to know the probability of clinical or practical significance between the sources so as to assert the beneficial source. As the probability of variations in the sample (both within and between samples) is most noteworthy for the relevance of better sources, smallest variations might be of significant assessment and also for the wide spread characterization study needed a multiple p value analysis. We have subjected p with: p value <0.05, p value <0.01, p value <0.005, p value <0.0001.
Results
Assorted cell surface marker expression in whole cell population (wcp) of BM, SF and OF
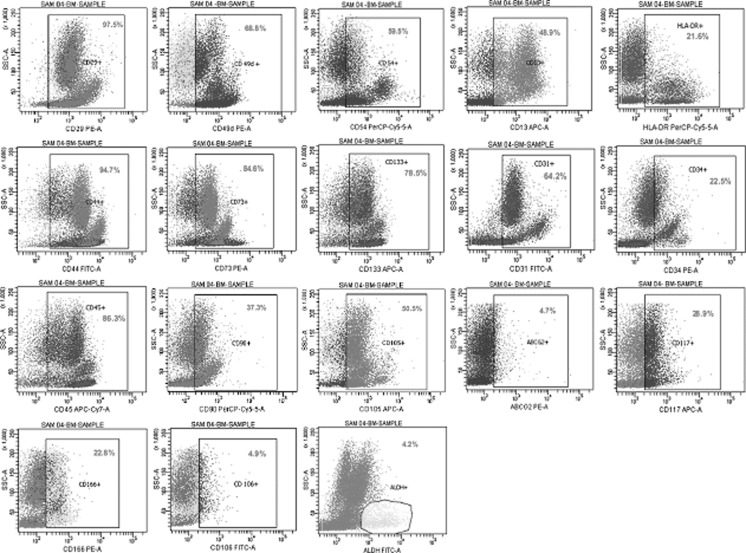
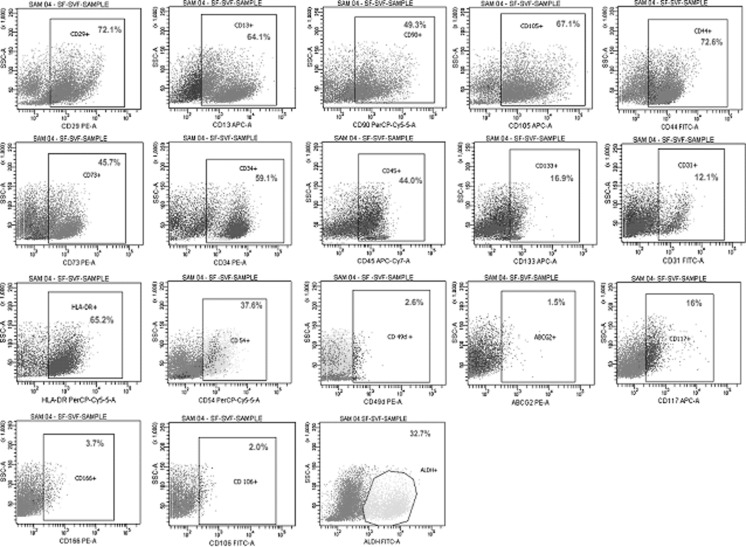
The cell surface profile (n = 5) with respect to sundry markers including Hematopoietic stem cells (HSC), Mesenchymal stem cells (MSC), Cell adhesion molecules (CAM) and other unique markers like ALDH, ABCG2 and CD 117 were analyzed using flow cytometry for WCP of BM, SF and OF. The graphical representation of the expression profile analysed using flow cytometry are illustrated (Figs. 1, 2, 3). The comparisons of Mean ± SEM values of all three sources are detailed in (Table 2). The portrayal on comparison of the entirety of these vibrant classes of cell surface markers of each source revealed that all sources exhibited positivity for wide spectrum of markers, thus correlating towards therapeutics. Surprisingly, it was also found that the stem cells obtained from omentum fat were found to be similar to subcutaneous fat and bone marrow, in facets of cell surface marker expression, thus adding triumph to the world of regenerative medicine.
Fig. 1.
Flowcytometric analysis of Bone marrow derived stem cells
Fig. 2.
Flowcytometric analysis of Subcutaneous fat derived stem cells
Fig. 3.
Flowcytometric analysis of Omentum fat derived stem cells
Table 2.
Cell surface antigenic profile of BM, SF and OF
| CD markers | BM | SF | OF |
|---|---|---|---|
| HSC markers | |||
| CD34 | 24.74 ± 4.2 | 59.58 ± 3.6 | 47.76 ± 3.3 |
| CD45 | 87.98 ± 2.0 | 38.04 ± 3.1 | 50.9 ± 10.0 |
| CD133 | 75.76 ± 6.0 | 18.02 ± 3.0 | 20.62 ± 4.0 |
| HLA-DR | 22.24 ± 3.4 | 60.34 ± 5.7 | 37.18 ± 4.8 |
| MSC markers | |||
| CD90 | 31.06 ± 3.7 | 57.02 ± 8.2 | 40.54 ± 6.3 |
| CD105 | 37.14 ± 4.9 | 63.34 ± 8.8 | 42.34 ± 3.8 |
| CD73 | 67.88 ± 5.6 | 51.08 ± 8.4 | 38.82 ± 2.6 |
| CAM markers | |||
| CD29 | 91.58 ± 1.7 | 68.28 ± 5.5 | 52.36 ± 2.5 |
| CD49d | 66.74 ± 4.0 | 7.28 ± 3.4 | 7.06 ± 1.2 |
| CD13 | 36 ± 3.7 | 60.54 ± 9.8 | 36.94 ± 6.6 |
| CD44 | 90.04 ± 1.5 | 67.72 ± 7 | 63.06 ± 6.9 |
| CD54 | 43.54 ± 6.4 | 44.9 ± 7.3 | 44.82 ± 3.0 |
| CD 31 | 63.28 ± 7.1 | 10.2 ± 1.6 | 12.06 ± 3.4 |
| CD 106 | 3.14 ± 1.0 | 3.62 ± 1.3 | 2.26 ± 0.3 |
| CD 166 | 31.04 ± 3.6 | 5.84 ± 1.8 | 5.92 ± 1.3 |
| Unique markers | |||
| ABCG2 | 3.88 ± 0.3 | 3.96 ± 1.2 | 1.8 ± 0.3 |
| ALDH | 3.28 ± 0.5 | 26.48 ± 4 | 27.76 ± 2.0 |
| CD 117 | 31.44 ± 1.8 | 24.88 ± 3.5 | 11.94 ± 3.7 |
However, there is a disparity in the representative values of these cell surface markers expression in every source, thus making comparison an arduous and challenging task. In lieu of assorted antigenic expression, the markers were categorized and collated in detail with corresponding range of expression as outlined (Table 3). The range of expression as interpreted in Fig. 4 manifests the intense elucidation of these vivacious markers relating to its divergence in expression. Hence, it is evident that omentum fat articulates moderate to high percentage of expressions for majority of cell surface markers including certain cell adhesion molecules and mesenchymal stem cell markers similar to subcutaneous fat and bone marrow. However, unlike omentum fat and subcutaneous fat, remarkable expressions of few cell surface markers were expressed in bone marrow.
Table 3.
Range of variations in % of cell surface markers
| Cell surface markers | % In BM-WCP | % In SF-WCP | % In OF-WCP |
|---|---|---|---|
| HSC | |||
| CD34 | + | +++ | ++ |
| CD45 | # | ++ | +++ |
| CD133 | # | + | + |
| HLA-DR | + | +++ | ++ |
| MSC | |||
| CD90 | ++ | +++ | ++ |
| CD105 | ++ | +++ | ++ |
| CD73 | +++ | +++ | ++ |
| CAM | |||
| CD29 | # | +++ | +++ |
| CD49d | +++ | □ | □ |
| CD13 | ++ | +++ | ++ |
| CD44 | # | +++ | +++ |
| CD54 | ++ | ++ | ++ |
| CD31 | +++ | □ | + |
| CD106 | □ | □ | □ |
| CD166 | ++ | □ | □ |
| Unique marker | |||
| ABCG2 | □ | □ | □ |
| ALDH | □ | ++ | ++ |
| CD117 | ++ | + | + |
□: Range from 1.8 to 10.2% (sparse expression); +: range from 11 to 24% (low expression); ++: range from 26 to 47% (moderate expression); +++: range from 50 to 68% (high expression); #: range from 75 to 92% (remarkable expression)
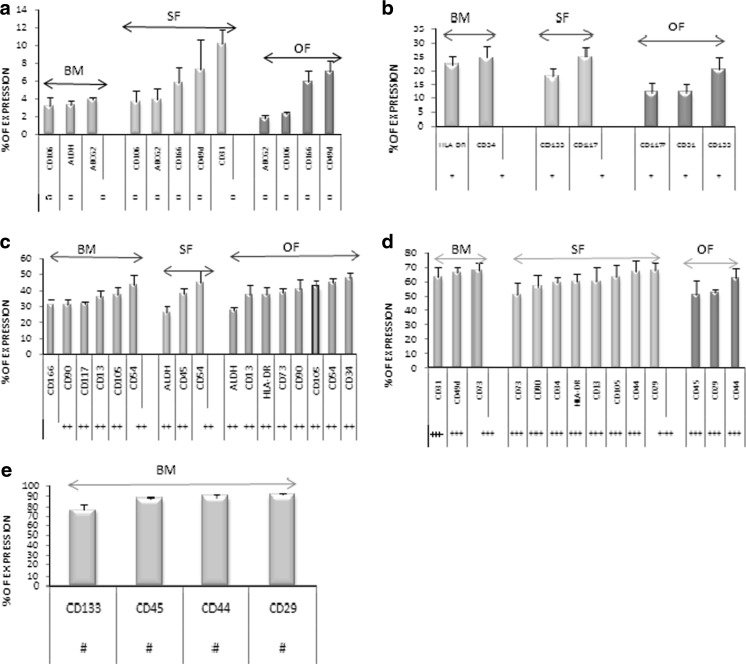
Fig. 4.
Ranges of Expression of cell surface markers among BM, SF and OF. The expression profiles of cell surface markers are categorized based on their range of expression using Mean ± SEM values. The categorization of expressions is as follows: The sparse expression of the cell surface markers (a); low expression of the cell surface markers (b); moderate expression of the cell surface markers (c); high expression of the cell surface markers (d); remarkable expression of the cell surface markers (e)
Regardless of the above results, it was not comprehensible to find out the ideal source of stem cells for clinical transplantation. Thus, the surface markers were analysed individually using paired student t test to identify any significant difference among samples of BM versus SF, BM versus OF and SF versus OF respectively as depicted (Table 4). The detailed values of these sources are represented in supplementary data (Table S1). It clearly emphasises the fact that there is no significant difference between the expression percentages of omentum fat in comparison to subcutaneous fat. However, some considerable difference between the expressions of omentum fat in comparison to bone marrow was apparent from the p value analysis (Table S1).
Table 4.
p value of 18 cell surface markers of BM, SF and OF
| CD markers | p value BM versus SF | p value BM versus OF | p value SF versus OF |
|---|---|---|---|
| HSC markers | |||
| CD34 | *** | **** | – |
| CD45 | $ | ** | – |
| CD133 | *** | **** | – |
| HLA-DR | **** | – | * |
| MSC markers | |||
| CD90 | * | – | – |
| CD105 | * | – | * |
| CD73 | – | *** | – |
| CAM markers | |||
| CD29 | ** | $ | – |
| CD49d | $ | $ | – |
| CD13 | * | – | ** |
| CD44 | ** | ** | – |
| CD54 | – | – | – |
| CD31 | ** | *** | – |
| CD106 | – | – | – |
| CD166 | *** | * | – |
| Other unique markers | |||
| ABCG2 | – | – | – |
| ALDH | *** | $ | – |
| CD117 | – | – | – |
–: no significant difference; *: significant difference (p < 0.05); **: significant difference (p < 0.01); ***: p < 0.005 significant difference; ****: p < 0.0001 significant difference; $: very highly significant difference
Lineage specific categorization of immunophenotype of whole cell population
Hematopoietic stem cells
In the literature was reported that bone marrow possesses a higher percentage of HSC population. We scrutinised the expression of key HSC markers CD34, CD45, CD133 and HLA-DR. Here in our study, we found that bone marrow possesses a lower percentage of CD34 and HLA-DR than SF and OF (Fig. 5a).
Fig. 5.
Lineage specific marker categorization. The cell surface marker expression is depicted based on their lineage using Mean ± SEM values. The lineage specific categorizations are as follows: Hematopoietic stem cell markers (a); mesenchymal stem cell markers (b); cell adhesion molecules (c); other unique markers (d)
Mesenchymal stem cells
To emphasize the presence of significant MSC population in these three sources, the expression of CD90, CD105 and CD73 was studied. The results were confounding in all three sources. It was identified that SF possess higher expression of MSC compared to other sources, whereas BM and OF possess almost a similar percentage of MSCs (Fig. 5b). This is another proof of evidence to reiterate our hypothesis that omentum fat is also an excellent alternative source for stem cell therapeutics.
Cell adhesion molecules
Cell Adhesion molecules, as the name suggests, are known to be responsible for cell- cell communication and migration through of chemo-attractive properties. Accordingly expression of CD29, CD49d, CD13, CD54, CD44, CD31, CD106 and CD166 was examined. Most of the cell adhesion molecules are widely expressed in BM compared to SF and OF. CD 106, which is said to be an ADSC specific marker showed no positivity for BM or for adipose tissue. Similarly, CD 49d, an ADSC specific marker, is not expressed in adipose tissue, rather showed significant expression in bone marrow. The rest of the cell adhesion molecules was similarly expressed in all three sources except CD 31 and CD 166, which showed higher expression in BM than the other sources (Fig. 5c).
Unique marker
Aldehyde Dehydrogenase, a pluripotent/progenitor stem cell surface marker (Hess et al. 2006) is found to be significantly expressed in both SF and OF than bone marrow which presents <5% of ALDH. This suggests that SF and OF might possess more properties of pluripotency than BM. CD117 on the other hand, was expressed to higher levels in BM than SF and OF (Fig. 5d).
Quantitative validation of surface antigenic expression pattern within the stem cell sources
The complete profiling of cell surface expression of all 18 markers of individual samples (n = 5) of bone marrow, subcutaneous fat and omentum fat was compared. These results can be used as a catalogue for future researchers in identification of unique cell surface markers of stem cells with its expression pattern in all sources of bone marrow, subcutaneous fat and omentum fat.
Bone marrow
The values of each marker manifested variation among samples. There was a significant difference among the 5 samples of all cell surface markers except CD 106, ABCG2 and ALDH (Fig. 6). The flowcytometric values obtained for each sample of bone marrow derived stem cells are detailed in supplementary data (Table S2).
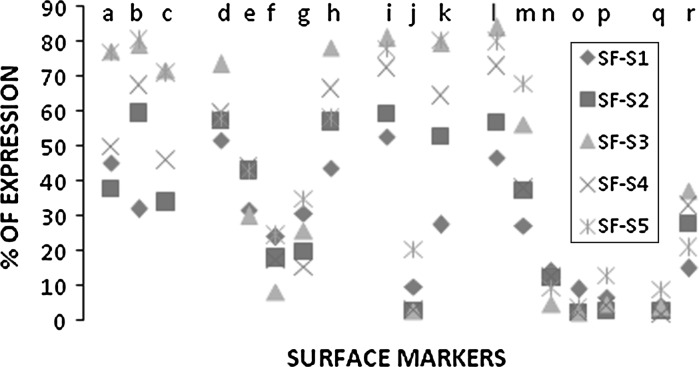
Fig. 6.
Cell surface marker profile (n = 18) of samples within bone marrow. This figure represents a systematic comparison of expression profile variation among 5 samples of bone marrow. The details of cell surface markers denoted by a–r are mentioned below: (a CD90; b CD105; c CD73; d CD34; e CD45; f CD133; g CD177; h HLADR; i CD29; j CD49d; k CD13; l CD54; m CD31; n CD44; o CD106; p CD166; q ABCG2; r ALDH)
Adipose tissue
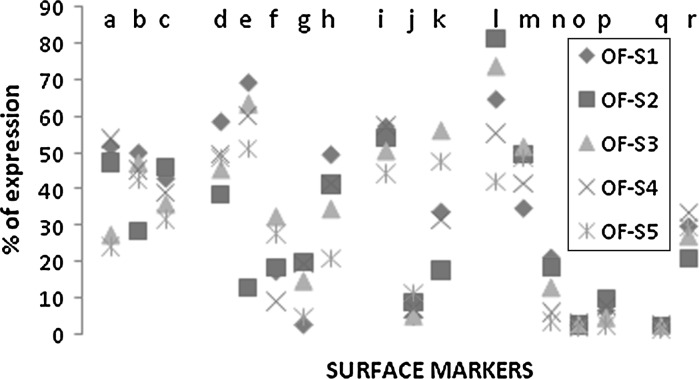
Similar to bone marrow, we found that even subcutaneous fat and omentum fat showed variations in cell surface marker expression among samples (n = 5). The cell surface markers CD 31, CD 106, CD 166 and ABCG2 do not show much variations among samples of subcutaneous adipose tissue (Fig. 7) and the cell surface markers CD 49d, CD 106, CD 166 and ABCG2 do not vary among samples of omentum fat (Fig. 8). The flowcytometric values obtained for each sample of SF and OF are detailed in supplementary datas (Tables S3, S4).
Fig. 7.
Cell surface marker profile (n = 18) of samples within subcutaneous fat. This figure represents a systematic comparison of expression profile variation among 5 samples of subcutaneous fat. The details of cell surface markers denoted by a–r are mentioned below: (a CD90; b CD105; c CD73; d CD34; e CD45; f CD133; g CD177; h HLADR; i CD29; j CD49d; k CD13; l CD54; m CD31; n CD44; o CD106; p CD166; q ABCG2; r ALDH)
Fig. 8.
Cell surface marker profile (n = 18) of samples within Omentum Fat. This figure represents a systematic comparison of expression profile variation among 5 samples of omentum fat. The details of cell surface markers denoted by a–r are mentioned below: (a CD90; b CD105; c CD73; d CD34; e CD45; f CD133; g CD177; h HLADR; i CD29; j CD49d; k CD13; l CD54; m CD31; n CD44; o CD106; p CD166; q ABCG2; r ALDH)
Discussion
On the frontline, unlike some literature references stating a higher expression of CD 133 and CD 45 in cord blood (Kern et al. 2006; Rebelatto et al. 2007), our sources of stem cells of interest possess significant expression of these markers. However, bone marrow showed remarkable expression of CD133 and CD45 in comparison to SF and OF. Similarly, unlike other data reported in literature (Zuk et al. 2002; Aust et al. 2004; Tholpady et al. 2003; Gronthos et al. 2001; Boquest et al. 2005), our results revealed that CD 34 and HLADR showed significant expression in subcutaneous fat and omentum fat in comparison to bone marrow. This is indicative for the fact that apart from bone marrow or cord blood being used as a therapeutic tool for blood related diseases (Dann et al. 1997; Lawson et al. 2003; Kumar et al. 2009; Ruggeri et al. 2010), SF and OF could also be used as a source of stem cell for efficient HSC transplantation.
Secondly, we found that there are controversies among various citations over MSC specific markers such as CD 90, CD 54, CD 44, CD 29, CD 105, CD 166, and CD 13 (De Ugarte et al. 2003a, b; Zuk et al. 2002; Kotaro et al. 2006; Aust et al. 2004; Rebelatto et al. 2007; Gronthos et al. 2001; Boquest et al. 2005; Katz et al. 2005; Mitchell et al. 2006). Thus, it is unambiguous that prevalence of marker specificity for MSC with respect to all sources is quite vague. There is hardly any distinct categorization towards MSCs and CAM populations available until now.
Hence, in order to avoid this uncertainty and to get a coherent clue of the presence of construed MSC in these sources, we have categorized only CD 90, CD 105 and CD 73 as positive markers, as reported and confirmed by ISCT (Dominici et al. 2006). It is widely apprehended in our previous work and several other literature references that majority of the MSC specific surface markers are exquisitely revealed in ex vivo conditions upon culturing (Wagner et al. 2005; Kern et al. 2006; Rebelatto et al. 2007). Several literature references failed to report CD 73 as an MSC specific marker (Varma et al. 2007; Boquest et al. 2005; Katz et al. 2005). However, we found that like SF and BM, OF expressed similarly higher levels of CD 90, CD 105 and CD 73 (Tables 3 and 4), proving its plasticity towards stem cell therapeutics. This indicates that omentum fat proves to be an efficient source of therapeutics even with regard to mesenchymal stem cells.
Subsequently, the molecular mechanisms that underlay the roots for migration of stem cells in vivo to the site of injury, necessitates a complex multistep cascade of events capable of resisting shear forces coupled with transendothelial migration. This clearly emphasizes the imminent activity of cell adhesion molecules in transendothelial migration and homing of stem cells. In lieu of this, we found that, expression profile of few cell adhesion molecules among these cell sources were inconsistent with the existing reports with respect to percentage expression as well as tissue specificity (De Ugarte et al. 2003a, b; Zuk et al. 2002; Gimble et al. 2007; Gronthos et al. 2001; Katz et al. 2005; Pittenger et al. 1999).
CD 49d being an ADSC specific marker (Zuk et al. 2002; Rebelatto et al. 2007; De Ugarte et al. 2003a, b; Katz et al. 2005), was found to be very sparsely expressed both in subcutaneous fat and omentum fat tissue, whereas surprisingly highly expressed in bone marrow. This suggests that CD 49d could not be an ADSC specific marker and was found to render a pivotal support in bone marrow derived stem cell migration. Similarly, CD 106 (VCAM) reported as an ADSC specific marker (De Ugarte et al. 2003a, b; Zuk et al. 2002; Gronthos et al. 2001; Katz et al. 2005; Pittenger et al. 1999), was neither expressed in ADSC nor in bone marrow. This is indicative of the clue that there is no significant role to be played by CD 106 in stem cell therapeutics. CD 54 (ICAM) on the other hand, is moderately expressed in all sources showing no significant difference in overall expression. This illustrates the significance of CD 54 among all sources.
Similarly, CD 29, an important integrin family member and CD 44 are remarkably expressed in bone marrow (Table 3) and bind to selectin as known from the in vivo mechanism of bone marrow niche (Zhu et al. 2006; Sackstein et al. 2008; Dimitroff et al. 2001; Brooke et al. 2008). CD 29 and CD 44 also are widely expressed in adipose tissue despite the absence of selectin binding niche mechanism in them (Zuk et al. 2002; Gimble et al. 2007; Aust et al. 2004; Gronthos et al. 2001; De Ugarte et al. 2003a, b; Katz et al. 2005). CD 13, rather than being an important monocyte/macrophage marker, plays a vital role in angiogenesis and migration (Mins-Osorio et al. 2006; Pasqualini et al. 2007). It is expressed in higher percentage in SF and moderately expressed in both BM and OF. This apparently proves the fact that these sources including stem cells from omentum fat can be used as a source for regeneration of treating vascular diseases and ischemic diseases as well.
Finally, omentum fat not only exhibits similarity to BM and SF with regard to expression pattern to HSC, MSC and CAM, but also shows similarity in CD 117 and ALDH expression. Despite being a bone marrow specific marker, both OF and SF showed clear expression of CD 117 (Wilson and Trumpp 2006; Felipe Prosper et al. 2001; Heissig et al. 2002), is found to be similar in both SF and OF. This is inconsistent with certain reports (Wagner et al. 2005; Gronthos et al. 2001; Boquest et al. 2005; Katz et al. 2005). The pluripotent marker ALDH, was highly expressed in SF and OF in comparison to BM (Hess et al. 2006; Lioznov et al. 2005). This is indicative of the fact that adipose tissue might possess more pluripotency than bone marrow. It is evident that the cell surface markers of omentum fat are highly similar to those of subcutaneous fat and bone marrow in all aspects. This enables us to audaciously suggest that omentum fat is an effective and alternative source for clinical transplantation.
Furthermore, this systematic study of the surface antigenic properties of all 18 surface markers of individual samples for three major stem cell sources (n = 5) will remain a catalogue for easy reference of expected surface expression, paving way for further in depth research.
Summary
We found from this study that, identification of an ideal source for stem cell therapeutics is strenuous. However, it can be concluded that omentum fat has emerged as an alternative source for stem cell therapeutics in addition to the existing widely accepted sources such as subcutaneous fat and bone marrow. Furthermore, this study will serve as a database for future research, possessing the expressions of wide range of markers (n = 18), specific for OF, SF and BM. Nonetheless, substantiation and imperativeness of this work requires execution of the same kind of research work upon culturing of every source. This might unveil the existence of uncertainty among sources and among cell surface markers.
Electronic supplementary material
References
- Aust L, Devlin B, Foster SJ, Halvorsen YD. Yield of human adipose derived adult stem cells from liposuction aspirates. Cytotherapy. 2004;6:7–14. doi: 10.1080/14653240310004539. [DOI] [PubMed] [Google Scholar]
- Baglioni S, Francalanci M, Squecco R, Lombardi A, Cantini G, Angeli R, Gelmini S, Guasti D, Benvenuti S, Annunziato F, Bani D, Liotta F, Francini F, Perigli G, Serio M, Luconi M. Characterization of human adult stem-cell populations isolated from visceral and subcutaneous adipose tissue. FASEB J. 2009;23:3494–3505. doi: 10.1096/fj.08-126946. [DOI] [PubMed] [Google Scholar]
- Bai X, Yan Y, Song Y-H, Seidensticker M, Rabinovich B, Metzele R, Bankson JA, Vykoukal D, Alt E. Both cultured and freshly isolated adipose tissue derived stem cells enhance cardiac function after acute myocardial infarction. Eur Heart J. 2010;31:489–501. doi: 10.1093/eurheartj/ehp568. [DOI] [PubMed] [Google Scholar]
- Boquest AC, Shahdadfar A, Fronsdal K, Sigurjonsson O, Tunheim SH, Collas P, Brichmann JE. Isolation and transcription profiling of purified uncultured human stromal stem cells: alteration of gene expression after in vitro cell culture. Mol Biol Cell. 2005;16:1131. doi: 10.1091/mbc.E04-10-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooke G, Tong H, Levesque J-P, Atkinson K. Molecular trafficking mechanisms of multipotent mesenchymal stem cells derived from human bone marrow and placenta. Stem Cells Dev. 2008;17:929–940. doi: 10.1089/scd.2007.0156. [DOI] [PubMed] [Google Scholar]
- Dann EJ, Daugherty CK, Larson RA. Allogeneic bone marrow transplantation for relapsed and refractory Hodgkin’s disease and non-Hodgkin’s lymphoma. Bone Marrow Transplant. 1997;20:369–374. doi: 10.1038/sj.bmt.1700904. [DOI] [PubMed] [Google Scholar]
- Ugarte DA, Alphonso Z, Zuk PA, Elbarbury A. Differentiation extension of stem cell mobilization associated-molecules on multi lineage cells from adipose tissue and bone marrow. Immunol Lett. 2003;89:267–270. doi: 10.1016/S0165-2478(03)00108-1. [DOI] [PubMed] [Google Scholar]
- Ugarte DA, Morizono K, Elbarbary A, Alphonso Z. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells tissue organs. 2003;174:101–109. doi: 10.1159/000071150. [DOI] [PubMed] [Google Scholar]
- Dimitroff CJ, Lee JY, Rafii S, Fuhlbrigge RC, Sackstein R. CD44 is a major E-selectin ligand on human hematopoietic progenitor cells. J Cell Biol. 2001;153:1277–1286. doi: 10.1083/jcb.153.6.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici M, Blanc K, Mueller I, Cortenbach IS, Marini FC, Krausc DS, Deans RJ, Keating A, Prockop DJ, Horwitz EM. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Garcia-Olmo D, Garcia-Arranz M, Herreros D. Expanded adipose-derived stem cells for the treatment of complex perianal fistula including Crohn’s disease. Expert Opin Biol Ther. 2008;8:1417–1423. doi: 10.1517/14712598.8.9.1417. [DOI] [PubMed] [Google Scholar]
- Gimble JM, Guilak F. Differentiation potential of adipose derived adult stem cell (ADAS) cells. Curr Top Dev Biol. 2003;58:137–160. doi: 10.1016/S0070-2153(03)58005-X. [DOI] [PubMed] [Google Scholar]
- Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimble JM, Guilak F, Bunnell BA. Clinical and preclinical translation of cell-based therapies using adipose tissue-derived cells. Stem Cell Res Ther. 2010;1:19. doi: 10.1186/scrt19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronthos S, Franklin DM, Leddy HA, Robey PG. Surface protein characterization of human adipose tissue-derived stormal cells. J Cell Physiol. 2001;189:54–63. doi: 10.1002/jcp.1138. [DOI] [PubMed] [Google Scholar]
- Hallam S, Gribben JG. Stem cell transplantation in chronic lymphocytic leukaemia—steering a safe course over shifting sands. Best Pract Res Clin Haematol. 2010;23:109–119. doi: 10.1016/j.beha.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, Crystal RG, Besmer P, Lyden D, Moore MAS, Werb Z, Raffi S. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–637. doi: 10.1016/S0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess DA, Writhlin L, Carft TP, Phillip EH, Hohm SA, Lahey R, Eades WC, Creer MH, Nolta JA. Selection based on CD133 and high aldehyde dehydrogenase activity isolates long-term reconstituting human hematopoietic stem cells. Blood. 2006;107:2162–2169. doi: 10.1182/blood-2005-06-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, Sussman M, Orchard P, Marx JC, Pveritz RE, Brenner MK. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309–313. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- Jurgens WJ, Oedayrajsingh-Varma MJ, Helder MN, Zandiehdoulabi B, Schouten TE, Kuik DJ, Ritt MJ, Milligen FJ. Effect of tissue-harvesting site on yield of stem cells derived from adipose tissue: implications for cell-based therapies. Cell Tissue Res. 2008;332:415–426. doi: 10.1007/s00441-007-0555-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz AJ, Tholpady A, Tholpady SS, Shang H, Ogle RC. Cell surface and transcriptional characterization of human adipose-derived adherent stromal (hADAS) cells. Stem Cells. 2005;23:412–423. doi: 10.1634/stemcells.2004-0021. [DOI] [PubMed] [Google Scholar]
- Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- Kopp HG, Avecilla ST, Hooper AT, Rafii S. The bone marrow vascular niche: home of HSC differentiation and mobilization. Physiology. 2005;20:349–356. doi: 10.1152/physiol.00025.2005. [DOI] [PubMed] [Google Scholar]
- Kotaro Y, Shigeura T, Matsumoto D, Sato T, Takaki Y, Aiba-Kojima E, Sato K, Inoue K, Nagase T, Koshima I, Gonda K. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J Cell Physiol. 2006;s208:64–76. doi: 10.1002/jcp.20636. [DOI] [PubMed] [Google Scholar]
- Kuethe F, Richartz BM, Kasper C, Sayer HG, Hoeffken K, Werner GS, Figulla HR. Autologous intracoronary mononuclear bone marrow cell transplantation in chronic ischemic cardiomyopathy in humans. Int J Cardiol. 2004;100:485–491. doi: 10.1016/j.ijcard.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Kuethe F, Richartz BM, Sayer HG, Kasper C, Werner GS, Höffken K, Figulla HR. Lack of regeneration of myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans with large anterior myocardial infarctions. Int J Cardiol. 2004;97:123–127. doi: 10.1016/j.ijcard.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Kumar AA, Kumar SR, Narayanan R, Arul K, Baskaran M. Autologous bone marrow derived mononuclear cell therapy for spinal cord injury: a phase I/II clinical safety and primary efficacy data. Exp Clin Transplant. 2009;7:241–248. [PubMed] [Google Scholar]
- Lawson SE, Roberts IAG, Amrolia P, Dokal I, Szydloand R, Darbyshire PJ. Bone marrow transplantation for b-thalassaemia major: the UK experience in two paediatric centres. Br J Haematol. 2003;120:289–295. doi: 10.1046/j.1365-2141.2003.04065.x. [DOI] [PubMed] [Google Scholar]
- Lioznov MV, Freiberger P, Kroger N, Zander AR, Fehse B. Aldehyde dehydrogenase activity as a marker for the quality of hematopoietic stem cell transplants. Bone Marrow Transplant. 2005;35:909–914. doi: 10.1038/sj.bmt.1704928. [DOI] [PubMed] [Google Scholar]
- Mesimaki K, Lindroos B, Tornwall J, Mauno J, Lindqvist C, Kontio R, Miettinen S, Suuronen R. Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. Oral Maxillofac Surg. 2009;38:201–209. doi: 10.1016/j.ijom.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Mins-Osorio P, Shapiro LH, Ortega E. CD 13 in cell adhesion: aminopeptidase N (CD 13) mediates homotypic aggregation of monocytic cells. J Leukoc Bio. 2006;79:719–730. doi: 10.1189/jlb.0705425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JB, McIntosh K, Zvonic S, Garrett S, Floyd ZE, Kloster A, Halvorsen Y, Storms RW, Goh B, Kilroy G, Wu X, Gimble JM. Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells. 2006;24:376–385. doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- Pasqualini R, Renate K, Lahdenranta J, Sakamoto M, Stryhn A, Ashmun RA, Shapiro LH, Arap W, Ruoslahti E. Aminopeptidase N is a receptor for tumor homing peptides and a target for inhibiting angiogenesis. Cancer Res. 2007;60:722–727. [PMC free article] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Pountos I, Corscadden D, Emery P, Giannoudis PV. Mesenchymal stem cell tissue engineering: techniques for isolation, expansion and application. Injury. 2007;S4:S23–S33. doi: 10.1016/S0020-1383(08)70006-8. [DOI] [PubMed] [Google Scholar]
- Rebelatto CK, Aguiar AM, Moretao MP, Senegaglia AC, Hansen P, Barchiki F, Oliveira J, Martins J, Kuligovski C, Mansur F, Christofis A, Amaral VF, Brofman PS, Goldenberg S, Nakao LS, Correa A. Dissimilar differentiation of mesenchymal stem cells from bone marrow, umbilical cord blood, and adipose tissue. Exp Biol Med. 2007;233:901–913. doi: 10.3181/0712-RM-356. [DOI] [PubMed] [Google Scholar]
- Ruggeri A, Ciceri F, Gluckman E, Labopin M, Rocha V. Alternative donors hematopoietic stem cells transplantation for adults with acute myeloid leukemia: umbilical cord blood or haploidentical donors? Best Pract Res Clin Haematol. 2010;23:207–216. doi: 10.1016/j.beha.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Sackstein R, Merzaban JS, Cain DW, Dagia NM, Spencer JA, Lin CP, Wohlgemuth R. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat Med. 2008;14:187. doi: 10.1038/nm1703. [DOI] [PubMed] [Google Scholar]
- Singh AK, Patel J, Litbarg NO, Gudehithlu KP, Sethupathi P, Arruda JA, Dunea G. Stromal cells cultured from omentum express pluripotent markers, produce high amount of VEGF, and engraft to injured sites. Cell Tissue Res. 2008;332:81–88. doi: 10.1007/s00441-007-0560-x. [DOI] [PubMed] [Google Scholar]
- Stolzing A, Jones E, McGonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 2008;129:163–173. doi: 10.1016/j.mad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Tholpady SS, Katz AJ, Ogle RC. Mesenchymal stem cells from rat visceral fat exhibit multipotential differentiation in vitro. Anat Rec Part A. 2003;272A:398–402. doi: 10.1002/ar.a.10039. [DOI] [PubMed] [Google Scholar]
- Toyoda M, Matsubara Y, Lin K, Sugimachi K, Furue M. Characterization and comparison of adipose tissue-derived cells from human subcutaneous and omental adipose tissues. Cell Biochem Funct. 2009;27:440–447. doi: 10.1002/cbf.1591. [DOI] [PubMed] [Google Scholar]
- Tsiftsoglou AS, Bonovolias ID, Tsiftsoglou SA. Multilevel targeting of hematopoietic stem cell self-renewal, differentiation and apoptosis for leukemia therapy. Pharmacol Ther. 2009;122:264–280. doi: 10.1016/j.pharmthera.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Varma MJ, Breuls RG, Schouten TE, Jurgens WJ, Bontkes HJ, Schuurhuis GL, Ham SM, Milligen FJ. Phenotypical and functional characterization of freshly isolated adipose tissue-derived stem cells. Stem Cells Dev. 2007;16:91–104. doi: 10.1089/scd.2006.0026. [DOI] [PubMed] [Google Scholar]
- Vater C, Kasten P, Stiehler M. Culture media for the differentiation of mesenchymal stromal cells. Acta Biomater. 2011;7:463–477. doi: 10.1016/j.actbio.2010.07.037. [DOI] [PubMed] [Google Scholar]
- Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, Blake J, Schwager C, Eckstein V, Ansorge W, Ho AD. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005;33:1402–1416. doi: 10.1016/j.exphem.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006;6:93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- Zhu H, Mitsuhashi N, Klein A, Barsky LW, Weinberg K, Barr ML, Demetriou A, Wu GD. The role of the hyaluronan receptor CD44 in mesenchymal stem cell migration in the extracellular matrix. Stem Cells. 2006;24:928–935. doi: 10.1634/stemcells.2005-0186. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Liu T, Song K, Fan X, Ma X, Cui Z. Adipose-derived stem cell: a better stem cell than BMSC. Cell Biochem Funct. 2008;26:664–675. doi: 10.1002/cbf.1488. [DOI] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser jk, Banhaim P, Hedrick MH (2002) Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13(12): 4279–95 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.