Abstract
In Latin America, onchocerciasis is targeted for elimination by 2012 through twice-yearly mass treatment of the eligible population with ivermectin. In Guatemala, two of the four historical endemic foci have demonstrated elimination of transmission, following World Health Organization guidelines. Using established guidelines ophthalmological, serological, and entomological evaluations were conducted in 2007-8 to determine the transmission status of onchocerciasis in the Huehuetenango focus. The prevalence of Onchocerca volvulus microfilariae in the anterior segment of the eye in 365 residents was 0% (95% confidence interval [CI] 0–0.8%), the prevalence of infection of O. volvulus in Simulium ochraceum among 8252 flies collected between November 2007 and April 2008 was 0% (95% CI 0–0.02%), and the prevalence of antibodies to a recombinant O. volvulus antigen in 3118 school age children was 0% (95% CI 0–0.1%). These results showed transmission interruption; thus, in 2009 mass treatment was halted and posttreatment surveillance began. To verify for potential recrudescence an entomological evaluation (from December 2010 to April 2011) was conducted during the 2nd and 3rd year of posttreatment surveillance. A total of 4587 S. ochraceum were collected, and the prevalence of infection of O. volvulus was 0% (95% CI 0–0.04%). Transmission of onchocerciasis in the Huehuetenango focus has been eliminated.
1. Introduction
Onchocerciasis is a vector-borne parasitic disease caused by infection with the filarial nematode Onchocerca volvulus and can result in eye or skin lesions. The parasite is transmitted to humans by certain black flies of the genus Simulium. Although adult worms can live for years under the skin in fibrous nodules that are often palpable, morbidity is caused by the body's immune reaction to the microfilariae (mf) that leave the nodules and migrate through the skin and sometimes enter the eye [1]. In 2007, The World Health Organization (WHO) estimated 37 million persons were infected with onchocerciasis in 37 endemic countries (30 in Africa, six in the Americas, and one in the Arabian Peninsula) [2].
In the Americas there are 13 geographically isolated endemic foci found within Mexico, Guatemala, Colombia, Venezuela, Brazil, and Ecuador [3, 4] where 470,222 persons were at risk of infection in 2011 [5]. The Onchocerciasis Elimination Program of the Americas (OEPA) was established in 1992 in response to a resolution of the Directing Council of the Pan American Health Organization (PAHO) calling for the elimination of onchocerciasis ocular morbidity in the Americas by 2007 [6]. A new resolution in 2008 by PAHO called for the interruption of transmission throughout the region by 2012 [7]. A subsequent 2009 PAHO Resolution (CD49.R19), calling for the elimination or drastic reduction of 12 neglected infectious diseases of poverty in the Americas by 2015, includes onchocerciasis as an elimination target [8]. The OEPA strategy is to support national programs in the six endemic countries to provide twice-yearly mass drug administration (MDA) of ivermectin (Mectizan, donated by Merck & Co.) to ≥85% of the eligible population at risk [6, 9, 10].
There are four endemic onchocerciasis foci within Guatemala (Figure 1). The Guatemala Ministry of Public Health (MOPH), with the assistance of OEPA, began MDA with ivermectin in 1996 and has achieved ≥85% coverage of the eligible population at risk in twice-yearly MDA rounds since 2002. Interruption of transmission was demonstrated in two of the four foci (Santa Rosa in 2007 and Escuintla-Guatemala in 2008) [11, 12]; in 2010 both foci completed the posttreatment surveillance, and the evaluations showed elimination of transmission [5]. The Huehuetenango focus has participated in 22 rounds of MDA with ivermectin over the past 13 years, with ≥85% coverage in 17 consecutive rounds of twice-yearly ivermectin treatment. E. W. Cupp and M. S. Cupp [9] calculated that the death of adult worms is accelerated in the presence of ivermectin and that the adult population should die (in the absence of ongoing transmission) after 6.5 years (13 rounds) of twice-yearly treatment. Thus, the treatment period appears more than adequate to have eliminated the parasite in the focus of Huehuetenango. We, therefore, report the assessment of the status of 2007-2008 pretreatment and 2010-2011 posttreatment onchocerciasis transmission evaluations in the Huehuetenango focus.
Figure 1.
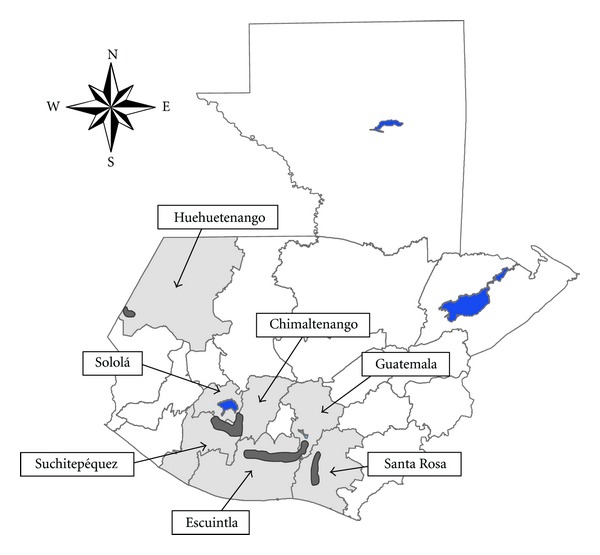
Onchocerciasis foci in Guatemala (in dark gray) in the endemic departments (light gray).
2. Methods
2.1. Evaluation Area
The Huehuetenango focus (15.35°N, 91.90°E) is situated in the western highlands of Guatemala along the border with Mexico and consists of eight historically endemic municipios (similar to counties) (Figure 2). Historical data from this area showed a gradual reduction of infection prevalence well before the initiation of MDA. Yamagata et al. [13], in an analysis of nodule prevalence data gathered since 1940 by MOPH nodulectomy teams working in the area, showed that nodule prevalence decreased from 41% (in 1940) to 21% in 1969. With the launching of ivermectin MDA, surveys conducted by the MOPH in 1987 and 1992 showed that the prevalence of nodules was 1.1% and 2.2%, respectively (F. O. Richards, Jr., unpublished data). The first surveys to measure the prevalence of infection with O. volvulus mf using skin snips in Huehuetenango demonstrated a prevalence of <1% (0.6% in 1987 and 0.8% in 1992). In 2006 an evaluation conducted by the MOPH reported a prevalence of 0% for skin mf and 0.4% for the presence of suspected onchocerciasis nodules (A. Dominguez, pers. comm).
Figure 2.
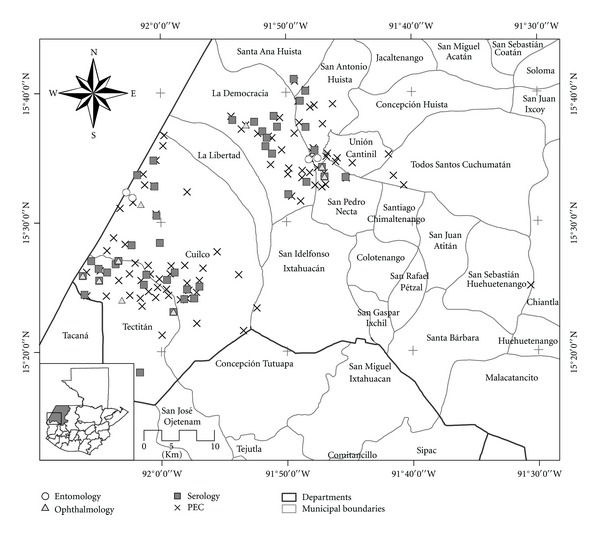
Map of the evaluation area in the Department of Huehuetenango, Guatemala.
Annual MDA with ivermectin was launched in the endemic municipios of Huehuetenango in 1996 (Figure 3). The program was strengthened in 2000, when twice per year treatment was instituted, and ≥85% coverage of the eligible population at risk was achieved for the first time. In 2008, there were 30,239 persons at risk of transmission, and the eligible population (nonpregnant, healthy, and older than five years of age) to receive treatment with ivermectin numbered 27,797. Between 2000 and 2008, there was sustained and adequate coverage (≥85% treatment coverage) for 17 consecutive twice-yearly MDA rounds.
Figure 3.
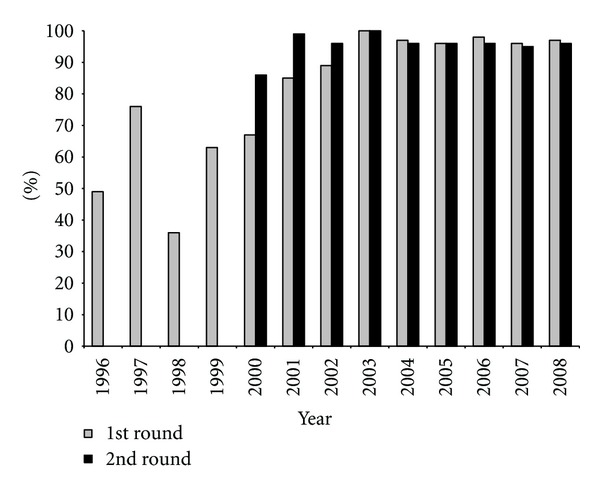
Ivermectin coverage of the eligible population at risk in the Huehuetenango focus from 1996 to 2008.
2.2. Identification of Potentially Endemic Communities
Using historical data from the MOPH from 1980 to 1990, we created a list of potentially endemic communities (PEC) for onchocerciasis using at least one of the following characteristics: (a) past evidence of onchocerciasis transmission (presence of at least one resident of the community with a palpable nodule and/or mf in the skin), (b) suspicion of past transmission (communities where a nodule survey took place, even if no transmission was detected), and/or (c) communities currently participating in MDA with the MOPH. We identified 94 PEC from the eight historically endemic municipios. Of these PECs, 43 were participating in MDA at the time of the evaluation; by the mid-1990s, the MOPH had removed the remaining communities from the list of endemic communities as they showed no evidence of infection.
2.3. Evaluation of Transmission Interruption
The evaluation of transmission interruption conducted in Huehuetenango consisted of three assessments: ophthalmological, serological and entomological. These assessments were conducted following the 2001 WHO guidelines as modified by Lindblade et al. [11], with the following criteria indicating that transmission had been interrupted: (1) <1% prevalence of mf in the anterior segment of the eye, (2) <0.1% cumulative incidence of infection in children (measured serologically), and (3) absence or near absence of infective (L3) blackflies (<1 infective fly per 2000 tested).
2.3.1. Ophthalmological Evaluation
To determine the presence of ocular morbidity associated with onchocerciasis, we measured the prevalence of mf in the anterior segment (MfAS) of the eye in a sample of residents from the PEC in Huehuetenango. A minimum sample size of 300 persons was needed to calculate a one-sided 95% confidence interval (CI) around a prevalence of zero that would exclude 1% [11]. Assuming a 15% nonresponse rate, our final sample size was 353. To increase the likelihood of finding cases with ocular morbidity, we identified all PECs (n = 19) where the prevalence of mf in the skin was >0% in any survey conducted between 1987 and 1992. Due to access problems, distance between communities, availability of the project ophthalmologist, and the number of persons who could be examined in one day, it was considered feasible to sample 40 residents from each of nine communities, which were selected from among the 19 eligible communities by probability proportional to size sampling. All nine selected communities were mapped and a census conducted on all households using a personal digital assistant (PDA) to identify all eligible persons (residents ≥7 years of age with at least five years living in the community) for the evaluation. We selected 13 houses (estimating three eligible individuals per household) at random from each of the selected communities after mapping, and all eligible individuals from these households were invited to participate in the evaluation.
An ophthalmologist with extensive experience in onchocerciasis-related ocular morbidity examined participants using a method described previously [11, 14]. Briefly, residents were examined with a slit lamp for the presence of microfilaria in the anterior chamber or the cornea of either eye.
2.3.2. Serological Evaluation
The cumulative incidence of infection with O. volvulus was determined serologically by measuring the prevalence of IgG4 antibodies to the recombinant antigen OV16 in school age children (6 to 12 years old) using methods previously described [11, 15]. Briefly, a sample size of 3000 children was required; assuming a 30% non-response rate, our final sample size was 4286 children. All schools in the PEC were identified, and the number of enrolled children 6 to 12 years old determined. The schools were ordered at random and then selected in order until the required sample size was achieved. Forty-three schools from 37 PECs (seven municipios) were selected by this procedure. All children aged 6 to 12 from each selected school were asked to participate in the evaluation, and children that were not present at school during the evaluation were followed up at their homes and asked to participate.
Finger-prick, filter paper blood samples were collected from all participating children as previously described [11]. IgG4 antibodies to the recombinant antigen OV16 were determined using the enzyme-linked immunosorbent assay (ELISA) method described previously [11, 15].
2.3.3. Entomological Evaluation
Black flies were collected twice per month from November 2007 to April 2008 (the peak black fly biting season), in four coffee plantations located in the same geographical areas of the PECs. Plantations were purposively selected to find those with high densities of S. ochraceum and owners willing to participate in the evaluation. We used a standard black fly collection method described in previous studies [11, 16]. Briefly, in each plantation, four collection sites, two near the residences and two in the coffee plantation, were identified. A team, comprised of a collector and a paid human attractant (male resident from 18 to 50 years of age), was employed at each collection site and worked two days per month per site. All human attractants received a dose of ivermectin before the evaluation and were assessed for the presence of antibodies to O. volvulus using the OV16 ELISA test before and after the evaluation. Flies were pooled and evaluated by a standard polymerase chain reaction (PCR) method to identify O. volvulus DNA [17–19].
2.4. Evaluation of Transmission Elimination
The evaluation of transmission elimination was conducted between the second and third year after treatment suspension, during peak black fly biting season. This evaluation consisted only of the entomological assessment since O. volvulus recrudescence should first be detected in the vectors [20]. The criteria to determine elimination of transmission is the absence or near absence of infective blackflies (<1 infective fly per 2000 tested).
2.4.1. Entomological Evaluation
Collection of backflies was carried out from December 2010 to April 2011 in the same four coffee plantations as the pretreatment evaluation and following the same procedures.
2.5. Statistical Analysis
The exact one-sided 95% CI for the prevalence of MfAS and IgG4 antibodies to OV16 was obtained as previously described [11] using SAS software (version 9.0, SAS Institute, Cary, NC, USA). The proportion of infective flies and the 95% CI were calculated using Poolscreen 2.0 [17, 18, 21]. Standard procedures were used to obtain geometric mean of the biting rate, arithmetic mean of the biting rate, biting density, and the seasonal transmission potential [11]. The seasonal transmission potential (STP) was calculated as the product of the seasonal biting density (SBD; number of bites per person during the transmission season), the proportion of flies with infective-stage O. volvulus larvae (PCR positive), and the mean number of infective larvae per infective fly (assumed to be one in an area of low transmission). Entomological analyses were performed using R 2.13.1 (2011-07-08) [22].
2.6. Human Subjects
The protocol for these evaluations was reviewed and approved by the Centers for Disease Control and Prevention (Atlanta, GA, USA), the Universidad del Valle de Guatemala (Guatemala City, Guatemala), and the Guatemala MOPH. Written informed consent was obtained from participants 18 years of age and older; parents or guardians of children less than 18 years provided written, informed consent for their participation. All children were asked to sign an assent form for participation.
3. Results
3.1. Evaluation of Transmission Interruption
Ophthalmological, serological, and entomological evaluations were conducted from June 2007 to April 2008. The results from these evaluations showed the transmission status of O. volvulus in the Huehuetenango focus, after 14 rounds of ivermectin treatment with coverages higher than 85% (Figure 3).
3.1.1. Ophthalmological Evaluation
The ophthalmological evaluation was carried out from 17 to 28 September 2007. A total of 365 eligible residents from nine selected PECs were evaluated. Table 1 shows the nine PECs with the eligible population and the number of individuals evaluated in each PEC (Figure 2). Of these individuals, 82% reported having lived in the community throughout their life, 55% were female, and 64% were between the age of seven and 29. Ophthalmological evaluation showed that 97% of the participants had a visual acuity between 20/20 and 20/70. No MfAS were found in any of the participants. The prevalence of MfAS was 0% (95% CI 0–0.8%).
Table 1.
Potentially endemic communities included in the ophthalmological evaluation. Nine communities were selected to carry out the ophthalmological evaluation, and a total of 10,183 persons were eligible to participate, of whom 365 were evaluated.
| Community | Municipio | Eligible population | Population evaluated |
|---|---|---|---|
| Michicoy | San Pedro Necta | 839 | 49 |
| Ixnul | San Pedro Necta | 1201 | 45 |
| Santa Rosa | Cuilco | 1550 | 41 |
| Agua Dulce | Cuilco | 2331 | 50 |
| El Rodeo | Cuilco | 1113 | 43 |
| Chiquihuil | Cuilco | 726 | 36 |
| Union Frontera | Cuilco | 194 | 42 |
| Cabecera Municipal Democracia | La Democracia | 1913 | 24 |
| El Tablon | Cuilco | 316 | 35 |
|
| |||
| Total | 10,183 | 365 | |
3.1.2. Serological Evaluation
Serological evaluation was carried out from June to July 2007. We identified 3910 children aged from 6 to 12 years old enrolled in the 43 selected schools (Table 2), of which 3118 (80%) participated in the evaluation. The mean age was 9.1 years (SD 2.1 years), 52% were male, and the average of years of living in the community was 9.3 years (SD 1.8 years). None of the children tested were found to be seropositive, resulting in a cumulative incidence of 0% (95% CI 0–0.1%).
Table 2.
Potentially endemic communities included in the serological evaluation. Forty-three schools were survey for the serological evaluation. A total of 3910 children were eligible to participate.
| School | Community | Municipio | Eligible children | Children sampled |
|---|---|---|---|---|
| 1 | Cumil | Cuilco | 145 | 139 |
| 2 | El Astillero | Cuilco | 105 | 84 |
| 3 | El Rodeo | Cuilco | 111 | 53 |
| 4 | El Zapotillo | Cuilco | 44 | 34 |
| 5 | La Laguna | Cuilco | 76 | 71 |
| 6 | Sabunul | Cuilco | 179 | 121 |
| 7 | Sosi | Cuilco | 149 | 118 |
| 8 | Yulva | Cuilco | 218 | 193 |
| 9 | Plan de las Vigas | Cuilco | 46 | 41 |
| 10 | Ampliacion Nueva Reforma | Cuilco | 60 | 44 |
| 11 | El Boqueron | Cuilco | 103 | 63 |
| 12 | Buenos Aires | Cuilco | 32 | 25 |
| 13 | Carrizal | Cuilco | 63 | 63 |
| 14 | Chiquihuil | Cuilco | 133 | 100 |
| 15 | Cruz de Pinapa | Cuilco | 109 | 69 |
| 16 | Ixtatilar | Cuilco | 43 | 33 |
| 17 | Jalapa | Cuilco | 64 | 53 |
| 18 | Cruz Miramar | Cuilco | 33 | 29 |
| 19 | La Soledad | Cuilco | 58 | 58 |
| 20 | Los Garcia | Cuilco | 41 | 35 |
| 21 | Salitre | Cuilco | 17 | 17 |
| 22 | Tablon (Sosi) | Cuilco | 64 | 57 |
| 23 | Union Frontera | Cuilco | 36 | 36 |
| 24 | Union Batal | Cuilco | 118 | 109 |
| 25 | Buena Vista | La Democracia | 236 | 228 |
| 26 | Cementerio | La Democracia | 111 | 88 |
| 27 | Norte | La Democracia | 49 | 40 |
| 28 | El Chorro | La Democracia | 24 | 14 |
| 29 | La Montañita | La Democracia | 76 | 70 |
| 30 | La Vega | La Democracia | 177 | 130 |
| 31 | Nuevo San Rafael | La Democracia | 85 | 33 |
| 32 | Plan Grande | La Democracia | 67 | 54 |
| 33 | Cerro Verde | La Libertad | 123 | 85 |
| 34 | Nojoya | San Antonio Huista | 69 | 60 |
| 35 | El Pajal | San Antonio Huista | 84 | 40 |
| 36 | Tablon Viejo | San Antonio Huista | 31 | 29 |
| 37 | Isnul | San Pedro Necta | 183 | 181 |
| 38 | Michicoy | San Pedro Necta | 97 | 74 |
| 39 | Rio Ocho | San Pedro Necta | 155 | 71 |
| 40 | Jolimex | San Pedro Necta | 49 | 35 |
| 41 | El Pajarito | San Pedro Necta | 53 | 53 |
| 42 | Finca Santa Cecilia | San Pedro Necta | 35 | 35 |
| 43 | Cabecera Departamental | Santa Ana Huista | 159 | 153 |
|
| ||||
| Total | 3910 | 3118 | ||
3.1.3. Entomological Evaluation
Entomological evaluation was carried out in four coffee plantations (two located in Agua Dulce, Cuilco and two in Marilandia, San Pedro Necta) which presented high densities of S. ochraceum. Thirty nine collections days, equivalent 1220 hours of collection, were completed between November 2007 and April 2008 (Table 3). A total of 8252 S. ochraceum and 11473 S. metallicum were collected As reported before [23], the biting density of S. ochraceum is higher in December and starts to decrease in February (Table 3). Overall, the geometric mean biting rate was 3.2 (95% CI 2.9–3.5) bites/person/hour, with the highest biting rate in November (16.7 bites/person/hour) and the lowest in April (0.7 bites/person/hour). The SBD indicating the total number of bites per person per season was 5765 (95% CI 5263–6300). The paid human attractants were tested at the beginning and end of the evaluation for antibodies to OV-16, but none was found positive.
Table 3.
Entomological collections in the Huehuetenango focus (from November 2007 to April 2008).
| Month | Hours collected | Days collected | Simulium ochraceum | Simulium metallicum | ||
|---|---|---|---|---|---|---|
| Number captured | Biting rate per hour∗ | Biting density§ | Number captured | |||
| November | 57 | 2 | 1641 | 16.7 (11.4–24.2) | 5019 (3432–7270) | 796 |
| December | 160 | 5 | 2745 | 12.7 (10.8–15) | 3951 (3346–4655) | 2639 |
| January | 252 | 8 | 1558 | 4.5 (3.9–5.1) | 1381 (1194–1589) | 3020 |
| February | 256 | 8 | 1083 | 2.5 (2.1–3) | 726 (602–865) | 2025 |
| March | 252 | 8 | 963 | 1.9 (1.6–2.4) | 600 (483–732) | 1753 |
| April | 243 | 8 | 262 | 0.7 (0.5–0.8) | 203 (158–252) | 1240 |
|
| ||||||
| Total | 1220 | 39 | 8252 | 3.2 (2.9–3.5) | 5765 (5263–6300) | 11473 |
*Geometric mean of biting rate per hour (95% CI).
§Number of black fly bites per person per month (geometric mean biting rate per hour ×10 hours per day × number of days in a month) (95% CI).
The S. ochraceum collected were grouped in 357 pools, and all were negative for O. volvulus infection. The prevalence of infection was 0% (95% CI 0–0.02%), and the maximum prevalence of infection was estimated to be 0.4/2000 flies. The STP was 0 infective larvae/person/season, but the maximum potential STP (using the upper boundary on the prevalence of infection in S. ochraceum) was 1.3 infective larvae/person/season.
3.2. Evaluation of Transmission Elimination
The results from the previous evaluations showed that transmission of O. volvulus through S. ochraceum was interrupted, thus in 2009 ivermectin MDA was suspended from the Huehuetenango focus. Following WHO guidelines, from December 2010 to April 2011, we conducted the entomological evaluation to evaluate the transmission of O. volvulus after treatment suspension.
3.2.1. Entomological Evaluation
The entomological evaluation was conducted in the same four coffee plantations as the previous entomological evaluation. Forty collection days corresponding to 1280 hours of collection were conducted. A total of 4587 S. ochraceum and 4912 S. metallicum were collected (Table 4), and a significant reduction in the density of both species was observed when compared to the 2007-2008 collection. However the biting density pattern of S. ochraceum was similar as that observed in 2007-2008. The overall geometric mean biting rate was 1.8 (95% CI 1.6–2.0) bites/person/hour, and the SBD was 2693 bites/person (95% CI 2454–2945). The paid human attractants did not present antibodies to OV16 before or after the evaluation.
Table 4.
Entomological collections in the Huehuetenango focus (from December 2010 to April 2011).
| Month | Hours collected | Days collected | Simulium ochraceum | Simulium metallicum | ||
|---|---|---|---|---|---|---|
| Number captured | Biting rate per hour∗ | Biting density§ | Number captured | |||
| December | 224 | 7 | 1743 | 4.2 (3.5–5.1) | 1309 (1075–1581) | 1603 |
| January | 288 | 9 | 1230 | 2.5 (2.1–2.9) | 769 (650–903) | 911 |
| February | 256 | 8 | 603 | 1.5 (1.2–1.8) | 420 (347–500) | 1055 |
| March | 256 | 8 | 775 | 1.6 (1.3–2) | 501 (403–611) | 864 |
| April | 256 | 8 | 236 | 0.4 (0.3–0.6) | 130 (93–170) | 479 |
|
| ||||||
| Total | 1280 | 40 | 4587 | 1.8 (1.6–2) | 2693 (2454–2945) | 4912 |
*Geometric mean of biting rate per hour (95% CI).
§Number of black fly bites per person per month (geometric mean biting rate per hour ×10 hours per day × number of days in a month) (95% CI).
The S. ochraceum collected were grouped in 147 pools, and all were negative for O. volvulus infection. The prevalence of infection was 0% (95% CI 0–0.04%), and the maximum prevalence of infection was estimated to be 0.8/2000 flies. The STP was 0 infective larvae/person/season, with the maximum potential STP (using the upper boundary on the prevalence of infection in S. ochraceum) being 1.1 infective larvae/person/season. As a result of low number of S. ochraceum collected, all S. metallicum were grouped in pools in the same manner as described for S. ochraceum and processed by PCR to determine if they were infected with O. volvulus. All S. metallicum were negative for O. volvulus; thus, there are no parasites circulating in these black flies.
4. Discussion
In 2001, the WHO established a set of guidelines to assist onchocerciasis programs to determine whether interruption of transmission had occurred and MDA with ivermectin could be stopped [16]. The process outlined by WHO involves four phases: (1) suppression of transmission, where new infective stage larvae are no longer introduced into the human population by the vectors, but the parasite population maintains the ability to recover if interventions are withdrawn; (2) interruption of transmission, when the parasite population is thought to be unable to recover and interventions (in this case, twice-yearly ivermectin treatment) can be halted; (3) precertification, during which time posttreatment surveillance is needed for three years with an in depth evaluation to take place during the second or third year, depending on the peak transmission season [20]; (4) if this evaluation was negative, a declaration of elimination. When all foci within a country reach the final phase, the country may request certification of elimination of onchocerciasis from WHO [16].
We conducted an assessment of the status of O. volvulus transmission in the third onchocerciasis focus of Guatemala to be so evaluated by this process. Data from evaluations conducted by the MOPH in Huehuetenango in the 1990s indicated that the parasite had, at best, a tenuous hold when MDA was launched in 1996. Our results in 2007-2008 confirmed that transmission of onchocerciasis had been successfully interrupted in Huehuetenango after 22 rounds of MDA over 13 years. We found no evidence of ocular lesions attributed to O. volvulus infection. Similarly, we found no serological evidence of recent exposure to the parasite among 6–12-year-old children residing in the endemic area, nor any entomological evidence of infected or infective black fly vectors. The maximum STP in this area was conservatively calculated at 1.2 infective larvae/person/season, which is not sufficient to sustain transmission in S. ochraceum areas [24]. The expert steering committee of OEPA (the Program Coordinating Committee (PCC)) reviewed the results of this evaluation and formally recommended to the Guatemala MOPH that MDA with ivermectin be suspended in Huehuetenango, beginning in 2009. This recommendation was accepted, and Huehuetenango joined the Santa Rosa and Escuintla-Guatemala foci in the posttreatment surveillance period.
One challenge to maintaining the Huehuetenango focus free of onchocerciasis is its proximity to the border with Mexico (Figure 1) and, in particular, to the South Chiapas onchocerciasis focus [19]. There has been conjecture that the two foci were linked through migrant coffee workers moving between the two countries and acquiring onchocerciasis in one or both foci. The unlikelihood of migrant workers being able to maintain onchocerciasis transmission in Guatemala and Mexico has been studied and discussed elsewhere [25, 26]. Of the two foci, South Chiapas historically had the highest levels of active onchocerciasis transmission in all of Mexico [19] whereas the evidence from Huehuetenango during the same time period showed significant decreases in onchocerciasis prevalence even before the MDA program, suggesting that South Chiapas had little effect on transmission in Huehuetenango. An MDA program requiring treatment four times per year was required to interrupt transmission in the South Chiapas focus; at the beginning of 2012 MDA was suspended and post treatment surveillance initiated there [27], thus it is unlikely that onchocerciasis could be reintroduced into Huehuetenango from Mexico.
Following PTS guidelines [20], from December 2010 to April 2011 an entomological evaluation was conducted in Huehuetenango to verify that O. volvulus transmission had not recrudesced. The results from this evaluation showed that O. volvulus L3 are not circulating in the primary vector S. ochraceum or in potentially a secondary vector, S. metallicum. A conservative calculation of STP using upper 95% confidence interval values for S. ochraceum was 1.1 infective larva/person/season, which is insufficient to maintain the parasite population. Thus we conclude that O. volvulus transmission has been eliminated from the Huehuetenango focus. Currently in Guatemala the three hypoendemic foci (Santa Rosa, Escuintla-Guatemala and Huehuetenango) have demonstrated, through completion of PTS, elimination of transmission. The only focus that is currently under post treatment surveillance is the hyperendemic central endemic focus, which only stopped MDA at the beginning of 2012. When the Central Endemic focus successfully completes PTS and attains elimination status, then the entire country of Guatemala will be able to request WHO certification of elimination in 2015.
In May 2009, the US President Barack Obama announced a new Global Health Initiative (GHI) to improve health outcomes in partner countries. Neglected tropical diseases feature prominently in the GHI, and a key target is elimination of onchocerciasis from the Americas (http://www.america.gov/st/texttrans-english/2010/August/20100817134101su0.3731152.html, accessed on September 1, 2010). As the elimination of onchocerciasis from the Americas by 2015 looks to be on target, this could be one of the early successes of the GHI and a significant public health success story that can help motivate and inform other elimination efforts.
Disclaimer
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Acknowledgments
Licenciada Nancy Cruz-Ortiz died on 3 August, 2012. Her professional expertise and personal commitment contributed significantly to the vision of an onchocerciasis-free Latin America, and her loss is deeply mourned by all of her coauthors. The authors are grateful to Jose Luis Boteo, Marvin Chiquita, Mynor Lopez, Aura Paniagua, Lisbeth Paniagua and Jorge Sincal for assisting with the field and laboratory work. They thank the Director of the Huehuetenango Health Area for his support and assistance with this project. They are grateful for the willing participation of the residents of Huehuetenango. The authors thank Margarita Vides for the development of the project map and to Oscar de Leon for the technical support. These studies were funded by the Centers for Disease Control and Prevention (Atlanta, GA, USA) and the Onchocerciasis Elimination Program for the Americas (Guatemala City, Guatemala). The OEPA funds were provided through a grant by the Bill and Melinda Gates Foundation (Seattle, WA, USA) to The Carter Center (Atlanta, GA, USA).
References
- 1.Boatin BA, Richards FO., Jr. Control of Onchocerciasis. Advances in Parasitology. 2006;61:349–394. doi: 10.1016/S0065-308X(05)61009-3. [DOI] [PubMed] [Google Scholar]
- 2.WHO. Onchocerciasis. Meeting of the International Task Force for Disease Eradication. Weekly Epidemiological Record. 2007;82(22/23):197–208. [Google Scholar]
- 3.WHO. Onchocerciasis. Report from the fourteenth InterAmerica Conference on Onchocerciasis. Weekly Epidemiological Record. 2005;30(80):257–260. [PubMed] [Google Scholar]
- 4.WHO. Report from the InterAmerican Conference on Onchocerciasis, Novermber 2007. Weekly Epidemiological Record. 2008;29(83):253–260. [Google Scholar]
- 5.WHO. InterAmerican Conference on Onchocerciasis, 2010: progress towards eliminating river blindness in the WHO Region of the Americas. Weekly Epidemiological Record. 2011;38(86):417–424. [PubMed] [Google Scholar]
- 6.Blanks J, Richards F, Beltrán F, et al. The Onchocerciasis Elimination Program for the Americas: a history of partnership. Revista Panamericana de Salud Publica/Pan American Journal of Public Health. 1998;3(6):367–374. doi: 10.1590/s1020-49891998000600002. [DOI] [PubMed] [Google Scholar]
- 7.WHO. Toward the Elimination of Onchocerciasis (River Blindness) in the Americas (CD48/10) Washintong, DC, USA: Pan American Health Organization, World Health Organization, 48th Direction Council; 2008. [Google Scholar]
- 8.WHO. Elimination of Neglected Diseases and Other Poverty-Related Infections. Resolution CD49.R19. Pan American Health Organization, World Health Organization; 2009. [Google Scholar]
- 9.Cupp EW, Cupp MS. Short report: impact of ivermectin community-level treatments on elimination of adult Onchocerca volvulus when individuals receive multiple treatments per year. American Journal of Tropical Medicine and Hygiene. 2005;73(6):1159–1161. [PubMed] [Google Scholar]
- 10.Sauerbrey M. The Onchocerciasis Elimination Program for the Americas (OEPA) Annals of Tropical Medicine and Parasitology. 2008;102(supplement 1):S25–S29. doi: 10.1179/136485908X337454. [DOI] [PubMed] [Google Scholar]
- 11.Lindblade KA, Arana B, Zea-Flores G, et al. Elimination of Onchocercia volvulus transmission in the Santa Rosa focus of Guatemala. American Journal of Tropical Medicine and Hygiene. 2007;77(2):334–341. [PubMed] [Google Scholar]
- 12.Gonzalez RJ, Cruz-Ortiz N, Rizzo N, et al. Successful interruption of transmission of Onchocerca volvulus in the Escuintla-Guatemala focus, Guatemala. PLoS Neglected Tropical Diseases. 2009;3(3, article e404) doi: 10.1371/journal.pntd.0000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamagata Y, Suzuki T, Garcia Manzo GA. Geographical distribution of the prevalence of nodules of Onchocerca volvulus in Guatemala over the last four decades. Tropical Medicine and Parasitology. 1986;37(1):28–34. [PubMed] [Google Scholar]
- 14.Winthrop KL, Proaño R, Oliva O, et al. The reliability of anterior segment lesions as indicators of onchocercal eye disease in Guatemala. American Journal of Tropical Medicine and Hygiene. 2006;75(6):1058–1062. [PubMed] [Google Scholar]
- 15.Lobos E, Weiss N, Karam M, Taylor HR, Ottesen EA, Nutman TB. An immunogenic Onchocerca volvulus antigen: a specific and early marker of infection. Science. 1991;251(5001):1603–1605. doi: 10.1126/science.2011741. [DOI] [PubMed] [Google Scholar]
- 16.WHO. Guidelines. Certification of elimination of human onchocerciasis: criteria and procedures. Geneva, Switzerland, 2001, WHO/CDC/CPE/CEE/2001.18a.
- 17.Katholi CR, Toe L, Merriweather A, Unnasch TR. Determining the prevalence of Onchocerca volvulus infection in vector populations by polymerase chain reaction screening of pools of black flies. Journal of Infectious Diseases. 1995;172(5):1414–1417. doi: 10.1093/infdis/172.5.1414. [DOI] [PubMed] [Google Scholar]
- 18.Unnasch TR, Meredith SE. The use of degenerate primers in conjunction with strain and species oligonucleotides to classify Onchocerca volvulus . Methods in Molecular Biology. 1996;50:293–303. doi: 10.1385/0-89603-323-6:293. [DOI] [PubMed] [Google Scholar]
- 19.Rodríguez-Pérez MA, Lilley BG, Domínguez-Vázquez A, et al. Polymerase chain reaction monitoring of transmission of Onchocerca volvulus in two endemic states in Mexico. American Journal of Tropical Medicine and Hygiene. 2004;70(1):38–45. [PubMed] [Google Scholar]
- 20.Program Coordinating Committee, staff OEPA. Guide to detecting a potential recrudescence of onchocerciasis during the posttreatment surveillance period: The American paradigm. Research and Reports in Tropical Medicine. 2012;3(1):21–33. doi: 10.2147/RRTM.S30482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katholi CR, Barker J. PoolScreen 2.0. Birmingham, AL, USA: University of Alabama at Birmingham; 2002. [Google Scholar]
- 22.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2011. [Google Scholar]
- 23.Porter CH, Collins RC. Seasonality of adult black flies and Onchocerca volvulus transmission in Guatemala. American Journal of Tropical Medicine and Hygiene. 1988;38(1):153–167. doi: 10.4269/ajtmh.1988.38.153. [DOI] [PubMed] [Google Scholar]
- 24.Wada Y. Theoretical approach to the epidemiology of onchocerciasis in Guatemala. Japanese Journal of Medical Science and Biology. 1982;35(4):183–196. doi: 10.7883/yoken1952.35.183. [DOI] [PubMed] [Google Scholar]
- 25.Lindblade KA, Richards M, Richards J, et al. Exposure of seasonal migrant workers to Onchocerca volvulus on coffee plantations in Guatemala. American Journal of Tropical Medicine and Hygiene. 2009;81(3):438–442. [PubMed] [Google Scholar]
- 26.Rodríguez-Pérez MA, Cabrera AS, Ortega CL, Basáñez MG, Davies JB. Contribution of migrant coffee labourers infected with Onchocerca volvulus to the maintenance of the microfilarial reservoir in an ivermectin-treated area of Mexico. Filaria Journal. 2007;6, article 16 doi: 10.1186/1475-2883-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arredondo J. Foco socunoso de Chiapas. in Meeting of the Program Coordinating Committee of Onchocerciasis Elimination Program for the Americas. Guatemala City, Guatemala: 2011. [Google Scholar]


