Abstract
Immunity to pathogens critically requires pattern recognition receptors (PRR) to trigger intracellular signaling cascades that initiate and direct innate and adaptive immune responses. For fungal infections, these responses are primarily mediated by members of the C-type lectin receptor family. In this review, we highlight recent advances in our understanding of the roles and mechanisms of these multifunctional receptors, explore how these PRR orchestrate anti-fungal immunity, and briefly discuss progress in the use of these receptors as targets for anti-fungal and other vaccines.
Introduction
Fungi are ubiquitous; we inhale several hundreds of Aspergillus spores each day, most of us are colonized with Candida and other fungal species, and in our lifetimes we will be exposed to hundreds of potentially infective fungal species1. With the prevalence of these microorganisms and relatively low incidence of pathogenicity, it is easy to overlook their threat to public health. In reality, however, fungi are robust pathogens that when given the opportunity to cause infection, particularly in immune-compromised individuals, establish lifelong or life-threatening diseases for which current diagnostic techniques and treatment options are unacceptably limited1. Dermatological infections of the skin, nails, and mucosa occur in an estimated 25% of the worldwide population and although the incidence of invasive fungal infections is considerably less, they are of greater concern due to their extremely high mortality rate1. Cryptococcal meningitis, disseminated candidiasis, and invasive pulmonary aspergillosis, for example, can result in 30-80% mortality during treatment and are 100% fatal if the diagnosis is missed1. Worryingly, recent decades have seen drastic increases in the incidence of invasive fungal infection, which is due primarily to modern medical practices, such as immunosuppressive therapy, and the HIV/AIDS pandemic.
The ability of healthy individuals to cope with the continual exposure to fungal pathogens, indicates that our immune system has effective mechanisms for preventing infections with these organisms. Although our understanding of these mechanisms has lagged behind those of other pathogens, substantial progress has been made over the last few years, and it is hoped that we will ultimately be able to use our growing knowledge to develop novel immunotherapeutic approaches for the treatment of these devastating diseases. One fundamental insight was the realization that C-type lectin receptors (CLR) play central roles in immunity to fungal pathogens. In this review, we will highlight the importance of CLR in antifungal immunity and explore the roles and mechanisms utilized by these receptors to induce and modulate innate and adaptive responses. We will also demonstrate how these receptors can collaborate with other PRR and discuss strategies used to target these receptors to drive tailored immune responses for vaccines.
The key role of CLRs
To date, four families of PRR have been shown to recognize pathogens and are capable of inducing cellular responses: the Toll-like (TLR), Nod-like (NLR), RIG-I like (RLR) and CLR receptor families. The RLR and NLR are not thought to contribute directly to fungal recognition, although certain NLRs can be activated by fungi through unknown mechanisms, as discussed below. The TLRs are the best-characterized family of PRR with regards to other types of pathogens, and they also have been implicated in fungal recognition. Mice lacking MyD88, a central signaling adaptor utilized by many TLRs (but also IL-1R) are susceptible to infections with several fungal species, including Candida albicans, Paracoccidioides brasiliensis, Aspergillus fumigatus and Cryptococcus neoformans. Furthermore, a number of TLRs have been implicated in fungal recognition, including TLR1, TLR2, TLR4, TLR6, TLR7 and TLR9 (2,3). However, there is contradictory evidence surrounding the individual role of these PRRs, particularly in mouse models, which may be due to variable recognition of different isolates of the same fungal pathogen, as shown for C. albicans4. Polymorphisms in TLRs have also been linked to human susceptibility in the context of immunosuppression5, but immunocompetent individuals which lack MyD88 and other critical downstream signaling components do not show a predisposition to fungal diseases6. Never the less, there is emerging evidence that the interaction of TLRs with other PRR is an integral component of anti-fungal immunity (discussed below).
In contrast to the TLRs, CLR and their signaling pathways are essential for anti-fungal immunity. CLR are part of a heterogeneous superfamily of soluble and transmembrane proteins defined by a characteristic C-type lectin domain7, and they bind to most, if not all, fungal species that cause disease in humans. These receptors recognize the major carbohydrate structures that are found in fungal cell walls, including β-glucan and mannan8 (Figure 1). Interestingly, no PRR has yet been identified which recognizes chitin, another major cell wall-component which has demonstrable immunomodulatory activities9.
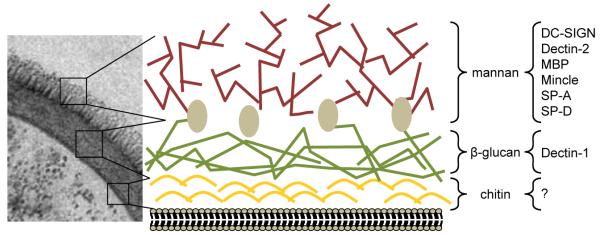
Figure 1.
Structure of the fungal cell wall. By EM, the various carbohydrate-rich layers of the fungal cell wall (in this example C. albicans) can be observed, which consist of mannan (mannosylated proteins), β-glucan and chitin, as indicated. Although providing a rigid framework, which gives these pathogens their shape and protection from the environment, the cell wall is a dynamic structure which changes significantly, particularly during the morphological transitions that many fungi can undergo (yeast to hyphae, for example). Furthermore, some of the internal components, such β-glucans, can be exposed on the fungal surface in specific areas, such as the bud scar in C. albicans32. The composition of the cell wall also varies between different fungal species. Several CLR have been identified which recognize these cell-wall structures, including transmembrane and soluble CLRs. The latter group, consisting of Surfactant Protein (SP)-A, SPD and mannose-binding lectin (MBP), opsonise fungi and facilitate their recognition, but were not discussed in the text (For a review of these molecules see Vautier et al. 201238). The micrograph was kindly provided by Jules Ene and Neil Gow.
Of particular interest are several CLR which can induce intracellular signaling upon fungal recognition, including Dectin-1, Dectin-2, the mannose receptor (MR), DC-SIGN, and Mincle10. While Dectin-1 recognizes β-glucan, the other receptors bind various, largely undefined, mannose-based structures found in the mannan layer of the fungal cell wall (Figure 1). The responses mediated by these receptors include fungal binding and phagocytosis, induction of antifungal effector mechanisms, and the production of various soluble mediators, including cytokines, chemokines and inflammatory lipids2. Notably, these receptors also direct and modulate the development of adaptive immunity, particularly TH1 and TH17 responses11-17 (see below).
Dectin-1, Dectin-2 and Mincle utilize the signal transduction kinase Syk, which activates MAPK, NFAT, and through the PKCδ-CARD9-Bcl10-MALT1 axis, NF-κB11,18-21 (Figure 2). Dectin-1 signals directly via Syk, whereas Dectin-2 and Mincle couple to Syk via the Fc receptor common γ-chain22-24. Both Dectin-1 and DC-SIGN can activate Raf-1 kinase to modulate NF-κB activity, although the proximal mechanisms involved are unclear12,13. Interestingly, Dectin-1 signaling is only activated following clustering into a “phagocytic synapse”, from which the regulatory tyrosine phosphatases, CD45 and CD148, are excluded25. The signal transduction pathway utilized by the MR is as yet undefined. In mouse models, genetic deletion of these CLR (including Dectin-1, Dectin-2, Mincle, MR) and their downstream signaling components (including PKCδ or CARD9) results in defective immunity to several fungal pathogens14,15,18,20,26-29. Importantly, in humans, polymorphisms in Dectin-1 and mutations in CARD9 have been identified that result in susceptibility to fungal infections, especially mucocutaneous candidiasis30,31. Interestingly, the phenotype of the CARD9-affected individuals was more severe than that of Dectin-130,31, indicative of an involvement of other PRRs.
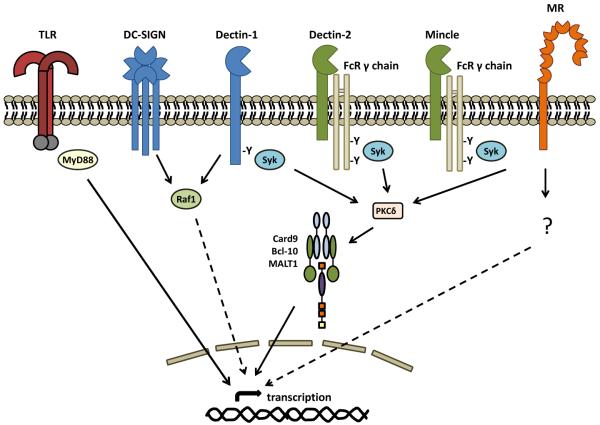
Figure 2.
Transmembrane CLRs involved in antifungal immunity and their intracellular signaling pathways. Dectin-1, Dectin-2 and Mincle induce intracellular signaling via tyrosine (Y)-based activation motifs (immunoreceptor tyrosine-based activation motifs or ITAMs) which recruit and activate Syk-kinase either directly, or indirectly through the FcγR adaptor chain. Signaling through protein-kinase C (PKC)δ, this pathway activates the Card9-Bcl10-Malt1 complex inducing gene transcription and the production of various inflammatory mediators. DC-SIGN and Dectin-1 can signal via the Raf-1 kinase pathway which modulates (dotted line) other signaling pathways, including those induced by the Toll-like receptors (TLR) and the Dectin-1/ Syk pathway. The mannose receptor (MR) can also induce intracellular signaling, but the mechanisms involved are unknown. CLR signaling can collaborate with that of the TLR (red bi-arrow), to synergistically induce or repress the induction of various cytokines and chemokines. CLR can also mediate fungal phagocytosis and induction of anti-microbial effector mechanisms (not shown).
Individual CLRs can recognize many fungal pathogens and there is clear overlap in the substrate specificities of some of these receptors. Yet, there is still some specificity in recognition by these receptors, due, in part, to the exposure of different carbohydrate structures by the different fungal species or morphological forms of the same organism. For example, Dectin-1 can only recognize the yeast form of Candida, because exposed β-glucans become masked by mannan upon formation of hyphae32. Another example is the MR, which has been implicated in the recognition of most fungi in vitro, yet in mice this receptor appears only to be required for protective immunity to infections with C. neoformans29,33. Furthermore, and similar to the TLRs, CLR recognition can be fungal strain dependent, such as occurs with Dectin-110 and Dectin-234, which has implications for our understanding of human susceptibility to these infections.
CLR-dependent control of anti-fungal immune responses
Phagocytic cells, particularly macrophages and neutrophils, are essential elements of protective antifungal immunity, and loss of these cells or defects in their antimicrobial effector mechanisms results in susceptibility (reviewed in 2). CLR mediate many of these effector functions and promote inflammatory responses, which are critically required for controlling fungal infections10. Dectin-1, for example, induces the respiratory burst and the production of inflammatory mediators, including eicosanoids, TNF, IL-1β, IL-6, IL-23, CCL2, CXCL1 and CCL310,35,36. In mouse models of infection with C. albicans and A. fumigatus, loss of Dectin-1 resulted in a failure to mount protective inflammatory responses (defective neutrophil and monocyte recruitment, defective production of cytokines such as IL-6, G-CSF etc) and a failure to control fungal growth26,37.
All of the signaling CLRs that recognize fungi (Dectin-1, Dectin-2, MR, DC-SIGN, MINCLE) are capable of inducing or modulating TH1 and TH17 responses, the latter of which is now considered to be a hallmark of antifungal adaptive immunity39 (Figure 3). Although TH1 responses are essential for the control of systemic fungal infections, in part through the activation of phagocytes by IFN-γ, TH17 responses appear to be primarily required for protection at the mucosa40,41. Indeed, various defects in TH17 immunity, including mutations in STAT1, STAT3, IL-17 and IL17RA have been linked to susceptibility to mucocutaneous infections in man, especially chronic mucocutaneous candidiasis (CMC)42-46. Furthermore, other diseases characterized by susceptibility to CMC, such as autoimmune polyendocrine syndrome 1, are also associated with alterations in TH17 immunity47,48. How TH17 responses drive protection at the mucosa is unclear, but they are thought to involve IL-17-mediated neutrophil recruitment and IL-22-mediated induction of antimicrobial peptide production by epithelial cells41. TH17 responses may also be involved in controlling systemic infections with some fungi, such as Candida (see Saijo et al, 201014 for an example), although there is evidence to suggest that these responses may contribute to pathology and susceptibility in certain settings49.
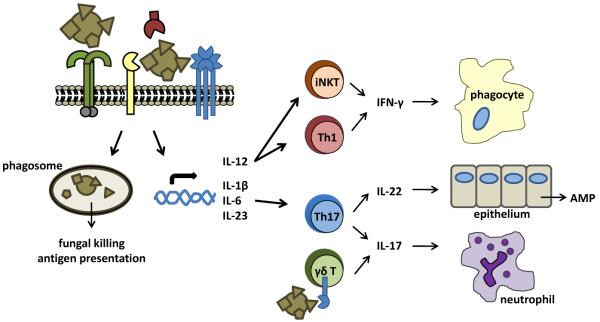
Figure 3.
Integration of CLR -mediated signaling directs adaptive immunity. CLR-mediated recognition of fungi drives their uptake and killing by phagocytes, and directs the development of protective Th1/Th17 responses. Induction of IL-12 drives IFN-γ production by Th1 and iNKT cells, which is critically required for the activation of phagocytes. Remarkably, production of IFN-γ by self-reactive iNKT cells occurs following CLR-mediated induction of IL-12 on antigen-presenting cells. On the other hand, induction of IL-1β, IL-6, and IL-23 promotes Th17 differentiation, which drives the production of IL-17 and IL-22. These cytokines are critically required for neutrophil recruitment and epithelial antimicrobial peptide (AMP) production and provide protection against fungal infections, particularly at the mucosa. Notably, the production of IL-17 can also be directly induced by CLRs expressed on γδ T cells, without TCR triggering.
CLRs and their signaling pathways play essential but varied roles in the development of these antifungal immune responses. Notably, CARD9 deficiency renders both mice and humans susceptible to infection with Candida and results in ablated TH17 and altered TH1 responses11,31. Reduced TH17 responses also correlated with susceptibility to mucocutaneous infections in Dectin-1-deficient humans30. In mice, deficiency of Dectin-1 or Dectin-2 results in susceptibility to infection with Candida, but only loss of Dectin-2 resulted in significant alterations in TH17 responses14,15. Loss of both Dectin-1 and Dectin-2, however, also led to profound reductions in TH1 responses, further demonstrating the importance of receptor cooperation15. Interestingly, Dectin-2 is capable of inducing TH2 immunity in response to house-dust mite allergens50, but whether this receptor, or other CLRs, are able to induce this type of response to fungi needs to be ascertained, as TH2 immunity is generally considered to contribute to fungal susceptibility40.
There have also been significant insights into the mechanisms that are utilized by CLRs to drive adaptive immunity. Stimulation of Dectin-1, for example, induces dendritic cell (DC) maturation and the expression of polarizing cytokines, such as IL-1β, IL-6 and IL-23, which favor TH17 differentiation11. Furthermore, Dectin-1 stimulated DC were able to convert a subset of Treg cells into IL-17 producers51, and Dectin-1 (along with TLR2) could amplify MR-induced TH17 responses17. The activation of the NF-kB subunit, c-Rel, by Malt1 (which forms a complex with CARD9 and Bcl-10, discussed above) induces the production of IL-1β and IL-23, and this is essential for Dectin-1 and Dectin-2-mediated TH17 differentiation34. In fact, Dectin-2 appears to selectively activate c-Rel34, whereas Dectin-1 also activates other NF-kB subunits, including the non-canonical RelB, promoting both TH1 and TH17 responses in vitro12,34. However, during pulmonary infection with Aspergillus, Dectin-1 actively suppresses IL-12 and IFN-γ production, favoring TH17 differentiation52.
CLRs can also influence the function of γδ and iNKT cells (Figure 3). γδ T cells are potent innate sources of IL-17 and produce this cytokine in response to IL-23 and IL-1β without TCR triggering53. Importantly, this IL-17 is produced in large amounts and prior to the development of adaptive TH17 responses53. CCR6+ γδ T cells express TLR1, TLR2 and Dectin-1, and triggering of these receptors directly induced IL-17 production in these cells; a response which could be amplified by IL-23 (ref 54). C. albicans hyphae, but not yeast, are able to recruit and stimulate γδ T cells in vivo54. iNKT cells, in contrast, do not respond directly to fungi, although they are required for the control of fungal pathogens in vivo55,56. Here, IL-12 production by DC, following stimulation of Dectin-1 or TLRs, enabled CD1d-restricted self-reactive iNKT cells to produce IFN-γ56. Although Dectin-1 normally suppresses IL-12 production by DC, as discussed above, co-culture with iNKT cells potently restored production of this cytokine56. Such iNKT responses could be initiated by several fungal species, including A. fumigatus, C. albicans, Histoplasma caspulatum and Alternaria alternata56. Thus, the CLR-mediated responses of γδ and iNKT cells may represent key early steps in the development of protective anti-fungal immune responses.
CLR and TLR collaboration
The recognition of intact pathogens involves multiple PRR and we are just starting to understand the “crosstalk” that can occur between these receptors57. As we have seen, fungi are recognized by several CLR and TLR, all of which are required for optimal antifungal responses58, and there are now several examples where direct interactions between these receptor families have been demonstrated to occur. Indeed, collaborative responses induced by Dectin-1 and TLR-2 were one of the first such interactions described59,60. Dectin-1 can collaborate with multiple MyD88-coupled TLRs to synergistically induce many cytokines, including TNF, IL-10 and IL-23, whilst repressing others, such as IL-1261-64. Other examples include MR-mediated production of IL-17, which was dependent on TLR2 and Dectin-1, and DC-SIGN, which does not directly induce cytokine responses but interacts with multiple TLRs to augment proinflammatory responses to fungi13,17. How these collaborative responses are mediated is still unclear, but may involve physical interactions upon ligand binding and modification of intracellular signaling cascades, by inclusion of components such as Raf-1, for example13,65,66. Having a better understanding of these mechanisms are likely to reveal novel targets for pharmacotherapy and the development of vaccine adjuvants.
The importance of PRRs crosstalk in the development of protective anti-fungal immunity is exemplified by our recent work with Fonsesaea pedrosoi. This pathogen is one of the main causative agents of chromoblastomycosis, a severe and chronic fungal disease of the skin which is very difficult to treat67. Notably, F. pedrosoi was recognized by CLRs, including Mincle, but was not detected by TLRs68. The lack of co-stimulation of both PRR pathways resulted in defective inflammatory responses68. However, exogenous administration of purified TLR ligands restored the cooperative inflammatory responses and led to pathogen clearance in mouse models, responses which were dependent on signaling cascades mediated through both Syk/ CARD9 and MyD88 pathways68. Remarkably, a similar approach also appears to work in humans; the topical application of TLR agonists to chromoblastomycosis lesions resulted in a rapid resolution of the infection when tested in a small group of patients (GDB unpublished results). Such defects in PRR cooperativity may also underlie chronic infections caused by other fungi, including Pneumocystis. In fact, treatment with heat-killed E. coli has been shown to clear P. carinii infection in mice, in part through the restoration of inflammatory responses69.
Fungi, CLRs and the inflammasome
The inflammasome is a cytoplasmic proteolytic multimeric protein complex, involving NLRs and several adaptors, which is required for the processing and activation IL-1β and IL-18 in response to pathogens70. Both cytokines are essential for protective anti-fungal immunity, particularly for driving the development of anti-fungal TH17 and TH1 responses71. Several inflammasome components are implicated in mediating these responses to fungi, including the adaptor ASC (associated speck-like protein), caspase-1 and two NLRs (NLRP3 and NLRC4)71-76 (Figure 4). Both NLRs are required for controlling mucosal infections with Candida, but only NLRP3 is involved in preventing subsequent dissemination of this pathogen75,77. While CLRs and TLRs can induce pro-IL1β in response to fungi, how the NLRs actually sense these pathogens to trigger caspase-1 activation is still unclear75. Activation of the NLRP3 inflammasome in response to both A. fumigatus and C. albicans requires Syk kinase, as well as the respiratory burst and potassium efflux, which is suggestive of a direct involvement of CLRs72,76.
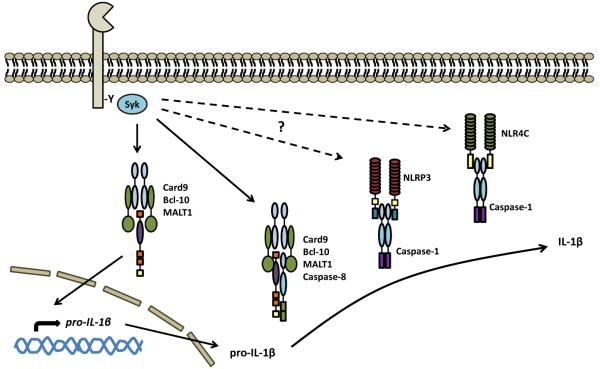
Figure 4.
CLRs mediate inflammasome activation. Fungi can activate the NLRP3 and NLRC4 inflammasomes, inducing the caspase-1-mediated cleavage of pro-IL-1β and production of bioactive IL-1β. While CLR, such as Dectin-1, can drive the induction of pro-IL-1β, it is unclear how the intracellularly-located NLR actually sense fungi. Activation of these receptors may directly involve CLR, as this process was found to require Syk kinase (as well as fungal uptake, the respiratory burst and potassium efflux). More recently, Dectin-1 has been shown to be able to activate the noncanonical caspase-8 inflammasome, which interacts directly with the Card9-Bcl-10-MALT1 complex. Activation of caspase-8 by recruitment to this signaling complex results in the cleavage of pro-IL-1β. Assembly of these inflammasomes also involves other components, including ASC (not shown).
More recently, a non-canonical inflammasome has been identified whose functioning is completely dependent on Dectin-178 (Figure 4). Here Dectin-1-mediated recognition of fungi induces both pro-IL1β and production of IL-1β through caspase-8 activation78. Syk-dependent signaling from this receptor induces a CARD9-Bcl-10-MALT1 scaffold which drives NF-κB activation, as described before, but this scaffold can also associate with ASC and caspase-8 to form the non-canonical inflammasome78. Unlike the NLR inflammasomes, activation of the caspase-8 inflammasome did not require particle internalization. In DC, various strains of A. fumigatus and C. albicans appeared to induce IL-1β primarily through this pathway, although some strains also induced the caspase-1 pathway in these cells78.
Enhancing vaccine efficacy
CLRs, especially those limited to particular DC subsets, have been of interest as vaccine targets. One approach has been antibody-mediated targeting of these receptors79,80 and Dectin-1, Dectin-2, MR and DC-SIGN have all been examined in this way and shown to variably enhance CD4+ and CD8+ T cell responses to experimental antigens81-84. However, any direct contribution of these receptors to the subsequent immune responses was not examined. Another strategy has been to use carbohydrate ligands of CLR to drive vaccine responses85,86.This has been tested with several carbohydrates, including various mannose-based ligands aimed at targeting the MR and DC-SIGN (although the actual receptor specificity in these studies is always questionable), as well as β-glucans aimed at targeting Dectin-1. In all cases, like antibody-mediated targeting, the complexing of these antigens with carbohydrates enhanced CD4+ and CD8+ T-cell responses80,87. Remarkably, β-glucan particles have also been used to systemically target macrophages following oral administration, but in this case complexed with siRNA88.
There has also been considerable interest in using these carbohydrates to develop anti-fungal vaccines, for which there is a desperate need as there are currently no vaccines that are available clinically1,89. Several approaches have been tested, including, for example, a conjugate vaccine composed of the β-glucan laminarin fused to diphtheria toxoid90. This vaccine induced anti-β-glucan antibodies which were protective against a range of fungi including Candida, Aspergillus and Cryptococcus90,91. Very recently, the particulate β-glucan, curdlan, was shown to act as a TH17- polarizing adjuvant when used with a novel epitope from C. albicans, which provided protection against infection with several species of Candida92. Interestingly, the acquisition of vaccine immunity using a live attenuated pathogen, Blastomyces dermatitidis, also required TH17 immunity; a response that was induced through MyD88 but not Dectin-1, although the role of other CLRs was not examined93. Mannose-based anti-fungal vaccines have also been tested in various models with various degrees of success, although mannosylation notably was essential for driving effective T cell responses against a recombinant C. neoformans protein85,94.
Conclusions
The data reviewed here highlight the central role of CLR in anti-fungal immunity. These receptors are essentially required for the binding and uptake of fungi by phagocytes, the induction of antifungal effector mechanisms, the production of inflammatory mediators, and the direction and modulation of adaptive immune responses. While the functions of CLR have been extensively studied in myeloid cells, it is likely that these receptors are also expressed in the epithelium. Indeed, a recent study has shown that expression of Dectin-1 can be induced in bronchial epithelial cells in vitro and stimulate antimicrobial and inflammatory responses in response to Aspergillus95. Having a better understanding of the function of CLR on non-myeloid cells is likely to provide important insights into protective host responses, given that the majority of our normal interactions with fungi occur at the epithelium96.
The ability of CLRs to drive adaptive immunity, particularly TH17 responses, has been of particular interest as these hallmark responses are essential for protection against fungal infections. The ability of CLR to stimulate adaptive immunity also represents a strategy for vaccination, and methodologies that utilize the antigenicity and adjuvanticity of CLR could provide powerful solutions for protecting against fungal and other diseases in the future. Further enhancement could potentially be achieved by taking advantage of the synergism that these receptors display when co-stimulated with other PRRs, particularly the TLRs.
Most CLR can recognize endogenous ligands (although few of these ligands have actually been identified) and these receptors are therefore likely to also have homeostatic roles. One of the best examples is the MR, which has a well established role as a regulator of serum glycoprotein homeostasis97. Furthermore, while largely associated with fungi, there is also evidence that CLR are involved in immunity to several other pathogens. DC-SIGN, MR, Dectin-1, Dectin-2 and Mincle, for example, have all been implicated in anti-mycobacterial immunity98. Considering the number of CLR in our genomes and their conservation during evolution99, it would not be surprising if these receptors are found to be far more extensively involved in anti-microbial immunity in the future.
Acknowledgements
The authors thank the Wellcome Trust for funding.
References
- 1.Brown GD, Denning DW, Levitz SM. Tackling Human Fungal Infections. Science. 2012;336:647. doi: 10.1126/science.1222236. [DOI] [PubMed] [Google Scholar]
- 2.Brown GD. Innate antifungal immunity: the key role of phagocytes. Annu. Rev. Immunol. 2011;29:1–21. doi: 10.1146/annurev-immunol-030409-101229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bourgeois C, et al. Conventional dendritic cells mount a type I IFN response against Candida spp. requiring novel phagosomal TLR7-mediated IFN-beta signaling. J. Immunol. 2011;186:3104–3112. doi: 10.4049/jimmunol.1002599. [DOI] [PubMed] [Google Scholar]
- 4.Netea MG, et al. Variable recognition of Candida albicans strains by TLR4 and lectin recognition receptors. Med. Mycol. 2010;48:897–903. doi: 10.3109/13693781003621575. [DOI] [PubMed] [Google Scholar]
- 5.Netea MG, van der Meer JW. Immunodeficiency and genetic defects of pattern-recognition receptors. N. Engl. J. Med. 2011;364:60–70. doi: 10.1056/NEJMra1001976. [DOI] [PubMed] [Google Scholar]
- 6.von Bernuth H, et al. Pyogenic bacterial infections in humans with MyD88 deficiency. Science. 2008;321:691–696. doi: 10.1126/science.1158298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zelensky AN, Gready JE. The C-type lectin-like domain superfamily. FEBS J. 2005;272:6179–6217. doi: 10.1111/j.1742-4658.2005.05031.x. [DOI] [PubMed] [Google Scholar]
- 8.Netea MG, Brown GD, Kullberg BJ, Gow NA. An integrated model of the recognition of Candida albicans by the innate immune system. Nat. Rev. Microbiol. 2008;6:67–78. doi: 10.1038/nrmicro1815. [DOI] [PubMed] [Google Scholar]
- 9.Reese TA, et al. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature. 2007;447:92–96. doi: 10.1038/nature05746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drummond RA, Saijo S, Iwakura Y, Brown GD. The role of Syk/CARD9 coupled C-type lectins in antifungal immunity. Eur. J. Immunol. 2011;41:276–281. doi: 10.1002/eji.201041252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leibundgut-Landmann S, et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat. Immunol. 2007;8:630–638. doi: 10.1038/ni1460. [This paper provides the first demonstration that C-type lectin receptors are able to induce T-cell differentiation and Th17 responses.] [DOI] [PubMed] [Google Scholar]
- 12.Gringhuis SI, et al. Dectin-1 directs T helper cell differentiation by controlling noncanonical NF-kappaB activation through Raf-1 and Syk. Nat. Immunol. 2009 doi: 10.1038/ni.1692. [DOI] [PubMed] [Google Scholar]
- 13.Gringhuis SI, et al. C-Type Lectin DC-SIGN Modulates Toll-like Receptor Signaling via Raf-1 Kinase-Dependent Acetylation of Transcription Factor NF-kappaB. Immunity. 2007;26:605–616. doi: 10.1016/j.immuni.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 14.Saijo S, et al. Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity. 2010;32:681–691. doi: 10.1016/j.immuni.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Robinson MJ, et al. Dectin-2 is a Syk-coupled pattern recognition receptor crucial for Th17 responses to fungal infection. J. Exp. Med. 2009;206:2037–2051. doi: 10.1084/jem.20082818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schoenen H, et al. Cutting edge: Mincle is essential for recognition and adjuvanticity of the mycobacterial cord factor and its synthetic analog trehalose-dibehenate. J. Immunol. 2010;184:2756–2760. doi: 10.4049/jimmunol.0904013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van de Veerdonk FL, et al. The macrophage mannose receptor induces IL-17 in response to Candida albicans. Cell Host Microbe. 2009;5:329–340. doi: 10.1016/j.chom.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Strasser D, et al. Syk kinase-coupled C-type lectin receptors engage protein kinase C-sigma to elicit Card9 adaptor-mediated innate immunity. Immunity. 2012;36:32–42. doi: 10.1016/j.immuni.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodridge HS, Simmons RM, Underhill DM. Dectin-1 stimulation by Candida albicans yeast or zymosan triggers NFAT activation in macrophages and dendritic cells. J. Immunol. 2007;178:3107–3115. doi: 10.4049/jimmunol.178.5.3107. [DOI] [PubMed] [Google Scholar]
- 20.Gross O, et al. Card9 controls a non-TLR signalling pathway for innate anti-fungal immunity. Nature. 2006;442:651–656. doi: 10.1038/nature04926. [Identification of CARD9 as a critical adaptor mediating the downstream signaling from CLRs. Identification of this molecule led to the discovery of mutations in CARD9 in humans which result in susceptibility to fungal infections (see Glocker et. al., 200931)] [DOI] [PubMed] [Google Scholar]
- 21.Sancho D, Reis e Sousa C. Signaling by myeloid C-type lectin receptors in immunity and homeostasis. Annu. Rev. Immunol. 2012;30:491–529. doi: 10.1146/annurev-immunol-031210-101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rogers NC, et al. Syk-dependent cytokine induction by dectin-1 reveals a novel pattern recognition pathway for C-type lectins. Immunity. 2005;22:507–517. doi: 10.1016/j.immuni.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Sato K, et al. Dectin-2 is a pattern recognition receptor for fungi that couples with the Fc receptor gamma chain to induce innate immune responses. J. Biol. Chem. 2006;281:38854–38866. doi: 10.1074/jbc.M606542200. [DOI] [PubMed] [Google Scholar]
- 24.Yamasaki S, Ishikawa E, Sakuma M, Ogata K, Saito T. Mincle is an ITAM-couples activating receptor that senses damaged cells. Nat. Immunol. 2008;9:1179–1188. doi: 10.1038/ni.1651. [DOI] [PubMed] [Google Scholar]
- 25.Goodridge HS, et al. Activation of the innate immune receptor Dectin-1 upon formation of a ‘phagocytic synapse’. Nature. 2011;472:471–475. doi: 10.1038/nature10071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor PR, et al. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat. Immunol. 2007;8:31–38. doi: 10.1038/ni1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saijo S, et al. Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nat. Immunol. 2007;8:39–46. doi: 10.1038/ni1425. [DOI] [PubMed] [Google Scholar]
- 28.Yamasaki S, et al. C-type lectin Mincle is an activating receptor for pathogenic fungus, Malassezia. Proc. Natl. Acad. Sci. U. S. A. 2009;106:1897–1902. doi: 10.1073/pnas.0805177106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dan JM, Kelly RM, Lee CK, Levitz SM. Role of the mannose receptor in a murine model of Cryptococcus neoformans infection. Infect. Immun. 2008;76:2362–2367. doi: 10.1128/IAI.00095-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferwerda B, et al. Human dectin-1 deficiency and mucocutaneous fungal infections. N. Engl. J. Med. 2009;361:1760–1767. doi: 10.1056/NEJMoa0901053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glocker EO, et al. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N. Engl. J. Med. 2009;361:1727–1735. doi: 10.1056/NEJMoa0810719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gantner BN, Simmons RM, Underhill DM. Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. EMBO J. 2005;24:1277–1286. doi: 10.1038/sj.emboj.7600594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee SJ, Zheng NY, Clavijo M, Nussenzweig MC. Normal Host Defense during Systemic Candidiasis in Mannose Receptor-Deficient Mice. Infect. Immun. 2003;71:437–445. doi: 10.1128/IAI.71.1.437-445.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gringhuis SI, et al. Selective C-Rel activation via Malt1 controls anti-fungal T(H)-17 immunity by dectin-1 and dectin-2. PLoS Pathog. 2011;7:e1001259. doi: 10.1371/journal.ppat.1001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Underhill DM, Rossnagle E, Lowell CA, Simmons RM. Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood. 2005;106:2543–2550. doi: 10.1182/blood-2005-03-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suram S, et al. Pathways regulating cytosolic phospholipase A2 activation and eicosanoid production in macrophages by Candida albicans. J. Biol. Chem. 2010 doi: 10.1074/jbc.M110.143800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Werner JL, et al. Requisite role for the dectin-1 beta-glucan receptor in pulmonary defense against Aspergillus fumigatus. J. Immunol. 2009;182:4938–4946. doi: 10.4049/jimmunol.0804250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vautier S, MacCallum DM, Brown GD. C-type lectin receptors and cytokines in fungal immunity. Cytokine. 2012;58:89–99. doi: 10.1016/j.cyto.2011.08.031. [DOI] [PubMed] [Google Scholar]
- 39.Acosta-Rodriguez EV, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat. Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 40.Romani L. Immunity to fungal infections. Nat. Rev. Immunol. 2011;11:275–288. doi: 10.1038/nri2939. [DOI] [PubMed] [Google Scholar]
- 41.Conti HR, Gaffen SL. Host responses to Candida albicans: Th17 cells and mucosal candidiasis. Microbes Infect. 2010;12:518–527. doi: 10.1016/j.micinf.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van de Veerdonk FL, et al. STAT1 mutations in autosomal dominant chronic mucocutaneous candidiasis. N. Engl. J. Med. 2011;365:54–61. doi: 10.1056/NEJMoa1100102. [These authors identified a noncanonical inflammasome which is triggered directly by Dectin-1.] [DOI] [PubMed] [Google Scholar]
- 43.Liu L, et al. Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. J. Exp. Med. 2011;208:1635–1648. doi: 10.1084/jem.20110958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Puel A, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332:65–68. doi: 10.1126/science.1200439. [Using fungal mutants lacking various cell wall carbohydrates and immune cells with PRR deficiencies, these authors provide the first demonstration of the importance of multiple receptor collaboration for inducing optimal anti-fungal immune responses.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma CS, et al. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J. Exp. Med. 2008;205:1551–1557. doi: 10.1084/jem.20080218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Milner JD, et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452:773–776. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Puel A, et al. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J. Exp. Med. 2010;207:291–297. doi: 10.1084/jem.20091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kisand K, et al. Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J. Exp. Med. 2010;207:299–308. doi: 10.1084/jem.20091669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zelante T, et al. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur. J. Immunol. 2007;37:2695–2706. doi: 10.1002/eji.200737409. [DOI] [PubMed] [Google Scholar]
- 50.Barrett NA, et al. Dectin-2 mediates Th2 immunity through the generation of cysteinyl leukotrienes. J. Exp. Med. 2011;208:593–604. doi: 10.1084/jem.20100793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Osorio F, et al. DC activated via dectin-1 convert Treg into IL-17 producers. Eur. J. Immunol. 2008;38:3274–3281. doi: 10.1002/eji.200838950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rivera A, et al. Dectin-1 diversifies Aspergillus fumigatus-specific T cell responses by inhibiting T helper type 1 CD4 T cell differentiation. J. Exp. Med. 2011;208:369–381. doi: 10.1084/jem.20100906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sutton CE, et al. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 54.Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–330. doi: 10.1016/j.immuni.2009.06.020. [In this paper, the authors show how a lack of TLR and CLR costimulation can result in chronic fungal infection. Importantly, they demonstrate that restoration of these costimulatory responses by treatment with exogenous PRR ligands can lead to resolution of the infection.] [DOI] [PubMed] [Google Scholar]
- 55.Kawakami K, et al. Monocyte chemoattractant protein-1-dependent increase of V alpha 14 NKT cells in lungs and their roles in Th1 response and host defense in cryptococcal infection. J. Immunol. 2001;167:6525–6532. doi: 10.4049/jimmunol.167.11.6525. [DOI] [PubMed] [Google Scholar]
- 56.Cohen NR, et al. Innate recognition of cell wall beta-glucans drives invariant natural killer T cell responses against fungi. Cell Host Microbe. 2011;10:437–450. doi: 10.1016/j.chom.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 58.Netea MG, et al. Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. J. Clin. Invest. 2006;116:1642–1650. doi: 10.1172/JCI27114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brown GD, et al. Dectin-1 mediates the biological effects of beta-glucan. J. Exp. Med. 2003;197:1119–1124. doi: 10.1084/jem.20021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM. Collaborative Induction of Inflammatory Responses by Dectin-1 and Toll-like Receptor 2. J. Exp. Med. 2003;197:1107–1117. doi: 10.1084/jem.20021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dennehy KM, et al. Syk kinase is required for collaborative cytokine production induced through Dectin-1 and Toll-like receptors. Eur. J. Immunol. 2008;38:500–506. doi: 10.1002/eji.200737741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dennehy KM, Willment JA, Williams DL, Brown GD. Reciprocal regulation of IL-23 and IL-12 following co-activation of Dectin-1 and TLR signaling pathways. Eur. J. Immunol. 2009;39:1379–1386. doi: 10.1002/eji.200838543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gerosa F, et al. Differential regulation of interleukin 12 and interleukin 23 production in human dendritic cells. J. Exp. Med. 2008;205:1447–1461. doi: 10.1084/jem.20071450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang H, et al. Distinct patterns of dendritic cell cytokine release stimulated by fungal beta-glucans and toll-like receptor agonists. Infect. Immun. 2009;77:1774–1781. doi: 10.1128/IAI.00086-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nagaoka K, et al. Association of SIGNR1 with TLR4-MD-2 enhances signal transduction by recognition of LPS in gram-negative bacteria. Int. Immunol. 2005;17:827–836. doi: 10.1093/intimm/dxh264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sato M, et al. Direct binding of toll-like receptor 2 to zymosan, and zymosan-induced NF-kappaB activation and TNF-alpha secretion are down-regulated by lung collectin surfactant protein A. J. Immunol. 2003;171:417–425. doi: 10.4049/jimmunol.171.1.417. [DOI] [PubMed] [Google Scholar]
- 67.Ameen M. Chromoblastomycosis: clinical presentation and management. Clin. Exp. Dermatol. 2009;34:849–854. doi: 10.1111/j.1365-2230.2009.03415.x. [DOI] [PubMed] [Google Scholar]
- 68.da Gloria Sousa M, et al. Restoration of pattern recognition receptor costimulation to treat chromoblastomycosis, a chronic fungal infection of the skin. Cell Host Microbe. 2011;9:436–443. doi: 10.1016/j.chom.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Empey KM, Hollifield M, Garvy BA. Exogenous heat-killed Escherichia coli improves alveolar macrophage activity and reduces Pneumocystis carinii lung burden in infant mice. Infect. Immun. 2007;75:3382–3393. doi: 10.1128/IAI.00174-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Franchi L, Munoz-Planillo R, Nunez G. Sensing and reacting to microbes through the inflammasomes. Nat. Immunol. 2012;13:325–332. doi: 10.1038/ni.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van de Veerdonk FL, et al. The inflammasome drives protective Th1 and Th17 cellular responses in disseminated candidiasis. Eur. J. Immunol. 2011;41:2260–2268. doi: 10.1002/eji.201041226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gross O, et al. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459:433–436. doi: 10.1038/nature07965. [DOI] [PubMed] [Google Scholar]
- 73.Netea MG, et al. Differential role of IL-18 and IL-12 in the host defense against disseminated Candida albicans infection. Eur. J. Immunol. 2003;33:3409–3417. doi: 10.1002/eji.200323737. [This paper provides a criticial molecular insight into the mechanisms behind CLRmediated induction of Th17 responses.] [DOI] [PubMed] [Google Scholar]
- 74.Vonk AG, et al. Endogenous interleukin (IL)-1 alpha and IL-1 beta are crucial for host defense against disseminated candidiasis. J. Infect. Dis. 2006;193:1419–1426. doi: 10.1086/503363. [These authors use one of the few fungal-specific TCR transgenics to explore the role of Dectin-1 in the development adaptive immunity during fungal infections, demonstrating how this CLR promotes Th17 development.] [DOI] [PubMed] [Google Scholar]
- 75.Hise AG, et al. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe. 2009;5:487–497. doi: 10.1016/j.chom.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Said-Sadier N, Padilla E, Langsley G, Ojcius DM. Aspergillus fumigatus stimulates the NLRP3 inflammasome through a pathway requiring ROS production and the Syk tyrosine kinase. PLoS ONE. 2010;5:e10008. doi: 10.1371/journal.pone.0010008. [This manuscript shows that γδ T-cells express CLR and can respond directly to fungi.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tomalka J, et al. A novel role for the NLRC4 inflammasome in mucosal defenses against the fungal pathogen Candida albicans. PLoS Pathog. 2011;7:e1002379. doi: 10.1371/journal.ppat.1002379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gringhuis SI, et al. Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1beta via a noncanonical caspase-8 inflammasome. Nat. Immunol. 2012;13:246–254. doi: 10.1038/ni.2222. [These authors identify a CLR-mediated mechanism of activation of self-reactive iNKT cell that induces IFNγ in response to fungi.] [DOI] [PubMed] [Google Scholar]
- 79.Roy RM, Klein B. Dendritic cells in anti-fungal immunity and vaccine design. Cell Host Microbe. 2012 doi: 10.1016/j.chom.2012.04.005. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tacken PJ, de Vries IJ, Torensma R, Figdor CG. Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nat. Rev. Immunol. 2007;7:790–802. doi: 10.1038/nri2173. [DOI] [PubMed] [Google Scholar]
- 81.Carter RW, Thompson C, Reid DM, Wong SY, Tough DF. Preferential induction of CD4+ T cell responses through in vivo targeting of antigen to dendritic cell-associated C-type lectin-1. J. Immunol. 2006;177:2276–2284. doi: 10.4049/jimmunol.177.4.2276. [DOI] [PubMed] [Google Scholar]
- 82.Carter RW, Thompson C, Reid DM, Wong SY, Tough DF. Induction of CD8+ T cell responses through targeting of antigen to Dectin-2. Cell. Immunol. 2006;239:87–91. doi: 10.1016/j.cellimm.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 83.Cruz LJ, et al. Comparison of antibodies and carbohydrates to target vaccines to human dendritic cells via DC-SIGN. Biomaterials. 2012;33:4229–4239. doi: 10.1016/j.biomaterials.2012.02.036. [DOI] [PubMed] [Google Scholar]
- 84.He LZ, et al. Antigenic targeting of the human mannose receptor induces tumor immunity. J. Immunol. 2007;178:6259–6267. doi: 10.4049/jimmunol.178.10.6259. [DOI] [PubMed] [Google Scholar]
- 85.Cutler JE, Deepe GS, Klein BS. Advances in combating fungal diseases: vaccines on the threshold. Nat. Rev. Microbiol. 2007;5:13–28. doi: 10.1038/nrmicro1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lang R, Schoenen H, Desel C. Targeting Syk-Card9-activating C-type lectin receptors by vaccine adjuvants: findings, implications and open questions. Immunobiology. 2011;216:1184–1191. doi: 10.1016/j.imbio.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 87.Huang H, Ostroff GR, Lee CK, Specht CA, Levitz SM. Robust stimulation of humoral and cellular immune responses following vaccination with antigen-loaded beta-glucan particles. MBio. 2010:1. doi: 10.1128/mBio.00164-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Aouadi M, et al. Orally delivered siRNA targeting macrophage Map4k4 suppresses systemic inflammation. Nature. 2009;458:1180–1184. doi: 10.1038/nature07774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Spellberg B. Vaccines for invasive fungal infections. F1000 Med Rep. 3:13–2011. doi: 10.3410/M3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Torosantucci A, et al. A novel glyco-conjugate vaccine against fungal pathogens. J. Exp. Med. 2005;202:597–606. doi: 10.1084/jem.20050749. [These authors demonstrate that vaccination with a β-glucan conjugate can drive protective responses towards several fungal species.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rachini A, et al. An anti-beta-glucan monoclonal antibody inhibits growth and capsule formation of Cryptococcus neoformans in vitro and exerts therapeutic, anticryptococcal activity in vivo. Infect. Immun. 2007;75:5085–5094. doi: 10.1128/IAI.00278-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bar E, et al. A Novel Th Cell Epitope of Candida albicans Mediates Protection from Fungal Infection. J. Immunol. 2012 doi: 10.4049/jimmunol.1200594. [DOI] [PubMed] [Google Scholar]
- 93.Wuthrich M, et al. Vaccine-induced protection against 3 systemic mycoses endemic to North America requires Th17 cells in mice. J. Clin. Invest. 2011;121:554–568. doi: 10.1172/JCI43984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Specht CA, Nong S, Dan JM, Lee CK, Levitz SM. Contribution of glycosylation to T cell responses stimulated by recombinant Cryptococcus neoformans mannoprotein. J. Infect. Dis. 2007;196:796–800. doi: 10.1086/520536. [DOI] [PubMed] [Google Scholar]
- 95.Sun WK, et al. Dectin-1 is inducible and plays a crucial role in Aspergillus-induced innate immune responses in human bronchial epithelial cells. Eur. J. Clin. Microbiol. Infect. Dis. 2012 doi: 10.1007/s10096-012-1624-8. [DOI] [PubMed] [Google Scholar]
- 96.Weindl G, Wagener J, Schaller M. Epithelial Cells and Innate Antifungal Defense. J. Dent. Res. 2010 doi: 10.1177/0022034510368784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee SJ, et al. Mannose receptor-mediated regulation of serum glycoprotein homeostasis. Science. 2002;295:1898–1901. doi: 10.1126/science.1069540. [DOI] [PubMed] [Google Scholar]
- 98.Kerrigan AM, Brown GD. Syk-coupled C-type lectins in immunity. Trends Immunol. 2011;32:151–156. doi: 10.1016/j.it.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sattler S, Ghadially H, Hofer E. Evolution of the C-Type Lectin-Like Receptor Genes of the DECTIN-1 Cluster in the NK Gene Complex. Scientific World Journal. 20122012:931386. doi: 10.1100/2012/931386. [DOI] [PMC free article] [PubMed] [Google Scholar]