SUMMARY
Deficits in prefrontal cholinergic function are implicated in cognitive impairment in many neuropsychiatric diseases, but acetylcholine’s specific role remains elusive. Rhesus monkeys with selective lesions of cholinergic input to prefrontal cortex were unimpaired in tests of decision-making and episodic memory that require intact prefrontal cortex, but were severely impaired on a spatial working memory task. These observations are consistent with a specific role for prefrontal acetylcholine in working memory.
Keywords: cholinergic, prefrontal, dorsolateral, ventrolateral, orbital, frontal, spatial memory, episodic, decision-making, reward
Impairments in memory, planning and decision-making are significant components of neurological diseases such as Alzheimer’s disease (AD). The prefrontal cortex (PFC) has been implicated in many of these functions 1,2, as has the neurochemical acetylcholine, which is reduced in AD3. The degree of cognitive impairment in patients with AD is closely correlated with the loss of basal forebrain cholinergic neurons that project to cortical structures involved in memory and executive function4 However, cholinesterase inhibitors produce little acute improvement in cognitive function5. One possibility is that cholinergic deficits only play a role in cognitive impairment when tasks require attentional modulation or reorientation6. However, a number of studies have found that selective cholinergic lesions spare many memory and executive function processes and instead impair specific aspects of attention or recognition memory7-9. We hypothesised that acetylcholine may play a role in specific functions of primate PFC beyond those which place demands on attention. To date no studies of prefrontal cortical cholinergic depletion in rhesus monkeys, with their large and highly differentiated PFC, have been carried out.
We tested rhesus monkeys with a selective cholinergic depletion of the lateral and orbital PFC, made using the immunotoxin ME20.4–saporin (Fig. 1; Supplementary Fig. 2; Supplementary Tables 1 and 2) on tasks which test the function of specific subregions of the PFC (see ref. 2 for review and Supplementary Information and Supplementary Fig. 1 for descriptions): object-in-place scene learning (an episodic memory task), strategy implementation, reinforcer devaluation (a reward–based decision making task) and spatial delayed response (a working memory task). Procedures were carried out under personal and project licences compliant with the UK Animals (Scientific Procedures) Act 1986.
Figure 1.
Cholinergic lesions.
The lesions were specific to cholinergic innervation of the prefrontal cortex. (a) Extent of intended lesions. (b) Cholinergic depletion for a representative monkey, PFC1. (c) Magnification of subregions of PFC in monkey PFC1, demonstrating ACh fibre loss (1– principal sulcus; 2– cingulate sulcus; 3– inferior convexity; 4– medial orbital sulcus). (d) Magnification of area indicated by solid box in a control brain, stained for (left to right) acetylcholinesterase, cresyl violet, tyrosine hydroxylase (dopaminergic fibres) and parvalbumin (GABAergic interneurons) in a control brain. (e) Magnification of the same region in a lesioned brain (with the same stains). These stains show that the cholinergic lesions were extensive and there was no non-specific cell body or noncholinergic fibre damage.
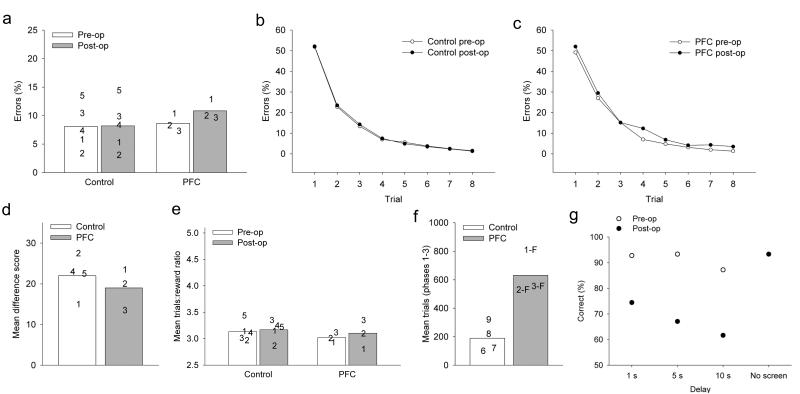
Monkeys learned new lists of object–in–place scene problems very rapidly, consistent with this task modeling human episodic memory (Fig. 2b), and the rate of learning was unaffected by PFC cholinergic depletions (Fig. 2a, 2c). A repeated-measures ANOVA of errors with pre/post surgery and trial (8 levels: each repetition) as within-subjects factors and group (controls and PFC) as the between-subjects factor revealed only a significant effect of trial, and no main effects of group, (F(1, 6) = 0.020, P = 0.892), pre/post surgery (F1, 6 = 0.329, P = 0.587), or significant interactions between pre/post and group (F1, 6 = 0.247, P = 0.635), or between pre/post, group, and trial (F7, 42 = 0.481, P = 0.836).
Figure 2.
Task performance.
(a-c) Object-in-place scene learning. (a) Mean data for each group across repetitions 2-8 (scores for the first repetition are at chance as the scenes are new on each day). Data points = individual scores. Monkeys with depletions of acetylcholine in lateral and orbital prefrontal cortex (PFC) were unimpaired. Mean numbers of errors on scene learning for each repetition (1-8, horizontal axis) in pre- and postoperative phases, for (b) controls, and (c) PFC. (d) Strategy implementation. Performance measured the ratio of rewards earned to trials completed; perfect performance = 2.5. Mean ratio of responses to rewards for control and PFC monkeys. Data points = individual scores. The PFC monkeys were unimpaired. (e) Reward devaluation. Mean difference scores across the two devaluation tests for control and PFC monkeys. Data points = individual scores. The PFC monkeys were unimpaired. (f-g) Spatial delayed response. (f) Mean errors to criterion for control and PFC monkeys that learned the task postoperatively. The PFC monkeys made significantly more errors than the controls, even though two of the PFC monkeys failed to reach the final phase of the task. (g) Performance of two additional monkeys that received PFC depletions following preoperative training on spatial delayed response. Mean scores are shown for each delay length both pre– and postoperatively. Their performance was worse at all delay lengths postoperatively (preoperative mean 91.1% correct; postoperative mean 67.7% correct), but not in the condition with no screen.
Monkeys with PFC cholinergic depletions were also unimpaired in postoperative performance of the strategy implementation task (Fig. 2d). A repeated-measures ANOVA of the ratio of rewards earned to trials performed was carried out, with pre/post surgery as the within-subjects factor and group (controls and lesioned) as the between-subjects factor. There was no significant main effect of group (F1, 6 = 0.266, P = 0.624), no main effect of pre/post (F1, 6 = 2.588, P = 0.159) and no group by pre/post interaction (F1, 6 = 2.190, P = 0.317). They were also unimpaired in reinforcer devaluation (Fig. 2e). One-way ANOVA revealed no main effect of group (F1, 6 = 0.038, P = 0.852).
In contrast to their intact performance on tests of episodic memory and executive function, monkeys with depletion of acetylcholine from PFC were strikingly impaired in learning the delayed response task, making many more errors than controls across all phases (Fig. 2f, Supplementary Table 3). Of the three PFC monkeys, one did not proceed beyond Phase 1 of the task, one did not succeed in reaching criterion on Phase 2, and the third reached Phase 3 but did not reach criterion. A one-way ANOVA revealed a significant difference between the controls and PFCs on total errors across phases (F(1, 5)=32.49, p=0.002) and mean number of trials completed in each phase (control mean=190 trials; PFC mean=631.67 trials), F(1, 5)=21.21, p=0.006. This result is very similar to the deficit following dorsolateral PFC ablations10, in which two of the three monkeys failed to reach the final phase of the task. The delayed response impairment was not one of acquisition, as two further monkeys (formerly controls) that received identical cholinergic depletions of PFC were dramatically impaired postoperatively (Fig. 2g). Even though all these monkeys showed impairment even when a brief (“0 sec”) delay with a screen occurred between baiting and choice, they performed normally with no delay, indicating that they could maintain attention to the baited location.
It should be noted that the role of attention in this task cannot be completely ruled out, even with pre-trained controls, but many of the unimpaired tasks were also attentionally demanding. Also, although spatial attention deficits have been reported after cholinergic depletion, and it is difficult to disentangle working memory from attentional performance, they are doubly dissociable from each other. Neurotoxic basal forebrain lesions in monkeys impair spatial attention but not delayed response performance whereas aged monkeys are impaired in delayed response but not in spatial attention11. Working memory tasks do not simply rely on attentional mechanisms and cannot always be explained by a failure of encoding. For example, a similar working memory deficit has been seen following cortical cholinergic depletion with scopolamine in rats, which was dissociable from attentional deficits7.
Our findings show, for the first time, an impairment following depletion of prefrontal acetylcholine in a specific aspect of memory (working memory), but not in other prefrontal-dependent cognitive tasks in primates. A similar role has been suggested for acetylcholine in the entorhinal cortex, another region important for active maintenance of mnemonic information12. Acetylcholine has been linked to sustained activity, which might be required for working memory maintenance13,14. Our new data complement these observations by showing that prefrontal cortical acetylcholine is essential for spatial working memory, even though it is not required for several other cognitive functions mediated by the PFC. As cholinergic signals have been proposed to act on multiple timescales, with both phasic and tonic components, they may therefore affect several different cognitive processes6. Therefore, we are inclined to hypothesize a specific role for acetylcholine in the maintenance of information in working memory by PFC, distinct from the other roles it may play in modulating cognition. Our results do not at all exclude a role for corticopetal cholinergic input in modulation of attentional processing, but we suggest that our observations also implicate prefrontal acetylcholine in working memory function. The finding that other mnemonic and executive functions of the PFC remain intact following cholinergic depletion is consistent with the relatively small acute effects of cholinesterase inhibitors in AD5. Until now, a plausible explanation of these limited effects has been that the cognitive enhancing effects of these drugs have been restricted by their pharmacokinetic properties and side effects. However, the loss of acetylcholine alone is not enough to lead to devastating impairments in all prefrontal function, perhaps because other neuromodulators may modulate different functions of the PFC, including a role in working memory15. It is likely that pharmacological strategies that stimulate multiple neuromodulatory systems may be necessary to produce generalized improvements in cortical function in dementia. In addition, our finding may help to explain the deficits in working memory seen in patients with other types of dementia in which the cholinergic loss is more extensive than in AD, such as Lewy body dementia. Further work will be necessary to elucidate the precise role of acetylcholine in prefrontal function.
Supplementary Material
Acknowledgments
We thank D. Gaffan for advice and support, and C. Bergmann, G. Daubney, C. Rae and K. Murphy for technical assistance. This research was supported by a grant from the Wellcome Trust (MGB).
References
- 1.Johnson JK, Head E, Kim R, Starr A, Cotman CW. Clinical and pathological evidence for a frontal variant of Alzheimer disease. Arch Neurol. 1999;56:1233–1239. doi: 10.1001/archneur.56.10.1233. [DOI] [PubMed] [Google Scholar]
- 2.Wilson CR, Gaffan D, Browning PG, Baxter MG. Functional localization within the prefrontal cortex: missing the forest for the trees? Trends Neurosci. 2010 doi: 10.1016/j.tins.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartus RT. On neurodegenerative diseases, models, and treatment strategies: lessons learned and lessons forgotten a generation following the cholinergic hypothesis. Exp Neurol. 2000;163:495–529. doi: 10.1006/exnr.2000.7397. [DOI] [PubMed] [Google Scholar]
- 4.Bierer LM, et al. Neurochemical correlates of dementia severity in Alzheimer’s disease: relative importance of the cholinergic deficits. J Neurochem. 1995;64:749–760. doi: 10.1046/j.1471-4159.1995.64020749.x. [DOI] [PubMed] [Google Scholar]
- 5.Bartus RT, Dean RL., 3rd Pharmaceutical treatment for cognitive deficits in Alzheimer’s disease and other neurodegenerative conditions: exploring new territory using traditional tools and established maps. Psychopharmacology (Berl) 2009;202:15–36. doi: 10.1007/s00213-008-1365-7. [DOI] [PubMed] [Google Scholar]
- 6.Parikh V, Sarter M. Cholinergic mediation of attention: contributions of phasic and tonic increases in prefrontal cholinergic activity. Ann N Y Acad Sci. 2008;1129:225–235. doi: 10.1196/annals.1417.021. [DOI] [PubMed] [Google Scholar]
- 7.Chudasama Y, Dalley JW, Nathwani F, Bouger P, Robbins TW. Cholinergic modulation of visual attention and working memory: dissociable effects of basal forebrain 192-IgG-saporin lesions and intraprefrontal infusions of scopolamine. Learn Mem. 2004;11:78–86. doi: 10.1101/lm.70904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turchi J, Saunders RC, Mishkin M. Effects of cholinergic deafferentation of the rhinal cortex on visual recognition memory in monkeys. Proc Natl Acad Sci U S A. 2005;102:2158–2161. doi: 10.1073/pnas.0409708102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Browning PG, Gaffan D, Croxson PL, Baxter MG. Severe Scene Learning Impairment, but Intact Recognition Memory, after Cholinergic Depletion of Inferotemporal Cortex Followed by Fornix Transection. Cereb Cortex. 2009 doi: 10.1093/cercor/bhp097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bachevalier J, Mishkin M. Visual recognition impairment follows ventromedial but not dorsolateral prefrontal lesions in monkeys. Behav Brain Res. 1986;20:249–261. doi: 10.1016/0166-4328(86)90225-1. [DOI] [PubMed] [Google Scholar]
- 11.Baxter MG, Voytko ML. Spatial orienting of attention in adult and aged rhesus monkeys. Behav Neurosci. 1996;110:898–904. doi: 10.1037//0735-7044.110.5.898. [DOI] [PubMed] [Google Scholar]
- 12.Egorov AV, Hamam BN, Fransen E, Hasselmo ME, Alonso AA. Graded persistent activity in entorhinal cortex neurons. Nature. 2002;420:173–178. doi: 10.1038/nature01171. [DOI] [PubMed] [Google Scholar]
- 13.Furey ML, Pietrini P, Haxby JV. Cholinergic enhancement and increased selectivity of perceptual processing during working memory. Science. 2000;290:2315–2319. doi: 10.1126/science.290.5500.2315. [DOI] [PubMed] [Google Scholar]
- 14.Schon K, et al. Scopolamine reduces persistent activity related to long-term encoding in the parahippocampal gyrus during delayed matching in humans. J Neurosci. 2005;25:9112–9123. doi: 10.1523/JNEUROSCI.1982-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robbins TW, Arnsten AF. The neuropsychopharmacology of fronto–executive function: monoaminergic modulation. Annu Rev Neurosci. 2009;32:267–287. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.