Abstract
G protein-coupled receptor kinase interacting protein 2 (GIT2) is a signaling scaffold protein involved in the regulation of cytoskeletal structure, membrane trafficking, and G protein-coupled receptor internalization. Since dynamic cytoskeletal reorganization plays key roles both in osteoblast differentiation and in the maintenance of osteoclast polarity during bone resorption, we hypothesized that skeletal physiology would be altered in GIT2−/− mice. We found that adult GIT2−/− mice have decreased bone mineral density and bone volume in both the trabecular and cortical compartments. This osteopenia was associated with decreased numbers of mature osteoblasts, diminished osteoblastic activity, and increased marrow adiposity, suggesting a defect in osteoblast maturation. In vitro, mesenchymal stem cells derived from GIT2−/− mice exhibited impaired differentiation into osteoblasts and increased adipocyte differentiation, consistent with a role for GIT2 in mesenchymal stem cell fate determination. Despite elevated osteoclast inducing cytokines and osteoclast numbers, GIT2−/− mice also exhibit impaired bone resorption, consistent with a further role for GIT2 in regulating osteoclast function. Collectively, these findings underscore the importance of the cytoskeleton in both osteoblast and osteoclast function and demonstrate that GIT2 plays essential roles in skeletal metabolism, affecting both bone formation and bone resorption in vivo.
Keywords: GIT2, osteoblastogenesis, mesenchymal stem cell, cytoskeleton, adipogenesis
INTRODUCTION
GPCRs exert their effects on bone and calcium metabolism through complex molecular mechanisms involving diverse downstream signaling events and multiple signaling complexes. Many GPCR-interacting proteins recruited during signal initiation and termination have been identified, including G proteins, arrestins and clathrin. Among the first of these proteins to be discovered were the GRKs, which are crucial for receptor phosphorylation and internalization. A search for proteins that interact with GRKs led to the identification of GITs [1].
GITs are signaling scaffold proteins comprising a family of ARF-GAPs. In cells, GITs exist in a oligomeric complexes with PIX/Cool proteins [2]. PIX/Cool proteins are GEFs for the cytoskeletal regulatory small GTPases, Rac1 and Cdc42. GIT/PIX complexes are known to function as scaffolds for a variety of signaling proteins, including GRKs, PAKs, FAK, the MEK1-ERK1/2 mitogen-activated proteins kinases, and phospholipase Cγ [3]. The 2 GIT family members, GIT1 and GIT2, are highly expressed in neurons, vascular smooth muscle, endothelial cells and bone. In addition to their roles as regulators of GPCR internalization and resensitization [1,4], in vitro, GIT proteins have been investigated for their participation in focal adhesion dynamics, cell migration [5,6], and as scaffolding proteins directing the spatial localization of signaling molecules such as MEK1 and ERK1/2 [7]. In vivo, GIT expression has been shown to regulate emotional function [8,9] vascular development [10] and mitochondrial biogenesis [11]. Little, however, is known about the role of GIT proteins in bone physiology.
Bone density is the product of complex interactions between OBs, which form new bone, and OCs, which resorb bone. OB differentiation and function is highly dependent upon cell adhesion and cytoskeletal organization. Recent studies have shown that extracellular cues, derived from interactions with the extracellular matrix and transmitted via cytoskeletal tension, direct MSC differentiation to either OB or adipocyte lineages [12,13,14,15,16], suggesting that proteins involved in regulating actin cytoskeletal dynamics, like GITs, may play a key role in the fate determination of MSCs. The contribution, however, of GIT proteins to OB lineage commitment has not been established.
GIT2 has been implicated in OC function. The nonreceptor tyrosine kinase c-Src is a known regulator of OC function, and Src−/− OCs exhibit impaired actin cytoskeletal organization, leading to diminished OC function and osteopetrosis. In a recent study to identify critical c-Src substrates in OCs, it was shown that Src-dependent phosphorylation and localization of GIT2 to OC sealing zones is essential for maintaining sealing zones and OC polarity for bone resorption in vitro [17]. There is also reported increased bone mass in GIT1−/− mice due to an OC defect [18].
As modulators of GPCR signaling, cytoskeletal rearrangement and cell adhesion, we hypothesized that GIT proteins are required for skeletal development and bone remodeling. To determine the contribution of the GIT family member GIT2 to bone metabolism in vivo, we evaluated GIT2−/− mice for alterations in bone formation and bone resorption. We found that the absence of GIT2 significantly decreases trabecular and cortical bone mass. Investigation of OC function confirmed impaired bone resorption, despite a marked increase in OC bone surface in GIT2−/− mice. The observed osteopenia in the setting of decreased bone resorption is explained by attenuated differentiation of MSC to OBs and increased adipogenesis. Together these findings highlight the complex skeletal effects of GIT2 expression, which are critical to the proliferation, differentiation and function of both OBs and OCs.
MATERIALS AND METHODS
Generation of GIT2 KO Mice
The derivation of the C57B6/129 GIT2−/− mice was previously described [19]. All animals were treated in accordance with NIH guidelines for the care and use of animals under a protocol approved by the Duke University Institutional Animal Care and Use Committee.
Bone densitometry and quantitative computed tomography
BMD and qCT were performed as previously described [20].
Histology and Histomorphometry
Quantitative histomorphometric analysis of vertebral spine trabecular bone was performed using techniques described previously [20] and data are expressed in units recommended by the American Society of Bone and Mineral Research [21].
Serum biochemistry and bone turnover markers
Serum osteocalcin, plasma PTH, urine DPD excretion and urine creatinine concentrations were measured as previously described [20]. Urine and Serum calcium measurements were determined using the Total Calcium LiquiColor colorometric assay (Stanbio Laboratory; Boerne, Texas) following the manufacturer's protocol.
Total DNA content
To control for potential viability differences between cells of different genotypes, total DNA concentration was determined. After 24 days of culture, media was aspirated and cells lysed by freeze-thaw in deionized water. DNA was stained with Hoechst 33258 and quantified (360nm excitation / 460nm emission).
Statistics
All values are expressed as means ± SEM. For comparisons between two groups, statistical significance was assessed using a two-tailed unpaired t-test. Additional methods are described in the supplementary section.
RESULTS
Decreased Bone Mineral Density in GIT2−/− Mice
Adult GIT2−/− mice are fertile and show no gross phenotypic abnormalities, skeletal deformities or defects when compared to WT mice. To examine the contributions of GIT2 expression on bone metabolism, BMD measurements were obtained by DEXA on 15-week old male and female mice (Figure 1A–B). Both male and female GIT2−/−mice had significantly decreased vertebral spine and femoral shaft BMD compared to their WT counterparts.
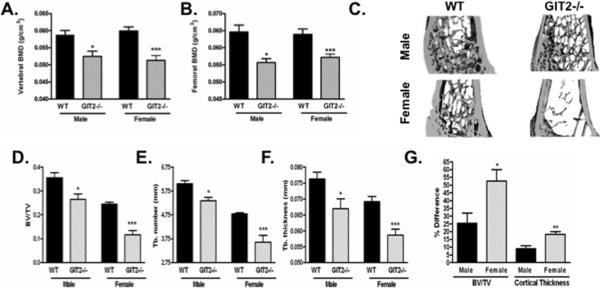
Fig. 1. GIT2−/− mice have decreased bone mass in trabecular and cortical compartments.
(A, B) Lumbar spine and femoral BMD of 15 week old male and female WT and GIT2−/− mice were determined by DEXA. (C) Representative qCT images of distal femur isolated from 15 week old male and female WT and GIT2−/− mice. (E, F, G) qCT of distal femur was used to determine the effects on trabecular bone (Tb.) volume fraction (BV/TV), Tb. number, and Tb. thickness. (G). Compares the % difference in trabecular BV/TV and cortical thickness from male and female cohorts of WT and GIT2−/− mice. Data represent the mean ± SEM of measurements taken from at least 7 mice. (***, P < 0.001; **, P<0.01; *, P<0.05 compared with WT).
GIT2−/− Mice Have Decreased Trabecular and Cortical Bone Morphometry
To determine the effects of GIT2 expression on trabecular bone morphometry, qCT measurements of the distal femur were acquired from 15-week old male and female WT and GIT2−/− mice (Figure 1C–F). GIT2−/− mice had a significantly decreased BV/TV, decreased trabecular number, and decreased trabecular thickness compared to WT mice (Figure 2A–C). Cortical bone indices were examined by qCT of the mid-femoral shaft (Supplementary Figure 1A–B). Both male and female GIT2−/− mice had significantly decreased cortical bone thickness and cortical bone area compared to WT mice. This loss of cortical bone mass caused a significant decrease in both periosteal circumference and CSA in the female GIT2−/− mice as well as significant increases in endosteal circumference and medullary area in the GIT2−/− male mice compared to WT (Supplementary Figure 1C–F). As shown in Figure 1G, the decrease in bone mass was more pronounced in the female GIT2−/− mice compared to the male GIT2−/− mice.
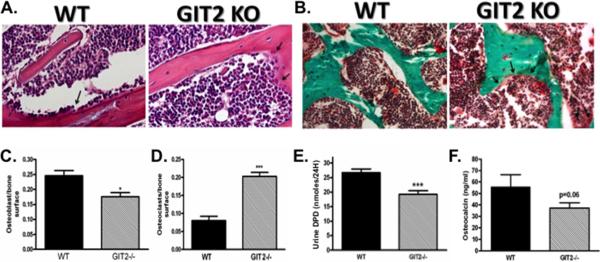
Fig. 2. GIT2−/− mice have decreased OB number, increased OC number and abnormalities in bone remodeling.
(A) Representative decalcified H and E stained sections of proximal tibia (40× magnification; arrows (→) designating multinucleated osteoclasts) and (B) non-decalcified trichrome stained sections of vertebral spine (20× magnification; arrows (→) designating multinucleated osteoclasts) isolated from WT and GIT2−/− mice. Quantitative histomorphometric analysis was used to determine (C) OB surface and OC surface (D). Markers of bone formation and resorption: (E) 24-hour urine DPD, and (F) serum osteocalcin. Data represent the mean ± SEM of measurements taken from at least 7 male mice. (***, P < 0.001; *, P<0.05 compared with WT).
Effect GIT2 expression on OB number, OC number and bone remodeling
Histomorphometric analysis revealed a significant increase in Oc.S and a significant decrease in Ob.S in the GIT2−/− mice compared to WT mice (Figure 2A–D). As shown in Figure 2E–F, these histomorphometric findings were associated with a decrease in serum and urine biomarkers of bone formation and bone resorption including: 1. Urine DPD, a marker of bone resorption, and 2. Serum osteocalcin levels, a marker of anabolic bone formation. Consistent with decreased osteoblastic activity, gene expression of osteocalcin in calvarial bone of neonatal mice was significantly decreased in the GIT2−/− mice compared to WT mice (Supplementary Figure 2A). Moreover, there was a significant increase in calvarial mRNA expression of the protein modulators of OC differentiation and proliferation, RANKL and OPG (Supplementary Figure 2B–C), suggesting a compensatory response to decreased osteoblastic activity. Consistent with decreased bone resorption, urine calcium excretion was significantly decreased in GIT−/− mice (Supplementary Figure 2D). Serum calcium and PTH levels were similar in WT and GIT−/− mice (Supplementary Figure 2E–F).
OB differentiation from MSC precursors is attenuated in the absence of GIT2
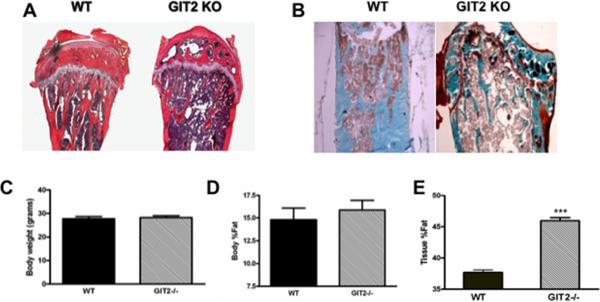
Histologic examination of proximal tibias revealed a significant increase in marrow fat (Figure 3A–B) in the GIT2−/− bone compared to WT. There was no significant difference in body weight or the percentage of whole body fat between the GIT2−/− mice and WT mice (Figure 3C–D). However, quantitation of fat in vertebrae isolated from GIT2−/− and WT mice by DEXA revealed significant increase in the fat percentage in the GIT2−/− compared to WT mice (Figure 3E). Examination of adipogenic gene expression in calvarial bone showed a significant increase in the marker of adipocyte differentiation LPL as well as a gene product of mature adipocytes aP2 in GIT2−/− mice compared to WT mice (Supplementary Figure 3A–B). In contrast, expression of PPARγ2 was unaffected by GIT2 expression (Supplementary Figure 3C).
Fig. 3. GIT2−/− mice have increased marrow fat.
Representative (A) decalcified H and E stained sections of proximal tibia and (B) non-decalcified trichrome stained sections of distal femur isolated from adult male WT and GIT2−/− mice. Comparison of (C) body weight, (D) percentage of whole body fat (Body %fat), and (E) percentage of bone tissue fat (Tissue %fat) between male WT and GIT2−/−. Data represent the mean ± SEM from at least 7 mice. (***, P < 0.001 compared with WT).
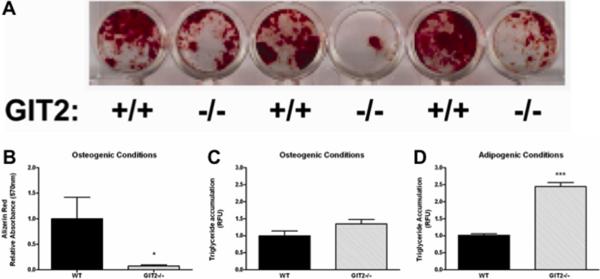
We next investigated the capacity of GIT2−/− bone marrow MSCs to differentiate into OBs or adipocytes ex vivo. MSCs were exposed to either OB differentiating media or adipocyte differentiating media and then stained with either alizarin red to assess OB mineralization or Oil Red O to assess adipogenesis. As shown in Figure 4A–B, in vitro OB differentiation is dramatically attenuated in MSCs derived from GIT2−/− mice compared to WT mice when cultured in OB differentiating media. There was no difference in adipocyte differentiation between WT and GIT2−/− MSCs grown in OB differentiating media (Figure 3C). In contrast, MSCs isolated from GIT2−/− mice exhibited significantly increased adipogenesis compared to WT mice when cultured in adipocyte differentiating media (Figure 6D). Consistent with a defect in osteoblastogenesis, gene expression of key transcriptional regulators of early OB differentiation (RUNX2 and Osterix) were significantly decreased in neonatal calvaria in GIT1−/− mice compared to WT mice (Supplementary Figure 4A–B). Moreover, markers of OB development and function including Col1a and alkaline phosphatase (Figure 6G–H) were both significantly decreased in GIT2−/− mice compared to WT mice.
Fig. 4. OB differentiation of MSCs is attenuated in GIT2−/− mice.
(A) Representative alizarin red stained MSCs cultured in OB differentiating media for 28 days. (B) MSCs cultured in OB differentiating media were assayed for OB differentiation by alizarin red staining. (C) MSCs cultured in OB differentiating media were assayed for adipocyte differentiation by measuring relative triglyceride content. (D) MSCs cultured in adipocyte differentiating media were assayed for adipocyte differentiation by measuring relative triglyceride content. Data represent the mean ± SEM from at least 5 mice. (***, P < 0.001; *, P<0.05 compared with WT).
Discussion
The results in this study provide evidence of a role for GIT2 in the modulation of both bone formation and resorption in vivo. The absence of GIT2 favors MSC differentiation into the adipocyte lineage at the expense of OB development, leading to reduced number and function of mature OBs and increased marrow adiposity. At the same time, OC function is impaired, reflected in decreased levels of bone turnover markers despite an increase in OC number. Despite impaired OC function, the net effect is osteopenia in both cortical and trabecular bone. The mechanistic basis for the OB and OC phenotype likely involves the role of GIT2 in the regulation of cytoskeletal dynamics.
GIT2 is an ARF-GAP scaffold protein known to regulate GPCR internalization and resensitization [1,4]. The involvement GPCRs, such as the PTH receptor, calcium sensing receptor, and prostaglandin receptors to bone and calcium metabolism is well recognized [22,23,24]. Modulators of GPCR internalization and desensitization such as β-arrestin2 have been shown to be critical to PTH receptor stimulated bone formation and bone resorption [20,25,26]. The involvement of GIT2 in β-arrestin mediated internalization and role of desensitization suggests a potential role for GIT2 in modulating bone metabolism. Despite gross similarities between β-arrestin2−/− [20] and GIT2−/− mice, such as significantly increased OC number compared to WT counterparts, the direct involvement of GIT2 in GPCR-stimulated effects on bone metabolism remains to be investigated.
In addition to regulating GPCR internalization and resensitization, GIT proteins have been extensively investigated for their participation in focal adhesion dynamics, cytoskeletal organization, and cell migration [5,6], processes now recognized as critical regulators of OB differentiation (10–12). Through the formation of GIT/PIX complexes, GIT proteins form a regulatory scaffold that controls both the activity and subcellular localization of the cytoskeletal regulatory small GTPases, Rac1 and Cdc42 [2,3]. We find that GIT2−/− mice have significantly decreased trabecular and cortical bone mass, suggesting a dominant bone formation defect. GIT2−/− mice have a significant decrease in OB number, which may be explained by decreased osteoblastogenesis. MSCs are multipotent cells isolated from bone marrow that possess the ability to differentiate into OBs, chondrocytes, or adipocytes [27,28]. A combination of physical, chemical, and biological cues present in the stem cell microenvironment have been implicated as directors of stem cell differentiation in vivo [29,30,31]. Our results demonstrate in vitro, that GIT2−/− MSC differentiation to OBs is significantly impaired, and in vivo GIT2−/− mice have a markedly decreased expression of key regulators of OB differentiation such as RUNX2 and OSX. Impaired OB differentiation may also explain the significant increase in RANKL and OPG expression, factors produced by immature OBs and stromal cells that control OC recruitment and differentiation [32,33]. Taken together, these results support the conclusion that GIT2 is essential to promote osteogenesis.
These findings are also consistent with recent studies showing that physical forces influenced by cytoskeletal organization directing cell shape and size are critical in determining the fate of adult stem cells [12,13,14,34,35,36,37]. For example, cell shape can regulates MSC commitment to an adipogenic or osteoblastic phenotype through actin-myosin- generated tension via RhoA signaling [14]. While the role of GIT2 in this process has not been studied directly, cellular tension required for motility and spreading is dependent on GIT2 via tyrosine phosphorylation of PKL/GIT2 via Src and FAK kinases [38,39]. In MSCs, the actin cytoskeleton appears to play a critical role in the transduction of mechanical signals into intracellular biochemical responses that control differentiation [40]. While the direct role of GIT2 in mechanotransduction remains to be determined, our current findings, taken together with previous studies showing that cytoskeletal tension or exogenous strain favor osteogenesis over adipogenesis, further support the notion that alterations in proteins that regulate cytoskeletal dynamics, such as GITs, may influence MSC fate.
It has long been known that c-Src−/− mice develop osteopetrosis [41]. Although OC development is normal, c-Src null OCs are functionally impaired due to defective ruffled border formation. Recent in vitro work has demonstrated that Src-dependent localization of GIT2 is essential for maintaining sealing zones and OC polarity [17]. Consistent with these findings, in vivo we show that GIT2−/− mice have decreased bone resorption compared to WT animals. However the GIT2−/− mice demonstrate numerous compensatory mechanisms to maintain bone integrity and calcium homeostasis. This is illustrated by the marked increase in OC surface, and decrease in urinary calcium excretion, factors which likely compensate in part for impaired OC function and help maintain normocalcemia.
In summary, we found that GIT2 regulates bone remodeling by inhibiting both osteoblastogenesis and bone resorption which, in turn, causes a decrease in bone mass. This skeletal phenotype is associated with an increase in bone marrow adipose tissue as well as a pattern of MSC differentiation favoring an adipocytic, rather than an osteoblastic, lineage. These data suggest that GIT2 plays an essential role in regulating bone remodeling, promoting differentiation of MSCs into obsteoblasts and maintenance of normal bone mass.
Supplementary Material
Highlights
-
➢
Mice lacking the scaffolding protein GIT2 (GIT2−/− mice) have decreased bone mass
-
➢
GIT2−/− mice exhibit a decrease in osteoblasts and reduced osteoblastic activity
-
➢
GIT2−/− mice exhibit an increase in osteoclasts but decreased osteoclastic activity
-
➢
Stem cells from GIT−/− mice have impaired differentiation into mature osteoblasts
Acknowledgements
The authors would like to thank Louis M. Luttrell for discussion, helpful suggestions and critical reading of the manuscript. This work was supported by an Arthritis Foundation Investigator Award (to D.G–P). R.F.S was supported by National Institutes of Health Grant DK75688. E.R.N was supported by the National Institutes of Health Grant DK48807. F.G. was supported by NIH Grants AR50245, AG15768, AR48182, and AR48852.
Abbreviations
- (GPCR)
G protein-coupled receptor
- (GRK)
GPCR kinase
- (GIT)
GRK-interacting protein
- (ARF-GAPs)
ADP-ribosylation factor GTPase-activating proteins
- (GEF)
guanine nucleotide exchange factor
- (MSC)
mesenchymal stem cell
- (qCT)
quantitative micro-computed tomography
- (WT)
wild type
- (GIT2−/−)
mice lacking GIT2
- (DEXA)
dual-energy X-ray absorptiometry
- (BV/TV)
bone volume/total volume
- (DPD)
deoxypyridinoline
- (LPL)
lipoprotein lipase
- (aP2)
adipocyte lipid binding protein 2
- (RUNX2)
runt-related transcription factor 2
- (Col1a)
collagen1a
- (PKL)
paxillin-kinase-linker
- (FAK)
focal adhesion kinase
- (BMD)
bone mineral density
- (Tb.)
trabecular bone
- (SEM)
standard error of the mean
- (qCT)
quantitative micro-computed tomography
- (CSA)
cross sectional area
- (Oc.S)
osteoclast/bone surface
- (Ob.S)
osteoblast/bone surface
- (Cool)
Cloned-out of library
- (PAK)
p21-activated kinase
- (PIX)
PAK-interactive exchange factor
- (ERK)
extracellular-signal regulated kinase
- (MEK)
mitogen activated ERK kinase
- (c-Src)
cellular-Src
- (OB)
osteoblast
- (OC)
osteoclast
- (PKL/GIT2)
paxillin-kinase-linker
- (OSX)
Osterix
- (Col1a)
collagen1a
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors have no conflicts of interest.
References
- [1].Premont RT, Claing A, Vitale N, Freeman JLR, Pitcher JA, Patton WA, Moss J, Vaughan M, Lefkowitz RJ. beta2-Adrenergic receptor regulation by GIT1, a G protein-coupled receptor kinase-associated ADP ribosylation factor GTPase-activating protein. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:14082–14087. doi: 10.1073/pnas.95.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Premont RT, Perry SJ, Schmalzigaug R, Roseman JT, Xing Y, Claing A. The GIT/PIX complex: an oligomeric assembly of GIT family ARF GTPase-activating proteins and PIX family Rac1/Cdc42 guanine nucleotide exchange factors. Cellular Signalling. 2004;16:1001–1011. doi: 10.1016/j.cellsig.2004.02.002. [DOI] [PubMed] [Google Scholar]
- [3].Hoefen RJ, Berk BC. The multifunctional GIT family of proteins. Journal of Cell Science. 2006;119:1469–1475. doi: 10.1242/jcs.02925. [DOI] [PubMed] [Google Scholar]
- [4].Premont RT, Claing A, Vitale N, Perry SJ, Lefkowitz RJ. The GIT Family of ADP-ribosylation Factor GTPase-activating Proteins. Journal of Biological Chemistry. 2000;275:22373–22380. doi: 10.1074/jbc.275.29.22373. [DOI] [PubMed] [Google Scholar]
- [5].Manabe R.-i., Kovalenko M, Webb DJ, Horwitz AR. GIT1 functions in a motile, multi-molecular signaling complex that regulates protrusive activity and cell migration. Journal of Cell Science. 2002;115:1497–1510. doi: 10.1242/jcs.115.7.1497. [DOI] [PubMed] [Google Scholar]
- [6].Zhao Z.-s., Manser E, Loo T-H, Lim L. Coupling of PAK-Interacting Exchange Factor PIX to GIT1 Promotes Focal Complex Disassembly. Molecular and Cellular Biology. 2000;20:6354–6363. doi: 10.1128/mcb.20.17.6354-6363.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yin G, Zheng Q, Yan C, Berk BC. GIT1 Is a Scaffold for ERK1/2 Activation in Focal Adhesions. Journal of Biological Chemistry. 2005;280:27705–27712. doi: 10.1074/jbc.M502271200. [DOI] [PubMed] [Google Scholar]
- [8].Schmalzigaug R, Rodriguiz RM, Phillips LE, Davidson CE, Wetsel WC, Premont RT. Anxiety-like behaviors in mice lacking GIT2. Neuroscience Letters. 2009;451:156–161. doi: 10.1016/j.neulet.2008.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Schmalzigaug R, Rodriguiz RM, Bonner PE, Davidson CE, Wetsel WC, Premont RT. Impaired fear response in mice lacking GIT1. Neuroscience Letters. 2009;458:79–83. doi: 10.1016/j.neulet.2009.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Pang J, Hoefen R, Pryhuber GS, Wang J, Yin G, White RJ, Xu X, O'Dell MR, Mohan A, Michaloski H, Massett MP, Yan C, Berk BC. G-Protein-Coupled Receptor Kinase Interacting Protein-1 Is Required for Pulmonary Vascular Development. Circulation. 2009;119:1524–1532. doi: 10.1161/CIRCULATIONAHA.108.823997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Pang J, Xu X, Getman MR, Shi X, Belmonte SL, Michaloski H, Mohan A, Blaxall BC, Berk BC. G protein coupled receptor kinase 2 interacting protein 1 (GIT1) is a novel regulator of mitochondrial biogenesis in heart. J Mol Cell Cardiol. 2011;51:769–776. doi: 10.1016/j.yjmcc.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Guilak F, Cohen DM, Estes BT, Gimble JM, Liedtke W, Chen CS. Control of Stem Cell Fate by Physical Interactions with the Extracellular Matrix. Cell Stem Cell. 2009;5:17–26. doi: 10.1016/j.stem.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kilian KA, Bugarija B, Lahn BT, Mrksich M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proceedings of the National Academy of Sciences. 2010;107:4872–4877. doi: 10.1073/pnas.0903269107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell Shape, Cytoskeletal Tension, and RhoA Regulate Stem Cell Lineage Commitment. Developmental Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- [15].Sen B, Xie Z, Case N, Ma M, Rubin C, Rubin J. Mechanical Strain Inhibits Adipogenesis in Mesenchymal Stem Cells by Stimulating a Durable {beta}-Catenin Signal. Endocrinology. 2008;149:6065–6075. doi: 10.1210/en.2008-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Luu YK, Capilla E, Rosen CJ, Gilsanz V, Pessin JE, Judex S, Rubin CT. Mechanical Stimulation of Mesenchymal Stem Cell Proliferation and Differentiation Promotes Osteogenesis While Preventing Dietary-Induced Obesity. Journal of Bone and Mineral Research. 2009;24:50–61. doi: 10.1359/JBMR.080817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Heckel T, Czupalla C, Expirto Santo AI, Anitei M, Arantzazu Sanchez-Fernandez M, Mosch K, Krause E, Hoflack B. Src-dependent repression of ARF6 is required to maintain podosome-rich sealing zones in bone-digesting osteoclasts. Proceedings of the National Academy of Sciences. 2009;106:1451–1456. doi: 10.1073/pnas.0804464106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Menon P, Yin G, Smolock EM, Zuscik MJ, Yan C, Berk BC. GPCR kinase 2 interacting protein 1 (GIT1) regulates osteoclast function and bone mass. Journal of Cellular Physiology. 225:777–785. doi: 10.1002/jcp.22282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Schmalzigaug R, Phee H, Davidson CE, Weiss A, Premont RT. Differential Expression of the ARF GAP Genes GIT1 and GIT2 in Mouse Tissues. Journal of Histochemistry and Cytochemistry. 2007;55:1039–1048. doi: 10.1369/jhc.7A7207.2007. [DOI] [PubMed] [Google Scholar]
- [20].Gesty-Palmer D, Flannery P, Yuan L, Corsino L, Spurney R, Lefkowitz RJ, Luttrell LM. A beta-arrestin-biased agonist of the parathyroid hormone receptor (PTH1R) promotes bone formation independent of G protein activation. Sci Transl Med. 2009;1:1ra1. doi: 10.1126/scitranslmed.3000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Parfitt A, Drezner M, Glorieux F, Kanis J, Malluche H, Meunier P, Ott S, Recker R. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- [22].Chang W, Tu C, Chen T-H, Bikle D, Shoback D. The Extracellular Calcium-Sensing Receptor (CaSR) Is a Critical Modulator of Skeletal Development. Science Signaling. 2008;1:ra1. doi: 10.1126/scisignal.1159945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Blackwell KA, Raisz LG, Pilbeam CC. Prostaglandins in bone: bad cop, good cop? Trends in Endocrinology & Metabolism. 21:294–301. doi: 10.1016/j.tem.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Qin L, Raggatt LJ, Partridge NC. Parathyroid hormone: a double-edged sword for bone metabolism. Trends in Endocrinology and Metabolism. 2004;15:60–65. doi: 10.1016/j.tem.2004.01.006. [DOI] [PubMed] [Google Scholar]
- [25].Bouxsein ML, Pierroz DD, Glatt V, Goddard DS, Cavat F, Rizzoli R, Ferrari SL. beta-Arrestin2 regulates the differential response of cortical and trabecular bone to intermittent PTH in female mice. J Bone Miner Res. 2005;20:635–643. doi: 10.1359/JBMR.041204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ferrari SL, Pierroz DD, Glatt V, Goddard DS, Bianchi EN, Lin FT, Manen D, Bouxsein ML. Bone response to intermittent parathyroid hormone is altered in mice null for {beta}-Arrestin2. Endocrinology. 2005;146:1854–1862. doi: 10.1210/en.2004-1282. [DOI] [PubMed] [Google Scholar]
- [27].Meirelles L.d.S., Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. Journal of Cell Science. 2006;119:2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- [28].Crisan M, Yap S, Casteilla L, Chen C-W, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng P-N, Traas J, Schugar R, Deasy BM, Badylak S, Buhring H-J, Giacobino J-P, Lazzari L, Huard J, Péault B. A Perivascular Origin for Mesenchymal Stem Cells in Multiple Human Organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- [29].Ohlstein B, Kai T, Decotto E, Spradling A. The stem cell niche: theme and variations. Current Opinion in Cell Biology. 2004;16:693–699. doi: 10.1016/j.ceb.2004.09.003. [DOI] [PubMed] [Google Scholar]
- [30].Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- [31].Derda R, Li LY, Orner BP, Lewis RL, Thomson JA, Kiessling LL. Defined substrates for human embryonic stem cell growth identified from surface arrays. Acs Chemical Biology. 2007;2:347–355. doi: 10.1021/cb700032u. [DOI] [PubMed] [Google Scholar]
- [32].Udagawa N, Takahashi N, Jimi E, Matsuzaki K, Tsurukai T, Itoh K, Nakagawa N, Yasuda H, Goto M, Tsuda E, Higashio K, Gillespie MT, Martin TJ, Suda T. Osteoblasts/stromal cells stimulate osteoclast activation through expression of osteoclast differentiation factor/RANKL but not macrophage colony-stimulating factor. Bone. 1999;25:517–523. doi: 10.1016/s8756-3282(99)00210-0. [DOI] [PubMed] [Google Scholar]
- [33].Thomas GP, Baker SU, Eisman JA, Gardiner EM. Changing RANKL/OPG mRNA expression in differentiating murine primary osteoblasts. Journal of Endocrinology. 2001;170:451–460. doi: 10.1677/joe.0.1700451. [DOI] [PubMed] [Google Scholar]
- [34].Connelly JT, Garcia AJ, Levenston ME. Interactions between integrin ligand density and cytoskeletal integrity regulate BMSC chondrogenesis. Journal of Cellular Physiology. 2008;217:145–154. doi: 10.1002/jcp.21484. [DOI] [PubMed] [Google Scholar]
- [35].Ward DF, Salasznyk RM, Klees RF, Backiel J, Agius P, Bennett K, Boskey A, Plopper GE. Mechanical strain enhances extracellular matrix-induced gene focusing and promotes osteogenic differentiation of human mesenchymal stem cells through an extracellular-related kinase-dependent pathway. Stem Cells and Development. 2007;16:467–479. doi: 10.1089/scd.2007.0034. [DOI] [PubMed] [Google Scholar]
- [36].McBride SH, Falls T, Knothe Tate ML. Modulation of Stem Cell Shape and Fate B: Mechanical Modulation of Cell Shape and Gene Expression. Tissue Engineering Part A. 2008;14:1573–1580. doi: 10.1089/ten.tea.2008.0113. [DOI] [PubMed] [Google Scholar]
- [37].Wall ME, Rachlin A, Otey CA, Loboa EG. Human adipose-derived adult stem cells upregulate palladin during osteogenesis and in response to cyclic tensile strain. AJP - Cell Physiology. 2007;293:C1532–1538. doi: 10.1152/ajpcell.00065.2007. [DOI] [PubMed] [Google Scholar]
- [38].Yu JA, Deakin NO, Turner CE. Paxillin-Kinase-Linker Tyrosine Phosphorylation Regulates Directional Cell Migration. Molecular Biology of the Cell. 2009;20:4706–4719. doi: 10.1091/mbc.E09-07-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Brown MC, Cary LA, Jamieson JS, Cooper JA, Turner CE. Src and FAK Kinases Cooperate to Phosphorylate Paxillin Kinase Linker, Stimulate Its Focal Adhesion Localization, and Regulate Cell Spreading and Protrusiveness. Molecular Biology of the Cell. 2005;16:4316–4328. doi: 10.1091/mbc.E05-02-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Patwari P, Lee RT. Mechanical Control of Tissue Morphogenesis. Circulation Research. 2008;103:234–243. doi: 10.1161/CIRCRESAHA.108.175331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Gregory RM. Cytokines and growth factors in the regulation of bone remodeling. Journal of Bone and Mineral Research. 1993;8:S505–S510. doi: 10.1002/jbmr.5650081315. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.