Abstract
Human depression is associated with cognitive deficits. It is critical to have valid animal models in order to investigate mechanisms and treatment strategies for these associated conditions. The goal of this study was to determine the association of cognitive dysfunction with depression-like behaviour in an animal model of depression and investigate the neural circuits underlying the behaviour. Mice that were exposed to social defeat for 14 d developed depression-like behaviour, i.e. anhedonia and social avoidance as indicated by reduced sucrose preference and decreased social interaction. The assessment of cognitive performance of defeated mice demonstrated impaired working memory in the T-maze continuous alternation task and enhanced fear memory in the contextual and cued fear-conditioning tests. In contrast, reference learning and memory in the Morris water maze test were intact in defeated mice. Neuronal activation following chronic social defeat was investigated by c-fos in-situ hybridization. Defeated mice exhibited preferential neural activity in the prefrontal cortex, cingulate cortex, hippocampal formation, septum, amygdala, and hypothalamic nuclei. Taken together, our results suggest that the chronic social defeat mouse model could serve as a valid animal model to study depression with cognitive impairments. The patterns of neuronal activation provide a neural basis for social defeat-induced changes in behaviour.
Keywords: c-fos mRNA expression, chronic social defeat, cognition, depression, fear memory, working memory
Introduction
Depression is one of the most common but serious mental illnesses. Persistent depressed mood and anhedonia are two core criteria for depression. In addition, cognitive deficits have been widely reported in depressed patients and are considered as a core element of this disorder according to DSM-IV criteria (APA, 1994). Impairments in cognitive performance in human depression have been identified across a number of cognitive domains including executive function (Baudic et al. 2004; Merriam et al. 1999; Nebes et al. 2003), working memory (Christopher & MacDonald, 2005; Rose & Ebmeier, 2006), emotional memory (Siegle et al. 2007; Surguladze et al. 2005), episodic memory (Butters et al. 2004; Porter et al. 2003) and semantic memory (Bhalla et al. 2005; Cataldo et al. 2005). Evidence suggests a positive correlation between depression severity and cognitive impairments (Austin et al. 2001; McDermott & Ebmeier, 2009). Major depression is associated with greater cognitive impairments, whereas minor depression has little or no effect on cognitive performance (Airaksinen et al. 2004). Neuroimaging studies have found that depressed patients show structural and functional changes in brain structures critical for learning and memory, such as the prefrontal cortex (Dolan et al. 1993; Drevets, 2000), and hippocampus and amygdala (Sheline et al. 1998; Videbech & Ravnkilde, 2004). For example, depressed patients showed higher amygdala activity in response to negative (but not positive) emotional stimuli that they memorized (Hamilton&Gotlib, 2008). In contrast, the response in cortical regions during a memory task is reduced in depression (Elliott et al. 1997). The neurobiological underpinnings of cognitive deficits in depression remain poorly understood. A better understanding and treatment of these disorders requires the development of valid animal models.
Stress is a predisposing risk factor for depression. Not surprisingly, a variety of experimental models of depression have utilized various stressors to induce depression-like behaviours (Bourin et al. 2001; Deussing, 2006; McArthur & Borsini, 2006). Chronic stress also impairs cognitive functions (Arnsten, 2009; Kim & Diamond, 2002; Sandi, 2004). However, very few studies have investigated the co-occurrence of depressive-like behaviour and cognitive deficits in animal models. A recent study reported that chronic mild stress induces a reduction of sucrose consumption and impairments in some aspects of learning and memory in rats (Henningsen et al. 2009). However, there are debates about the validity of the use of sucrose consumption instead of sucrose preference as a putative index of anhedonia (Forbes et al. 1996; Willner, 1997, 2005). Psychosocial stress plays an important role in many cases of human depression (Coyne & Downey, 1991; Gilbert et al. 2002; Kessler, 1997). Recent studies have validated chronic social defeat in mice as an animal model of depression (Malatynska & Knapp, 2005; Martinez et al. 1998a; Tsankova et al. 2006). In this model, mice are exposed to repeated social defeat which consistently leads to reduced sucrose preference and social interaction, mimicking anhedonia and social avoidance symptoms associated with human depression (Berton et al. 2006; Tsankova et al. 2006). Although this paradigm has been used as a valid animal model of depression by different research groups, cognitive profiling of socially defeated mice has not been reported in the literature. The goal of the present study was to determine whether chronic social defeat in mice can induce cognitive dysfunction associated with depression-like behaviour, and to investigate the neural circuits linked with depressive and cognitive behavioural changes.
Materials and methods
Animals
Male 8-wk-old C57BL/6J mice were obtained from Jackson Laboratory (USA) and male CD1 retired breeder mice were obtained from Charles River Laboratories (USA). Mice were housed in groups of five under a 12-h light/dark cycle (lights on 07:00 hours) with food and water available ad libitum, and were allowed to acclimate for at least 1 wk before beginning the experiments. All animal procedures were conducted in accordance with NIH guidelines and approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio.
Experimental design
Two sets of experimental animals were used in this study. Each set consisted of one group of animals subjected to chronic social defeat for 14 d and one group of non-defeated control mice. The first set was used to evaluate behavioural effects induced by chronic social defeat (non-defeated control group, n = 8; defeated group, n = 16). The second set was used to evaluate c-fos mRNA induction in response to social defeat (non-defeated control group, n = 3; defeated group, n = 4).
Social defeat
Social defeat was performed between 17:00 and 19:00 hours before the onset of the dark phase from days 1 to 14 using a resident-intruder paradigm as previously reported (Berton et al. 2006; Rygula et al. 2006). Retired breeder CD1 mice were used as resident aggressors. These mice were screened for consistent attack latencies in which mice were chosen only if their attack latencies were <30 s upon three consecutive screenings with a C57 intruder. CD1 resident mice were placed in each cage for 3 d to establish residency. Intruder mice were introduced to the home cage of an unfamiliar aggressive CD1 resident mouse for 10 min and physically defeated. When a serious attack occurred, we separated the C57 mouse from the CD1 aggressor by removing the C57 mouse for 1 min and then returning it to the cage with the CD1 mouse. After the 10-min social defeat session, the resident CD1 mouse and the intruder mouse were housed in one half of the cage separated by a perforated Plexiglas divider to allow visual, olfactory and auditory contact for the remainder of the 24-h period. Mice were exposed to a new resident CD1 mouse cage and subjected to social defeat each day for 14 consecutive days. Non-defeated control mice were housed two per cage in cages identical to those used for the socially defeated mice. Body weight was measured daily during the social defeat procedure. After completion of the 14-d social defeat paradigm, control and defeated C57 mice were singly housed until the end of the study.
Behavioural tests
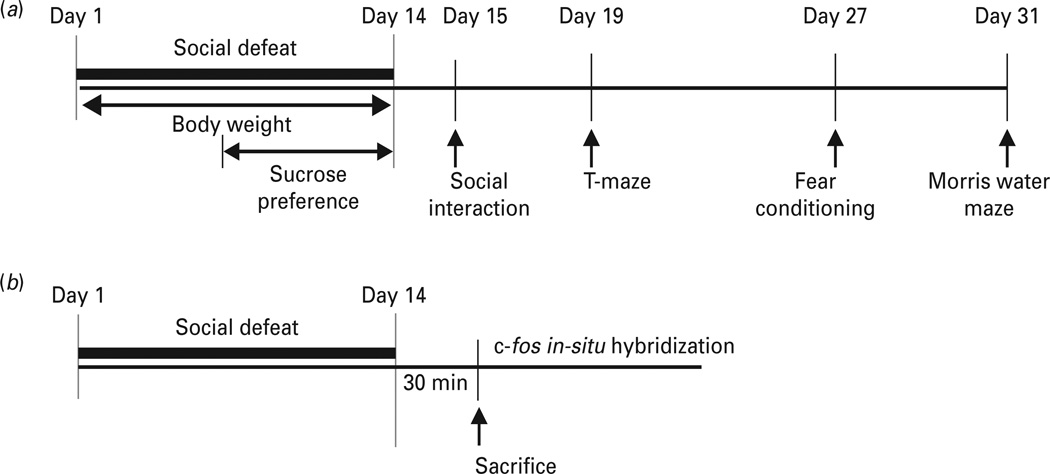
All behavioural tests were performed during the late light phase between 16:00 and 19:00 hours, except sucrose preference that was measured daily. For the same animals, depression-like behaviours were evaluated first to confirm the development of depression-like behaviour following social defeat (days 1–14). The sequence of behavioural tests were arranged to minimize the effect of one test influencing subsequent evaluation of the next test as illustrated in Fig. 1: days 7–14 for sucrose preference, day 15 for social interaction, day 19 for T-maze, day 27 for fear conditioning, day 29 for foot-shock sensitivity and hot-plate test, day 31 for Morris water maze. On the testing day, animals were transferred to the procedure room and habituated for 2 h prior to the beginning of the behavioural test.
Fig. 1.
Timeline of experimental procedures. (a) Behavioural tests. (b) c-fos mRNA study.
Sucrose preference
The sucrose preference test was performed in the housing room. Mice were first habituated to drinking from two bottles of water for 3 d in their home cage. A free choice of 1% sucrose solution and plain water was provided to the mice for 7 d starting from day 8 after the beginning of the social defeat procedure and continued until the end of social defeat. Water and sucrose intake was measured daily, and the positions of two bottles were switched daily to reduce confounds produced by side biases. Sucrose preference was calculated as a percentage of the volume of sucrose intake over the total volume of fluid intake and averaged over 7 d of testing.
Social interaction
At 24 h after the last social defeat session, a two-trial social interaction test was used to evaluate social interaction/avoidance behaviours towards an unfamiliar social target as described previously (Krishnan et al. 2008; Tsankova et al. 2006). This was performed in a 40 × 40 cm arena with infrared light illumination. The approach-avoidance behaviour of mice was digitally recorded and analysed with a video-tracking system (Noldus Information Technology, USA). Each 5-min test consisted of two sessions. In the first 2.5-min session, the mouse was allowed to explore freely in the open arena having an empty wire mesh cage (10 × 6.5 × 5 cm) apposed to one side; referred as ‘no target’. The mouse was removed and reintroduced into this area after an unfamiliar CD1 male mouse was placed into the wire mesh cage; referred as ‘with target’. The wire mesh cage allowed visual and olfactory interactions between the defeated mouse and the target, but prevented direct physical contact. The exploratory behaviour was recorded for an additional 2.5 min. The time spent in the ‘interaction zone’ (25 × 14 cm) located around where the wire mesh cage was located and the time spent in the ‘corners’ of the open area opposite to the ‘interaction zone’ were scored.
T-maze continuous alternation task
Working memory was assessed in the T-maze continuous alternation task. The T-maze consisted of a start arm (35 × 10 cm) and two identical goal arms (35 × 10 × 16 cm) arranged in the form of a T. At the beginning of the start arm, a removable wooden door separated a 10-cm-long start box from the rest of the arm. The test session consists of one forced trial and 14 subsequent free-choice trials. In the first trial, the mouse was placed in the start box and after 5 s of confinement, the door was lifted and the mouse allowed to explore the start arm and one of the goal arms. At this point, entry to the other goal arm was blocked. During 14 ‘free-choice’ trials, the mouse was able to choose freely between the left and right goal arms. After the door of the start arm was opened, the mouse was free to choose between both goal arms (all doors open). As soon as the mouse entered one goal arm (including all four feet and the tail tip), the other goal arm was closed. The mouse was returned to the closed start box, and the next free-choice trial was started after a 5-s confinement. The T-maze was thoroughly cleaned with a 20% ethanol solution before each new mouse started its session. The consecutive choices made by the mice were recorded. The overall alternation rate was calculated as a percentage (100 × number of alternations/total number of choices).
Contextual and cued fear-conditioning
Emotional memory was assessed using cued and contextual fear-conditioning. Each test consisted of a training phase followed 24 h later by the testing phase. During the training phase, mice were individually placed into the conditioning chamber inside a soundproof box and allowed to explore for 3 min after which a conditioned stimulus (CS) (an 80-dB tone) was played for 20 s. The last 2 s of the CS was paired with a 0.5-mA foot-shock. The mice were given three tone-shock pairings at 1-min intervals and then returned to their home cage. Twenty-four hours after training, contextual and cued fear-conditioning were tested. For contextual learning and memory, mice were placed back into the same conditioning chamber that was used during training for 5 min, and freezing behaviour during the 5-min re-exposure to the fear-conditioning chamber was scored. For cued learning and memory, mice were placed in a novel chamber 3 h after testing for contextual memory and allowed to explore for 3 min. The auditory cue was given after 3 min, and freezing behaviour was measured. Freezing, defined as a complete lack of movement besides respiration, was used as an indication of learning in this task and was assessed at 5-s intervals throughout the entire duration of the test.
Foot-shock sensitivity test
The sensitivity to foot-shock was evaluated in the same apparatus used for fear-conditioning training. Each mouse was placed in the same fear-conditioning chamber for 2 min to habituate. Then the animals were exposed to 1-s shocks at 30-s intervals in an order of increasing shock intensity. Electrical shocks were delivered starting at 0.08 mA and increased by 0.02 mA after each interval until reaching the maximum of 0.7 mA. The sensitivity to foot-shock was evaluated by measuring the levels of shock required to evoke their responses, including running, jumping and vocalization.
Hot-plate test
Pain sensitivity was assessed by detecting the reflexes in response to a thermal stimulus using a hot-plate test. The surface of the hot plate was heated to a constant temperature and maintained at 50 °C, 52 °C or 55 °C, respectively. Each trial began with the lower temperature and progressed towards higher temperatures. There was a 30-min interval between each hotplate trial. Mice were placed on the hot plate with a surrounding wall. The duration of the test session was a maximum of 30 s. The latency to respond with hindpaw lick, hindpaw flick, or jump was recorded. If the mouse did not respond, the test was terminated after 30 s.
Morris water maze
The water maze consisted of a circular tank of 120 cm in diameter that was filled with opaque water containing white non-toxic paint. A circular escape platform 10 cm in diameter was hidden 1 cm below the surface of the water. The pool was divided into four quadrants: north, south, east and west. A video camera was placed above the centre of the pool to record animals’ behaviour in the swimming tank. The water maze task was performed with three phases as reported previously (Malleret et al. 1999): the visible platform phase (days 1–2), the hidden platform phase (days 3–12), and the transfer phase (days 13–17). In the visible platform phase, the platform was made visible by attaching a coloured cube (3 × 3 × 20 cm) to the top of the platform. Mice were trained over 2 d with four trials per day and a 30-min inter-trial interval. The location of the platform was varied in each trial and mice were released from the wall opposite to the platform and allowed to swim for 60 s. In the hidden platform phase, mice learned the location of the hidden platform placed in a fixed location. Each mouse was subjected to four trials per day with 30-min inter-trial intervals for 10 d. On each day, mice were started in different quadrants of the swimming pool. Mice were released in a designated start position facing the tank wall and the time taken to reach the platform was recorded. In the transfer phase, the location of the hidden platform was relocated to the opposite quadrant. Animals were subjected to four trials per day with 30-min inter-trial intervals. To assess retention of the acquired information, probe trials were performed for 60 s on days 12 and 17, during which the platform was removed. Swimming of each mouse was monitored by a video camera and analysed with EthoVision software (Noldus Information Technology). Time spent in each quadrant was scored.
In situ hybridization for c-fos mRNA
Tissue preparation
Mice were subjected to 14 d of social defeat as described above. c-fos mRNA accumulation peaks at 30 min following a stressful stimulus (Cullinan et al. 1995). To measure c-fos mRNA expression in response to social defeat, mice were sacrificed by decapitation 30 min after the last social defeat. Brains were removed and snap-frozen in an isopentane/dry ice bath at −35 °C and stored at −80 °C. Brain sections were cut at a thickness of 14 µm in a cryostat and stored at −80 °C until performing in-situ hybridization.
A 375-bp cRNA probe was designed against the mouse c-fos mRNA corresponding to nucleotides 410–785 and labelled with [35S]UTP and [35S]CTP using a procedure reported in our previous studies (Garza et al. 2008; Liu et al. 2007). In-situ hybridization on brain tissue sections was performed as described previously (Garza et al. 2008; Liu et al. 2007).
A series of brain sections, 98 µm apart and anatomically matched, from each animal was used for quantification of c-fos mRNA levels. The integrated optical density of in-situ hybridization signals in various brain regions was determined using a MCID system (Imaging Research Inc., Canada). Optical density measures were defined as being 3.5 s.d. above background and were multiplied by the target area, yielding integrated optical density. Measurements were taken bilaterally using a standardized sampling box for each brain region to ensure that equivalent areas were analysed between animals. c-fos mRNA levels were quantified bilaterally in various brain regions.
Statistical analysis
Results are expressed as mean ± standard error of the mean (s.e.m.). Two-tailed Student’s t test was used for the analysis of sucrose preference, T-maze, contextual memory and c-fos induction. One-way ANOVA with repeated measures was performed on the hot-plate test, shock thresholds, and the acquisition of the Morris water maze. Two-way ANOVA was performed on social interaction, cued memory, and probe tests in the Morris water maze. Bonferroni/Dunn post-hoc comparisons followed ANOVAs. p < 0.05 was considered statistically significant.
Results
Social defeat-induced depression-like behaviour
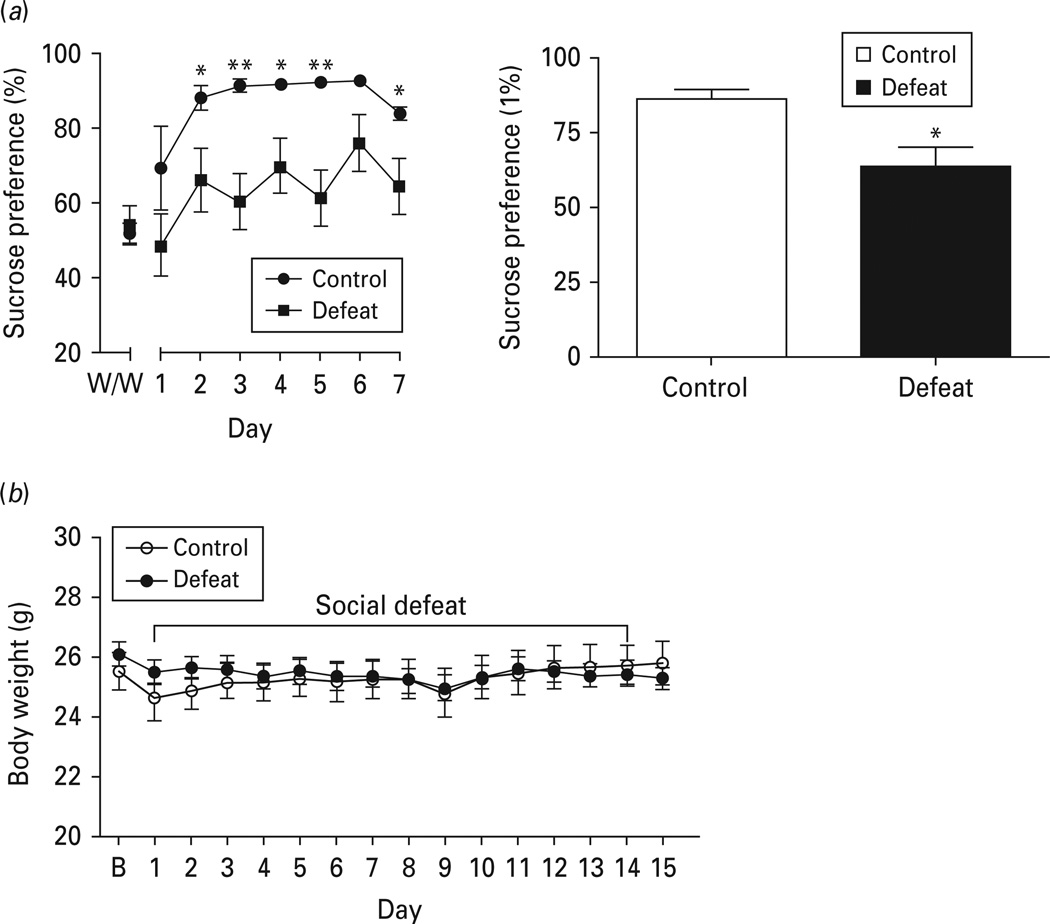
Although social defeat in mice has been previously shown to induce anhedonia as measured by sucrose preference (Berton et al. 2006; Tsankova et al. 2006), a detailed time-course of sucrose preference in response to social defeat has not been reported in the literature. The present study used a 14-d social defeat paradigm and performed a detailed analysis of the temporal effects of social defeat on hedonic responses to sucrose. Sucrose preference was measured by offering a free choice of water or 1% sucrose solution to mice for seven consecutive days beginning on day 7 of social defeat. ANOVA analysis showed a significant effect of social defeat on sucrose preference [F(1, 132)= 5.506, p < 0.05]. Post-hoc analysis revealed a significant decrease in sucrose preference measured on days 2–5 and day 7. Sucrose preference was averaged for the 7-d test (Fig. 2a). Defeated mice displayed an overall significant reduction of sucrose preference measured over 7 d compared to non-defeated control mice (p < 0.05) (Fig. 2a). Body weight was measured daily along the entire course of social defeat. We found that body weight in socially defeated mice was not altered in comparison with non-defeated controls during the social defeat period [F(1, 330) = 0.044, p = 0.836] (Fig. 2b).
Fig. 2.
Chronic social defeat induces depression-like behaviour. Mice were subjected to social defeat for 14 d. (a) Hedonic responses. Left panel: time-course of the effect of social defeat on sucrose preference. W/W (water/water), side preference for water before the sucrose preference test. Right panel: the averaged total sucrose preference over 7 d of testing. (b) Body-weight changes during social defeat. Data are expressed as mean ± s.e.m. (n = 8 for control group, n = 16 for defeated group). B, Baseline. * p < 0.05, ** p < 0.01 compared to non-defeated control mice.
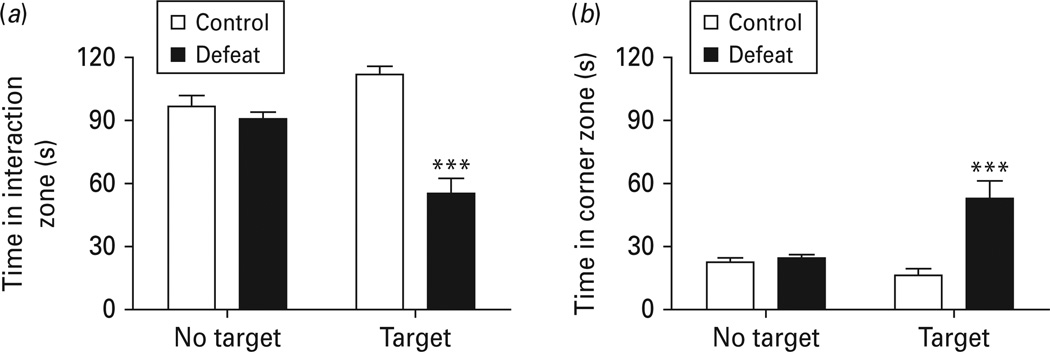
Social interaction was tested 24 h after the last social defeat session. In the absence of an aggressive CD1 mouse (no target), defeated mice and non-defeated mice spent equivalent amounts of time in the interaction zone (Fig. 3a), suggesting that social defeat does not affect general exploratory activity. In the presence of an aggressive CD1 mouse, defeated mice showed a significant reduction of the time spent in the interaction zone [F(1, 22) = 37.154, p < 0.001] and a significant increase in the time spent in the corner zone [F(1, 22) = 11.082, p < 0.005] (Fig. 3a, b). This suggests that the social defeat paradigm used in the present study effectively induces social avoidance. In some previous studies using a 10-d social defeat paradigm, defeated mice were segregated into susceptible and unsusceptible populations based upon the ratios of time spent in the interaction zone with the target to the time without the target, in which ‘susceptible’ referred to mice with an interaction ratio >100, and ‘unsusceptible’ referred to mice with an interaction ratio <100. Among 16 mice defeated for 14 d in the present study, we found 12 of them had an interaction ratio >100. This percentage of ‘ susceptiblility ’ could be attributed to the longer period of social defeat exposure in this study.
Fig. 3.
Chronic social defeat induces social withdraw. (a) Time spent in the interaction zone in the absence or presence of an aggressive CD1 social target. (b) Time spent in the corner zones in the absence or presence of an aggressive CD1 social target. Data are expressed as mean ± s.e.m. (n = 8 for control group, n = 16 for defeated group). *** p < 0.001 compared to non-defeated control mice.
Social defeat-induced cognitive dysfunction
The cognitive profile of defeated mice showing depression-like behaviour using the T-maze, fear conditioning, and Morris water maze
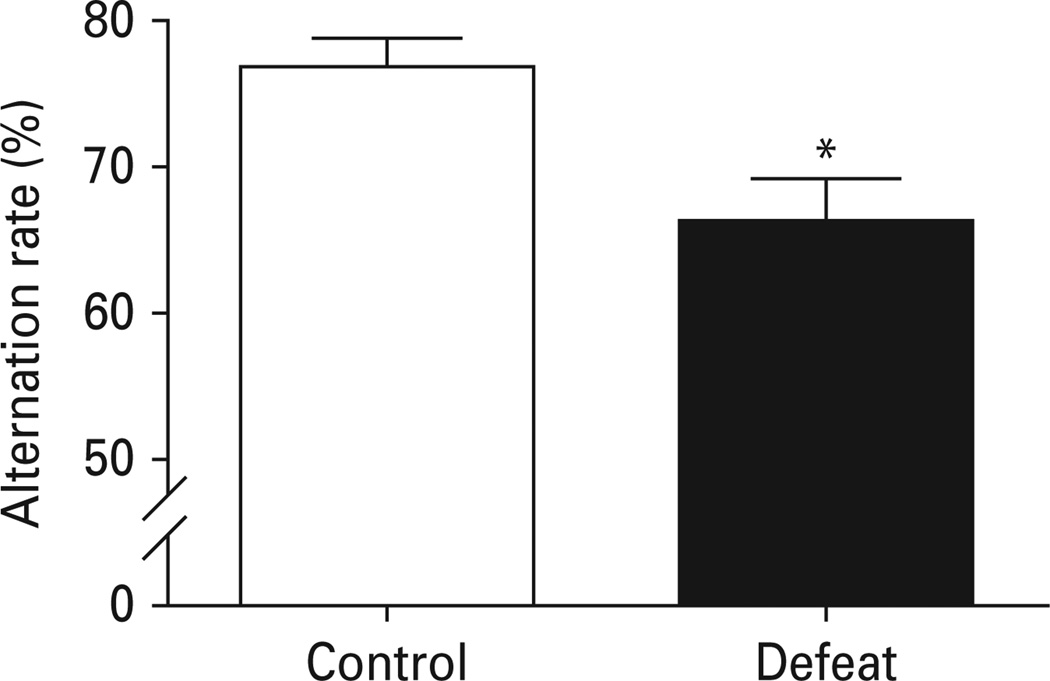
The T-maze continuous alternation task was used for assessing spatial working memory. The present study used retention intervals (5 s) within the working-memory range. We found that defeated mice exhibited a significant decrease in the alternation rate in the T-maze (p < 0.05) (Fig. 4), suggesting impairments in spatial working memory in defeated mice.
Fig. 4.
Effect of chronic social defeat on the performance of the T-maze continuous alternation task. The task consisted of one forced-choice trial and 14 free-choice trials. The percent of alternations was scored. Data are expressed as mean ± s.e.m. (n = 8 for control group, n = 16 for defeated group). * p < 0.05 compared to non-defeated control mice.
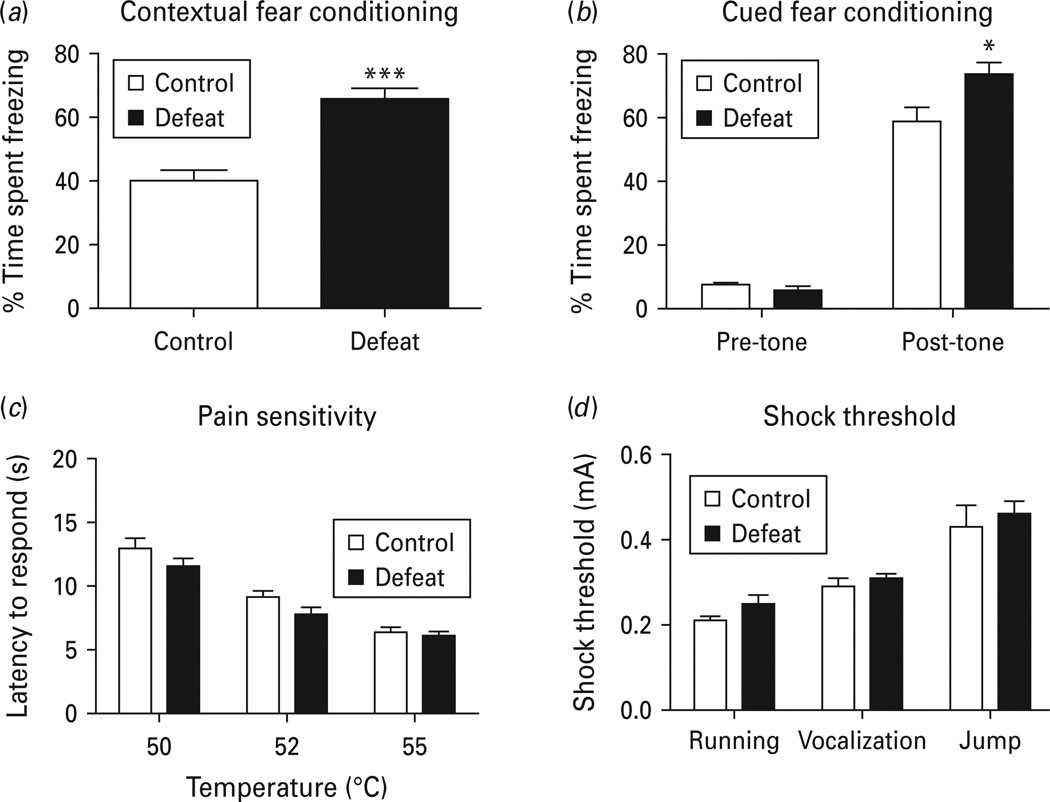
Emotional memory in defeated mice was assessed using fear conditioning, a behavioural paradigm in which the animal learns to predict aversive events. The defeated and non-defeated mice received three pairs of conditioned tone and unconditioned foot-shock in the shock chamber on the training day and were tested for the contextual conditioning and cued conditioning 24 h after the training. Defeated mice exhibited significant increases in time spent freezing in the shock chamber (p < 0.001), indicating enhanced contextual fear conditioning (Fig. 5a). Freezing behaviour in defeated mice and non-defeated controls was measured in a novel environment before and after an auditory tone was delivered. While the time of freezing before the tone did not differ between the treatments, social defeat significantly increased the time of freezing (p < 0.05), indicating enhanced cued fear conditioning (Fig. 5b). To rule out the confounding effects of pain and shock sensitivity we examined hot-plate latencies and shock thresholds in defeated mice in comparison with non-defeated controls. In the hot-plate test, the latencies to jump, lick or shake the hindpaws was not different between the control and social defeat groups (Fig. 5c) [F(1, 40) = 3.396, p = 0.08)], suggesting that pain sensitivity is not affected by chronic social defeat. In the foot-shock threshold test, defeated mice and non-defeated controls showed no difference in the thresholds of shock to evoke running, vocalization and jumping (Fig. 5d) [F(1, 26) = 1.377, p = 0.262)], suggesting the sensitivity to shock stimulus is not altered by chronic social defeat. Taken together, the results suggest that chronic social defeat predisposes mice to develop potentiated contextual fear memory and cued fear memory.
Fig. 5.
Effect of chronic social defeat on contextual and auditory-cued fear conditioning. (a) Contextual fear conditioning. Time spent freezing in response to the familiar context was measured 24 h after training. (b) Cued fear conditioning. Time spent freezing was measured in a novel environment before and after an auditory cue (a tone) was given. (c) Hot-plate test. (d) Shock sensitivity test. Data are expressed as mean ± s.e.m. (n = 8 for control group, n = 16 for defeated group). * p < 0.05, *** p < 0.001.
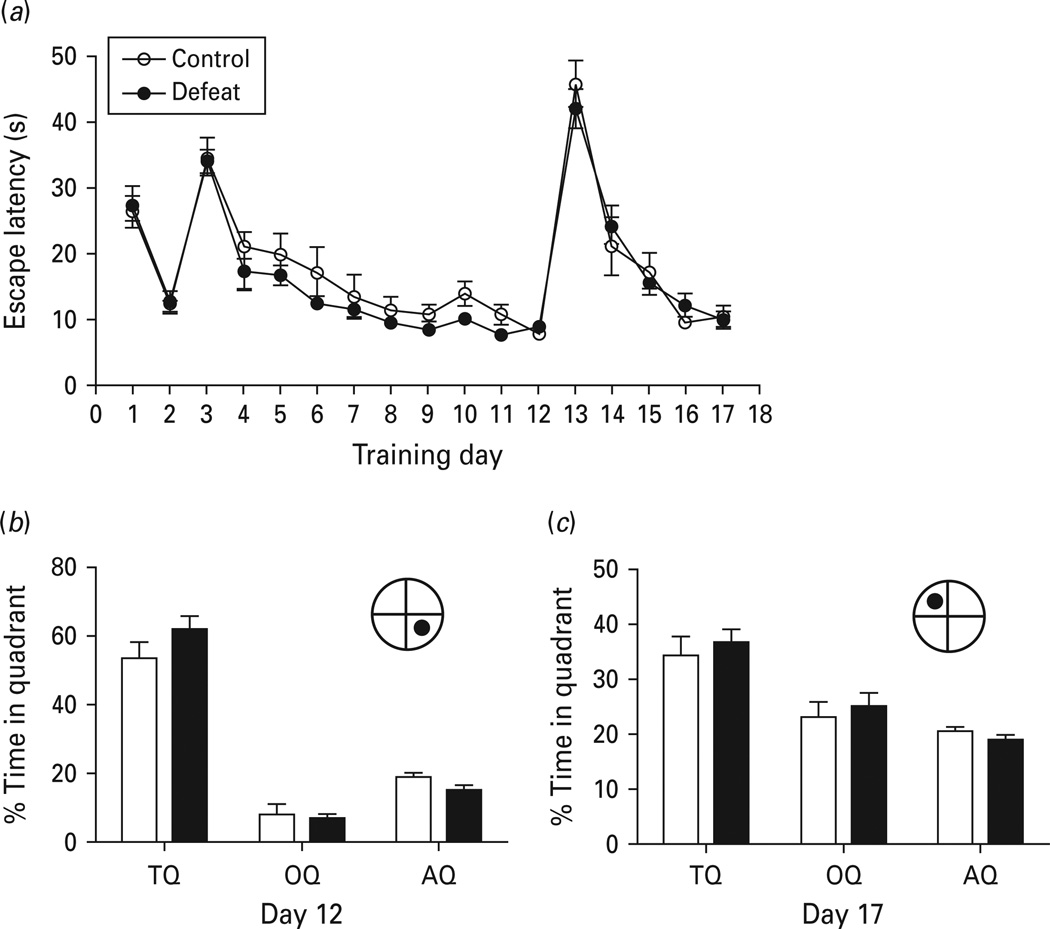
Previous studies have reported that chronic stress affects spatial reference memory (Alzoubi et al. 2009; Song et al. 2006; Sandi & Touyarot, 2006; Sterlemann et al. 2010). To determine the effect of social defeat on reference learning and memory, mice were subjected to the Morris water maze task. Mice were first subjected to the visible platform training test for 2 d to assess the performance skills necessary for the water maze tasks in defeated mice compared to non-defeated controls. Defeated mice in the visible platform training showed normal escape latencies (Fig. 6a). Subsequently, mice were assessed in the hidden platform training. The acquisition and performance of defeated mice during 10 d of hidden platform training were indistinguishable from non-defeated control [F(1, 88) = 0.002, p = 0.966]. Probe tests were performed on day 12 with the platform removed. Reference memory was not significantly affected by chronic social defeat (Fig. 6b). In addition, cognitive flexibility was assessed using a reversal learning paradigm, in which, after one location of the hidden platform was trained, the platform was moved to a different quadrant of pool. The reaction of the mouse to this change is an index of behavioural flexibility. We found that defeated mice and non-defeated controls showed similar acquisition time. The probe test on day 17 demonstrated no effect of social defeat (Fig. 6c).
Fig. 6.
Effect of chronic social defeat on reference memory and behavioural flexibility. (a) The escape latencies in Morris water maze training during visible platform (days 1–2), hidden platform (days 3–12) and reversal (days 13–17) phases. (b) Probe test on day 12. Time spent in the initial training quadrant (target quadrant, TQ), the opposite quadrant (OQ), and average of two adjacent quadrants (AQ) during probe trials on day 12 of experiment shown in panel (a). (c) Probe test on day 17 of experiment as shown in panel (a). Data are expressed as mean ± s.e.m. (n = 8 for control group, n = 16 for defeated group).
Neural activation in the brain induced by social defeat
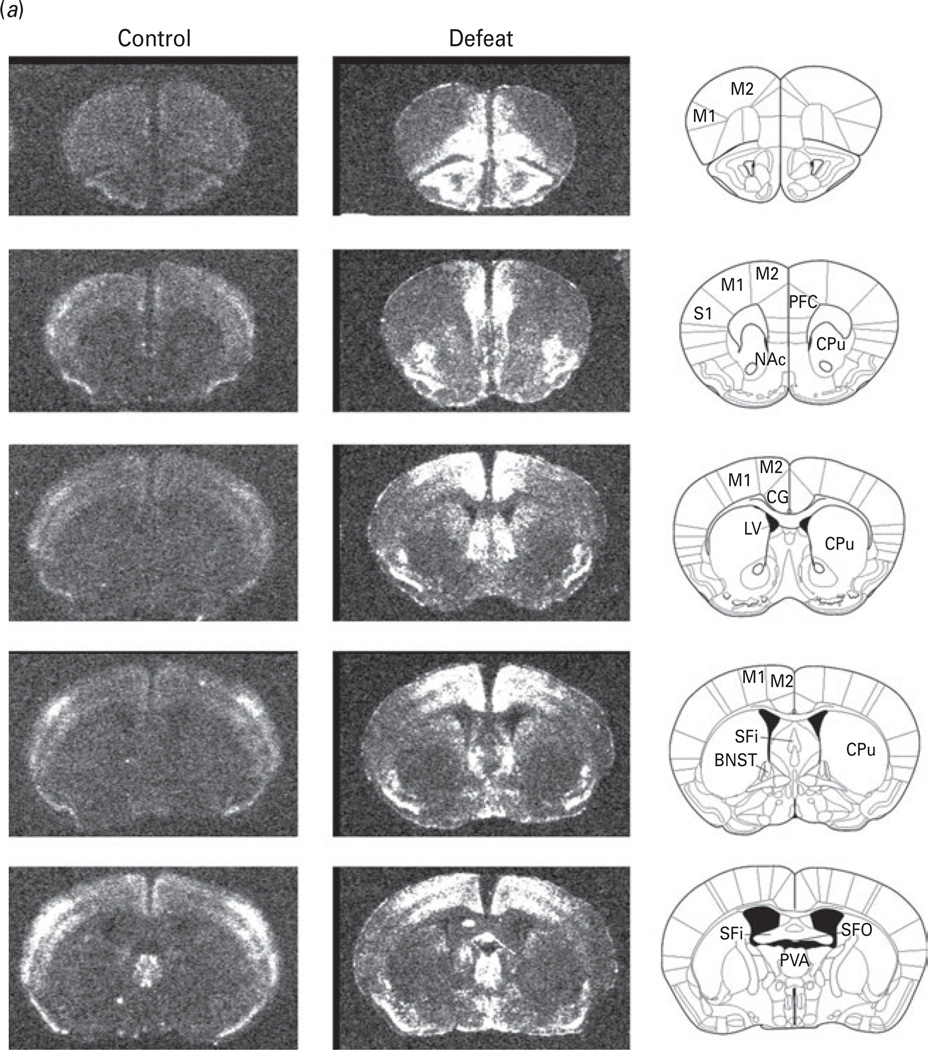
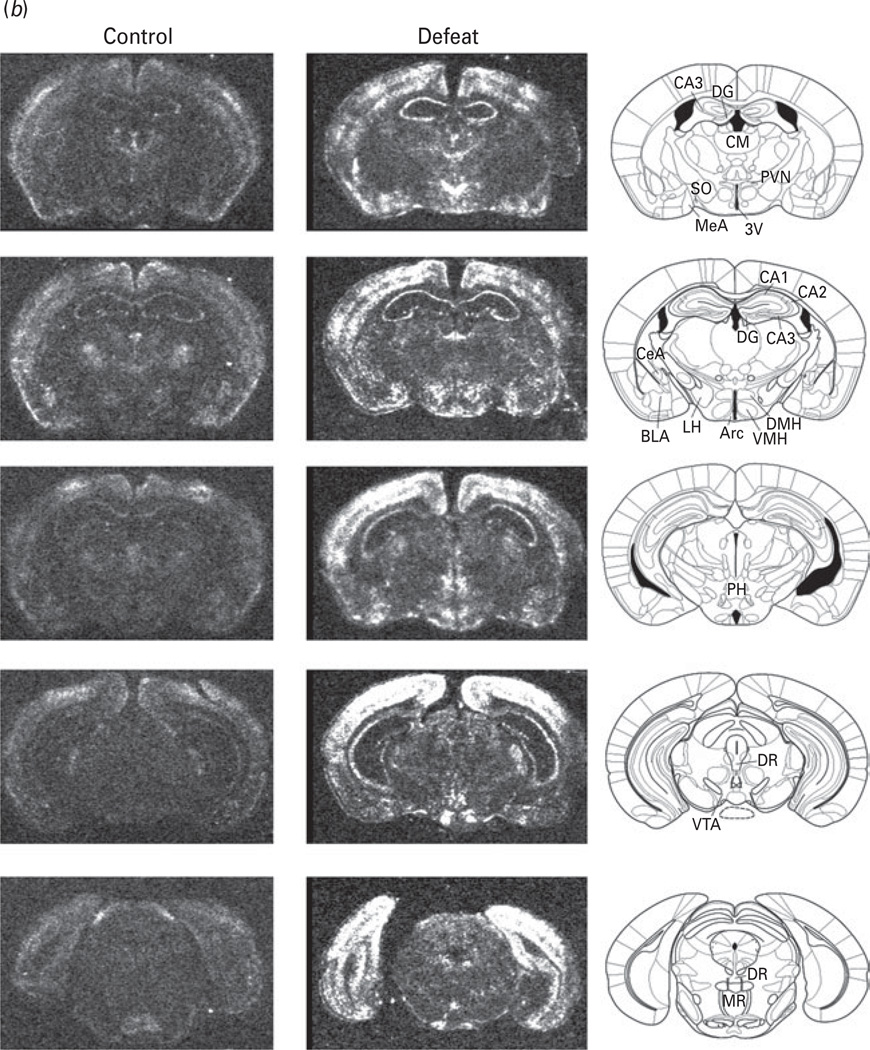
To determine neural correlates of behavioural changes induced by social defeat, we assessed mRNA expression of c-fos, a neuronal activation marker, in the mice following 14 d of social defeat compared to non-defeated controls using in-situ hybridization. c-fos mRNA distribution in the brain in response to chronic social defeat is illustrated in Fig. 7.
Fig. 7.
(a, b) Autoradiograms illustrating the distribution of c-fos mRNA expression within the selected brain regions in non-defeated control and defeated groups. Left panel, autoradiograms of coronal brain sections from non-defeated control ; middle panel, autoradiograms of coronal brain sections from defeated mice; right panel, the corresponding mouse brain atlas illustrating brain structures. 3V, 3rd ventricle ; NAc, nucleus accumbens; Arc, arcuate hypothalamic nucleus; BLA, basolateral amygdaloid nucleus; BNST, Bed nucleus of the stria terminalis ; CA1, field CA1 of hippocampus; CA2, field CA2 of hippocampus ; CA3, field CA3 of hippocampus ; CeA, central amygdaloid nucleus; CG, central grey; CM, central medial nucleus of the thalamus; CPu, caudate putamen; DG, dentate gyrus; DMH, dorsomedial nucleus of the hypothalamus; DR, dorsal raphe nucleus; LH, lateral habenula; LV, lateral ventricle ; M1, primary motor cortex ; M2, secondary motor cortex ; MeA, medial nucleus amygdala; MR, median raphe nucleus; PH, posterior hypothalamic area ; PVA, anterior periventricular nucleus of the thalamus; PVN, paraventricular nucleus; S1, primary somatosensory cortex ; Sfi, septofimbrial nucleus; SFO, subfornical organ; SO, substantia innominata; VMH, ventromedial hypothalamic nucleus; VTA, ventral tegmental area.
Non-defeated control mice showed very low or undetectable levels of c-fos expression in most brain regions, while a moderate level of c-fos mRNA expression was observed in the cerebral cortex and central medial thalamus (Table 1). c-fos mRNA levels were elevated in many forebrain structures, implicated in depression and cognitive deficits (Fu et al. 2004; Kumari et al. 2003; Sheline et al. 2001; Surguladze et al. 2005), of defeated mice compared to non-defeated controls (Table 1). An increase in c-fos mRNA was observed in the prefrontal cortex and cingulate cortex as well as in the primary motor cortex and somatosensory cortex in defeated mice. The induction of c-fos expression within the amygdaloid complex was heterogeneous. Elevation of c-fos mRNA in the medial amygdala was higher than that in the central amygdala, whereas c-fos mRNA expression was not significantly altered in the basolateral amygdala (Table 1). Within the hippocampal formation, the fields CA1 of Ammon’s horn expressed high levels of c-fos mRNA. A moderate level was observed in CA2 and dentate gyrus. Strong induction of c-fos expression was detected in the septum, especially in the lateral sector of the nucleus. In contrast, weak signals for c-fos mRNA were found in the nucleus accumbens.
Table 1.
c-fos mRNA expression in the brain in socially defeated mice and non-defeated controls
| Brain areas | Control | Social defeat | |
|---|---|---|---|
| Cortex | Prefrontal cortex | 1467 ± 527 | 35441 ± 10403* |
| Cingulate cortex | 591 ± 181 | 30365 ± 8689* | |
| Motor cortex | 444 ± 366 | 3652 ± 1804* | |
| Somatosensory cortex | 343 ± 97 | 143 ± 35 | |
| Hippocampus | CA1 | 22 ± 12 | 1457 ± 794* |
| CA2 | 12 ± 5 | 676 ± 289* | |
| CA3 | 71 ± 36 | 858 ± 472 | |
| DG | 28 ± 12 | 416 ± 135* | |
| Septum | Lateral septum | 27 ± 6 | 11000 ± 2982* |
| Medial septum | 4 ± 1 | 291 ± 29* | |
| Nucleus accumbens | 25 ± 7 | 418 ± 238 | |
| Bed nucleus of stria terminalis | 56 ± 10 | 611 ± 273 | |
| Thalamus | Central medial thalamus | 1023 ± 442 | 6842 ± 1577* |
| Paraventricular thalamic nucleus | 1533 ± 595 | 8796 ± 2837* | |
| Hypothalamus | Paraventricular nucleus | 35 ± 14 | 8764 ± 1797* |
| Lateral hypothalamus | 145 ± 114 | 5763 ± 2159 | |
| Dorsomedial hypothalamus | 5 ± 3 | 3590 ± 772* | |
| Arcuate nucleus | 4 ± 3 | 1323 ± 407* | |
| Posterior hypothalamus | 33 ± 14 | 4405 ± 1823 | |
| Supraoptic nucleus | 45 ± 33 | 4015 ± 600* | |
| Amygdala | Medial amygdala | 99 ± 62 | 4073 ± 771* |
| Central amygdala | 43 ± 28 | 743 ± 212* | |
| Basolateral amygdala | 357 ± 194 | 586 ± 284 | |
| Ventral tegmental area | 7 ± 4 | 91 ± 12 | |
| Raphe | Dorsal raphe | 25 ± 12 | 2805 ± 448* |
| Median raphe | 39 ± 11 | 864 ± 128* | |
| Pontine nucleus | 998 ± 387 | 8985 ± 1138 |
Data (integrated optical density) are expressed as mean ± s.e.m. (n = 3 for control group, n = 4 for defeated group).
p < 0.05 compared to non-defeated control mice.
Within the thalamus, only a few nuclei exhibited significant increase in c-fos mRNA expression. These nuclei included central medial thalamus and paraventricular thalamic nucleus. A number of hypothalamic subregions of defeated mice showed elevation of c-fos, including the paraventricular nucleus (PVN), dorsomedial hypothalamus, arcuate nucleus, and supraoptic nucleus. In the midbrain, the ventral tegmental area exhibited only a slight increase in c-fos mRNA expression in defeated mice. In addition, social defeat-induced c-fos mRNA expression was observed in the dorsal raphe and median raphe nuclei, and pontine nuclei.
Discussion
In this study, we have demonstrated that social defeat for 14 d produced depression-like symptoms as indicated by decreased sucrose preference and increased social avoidance. The assessment of cognitive functions in defeated mice revealed impairments in spatial working memory and potentiated contextual and cued fear memory, while spatial reference memory in defeated mice was not affected. These results suggest that chronic social defeat for 14 d can be used to model cognitive impairments associated with human depression. Furthermore, our c-fos in-situ hybridization data displayed the distribution of neuronal populations in the brain that were activated by chronic social defeat. These data provide possible mechanisms underlying depressive and cognitive behavioural changes.
Chronic social defeat has been used to induce depression-like behaviours in mice. We confirmed that mice exposed to chronic social defeat for 14 d developed depression-like symptoms indicated by social avoidance and anhedonia. One major finding of the present study was that depression-like behaviour was linked to distinct patterns of cognitive impairment in socially defeated mice. The T-maze is a widely used task which provides a measure of working-memory performance and encompasses spatial components (Deacon & Rawlins, 2006; Gerlai, 1998). During 14 free-choice trials, mice exposed to chronic social defeat displayed a decreased spontaneous alternation rate, reflecting an impairment of spatial working memory. This finding is in line with previous studies in which rats exposed to chronic mild stress or chronic water immersion stress showed impaired performance in the T-maze (Gerlai, 1998; Lai et al. 2006; Wang & Cai, 2006; Wenk, 2001). Fear conditioning is widely used to measure emotional learning and memory (Selcher et al. 2001; Shumyatsky et al. 2005; Wehner & Radcliffe, 2004). In this behavioural paradigm, animals learn the relationship between aversive events (foot-shock) and a context (shock chamber) or a stimulus (tone), and subsequently express fear responses to neutral context or stimulus. Chronic social defeat potentiated contextual and cued freezing, indicating facilitated contextual fear memory and cued fear memory. This is the first report showing that chronic social defeat potentiates fear memory. This finding is consistent with previous findings using other chronic stress paradigms in rats. It has been demonstrated that chronic mild stress for 7 wk or chronic restraint stress for 3 wk enhances fear conditioning (Henningsen et al. 2009; Sandi et al. 2001). A possible confound for interpreting fear conditioning results is pain sensitivity. This phenomenon has previously been reported in mice after exposure to chronic restraint stress, which resulted in a hyperalgesic effect (Imbe et al. 2004). However, this is unlikely to confound the results of the fear-conditioning test in the present study because pain sensitivity in chronically defeated mice was indistinguishable from non-defeated controls as measured in the hot-plate test. In addition, there was no difference in the sensitivity to foot-shock between chronically defeated mice and non-defeated mice, as indicated by the jump thresholds in response to the same foot-shock intensities. However, the possible influence of anxiety in fear responses cannot be ruled out. In contrast to impairment of spatial working memory in the T-maze continuous alternation and enhancement of fear conditioning in defeated mice, reference learning and memory in the Morris water maze test was not affected by chronic social defeat. Although depression-related behaviours persist up to 4 wk after social defeat (Berton et al. 2006; Krishnan et al. 2007), it is possible that the effects of social defeat on spatial reference learning and memory may diminish in time after the cessation of social defeat and become undetectable after a certain time period. To our knowledge, the effect of repeated social defeat on reference learning and memory has not been previously reported in the literature. However, impairments in reference learning and memory have been demonstrated in rats or mice subjected to other stress paradigms. For instance, exposure of rats or mice to chronic unpredictable/variable/mild stress for 28 d or 40 d results in impairments in spatial memory in the water maze (Sandi & Touyarot, 2006; Song et al. 2006; Vasconcellos et al. 2003). Moreover, rats that experienced maternal deprivation for the first 3 wk of life have been shown to have learning impairments in the water maze (Aisa et al. 2007). Nonetheless, stress can produce diverse effects on cognitive functions depending on stress paradigms, intensity and duration as well as learning tasks and intervals between stress exposure and learning/memory tests (Sandi & Pinelo-Nava, 2007).
The neuronal activity in response to repeated exposure to social defeat was investigated by c-fos in-situ hybridization. In comparison to control mice, c-fos mRNA levels were elevated in selective brain regions of chronically defeated mice following an acute challenge. Such brain areas include the prefrontal cortex, cingulated cortex, hippocampus and subdivisions of the amygdala; these limbic structures have been implicated in a wide variety of emotional, cognitive and behavioural control processes. Maintaining high levels of c-fos mRNA in these areas may reflect that their neural responses do not adapt to repeated exposure to social defeat. Several studies have examined the region-specific patterns of adaptation by comparing c-fos expression after an acute defeat with that following chronic social defeat (Kollack-Walker et al. 1999; Martinez et al. 1998b; Matsuda et al. 1996). Acute defeat in rats or hamsters induces a widespread pattern of neuronal activation (Kollack-Walker et al. 1999; Martinez et al. 1998b), similar to those brain areas activated by other types of stressors such as restraint, immobilization, electrical foot-shock, swimming and noise (Martinez et al. 2002). However, c-fos expression patterns following repeated social defeat differ. For example, the PVN and medial amygdala, but not lateral septum, show increased c-fos expression following 10 d of social defeat in comparison to control groups (Martinez et al. 1998b). In contrast, an increase in c-fos mRNA in the lateral septum, but not in the PVN and medial amygdala, was found in hamsters after 7 d of social defeat (Kollack-Walker et al. 1999). In the present study, mice that were exposed to 14 d of social defeat displayed an increase in c-fos expression in all three areas. The discrepancies between these studies are not clear, but variations in the length and procedures of defeat, species, housing conditions and intervals between the last defeat and detection of c-fos levels are possible underlying factors.
The neuronal activation pattern after chronic social defeat may represent evidence for a prerequisite for behavioural changes induced by social defeat. It is known that different limbic brain regions are involved in mediating distinct cognitive dysfunctions. For instance, T-maze continuous alternation can be attributed to the prefrontal cortex as well as hippocampus (Gerlai, 1998; Le Marec et al. 2002; Wang & Cai, 2006; Wenk, 2001), while fear conditioning is engaged with the hippocampus and amygdala (Kim & Jung, 2006; Wehner & Radcliffe, 2004). The amygdala is believed to be involved in both contextual and cued memory, whereas the hippocampus is associated with contextual memory only (Rudy et al. 2004; Sandi et al. 2001). These structures have also been implicated in depression (Davidson et al. 2002; Drevets, 2003; Marvel & Paradiso, 2004) and they may represent common brain areas that mediate both depression-like behaviours and cognitive dysfunctions induced by social defeat. However, given the interval between c-fos mRNA detection and different behavioural tests, further investigations are needed to confirm the link between social defeat-induced neuronal activation and various behavioural deficits. It is noteworthy to point out that the absence of c-fos expression may not necessarily mean that neurons are not involved in the behavioural response. It is possible that other species of immediate early genes may be expressed in these neurons.
Cognitive dysfunction is common in patients with major depression (Castaneda et al. 2008; Thomas & Elliott, 2009; Thomas & O’Brien, 2008). Working memory is impaired in depression (Austin et al. 2001; Rose & Ebmeier, 2006); however, individuals with major depression often have better memory for negative stimuli than for neutral or positive ones (Davidson et al. 2002; Drevets, 2001, 2003). Brain-imaging studies have found that depressed patients show a greater amygdala response to negative stimuli than controls (Fu et al. 2004; Sheline et al. 2001; Surguladze et al. 2005). In contrast, decreased responses in the medial frontal gyrus, anterior cingulate cortex, and hippocampus for positive stimuli have been observed in depressed patients (Kumari et al. 2003). Our findings of impaired working memory and enhanced fear memory in defeated mice with anhedonia and social withdrawal suggest that chronic social defeat in mice can serve as a valid model to study cognitive dysfunction in depression and their neurobiological underpinnings. In addition, our results of neuronal activation patterns in the brain provide a neural basis for affective and cognitive behavioural abnormalities.
Acknowledgements
This work was supported by NIH grants MH073844 and MH076929 (X.Y.L.), NS056237 (W.Z.) and the China Scholarship Council (T.Y.).
Footnotes
Statement of Interest
None.
References
- Airaksinen E, Larsson M, Lundberg I, Forsell Y. Cognitive functions in depressive disorders : evidence from a population-based study. Psychological Medicine. 2004;34:83–91. doi: 10.1017/s0033291703008559. [DOI] [PubMed] [Google Scholar]
- Aisa B, Tordera R, Lasheras B, Del Rio J, et al. Cognitive impairment associated to HPA axis hyperactivity after maternal separation in rats. Psychoneuroendocrinology. 2007;32:256–266. doi: 10.1016/j.psyneuen.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Alzoubi KH, Abdul-Razzak KK, Khabour OF, Al-Tuweiq GM, et al. Adverse effect of combination of chronic psychosocial stress and high fat diet on hippocampus-dependent memory in rats. Behavioural Brain Research. 2009;204:117–123. doi: 10.1016/j.bbr.2009.05.025. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT. Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews Neuroscience. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin MP, Mitchell P, Goodwin GM. Cognitive deficits in depression: possible implications for functional neuropathology. British Journal of Psychiatry. 2001;178:200–206. doi: 10.1192/bjp.178.3.200. [DOI] [PubMed] [Google Scholar]
- Baudic S, Tzortzis C, Barba GD, Traykov L. Executive deficits in elderly patients with major unipolar depression. Journal of Geriatric Psychiatry and Neurology. 2004;17:195–201. doi: 10.1177/0891988704269823. [DOI] [PubMed] [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Bhalla RK, Butters MA, Zmuda MD, Seligman K, et al. Does education moderate neuropsychological impairment in late-life depression? International Journal of Geriatric Psychiatry. 2005;20:413–417. doi: 10.1002/gps.1296. [DOI] [PubMed] [Google Scholar]
- Bourin M, Fiocco AJ, Clenet F. How valuable are animal models in defining antidepressant activity? Human Psychopharmacology : Clinical and Experimental. 2001;16:9–21. doi: 10.1002/hup.178. [DOI] [PubMed] [Google Scholar]
- Butters MA, Whyte EM, Nebes RD, Begley AE, et al. The nature and determinants of neuropsychological functioning in late-life depression. Archives of General Psychiatry. 2004;61:587–595. doi: 10.1001/archpsyc.61.6.587. [DOI] [PubMed] [Google Scholar]
- Castaneda AE, Tuuio-Henriksson A, Marttunen M, Suvisaari J, et al. A review on cognitive impairments in depressive and anxiety disorders with a focus on young adults. Journal of Affective Disorders. 2008;106:1–27. doi: 10.1016/j.jad.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Cataldo MG, Nobile M, Lorusso ML, Battaglia M, et al. Impulsivity in depressed children and adolescents : a comparison between behavioral and neuropsychological data. Psychiatry Research. 2005;136:123–133. doi: 10.1016/j.psychres.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Christopher G, MacDonald J. The impact of clinical depression on working memory. Cognitive Neuropsychiatry. 2005;10:379–399. doi: 10.1080/13546800444000128. [DOI] [PubMed] [Google Scholar]
- Coyne JC, Downey G. Social factors and psychopathology : stress, social support, and coping processes. Annual Review of Psychology. 1991;42:401–425. doi: 10.1146/annurev.ps.42.020191.002153. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Battaglia DF, Akil H, et al. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience. 1995;64:477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Lewis DA, Alloy LB, Amaral DG, et al. Neural and behavioral substrates of mood and mood regulation. Biological Psychiatry. 2002;52:478–502. doi: 10.1016/s0006-3223(02)01458-0. [DOI] [PubMed] [Google Scholar]
- Deacon RM, Rawlins JN. T-maze alternation in the rodent. Nature Protocols. 2006;1:7–12. doi: 10.1038/nprot.2006.2. [DOI] [PubMed] [Google Scholar]
- Deussing JM. Animal models of depression. Drug Discovery Today: Disease Models. 2006;3:375–383. [Google Scholar]
- Dolan RJ, Bench CJ, Liddle PF, Friston KJ, et al. Dorsolateral prefrontal cortex dysfunction in the major psychoses; symptom or disease specificity? Journal of Neurology, Neurosurgery & Psychiatry. 1993;56:1290–1294. doi: 10.1136/jnnp.56.12.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC. Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Progress in Brain Research. 2000;126:413–431. doi: 10.1016/S0079-6123(00)26027-5. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Current Opinion in Neurobiology. 2001;11:240–249. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging abnormalities in the amygdala in mood disorders. Annals of the New York Academy of Sciences. 2003;985:420–444. doi: 10.1111/j.1749-6632.2003.tb07098.x. [DOI] [PubMed] [Google Scholar]
- Elliott R, Sahakian BJ, Herrod JJ, Robbins TW, et al. Abnormal response to negative feedback in unipolar depression: evidence for a diagnosis specific impairment. Journal of Neurology, Neurosurgery & Psychiatry. 1997;63:74–82. doi: 10.1136/jnnp.63.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes NF, Stewart CA, Matthews K, Reid IC. Chronic mild stress and sucrose consumption: Validity as a model of depression. Physiology & Behavior. 1996;60:1481–1484. doi: 10.1016/s0031-9384(96)00305-8. [DOI] [PubMed] [Google Scholar]
- Fu CH, Williams SC, Cleare AJ, Brammer MJ, et al. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Archives of General Psychiatry. 2004;61:877–889. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- Garza JC, Kim CS, Liu J, Zhang W, et al. Adeno-associated virus-mediated knockdown of melanocortin-4 receptor in the paraventricular nucleus of the hypothalamus promotes high-fat diet-induced hyperphagia and obesity. Journal of Endocrinology. 2008;197:471–482. doi: 10.1677/JOE-08-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlai R. A new continuous alternation task in T-maze detects hippocampal dysfunction in mice. A strain comparison and lesion study. Behavioural Brain Research. 1998;95:91–101. doi: 10.1016/s0166-4328(97)00214-3. [DOI] [PubMed] [Google Scholar]
- Gilbert P, Allan S, Brough S, Melley S, et al. Relationship of anhedonia and anxiety to social rank, defeat and entrapment. Journal of Affective Disorders. 2002;71:141–151. doi: 10.1016/s0165-0327(01)00392-5. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Gotlib IH. Neural substrates of increased memory sensitivity for negative stimuli in major depression. Biological Psychiatry. 2008;63:1155–1162. doi: 10.1016/j.biopsych.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningsen K, Andreasen JT, Bouzinova EV, Jayatissa MN, et al. Cognitive deficits in the rat chronic mild stress model for depression: relation to anhedonic-like responses. Behavioural Brain Research. 2009;198:136–141. doi: 10.1016/j.bbr.2008.10.039. [DOI] [PubMed] [Google Scholar]
- Imbe H, Murakami S, Okamoto K, Iwai-Liao Y, et al. The effects of acute and chronic restraint stress on activation of ERK in the rostral ventromedial medulla and locus coeruleus. Pain. 2004;112:361–371. doi: 10.1016/j.pain.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Kessler RC. The effects of stressful life events on depression. Annual Review of Psychology. 1997;48:191–214. doi: 10.1146/annurev.psych.48.1.191. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nature Reviews Neuroscience. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Jung MW. Neural circuits and mechanisms involved in Pavlovian fear conditioning: A critical review. Neuroscience and Biobehavioral Reviews. 2006;30:188–202. doi: 10.1016/j.neubiorev.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollack-Walker S, Don C, Watson SJ, Akil H. Differential expression of c-fos mRNA within neurocircuits of male hamsters exposed to acute or chronic defeat. Journal of Neuroendocrinology. 1999;11:547–559. doi: 10.1046/j.1365-2826.1999.00354.x. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Mazei-Robison M, Iniguez SD, et al. AKT Signaling within the ventral tegmental area regulates cellular and behavioral responses to stressful stimuli. Biological Psychiatry. 2008;64:691–700. doi: 10.1016/j.biopsych.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari V, Mitterschiffthaler MT, Teasdale JD, Malhi GS, et al. Neural abnormalities during cognitive generation of affect in treatment-resistant depression. Biological Psychiatry. 2003;54:777–791. doi: 10.1016/s0006-3223(02)01785-7. [DOI] [PubMed] [Google Scholar]
- Lai WS, Xu B, Westphal KGC, Paterlini M, et al. Akt1 deficiency affects neuronal morphology and predisposes to abnormalities in prefrontal cortex functioning. Proceedings of the National Academy of Sciences USA. 2006;103:16906–16911. doi: 10.1073/pnas.0604994103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Marec N, Ethier K, Rompre PP, Godbout R. Involvement of the medial prefrontal cortex in two alternation tasks using different environments. Brain and Cognition. 2002;48:432–436. [PubMed] [Google Scholar]
- Liu J, Garza JC, Truong HV, Henschel J, et al. The melanocortinergic pathway is rapidly recruited by emotional stress and contributes to stress-induced anorexia and anxiety-like behavior. Endocrinology. 2007;148:5531–5540. doi: 10.1210/en.2007-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malatynska E, Knapp RJ. Dominant-submissive behavior as models of mania and depression. Neuroscience and Biobehavioral Reviews. 2005;29:715–737. doi: 10.1016/j.neubiorev.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Malleret G, Hen R, Guillou JL, Segu L, et al. 5-HT1B receptor knock-out mice exhibit increased exploratory activity and enhanced spatial memory performance in the Morris water maze. Journal of Neuroscience. 1999;19:6157–6168. doi: 10.1523/JNEUROSCI.19-14-06157.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez M, Calvo-Torrent A, Herbert J. Mapping brain response to social stress in rodents with c-fos expression : a review. Stress. 2002;5:3–13. doi: 10.1080/102538902900012369. [DOI] [PubMed] [Google Scholar]
- Martinez M, Calvo-Torrent A, Pico-Alfonso MA. Social defeat and subordination as models of social stress in laboratory rodents: A review. Aggressive Behavior. 1998a;24:241–256. [Google Scholar]
- Martinez M, Phillips PJ, Herbert J. Adaptation in patterns of c-fos expression in the brain associated with exposure to either single or repeated social stress in male rats. European Journal of Neuroscience. 1998b;10:20–33. doi: 10.1046/j.1460-9568.1998.00011.x. [DOI] [PubMed] [Google Scholar]
- Marvel CL, Paradiso S. Cognitive and neurological impairment in mood disorders. Psychiatric Clinics of North America. 2004;27:19–36. doi: 10.1016/S0193-953X(03)00106-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda S, Peng H, Yoshimura H, Wen TC, et al. Persistent c-fos expression in the brains of mice with chronic social stress. Neuroscience Research. 1996;26:157–170. [PubMed] [Google Scholar]
- McArthur R, Borsini F. Animal models of depression in drug discovery : a historical perspective. Pharmacology Biochemistry and Behavior. 2006;84:436–452. doi: 10.1016/j.pbb.2006.06.005. [DOI] [PubMed] [Google Scholar]
- McDermott LM, Ebmeier KP. A meta-analysis of depression severity and cognitive function. Journal of Affective Disorders. 2009;119:1–8. doi: 10.1016/j.jad.2009.04.022. [DOI] [PubMed] [Google Scholar]
- Merriam EP, Thase ME, Haas GL, Keshavan MS, et al. Prefrontal cortical dysfunction in depression determined by Wisconsin Card Sorting Test performance. American Journal of Psychiatry. 1999;156:780–782. doi: 10.1176/ajp.156.5.780. [DOI] [PubMed] [Google Scholar]
- Nebes RD, Pollock BG, Houck PR, Butters MA, et al. Persistence of cognitive impairment in geriatric patients following antidepressant treatment: a randomized, double-blind clinical trial with nortriptyline and paroxetine. Journal of Psychiatric Research. 2003;37:99–108. doi: 10.1016/s0022-3956(02)00085-7. [DOI] [PubMed] [Google Scholar]
- Porter RJ, Gallagher P, Thompson JM, Young AH. Neurocognitive impairment in drug-free patients with major depressive disorder. British Journal of Psychiatry. 2003;182:214–220. doi: 10.1192/bjp.182.3.214. [DOI] [PubMed] [Google Scholar]
- Rose EJ, Ebmeier KP. Pattern of impaired working memory during major depression. Journal of Affective Disorders. 2006;90:149–161. doi: 10.1016/j.jad.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Huff NC, Matus-Amat P. Understanding contextual fear conditioning: insights from a two-process model. Neuroscience and Biobehavioral Reviews. 2004;28:675–685. doi: 10.1016/j.neubiorev.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Rygula R, Abumaria N, Flugge G, Hiemke C, et al. Citalopram counteracts depressive-like symptoms evoked by chronic social stress in rats. Behavioural Pharmacology. 2006;17:19–29. doi: 10.1097/01.fbp.0000186631.53851.71. [DOI] [PubMed] [Google Scholar]
- Sandi C. Stress, cognitive impairment and cell adhesion molecules. Nature Reviews Neuroscience. 2004;5:917–930. doi: 10.1038/nrn1555. [DOI] [PubMed] [Google Scholar]
- Sandi C, Merino JJ, Cordero MI, Touyarot K, et al. Effects of chronic stress on contextual fear conditioning and the hippocampal expression of the neural cell adhesion molecule, its polysialylation, and L1. Neuroscience. 2001;102:329–339. doi: 10.1016/s0306-4522(00)00484-x. [DOI] [PubMed] [Google Scholar]
- Sandi C, Pinelo-Nava MT. Stress and memory: behavioral effects and neurobiological mechanisms. Neural Plasticity. 2007 doi: 10.1155/2007/78970. Published online: 15 April 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandi C, Touyarot K. Mid-life stress and cognitive deficits during early aging in rats : individual differences and hippocampal correlates. Neurobiology of Aging. 2006;27:128–140. doi: 10.1016/j.neurobiolaging.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Selcher JC, Nekrasova T, Paylor R, Landreth GE, et al. Mice lacking the ERK1 isoform of MAP kinase are unimpaired in emotional learning. Learning & Memory. 2001;8:11–19. doi: 10.1101/lm.37001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, et al. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biological Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Gado MH, Price JL. Amygdala core nuclei volumes are decreased in recurrent major depression. Neuroreport. 1998;9:2023–2028. doi: 10.1097/00001756-199806220-00021. [DOI] [PubMed] [Google Scholar]
- Shumyatsky GP, Malleret G, Shin RM, Takizawa S, et al. Stathmin, a gene enriched in the amygdala, controls both learned and innate fear. Cell. 2005;123:697–709. doi: 10.1016/j.cell.2005.08.038. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Thompson W, Carter CS, Steinhauer SR, et al. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biological Psychiatry. 2007;61:198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Song L, Che W, Min-Wei W, Murakami Y, et al. Impairment of the spatial learning and memory induced by learned helplessness and chronic mild stress. Pharmacology Biochemistry and Behavior. 2006;83:186–193. doi: 10.1016/j.pbb.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Sterlemann V, Rammes G, Wolf M, Liebl C, et al. Chronic social stress during adolescence induces cognitive impairment in aged mice. Hippocampus. 2010;20:540–549. doi: 10.1002/hipo.20655. [DOI] [PubMed] [Google Scholar]
- Surguladze S, Brammer MJ, Keedwell P, Giampietro V, et al. A differential pattern of neural response toward sadvs. happy facial expressions in major depressive disorder. Biological Psychiatry. 2005;57:201–209. doi: 10.1016/j.biopsych.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Thomas AJ, O’Brien JT. Depression and cognition in older adults. Current Opinion in Psychiatry. 2008;21:8–13. doi: 10.1097/YCO.0b013e3282f2139b. [DOI] [PubMed] [Google Scholar]
- Thomas EJ, Elliott R. Brain imaging correlates of cognitive impairment in depression. Frontiers in Molecular Neuroscience. 2009;3:30. doi: 10.3389/neuro.09.030.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsankova NM, Berton O, Renthal W, Kumar A, et al. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nature Neuroscience. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- Vasconcellos APS, Tabajara AS, Ferrari C, Rocha E, et al. Effect of chronic stress on spatial memory in rats is attenuated by lithium treatment. Physiology & Behavior. 2003;79:143–149. doi: 10.1016/s0031-9384(03)00113-6. [DOI] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. American Journal of Psychiatry. 2004;161:1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- Wang GW, Cai JX. Disconnection of the hippocampal-prefrontal cortical circuits impairs spatial working memory performance in rats. Behavioural Brain Research. 2006;175:329–336. doi: 10.1016/j.bbr.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Wehner JM, Radcliffe RA. Cued and contextual fear conditioning in mice. Current Protocols in Neuroscience. 2004;Chapter 8(Unit 8) doi: 10.1002/0471142301.ns0805cs27. 5C. [DOI] [PubMed] [Google Scholar]
- Wenk GL. Assessment of spatial memory using the T maze. Current Protocols in Neuroscience. 2001;Chapter 8(Unit 8) doi: 10.1002/0471142301.ns0805bs04. 5B. [DOI] [PubMed] [Google Scholar]
- Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berlin) 1997;134:319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- Willner P. Chronic mild stress (CMS) revisited : consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]