Abstract
Leptin receptors (Lepr) are expressed on midbrain dopamine neurons. However, the specific role of Lepr signaling in dopamine neurons remains to be clarified. In the present study, we generated a line of conditional knockout mice lacking functional leptin receptors selectively on dopamine neurons (LeprDAT-Cre). These mice exhibit normal body weight and feeding. Behaviorally, LeprDAT-Cre mice display an anxiogenic-like phenotype in the elevated plus-maze, light-dark box, social interaction and novelty-suppressed feeding tests. Depression-related behaviors in the chronic stress-induced anhedonia, forced swim and tail-suspension tests were not affected by deletion of Lepr in dopamine neurons. In vivo electrophysiological recordings of dopamine neurons in the ventral tegmental area (VTA) revealed an increase in burst firing in LeprDAT-Cre mice. Moreover, blockade of D1-dependent dopamine transmission in the central amygdala by local microinjection of the D1 antagonist SCH23390 attenuated the anxiogenic phenotype of LeprDAT-Cre mice. These findings suggest that leptin receptor signaling in midbrain dopamine neurons has a crucial role for the expression of anxiety and for the dopamine modulation of amygdala function.
Keywords: Leptin receptor, dopamine neurons, feeding, anxiety, ventral tegmental area, central amygdala
Introduction
The adipocyte-derived hormone leptin is a pleiotropic hormone that affects multiple physiological processes including appetite, body weight, neuroendocrine function and emotional behaviors1–6. Leptin exerts its effects by acting on the full-length functional leptin receptor (Lepr), which is expressed in various brain regions7. Whether the different functional roles of leptin are mediated by discrete neuronal populations, however, remains to be characterized. A great deal of attention has focused on the Lepr-containing neurons within the subdivisions of the hypothalamus8–11 and suggested that the hypothalamic targets are essential for the regulation of leptin action on food intake and body weight. The cell populations associated with limbic functions of leptin, however, remain to be clarified. Recent studies suggest that midbrain dopamine neurons are direct targets for leptin7, 12–15.
Midbrain dopamine neurons of the ventral tegmental area (VTA), projecting to limbic regions including the amygdala, nucleus accumbens and prefrontal cortex, are important neural substrates for rewarding and emotional behaviors16–23. Previous studies have demonstrated that leptin modulates the mesolimbic dopamine system at multiple levels including tyrosine hydroxylase (the rate-limiting enzyme in dopamine biosynthesis), vesicular somatodendritic stores, neuronal excitability, dopamine transporter and dopamine release in wild-type rats or leptin-deficient ob/ob mice, although the results across studies have not always been consistent11, 13, 14, 24–26. Moreover, it has been reported that administration of leptin into the VTA decreases normal feeding, and adeno-associated virus (AAV)-mediated Lepr knockdown in the VTA increases food intake and the sensitivity to highly palatable foods14, 27. However, the specific functional role of Lepr signaling in dopamine neurons in appetite control and energy homeostasis remains unclear. On the other hand, leptin has been found to regulate depression- and anxiety-related behaviors in rats or mice28–31. Neuronal circuits that mediate mood-regulating effects of leptin are currently unknown. Given the important role of the mesolimbic dopamine system in mood, motivation and responses to rewarding stimuli, we hypothesized that midbrain dopamine neurons mediate the actions of leptin on feeding and emotional behaviors. To assess the functional role of Lepr in dopamine neurons, we utilized the Cre/loxP system and generated a line of conditional knockout mice that lack Lepr selectively on dopamine neurons. Using this mouse model, we examined the effects of ablation of Lepr in dopamine neurons on feeding, anxiety- and depression-related behaviors, and further characterized possible neural mechanisms underlying behavioral changes.
Materials and Methods
Animals
Mice were housed on a 12 h light/12 h dark cycle (lights on at 7:00 h) with ad libitum access to food and water. All procedures were approved by the Institutional Animal Care and Use Committee and carried out in accordance with the National Institutes of Health Guide.
Generation and characterization of mice lacking Lepr on dopamine neurons
Generation of mice lacking Lepr on dopamine neurons
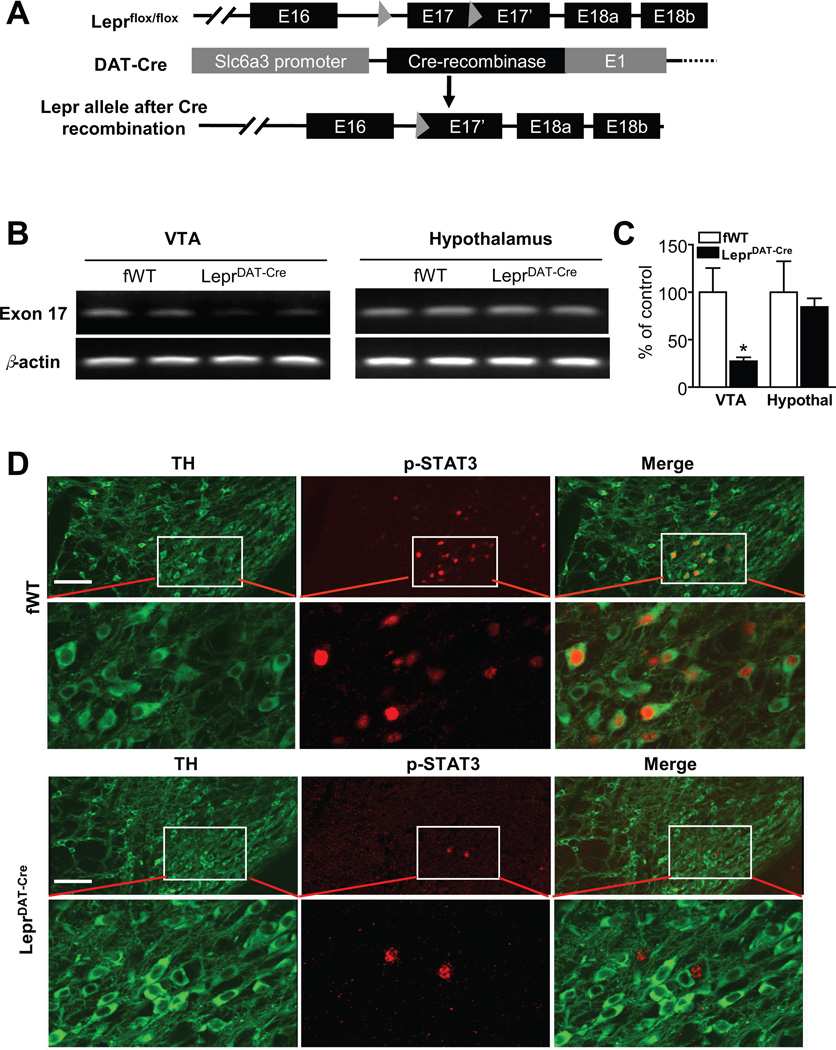
To generate conditional knockout mice lacking Lepr in dopamine neurons, Lepr-floxed (Leprflox/flox) mice (obtained from Dr. Streamson. Chua, Albert Einstein College of Medicine), in which exon 17, a critical exon involved in Lepr signaling, is floxed32, were crossed with a dopamine transporter (DAT, Slc6a3) promoter-driven Cre transgenic mouse line (DAT-Cre)33 (Figure 1A). Cre is expressed in virtually all midbrain dopamine neurons in this line of DAT-Cre transgenic mice33. The Leprflox/+, DAT-Cre offspring were back-crossed with Leprflox/flox to generate conditional knockout mice, i.e. Leprflox/flox, DAT-Cre (LeprDAT-Cre) and Leprflox/flox littermates. DAT expression is restricted to dopamine neurons, and it is highly expressed in the ventral midbrain34. The mice were maintained by crossing LeprDAT-Cre with Leprflox/flox mice. Animals from generations F5–6 were used for the experiments in this study.
Figure 1.
Generation of mice lacking Lepr selectively in dopamine neurons. (A) Schematic diagram depicting the floxed Lepr allele, the Slc6a3 (or DAT) Cre allele, and the Lepr floxed allele after recombination. (B) RT-PCR detection of exon 17 of the leptin receptor in the ventral tegmental area (VTA) versus hypothalamus in LeprDAT-Cre mice and Leprflox/flox littermate control (fWT) mice. (C) Real-time quantitative PCR analysis showing a Cre-mediated deletion of exon 17 of Lepr in the VTA of LeprDAT-Cre mice. Values are expressed as a percentage change from fWT control mice. Data are expressed as mean ± SEM. n = 4 per group. *p < 0.05 compared with fWT control mice. (D) Double-labeling immunohistochemistry showing the colocalization of phosphorylated STAT3 in dopamine neurons, positive for tyrosine hydroxylase (TH), in fWT control mice and LeprDAT-Cre mice. The loss of Lepr in dopamine neurons eliminates leptin-stimulated phosphorylation of STAT3 in dopamine neurons in LeprDAT-Cre mice. Scale bar = 100 µM.
X-gal staining
To evaluate the specificity of DAT-Cre recombinase activity in dopamine neurons, DAT-Cre mice were mated with Rosa-26 reporter mice carrying the Gt(Rosa)26Sortm1Sor allele, in which lacZ expression is driven by the ROSA26 promoter35. Double-transgenic mice expressing the Rosa-26 reporter allele and the DAT-Cre allele were identified using PCR-based genotyping. Mice that were positive for both transgenes were transcardially fixed with 4% paraformaldehyde (PFA). The brains were removed, cryoprotected in 30% sucrose, and sectioned at 40 µm. X-gal staining was processed with free-floating tissue sections by incubating in X-gal staining solution (0.1% X-gal, 5 mM K3Fe(CN)6, 5 mM K4Fe(CN)6, 2 mM MgCl2 in PB, pH = 7.4) for 4 h at 37°C. The staining was examined underneath a light microscope.
RNA extraction and RT-PCR
Tissue micropunches of the VTA and the entire hypothalamus of Leprflox/flox mice and LeprDAT-Cre mice were homogenized, and total RNA was extracted. SuperScript™ first-strand synthesis system (Invitrogen) was used to generate cDNA using the oligo(dT)25 as the template primer. The reaction mixture consisted of 1 µg of total RNA, 500 ng oligo(dT)25, 2 µl of 10× First-Strand buffer, 10 mM DTT, 40 units of RNaseOUT™, and 50 units of SuperScript™ II reverse transcriptase. After incubation at 42°C for 50 minutes, the reaction was inactivated by heating at 70°C for 15 minutes. The resulting cDNA was used for PCR amplification of Lepr exon 17 or β-actin with Accuprime pfx Supermix (Invitrogen). The conditions for PCR were 94°C for 5 min, followed by 31 cycles of 94°C for 1 min, 60°C for 1 min and 72°C for 1 min followed by a final incubation at 72°C for 10 minutes. The primer sequences used to amplify each product are as follows: Lepr exon 17, forward: 5’-GGGACGATGTTCCAAACCCCA-3’ and reverse: 5’-AGGCTCCAGAAGAAGAGGACC-3’; β-actin, forward -AGCCATGTACGTAGCCATCC and reverse - TGTGGTGGTGAAGCTGTAGC. The PCR products were analyzed on a 1% agarose gel stained with ethidium bromide.
Real-time PCR was performed on a Realplex2 Mastercycler (Eppendorf). The Ct values for each duplicate were averaged and used for quantification. The amount of mRNA for exon17 for each sample was normalized to β-actin mRNA using the following formula: 2(CTexon 17 – CTβ-actin).
Immunohistochemistry
To confirm the expression of Cre recombinase in dopamine neurons, LeprDAT-Cre mice were perfused with 4% PFA. The brains were removed, post-fixed overnight, and then cryoprotected in 30% sucrose and cut into 40-µm coronal sections. Double immunohistochemistry was performed to detect Cre immunoreactivity in neurons positive for tyrosine hydroxylase (TH), a marker for dopamine neurons. Briefly, sections were rinsed three times in PBS, and incubated in blocking buffer (1% BSA, 3% goat serum, 0.3% triton X-100 in PBS) for 1 h. The sections were then incubated with rabbit-anti-Cre antibody (1:500; EMD Biosciences, Madison, WI) and mouse anti-TH antibody (1:1000, Pel-Freez Biologicals, Rogers, AR). After washing in PBS buffer, sections were incubated for 4 h with fluorescent secondary antibodies: Alexa Fluor 488 goat anti-rabbit IgG (1:400, Molecular Probes, Eugene, Oregon) to reveal immunoreactivity for Cre and Alexa Fluor 546 goat anti-mouse IgG (1:400) to reveal immunoreactivity for TH. Finally, the sections were washed in PBS, mounted onto poly-lysine coated glass slides, and coversliped with fluorescence mounting medium.
To confirm the loss of functional Lepr in dopamine neurons, LeprDAT-Cre mice and Leprflox/flox control mice were food deprived overnight received injections with recombinant mouse leptin (5 mg/kg, i.p., R&D systems, Minneapolis, MN). Two hours after leptin injection, mice were transcardially perfused and processed as described above. The brain sections were incubated with rabbit anti-phosphorylated STAT3 (tyr705) (1:3000, Cell Signal Technology) antibody and mouse anti-TH antibody, for 48 h at 4°C, followed by incubation with Alexa Fluor 488-conjugated goat anti-mouse to reveal immunoreactivity of TH and HRP-conjugated goat anti-rabbit antibodies (1:1000, Progema, Madison, WI). The sections were then incubated in the TSA Plus Cy3 reagent (Perkin-Elmer) for visualization of phosphorylated STAT3. The colocalization of phosphorylated STAT3 with TH was visualized using an Olympus FV1000 confocal microscope. The numbers of the phosphorylated-STAT3 labeled cells were counted bilaterally in the VTA from 3 animals per group.
Behavioral procedures
Motor and sensory performance
On the testing day, the mice were transferred to the procedure room, separated into individual clean cages containing a mixture of new bedding and bedding from the original cage and habituated for 2 h. For the assessment of locomotor activity, the cage lid of the mouse cage was replaced by a clear Plexiglass cover, and the cage was placed in the center of a box with an open top. A CCD camera was mounted above the open box for recording locomotor activity of mice for 30 min. The distance travelled every 2-min interval and the total distance travelled in 30 min were measured by using the Noldus EthoVision 3.0 system (Noldus Information Technology Inc., Leesburg, VA). To evaluate motor coordination, mice were tested on the accelerating rotarod (Ugo Basile Biological Research Apparatus Company, PA). Mice were placed on the rotarod apparatus and the rotarod was programmed to accelerate from 0 to 45 rpm over 5 min. The latency to fall from the rod was recorded. Each mouse was tested four times a day for two consecutive days with a 15–30 min inter-trial interval, for a total of 8 trials. The visual ability was measured using the visual cliff test by evaluating their avoidance of a drop-off at the edge of a horizontal surface36. A box with an open side and a black floor was placed adjacent to a vertical drop of 0.7 m. A sheet of clear Plexiglas covered the drop off and provided a solid horizontal surface in spite of the visual appearance of a cliff. The mouse was placed at the edge of the black floor and the direction in which the mouse stepped was recorded. Normal mice step toward the black surface more often than the clear surface whereas mice with impaired vision will step toward the black and clear side with equal frequency. Each mouse was subjected 10 consecutive trials. The olfactory function was examined using a buried food pellet test37. Mice were food deprived for 24 h prior to the test. On the test day, a single mouse was placed in a clean cage to recover a food pellet that was buried 0.5 cm below the surface of a 3 cm deep layer of mouse bedding material. The location of the pellet was changed for different animals at random and the latency to find the pellet was recorded. This was defined as the time between placement of the mouse in the cage and grasping the food pellet with its forepaws or teeth.
Anxiety-related behavioral tests
A battery of behavioral tests was used to evaluate anxiety-like behaviors. The elevated plus maze is a validated and widely used test of anxiety in rodents38. The plus maze consisted of four arms (30-cm long and 5-cm wide) arranged in the shape of a "plus" sign and elevated to a height of 70 cm from the floor. Two arms had no side or end walls (open arms), and the other two arms had side walls and end walls (12-cm high) but were open on top (closed arms). The arms intersected at a central platform (5×5 cm) that allowed access to all of the arms. Mice were placed in the central square facing the corner between a closed arm and an open arm, and allowed to explore the elevated plus-maze for 5 min. The time spent on the open and closed arms and the numbers of entries made into each arm were measured. Entry was defined as all four paws entering one arm. The degree of anxiety was assessed by calculating the percentage of open arm entries (entries into the open arms/total entries into all arms) and percentage of open arm time (time spent in the open arms/total time spent in all arms). The light dark test procedure is based on a natural conflict of a mouse between the innate aversion to brightly illuminated areas and the exploration of a novel environment39. The apparatus consists of two compartments (27 × 27 × 30 cm for the light compartment and 18 × 27 × 30 cm for the dark compartment) divided by a wall with a door between the two compartments. The light compartment was brightly illuminated with light intensity of 900 lux; and the dark compartment was black-walled and covered at the top with black Plexiglas. Mice were placed individually in the center of the dark compartment facing away from the opening. The latency for the mice to move to the light side and time spent in the light compartment were recorded for 5 min. Entry to the light compartment was defined as all four paws entering the light side of the box. The social interaction test was carried out with a modification of the method used by File and Hyde40. The test apparatus consisted of a white box (40 × 40 × 40 cm) with an open top. The illumination in the test arena was adjusted to 250 lux. Animals were not habituated to the test box prior to testing. Mice were tested for social interaction with an unknown test partner from a different cage but with the same genotype and approximately the same body weight. Two mice were placed simultaneously in the opposite corners of the arena. Their social activity was recorded for 10 min. The active social behaviors were scored including nosing, following, and non-aggressive physical contacts (body sniffing, anogenital sniffing, body crossing). The novelty suppressed feeding test assesses stress-induced anxiety by measuring the latency of an animal to approach and eat a familiar food in a novel environment41. Mice were food deprived for 24 h prior to the test. At the time of testing 1 food pellet was placed on a white filter paper located in the middle of the test arena (60 × 60 × 40 cm) and covered with 2 cm of fresh bedding. Each mouse was placed in one corner and allowed to explore for a maximum of 10 min. The latency to approach the pellet was recorded. Immediately after an eating event, the mouse was transferred to its home cage and allowed to free-feed for 5 minutes. The amount of food consumption was recorded.
Depression-related tests
The sucrose preference test has been used as a procedure to measure hedonic response to natural reward in mice42. For the basal sucrose preference, mice were habituated to drinking from two bottles of water for one week prior to testing. To measure the preference for sucrose, the animals were singly housed and provided with a free choice of water or sucrose solution (0.2% or 1%, w/v) for 4 consecutive days. Water and sucrose intake was measured daily, and the position of the two bottles was switched every day. Sucrose preference was calculated as a percentage of consumed sucrose solution of the total amount of liquid that the mice drank. For the measurement of sucrose preference in response to chronic unpredictable stress, animals were singly housed and subjected to a variety of stressors applied randomly and at varying times of the day for 14 consecutive days as described previously29. The stressors included 30 min restraint, 10 min tail pinch, 1h shaking, 24 h constant light, 24h wet bedding/45° cage tilt, electric footshock, and 2 h social stress. Control non-stressed mice were group housed and handled daily in the housing room. Two bottles of water were placed in each animal cage 2 days prior to and during the chronic unpredictable stress procedure. After 14 days of chronic unpredictable stress, the animals were provided with a free choice between plain water and 1% sucrose. Water and sucrose intake was measured for 2 days and the position of the two bottles was switched every 24 h.
The tail suspension and forced swim tests are widely used for screening antidepressant properties of drugs43, 44. In both tests, animals first struggle vigorously in an attempt to escape. However, over time animals become immobile, which is believed to reflect a state of “behavioral despair”. For the tail suspension test, the apparatus was constructed of a wooden box (30×30×30 cm) with an open front. A horizontal bar was placed 1 cm from the top and a vertical 9 cm bar hanging down in the center. Mice were individually suspended by the tail to the vertical bar with adhesive tape affixed 2 cm from the tip of the tail. The animal’s behavior was recorded for 6 min. The immobility and escape oriented behaviors scored by a trained observer who was blind to the treatments. For the forced swim test, mice were placed in a clear Plexiglas cylinder (25 cm high; 10 cm in diameter) filled to a depth of 15 cm with 24°C water. Animals’ behavior in a 6-min test session was recorded. The first 2 min were designated as a habituation period, and the duration of immobility was measured during last 4 min using Noldus EthoVision 3.0 system (Noldus Information Technology Inc., Leesburg, VA).
Adult male mice (8–12 weeks) were used for the anxiety- and depression-related behavioral tests. All behavioral tests were performed in the light cycle, and were scored by experimenters who were blind to the treatments or genotypes. New animals were used for the elevated plus-maze, light dark box, social interaction, novelty suppressed feeding, sucrose preference, tail suspension test and forced swim test. The animals used for locomotor activity were re-tested for rotarod performance. The mice that were tested for sucrose preference under the basal condition were used for visual cliff and olfactory tests.
Electrophysiology
Mice were anesthetized with 4% chloral hydrate (400 mg/kg, i.p.) and placed in a stereotaxic apparatus. It has been reported that, under chloral hydrate anesthesia, dopamine neuron activity states more closely resemble that observed in freely moving animals45. Anesthesia was maintained by supplemental administration of chloral hydrate as required to maintain suppression of limb compression withdrawal reflex. A core body temperature of 37°C was sustained by a thermostatically controlled heating pad. An incision was made in the midline and a burr hole drilled above the VTA. Recording electrodes were pulled from borosilicate glass capillaries and filled with 2M NaCl containing 2% Pontamine sky blue dye (impedance 6–14 MΩ). Electrodes were lowered into the VTA (Coordinates: 3.2 mm posterior to the bregma, 0.4 mm lateral to the midline, and -5.0 mm ventral to the brain surface) using a hydraulic microdrive and 6–12 vertical passes were made throughout the VTA, separated by 100 µm. Spontaneously active dopamine neurons were identified with open filter settings (30 Hz – 30 kHz) using established electrophysiological criteria46, 47. Once isolated, two distinct activity states were measured over a period of 3 min: i) basal firing rate (Hz), and (ii) the proportion of action potentials occurring in bursts (defined as the occurrence of two spikes with an interspike interval of < 80 ms, and the termination of the burst defined as the occurrence of an interspike interval of > 160 ms48. At the cessation of the experiments, mice were decapitated and their brains processed for histological verification of electrode tracks.
Intra-central amygdala microinjection
Mice were anesthetized as described above and implanted unilaterally or bilaterally with a guide cannula (26-gauge; C315GS; Plastics One, Roanoke, VA) positioned 2 mm above the central amygdala (Coordinates: −0.94 mm posterior to bregma, ±2.8 mm lateral to the midline, and −2.8 mm ventral to bregma). A stainless steel dummy cannula was used to seal the guide cannula after surgery. Following cannula implantation, animals were housed individually and allowed to recover for 7 d before performing microinjection. During this recovery period, animals were handled daily to minimize stress caused by the microinjection procedure. All microinjections were performed on conscious, unrestrained, freely moving mice in their home cage. On experimental day, a 33-gauge stainless steel injector connected to a 5-µl syringe was inserted into the guide cannula and extended 2 mm beyond the tip. The D1 dopamine antagonist SCH23390 (Sigma, St. Louis, MO) was dissolved in isotonic sterile saline. SCH23390 (0.3 µg per mouse) or vehicle (saline) was infused in a volume of 0.2 µl per side over 2 min. An additional minute was allowed for diffusion and prevention of backflow through the needle track before the injector was withdrawn. Animals were tested on the elevated plus-maze 30 min after microinjections. After completion of behavioral testing, mice were injected with 0.2 µl of India ink via an injector under anesthesia and sacrificed by decapitation. The fresh frozen brains were sectioned at 20 µm and stained with toluidine blue to verify the placement of the injection needle. Animals with misplacement of cannula were excluded from data analysis.
Western blot analysis
Mice were decapitated rapidly, and tissue micropunches from the central amygdala, nucleus accumbens, striatum and VTA were homogenized in lysis buffer (50 mM Hepes, pH 7.6, 1% Triton X-100, 150 mM NaCl, 20 mM sodium pyrophosphate, 20 mM β-glycerophosphate, 10 mM NaF) containing a mixture of phosphatase inhibitors (leupeptin, aprotinin, sodium orthovanadate, phenylmethylsulfonyl fluoride, Ser/Thr phosphatase inhibitor mixture, Tyr phosphatase inhibitor mixture). A total amount of 40 µg protein was separated on an SDS-PAGE gel, transferred to a nitrocellulose membrane, and subsequently incubated with rat anti-DAT (1:3000, Chemicon) and mouse anti-β-tubulin antibodies (1: 1000, Chemicon) diluted in a solution of 1% bovine serum albumin and 0.1% Tween 20 in Tris-buffered saline overnight at 4 °C. Next, the membrane was washed and incubated in secondary antibody conjugated to horseradish peroxidase in a blocking solution for 1 h. Immunoblotting results were visualized using an electrogenerated chemiluminescence reaction and exposed to X-ray film.
Statistical analysis
Results are expressed as mean ± standard error of the mean (SEM). Statistical analyses were performed by using one-factor ANOVA with repeated measures on food intake, body weight gain, sucrose preference and motor functions, two-way ANOVA on chronic unpredictable stress-induced anhedonia, and behavioral effects of SCH23390, followed by a post hoc Bonferroni/Dunn or Tukey/Kramer (for unequal n) test. The proportions of high burst-firing cells and low burst-firing neurons were analyzed using Chi-squared test. The rest of experimental results were analyzed using Student t-test.
Results
Generation of mice lacking Lepr in dopamine neurons
The specificity of DAT-Cre-mediated recombination was confirmed by crossing DAT-Cre mice with Rosa26 reporter mice. X-gal staining indicated that the Cre recombinase activity was restricted to dopamine neuron regions including the VTA and SNc (Figure S1A). Furthermore, double-labeling immunohistochemistry was performed on brain sections of LeprDAT-Cre mice and confirmed that Cre immunoreactivity was exclusively localized in neurons positive for the dopamine neuron marker TH (Figure S1B). The effectiveness of deletion of exon 17 was confirmed by RT-PCR and real-time quantitative PCR analyses, which detected a significant reduction of exon 17 of Lepr in the VTA (t(6) = 2.82, p < 0.05) (Figure 1B,C). In contrast, the hypothalamus, another brain region containing dopamine neurons49, showed no difference in the levels of Lepr exon 17 mRNA (Figure 1B,C), suggesting that a Cre-mediated recombination of the Lepr-flox allele occur specifically in the midbrain. This is consistent with the report that dopamine neurons that express Lepr are restricted to the midbrain7. To validate that the deletion of exon 17 leads to the functional loss of leptin receptors in dopamine neurons, we assessed STAT3 phosphorylation, a functional readout for leptin receptor activation, in LeprDAT-Cre mice. Following intraperitoneal (i.p.) injection of leptin (5 mg/kg), phosphorylated STAT3 was observed in VTA dopamine neurons in Leprflox/flox control mice. In Leprflox/flox control mice, the majority of the cells positive for phosphorylated STAT3 were also stained for TH immunoreactivity. However, none of the scattered phosphorylated STAT3 cells in LeprDAT-Cre mice were observed to colocalize with TH (Figure 1D), supporting complete ablation of the functional Lepr in dopamine neurons.
LeprDAT-Cre mice display normal body weight, food intake and hedonic responses
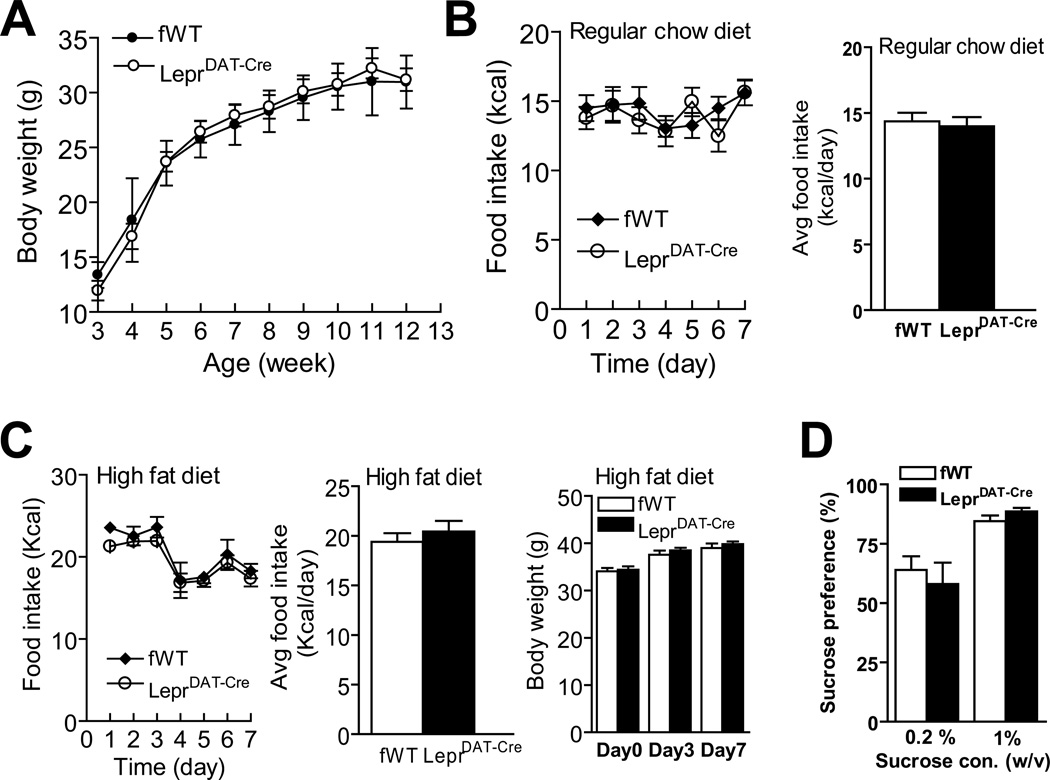
Given the role of the mesolimbic dopamine system in food intake and reward, we assessed the effects of inactivation of Lepr on dopamine neurons on appetite, weight regulation and hedonic responses. Body weight was monitored in mice from 3 to 12 weeks of age. There was no significant difference between LeprDAT-Cre mice and Leprflox/flox littermate controls (F(1, 231) = 0.004, p > 0.5) (Figure 2A). At 13 weeks of age, the intake of standard chow was measured for 7 days. Daily food intake over the 7-day testing period showed no significant effect of genotype (F(1, 102) = 0.14, p > 0.5 for daily food intake; t(17) = 0.39; p > 0.5 for average daily food intake) (Figure 2B). Subsequently, LeprDAT-Cre mice and Leprflox/flox mice were tested for the response to a palatable, high fat diet (45% calories from fat) for 7 days. The two genotype groups showed similar daily intake of high fat diet over the 7-day testing period (F(1, 90) = 1.60, p < 0.1 for daily food intake; t(15) = 0.75, p > 0.1 for average daily food intake) (Figure 2C). Body weight was measured before and 3 or 7 days after the high fat diet switch. LeprDAT-Cre mice and Leprflox/flox littermate control mice showed similar body weight gain during the high fat diet feeding (F(1,28) = 0.34, p > 0.5).
Figure 2.
Normal body weight gain, food intake and hedonic responses in LeprDAT-Cre mice. (A) Body weight. (B) Intake of regular chow diet. Left panel: Daily food intake. Right panel: Average food intake over 7 days. n = 9–10 per group. (C) Intake of high fat diet. Left panel: Daily food intake. Middle panel: Average food intake over 7 days. Right panel: Body weight. n = 8–9 per group. (D) Sucrose preference. The preference for sucrose was expressed as a percentage of the volume of sucrose solution intake to the volume of total fluid intake within a 4-day test. n = 8–9 per group. All data are presented as mean ± SEM.
Next, we examined the intake and preference for sucrose, a well established natural reward that involves activation of dopamine neurons50–53. A free choice between plain water and 0.2% sucrose was first presented to LeprDAT-Cre mice and Leprflox/flox littermate control mice. Then, a higher concentration (1%) of sucrose solution was given to the same mice. The statistical analysis revealed a significant effect of sucrose concentration (F(1,15) = 21.20, p < 0.001) but no main effect of genotype (F(1,15) = 0.015, p > 0.5). The interaction between genotype and sucrose concentration was not significant (F(1,15) = 0.89, p > 0.3) (Figure 2D). In addition, LeprDAT-Cre mice and Leprflox/flox littermate controls showed no significant difference in daily sucrose intake for either 0.2% sucrose solution (Leprflox/flox: 2.13 ± 0.22 ml; LeprDAT-Cre: 2.40 ± 0.42 ml) or for 1% sucrose solution (Leprflox/flox:3.33 ± 0.48 ml; LeprDAT-Cre: 3.83 ± 0.39 ml). Taken together, these data suggest that leptin receptor signaling in dopamine neurons may not be required for appetitive behavior and the reward processes manifested by palatable high fat food or sucrose solution.
To rule out the possible confounding effect of the Cre transgene in LeprDAT-Cre mice, body weight gain, food intake, including regular chow and high fat diets, and sucrose preference were evaluated in DAT-Cre transgenic mice in comparison with wild-type littermate controls. None of these measures showed a significant difference between Cre mice and wild-type littermate controls (Figure S2).
LeprDAT-Cre mice display normal motor and sensory performance
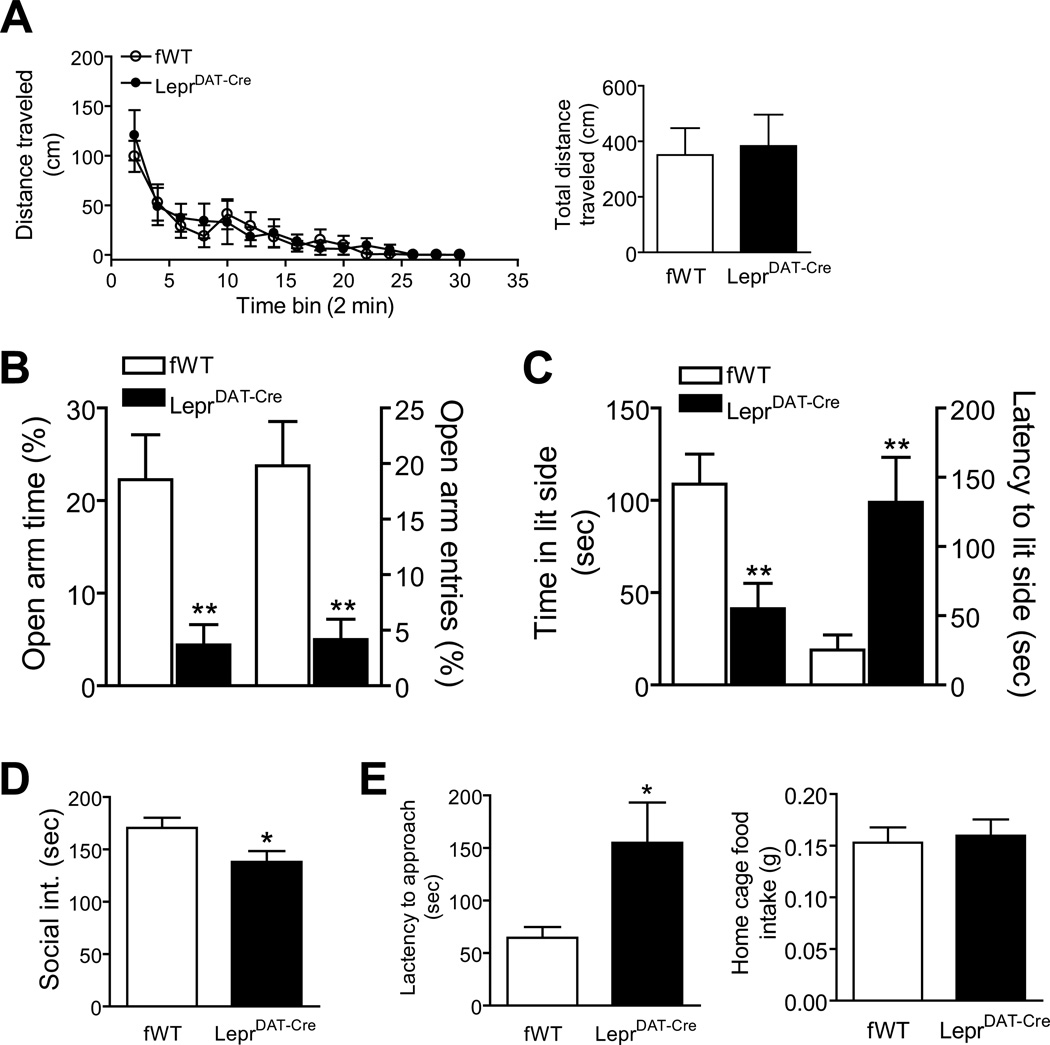
Given that most behavioral evaluations are dependent on locomotor activity, we examined locomotor activity in the late light cycle when most behavioral tests were performed. LeprDAT-Cre mice and Leprflox/flox littermate controls exhibited similar locomotor activity during the 30-min test [(F(1, 252) = 0.046, p > 0.5) for the time course of distance traveled every 2 min; t(18) = 1.44; p > 0.1 for the total distance traveled] (Figure 3A). In addition, motor coordination was evaluated using a rotarod test. The ability to perform or learn an accelerating rotarod task was unaltered in LeprDAT-Cre mice (Figure S3A). These results suggest that a loss of leptin signaling in dopamine neurons does not affect motor functions. In addition, no gross impairments in their visual and olfactory capacities in LeprDAT-Cre mice were observed at the age when behavioral tests were performed (Figure S3B, C).
Figure 3.
Anxiogenic-like behavioral phenotype of LeprDAT-Cre mice. (A) Locomotor activity. Left panel: locomotor activity monitored for 30 min and analyzed at 2 min intervals. Right panel: Total distance traveled within 30 min. n = 10 per group. (B) Elevated plus-maze test. Left axis, the percentage of open arm time. Right axis, the percentage of open arm entries. n = 10 per group. (C) Light/dark choice test. Left axis, latency to enter the light side. Right axis, time spent in the light compartment. n = 12 per group. (D) Social interaction test. n = 9–12 pairs per group. (E) Novelty-suppressed feeding test. Left panel, latency to approach food pallets in the center of an open arena. Right panel, home cage food intake for 5 min immediately following the test. n = 12 per group. All data are expressed as mean ± SEM. *p < 0.05; **p < 0.01 compared with Leprflox/flox littermate control (fWT) mice by two-tailed Student’s t test.
LeprDAT-Cre mice exhibit an anxiogenic phenotype
To evaluate the effects of Lepr deletion in dopamine neurons on anxiety-related behaviors, mice were examined in a series of behavioral tests. In the elevated plus-maze test, LeprDAT-Cre mice displayed a significantly lower percentage of open arm entries (t(18) = 3.35; p < 0.01) and time spent in the open arms (t(18) = 3.57; p < 0.01) without affecting total arm entries (Leprflox/flox: 13.60 ± 1.431; LeprDAT-Cre: 11.60 ± 1.108; t(18) = 1.11; p > 0.2), suggesting increased anxiety levels in LeprDAT-Cre mice (Figure 3B). In the light dark box, LeprDAT-Cre mice explored the light compartment to a significantly lesser extent than Leprflox/flox littermate controls, as measured by increased latency to enter the lit side (t(22) = 3.19; p < 0.01) and decreased time spent in the lit side (t(22) = 3.12; p < 0.01) (Figure 3C). In the social interaction test, two mice with the same genotype that had similar body weight and were unfamiliar to each other were simultaneously placed in a novel test arena28. LeprDAT-Cre mice spent significantly less time in active social interaction compared to Leprflox/flox littermate controls (t(19) = 2.21; p < 0.05) (Figure 3D). The novelty suppressed feeding test required food deprivation for 24 h followed by testing the animal’s latency to approach food pellets that were placed in the center of a novel, open and brightly lit arena. LeprDAT-Cre mice exhibited significantly longer latencies to begin feeding when compared with Leprflox/flox littermate controls (t(22) = 2.28; p < 0.05), while feeding activity in the home cage was similar (Figure 3E).
Depression-related behaviors are unaltered in LeprDAT-Cre mice
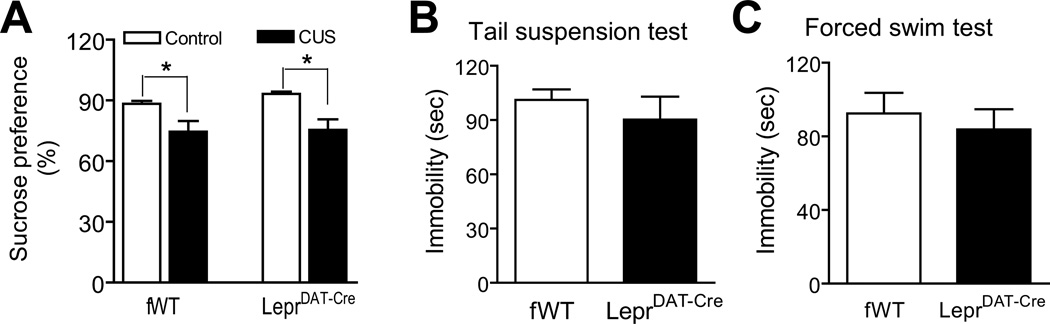
Sucrose preference has been widely used for assessing ‘anhedonia’, a symptom of human depression, which can be induced by chronic stress in rodents29, 54–57. To determine if deletion of Lepr signaling in dopamine neurons influences the sensitivity to chronic stress-induced anhedonia, mice were exposed to a 2-week chronic unpredictable stress procedure. Sucrose preference in LeprDAT-Cre mice and Leprflox/flox littermate controls was tested using 1% sucrose solution. The statistical analysis indicated a significant effect of chronic unpredictable stress (F(1, 33) = 17.34; p < 0.001)., but not genotype (F(1, 33) = 0.58; p > 0.1). There was no significant effect of genotype × chronic unpredictable stress (F(1, 33) = 0.28; p > 0.1) (Figure 4A). In the tail suspension test and forced swim test, immobility time, an index of ‘behavioral despair’, was unaltered in LeprDAT-Cre mice in comparison with Leprflox/flox littermate controls (Tail suspension: t(17) = 0.81; p > 0.1; Forced swim: t(15) = 0.56; p > 0.5) (Figure 4B, C). Taken together, these behavioral data indicate that a loss of Lepr in dopamine neurons has no effect on depression-related behaviors.
Figure 4.
Normal depression-related behavior in LeprDAT-Cre mice. (A) Sucrose preference following 2 weeks of chronic unpredictable stress (CUS). n = 8–12 per group.*p < 0.05 compared with non-stressed groups. (B) Tail suspension test. Immobility time was measured during a 6-min test period. n = 9–10 per group. (C) Forced swim test. Immobility time was measured during the last 4 min of a 6-min test period. n = 8–9 per group. All data are expressed as mean ± SEM.
To rule out the possible confounding effects of the Cre transgene on emotional behaviors, we tested DAT-Cre mice in comparison with wild-type littermate controls. The DAT-Cre transgenic mice and wild-type mice were indistinguishable in the behavioral tasks as described above (Figure S4). Furthermore, to rule out the possibility that a change in DAT protein might contribute to the behavioral outcome in LeprDAT-Cre mice, we measured the levels of DAT in different terminal regions of dopamine neurons, including the central amygdala, nucleus accumbens, striatum, and VTA of LeprDAT-Cre mice at 9 weeks of age. DAT protein levels were similar in LeprDAT-Cre mice and Leprflox/flox littermate control mice (Figure S5).
LeprDAT-Cre mice show increased burst firing activity of dopamine neurons in the VTA
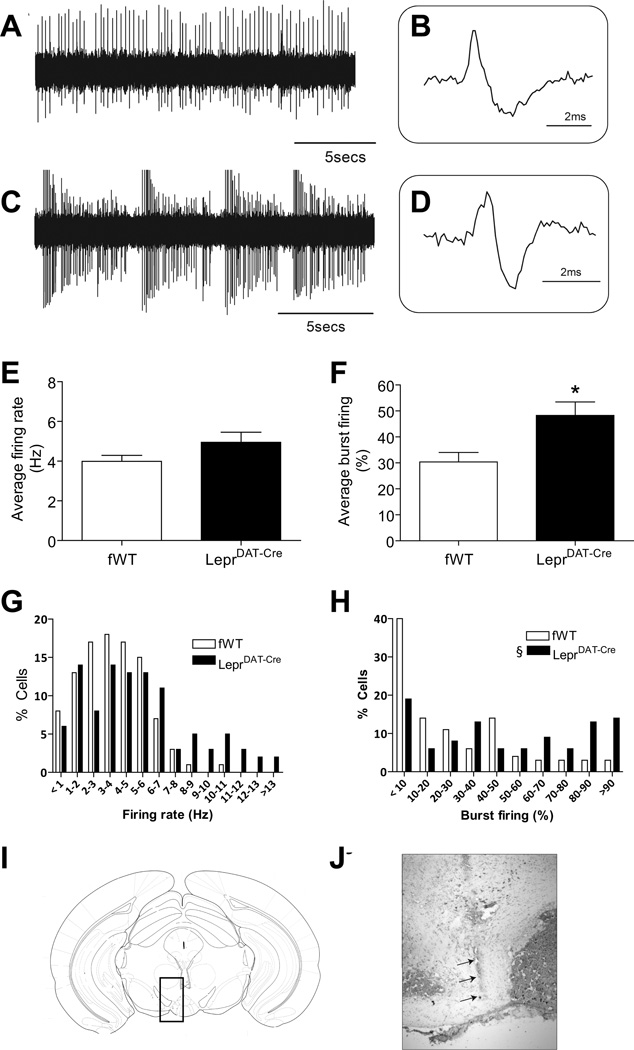
To understand the mechanisms underlying the behavioral effects of dopamine neuron-specific Lepr deletion, we investigated the electrophysiological profiles of dopamine neurons in the VTA. Single-unit extracellular recordings were performed in chloral hydrate anesthetized LeprDAT-Cre mice and Leprflox/flox littermate controls. Spontaneously active dopamine neurons were identified with open filter settings (low pass: 30 Hz, high pass: 30 kHz) using previously established electrophysiological criteria, including an action potential duration > 2 ms (Leprflox/flox: 2.9 ± 0.1 ms; LeprDAT-Cre: 3.2 ± 0.3 ms)46. Leprflox/flox mice displayed an average firing rate of 3.99 ± 0.30 Hz with 30.4 ± 3.7 % of action potentials occurring in bursts (Figure 5A, B, E, F), consistent with previous findings in untreated animals58, 59. LeprDAT-Cre mice showed no difference in the average firing rate (4.95 ± 0.51 Hz) (Figure 5E), however, these mice exhibited a significantly greater burst firing (48.3 ± 5.2 %; p < 0.05. Figure 5C, D, F). Burst-firing frequency distribution of cells recorded showed a significant increase in the number of high burst-firing cells with a concomitant decrease in low burst-firing neurons in LeprDAT-Cre mice (p < 0.05; Figure 5G, H). The electrode tract throughout the VTA was confirmed with histological staining (Figure 5I, J), targeting the caudal aspect of the VTA where a high degree of colocalization of Lepr and TH was reported7. These observations suggest that a loss of leptin signaling in dopamine neurons results in an augmented dopamine neuronal activity.
Figure 5.
LeprDAT-Cre mice display enhanced VTA dopamine neuronal activity. Representative voltage traces from identified dopamine neurons demonstrate an irregular, single spike firing pattern of dopamine neurons in the VTA of a Leprflox/flox mouse (fWT, A & B) and a high burst-firing activity state in a LeprDAT-Cre mouse (C & D). (E) Average firing rate. (F) Burst firing (G) Firing rate distributions. (H) Burst-firing frequency distributions. Data are expressed as mean ± SEM. n = 7–10 mice, 64–72 neurons per group. (I–J) A representative electrode track throughout the VTA. *p < 0.05 compared to Leprflox/flox littermate controls. § representing a significant difference in proportions of high burst-firing cells and low burst-firing neurons, p < 0.05.
Dopamine transmission via D1 receptors in the central amygdala mediates anxiogenic-like behavior in LeprDAT-Cre mice
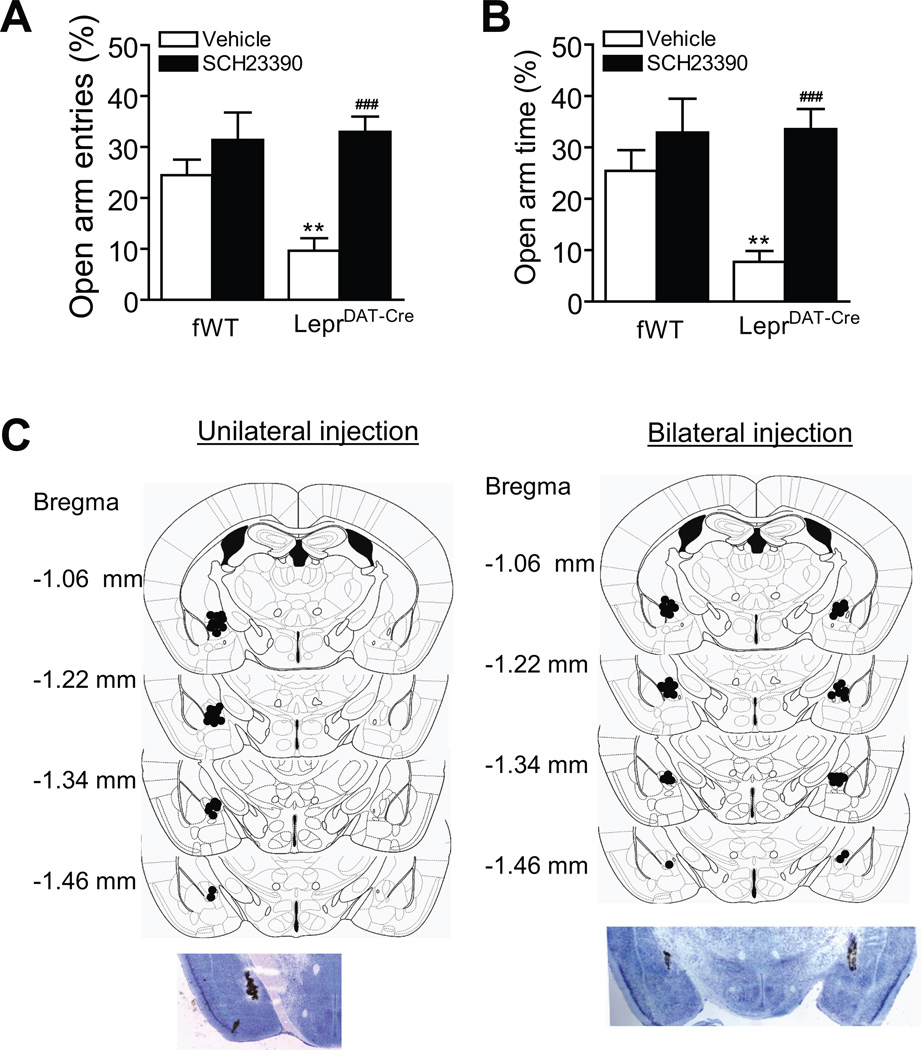
To test whether dopamine transmission in the central amygdala contributes the anxiogenic phenotype of LeprDAT-Cre mice, we examined whether blockade of dopaminergic transmission in the central amygdala would alleviate the anxiogenic-like behavior in the elevated plus maze test. The D1 antagonist SCH23390 or vehicle was infused unilaterally or bilaterally into the central amygdala of freely moving mice. Thirty min after drug injection, mice were tested on the elevated plus maze for 5 min. Similar results were observed in mice that received unilateral or bilateral injection of SCH23390 into the central amygdala, the data were combined. Statistical analysis revealed no significant main effect of genotype (F(1,57) = 3.56, p > 0.05 for percentage of open arm entries; F(1,57) = 3.90, p > 0.05 for percentage of open arm time), but showed significant effects of treatment (F(1,57) = 18.62, p < 0.001 for percentage of open arm entries; F(1,57) = 14.97, p < 0.001 for percentage of open arm time) and interaction between genotype and treatment (F(1,57) = 5.47, p < 0.05 for percentage of open arm entries; F(1,57) = 4.57, p < 0.05 for percentage of open arm time). Post-hoc analyses showed that percentage of open arm entries and open arm time in vehicle-treated LeprDAT-Cre mice was significantly lower than that of vehicle-treated Leprflox/flox control mice (p < 0.01), and SCH23390 treatment significantly increased the percentage of open arm entries and open arm time in LeprDAT-Cre mice (p < 0.001) (Figure 6). Together, these results suggest that the anxiogenic effect of ablation of Lepr signaling in dopamine neurons may be mediated by D1 dopamine transmission in the central amygdala.
Figure 6.
Effect of unilateral and bilateral intra-central amygdala microinjection of the D1 dopamine receptor antagonist SCH23390 on anxiety-like behavior in the elevated plus-maze test. (A) The percentage of open arm entries. (B) The percentage of time spent in the open arms. n = 13–16 mice per group. **p < 0.01 compared with the vehicle-treated fWT littermate control group;##p < 0.01,###p < 0.001 compared with the vehicle-treated LeprDAT-Cre group. (C) Schematic illustration of unilateral (left panel) and bilateral (right panel) injection sites in the central amygdala (top) and representative ink injections showing the deposition site within the central amygdala (bottom). All data are expressed as mean ± SEM.
Discussion
This study demonstrates that the selective inactivation of Lepr in dopamine neurons in mice results in enhanced anxiogenic-like behaviors and increased excitability of dopamine neurons in the VTA. The anxiogenic-like phenotype of mutant mice is likely mediated by dopamine D1 transmission in the central amygdala as blockade of D1 dopamine receptors in this area attenuates the anxiogenic-like behavior. By contrast, depression-related behavior and feeding behavior are not affected by ablation of Lepr in dopamine neurons. These data suggest that leptin receptor signaling in dopamine neurons plays a critical role in modulating anxiety-related behaviors.
Although previous studies have suggested that the midbrain is one extra-hypothalamic site where leptin regulates normal food intake and hedonic feeding14, 27, the specific role of Lepr signaling in midbrain dopamine neurons in feeding behavior was not clear. In this study, we showed that LeprDAT-Cre mice lacking Lepr selectively in dopamine neurons exhibited normal body weight gain and feeding behavior. The intake of standard chow and high palatable foods including high fat diet and sucrose solutions (0.2% and 1%) was unaltered in LeprDAT-Cre mice, suggesting that leptin receptor signaling in dopamine neurons is not required for homeostatic or hedonic feeding. The difference between our results and the previous report by Hommel et al. using AAV-mediated Lepr knockdown in rats, which targeted both dopamine and non-dopamine neurons in the midbrain14, implies that Lepr on non-dopamine neurons might account for feeding behavior. However, this difference could also be due to species differences between mice and rats. While we cannot exclude the possibility of developmental compensation that might have occurred in LeprDAT-Cre mice, the lack of feeding and body weight phenotypes in these mice is in contrast to other lines of Lepr conditional knockout mice, such as those mice with loss of Lepr in POMC neurons, SF1 neurons and Nkx2.1 neurons, that develop obesity despite having neuron-specific Lepr deficiency at early stages of development8, 10, 60, 61. Recent studies in leptin-deficient subjects suggest that neural circuits through which leptin regulates hedonic feeding behavior might involve specific limbic cortical and subcortical areas. Using functional magnetic resonance imaging (fMRI), Baicy et al. reported that leptin replacement in adults with congenital leptin deficiency reduced activation of the brain regions involved with hunger (insula, parietal and temporal cortex) and increased activation of regions involved in cognitive inhibition and satiety (prefrontal cortex) in response to food cues62. Another fMRI study demonstrated that leptin-deficient adolescents displayed activation of the ventral striatum to images of food, which was attenuated after 1 week of leptin treatment63. Whether these brain regions are direct leptin targets and whether they mediate leptin action on hedonic feeding requires further investigation. Moreover, leptin replacement in leptin-deficient patients has been shown to exert a sustained effect on cortical structural organization64 and promote cognitive development65. How these structure and functional changes induced by leptin contribute to rewarding responses to food stimuli remain to be explored.
A robust anxiogenic phenotype was observed in LeprDAT-Cre mice. This was consistently revealed in multiple behavioral tests including the elevated plus-maze, light-dark box, social interaction and novelty suppressed feeding tests. The anxiogenic behavior is unlikely to be a result of general hypolocomotion, sensory abnormalities or altered feeding activity as motor and sensory abilities as well as food consumption in LeprDAT-Cre mice were not different from Leprflox/flox littermate controls. Although anxiety and depression often co-occur66, depression-related behaviors were not affected in LeprDAT-Cre mice, as evidenced by the absence of an anhedonic phenotype in the sucrose preference test under both basal and chronic stress conditions. In addition, the LeprDAT-Cre mice exhibited normal performance in the tail suspension test and forced swim test, two procedures widely used for evaluating "behavioral despair”. These findings suggest that the modulation of anxiety- and depression-related behaviors by leptin may be mediated via distinct neural circuits. The data from anxiety- and depression-related behavioral tests in LeprDAT-Cre mice were unlikely to be confounded by the DAT-Cre transgene because DAT-Cre mice did not significantly differ from wild-type littermate mice in these behavioral tests.
Dopamine neuronal activity in the VTA has been implicated in fear and anxiety-like states. A considerable number of dopamine neurons are excited by aversive stimuli67, and stressful events are associated with mesolimbic dopamine release68–72. On the other hand, electrical stimulation of VTA neurons produces fear and anxiogenic responses, whereas lesions of the VTA or inhibition of VTA dopamine neurons by dopamine D2 agonists have anxiolytic effects73–76. Anatomical studies have demonstrated that the localization of Lepr in dopamine neurons is restricted to the midbrain with the majority of Lepr-expressing dopamine neurons distributed in the VTA7. To understand the mechanisms underlying the anxiogenic phenotype of mice lacking Lepr in dopamine neurons, we recorded the firing activity of dopamine neurons from the VTA in vivo and found that the burst firing of VTA dopamine neurons was increased in LeprDAT-Cre mice. The distribution of burst-firing frequency indicated that a subset of dopamine neurons (20~30%) in the VTA were affected in the LeprDAT-Cre mice. VTA dopamine neurons are heterogeneous in cortical and sub-cortical projections and functional properties77. Our electrophysiological recordings were not limited to dopamine neurons projecting a specific mesolimbic structure. A very recent study by Leshan et al. demonstrates that Lepr neurons in the VTA densely innervate the central amygdala15, a target area of VTA dopamine neurons78–80. Given that the majority of Lepr neurons in the VTA are dopaminergic, we thus posit that altered VTA dopamine neuronal firing properties in LeprDAT-Cre mice may lead to changes in dopamine transmission in the central amygdala.
The amygdala is a key component essential for the processing of emotions81, 82. This structure consists of a group of anatomically and functionally distinct nuclei83. Especially, the central nucleus of the amygdala receive convergent information from other amygdaloid subnuclei and elicit an output that reflects the sum of amygdaloid activity84. Mesoamygdaloid dopamine neurons have been considered as a neurologic substrate in anxiety and stress responses85–87. Indeed, midbrain dopamine neurons increase firing in response to aversive stimuli88–90, and dopamine transmission in the amygdala is elevated in response to a variety of stress conditions such as inescapable electrical footshock, conditioned fear and chronic restraint stress86, 87, 91, 92. Both dopamine D1 and D2 receptors are expressed in the central amygdala93–97. However, D1 receptors have been consistently reported to modulate anxiety behaviors98–101. Blockade of D1 receptors in the central amygdala by local infusion of the D1 selective antagonist SCH23390 attenuated the anxiogenic phenotype of LeprDAT-Cre mice in the elevated plus-maze test. The effect of SCH23390 could include a mixture of postsynaptic and presynaptic mechanisms, blocking D1 receptor activity localized at postsynaptic elements of central amygdala neurons as well as at presynaptic nerve terminals. Indeed, in addition to sending prominent efferents to the central amygdala, VTA Lepr neurons were also found to project to the other components of the extended amygdala namely the bed nucleus of stria terminalis (BNST) and the interstitial nucleus of the posterior limb of the anterior commissure (IPAC), where dopamine D1 receptors are expressed78, 102, 103. These two areas in turn project to the central amygdala104–106, thereby possibly providing presynaptic axon terminals equipped with D1 receptors. However, given the anatomical proximity and functional connectivity of these two areas with the amygdala, their direct involvement in dopamine regulation of anxiety behavior cannot be ruled out. Nonetheless, the attenuation of the anxiogenic phenotype by blockade of D1 dopaminergic transmission in the central amygdala of LeprDAT-Cre mice, together with the finding of increased burst firing of VTA dopamine neurons, supports that increased VTA dopaminergic input to the amygdala is likely an underlying mechanism of anxiogenesis.
In summary, this study demonstrates that Lepr signaling in midbrain dopamine neurons has important actions on anxiety-related behavior that are distinct from the neuronal targets by which leptin regulates feeding behavior or depression-related behavior. Our results provide evidence that the modulation of dopamine neuronal activity by Lepr signaling may represent a novel mechanism for the genesis of anxiety disorders.
Supplementary Material
Acknowledgement
This work was supported by NIH grants NIMH 076929 and NIMH 073844 (to XYL), a NARSAD award from the Maltz Family Foundation (to DJL) and a NIH grant NS056237 (to WZ). The authors would like to thank Dr. Streamson Chua for the Leprflox/flox mice, Dr. Xiaoxi Zhuang for the Slc6a3-Cre mice.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
Supplementary information is available at Molecular Psychiatry’s website.
References
- 1.Berthoud HR, Morrison C. The brain, appetite, and obesity. Annu Rev Psychol. 2008;59:55–92. doi: 10.1146/annurev.psych.59.103006.093551. [DOI] [PubMed] [Google Scholar]
- 2.Elmquist JK, Coppari R, Balthasar N, Ichinose M, Lowell BB. Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. J Comp Neurol. 2005;493(1):63–71. doi: 10.1002/cne.20786. [DOI] [PubMed] [Google Scholar]
- 3.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395(6704):763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 4.Lu XY. The leptin hypothesis of depression: a potential link between mood disorders and obesity? Curr Opin Pharmacol. 2007;7(6):648–652. doi: 10.1016/j.coph.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Myers MG, Jr, Munzberg H, Leinninger GM, Leshan RL. The geometry of leptin action in the brain: more complicated than a simple ARC. Cell Metab. 2009;9(2):117–123. doi: 10.1016/j.cmet.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Licinio J, Ribeiro L, Busnello JV, Delibasi T, Thakur S, Elashoff RM, et al. Effects of leptin replacement on macro- and micronutrient preferences. Int J Obes (Lond) 2007;31(12):1859–1863. doi: 10.1038/sj.ijo.0803703. [DOI] [PubMed] [Google Scholar]
- 7.Scott MM, Lachey JL, Sternson SM, Lee CE, Elias CF, Friedman JM, et al. Leptin targets in the mouse brain. J Comp Neurol. 2009;514(5):518–532. doi: 10.1002/cne.22025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42(6):983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411(6836):480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 10.Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49(2):191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 11.Leinninger GM, Jo YH, Leshan RL, Louis GW, Yang H, Barrera JG, et al. Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell Metab. 2009;10(2):89–98. doi: 10.1016/j.cmet.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Figlewicz DP, Evans SB, Murphy J, Hoen M, Baskin DG. Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat. Brain Res. 2003;964(1):107–115. doi: 10.1016/s0006-8993(02)04087-8. [DOI] [PubMed] [Google Scholar]
- 13.Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, et al. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006;51(6):811–822. doi: 10.1016/j.neuron.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 14.Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, et al. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51(6):801–810. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 15.Leshan RL, Opland DM, Louis GW, Leinninger GM, Patterson CM, Rhodes CJ, et al. Ventral tegmental area leptin receptor neurons specifically project to and regulate cocaine- and amphetamine-regulated transcript neurons of the extended central amygdala. J Neurosci. 2010;30(16):5713–5723. doi: 10.1523/JNEUROSCI.1001-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Willner P. Dopamine and depression: a review of recent evidence. I. Empirical studies. Brain Res. 1983;287(3):211–224. doi: 10.1016/0165-0173(83)90005-x. [DOI] [PubMed] [Google Scholar]
- 17.Zacharko RM, Bowers WJ, Kokkinidis L, Anisman H. Region-specific reductions of intracranial self-stimulation after uncontrollable stress: possible effects on reward processes. Behav Brain Res. 1983;9(2):129–141. doi: 10.1016/0166-4328(83)90123-7. [DOI] [PubMed] [Google Scholar]
- 18.Willner P. Dopamine and depression: a review of recent evidence. III. The effects of antidepressant treatments. Brain Res. 1983;287(3):237–246. doi: 10.1016/0165-0173(83)90007-3. [DOI] [PubMed] [Google Scholar]
- 19.Berridge KC. Motivation concepts in behavioral neuroscience. Physiol Behav. 2004;81(2):179–209. doi: 10.1016/j.physbeh.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Pezze MA, Feldon J. Mesolimbic dopaminergic pathways in fear conditioning. Prog Neurobiol. 2004;74(5):301–320. doi: 10.1016/j.pneurobio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5(6):483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 22.Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59(12):1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 23.Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80(1):1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- 24.Roseberry AG, Painter T, Mark GP, Williams JT. Decreased vesicular somatodendritic dopamine stores in leptin-deficient mice. J Neurosci. 2007;27(26):7021–7027. doi: 10.1523/JNEUROSCI.1235-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perry ML, Leinninger GM, Chen R, Luderman KD, Yang H, Gnegy ME, et al. Leptin promotes dopamine transporter and tyrosine hydroxylase activity in the nucleus accumbens of Sprague-Dawley rats. J Neurochem. 2010 doi: 10.1111/j.1471-4159.2010.06757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krugel U, Schraft T, Kittner H, Kiess W, Illes P. Basal and feeding-evoked dopamine release in the rat nucleus accumbens is depressed by leptin. Eur J Pharmacol. 2003;482(1–3):185–187. doi: 10.1016/j.ejphar.2003.09.047. [DOI] [PubMed] [Google Scholar]
- 27.Morton GJ, Blevins JE, Kim F, Matsen M, Figlewicz DP. The action of leptin in the ventral tegmental area to decrease food intake is dependent on Jak-2 signaling. Am J Physiol Endocrinol Metab. 2009;297(1):E202–E210. doi: 10.1152/ajpendo.90865.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu J, Garza JC, Bronner J, Kim CS, Zhang W, Lu XY. Acute administration of leptin produces anxiolytic-like effects: a comparison with fluoxetine. Psychopharmacology (Berl) 2010;207(4):535–545. doi: 10.1007/s00213-009-1684-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu XY, Kim CS, Frazer A, Zhang W. Leptin: a potential novel antidepressant. Proc Natl Acad Sci U S A. 2006;103(5):1593–1598. doi: 10.1073/pnas.0508901103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asakawa A, Inui A, Inui T, Katsuura G, Fujino MA, Kasuga M. Leptin treatment ameliorates anxiety in ob/ob obese mice. J Diabetes Complications. 2003;17(2):105–107. doi: 10.1016/s1056-8727(02)00185-x. [DOI] [PubMed] [Google Scholar]
- 31.Finger BC, Dinan TG, Cryan JF. Leptin-deficient mice retain normal appetitive spatial learning yet exhibit marked increases in anxiety-related behaviours. Psychopharmacology (Berl) 2010 doi: 10.1007/s00213-010-1858-z. [DOI] [PubMed] [Google Scholar]
- 32.McMinn JE, Liu SM, Dragatsis I, Dietrich P, Ludwig T, Eiden S, et al. An allelic series for the leptin receptor gene generated by CRE and FLP recombinase. Mamm Genome. 2004;15(9):677–685. doi: 10.1007/s00335-004-2340-1. [DOI] [PubMed] [Google Scholar]
- 33.Zhuang X, Masson J, Gingrich JA, Rayport S, Hen R. Targeted gene expression in dopamine and serotonin neurons of the mouse brain. J Neurosci Methods. 2005;143(1):27–32. doi: 10.1016/j.jneumeth.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 34.Augood SJ, Westmore K, McKenna PJ, Emson PC. Co-expression of dopamine transporter mRNA and tyrosine hydroxylase mRNA in ventral mesencephalic neurones. Brain Res Mol Brain Res. 1993;20(4):328–334. doi: 10.1016/0169-328x(93)90059-x. [DOI] [PubMed] [Google Scholar]
- 35.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21(1):70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 36.Crawley JN, Paylor R. A proposed test battery and constellations of specific behavioral paradigms to investigate the behavioral phenotypes of transgenic and knockout mice. Horm Behav. 1997;31(3):197–211. doi: 10.1006/hbeh.1997.1382. [DOI] [PubMed] [Google Scholar]
- 37.Scott JW, Davis LM, Shannon D, Kaplan C. Relation of chemical structure to spatial distribution of sensory responses in rat olfactory epithelium. J Neurophysiol. 1996;75(5):2036–2049. doi: 10.1152/jn.1996.75.5.2036. [DOI] [PubMed] [Google Scholar]
- 38.Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14(3):149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 39.Crawley J, Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav. 1980;13(2):167–170. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- 40.File SE, Hyde JR. Can social interaction be used to measure anxiety? Br J Pharmacol. 1978;62(1):19–24. doi: 10.1111/j.1476-5381.1978.tb07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shephard RA, Broadhurst PL. Hyponeophagia and arousal in rats: effects of diazepam, 5-methoxy-N,N-dimethyltryptamine, d-amphetamine and food deprivation. Psychopharmacology (Berl) 1982;78(4):368–372. doi: 10.1007/BF00433744. [DOI] [PubMed] [Google Scholar]
- 42.Willner P, Muscat R, Papp M. Chronic mild stress-induced anhedonia: a realistic animal model of depression. Neurosci Biobehav Rev. 1992;16(4):525–534. doi: 10.1016/s0149-7634(05)80194-0. [DOI] [PubMed] [Google Scholar]
- 43.Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266(5604):730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 44.Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 1985;85(3):367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- 45.Hyland BI, Reynolds JN, Hay J, Perk CG, Miller R. Firing modes of midbrain dopamine cells in the freely moving rat. Neuroscience. 2002;114(2):475–492. doi: 10.1016/s0306-4522(02)00267-1. [DOI] [PubMed] [Google Scholar]
- 46.Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons--1. Identification and characterization. Neuroscience. 1983;10(2):301–315. doi: 10.1016/0306-4522(83)90135-5. [DOI] [PubMed] [Google Scholar]
- 47.Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30(5):220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 48.Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci. 1984;4(11):2877–2890. doi: 10.1523/JNEUROSCI.04-11-02877.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meister B, Elde R. Dopamine transporter mRNA in neurons of the rat hypothalamus. Neuroendocrinology. 1993;58(4):388–395. doi: 10.1159/000126568. [DOI] [PubMed] [Google Scholar]
- 50.Smith A, Roberts DC. Oral self-administration of sweetened nicotine solutions by rats. Psychopharmacology (Berl) 1995;120(3):341–346. doi: 10.1007/BF02311182. [DOI] [PubMed] [Google Scholar]
- 51.Pecina S, Berridge KC, Parker LA. Pimozide does not shift palatability: separation of anhedonia from sensorimotor suppression by taste reactivity. Pharmacol Biochem Behav. 1997;58(3):801–811. doi: 10.1016/s0091-3057(97)00044-0. [DOI] [PubMed] [Google Scholar]
- 52.Hajnal A, Norgren R. Accumbens dopamine mechanisms in sucrose intake. Brain Res. 2001;904(1):76–84. doi: 10.1016/s0006-8993(01)02451-9. [DOI] [PubMed] [Google Scholar]
- 53.Hajnal A, Norgren R. Repeated access to sucrose augments dopamine turnover in the nucleus accumbens. Neuroreport. 2002;13(17):2213–2216. doi: 10.1097/00001756-200212030-00010. [DOI] [PubMed] [Google Scholar]
- 54.Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl) 1997;134(4):319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- 55.Elizalde N, Gil-Bea FJ, Ramirez MJ, Aisa B, Lasheras B, Del Rio J, et al. Long-lasting behavioral effects and recognition memory deficit induced by chronic mild stress in mice: effect of antidepressant treatment. Psychopharmacology (Berl) 2008;199(1):1–14. doi: 10.1007/s00213-007-1035-1. [DOI] [PubMed] [Google Scholar]
- 56.Monleon S, D'Aquila P, Parra A, Simon VM, Brain PF, Willner P. Attenuation of sucrose consumption in mice by chronic mild stress and its restoration by imipramine. Psychopharmacology (Berl) 1995;117(4):453–457. doi: 10.1007/BF02246218. [DOI] [PubMed] [Google Scholar]
- 57.Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl) 1987;93(3):358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- 58.Lodge DJ, Grace AA. The laterodorsal tegmentum is essential for burst firing of ventral tegmental area dopamine neurons. Proc Natl Acad Sci U S A. 2006;103(13):5167–5172. doi: 10.1073/pnas.0510715103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mameli-Engvall M, Evrard A, Pons S, Maskos U, Svensson TH, Changeux JP, et al. Hierarchical control of dopamine neuron-firing patterns by nicotinic receptors. Neuron. 2006;50(6):911–921. doi: 10.1016/j.neuron.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 60.Ring LE, Zeltser LM. Disruption of hypothalamic leptin signaling in mice leads to early-onset obesity, but physiological adaptations in mature animals stabilize adiposity levels. J Clin Invest. 2010;120(8):2931–2941. doi: 10.1172/JCI41985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van de Wall E, Leshan R, Xu AW, Balthasar N, Coppari R, Liu SM, et al. Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology. 2008;149(4):1773–1785. doi: 10.1210/en.2007-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baicy K, London ED, Monterosso J, Wong ML, Delibasi T, Sharma A, et al. Leptin replacement alters brain response to food cues in genetically leptin-deficient adults. Proc Natl Acad Sci U S A. 2007;104(46):18276–18279. doi: 10.1073/pnas.0706481104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Farooqi IS, Bullmore E, Keogh J, Gillard J, O'Rahilly S, Fletcher PC. Leptin regulates striatal regions and human eating behavior. Science. 2007;317(5843):1355. doi: 10.1126/science.1144599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Matochik JA, London ED, Yildiz BO, Ozata M, Caglayan S, DePaoli AM, et al. Effect of leptin replacement on brain structure in genetically leptin-deficient adults. J Clin Endocrinol Metab. 2005;90(5):2851–2854. doi: 10.1210/jc.2004-1979. [DOI] [PubMed] [Google Scholar]
- 65.Paz-Filho GJ, Babikian T, Asarnow R, Delibasi T, Esposito K, Erol HK, et al. Leptin replacement improves cognitive development. PLoS One. 2008;3(8):e3098. doi: 10.1371/journal.pone.0003098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sartorius N, Ustun TB, Lecrubier Y, Wittchen HU. Depression comorbid with anxiety: results from the WHO study on psychological disorders in primary health care. Br J Psychiatry Suppl. 1996;(30):38–43. [PubMed] [Google Scholar]
- 67.Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc Natl Acad Sci U S A. 2009;106(12):4894–4899. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kalivas PW, Duffy P. Selective activation of dopamine transmission in the shell of the nucleus accumbens by stress. Brain Res. 1995;675(1–2):325–328. doi: 10.1016/0006-8993(95)00013-g. [DOI] [PubMed] [Google Scholar]
- 69.Pruessner JC, Champagne F, Meaney MJ, Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C]raclopride. J Neurosci. 2004;24(11):2825–2831. doi: 10.1523/JNEUROSCI.3422-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Imperato A, Puglisi-Allegra S, Casolini P, Angelucci L. Changes in brain dopamine and acetylcholine release during and following stress are independent of the pituitary-adrenocortical axis. Brain Res. 1991;538(1):111–117. doi: 10.1016/0006-8993(91)90384-8. [DOI] [PubMed] [Google Scholar]
- 71.Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MJ. Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J Neurochem. 1989;52(5):1655–1658. doi: 10.1111/j.1471-4159.1989.tb09224.x. [DOI] [PubMed] [Google Scholar]
- 72.Tidey JW, Miczek KA. Social defeat stress selectively alters mesocorticolimbic dopamine release: an in vivo microdialysis study. Brain Res. 1996;721(1–2):140–149. doi: 10.1016/0006-8993(96)00159-x. [DOI] [PubMed] [Google Scholar]
- 73.Borowski TB, Kokkinidis L. Contribution of ventral tegmental area dopamine neurons to expression of conditional fear: effects of electrical stimulation, excitotoxin lesions, and quinpirole infusion on potentiated startle in rats. Behav Neurosci. 1996;110(6):1349–1364. doi: 10.1037//0735-7044.110.6.1349. [DOI] [PubMed] [Google Scholar]
- 74.de Oliveira AR, Reimer AE, Brandao ML. Dopamine D2 receptor mechanisms in the expression of conditioned fear. Pharmacol Biochem Behav. 2006;84(1):102–111. doi: 10.1016/j.pbb.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 75.Gifkins A, Greba Q, Kokkinidis L. Ventral tegmental area dopamine neurons mediate the shock sensitization of acoustic startle: a potential site of action for benzodiazepine anxiolytics. Behav Neurosci. 2002;116(5):785–794. [PubMed] [Google Scholar]
- 76.Munro LJ, Kokkinidis L. Infusion of quinpirole and muscimol into the ventral tegmental area inhibits fear-potentiated startle: implications for the role of dopamine in fear expression. Brain Res. 1997;746(1–2):231–238. doi: 10.1016/s0006-8993(96)01225-5. [DOI] [PubMed] [Google Scholar]
- 77.Lammel S, Hetzel A, Hackel O, Jones I, Liss B, Roeper J. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57(5):760–773. doi: 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 78.Hasue RH, Shammah-Lagnado SJ. Origin of the dopaminergic innervation of the central extended amygdala and accumbens shell: a combined retrograde tracing and immunohistochemical study in the rat. J Comp Neurol. 2002;454(1):15–33. doi: 10.1002/cne.10420. [DOI] [PubMed] [Google Scholar]
- 79.Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9(1–6):321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- 80.Ungerstedt U. Stereotaxic mapping of the monoamine pathways in the rat brain. Acta Physiol Scand Suppl. 1971;367:1–48. doi: 10.1111/j.1365-201x.1971.tb10998.x. [DOI] [PubMed] [Google Scholar]
- 81.Aggleton JP. The contribution of the amygdala to normal and abnormal emotional states. Trends Neurosci. 1993;16(8):328–333. doi: 10.1016/0166-2236(93)90110-8. [DOI] [PubMed] [Google Scholar]
- 82.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 83.Amaral DG, Insausti R. Retrograde transport of D-[3H]-aspartate injected into the monkey amygdaloid complex. Exp Brain Res. 1992;88(2):375–388. doi: 10.1007/BF02259113. [DOI] [PubMed] [Google Scholar]
- 84.Pitkanen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci. 1997;20(11):517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- 85.Coco ML, Kuhn CM, Ely TD, Kilts CD. Selective activation of mesoamygdaloid dopamine neurons by conditioned stress: attenuation by diazepam. Brain Res. 1992;590(1–2):39–47. doi: 10.1016/0006-8993(92)91079-t. [DOI] [PubMed] [Google Scholar]
- 86.Yokoyama M, Suzuki E, Sato T, Maruta S, Watanabe S, Miyaoka H. Amygdalic levels of dopamine and serotonin rise upon exposure to conditioned fear stress without elevation of glutamate. Neurosci Lett. 2005;379(1):37–41. doi: 10.1016/j.neulet.2004.12.047. [DOI] [PubMed] [Google Scholar]
- 87.Torres IL, Gamaro GD, Vasconcellos AP, Silveira R, Dalmaz C. Effects of chronic restraint stress on feeding behavior and on monoamine levels in different brain structures in rats. Neurochem Res. 2002;27(6):519–525. doi: 10.1023/a:1019856821430. [DOI] [PubMed] [Google Scholar]
- 88.Guarraci FA, Kapp BS. An electrophysiological characterization of ventral tegmental area dopaminergic neurons during differential pavlovian fear conditioning in the awake rabbit. Behav Brain Res. 1999;99(2):169–179. doi: 10.1016/s0166-4328(98)00102-8. [DOI] [PubMed] [Google Scholar]
- 89.Horvitz JC. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience. 2000;96(4):651–656. doi: 10.1016/s0306-4522(00)00019-1. [DOI] [PubMed] [Google Scholar]
- 90.Ungless MA, Magill PJ, Bolam JP. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science. 2004;303(5666):2040–2042. doi: 10.1126/science.1093360. [DOI] [PubMed] [Google Scholar]
- 91.Inglis FM, Moghaddam B. Dopaminergic innervation of the amygdala is highly responsive to stress. J Neurochem. 1999;72(3):1088–1094. doi: 10.1046/j.1471-4159.1999.0721088.x. [DOI] [PubMed] [Google Scholar]
- 92.Suzuki T, Ishigooka J, Watanabe S, Miyaoka H. Enhancement of delayed release of dopamine in the amygdala induced by conditioned fear stress in methamphetamine-sensitized rats. Eur J Pharmacol. 2002;435(1):59–65. doi: 10.1016/s0014-2999(01)01563-1. [DOI] [PubMed] [Google Scholar]
- 93.Dawson TM, Gehlert DR, McCabe RT, Barnett A, Wamsley JK. D-1 dopamine receptors in the rat brain: a quantitative autoradiographic analysis. J Neurosci. 1986;6(8):2352–2365. doi: 10.1523/JNEUROSCI.06-08-02352.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xiang L, Szebeni K, Szebeni A, Klimek V, Stockmeier CA, Karolewicz B, et al. Dopamine receptor gene expression in human amygdaloid nuclei: elevated D4 receptor mRNA in major depression. Brain Res. 2008;1207:214–224. doi: 10.1016/j.brainres.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mansour A, Meador-Woodruff JH, Zhou QY, Civelli O, Akil H, Watson SJ. A comparison of D1 receptor binding and mRNA in rat brain using receptor autoradiographic and in situ hybridization techniques. Neuroscience. 1991;45(2):359–371. doi: 10.1016/0306-4522(91)90233-e. [DOI] [PubMed] [Google Scholar]
- 96.Fuxe K, Jacobsen KX, Hoistad M, Tinner B, Jansson A, Staines WA, et al. The dopamine D1 receptor-rich main and paracapsular intercalated nerve cell groups of the rat amygdala: relationship to the dopamine innervation. Neuroscience. 2003;119(3):733–746. doi: 10.1016/s0306-4522(03)00148-9. [DOI] [PubMed] [Google Scholar]
- 97.Boyson SJ, McGonigle P, Molinoff PB. Quantitative autoradiographic localization of the D1 and D2 subtypes of dopamine receptors in rat brain. J Neurosci. 1986;6(11):3177–3188. doi: 10.1523/JNEUROSCI.06-11-03177.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Guarraci FA, Frohardt RJ, Kapp BS. Amygdaloid D1 dopamine receptor involvement in Pavlovian fear conditioning. Brain Res. 1999;827(1–2):28–40. doi: 10.1016/s0006-8993(99)01291-3. [DOI] [PubMed] [Google Scholar]
- 99.Guarraci FA, Frohardt RJ, Young SL, Kapp BS. A functional role for dopamine transmission in the amygdala during conditioned fear. Ann N Y Acad Sci. 1999;877:732–736. doi: 10.1111/j.1749-6632.1999.tb09312.x. [DOI] [PubMed] [Google Scholar]
- 100.Lamont EW, Kokkinidis L. Infusion of the dopamine D1 receptor antagonist SCH 23390 into the amygdala blocks fear expression in a potentiated startle paradigm. Brain Res. 1998;795(1–2):128–136. doi: 10.1016/s0006-8993(98)00281-9. [DOI] [PubMed] [Google Scholar]
- 101.de la Mora MP, Cardenas-Cachon L, Vazquez-Garcia M, Crespo-Ramirez M, Jacobsen K, Hoistad M, et al. Anxiolytic effects of intra-amygdaloid injection of the D1 antagonist SCH23390 in the rat. Neurosci Lett. 2005;377(2):101–105. doi: 10.1016/j.neulet.2004.11.079. [DOI] [PubMed] [Google Scholar]
- 102.Scibilia RJ, Lachowicz JE, Kilts CD. Topographic nonoverlapping distribution of D1 and D2 dopamine receptors in the amygdaloid nuclear complex of the rat brain. Synapse. 1992;11(2):146–154. doi: 10.1002/syn.890110208. [DOI] [PubMed] [Google Scholar]
- 103.Jacobsen KX, Hoistad M, Staines WA, Fuxe K. The distribution of dopamine D1 receptor and mu-opioid receptor 1 receptor immunoreactivities in the amygdala and interstitial nucleus of the posterior limb of the anterior commissure: relationships to tyrosine hydroxylase and opioid peptide terminal systems. Neuroscience. 2006;141(4):2007–2018. doi: 10.1016/j.neuroscience.2006.05.054. [DOI] [PubMed] [Google Scholar]
- 104.Shammah-Lagnado SJ, Beltramino CA, McDonald AJ, Miselis RR, Yang M, de Olmos J, et al. Supracapsular bed nucleus of the stria terminalis contains central and medial extended amygdala elements: evidence from anterograde and retrograde tracing experiments in the rat. J Comp Neurol. 2000;422(4):533–555. doi: 10.1002/1096-9861(20000710)422:4<533::aid-cne5>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 105.Veinante P, Freund-Mercier MJ. Intrinsic and extrinsic connections of the rat central extended amygdala: an in vivo electrophysiological study of the central amygdaloid nucleus. Brain Res. 1998;794(2):188–198. doi: 10.1016/s0006-8993(98)00228-5. [DOI] [PubMed] [Google Scholar]
- 106.Shammah-Lagnado SJ, Alheid GF, Heimer L. Striatal and central extended amygdala parts of the interstitial nucleus of the posterior limb of the anterior commissure: evidence from tract-tracing techniques in the rat. J Comp Neurol. 2001;439(1):104–126. doi: 10.1002/cne.1999. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.