Abstract
Objective To determine the effectiveness of interventions designed to improve outcomes in patients with multimorbidity in primary care and community settings.
Design Systematic review.
Data sources Medline, Embase, CINAHL, CAB Health, Cochrane central register of controlled trials, the database of abstracts of reviews of effectiveness, and the Cochrane EPOC (effective practice and organisation of care) register (searches updated in April 2011).
Eligibility criteria Randomised controlled trials, controlled clinical trials, controlled before and after studies, and interrupted time series analyses reporting on interventions to improve outcomes for people with multimorbidity in primary care and community settings. Multimorbidity was defined as two or more chronic conditions in the same individual. Outcomes included any validated measure of physical or mental health and psychosocial status, including quality of life outcomes, wellbeing, and measures of disability or functional status. Also included were measures of patient and provider behaviour, including drug adherence, utilisation of health services, acceptability of services, and costs.
Data selection Two reviewers independently assessed studies for eligibility, extracted data, and assessed study quality. As meta-analysis of results was not possible owing to heterogeneity in participants and interventions, a narrative synthesis of the results from the included studies was carried out.
Results 10 studies examining a range of complex interventions totalling 3407 patients with multimorbidity were identified. All were randomised controlled trials with a low risk of bias. Two studies described interventions for patients with specific comorbidities. The remaining eight studies focused on multimorbidity, generally in older patients. Consideration of the impact of socioeconomic deprivation was minimal. All studies involved complex interventions with multiple components. In six of the 10 studies the predominant component was a change to the organisation of care delivery, usually through case management or enhanced multidisciplinary team work. In the remaining four studies, intervention components were predominantly patient oriented. Overall the results were mixed, with a trend towards improved prescribing and drug adherence. The results indicated that it is difficult to improve outcomes in this population but that interventions focusing on particular risk factors in comorbid conditions or functional difficulties in multimorbidity may be more effective. No economic analyses were included, although the improvements in prescribing and risk factor management in some studies could provide potentially important cost savings.
Conclusions Evidence on the care of patients with multimorbidity is limited, despite the prevalence of multimorbidity and its impact on patients and healthcare systems. Interventions to date have had mixed effects, although are likely to be more effective if targeted at risk factors or specific functional difficulties. A need exists to clearly identify patients with multimorbidity and to develop cost effective and specifically targeted interventions that can improve health outcomes.
Introduction
Healthcare systems are placing increasing emphasis on the management of chronic diseases. Despite the increasing numbers of patients with two or more chronic conditions, or multimorbidity, the delivery of care is usually built around single diseases.1 A well established evidence base highlights the impact and costs associated with multimorbidity in both younger and older patients.2 3 4 5 6 Patients with multimorbidity are more likely to die prematurely than those with single conditions, be admitted to hospital, and have longer hospital stays.6 7 They have poorer quality of life, have loss of physical functioning, and are more likely to experience depression and to be receiving multiple drugs with consequent difficulties with adherence.8 9 Evidence of the impact of socioeconomic deprivation is also clear as onset of multimorbidity occurs 10-15 years earlier in people living in the most deprived areas compared with those living in the least deprived areas.10
Despite the increasing numbers of patients with multimorbidity, evidence on the effectiveness of interventions to improve outcomes in such patients is limited. The clinical care of these patients is complex and the evidence base for managing chronic conditions is based largely on trials of interventions for single conditions, which too often exclude patients with multimorbidity.11 12 Clinical care is often fragmented, involving both primary care and multiple secondary care specialists who may not be communicating effectively, and there is a clear need for integrated care of multiple conditions.13 14 15 16
We determined the effectiveness of interventions designed to improve physical, psychosocial, and health service utilisation outcomes in patients with multimorbidity in primary care and community settings.
Methods
We used the methodology of the Cochrane collaboration. The protocol and full review are available on the Cochrane Library.17
Inclusion criteria
The types of studies we considered eligible were randomised controlled trials, controlled clinical trials, controlled before and after studies, and interrupted time series analyses (meeting the quality criteria of the Cochrane Effective Practice And Organisation of Care Review Group). Studies published in all languages were eligible for inclusion.
Participants included people or populations with multimorbidity. We adopted the most widely used definition of multimorbidity—that is, the coexistence of multiple chronic diseases and medical conditions in the same individual (usually defined as two or more conditions).2 18 We used the World Health Organization definition of chronic disease, which is “health problems that require ongoing management over a period of years or decades.”19 We included studies that only specifically identified participants or subgroups of participants as having multimorbidity.
We included any type of intervention that was specifically directed towards a group of patients defined as having multimorbidity. Interventions based only in primary care or community settings were included and we adopted the definition of primary healthcare as providing “integrated, easy to access, health care services by clinicians who are accountable for addressing a large majority of personal health care needs, developing a sustained and continuous relationship with patients, and practicing in the context of family and community.”20 We included care delivered in community settings by practitioners fulfilling the basic criteria for primary care—that is, they are available to treat all common conditions in all age groups and have an ongoing relationship with their patients.
To group interventions for this review we used the taxonomy of interventions developed by the Cochrane Effective Practice And Organisation of Care Review Group (box).
Taxonomy of interventions according to Cochrane Effective Practice And Organisation of Care Review Group
Professional interventions
For example, education designed to change the behaviour of clinicians
Organisational interventions
For example, any change to care delivery such as case management (defined as the “explicit allocation of co-ordination tasks to an appointed individual or group”)21, or the addition of different healthcare workers, such as a pharmacist, to the healthcare team
Patient oriented interventions
For example, patient education or support for self management
Financial interventions
For example, financial incentives to providers to reach treatment targets
Regulatory interventions
For example, changes to local or national regulations designed to alter care delivery to improve outcomes
Where interventions had multiple components we defined each using the taxonomy and highlighted the predominant one of the intervention for each study. We excluded professional educational interventions or research initiatives where there was no specified structured clinical care delivered to an identified group of patients with multimorbidity and interventions targeted at comorbid conditions where the intervention was targeted solely at one of the conditions.
Outcome measures
We included validated outcome measures reporting on physical health; patient reported measures reflecting psychological and functional status , including wellbeing, quality of life, and disability or pain; utilisation of health services, including hospital admissions and visits to doctors; patient behaviour, including diet and exercise and measures of drug adherence; and provider behaviour, including prescribing. We also included any available economic outcomes reported in included studies.
Search strategy
The search strategy was particularly challenging as multimorbidity is a relatively new term and is sometimes used synonymously with the term comorbidity, although this tends to be used in relation to diseases that coexist with an index condition. Comorbidity is a MeSH term, whereas multimorbidity is not, so we included both terms in our search. For pragmatic reasons and because multimorbidity is a relatively new concept we limited the Medline search to articles indexed from 1990 onwards.
We searched the following electronic databases: Medline, Embase, CINAHL, CAB Health, the Cochrane central register of controlled trials (CENTRAL), the database of abstracts of reviews of effectiveness, AMED—Allied and Complimentary Medicine (1985 to current), and the Cochrane EPOC (effective practice and organisation of care) register (searches updated in April 2011). We also searched reference lists of all included papers and contacted the authors of relevant papers for any further published or unpublished work (see the supplementary file for the full Medline search strategy).
Data extraction
Potentially relevant studies were determined by review of the abstracts of search results. We retrieved the full text copies of all articles identified as potentially relevant. Two authors (SS and HS) independently assessed each of these 30 retrieved articles for inclusion, extracted data, and cross checked data abstraction forms using a modified version of the Cochrane Effective Practice And Organisation of Care data collection checklist. The review authors resolved disagreements about eligibility and quality by consensus.
Assessment of risk of bias
Two authors (SS and HS) independently assessed risk of bias using standard Cochrane criteria, including sequence generation, allocation concealment, blinding, protection against contamination, reliability of primary outcomes, follow up of patients, and performance of baseline measurement.
Data analysis
For each trial we calculated results in terms of absolute difference (mean or proportion of outcome in intervention group minus control at study completion) and relative percentage difference (absolute difference divided by post-intervention score in the control group). We contacted authors for missing data. If studies were identified that were similar in terms of settings, patients, interventions, outcome assessment, and study methods we planned to carry out meta-analysis. This was not, however, possible owing to noticeable clinical heterogeneity. We therefore carried out a narrative synthesis of the results from the included studies.
Results
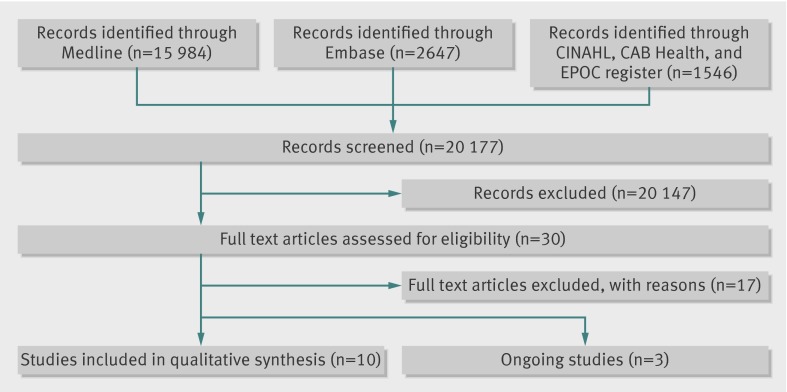
Figure 1 outlines the search. Overall, 20 177 potentially eligible titles were identified.
Fig 1 Study flow of papers through review
Overall, 17 of 30 full text articles screened for eligibility were excluded on the basis of study design and intervention type. Three ongoing studies led to the identification of 10 studies included in the narrative synthesis. All 10 were randomised controlled trials, totalling 3407 patients (table 1) The studies varied in duration from eight weeks to two years, with most lasting 6-12 months. Eight of the 10 studies included patients with a broad range of conditions,22 23 24 25 26 27 28 29 whereas the remaining two focused on comorbidities: coexisting depression and hypertension30 and coexisting depression and diabetes or heart disease.31 Most were based in the United States, apart from one study set in the United Kingdom27 and one in Canada.26 All were funded by a government or university grant or charitable foundation.
Table 1.
Characteristics of studies included in systematic reviews
| Study | Study participants | Duration and follow-up | Intervention elements; (theoretical framework, where specified) | Outcomes | Results (primary outcomes, where specified): intervention versus control |
|---|---|---|---|---|---|
| Predominantly organisational interventions: | |||||
| Bognor 200830 | Aged >50, depression and hypertension (n=64) | Intervention six weeks, follow-up two weeks later | Care manager, structured visits, telephone contact, and patient care plans (adherence based model) | Depression scores (CES-D score); systolic blood pressure; drug adherence | CES-D score 9.9 v 19.3, P=0.006; systolic blood pressure (mm Hg) 127.3 v 141.3, P=0.003; ≥80% adherence to antidepressants 23% v 10%, P=0.001, ≥80% adherence to antihypertensives 25% v 10%, P<0.001 |
| Boult 201122 | Aged >65, multiple conditions and high service use (n=904) | Intervention 18 months, follow-up at six and 18 months | Organisational: guided care nurse managers, enhanced multidisciplinary team, home assessments and monthly monitoring, patient care plans. Professional: education of nurse managers. Patient: self management support | Primary outcome: health service use hospital admissions, nursing facility use, visits, and home healthcare episodes. Secondary outcomes: quality of chronic care (PACIC) scores | Adjusted intervention:control ratio of service use: hospital 30 day readmissions 1.01 (95% CI 0.83 to 1.23); hospital days 0.79 (0.53 to 1.16); skilled nursing facility admissions 1.00 (0.77 to 1.30); skilled nursing facilities days 0.92 (0.6 to 1.4); emergency department visits 0.84 (0.48 to 1.47); primary care visits 1.04 (0.81 to 1.34); speciality care visits 1.02 (0.91 to 1.14); home healthcare episodes 1.07 (0.93 to 1.23); (PACIC) scores 0.70 (0.53 to 0.93) |
| Hogg 200826 | Aged >50, at least two conditions and at risk of experiencing adverse outcome (n=241) | Intervention 15 months, follow-up on completion of intervention | Enhanced multidisciplinary team with structured home visit, drug review, and patient care plans | Primary outcome: chronic disease management score. Secondary outcomes included preventive care delivery score, physical health outcomes, health service use, psychosocial measures, quality of life, and activities of daily living | Difference in chronic disease management score after intervention 0.091 (95% CI 0.037 to 0.144) |
| Katon 201031 | Depression and diabetes or coronary heart disease, or both (n=214) | Intervention 12 months, follow-up at 12 months | Organisational: TEAMcare nurses, structured visits, patient care plans and treatment targets, weekly team meetings, and use of electronic registry to track patient progress. Professional: education of nurse managers. Patient: support for self care (behavioural activation theory) | Primary outcomes: depression scores (SCL-20); diabetes (glycated haemoglobin); systolic blood pressure; and low density lipoprotein cholesterol. Secondary outcomes: increases in drug adjustments, quality of life, and satisfaction with care | Adjusted between group difference (95% CI): depression scores SCL-20) −0.41 (−0.56 to −0.26); glycated haemoglobin −0.56% (−0.85% to −0.27%); systolic blood pressure (mm Hg) −3.4 (−6.9 to 0.1); low density lipoprotein cholesterol (mg/dL) −9.1 (−17.5 to −0.8) |
| Krska 200127 | Aged >65, at least two conditions (n=332) | Intervention three months, follow-up three months after drug review | Senior care connections: structured visit with pharmaceutical patient care plan created by pharmacist and implemented by practice team | Primary outcome: pharmaceutical care issues. Secondary outcomes: medicine costs, quality of life, and health service use | Pharmaceutical care issues (%) resolved after intervention: 82.7% v 41.2%, P<0.001 |
| Sommers 200029 | Aged >65, at least two conditions (n=543) | Intervention two years, follow-up 12 months after intervention | Organisational: enhanced multidisciplinary team including social worker, home assessment, and patient care plans, professional: training of care coordinators | Health service use including admissions, office visits, emergency department visits, home care visits, and nursing home visits. Patient reported health status: social activities count, quality of life, depression scores, nutrition checklists, and drug adherence | Odds ratio admissions/patient/year 0.63 (95% CI 0.41 to 0.96); ≥1 60 day readmissions 0.26 (0.08 to 0.84). Not fully reported for seven other outcomes, non-significant for six. Difference in adjusted mean scores, social activities count 0.50 (95% CI 0.02 to 1.00). Symptom scale 0.50 (−3.20 to 0.16), SF-36 self rated health 0.10 (−0.27 to 0.02), not reported for four other outcomes, non-significant |
| Predominantly patient oriented: | |||||
| Eakin 200723 | Multimorbidity defined as at least two conditions (n=175) (data for multimorbidity group from authors) | Intervention 16 weeks, follow-up six months after intervention | Patient: self management support, diet, and exercise intervention delivered by health educator; organisational: structured visits and telephone contact (chronic care model: patient self management) | Dietary behaviour, support for healthy lifestyles, and physical activity | Adjusted mean (SE): dietary behaviour (lower score better) 2.20 (0.05) v 2.41 (0.05), P<0.5; support for healthy lifestyle (higher score better) 2.98 (0.06) v 2.68 (0.06), P<0.05; change minutes walking/week 8 (22) v −10 (27), P>0.5 |
| Gitlin 200624 32 | Aged >70, multiple conditions and reported difficulties with activities of daily living (n=319) | 12 months intervention, follow-up at completion of intervention, four year mortality follow-up | Patient (Advancing Better Living for Elders, ABLE): occupational therapy and physiotherapy home based intervention including balance and muscle strengthening and fall recovery techniques, patient: problem solving techniques (lifespan theory of control) | Primary outcomes: functional difficulty (activities of daily living, activities of daily living, instrumental activities of daily living, and mobility), self efficacy and fear of falling (self efficacy for falls). Secondary outcomes: adaptive strategy use and presence of home hazards. Four year follow-up: mortality | Difference in adjusted means at 12 months: activities of daily living −0.10 (95% CI −0.21 to 0.02); instrumental activities of daily living −0.12 (−0.26 to 0.03); mobility −0.14 (−0.29 to 0.01); overall self efficacy 0.09 (−0.06 to 0.23); fear of falling 0.56 (0.15 to 0.97); mortality at two years 5.6% (9 deaths) v 13.2% (21 deaths), P=0.02. Mortality at four years no significant difference, intervention increased survivorship by 3.5 years |
| Hochhalter 201025 | Aged >65, at least two of seven chronic conditions (n=79) | Intervention three months, follow-up three months after intervention | Patient engagement intervention led by “coaches” with focus on making most of healthcare (chronic care model: patient self management) | Primary outcome: patient activation measure. Secondary outcomes: total unhealthy days, self efficacy, and self rated health | Patient activation measure: reported as no significant difference between intervention and control at follow-up |
| Lorig 199928 | Aged >40, at least two of heart disease, lung disease, arthritis, or stroke (n=536) (subgroup of patients with comorbidities) | Intervention seven weeks, follow-up at six months | Patient (weekly community based meetings led by trained volunteer lay leaders focusing on self management and peer support) (Bandura’s self efficacy theory) | Health service use: admissions, emergency department plus visits to physician. Health behaviours: four measures. Health status: eight measures | Adjusted mean difference (SD). Number of admissions 0.19 (0.73) v 0.33 (1.2), P<0.5; nights in hospital 1.05 (6.3) v 2.1 (6.8), P<0.5; number of physician visits 4.96 (6.1) v 6.87 (7.2), P>0.5. Significance of 12 measures relating to health behaviours and health status in comorbidity subgroup not reported |
In most of the included studies the comparator was usual medical care, which was supplemented by a newsletter or leaflet23 24 or assessment but no follow-on intervention.27 30 31 One study used an attention control based on an unrelated topic, with control patients also attending a group session.25 No study specifically reported patient or family involvement in the intervention design. Whereas only two of the six organisational studies were based on explicit theoretical frameworks, all four of the patient oriented interventions were guided by a variety of frameworks (table 1). In the four organisational studies that did not specify a theoretical framework, the intervention was presented as enhancing multidisciplinary teamwork. Only one of the 10 studies specifically targeted disadvantaged patients or those on low incomes.23 Socioeconomic status was recorded at baseline in three of the remaining studies, but no study considered a differential effect of the intervention based on socioeconomic status of participants.
Description of interventions
The interventions were all multifaceted (table 1). Although the interventions identified all multiple components they could be divided into two main groups: predominantly organisational22 26 27 29 30 31 and predominantly patient oriented.23 24 25 28 No study involved financial incentives or regulatory interventions. Table 2 outlines the elements of the interventions in the included studies.
Table 2.
Intervention elements in included studies
| Intervention element | Studies |
|---|---|
| Professional | |
| Education or training of care coordinators | Boult22, Katon31, Sommers29 |
| Financial | |
| Patient | |
| Self management support and patient education | Eakin23*, Boult, Katon, Lorig28*, Hochhalter25* |
| Peer support | Lorig |
| Organisational | |
| Provider: | |
| Care coordination or management | Bognor30*, Boult*, Katon*, Sommers29 |
| Enhanced multidisciplinary team (for example, addition of pharmacist or social worker) | Hogg26*, Katon, Krska27*, Sommers* |
| Patient: | |
| Individual care plans | Bognor, Boult, Hogg, Katon, Krska, Sommers |
| Structural: | |
| Structured visits | Eakin, Bognor, Boult, Hogg, Katon, Krska |
| Structured telephone contact | Bognor, Eakin, Hochhalter, Hogg |
| Regulatory† | |
*No studies reported on this element.
Risk of bias in included studies
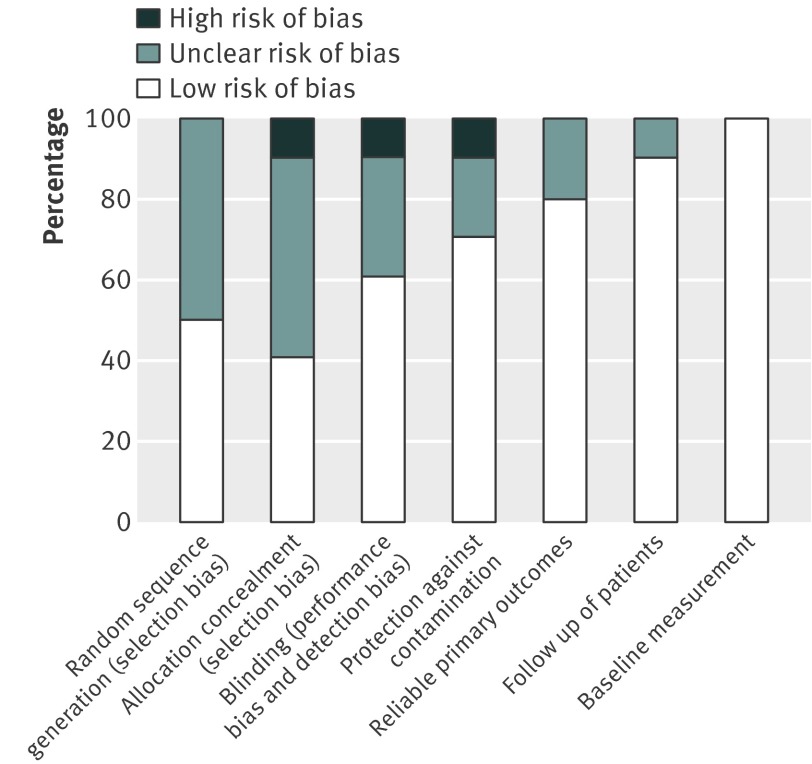
Overall the studies had low risk of bias (fig 2). Baseline measurement, use of reliable outcomes, and follow-up of participants was done in most studies. Randomisation, allocation concealment, and blinding of outcome assessment was less reliably reported or carried out. Only two of the 10 studies had a cluster design ensuring no contamination of control patients.22 29 The patient oriented interventions were less likely to result in contamination of control patients as the intervention was not delivered through healthcare providers. The two cluster randomised controlled trials had accounted for design effects in their analysis, with no unit of analysis errors.22 29
Fig 2 Risk of bias in included studies
Effects by type of intervention
As interventions could be classified generally as organisational or patient oriented, results are presented in these groupings. It must be stressed that all interventions were complex and multifaceted, with overlapping components across these two classifications. No study attempted to link outcomes to specific intervention components.
Six studies had organisational interventions.22 26 27 29 30 31 These predominantly involved case management and coordination of care or the enhancement of skill mix in multidisciplinary teams. Overall the results indicated that organisational interventions targeted at specific risk factor management or that focused on areas where patients have difficulties, such as with the management of medicines, were more likely to be effective. Organisational interventions with a broader focus, such as case management or changes in delivery of care, seemed less effective.
Four studies had predominantly patient oriented interventions.23 24 25 28 All aimed to deal with patient health related behaviour and did not engage or intervene with healthcare providers directly. In general, patient oriented interventions not linked to healthcare delivery seemed less effective, although the exception was a professional led patient oriented intervention focused on functional difficulties, which was associated with significant improvements including a reduction in mortality in the intervention group.24 32
Physical health outcomes
Four of the six studies with organisational interventions reported physical health outcomes. Two of these reported statistically significant improvements in blood pressure.30 31 One also reported statistically significant improvements in glycated haemoglobin and low density lipoprotein cholesterol levels and in their composite primary outcome, which included these three physical health outcomes and a depression score.31
Two studies with patient oriented interventions reported physical health outcomes. One of these reported mixed results for three outcomes, with significant improvements in dietary behaviour and support for healthy lifestyle but no improvement in physical activity.23 The other reported a follow-up study, which found significantly reduced mortality in the first two years after a focused occupational therapy and physiotherapy led intervention targeting functional difficulty and fall prevention (table 1)
Mental health outcomes
Three organisational studies presented data on mental health outcomes.29 30 31 Two of these showed significant improvements in depression related outcomes.30 31 One of the four patient oriented studies presented data on mental health outcomes and found no difference in the management of cognitive symptoms between groups at study completion.28
Patient reported functional health outcomes
Four organisational type studies involved patient reported functional health outcomes.26 27 29 31 One found significant improvements,31 whereas another found significant improvements in social activities but no significant differences in scores on the short form 36 health survey (SF-36) or health assessment questionnaire.29 All four of the patient oriented studies reported a range of these outcomes (table 1) but only one reported improvements, which occurred in two of the six psychosocial outcomes reported.24
Utilisation of health services
Five of the six organisational studies reported outcomes on utilisation of health services.22 26 27 29 31 Only one of these reported significant improvements across a variety of measures related to hospital admissions, although numbers of admissions were small in most studies. Three studies reported on visits to a range of health service providers and none showed significant changes in health service use.22 26 29 One of the four patient oriented studies reported outcomes on health services utilisation and found significant improvements across a variety of measures related to hospital admissions.28
Patient behaviour
Only one organisational study reported measures relating to drug use and adherence and found significant improvements in proportions of intervention patients adhering to both antidepressants and antihypertensives, as measured using automated counting systems in the caps of medicine bottles (MEMS caps).30 Two organisational studies provided data on a variety of outcomes related to health behaviours by patients and found no significant improvement.29 31 Two patient oriented studies also reported on patient health behaviours,23 28 although only one reported significant improvements in diet behaviour scores and in minutes of walking per week.23
Provider behaviour
Two organisational studies reported measures relating to prescribing by practitioners or the management of medicines, both of which indicated significant benefits for intervention patients. One study reported drug adjustments for five classes of drugs related to the comorbid conditions being studied and found statistically significant differences for four of these five groups.31 The other study reported a significant reduction in pharmaceutical care issues in intervention patients, which would have resulted from changes in both intervention by pharmacists and prescribing by general practitioners.27
Two other organisational studies reported on the behaviours of providers related to the management of chronic disease or preventive care.22 26 The guided care study included a secondary outcome of a validated patient measure called the patient assessment of chronic illness care (PACIC) score.22 The aggregate quality score was significantly improved. The other study reported measures relating to chronic disease management and preventive care based on data from charts.26 Both outcomes were significantly improved in the intervention group (table 1).
Acceptability of services
Only two organisational studies reported satisfaction with treatment as secondary outcomes. One study reported that significantly more intervention patients were satisfied with their care at study completion compared with those experiencing usual care.31 The other study reported on the changes in satisfaction for providers as part of an overall examination of the effect of the intervention on providers.22 Five of the 11 domains relating to satisfaction with service provision did not improve significantly.22
Costs
Four organisational studies provided data on costs.22 27 29 31 These data were difficult to compare across the studies and were often presented in relation to non-significant results, indicating that no study had identified a significantly cost effective intervention.
Two patient oriented studies provided data on direct costs of providing the intervention.24 28 One calculated that the reduction in hospital admissions led to a saving in healthcare costs per participant of $750 (£478; €611), which was 10 times the cost of the intervention.
Comorbidity versus multimorbidity studies
Two of the 10 included studies focused on comorbidity rather than on multimorbidity in general.30 31 Both of these studies had organisational type interventions and were able to use more disease focused interventions and outcome measures. Both showed more significant intervention effects than the multimorbidity studies.
Discussion
Multimorbidity presents an important clinical and organisational challenge to the current single system approach to the management of chronic disease. In this review we identified 10 relatively recent randomised trials (up to April 2011). Even within this small number of studies the variation in participants and interventions was substantial and a variety of theoretical frameworks was applied. In two studies the focus was on comorbid conditions, whereby the interventions were directed at prespecified conditions. In the other studies, which included patients with any combination of conditions, the focus tended to be on older patients and the interventions had multiple components, making comparison between studies and between intervention components difficult. Overall these studies suggest that organisational interventions targeted at the management of specific risk factors or focused on areas where patients have difficulties, such as with functional ability or the management of medicines, seem more likely to be effective. Organisational interventions that have a broader focus, such as case management or changes in delivery of care, seem less effective. The patient oriented interventions that were not linked to healthcare delivery or specific functional difficulties were also less effective.
In general the results were mixed and inconclusive, particularly those relating to physical health outcomes. The lack of focus on physical health measures may reflect the challenge in research on multimorbidity, as disease specific clinical outcomes cannot be used. Three studies measured outcomes relating to prescribing, use of drugs, and drug adherence and all found significant benefits. The studies, however, may be too short for these benefits to translate into improvements in physical health outcomes. Costs were presented in six studies but data were only provided on direct costs. The results relating to improved prescribing and risk factor management, particularly in the comorbidity trials, indicate a potential for these interventions to reduce health service costs over longer periods.
Unsurprisingly most of the studies in this review were relatively recent, as research on multimorbidity is a conceptually new area and to date has focused on description and impact rather than on interventions. Earlier studies tended to focus on comorbidity rather than multimorbidity. The improvements found in studies with a focus on comorbid conditions are likely to be related to the strong focus in these interventions of targeting specific conditions. Although it is more challenging to design interventions for people with a broad range of conditions, interventions that seemed more effective in this group were those that targeted specific areas of concern for patients. One of the more recent included studies was a large well designed and executed randomised trial of a broad organisational intervention, the “guided care” model, targeted at high risk people with multimorbidity, but which found no significant benefit overall.22 However, a preplanned subgroup analysis within this trial indicated significant improvements in the use of some health services in the patients enrolled in one of the participating care plans (Kaiser-Permanente, n=365, 43% of full sample). This difference highlights the importance of the healthcare delivery setting into which new interventions are added. Indeed, some of the patient oriented interventions were delivered independently of patients’ healthcare, and most of these studies had limited effectiveness, suggesting that interventions should be integrated into the healthcare system. The results of the patient oriented intervention studies were consistent with those of the Cochrane review on lay led self management support programmes, which found no evidence that these interventions improve psychological health, symptoms, or health related quality of life, or that they significantly alter healthcare use.33
Strengths and weaknesses of this review
To our knowledge this is the first systematic review of interventions for patients with multimorbidity. Potential limitations in the search process related to the lack of a MeSH term for multimorbidity. We therefore used broad search terms, which led to a high yield of citations to be searched and a need to limit the Medline search to 1990 onwards. The review authors are, however, active researchers in the discipline of multimorbidity and we are unaware of any potentially eligible studies missed by the search. We were also unable to retrieve missing data from some authors. As no meta-analysis was undertaken this did not lead to any appreciable bias. We were unable to group sufficient numbers of studies with similar interventions to comment on which elements of interventions (for example, the use of community pharmacists) seemed most effective and to compare our review to other reviews of these interventions.
Implications for clinical practice and future research
Multimorbidity is common in clinical practice, and evidence supporting specific interventions is limited. Most of the studies in this review focused on older patients; however, it is also important to deal with the needs of younger patients with multimorbidity, which are likely to be different and to include problems related to employment and absenteeism.34 Absolute numbers of patients with multimorbidity are higher in those aged less than 65 years,10 and a recent systematic review on prevalence studies on multimorbidity reported a low prevalence before age 40 followed by a steep increase and a plateau after age 70. This would suggest that interventions should target patients across the age spectrum.35 There was minimal consideration of the impact of socioeconomic deprivation in the included studies, and no study considered the possibility of a differential effect of interventions in different socioeconomic groups. Research in Scotland has found that people in the poorest socioeconomic groups are more likely to develop multimorbidity at a younger age and to have greater mental health problems compounding difficulties in their management.10
Research on multimorbidity would be facilitated by the inclusion of multimorbidity as a MeSH term. Otherwise, related literature searches are particularly complex and time consuming. An additional challenge for researchers is to define a set of generic outcome measures that incorporate physical functioning and quality of life and that are responsive to change over time. The recent work of PROMIS (patient reported outcomes measurement information system) provides validated and useful patient reported outcomes that will be particularly relevant for those researching interventions to improve outcomes for patients with multimorbidity.36
Future research should be planned in collaboration with policy makers to ensure applicability and successful integration of interventions into current delivery systems. Studies must include clear conceptual definitions of multimorbidity and the differentiation of multimorbidity from other related concepts such as comorbidity, complexity, frailty, and vulnerability. Without these definitions and consideration of related theoretical concepts, the generalisability or applicability of studies for patients with multimorbidity (with a broader definition than only two or three diseases) will be uncertain, as is the case for many of the studies in this review, particularly those with a specific focus on comorbidity.
The review has highlighted the lack of a clear theoretical framework guiding interventions for multimorbidity at present. There is still a need for prospective cohort studies that will give more information on the trajectory of patients with multimorbidity across all ages and socioeconomic groups.37 Researchers in this area need to collaborate and develop consensus on which models are likely to be most appropriate for multimorbidity. Once identified, authors who develop interventions should consider the individual components of the interventions and the evidence base behind each and link these to relevant outcomes, including health economic analyses.
Conclusion
This review highlights the paucity of research into interventions to improve outcomes for patients with multimorbidity, with the focus to date being on comorbid conditions or multimorbidity in older patients. The review indicates that interventions targeted either at specific combinations of common conditions or at specific problems for patients with multiple conditions, may be more effective. Further research is, however, needed and future interventions should be developed in ways that allow rigorous evaluations that will add to the evidence. There is a need for clear and broader definitions of participants, consideration of appropriate outcomes, and further pragmatic studies based in primary care settings.
What is already known on this topic
Patients with multimorbidity have poorer health outcomes than those with single chronic conditions
Despite the increasing numbers of patients with multimorbidity, the delivery of care is usually built around single diseases
Existing evidence on the effectiveness of interventions to improve outcomes in patients with multimorbidity is limited
What this study adds
This review identified 10 randomised trials, highlighting the paucity of research into interventions to improve outcomes for patients with multimorbidity
The focus to date has been on comorbid conditions or multimorbidity in older patients
The review indicates that interventions targeted either at specific combinations of common conditions or at specific problems for patients with multiple conditions, may be more effective
We thank Alain Mayhew, Sasha Shepperd, Merrick Zwarenstein, Jeremy Grimshaw, Doug Salzwedel, and Michelle Fiander of the Cochrane EPOC review group; Ailbhe Mealy who provided administrative support during the initial drafting of the review; Sophie Hill, Craig Ramsay, Sarah Dennis, Luciana Ballini, and Martin Eccles for their suggestions and comments during the Cochrane review process; Chris Salisbury, Stewart Mercer and Frances Mair during the BMJ review process; and Emma Wallace who provided additional comments and feedback.
Contributors: SMS conceived, coordinated, and designed the review. HS helped coordinate the review, assessed studies for inclusion, and extracted data from included studies. MF, CH, and TO’D, along with SMS and HS, contributed to all stages of the protocol and review and were involved in writing all review drafts and responding to peer review comments.
Funding: This review was not directly funded by any external organisation. SMS and TO’D are affliliated to the HRB Centre for Primary Care Research, which is funded by the Health Research Board of Ireland (http://www.hrbcentreprimarycare.ie/).
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; and no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: Not required.
Data sharing: No additional data available.
Cite this as: BMJ 2012;345:e5205
Web Extra. Extra material supplied by the author
Medline search strategy
References
- 1.Fortin M, Bravo G, Hudon C, Vanasse A, Lapointe L. Prevalence of multimorbidity among adults seen in family practice. Ann Fam Med 2005;3:223-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fortin M, Soubhi H, Hudon C, Bayliss EA, van den Akker M. Multimorbidity’s many challenges. BMJ 2007;334:1016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marengoni A, Angleman S, Melis R, Mangialasche F, Karp A, Garmen A, et al. Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev 2011;10:430-9. [DOI] [PubMed] [Google Scholar]
- 4.Smith SM, O’Dowd T. Chronic diseases: what happens when they come in multiples? Br J Gen Pract 2007;57:268-70. [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor AW, Price K, Gill TK, Adams R, Pilkington R, Carrangis N, et al. Multimorbidity—not just an older person’s issue. Results from an Australian biomedical study. BMC Public Health 2010;10:718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vogeli C, Shields AE, Lee TA, Gibson TB, Marder WD, Weiss KB, et al. Multiple chronic conditions: prevalence, health consequences, and implications for quality, care management, and costs. J Gen Intern Med 2007;22(suppl 3):391-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menotti A, Mulder I, Nissinen A, Giampaoli S, Feskens EJ, Kromhout D. Prevalence of morbidity and multimorbidity in elderly male populations and their impact on 10-year all-cause mortality: the FINE study (Finland, Italy, Netherlands, Elderly). J Clin Epidemiol 2001;54:680-6. [DOI] [PubMed] [Google Scholar]
- 8.Fortin M, Lapointe L, Hudon C, Vanasse A, Ntetu AL, Maltais D. Multimorbidity and quality of life in primary care: a systematic review. Health Qual Life Outcomes 2004;2:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Townsend A, Hunt K, Wyke S. Managing multiple morbidity in mid-life: a qualitative study of attitudes to drug use. BMJ 2003;327:837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. The epidemiology of multimorbidity in a large cross-sectional dataset: implications for health care, research and medical education. Lancet 2012: published online 9 May. [DOI] [PubMed]
- 11.Fortin M, Dionne J, Pinho G, Gignac J, Almirall J, Lapointe L. Randomized clinical trials: do they have external validity for patients with multiple comorbidities? Ann Fam Med 2006;4:104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Starfield B. New paradigms for quality in primary care. Br J Gen Pract 2001;51:303-9. [PMC free article] [PubMed] [Google Scholar]
- 13.Bayliss EA. Simplifying care for complex patients. Ann Fam Med 2012;10:3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamerow D. How can we treat multiple chronic conditions? BMJ 2012;344:e1487. [DOI] [PubMed] [Google Scholar]
- 15.Smith SM, O’Kelly S, O’Dowd T. GPs’ and pharmacists’ experiences of managing multimorbidity: a “Pandora’s box.” Br J Gen Pract 2010;60:285-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stange KC. In this issue: challenges of managing multimorbidity. Ann Fam Med 2012;10:2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith SM, Soubhi H, Fortin M, Hudon C, O’Dowd T. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database Syst Rev 2012;4:CD006560. [DOI] [PubMed] [Google Scholar]
- 18.Van den Akker M, Buntinx F, Metsemakers JF, Roos S, Knottnerus JA. Multimorbidity in general practice: prevalence, incidence, and determinants of co-occurring chronic and recurrent diseases. J Clin Epidemiol 1998;51:367-75. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. Innovative care for chronic conditions: building blocks for action. Global report. World Health Organization, 2002.
- 20.Vanselow N, Donaldson M, Yordy K. A new definition of primary care. JAMA 1995;273:192. [DOI] [PubMed] [Google Scholar]
- 21.Zwarenstein M, Reeves S, Straus S, Pinfold PJG. Case management: effects on professional practice and health care outcomes (protocol). Cochrane Database Syst Rev 2000;4:CD002797. [Google Scholar]
- 22.Boult C, Reider L, Leff B, Frick KD, Boyd CM, Wolff JL, et al. The effect of guided care teams on the use of health services: results from a cluster-randomized controlled trial. Arch Intern Med 2011;171:460-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eakin E, Bull S, Riley K, Reeves M, McLaghlin M, Gutierrez S. Resources for health: a primary-care-based diet and physical activity intervention targeting urban Latinos with multiple chronic conditions. Health Psychol 2007;26:392-400. [DOI] [PubMed] [Google Scholar]
- 24.Gitlin LN, Winter L, Dennis MP, Corcoran M, Schinfeld S, Hauck WW. A randomized trial of a multicomponent home intervention to reduce functional difficulties in older adults. J Am Geriatr Soc 2006;54:809-16. [DOI] [PubMed] [Google Scholar]
- 25.Hochhalter AK, Song J, Rush J, Sklar L, Stevens A. Making the Most of Your Healthcare intervention for older adults with multiple chronic illnesses. Patient Educ Couns 2010;81:207-13. [DOI] [PubMed] [Google Scholar]
- 26.Hogg W, Lemelin J, Moroz I, Soto E, Russell G. Improving prevention in primary care: evaluating the sustainability of outreach facilitation. Can Fam Physician 2008;54:712-20. [PMC free article] [PubMed] [Google Scholar]
- 27.Krska J, Cromarty JA, Arris F, Jamieson D, Hansford D, Duffus PR, et al. Pharmacist-led medication review in patients over 65: a randomized, controlled trial in primary care. Age Ageing 2001;30:205-11. [DOI] [PubMed] [Google Scholar]
- 28.Lorig K, Sobel D, Stewart A, Brown B, Bandura A, Ritter P, et al. Evidence suggesting that a chronic disease self-management program can improve health status while reducing hospitalization: a randomised trial. Med Care 1999;37:5-14. [DOI] [PubMed] [Google Scholar]
- 29.Sommers LS, Marton KI, Barbaccia JC, Randolph J. Physician, nurse, and social worker collaboration in primary care for chronically ill seniors. Arch Intern Med 2000;160:1825-33. [DOI] [PubMed] [Google Scholar]
- 30.Bogner HR, de Vries HF. Integration of depression and hypertension treatment: a pilot, randomized controlled trial. Ann Fam Med 2008;6:295-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katon WJ, Lin EHB, Von Korff M, Ciechanowski P, Ludman EJ, Young B, et al. Collaborative care for patients with depression and chronic illnesses. N Engl J Med 2010;363:2611-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gitlin LN, Hauck WW, Dennis MP, Winter L, Hodgson N, Schinfeld S. Long-term effect on mortality of a home intervention that reduces functional difficulties in older adults: results from a randomized trial. JAGS 2009;57:476-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Foster G, Taylor SJ, Eldridge SE, Ramsay J, Griffiths CJ. Self-management education programmes by lay leaders for people with chronic conditions. Cochrane Database Syst Rev 2007;4:CD005108. [DOI] [PubMed] [Google Scholar]
- 34.Turner BJ, Cuttler L. The complexity of measuring clinical complexity. Ann Intern Med 2011;155:851-2. [DOI] [PubMed] [Google Scholar]
- 35.Fortin M, Stewart M, Poitras M-E, Almirall J, Maddocks H. A systematic review of prevalence studies on multimorbidity: toward a more uniform methodology. Ann Fam Med 2012;10:142-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.PROMIS. Home page. 2012. www.nihpromis.org.
- 37.France EF, Wyke S, Gunn JM, Mair FS, McLean G, Mercer SW. Multimorbidity in primary care: a systematic review of prospective cohort studies. BJGP 2012;62:e297-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Medline search strategy