Abstract
Breast cancer is the second leading cause of cancer death in women in the United States. While mammography and breast magnetic resonance imaging (MRI) improve detection of early disease, there remains an Ullmet need for biomarkers for risk stratification, early detection, prediction, and disease prognosis. A number of early breast lesions, from atypical hyperplasias to carcinomas in situ, are associated with an increased risk of developing subsequent invasive breast carcinoma. The recent development of genomic, epigenomic, and proteomic tools for tissue biomarker detection, including array CGH, RNA expression microarrays, and proteomic arrays have identified a number of potential biomarkers that both identify patients at increased risk, as well as provided insights into the pathology of early breast cancer development. This chapter focuses on the detection and application of tissue and serum biomarkers for the identification and risk stratification of early breast cancer lesions.
Keywords: Breast cancer, benign breast disease, biomarkers
1. Introduction
Invasive breast cancer remains a common cancer and significant public health problem, with over 192,370 new cases annually in the United States [81] and 1.15 million worldwide [133]. In 2009, it is estimated that over 40,170 people in the U.S. will die of breast cancer. Biomarkers for early detection, disease monitoring, and prognosis would have great potential clinical impact. With the advent of molecularly-targeted therapeutics, biomarkers that distinguish biological subtypes of cancer will be critical for predicting responses to therapeutic interventions.
There are many histologically defined premalignant lesions in the breast, but only a subset of these lesions are true precursor lesions that will progress to invasive cancer. The first model of breast cancer progression by Wellings and Jenson in 1973 proposed a gradual continuous histologic progression of defined histologic subtypes [188,189]. These subtypes were thought to develop from the terminal duct lobular unit (TDLU), and include columnar cell hyperplasia (CCH), atypical ductal hyperplasia (ADH), ductal carcinoma in situ (DCIS), and invasive ductal carcinoma (IDC). More recently, the WHO classification includes a neoplastic intraepithelial breast lesion, flat epithelial atypia (FEA) or flat ductal intraepithelial neoplasia (DIN), which is thought to precede ADH in the progression of neoplasia [173]. Atypical lobular hyperplasia (ALH) and lobular carcinoma in situ (LCIS) represent a second, less common lineage also originating from the TDLU, leading to invasive lobular carcinoma (ILC) [70]. In support of this hypothesis, early studies demonstrated that precursor lesions are more common in cancerous breasts than noncancerous [8]. While this model has remained fundamentally intact, the critical identification of which precursors will evolve to invasive cancer remains largely unsolved.
Seminal work by Dupont and Page classified the risk associated with premalignant breast lesions [51] and reviewed by Schnitt and Collins in [70]. Using a large retrospective cohort, they determined that the risk of subsequent invasive cancer increased from 1.5–2.0% for proliferative lesions without atypia, to 3.5–5.0% for hyperplastic lesions with atypia [127]. One limitation to studies of benign breast disease is that there remains significant inter-observer variability in categorizing these lesions [151,156]. The advent of new molecular technologies represents a unique opportunity for the standardization of disease classification, and to identify the earliest molecular changes of preinvasive breast disease that lead to breast cancer development.
2. Molecular pathology of early breast lesions
2.1. Atypical lobular hyperplasia and lobular carcinoma in situ
The development of mammographic screening has led to significant increases in the detection of incidental atypical hyperplasias, found in less than 10% of benign breast biopsies [152]. ALH is associated with a significant increased risk (4–5 fold) for subsequent breast cancer, which occur on average more than 10 years after diagnosis [51,71,98,109,112,113,129]. The risk for breast cancer is especially high for premenopausal women [39,98,109]. In comparison to ALH, LCIS carries twice the risk for the development of invasive breast cancer and an increased risk of in-breast tumor recurrence [98,114,128].
ALH and LCIS are frequently multicentric and bilateral. Because there is an increased risk of bilateral breast cancers for both ALH and LCIS, these lesions have been thought to represent biomarkers of risk, rather than true precursor lesions for invasive breast cancer. Local clinical management for both ALH and low grade LCIS is focused on obtaining sufficient tissue for definitive diagnosis, but not excision to clear margins [10,121]. However, several lines of evidence suggest that, in part, ALH represents early, potentially reversible, biologic changes of neoplastic progression. First, the risk of subsequent breast cancers is increased more in the ipsilateral breast after the diagnosis of ALH [129]. Second, in the setting of concurrent ALH and invasive lobular carcinomas, similar molecular changes have been observed.
2.1.1. Histopathology
Preinvasive lobular neoplasias represent a pathologic continuum from ALH to the more extensive LCIS. ALH is characterized by small, well-differentiated epithelial cells that grow in discohesive clusters in terminal ductai/lobular units (TDLUS) [70]. According to the criteria of Page et. al. [127], the cells occupy less than half the acini, but do not have the defining features of LCIS, which is characterized by extensive distention and filling of a lobular unit [70,128]. Immunohistochemical (IHC) analysis shows strong ER-α in lobular neoplasia, compared to adjacent normal breast tissue [123].
Originally described by Foote and Stewart in 1941 [61], many variants ofLCIS have now been identified, based on nuclear grade, pleomorphism and necrosis [62]. Typically, LCIS is characterized by minimal Ki-67 staining, negative p53 staining, and low erb-B2 expression (Table 1, [47,115,118]). Most histological SUbtypes of LCIS, particularly classical, show expression of ER-α, ER-β and progesterone receptor (PR) in the majority of cells. Higher-grade lesions have increased frequencies of concurrent AD Hand DCIS [63].
Table 1.
Tissue biomarkers of preinvasive breast disease
| Lesion | Protein markers | Genetic markers | RNA expression markers |
|---|---|---|---|
| ALH/LCIS | ERα, PR: positive |
CDHJ (e-cadherin) -inactivation -mutations -promoter methylation |
Claudin 4↓ MMP-9↑ |
| HER2 – negative Ki-67 – low p53 – negative e-cadherin – negative pl20 – cytoplasmic α – & β-catenins – negative or low |
Loss 16q21-q23.1 (CDHl) 17p13.1 (p53 ) Gain 14q32.33 (AKTJ) 20q13.13 (CEBPB) 5q32-33.1 (CSFlR) 11q13 (cyclin D1) |
TGF-β, Snail, Slug, & ZEB1↑ | |
| PLCIS | ERα, ERβ, & PR: usually positive |
Loss 16q |
|
| HER2 – subset positive Ki-67 – high p53 – positive |
Gain Iq Sq24 (MYC) 17q12 (HER2/neu) |
||
| ADH | ER, PR: positive HER2 – negative Ki-67 – low (5%) p53 – negative |
Loss (ADHlDCIS) Sp, 16q & 17q Loss (DCIS) 1 q, 17p (p53) |
EGF ↓ Amphireulin (AREG)↑ |
| DCIS (low grade) | ER, PR: positive HER2 – negative Ki-67 – low (5%) p53 – negative hFATl – negative |
p53 – not mutated HER2/neu – not amplified |
BCL2 downregulated |
| DCIS (high grade) | ER & PR – negative Ki-67 intermediate (35%) p53 – often positive HER2 – subset positive hFATl – positive |
p53 – frequent mutations HER2/neu – subset amplified PIK3CA – mutated Promoter hypermethylation: RASSFlA HIN-l RARβ Cyclin D2 |
BCL2↓ S 1 OOA 7 (psoriasin)↑ SlOOA9↑ TFF3 (trefoil factor)↑ KRTl9 (Keratin 19)↑ APOD(Apolipoprotein D)↑ |
Pleomorphic LCIS (PLCIS) is a variant of LCIS that is histologically distinct from classical LCIS [149]. Whereas classical LCIS shows discohesive, monotonous, and relatively small tumor cells, PLCIS shows larger, pleomorphic nuclei, often associated with necrosis [33], and is occasionally ER and PR negative [33]. PLCIS is associated with a high proliferation rate, p53 overexpression, and occasional HER2/neu overexpression/amplification [33,160].
2.1.2. Loss of E-cadherin in lobular neoplasia
The hallmark loss of e-cadherin in ALH and LCIS, which gives lobular neoplasia its characteristic dishesive appearance, is thought to play a fundamental role in the infiltrative growth pattern seen in ILC [94]. The e-cadherin gene encodes a cell adhesion molecule that interacts with the actin cytoskeleton via binding of α-, β-, and γ-catenins, as well as p120 catenin. Loss of e-cadherin leads epithelial discohesion, the characteristic histologic feature of lobular neoplasia. Both α- and, β-catenins are also downregulated in ALH and LCIS [110], while p120 catenin localizes to the cytoplasm in both ALH and LCIS with loss of e-cadherin [153]. E-cadherin’s ability to bind to beta-catenin links it to the Wntlbeta-catenin signaling pathway, an important regulator of proliferation and differentiation that is implicated in a variety of human malignancies.
The mechanism of e-cadherin inactivation in lobular neoplasia has been shown to occur through multiple mechanisms, including inactivating gene mutations, loss of heterozygosity, transcriptional regulation, and abnormal promoter methylation. These changes result in functional loss of both alleles and loss of protein transcription [22,50]. By array comparative genomic hybridization (CGH), both ALH and LCIS show a loss of 16q21-q23.1 [111], which contains the e-cadherin gene (CDH1) and is associated with a chromosome 1;16 fusion and gain of 1 q [77,178]. Inactivating mutations in CDH1 are frequently observed in LCIS but not in ALH [110]. These truncation mutations of CDH1 have been found in LCIS and synchronous ILC, supporting the hypothesis that LCIS can be a precursor lesion of ILC [70,182].
The fact that loss of protein expression is accompanied by genetic alterations in LCIS but not in ALH suggests that another mechanism, such as silencing of the e-cadherin promoter by methylation, may be involved in loss of expression of the e-cadherin complex in ALH [110]. Recent studies have shown that hypermethylation of the CDH1 promoter occurs in both ALH/LCIS and invasive lobular carcinomas, as well as in adjacent non-neoplastic epithelia [199]. Downregulation of e-cadherin can also occur through transcriptional regulation, via activation of pathways involving TGF-β, Snail, and Slug [41]. Activation of transcription factor path ways of zinc finger E-box binding homeoboxl (ZEBl) is also known to silence e-cadherin expression [2]. These data suggest that epigenetic andlor transcriptional CDH1 downregulation may be an early event in the neoplastic process of lobular carcinomas.
2.1.3. Genomic alterations
Studies of genetic alterations in lobular hyperplasias, in particular ALH, have been limited by the small, amount of tissue that can be micro dissected from these lesions. In addition to the hallmark loss of CDH1 at 16q21-q23.1 described above, early loss of heterozygosity (LOH) studies observed allelic loss at 11q13 in LCIS and invasive lobular carcinomas, but not in ALH [122]. LOH has been observed in 80% of LCIS primarily at loci on 9p, 16q, 17p, and 17q [118]. Studies using micro array CGH have demonstrated gain at 2p11.2 and loss at 7pll-p11.1 and 22q11.1 in ALH. In comparison, in LCIS gain at 20q13.13 and loss at 19q13.2-q13.31 have been observed [111]. The region at 20q13.13 contains the CCAAT/enhancer binding protein beta (CEBPB), which has been implicated in cellular proliferation and differentiation [66].
Other genomic areas of alterations in ALH/LCIS include 1p32.1, 16p13.3, 17q21.32, and 17q25.3 (including the candidate genes JUN, AXINI, HOXB, and RAC3, respectively) [111]. Two areas of copy number gain in both ALH and LCIS are 14q32.33 (including AKT1) and 5q32-33.1 (including CSFlR), both implicated in mammary epithelial proliferation [43,194].
Comparison studies using ALH, LCIS and invasive lobular carcinomas that were microdissected from synchronous lesions have been used to investigate whether a genetic relationship exists between ALH and LCIS and, if so, whether it differs in unilateral and bilateral cases. Genetic losses at 16p, 16q, 17p, and 22q, as well as gain at 6q have been observed at high frequency in both ALH and LCIS with no significant differences between the alterations found in the unilateral and bilateral cases [101]. These studies also show that gains of 1q and loss of 16q occur in high frequency in ILC as well as synchronous LCIS, indicating they may represent a precursor lesion of ILC [77]. In addition, the region of 17p13.1 (containingp53) was lost in 30% of LCIS, while llq13 (containing cyclin D1) was gained [78]. Co-existentDCIS and LCIS may also have similar LOH, suggesting a clonal relationship [183].
2.1.4. Pleomorphic LCIS (PLCIS)
Using array CGH to evaluate genetic alterations in PLCIS, it has been shown thatPLCIS lesions also have loss of 16q and gain of 1q, but have more genomic alterations than classical LCIS [33]. Based on CGH, array CGH, and chromogenic in situ hybridization (CISH), PLCIS and PILC appear genetically related, suggesting that PLCIS may be a non-obligate precursor lesion of PILC [149]. Additional loci may be amplified in PLCIS, including 8q24, containing the MYC gene, and 17q12, containing HER2/neu [149,160].
2.1.5. RNA expression analysis
Gene expression profiling of LCIS lesions using serial analysis of gene expression (SAGE) has shown downregulation of claudin 4 and upregulation of matrix metalloproteinase 9 (MMP-9) compared with benign breast epithelium and stroma [27]. Downregulation of claudin 4, a cell-junction protein, may, in addition to loss of e-cadherin, playa role in the disco hesion seen in LCIS. Urinary metalloproteinases (MMP-9 and ADAMI2) are significantly elevated in women with atypical hyperplasia and LCIS, and have been proposed as non-invasive biomarkers for breast cancer risk assessment [140].
2.2. Atypical ductal hyperplasia
2.2.1. Histopathology
Like ALH, the presence of ADH is associated with an increased risk of breast cancer, especially among premenopausal women (OR, 3.1; [39]). Architecturally, ADH is distinguished from columnar cell hyperplasia by tufting of the epithelial cells and architectural complexity, with separation of the cells from the basement membrane of the duct or lobule. This histologic transformation from CCH to ADH is thought to represent progression from a polyclonal to a clonal or neoplastic lesion [86,88] and reviewed by Allred in [70].
Nearly all ADH lesions express high levels of ER andPR in the majority of the cells, approximately three to four times that seen in normal breast cells [17,155]. Compared with normal breast tissue, the proliferation index of ADH is 2–3 fold higher (as measured by Ki-67), while the rate of apoptosis is decreased. Downregulation of EGF expression and upregulation of AREG (amphireulin), critical to embryonic breast development, have been demonstrated in ADH [90].
2.2.2. Genetic alterations
Supporting the hypothesis that the transition from CCH to ADH represents a transition to monoclonality, multiple studies have shown a relatively high incidence of clonal allelic imbalances in ADH when compared with CCH. There is frequent loss of heterozygosity at 8p, 16q, 17q, and 17p, which is also observed in ductal carcinoma in situ (DCIS) and invasive ductal carcinoma (IDC) [9,86,88,124]. Gene expression analysis using DNA micro arrays has shown that premalignant (ADH), preinvasive (DCIS), and IDC breast lesions share similar gene expression signatures [106]. This suggests that ADH is a precursor lesion to DCIS and IDC, and that the genetic changes that lead to invasive cancer may already be in place in premalignant lesions [70,106]. Preliminary studies show that two thirds of ADH and ALH in the same breast share allelic imbalances, providing support for the hypothesis that these two lesions are genetically related [120]. Several studies have shown that amplification of the oncogene erb-b2, which is typically seen in a significant proportion of DCIS and invasive breast cancers, is not seen in ADH [6]. Additionally, mutations in the p53 tumor suppressor gene, also found in a subset of DCIS and invasive cancers, are not seen in ADH [37,179].
2.3. Ductal carcinoma in situ
2.3.1. Histopathology
Histologically, DCIS has typically been classified either by architecture (comedo, cribiform, micropapillary, papillary, and solid), or by nuclear grade (low, intermediate, and high) [70]. In low-grade DCIS, there is a monotonous intraductal proliferation of epithelial cells filling the ductal spaces, whereas high-grade DCIS shows greater nuclear atypia and mitotic activity often with areas of necrosis (reviewed in [70]). There is no uniformly accepted classification system to date, though reports typically comment on grade, architectural pattern and the presence of necrosis. The Van Nuys Prognostic Index (VNPI), combining tumor size, margin width and pathologic classification, has’ been used for predicting the risk of local recurrence in patients with DCIS [45].
DCIS, like IDC, has a broad range of histopathologic features and biomarkers, from well to poorly differentiated tumors [?,?]. Most cases oflow-grade DCIS show high levels of ER and PR expression in nearly all cells. Conversely, most cases of high-grade DCIS do not express ER or PR [7,126]. There is also a significant correlation between large nuclear size, comedonecrosis, amplification and overexpression of Her2/neu, and mutations and overexpression of p53 as features of high-grade DCIS [126]. The proliferation index increases from approximately 5% in low-grade DCIS to about 35% in high grade DCIS [7]. hFATl protein, a member of the cadherin superfamily, is strongly expressed in high-grade DCIS [85]. These data suggest divergent pathways of low- and high-grade DCIS and IDC development, with different associated risk ofIDC [162].
2.3.2. Genetic alterations
Studies looking at allelic imbalances in DCIS by LOH and comparative genomic hybridization have identified multiple genetic abnormalities in DCIS. Most of these genetic imbalances are complex, spanning 17 chromosomes and involve up to 100 different loci [78,124,143,184], with higher frequencies in high-grade lesions [78,124]. Low- and high-grade lesions may already be committed to distinct pathways [24,25], with lobular and ductal neoplasias in the same pathway. For example, chromosomal gain of lq and loss of 16q occurs in both low grade DCIS and LCIS [158]. Chromosomes 16q, 17p (encompassing the p53 locus) and 17q are frequent sites of abnormalities in DCIS, with incidences exceeding 40% in some studies [7]. Studies using array CGH have demonstrated that DCIS lesions have genetic alterations similar to adjacent invasive cancers [14,25]. Similarly, mutations in PIK3CA have been detected in both DCIS and IDC [95]. The transition from DCIS to IDC is also affected by the tumor microenvironment, and can be promoted by fibroblasts and inhibited by myoepithelial cells [75].
2.3.3. RNA and protein expression analysis
Differential gene expression has been observed between histologically well- and poorly-differentiated lesions, but no expression profiles unique to ADH, DCIS, and IDC have been observed [99], suggesting that two distinct pathways exist based on histologic grade rather than stage of progression [69,106,141]. 43 genes were differentially expressed in well- and poorly-differentiated DCIS, including genes involved in metabolism (BID, ETFA, GMFG, and PLAT), cell communication (ESRl, ACKl, CELSR2, andCCL19), and apoptosis (BCL2) [69]. SAGE expression analysis has identified multiple genes that are more highly expressed in DCIS than in normal or invasive breast cancer, including S100A7 (psoriasin), SlOOA9, trefoil factor protein (TFF3), keratin 19 (KRTl9), and apolipoproteinD (APOD) [141].
SlOOA7 has been demonstrated to act as a chemoattractant for T-lymphocytes and neutrophils, and induces serum antibody responses [11]. SlOOA7 is highly expressed in high-grade DCIS with comedonecrosis, and in ER-negative, poorly differentiated, lymph node positive IDC, suggesting that it may be a biomarker of high risk of progression in DCIS [141]. Stromal CDlO and SPARC expression measured by IHC are both associated with a decreased time to recurrence. Therefore, expression levels of CDlO and SPARC are potential markers of DCIS recurrence [193].
3. Early invasive carcinomas
3.1. Histopathology
The standard IHe biomarkers of ER, PR, and Her2/neu are both prognostic and predictive factors that have been established in multiple studies [40,125, 145,147,163]. The most common subtype, ER+fPR+, have both the best prognosis and the highest rates of response to hormonal therapy [28]. The ER−/PR− subtype is associated with higher grade lesions, recurrence rates and mortality [16,36,52,142]. Her2!neu defines a subset of tumors with increased sensitivity to Her2-targeted therapeutics, such as trastuzumab.
3.2. Tumor classification
By RNA expression micro array analysis, breast cancers can be divided into distinct subgroups with distinct biologic behavior and clinical outcome [136,164]. These groups are comprised of Luminal A, Luminal B, Her2-like, and Basal-like breast cancers. These subgroups correspond, in general, to standard histopathologic criteria, such as hormone receptor positive cancers (Luminal A and B, 96% ER+), Her2+ cancer (Her2-like, 100% Her2+), and triple negative cancers (Basal-like; 10% Her2+, 12% ER+) [165]. Luminal B cancers, in contrast to Luminal A cancers are poorly differentiated. Nineteen percent of Luminal A cancers are grade III, while 53% of Luminal B cancers are grade III and highly proliferative as measured by Ki-67. Her2!neu can be detected by IRC in 10–20% of non-Her2-like subgroups.
3.3. Prognostic biomarkers
Perhaps the greatest impact of molecular pathology on clinical practice over the past 5 years has been the advent of molecular signatures to predict the risk of systemic disease recurrence in breast cancer. Using RNA micro array analysis, multiple prognostic gene signatures have been identified, many of which are available for clinical use in the U.S. and in Europe (reviewed in [165]; Table 2). Now widely integrated into clinical practice, these prognostic signatures are primarily used to identify patients with low-risk hormone-receptor positive cancers that would be unlikely to benefit from adjuvant chemotherapy. For the development of the Oncotype DX signature, 384 potential candidate genes were selected from the literature, RNA expression analysis, and databases [30,87, 154,166,169]. From this set, 16 cancer-related and 5 reference genes were selected for a continuous variable prognostic signature (Recurrence Score) for hormone receptor positive node-negative breast cancer. These were tested and validated on archived samples from NSABP B14 [131] and NSABP B20 [130]. These results have been extended to early node-positive cancers [4]. Intermediate-risk cohorts are being tested in the TAILORx clinical trial [168]. OncotypeDx testing has now been incorporated into the NCCN guidelines for breast cancer treatment, as well as ASCO guidelines http://jco.ascopubs.org/cgi/contentlfull/25/33/5287. Currently, the Amsterdam 70-gene signature (Mamma-print) is being tested in the EORTC-BIG Mindact trial [180]. Multiple other expression signatures have been developed, including the Rotterdam 76-gene signature [186], the ’stem cell’ profiles (CD44+/CD24−) 184-geneInvasiveness Gene Signature [96], the Wound Response Signature [29], the Proliferation Signature [42], the 97-gene Grade Signature [167], and the HoxB 13!IL17R ratio [105]. The precise clinical utility and appropriate application for these other assays at this time is being defined.
Table 2.
Prognostic biomarkers for early stage breast cancer
| Biomarker | Name | Tissue source | Technique | Reference |
|---|---|---|---|---|
| 21-gene Recurrence Score | Oncotype Dx | FFPE | Q-RT-PCR | [131] |
| Amsterdam 70-gene assay | MammaPrint | Fresh or frozen | RNA microarray | [180] |
| 97-gene grade signature | MapQuantDx | Fresh or frozen | RNA microarray | [167] |
| Rotterdam 76-gene signature | Veridex | Fresh or frozen | RNA microarray | [186] |
| Proliferation Signature | Fresh or frozen | RNA microarray | [42] | |
| Stem Cell/Invasiveness Gene Signature | Fresh or frozen | RNA microarray | [96] | |
| Wound Response Signature | Fresh or frozen | RNA microarray | [29] | |
| HoxB l31/L17BR | Theros | FFPE | Q-RT-PCR | [105] |
FFPE: Formalin Fixed Paraffin Embedded.
4. Emerging topics in molecular breast pathology
4.1. Epigenetics and early breast cancer development
Epigenetic alterations, including DNA methylation, histone modifications, and chromatin remodeling, result in aberrant expression of genes involved in early breast cancer development. DNA methylation at CpG dinucleotides located in the promoter regions of tumor suppressor genes is an early, reversible, and specific hallmark of many cancers. Persistent estrogen exposure of breast progenitor cells results in methylation-induced silencing of tumor suppressor genes [34]. The majority of both LCIS and DCIS show common patterns of promoter hypermethylation of CDH1, RASSF1A, HlN-1, RARβ, and Cyclin D2, but differential methylation of Twist. This suggests that early gene silencing is a common early event in both ductal and lobular neoplasias [56]. Promotor methylation can functionally inactivate the remaining allele of tumor suppressor genes, such as BRCA1 [55], and can silence tumor suppressive micro-RNAs [92].
In addition to locus-specific hypermethylation, global hypomethylation occurs during tumorigenesis and leads to genomic instability, inactivation of tumor suppressor genes and activation of oncogenes [97]. Global hypomethylation in breast cancer is reportedly associated with features such as stage, tumor size, and histologic grade [161]. Hypomethylation of the promotors of proto-oncogenes involved in breast cancer proliferation, metastasis, and drug resistance (synuclein γ, urokinase, N-cadherin, β-catenin and WNTll genes) have been identified [58,67,132]. Other genes identified from breast neoplasms that have alterations in methylation, include genes involved in cell cycle regulation (p16IN4α, pI4ARF, 14-3-3σ, cyclin D2, p57KIP2), apoptosis (APC, DAPKI, HICI, HOXA5, TWIST, TMSI), DNA repair (GSTPI, MGMT), hormone regulation (ERα, PR), cell adhesion and invasion (CDHI, APC, TIMP3), angiogenesis (maspin, THBSI), and growth inhibition (RARβ, RASSFIA, SYK, SYK, TGJBRII, HlNI, NESI, SOCSI, SFRPI, and WIFl) (reviewed in [97]).
Because of the high specificity of methylation patterns in cancer, global analysis of the breast methylome can identify biomarkers for early detection of breast cancers, for risk assessment, prognosis, and therapeutic strategies [97,191]. Assays such as quantitative multiplex methylation-specific PCR (QM-MSP) allow for quantitative detection of targeted methylated DNA [57]. Novel techniques for the identification of global methylation profiles include differential methylation hybridization (DMH), methylated DNA immunoprecipitation (methyl-DIP) [187], and methylation-specific digital karyotyping (MSDK) [74]. Post-translational histone modifications can be detect-. ed with ChIP-SAGE [150] and ChiP-SEQ [18,116]. These emerging methods will enable detailed analyses of the epigenetic changes that occur in early breast cancer development, both for the identification of high-risk patients, as well as early interventions of potentially reversible changes of pre-malignant lesions.
4.2. The breast cancer stem cell
Within the heterogeneous population of breast cancer tumor cells is a subset of cancer cells with stemcell like properties that have the ability to self-renew and proliferate differentiation. According to the cancer stem cell hypothesis, this subset of undifferentiated stem cells can differentiate into a tumorigenic cell population [38,59,103,137,190].
To identify tumor markers associated with a breast cancer stem cell phenotype, AI-Hajj et al. identified a subpopulation of human breast cancer cells that were able to generate tumors in NOD/SCID mice [3]. Tumorigenic cells were CD44+, CD24lo, ESA+ and lineage-negative (cells did not express CD2, CD3, CDlO, CDI6, CDI8, CD31). Resulting tumors contained not only the original CD44+, CD24lo cells, but also a phenotypically diverse population of non-tumorigenic cells. To further define the subpopulation of putative stem cells, nonadherent mammosphere cultures were established that had the capacity to self-renew and to differentiate into breast lineages [49]. Approximately, 96% of the cells were CD44+/CD24−, however, only about 20% of these had the capacity to self-renew [139]. Putative breast cancer stem cells can also be enriched by flow cytometry based on increased aldehyde dehydrogenase (ALDH) activity, which is associated with poor prognosis [5,32]. Even standard cell lines, such as MCF-7, contain subpopulations with greater tumorigenic capacity, and higher levels ofN otch 1 and β-catenin expression when implanted subcutaneously into NOD/SCm mice [134].
Gene expression analysis of purified CD44+ cells from primary and metastatic breast cancers shows signatures associated with cell motility, invasion, apoptosis and matrix remodeling [157]. TGF-β pathway genes, in particular, had high expression [13]. TGF-β has also been shown to regulate breast cancer stem cells and maintain the pluripotency of embryonic stem cells [80,108,119].
4.3. The epithelial-mesenchymal transition
The epithelial-mesenchymal transition (EMT) is a reversible process in embryogenesis that is necessary for cellular differentiation and organogenesis. Coordinated EMT and the reverse mesenchymal-epithelial transition (MET) occurs between the tightly adherent epithelial cells and the loosely adherent, invasive, and migratory mesenchymal cells [19,76,176,196]. Mesoderm that is generated by EMT can develop into different tissue types and organs, and can undergo the reverse process and develop into epithelium. EMT also contributes to tissue remodeling and wound healing [72, 175].
In the tumor microenvironment, a number of factors are associated with induction of EMT. Inflammatory pathways induce the NF-κB and TGF-β pathways, while tumor hypoxia induce the HIF 112 and Notch pathways. These, in turn, induce Twist, Snail 112, and Zeb 112. Loss of E-cadherin expression is crucial step in EMT, and is regulated by Snail, Twist, and Zeb. Snail expression is also associated with immunosuppression and impairment of dendritic cells [84]. Overall, induction of EMT is associated with tumor dissemination, invasion, and acquisition of stem-cell like properties. A number of transcription factors that are involved in embryogenesis also regulate EMT and confer characteristics found in malignancy, i.e., invasiveness, resistance to apoptosis, motility, and metastatic potential [23,35, 107,175]. This evidence suggests that the process of EMT may also impart a stem cell-like phenotype on cancer cells which allows them to proliferate and then differentiate [108].
In support of the link between EMT and mammary stem cells, isolated CD44hi/CD24lo stem-cell like sub-populations from cultured normal human mammary epithelial cells show a characteristic mesenchymal morphology and express mesenchymal markers [108,119]. This population also has mammosphere-forming ability and can differentiate into cells expressing lineage-specific markers of myoepithelial or luminal epithelial cells. Analysis of stem cells from mouse mammary glands has shown that the regenerated cells after injection into the mouse mammary fat pads displayed a mesenchymal morphology and expressed mesenchymal markers. Normal mammary epithelial cells adopt the CD44hi/CD24lo phenotype when exposed to TGF-β1, Snail or Twist [108]. These experiments demonstrate that the process ofEMT may cause cells to adopt a stem cell phenotype [138,144].
4.4. MicroRNAs and early breast cancer development
MicroRNAs (miRNA) are a large class of short (20–25 nucleotides) noncoding RNAs that bind to complementary sequences in target mRNAs, primarily in the 3′ UTR. Binding of miRNAs to cognate sequences are primarily associated with RNA degradation and loss of translation of the target genes [117]. Originally identified in C. elegans and in zebrafish as moderators of developmental timing [79,89,148,192], recent evidence has linked certain miRNA families (miR-141, miR-200b, and miR-205) with EMT through regulation of ZEBI and ZEB2 [26,68,159]. miR-141 and miR-200b miRNAs are downregulated in metaplastic breast cancers [65]. The translational control by miRNAs can function as oncogenic or anti-oncogenic; miR-I0b is upregulated by Twist to increase breast cancer cell invasion [104], miR-21 stimulates cell invasion and metastasis in tumor models [198], while miR-335 inhibits metastasis [174]. Patterns of miRNA expression are associated with hormone-receptor status [100], and the breast cancer stem-cell phenotype [48]. A miRNA hypermethylation profile associated with cancer metastasis has been identified [102].
5. Serum biomarker detection
Biologic changes in premalignant and malignant breast tissue are associated with systemic changes in biomarkers. The advent of genomics- and proteomics-based technologies has markedly expanded the detection of serum biomarkers for early breast cancer detection. For example, specific miRNAs have been detected in the sera of breast cancer patients [73]. Many autoantibodies have been detected in the blood of patients with DCIS and early-stage breast cancers, corresponding to known tumor antigens (Table 3). Assays such as methylation assays, miRNA detection, protein microarrays, reverse phase protein arrays, phage display, and glycan arrays have all been used to discover cancer-associated biomarkers.
Table 3.
Breast cancer serum autoantibody biomarkers
| Analysis | Function | Marker | Cases/controls | Sensitivity | Specificity | Reference |
|---|---|---|---|---|---|---|
| Apoptosis | SURVIVIN | 46/10 | 24% | - | [12,195] | |
| LIVIN | 46/10 | 33% | - | |||
| P53 | 165/230 | 7%-21% | 95%-100% | [12,64,93,146,181] | ||
| 165/0 | ||||||
| 144/242 | ||||||
| Single Markers | ANNEXIN XIA | 90/51 | 19%(PBC) (60%DCIS) |
98% | [60] | |
| Growth | RPA32 | 801165 | 11% | 100% | [177] | |
| Signaling | SPAG9 | 94 | 80% | - | [82] | |
| HER2 | 107/200 | 11% | 100% | [20,46] | ||
| Adhesion | CYCLOPHILIN | 28/21 | 86% | 52% | [170] | |
| Other | GLOBOH | 58/47 | 58% | 73% | [185] | |
| FXBP52 | 142/93 | - | 95% | [44] | ||
| PPIA | ||||||
| PRDX2 | ||||||
| HPSO | ||||||
| MUCI | ||||||
| AAb Panel | ASB-9 | 87/87 | 80% | 100% | [197] | |
| SERACI | ||||||
| RELT | ||||||
| BRCAI | 133/94 | 64%(PBC) | 85% | [31] | ||
| C-MYC | 45%(DCIS) | |||||
| HER2 | ||||||
| MUCI | ||||||
| NY-ESOI | ||||||
| P53 | ||||||
| BRCA2 | ||||||
| P53 mutants | 55/8 | 16% | 100% | [15] | ||
| C-MYC | 64/82 | 77%-92% | 85%-91% | [83] | ||
| CYCLINBI | ||||||
| IMPI | ||||||
| KOC | ||||||
| P53 | ||||||
| P62 | ||||||
| SURVIVIN |
PBC: Primary Breast Carcinoma; DCIS: Ductal Carcinoma In Situ.
These assays are excellent discovery tools, approaching the ultimate goal of genome-wide and proteome-wide molecular pathology. However, multiplexed assays for clinical validation studies have lagged behind in development, so that the majority of these biomarkers have not yet undergone phase III or later clinical validation studies [135]. The National Cancer Institute Early Detection Research Network (NCI EDRN) is developing a multi-center Breast Cancer Reference Set of plasma and sera for investigator use, which will be an invaluable resource for blinded validation studies of potential serum biomarkers.
6. Validation studies of biomarkers
Currently, standard histopathologic criteria for the diagnosis of early breast lesions are inadequate predictors of risk of the subsequent development of invasive breast cancers. In particular, high-grade breast cancers are associated with both decreased detection by routine mammographic screening and increased mortality. Despite promising early studies, no new tissue or serum biomarkers have been validated for early detection or prognosis of pre-invasive disease, although a number of prognostic tissue biomarkers have been incorporated into clinical practice for invasive cancers. This is likely due to several factors.
First, most genomic and proteomic-based discovery platforms are not technically amenable to large-scale multicenter tissue screening for clinical validation studies, in particular from archived fixed paraffin-embedded tissue microarrays. These studies require both tightly controlled coefficients of variation and cost-effective assay development. Clinical-grade platforms designed for multiplexed detection of selected markers using limited quantities of tissue need to be developed and validated.
Second, most biomarker discovery projects rely on single-institution collections. Many biomarkers of risk may be dependent on age, gender, or other clinical factors. Cases and controls must be collected with identical sample handling protocols, in comparable time frames, ideally in the exact clinical setting for which the biomarker is designed (i.e. assessment of future risk from ADH). Due to the long time delay to the development of IDC, all samples need to be well annotated for clinical outcome. Potential confounding variables, including co-morbidities, hormone use, alcohol use, menopausal status, medications, and parity, need to be assessed.
One key challenge for the systematic molecular analysis of early breast lesions is the need for well-annotated tumor specimens that are coordinately linked with data on global molecular signatures of DNA, RNA, and protein levels, as well as data on detailed epidemiologic variables and long-term clinical outcome. Studies of genomic DNA (amplifications/deletions, mutations), single nucleotide polymorphisms (SNPs), epigenetic changes, RNA and miRNA expression, as well as protein expression and post-translational modifications all require differential tissue processing and assay requirements. Techniques requiring frozen samples, in particular, are difficult due to limited tissue availability, since most of the involved tissue is used for clinical pathologic diagnosis. Retrospective studies of such banked tumor specimens and data mining by bioinformatics provide invaluable information on tumor biology and potential biomarkers for invasive cancer risk.
Molecular signatures identified at this stage need to be validated and incorporated into prospective clinical trials alongside existing risk assessment tools and imaging modalities such as mammography. Because of the low overall risk of subsequent invasive breast cancers from lesions such as ALH, the small amount of disease tissue present for many of these lesions and the many years to invasive cancer development, large multicenter tissue cohorts are required for both biomarker development and validation. NCI initiatives, such as PAC-CT (Program for the Assessment of Clinical Cancer Tests), SPECS (Strategic Partnering to Evaluate Cancer Signatures), TRWG (Translational Research Working Group) and the EDRN (Early Detection Research Network) are all designed to support the infrastructure needs both for biomarker detection and validation.
7. Applications for clinical development
Tissue-based biomarkers have a tremendous potential for risk assessment of early breast lesions, but the application of these markers for a global assessment of risk across multiple platforms will be challenging. Incorporation of serum-based biomarkers will also need to incorporate radiographic screening (mammography, ultrasound, and/or MRI) for tumor localization. Recent guidelines from the U.S. Preventive Services Task Force recommend biennial mammographic screening for average-risk women between ages 50 and 74 [1]. For high-risk women (> 20% lifetime risk), the National Comprehensive Cancer Network (NCCN) guidelines recommend annual mammography and breast MRI screening [91]. Statistical modeling by the Cancer Intervention and Surveillance Modeling Network (CISNET) has estimated that the proportion of the total reduction in the rate of death from breast cancer attributed to screening is 28 to 65 percent (median, 46 percent) [21].
The use of mammography as a primary screening tool has limitations. High-grade, rapidly-proliferative cancers that are associated with higher mortality frequently present as interval cancers, which are not detected by routine screening. In the neoadjuvant 1-SPY clinical trial of locally advanced breast cancers, 85% of the malignancies were interval cancers [53]. Increased breast density both obscures the detection of breast cancers by mammography, but is one of the strongest predictors of breast cancer risk, especially for premenopausal women [171,172]. Women in the highest category of mammographic density have a four- to six-fold increased risk of breast cancer as compared with women in the lowest category of risk.
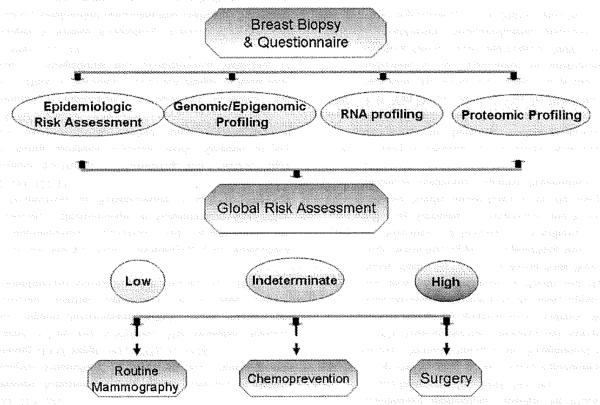
Potential clinical applications for breast cancer tissue biomarkers for risk assessment are shown in Fig. 1. At the time of initial breast biopsy for any pre-invasive pathology, a global risk assessment based on genetic and proteomic profiling, as well as epidemiologic risk factors, could be performed. Ultimately, replacement of screening mammography with a baseline breast biopsy in healthy women may provide more valuable molecular information for risk assessment and screening. Blood-based biomarkers could be incorporated into that assessment of risk, and used to replace mammography, enhance detection by mammography, or for risk stratification for further imaging, such as MRI screening. More intensive screening of these high-risk populations may involve MRI, serum biomarkers, or chemoprevention studies as an adjunct to yearly mammographic detection are likely to have the greatest clinical impact [54].
Fig. 1.
Tailored biomarker-based risk assessment for breast cancer. Top. At the time of biopsy for benign breast disease (or for a baseline breast biopsy), a global risk assessment would be performed. This will incorporate epidemiologic risk factors, as well as genomic/epigenomic, RNA profiling, and proteomic profiling. The challenge will be to assess risk from breast tissue across different technologic platforms. Bottom. Based on a quantitative level of risk, increasing levels of screening (both frequency of mammography and use of MRl) would be performed. For patients at higher risk, chemoprevention studies and therapeutic intervention can be considered. Serum-based biomarkers can be used as an adjunct to identify higher-risk patients. Repeat biopsies to determine changes in biomarker levels could be incorporated.
8. Summary and future directions
Modern methods in molecular biology have led to the identification of multiple tissue-based biomarkers to aid in both the diagnosis and prognosis of benign breast disease and early breast cancers. The early identification of patients at risk for subsequent invasive breast cancers allows for interventions with increased imaging and chemoprevention. These studies will require the retrospective and prospective collection of multi-center, annotated tissue collections for assay validation. Adoption of global risk stratification models that incorporate both standard histopathologic structural analysis as well as molecular changes in the breast tissue will enable future multicenter intervention trials. Tailoring both diagnostics and therapies will minimize unnecessary biopsies, surgeries, and radiation therapy.
Acknowledgements
This work is reproduced in part with permission from a review by Tabernero, Lv, and Anderson in Cancer Biomarkers, 2010. This work was supported in part by grants from the NCI/Early Detection Research Network (K.S.A.; 5U01CAl17374).
References
- [1].Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement Ann Intern Med. 2009;151:716–726. W–236. doi: 10.7326/0003-4819-151-10-200911170-00008. [DOI] [PubMed] [Google Scholar]
- [2].Aigner K, et al. The transcription factor ZEB 1 (deltaEFl) promotes tumour cell dedifferentiation by repressing master regulators of epithelial polarity. Oncogene. 2007;26:6979–6988. doi: 10.1038/sj.onc.1210508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].AI-Hajj M, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Albain KS, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncolll. :55–65. doi: 10.1016/S1470-2045(09)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Alison MR, et al. Finding cancer stem cells: are aldehyde dehydrogenases fit for purpose? J Pathol. 222:335–344. doi: 10.1002/path.2772. [DOI] [PubMed] [Google Scholar]
- [6].Allred DC, et al. Overexpression of HER-2/neu and its relationship with other prognostic factors change during the progression of in situ to invasive breast cancer. Hum Pathol. 1992;23:974–979. doi: 10.1016/0046-8177(92)90257-4. [DOI] [PubMed] [Google Scholar]
- [7].Allred DC, et al. Ductal carcinoma in situ and the emergence of diversity during breast cancer evolution. Clin Cancer Res. 2008;14:370–378. doi: 10.1158/1078-0432.CCR-07-1127. [DOI] [PubMed] [Google Scholar]
- [8].Alpers CE, Wellings SR. The prevalence of carcinoma in situ in normal and cancer-associated breasts. Hum Pathol. 1985;16:796–807. doi: 10.1016/s0046-8177(85)80251-3. [DOI] [PubMed] [Google Scholar]
- [9].Amari M, et al. LOH analyses of premalignant and malignant lesions of human breast: frequent LOH in 8p, 16q, and 17q in atypical ductal hyperplasia. Oncol Rep. 1999;6:1277–1280. doi: 10.3892/or.6.6.1277. [DOI] [PubMed] [Google Scholar]
- [10].Anderson BO, et al. Evolving concepts in the management of lobular neoplasia. J Natl Compr Canc Netw. 2006;4:511–522. doi: 10.6004/jnccn.2006.0041. [DOI] [PubMed] [Google Scholar]
- [11].Anderson KS, et al. Detection of psoriasinlSIOOA7 in the sera of patients with psoriasis. Br J Dermatol. 2009;160:325–332. doi: 10.1111/j.1365-2133.2008.08904.x. [DOI] [PubMed] [Google Scholar]
- [12].Anderson KS, et al. Application of protein microarrays for multiplexed detection of antibodies to tumor antigens in breast cancer. J Proteome Res. 2008;7:1490–1499. doi: 10.1021/pr700804c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Arteaga CL. Inhibition ofTGFbeta signaling in cancer therapy. Curr Opin Genet Dev. 2006;16:30–37. doi: 10.1016/j.gde.2005.12.009. [DOI] [PubMed] [Google Scholar]
- [14].Aubele M, et al. Extensive ductal carcinoma In situ with small foci of invasive ductal carcinoma: evidence of genetic resemblance by CGH. Int J Cancer. 2000;85:82–86. doi: 10.1002/(sici)1097-0215(20000101)85:1<82::aid-ijc15>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- [15].Balogh GA, et al. Mutant p53 protein in serum could be used as a molecular marker in human breast cancer. Int J Oncol. 2006;28:995–1002. doi: 10.3892/ijo.28.4.995. [DOI] [PubMed] [Google Scholar]
- [16].Bardou VI, et al. Progesterone receptor status significantly improves outcome prediction over estrogen receptor status alone foradjuvant endocrine therapy in two large breast cancer databases. J Clin Oncol. 2003;21:1973–1979. doi: 10.1200/JCO.2003.09.099. [DOI] [PubMed] [Google Scholar]
- [17].Barnes R, Masood S. Potential value of hormone receptor assay in carcinoma in situ of breast. Am J Clin Pathol. 1990;94:533–537. doi: 10.1093/ajcp/94.5.533. [DOI] [PubMed] [Google Scholar]
- [18].Barski A, et al. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- [19].Baum B, et al. Transitions between epithelial and mesenchymal states in development and disease. Semin Cell Dev BioI. 2008;19:294–308. doi: 10.1016/j.semcdb.2008.02.001. [DOI] [PubMed] [Google Scholar]
- [20].Beenken SW, et al. Molecular biomarkers for breast cancer prognosis: coexpression of c-erbB-2 and p53. Ann Surg. 2001;233:630–638. doi: 10.1097/00000658-200105000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Berry DA, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784–1792. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- [22].Berx G, et al. E-cadherin is inactivated in a majority of invasive human lobular breast cancers by truncation mutations throughout its extracellular domain. Oncogene. 1996;13:1919–1925. [PubMed] [Google Scholar]
- [23].Briegel KI. Embryonic transcription factors in human breast cancer. IUBMB Life. 2006;58:123–132. doi: 10.1080/15216540600686870. [DOI] [PubMed] [Google Scholar]
- [24].Buerger H, et al. Ductal invasive G2 and G3 carcinomas of the breast are the end stages of at least two different lines of genetic evolution. J Pathol. 2001;194:165–170. doi: 10.1002/path.875. [DOI] [PubMed] [Google Scholar]
- [25].Buerger H, et al. Comparative genomic hybridization of ductal carcinoma in situ of the breast-evidence of multiple genetic pathways. J Pathol. 1999;187:396–402. doi: 10.1002/(SICI)1096-9896(199903)187:4<396::AID-PATH286>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- [26].Calin GA, Croce e.M. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- [27].Cao D, et al. Serial analysis of gene expression of lobular carcinoma in situ identifies down regulation of c1audin 4 and overexpression of matrix metalloproteinase 9. Breast Cancer Res. 2008;10:R91. doi: 10.1186/bcr2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Carey LA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. Jama. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- [29].Chang HY, et al. Robustness, scalability, and integration of a wound-response gene expression signature in predicting breast cancer survival. Proc Natl Acad Sci USA. 2005;102:3738–3743. doi: 10.1073/pnas.0409462102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chang JC, et al. Gene expression profiling for the prediction of therapeutic response to docetaxel in patients with breast cancer. Lancet. 2003;362:362–369. doi: 10.1016/S0140-6736(03)14023-8. [DOI] [PubMed] [Google Scholar]
- [31].Chapman C, et al. Autoantibodies in breast cancer: their use as an aid to early diagnosis. Ann Oncol. 2007;18:868–873. doi: 10.1093/annonc/mdm007. [DOI] [PubMed] [Google Scholar]
- [32].Charafe-Jauffret E, et al. Aldehyde dehydrogenase 1-positive cancer stem cells mediate metastasis and poor clinical outcome in inflammatory breast cancer. Clin Cancer Res. 16:45–55. doi: 10.1158/1078-0432.CCR-09-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chen YY, et al. Genetic and phenotypic characteristics of pleomorphic lobular carcinoma in situ of the breast. Am J Surg Pathol. 2009;33:1683–1694. doi: 10.1097/PAS.0b013e3181b18a89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Cheng AS, et al. Epithelial progeny of estrogen-exposed breast progenitor cells display a cancer-like methylome. Can-cer Res. 2008;68:1786–1796. doi: 10.1158/0008-5472.CAN-07-5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Cheng GZ, et al. Twist transcriptionally up-regulates AKT2 in breast cancer cells leading to increased migration, invasion, and resistance to paclitaxe1. Cancer Res. 2007;67:1979–1987. doi: 10.1158/0008-5472.CAN-06-1479. [DOI] [PubMed] [Google Scholar]
- [36].Chin K, et al. Genomic and transcriptional aberrations linked to breast cancer pathophysiologies. Cancer Cell. 2006;10:529–541. doi: 10.1016/j.ccr.2006.10.009. [DOI] [PubMed] [Google Scholar]
- [37].Chitemerere M, et al. TP53 alterations in atypical ductal hyperplasia and ductal carcinoma in situ of the breast. Breast Cancer Res Treat. 1996;41:103–109. doi: 10.1007/BF01807155. [DOI] [PubMed] [Google Scholar]
- [38].Clarke ME, Fuller M. Stem cells and cancer: two faces of eve. Cell. 2006;124:1111–1115. doi: 10.1016/j.cell.2006.03.011. [DOI] [PubMed] [Google Scholar]
- [39].Collins LC, et al. Magnitude and laterality of breast cancer risk according to histologic type of atypical hyperplasia: results from the Nurses’ Health Study. Cancer. 2007;109:180–187. doi: 10.1002/cncr.22408. [DOI] [PubMed] [Google Scholar]
- [40].Colomer R, et al. It is not time to stop progesterone receptor testing in breast cancer. J Clin Oncol. 2005;23:3868–3869. doi: 10.1200/JCO.2005.05.203. author reply 3869-3870. [DOI] [PubMed] [Google Scholar]
- [41].Da Silva L, et al. Aberrant expression of E-cadherin in lobular carcinomas of the breast. Am J Surg Pathol. 2008;32:773–783. doi: 10.1097/PAS.0b013e318158d6c5. [DOI] [PubMed] [Google Scholar]
- [42].Dai H, et al. A cell proliferation signature is a marker of extremely poor outcome in a subpopulation of breast cancer patients. Cancer Res. 2005;65:4059–4066. doi: 10.1158/0008-5472.CAN-04-3953. [DOI] [PubMed] [Google Scholar]
- [43].Debnath J, et al. The role of apoptosis in creating and maintaining luminal space within normal and oncogene-expressing mammary acini. Cell. 2002;111:29–40. doi: 10.1016/s0092-8674(02)01001-2. [DOI] [PubMed] [Google Scholar]
- [44].Desmetz e., et al. Identification of a new panel of serum autoantibodies associated with the presence of in situ carcinoma of the breast in younger women. Clin Cancer Res. 2009;15:4733–4741. doi: 10.1158/1078-0432.CCR-08-3307. [DOI] [PubMed] [Google Scholar]
- [45].Di Saverio S, et al. 259 patients with DCIS of the breast applying USCNan Nuys prognostic index: a retrospective review with long term follow up. Breast Cancer Res Treat. 2008;109:405–416. doi: 10.1007/s10549-007-9668-7. [DOI] [PubMed] [Google Scholar]
- [46].Disis ML, et al. High-titer HER-2/neu protein-specific antibody can be detected in patients with early-stage breast cancer. J Clin Oncol. 1997;15:3363–3367. doi: 10.1200/JCO.1997.15.11.3363. [DOI] [PubMed] [Google Scholar]
- [47].Domagala W, et al. Immunohistochemical profile of invasive lobular carcinoma of the breast: predominantly vimentin and p53 protein negative, cathepsin 0 and oestrogen receptor positive. Virchows Arch A Pathol Anat Histopathol. 1993;423:497–502. doi: 10.1007/BF01606541. [DOI] [PubMed] [Google Scholar]
- [48].Dontu G, de Rinaldis E. MicroRNAs: shortcuts in dealing with molecular complexity? Breast Cancer Res. 12:301. doi: 10.1186/bcr2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Dontu G, et al. In vitro propagation and transcriptional profiling of human mammary stern/progenitor cells. Genes Dev. 2003;17:1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Droufakou S, et al. Multiple ways of silencing E-cadherin gene expression in lobular carcinoma of the breast. Int J Cancer. 2001;92:404–408. doi: 10.1002/ijc.1208. [DOI] [PubMed] [Google Scholar]
- [51].Dupont WO, Page DL. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med. 1985;312:146–151. doi: 10.1056/NEJM198501173120303. [DOI] [PubMed] [Google Scholar]
- [52].Elledge RM, et al. Estrogen receptor (ER) and progesterone receptor (PgR), by ligand-binding assay compared with ER, PgR and pS2, by immuno-histochemistry in predicting response to tamoxifen in metastatic breast cancer: a Southwest Oncology Group Study. Int J Cancer. 2000;89:111–117. [PubMed] [Google Scholar]
- [53].Esserman L, et al. Rethinking screening for breast cancer and prostate cancer. Jama. 2009;302:1685–1692. doi: 10.1001/jama.2009.1498. [DOI] [PubMed] [Google Scholar]
- [54].Esserman LJ, et al. A role for biomarkers in the screening and diagnosis of breast cancer in younger women. Expert Rev Mol Diagn. 2007;7:533–544. doi: 10.1586/14737159.7.5.533. [DOI] [PubMed] [Google Scholar]
- [55].Esteller M. Epigenetic lesions causing genetic lesions in human cancer: promoter hypermethylation of DNA repair genes. Eur J Cancer. 2000;36:2294–2300. doi: 10.1016/s0959-8049(00)00303-8. [DOI] [PubMed] [Google Scholar]
- [56].Fackler MJ, et al. DNA methylation of RASSFIA, HIN-l, RAR-beta, Cyclin D2 and Twist in in situ and invasive lobular breast carcinoma. Int J Cancer. 2003;107:970–975. doi: 10.1002/ijc.11508. [DOI] [PubMed] [Google Scholar]
- [57].Fackler MI, et al. Quantitative multiplex methylation-specific PCR assay for the detection of promoter hypermethylation in multiple genes in breast cancer. Cancer Res. 2004;64:4442–4452. doi: 10.1158/0008-5472.CAN-03-3341. [DOI] [PubMed] [Google Scholar]
- [58].Fan M, et al. Diverse gene expression and DNA methylation profiles correlate with differential adaptation of breast cancer cells to the antiestrogens tamoxifen and fulvestrant. Cancer Res. 2006;66:11954–11966. doi: 10.1158/0008-5472.CAN-06-1666. [DOI] [PubMed] [Google Scholar]
- [59].Famie G, Clarke RB. Mammary stem cells and breast cancer - role of Notch signalling. Stem Cell Rev. 2007;3:169–175. doi: 10.1007/s12015-007-0023-5. [DOI] [PubMed] [Google Scholar]
- [60].Fernandez-Madrid E, et al. Autoantibodies to Annexin XI-A and Other Autoantigens in the Diagnosis of Breast Cancer. Cancer Res. 2004;64:5089–5096. doi: 10.1158/0008-5472.CAN-03-0932. [DOI] [PubMed] [Google Scholar]
- [61].Foote EW, Stewart EW. Lobular carcinoma in situ: A rare form of mammary cancer. Am J Pathol. 1941;17:491–496. doi: 10.3322/canjclin.32.4.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Frykberg ER. Lobular Carcinoma In Situ of the Breast. Breast J. 1999;5:296–303. doi: 10.1046/j.1524-4741.1999.00002.x. [DOI] [PubMed] [Google Scholar]
- [63].Gao E, et al. Clinical importance of histologic grading of lobular carcinoma in situ in breast core needle biopsy specimens: current issues and controversies. Am J Clin Pathol. 133:767–771. doi: 10.1309/AJCP04ZJQTJHQYVY. [DOI] [PubMed] [Google Scholar]
- [64].Gao RI, et al. The presence of serum anti-p53 antibodies from patients with invasive ductal carcinoma of breast: correlation to other clinical and biological parameters. Breast Cancer Res Treat. 2005;93:111–115. doi: 10.1007/s10549-005-4321-9. [DOI] [PubMed] [Google Scholar]
- [65].Gregory PA, et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB 1 and SIPl. Nat Cell Bioi. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- [66].Grimm SL, Rosen JM. The role of CIEBPbeta in mammary gland development and breast cancer. J Mammary Gland Bioi Neoplasia. 2003;8:191–204. doi: 10.1023/a:1025900908026. [DOI] [PubMed] [Google Scholar]
- [67].Gupta A, et al. Hypomethylation of the synuclein gamma gene CpG island promotes its aberrant expression in breast carcinoma and ovarian carcinoma. Cancer Res. 2003;63:664–673. [PubMed] [Google Scholar]
- [68].Hammond SM. MicroRNAs as tumor suppressors. Nat Genet. 2007;39:582–583. doi: 10.1038/ng0507-582. [DOI] [PubMed] [Google Scholar]
- [69].Hannemann J, et al. Classification of ductal carcinoma in situ by gene expression profiling. Breast Cancer Res. 2006;8:R61. doi: 10.1186/bcr1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Harris JR, Lippman ME, Morrow M, Osborne CK. Diseases of the Breast (ed4th) 2010;69-85:321–340. [Google Scholar]
- [71].Hartmann L.c., et al. Benign breast disease and the risk of breast cancer. N Engl J Med. 2005;353:229–237. doi: 10.1056/NEJMoa044383. [DOI] [PubMed] [Google Scholar]
- [72].Hay ED. The mesenchymal cell, its role in the embryo, and the remarkable signaling mechanisms that create it. Dev Dyn. 2005;233:706–720. doi: 10.1002/dvdy.20345. [DOI] [PubMed] [Google Scholar]
- [73].Heneghan HM, et al. MicroRNAs as Novel Biomarkers for Breast Cancer. J Oncol. 2009;2009:950201. doi: 10.1155/2010/950201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Hu M, et al. Methylation-specific digital karyotyping. Nat Pro toe. 2006;1:1621–1636. doi: 10.1038/nprot.2006.278. [DOI] [PubMed] [Google Scholar]
- [75].Hu M, et al. Regulation of in situ to invasive breast carcinoma transition. Cancer Cell. 2008;13:394–406. doi: 10.1016/j.ccr.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Hugo H, et al. Epithelial-mesenchymal and mesenchymalepithelial transitions in carcinoma progression. J Cell Physiol. 2007;213:374–383. doi: 10.1002/jcp.21223. [DOI] [PubMed] [Google Scholar]
- [77].Hwang ES, et al. Clonality of lobular carcinoma in situ and synchronous invasive lobular carcinoma. Cancer. 2004;100:2562–2572. doi: 10.1002/cncr.20273. [DOI] [PubMed] [Google Scholar]
- [78].Hwang ES, et al. Patterns of chromosomal alterations in breast ductal carcinoma in situ. Clin Cancer Res. 2004;10:5160–5167. doi: 10.1158/1078-0432.CCR-04-0165. [DOI] [PubMed] [Google Scholar]
- [79].Inui M, et al. MicroRNA control of signal transduction. Nat Rev Mol Cell Bioi. 11:252–263. doi: 10.1038/nrm2868. [DOI] [PubMed] [Google Scholar]
- [80].James D, et al. TGF beta/activ/nlnodal signaling is necessary for the maintenance of pluripotency in human embryonic stem cells. Development. 2005;132:1273–1282. doi: 10.1242/dev.01706. [DOI] [PubMed] [Google Scholar]
- [81].Jemal A, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- [82].Kanojia D, et al. Sperm-associated antigen 9, a novel biomarker for early detection of breast cancer. Cancer Epidemiol Biomarkers Prey. 2009;18:630–639. doi: 10.1158/1055-9965.EPI-08-0629. [DOI] [PubMed] [Google Scholar]
- [83].Koziol JA, et al. Recursive partitioning as an approach to selection of immune markers for tumor diagnosis. Clin Cancer Res. 2003;9:5120–5126. [PubMed] [Google Scholar]
- [84].Kudo-Saito C, et al. Cancer metastasis is accelerated through immunosuppression during Snail-induced EMT of cancer cells. Cancer Cell. 2009;15:195–206. doi: 10.1016/j.ccr.2009.01.023. [DOI] [PubMed] [Google Scholar]
- [85].Kwaepila N, et al. Immunohistological localisation of human FATI (hFAT) protein in 326 breast cancers. Does this adhesion molecule have a role in pathogenesis? Pathology. 2006;38:125–131. doi: 10.1080/00313020600559975. [DOI] [PubMed] [Google Scholar]
- [86].Lakhani SR, et al. Atypical ductal hyperplasia of the breast: clonal proliferation with loss of heterozygosity on chromosomes 16q and 17p. J Clin Pathol. 1995;48:611–615. doi: 10.1136/jcp.48.7.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Lamendola DE, et al. Molecular description of evolving paclitaxel resistance in the SKOV-3 human ovarian carcinoma cell line. Cancer Res. 2003;63:2200–2205. [PubMed] [Google Scholar]
- [88].Larson PS, et al. Quantitative analysis of allele imbalance supports atypical ductal hyperplasia lesions as direct breast cancer precursors. J Pathol. 2006;209:307–316. doi: 10.1002/path.1973. [DOI] [PubMed] [Google Scholar]
- [89].Lee RC, et al. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- [90].Lee S, et al. Alterations of gene expression in the development of early hyperplastic precursors of breast cancer. Am J Pathol. 2007;171:252–262. doi: 10.2353/ajpath.2007.061010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Lehman CD, Smith RA. The role of MRI in breast cancer screening. J Natl Compr Cane Netw. 2009;7:1109–1115. doi: 10.6004/jnccn.2009.0072. [DOI] [PubMed] [Google Scholar]
- [92].Lehmann U, et al. Epigenetic inactivation of micro RNA gene hsa-mir-9-1 in human breast cancer. J Pathol. 2008;214:17–24. doi: 10.1002/path.2251. [DOI] [PubMed] [Google Scholar]
- [93].Lenner P, et al. Serum antibodies against p53 in relation to cancer risk and prognosis in breast cancer: a population-based epidemiological study. Br J Cancer. 1999;79:927–932. doi: 10.1038/sj.bjc.6690148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Lerwill ME. The evolution of lobular neoplasia. Adv Anat Pathol. 2006;13:157–165. doi: 10.1097/00125480-200607000-00002. [DOI] [PubMed] [Google Scholar]
- [95].Li H, et al. PIK3CA mutations mostly begin to develop in ductal carcinoma of the breast. Exp Mol Pathol. 88:150–155. doi: 10.1016/j.yexmp.2009.09.016. [DOI] [PubMed] [Google Scholar]
- [96].Liu R, et al. The prognostic role of a gene signature from tumorigenic breast-cancer cells. N Engl J Med. 2007;356:217–226. doi: 10.1056/NEJMoa063994. [DOI] [PubMed] [Google Scholar]
- [97].Lo PK, Sukumar S. Epigenomics and breast cancer. Pharmacogenomics. 2008;9:1879–1902. doi: 10.2217/14622416.9.12.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].London SI, et al. A prospective study of benign breast dis-ease and the risk of breast cancer. JAMA. 1992;267:941–944. [PubMed] [Google Scholar]
- [99].Lopez-Garcia MA, et al. Breast cancer precursors revisited: molecular features and progression pathways. Histopathology. 57:171–192. doi: 10.1111/j.1365-2559.2010.03568.x. [DOI] [PubMed] [Google Scholar]
- [100].Lowery AJ, et al. MicroRNA signatures predict oestrogen receptor, progesterone receptor and HER2/neu receptor status in breast cancer. Breast Cancer Res. 2009;11:R27. doi: 10.1186/bcr2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Lu YJ, et al. Comparative genomic hybridization analysis oflobular carcinoma in situ and atypical lobular hyperplasia and potential roles for gains and losses of genetic material in breast neoplasia. Cancer Res. 1998;58:4721–4727. [PubMed] [Google Scholar]
- [102].Lujambio A, et al. A microRNA DNA methylation signature for human cancer metastasis. Proc Natl Acad Sci USA. 2008;105:13556–13561. doi: 10.1073/pnas.0803055105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Lynch MD, et al. Breast cancer, stem cells and prospects for therapy. Breast Cancer Res. 2006;8:211. doi: 10.1186/bcr1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Ma L, et al. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449:682–688. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- [105].Ma XJ, et al. The HOXBI3:IL17BR expression index is a prognostic factor in early-stage breast cancer. J Clin Oncol. 2006;24:4611–4619. doi: 10.1200/JCO.2006.06.6944. [DOI] [PubMed] [Google Scholar]
- [106].Ma XJ, et al. Gene expression profiles of human breast cancer progression. Proc Natl Acad Sci USA. 2003;100:5974–5979. doi: 10.1073/pnas.0931261100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Mani SA, et al. Mesenchyme Forkhead 1 (FOXC2) plays a key role in metastasis and is associated with aggressive basal-like breast cancers. Proc Natl Acad Sci USA. 2007;104:10069–10074. doi: 10.1073/pnas.0703900104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Mani SA, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Marshall LM, et al. Risk of breast cancer associated with atypical hyperplasia of lobular and ductal types. Cancer Epidemiol Biomarkers Prev. 1997;6:297–301. [PubMed] [Google Scholar]
- [110].Mastracci TL, et al. E-cadherin alterations in atypical lobular hyperplasia and lobular carcinoma in situ of the breast. Mod Pathol. 2005;18:741–751. doi: 10.1038/modpathol.3800362. [DOI] [PubMed] [Google Scholar]
- [111].Mastracci TL, et al. Genomic alterations in lobular neoplasia: a microarray comparative genomic hybridization signature for early neoplastic proliferationin the breast. Genes Chromosomes Cancer. 2006;45:1007–1017. doi: 10.1002/gcc.20368. [DOI] [PubMed] [Google Scholar]
- [112].McDivitt RW, et al. The Cancer and Steroid Honnone Study Group Histologic types of benign breast disease and the risk for breast cancer. Cancer. 1992;69:1408–1414. doi: 10.1002/1097-0142(19920315)69:6<1408::aid-cncr2820690617>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- [113].McLaren BK, et al. Excellent survival, cancer type, and Nottingham grade after atypical lobular hyperplasia on initial breast biopsy. Cancer. 2006;107:1227–1233. doi: 10.1002/cncr.22113. [DOI] [PubMed] [Google Scholar]
- [114].Mechera R, et al. Factors predicting in-breast tumor recurrence after breast-conserving surgery. Breast Cancer Res Treat. 2009;116:171–177. doi: 10.1007/s10549-008-0187-y. [DOI] [PubMed] [Google Scholar]
- [115].Middleton LP, et al. Expression of ERalpha and ERbeta in lobular carcinoma in situ. Histopathology. 2007;50:875–880. doi: 10.1111/j.1365-2559.2007.02689.x. [DOI] [PubMed] [Google Scholar]
- [116].Mikkelsen TS, et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature. 2007;448:553–560. doi: 10.1038/nature06008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Miska EA. MicroRNAs-keeping cells in fonnation. Nat Cell Bioi. 2008;10:501–502. doi: 10.1038/ncb0508-501. [DOI] [PubMed] [Google Scholar]
- [118].Mohsin SK, et al. Biomarker profile and genetic abnonnalities in lobular carcinoma in situ. Breast Cancer Res Treat. 2005;90:249–256. doi: 10.1007/s10549-004-4493-8. [DOI] [PubMed] [Google Scholar]
- [119].Morel AP, et al. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS ONE. 2008;3:e2888. doi: 10.1371/journal.pone.0002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Moshin Modern Pathology. 2005 [Google Scholar]
- [121].Nagi CS, et al. Lobular neoplasia on core needle biopsy does not require excision. Cancer. 2008;112:2152–2158. doi: 10.1002/cncr.23415. [DOI] [PubMed] [Google Scholar]
- [122].Nayar R, et al. Loss of heterozygosity on chromosome 11q13 in lobular lesions of the breast using tissue microdissection and polymerase chain reaction. Hum Pathol. 1997;28:277–282. doi: 10.1016/s0046-8177(97)90124-6. [DOI] [PubMed] [Google Scholar]
- [123].Nonni A, et al. Immunohistochemical expression of estrogen receptors alpha and beta in lobular neoplasia. Virchows Arch. 2007;451:893–897. doi: 10.1007/s00428-007-0504-6. [DOI] [PubMed] [Google Scholar]
- [124].O’Connell P, et al. Analysis ofloss of heterozygosity in 399 premalignant breast lesions at 15 genetic loci. J Natl Cancer Inst. 1998;90:697–703. doi: 10.1093/jnci/90.9.697. [DOI] [PubMed] [Google Scholar]
- [125].Oh DS, et al. Estrogen-regulated genes predict survival in honnone receptor-positive breast cancers. J Clin Oncol. 2006;24:1656–1664. doi: 10.1200/JCO.2005.03.2755. [DOI] [PubMed] [Google Scholar]
- [126].Ottesen GL, et al. Carcinoma in situ of the breast: correlation of histopathology to immnnohistochemical markers and DNA ploidy. Breast Cancer Res Treat. 2000;60:219–226. doi: 10.1023/a:1006453420088. [DOI] [PubMed] [Google Scholar]
- [127].Page DL, et al. Atypical hyperplastic lesions of the female breast. A long-tenn follow-up study. Cancer. 1985;55:2698–2708. doi: 10.1002/1097-0142(19850601)55:11<2698::aid-cncr2820551127>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- [128].Page DL, et al. Lobular neoplasia of the breast: higher risk for subsequent invasive cancer predicted by more extensive disease. Hum Pathol. 1991;22:1232–1239. doi: 10.1016/0046-8177(91)90105-x. [DOI] [PubMed] [Google Scholar]
- [129].Page DL, et al. Atypical lobular hyperplasia as a unilateral predictor of breast cancer risk: a retrospective cohort study. Lancet. 2003;361:125–129. doi: 10.1016/S0140-6736(03)12230-1. [DOI] [PubMed] [Google Scholar]
- [130].Paik S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- [131].Paik S, et al. A mUltigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J M ed. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- [132].Pakneshan P, et al. Reversal of the hypomethylation status of urokinase (uPA) promoter blocks breast cancer growth and metastasis. J Bioi Chem. 2004;279:31735–31744. doi: 10.1074/jbc.M401669200. [DOI] [PubMed] [Google Scholar]
- [133].Parkin DM, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- [134].Patrawala L, et al. Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2-cancer cells are similarly tumorigenic. Cancer Res. 2005;65:6207–6219. doi: 10.1158/0008-5472.CAN-05-0592. [DOI] [PubMed] [Google Scholar]
- [135].Pepe MS, et al. Phases of biomarker development for early detection of cancer. J Natl Cancer Inst. 2001;93:1054–1061. doi: 10.1093/jnci/93.14.1054. [DOI] [PubMed] [Google Scholar]
- [136].Perou CM, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- [137].Polyak K, Hahn WC. Roots and stems: stem cells in cancer. Nat Med. 2006;12:296–300. doi: 10.1038/nm1379. [DOI] [PubMed] [Google Scholar]
- [138].Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- [139].Ponti D, et al. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65:5506–5511. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- [140].Pories SE, et al. Urinary metalloproteinases: noninvasive biomarkers for breast cancer risk assessment. Cancer Epidemiol Biomarkers Prey. 2008;17:1034–1042. doi: 10.1158/1055-9965.EPI-07-0365. [DOI] [PubMed] [Google Scholar]
- [141].Porter D, et al. Molecular markers in ductal carcinoma in situ of the breast. Mol Cancer Res. 2003;1:362–375. [PubMed] [Google Scholar]
- [142].Precht LM, et al. Neoadjuvant Chemotherapy of Breast Cancer: Tumor Markers as Predictors of Pathologic Response, Recurrence, and Survival. Breast J. doi: 10.1111/j.1524-4741.2010.00935.x. [DOI] [PubMed] [Google Scholar]
- [143].Radford DM, et al. Allelic loss and the progression of breast cancer. Cancer Res. 1995;55:5180–5183. [PubMed] [Google Scholar]
- [144].Radisky DC, LaBarge MA. Epithelial-mesenchymal transition and the stem cell phenotype. Cell Stem Cell. 2008;2:511–512. doi: 10.1016/j.stem.2008.05.007. [DOI] [PubMed] [Google Scholar]
- [145].Rakha EA, et al. Biologic and clinical characteristics of breast cancer with single hormone receptor positive phenotype. J Clin Oncol. 2007;25:4772–4778. doi: 10.1200/JCO.2007.12.2747. [DOI] [PubMed] [Google Scholar]
- [146].Ramachandran N, et al. Tracking humoral responses using self assembling protein microarrays. Proteomics-Clinical Applications. 2008;2:1518–1527. doi: 10.1002/prca.200800034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Ravdin PM, et al. Prognostic significance of progesterone receptor levels in estrogen receptor-positive patients with metastatic breast cancer treated with tamoxifen: results of a prospective Southwest Oncology Group study. J Clin Oneal. 1992;10:1284–1291. doi: 10.1200/JCO.1992.10.8.1284. [DOI] [PubMed] [Google Scholar]
- [148].Reinhart BJ, et al. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403:901–906. doi: 10.1038/35002607. [DOI] [PubMed] [Google Scholar]
- [149].Reis-Filho JS, et al. Pleomorphic lobular carcinoma of the breast: role of comprehensive molecular pathology in characterization of an entity. J Pathol. 2005;207:1–13. doi: 10.1002/path.1806. [DOI] [PubMed] [Google Scholar]
- [150].Roh T.y., et al. High-resolution genome-wide mapping of histone modifications. Nat Biotechnol. 2004;22:1013–1016. doi: 10.1038/nbt990. [DOI] [PubMed] [Google Scholar]
- [151].Rosai J. Borderline epithelial lesions of the breast. Am J Surg Pathol. 1991;15:209–221. doi: 10.1097/00000478-199103000-00001. [DOI] [PubMed] [Google Scholar]
- [152].Rubin E, et al. Proliferative disease and atypia in biopsies performed for nonpalpable lesions detected mammographically. Cancer. 1988;61:2077–2082. doi: 10.1002/1097-0142(19880515)61:10<2077::aid-cncr2820611024>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- [153].Sarrio D, et al. Cytoplasmic localization of p120ctn and E-cadherin loss characterize lobular breast carcinoma from preinvasive to metastatic lesions. Oncogene. 2004;23:3272–3283. doi: 10.1038/sj.onc.1207439. [DOI] [PubMed] [Google Scholar]
- [154].Scherf U, et al. A gene expression database forthe molecular pharmacology of cancer. Nat Genet. 2000;24:236–244. doi: 10.1038/73439. [DOI] [PubMed] [Google Scholar]
- [155].Schmitt EC. Multistep progression from an oestrogen-dependent growth towards an autonomous growth in breast carcinogenesis. Eur J Cancer. 1995;3lA:2049–2052. doi: 10.1016/0959-8049(95)00430-0. [DOI] [PubMed] [Google Scholar]
- [156].Schnitt SJ, et al. Interobserver reproducibility in the diagnosis of ductal proliferative breast lesions using standardized criteria. Am J Surg Pathol. 1992;16:1133–1143. doi: 10.1097/00000478-199212000-00001. [DOI] [PubMed] [Google Scholar]
- [157].Shipitsin M, et al. Molecular definition of breast tumor heterogeneity. Cancer Cell. 2007;11:259–273. doi: 10.1016/j.ccr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- [158].Simpson PT, et al. Molecular evolution of breast cancer. J Pathol. 2005;205:248–254. doi: 10.1002/path.1691. [DOI] [PubMed] [Google Scholar]
- [159].Slack EJ, Weidhaas JB. MicroRNAs as a potential magic bullet in cancer. Future Oncol. 2006;2:73–82. doi: 10.2217/14796694.2.1.73. [DOI] [PubMed] [Google Scholar]
- [160].Sneige N, et al. Clinical, histopathologic, and biologic features of pleomorphic lobular (ductal-lobular) carcinoma in situ of the breast: a report of 24 cases. Mod Pathol. 2002;15:1044–1050. doi: 10.1097/01.MP.0000027624.08159.19. [DOI] [PubMed] [Google Scholar]
- [161].Soares J, et al. Global DNA hypomethylation in breast carcinoma: correlation with prognostic factors and tumor progression. Cancer. 1999;85:112–118. [PubMed] [Google Scholar]
- [162].Solin LJ. Is excision alone adequate treatment for low-risk ductal carcinoma-in-situ of the breast? J Clin Oncol. 2006;24:1017–1019. doi: 10.1200/JCO.2005.04.4610. [DOI] [PubMed] [Google Scholar]
- [163].Sorlie T, et al. Repeated observation of breast tumor SUbtypes in independent gene expression data sets. Proc Natl Acad Sci USA. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Sorlie T, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Sotiriou C, Pusztai L. Gene-expression signatures in breast cancer. N Engl J Med. 2009;360:790–800. doi: 10.1056/NEJMra0801289. [DOI] [PubMed] [Google Scholar]
- [166].Sotiriou C, et al. Gene expression profiles derived from fine needle aspiration correlate with response to systemic chemotherapy in breast cancer. Breast Cancer Res. 2002;4:R3. doi: 10.1186/bcr433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Sotiriou C, et al. Gene expression profiling in breast cancer: understanding the molecular basis of histologic grade to improve prognosis. J Natl Cancer Inst. 2006;98:262–272. doi: 10.1093/jnci/djj052. [DOI] [PubMed] [Google Scholar]
- [168].Sparano JA, Paik S. Development of the 21-gene assay and its application in clinical practice and clinical trials. J Clin Oncol. 2008;26:721–728. doi: 10.1200/JCO.2007.15.1068. [DOI] [PubMed] [Google Scholar]
- [169].Staunton JE, et al. Chemosensitivity prediction by transcriptional profiling. Proc Natl Acad Sci USA. 2001;98:10787–10792. doi: 10.1073/pnas.191368598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Tamesa MS, et al. Detection of autoantibodies against cyc10philin A and triosephosphate isomerase in sera from breast cancer patients by proteomic analysis. Electrophoresis. 2009;30:2168–2181. doi: 10.1002/elps.200800675. [DOI] [PubMed] [Google Scholar]
- [171].Tamimi RM, et al. Endogenous sex hormone levels and mammographic density among postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2005;14:2641–2647. doi: 10.1158/1055-9965.EPI-05-0558. [DOI] [PubMed] [Google Scholar]
- [172].Tamimi RM, et al. Endogenous hormone levels, mammographic density, and subsequent risk of breast cancer in post-menopausal women. J Natl Cancer Inst. 2007;99:1178–1187. doi: 10.1093/jnci/djm062. [DOI] [PubMed] [Google Scholar]
- [173].Tavassoli EA. Breast pathology: rationale for adopting the ductal intraepithelial neoplasia (DIN) classification. Nat Clin Pract Oncol. 2005;2:116–117. doi: 10.1038/ncponc0109. [DOI] [PubMed] [Google Scholar]
- [174].Tavazoie S,E, et al. Endogenous human microRNAs that suppress breast cancer metastasis. Nature. 2008;451:147–152. doi: 10.1038/nature06487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Bioi. 2003;15:740–746. doi: 10.1016/j.ceb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- [176].Thiery J,P, et al. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- [177].Tomkiel JE, et al. Autoimmunity to the M(r) 32,000 subunit of replication protein A in breast cancer. Clin Cancer Res. 2002;8:752–758. [PMC free article] [PubMed] [Google Scholar]
- [178].Tsuda H, et al. Correlation of numerical and structural status of chromosome 16 with histological type and grade of non-invasive and invasive breast carcinomas. Int J Cancer. 1999;84:381–387. doi: 10.1002/(sici)1097-0215(19990820)84:4<381::aid-ijc9>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- [179].Umekita Y, et al. Expression of p53 protein in benign epithelial hyperplasia, atypical ductal hyperplasia, non-invasive and invasive mammary carcinoma: an immunohistochemical study. Virchows Arch. 1994;424:491–494. doi: 10.1007/BF00191434. [DOI] [PubMed] [Google Scholar]
- [180].van de Vijver MJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- [181].Volkmann M, et al. Anti-p53 in.breast cancer: concordance of different assay procedures and association with p53 antigen expression. Oncology. 2002;63:297–305. doi: 10.1159/000065472. [DOI] [PubMed] [Google Scholar]
- [182].Vos CB, et al. E-cadherin inactivation in lobular carcinoma in situ of the breast: an early event in tumorigenesis. Br J Cancer. 1997;76:1131–1133. doi: 10.1038/bjc.1997.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Wagner PL, et al. Clonal relationship between closely approximated low-grade ductal and lobular lesions in the breast: a molecular study of 10 cases. Am J Clin Pathol. 2009;132:871–876. doi: 10.1309/AJCP7AK1VWFNMCSW. [DOI] [PubMed] [Google Scholar]
- [184].Waldman EM, et al. Chromosomal alterations in ductal carcinomas in situ and their in situ recurrences. J Natl Cancer Inst. 2000;92:313–320. doi: 10.1093/jnci/92.4.313. [DOI] [PubMed] [Google Scholar]
- [185].Wang CC, et al. Glycan microarray of Globo H and related structures for quantitative analysis of breast cancer. Proc Natl Acad Sci USA. 2008;105:11661–11666. doi: 10.1073/pnas.0804923105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Wang Y, et al. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet. 2005;365:671–679. doi: 10.1016/S0140-6736(05)17947-1. [DOI] [PubMed] [Google Scholar]
- [187].Weber M, et al. Chromosome-wide and promoter-specific analyses identify sites of differential DNA methylation in normal and transformed human cells. Nat Genet. 2005;37:853–862. doi: 10.1038/ng1598. [DOI] [PubMed] [Google Scholar]
- [188].Wellings SR, Jensen HM. On the origin and progression of ductal carcinoma in the human breast. J Natl Cancer Inst. 1973;50:1111–1118. doi: 10.1093/jnci/50.5.1111. [DOI] [PubMed] [Google Scholar]
- [189].Wellings SR, et al. An atlas of subgross pathology of the human breast with special reference to possible precancerous lesions. J Natl Cancer Inst. 1975;55:231–273. [PubMed] [Google Scholar]
- [190].Wicha MS, et al. Cancer stem cells: an old idea-a paradigm shift. Cancer Res. 2006;66:1883–1890. doi: 10.1158/0008-5472.CAN-05-3153. discussion 1895-1886. [DOI] [PubMed] [Google Scholar]
- [191].Widschwendter M, Jones PA. DNA methylation and breast carcinogenesis. Oncogene. 2002;21:5462–5482. doi: 10.1038/sj.onc.1205606. [DOI] [PubMed] [Google Scholar]
- [192].Wightman B, et al. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell. 1993;75:855–862. doi: 10.1016/0092-8674(93)90530-4. [DOI] [PubMed] [Google Scholar]
- [193].Witkiewicz AK, et al. Stromal CDI0 and SPARC expression in ductal carcinoma in situ (DCIS) patients predicts disease recurrence. Cancer Bioi Ther. 10:391–396. doi: 10.4161/cbt.10.4.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Wrobel CN, et al. Autocrine CSF-IR activation promotes Src-dependent disruption of mammary epithelial architecture. J Cell Bioi. 2004;165:263–273. doi: 10.1083/jcb.200309102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Yagihashi A, et al. Detection of autoantibodies to survivin and livin in sera from patients with breast cancer. Clin Chim Acta. 2005;362:125–130. doi: 10.1016/j.cccn.2005.06.009. [DOI] [PubMed] [Google Scholar]
- [196].Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- [197].Zhong L, et al. Autoantibodies as potential biomarkers for breast cancer. Breast Cancer Res. 2008;10:R40. doi: 10.1186/bcr2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Zhu S, et al. MicroRNA-21 targets tumor suppressor genes in invasion and metastasis. Cell Res. 2008;18:350–359. doi: 10.1038/cr.2008.24. [DOI] [PubMed] [Google Scholar]
- [199].Zou D, et al. Epigenetic silencing in non-neoplastic epithelia identifies E-cadherin (CD HI) as a target for chemoprevention of lobular neoplasia. J Pathol. 2009;218:265–272. doi: 10.1002/path.2541. [DOI] [PubMed] [Google Scholar]