SUMMARY
The goal of cancer vaccines and immunotherapies is to train the immune system to recognize cancer cells and destroy them. Immune responses play a dynamic role in the development of cancers, from immunosurveillance to immune escape; from in situ immune dysregulation to metastatic spread. The systematic identification and targeting of molecules involved in the immune response has led to a wide variety of potential immunotherapeutic targets for the treatment of breast cancer. Extraordinary advances in molecular immunology have led to a detailed understanding of tumor antigens, antigen presentation, innate immunity, cytokine and chemokine pathways, and immunoregulation. Many of these vaccine therapies are already in clinical development. It is the rational and rapid translation of these scientific discoveries into effective therapies for patients with breast cancer that poses the greatest challenge, and opportunity, to realize the potential of tumor vaccine therapy for breast cancer.
CANCER AND THE IMMUNE RESPONSE
The immune system is a complex multi-cellular network, which can quickly accommodate or combat novel pathogens. This network of activating and inhibitory cells and molecules result in a tight balance between immunity and autoimmunity. It is the ability of the immune system to distinguish self from non-self that results in effective clearance of pathogens and immunologic memory. The primary challenge facing the field of tumor immunology is that, unlike infections, all tumor cells contain self-antigens that vary from normal tissue, primarily by mutation or by expression level. Many of these self-antigens are critical for biologic processes, such as DNA replication, or are expressed at some level on normal tissues. Thus, effective tumor immunity carries the risk of clinically significant autoimmunity.
There are several lines of evidence suggesting that breast cancer is subject to immunosurveillance. A case–control study of 176 women with breast cancer showed a genetic association with protective human leukocyte antigen (HLA) class II alleles (1). MHC molecules are down regulated in 20% to 50% of primary breast tumors and cell lines, and class II molecules have been detected in around 30% of breast carcinoma lesions (2, 3), but this is of unclear clinical significance. As with ovarian cancer, melanoma, and colon cancer, lymphocytic infiltrates have been shown to be associated with improved overall survival in breast cancer (4, 5). T-cells recognizing MUC-1 and HER2/neu-derived antigens have been isolated from the blood of breast cancer patients (6, 7). Evidence that T-lymphocytes can effectively target breast cancer tumor cells is demonstrated by the small, but measurable graft-versus-tumor effects that have been shown in patients, undergoing donor-lymphocyte infusion after allogeneic stem-cell transplantation (8–10).
Innate immunity
The identification of the molecular pathways involved in the innate immune response has led to numerous clinical trials of immune adjuvants. The innate immune response represents the first line of defense against pathogens, and includes natural barriers (skin, mucosa, and the blood–brain barrier), cytokines, complement, and cellular immunity including natural killer cells (NK cells), neutrophils, and macrophages (11). This response is primarily mediated by activation of the family of toll-like receptors (TLRs) on macrophages. There are at least 10 known human TLRs, each of which is stimulated by specific molecular structures. These agonists are potent immunostimulants and include double-stranded RNA (which activates TLR3), lipo-polysaccharide (which activates TLR4), and CpG DNA (which activates TLR9). TLR stimulation leads to the destruction of pathogens by means of activated macrophages or natural killer (NK) cells as well as cytokine release for immune amplification and dendritic cell maturation (12). As a result, TLR agonists are being developed as adjuvants in both infectious and cancer vaccine trials. For example, CpGs are synthetic 8 to 30 base-long oligonucleotides that mimic pathogenic DNA, and activate TLR9 on dendritic cells to augment T-cell responses to vaccination (13, 14).
In addition to TLRs, NKG2D is an activating receptor expressed on NK cells and macrophages. NKG2D can interact with ligands expressed by tumor cells, causing alteration of innate immunity (15). In animal models, NKG2D ligand expression early in tumor development protects the host from tumor initiation (16). These ligands include major histocompatibility complex (MHC) class I chain-related protein A and B (MICA and MICB). MIC proteins are overexpressed in most epithelial cancers, including breast tumors (17, 18), and soluble major histocomputibility complex (MHC) antigens secreted by tumors down regulate T-cell activity (19). In addition, the inhibitory NK cell ligands HLA-E and -F have been detected on a subset of breast tumor cell lines (3), and soluble HLA-G, which induces apoptosis of T-cells that has been detected in malignant ascites (20). The mechanisms used by tumor cells to regulate the innate immune response are all potential targets for therapeutic intervention.
Adaptive immunity
The adaptive immune response, which involves T- and B-lymphocytes, is required for immunologic memory. This response is initially slower than the innate response but leads to rapid and highly specific memory responses on subsequent challenge. Antigens may be either directly presented by tumor cells, or cross-presented by antigen-presenting cells (APCs). Either way, the antigens are degraded to peptide epitopes, which are then bound to MHC molecules, for presentation to T-cells (Fig. 1). MHC class I peptide epitopes that are created by proteasomal cleavage are structurally limited by the size of the MHC peptide-binding groove. Therefore, for a given antigenic sequence, potential MHC class I-binding epitopes, such as the E75 peptide of HER2/neu (21) or the I540 peptide of telomerase (22), can be predicted with some accuracy using algorithms based on the primary sequence of the protein. A limited number of tumor antigenic epitopes have been directly sequenced from purified class I MHC molecules (23, 24), but the low concentration of specific peptides has made direct identification of tumor antigenic sequences by mass spectrometry difficult.
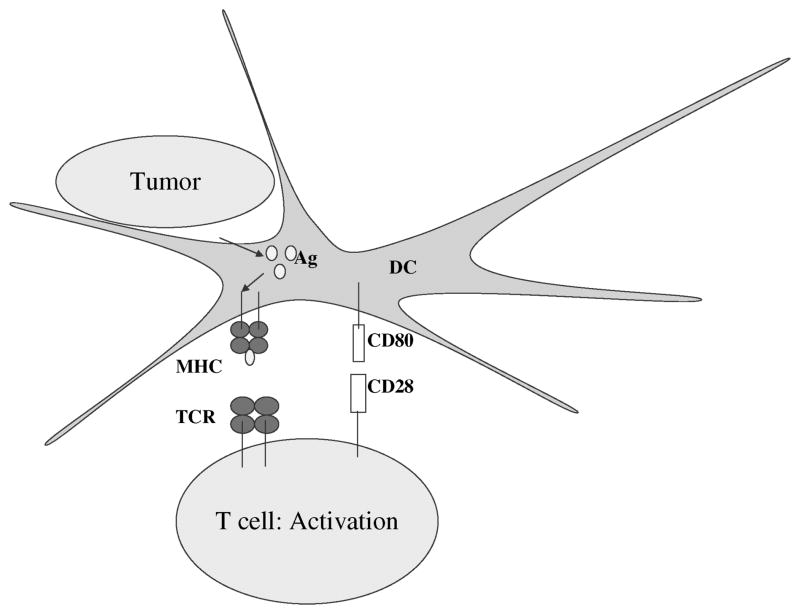
Figure 1.
Induction of T-cell immunity. Tumor antigens are endo-cytosed by immature dendritic cells, processed by proteases, and presented as peptides by MHC molecules to T cells. Expression of costimulatory molecules such as CD80 and CD86 are required for efficient priming of T cells via activation of CD28. Abbreviations: DC, dendritic cells; MHC, major histocompatibility complex; TCR, T-cell receptor.
In contrast, MHC class II molecules primarily bind peptides derived from exogenous antigen that is endocytosed by APCs for presentation to CD4+ T-cells. Since the peptide-binding groove of class II molecules is structurally more flexible than that of class I molecules, prediction of antigenic peptides is more difficult and requires systematic empirical identification with the use of overlapping peptide sets. As a result, fewer class II peptide epitopes from tumor antigens have been identified and tested in clinical trials (25, 26).
Antibody immunity
The natural development of B cell antibody responses to tumor antigens is dependent on antigen overexpression, mutation, apoptosis, changes structural, and aberrant glycosylation (27). Aberrently glycosylated carbohydrate antigens, such as Tn, are expressed by tumor cells and have been used in clinical vaccine trials in breast cancer (28–31) with evidence of immunogenicity. Serologic expression cloning has been used to detect antibodies to multiple breast cancer protein antigens, including HER2/neu, p53, MUC1, and NY-ESO-1 (32–35). In prostate cancer, patterns of autoantibody production correlate with disease outcome (36), suggesting that autoantibodies may be useful as proteomic biomarkers both for diagnosis and prognosis (27). Similarly, antibodies to HER2/neu have been detected in serum samples from 20% of patients with HER2+ early-stage of breast cancer (32). Although HER2 antibody titers of exceeding 1:5000 have been reported, it is unclear whether they confer a protective immune response. Since B-cell immunity often correlates with T-cell immunity, autoantigen identification has led to the identification of T-cell antigens for vaccine development (37, 38). Since tumor antigen-specific antibodies can enhance tumor antigen cross-presentation, combined vaccine and antibody therapy, such as HER2-vaccines and trastuzumab, may augment anti-tumor immunity.
Cytokine dysregulation
In the tumor microenvironment, tumor cells both actively down regulate the immune function and co-opt the immune molecules for tumor activation, invasion, and metastasis (39–41). Molecules such as vascular endothelial growth factor (VEGF), interleukin-6, macrophage-colony stimulating factor (M-CSF), cyclooxygenase 2 (COX-2), interleukin-10, stem cell factor-1, and transforming growth factor (TGF β) are abundant in the tumor microenvironment, resulting in altered dendritic cell and T-cell function (Fig. 2) (39). In addition to secreted molecules, transmembrane molecules such as FasL (CD95L), B7-H1/PD-L1 and B7-H4 are potent inhibitors of T-lymphocyte function (42). FasL (43), B7-H1 (44), and B7-H4 (45) are all expressed by subsets of breast tumors and are potential targets of immune intervention.
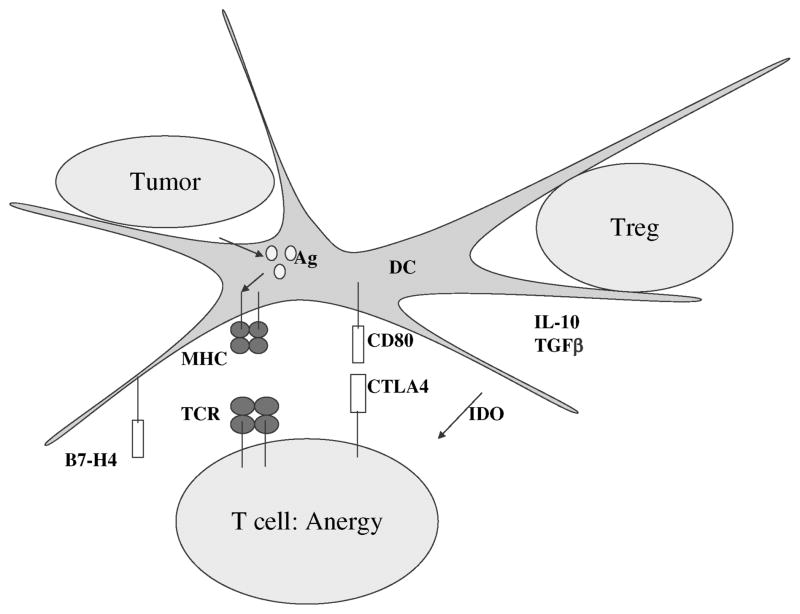
Figure 2.
Regulation of T-cell immunity. CD4+CD25+ regulatory T cells and cytokines in the tumor microenvironment such as IL-10 and TGF-β result in altered dendritic cell and effector T-cell function. This includes overexpression of inhibitory molecules, such as B7-H4 and indoleamine 2,3-dioxygenase, and activation of the T-cell inhibitory molecule, CTLA-4. These pathways result in T-cell anergy or tolerance. Abbreviations: CTLA4, cyto-toxic T-lymphocyte-associated antigen; DC, dendritic cell; IDO, in-doleamine 2,3-dioxygenase; MHC, major histocompatibility complex; TCR, T-cell receptor; TGF β, transforming growth factor.
Antigen presentation and dendritic cells
Although tumor antigenic peptides can be presented directly from tumor cells, professional APCs, in particular dendritic cells, are essential for priming naïve T-cells and activating the immune response (46). Tumors of epithelial origin generally do not express costimulatory signals, such as B7, CD40, 4-1BBL (47), and OX40L (48) that are required for activation of effective T-cell responses. Immature DC’s may actively endocytose necrotic, apoptotic, or antibody-coated tumor cells (“cross-presentation”), and then undergo maturation upon activation of TLRs, CD40 ligand, or cytokine signals such as TNF-alpha (Fig. 1). Upon maturation, dendritic cells upregulate MHC molecules and costimulatory molecules, secrete cytokines and chemokines to enhance the migration of lymphocytes, and express chemokine receptors for migration to lymph nodes (49).
There is mounting evidence that dendritic cells have abnormal function in cancer patients (Fig. 2) (50). Indoleamine 2,3-dioxygenase (IDO) is involved in tryptophan catabolism and is thought to play a role in placental-based maternal immune tolerance. Accumulation of IDO in dendritic cells correlates with impairment of T-cell function in vitro and has been observed in lymph nodes of patients with melanoma and breast cancer, and other tumors (51, 52). IDO accumulation in dendritic cells can predate the development of overt lymphnode metastases. Inhibitors of IDO are now being developed as potential immune adjuvants.
There have been multiple clinical trials of vaccine delivery with the use of dendritic cells. The cells are usually isolated from peripheral blood by means of leukapheresis. They are cultured in vitro with the cytokines GM-CSF and interleukin-4, loaded with antigen, and matured ex vivo to enhance antigen presentation and costimulation of T-cells before being injected into patients. Antigen may be delivered as peptide, protein, RNA (53), or tumor lysates (54) (Table 1). In addition, dendritic cells have been directly fused with autologous breast cancer tumor cells, which allows for the presentation of multiple tumor antigens (55). Although dendritic cell-based vaccines have had minimal side effects and have induced measurable T-cell immunity, few durable clinical responses have been reported (55–57). However, a dendritic cell-based vaccine was shown to confer a modest survival benefit in hormone-refractory prostate cancer (58). Because dendritic cell production must be performed in specialized clinical laboratories, alternative strategies, including using artificial APCs or targeting antigen directly to dendritic cells in vivo using DC-targeted antibodies, microparticles, electroporation, or nanotechnology are being explored.
Table 1.
Breast Cancer Vaccination Strategies
| Antigen | Antigen delivery | Adjuvant |
|---|---|---|
| HER2/neu | Peptide | GM-CSF |
| MUC-1 | Protein | CpG |
| CEA | RNA | TRICOM™ |
| Survivin | DNA | Dendritic cells |
| Telomerase | Vaccinia virus | QS-21 |
| NY-ESO-1 | Fowlpox virus | |
| Cyp1B1 | Nanoparticles | |
| Cyclin B1 | Expanded T cells | |
| Mammaglobin A | Cell fusion | |
| Carbohydrate antigens | ||
| Autologous cells | ||
| Allogeneic cells |
TARGETING TUMOR ANTIGENS
Antigen-specific vaccines
The ideal breast cancer vaccine would induce broadly reactive immunity to multiple types of breast cancer without causing clinically significant autoimmunity and, most important, be clinically effective. One approach to minimize autoimmunity and enhance specificity of vaccines is to target them to specific protein antigens that are overexpressed on the tumor cells but that have limited distribution in normal tissue. Many breast cancer tumor antigens are also expressed on tumor cells in other epithelial-derived cancers, such as ovarian cancer and colon cancer, and have been targeted in early-phase clinical trials in breast cancer and other solid tumors. In addition to MUC-1, HER2/neu, and telomerase (see subsequently), target antigens include CEA (59, 60), cyp1B1 (61), survivin (62, 63), and others (Table 1).
MUC-1
Overexpression and aberrant glycosylation of mucin-1 (MUC-1) antigen by epithelial tumors results in endogenous antibody responses in cancer patients to MUC-1 antigen (64). This finding has led to the identification of MUC-1-derived peptide epitopes that induce T-cell responses. MUC-1-based clinical trials have used peptides (65–69), protein (70), pulsed dendritic cells (61), or keyhole limpet hemocyanin (KLH) adjuvant (31).
HER2/neu
The HER2/neu antigen is a well-known target of antibody-mediated immunotherapy in breast cancer. The initial demonstration of multiple HLA-A2-binding peptides derived from the HER2/neu protein has led to multiple clinical vaccine trials. Initial studies using peptide and adjuvant have demonstrated safety with minimal toxicity (61, 71–73), but induced cytotoxic T-cells that failed to lyse tumor cells (21). To augment CD4+ T-cell immunity, HER2-derived class II peptides (26, 74), or the HER2 intracellular domain (75, 76), have been used for vaccination. A recent study of vaccination of high-risk patients in the adjuvant setting showed a trend toward improved disease-free survival in patients who received a HER2 peptide-based vaccine (85.7% vs. 59.8% in unvaccinated patients).
hTERT
The catalytic subunit of telomerase, hTERT, is a widely expressed tumor antigen, present in more than 85% of all human cancers (22). Initial clinical trials of dendritic cells pulsed with hTERT-derived peptides or hTERT RNA resulted in measurable hTERT-specific immunity (78, 79), but hTERT peptide vaccination with adjuvant generated T-cells that did not recognize endogenously-processed telomerase (80).
Overall, these antigen-specific therapies have been well tolerated, with minimal toxicity, but only sporadic disease responses have been observed. The majority of these vaccines have been tested in the advanced disease setting. The optimal method of delivery of tumor antigens is not yet known, although many approaches have been tried (Table 1). These include adoptive immunotherapy with ex vivo expanded T-cells (81), peptide-based vaccines, proteins, RNA, DNA, and viral vectors such as vaccinia and fowlpox, that also encode three costimulatory molecules [CD80/B7.1, ICAM-1, and LFA-3; designated TRICOM™ (59)].
Cellular-based vaccines
Vaccines based on whole autologous or allogeneic tumor cells have been combined with strong adjuvants or cytokines, since tumor cells themselves generally stimulate poor antigen presentation (82). Both autologous tumor cells (83–85) and allogeneic cell lines (86–88) have been used in clinical trials in breast cancer, with isolated clinical responses reported. Whole tumor cells have also been fused with dendritic cells (89). In murine models, GM-CSF was the most potent cytokine adjuvant for vaccination (90), and GM-CSF-secreting autologous and allogeneic vaccines are currently being evaluated in clinical trials in breast cancer.
TARGETING IMMUNE REGULATION
The focus of tumor immunology is shifting from targeting specific antigens to targeting the regulation of immune responses that result in impaired host immunity and tolerance to tumor antigens (Fig. 2 and Table 2). By activating co-stimulatory molecules and inhibiting molecules that down regulate immunity, effective T-cell immunity can be generated. By combining these approaches of targeted antigenic vaccination with “regulating the regulators,” it is hoped that specific anti-tumor T-cell-immunity can be generated.
Table 2.
Targets of Immune Regulation
| Activating targets | Inhibitory targets |
|---|---|
| B7.1/CD80 | Regulatory T cells |
| B7.2/CD86 | FoxP3 |
| CD40 | CTLA-4 |
| 4-1BB | PD-L1/B7-H1 |
| OX40 | B7-H4 |
| MICA, MICB | IDO |
| Toll-like receptors | TGF-β |
| TNF-alpha | IL-10 |
| FasL/CD95L |
Abbreviations: TGF-β, transforming growth factor-β; TNF-alpha, tumor necrosis factor-alpha; CTLA-4, cytotoxic T-lymphocyte-associated antigen-4; IDO, indoleamine 2,3-dioxygenase.
Regulatory T cells
One mechanism of immune regulation is the activity of regulatory T-cells. These CD4+CD25+FoxP3+ T-cells inhibit other cellular immune responses and the development of autoimmunity (91, 92). Regulatory T-cells normally account for 5% to 10% of CD4+ lymphocytes in peripheral blood. In patients with breast cancer, however, regulatory T-cells are increased both in peripheral blood and in malignant effusions (93, 94). In a study of patients with ovarian cancer, elevated levels of regulatory T-cells in the tumor and in ascites were associated with poor survival (95).
Targeted therapeutics that specifically inhibit regulatory T-cells have been developed and are being tested in clinical trials. Denileukin diftitox (Ontak), which is a fusion of the full-length of interleukin-2 and the active portion of diphtheria toxin, can deplete regulatory T-cells by directly binding to CD25 (interleukin-2 receptor). In combination with RNA-transfected dendritic cells, Ontak augments T-cell responses in patients with renal-cell carcinoma (96) and in those with ovarian carcinoma (97).
Cytotoxic T-Lymphocyte-associated antigen-4 blockade
Cytotoxic T-lymphocyte-associated antigen (CTLA-4) is an inhibitory transmembrane molecule expressed on T-lymphocytes (Fig. 2). Activation of CTLA-4 strongly inhibits memory T-cell responses, as demonstrated by the development of lethal lymphoproliferative disease in CTLA-4 knockout mice (98, 99). The observation that CTLA-4 blockade can augment anti-tumor immunity in mouse models, has prompted an intense effort to develop antibody therapeutics that target CTLA-4.
Early-phase clinical trials have used anti-CTLA-4 mono-clonal antibodies in melanoma, and in ovarian, renal-cell, colon, and prostate cancers (101–106). CTLA-4 blockade has been associated with the development of significant Grade III/IV autoimmunity (dermatitis, colitis, hypophysitis) (106), but also with clinical responses in melanoma patients, including tumor necrosis (101–103). Notably, 9 of 29 patients with melanoma had either stable disease or extended periods without disease progression (23 to 36+ months) (102). Hodi et al. (101) used autologous vaccination to prime T-cells and subsequent administration of an antibody that blocks CTLA-4 (MDX-010) to boost memory T-cell responses. This vaccination strategy resulted in significant tumor necrosis in three of seven patients with melanoma. Although these studies have focused primarily on malignant melanoma, CA-125 responses have been reported in patients with ovarian cancer (107) and PSA responses have been reported in hormone-refractory prostate cancer patients (105), arguing that augmentation of memory T-cell responses can be clinically effective in adenocarcinomas. It is not yet known whether the development of autoimmunity can be separated from the antitumor effects of this potent immunotherapeutic target.
CLINICAL ISSUES IN VACCINATION
There are several challenges that affect the development of breast cancer immunotherapies. Molecular typing of breast cancer (108) and genomic identification of breast cancer antigens (109) have made it clear that there are specific biologic types of breast cancer with different levels and patterns of tumor antigen expression. The identification of multiple antigenic targets in breast cancer (Table 1) has required the development of immunologic assays for careful monitoring of antigen-specific immune responses. There is no global assay for assessing immunocompetence, but antigen-specific T-cell responses can now be quantitatively measured with the use of flow cytometry using recombinant tetrameric HLA molecules (110), and plate-based ELISA and Elispot assays for T-celldependent cytokine secretion. Whole-cell based vaccines and modulators of immune regulation are more difficult to assess. Delayed-type hypersensitivity to vaccine can be tested in skin-biopsy specimens, and tumor-biopsy specimens can be examined for evidence of infiltrating lymphocytes, but identification of target antigens in complex vaccines and after targeted anti-immunoregulation remains difficult. Ideally, genome-wide and proteome-wide approaches to monitor immune responses will prove useful (27, 36).
Timing of vaccination
The timing of prior chemotherapy may be critical to the successful development of tumor-specific immunity. Cytotoxic chemotherapy has several effects on immune responses [reviewed in (111)]. It can abrogate existing immune responses, deplete regulatory T-cells, and induce a minimal residual disease state, thereby enhancing the potential effectiveness of immunotherapies. Specific chemotherapeutic agents, such as doxorubicin, paclitaxel, 5-fluorouracil, and cisplatin, have effects on the local tumor microenvironment, enhancing apoptosis, antigen presentation, or sensitivity to cytotoxic T-lymphocyte-mediated killing (112, 113). Cyclophosphamide, in particular, may decrease the function of CD4+CD25+ T-regulatory cells that inhibit immune responses (114). In addition, immunotherapy may enhance the efficacy of subsequent chemotherapies (115). These findings point to potential synergistic effects of chemotherapies and immunotherapies. Similarly, immunotherapies may be synergistic with other targeted therapeutics.
CONCLUSION
The development of cancer requires inhibition of effective immunity at multiple levels, from dysregulation of innate immune responses to active inhibition of adaptive immunity at the tumor microenvironment. As cancer progresses, so does the extent of immune dysregulation. Shifting the timing of vaccine delivery from the metastatic to the adjuvant setting (or earlier) should facilitate more effective anti-tumor immunity. To date, most vaccine strategies have focused on immune activation such as antigenic delivery, TLR activation by CpGs and adjuvant, and cytokine stimulation. However, the identification of immune regulatory pathways, such as B7-H1, B7-H4, CTLA-4, IDO, and regulatory T-cells has demonstrated that inhibition of immune regulation will be critical to establish effective anti-tumor immunity. The successful development of breast cancer vaccines will require combinatorial therapies that target both breast-cancer specific immune activation and inhibition of immune tolerance.
Acknowledgments
This work is supported by grants from the NIH, NCI (3 P30 CA006516-41S4 and U01 CA117374), and the AVON Foundation. The author thanks Dr. Ellis Reinherz and Dr. Glenn Dranoff for critical review, and Victoria Alexander for editorial assistance.
References
- 1.Chaudhuri S, Cariappa A, Tang M, et al. Genetic susceptibility to breast cancer: HLA DQB*03032 and HLA DRB1*11 may represent protective alleles. Proc Natl Acad Sci USA. 2000;97:11451–11454. doi: 10.1073/pnas.97.21.11451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marincola FM, Jaffee EM, Hicklin DJ, et al. Escape of human solid tumors from T-cell recognition: molecular mechanisms and functional significance. Adv Immunol. 2000;74:181–273. doi: 10.1016/s0065-2776(08)60911-6. [DOI] [PubMed] [Google Scholar]
- 3.Campoli M, Chang CC, Oldford SA, et al. HLA antigen changes in malignant tumors of mammary epithelial origin: molecular mechanisms and clinical implications. Breast Dis. 2004;20:105–125. doi: 10.3233/bd-2004-20112. [DOI] [PubMed] [Google Scholar]
- 4.Aaltomaa S, Lipponen P, Eskelinen M, et al. Lymphocyte in-filtrates as a prognostic variable in female breast cancer. Eur J Cancer. 1992;28A:859–864. doi: 10.1016/0959-8049(92)90134-n. [DOI] [PubMed] [Google Scholar]
- 5.Menard S, Tomasic G, Casalini P, et al. Lymphoid infiltration as a prognostic variable for early-onset breast carcinomas. Clin Cancer Res. 1997;3:817–819. [PubMed] [Google Scholar]
- 6.Jerome KR, Domenech N, Finn OJ. Tumor-specific cytotoxic T -cell clones from patients with breast and pancreatic adenocarcinoma recognize EBV-immortalized B cells transfected with polymorphic epithelial mucin complementary DNA. J Immunol. 1993;151:1654–1662. [PubMed] [Google Scholar]
- 7.Disis ML, Calenoff E, McLaughlin G, et al. Existent T-cell and antibody immunity to HER-2/neu protein in patients with breast cancer. Cancer Res. 1994;54:16–20. [PubMed] [Google Scholar]
- 8.Bishop MR, Fowler DH, Marchigiani D, et al. Allogeneic lymphocytes induce tumor regression of advanced metastatic breast cancer. J Clin Oncol. 2004;22:3886–3892. doi: 10.1200/JCO.2004.01.127. [DOI] [PubMed] [Google Scholar]
- 9.Lundqvist A, Childs R. Allogeneic hematopoietic cell transplantation as immunotherapy for solid tumors: current status and future directions. J Immunother. 2005;28:281–288. doi: 10.1097/01.cji.0000165354.19171.8f. [DOI] [PubMed] [Google Scholar]
- 10.Carella AM, Beltrami G, Corsetti MT, et al. Reduced intensity conditioning for allograft after cytoreductive autograft in metastatic breast cancer. Lancet. 2005;366:318–320. doi: 10.1016/S0140-6736(05)66989-9. [DOI] [PubMed] [Google Scholar]
- 11.Janeway CTP, Walport M, Shlomchikm M. Immunobiology: The Immune System in Health and Disease. 6. New York, NY: Garland Science Publishing; 2005. [Google Scholar]
- 12.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 13.Vollmer J. Progress in drug development of immunostimulatory CpG oligodeoxynucleotide ligands for TLR9. Expert Opin Biol Ther. 2005;5:673–682. doi: 10.1517/14712598.5.5.673. [DOI] [PubMed] [Google Scholar]
- 14.Speiser DE, Lienard D, Rufer N, et al. Rapid and strong human CD8+ T cell responses to vaccination with peptide, IFA, and CpG oligodeoxynucleotide 7909. J Clin Invest. 2005;115:739–746. doi: 10.1172/JCI23373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diefenbach A, Raulet DH. The innate immune response to tumors and its role in the induction of T-cell immunity. Immunol Rev. 2002;188:9–21. doi: 10.1034/j.1600-065x.2002.18802.x. [DOI] [PubMed] [Google Scholar]
- 16.Smyth MJ, Swann J, Cretney E, et al. NKG2D function protects the host from tumor initiation. J Exp Med. 2005;202:583–588. doi: 10.1084/jem.20050994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bauer S, Groh V, Wu J, et al. Activation of NK cells and Tcells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 18.Groh V, Rhinehart R, Secrist H, et al. Broad tumor-associated expression and recognition by tumor-derived gamma delta T cells of MICA and MICB. Proc Natl Acad Sci USA. 1999;96:6879–6884. doi: 10.1073/pnas.96.12.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groh V, Wu J, Yee C, et al. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419:734–738. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 20.Singer G, Rebmann V, Chen YC, et al. HLA-G is a potential tumor marker in malignant ascites. Clin Cancer Res. 2003;9:4460–4464. [PubMed] [Google Scholar]
- 21.Zaks TZ, Rosenberg SA. Immunization with a peptide epitope (p369–377) from HER-2/neu leads to peptide-specific cytotoxic T lymphocytes that fail to recognize HER-2/neu+ tumors. Cancer Res. 1998;58:4902–4908. [PubMed] [Google Scholar]
- 22.Vonderheide RH, Hahn WC, Schultze JL, et al. The telomerase catalytic subunit is a widely expressed tumor-associated antigen recognized by cytotoxic T lymphocytes. Immunity. 1999;10:673–679. doi: 10.1016/s1074-7613(00)80066-7. [DOI] [PubMed] [Google Scholar]
- 23.Maecker B, Sherr DH, Vonderheide RH, et al. The shared tumor-associated antigen cytochrome P450 1B1 is recognized by specific cytotoxic T cells. Blood. 2003;102:3287–3294. doi: 10.1182/blood-2003-05-1374. [DOI] [PubMed] [Google Scholar]
- 24.Kao H, Marto JA, Hoffmann TK, et al. Identification of cyclin B1 as a shared human epithelial tumor-associated antigen recognized by T cells. J Exp Med. 2001;194:1313–1323. doi: 10.1084/jem.194.9.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khong HT, Yang JC, Topalian SL, et al. Immunization of HLA-A 0201 and/or HLADPbeta1*04 patients with metastatic melanoma using epitopes from the NY-ESO-1 antigen. J Immunother. 2004;27:472–477. doi: 10.1097/00002371-200411000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salazar LG, Fikes J, Southwood S, et al. Immunization of cancer patients with HER-2/neu-derived peptides demonstrating high-affinity binding to multiple class II alleles. Clin Cancer Res. 2003;9:5559–5565. [PubMed] [Google Scholar]
- 27.Anderson KS, LaBaer J. The sentinel within: exploiting the immune system for cancer biomarkers. J Proteome Res. 2005;4:1123–1133. doi: 10.1021/pr0500814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacLean GD, Miles DW, Rubens RD, et al. Enhancing the effect of THERATOPE STn-KLH cancer vaccine in patients with metastatic breast cancer by pretreatment with low-dose intravenous cyclophosphamide. J Immunother Emphasis Tumor Immunol. 1996;19:309–316. doi: 10.1097/00002371-199607000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Sandmaier BM, Oparin DV, Holmberg LA, et al. Evidence of a cellular immune response against sialyl-Tn in breast and ovarian cancer patients after high-dose chemotherapy, stem cell rescue, and immunization with Theratope STn-KLH cancer vaccine. J Immunother. 1999;22:54–66. doi: 10.1097/00002371-199901000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Holmberg LA, Oparin DV, Gooley T, et al. Clinical outcome of breast and ovarian cancer patients treated with high-dose chemotherapy, autologous stem cell rescue and THERATOPE STn-KLH cancer vaccine. Bone Marrow Transplant. 2000;25:1233–1241. doi: 10.1038/sj.bmt.1702430. [DOI] [PubMed] [Google Scholar]
- 31.Musselli C, Livingston PO, Ragupathi G. Keyhole limpet hemocyanin conjugate vaccines against cancer: the Memorial Sloan Kettering experience. J Cancer Res Clin Oncol. 2001;2(127 Suppl):R20–R26. doi: 10.1007/BF01470995. [DOI] [PubMed] [Google Scholar]
- 32.Disis ML, Pupa SM, Gralow JR, et al. High-titer HER-2/neu protein-specific antibody can be detected in patients with early-stage breast cancer. J Clin Oncol. 1997;15:3363–3367. doi: 10.1200/JCO.1997.15.11.3363. [DOI] [PubMed] [Google Scholar]
- 33.Stockert E, Jager E, Chen YT, et al. A survey of the humoral immune response of cancer patients to a panel of human tumor antigens. J Exp Med. 1998;187:1349–1354. doi: 10.1084/jem.187.8.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sioud M, Hansen MH. Profiling the immune response in patients with breast cancer by phage-displayed cDNA libraries. Eur J Immunol. 2001;31:716–725. doi: 10.1002/1521-4141(200103)31:3<716::aid-immu716>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 35.Sugita Y, Wada H, Fujita S, et al. NY-ESO-1 expression and immunogenicity in malignant and benign breast tumors. Cancer Res. 2004;64:2199–2204. doi: 10.1158/0008-5472.can-03-3070. [DOI] [PubMed] [Google Scholar]
- 36.Wang X, Yu J, Sreekumar A, et al. Autoantibody signatures in prostate cancer. N Engl J Med. 2005;353:1224–1235. doi: 10.1056/NEJMoa051931. [DOI] [PubMed] [Google Scholar]
- 37.Hodi FS, Schmollinger JC, Soiffer RJ, et al. ATP6S1 elicits potent humoral responses associated with immune-mediated tumor destruction. Proc Natl Acad Sci USA. 2002;99:6919–6924. doi: 10.1073/pnas.102025999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gnjatic S, Atanackovic D, Jager E, et al. Survey of naturally occurring CD4+ T cell responses against NY-ESO-1 in cancer patients: correlation with antibody responses. Proc Natl Acad Sci USA. 2003;100:8862–8867. doi: 10.1073/pnas.1133324100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 40.Zou W, Machelon V, Coulomb-L’Hermin A, et al. Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nat Med. 2001;7:1339–1346. doi: 10.1038/nm1201-1339. [DOI] [PubMed] [Google Scholar]
- 41.Rollins BJ. Inflammatory chemokines in cancer growth and progression. Eur J Cancer. 2006;42:760–767. doi: 10.1016/j.ejca.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 42.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 43.Muschen M, Moers C, Warskulat U, et al. CD95 ligand expression as a mechanism of immune escape in breast cancer. Immunology. 2000;99:69–77. doi: 10.1046/j.1365-2567.2000.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown JA, Dorfman DM, Ma FR, et al. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170:1257–1266. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- 45.Tringler B, Zhuo S, Pilkington G, et al. B7-h4 is highly expressed in ductal and lobular breast cancer. Clin Cancer Res. 2005;11:1842–1848. doi: 10.1158/1078-0432.CCR-04-1658. [DOI] [PubMed] [Google Scholar]
- 46.Schuler G, Schuler-Thurner B, Steinman RM. The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol. 2003;15:138–147. doi: 10.1016/s0952-7915(03)00015-3. [DOI] [PubMed] [Google Scholar]
- 47.Wilcox RA, Tamada K, Flies DB, et al. Ligation of CD137 receptor prevents and reverses established anergy of CD8+ cytolytic T lymphocytes in vivo. Blood. 2004;103:177–184. doi: 10.1182/blood-2003-06-2184. [DOI] [PubMed] [Google Scholar]
- 48.Bansal-Pakala P, Jember AG, Croft M. Signaling through OX40 (CD134) breaks peripheral T-cell tolerance. Nat Med. 2001;7:907–912. doi: 10.1038/90942. [DOI] [PubMed] [Google Scholar]
- 49.O’Neill DW, Adams S, Bhardwaj N. Manipulating dendritic cell biology for the active immunotherapy of cancer. Blood. 2004;104:2235–2246. doi: 10.1182/blood-2003-12-4392. [DOI] [PubMed] [Google Scholar]
- 50.Gervais A, Leveque J, Bouet-Toussaint F, et al. Dendritic cells are defective in breast cancer patients: a potential role for polyamine in this immunodeficiency. Breast Cancer Res. 2005;7:R326–R335. doi: 10.1186/bcr1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Munn DH, Sharma MD, Lee JR, et al. Potential regulatory function of human dendritic cells expressing indoleamine 2:3-dioxygenase. Science. 2002;297:1867–1870. doi: 10.1126/science.1073514. [DOI] [PubMed] [Google Scholar]
- 52.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 53.Nair SK, Morse M, Boczkowski D, et al. Induction of tumor-specific cytotoxic T lymphocytes in cancer patients by autologous tumor RNA-transfected dendritic cells. Ann Surg. 2002;235:540–549. doi: 10.1097/00000658-200204000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nestle FO, Alijagic S, Gilliet M, et al. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 55.Avigan D, Vasir B, Gong J, et al. Fusion cell vaccination of patients with metastatic breast and renal cancer induces immunological and clinical responses. Clin Cancer Res. 2004;10:4699–4708. doi: 10.1158/1078-0432.CCR-04-0347. [DOI] [PubMed] [Google Scholar]
- 56.Holtl L, Zelle-Rieser C, Gander H, et al. Immunotherapy of metastatic renal cell carcinoma with tumor lysate-pulsed autologous dendritic cells. Clin Cancer Res. 2002;8:3369–3376. [PubMed] [Google Scholar]
- 57.O’Rourke MG, Johnson M, Lanagan C, et al. Durable complete clinical responses in a phase I/II trial using an autologous melanoma cell/dendritic cell vaccine. Cancer Immunol Immunother. 2003;52:387–395. doi: 10.1007/s00262-003-0375-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Small EJSPF, Higano C, Neumanaitis J, Valone F, Herschberg RM. Immunotherapy (APC8015) for androgen independent prostate cancer (AIPC): Final survival data from a phase 3 randomized placebo-controlled trial. 2005 ASCO Prostate Cancer Symposium; 2005. [Google Scholar]
- 59.Morse MA, Clay TM, Hobeika AC, et al. Phase I study of immunization with dendritic cells modified with fowlpox encoding carcinoembryonic antigen and costimulatory molecules. Clin Cancer Res. 2005;11:3017–3024. doi: 10.1158/1078-0432.CCR-04-2172. [DOI] [PubMed] [Google Scholar]
- 60.Morse MA, Nair SK, Mosca PJ, et al. Immunotherapy with autologous, human dendritic cells transfected with carcinoembryonic antigen mRNA. Cancer Invest. 2003;21:341–349. doi: 10.1081/cnv-120018224. [DOI] [PubMed] [Google Scholar]
- 61.Brossart P, Wirths S, Stuhler G, et al. Induction of cytotoxic T-lymphocyte responses in vivo after vaccinations with peptide-pulsed dendritic cells. Blood. 2000;96:3102–3108. [PubMed] [Google Scholar]
- 62.Wobser M, Keikavoussi P, Kunzmann V, et al. Complete remission of liver metastasis of pancreatic cancer under vaccination with a HLA-A2 restricted peptide derived from the universal tumor antigen survivin. Cancer Immunol Immunother. 2006;55:1294–1298. doi: 10.1007/s00262-005-0102-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Otto K, Andersen MH, Eggert A, et al. Lack of toxicity of therapy-induced T cell responses against the universal tumour antigen survivin. Vaccine. 2005;23:884–889. doi: 10.1016/j.vaccine.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 64.Taylor-Papadimitriou J, Finn OJ. Biology, biochemistry and immunology of carcinomaassociated mucins. Immunol Today. 1997;18:105–107. doi: 10.1016/s0167-5699(97)01028-1. [DOI] [PubMed] [Google Scholar]
- 65.Goydos JS, Elder E, Whiteside TL, et al. A phase I trial of a synthetic mucin peptide vaccine. Induction of specific immune reactivity in patients with adenocarcinoma. J Surg Res. 1996;63:298–304. doi: 10.1006/jsre.1996.0264. [DOI] [PubMed] [Google Scholar]
- 66.Reddish M, MacLean GD, Koganty RR, et al. Anti-MUC1 class I restricted CTLs in metastatic breast cancer patients immunized with a synthetic MUC1 peptide. Int J Cancer. 1998;76:817–823. doi: 10.1002/(sici)1097-0215(19980610)76:6<817::aid-ijc9>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 67.Gilewski T, Adluri S, Ragupathi G, et al. Vaccination of high-risk breast cancer patients with mucin-1 (MUC1) keyhole limpet hemocyanin conjugate plus QS-21. Clin Cancer Res. 2000;6:1693–1701. [PubMed] [Google Scholar]
- 68.Karanikas V, Thynne G, Mitchell P, et al. Mannan Mucin-1 Peptide Immunization: Influence of Cyclophosphamide and the Route of Injection. J Immunother. 2001;24:172–183. [PubMed] [Google Scholar]
- 69.Snijdewint FG, von Mensdorff-Pouilly S, Karuntu-Wanamarta AH, et al. Antibodydependent cell-mediated cytotoxicity can be induced by MUC1 peptide vaccination of breast cancer patients. Int J Cancer. 2001;93:97–106. doi: 10.1002/ijc.1286. [DOI] [PubMed] [Google Scholar]
- 70.Karanikas V, Hwang LA, Pearson J, et al. Antibody and Tcell responses of patients with adenocarcinoma immunized with mannan-MUC1 fusion protein. J Clin Invest. 1997;100:2783–2792. doi: 10.1172/JCI119825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Murray JL, Gillogly ME, Przepiorka D, et al. Toxicity, immunogenicity, and induction of E75-specific tumorlytic CTLs by HER-2 peptide E75 (369–377) combined with granulocyte macrophage colony-stimulating factor in HLA-A2+ patients with metastatic breast and ovarian cancer. Clin Cancer Res. 2002;8:3407–3418. [PubMed] [Google Scholar]
- 72.Knutson KL, Schiffman K, Cheever MA, et al. Immunization of cancer patients with a HER-2/neu, HLA-A2 peptide, p369–377: results in short-lived peptide-specific immunity. Clin Cancer Res. 2002;8:1014–1018. [PubMed] [Google Scholar]
- 73.Disis ML, Gooley TA, Rinn K, et al. Generation of T-cell immunity to the HER-2/neu protein after active immunization with HER-2/neu peptide-based vaccines. J Clin Oncol. 2002;20:2624–2632. doi: 10.1200/JCO.2002.06.171. [DOI] [PubMed] [Google Scholar]
- 74.Knutson KL, Schiffman K, Disis ML. Immunization with a HER-2/neu helper peptide vaccine generates HER-2/neu CD8 T-cell immunity in cancer patients. J Clin Invest. 2001;107:477–484. doi: 10.1172/JCI11752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Disis ML, Schiffman K, Guthrie K, et al. Effect of dose on immune response in patients vaccinated with an her-2/neu in-tracellular domain protein-based vaccine. J Clin Oncol. 2004;22:1916–1925. doi: 10.1200/JCO.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 76.Morse MA, Clay TM, Colling K, et al. HER2 dendritic cell vaccines. Clin Breast Cancer. 2003;4(3 Suppl):S164–S172. doi: 10.3816/cbc.2003.s.007. [DOI] [PubMed] [Google Scholar]
- 77.Peoples GE, Gurney JM, Hueman MT, et al. Clinical trial results of a HER2/neu (E75) vaccine to prevent recurrence in high-risk breast cancer patients. J Clin Oncol. 2005;23:7536–7545. doi: 10.1200/JCO.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 78.Vonderheide RH, Domchek SM, Schultze JL, et al. Vaccination of cancer patients against telomerase induces functional antitumor CD8+ T lymphocytes. Clin Cancer Res. 2004;10:828–839. doi: 10.1158/1078-0432.ccr-0620-3. [DOI] [PubMed] [Google Scholar]
- 79.Su Z, Dannull J, Yang BK, et al. Telomerase mRNA-transfected dendritic cells stimulate antigen-specific CD8+ and CD4+ T cell responses in patients with metastatic prostate cancer. J Immunol. 2005;174:3798–3807. doi: 10.4049/jimmunol.174.6.3798. [DOI] [PubMed] [Google Scholar]
- 80.Parkhurst MR, Riley JP, Igarashi T, et al. Immunization of patients with the hTERT:540–548 peptide induces peptide-reactive T lymphocytes that do not recognize tumors endogenously expressing telomerase. Clin Cancer Res. 2004;10:4688–4698. doi: 10.1158/1078-0432.CCR-04-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ho WY, Blattman JN, Dossett ML, et al. Adoptive immunotherapy: engineering T cell responses as biologic weapons for tumor mass destruction. Cancer Cell. 2003;3:431–437. doi: 10.1016/s1535-6108(03)00113-2. [DOI] [PubMed] [Google Scholar]
- 82.Mach N, Dranoff G. Cytokine-secreting tumor cell vaccines. Curr Opin Immunol. 2000;12:571–575. doi: 10.1016/s0952-7915(00)00144-8. [DOI] [PubMed] [Google Scholar]
- 83.Ahlert T, Sauerbrei W, Bastert G, et al. Tumor-cell number and viability as quality and efficacy parameters of autologous virus-modified cancer vaccines in patients with breast or ovarian cancer. J Clin Oncol. 1997;15:1354–1366. doi: 10.1200/JCO.1997.15.4.1354. [DOI] [PubMed] [Google Scholar]
- 84.Wood G, Baynes R. Vaccination of Stage IV Breast Cancer Patients with Whole Autologous Malignant Cells and Granulocyte Mactrophage Colony Stimulating Factor. Proc Am Soc Clin Oncol. 1999:abstr 168. [Google Scholar]
- 85.Dillman RO, Beutel LD, Barth NM, et al. Irradiated cells from autologous tumor cell lines as patient-specific vaccine therapy in 125 patients with metastatic cancer: induction of delayed-type hypersensitivity to autologous tumor is associated with improved survival. Cancer Biother Radiopharm. 2002;17:51–66. doi: 10.1089/10849780252824073. [DOI] [PubMed] [Google Scholar]
- 86.Wiseman CL. Inflammatory breast cancer. 10-year follow-up of a trial of surgery, chemotherapy, and allogeneic tumor cell/BCG immunotherapy . Cancer Invest. 1995;13:267–271. doi: 10.3109/07357909509094460. [DOI] [PubMed] [Google Scholar]
- 87.Schoof DD, Smith JW, II, Disis ML, et al. Immunization of metastatic breast cancer patients with CD80-modified breast cancer cells and GM-CSF. Adv Exp Med Biol. 1998;451:511–518. doi: 10.1007/978-1-4615-5357-1_79. [DOI] [PubMed] [Google Scholar]
- 88.Jiang XP, Yang DC, Elliott RL, et al. Vaccination with a mixed vaccine of autogenous and allogeneic breast cancer cells and tumor associated antigens CA15–3: CEA and CA125–results in immune and clinical responses in breast cancer patients. Cancer Biother Radiopharm. 2000;15:495–505. doi: 10.1089/cbr.2000.15.495. [DOI] [PubMed] [Google Scholar]
- 89.Avigan D. Fusions of breast cancer and dendritic cells as a novel cancer vaccine. Clin Breast Cancer. 2003;4(3 Suppl):S158–S163. doi: 10.3816/cbc.2003.s.006. [DOI] [PubMed] [Google Scholar]
- 90.Dranoff G, Jaffee E, Lazenby A, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang RF. Functional control of regulatory T cells and cancer immunotherapy. Semin Cancer Biol. 2006;16:106–114. doi: 10.1016/j.semcancer.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 92.Khazaie K, von Boehmer H. The impact of CD4(+)CD25(+) Treg on tumor specific CD8(+) T cell cytotoxicity and cancer. Semin Cancer Biol. 2006;16:124–136. doi: 10.1016/j.semcancer.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 93.Liyanage UK, Moore TT, Joo HG, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 94.DeLong P, Carroll RG, Henry AC, et al. Regulatory T cells and cytokines in malignant pleural effusions secondary to mesothelioma and carcinoma. Cancer Biol Ther. 2005;4:342–346. doi: 10.4161/cbt.4.3.1644. [DOI] [PubMed] [Google Scholar]
- 95.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 96.Dannull J, Su Z, Rizzieri D, et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115:3623–3633. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Barnett B, Kryczek I, Cheng P, et al. Regulatory T cells in ovarian cancer: biology and therapeutic potential. Am J Reprod Immunol. 2005;54:369–377. doi: 10.1111/j.1600-0897.2005.00330.x. [DOI] [PubMed] [Google Scholar]
- 98.Waterhouse P, Penninger JM, Timms E, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 99.Tivol EA, Borriello F, Schweitzer AN, et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 100.Leach DR, Krummel MF, Allison JP. Enhancement of anti-tumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 101.Hodi FS, Mihm MC, Soiffer RJ, et al. Biologic activity of cytotoxic T lymphocyteassociated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci USA. 2003;100:4712–4717. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ribas A, Camacho LH, Lopez-Berestein G, et al. Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675:206. J Clin Oncol. 2005;23:8968–8977. doi: 10.1200/JCO.2005.01.109. [DOI] [PubMed] [Google Scholar]
- 103.Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA. 2003;100:8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sanderson K, Scotland R, Lee P, et al. Autoimmunity in a phase I trial of a fully human anti-cytotoxic T-lymphocyte antigen-4 monoclonal antibody with multiple melanoma peptides and Montanide ISA 51 for patients with resected stages III and IV melanoma. J Clin Oncol. 2005;23:741–750. doi: 10.1200/JCO.2005.01.128. [DOI] [PubMed] [Google Scholar]
- 105.Davis TATS, Korman A, et al. MDX-010 (human anti-CTLA4): a phase 1 trial in hormone refractory prostate carcinoma (HRPC) Proc Am Soc Clin Oncol. 2002;21:2002, abstr 74. [Google Scholar]
- 106.Attia P, Phan GQ, Maker AV, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23:6043–6053. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hodi FS, Seiden M, Butler M, et al. Cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) antibody blockade in patients previously vaccinated with irradiated, autologous tumor cells engineered to secrete granulocyte-macrophage colony stimulating factor (GM-CSF) J Clin Oncol 2004 ASCO Annual Meeting Proceedings. 2004;22(145):2536. [Google Scholar]
- 108.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 109.Porter DA, Krop IE, Nasser S, et al. A SAGE (serial analysis of gene expression) view of breast tumor progression. Cancer Res. 2001;61:5697–5702. [PubMed] [Google Scholar]
- 110.Altman JD, Moss PA, Goulder PJ, et al. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 111.Emens LA, Jaffee EM. Leveraging the activity of tumor vaccines with cytotoxic chemotherapy. Cancer Res. 2005;65:8059–8064. doi: 10.1158/0008-5472.CAN-05-1797. [DOI] [PubMed] [Google Scholar]
- 112.Yang S, Haluska FG. Treatment of melanoma with 5-fluorouracil or dacarbazine in vitro sensitizes cells to antigen-specific CTL lysis through perforin/granzyme- and Fasmediated pathways. J Immunol. 2004;172:4599–4608. doi: 10.4049/jimmunol.172.7.4599. [DOI] [PubMed] [Google Scholar]
- 113.Keane MM, Ettenberg SA, Nau MM, et al. Chemotherapy augments TRAIL-induced apoptosis in breast cell lines. Cancer Res. 1999;59:734–741. [PubMed] [Google Scholar]
- 114.Lutsiak ME, Semnani RT, De Pascalis R, et al. Inhibition of CD4(+)25+ Tregulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105:2862–2868. doi: 10.1182/blood-2004-06-2410. [DOI] [PubMed] [Google Scholar]
- 115.Gribben JG, Ryan DP, Boyajian R, et al. Unexpected association between induction of immunity to the universal tumor antigen CYP1B1 and response to next therapy. Clin Cancer Res. 2005;11:4430–4436. doi: 10.1158/1078-0432.CCR-04-2111. [DOI] [PubMed] [Google Scholar]