Ask any clinician or basic scientist who has studied aqueous outflow for many years “What is the etiology of IOP elevation in POAG?” Each will have their pet theory but each will also tell you that we do not know. This review is thus a summary of existing clues rather than a direct answer to the question, “Where does aqueous outflow resistance reside and how is it generated?” We simply do not know. Equally important, we do not know whether the added resistance that elevates intraocular pressure (IOP) in primary open angle glaucoma (POAG) is due to more resistance in the same location as that in which the resistance of the normal eye is found, or an additional resistance that is added downstream in the glaucomatous eye.
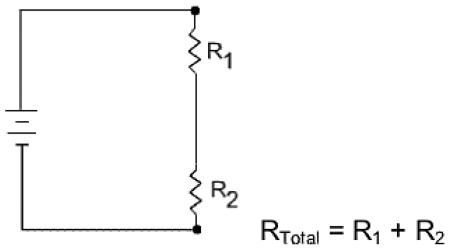
In this regard it is important to recall the corollary to Kirchhoff’s law that resistances placed in series are additive.
Only recently has evidence arisen that the added resistance of the POAG eye might lie, at least in part, in a location other than that which appears to harbor most of the resistance in the normal eye. In this review, we will consider some of the principal theories of outflow resistance, concluding with recent data implicating an added resistance in a previously unappreciated location.
OUTFLOW vs RESISTANCE
Knowing where aqueous leaves the eye is quite different from knowing where the resistance to outflow resides or how it is generated. We know that aqueous humor leaves the human eye by two principal pathways. The major outflow pathway is through the trabecular meshwork, into Schlemm’s canal (SC) and external collector channels, ultimately reaching the venous blood of the episclera. The other pathway is the uveoscleral pathway, which leads through the interstices of the ciliary muscle to the supraciliary and suprachoroidal space to leave the eye. Most current data suggests that uveoscleral outflow contributes little more than 10% of total outflow in the normal eye, notwithstanding our ability to enhance outflow via this pathway in glaucoma using prostanoids (1,2). As such, this review will only consider resistances in the major outflow pathway, beginning with the trabecular meshwork.
In the absence of a pumping mechanism, the flow of fluid through a porous tissue such as the trabecular meshwork is driven passively by gradients in osmotic and/or hydrostatic pressures. Because there is no osmotic difference between aqueous and the blood into which it flows (3), we are left with hydrostatic pressure as the dominant force. So, it is the pressure difference (P) between the IOP in the anterior chamber and that in the episcleral veins (P = 5 mmHg) that drives aqueous humor through this system. The ratio of this pressure difference to that of aqueous flow (assuming a typical flow rate (Q) of 2μl/min) equals the flow resistance (R).
The inverse of the outflow resistance is known as the outflow facility (Ctm).
where Pi equals IOP and Pe equals episcleral venous pressure.
WHERE DOES NORMAL OUTFLOW RESISTANCE RESIDE?
Enroute to SC, aqueous humor leaving the anterior chamber enters the trabecular meshwork, a nonvascularized tissue that is separated on anatomical grounds into the uveal meshwork, the deeper corneoscleral meshwork and the still deeper juxtacanalicular connective tissue (JCT). The uveal and corneoscleral meshwork are configured as intersecting beams of collagen and elastin in their cores, with an enveloping layer of thin endothelial cells. The open spaces between the beams become progressively smaller as aqueous moves through these tissues towards SC (Figure 1). By contrast, the juxtacanalicular tissue (JCT), just internal to SC is an open connective tissue matrix with fibroblast-like cells in a collagen and elastin matrix (4) (Figure 2).
Figure 1.
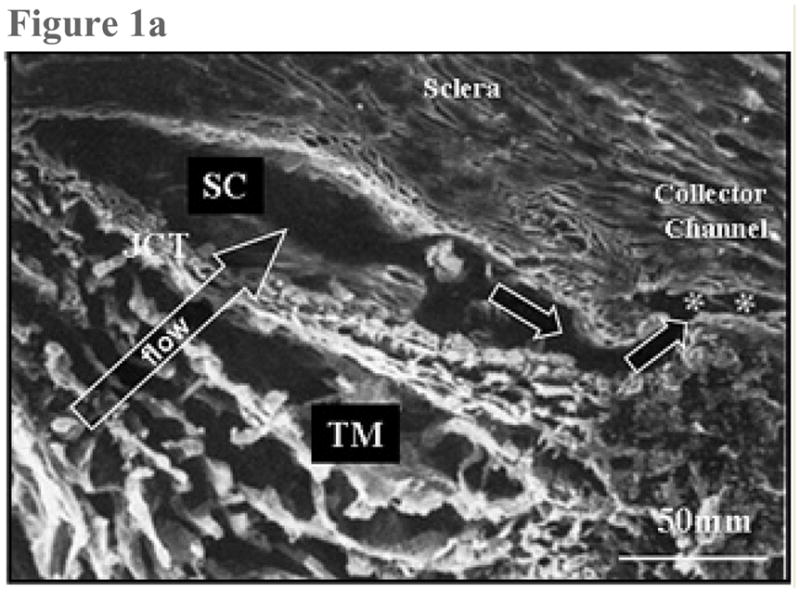
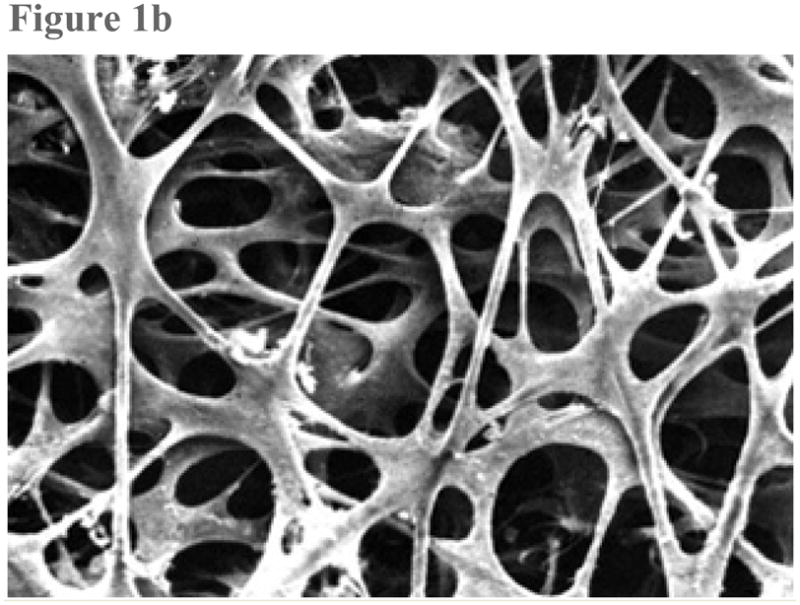
Figure 1a: Scanning electron micrograph. This sagittal section traverses the trabecular meshwork (TM), the juxtacanalicular region (JCT), Schlemm’s canal (SC) and one of the external collector channels (asterisks) that leads from Schlemm’s canal to the episcleral venous system. (From Freddo T. Chapter 3. Ocular anatomy and physiology related to aqueous production and outflow. In: Primary Care of the Glaucomas. Lewis T, Fingeret M, eds, Appleton and Lange, 1993. With kind permission of The McGraw-Hill Companies Inc.)
Figure 1b: Scanning electron micrograph shows the uveal face of the trabecular meshwork. The intersecting trabecular beams are covered by a uniform, thin layer of endothelial cells, surrounding an avascular core of collagen and elastin. The larger open spaces seen at the surface get progressively smaller in deeper layers. (From TF Freddo, MM Patterson, DR Scott, and DL Epstein. Influence of mercurial sulfhydryl agents on aqueous outflow pathways in enucleated eyes. Invest Ophthalmol. Vis. Sci. 1984; 25:278–285. With kind permission of copyright holder, the Association for Research in Vision and Opthalmology.)
Figure 2.
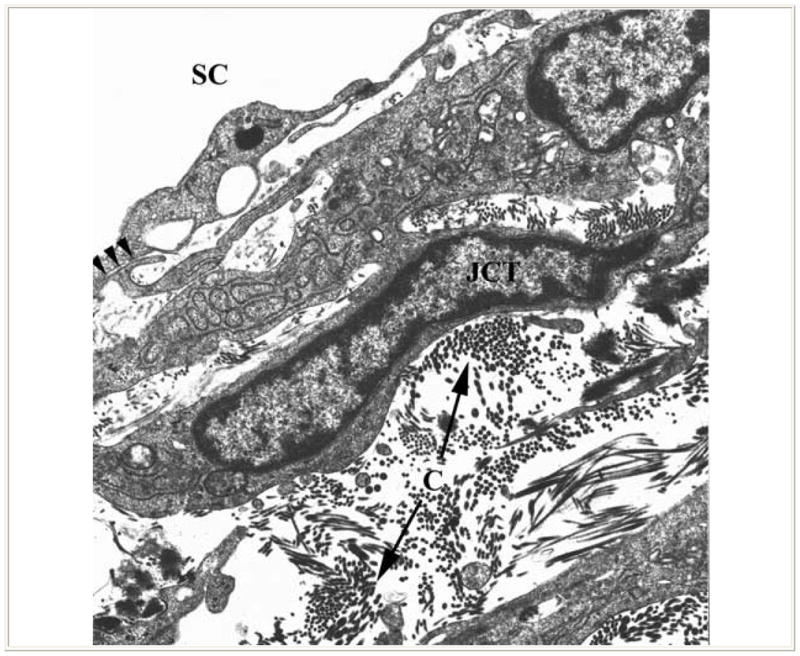
Transmission electron micrograph shows juxtacanlicular region (JCT) of the trabecular meshwork and inner wall of Schlemm’s canal (SC). The JCT region exhibits an open matrix including collagen (C) and elastin. The fibroblast-like cells of the region extend slender connections to the endothelial cells lining Schlemm’s canal (arrows). (From Freddo T. Chapter 3. Ocular anatomy and physiology related to aqueous production and outflow. In: Primary Care of the Glaucomas. Lewis T, Fingeret M, eds, Appleton and Lange, 1993. With kind permission of The McGraw-Hill Companies Inc.)
The Uveal and Corneoscleral Meshwork
Early studies of Bárány (5) showed that perfusion of enucleated bovine eyes with hyaluronidase reduced outflow resistance. From these studies the authors concluded that the glycosaminoglycan substrate for this enzyme, hyaluronan or hyaluronic acid, was the principal resistive material in the outflow pathways and presumed that it was distributed in the “open-spaces” between the trabecular beams of the uveal and corneoscleral meshwork. Only four years later, McEwen (6) showed that there is negligible flow resistance in the uveal and corneoscleral meshwork. Nonetheless, for many years, the notion that glycosaminoglycans (GAGs) were the resistive material in the outflow pathway persisted. More recently, immunohistochemical studies have demonstrated the absence of GAGs in the “open-spaces” of the meshwork (7,8) and intracameral injection of purified testicular hyaluronidase in living monkey eyes failed to produce either a reduction in IOP or a change in outflow facility (9). Most glaucoma investigators now agree that virtually none of the resistance to outflow resides in the uveal or corneoscleral meshwork of the normal eye.
The Juxtacanalicular Region (JCT)
The tortuous and much smaller “open-spaces” for flow within the JCT have made this region an attractive candidate as the tissue that generates the bulk of outflow resistance. But, hydrodynamic analyses have failed to support this view. (10–12). In a particularly innovative approach, Mäepea and Bill (13) passed a micropressure sensor through the inner wall of SC and into the meshwork, to measure the point at which the pressure drop occurred. They found that the pressure drop occurred within 14 μm of the inner wall of SC, a position that should correspond to a point within the JCT. These findings notwithstanding, calculations of hydraulic conductivity in the JCT, using measurements of “open space” from electron micrographs, show a JCT that is simply too porous to account for the outflow resistance of the normal eye. (10–12,14–17). Indeed, even using the challenging technique of quick-freeze/deep-etch (QF/DE) to obtain a more fully preserved extracellular matrix in the JCT, too much open space remained for the JCT to account for even normal outflow resistance (18).
An important caveat of this work is that even the QF/DE technique results in collapse of most of the glycosaminoglycans (GAGs). If these GAGs were not collapsed, the question remains whether measurements of “open-space” in such images (if they could be obtained) would finally give us a calculated hydraulic conductivity that would allow us to attribute most of the outflow resistance to the JCT.
Schlemm’s Canal
The inner wall of SC is composed of endothelial cells, joined by tight junctions that simplify in complexity and tightness as IOP is raised. (19) The endothelium of the inner wall encounters a direction of flow toward the lumen of the canal, similar to that seen in post-capillary venules and lymphatics. (20). There are a couple of specific features that distinguish the behavior of the inner wall from the behavior of post-capillary venues and lymphatics. When the inner wall is chemically fixed for microscopic examination while under conditions of flow, large blebs are seen along the inner wall, which are termed giant vacuoles (4). We know that the size and number of these vacuoles increases with IOP (21,22). Similar structures are also found in the choroid plexus of the brain where cerebrospinal fluid is reabsorbed (23).
In addition to giant vacuoles, the inner wall endothelium of SC exhibits two types of pores. Some occur at the border between adjacent endothelial cells (“B” pores) and others are found away from the cell borders (“I” intracellular pores) (24) (Figure 3). The issue of whether one or both of these pores might be artifactual remains undetermined. One theory is that B pores might correspond to areas in which intercellular tight junctions have been focally interrupted, creating a paracellular pathway for aqueous to enter SC (19,25).
Figure 3.
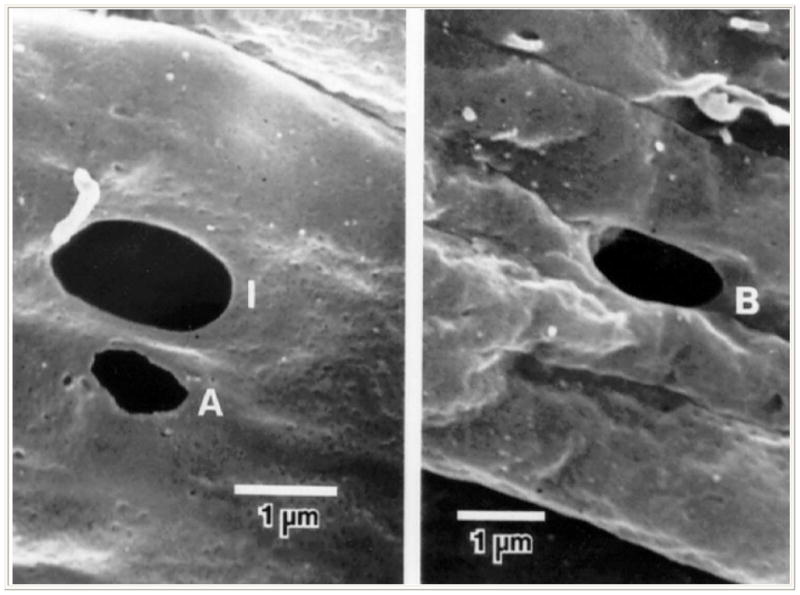
Scanning electron micrograph showing pores within the inner wall of Schlemm’s canal as viewed from inside the lumen of Schlemm’s canal. Left, an intracellular or I-pore (I) and an artifactual pore with ragged edge (A); right, an intercellular or B-pore (B) (From Ethier CR, Coloma FM, Sit AJ, Johnson M. Two pore types in the inner-wall endothelium of Schlemm’s canal. Invest Ophthalmol Vis Sci. 1998; 39:2041–2048. With kind permission of copyright holder, the Association for Research in Vision and Opthalmology.)
If pores were the “bottleneck” that creates resistance in the outflow pathway one might expect to find their numbers to be inversely related to resistance. In fact, Allingham, et al. (1992) found that the density of pores in the inner wall was inversely correlated with resistance under conditions of perfusion at constant pressure and variable flow. But when these studies were repeated under conditions of constant flow with variable pressure, no such relationship was found. (24,27,28). This leaves uncertain whether pores might be a source of resistance in the normal eye. But importantly, in both studies, regardless of perfusion conditions, glaucomatous eyes exhibited fewer pores than normal eyes (28). Could it be that a change in the capacity of the endothelium to produce pores in response to changes in pressure and flow could create an added resistance?
THE FUNNELING EFFECT
There is evidence to support the notion that pores and the JCT may interact in a way that would not be predicted from anatomy alone. Based upon fluid dynamic modeling, even if pores and vacuoles contribute minimal resistance themselves, they could force fluid to flow in limited patterns to reach them. This phenomenon has been referred to as the “funneling effect”. This model predicts that with flow headed for a small population of pores, only a limited portion of the JCT is realistically available for flow, thus increasing resistance (29) (Figure 4).
Figure 4.
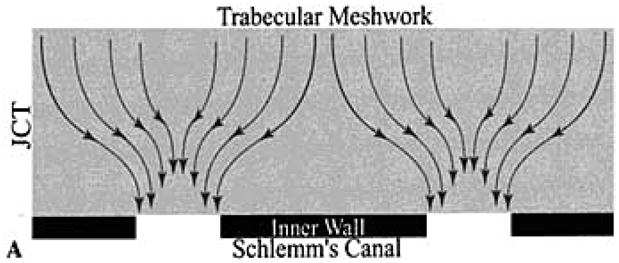
Schematic representation of the flow pattern predicted by the “funneling” hypothesis as aqueous moves through the JCT region towards the limited population of pores in the inner wall of Schlemm’s canal. (From Overby D, Gong H, Qiu, G., Freddo T.F., Johnson, M.. “The mechanism of increasing outflow facility during washout in the bovine eye.” Invest Ophthalmol Vis Sci. 2002; 43: 3455–3464. With kind permission of copyright holder, the Association for Research in Vision and Ophthalmology.)
External Collector Channels
Because the external collector channels are tens of microns in diameter, calculations indicate that these vessels should have negligible flow resistance (30–33). Still, smooth muscle cells have been found within the walls of these vessels at their point of origin, raising the issue of whether they can be regulated at these points in a way that could increase resistance by contracting (34). Moreover, several investigators have perfused enucleated eyes following 360 degree trabeculotomy, which should eliminate all resistance internal to the collector channels. Results consistently show that only 75% of resistance is eliminated (35). More study in this area is needed because it suggests that total resistance, even in the normal eye, is the result of more than one resistance coupled in series.
WHAT CAUSES THE ADDED RESISTANCE IN POAG AND WHERE IS IT?
Speculating on the source of added resistance in POAG is daunting, especially when one begins with uncertainty as to the source(s) and location(s) of the resistance in the normal eye. While there is general presumption that the resistance of the normal eye resides within the JCT and/or inner wall of SC, or some dynamic combination of both, this does not guarantee that the additional resistance found in the eye with glaucoma is the result of higher resistance in the same location(s). As mentioned earlier, resistances in series are additive. Thus it is possible that a resistance could be created in the glaucomatous eye that does not exist at all in the normal eye. Based upon the discussion above, a host of candidates present for consideration, either singly or in combination.
Extracellular Matrix, Sheath-derived plaques and the Cribriform Plexus
It has long been noted that there is a progressive accumulation of extracellular matrix in the JCT region with age. Much of this material, at least in POAG, is associated with the sheaths of the elastic fibers of the cribriform plexus (36). Notwithstanding this progressive accumulation of material, available data do not appear to support the notion that this added material is sufficient in amount to have any hydrodynamic consequences (16,37). It is important to note here that this conclusion is based upon the assumption that the microscopically discernable open space in these specimens truly reflects the open space present in-vivo.
The cribriform plexus mentioned above is the system of tendons from the smooth muscle cells of the longitudinal bundle of the ciliary muscle extending into the meshwork and applying tension to the JCT region and directly to the inner wall (38). We know that as IOP increases, SC collapses (39,40) and in doing so additional resistance is created (41,42). Preventing collapse of SC is likely the mechanism whereby muscarinic agents, such as pilocarpine, act to decrease outflow resistance (14). These agents cause the longitudinal fibers of the ciliary muscle to contract, thus pulling on the cribriform plexus and tethering the wall of SC open in the face of pressure increases. In this way, the canal remains open and normal resistance is maintained. (38,43). Could it be that the documented increase in “sheath-like material” compromises the ability of the cribriform plexus to contract, and therefore to hold the canal open in the absence of the stronger pull of a muscarinic agent such as pilocarpine?
Contractility and Matrix Interactions
Even if the source and location of the added resistance in POAG remain unknown, interventions that reduce resistance and therefore reduce intraocular pressure can provide us with clues.
Y-27632 is a protein kinase inhibitor selective for Rho associated kinase (ROCK) (44–46). ROCK regulates the phosphorylation of the regulatory myosin light chain (MLC) to promote actomyosin-driven cell contractility. By inhibiting ROCK with Y-27632, MLC phosphorylation is decreased (47,48), resulting in cell relaxation and disassembly of actin stress fibers and focal adhesions in many cell types (49), Included among these cell types are human trabecular meshwork (TM) and SC endothelial cells in-vitro (49,50). Relaxation of stress fiber and focal adhesions would be expected to facilitate aqueous outflow.
H-7, a serine–threonine kinase inhibitor, also induces relaxation of cells in the trabecular meshwork. It is hypothesized that H-7-induced cellular relaxation in the TM may be partially related to its ability to inhibit Rho kinase (51).
Another group of cell/matrix active agents, latrunculins, also alter outflow resistance. Latrunculins disrupt cellular F-actin filaments (52,53). The two most common latrunculins, latrunculin (LAT)-A and -B, cause reversible dose- and incubation time-dependent destruction of actin bundles and associated proteins in cultured trabecular meshwork cells. (52–57). These agents also increase outflow facility in live monkeys, presumably by disrupting the actin cytoskeleton in meshwork cells, leading to relaxation of the meshwork and/or alteration of cell–cell and cell–extracellular matrix adherens junctions (55,58).
It is of interest that the effects of these various agents are to produce changes in the meshwork that are strikingly similar to those resulting from a phenomenon called the wash-out effect (59). When eyes of all species but humans are perfused, the resistance to outflow decreases with the volume of fluid (60). The structural correlate for this increase in facility appears to be similar to that reported following intervention with these various agents. Common features include expansion of the subendothelial JCT matrix and distention of the inner wall of SC. Such changes were reported following Y-27632 perfusion in porcine and bovine eyes (49), H-7 (61) and latrunculin-B (62) in monkeys, and these changes are morphologically similar to the “matrix-matrix” type of inner wall/JCT separation previously shown to correlate with increasing outflow facility during “washout” in bovine eyes (59,63). Combining these results it would appear that inner wall/JCT separation appears to be a critical change underlying both drug-induced and washout-induced increases in outflow facility (59,63). What is unclear from these studies is whether these agents are pointing us toward “the” source of resistance or “a” source of resistance in the normal eye and the eye with POAG.
DOWNSTREAM EFFECTS?
It is clear that most of the research in the area of trabecular outflow resistance, at least in the normal eye, is now focused squarely on the JCT and inner wall of SC, either separately or in combination. And yet, despite decades of research, we remain at a loss to convincingly identify morphological differences in the outflow pathway that would seem to account for the change in intraocular pressure between normal eyes and those with early or moderate glaucoma, using existing methods. Logically, there are several possible explanations. One is that we simply have not yet found the right methods that will give us a “true picture” of the open-spaces in the meshwork and the extent to which the spaces open to flow are reduced in glaucoma. But another possibility is that a site that offers little or no resistance in the normal eye, becomes an added resistance in glaucoma because resistances in series are additive!
A recent line of evidence by Gong, Freddo and Zhang (64), points to the possibility that the normally low resistance openings from SC into the external collector channels may become occluded in glaucomatous eyes. They have documented that as pressure is elevated, areas of the inner wall of SC progressively collapse and the attached portions of the JCT that face the openings of the external collector channels herniate into the openings. In normal eyes, as pressure is reduced, these herniations withdraw from the openings, relieving them of this partial occlusion (65) (Figure 5). Of particular interest, however, when enucleated eyes from patients with POAG were examined, seemingly permanent herniations into the openings of the collector channels were found, even at a pressure of zero. This line of investigation has opened up the possibility that glaucoma could be the result of a series of events, affecting resistance at more than one site along the outflow pathway.
Figure 5.
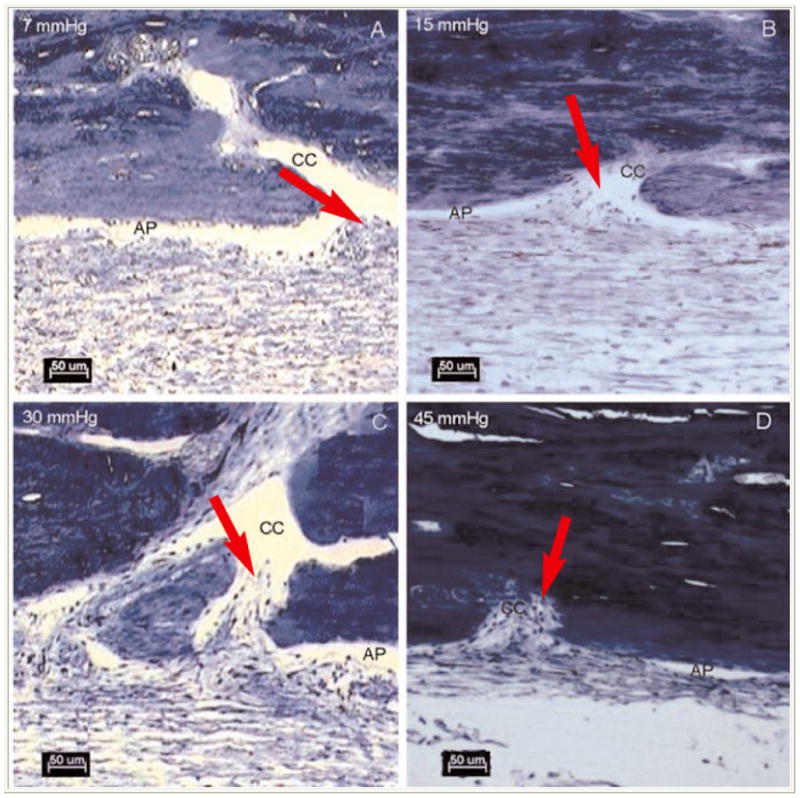
At 7 mmHg, the aqueous plexus (AP) is more open compared to the tissue perfused at higher pressures. At 15mmHg, there is focal herniation (arrows) of the inner wall and JCT at the collector channel (CC) ostium. At 30 and 45mmHg more dramatic herniations of the inner wall and JCT into the collector channel ostia were found. (From Battista, S.A., Lu Z., Hofmann, S., Freddo, T.F., Overby, D.R., Gong, H. “Acute IOP elevation reduces the available area for aqueous humor outflow and induces meshwork herniations into collector channels of bovine eyes”. Invest. Ophthalmol. Vis. Sci. 2008, 49:5346–52. With kind permission of copyright holder, the Association for Research in Vision and Opthalmology.)
SUMMARY
Candidates for the site or sites of outflow resistance in the normal eye are being narrowed but the mechanisms that regulate outflow facility remain elusive. The same remains true for the added resistance in glaucoma, but recent studies suggest that both trabecular and non-trabecular elements of the outflow pathway deserve consideration as we continue to unravel this elusive mystery.
Acknowledgments
Supported by: National Glaucoma Research, a program of the American Health Assistance Foundation, NIH EY-09699, The Massachusetts Lions Eye Research Fund, Inc., The University of Waterloo, School of Optometry.
Contributor Information
Thomas F. Freddo, School of Optometry, University of Waterloo, Waterloo, Ontario, Canada.
Haiyan Gong, Boston University School of Medicine, Boston, MA.
References
- 1.Tripathi RC. Uveoscleral drainage of aqueous humour. Exp Eye Res. 1977a;25 (suppl):305–308. doi: 10.1016/s0014-4835(77)80026-2. [DOI] [PubMed] [Google Scholar]
- 2.Gabelt B, Kaufman P. Prostaglandin F increases uveoscleral outflow inthe cynomolgus monkey. Exp Eye Res. 1989;49:389–402. doi: 10.1016/0014-4835(89)90049-3. [DOI] [PubMed] [Google Scholar]
- 3.Bárány E. A mathematical formulation of intraocular pressure as dependent on secretion, ultrafiltration, bulk outflow, and osmotic readsorption of fluid. Invest Ophthalmol. 1963;2:584–590. [PubMed] [Google Scholar]
- 4.Gong H, Tripathi RC, Tripathi BJ. Morphology of the aqueous outflow pathway. Microscop Res Tech. 1996;33:336–367. doi: 10.1002/(SICI)1097-0029(19960301)33:4<336::AID-JEMT4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 5.Bárány EH. The action of different kinds of hyaluronidase on the resistance to flow through the anterior chamber. Acta Ophthalmol. 1956;34:397–403. [PubMed] [Google Scholar]
- 6.McEwen WK. Application of Poiseuille’s law to aqueous outflow. Arch Ophthamol. 1958;60:290. doi: 10.1001/archopht.1958.00940080306017. [DOI] [PubMed] [Google Scholar]
- 7.Knepper PA, Goossens W, Hvizd M, Palmberg PF. Glycosaminoglycans of the human trabecular meshwork in primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 1996a;37:1360–1367. [PubMed] [Google Scholar]
- 8.Gong H, Underhill CB, Freddo TF. Hyaluronan in the bovine ocular anterior segment, with emphasis on the outflow pathways. Invest Ophthalmol Vis Sci. 1994;35:4328–4332. [PubMed] [Google Scholar]
- 9.Hubbard WC, Johnson M, Gong H, Gabelt BT, Peterson JA, Sawhney R, Freddo T, Kaufman PL. Intraocular pressure and outflow facility are unchanged following acute and chronic intracameral chondroitinase ABC and hyaluronidase in monkeys. Exp Eye Res. 1997;65:177–90. doi: 10.1006/exer.1997.0319. [DOI] [PubMed] [Google Scholar]
- 10.Kamm RD, Palaszewski BA, Johnson MC. Calculation of flow resistance in the juxtacanalicular meshwork. ARVO Abstracts. Invest Ophthalmol Vis Sci. 1983;24:135. [PubMed] [Google Scholar]
- 11.Seiler T, Wollensak J. The resistance of the trabecular meshwork to aqueous humor outflow. Albrecht von Graefe’s Arch Clin Exp Ophthalmol. 1985;223:88–91. doi: 10.1007/BF02150951. [DOI] [PubMed] [Google Scholar]
- 12.Ethier CR, Kamm RD, Palaszewski BA, Johnson MC, Richardson TM. Calculations of flow resistance in the juxtacanalicular meshwork. Invest Ophthalmol Vis Sci. 1986;27:1741–1750. [PubMed] [Google Scholar]
- 13.Mäepea O, Bill A. Pressures in the juxtacanalicular tissue and Schlemm’s canal in monkeys. Exp Eye Res. 1992;54:879–883. doi: 10.1016/0014-4835(92)90151-h. [DOI] [PubMed] [Google Scholar]
- 14.Johnson M, Erickson K. Mechanisms and routes of aqueous humor drainage. In: Albert DM, Jakobiec FA, editors. Principles and Practice of Ophthalmology. Vol. 4. Philadelphia: WB Saunders Co; 2000. pp. 2577–2595. Glaucoma, Chapter 193B. [Google Scholar]
- 15.Johnson M. What controls aqueous humour outflow resistance? Exp Eye Res. 2006;82(4):545–57. doi: 10.1016/j.exer.2005.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy CG, Johnson M, Alvarado JA. Juxtacanalicular tissue in pigmentary and primary open angle glaucoma. The hydrodynamic role of pigment and other constituents. Arch Ophthamol. 1992;110:1779–1785. doi: 10.1001/archopht.1992.01080240119043. [DOI] [PubMed] [Google Scholar]
- 17.Ten Hulzen RD, Johnson DH. Effect of fixation pressure on juxtacanalicular tissue and Schlemm’s canal. Invest Ophthalmol Vis Sci. 1996;37:114–124. [PubMed] [Google Scholar]
- 18.Gong H, Ruberti J, Overby D, Johnson M, Freddo TF. A new view of the human trabecular meshwork using quick-freeze, deep-etch electron microscopy. Exp Eye Res. 2002;75:347–358. [PubMed] [Google Scholar]
- 19.Ye W, Gong H, Sit A, Johnson M, Freddo TF. Interendothelial junctions in normal human Schlemm’s canal respond to changes in pressure. Invest Ophthalmol Vis Sci. 1997;38:2460–2468. [PubMed] [Google Scholar]
- 20.Ramos RF, Hoying JB, Witte MH, Stamer WD. Schlemm’s canal endothelia, lymphatic or blood vasculature? J Glaucoma. 2007;16:391–405. doi: 10.1097/IJG.0b013e3180654ac6. [DOI] [PubMed] [Google Scholar]
- 21.Grierson I, Lee WR. Light microscopic quantitation of the endothelial vacuoles in Schlemm’s canal. Amer J Ophthalmol. 1977;84:234–246. doi: 10.1016/0002-9394(77)90857-1. [DOI] [PubMed] [Google Scholar]
- 22.Grierson I, Lee WR. Pressure effects on flow channels in the lining endothelium of Schlemm’s canal. Acta Ophthamologica. 1978;56:935–952. doi: 10.1111/j.1755-3768.1978.tb03813.x. [DOI] [PubMed] [Google Scholar]
- 23.Tripathi RC. The functional morphology of the outflow systems of ocular and cerbrospinal fluids. Exp Eye Res. 1977b;25 (suppl):65–116. doi: 10.1016/s0014-4835(77)80010-9. [DOI] [PubMed] [Google Scholar]
- 24.Ethier CR, Coloma FM, Sit AJ, Johnson M. Two pore types in the inner wall endothelium of Schlemm’s canal. Invest Ophthalmol Vis Sci. 1998;39:2041–2048. [PubMed] [Google Scholar]
- 25.Epstein DL, Rohen JW. Morphology of the trabecular meshwork and inner-wall endothelium after cationized ferritin perfusion in the monkey eye. Invest Ophthalmol Vis Sci. 1991;32:160–71. [PubMed] [Google Scholar]
- 26.Allingham RR, de Kater AW, Ethier CR, Anderson PJ, Hertzmark E, Epstein DL. The relationship between pore density and outflow facility in human eyes. Invest Ophthalmol Vis Sci. 1992;33:1661–1669. [PubMed] [Google Scholar]
- 27.Sit AJ, Coloma FM, Ethier CR, Johnson M. Factors affecting the pores of the inner wall endothelium of Schlemm’s canal. Invest Ophthalmol Vis Sci. 1997;38:1517–1525. [PubMed] [Google Scholar]
- 28.Johnson M, Chan D, Reed A, Christensen C, Sit A, Ethier C. Glaucomatous eyes have a reduced pore density in the inner wall endothelium of Schlemm’s canal. Invest Ophthalmol Vis Sci. 2002;43:2950–2955. [PubMed] [Google Scholar]
- 29.Johnson M, Shapiro A, Ethier CR, Kamm RD. Modulation of outflow resistance by the pores of the inner wall endothelium. Invest Ophthalmol Vis Sci. 1992;33:1670–1675. [PubMed] [Google Scholar]
- 30.Dvorak-Theobald G. Schlemm’s canal: its anastomoses and anatomic relations. Trans Am Ophthalmol Soc. 1934;32:574–585. [PMC free article] [PubMed] [Google Scholar]
- 31.Batmanov IuE. The structure of the drainage system of the human eye. [Russian] Vestnik Oftalmologii. 1968;81:27–31. [PubMed] [Google Scholar]
- 32.Rohen JW, Rentsch FJ. Morphology of Schlemm’s canal and related vessels in the human eye. [German] Albrecht Von Graefes Archiv Klin Exp Ophthalmol. 1968;176:309–29. doi: 10.1007/BF00421587. [DOI] [PubMed] [Google Scholar]
- 33.Rosenquist RC, Jr, Melamed S, Epstein DL. Anterior and posterior axial lens displacement and human aqueous outflow facility. Invest Ophthalmol Vis Sci. 1988;29:1159–64. [PubMed] [Google Scholar]
- 34.deKater AW, Spurr-Michaud SJ, Gipson IK. Localization of smooth muscle myosin-containing cells in the aqueous outflow pathway. Invest Ophthalmol Vis Sci. 1990;31:347–353. [PubMed] [Google Scholar]
- 35.Ellingson BA, Grant WM. Trabeculectomy and sinusotomy in enucleated human eyes. Invest Ophthalmol. 1972;11:21–28. [PubMed] [Google Scholar]
- 36.Lütjen-Drecoll E, Futa R, Rohen JW. Ultrahistochemical studies on tangential sections of trabecular meshwork in normal and glaucomatous eyes. Invest Ophthalmol Vis Sci. 1981;21:563–573. [PubMed] [Google Scholar]
- 37.Alvarado JA, Yun AJ, Murphy CG. Juxtacanalicular tissue in primary open angle glaucoma and in nonglaucomatous normals. Arch Ophthalmol. 1986;104:1517–28. doi: 10.1001/archopht.1986.01050220111038. [DOI] [PubMed] [Google Scholar]
- 38.Rohen JW. Why is intraocular pressure elevated in chronic simple glaucoma? Anatomical considerations. Ophthalmol. 1983;90:758–765. doi: 10.1016/s0161-6420(83)34492-4. [DOI] [PubMed] [Google Scholar]
- 39.Johnstone MA, Grant WM. Pressure dependent changes in the structures of the aqueous outflow system of human and monkey eyes. Am J Ophthalmol. 1973;75:365. doi: 10.1016/0002-9394(73)91145-8. [DOI] [PubMed] [Google Scholar]
- 40.Johnson M, Kamm RD. The role of Schlemm’s canal in aqueous outflow from the human eye. Invest Ophthalmol Vis Sci. 1983;24:320–325. [PubMed] [Google Scholar]
- 41.Moses RA. Circumferential flow in Schlemm’s canal. Am J Ophthalmol. 1979;88:585–591. doi: 10.1016/0002-9394(79)90519-1. [DOI] [PubMed] [Google Scholar]
- 42.Van Buskirk EM. Anatomic correlates of changing aqueous outflow facility in excised human eyes. Invest Ophthalmol Vis Sci. 1982;22:625–632. [PubMed] [Google Scholar]
- 43.Gong H, Trinkaus-Randall V, Freddo TF. Ultrastructural immunocytochemical localization of elastin in normal human trabecular meshwork. Curr Eye Res. 1989;8:1071–1082. doi: 10.3109/02713688908997400. [DOI] [PubMed] [Google Scholar]
- 44.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. J Biochem. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ishazaki T, Uehata M, Tamocihka I, Keel J, Nonomura K, Mackawa M, Narumiya S. Pharmacological properties of Y-27632, a specific inhibitor of rho-assocatied kinases. Mol Pharmacol. 2000;57:976–983. [PubMed] [Google Scholar]
- 46.Uehata M, Ishizaki T, Satoh H, Ono T, Kawahura T, Morishita T, Tamakawa H, Yamagami K, Inui J, Mackawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a rho-associated protein kinase in hypertension. Nature. 1997;389 (6554):990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 47.Kaibuchi K, Kuronda S, Amano M. Regulation of the cytoskeleton and cell adhesion of the rho family GTPases in mammalian cells. Annu Rev Biochem. 1999;68:459–486. doi: 10.1146/annurev.biochem.68.1.459. [DOI] [PubMed] [Google Scholar]
- 48.Rosenthal R, Choritz L, Schlott S, Bechrakis NE, Jaroszewski J, Wiederholt M, Thieme H. Effects of ML-7 and Y-27632 on carbachol and endothelin-1 induced contraction of bovine trabecular meshwork. Exp Eye Res. 2005;80:837–845. doi: 10.1016/j.exer.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 49.Rao PV, Deng PF, Kumar J, Epstein DJ. Modulation of aqueous humor outflow facility by the rho-kinase-specific inhibitor Y-27632. Invest Ophthalmol Vis Sci. 2001;42:1029–1037. [PubMed] [Google Scholar]
- 50.Honjo M, Tanihara H, Inatani M, Kido N, Sawamura T, Yue BJ, Narumiya S, Honda Y. Effects of rho-associated kinase inhibitor Y-27632 on intraocular pressure and outflow facility. Invest Ophthalmol Vis Sci. 2001;42:137–144. [PubMed] [Google Scholar]
- 51.Tian B, Gabelt TB, Geiger B, Kaufman PL. Combined effects of H-7 and cytochalasin-B on outflow facility in monkey eyes. Exp Eye Res. 1999;68:649–655. doi: 10.1006/exer.1998.0647. [DOI] [PubMed] [Google Scholar]
- 52.Coue M, Brenner SL, Spector I, Korn ED. Inhibition of actin polymerization by latrunculin-A. FEBS Lett. 1987;213:316–318. doi: 10.1016/0014-5793(87)81513-2. [DOI] [PubMed] [Google Scholar]
- 53.Lyubimova A, Bershadsky AD, Ben-Ze’ev A. Autoregulation of actin synthesis responds to monomeric actin levels. J Cell Biochem. 1997;65:469–478. [PubMed] [Google Scholar]
- 54.Epstein DL, Rowlette LL, Roberts BC. Acto-myosin drug effects and aqueous outflow function. Invest Ophthalmol Vis Sci. 1999;40:74–81. [PubMed] [Google Scholar]
- 55.Peterson JA, Tian B, Bershadsky AD, Volberg T, Gangnon RE, Spector I, Geiger B, Kaufman PL. Latrunculin-A increases outflow facility in the monkey. Invest Ophthalmol Vis Sci. 1999;40:931–941. [PubMed] [Google Scholar]
- 56.Spector I, Shochet NR, Blasberger D, Kashman Y. Latrunculins--novel marine macrolides that disrupt microfilament organization and affect cell growth: I. Comparison with cytochalasin D. Cell Motil Cytoskeleton. 1989;13:127–144. doi: 10.1002/cm.970130302. [DOI] [PubMed] [Google Scholar]
- 57.Spector I, Shochet NR, Kashman Y, Groweiss A. Latrunculins: novel marine toxins that disrupt microfilament organization in cultured cells. Science. 1983;219:493–495. doi: 10.1126/science.6681676. [DOI] [PubMed] [Google Scholar]
- 58.Peterson JA, Tian B, McLaren JW, Hubbard WC, Geiger B, Kaufman PL. Latrunculins’ effects on intraocular pressure, aqueous humor flow, and corneal endothelium. Invest Ophthalmol Vis Sci. 2000;41:1749–1758. [PubMed] [Google Scholar]
- 59.Overby D, Gong H, Qiu G, Freddo TF, Johnson M. The mechanism of increasing outflow facility during washout in the bovine eye. Invest Ophthalmol Vis Sci. 2002;43:3455–3464. [PubMed] [Google Scholar]
- 60.Johnson M, Chen A, Epstein DL, Kamm RD. The pressure and volume dependence of the rate of wash-out in the bovine eye. Curr Eye Res. 1991;10:373–375. doi: 10.3109/02713689108996343. [DOI] [PubMed] [Google Scholar]
- 61.Sabanay I, Tian B, Gabelt TB, Geiger B, Kaufman PL. Functional and structural reversibility of H-7 effects on the conventional aqueous outflow pathway in monkeys. Exp Eye Res. 2004;78:137–150. doi: 10.1016/j.exer.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 62.Sabanay I, Tian B, Gabelt TB, Geiger B, Kaufman PL. Latrunculin-B effects on trabecular meshwork and corneal endothelial morphology in monkeys. Exp Eye Res. 2006;82:236–246. doi: 10.1016/j.exer.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 63.Scott PA, Overby DR, Freddo TF, Gong H. Comparative studies between species that do and do not exhibit the washout effect. Exp Eye Res. 2007;84:435–443. doi: 10.1016/j.exer.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gong H, Freddo TF, Zhang Y. New morphological findings in primary open angle glaucoma. Invest Ophthalmol Vis Sci. 2007;48:E-Abstract 2079. [Google Scholar]
- 65.Battista SA, Lu Z, Hofmann S, Freddo TF, Overby DR, Gong H. Acute IOP elevation reduces the available area for aqueous humor outflow and induces meshwork herniations into collector channels of bovine eyes. Invest Ophthalmol Vis Sci. 2008;49:5346–52. doi: 10.1167/iovs.08-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]


