Abstract
Currently, two major classification systems segregate diffuse large B-cell lymphoma (DLBCL) into subtypes based on gene expression profiles and provide great insights about the oncogenic mechanisms that may be crucial for lymphomagenesis as well as prognostic information regarding response to current therapies. However, these current classification systems primarily look at expression and not dependency and are thus limited to inductive or probabilistic reasoning when evaluating alternative therapeutic options. The development of a deductive classification system that identifies subtypes in which all patients with a given phenotype require the same oncogenic drivers, and would therefore have a similar response to a rational therapy targeting the essential drivers, would significantly advance the treatment of DLBCL. This review highlights the putative drivers identified as well as the work done to identify potentially dependent populations. These studies integrated genomic analysis and functional screens to provide a rationale for targeted therapies within defined populations. Personalizing treatments by identifying patients with oncogenic dependencies via genotyping and specifically targeting the responsible drivers may constitute a novel approach for the treatment of DLBCL.
INTRODUCTION
DLBCL is a highly malignant form of non-Hodgkin’s lymphoma (NHL) that constitutes about 40% of all lymphoma diagnoses (1). In 1966 the chemotherapy regimen of cyclophosphamide, hydroxyldaunorubicin, vincristine and prednisone (CHOP) was introduced to manage lymphoma. This regimen improved the response rates previously achieved by single chemotherapeutic regimens from 50% to 69% as well as providing superior survival (2–6). Numerous efforts were made over prior decades to improve on the results obtained with CHOP, including complicated multi-drug regimens such as MACOP-B and COPLAM, unfortunately with no success. It was not until 1997 that CHOP was modified with the addition of chimeric anti-CD20 monoclonal antibody rituximab resulting in significantly improved cure rates (7, 8). However, with the exception of rituximab, no other targeted therapy has been utilized extensively as a front line therapy for DLBCL. This is felt to be in large part due to the heterogeneity of DLBCL and the inability to identify the oncogenic drivers responsible for a patient’s disease.
The phenomenon of oncogene ‘‘addiction’’ has been defined as a tumor requirement for constitutive expression and activity of a single aberrant gene, regardless of other tumor-related alterations (9). BCR-ABL-expressing chronic myelogenous leukemia (CML) exemplifies this phenomenon as the direct targeting of BCR-ABL with the specific kinase inhibitor Imatinib results in a 95% response rate despite the presence of other genetic lesions. This strong clinical evidence for oncogene addiction is further supported by the BCR-ABL reactivation that occurs in patients that develop resistance to Imatinib with the most common mechanism of resistance being a de novo T315I mutation in the ATP-binding pocket (10–12).
Unlike CML, other malignancies have poorly defined addictions to oncogenic drivers. This is primarily due to the fact that BCR-ABL is a unique fusion protein that is exclusive to CML, while many other malignancies are dependent on the overexpression of oncogenic signaling cascades that are not cancer specific. Conversely, it has been demonstrated that the overexpression of an oncogene does not necessarily predict for an oncogenic dependency. A recent study sought to stratify human cancer cell lines with K-Ras mutations into two groups. They examined the requirement for constitutive K-Ras function in maintaining viability and established distinct phenotypes for the dependent and independent groups (13). Their findings demonstrated a correlation between K-Ras dependent cell lines and a well-differentiated epithelial phenotype. RNA interference synthetic lethality screens have been utilized to identify and dissect oncogene addictions within DLBCL. Synthetic lethality arises when the ablation of two different genes does not reduce cell viability but the ablation of both is highly lethal. This is a particularly attractive concept in the treatment of cancer, as targeting a gene that is related to a recurrent cancer mutation should selectively kill malignant cells only. This concept is nicely reviewed in Kaelin WG Jr. (14). One such synthetic lethality screen in DLBCL identified CARD11 as crucial mediator between BCR and NF-κB signaling in a subtype of DLBCL (15).
B-CELL MATURATION, LYMPHOMAGENESIS & SUBTYPE CLASSIFICATION
B-cells primarily originate from common lymphoid progenitor cells in the bone marrow and undergo recombinase-activating gene (RAG1 and RAG2) mediated gene rearrangement during B-cell receptor (BCR) development (16). During this process, double-strand breaks (DSB) are introduced into the V, D, J gene segments to facilitate recombination and formation of immunoglobulin heavy-chain (IgH) and light chain (IgL) genes (17). The DSBs introduced are repaired by homologous recombination but have been shown to contribute to chromosomal translocations involving immunoglobulin loci (18).
Until B-cells encounter an antigen, they are considered mature naïve B-cells. Upon antigen-induced B-cell activation, B-cells proliferate and can differentiate into centroblasts or plasma cells via the follicular or extrafollicular pathway, respectively (19). The centroblasts go through the dark zone of the germinal center (GC) where they rapidly proliferate, differentiate and revise their antigen receptors via immunoglobulin somatic hypermutation (SHM) of the immunoglobulin heavy chain variable region (IGHV) genes as well as undergo class switch recombination (CSR) (20–23). Both of these processes are mediated by activation-induced cytidine deaminase (AID), which deaminates cytosine to uracil (24, 25). SHM modulates the affinity of the antibodies to a specific antigen and it is believed that the mistargeting of SHM can also result in the translocation of oncogenes. Mouse models have firmly established the cause and effect link between AID enzymatic activity and IgH-cMyc translocations (26). Mouse models have also demonstrated that AID is necessary for BCL6 mediated lymphomagenesis in some DLBCL populations (27).
Germinal center B-cell lymphomas (GCB-DLBCL) are DLBCLs that resemble germinal-center B-cells (28). They generally have switched IgH classes and continue to undergo somatic hypermutation. Translocations of BCL-2 and Myc with IGHV are commonly observed in this population (29, 30). These translocations may lead to a malignant transformation by preventing apoptosis or blocking terminal differentiation by placing these genes under the IGHV promoter, which results in their constitutive expression. Usually normal GC B-cells are primed for apoptosis as they lowly express the anti-apoptotic protein BCL2, and require selection to progress and further differentiate (31). The t(14;18) translocation results in an increased expression of BCL2 and provides a mechanism for GCB-DLBCL to evade apoptosis. The differentiation of germinal center B-cells can be inhibited by BCL6 proteins, which work along with other transcription, factors such as B-lymphocyte-induced maturation protein (BLIMP1), PAX4 and XBP1, to regulate and coordinate germinal center B-cell to plasma cell differentiation (32, 33).
Activated peripheral blood B-cell (ABC-DLBCL) origination is less understood but this subtype is characterized by a gene expression pattern similar to that of normal plasma cells including the expression of the transcription factor XBP1, which regulates immunoglobulin secretion (34, 35). The ABC subtype is also characterized by constitutive NF-κB activity, which induces IRF4 expression (36). Generally this would drive B-cells towards plasmacytic differentiation but some data suggests that inactivation of BLIMP-1 in IgM positive post-GC memory cells inhibits plasmacytic differentiation and may be crucial for lymphomagenesis in the ABC subtype (37). Other data suggests that ABC-DLBCL may develop from extrafollicular B-cells with high levels of AID (38). Regardless of its origin, this subtype accumulates chromosomal translocations involving the IgH switch regions and may be associated with high levels of AID generating DSB.
Gene expression signatures have been used to identify relevant diffuse large B-cell lymphoma (DLBCL) subsets. The cell of origin (COO) classification system is a developmentally defined system that divides DLBCL into two main subsets based on how closely the tumor resembles either a GCB-DLBCL or an ABC-DLBCL, with tumors that do not resemble either being left unassigned or referred to as type III (28). The Hans classification system, albeit imperfect is a widely accepted method utilized for research studies to distinguishing the ABC versus GCB-DLBCL subtypes, which are not yet recognized by the WHO 2008 classification, but are distinct lymphomas in regards to their biological and clinical presentation (39). The method utilizes immunoperoxidase tissue microarrays (TMA) and classifies CD10 positive and BCL6 positive/MUM1 negative tumors as GCB tumors. Both CD10 and BCL6 are GC markers while MUM1 is expressed by plasma cells and later stage B-cells (40, 41). The remaining BCL6 negative and BCL6 positive/MUM1 positive tumors are then classified as non-GC. One group observed that DLBCLs harboring the t(3;14) translocation between BCL-6 with IGHV are predominantly MUM1 positive and therefore classified as non-GC (42). This classification system is quite useful in understanding the mechanisms for lymphomagenesis in DLBCL, as many B-cell lymphomas hijack regulatory processes during B-cell development and are functionally defined by their differentiation state. One of the caveats to this approach is its limited accuracy. Antibodies that are more specific to germinal center B-cells are now being employed in an immunohistochemical algorithm to increase the 80% accuracy of the Hans classification system to 93% (43). Shaknovich et al., employing a DNA methylation signature of 16 genes had a 98% accuracy in predicting ABC versus GCB subtypes from the same cohort of DLBCL samples that were previously classified by the Staudt group using gene expression profiling (44, 45).
Recently, a consensus clustering system has been used to classify DLBCL subsets in an unbiased manner. This highly reproducible method has identified B-cell receptor/proliferation (BCR), oxidative phosphorylation (OxPhos) and host response (HR) populations (46). The BCR subtype has higher expression of BCR signaling components such as CD19, IgH, CD79a, BLK, SYK, PLCγ2 and MAP4K and higher expression of cell-cycle regulatory genes and DNA repair genes such as CDK2, H2AX and p53. The BCR subtype also displays a higher expression of PAX5, OBF-1, E2A, STAT6, Myc and BCL6, which have been implicated in neoplastic transformation and tumorigenesis. The OxPhos phenotype has increased expression of mitochondrial-associated proteins, such as nicotinamide adenine dinucleotide dehydrogenase (NADH) complex, cytochrome c, cytochrome c oxidase (COX), and adenosine triphosphate (ATP) synthase components as well as the anti-apoptotic protein BFL-1. Finally, their high levels of CD2+/CD3+ infiltrating lymphocytes and CD1a-/CD123-dendritic cells as well as their lower expression of genetic aberrations generally characterize the HR tumors.
BCL6
The most commonly involved oncogene in B-cell lymphomagenesis is BCL6 (B-cell lymphoma 6). Its gene rearrangements at 3q27 have been reported in 30–40% of DLBCL cases with a higher percentage being observed in the ABC subtype (47). Point mutations in the 5′ non-coding region occur independently of the chromosome translocation in roughly 75% of DLBCL cases with a higher frequency in GCB patients (48).
BCL6 is a bric-a-brac, tramtrack, broad complex/Pox virus zinc finger (BTB/POZ) transcriptional repressor. Under normal conditions B-cells express BCL6 exclusively during germinal center differentiation, as B-cells require BCL6 expression for germinal center and immunoglobulin affinity development (49). BCL6 regulates survival and differentiation via distinct corepressor complexes (50). Survival is regulated through both the SMRT and NCoR corepressors, which are found in large multi protein histone deacetylase (HDAC)-containing complexes. Both corepressors contain a highly conserved 17-residue BCL6 binding domain (BBD) that binds to the homodimeric BCL6 BTB domain (51). The complexes mediate survival by repressing transcription of ATR, TP53 and CDKN1A, which are involved in DNA damage and cell cycle regulation (52, 53). Differentiation is regulated through BCL6 interactions with the MTA3/NuRD corepressor complex, which represses a regulator of plasmacytic differentiation, BLIMP1 (54).
BCL6 is believed to contribute to lymphomagenesis when its downregulation that usually occurs after affinity maturation is disrupted. One proposed mechanism for BCL6 downregulation disruption is the loss of IRF4 binding sites of the BCL6 gene. IRF4 expression is induced by sustained CD40 stimulation of the NF-κB pathway in germinal center cells. IRF4 usually binds to exon 1 and intron 1 of the BCL6 gene and represses BCL6 expression but chromosome translocations or point mutations introduced during SHM, which commonly targets the 5′ non-coding promoter region of BCL6, may prevent this repressive effect (55). BCL6 promoter binding and gene repression has also been shown to vary between normal and malignant cells. The repression of genes implicated in malignant transformation such as BCL2 and Myc is lost in DLBCL demonstrating the role of deregulation of the BCL6 transcriptional network in lymphomagenesis (56). For a more comprehensive review on BCL6 and lymphomagenesis, please refer to Basso and Dalla-Favera (57).
BCL6 dependency has no correlation to the COO classification system as dependency occurs in both ABC and GCB cell lines. Also, response to BCL6 inhibition is independent of the mechanism for BCL6 deregulation. Recently, a study demonstrated the differential regulation of BCL6 target genes in BCL6 dependent versus BCL6 independent cell lines. The transcriptional profiles of cells lines were used to identify BCR and OxPhos representative cell lines according to the comprehensive clustering method previously established. Chromatin immunoprecipitation (ChIP)-on-chip analysis identified BCL6 target genes. Gene set enrichment analysis (GSEA) was then used to determine the differentially expressed BCL6 target genes using the cell line gene list (58).
Subsequent experiments demonstrated that treatment with a BCL6 peptide inhibitor (BPI) selectively increased the expression of the BCL6 target genes in BCR cell lines but not OxPhos cell lines. The data suggests that BCL6 represses target genes in BCR DLBCLs. BPI treatment also inhibited growth of BCR cells in vitro and in vivo. These findings demonstrated a correlation between BCL6 dependent cell lines and the BCR subtype of DLBCL.
Targeting the BTB domain of BCL6 by mimicking the structure of SMRT BBD appears to be a rational and effective approach in preventing BCL6 dependent DLBCL survival (59). The cell-penetrating BPI is highly selective in that DLBCLs are killed in vitro and in vivo without any negative side effects including inflammatory disease that could potentially result from BCL6 depletion (60). The use of this specific peptide interference to identify the BCL6 transcriptional network in BCL6 dependent populations also identified HDAC and Hsp90 as synergistic targets. These findings have significant translational implications since small inhibitory molecules targeting HDAC and Hsp90 are already approved for clinical use or in clinical trials (61). Additional therapies including peptide aptamers and small molecule inhibitors that antagonize BLC6 function also show promise for the treatment of BCL6 dependent DLBCLs (62, 63).
BCL2
BCL2 (B-cell lymphoma 2) was initially described by the t(14;18) chromosome translocation observed in 90% of follicular lymphomas (64–66). In roughly 20% of DLBCL cases BLC2 is overexpressed due to the BCL2/IgH t(14;18) chromosome translocation, which constitutively activates the anti-apoptotic protein by placing it under the immunoglobulin heavy chain gene transcriptional elements (67, 29). BCL2 gene amplifications at 18q21 have also been observed in 10% of GCB-DLBCLs as well as 34% of ABC DLBCLs irrespective of t(14;18) status. B-cells without a higher affinity to an antigen would generally not be selected out by follicular dendritic cells and T-cells for further differentiation into plasma cells and memory cells. Usually the bulk of the germinal center B-cells undergo apoptosis when they acquire somatic mutations that reduce antigen binding, unless the mutation provides a mechanism to evade this process (68).
The BCL2 family of apoptosis regulating proteins contains key regulators of the mitochondrial-dependent (intrinsic) apoptosis pathway (69). There are three subgroups of BCL2 proteins: multi-domain anti-apoptotic, multi-domain pro-apoptotic, and BH3-only pro-apoptotic, which are segregated by both their function as well as their regions of BCL2 homology (BH) domains (70). The BH3-only pro-apoptotic proteins can respond to transcriptional or posttranslational modifications and become activators initiating the oligomerization of BAX and/or BAD (71, 72). This rapid oligomerization process leads to mitochondrial outer membrane permeabilization (MOMP), which allows for the release of pro-apoptotic proteins including cytochrome c (73). Once released into the cytoplasm, cytochrome c can bind to APAF-1 and unfold it forming a heteroheptameric complex called the apoptosome (74). APAF-1 is then able to activate widespread proteolysis through the activation of cysteine proteases, known as caspases. This induces cellular dysfunction and the apoptotic cell is tagged for phagocytosis.
Multiple mechanisms can be utilized by malignant cells to evade programmed cell death (PCD). Reduction or elimination of BH3-only pro-apoptotic proteins through gene deletion or inactivation results in a class A block (75). An apoptotic block may be employed by tumors with inactivated tumor suppressor p53, which regulates both the PUMA and NOXA BH3-only pro-apoptotic proteins (76, 77). Downregulation of multi-domain pro-apoptotic proteins such as BAX and BAK provides an alternative mechanism for defying apoptosis and is referred to as a class B block (78). Finally, a class C block can occur through increased expression of multidomain anti-apoptotic proteins such as BCL2 and BCL-XL (79).
Multi-domain anti-apoptotic proteins such as BCL2, BCL-XL, BCL-w and BFL1 have sequence homology in four α-helical BH regions, BH1-4; MCL-1 is the exception, sharing homology in only BH1-3 (80–83). The BH1-3 domains in the hydrophobic groove of these anti-apoptotic proteins can bind the hydrophobic α-helical BH3 region of pro-apoptotic proteins. This interaction provides an anti-apoptotic mechanism by binding and sequestering activator BH3-only pro-apoptotic proteins or by binding and preventing oligomerization of multi-domain pro-apoptotic proteins and subsequent MOMP.
Many DLBCLs may be dependent on BCL2 as BCL2 provides an advantageous class C block, but the mechanisms that result in this survival advantage also produce many confounding factors. This has made elucidating the mechanism for dependency as well as describing the BCL2 dependent phenotype quite difficult. BCL6 suppresses BCL2 expression by interacting with the transcriptional activator Miz1, which inhibits Miz1-induced expression of BCL2 (84). Both chromosome translocations and amplifications can evade the tumor suppressor function of BCL6, which explains how high levels of both BCL2 and BCL6 can be observed in some cases of DLBCL. BCL2 can also be upregulated by constitutive NF-κB activation as BCL2 is a target gene of NF-κB (85).
BCL2 has been specifically targeted with the antisense DNA agent, oblimersen, as well as the small molecule inhibitors ABT-263 and ABT-737. A phase II clinical trial for oblimersen, in combination with rituximab, demonstrated an overall response rate of 42% in relapsed/refractory B-NHL, but oblimersen has yet to fulfill its early promise (86). On the other hand ABT-263, the orally available form of ABT-737, has been tested in clinical trials for small cell lung cancer, CLL and lymphoma (87). This drug was designed to bind the binding pockets of BCL2 and BCL-XL by using a strategy called structure-activity relationships by NMR (SAR by NMR). Although these three multi-domain anti-apoptotic proteins are inhibited by ABT-263 with sub-nanomolar affinity, it only shows a low affinity for MCL1 and BFL1. Acquired resistance to ABT-737 has been demonstrated in lymphoma cells that upregulate MCL1 and BFL1 and is one of the main mechanisms for intrinsic resistance (88).
To determine the dependency on BCL2 one group used BH3 profiling to detect the class of apoptotic block in a panel of 18 DLBCL cell lines (89). Briefly, BH3 profiling tests a panel of sensitizer peptides for their ability to induce MOMP from mitochondria isolated from the lymphoma cell lines. MOMP is determined by measuring cytochrome c release with ELIZA. The group identified that 9 out of 10 DLBCLs harboring the t(14;18) translocation generally have a “primed” phenotype with a class C block. Their study also demonstrated that t(14;18) is positively correlated with sensitivity to ABT-737. Interestingly, BFL-1 expression in at(14;18) positive cell line conferred resistance to ABT-737 treatment, likely by providing an alternative mechanism for evading apoptosis (89).
NF-κB
The NF-κB (nuclear factor kappa light chain enhancer of activated B-cells) system was first linked to lymphomagenesis in an avian reticuloendotheliosis viral study. The v-Rel oncogene, which is a member of the Rel/NF-κB transcription factor family, induced rapidly fatal hematological malignancies in birds with numerous studies thereafter making similar observations in human cells (90). Gene signature analysis has shown increased expression of NF-κB target genes such as BCL2 family proteins, IRF-4, c-FLIP and cyclin D2 in ABC-DLBCL (36). Constitutive NF-κB activity characterized by high NF-κB DNA binding activity, IKK activity and IκB degradation have also been observed exclusively in ABC-DLBCL and has become the hallmark of this subtype (36). It is interesting to note that the IκB gene is rarely mutated in DLBCL (91).
The NF-κB protein family shares an N-terminal Rel homology domain, which appears to form DNA-binding complexes. The class I NF-κB proteins are NF-κB1/p105 and NF-κB2/p100 and undergo ubiquitin/proteasome mediated partial degradation of their C-terminus to produce active p50 and p52 subunits, respectively (92, 93). Class II NF-κB proteins include RelA/p65, RelB and c-Rel which all have transactivation domains in their C-terminus and generally form heterodimers with class I NF-κB proteins to initiate transcription activation (94–96). NF-κB activity is regulated by the IκB family of inhibitors, which bind to NF-κB dimers and sequester them in the cytoplasm (97). IκB phosphorylation by the IκB kinase (IKK) complex results in IκB ubiquitination and proteasomal degradation, thus releasing the NF-κB dimer for nuclear translocation and gene transcription (98). This mechanism makes NF-κB a rapidly acting primary transcription factor, as it does not require new protein synthesis for activation.
In normal B-cells, NF-κB signaling is activated upon antigen binding to the BCR and is involved in numerous functions such as proliferation, isotype switching, cytokine production, and mature B-cell maintenance (99–101). The BCR complex is composed of a ligand-binding moiety, which is a transmembrane form of immunoglobulin and a signal transduction moiety composed of the Ig-α/Ig-β (CD79a/b) heterodimer. The cytoplasmic tails of the CD79 heterodimer contain immunoreceptor tyrosine-based activation motifs (ITAM), which recruit Src homology 2 (SH2) containing proteins upon tyrosine phosphorylation. Upon antigen binding and BCR oligomerization Lyn or Fyn tyrosine kinases phosphorylate the ITAMs and initiate downstream signaling (102). Spleen tyrosine kinase (Syk) and B-cell linker protein (BLNK) are recruited to the ITAMs via their SH2 domains. Phosphorylation of BLNK by Syk recruits phospholipase C (PLCγ2), which is also phosphorylated by Syk but not fully activated until phosphorylation by Bruton’s tyrosine kinase (BTK). PLCγ2 cleaves PIP2 into IP3 and DAG, which ultimately activates protein kinase C (PKCβ). One of PKCβ’s substrates is CARD11, which functions upstream of the IKK complex (103).
In addition to BCR signaling, both Toll-like receptors (TLRs), an ancient and highly conserved component of the immune system found in vertebrates and invertebrates, and interleukin-1 receptor (IL-1R) family members can activate NF-κB signaling in B-cells. This signal is transduced by a myeloid differentiation primary response gene 88 (MYD88), IL-R1-associated kinase (IRAK) and TNFR-associated factor 6 (TRAF6) mediated mechanism (104, 105). Upon ligand binding, MyD88 is recruited to TLR/IL-1R through a homotypic interaction between TLR/IL-1R (TIR) domains. Both IRAK1 and IRAK4 are recruited by MyD88 to the complex, resulting in phosphorylation of IRAK1 by IRAK4 and subsequent IRAK1 autophosphorylation (106). Once activated, IRAK1 can interact with TRAF6 to mediate NF-κB signaling (107).
Numerous mechanisms for constitutive NF-κB signaling have been implicated in DLBCL, which has made defining a dependent population rather difficult. Upstream activation of IKKβ via CARD11, BCL10 and MALT dependent mechanisms, which form the CBM complex, are commonly observed in ABC-DLBCL. Activating missense mutations in CARD11 have been observed in 10% of ABC-DLBCLs while many ABC-DLBCL tumors without CARD11 mutations display chronic activation of BCR signaling which activates the CBM complex (108, 109). Amplification of c-Rel at 2p13 has also been observed in roughly 25% of DLBCL (110, 111). Recent studies also found that 30% of ABC-DLBCLs have biallelic inactivation of the A20 deubiquitinase, which negatively regulates NF-κB, suggesting another mechanism for constitutive NF-κB expression (112, 113). Mutations in oncogenic MyD88, an adaptor protein that mediates toll and interleukin receptor signaling, occur in 39% of ABC-DLBCL and also activates NF-κB signaling (114). The recurrence of aberrant NF-κB signaling through the hijacking of signaling pathways essential for normal B-cell homeostasis strongly suggests a dependency within the ABC-DLBCL subtype.
The NF-κB pathway has been targeted through IKK specific kinase inhibitors, heat shock protein 90 (HSP90) inhibitors, IκB super-repressor molecules and proteasome inhibitors. The small molecular IKK inhibitors PS-1145 and MLX015 are highly specific kinase inhibitors that were found to be very effective at inducing cell death in ABC-DLBCL cells after reducing NF-κB target genes and inducing activation of pro-apoptotic caspases (115). The HSP90 inhibitor Geldanamycin (GA) has also shown efficacy by disrupting the formation of the HSP90/IKK complex (116). The use of the super-repressor IκBα (S32G/S36A) results in selective killing of ABC-DLBCL cell lines (36). The super-repressor cannot be phosphorylated by IKK, preventing its degradation and the activation of NF-κB, which is critical for ABC-DLBCL survival. Indirect inhibition of NF-κB has been accomplished through the use of the proteasome inhibitor bortezomib, which prevents IκBα degradation. Phase 2 clinical trials for bortezomib in combination with chemotherapy demonstrated response rates of 85% in refractory ABC-DLBCL but only 13% in GCB-DLBCL, again demonstrating a NF-κB dependency in the ABC subtype (117).
ALK
DLBCLs positive for ALK (anaplastic lymphoma kinase) fusion proteins were first described in 1997 and generally feature a t(2;17) resulting in a clathrin (CLTC)-ALK fusion protein (118). Approximately 50 cases of this rare and distinct form of DLBCL have been reported and a poor prognosis with a 5-year survival rate of only 25% has been observed in these patients (119,120). Studies indicate that virtually all patients belonging to this subgroup are CD20 negative and R-CHOP is unlikely to significantly improve survival.
ALK is an orphan receptor tyrosine kinase in the insulin receptor family and is commonly activated in cancer through the generation of ALK fusion proteins. Fusion proteins resulting in the constitutive activation of the ALK kinase domain have an oncogenic potential by enhancing cell proliferation and survival via numerous redundant and interconnected pathways. This subtype’s immunophenotype has been characterized by the positive expression of plasma cell associated antigens CD38 and CD138, epithelial membrane antigen (EMA) and immunoglobulin light chain kappa or lambda and most closely resemble post germinal center B-cell lymphomas. ALK-DLBCL also lacks T-cell-related antigen (CD2, CD3, CD5, CD43 and CD8), B-cell related antigen (CD20, and CD79a) and CD30 expression(121,122). Although the reported incidence of ALK-DLBCL is very low, this may be due to being misdiagnosed as ALCL which is typically CD30+, or plasmablastic lymphoma, plasmablastic myeloma, immunoblastic DLBCL and anaplastic carcinoma which are all generally ALK negative.
One group recently developed the first CLTC-ALK positive DLBCL cell line and evaluated its dependency on ALK kinase function (123). The selective ALK inhibitor TAE-684 demonstrated a reduction in the activation of downstream pathways associated with lymphomagenesis such as constitutive JAK-STAT3, ERK and PI3K-AKT activation (124–126). This inhibitor also induced cell death in vitro as well as in complete regression in xenograft models. The dual c-Met/ALK inhibitor crizotinib (PF-02341066), which is structurally unrelated to TAE-684, has also been explored as a therapeutic option for ALK dependent malignancies.
FUTURE DIRECTIONS
The COO classification system and subsequent functional studies have defined the ABC subtype as highly dependent on NF-κB signaling. Unfortunately, at each differentiation state, there are multiple mechanisms for lymphomagenesis and identifying the dependency in the GCB subtypes can be limited using this method. In addition, multiple lesions can lead to NF-κB activation but differential dependencies on these lesions result in variable responses to targeted therapies. This is exemplified by the documented resistance to both BTK and PKC inhibitors in ABC-DLBCL cell lines harboring CARD11 or A20 mutations (127). Consensus clustering and subsequent functional studies have identified the BCR subtype as a superb candidate for BCL6 targeting therapies. At the same time, much work still remains in determining a dependency and therapy for both the OxPhos and HR subtypes. The HR subtype is characterized by a heterogeneous population with significant T-cell infiltration, which poses additional complications for analysis, as the tumor is not readily recapitulated for in vitro studies. FISH can also identify DLBCLs harboring t(14;18) and t(2;17) translocations as BCL2 and ALK dependent populations, respectively. The subtypes identified from classification systems and FISH can be merged to stratify DLBCL subtypes by oncogenic dependencies. This is essential for effectively designing and interpreting clinical trials exploring novel therapeutic options selectively targeting the responsible drivers. Even after merging these classification systems, there remain large gaps in information vis-à-vis defining the epidemiology of both the subtypes with known dependencies and those that remain undefined.
Other mechanisms, which may include putative drivers through direct or indirect mechanisms, are being explored and may address some of the current gaps in the field. Recently, coding genome analysis by next generation sequencing and copy number analysis revealed a role of aberrant epigenetic regulation in lymphomagenesis (128). Specifically, alterations in Histone-lysine N-methyltransferase (MLL2), CREB binding protein (CREBBP), E1A binding protein p300 (EP300) and other chromatin modifying enzymes have been observed in over one third of DLBCL cases, regardless of COO status (128–130). EP300 has recently been proposed as a mechanism for BCL6 dependency as BCL6 represses EP300 expression and lymphomas insensitive to BCL6 inhibitors often have p300 mutations (61). In addition, p300 has been shown to be necessary for BCL6 acetylation and recruitment of HDACs (131). This BCL6-p300 axis as well as the newly identified lesions suggests that HDAC inhibitors may be rational therapies for discreet populations of DLBCL. Characterization of populations dependent on these mechanisms versus populations where these abnormalities are passenger mutations may further elucidate their role in lymphomagenesis.
Some of these epigenetic alterations may lead to the disruption of tumor suppressor genes and contribute to lymphomagenesis. One study recently observed the inactivation of CD58 in 21% of DLBCL cases which serves as a means for evading T and natural killer (NK) cell mediated cytolysis (132–134). In the same study, B2M mutations or deletions were also observed in 29% of DLBCL cases, thus preventing surface expression of human leukocyte antigen (HLA) class I molecule, which is essential for CD8+ cytotoxic T-cell (CTL) recognition and cytolysis (135). Roughly 61% of DLBCL cases lack both CD58 and B2M expression on the plasma membrane, which likely results in tumor evasion of both CTL and NK cells (132). The high incidence of DLBCL tumors co-selecting these complementary mechanisms suggests an essential role of immune evasion in DLBCL development and/or progression stressing the need for further characterization and development of treatment options targeting these mechanisms.
In addition, the emergence of microRNA profiling will almost certainly reveal additional oncogenic mechanisms thereby promoting additional stratification of heterogeneous populations (136). MicroRNAs have a repertoire of mechanisms to regulate both gene transcription and translation and the concept of onco-miRs has been recently proposed (137, 138). Specifically, the oncogenic nature of miR-155 has been implicated in DLBCL by the Aguiar group, which observed the segregation of miR-155 expression with NF-κB activity in DLBCL cell lines as well as increased expression in primary samples defined as ABC-DLBCL (139). Such miRs often regulate a cluster of functionally related genes and may hold great potential as therapeutic targets (140).
It is expected that even after drivers are identified and targeted, responses will be variable as de novo mutations, oncogene switching and inactivation of tumor suppressors will likely appear providing resistance mechanisms to therapies. The revelation of these secondary events may identify additional tractable and rational targets that could greatly improve the survival of discrete populations where they are essential for tumor survival. Defining dependent phenotypes in DLBCL will ultimately benefit patients by personalizing treatments through the specific targeting of the responsible drivers and has the potential to significantly advance the treatment of DLBCL.
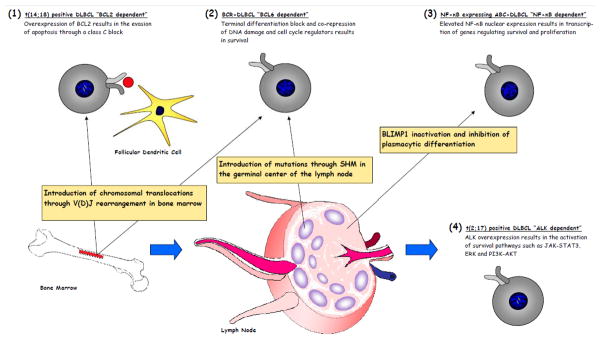
Figure 1. An illustration of putative oncogene dependencies in DLBCL.
(1) BCL2 dependency is observed within t(14;18) positive populations, which are generally described as GCB-DLBCL, and is generally attributed to aberrant SHM. (2) BCL6 dependency is observed within the BCR-DLBCL population and may be the result of aberrant V(D)J rearrangement, SHM or other mechanisms. (3) NF-κB dependency is primarily observed within the ABCDLBCL population that is believed to be the result of NF-κB activating mutations along with BLIMP1 inhibition, thus preventing plasmacytic differentiation. (4) ALK dependency is very rare and the mechanism for acquiring the t(2;17) translocation generally observed within this population is poorly understood.
Acknowledgments
This work was supported in part by an R01AA017972 from the NIH (R.B.G.) and a Merit Review Award from the Department of Veterans Affairs (R.B.G.). We thank Dr. Martin Flajnik for helpful and insightful discussion. We apologize to those authors whose original articles could not be cited due to space constraints.
References
- 1.Abramson JS, Shipp MA. Advances in the biology and therapy of diffuse large B-cell lymphoma: moving toward a molecularly targeted approach. Blood. 2005;106:1164–74. doi: 10.1182/blood-2005-02-0687. [DOI] [PubMed] [Google Scholar]
- 2.Luce JK, Gamble JF, Wilson HE, Monto RW, Isaacs BL, Palmer RL, et al. Combined cyclophosphamide, vincristine and prednisone therapy of malignant lymphoma. Cancer. 1971;28:306–17. doi: 10.1002/1097-0142(197108)28:2<306::aid-cncr2820280208>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez V, Cabanillas F, Burgess MA, McKelvey EM, Valdivieso M, Bodey G, et al. Combination Chemotherapy (“CHOP-Bleo”) in Advanced (Non-Hodgkin) Malignant Lymphoma. Blood. 1977;49:325–33. [PubMed] [Google Scholar]
- 4.Carbone PP, Spurr C. Management of patients with malignant lymphoma: A comparative study with cyclophosphamide and vinca alkaloids. Cancer Res. 1968;28:811–22. [PubMed] [Google Scholar]
- 5.Carbone PP, Bono V, Frei E, III, Brindley CO. Clinical studies with vincristine. Blood. 1967;21:640–7. [PubMed] [Google Scholar]
- 6.Hall TC, Choi OS, Abadi A, Krant MJ. High-dose corticoid therapy in Hodgkin’s disease and other lymphomas. Ann Intern Med. 1967;66:1144–53. doi: 10.7326/0003-4819-66-6-1144. [DOI] [PubMed] [Google Scholar]
- 7.Coiffier B, Lepage E, Herbrecht R, Tilly H, Solal-Celigny P, Munck JN, et al. MabThera (rituximab) plus CHOP is superior to CHOP alone in elderly patients with diffuse large B-cell lymphoma (DLCL): interim results of a randomized GELA trial. Blood. 2000;96:223A–223A. [Google Scholar]
- 8.Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S, et al. Long-term outcome of patients in the LNH-98. 5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood. 2010;116:2040–5. doi: 10.1182/blood-2010-03-276246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinstein IB. Addiction to oncogenes – the Achilles heel of cancer. Science. 2002;297:63–4. doi: 10.1126/science.1073096. [DOI] [PubMed] [Google Scholar]
- 10.Soverini S, Colarossi S, Gnani A, Castagnetti F, Rosti G, Bosi C, et al. Resistance to dasatinib in Philadelphia-positive leukemia patients is mainly mediated by the presence or the selection of mutations at residues 315 and 317 in the Bcr-Abl kinase domain. Haematologica. 2007;92:401–4. doi: 10.3324/haematol.10822. [DOI] [PubMed] [Google Scholar]
- 11.Gorre ME, Mohammed M, Ellwood K, Hsu N, Paquette R, Rao PN, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293:876–80. doi: 10.1126/science.1062538. [DOI] [PubMed] [Google Scholar]
- 12.Nagar B, Bornmann WG, Pellicena P, Schindler T, Veach DR, Miller WT, et al. Crystal structures of the kinase domain of c-Abl in complex with the small molecule inhibitors PD173955 and imatinib (STI-571) Cancer Res. 2002;62:4236–43. [PubMed] [Google Scholar]
- 13.Singh A, Greninger P, Rhodes D, Koopman L, Violette S, Bardeesy N, et al. A Gene Expression Signature Associated with “K-Ras Addiction” Reveals Regulators of EMT and Tumor Cell Survival. Cancer Cell. 2009;15:489–500. doi: 10.1016/j.ccr.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaelin WG., Jr The concept of synthetic lethality in the context of anticancer therapy. Nat Rev Cancer. 2005;5:689–98. doi: 10.1038/nrc1691. [DOI] [PubMed] [Google Scholar]
- 15.Ngo VN, Davis RE, Lamy L, Yu X, Zhao H, Lenz G, et al. A loss-of-function RNA interference screen for molecular targets in cancer. Nature. 2006;441:106–10. doi: 10.1038/nature04687. [DOI] [PubMed] [Google Scholar]
- 16.Oettinger MA, Schatz DG, Gorka C, Baltimore D. RAG-1 and RAG-2, adjacent genes that synergistically activate V(D)J recombination. Science. 1990;248:1517–23. doi: 10.1126/science.2360047. [DOI] [PubMed] [Google Scholar]
- 17.Hozumi N, Tonegawa S. Evidence for somatic rearrangement of immunoglobulin genes coding for variable and constant regions. Proc Natl Acad Sci U S A. 1976;73:3628–32. doi: 10.1073/pnas.73.10.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Callén E, Jankovic M, Difilippantonio S, Daniel JA, Chen HT, Celeste A, et al. ATM prevents the persistence and propogation of chromosome breaks in lymphocytes. Cell. 2007;130:63–75. doi: 10.1016/j.cell.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 19.MacLennan IC. Germinal Centers. Annu Rev Immunol. 1994;12:117–39. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 20.Berek C, Berger A, Apel M. Maturation of the immune response in germinal centers. Cell. 1991;67:1121–9. doi: 10.1016/0092-8674(91)90289-b. [DOI] [PubMed] [Google Scholar]
- 21.Jacob J, Kelsoe G, Rajewsky K, Weiss U. Intraclonal generation of antibody mutants in germinal centres. Nature. 1991;354:389–92. doi: 10.1038/354389a0. [DOI] [PubMed] [Google Scholar]
- 22.Kim S, Davis M, Sinn E, Patten P, Hood L. Antibody diversity: somatic hypermutation of rearranged VH genes. Cell. 1981;27:573–81. doi: 10.1016/0092-8674(81)90399-8. [DOI] [PubMed] [Google Scholar]
- 23.Kataoka T, Kawakami T, Takahashi N, Honjo T. Rearrangement of immunoglobulin gamma 1-chain gene and mechanism for heavy-chain class switch. Proc Natl Acad Sci U S A. 1980;77:919–23. doi: 10.1073/pnas.77.2.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muramatsu M, Sankaranand VS, Anant S, Sugai M, Kinoshita K, Davidson NO, et al. Specific expression of activation-induced cytidine deaminase (AID), a novel member of the RNA-editing deaminase family in germinal center B cells. J Biol Chem. 1999;274:18470–6. doi: 10.1074/jbc.274.26.18470. [DOI] [PubMed] [Google Scholar]
- 25.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermuation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–63. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 26.Takizawa M, Tolarová H, Li Z, Dubois W, Lim S, Callen E, et al. AID expression levels determine the extent of cMyc oncogenic translocations and the incidence of B cell tumor development. J Exp Med. 2008;205:1949–57. doi: 10.1084/jem.20081007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pasqualucci L, Bhagat G, Jankovic M, Compagno M, Smith P, Muramatsu M, et al. AID is required for germinal center-derived lymphomagenesis. Nat Genet. 2008;40:108–12. doi: 10.1038/ng.2007.35. [DOI] [PubMed] [Google Scholar]
- 28.Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–11. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 29.Huang JZ, Sanger WG, Greiner TC, Staudt LM, Weisenburger DD, Pickering DL, et al. The t(14;18) defines a unique subset of diffuse large B-cell lymphoma with a germinal center B-cell gene expression profile. Blood. 2002;99:2285–90. doi: 10.1182/blood.v99.7.2285. [DOI] [PubMed] [Google Scholar]
- 30.Kikuchi A, Nakamura N, Kuze T, Sasaki Y, Abe M, Ohno H, et al. Characterization of de novo diffuse large B-cell lymphoma with a translocation of c-myc and immunoglobulin genes. Leuk Res. 2008;32:1176–82. doi: 10.1016/j.leukres.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 31.Liu YJ, Mason DY, Johnson GD, Abbot S, Gregory CD, Hardie DL, et al. Germinal center cells express bcl-2 protein after activation by signals which prevent their entry into apoptosis. Eur J Immunol. 1991;21:1905–10. doi: 10.1002/eji.1830210819. [DOI] [PubMed] [Google Scholar]
- 32.Shapiro-Shelef M, Lin KI, McHeyzer-Williams LJ, Liao J, McHeyzer-Williams MG, Calame K. Blimp-1 is required for the formation of immunoglobulin secreting plasma cells and pre-plasma memory B cells. Immunity. 2003;19:607–20. doi: 10.1016/s1074-7613(03)00267-x. [DOI] [PubMed] [Google Scholar]
- 33.Reimold AM, Iwakoshi NN, Manis J, Vallabhajosyula P, Szomolanyi-Tsuda E, Gravallese EM, et al. Plasma cell differentiation requires the transcription factor XBP-1. Nature. 2001;12:300–7. doi: 10.1038/35085509. [DOI] [PubMed] [Google Scholar]
- 34.Shaffer AL, Shapiro-Shelef M, Iwakoshi NN, Lee AH, Qian SB, Zhao H, et al. XBP1, downstream of BLIMP-1, expands the secretory apparatus and other organelles, and increases protein synthesis in plasma cell differentiation. Immunity. 2004;21:81–93. doi: 10.1016/j.immuni.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 35.Wright G, Tan B, Rosenwald A, Hurt EH, Wiestner A, Staudt LM. A gene expression-based method to diagnose clinically distinct subgroups of diffuse large B cell lymphoma. Proc Natl Acad Sci U S A. 2003;100:9991–6. doi: 10.1073/pnas.1732008100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis RE, Brown KD, Siebenlist U, Staudt LM. Constitutive nuclear factor kappaB activity is required for survival of activated B cell-like diffuse large B cell lymphoma cells. J Exp Med. 2001;194:1861–74. doi: 10.1084/jem.194.12.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pasqualucci L, Compagno M, Houldsworth J, Monti S, Grunn A, Nandula SV, et al. Inactivation of the PRDM1/BLIMP1 gene in diffuse large B cell lymphoma. J Exp Med. 2006;203:311–7. doi: 10.1084/jem.20052204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cattoretti G, Shaknovich R, Smith PM, Jäck HM, Murty VV, Alobeid B. Stages of germinal center transit are defined by B cell transcription factor coexpression and relative abundance. J Immunol. 2006;177:6930–9. doi: 10.4049/jimmunol.177.10.6930. [DOI] [PubMed] [Google Scholar]
- 39.Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;1:275–82. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 40.Dogan A, Bagdi E, Munson P, Isaacson PG. CD10 and BCL-6 expression in paraffin sections of normal lymphoid tissue and B-cell lymphomas. Am J Surg Pathol. 2000;24:846–52. doi: 10.1097/00000478-200006000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Falini B, Fizzotti M, Pucciarini A, Bigerna B, Marafioti T, Gambacorta M, et al. A monoclonal antibody (MUM1p) detects expression of the MUM1/IRF4 protein in a subset of germinal center B cells, plasma cells, and activated T cells. Blood. 2000;95:2084–92. [PubMed] [Google Scholar]
- 42.Jardin F, Sahota SS, Ruminy P, Parmentier F, Picquenot JM, Rainville V, et al. Novel Ig V gene features of t(14;18) and t(3;14) de novo diffuse large B-cell lymphoma displaying germinal center-B cell like and non-germinal center-B cell like markers. Leukemia. 2006;20:2070–4. doi: 10.1038/sj.leu.2404370. [DOI] [PubMed] [Google Scholar]
- 43.Choi WW, Weisenburger DD, Greiner TC, Piris MA, Banham AH, Delabie J, et al. A New Immunostain Algorithm Classifies Diffuse Large B-Cell Lymphoma into Molecular Subtypes with High Accuracy. Clin Cancer Res. 2009;15:5494–502. doi: 10.1158/1078-0432.CCR-09-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shaknovich R, Geng H, Johnson NA, Tsikitas L, Cerchietti L, Greally JM, et al. DNA methylation signatures define molecular subtypes of diffuse large B-cell lymphoma. Blood. 2010;116:e81–9. doi: 10.1182/blood-2010-05-285320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lenz G, Wright GW, Emre NC, Kohlhammer H, Dave SS, Davis RE, et al. Molecular subtypes of diffuse large B-cell lymphoma arise by distinct genetic pathways. Proc Natl Acad Sci U S A. 2008;105:13520–5. doi: 10.1073/pnas.0804295105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Monti S, Savage KJ, Kutok JL, Feuerhake F, Kurtin P, Mihm M, et al. Molecular profiling of diffuse large B-cell lymphoma identifies robust subtypes including one characterized by host inflammatory response. Blood. 2004;105:1851–61. doi: 10.1182/blood-2004-07-2947. [DOI] [PubMed] [Google Scholar]
- 47.Küppers R, Dalla-Favera R. Mechanisms of chromosomal translocations in B cell lymphomas. Oncogene. 2001;20:5580–94. doi: 10.1038/sj.onc.1204640. [DOI] [PubMed] [Google Scholar]
- 48.Pasqualucci L, Breschenko O, Niu H, Klein U, Basso K, Guglielmino R, et al. Molecular pathogenesis of non-Hodgkin’s lymphoma: the role of Bcl-6. Leuk Lymphoma. 2003;44:S5–12. doi: 10.1080/10428190310001621588. [DOI] [PubMed] [Google Scholar]
- 49.Allman D, Jain A, Dent A, Maile RR, Selvaggi T, Kehry MR, et al. BCL6 expression during B-cell activation. Blood. 1996;87:5257–68. [PubMed] [Google Scholar]
- 50.Parekh S, Polo JM, Shaknovich R, Juszcynski P, Ranucolo SM, Yin Y, et al. BCL6 programs lymphoma cells for survival and differentiation through distinct biochemical mechanisms. Blood. 2007;6:2067–74. doi: 10.1182/blood-2007-01-069575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ahmad KF, Melnick A, Lax S, Bouchard D, Liu J, Kiang CL, et al. Mechanism of SMRT corepressor recruitment by the BCL6 BTB domain. Mol Cell. 2003;12:1551–64. doi: 10.1016/s1097-2765(03)00454-4. [DOI] [PubMed] [Google Scholar]
- 52.Ranuncolo SM, Polo JM, Dierov J, Singer M, Kuo T, Greally J, et al. Bcl-6 mediates the germinal center B cell phenotype and lymphomagenesis through transcriptional repression of the DNA-damage sensor ATR. Nature Immunology. 2007;8:705–14. doi: 10.1038/ni1478. [DOI] [PubMed] [Google Scholar]
- 53.Phan RT, Dalla-Favera R. The BCL6 proto-oncogene suppresses p53 expression in germinal-center B cells. Nature. 2004;432:635–9. doi: 10.1038/nature03147. [DOI] [PubMed] [Google Scholar]
- 54.Fujita N, Jaye DL, Geigerman C, Akyildiz A, Mooney MR, Boss JM, et al. MTA3 and Mi-2/NuRD Complex Regulate Cell Fate During B-Lymphocyte Differentiation. Cell. 2004;119:75–86. doi: 10.1016/j.cell.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 55.Saito M, Gao J, Basso K, Kitagawa Y, Smith PM, Bhagat G, et al. A signaling pathway mediating downregulation of BCL6 in germinal center B cells is blocked by BCL6 gene alterations in B cell lymphoma. Cancer Cell. 2007;12:280–92. doi: 10.1016/j.ccr.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 56.Ci W, Polo JM, Cerchietti L, Shaknovich R, Wang L, Yang SN, et al. The BCL6 transcriptional program features repression of multiple oncogenes in primary B cells and is deregulated in DLBCL. Blood. 2009;113:5536–48. doi: 10.1182/blood-2008-12-193037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Basso K, Dalla-Favera R. BCL6: Master Regulator of the Germinal Center Reaction and Key Oncogene in B Cell Lymphomagenesis. Adv Immunol. 2010;105:193–210. doi: 10.1016/S0065-2776(10)05007-8. [DOI] [PubMed] [Google Scholar]
- 58.Polo JM, Juszczynski P, Monti S, Cerchietti L, Ye K, Greally JM, et al. Transcriptional signature with differential expression of BCL6 target genes accurately identifies BCL6-dependent diffuse large B cell lymphomas. Proc Natl Acad Sci U S A. 2007;104:3207–12. doi: 10.1073/pnas.0611399104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Polo JM, Dell’Oso T, Ranuncolo SM, Cerchietti L, Beck D, Da Silva GF, et al. Specific peptide interference reveals BCL6 transcriptional and oncogenic mechanisms in B-cell lymphoma cells. Nat Med. 2004;10:1329–35. doi: 10.1038/nm1134. [DOI] [PubMed] [Google Scholar]
- 60.Toney LM, Cattoretti G, Graf JA, Merghoub T, Pandolfi PP, Dalla-Favera R, et al. BCL-6 regulates chemokine gene transcription in macrophages. Nature Immunology. 2007;1:214–20. doi: 10.1038/79749. [DOI] [PubMed] [Google Scholar]
- 61.Cerchietti LC, Hatzi K, Caldas-Lopes E, Yang SN, Figueroa ME, Morin RD, et al. BCL6 repression of EP300 in human diffuse large B cell lymphoma cells provides a basis for rational combinatorial therapy. J Clin Invest. 2010;120:4569–82. doi: 10.1172/JCI42869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chattopadhyay A, Tate SA, Beswick RW, Wagner SD, Ko Ferrigno P. A peptide aptamer to antagonize BCL-6 function. Oncogene. 2006;25:2223–33. doi: 10.1038/sj.onc.1209252. [DOI] [PubMed] [Google Scholar]
- 63.Parekh S, Prive GG, Melnick A. Therapeutic targeting of the BCL6 oncogene for diffuse large B-cell lymphomas. Leuk Lymphoma. 2008;49:874–82. doi: 10.1080/10428190801895345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bakhshi A, Jensen JP, Goldman P, Wright JJ, McBride OW, Epstein AL, et al. Cloning the chromosomal breakpoint of t(14;18) human lymphomas: clustering around JH on chromosome 14 and near a transcriptional unit on 18. Cell. 1985;41:899–906. doi: 10.1016/s0092-8674(85)80070-2. [DOI] [PubMed] [Google Scholar]
- 65.Cleary ML, Sklar J. Nucleotide sequence of a t(14;18) chromosomal breakpoint in follicular lymphoma and demonstration of a breakpoint-cluster region near a transcriptionally active locus on chromosome 18. Proc Natl Acad Sci U S A. 1985;82:7439–43. doi: 10.1073/pnas.82.21.7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tsujimoto Y, Cossman J, Jaffe E, Croce CM. Involvement of the bcl-2 gene in human follicular lymphoma. Science. 1985;228:1440–3. doi: 10.1126/science.3874430. [DOI] [PubMed] [Google Scholar]
- 67.Iqbal J, Sanger WG, Horsman DE, Rosenwald A, Pickering DL, Dave B, et al. BCL2 translocation defines a unique tumor subset within the germinal center B-cell-like diffuse large B-cell lymphoma. Am J Pathol. 2004;165:159–66. doi: 10.1016/s0002-9440(10)63284-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shaffer AL, Rosenwald A, Staudt LM. Decision making in the immune system: Lymphoid Malignancies: the dark side of B-cell differentiation. Nature Reviews Immunology. 2002;2:920–32. doi: 10.1038/nri953. [DOI] [PubMed] [Google Scholar]
- 69.Hockenbery D, Nuñez G, Milliman C, Schreiber RD, Korsmeyer SJ. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990;348:334–6. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- 70.Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15:2922–33. [PubMed] [Google Scholar]
- 71.Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–19. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 72.Yang E, Zha J, Jockel J, Boise LH, Thompson CB, Korsmeyer SJ. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995;80:285–91. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]
- 73.Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–57. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 74.Zou H, Henzel WJ, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase 3. Cell. 1997;90:405–13. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]
- 75.Wang K, Yin XM, Chao DT, Milliman CL, Korsmeyer SJ. BID: a novel BH3 domain-only death agonist. Genes Dev. 1996;10:2859–69. doi: 10.1101/gad.10.22.2859. [DOI] [PubMed] [Google Scholar]
- 76.Yu J, Zhang L, Hwang PM, Kinzler KW, Vogelstein B. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol Cell. 2001;7:673–82. doi: 10.1016/s1097-2765(01)00213-1. [DOI] [PubMed] [Google Scholar]
- 77.Oda E, Ohki R, Murasawa H, Nemoto J, Shibue T, Yamashita T, et al. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science. 2000;288:1053–8. doi: 10.1126/science.288.5468.1053. [DOI] [PubMed] [Google Scholar]
- 78.Zong WX, Lindsten T, Ross AJ, MacGregor GR, Thompson CB. BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes Dev. 2001;15:1481–6. doi: 10.1101/gad.897601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, et al. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX-and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001;8:705–11. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- 80.Cheng EH, Levine B, Boise LH, Thompson CB, Hardwick JM. Bax-Independent inhibition of apoptosis by Bcl-XL. Nature. 1996;379:554–6. doi: 10.1038/379554a0. [DOI] [PubMed] [Google Scholar]
- 81.Kelekar A, Chang BS, Harlan JE, Fesik SW, Thompson CB. Bad is a BH3 domain-containing protein that forms an inactivating dimer with Bcl-XL. Mol Cell Biol. 1997;17:7040–6. doi: 10.1128/mcb.17.12.7040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kelekar A, Thompson CB. Bcl-2-family proteins: the role of the BH3 domain in apoptosis. Trends Cell Biol. 1998;8:324–30. doi: 10.1016/s0962-8924(98)01321-x. [DOI] [PubMed] [Google Scholar]
- 83.Sattler M, Liang H, Nettesheim D, Meadows RP, Harlan JE, Erbstadt, et al. Structure of Bcl-XL-Bak peptide complex: recognition between regulators of apoptosis. Science. 1997;275:983–6. doi: 10.1126/science.275.5302.983. [DOI] [PubMed] [Google Scholar]
- 84.Saito M, Novak U, Piovan E, Basso K, Sumazin P, Schneider C, et al. BCL6 suppression of BCL2 via Miz1 and its disruption in diffuse large B cell lymphoma. Proc Natl Acad Sci U S A. 2009;27:11294–9. doi: 10.1073/pnas.0903854106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Viatour P, Bentires-Alj M, Chariot A, Deregowski V, de Leval L, Merville MP, et al. NF-kappa B2/p100 induces Bcl-2 expression. Leukemia. 2003;17:1349–56. doi: 10.1038/sj.leu.2402982. [DOI] [PubMed] [Google Scholar]
- 86.Pro B, Leber B, Smith M, Fayad L, Romaguera J, Hagemeister F, et al. Phase II multicenter study of oblimersen sodium, a Bcl-2 antisense oligonucleotide, in combination with rituximab in patients with recurrent B-cell non-Hodgkin lymphoma. Br J Haematol. 2008;143:355–60. doi: 10.1111/j.1365-2141.2008.07353.x. [DOI] [PubMed] [Google Scholar]
- 87.Wilson W, O’Connor O, Roberts AW, Czuczman M, Brown J, Xiong H, et al. ABT-263 activity and safety in patients with relapsed or refractory lymphoid malignancies in particular chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) J Clin Oncol. 2009;27:15S. [Google Scholar]
- 88.Yecies D, Carlson NE, Deng J, Letai A. Acquired resistance to ABT-737 in lymphoma cells that up-regulate MCL-1 and BFL-1. Blood. 2010;115:3304–13. doi: 10.1182/blood-2009-07-233304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Deng J, Carlson N, Taleyama K, Cin PD, Shipp MA, Letai A. BH3 Profiling Identifies Three Distinct Classes of Apoptotic Blocks to Predict Response to ABT-737 and Conventional Chemotherapeutic Agents. Cancer Cell. 2007;12:171–85. doi: 10.1016/j.ccr.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 90.Gilmore TD. Multiple mutations contribute to the oncogenicity of the retroviral oncoprotein v-Rel. Oncogene. 1999;18:6925–37. doi: 10.1038/sj.onc.1203222. [DOI] [PubMed] [Google Scholar]
- 91.Thomas RK, Wickenhauser C, Tawadros S, Diehl V, Küppers R, Wolf J, et al. Mutational analysis of the IkappaBalpha gene in activated B cell-like diffuse large B-cell lymphoma. Br J Haematol. 2004;126:50–4. doi: 10.1111/j.1365-2141.2004.05000.x. [DOI] [PubMed] [Google Scholar]
- 92.Fan CM, Maniatis T. Generation of p50 subunit of NF-kappa B by processing of p105 through an ATP-dependent pathway. Nature. 1991;354:395–8. doi: 10.1038/354395a0. [DOI] [PubMed] [Google Scholar]
- 93.Mercurio F, DiDonato J, Rosette C, Karin M. Molecular cloning and characterization of a novel Rel/NF-kappa B family member displaying structural and functional homology to NF-kappa B p50/p105. DNA Cell Biol. 1992;11:523–37. doi: 10.1089/dna.1992.11.523. [DOI] [PubMed] [Google Scholar]
- 94.Baeuerle PA, Baltimore D. A 65-kappaD subunit of active NF-kappaB is required for inhibition of NF-kappaB by I kappaB. Cell. 1989;3:1689–98. doi: 10.1101/gad.3.11.1689. [DOI] [PubMed] [Google Scholar]
- 95.Ryseck RP, Bull P, Takamiya M, Bours V, Siebenlist U, Dobrzanski P, et al. RelB, a new Rel family transcription activator that can interact with p50-NF-kappa B. Mol Cell Biol. 1992;12:674–84. doi: 10.1128/mcb.12.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bull P, Morley KL, Hoekstra MF, Hunter T, Verma IM. The mouse c-rel protein has an N-terminal regulatory domain and a C-terminal transcriptional transactivation domain. Mol Cell Biol. 1990;10:5473–85. doi: 10.1128/mcb.10.10.5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Baeuerle PA, Baltimore D. I kappa B: a specific inhibitor of the NF-kappa B transcription factor. Science. 1988;242:540–6. doi: 10.1126/science.3140380. [DOI] [PubMed] [Google Scholar]
- 98.Régnier CH, Song HY, Gao X, Goeddel DV, Cao Z, Rothe M. Identification and characterization of an IkappB kinase. Cell. 1997;90:373–83. doi: 10.1016/s0092-8674(00)80344-x. [DOI] [PubMed] [Google Scholar]
- 99.Gerondakis S, Grumont R, Rourke I, Grossmann M. The regulation and roles of Rel/NF-kappa B transcription factors during lymphocyte activation. Curr Opin Immunol. 1998;10:353–9. doi: 10.1016/s0952-7915(98)80175-1. [DOI] [PubMed] [Google Scholar]
- 100.Grossmann M, Nakamura Y, Grumont R, Gerondakis S. New insights into the roles of ReL/NF-kappa B transcription factors in immune function, hemopoiesis and human disease. Int J Biochem Cell Biol. 1999;31:1209–19. doi: 10.1016/s1357-2725(99)00068-0. [DOI] [PubMed] [Google Scholar]
- 101.Gugasyan R, Grumont R, Grossmann M, Nakamura Y, Pohl T, Nesic D, et al. Rel/NF-kappaB transcription factors: key mediators of B-cell activation. Immunol Rev. 2000;176:134–40. doi: 10.1034/j.1600-065x.2000.00615.x. [DOI] [PubMed] [Google Scholar]
- 102.Monroe JG. ITAM-mediated tonic signalling through pre-BCR and BCR complexes. Nat Rev Immunol. 2006;6:283–94. doi: 10.1038/nri1808. [DOI] [PubMed] [Google Scholar]
- 103.Sommer K, Guo B, Pomerantz JL, Bandaranayake AD, Moreno-Garcia ME, Ovechkina YL, et al. Phosphorylation of the CARMA1 linker controls NF-kappaB activation. Immunity. 2005;23:561–74. doi: 10.1016/j.immuni.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 104.Janssens S, Beyaert R. Functional diversity and regulation of different interleukin-1 receptor-associated kinase (IRAK) family members. Mol Cell. 2003;11:293–302. doi: 10.1016/s1097-2765(03)00053-4. [DOI] [PubMed] [Google Scholar]
- 105.Fujihara M, Muroi M, Tanamoto K, Suzuki T, Azuma H, Ikeda H. Molecular mechanisms of macrophage activation and deactivation by lipopolysaccharide: roles of the receptor complex. Pharmacol Ther. 2003;100:171–94. doi: 10.1016/j.pharmthera.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 106.Li S, Strelow A, Fontana EJ, Wesche H. IRAK-4: a novel member of the IRAK family with the properties of an IRAK-kinase. Proc Natl Acad Sci U S A. 2002;99:5567–72. doi: 10.1073/pnas.082100399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cao Z, Xiong J, Takeuchi M, Kurama T, Goeddel DV. TRAF6 is a signal transducer for interleukin-1. Nature. 1996;383:443–6. doi: 10.1038/383443a0. [DOI] [PubMed] [Google Scholar]
- 108.Lenz G, Davis RE, Ngo VN, Lam L, George TC, Wright GW, et al. Oncogenic CARD11 mutations in human diffuse large B cell lymphoma. Science. 2008;319:1676–9. doi: 10.1126/science.1153629. [DOI] [PubMed] [Google Scholar]
- 109.Davis RE, Ngo VN, Lenz G, Tolar P, Young RM, Romesser PB, et al. Chronic activate B-cell-receptor signaling in diffuse large B-cell lymphoma. Nature. 2010;463:88–92. doi: 10.1038/nature08638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Houldsworth JS, Mathew PH, Rao K, Dyomina DC, Louie N, Parsa K, et al. REL protooncogene is frequently amplified in extranodal diffuse large cell lymphoma. Blood. 1996;87:25–29. [PubMed] [Google Scholar]
- 111.Jardin F, Ruminy P, Kerckaert JP, Parmentier F, Picquenot JM, Quief S, et al. Detection of somatic quantitative genetic alterations by multiplex polymerase chain reaction for the prediction of outcome in diffuse large B-cell lymphomas. Haematologica. 2008;93:543–50. doi: 10.3324/haematol.12251. [DOI] [PubMed] [Google Scholar]
- 112.Compagno M, Lim WK, Grunn A, Nandula SV, Brahmachary M, Shen Q, et al. Mutations of multiple genes cause deregulation of NF-kappaB in diffuse large B-cell lymphoma. Nature. 2009;459:717–21. doi: 10.1038/nature07968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kato M, Sanada M, Kato I, Sato Y, Takita J, Takeuchi K, et al. Frequent inactivation of A20 in B-cell lymphomas. Nature. 2009;459:712–6. doi: 10.1038/nature07969. [DOI] [PubMed] [Google Scholar]
- 114.Ngo VN, Young RM, Schmitz R, Jhavar S, Xiao W, Lim KH, et al. Oncogenically active MYD88 mutations in human lymphoma. Nature. 2011;470:115–9. doi: 10.1038/nature09671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lam LT, Davis RE, Pierce J, Hepperle M, Xu Y, Hottelet M, et al. Small molecule inhibitors of IkappaBkinase are selectively toxic for subgroups of diffuse large B-cell lymphoma defined by gene expression profiling. Clin Cancer Res. 2005;1:28–40. [PubMed] [Google Scholar]
- 116.Chen G, Cao P, Goeddel DV. TNF-induced recruitment and activation of the IKK complex require Cdc37 and Hsp90. Mol Cell. 2002;2:9401–10. doi: 10.1016/s1097-2765(02)00450-1. [DOI] [PubMed] [Google Scholar]
- 117.Dunleavy K, Pittaluga S, Czuczman MS, Dave SS, Wright G, Grant N, et al. Differential efficacy of bortezomib plus chemotherapy within molecular subtypes of diffuse large B-cell lymphoma. Blood. 2009;113:6069–79. doi: 10.1182/blood-2009-01-199679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Delsol G, Lamant L, Mariamé B, Pulford K, Dastugue N, Brousset P, et al. A new subtype of large B-cell lymphoma expressing the ALK kinase and lacking the 2;5 translocation. Blood. 1997;89:1483–90. [PubMed] [Google Scholar]
- 119.Li K, Tipps A, Wang H. Anaplastic lymphoma kinase-positive diffuse large B-cell lymphoma presenting as an isolated nasopharyngeal mass: a case report and review of literature. Int J Clin Exp Pathol. 2010;4:190–6. [PMC free article] [PubMed] [Google Scholar]
- 120.Laurent C, Do C, Gascoyne R, Lamant L, Ysebaert L, Laurent G, et al. Anaplastic Lymphoma Kinase-Positive Diffuse Large B-Cell Lymphoma: A Rare Clinicopathologic Entity with Poor Prognosis. J Clin Oncol. 2009;27:4211–6. doi: 10.1200/JCO.2008.21.5020. [DOI] [PubMed] [Google Scholar]
- 121.De Paepe P, Baens M, van Krieken H, Verhasselt B, Stul M, Simons A, et al. ALK activation by the CLTC-ALK-Clathrin fusion is a recurrent event in large B-cell lymphoma. Blood. 2003;102:2638–41. doi: 10.1182/blood-2003-04-1050. [DOI] [PubMed] [Google Scholar]
- 122.Gascoyne RD, Lamant L, Martin-Subero JI, Lestou VS, Harris NL, Müller-Hermelink HK, et al. ALK-positive diffuse large B-cell lymphoma is associated with Clathrin-ALK rearrangements: report of 6 cases. Blood. 2003;102:2568–73. doi: 10.1182/blood-2003-03-0786. [DOI] [PubMed] [Google Scholar]
- 123.Cerchietti L, Damm-Welk C, Vater I, Klapper W, Harder L, Pott C, et al. Inhibition of Anaplastic Lymphoma Kinase (ALK) Activity Provides a Therapeutic Approach for CLTC-ALK-Positive Human Diffuse Large B Cell Lymphomas. PLoS One. 2011;6:e18436. doi: 10.1371/journal.pone.0018436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kasprzycka M, Marzec M, Liu X, Zhang Q, Wasik MA. Nucleophosmin/anaplastic lymphoma kinase (NPM/ALK) oncoprotein induces the T regulatory cell phenotype by activating STAT3. Proc Natl Acad Sci U S A. 2006;103:9964–9. doi: 10.1073/pnas.0603507103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chiarle R, Simmons WJ, Cai H, Dhall G, Zamo A, Raz R, et al. Stat3 is required for ALK-mediated lymphomagenesis and provides a possible therapeutic target. Nat Med. 2005;11:623– 9. doi: 10.1038/nm1249. [DOI] [PubMed] [Google Scholar]
- 126.Bai RY, Ouyang T, Miething C, Morris SW, Peschel C, Duyster J. Nucleophosmin-anaplastic lymphoma kinase associated with anaplastic large-cell lymphoma activates the phosphatidylinositol 3-kinase/Akt antiapoptotic signaling pathway. Blood. 2000;96:4319–27. [PubMed] [Google Scholar]
- 127.Naylor TL, Tang H, Ratsch BA, Enns A, Loo A, Chen L, et al. Protein Kinase C Inhibitor Sotrastaurin Selectively Inhibits the Growth of CD79 Mutant Diffuse Large B-Cell Lymphomas. Cancer Res. 2011;71:2643–53. doi: 10.1158/0008-5472.CAN-10-2525. [DOI] [PubMed] [Google Scholar]
- 128.Pasqualucci L, Trifonov V, Fabbri G, Ma J, Rossi D, Chiarenza A, et al. Analysis of the coding genome of diffuse large B-cell lymphoma. Nat Genet. 2011;43:830–7. doi: 10.1038/ng.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Morin RD, Mendez-Lago M, Mungall AJ, Goya R, Mungall K, Corbett RD, et al. Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma. Nature. 2011;476:298–303. doi: 10.1038/nature10351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pasqualucci L, Dominguez-Sola D, Chiarenza A, Fabbri G, Grunn A, Trifonov V, et al. Inactivating mutations of acetyltransferase genes in B-cell lymphoma. Nature. 2011;471:189–95. doi: 10.1038/nature09730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bereshchencko OR, Gu W, Dalla-Favera R. Acetylation inactivates the transcriptional repressor BCL6. Nat Genet. 2002;32:606–13. doi: 10.1038/ng1018. [DOI] [PubMed] [Google Scholar]
- 132.Challa-Malladi M, Lieu YK, Califano O, Holmes AB, Bhagat G, Murty VV, et al. Combined Genetic Inactivation of β2-Microglobulin and CD58 Reveals Frequent Escape from Immune Recognition in Diffuse Large B Cell Lymphoma. Cancer Cell. 2011;20:728–40. doi: 10.1016/j.ccr.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sanchez-Madrid F, Krensky AM, Ware CF, Robbins E, Strominger JL, Burakoff SJ, et al. Three distinct antigens associated with human T-lymphocyte-mediated cytolysis: LFA-1, LFA-2, and LFA-3. Proc Natl Acad Sci U S A. 1982;79:7489–93. doi: 10.1073/pnas.79.23.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Selvaraj P, Plunkett ML, Dustin M, Sanders ME, Shaw S, Springer TA. The T lymphocyte glycoprotein CD2 binds the cell surface ligand LFA-3. Nature. 1987;326:400–3. doi: 10.1038/326400a0. [DOI] [PubMed] [Google Scholar]
- 135.Poulik MD, Reisfeld RA. Beta2-Microglobulins. Contemp Top Mol Immunol. 1975;4:157–204. [PubMed] [Google Scholar]
- 136.Li C, Kim S, Rai D, Bolla AR, Adhvaryu S, Kinney MC, et al. Copy number abnormalities, MYC activity, and the genetic fingerprint of normal B cells mechanistically define the microRNA profile off diffuse large B-cell lymphoma. Blood. 2009;113:6681–90. doi: 10.1182/blood-2009-01-202028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cho WC. OncomiRs: the discovery and progress of microRNAs in cancers. Mol Cancer. 2007;6:60. doi: 10.1186/1476-4598-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Medina P, Nolde M, Slack F. OncomiR addiction in an in vivo model of microRNA-21-induced pre-B-cell lymphoma. Nature. 2010;467:86–90. doi: 10.1038/nature09284. [DOI] [PubMed] [Google Scholar]
- 139.Rai D, Karanti S, Jung I, Dahia P, Aguiar R. Coordinated Expression of MicroRNA-155 and Predicted Target Genes in Diffuse Large B-cell Lymphoma. Cancer Genet Cytogenet. 2009;181:8–15. doi: 10.1016/j.cancergencyto.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lowery AJ, Miller N, McNeill RE, Kerin MJ. MicroRNAs as prognostic indicators and therapeutic targets: potential effect on breast cancer management. Clin Cancer Res. 2008;14:360–5. doi: 10.1158/1078-0432.CCR-07-0992. [DOI] [PubMed] [Google Scholar]