Abstract
The mechanistic target of rapamycin (mTOR) kinase controls growth and metabolism, and its deregulation underlies the pathogenesis of many diseases, including cancer, neurodegeneration, and diabetes. mTOR complex 1 (mTORC1) integrates signals arising from nutrients, energy, and growth factors, but how exactly these signals are propagated await to be fully understood. Recent findings have placed the lysosome, a key mediator of cellular catabolism, at the core of mTORC1 regulation by amino acids. A multiprotein complex that includes the Rag GTPases, Ragulator, and the v-ATPase forms an amino acid-sensing machinery on the lysosomal surface that affects the decision between cell growth and catabolism at multiple levels. The involvement of a catabolic organelle in growth signaling may have important implications for our understanding of mTORC1-related pathologies.
mTOR in growth control
Cell growth, defined as the increase in cellular mass, is central to life in both unicellular and multicellular organisms. At the single cell level, it precedes and allows proliferation, and is important for building energy stores. At the level of a whole organism, it is critical for development and for the complex coordination of whole body homeostasis. Cells and organisms grow by executing a series of anabolic processes that include protein synthesis, lipid synthesis, organelle biogenesis, and DNA replication. Conversely, under certain conditions such as starvation and stress, cells trigger degradative processes that allow them to obtain energy at the expense of consuming their internal stores [1]. Logically, to avoid futile cycles of synthesis and catabolism, these processes are controlled and tightly coordinated. Growth poses intensive demands for energy and basic building blocks, both of which are provided by amino acids, glucose, and other carbon sources, and cells have evolved mechanisms to ensure that growth is triggered only when these basic nutrients are plentiful. When growth becomes uncoupled from appropriate signals of nutrient status, it can drive progression of multiple pathological processes (Box 1), including cancer, type 2 diabetes, and neurodegeneration. Cancer is characterized by the unrestrained growth and proliferation of cells under suboptimal nutrient and environmental conditions. In type 2 diabetes, aberrant growth signals derange the ability of the body to respond to nutrients as well as to use and store energy. Finally, chronic impairment of cellular clearance may be the driving force behind aging and neurodegenerative diseases.
Box 1. Aberrant cell growth in diabetes and cancer.
Cellular processes that drive growth are subjected to tight regulation by converging inputs from mTOR complexes 1 and 2, together with numerous other signaling pathways. This tight regulation ensures that growth and division are triggered not only upon favorable local conditions of energy and nutrient availability but also as required by the nutritional state of the organism as a whole. Hence, it is not surprising that deregulated cell growth downstream of mTOR activity can derange cellular homeostasis in ways that impact the entire body. In particular, aberrant mTORC1 activation in different tissues may underlie the pathogenesis of type 2 diabetes (T2D).
T2D is a chronic disease that is generally hastened by long-term overfeeding and insulin resistance, two conditions related to excess mTORC1 activation. Overfeeding causes abnormally high levels of glucose and amino acids in the blood [71], which trigger insulin release by the pancreas. In turn, chronically high nutrients and insulin lead to sustained mTORC1 activation, which desensitizes the cell to insulin through a series of inhibitory loops converging onto the insulin receptor [72–74]. Thus, mTORC1 worsens the metabolic derangements driven by overfeeding in almost every metabolic tissue. In the liver, it contributes to excess gluconeogenesis and glucose export to the bloodstream [72]. In skeletal muscle, glucose import is suppressed and skeletal muscle waste ensues as a consequence (reviewed in [75]). In white adipose tissue, excess mTOR activity increases lipid synthesis and fat storage (reviewed in [76]). Collectively, the disparate tissue-specific alterations in mTORC1 signaling synergize to hasten the onset of T2D.
The same anabolic processes through which mTOR promotes the growth of normal cells also fuel the abnormal behavior and proliferation of cancer cells. As part of mTORC1, mTOR activity drives translation of a subset of genes that activate the cell division programs and block the induction of programmed cell death [77,78]. As mentioned previously, mTORC1 is also involved in lipid synthesis, a key process for the rapid growth and proliferation of cancer cells. Furthermore, aberrant mTORC1 potently suppresses autophagy, which may play a role in tumor suppression [62,63,79]. These observations have motivated the design of mTOR inhibitors for therapy. The naturally occurring mTORC1 allosteric inhibitor rapamycin, which blocks mTORC1 activity towards some targets while sparing others [80,81], is an FDA-approved drug for treating renal cell carcinoma and other malignancies. Recent evidence also suggests that aberrant mTORC2 activity may contribute to tumor formation [82], and several efforts were undertaken to generate ATP-competitive inhibitors able to block all mTOR-related activity [81,83–86], with most yielding promising results in preclinical trials. However, further efforts are required to prove the efficacy of these compounds in human malignancies.
The large protein kinase mechanistic target of rapamycin (mTOR) (previously referred to as mammalian target of rapamycin) (see Glossary) plays a key role in coupling cell growth with the nutritional status of the cell. mTOR is a serine–threonine kinase that belongs to the superfamily of phosphatidylinositol-3 kinase related-kinases (PI3KK). The mTOR kinase nucleates two distinct core complexes that have different kinase specificity and distinct protein partners (reviewed in [2]). mTOR complex 1 (mTORC1) contains regulatory associated protein of mTOR (raptor), mTOR associated protein LST8 homolog (mLST8, also known as GβL) and DEP domain containing mTOR-interacting protein (Deptor). The second complex, mTORC2, is defined by association with RPTOR-independent companion of mTOR (rictor), Sin1, GβL, and Deptor.
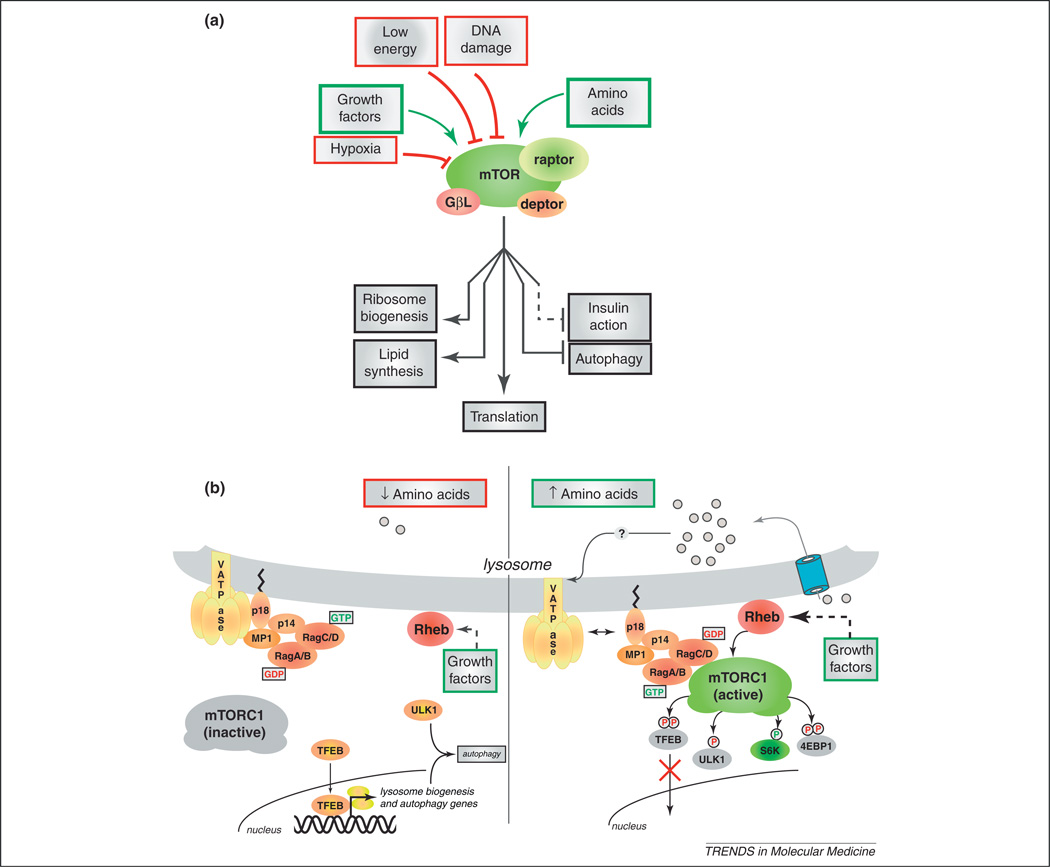
In this review, we focus on mTORC1 due to its prominent role as a driver of cellular and organismal growth, both in normal and disease states. mTORC1 functions as a signal integrator, combining regulatory inputs from nutrients, growth factors, energy levels, and stress signals (Figure 1a). Thus, in multicellular organisms, mTORC1 not only detects nutrients present inside the cell and in the cell surroundings, but it also senses long-range hormonal or growth factor signals that communicate the nutritional status of the organism as a whole.
Figure 1.
(a) Inputs and outputs of mechanistic target of rapamycin complex 1 (mTORC1) signaling. mTORC1 is formed by mTOR (target of rapamycin), raptor (regulatory associated protein of mTOR), GβL (mTOR associated protein LST8 homolog), and Deptor (DEP domain containing mTOR-interacting protein). mTORC1 integrates positive growth signals arising from amino acids and growth factors with inhibitory signals from hypoxia, low energy, and DNA damage. Upon activation, mTORC1 promotes several cellular anabolic processes, such as mRNA translation and ribosome biogenesis, lipid synthesis, whereas it blocks autophagy and other catabolic processes. mTORC1 activation also unleashes a negative feedback loop to the insulin receptor, which tends to dampen insulin/PI3K (phosphatidylinositol 3-kinase) signaling with profound physiological consequences. (b) mTORC1 and the lysosomal surface. Amino acids regulate the recruitment of mTORC1 to the lysosomal surface, where mTORC1 is activated. Under low amino acids (left) the v-ATPase (vacuolar H+-ATPase)–Ragulator (LAMTOR1–3)–Rag GTPase complex is in the inactive conformation and is unable to bind to mTORC1, resulting in its cytoplasmic localization. Amino acids (right), acting at least in part via a lysosomal ‘inside-out’ mechanism, signal to the v-ATPase–Ragulator complex and through them to the Rag GTPases, which switch their nucleotide loading and become activated. In turn, active Rag GTPases recruit mTORC1 to the lysosomal surface, where the small GTPase Rheb (Ras homolog enriched in brain) turns on the kinase activity of mTORC1. Active mTORC1 phosphorylates several targets, including S6K, 4E-BP1, the autophagy regulator ULK1 and the transcription factor TFEB. Phosphorylated S6K and 4E-BP1 favor protein synthesis; phosphorylation of ULK1 blocks autophagosome formation, whereas phosphorylation of TFEB prevents it from entering the nucleus and activating a catabolic transcriptional program.
mTORC1 integrates multiple inputs
The pathway that connects growth factors to mTORC1 has been extensively characterized and is activated when insulin and other ligands bind their tyrosine kinase receptors at the plasma membrane. Receptor activation leads to the activation of phosphatidylinositol 3-kinase (PI3K) type I, which generates the lipid second messenger PI(3,4,5)P3 and leads to activation of the Akt/PKB protein kinase. Among other targets, Akt phosphorylates and inhibits two distinct substrates that suppress mTORC1 activity. One is the tuberous sclerosis complex protein 2 (TSC2, or tuberin), which heterodimerizes with TSC1 and acts as a GTPase-activating protein for the small GTPase Ras homolog enriched in brain (Rheb) (reviewed in [3]). The key function of the Rheb GTPase is to bind mTORC1 and promote its kinase activity; thus, by blocking TSC, Akt drives mTORC1 activity. Akt also phosphorylates proline-rich Akt substrate 40 kDa (PRAS40) [4–7], an inhibitor of mTORC1 that binds to raptor and prevents mTORC1 activation by Rheb. Phosphorylation by Akt prevents PRAS40 from binding to mTORC1, thus enhancing the activity of the complex.
mTORC1 kinase activity is further regulated by additional, mostly inhibitory, inputs arising from disparate forms of stress, which converge on the TSC complex. The AMP-activated protein kinase (AMPK) is allosterically activated by the high AMP and ADP levels that occur under low energetic states [8,9]. Among its many targets, AMPK directly phosphorylates TSC2 [10,11]; but in contrast to Akt, AMPK-dependent phosphorylation of TSC2 stimulates the GTPase activity of TSC, inhibiting Rheb. In parallel, AMPK phosphorylates Raptor [12], leading to direct inhibition of mTORC1, possibly through structural destabilization of the complex.
In addition to energetic status, DNA integrity also affects mTORC1 activity. The DNA damage response, a signal cascade that is initiated by the detection of DNA double-strand breaks and other genetic insults, culminates in activation of the tumor suppressor transcription factor p53, which transactivates AMPK and TSC2, contributing to mTORC1 inhibition [13,14].
Hypoxia is another form of stress that deeply impacts cellular viability and growth that, by suppressing mitochondrial respiration, limits energetic availability to the cell. In response to low oxygen, the transcription factor hypoxia inducible factor 1α (HIF-1α) drives an adaptive cellular program that induces, among other targets, the expression of Redd1, an activator of TSC2 and, hence, an mTORC1 inhibitor [15].
Amino acids and mTORC1
Among regulators of mTORC1, amino acids have until very recently been shrouded in mystery. Amino acids are the basic building blocks for protein synthesis, in addition to providing substrates for energy production; for instance, deamination of glutamate generates α-ketoglutarate, a Krebs cycle intermediate that fuels the production of ATP. Thus, both unicellular and multicellular organisms have evolved mechanisms to sense amino acids, import them into the cell when they are available, and synthesize new ones when they are lacking. A great diversity of amino acid sensing mechanisms is found in unicellular organisms, which experience drastic changes of nutrient concentration in their surroundings. Many prokaryotes possess proteins dedicated to sensing amino acids, which allow them to couple nutrient availability to the regulation of multiple physiological processes [16,17]. Moreover, cells can indirectly sense a drop in amino acid levels through the accumulation of uncharged tRNAs and other stalled translation intermediates [18]. Sensing of uncharged tRNAs is conserved from yeast to man and potently regulates cellular physiology (reviewed in [19]).
The importance of amino acids in the growth and homeostasis of organisms was recognized decades ago. In a striking series of early experiments, depriving rats of a single amino acid, leucine, caused profound weight loss and muscle waste, followed by death [20]. Moreover, it was observed that amino acid withdrawal from cells and organisms triggered autophagy, a process of cellular self-eating where pre-existing proteins and organelles are broken down into simpler metabolites via lysosomal degradation [21]. Following the discovery of mTORC1, it was observed that withdrawal of amino acids from the culture media potently suppressed mTORC1 signaling in mammalian cells and yeast alike; moreover, suppressing mTORC1 by starvation or using its chemical inhibitor rapamycin strongly induced autophagy [21]. Thus, a feedback loop began to emerge, connecting amino acids, mTORC1, and autophagy in a mechanism that drives growth under nutrient abundance and mediates growth arrest under starvation conditions, allowing amino acid stores to be replenished.
For a long time, our understanding of amino acid regulation of mTORC1 remained confined to a few circumstantial observations, above all the fact that amino acids acted independently of insulin and TSC, and thus appeared to be distinct from the insulin/PI3K pathway [22–24]. These hints were followed by a major leap in our understanding of amino acid regulation of mTORC1 when a search for novel mTORC1 regulators using both biochemical methods in mammalian cells and genetic screens in Drosophila melanogaster uncovered a group of small GTPases as key mediators of amino acid signaling to mTORC1 [25,26]. These small GTPases, the Rags, belong to the Ras superfamily and are quite unusual: they exist as heterodimers where the highly similar RagA and RagB bind to either RagC or RagD, which are also similar to one another [27], leading to four possible dimer combinations. Crucially, amino acids regulate the nucleotide loading of the Rags, causing them to switch to an active conformation in which they physically bind and activate mTORC1.
The Rag GTPases and amino acid sensing
Based on sequence homology to other GTPases, Rag mutants can be engineered to be fixed in either a GTP-bound or GDP-bound state. For example, a complex can be constructed where RagA/B is fixed in the GTP-bound conformation, whereas RagC/D is GDP-bound [28]. These RagA/BGTP–RagC/DGDP mutants display maximum binding to mTORC1; they also potently activate mTORC1 signaling and render it insensitive to amino acid starvation. Conversely, the ‘inactive’ RagA/BGDP–RagC/DGTP mutants are unable to bind to mTORC1 and potently suppress mTORC1 activity, even in the presence of amino acids. These results imply that the binding of Rag GTPases to mTORC1 should be regulated by amino acids and that, crucially, amino acids should regulate the nucleotide state of the Rags. Both predictions turned out to be correct and firmly placed the Rags as key mediators of amino acid signals to mTORC1 [25,26].
Unlike Rheb, the Rags do not directly stimulate the kinase activity of mTORC1 [26]. Instead, and quite surprisingly, the Rags control the subcellular localization of mTORC1. Using new antibodies that successfully detect the mTOR kinase in a variety of cell lines, Sancak et al. [26] demonstrated that the subcellular distribution of mTOR changes dramatically as a function of amino acid abundance: mTOR is diffuse under starvation but quickly clusters to intracellular puncta upon addition of amino acids. Moreover, this redistribution could be faithfully recapitulated by overexpressing the active Rag mutants and was completely blocked by either the inactive Rags or Rag knockdown. These observations have since been confirmed by numerous groups [29–33]. Detailed immunofluorescence studies have shown that the intracellular puncta where mTOR localizes as a function of amino acids or active Rags are lysosomes and late endosomes [34,35].
These findings are consistent with earlier observations made in yeast. Genetic screens in this organism identified genes that are required for yeast cells to recover from amino acid starvation. Remarkably, many of these genes were membrane traffic regulators, including lipid kinases such as the type III PI3-Kinase Vps34 as well as small GTPases and endosomal tethering complexes [36,37]. In mammalian cells, the Vps34 homolog has been implicated in amino acid signaling to mTORC1 [38]. Concurrent with the genetic screens, studies in yeast began to demonstrate TOR association with intracellular membranes, including elements of the late endosomal/lysosomal pathways [39–41].
The Rags and mTOR at the lysosome
The Rag GTPases also localize to the lysosomal surface, but unlike other Ras family GTPases they lack canonical lipid modification motifs. Thus, it was hypothesized that unidentified Rag-binding proteins should mediate their docking to the lysosomal surface. Indeed, mass spectrometry analysis of Rag immunoprecipitates identified a complex of three small proteins, LAMTOR1–3, collectively known as Ragulator, which reside on the lysosome and dock the Rags to the lysosomal surface [34]. When Ragulator is genetically deleted, the Rags become cytoplasmic and, as is observed with loss of Rag function, the amino acid-induced translocation of mTOR to lysosomes is impaired. Thus, Ragulator is an essential component of the docking complex that recruits mTORC1 to the lysosome in response to amino acids. Interestingly, a partial loss of function of LAMTOR2 in humans underlies the first identified disease of hypoactive mTORC1 signaling, characterized by stunted growth, immunodeficiency, and albinism [42] (Box 2 and Figure 2).
Box 2. The mTORC1 pathway in human disease syndromes.
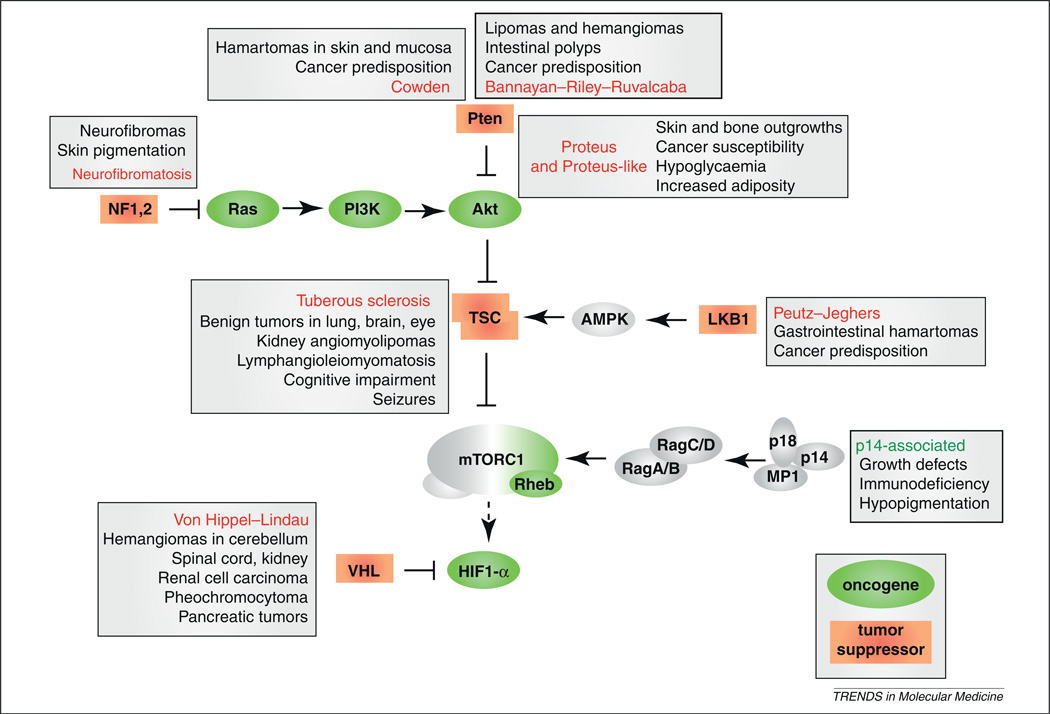
Germline mutations of genes coding for upstream regulators of mTOR cause several distinct human syndromes, all characterized by pathologically deregulated cell growth (Figure 2). A prominent example is tuberous sclerosis [87,88], a syndrome caused by inactivating mutations of the TSC1 or TSC2 proteins (also known as hamartin and tuberin, respectively), which are inhibitors of Rheb. Tuberous sclerosis is characterized by spontaneous formation of renal, brain, liver, and lung tumors, benign outgrowths known as lymphangioleiomyomatosis and angiomyolipomas, and is often associated with cognitive impairment. Two syndromes, Peutz–Jeghers syndrome, named after its discoverers, and PMSE (polyhydramnios, megalencephaly, and symptomatic epilepsy) indirectly affect the function of the TS complex by inactivating the upstream kinase LKB1 or its activator, STRADα [89,90]. Peutz–Jeghers patients develop gastrointestinal hamartomas and are predisposed to the development of malignancies, whereas individuals with PMSE suffer severe developmental and neurological abnormalities. Neurofribomatosis [91,92], characterized by aberrant skin pigmentation and neurofibromas, is driven by aberrant PI3K/Akt activation caused by inactivating mutations of the NF1 or NF2 genes, which encode inhibitors of the Ras signaling pathway. Mutations in the Von Hippel–Lindau (VHL) gene [93], which opposes mTORC1 inactivation by HIF1-α, causes widespread hemangiomas, renal cell carcinomas, pheochromocytomas, and pancreatic tumors. A series of different genetic alterations that collectively block the action of the tumor suppressor Pten cause Cowden [94], Proteus [95], and Bannayan–Riley–Ruvalcaba syndromes [96], characterized by cancer predisposition and by the development of benign tumors and outgrowths of various types (reviewed in [97]). The prime function of Pten is the inactivation of the oncogene Akt and, recently, early somatic mutations in the Akt1 gene were found in Proteus syndrome patients [98]. Interestingly, somatic mutations in the Akt2 gene do not cause cancer, but are the cause of a syndrome characterized by severe hypoglycemia, asymmetrical growth, and increased adiposity [99]. Whereas most of the aforementioned syndromes are the consequences of mutations affecting growth factor-dependent activation of mTORC1, dysregulation of amino acid-dependent mTORC1 activation also leads to human disease: a rare germline mutation in a gene encoding for LAMTOR2, one of the Ragulator proteins [42], leads to delayed growth, immunosuppression, and hypopigmentation. Unraveling additional players in amino acid signaling via mTORC1 will probably reveal the involvement of this signaling cascade in other human syndromes related to growth defects and cancer predisposition.
It is surprising that mutations in regulators of mTOR cause so many syndromes but no mutations in the gene encoding mTOR itself have been reported. A potential explanation is that affecting the mTOR gene directly would be incompatible with embryonic development. In addition, it is intriguing that only a very few mutations in mTOR have been found in human sporadic cancers [100,101].
Figure 2.
Involvement of the target of rapamycin (mTOR) pathway in human disease syndromes. Germline mutations that affect target of rapamycin complex 1 (mTORC1) activity are found in syndromes associated with deregulated cell growth. Tuberous sclerosis, Von Hippel–Lindau disease (VHL), and Peutz–Jeghers syndrome directly affect mTORC1 activation without impacting PI3K (phosphatidylinositol 3-kinase)-Akt, whereas neurofibromatosis and the Cowden, Proteus, and Bannayan–Riley–Ruvalcaba syndromes involve PI3K-Akt dysregulation, indirectly leading to hyperactivation of mTORC1 and also affecting other downstream effectors. Remarkably, a rare syndrome associated with an almost complete loss of function of the Ragulator component LAMTOR2/p14 is the only known syndrome that affects amino acid-dependent activation of mTORC1, and the only syndrome that leads to mTORC1 hypoactivity.
This lysosomal docking mechanism, discovered in mammalian cells, has many similarities to yeast, but also some potentially important differences. The Rags are the mammalian homologs of the yeast proteins Gtr1p and Gtr2p, which also exist as heterodimers of Gtr1 and Gtr2 [28]. The Gtrs localize to the vacuole, the yeast equivalent of the lysosome, where they play a role in trafficking amino acid transporters to and from the plasma membrane [43,44]. As in mammalian cells, Gtr1–Gtr2 is essential for mTORC1 activation by amino acids [45]. Ragulator is not conserved in yeast, but a complex of three small proteins, Ego1–3, interacts with the Gtrs and is required for their targeting to the vacuole. Interestingly, the crystal structure of Ego2–3 was recently solved and found to be very similar to that of LAMTOR2–3 [46,47]. In both cases, two mirror image proteins interact with each other via roadblock domains. Even more intriguingly, the recently reported crystal structure of the Gtr1–Gtr2 C-terminal domain displays a remarkable similarity to both the Ego2–3 and the LAMTOR2–3 dimer [48], and the same structural feature is predicted to exist in the C-terminal domain of the mammalian RagA–RagC heterodimer. Collectively, these observations indicate that although the Rags are conserved from mammals to yeast, different protein complexes have been repurposed to facilitate Rag docking. There may be organism-specific functions of each complex encoded in their primary amino acid sequence, whereas their Rag docking function appears to be carried out by their identical structure. Moreover, the roadblock domain may represent the basic architectural element of the Ragulator–Rag complex and may be endowed with regulatory functions in addition to structural ones.
A key remaining question is what role lysosomal/vacuolar localization plays in activating mTORC1. Microscopy studies in mammalian cells have shown that the lysosomal membrane contains Rheb [34,49,50], the small GTPase that is the endpoint of insulin and growth factor inputs via the PI3K pathway. Thus, amino acids may ‘gate’ insulin-derived signals by bringing mTORC1 to the location where it can bind to Rheb. Supporting this hypothesis, overexpression of Rheb, which causes its mislocalization across the cell, renders amino acids dispensable for mTORC1 signaling, probably because under these conditions mTORC1 and Rheb can bind independently of the lysosomal surface [34,49]. Even more compelling evidence is that expression of a lysosomally anchored mTORC1, created by adding a lysosomal lipid modification signal to Raptor, rendered amino acids, the Rags, and Ragulator completely dispensable for activation of the pathway [34]. However, knocking down Rheb completely abolished the constitutive signaling of lysosomally anchored mTORC1. Conversely, cotargeting mTORC1 and Rheb to the plasma membrane, where neither is normally found, caused strong, amino acid-independent activation of the pathway [34]. Thus, the primary function of the Rag–Ragulator scaffold appears to be enabling the amino acid-dependent binding of mTORC1 to Rheb, which serves as the ‘ignition key’ for the kinase activity of the complex. From a clinical perspective, generating small molecules that block mTORC1 localization, and hence impair its activation, emerges as a novel therapeutic approach against mTORC1 (Box 3).
Box 3. Targeting amino acid signaling with small molecules.
Rapamycin, an FDA-approved mTOR inhibitor, has two defining characteristics: it is highly selective towards mTORC1 and it blocks phosphorylation of some mTORC1 targets but not others. Despite minimal side effects and outstanding pharmacokinetics, rapamycin has efficacy against only a few pathologies associated with high levels of mTORC1 activity, such as renal cell carcinoma [102] and tuberous sclerosis [103–105]. These limited anticancer effects have motivated the pursuit of improved small molecules specifically targeting mTORC1 and ATP-competitive inhibitors that can block all mTORC1 and mTORC2 kinase activity [81,83–86] (Box 1). Inhibition of both mTORC1 and mTORC2 dampens the PI3K/Akt pathway, simultaneously inhibiting two key oncogenic signaling pathways.
There are scenarios, however, where completely blocking mTORC1 activity is desired but inhibiting the PI3K/Akt pathway could be harmful, as in T2D, where preserving insulin signaling is key. An alternative therapeutic approach would be to target amino acid-dependent recruitment of mTORC1 to the lysosomal surface. This would lead to complete inhibition of mTORC1, unlike rapamycin, without affecting mTORC2 activity, unlike ATP-competitive inhibitors. Given that suppressing mTORC1 activity leads to activation of the PI3K/Akt pathway, the effects associated with this activation must be considered in depth.
A complex disease such as T2D exemplifies an apparent paradox of high mTORC1 activity coexisting with decreased insulin signaling. In this case, targeting amino acid-dependent activation of mTORC1 could constitute an optimal approach. Complete and selective inhibition of mTORC1 would bring its deregulated anabolic program under control and help restore insulin sensitivity by disengaging the inhibitory feedback loops (Box 1); in turn, restored insulin sensitivity would help correct hyperglycemia. Manipulating mTORC1 activity in this way may also prove important for anti-aging purposes, as suggested by the fact that rapamycin delays aging in mammals [106,107]. Basic research with animal models will shed light on the effectiveness of this approach and the rationale of searching for small molecules that inhibit mTORC1 only at the lysosome.
Patients with disease syndromes such as tuberous sclerosis, Peutz–Jeghers syndrome, or VHL could benefit from direct inhibition of mTORC1, whereas inhibiting PI3K/Akt would bring no additional benefit. In sharp contrast to this, tumor syndromes that arise owing to deregulated Akt activity downstream of PI3K, such as Cowden or Proteus syndromes, would require simultaneous inhibition of mTOR and PI3K/Akt.
Successful and specific therapeutic strategies generally depend on developing suitable inhibitors of an enzyme active site, because active sites are protein cavities most often suitable for binding small molecules. Hence, blocking mTORC1 recruitment to the lysosomal surface does not immediately appear to be an easy avenue. However, preventing recruitment could be achieved by designing inhibitors against the active sites of the Rag GTPases. In addition, targeting the Rag/Ragulator interaction or Ragulator/v-ATPase interaction may be possible. Increasing our understanding of amino acid signaling to mTORC1 is likely to reveal additional targeting strategies.
Although compelling evidence exists for this mechanism in mammals, the shuttling model of mTORC1 does not explain the pathway in budding yeast, which have no PI3K or Rheb equivalents. Indeed, in Saccharomyces cerevisiae, TORC1 appears to remain bound to the vacuole even following amino acid withdrawal [45]. Again, this key mechanistic difference could reflect evolutionary divergence: in the absence of a Rheb homolog, Gtr1/2 may directly regulate TORC1 kinase activity, rather than controlling its subcellular localization.
The specific localization of TORC1 to the yeast vacuole and the metazoan lysosome suggests that the lysosome/vacuole may play a more profound function in the amino acid pathway than merely serving as a scaffold. In yeast, the vacuole was recognized early on as a storage site for amino acids (reviewed in [51]). Basic amino acids arginine, lysine, and histidine preferentially accumulate in the vacuole, whereas they are relatively less abundant in the cytoplasm [52]. Other amino acids also display varying degrees of vacuolar accumulation. Similarly, there is evidence that mammalian lysosomes may maintain a stable pool of luminal amino acids [53]. Moreover, both in yeast and mammals the lysosome/vacuole is the end point of autophagy, which by degrading proteins and organelles generates a fresh supply of amino acids during starvation. Over time, amino acids generated via autophagy reactivate mTORC1 [54], indicating that the lysosome/vacuole may not only be the end point but also the starting point of amino acid signaling to mTORC1.
A possible role for the lysosome in sensing amino acids was tested using a cell-free system, in which a preparation of intact lysosomes was mixed with purified mTORC1 and binding of mTORC1 to these lysosomes was measured. In this assay, treatment with amino acids was sufficient to induce mTORC1 binding to lysosomes, indicating that the lysosome contains all the machinery required for sensing amino acids and activating the Rag GTPases [35]. Moreover, in this system alcohol esters of amino acids, which freely cross membranes and then accumulate inside lysosomes, were more potent than native amino acids in inducing mTORC1 binding to Rag-containing organelles. Furthermore, making these lysosomes ‘leaky’ strongly suppressed mTORC1 recruitment by amino acids or their esters. Together with data gathered from intact cells, these results suggest that amino acids may be sensed, at least in part, inside the lysosomal lumen, where they generate an ‘inside-out’ signal that leads to Rag activation. This study also identified the vacuolar H+-ATPase (v-ATPase) as an essential mediator of the inside-out signal that engages in extensive, amino acid-regulated interactions with Ragulator and Rags [35]. Thus, the v-ATPase appears to play a direct, physical role in amino acid signaling to mTORC1.
In light of these results, it is noteworthy that the yeast vacuole contains multiple transporters that ferry amino acids between the lumen and the cytoplasm (reviewed in [51,55]). Several such transporters, including those belonging to the Avt family, utilize the proton gradient established by the v-ATPase in symport or antiport mechanisms [56]. Thus, the v-ATPase may play multiple roles in amino acid sensing: it enables transport of amino acids in and out of the lysosome by establishing the proton gradients, and it helps relay information on amino acid abundance via its physical interactions with Ragulator and the Rags. Several amino acid transporters have also been identified in the mammalian lysosome, and some of them may play a role in regulating mTORC1 signaling [57–59]; however, it is likely that others remain to be identified. Understanding how the transport of lysosomal amino acids is orchestrated is an area of significant interest for future research.
Two recent reports suggest that, in parallel to lysosome-based sensing, a dedicated mechanism for detecting leucine availability may exist in the cytoplasm [60,61]. This mechanism centers around leucyl-tRNA synthetase (LRS), an enzyme that couples leucine to its cognate tRNA and thus plays a key role in protein synthesis. In mammalian cells, LRS was proposed to bind to GTP-bound RagD in a leucine-dependent way and to promote its conversion to the GDP-bound form, which activates the pathway [61]. In yeast, LRS was shown to act as a positive regulator of TORC1 downstream of leucine: when leucine is present, LRS binds to Gtr1 (the RagA/B homolog) and prevents its inactivation by an unidentified negative regulator [60]. It will be interesting to understand how and to what extent the lysosomal and cytoplasmic sensing mechanisms are integrated.
mTORC1 localization and autophagy
The presence of mTORC1 at the vacuole/lysosome has important implications for its ability to control autophagy. In non-starving cells, mTORC1 suppresses the formation of the phagophore by phosphorylating and inhibiting the kinase ULK1 and its interacting partner, ATG13 [62,63]. Upon nutrient withdrawal and consequent mTORC1 inhibition, phagophore formation is triggered, followed by the massive fusion of autophagosomes with lysosomes to generate a hybrid organelle that enables cargo digestion. Importantly, these effects depend on the Rag GTPases; expression of the active Rags suppresses autophagy under starvation conditions, whereas expressing the inhibitory mutants results in constitutive autophagosome formation [25].
A recent report showed that during starvation, autophagy restores cellular amino acid levels and leads to the recruitment of mTORC1 to the surface of autophagolysosomes [54]. Intriguingly, mTORC1 then promotes the reformation of primary lysosomes, which bud from the hybrid organelle in a way that requires mTORC1 kinase activity. Thus, although mTORC1 antagonizes autophagy in the short term, it may be essential for the continued ability of cells to trigger this degradative process. A similar idea is supported by the observation that, in yeast, the Gtrs and EGO complex are required to restore vacuolar morphology after rapamycin-induced or starvation-induced autophagy [64]. Finally, a recent report shows that mTORC1 activation and autophagy can coexist in cells undergoing oncogene-induced senescence. In these cells, spatial coupling of autophagy and mTORC1 enables the massive synthesis and secretion of cytokines that maintain the senescent state [33]. Thus, the relationship between mTORC1 and autophagy may be more complex than previously thought. The presence of mTORC1 and the autophagic machinery on the same organelle suggests novel mechanisms to coordinate cellular growth and clearance that may have important implications not only in normal cells but also in cancer and neurodegeneration (Box 1 and Box 4).
Box 4. Targeting mTORC1 in aging and neurodegeneration.
An important function of the lysosome is to maintain cellular homeostasis by eliminating aged or damaged cellular components in the process known as autophagy. The efficiency of this quality control program appears to decline over time, likely contributing to aging and age-related diseases [108] (reviewed in [109]). Moreover, disruption of lysosomal and autophagic function by the accumulation of misfolded protein aggregates may drive progression of Huntington’s, Parkinson’s, and Alzheimer’s diseases (reviewed in [110]). The mTORC1 inhibitor rapamycin has attracted significant interest as a therapeutic avenue for treating aging and neurodegeneration, due to the ability of rapamycin to cause deinhibition of ULK1 and boost autophagosome formation both in cells and in whole organisms. In a paradigm study, treatment with rapamycin reduced the toxicity of polyglutamine expansions in cellular and animal models of Huntington’s disease [111]. The identification of TFEB as a substrate that is negatively regulated by mTORC1 further supports mTORC1 inhibition as a strategy for increasing cellular clearance [66,68]. TFEB upregulates the catabolic capacity of the cell by activating a wide-ranging, coherent transcriptional program that may exert long-lasting, protective effects [65,66]. However, unlike ULK1, TFEB is a rapamycin-insensitive mTORC1 substrate, and TFEB phosphorylation and nuclear localization are only minimally affected by this drug [68]. Thus, stimulating TFEB function by manipulating mTORC1 activity would require using more potent but less well-tested mTOR catalytic inhibitors; the benefits of these inhibitors in contrast to their potentially harmful side effects remain to be determined in vivo.
Using mTORC1 inhibitors to boost cellular clearance must be reviewed in light of recent reports indicating that mTORC1 signaling and autophagy may be interdependent, at least to some extent. The requirement for mTOR in lysosome reformation suggests that chronic mTORC1 inhibition may hamper autophagy in the long term [54]. Moreover, reports that constitutive mTORC1 activation upregulates the expression of important lysosomal genes suggests that mTORC1 may affect cellular clearance through multiple pathways that involve both positive and negative regulation of substrates [69,70]. Thus, further investigation is required to determine the cost-effectiveness of this strategy.
An alternative approach would be to identify compounds that cause ULK1 and TFEB activation independent of mTORC1 inhibition. This strategy may enhance autophagy without eliminating the positive contributions of mTORC1 signaling to this process.
Very recently, a novel paradigm has begun to emerge where the amino acid/mTORC1 pathway centered at the lysosome may be part of a novel signaling mechanism that controls lysosomal gene expression and, through this process, affects cellular clearance and metabolism. A bioinformatics search for consensus binding sites in the promoters of lysosomal genes identified the coordinated lysosomal expression and regulation (CLEAR) element, which is bound by the MiT/TFE subfamily of helix–loop–helix (bHLH) transcription factors. One member of the MiT/TFE family, known as transcription factor EB (TFEB), physically binds the CLEAR motif in the promoter of multiple lysosomal genes, including luminal hydrolases and membrane transporters, to upregulate their expression [65]. Overexpressing TFEB in cells led to a striking expansion of the lysosomal compartment, both in terms of size and number. This, in turn, resulted in enhanced clearance capacity towards multiple lysosomal substrates.
Shuttling between the nucleus and the cytoplasm regulates the activity of TFEB. A key observation was that withdrawal of nutrients from the culture media induced the nuclear translocation of TFEB in cells [66]. Among the transcriptional targets of TFEB are several autophagy-mediating genes and, accordingly, TFEB overexpression resulted in enhanced formation of LC3-positive autophagosomes. Conversely, siRNA-mediated TFEB depletion resulted in a defective autophagic response to nutrient starvation. These findings, which were confirmed in mice [66], support a model where TFEB is a key component of a transcriptional starvation-response program. By expanding the lysosomal and autophagic compartments, this program increases the ability of cells to degrade and recycle their substrates, and thus to sustain adequate levels of energy and metabolites.
Two kinases, mTORC1 and ERK, control the nuclear/cytoplasmic shuttling of TFEB. In particular, mTOR exerts a tight control over the subcellular localization of TFEB: when cells are replete with nutrients, mTOR phosphorylates TFEB at two critical serines, sequestering TFEB in the cytoplasm [67,68]. A series of observations strongly suggest that the amino acids/mTORC1 pathway is especially important in controlling TFEB nuclear localization. Treatments that cause starvation or lysosomal stress, including amino acid withdrawal, v-ATPase inactivation, and overexpression of transporters that empty the lysosome of its amino acid content, caused a massive translocation of TFEB to the nucleus. Moreover, the effect of these stressors could be completely prevented by expressing the active Rag GTPase mutants, which maintained TFEB in the cytoplasm by constitutively activating mTORC1. Conversely, the inactive Rag mutants caused constitutive localization of TFEB to the nucleus, even in the presence of nutrients [68]. Finally, TFEB phosphorylation occurs on the lysosomal membrane, where mTORC1 and TFEB physically bind to each other [67,68]. Thus, the lysosome seems to operate as a ‘gate’ that controls the amount of TFEB allowed to reach the nucleus. In fully fed cells, active mTORC1 meets TFEB at the lysosome, phosphorylates it, and releases it back into the cytoplasm. When mTORC1 is inactivated, it detaches from the lysosomal membrane, allowing TFEB to become unphosphorylated and move to the nucleus.
This lysosome-to-nucleus signaling system may play a key role in coordinating cellular adaptation to growth-promoting, versus starvation, conditions. When nutrients are plentiful and stressors are absent, mTORC1 is closely associated with the lysosomal system, where it promotes biosynthetic and anabolic reactions. In turn, the lysosomal system provides basal levels of cellular turnover that is compatible with growth and, in parallel, operates as a monitor for the levels of important nutrients. Nutrient depletion and lysosomal stress converge on the v-ATPase–Ragulator–Rag GTPase system, causing mTORC1 detachment from the lysosome and inactivation. mTORC1 inactivation arrests anabolic reactions and boosts cellular degradation via two complementary, parallel mechanisms: acute deinhibition of ULK1, which directly stimulates autophagosome formation, and nuclear translocation of TFEB, which activates transcriptional networks that subsequently expand the size and activity of the lysosomal/autophagic compartments.
This simple model seems optimally designed to enable cells to switch between growth and maintenance modes. Nonetheless, additional crosstalk between mTORC1 and the autophagic/lysosomal system may enable a more nuanced and fine-tuned control. As previously mentioned, mTORC1 may play a positive role in catabolism by mediating lysosome reformation following autophagy. Moreover, certain conditions that result in overactivation of mTORC1 may actually increase the expression of lysosomal genes (likely in a TFEB-independent way) [69,70]. As the range of substrates and cellular actions of mTORC1 expands at an increasing rate, we shall achieve a more profound understanding of the interplay between nutrient sensing, growth control, and cellular degradation in the foreseeable future.
Acknowledgments
The authors acknowledge support from the US National Institutes of Health (R01 CA129105, R01 CA103866, and R37 AI047389) and awards from the American Federation for Aging, Starr Foundation, Koch Institute Frontier Research Program, and the Ellison Medical Foundation to D.M.S.; fellowships from the Jane Coffin Childs Memorial Fund for Medical Research and the LAM Foundation to R.Z., and Human Frontier Science Program to A.E. D.M.S. in an investigator of the Howard Hughes Medical Institute.
Glossary
- Anabolism
series of biochemical reactions that lead to the synthesis of larger molecules from smaller units, at the expense of energy. Is the opposite of catabolism, which produces energy by breaking them down
- Cellular senescence
an irreversible state of cell cycle arrest triggered by various different types of stress: replicative, telomeric, oncogenic, and DNA damage
- GTPase
enzyme that hydrolyzes guanosine triphosphate (GTP) into guanosine diphosphate (GDP) and inorganic phosphate (Pi). The guanosine loading state of GTPase dictates its activity
- Lysosome
vesicular organelle rich in degradative enzymes (hydrolases, proteases, lipases, etc.) where macromolecules are broken down. It is the endpoint of endocytosis, phagocytosis, and autophagy
- Phagophore
double membrane structure that engulfs components of the cytoplasm during macroautophagy
- Rapamycin
an antifungal macrolide synthesized by the bacterium Streptomyces hygroscopicus originally found in Rapa Nui Island. Its biological properties prompted intensive research and led to the discovery of its cellular target: mTOR
- Roadblock domain
protein domain of undefined function containing a conserved secondary and tertiary structure that facilitates dimerization of proteins containing the domain
References
- 1.Rabinowitz JD, White E. Autophagy and metabolism. Science. 2010;330:1344–1348. doi: 10.1126/science.1193497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zoncu R, et al. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 2010;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fonseca BD, et al. PRAS40 is a target for mammalian target of rapamycin complex 1 and is required for signaling downstream of this complex. J. Biol. Chem. 2007;282:24514–24524. doi: 10.1074/jbc.M704406200. [DOI] [PubMed] [Google Scholar]
- 5.Oshiro N, et al. The proline-rich Akt substrate of 40 kDa (PRAS40) is a physiological substrate of mammalian target of rapamycin complex 1. J. Biol. Chem. 2007;282:20329–20339. doi: 10.1074/jbc.M702636200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sancak Y, et al. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol. Cell. 2007;25:903–915. doi: 10.1016/j.molcel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Vander Haar E, et al. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat. Cell Biol. 2007;9:316–323. doi: 10.1038/ncb1547. [DOI] [PubMed] [Google Scholar]
- 8.Xiao B, et al. Structural basis for AMP binding to mammalian AMP-activated protein kinase. Nature. 2007;449:496–500. doi: 10.1038/nature06161. [DOI] [PubMed] [Google Scholar]
- 9.Xiao B, et al. Structure of mammalian AMPK and its regulation by ADP. Nature. 2011;472:230–233. doi: 10.1038/nature09932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inoki K, et al. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 11.Shaw RJ, et al. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc. Natl. Acad. Sci. U.S.A. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gwinn DM, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng Z, et al. The regulation of AMPK beta1, TSC2, and PTEN expression by p53: stress, cell and tissue specificity, and the role of these gene products in modulating the IGF-1-AKT-mTOR pathways. Cancer Res. 2007;67:3043–3053. doi: 10.1158/0008-5472.CAN-06-4149. [DOI] [PubMed] [Google Scholar]
- 14.Jones RG, et al. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol. Cell. 2005;18:283–293. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 15.Brugarolas J, et al. Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 2004;18:2893–2904. doi: 10.1101/gad.1256804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gardina PJ, Manson MD. Attractant signaling by an aspartate chemoreceptor dimer with a single cytoplasmic domain. Science. 1996;274:425–426. doi: 10.1126/science.274.5286.425. [DOI] [PubMed] [Google Scholar]
- 17.Levdikov VM, et al. The structure of CodY, a GTP- and isoleucine-responsive regulator of stationary phase and virulence in gram-positive bacteria. J. Biol. Chem. 2006;281:11366–11373. doi: 10.1074/jbc.M513015200. [DOI] [PubMed] [Google Scholar]
- 18.Dong J, et al. Uncharged tRNA activates GCN2 by displacing the protein kinase moiety from a bipartite tRNA-binding domain. Mol. Cell. 2000;6:269–279. doi: 10.1016/s1097-2765(00)00028-9. [DOI] [PubMed] [Google Scholar]
- 19.Hinnebusch AG. Translational regulation of GCN4 and the general amino acid control of yeast. Annu. Rev. Microbiol. 2005;59:407–450. doi: 10.1146/annurev.micro.59.031805.133833. [DOI] [PubMed] [Google Scholar]
- 20.Said AK, Hegsted DM. Response of adult rats to low dietary levels of essential amino acids. J. Nutr. 1970;100:1363–1375. doi: 10.1093/jn/100.11.1363. [DOI] [PubMed] [Google Scholar]
- 21.Kroemer G, et al. Autophagy and the integrated stress response. Mol. Cell. 2010;40:280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Long X, et al. Rheb binding to mammalian target of rapamycin (mTOR) is regulated by amino acid sufficiency. J. Biol. Chem. 2005;280:23433–23436. doi: 10.1074/jbc.C500169200. [DOI] [PubMed] [Google Scholar]
- 23.Hara K, et al. Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. J. Biol. Chem. 1998;273:14484–14494. doi: 10.1074/jbc.273.23.14484. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, et al. Amino acid availability regulates p70 S6 kinase and multiple translation factors. Biochem. J. 1998;334:261–267. doi: 10.1042/bj3340261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim E, et al. Regulation of TORC1 by Rag GTPases in nutrient response. Nat. Cell Biol. 2008;10:935–945. doi: 10.1038/ncb1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sancak Y, et al. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sekiguchi T, et al. Novel G proteins, Rag C and Rag D, interact with GTP-binding proteins, Rag A and Rag B. J. Biol. Chem. 2001;276:7246–7257. doi: 10.1074/jbc.M004389200. [DOI] [PubMed] [Google Scholar]
- 28.Hirose E, et al. RagA is a functional homologue of S. cerevisiae Gtr1p involved in the Ran/Gsp1–GTPase pathway. J. Cell Sci. 1998;111:11–21. doi: 10.1242/jcs.111.1.11. [DOI] [PubMed] [Google Scholar]
- 29.Korolchuk VI, et al. Lysosomal positioning coordinates cellular nutrient responses. Nat. Cell Biol. 2011;13:453–460. doi: 10.1038/ncb2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flinn RJ, et al. The late endosome is essential for mTORC1 signaling. Mol. Biol. Cell. 2010;21:833–841. doi: 10.1091/mbc.E09-09-0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoon MS, et al. Class III PI-3-kinase activates phospholipase D in an amino acid-sensing mTORC1 pathway. J. Cell Biol. 2011;195:435–447. doi: 10.1083/jcb.201107033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalender A, et al. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab. 2010;11:390–401. doi: 10.1016/j.cmet.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Narita M, et al. Spatial coupling of mTOR and autophagy augments secretory phenotypes. Science. 2011;332:966–970. doi: 10.1126/science.1205407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sancak Y, et al. Ragulator–Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141:290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zoncu R, et al. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H-ATPase. Science. 2011;334:678–683. doi: 10.1126/science.1207056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Puria R, et al. Nuclear translocation of Gln3 in response to nutrient signals requires Golgi-to-endosome trafficking in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U.S.A. 2008;105:7194–7199. doi: 10.1073/pnas.0801087105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zurita-Martinez SA, et al. Efficient Tor signaling requires a functional class C Vps protein complex in Saccharomyces cerevisiae. Genetics. 2007;176:2139–2150. doi: 10.1534/genetics.107.072835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nobukuni T, et al. Amino acids mediate mTOR/raptor signaling through activation of class 3 phosphatidylinositol 3OH-kinase. Proc. Natl. Acad. Sci. U.S.A. 2005;102:14238–14243. doi: 10.1073/pnas.0506925102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wedaman KP, et al. Tor kinases are in distinct membrane-associated protein complexes in Saccharomyces cerevisiae. Mol. Biol. Cell. 2003;14:1204–1220. doi: 10.1091/mbc.E02-09-0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sturgill TW, et al. TOR1 and TOR2 have distinct locations in live cells. Eukaryot. Cell. 2008;7:1819–1830. doi: 10.1128/EC.00088-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berchtold D, Walther TC. TORC2 plasma membrane localization is essential for cell viability and restricted to a distinct domain. Mol. Biol. Cell. 2009;20:1565–1575. doi: 10.1091/mbc.E08-10-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bohn G, et al. A novel human primary immunodeficiency syndrome caused by deficiency of the endosomal adaptor protein p14. Nat. Med. 2007;13:38–45. doi: 10.1038/nm1528. [DOI] [PubMed] [Google Scholar]
- 43.Bun-Ya M, et al. Putative GTP-binding protein, Gtr1, associated with the function of the Pho84 inorganic phosphate transporter in Saccharomyces cerevisiae. Mol. Cell. Biol. 1992;12:2958–2966. doi: 10.1128/mcb.12.7.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao M, Kaiser CA. A conserved GTPase-containing complex is required for intracellular sorting of the general amino-acid permease in yeast. Nat. Cell Biol. 2006;8:657–667. doi: 10.1038/ncb1419. [DOI] [PubMed] [Google Scholar]
- 45.Binda M, et al. The Vam6 GEF controls TORC1 by activating the EGO complex. Mol. Cell. 2009;35:563–573. doi: 10.1016/j.molcel.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 46.Kurzbauer R, et al. Crystal structure of the p14/MP1 scaffolding complex: how a twin couple attaches mitogen-activated protein kinase signaling to late endosomes. Proc. Natl. Acad. Sci. U.S.A. 2004;101:10984–10989. doi: 10.1073/pnas.0403435101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kogan K, et al. Structural conservation of components in the amino acid sensing branch of the TOR pathway in yeast and mammals. J. Mol. Biol. 2010;402:388–398. doi: 10.1016/j.jmb.2010.07.034. [DOI] [PubMed] [Google Scholar]
- 48.Gong R, et al. Crystal structure of the Gtr1p–Gtr2p complex reveals new insights into the amino acid-induced TORC1 activation. Genes Dev. 2011;25:1668–1673. doi: 10.1101/gad.16968011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buerger C, et al. Localization of Rheb to the endomembrane is critical for its signaling function. Biochem. Biophys. Res. Commun. 2006;344:869–880. doi: 10.1016/j.bbrc.2006.03.220. [DOI] [PubMed] [Google Scholar]
- 50.Saito K, et al. Novel role of the small GTPase Rheb: its implication in endocytic pathway independent of the activation of mammalian target of rapamycin. J. Biochem. 2005;137:423–430. doi: 10.1093/jb/mvi046. [DOI] [PubMed] [Google Scholar]
- 51.Klionsky DJ, et al. The fungal vacuole: composition, function, and biogenesis. Microbiol. Rev. 1990;54:266–292. doi: 10.1128/mr.54.3.266-292.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kitamoto K, et al. Dynamic aspects of vacuolar and cytosolic amino acid pools of Saccharomyces cerevisiae. J. Bacteriol. 1988;170:2683–2686. doi: 10.1128/jb.170.6.2683-2686.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harms E, et al. Lysosomal pool of free-amino acids. Biochem. Biophys. Res. Commun. 1981;99:830–836. doi: 10.1016/0006-291x(81)91239-0. [DOI] [PubMed] [Google Scholar]
- 54.Yu L, et al. Termination of autophagy and reformation of lysosomes regulated by mTOR. Nature. 2010;465:942–946. doi: 10.1038/nature09076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li SC, Kane PM. The yeast lysosome-like vacuole: endpoint and crossroads. Biochim. Biophys. Acta. 2009;1793:650–663. doi: 10.1016/j.bbamcr.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Russnak R, et al. A family of yeast proteins mediating bidirectional vacuolar amino acid transport. J. Biol. Chem. 2001;276:23849–23857. doi: 10.1074/jbc.M008028200. [DOI] [PubMed] [Google Scholar]
- 57.Heublein S, et al. Proton-assisted amino-acid transporters are conserved regulators of proliferation and amino-acid-dependent mTORC1 activation. Oncogene. 2010;29:4068–4079. doi: 10.1038/onc.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ruivo R, et al. Mechanism of proton/substrate coupling in the heptahelical lysosomal transporter cystinosin. Proc. Natl. Acad. Sci. U.S.A. 2012;109:E210–E217. doi: 10.1073/pnas.1115581109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sagne C, et al. Identification and characterization of a lysosomal transporter for small neutral amino acids. Proc. Natl. Acad. Sci. U.S.A. 2001;98:7206–7211. doi: 10.1073/pnas.121183498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bonfils G, et al. Leucyl-tRNA synthetase controls TORC1 via the EGO complex. Mol. Cell. 2012;46:105–110. doi: 10.1016/j.molcel.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 61.Han JM, et al. Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway. Cell. 2012;149:410–424. doi: 10.1016/j.cell.2012.02.044. [DOI] [PubMed] [Google Scholar]
- 62.Hosokawa N, et al. Nutrient-dependent mTORC1 association with the ULK1–Atg13–FIP200 complex required for autophagy. Mol. Biol. Cell. 2009;20:1981–1991. doi: 10.1091/mbc.E08-12-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim J, et al. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dubouloz F, et al. The TOR and EGO protein complexes orchestrate microautophagy in yeast. Mol. Cell. 2005;19:15–26. doi: 10.1016/j.molcel.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 65.Sardiello M, et al. A gene network regulating lysosomal biogenesis and function. Science. 2009;325:473–477. doi: 10.1126/science.1174447. [DOI] [PubMed] [Google Scholar]
- 66.Settembre C, et al. TFEB links autophagy to lysosomal biogenesis. Science. 2011;332:1429–1433. doi: 10.1126/science.1204592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martina JA, et al. MTORC1 functions as a transcriptional regulator of autophagy by preventing nuclear transport of TFEB. Autophagy. 2012 doi: 10.4161/auto.19653. PMID: 22576015; ( http://dx.doi.org/10.4161/auto.19653) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Settembre C, et al. A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. EMBO J. 2012;31:1095–1108. doi: 10.1038/emboj.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Duvel K, et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol. Cell. 2010;39:171–183. doi: 10.1016/j.molcel.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pena-Llopis S, et al. Regulation of TFEB and V-ATPases by mTORC1. EMBO J. 2011;30:3242–3258. doi: 10.1038/emboj.2011.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Newgard CB, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Khamzina L, et al. Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involvement in obesity-linked insulin resistance. Endocrinology. 2005;146:1473–1481. doi: 10.1210/en.2004-0921. [DOI] [PubMed] [Google Scholar]
- 73.O’Reilly KE, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Um SH, et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- 75.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Laplante M, Sabatini DM. An emerging role of mTOR in lipid biosynthesis. Curr. Biol. 2009;19:R1046–R1052. doi: 10.1016/j.cub.2009.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wendel HG, et al. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature. 2004;428:332–337. doi: 10.1038/nature02369. [DOI] [PubMed] [Google Scholar]
- 78.Wendel HG, et al. Dissecting eIF4E action in tumorigenesis. Genes Dev. 2007;21:3232–3237. doi: 10.1101/gad.1604407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Noda T, Ohsumi Y. Tor, a phosphatidylinositol kinase homologue, controls autophagy in yeast. J. Biol. Chem. 1998;273:3963–3966. doi: 10.1074/jbc.273.7.3963. [DOI] [PubMed] [Google Scholar]
- 80.Choo AY, et al. Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. Proc. Natl. Acad. Sci. U.S.A. 2008;105:17414–17419. doi: 10.1073/pnas.0809136105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thoreen CC, et al. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J. Biol. Chem. 2009;284:8023–8032. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Guertin DA, et al. mTOR complex 2 is required for the development of prostate cancer induced by Pten loss in mice. Cancer Cell. 2009;15:148–159. doi: 10.1016/j.ccr.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chresta CM, et al. AZD8055 is a potent, selective, and orally bioavailable ATP-competitive mammalian target of rapamycin kinase inhibitor with in vitro and in vivo antitumor activity. Cancer Res. 2010;70:288–298. doi: 10.1158/0008-5472.CAN-09-1751. [DOI] [PubMed] [Google Scholar]
- 84.Feldman ME, et al. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol. 2009;7:e38. doi: 10.1371/journal.pbio.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Garcia-Martinez JM, et al. Ku-0063794 is a specific inhibitor of the mammalian target of rapamycin (mTOR) Biochem. J. 2009;421:29–42. doi: 10.1042/BJ20090489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yu K, et al. Biochemical, cellular, and in vivo activity of novel ATP-competitive and selective inhibitors of the mammalian target of rapamycin. Cancer Res. 2009;69:6232–6240. doi: 10.1158/0008-5472.CAN-09-0299. [DOI] [PubMed] [Google Scholar]
- 87.European Chromosome 16 Tuberous Sclerosis Consortium. Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell. 1993;75:1305–1315. doi: 10.1016/0092-8674(93)90618-z. [DOI] [PubMed] [Google Scholar]
- 88.van Slegtenhorst M, et al. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science. 1997;277:805–808. doi: 10.1126/science.277.5327.805. [DOI] [PubMed] [Google Scholar]
- 89.Hemminki A, et al. A serine/threonine kinase gene defective in Peutz–Jeghers syndrome. Nature. 1998;391:184–187. doi: 10.1038/34432. [DOI] [PubMed] [Google Scholar]
- 90.Orlova KA, et al. STRADalpha deficiency results in aberrant mTORC1 signaling during corticogenesis in humans and mice. J. Clin. Invest. 2010;120:1591–1602. doi: 10.1172/JCI41592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xu GF, et al. The neurofibromatosis type 1 gene encodes a protein related to GAP. Cell. 1990;62:599–608. doi: 10.1016/0092-8674(90)90024-9. [DOI] [PubMed] [Google Scholar]
- 92.Trofatter JA, et al. A novel moesin-, ezrin-, radixin-like gene is a candidate for the neurofibromatosis 2 tumor suppressor. Cell. 1993;75:826. doi: 10.1016/0092-8674(93)90501-g. [DOI] [PubMed] [Google Scholar]
- 93.Latif F, et al. Identification of the von Hippel–Lindau disease tumor suppressor gene. Science. 1993;260:1317–1320. doi: 10.1126/science.8493574. [DOI] [PubMed] [Google Scholar]
- 94.Liaw D, et al. Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat. Genet. 1997;16:64–67. doi: 10.1038/ng0597-64. [DOI] [PubMed] [Google Scholar]
- 95.Zhou X, et al. Association of germline mutation in the PTEN tumour suppressor gene and Proteus and Proteus-like syndromes. Lancet. 2001;358:210–211. doi: 10.1016/s0140-6736(01)05412-5. [DOI] [PubMed] [Google Scholar]
- 96.Marsh DJ, et al. Germline mutations in PTEN are present in Bannayan–Zonana syndrome. Nat. Genet. 1997;16:333–334. doi: 10.1038/ng0897-333. [DOI] [PubMed] [Google Scholar]
- 97.Orloff MS, Eng C. Genetic and phenotypic heterogeneity in the PTEN hamartoma tumour syndrome. Oncogene. 2008;27:5387–5397. doi: 10.1038/onc.2008.237. [DOI] [PubMed] [Google Scholar]
- 98.Lindhurst MJ, et al. A mosaic activating mutation in AKT1 associated with the Proteus syndrome. N. Engl. J. Med. 2011;365:611–619. doi: 10.1056/NEJMoa1104017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hussain K, et al. An activating mutation of AKT2 and human hypoglycemia. Science. 2011;334:474. doi: 10.1126/science.1210878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gerlinger M, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sato T, et al. Single amino-acid changes that confer constitutive activation of mTOR are discovered in human cancer. Oncogene. 2010;29:2746–2752. doi: 10.1038/onc.2010.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hudes G, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N. Engl. J. Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 103.Bissler JJ, et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N. Engl. J. Med. 2008;358:140–151. doi: 10.1056/NEJMoa063564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Davies DM, et al. Sirolimus therapy in tuberous sclerosis or sporadic lymphangioleiomyomatosis. N. Engl. J. Med. 2008;358:200–203. doi: 10.1056/NEJMc072500. [DOI] [PubMed] [Google Scholar]
- 105.Franz DN, et al. Rapamycin causes regression of astrocytomas in tuberous sclerosis complex. Ann. Neurol. 2006;59:490–498. doi: 10.1002/ana.20784. [DOI] [PubMed] [Google Scholar]
- 106.Miller RA, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J. Gerontol. A: Biol. Sci. Med. Sci. 2011;66:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Harrison DE, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Demontis F, Perrimon N. FOXO/4E-BP signaling in Drosophila muscles regulates organism-wide proteostasis during aging. Cell. 2010;143:813–825. doi: 10.1016/j.cell.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rubinsztein DC, et al. Autophagy and aging. Cell. 2011;146:682–695. doi: 10.1016/j.cell.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 110.Menzies FM, et al. Protein misfolding disorders and macroautophagy. Curr. Opin. Cell Biol. 2011;23:190–197. doi: 10.1016/j.ceb.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ravikumar B, et al. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat. Genet. 2004;36:585–595. doi: 10.1038/ng1362. [DOI] [PubMed] [Google Scholar]