Abstract
In contrast with pathological hypertrophy, exercise-induced physiological hypertrophy is not associated with electrical abnormalities or increased arrhythmia risk. Recent studies have shown that increased cardiac-specific expression of phosphoinositide-3-kinase-α (PI3Kα), the key mediator of physiological hypertrophy, results in transcriptional upregulation of ion channel subunits in parallel with the increase in myocyte size (cellular hypertrophy) and the maintenance of myocardial excitability. The experiments here were undertaken to test the hypothesis that Akt1, which underlies PI3Kα-induced cellular hypertrophy, mediates the effects of augmented PI3Kα signaling on the transcriptional regulation of cardiac ion channels. In contrast to wild-type animals, chronic exercise (swim) training of mice (Akt1−/−) lacking Akt1 did not result in ventricular myocyte hypertrophy. Ventricular K+ current amplitudes and the expression of K+ channel subunits, however, were increased markedly in Akt1−/− animals with exercise training. Expression of the transcripts encoding inward (Na+ and Ca2+) channel subunits were also increased in Akt1−/− ventricles following swim training. Additional experiments in a transgenic mouse model of inducible cardiac-specific expression of constitutively active PI3Kα (icaPI3Kα) revealed that short-term activation of PI3Kα signaling in the myocardium also led to the transcriptional upregulation of ion channel subunits. Inhibition of cardiac Akt activation with triciribine in this (inducible caPI3Kα expression) model did not prevent the upregulation of myocardial ion channel subunits. These combined observations demonstrate that chronic exercise training and enhanced PI3Kα expression/activity result in transcriptional upregulation of myocardial ion channel subunits independent of cellular hypertrophy and Akt signaling.
Keywords: PI3Kα signaling, electrical remodeling, ion channel, Akt
1.Introduction
Pathological cardiac hypertrophy, a maladaptive response of the myocardium to increased biomechanical stresses, is associated with increased risk of heart failure and life-threatening ventricular arrhythmias [1, 2]. Several previous studies in animal models and in patients with left ventricular hypertrophy (LVH) and heart failure have demonstrated that repolarizing K+ current densities are decreased, resulting in action potential and QT prolongation, both of which are arrhythmogenic and predispose individuals to life-threatening arrhythmias [3, 4]. Recent studies in a mouse model of pressure overload-induced LVH, produced by transverse aortic constriction, revealed that reduced repolarizing K+ current densities result directly from the failure to upregulate the expression of the underlying K+ channel subunits in proportion to the increases in LV myocyte size (hypertrophy) [5], resulting in reduced LV K+ current densities and action potential prolongation.
Chronic exercise training, particularly in elite athletes, also produces cardiac hypertrophy, but this physiological hypertrophy is not typically associated with electrical abnormalities or increased risk of life-threatening ventricular arrhythmias [6–8]. It is important to note, however, that exercise, even routine exercise, can trigger lethal arrhythmias in individuals with previously unrecognized congenital cardiac electrical or structural defects [6–8]. These events, however, appear to reflect the impact of exercise-induced neurohumoral changes on the effects of the underlying congenital defect on the regulation of cardiac electrical functioning. In recent studies, we demonstrated that physiological hypertrophy, induced by exercise training or by cardiac-specific expression of constitutively active phosphoinositide-3-kinase p110α (caPI3Kα), is associated with transcriptional upregulation of the subunits encoding the K+, Ca2+ and Na+ channels that underlie action potential generation in the ventricular myocardium [9]. This balanced upregulation of ion channel subunits results in increased repolarizing (outward) and depolarizing (inward) current amplitudes in ventricular myocytes in proportion to the cellular hypertrophy, thereby normalizing current densities, action potential waveforms and QT intervals. It has also been demonstrated recently that enhancing PI3Kα signaling in the setting of pathological hypertrophy or heart failure similarly results in the transcriptional upregulation of K+ channel subunits, normalizing K+ current densities and preserving ventricular functioning [10]. Despite the marked effects of enhanced PI3Kα signaling on myocardial ion channel gene expression, the molecular mechanisms linking PI3Kα signaling to transcriptional regulation of ion channel subunits have not been explored.
PI3Kα, the Class IA component of the PI3K enzyme family, converts the plasma membrane lipid phosphatidylinositol-4,5-bisphosphate (PIP2) to phosphatidylinositol-3,4,5-trisphosphate (PIP3), which initiates the activation of downstream signaling molecules containing pleckstrin-homology (PH) domains such as phosphatidylinositol-dependent kinase 1 (PDK1), Bruton’s tyrosine kinase (Btk), ADP-ribosylating factor 6 (ARF6) and Akt [11]. Akt is a well-characterized serine threonine kinase downstream of PI3Kα, and consists of three isoforms, Akt1, Akt2 and Akt3, two of which, Akt1 and Akt2, are highly expressed in the heart [12]. Importantly, Akt1 has been shown to be required for the development of physiological hypertrophy induced by chronic exercise training [13], as well as the regulation of normal cardiac growth [14]. Akt2, on the other hand, plays a critical role in insulin-regulated glucose homeostasis, as well as in cardiomyocyte survival [15–17]. Because of the pivotal role of PI3Kα-Akt1 signaling axis in mediating physiological hypertrophy, we hypothesized that the increased expression of ion channel subunits seen with enhanced PI3Kα signaling also depends on Akt1. Genetic and pharmacological approaches were utilized here in experiments designed to explore this hypothesis directly. Unexpectedly, these experiments revealed that PI3Kα-mediated electrical remodeling, reflecting transcriptional upregulation of ion channel subunits, is independent of cellular hypertrophy and Akt signaling.
2.Methods
2.1 Experimental animals
Animals were handled in accordance with the NIH Guide for the Care and Use of Laboratory Animals. All protocols involving animals were approved by the Animal Studies Committee at Washington University Medical School.
2.1.1 Chronic Swim Training in Akt1−/− and WT mice
Experiments were performed on adult (8–10 week) Akt1−/− mice (in the C57Bl/6 background) [13, 14] with (n=14) and without (n=15) chronic exercise (swim) training [9, 18]. For swim training, animals were placed in a small tank (surface area of 225 cm2) filled with still water maintained at 30–32°C to avoid thermal stress. Multiple (6–8) mice were placed in the same tank, where they swam in group and floating was prevented. The initial swim time was 20 min, and was increased by 10 min per day until 90 min sessions were reached. Once attained, the 90 min training schedule was continued twice a day (separated by 4–5 hr), 7 days a week, for 4 weeks. We and others have previously demonstrated that this chronic swim training protocol induces robust physiological cardiac hypertrophy in wild-type mice [9, 13, 18]. Similar experiments were carried out here on adult WT (C57Bl/6) mice with and without swim training (n=5).
2.1.2 Transgenic Mouse Model of Inducible Cardiac-Specific Expression of Constitutively Active PI3Kα (icaPI3Kα)
Additional experiments were conducted on a mouse model of cardiac-specific expression of caPI3Kα transgene driven by a tet-responsive (tet-off) α-MHC promoter [19]. Double transgenic (FVB/N) animals, carrying both the tTA and caPI3Kα transgenes (icaPI3Kα), were maintained on a doxycycline-containing (200mg/kg) diet to suppress transgene expression. The expression of the caPI3Kα transgene was induced by removing the doxycycline-containing diet in adult (8–10 week) icaPI3Kα animals [19]. The activation of PI3Kα signaling was confirmed by Western blot analyses of Akt phosphorylation in LV extracts from icaPI3Kα animals 4 weeks after removal of the doxycycline-containing diet.
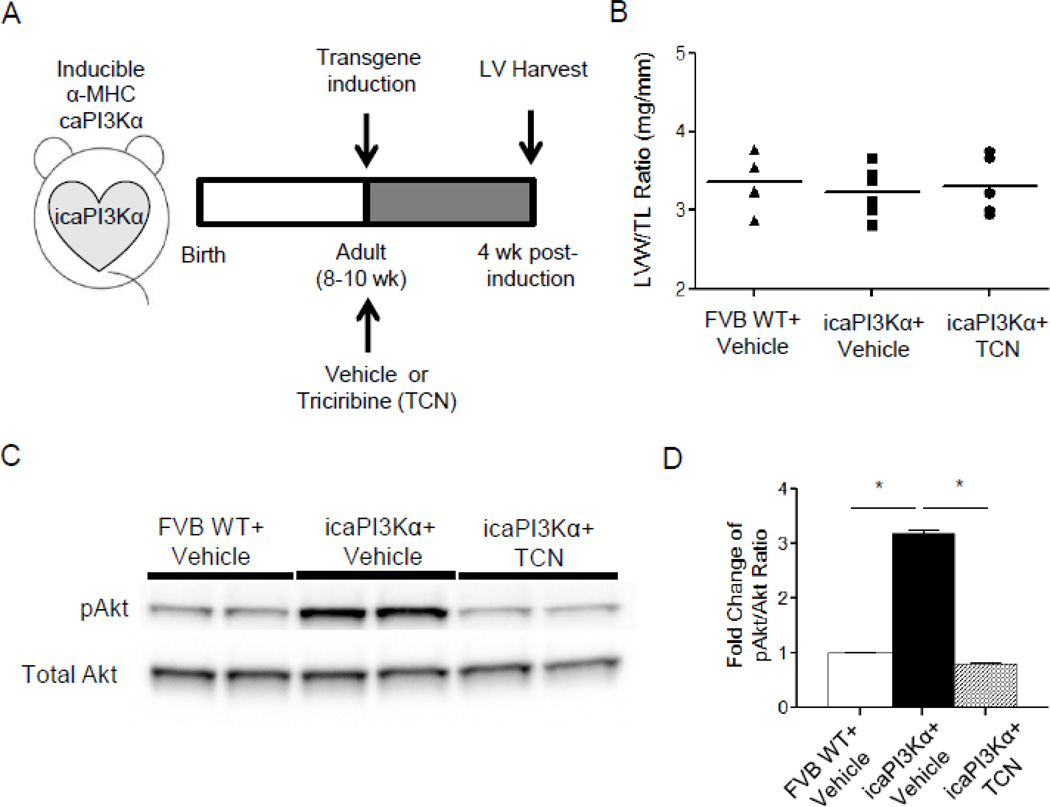
To inhibit the activation of the downstream effectors of PI3Kα, Akt1 and Akt2, a pan-Akt inhibitor triciribine (TCN, Sigma Aldrich, St Louis, MO) was given (0.5 mg/kg/day in 200 µL 20% DMSO saline solution, intraperitoneally [i.p.]) [20] to icaPI3Kα animals (n=15) simultaneous with the removal of the doxycycline-containing diet (and the activation of caPI3Kα transgene); TCN injections were administered daily for 4 weeks (see Figure 5A for a schematic illustration of the caPI3Kα transgene induction and the TCN injection protocol). Vehicle-treated icaPI3Kα and wild-type (FVB/N) animals (n=15 in each group) were compared.
Figure 5. Administration of triciribine (TCN) in icaPI3Kα animals blocks the hyperphosphorylation of cardiac Akt.
(A) Schematic illustration of the caPI3Kα transgene induction and the pan-Akt inhibitor triciribine (TCN) injection protocol in icaPI3Kα animals. (B) LVW/TL ratios were similar in WT+Vehicle, icaPI3Kα+Vehicle and icaPI3Kα+TCN animals. (C) Representative Western blots of fractionated LV proteins from WT+Vehicle, icaPI3Kα+Vehicle and icaPI3Kα+TCN animals (n=4 in each group) probed with anti-pAkt and anti-total Akt antibodies. The expression level of pAkt in each lane on each blot was measured and normalized to the expression of total-Akt in the same lane on the same blot. Mean pAkt/Akt ratios in the LV from icaPI3Kα animals, vehicle- or TCN-treated, were expressed relative to the mean value in the control (WT+Vehicle) LV samples. (D) The mean ± SEM pAkt/Akt ratio is significantly (*P<0.001) higher in icaPI3Kα+Vehicle (n=4), compared with WT+Vehicle (n=4), LV. In addition, 4 week administration of TCN significantly (*P<0.001) reduced the phosphorylation of Akt in icaPI3Kα LV (see text).
2.3 Electrophysioloigcal Recordings
Surface electrocardiograms (ECG) were recorded from anesthetized (Tribromoethanol, 0.25 mg/kg, i.p.) swim-trained and untrained Akt1−/− animals, with needle electrodes connected to a dual bioamplifier (AD Instrument, PowerLab 26T) using described methods [9, 10].
Body weights, tibia lengths and LV weights were measured and recorded at the time of tissue harvesting. Hearts were removed from anesthetized animals, mounted on a Langendorfapparatus and perfused retrogradely through the aorta with 25 ml of (0.8 mg/ml) collagenase-containing (type II, Worthington) solution [5, 9]. Following perfusion, the LV apex was separated, mechanically dispersed, plated on laminin-coated coverslips and maintained in a 95% air-5% CO2 incubator. Whole-cell voltage-clamp recordings were obtained from LV apex myocytes within 24 hr of isolation at room temperature (22–24°C). All voltage-clamp experiments were performed using an Axopatch 1B patch clamp amplifier (Molecular Devices) interfaced to a microcomputer with a Digidata 1332 analog/digital interface and the pCLAMP9 software package (Molecular Devices) as described previously [9, 10].
2.4 Transcript analyses
Total RNA from the LV of individual animals was isolated and treated with DNase using described methods [5, 9]. Using equal amounts of RNA, transcript analyses of genes encoding K+, Na+ and Ca2+ channel pore-forming (α) and accessory subunits, atrial natriuretic factor (ANF) and hypoxanthine-guanine phosphoribosyltransferase (Hprt) were carried out using SYBR green RT-PCR in a two-step process [5, 9]. Data were analyzed using the threshold cycle (CT) relative quantification method and normalized to Hprt. For each transcript, the normalized values were then expressed relative to the mean of the control (untrained Akt1−/− or WT+Vehicle) LV samples.
2.5 Citrate Synthase Activity
Citrate synthase (CS) activity, an indicator of aerobic muscle activity, was measured in gastrocnemius muscles dissected from swim-trained (n=4) and untrained (n=4) Akt1−/− animals [21], weighed and frozen in liquid nitrogen. Frozen samples were homogenized on ice in 100 mM Tris-HCl, and protein concentrations were determined using the BCA protein Assay Kit (Pierce). Individual tissue homogenates (5µl) were then added to a (1 ml) reaction mix containing: 100 mM Tris-HCl, 1.0 mM dithiobis-(2-nitrobenzoic acid), 10 mM oxaloacetate and 0.2 mM acetyl CoA. The absorbance of each sample at 412 nm was recorded at 25 °C every 30 seconds for 5 minutes. Mean absorbance change per minute was determined and citrate synthase activity (in µmol*mg protein−1*min−1) was calculated using the extinction coefficient (13.6 mM−1*cm−1) of 5-thio-2-nitrobenzoic acid at 412 nm [21].
2.6 Biochemical Analyses
Protein lysates were prepared from the LV of FVB WT+Vehicle, icaPI3Kα+Vehicle and icaPI3Kα+TCN animals using described methods [9]. Protein concentrations were determined using the BCA protein Assay Kit (Pierce). For Western blot analyses, equal amounts of total proteins prepared from individual control and experimental animals were loaded on 7.5–12% SDS-PAGE gels and transferred to PVDF membranes. The PVDF membrane strips were incubated in 5% skim milk in PBS containing 0.1% Tween 20 (blocking buffer) for 1 hr at room temperature, followed by overnight incubation at 4°C with a rabbit polyclonal anti-phospho-Akt (S473) or anti-total Akt primary antibody (Cell Signaling Technology, Danvers, MA, USA).
After washing, the membrane strips were incubated for 1 hr at room temperature with an alkaline phosphatase-conjugated secondary antibody (GE Healthcare, Buckinghamshire, UK) diluted in blocking buffer, and bound antibodies were detected using a chemiluminescent alkaline phosphate substrate. Protein band intensities were quantified by densitometry (Quantity One Basic, Bio-Rad Laboratory, Hercules, CA), and expression of phospho-Akt (p-Akt) in each sample was normalized to the expression of total Akt in the same sample on the same blot. Mean p-Akt protein expression data in icaPI3Kα ventricles were expressed relative to the mean value in the control (WT+Vehicle) LV samples.
2.7 Statistical analyses
All averaged electrophysiological, transcript and Western blot data are presented as means ± SEM. The statistical significance of differences between experimental groups was evaluated by the Student’s t test or the Mann-Whitney U test.
3. Results
3.1 Upregulation of repolarizing K+ Currents with Chronic Swim Training Does Not Require Akt1
In recent studies, we demonstrated that chronic exercise (swim) training-induced physiological hypertrophy, which results in increased PI3Kα signaling [9, 18], leads to the upregulation of ionic currents in left ventricular (LV) myocytes in parallel with the increase in size [9]. The critical downstream effector of PI3Kα-mediated physiological hypertrophy in response to exercise training is the serine-threonine kinase Akt1 [13]. Indeed, chronic swim training has been shown previously [9, 18, 22], to increase the activation (hyperphosphorylation) of Akt as a result of increased PI3Kα activity. It has also been demonstrated that, in contrast with WT animals, mice lacking Akt1 (Akt1−/−) [13, 14] fail to develop LV hypertrophy following exercise training, revealing the critical role of Akt in hypertrophic cell growth [13].
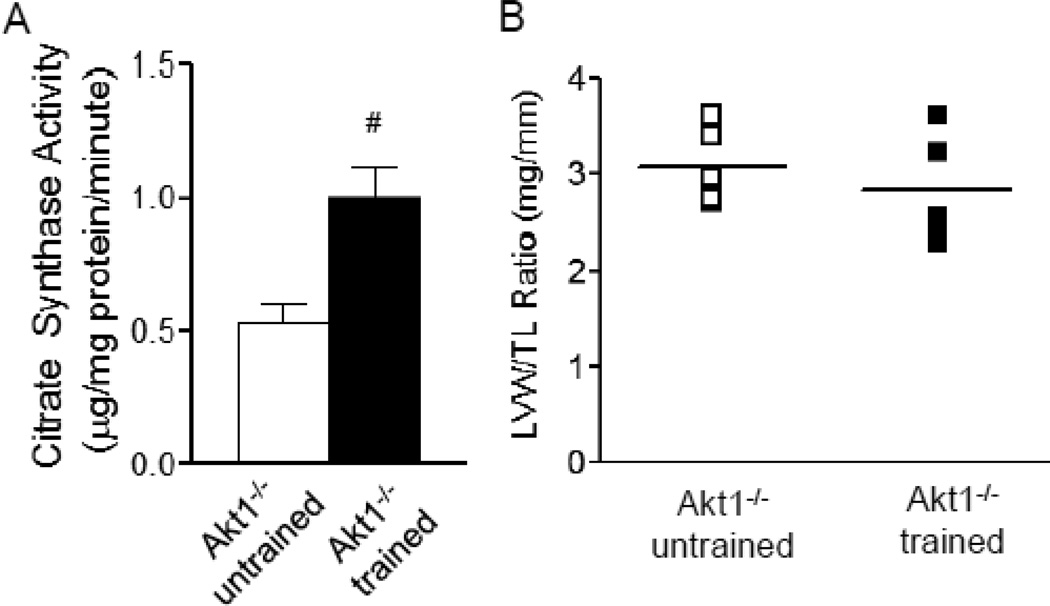
To test the hypothesis that Akt1 is also required for exercise (PI3Kα)-induced electrical remodeling, adult (8–10 week) Akt1−/− mice were subjected to chronic swim training for 4 weeks. Importantly, it has previously been reported that Akt1−/− mice are fertile with normal life span [16] and that Akt2 and Akt3 expression levels in adult Akt1−/− and WT mouse hearts [13] are indistinguishable. In contrast to WT animals, for which similar chronic swim training increases LVW/TL ratios by ~20% [13], however, no LV hypertrophy was evident in Akt1−/− animals: LVW/TL ratios (Figure 1B), as well as heart weight/body weight and LVW/body weight ratios in swim-trained and untrained Akt1−/− animals (Supplemental Figure 1A,B) were not significantly different. These observations are consistent with previous suggestions that Akt1 is required for exercise training-induced physiological hypertrophy [13]. Following 4 weeks of swim training (see Methods), however, mean ± SEM citrate synthase activity in the gastrocnemius muscles was increased significantly (P<0.01) to 1.01±0.12 µmol/mg protein/min (n=4), compared with the value of 0.53±0.07 µmol/mg protein/min (n=4) measured in muscles from untrained Akt1−/− animals (Figure 1A), indicating increased metabolic activity and, therefore, the adequacy of exercise produced with chronic swim training [23].
Figure 1. Chronic swim training did not result in cardiac hypertrophy in Akt1−/− animals.
(A) Citrate synthase activity was increased significantly (P<0.01) in gastrocnemius muscles from swim-trained, compared with untrained, Akt1−/− animals (n=4 in each group), indicating increased muscle metabolic activities with exercise training [23]. (B) LV mass/tibia length (LVM/TL) ratios were determined in swim trained and untrained Akt1−/− animals (n=6 in each group); individual and mean ± SEM values are plotted.
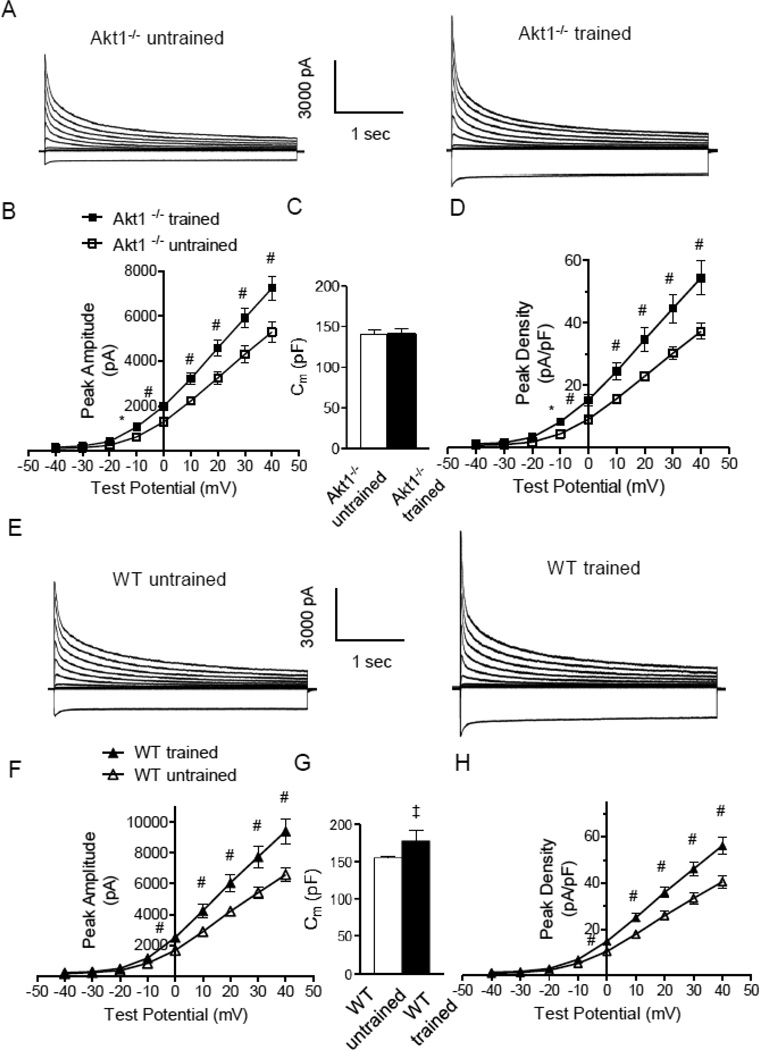
Whole-cell voltage-clamp recordings were obtained from LV myocytes isolated from swim-trained and untrained Akt1−/− mice to assess effects of the loss of Akt1 on the remodeling of repolarizing K+ channels associated with exercise that is observed in WT mice [9]. As illustrated in Figure 2, these experiments revealed that the amplitudes of the peak (IK,peak) outward voltage-gated (Kv) and inwardly rectifying (Kir) K+ currents were significantly higher in LV myocytes from swim-trained, compared with untrained, Akt1−/− animals (P<0.05; Figure 2A,B). The mean ± SEM whole-cell membrane capacitances (Cm) of LV myocytes isolated from Akt1−/− mice with and without swim training (Figure 2C), however, were similar, observations consistent with the absence of hypertrophic growth of LV myocytes with exercise in the absence of Akt [12, 13]. Normalization of the measured K+ current amplitudes in individual cells to the whole cell capacitance (myocyte size) in the same cell revealed that, similar to the current amplitudes, mean ± SEM repolarizing IK,peak and IK1 densities were significantly (P<0.05) higher in Akt1−/− LV myocytes following swim training (Figure 2D and Table 1).
Figure 2. Repolarizing K+ current amplitudes and densities were increased in LV myocytes isolated from swim-trained Akt1−/− animals.
(A) Representative whole-cell K+ currents, recorded from myocytes isolated from the LV apex of swim-trained and untrained C57Bl/6 Akt1−/− mice, are illustrated. Currents were evoked in response to (4.5 s) voltage steps to test potentials between −120 and +40 mV from a holding potential (HP) of −70 mV. (B) The mean ± SEM amplitudes of the peak outward K+ currents were significantly (#P<0.01,*P<0.001) higher in LV apex myocytes from swim-trained, compared with untrained Akt1−/− animals. (C) Mean ± SEM whole-cell membrane capacitances (Cm) were similar in LV myocytes isolated from swim-trained and untrained Akt1−/− animals, consistent with the absence of hypertrophic growth in response to exercise training. (D) Normalizing current amplitudes to cell size (Cm) revealed that mean ± SEM IK,peak densities were also significantly higher in LV apex myocytes from swim-trained, compared with untrained, Akt1−/− animals. (E) Representative whole-cell K+ currents, recorded from myocytes isolated from the LV apex of swim-trained and untrained WT (C57Bl/6) mice, are illustrated. (F) The mean ± SEM amplitudes of the peak outward K+ currents (IK,peak) were significantly (#P<0.01) higher in LV apex myocytes from swim-trained, compared with untrained, WT (C57Bl/6) animals. (G) Mean ± SEM whole-cell Cm were significantly (‡P<0.05) higher in LV myocytes isolated from swim-trained, compared to untrained, WT (C57Bl/6) animals, consistent with the development of hypertrophic growth in response to chronic swim training. (H) Normalizing current amplitudes to whole-cell membrane capacitance revealed that mean ± SEM IK,peak densities were also significantly higher in LV apex myocytes from swim-trained, compared with untrained, WT animals.
Table 1.
Kv and Kir Currents in Swim-trained and Untrained Akt1−/−, WT+Vehicle, icaPI3Kα+Vehicle and icaPI3Kα+TCN LV Myocytes.*
| LV Cells | Cm (pF) | IK,peak | Ito,f | IK,slow | Iss | IK1 | |
|---|---|---|---|---|---|---|---|
| Akt1−/− untrained | 141 ± 6 | ||||||
| (n=23) | τ (ms) | — | 89 ± 6 | 1597 ± 112 | — | — | |
| Amplitude (pA) | 5289 ± 436 | 2103 ± 225 | 2118 ± 194 | 1067 ± 68 | −1276 ± 80 | ||
| Density (pA/pF) | 37.2 ± 2.5 | 14.7 ± 1.4 | 14.9 ± 1.1 | 7.6 ± 0.5 | −8.4 ± 0.9 | ||
| Akt1−/− trained | 141 ±7 | ||||||
| (n=20) | τ (ms) | — | 75 ± 6 | 1303 ± 61 | — | — | |
| Amplitude (pA) | 7235 ± 523# | 3387 ± 385# | 2557 ± 198 | 1291 ± 91‡ | −2471 ± 139* | ||
| Density (pA/pF) | 54.3 ± 5.4# | 25.9 ± 3.4# | 19.2 ± 2.1 | 9.3 ± 0.6‡ | −12.5 ± 0.6* | ||
| WT+Vehicle | 181 ± 9 | ||||||
| (n=33) | τ (ms) | — | 121 ± 9 | 1097 ± 95 | — | — | |
| Amplitude (pA) | 11485 ± 659 | 4159 ± 314 | 5399 ± 391 | 1599 ± 91 | −2677 ± 173 | ||
| Density (pA/pF) | 67.2 ± 4.0 | 24.8 ± 2.1 | 31.1 ± 2.0 | 9.1 ± 0.4 | −14.9 ± 1.0 | ||
| icaPI3Kα+Vehicle | 177 ± 9 | ||||||
| (n=24) | τ (ms) | — | 109 ± 55 | 981 ± 55 | — | — | |
| Amplitude (pA) | 16087 ± 1139* | 6424 ± 734# | 6780 ± 471‡ | 1837 ± 104 | −3457 ± 284‡ | ||
| Density (pA/pF) | 92.2 ± 5.9# | 38.8 ± 4.4# | 41.2 ± 3.1‡ | 11.2 ± 0.7 | −19.1 ± 1.4‡ | ||
| icaPI3Kα+TCN | 171 ± 6 | ||||||
| (n=21) | τ (ms) | — | 90 ± 36 | 949 ± 30 | — | — | |
| Amplitude (pA) | 15394 ± 903* | 6199 ± 493* | 6563 ± 367‡ | 1751 ± 94 | −3077 ± 188‡ | ||
| Density (pA/pF) | 96.1 ± 6.0* | 35.0 ± 2.5# | 37.4 ± 2.1‡ | 10.0 ± 0.5 | −18.8 ± 1.4‡ | ||
All values are means ± SEM. Kv and Kir current amplitudes/densities reported here were determined at +40 mV and −120mV, respectively. Measured K+ current amplitudes/densities in swim-trained and untrained Akt1−/− LV myocytes were compared and K+ currents in icaPI3Kα+Vehicle and icaPI3Kα+TCN LV myocytes were both compared to results from WT+Vehicle animals.
Values that are significantly (‡P<0.05, #P<0.01, *P<0.001) different are indicated.
Kv indicates voltage-gated K+ currents; Kir, inwardly rectifying K+ currents; TCN: triciribine; Cm, cell membrane capacitance; τ, time constant of inactivation.
Parallel experiments conducted on adult (8–10 week) WT (C57Bl/6) animals revealed that, similar to previous findings in WT (FVB/N) animals [9, 10, 18], chronic swim training results in marked LV myocyte hypertrophy, clearly evident in the increase in the mean ± SEM whole-cell membrane capacitance (Cm) of the LV myocytes from trained, compared with untrained, WT (C57Bl/6) animals (Figure 2G). As expected, swim training resulted in increased repolarizing ventricular K+ current amplitude in WT C57Bl/6 LV myocytes (Figure 2E,F). The magnitude of the increase in current amplitudes is similar to the Akt1−/− LV myocytes (Figure 2B), as well as to that observed in LV myocytes from WT FVB/N mice [9, 10], revealing that the cellular response to chronic exercise is the same in WT animals in the C57Bl/6 and FVB/N genetic backgrounds. Normalization of the measured peak outward K+ current amplitudes (Figure 2F) for differences in myocyte size (Cm) (Figure 2G) revealed that, similar to the observations in WT FVB/N mice [9], peak K+ current densities are actually higher in LV cells from swim-trained, compared to untrained, WT C57Bl/6 animals (Figure 2H).
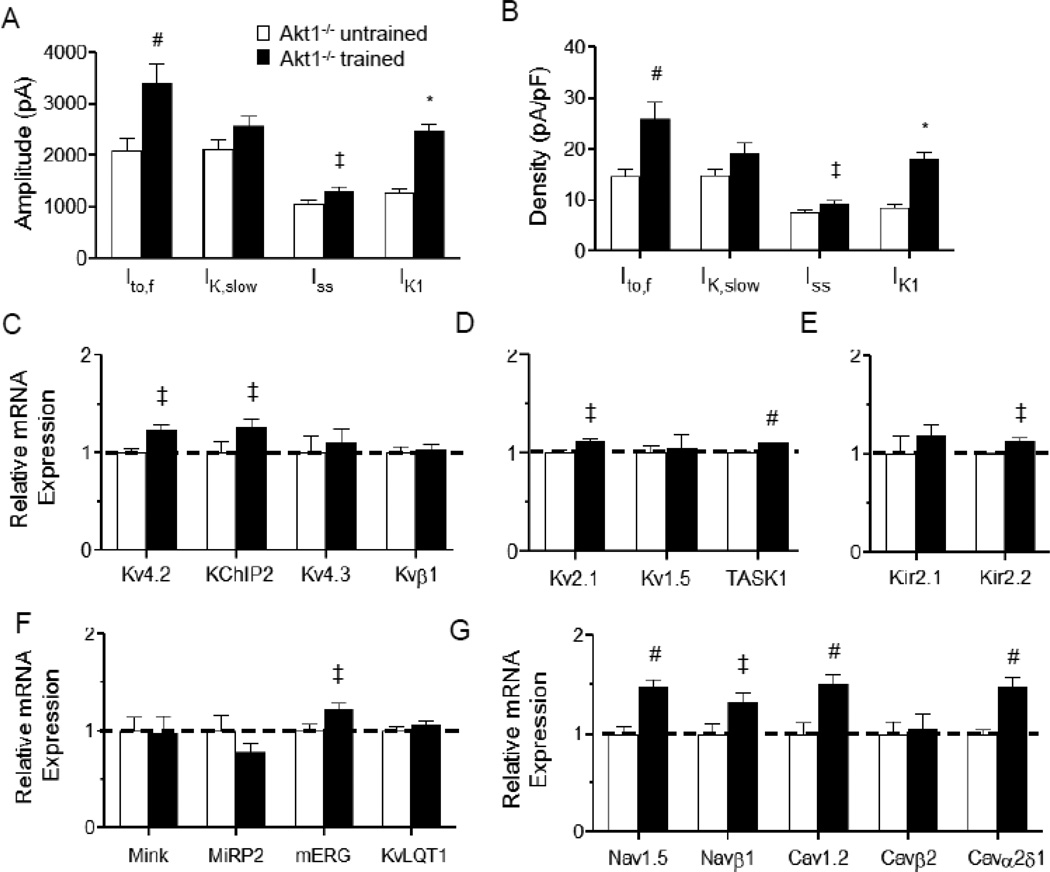
Kinetic analyses of the decay phases of the outward K+ currents revealed that the amplitudes of the individual Kv current components, Ito,f and Iss, were significantly (P<0.05) higher in LV myocytes from swim-trained, than in untrained, Akt1−/− animals (Figure 3A). The amplitudes of IK,slow were also higher in Akt1−/− LV myocytes with swim training, although the differences did not reach statistical significance. In contrast, there were no measurable differences in the time- or the voltage-dependent properties of the Kv currents in LV cells from Akt1−/− mice with and without swim training (Table 1). Normalizing the measured K+ current amplitudes (Figure 3A) to the whole-cell membrane capacitance (in the same cell) revealed that Ito,f and Iss current densities are significantly (P<0.05) higher in the LV myocytes from swim-trained, compared to untrained, Akt1−/− mice (Figure 3B).
Figure 3. Increased ventricular K+ current amplitudes with chronic exercise training in animals lacking Akt1 is accompanied by transcriptional upregulation of channel subunits.
The amplitudes of the individual Kv current components, Ito,f, IK,slow, and Iss, as well as of IK1, in the LV apex myocytes from swim-trained and untrained Akt1−/− animals were determined (as described in Methods). Mean ± SEM Ito,f and Iss (at +40 mV) and IK1 (at −120 mV) amplitudes (A) and densities (B) were significantly (‡P<0.05, #P<0.01, *P<0.001) higher in LV apex myocytes from swim-trained, compared with untrained, Akt1−/− animals. Expression levels of the transcripts encoding repolarizing K+ (C–F), as well as depolarizing Na+ and Ca2+ (G), channel subunits were measured in individual LV samples from swim-trained (n=6) and untrained (n=6) Akt1−/− LV, normalized to Hprt and subsequently to the mean value of the untrained Akt1−/− LV samples. The mean ± SEM relative expression levels of many ion channel subunit transcripts were significantly (‡P<0.05, #P<0.01,*P<0.001) higher in swim-trained, than in untrained, Akt1−/− LV.
3.2 Transcriptional Upregulation of Ion Channel Subunits with Chornic Exercise Training Does Not Require Akt1
Recent studies demonstrated that the increases in ventricular K+ current amplitudes with chronic exercise training reflect the upregulation of the transcripts encoding the underlying K+ channel pore forming and accessory subunits [9]. Subsequent experiments here, therefore, were aimed at determining if the observed increases in K+ current amplitudes in Akt1−/− LV myocytes with swim training (Figure 2B) also reflect the transcriptional upregulation of K+ channel subunits.
As illustrated in Figure 3C–G, quantitative RT-PCR revealed that the expression levels of the transcripts encoding the Ito,f channel pore-forming (α) subunit, Kcnd2 (Kv4.2) [24], and the Ito,f channel accessory subunit, Kcnip2 (KChIP2) [25, 26], were increased significantly (P<0.05) in the LV of swim-trained, compared with untrained Akt1−/− animals (Figure 3C). The expression levels of Kcnb1 (Kv2.1), which encodes IK,slow2 [27], and of the K2P channel subunit, Kcnk3 (TASK1), which has been suggested to underlie Iss in rat cardiomyocytes [28], were also significantly (P<0.05) higher in swim-trained Akt1−/− LV (Figure 3D). Similarly, the expression levels of the IK1 channel subunit, Kcnj12 (Kir2.2) [29], as well as of Kcnh2 (mERG) [30], the α subunit encoding the rapid cardiac delayed rectifiers, IKr, in large mammals, were also elevated in Akt1−/− LV following swim training (Figure 3E,F). In addition, the transcripts encoding depolarizing voltage-gated Na+ and Ca2+ channel pore-forming and accessory subunits were also significantly (P<0.05) higher in swim-trained, compared with untrained, Akt1−/− LV (Figure 3G). Similar to WT animals [9], therefore, chronic exercise training leads to the transcriptional upregulation of the subunits encoding both repolarizing and depolarizing cardiac ion channels. Importantly, the results here demonstrated that transcriptional remodeling of channel subunits is independent of cellular hypertrophy and the presence of Akt1. Also similar to previous findings in WT mice [9], this parallel upregulation of the subunits encoding depolarizing and repolarizing myocardial ion channels in response to exercise training results in the maintenance of normal myocardial excitability: ECG waveforms in swim-trained and untrained Akt1−/− animals were indistinguishable (Figure 4A, B).
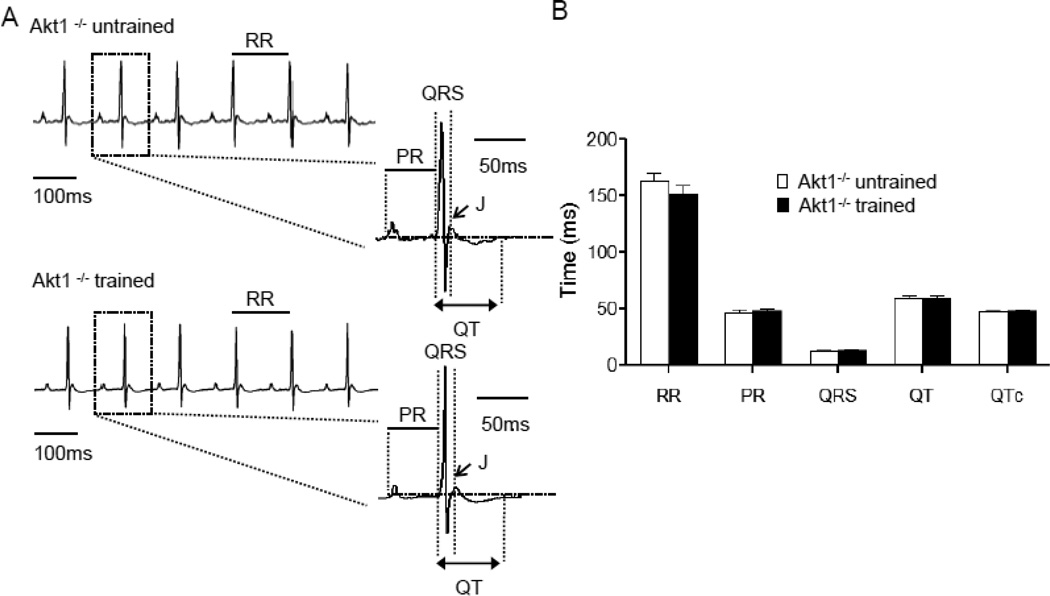
Figure 4. ECG waveforms in Akt1−/− animals with and without chronic swim-training are indistinguishable.
(A) Representative ECG (lead II) waveforms from anesthetized adult Akt1−/− mice, with and without swim-training, are illustrated. (B) Mean ± SEM RR, PR, QRS, QT and QTc intervals in Akt1−/− animals with and without swim-training were not significantly different.
3.3 Short-term Activation of Cardiac PI3Kα Signaling Upregulates Repolarizing K+ Currents Independent of Akt
It has previously been reported that cardiac specific expression of constitutively active PI3Kα (caPI3Kα) mimicks the effects of exercise training, increasing repolarizing ventricular K+ current amplitudes and normalizing K+ current densities to the increase in myocyte size (hypertrophy) [9, 10]. Also similar to exercise training, cardiac-specific expression of caPI3Kα results in transcriptional upregulation of the subunits encoding repolarizing (K+) and depolarizing (Na+/Ca2+) channels [9, 10]. Additional experiments here, therefore, were designed to test the hypothesis that Akt activation is required for the upregulation of ventricular K+ currents and ion channel subunits in response to increased PI3Kα signaling.
For these experiments, a mouse model previously developed and described by Yano and colleagues [19] with tetracycline transactivator (tet-off) controlled, cardiac-specific expression of caPI3Kα (icaPI3Kα), was utilized. In this transgenic mouse line, cardiac PI3Kα activity and Akt phosphorylation are significantly increased with transgene induction following the removal of dietary doxycycline for 2 weeks [19]. Interestingly, short-term (2–8 week) activation of cardiac PI3Kα in this model does not produce measurable cardiac hypertrophy, despite the marked increase in PI3Kα activity [19]. This transgenic mouse model, therefore, allows a unique opportunity to explore the phenotypic effects of increased PI3Kα signaling in vivo before the development of measurable ventricular hypertrophy.
Using the protocol schematized in Figure 5A, expression of the caPI3Kα transgene was induced in adult (8–10 week) icaPI3Kα animals by removing the doxycycline-containing diet. Similar to the findings reported in the original description of the icaPI3Kα mouse line [19], removal of the doxycycline-containing diet for 4 weeks, which results in increased expression of the caPI3Kα transgene [19], did not produce measurable cardiac hypertrophy (Figure 5B). Ventricular PI3Kα signaling, however, was increased significantly (P<0.001), evident in the ~3 fold increase in phospho-Akt (pAkt) and in the pAkt/total Akt ratio in icaPI3Kα, compared with WT, LV (Figure 5C,D).
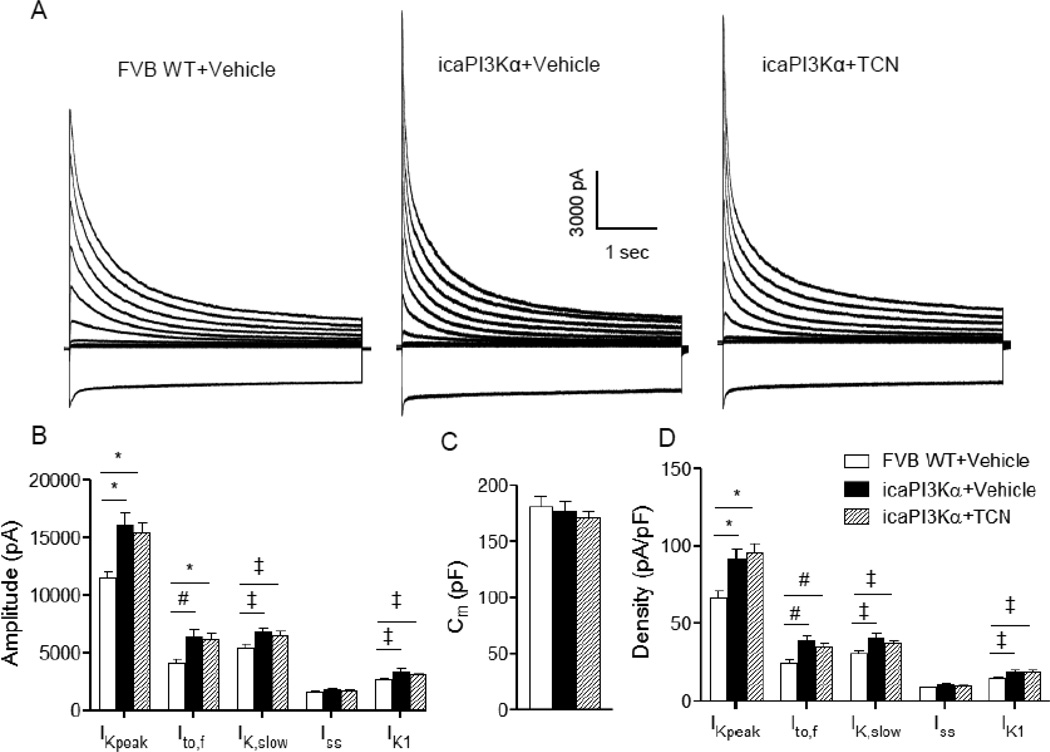
Voltage-clamp recordings revealed that the amplitudes of IK,peak and IK1, as well as of the Kv current components, Ito,f and IK,slow, were increased significantly (P<0.05) in icaPI3Kα, compared with WT, LV myocytes (Figure 6A, B); the mean ± SEM amplitude of Iss was also higher in icaPI3Kα LV myocytes, although this increase was not statistically significant. Although the amplitudes were increased, the time- and voltage-dependent properties of ITO,F and IK,slow in icaPI3Kα and WT cells were not significantly different (Table 1). In addition, the mean Cm (Figure 6C) values determined in icaPI3Kα and WT LV myocytes were similar, revealing that cellular hypertrophy is not evident following 4 weeks of induced caPI3Kα expression. These observations are consistent with the absence of detectable LV hypertrophy in icaPI3Kα animals (Figure 5B) in spite of the increase in PI3Kα signaling (Figure 5C,D). The markedly higher K+ current amplitudes (Figure 6B) in icaPI3Kα LV myocytes, therefore, translates directly into increased K+ current densities (Figure 6D). Repolarizing Ito,f, IK,slow and IK1 densities were significantly (P<0.05) higher in icaPI3Kα, compared with WT, LV cells (Figure 6D).
Figure 6. Short-term activation of cardiac PI3Kα signaling upregulates repolarizing K+ currents in an Akt-independent manner.
(A) Representative whole-cell K+ currents, recorded from LV apex myocytes isolated from WT+Vehicle, icaPI3Kα+Vehicle and icaPI3Kα+TCN animals, are shown. (B) Mean ± SEM IK,peak, Ito,f, IK,slow and IK1 amplitudes in icaPI3Kα+Vehicle (n=24) were markedly higher than in WT+Vehicle (n=33) LV apex myocytes; K+ current (IK,peak, Ito,f, IK,slow and IK1) amplitudes were also significantly higher in icaPI3Kα LV apex myocytes treated with TCN (n=21). (C) Mean ± SEM Cm values were similar in WT+Vehicle, icaPI3Kα+Vehicle and icaPI3Kα+TCN LV apex myocytes, consistent with the absence of cellular (LV) hypertrophy with short term caPI3Kα induction (see text). (D) Normalization of current amplitudes for differences in cell size (Cm) revealed that mean ± SEM IK,peak, Ito,f, IK,slow and IK1 densities were significantly (‡P<0.05, #P<0.01, *P<0.001) higher in icaPI3Kα+vehicle, than in WT+Vehicle, LV apex myocytes. The higher K+ current densities observed in icaPI3Kα LV apex myocytes were unaffected by the TCN treatment.
To block Akt activation (hyperphosphorylation) in parallel with the induction of caPI3Kα transgene expression, a pan-Akt inhibitor triciribine (TCN) was administered (0.5 mg/kg/day, i.p.) daily (for four weeks) to icaPI3Kα animals simultaneous with the removal of the doxycycline-containing diet (see schematic in Figure 5A). The hyperphosphorylation of Akt in icaPI3Kα LV was completely abrogated with 4 weeks of TCN treatment (Figure 5C, D), indicating the successful blockade of Akt activation. As illustrated in Figure 6, however, the amplitudes of the repolarizing K+ currents, Ito,f, IK,slow, and IK1 were significantly (P<0.05) higher in LV myocytes isolated from icaPI3Kα+TCN, than from WT+vehicle, animals (Figure 6A, B). Normalizing the current amplitudes to the measured Cm values revealed that mean ± SEM IK,peak, Ito,f, IK,slow and IK1 densities were also significantly (P<0.05) higher (Figure 6D) in icaPI3Kα+TCN, compared to WT, LV cells.
3.4 Transcriptional Upregulation of Ion Channel Subunits with Short-term Activation of Cardiac PI3Kα Signaling is Akt-Independent
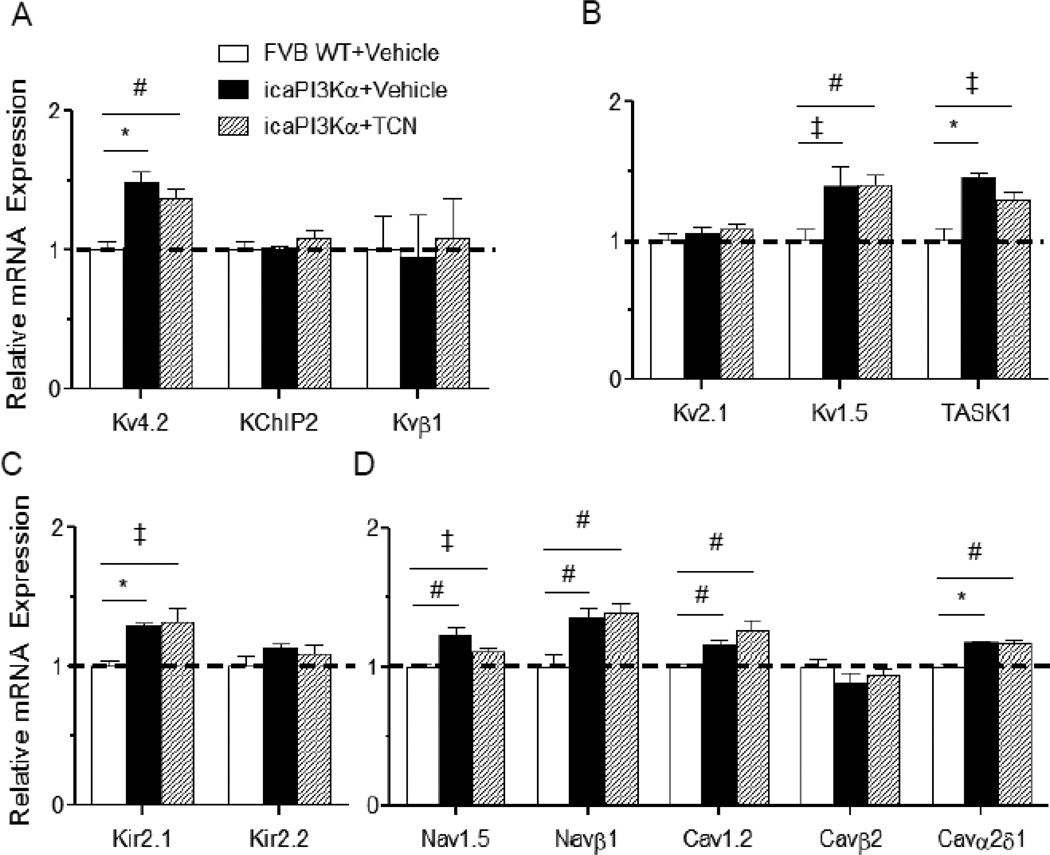
Quantitative RT-PCR experiments were conducted to determine ion channel subunit expression levels in the LV of WT+Vehicle, icaPI3Kα+Vehicle and icaPI3Kα+TCN mice. Consistent with the observed increases in K+ current amplitudes, the expression levels of the Ito,f channel subunit, Kv4.2, the IK,slow1 pore-forming subunit Kv1.5, the putative Iss subunit, TASK1, and the IK1 channel subunit, Kir2.1, were also higher in icaPI3Kα+Vehicle, compared to WT+Vehicle, LV (Figures 7A–C). In addition, the expression levels of transcripts encoding voltage-gated Na+ and Ca2+ channel subunits were higher in the LV of icaPI3Kα+Vehicle, than in WT+Vehicle, animals (Figure 7D). The observed transcriptional upregulation of these ion channel subunits with enhanced PI3Kα signaling, however, was not affected by TCN treatment (Figure 7A–D), revealing that PI3Kα-mediated transcriptional upregulation of myocardial ion channel subunits is independent of Akt.
Figure 7. Transcriptional upregulation of ion channel subunits with activation of PI3Kα signaling is independent of Akt.
Channel subunit transcript expression levels were measured in individual LV samples from WT+Vehicle, icaPI3Kα+Vehicle and icaPI3Kα+TCN LV (n=6 in each group), normalized to Hprt in the same sample and to the mean value of the WT+Vehicle control LV samples. The expression levels of the transcripts encoding several K+ (A,B,C), as well as Na+ and Ca2+ (D), channel subunits were significantly (‡P<0.05, *P<0.001) higher in icaPI3Kα+Vehicle, compared with WT+Vehicle, LV. In addition, increased expression of the channel subunit transcripts with PI3Kα induction was unaffected by the addition of TCN and the resulting inhibition of Akt.
4. Discussion
The phosphoinositide-3-kinases (PI3Ks) are a family of enzymes with both protein and lipid kinase activity that are known to be involved in many cellular processes [11]. PI3Kα is a heterodimeric lipid kinase with a 110 kDa catalytic subunit (p110α) and an 85 kDa regulatory subunit (p85) [11] that is activated upon stimulation of insulin or growth factor receptor-coupled tyrosine kinases [11]. Activation of PI3Kα catalyzes the conversion of the membrane lipid PIP2 to PIP3 which, in turn, recruits and activates downstream signaling cascades, most notably those involving Akt, which link PI3Kα to myocyte growth, hypertrophy, proliferation, survival, metabolism, aging and regeneration [31]. Surprisingly, the results of the experiments detailed here, however, revealed that Akt, despite its ubiquitous importance across a wide range of cellular processes, is not required for PI3Kα-mediated myocardial electrical remodeling.
4.1 Exercise Training-induced Electrical Remodeling is Independent of Cellular Hypertrophy and Akt1
Consistent with previous findings [13], the results here demonstrate that genetic deletion of Akt1 disrupts hypertrophic growth in response to chronic exercise training (Figure 1B,2C). The observation that repolarizing K+ current amplitudes and the expression of multiple ion channel subunits are upregulated in swim-trained Akt1−/− animals suggests that the electrical remodeling induced by exercise training does not require the presence of Akt1 and is, therefore, also unrelated to PI3Kα-induced, Akt1-dependent cellular hypertrophy. The observation that ion channel subunit transcripts are upregulated with exercise training independent of cardiac myocyte hypertrophy clearly suggests that this transcriptional upregulation reflects a mechanism that is distinct from that which produces cell growth, i.e., hypertrophy. The suggestion that ion channel subunit expression is coordinately regulated independent of the hypertrophy pathways is supported by the observation that in pressure overload-induced pathological hypertrophy, most cellular genes are upregulated in response to hypertrophic stimuli (i.e. pressure overload in this case), but that the genes encoding ion channel subunits are not [5]. Thus, both hypertrophy models (physiological and pathological) suggest the presence of regulatory mechanism(s) that coordinate myocardial ion channel gene expression, which is(are) distinct from and independent of the pathways(s) leading to cellular hypertrophy and global increases in transcripts/proteins.
Although the observed increases in repolarizing K+ current amplitudes in response to exercise training are correlated with the transcriptional upregulation of the underlying channel subunits, it is certainly possible that additional post-transcriptional, as well as post-translational, mechanisms contribute to the observed increases in current amplitudes in response to exercise training. Reactive oxygen species (ROS), for example, has been shown previously to reduce outward K+ currents in cardiomyocytes [32, 33]. Although myocardial ROS, the physiological by-product of aerobic metabolism, may increase in response to acute exercise [34], chronic exercise training actually reduces cardiac ROS by upregulating myocardial antioxidants such as manganese superoxide dismutase [35, 36]. It is possible, therefore, that reductions in cardiac ROS with chronic exercise training also contribute to increasing myocardial repolarizing K+ current amplitudes. In addition, although we have demonstrated that enhanced PI3Kα signaling upregulates ion channel subunits, including Cav1.2 and Cavα2δ1, as well as Nav1.5 and Navβ1 (Figure 3; references 9 and 19), which encode depolarizing voltage-gated Ca2+ and Na+channels, respectively, increased PI3Kα signaling might also affect the functional expression of depolarizing Ca2+ and/or Na+ currents through post-translational mechanisms. Myocardial L-type Ca2+ currents, for example, have been shown to be regulated post-translationally by PI3Kα without measureable changes in the expression levels of the L-type alpha1C Ca2+ channel subunit protein [37, 38].
Although it would be of interest to also utilize a targeted deletion strategy to explore the hypothesis that if Akt2, the other Akt isoform that is robustly expressed in the myocardium [12], is required for exercise training-induced ion channel upregulation, it has been reported previously that adult Akt2−/− animals have severe hyperglycemia [16] and evidence of diabetic cardiomyopathy [17]. Importantly, several previous studies have demonstrated alterations in ionic currents and ion channel subunit expression levels in rodent models of diabetic cardiomyopathy [39, 40]. The diabetic phenotype of the Akt2−/− mice, therefore, might impact myocardial ionic currents and ion channel subunit expression levels at baseline, as well as in response to exercise, effects that could compromise the interpretation of experiments aimed at determining the functional impact of the loss of Akt2 on electrical remodeling. As an alternative approach, therefore, the effects of triciribine (TCN), which blocks both Akt1 and Akt2, on PI3Kα-induced electrical remodeling were examined directly in the experiments here (see sections 3.3 and 3.4, and further discussion below.
4.2 Increased PI3Kα Activity Upregulates K+ Current and Ion Channel Subunit Expression Independent of Akt Signaling
The experiments conducted using the tet-off inducible caPI3Kα mouse model demonstrate that short-term activation of cardiac PI3Kα signaling also upregulates K+ currents and the expression of ion channel subunit transcripts. Inhibition of cardiac Akt (both Akt1 and Akt2) activation in the context of enhanced PI3Kα signaling did not prevent the upregulation of myocardial K+ currents or ion channel subunit transcripts, suggesting that the impact of PI3Kα signaling on myocardial electrical remodeling is independent of Akt signaling. These results extend the findings obtained in the Akt1−/− exercise training experiments and suggest that both Akt1 and Akt2 are dispensable for PI3Kα-mediated electrical remodeling. The pharmacological approach of simultaneously blocking both Akt1 and Akt2 avoids several potential caveats in the interpretation of data obtained from animals lacking Akt1 or Akt2. First, this approach ruled out the role of Akt2 in PI3Kα-mediated electrical remodeling without using Akt2−/− animals, for which, as noted above, the quantification of ionic currents and channel subunit expression might be complicated by the presence of the diabetic phenotype. Secondly, the interpretation of negative results obtained from either Akt1−/− or Akt2−/− animals requires taking into consideration the potential functional redundancy between Akt1 and Akt2. Theoretically, using animals lacking both Akt1 and Akt2 (Akt1−/−/Akt2−/−) might solve this issue. This, however, is not feasible as mice lacking both Akt1 and Akt2 die shortly after birth [41].
Interestingly, it has been shown that PI3Kα activation is also critical in mediating myocardial metabolic remodeling in physiological hypertrophy, including increased capacity to oxidize fatty acids/glucose and increased mitochondrial biogenesis, and that this metabolic remodeling mediated by PI3Kα is also Akt-independent [22]. Taken together, these results demonstrate that PI3Kα signaling exerts distinct biological effects on the myocardium through divergent downstream pathways: Akt1-dependent physiological cardiac growth, Akt2-dependent insulin-sensitization and cellular survival, as well as Akt-independent effects on metabolic and electrical remodeling. The signaling mechanisms linking PI3Kα to metabolic and electrical remodeling have not been defined, although it has been suggested that PKCλ/ζ could be the potential downstream mediator that is required for PI3Kα-dependent metabolic remodeling [22]. Interestingly, PI3Kα has also been shown previously to modulate the activities of a number of transcription factors, such as FOXO [42] or NFκB [43, 44], that could potentially affect cardiac ion channel expression. It is certainly also possible that PI3Kα mediates electrical remodeling through other downstream effectors such as PDK1, Btk or ARF6. Further studies are needed to explore these possibilities directly and to identify the downstream signaling effectors that mediate myocardial electrical remodeling in response to PI3Kα activation.
Supplementary Material
Highlights.
We examined the role of Akt in exercise/PI3Kα-mediated cardiac electrical remodeling
We revealed that exercise/PI3Kα activation transcriptionally upregulate ion channels
We showed that exercise/PI3Kα-mediated ion channel upregulation is Akt-independent
These data suggest ion channel gene regulation is independent of hypertrophic pathway
Acknowledgments
Funding
This work was supported by the National Institutes of Health [HL-034161 to J.M.N., 1-P20-RR018728 to Y.T.T.] and the Midwest Affiliate of the American Heart Association [Predoctoral Fellowship to K.C.Y.].
Abbreviations
- caPI3Kα
constitutively active phosphoinositide-3-kinase p110α
- Cav
voltage-gated Ca2+ channel
- Cm
cell membrane capacitance
- GAPDH
glyceraldehydes 3-phosphate dehydrogenase
- icaPI3Kα
inducible constitutively active phosphoinositide-3-kinase p110α
- IK1
inwardly rectifying K+ current
- IK,slow
slow-inactivating outward K+ current
- Iss
non-inactivating steady state outward K+ current
- Ito,f
fast transient outward K+ current
- K2P
two-pore domain K+ channel
- Kir
inward rectifier K+ channel
- Kv
voltage-gated K+ channel
- LV
Left ventricle
- LVH
Left ventricular hypertrophy
- LVW/TL
LV weight to tibia length ratio
- Nav
voltage-gated Na+ channel
- qRT-PCR
quantitative real time polymerase chain reaction
- TCN
triciribine
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
None.
References
- 1.Haider AW, Larson MG, Benjamin EJ, Levy D. Increased left ventricular mass and hypertrophy are associated with increased risk for sudden death. J Am Coll Cardiol. 1998;32:1454–1459. doi: 10.1016/s0735-1097(98)00407-0. [DOI] [PubMed] [Google Scholar]
- 2.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 3.McIntyre H, Fry CH. Abnormal action potential conduction in isolated human hypertrophied left ventricular myocardium. J Cardiovasc Electrophysiol. 1997;8:887–894. doi: 10.1111/j.1540-8167.1997.tb00850.x. [DOI] [PubMed] [Google Scholar]
- 4.Oikarinen L, Nieminen MS, Viitasalo M, Toivonen L, Jern S, Dahlof B, et al. QRS duration and QT interval predict mortality in hypertensive patients with left ventricular hypertrophy: the Losartan Intervention for Endpoint Reduction in Hypertension Study. Hypertension. 2004;43:1029–1034. doi: 10.1161/01.HYP.0000125230.46080.c6. [DOI] [PubMed] [Google Scholar]
- 5.Marionneau C, Brunet S, Flagg TP, Pilgram TK, Demolombe S, Nerbonne JM. Distinct cellular and molecular mechanisms underlie functional remodeling of repolarizing K+ currents with left ventricular hypertrophy. Circ Res. 2008;102:1406–1415. doi: 10.1161/CIRCRESAHA.107.170050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayet J, Kanagaratnam P, Shahi M, Senior R, Doherty M, Poulter NR, et al. QT dispersion in athletic left ventricular hypertrophy. Am Heart J. 1999;137:678–681. doi: 10.1016/s0002-8703(99)70222-x. [DOI] [PubMed] [Google Scholar]
- 7.Biffi A, Maron BJ, Di Giacinto B, Porcacchia P, Verdile L, Fernando F, et al. Relation between training-induced left ventricular hypertrophy and risk for ventricular tachyarrhythmias in elite athletes. Am J Cardiol. 2008;101:1792–1795. doi: 10.1016/j.amjcard.2008.02.081. [DOI] [PubMed] [Google Scholar]
- 8.Pelliccia A, Di Paolo FM, Quattrini FM, Basso C, Culasso F, Popoli G, et al. Outcomes in athletes with marked ECG repolarization abnormalities. N Engl J Med. 2008;358:152–161. doi: 10.1056/NEJMoa060781. [DOI] [PubMed] [Google Scholar]
- 9.Yang KC, Foeger NC, Marionneau C, Jay PY, McMullen JR, Nerbonne JM. Homeostatic regulation of electrical excitability in physiological cardiac hypertrophy. J Physiol. 2010;588:5015–5032. doi: 10.1113/jphysiol.2010.197418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang KC, Jay PY, McMullen JR, Nerbonne JM. Enhanced cardiac PI3Kalpha signaling mitigates arrhythmogenic electrical remodeling in pathological hypertrophy and heart failure. Cardiovasc Res. 2012;93:252–262. doi: 10.1093/cvr/cvr283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 12.Muslin AJ, DeBosch B. Role of Akt in cardiac growth and metabolism. Novartis Found Symp. 2006;274:118–126. discussion 26–31, 52–5, 272–6. [PubMed] [Google Scholar]
- 13.DeBosch B, Treskov I, Lupu TS, Weinheimer C, Kovacs A, Courtois M, et al. Akt1 is required for physiological cardiac growth. Circulation. 2006;113:2097–2104. doi: 10.1161/CIRCULATIONAHA.105.595231. [DOI] [PubMed] [Google Scholar]
- 14.Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem. 2001;276:38349–38352. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- 15.Garofalo RS, Orena SJ, Rafidi K, Torchia AJ, Stock JL, Hildebrandt AL, et al. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKB beta. J Clin Invest. 2003;112:197–208. doi: 10.1172/JCI16885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB, 3rd, et al. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta) Science. 2001;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 17.Etzion S, Etzion Y, DeBosch B, Crawford PA, Muslin AJ. Akt2 deficiency promotes cardiac induction of Rab4a and myocardial beta-adrenergic hypersensitivity. J Mol Cell Cardiol. 2010;49:931–940. doi: 10.1016/j.yjmcc.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McMullen JR, Shioi T, Zhang L, Tarnavski O, Sherwood MC, Kang PM, et al. Phosphoinositide 3-kinase(p110alpha) plays a critical role for the induction of physiological, but not pathological, cardiac hypertrophy. Proc Natl Acad Sci U S A. 2003;100:12355–12360. doi: 10.1073/pnas.1934654100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yano N, Tseng A, Zhao TC, Robbins J, Padbury JF, Tseng YT. Temporally controlled overexpression of cardiac-specific PI3Kalpha induces enhanced myocardial contractility--a new transgenic model. Am J Physiol Heart Circ Physiol. 2008;295:H1690–H1694. doi: 10.1152/ajpheart.00531.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu CH, Wyszomierski SL, Tseng LM, Sun MH, Lan KH, Neal CL, et al. Preclinical testing of clinically applicable strategies for overcoming trastuzumab resistance caused by PTEN deficiency. Clin Cancer Res. 2007;13:5883–5888. doi: 10.1158/1078-0432.CCR-06-2837. [DOI] [PubMed] [Google Scholar]
- 21.Harrison BC, Bell ML, Allen DL, Byrnes WC, Leinwand LA. Skeletal muscle adaptations in response to voluntary wheel running in myosin heavy chain null mice. J Appl Physiol. 2002;92:313–322. doi: 10.1152/japplphysiol.00832.2001. [DOI] [PubMed] [Google Scholar]
- 22.O'Neill BT, Kim J, Wende AR, Theobald HA, Tuinei J, Buchanan J, et al. A conserved role for phosphatidylinositol 3-kinase but not Akt signaling in mitochondrial adaptations that accompany physiological cardiac hypertrophy. Cell Metab. 2007;6:294–306. doi: 10.1016/j.cmet.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allen DL, Harrison BC, Maass A, Bell ML, Byrnes WC, Leinwand LA. Cardiac and skeletal muscle adaptations to voluntary wheel running in the mouse. J Appl Physiol. 2001;90:1900–1908. doi: 10.1152/jappl.2001.90.5.1900. [DOI] [PubMed] [Google Scholar]
- 24.Guo W, Jung WE, Marionneau C, Aimond F, Xu H, Yamada KA, et al. Targeted deletion of Kv4.2 eliminates I(to,f) and results in electrical and molecular remodeling, with no evidence of ventricular hypertrophy or myocardial dysfunction. Circ Res. 2005;97:1342–1350. doi: 10.1161/01.RES.0000196559.63223.aa. [DOI] [PubMed] [Google Scholar]
- 25.Guo W, Li H, Aimond F, Johns DC, Rhodes KJ, Trimmer JS, et al. Role of heteromultimers in the generation of myocardial transient outward K+ currents. Circ Res. 2002;90:586–593. doi: 10.1161/01.res.0000012664.05949.e0. [DOI] [PubMed] [Google Scholar]
- 26.Kuo HC, Cheng CF, Clark RB, Lin JJ, Lin JL, Hoshijima M, et al. A defect in the Kv channel-interacting protein 2 (KChIP2) gene leads to a complete loss of I(to) and confers susceptibility to ventricular tachycardia. Cell. 2001;107:801–813. doi: 10.1016/s0092-8674(01)00588-8. [DOI] [PubMed] [Google Scholar]
- 27.Xu H, Barry DM, Li H, Brunet S, Guo W, Nerbonne JM. Attenuation of the slow component of delayed rectification, action potential prolongation, and triggered activity in mice expressing a dominant-negative Kv2 alpha subunit. Circ Res. 1999;85:623–633. doi: 10.1161/01.res.85.7.623. [DOI] [PubMed] [Google Scholar]
- 28.Putzke C, Wemhoner K, Sachse FB, Rinne S, Schlichthorl G, Li XT, et al. The acid-sensitive potassium channel TASK-1 in rat cardiac muscle. Cardiovasc Res. 2007;75:59–68. doi: 10.1016/j.cardiores.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 29.Zaritsky JJ, Redell JB, Tempel BL, Schwarz TL. The consequences of disrupting cardiac inwardly rectifying K(+) current (I(K1)) as revealed by the targeted deletion of the murine Kir2.1 and Kir2.2 genes. J Physiol. 2001;533:697–710. doi: 10.1111/j.1469-7793.2001.t01-1-00697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanguinetti MC, Curran ME, Zou A, Shen J, Spector PS, Atkinson DL, et al. Coassembly of K(V)LQT1 and minK (IsK) proteins to form cardiac I(Ks) potassium channel. Nature. 1996;384:80–83. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- 31.Sussman MA, Volkers M, Fischer K, Bailey B, Cottage CT, Din S, et al. Myocardial AKT: the omnipresent nexus. Physiol Rev. 2011;91:1023–1070. doi: 10.1152/physrev.00024.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu Z, Patel KP, Lou MF, Rozanski GJ. Up-regulation of K(+) channels in diabetic rat ventricular myocytes by insulin and glutathione. Cardiovasc Res. 2002;53:80–88. doi: 10.1016/s0008-6363(01)00446-1. [DOI] [PubMed] [Google Scholar]
- 33.Cerbai E, Ambrosio G, Porciatti F, Chiariello M, Giotti A, Mugelli A. Cellular electrophysiological basis for oxygen radical-induced arrhythmias. A patch-clamp study in guinea pig ventricular myocytes. Circulation. 1991;84:1773–1782. doi: 10.1161/01.cir.84.4.1773. [DOI] [PubMed] [Google Scholar]
- 34.Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88:1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Demirel HA, Powers SK, Zergeroglu MA, Shanely RA, Hamilton K, Coombes J, et al. Short-term exercise improves myocardial tolerance to in vivo ischemia-reperfusion in the rat. J Appl Physiol. 2001;91:2205–2212. doi: 10.1152/jappl.2001.91.5.2205. [DOI] [PubMed] [Google Scholar]
- 36.Yamashita N, Hoshida S, Otsu K, Asahi M, Kuzuya T, Hori M. Exercise provides direct biphasic cardioprotection via manganese superoxide dismutase activation. J Exp Med. 1999;189:1699–1706. doi: 10.1084/jem.189.11.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun H, Kerfant BG, Zhao D, Trivieri MG, Oudit GY, Penninger JM, et al. Insulin-like growth factor-PTEN deletion enhance cardiac L-type Ca2+ currents via increased PI3Kalpha/PKB signaling. Circ Res. 2006;98:1390–1397. doi: 10.1161/01.RES.0000223321.34482.8c. [DOI] [PubMed] [Google Scholar]
- 38.Lu Z, Jiang YP, Wang W, Xu XH, Mathias RT, Entcheva E, et al. Loss of cardiac phosphoinositide 3-kinase p110 alpha results in contractile dysfunction. Circulation. 2009;120:318–325. doi: 10.1161/CIRCULATIONAHA.109.873380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qin D, Huang B, Deng L, El-Adawi H, Ganguly K, Sowers JR, et al. Downregulation of K(+) channel genes expression in type I diabetic cardiomyopathy. Biochem Biophys Res Commun. 2001;283:549–553. doi: 10.1006/bbrc.2001.4825. [DOI] [PubMed] [Google Scholar]
- 40.Marionneau C, Aimond F, Brunet S, Niwa N, Finck B, Kelly DP, et al. PPARalpha-mediated remodeling of repolarizing voltage-gated K+ (Kv) channels in a mouse model of metabolic cardiomyopathy. J Mol Cell Cardiol. 2008;44:1002–1015. doi: 10.1016/j.yjmcc.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng XD, Xu PZ, Chen ML, Hahn-Windgassen A, Skeen J, Jacobs J, et al. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev. 2003;17:1352–1365. doi: 10.1101/gad.1089403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Philip-Couderc P, Tavares NI, Roatti A, Lerch R, Montessuit C, Baertschi AJ. Forkhead transcription factors coordinate expression of myocardial KATP channel subunits and energy metabolism. Circ Res. 2008;102:e20–e35. doi: 10.1161/CIRCRESAHA.107.166744. [DOI] [PubMed] [Google Scholar]
- 43.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 44.Panama BK, Latour-Villamil D, Farman GP, Zhao D, Bolz SS, Kirshenbaum LA, et al. Nuclear factor kappaB downregulates the transient outward potassium current I(to,f) through control of KChIP2 expression. Circ Res. 2011;108:537–543. doi: 10.1161/CIRCRESAHA.110.229112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.