Abstract
Human immunodeficiency virus type 1 (HIV-1) mostly owes its success to its ability to evade host immune responses. Understanding viral immune escape mechanisms is prerequisite to improve future HIV-1 vaccine design. This review focuses on the strategies that HIV-1 has evolved to evade recognition by natural killer (NK) cells.
Keywords: HIV-1, NK cells, escape, innate immunity, vaccine
1. Introduction
Since the first reported cases of acquired immunodeficiency syndrome (AIDS) in 1982, about 30 million AIDS-related deaths have occurred globally [1]. In its most recent update, UNAIDS estimated that worldwide there were 34 million adults and children living with human immunodeficiency virus type 1 (HIV-1) infection. Further, a total of about 2.7 million new infections occurred and 1.8 million people died from AIDS in 2010. Means to prevent the transmission of HIV-1 are urgently needed to stop the epidemic.
In the history of global human health, preventive vaccines have been the most effective approaches to reduce the transmission of infections and to increase human health. However, the design of a preventive HIV-1 vaccine has been proven largely elusive to date. Two major vaccine trials, one aimed at inducing protective antibody responses and one aimed at inducing antiviral T cell responses, have failed, while in a more recent trial the use of a candidate vaccine combining both approaches lowered the rate of infection by about 30% with marginal significance [2]. The failure or modest success of previous vaccine approaches to protect from HIV-1 infection have highlighted that our understanding of protective immunity in HIV-1 infection is very limited, and that novel, innovative approaches are needed to overcome the tremendous challenges we are facing in HIV-1 vaccine design.
The immune response to pathogens depends on the interplay of different cell subsets each with their own role in pathogen uptake, recognition, clearance, and generation of an effective memory response. Following infections, the first line of defense consists of a highly organized innate immune response, which precedes the induction of the adaptive immune response and plays a central role in determining its quality. While traditional vaccine strategies have largely focused on the induction of adaptive immunity, only recently attention has been directed towards rationally exploring ways to harness the antiviral activity of innate immune cells [3].
Natural killer (NK) cells represent the major antiviral effector cell population of the innate immune system. They play an important role in containing viral replication in the very early stages of the infection, and in shaping the subsequent adaptive immune response by interacting with other immune cells, including dendritic cells (DCs) and CD4 T cells [4–7]. Mounting evidence supports the importance of NK cell function in determining the outcome of HIV-1 infection [8, 9]. In addition, NK cell contribution to the immune control of HIV-1 infection has been further highlighted by several studies describing strategies evolved by HIV-1 to specifically evade NK cell-mediated immune responses, and the demonstration that NK cells significantly impact HIV-1 evolution [10]. Here, we review known and recently discovered mechanisms employed by HIV-1 to evade NK cell immunity. Understanding these HIV-1 immune escape schemes will enable identifying novel targets to manipulate NK cell responses to HIV-1.
2. NK cell receptors
The precise mechanisms by which NK cells recognize and eliminate malignant or virally infected cells are complex and still not entirely understood. Unlike other lymphocytes, NK cells lack specific antigen receptors but lyse target cells following the integration of inhibitory and activating signals generated by an arsenal of cell surface receptors, with effector functions taking place when activating signals overcome inhibitory ones [11]. Receptors expressed at the surface of NK cells mostly belong to three major groups of cell surface signaling molecules, namely the killer cell immunoglobulin-like receptors (KIR), C-type lectins (NKG2) and natural cytotoxicity receptors (NCR), the latter representing a family of NK cell receptors that are characteristic markers for NK cells.
2.1. NCRs
NCRs are activating receptors and include NKp46 and NKp30, which are constitutively expressed on NK cells, and NKp44, which is up-regulated upon IL-2-mediated NK cell activation [12]. The precise ligands for these receptors remain largely undefined, yet several viral or tumor-associated molecules that can interact with NCRs have been identified so far (Table 1).
Table 1.
Pathogen- and tumor-associated ligands for NCRs
| NKp30 | NKp44 | NKp46 | References | |
|---|---|---|---|---|
| Heparan sulfates | + | + | + | [13–16] |
| Influenza virus haemagglutinin Sendai virus haemagglutinin-neuraminidase | − | + | + | [17, 18] |
| HCMV tegument protein pp65 | + | − | − | [19] |
| Duffy-binding-like (DBL)-1α of Plasmodium falciparum erythrocyte membrane protein-1 (PfEMP-1) | + | ND | + | [20] |
| Leukocyte antigen-B-associated transcript 3 (BAT3) | + | ND | − | [21, 22] |
| B7-H6 | + | ND | − | [23] |
| Mycobacteria, Nocardia farcinica and Pseudomonas aeruginosa surface protein | − | + | − | [24] |
| West Nile virus and Dengue virus E glycoprotein | − | + | − | [25] |
ND: not determined
2.2. KIRs
A second group of receptors that has recently received much attention includes members of the KIR family. Genes encoding KIRs are located on human chromosome 19q13.4 and consist of the second most diverse family of genes after the HLA class I locus [26]. The number and the type of KIR genes present in the human genome differ from one individual to another and an important allelic polymorphism further contributes to the variability of the KIR locus. KIRs can be either inhibitory or activating, a characteristic that depends on the size of their intracellular tail. Inhibitory KIRs usually have a long (L) intraplasmatic tail with two immunoreceptor tyrosine-based inhibition motifs (ITIMs) that can recruit the phosphatases SHP-1 and -2 upon engagement with their ligands, while activating KIRs have a short (S) tail that interacts with immunoreceptor tyrosine-based activation motif (ITAM)-containing adaptor proteins via a transmembrane lysine residue when they are bound to their ligand [11]. KIRs primarily recognize specific allotypes of the classical (-A, -B, -C) and non-classical (-G) HLA class I receptors, and engagement of inhibitory KIRs to their HLA class I ligands protects cells from NK cell-mediated lysis. Extracellular domains of activating KIRs are very similar to those of their inhibitory counterparts, however they appear to have less avidity for their ligands. Thus far only KIR2DS1 has been shown to directly interact with HLA-C, while the ligands for other activating KIRs still remain to be identified [27].
2.3. NKG2 receptors
NK cells also express c-type lectins. These are highly conserved receptors that can deliver activating or inhibitory signals. The activating receptor NKG2D recognizes human cytomegalovirus (HCMV) glycoprotein UL16-binding proteins (ULBPs) as well as the MHC class I chain-related molecules MICA and MICB that are expressed upon cell stress. Inhibitory CD94/NKG2A and activating CD94/NKG2C heterodimers bind the non-classical HLA-E loaded with peptides derived from other HLA class I molecules, thereby monitoring the overall expression level of HLA class I.
2.4. LILRB1
Leucocyte immunoglobulin-like receptors (LILRs) are mostly expressed on cells of myeloid lineage, but one of these immunomodulatory molecules, LILRB1 (LIR1/CD85j/ILT2), is also present on NK cells [28]. The cytoplasmic tail of LILRB1 carries ITIMs, and therefore this particular LILR probably functions as an inhibitory receptor. Moreover, similarly to other members of this family, LILRB1 has the ability to broadly interact with HLA class I molecules, yet it has an increased affinity for the non-classical HLA-G [29], which is highly expressed by trophoblasts during pregnancy, and for the HCMV protein UL18. The LILRB1 receptor on NK cells might thus contribute to monitoring the HLA class I expression status of cells, and act in concert with KIRs to regulate NK cell function. Although a wide range of HLA class I can bind LILRB1, its interaction with HLA-G probably plays a dominant role in modulating NK cell activity during pregnancy to ensure immune tolerance.
2.5. Other NK cell receptors
Almost 90% of NK cells in the peripheral blood express CD16, which is the low-affinity FcγRIIIa receptor that allows recognition and lysis of antibody-coated cells via antibody-dependent cellular cytotoxicity (ADCC) [30, 31]. NK cells also express Toll-like receptors (TLRs), including TLR2, TLR3, TLR7/8 and TLR9, which can sense pathogen-associated molecular patterns, with the TLR3, 7/8 and 9 being specific for nucleic acids [32–34]. For instance, several HIV-1-derived single stranded RNAs can be recognized by the intracellular TLR7/8 [35–37]. However, it has been demonstrated that a direct contact with other immune cells, including plasmacytoid DCs (pDCs) and monocytes, is required to potently stimulate NK cells with TLR7/8 ligands [38]. Finally, immunoregulatory molecules such as 2B4, Tim-3, and CD160 are highly expressed on NK cells. While these immune receptors have been associated with negative regulation of T cell activity, their relevance for NK cell function and/or and development is not completely understood.
2.6. NK cell subsets
Human NK cells have been classically defined as CD3negCD56pos lymphocytes, representing about 15% of peripheral blood lymphocytes. Based on the expression of CD16 and the expression levels of CD56, NK cells can be further subdivided into three subsets with distinct functional properties, namely CD56bright, CD56dim and CD56neg NK cells. CD56bright lack the expression of CD16 and KIRs, and have a high proliferation potential. These cells might have important immunoregulatory functions as they are able to secrete large amounts of pro-inflammatory cytokines [39]. The most abundant NK cell subpopulation in the peripheral blood consists of CD56dim NK cells (~90%), which express CD16 and KIRs, have strong cytotoxic activity and a modest ability to secrete cytokines [30, 40]. It is still controversial whether CD56bright NK cells mature into CD56dim NK cells, or if these are two independent lineages [41, 42]. Finally, a subset of CD56negCD16pos NK cells has been described [43]. This subpopulation of NK cells appears to be expanded in HIV-1 infection and other chronic viral infections, and does not respond to stimulation with HLA class I-devoid target cells or mitogens, despite the expression of KIRs [44, 45], suggesting that these NK cells represent an exhausted/anergic subset of NK cells.
3. Role of NK cells in the host defense against HIV-1 infection
A number of epidemiological and functional studies have put forth evidence that NK cells may be directly involved in limiting HIV-1 replication, and have suggested that specific KIRs play an important role in this process.
3.1. Impact of KIR3DS1 on HIV-1 disease outcome
First, data from Dr. Carrington’s group have shown a strong association between the genomic presence of KIR3DS1 along with HLA-Bw4 alleles that encode isoleucine at position 80 (Bw4 80I), and slower HIV-1 disease progression [8]. KIR3DS1+ NK cells can not only potently inhibit HIV-1 replication in Bw4-80I+ cells in vitro [46], but KIR3DS1+ NK cells also preferentially expand during HIV-1 acute infection in individuals bearing at least one HLA-B Bw4 allele [47]. This indicates that NK cells expressing KIR3DS1 might be preferentially activated by HIV-1-infected cells expressing HLA-B Bw4. However, there is no demonstration to date that KIR3DS1 can physically interact with HLA-Bw4 molecules, despite more than 97% similarity to KIR3DL1 in its extracellular domain [48], and the mechanisms by which KIR3DS1+ NK cells might eliminate HIV-1-infected cells remain unclear. NK cells from KIR3DS1+ subjects with recent untreated HIV-1 infection were found to display enhanced functional activity, and particularly produced increased amounts of IFN-γ in response to HLA class I-devoid target cells, corroborating the idea that KIR3DS1+ NK cells might have a higher capacity to kill HIV-1-infected targets [49]. However, this effect was largely independent of the presence of the putative HLA-Bw4 ligand, with the exception of the protective alleles HLA-B57 and HLA-B58, which appeared to contribute to the augmented cytotoxic function of NK cells in KIR3DS1+ patients. In contrast, other studies focusing on NK cells from HIV-1 elite controllers, who are naturally able to maintain HIV-1 viral loads below the level of detection, as well as high CD4 cell counts without any antiretroviral treatment, suggested that NK cells and KIR3DS1 do not play a significant role in the control of HIV-1 infection [50]. Of note, the presence of other activating KIR receptors, including KIR2DS2, has been associated with a detrimental outcome of HIV-1 infection [51]. Therefore, the significance of KIR3DS1 and its putative HLA-Bw4 ligand and that of other activating KIRs in the control of HIV-1 infection remains to be fully elucidated.
3.2. Impact of KIR3DL1 on HIV-1 disease outcome
In addition to the activating KIR3DS1 receptor, various distinct combinations of inhibitory KIR3DL1 and HLA-Bw4 alleles also have an influence on HIV-1 disease. In particular, KIR3DL1 alleles associated with high expression of KIR3DL1 on the cell surface (KIR3DL1*h alleles) significantly enhance protection conferred by Bw4-80I alleles [9]. Co-expression of KIR3DL1*h alleles and one particular HLA-Bw4-80I molecule, HLA-B57, is not only strongly associated with reduced risk to progress towards AIDS, but has also been shown to have a protective effect against HIV-1 acquisition [52]. In accordance with these epidemiological data, KIR3DL1+ NK cells from individuals carrying both HLA-B57 and KIR3DL1*h have an increased functional potential in response to HLA class I-devoid target cells compared to NK cells from subjects expressing other HLA-Bw4 alleles or being homozygous for HLA-Bw6 [53]. Furthermore, among HIV-1-infected individuals with a slow progression towards AIDS, polyfunctional NK cells, namely NK cells that can produce large amounts of CD107a, IFN-γ and TNF-α upon stimulation, were only present in subject expressing both KIR3DL1 and HLA-Bw4 [54]. Interestingly, the genes encoding KIR3DL1 and KIR3DS1 are found in variable numbers of copies among individuals as a result of gene duplication or deletion. It appears that protection against HIV-1 increases with the number of gene copies of KIR3DL1, provided that at least one copy of the KIR3DS1 gene and the ligands for both receptors are also present [55]. Further investigations to assess the activity of KIR3DL1+ NK cells against HIV-1, and to understand the mechanisms by which they might mediate protection from HIV-1 disease, are warranted.
3.3. Enhanced NK cell function in HIV-1-exposed uninfected individuals
Studies looking into the innate immune responses in individuals who are persistently exposed to HIV-1 but remain uninfected also support a critical role for NK cells in the protection from HIV-1 acquisition. NK cells from exposed uninfected intravenous drug users (IDUs) display increased levels of cytolytic activity and enhanced production of cytokines before and after in vitro stimulation with target cell lines [56]. The presence of pDCs with a higher maturation status compared to normal blood donors [57], and a distinct expression pattern of NK cell receptors [58] have both been proposed to be associated with enhanced NK cell activation in HIV-1-exposed uninfected IDUs. NK cells from sexually HIV-1-exposed individuals who remain seronegative also display a higher capacity to produce IFN-γ than those from low-risk uninfected subjects [59]. While most studies did not report any enrichment in protective KIR alleles or their putative ligands in HIV-1-exposed uninfected cohorts [60], homozygosity for the activating receptor KIR3DS1 might contribute to the enhanced NK cell activity observed in these individuals [61]. Less suppression of NK cell activation by inhibitory KIRs might provide another explanation. Along those lines, the presence of inhibitory KIRs that lack their cognate HLA class I ligand, and therefore of NK cells that can be more easily activated, has been associated with resistance to HIV-1 among sex workers [62]. Differences in the characteristics and sizes of HIV-1-exposed uninfected cohorts studied might account for the discrepancies between some of the abovementioned reports. Nevertheless, altogether, these data provide evidence that NK cells can have an important role in preventing HIV-1 infection and in eliminating HIV-1-infected cells.
4. HIV-1 infection affects NK cell phenotype and function
Many studies have linked HIV-1 infection to severe NK cell dysfunction and changes in NK cell subsets distribution, and a comprehensive review of these major observations was recently published by Ianello et al., and will not be discussed in great detail here [63] (Table 2 and Fig. 1, left panel). While these deregulations might largely reflect the overall impairment of the immune system during chronic HIV-1 infection rather than specific NK cell evasion mechanisms, they contribute importantly to dampening the NK cell responses to HIV-1 and as such can be viewed as part of HIV-1 evasion strategies.
Table 2.
Dysregulation of NK cell phenotype and function in HIV-1 infection and HIV-1 proteins contributing to these defects
| Major observations | HIV-1 proteins involved |
|---|---|
| Redistribution of NK cell subsets
| |
| - Depletion of NK cells and particularly immunoregulatory | |
| CD56bright NK cells | |
| - Increase in dysfunctional CD56neg NK cells | |
|
| |
| Dysregulation of NK cell receptor expression | Vpu |
|
| |
| - Increase in inhibitory receptors | |
| - Decrease in activating receptors | |
|
| |
| Decreased production of cytokines and chemokines
| |
| Decreased cytotoxic activity | Tat, Vpu |
|
| |
| Impaired ability to perform ADCC | Tat |
|
| |
| Impaired interactions with other immune cells, including DCs | Tat, Nef |
|
| |
| Enhanced apoptosis | gp120, Tat |
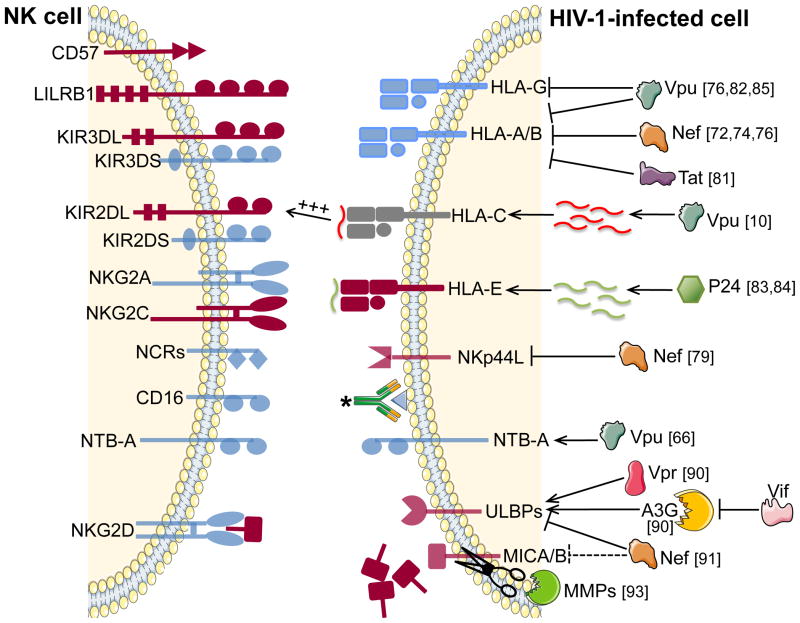
Figure 1. Major changes in the expression of NK cell receptors and their ligands in HIV-1 infection.
The left panel represents major reported changes in NK cell receptors expression in HIV-1 chronic infection. The right panel depicts how viral or HIV-1-induced host proteins affect the expression of ligands for NK cell receptors on HIV-1-infected cells, thereby impairing NK cell recognition. Relevant references are indicated next to each protein involved in HIV-1 escape from NK cell recognition. Dark red: increased expression; Faded dark red: induced expression counteracted by HIV-1 proteins or HIV-1-induced host factors; Faded blue: decreased expression; Grey: stable expression. *: HIV-1 specific antibody
4. 1. HIV-1-associated changes in NK cell receptor expression
Progressive HIV-1 infection is associated with a preferential depletion of cytotoxic CD56dim NK cells, which is counterbalanced by a parallel increase in the dysfunctional subset of CD56neg NK cells (reviewed in [64]). HIV-1 infection of NK cells, as well as enhanced apoptosis of NK cells might account for their depletion in HIV-1 infection, although alternate mechanisms might be involved. Important changes in NK cell receptor expression co-occur with a major redistribution in NK cell subsets and have been connected to altered NK cell activity (reviewed in [65])(Fig. 1, left panel). These phenotypic perturbations have been essentially described in viremic chronic HIV-1 infection and usually correlate with viral loads.
4. 2. HIV-1 associated changes in NK cell functions
Chronic HIV-1 infection can significantly affect NK cell function, leading to a reduced ability to lyse target cells and to produce cytokines and chemokines [63] (Table 2). Several factors might contribute to impairing NK cell activity. This includes persistent generalized immune activation, NK cell subsets redistribution, and also direct effects of HIV-1 viral proteins on NK cells, including Tat and Vpu [63, 66]. HIV-1 Vpu has been recently proposed to down-regulate the expression of NTB-A, a receptor previously described for its ability to enhance NK-cell mediated killing of HIV-1-infected CD4 cells [67]. Removal of NTB-A, which might act as an activating co-receptor for NKG2D, from the cell surface, protects HIV-1 infected cells from NK cell lysis.
As other NK cell-mediated cytolytic functions, ADCC activity also declines with progressive HIV-1 infection [63]. Interesting novel data show that KIR3DL1+ NK cells, which normally display stronger ADCC activity compared to KIR3DL1- NK cells in HLA-Bw4+ individuals, lose their increased ability to respond to ADCC target cells in HIV-1 infection [68]. While further studies are needed to elucidate how HIV-1 affects the efficiency of NK cells to perform ADCC, low expression levels of CD16 on NK cells from subjects with chronic HIV-1 infection have been linked to a reduced ADCC activity [69]. Viral escape from HIV-1-specific non-neutralizing ADCC antibodies could also account for defects in ADCC activity. Recent data demonstrate that chronic HIV-1 infection is associated with the presence of ADCC antibodies that are unable to recognize autologous HIV-1 strains, suggesting that NK-cell mediated ADCC activity exerts a significant selective pressure to drive HIV-1 evolution [70].
4. 3. Impaired NK-DC cross-talks in HIV-1 infection
HIV-1 infection also impairs crucial interactions between NK cells and other immune cells, including DCs. Aberrant killing of mature DCs by NK cells during chronic HIV-1 infection has been described [63, 71]. In addition, it has been suggested that the viral proteins Tat and Nef can both directly impair NK-DC cross-talks [72]. Understanding how the interactions between dysfunctional NK cells and other immunocytes impact the quality of the adaptive immune response and HIV-1 disease outcome is warranted to become an important area of study in the upcoming years. Finally, while NK cells undergo many functional and phenotypic changes as HIV-1 disease progress, most studies describe largely normalized NK cell numbers and function following effective antiretroviral therapy. Overall, these data suggest that active HIV-1 replication and associated immunopathology result in significant perturbation in the proportion and activity of NK cell subpopulations.
5. Selective dysregulation of HLA-A and HLA-B expression by HIV-1: how to evade both CD8 T cell and NK cell recognition
In an effort to avoid recognition by CD8+ T cells, several viruses, including HIV-1, down-regulate HLA class I molecules on the surface of infected cells [73–75]. However, this immune evasion strategy can result in increased susceptibility to NK cell lysis, as NK cells sense changes in HLA class I molecules via inhibitory KIRs. Viruses have therefore developed ways to counterbalance this enhanced sensitivity. Two main strategies, exploited notably by human cytomegalovirus to efficiently escape NK cell recognition, have been extensively described in the literature (reviewed in [76]). They consist on the one hand in encoding for alternate ligands for inhibitory NK cell receptors, and on the other hand in down-modulating preferentially the HLA class I molecules that present peptides to CD8 T cells, while sparing the expression of others in order to keep a sufficient level of NK cell inhibition.
5.1. Down-regulation of HLA-A and -B by HIV-1 Nef
In the case of HIV-1, Nef activity leads to the selective down-regulation of HLA-A and HLA-B, the two families of HLA class I molecules restricting most of the virus-specific CD8+ T cells responses, but not that of HLA-C and HLA-E (Fig. 1, right panel). This results in maintaining the surface expression of important ligands for inhibitory NK cell receptors, including several inhibitory KIRs of the KIR2DL family and NKG2s. In contrast, HLA-A and -B are only recognized by KIR3DL1 in the context of HLA-Bw4 [73, 77].
The fact that Nef does not affect the expression of HLA-C and HLA-E might help to explain why bulk peripheral blood NK cells are not able to efficiently kill autologous infected CD4 cells in vitro [78, 79]. However, HLA-A and -B are not the only molecules that are down-modulated by Nef, as this viral protein has also been suggested to decrease the surface expression of the cellular ligand for NKp44 on infected CD4 cells [80], thereby providing an alternative way of protecting HIV-1-infected cells from NK cell lysis. Interestingly, HIV-1 infection might also trigger overexpression of NKp44 ligands on uninfected CD4 cells, rendering healthy functional CD4 cells susceptible to NK cell-mediated killing in chronically infected individuals [81].
5. 2. Dysregulation of HLA-A and -B expression by other HIV-1 proteins
Besides Nef, two other HIV-1 accessory proteins, Tat and Vpu, have also been suggested to impair the expression of HLA class I. Tat can bind to the basal promoters of the HLA class I heavy chain and β2-microglobulin genes, thereby repressing their transcription [82]. In the same study, the authors show that TAR, an element of HIV-1 RNA that is essential to initiate HIV-1 transcription upon its interaction with Tat, enhances Tat-mediated repression of β2-microglobulin expression. Vpu plays a role in HIV-1 pathogenesis by increasing the amount of released viral particles from infected cells and by enhancing CD4 degradation in the endoplasmic reticulum. Vpu has also been proposed to contribute to the decreased expression of HLA class I on HIV-1-infected CD4 cells, probably by inducing the proteolysis of newly synthesized HLA class I molecules in the endoplasmic reticulum, and as such probably contributes to hide HIV-1 infected cells from NK cell surveillance [83].
5. 3. Modulated expression of non-A non-B HLA class I molecules in HIV-1 infection
Despite Nef, Tat and Vpu activities, HIV-1-infected CD4 cells maintain significant levels of HLA class I surface expression. This is in part explained by the fact that Nef does not trigger the degradation of HLA-E and HLA-C but also by a potential HIV-1-mediated enhanced expression and stabilization of the non-classical HLA-E molecule, further favoring inhibition of NK cell cytotoxicity [84]. Martini et al. demonstrated that HLA-E expression levels on CD4 cells from chronic HIV-1-infected individuals correlate with viremia. One proposed mechanism to explain these observations is the presentation of an HIV-1 p24 epitope, which has been suggested to stabilize HLA-E at the surface of infected cells [85]. Further research in this area is needed to reproduce these findings, and to understand their contribution to the ability of the virus to evade NK cell recognition. Expression levels of HLA-G on HIV-1-infected CD4 is still controversial, as this non-classical molecule has been reported to be down-modulated by Vpu [77, 86], yet it is still significantly expressed in HIV-1-infected patients [87, 88].
Taken together, the results of these studies show that modulating the expression of the different HLA class I molecules at the surface of infected cells is a major mechanism employed by HIV-1 to evade recognition by inhibitory NK cell receptors. However, another strategy consists in avoiding engagement of NK cell activating receptors, and particularly that of NKG2D.
6. HIV-1 escape from NKG2D recognition
Viral infections do not only affect HLA class I expression but also generally trigger the up-regulation of stress receptors that can serve as NKG2D ligands (reviewed in [89]), resulting in increased recognition by NK cells. Upon HIV-1 infection, NKG2D ligands, and particularly ULPBs, are up-regulated, and their expression has been suggested to account for the ability of NK cells to kill HIV-1-infected CD4 cells [67, 90]. Moreover, induction of significant levels of NKG2D ligands can trigger NK cell lysis of infected cells, even in the presence of normal amounts of HLA class I [91]. Given the high capacity of NKG2D ligands to activate NK cells, HIV-1, as other viruses, have evolved mechanisms to reduce the expression of NKG2D ligands on infected cells. Several strategies have been suggested by which HIV-1 might escape from NKG2D recognition (Fig. 1, right panel).
6. 1. Degradation of NKG2D ligands by HIV-1 Nef
HIV-1 Nef limits the expression of NKG2D ligands by mediating the degradation of ULBP-1 and -2, and to a lesser extent, that of MICA [92]. Indeed, CD4+ T cells infected with HIV-1 viruses lacking Nef are killed very strongly by NK cells in vitro through NKG2D-dependent mechanisms (Alter and Altfeld, unpublished data), and this mechanism might account to some extend for the observed protection from disease in delta-Nef SIV-infected rhesus macaques, as well as delayed disease progression in humans infected with delta-Nef HIV-1 viruses (reviewed in [93]).
6. 2. Shedding of MICA from the surface of HIV-1-infected cells
Alternative ways for HIV-1 to impair NKG2D-mediated NK cell activation have been recently suggested. Enzymatique cleavage of MICA and MICB from the surface of cancer cells leads to engagement of NKG2D by the soluble forms of MICA/B and its subsequent down-regulation, thus promoting the generation of anergic NK cells and tumor immune evasion (reviewed in [89]). A similar mechanism might be used by HIV-1 to reduce susceptibility of HIV-1-infected cells to NKG2D-dependent elimination as elevated plasma levels of soluble MICA and compromised NKG2D-mediated NK cell responses were both found to be associated with chronic HIV-1 infection [94]. The matrix metalloproteinases MMP-2 and -7 might be responsible for the shedding of MICA, as transcription of these proteolytic enzymes is up-regulated upon HIV-1 infection. Differential expression of such proteolytic enzymes would explain why studies reported that following HIV-1 infection, MICA is barely detectable at the surface of CD4 cells [67, 90], while it appears to be up-regulated on cell lines, including Jurkat cells [92].
6. 3. Inhibition of APOBEC3G-mediated up-regulation of NKG2D ligands
NKG2D ligands are not exclusively up-regulated in response to viral infection but rather upon the development of any general cell stress. HIV-1 Vpr has long been identified as a viral factor able to induce cell cycle arrest. However it is only recently that this observation has been linked to DNA damage responses, notably through activation of the ATR pathway (reviewed in [95]), and to the overexpression of NKG2D ligands [96, 97]. Novel data from Norman et al. shows that Vpr affects the DNA repair machinery in response to viral DNA editing by intracellular innate immune effectors, such as APOBEC3G. This study describes an as yet unidentified consequence of the antiviral activity of these DNA editing enzymes, which leads to the expression of NKG2D ligands on HIV-1-infected cells, and is counteracted by HIV-1 Vif [91].
APOBEC3G is an editing enzyme that belongs to a family of polynucleotide cytidine deaminases initially recognized for its ability to inhibit retroviruses, including HIV-1, and as the target of HIV-1 Vif (reviewed in [98]). APOBEC3G is packaged into the virion and once in the target cell, it remains associated with the reverse transcription complex and deaminates cytidine residues into uridine residues in the growing minus-strand viral DNA, leading to G-to-A changes in the plus strand synthesized from this template. U-rich transcripts can become integrated into the host genome but yield proviruses that are largely nonfunctional due to G-to-A hypermutation. In addition to the lethal editing of the nascent viral reverse transcript, APOBEC3G activities trigger a dramatic decrease in viral cDNA products, possibly resulting from their degradation by DNA repair enzymes, as well as defects in all the reverse transcription steps. Most lentiviruses have evolved strategies to escape APOBEC3G restriction, notably via the expression of the Vif protein, which induces the proteasome-mediated degradation of APOBEC3G. In addition, Vif decreases the translation of the APOBEC3G mRNA, further reducing the levels of cytidine deaminases in the cell.
Therefore, Vif allows HIV-1 to replicate in target cells which express APOBEC3G or other members of the polynucleotide cytidine deaminase family. However, recent data show that Vif activity has another crucial function in protecting HIV-1 replication against innate immune effectors, and acts in tandem with Nef to help HIV-1-infected cells escaping NKG2D-mediated NK cell lysis [91]. Indeed, it is known that DNA damage leads to the up-regulation of NKG2D ligands [99]. Similarly, in the absence of Vif, DNA editing by APOBEC3G activates DNA damage pathways, leading to the up-regulation of NKG2D ligands at the surface of HIV-1-infected T cells, thereby increasing their susceptibility to NK cell mediated lysis [91]. Corroborating this observation, the amount of NKG2D ligands at the surface of HIV-1-infected cells directly depends on the expression levels of APOBEC3G.
Interestingly, in accordance with previous data showing that production of Vpr in HIV-1 infected cells leads to an increased surface expression of NKG2D ligands [96, 97], APOBEC3G-mediated up-regulation of NKG2D ligands depends on the binding of HIV-1 Vpr to Uracil DNA glycosylase 2 (UNG2), an nuclear enzyme that removes uracil from single- and double-stranded DNA, and that has previously been shown to interact with Vpr (reviewed in [100]). The elegant study from Norman et al. suggests that Vpr is required to enhance DNA repair by UNG2 of APOBEC3G-induced mutations, a process which generates DNA breaks, thereby activating DNA damage pathways, and results in the up-regulation of NKG2D ligands. It is of prime importance for HIV-1 to be able to repair uridines incorporated into their genome by host intracellular immune factors as it would seriously impair infectivity of the progeny virions. The critical relevance of this pathway is highlighted by the fact that two HIV-1 viral proteins, Vif and Vpr, are involved in limiting uridine incorporation into the HIV-1 genome.
7. Selection of viral sequence polymorphisms to escape from NK cell-mediated recognition
Loss or reduction of HLA class I expression is one mechanism underlying the increased sensitivity of virally-infected cells to NK cell-mediated lysis. However, the nature of the presented peptide can also significantly impact the ability of KIR to bind to the HLA class I/peptide complex, thereby affecting NK cell function [101, 102]. Recent data furthermore suggest that changes in peptide repertoire can be more potent at activating NK cells than HLA class I down-regulation alone. Indeed, peptides that can bind to HLA class I molecules but not KIRs can disrupt KIR-mediated inhibition of NK cell activation. Peptides conferring weak recognition by KIRs can antagonize the inhibitory effect of other peptides inducing strong KIR binding to the HLA class I ligand, and trigger NK cell degranulation [103]
7.1. Peptide-specific recognition of HLA ligands by KIRs
Peptide-specificity in the recognition of HLA class I molecules by NK cells has been described for a number of inhibitory KIR receptors. For instance, it has been long known that binding of KIR2DL1 to HLA-Cw4 only occurs when the HLA class I molecule is loaded with peptides, and certain substitutions in position 8 abolish the interaction with KIR while still allowing the epitope to bind to HLA class I [104]. More recently, it was reported that amino acid changes at position 8 in an HLA-A3/A11-restricted epitope can abolish binding of KIR3DL2-expressing NK cells [105]. Similarly, HLA-Cw*0304 and -Cw7 interaction with KIR2DL2 is modulated by the sequence of the peptide presented by the HLA class I molecules [106, 107]. This data have been supported by the analysis of the crystal structure of KIR2DL2 in complex with HLA-Cw3, demonstrating that KIR contact requires the amino acid in position 8 to be a small residue, otherwise binding does not occur [108].
7.2. Impact of the presented peptide on KIR3DL1 binding to its HLA ligand
The combination of KIR3DL1*h and HLA-Bw4 is associated with a protective effect in HIV-1 infection [9]. Interestingly, as for other KIRs, there is accumulating evidence that the nature of the presented peptide influences the binding of KIR3DL1 to its HLA class I ligand. It was initially described that HLA-B*2705 interaction with KIR3DL1 shows some degree of peptide-specificity with residues at position 7 and 8 [109–111] (Fig. 2). Furthermore, various KIR3DL1 allotypes were reported to have a differential ability to bind to HLA-A2402, and their binding to HLA class-I ligands is affected by the sequence of the loaded peptide [112]. Finally, the importance of amino acid 8 of the HLA class I-presented epitope has been further emphasized by the recent resolution of the crytal structure of KIR3DL1 bound to HLA-B5701 complexed with a self-peptide, which revealed that position 8 (and 9) is directly involved in the contact between KIR3DL1 and its ligand [48].
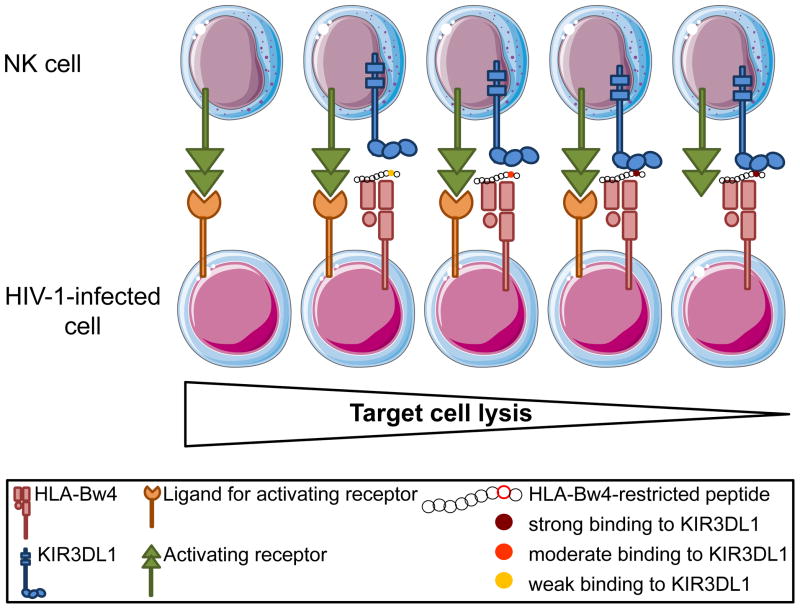
Figure 2. Influence of the presented peptide on KIR3DL1-HLA-Bw4 interaction and NK cell activation.
Single amino acid changes in the C-terminus of the peptide presented by HLA-Bw4 can significantly impact KIR3DL1-HLA-Bw4 interactions, and therefore NK cell function. Presentation of an endogenous or a viral peptide that can bind to KIR3DL1 with a high affinity might result in a strong inhibitory signal to NK cells. In such cases, NK cell activation, and potentially killing of a target cell, might only occur in the presence of strong concomitant activating signals delivered via other NK cell receptors. In contrast, mutations leading to a weaker binding between KIR3DL1 and its HLA-Bw4 ligand might lower the threshold of NK cell activation and favor target cell lysis.
Increased recognition of HIV-1-infected cells by NK cells, mediated by an HIV-1-driven change in peptide presentation, could provide a possible explanation for the protective effect associated with the expression of KIR3DL1 in conjunction with its HLA-Bw4 ligand. This hypothesis is supported by the fact that KIR3DL1 can discriminate between common HIV-1 peptide variants presented by HLA-B57, leading to promotion or abrogation of KIR3DL1 binding to HLA-B57 [113, 114]. Presentation of HIV-1-derived epitopes that leads to weak KIR3DL1/HLA-B57 binding might promote NK cell lysis. On the other hand, HIV-1 peptides that trigger increased binding between the inhibitory KIR3DL1 receptor and HLA-B57 might allow the virus to escape NK cell recognition. Numerous single amino acid mutations in the HIV-1 genome have been identified thus far that promote virus escape from TCR-mediated recognition by CD8 T cells (Reviewed in [115]). Among these, mutations in the HLA-B57 restricted epitope TW10 have been described and allow the virus to rapidly escape adaptive immune responses in HLA-B57+ individuals [116]. Interestingly, TW10 CTL escape mutations abrogate binding to KIR3DL1, suggesting that although such mutants escape HIV-1-specific CD8 recognition, they are able to activate NK cell function, allowing to maintain some control of HIV-1 replication. The impact of CTL escape mutations on NK cell responses to HIV-1 was further illustrated by another study describing two TW10 CTL escape mutations (G9E and T3N) that appeared very early in viruses isolated from two KIR3DL1+ HLA-B57+ subjects with acute HIV-1 infection [113]. In contrast to the T3N mutation, the C-terminal G9E change did not abrogate recognition of the TW10 epitope by CD8 T cells, but rather significantly impaired the binding of KIR3DL1 to HLA-B57. A decreased affinity between KIR3DL1 and its ligand is expected to increase NK cells lysis of the infected cells and would be a potential disadvantage to the virus. However, the appearance of this mutation could benefit viral replication at other levels. For example, expression of KIR3DL1 on CD8 may act as a coreceptor stabilizing the interaction between TCR and HLA class I. In this instance, G9E mutation could impair CD8 T cell function by impairing TCR engagement. Further investigations will be required to fully understand the impact of HIV-1 escape mutations on NK cell responses.
7.3. Identification of KIR footprints in the HIV-1 genome
Overall, these studies demonstrate that changes in the sequences of the peptides presented on HLA class I molecules have an important influence on the affinity of the binding between KIRs and their respective HLA class I ligands, and potentially on NK cell recognition of HIV-1-infected cells. If the HLA class I-presented viral peptide impacts the subsequent NK cell responses, the virus might evolve to either escape NK cell recognition by activating KIRs and/or repress NK cell function by increasing binding of inhibitory KIRs. While numerous studies have focused on the emergence of amino acid changes involved in CTL escape, it is only recently that mutations arising in the HIV-1 genome and associated with KIR recognition were described, further supporting the involvement of this family of receptors in the response to HIV-1 infection. Screening of 91 HIV-1 sequences from individuals with untreated chronic HIV-1 infection led to the identification of 22 amino acid polymorphisms in HIV-1 that were significantly more represented in individuals carrying a specific KIR gene [10]. Of particular interest, patients possessing at least one copy of the KIR2DL2 gene were more likely to have two characteristic amino acid changes (Vpu(71) and Vpu(74)) at positions located in the region overlapping the c-terminal region of Vpu and the n-terminal region of Env compared to individuals who do not possess any copy of the gene encoding KIR2DL2. Consistent with these observations, in vitro, NK cells lose their ability to inhibit the replication of an engineered HIV-1 virus expressing the two mutations commonly found in KIR2DL2+ subjects. One potential mechanism underlying this observation is that presentation of peptides derived from the HIV-1 sequence carrying the observed polymorphisms increases the binding affinity between the inhibitory KIR2DL2 receptor and its HLA ligand, thereby increasing the inhibitory signal delivered to NK cells. Overall, these findings reflect the evolutionary pressure exerted by NK cells on HIV-1.
8. Future perspectives
A better understanding of the interplay between HIV-1 infection and NK cell function will allow to identifying novel approaches to restore and/or enhance NK cell function in HIV-1 infected patients, and potentially harness NK cell activity to improve future HIV-1 vaccine strategies. Notably, there is mounting evidence emerging from studies conducted in the mouse model that NK cells can be antigen-specific and can mediate immunological memory [117–121]. While the existence of similar properties in humans has not been demonstrated so far, the identification of potential memory human NK cells would open the possibility to use NK cells with activity against HIV-1 to enhance viral control in infected individuals, or to prevent infection by inducing NK cells at mucosal sites. However, many aspects of the NK cell immune response remain to be understood in order to comprehend how to harness their activity for HIV-1 vaccine design. First, the physiological ligands for NCRs have not been characterized yet, and it will be of paramount importance to identify whether NCRs can recognize HIV-1-infected cells. Then, due to the lack of commercially available reagents to specifically assess the expression of most activating KIRs and some inhibitory KIRs, the role played by these receptors in the NK cell response to HIV-1 is difficult to study. The development of tools to characterize KIR-HLA class I interactions for each of these receptors is urgently needed. Finally, the cross talks between NK cells and other immune cells, such as DCs and CD4 cells, is becoming an area of intense investigation and will probably uncover new pathways that can be manipulated in the context of vaccine design.
9. Conclusion
Overall, HIV-1 accessory proteins seem to have evolved to counteract immune responses, and notably NK cell responses. These viral escape mechanisms reflect the strong evolutionary pressure exerted by NK cells on HIV-1, and indicate that NK cells represent an important component to consider in future interventions to harness antiviral immune responses against HIV-1.
Acknowledgments
We apologize to all the authors who contributed to understanding the role played by NK cells in HIV-1 infection and who could not be cited due to space restrictions. Figures were produced using Servier Medical Art.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.UNAIDS/WHO. World AIDS day report 2011. 2011 http://www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2011/JC2216_WorldAIDSday_report_2011_en.pdf.
- 2.Kim JH, Rerks-Ngarm S, Excler JL, Michael NL. HIV vaccines: lessons learned and the way forward. Curr Opin HIV AIDS. 2010;5:428–434. doi: 10.1097/COH.0b013e32833d17ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pulendran B, Ahmed R. Immunological mechanisms of vaccination. Nat immunol. 2011;12:509–517. doi: 10.1038/ni.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrews DM, Estcourt MJ, Andoniou CE, Wikstrom ME, Khong A, Voigt V, Fleming P, Tabarias H, Hill GR, van der Most RG, Scalzo AA, Smyth MJ, Degli-Esposti MA. Innate immunity defines the capacity of antiviral T cells to limit persistent infection. J Exp Med. 2010;207:1333–1343. doi: 10.1084/jem.20091193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper MA, Fehniger TA, Fuchs A, Colonna M, Caligiuri MA. NK cell and DC interactions. Trends Immunol. 2004;25:47–52. doi: 10.1016/j.it.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Robbins SH, Bessou G, Cornillon A, Zucchini N, Rupp B, Ruzsics Z, Sacher T, Tomasello E, Vivier E, Koszinowski UH, Dalod M. Natural killer cells promote early CD8 T cell responses against cytomegalovirus. PLoS pathog. 2007;3:e123. doi: 10.1371/journal.ppat.0030123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waggoner SN, Cornberg M, Selin LK, Welsh RM. Natural killer cells act as rheostats modulating antiviral T cells. Nature. 2012;481:394–398. doi: 10.1038/nature10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin MP, Gao X, Lee JH, Nelson GW, Detels R, Goedert JJ, Buchbinder S, Hoots K, Vlahov D, Trowsdale J, Wilson M, O’Brien SJ, Carrington M. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat Genet. 2002;31:429–434. doi: 10.1038/ng934. [DOI] [PubMed] [Google Scholar]
- 9.Martin MP, Qi Y, Gao X, Yamada E, Martin JN, Pereyra F, Colombo S, Brown EE, Shupert WL, Phair J, Goedert JJ, Buchbinder S, Kirk GD, Telenti A, Connors M, O’Brien SJ, Walker BD, Parham P, Deeks SG, McVicar DW, Carrington M. Innate partnership of HLA-B and KIR3DL1 subtypes against HIV-1. Nat Genet. 2007;39:733–740. doi: 10.1038/ng2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alter G, Heckerman D, Schneidewind A, Fadda L, Kadie CM, Carlson JM, Oniangue-Ndza C, Martin M, Li B, Khakoo SI, Carrington M, Allen TM, Altfeld M. HIV-1 adaptation to NK-cell-mediated immune pressure. Nature. 2011;476:96–100. doi: 10.1038/nature10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 12.Arnon TI, Markel G, Mandelboim O. Tumor and viral recognition by natural killer cells receptors. Semin Cancer Biol. 2006;16:348–358. doi: 10.1016/j.semcancer.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Hershkovitz O, Jivov S, Bloushtain N, Zilka A, Landau G, Bar-Ilan A, Lichtenstein RG, Campbell KS, van Kuppevelt TH, Porgador A. Characterization of the recognition of tumor cells by the natural cytotoxicity receptor, NKp44. Biochemistry (Mosc) 2007;46:7426–7436. doi: 10.1021/bi7000455. [DOI] [PubMed] [Google Scholar]
- 14.Hershkovitz O, Jarahian M, Zilka A, Bar-Ilan A, Landau G, Jivov S, Tekoah Y, Glicklis R, Gallagher JT, Hoffmann SC, Zer H, Mandelboim O, Watzl C, Momburg F, Porgador A. Altered glycosylation of recombinant NKp30 hampers binding to heparan sulfate: a lesson for the use of recombinant immunoreceptors as an immunological tool. Glycobiology. 2008;18:28–41. doi: 10.1093/glycob/cwm125. [DOI] [PubMed] [Google Scholar]
- 15.Hecht ML, Rosental B, Horlacher T, Hershkovitz O, De Paz JL, Noti C, Schauer S, Porgador A, Seeberger PH. Natural cytotoxicity receptors NKp30, NKp44 and NKp46 bind to different heparan sulfate/heparin sequences. J Proteome Res. 2009;8:712–720. doi: 10.1021/pr800747c. [DOI] [PubMed] [Google Scholar]
- 16.Bloushtain N, Qimron U, Bar-Ilan A, Hershkovitz O, Gazit R, Fima E, Korc M, Vlodavsky I, Bovin NV, Porgador A. Membrane-associated heparan sulfate proteoglycans are involved in the recognition of cellular targets by NKp30 and NKp46. J Immunol. 2004;173:2392–2401. doi: 10.4049/jimmunol.173.4.2392. [DOI] [PubMed] [Google Scholar]
- 17.Mandelboim O, Lieberman N, Lev M, Paul L, Arnon TI, Bushkin Y, Davis DM, Strominger JL, Yewdell JW, Porgador A. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature. 2001;409:1055–1060. doi: 10.1038/35059110. [DOI] [PubMed] [Google Scholar]
- 18.Arnon TI, Lev M, Katz G, Chernobrov Y, Porgador A, Mandelboim O. Recognition of viral hemagglutinins by NKp44 but not by NKp30. Eur J Immunol. 2001;31:2680–2689. doi: 10.1002/1521-4141(200109)31:9<2680::aid-immu2680>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 19.Arnon TI, Achdout H, Levi O, Markel G, Saleh N, Katz G, Gazit R, Gonen-Gross T, Hanna J, Nahari E, Porgador A, Honigman A, Plachter B, Mevorach D, Wolf DG, Mandelboim O. Inhibition of the NKp30 activating receptor by pp65 of human cytomegalovirus. Nat Immunol. 2005;6:515–523. doi: 10.1038/ni1190. [DOI] [PubMed] [Google Scholar]
- 20.Mavoungou E, Held J, Mewono L, Kremsner PG. A Duffy binding-like domain is involved in the NKp30-mediated recognition of Plasmodium falciparum-parasitized erythrocytes by natural killer cells. J Infect Dis. 2007;195:1521–1531. doi: 10.1086/515579. [DOI] [PubMed] [Google Scholar]
- 21.Simhadri VR, Reiners KS, Hansen HP, Topolar D, Simhadri VL, Nohroudi K, Kufer TA, Engert A, Pogge von Strandmann E. Dendritic cells release HLA-B-associated transcript-3 positive exosomes to regulate natural killer function. PloS One. 2008;3:e3377. doi: 10.1371/journal.pone.0003377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pogge von Strandmann E, Simhadri VR, von Tresckow B, Sasse S, Reiners KS, Hansen HP, Rothe A, Boll B, Simhadri VL, Borchmann P, McKinnon PJ, Hallek M, Engert A. Human leukocyte antigen-B-associated transcript 3 is released from tumor cells and engages the NKp30 receptor on natural killer cells. Immunity. 2007;27:965–974. doi: 10.1016/j.immuni.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 23.Brandt CS, Baratin M, Yi EC, Kennedy J, Gao Z, Fox B, Haldeman B, Ostrander CD, Kaifu T, Chabannon C, Moretta A, West R, Xu W, Vivier E, Levin SD. The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J Exp Med. 2009;206:1495–1503. doi: 10.1084/jem.20090681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esin S, Batoni G, Counoupas C, Stringaro A, Brancatisano FL, Colone M, Maisetta G, Florio W, Arancia G, Campa M. Direct binding of human NK cell natural cytotoxicity receptor NKp44 to the surfaces of mycobacteria and other bacteria. Infect Immun. 2008;76:1719–1727. doi: 10.1128/IAI.00870-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hershkovitz O, Rosental B, Rosenberg LA, Navarro-Sanchez ME, Jivov S, Zilka A, Gershoni-Yahalom O, Brient-Litzler E, Bedouelle H, Ho JW, Campbell KS, Rager-Zisman B, Despres P, Porgador A. NKp44 receptor mediates interaction of the envelope glycoproteins from the West Nile and dengue viruses with NK cells. J Immunol. 2009;183:2610–2621. doi: 10.4049/jimmunol.0802806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bashirova AA, Thomas R, Carrington M. HLA/KIR restraint of HIV: surviving the fittest. Annu Rev Immunol. 2011;29:295–317. doi: 10.1146/annurev-immunol-031210-101332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stewart CA, Laugier-Anfossi F, Vely F, Saulquin X, Riedmuller J, Tisserant A, Gauthier L, Romagne F, Ferracci G, Arosa FA, Moretta A, Sun PD, Ugolini S, Vivier E. Recognition of peptide-MHC class I complexes by activating killer immunoglobulin-like receptors. Proc Natl Acad Sci U S A. 2005;102:13224–13229. doi: 10.1073/pnas.0503594102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown D, Trowsdale J, Allen R. The LILR family: modulators of innate and adaptive immune pathways in health and disease. Tissue Antigens. 2004;64:215–225. doi: 10.1111/j.0001-2815.2004.00290.x. [DOI] [PubMed] [Google Scholar]
- 29.Shiroishi M, Tsumoto K, Amano K, Shirakihara Y, Colonna M, Braud VM, Allan DS, Makadzange A, Rowland-Jones S, Willcox B, Jones EY, van der Merwe PA, Kumagai I, Maenaka K. Human inhibitory receptors Ig-like transcript 2 (ILT2) and ILT4 compete with CD8 for MHC class I binding and bind preferentially to HLA-G. Proc Natl Acad Sci U S A. 2003;100:8856–8861. doi: 10.1073/pnas.1431057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640. [Google Scholar]
- 31.Trinchieri G, Valiante N. Receptors for the Fc fragment of IgG on natural killer cells. Nat Immun. 1993;12:218–234. [PubMed] [Google Scholar]
- 32.Hart OM, Athie-Morales V, O’Connor GM, Gardiner CM. TLR7/8-mediated activation of human NK cells results in accessory cell-dependent IFN-gamma production. J Immunol. 2005;175:1636–1642. doi: 10.4049/jimmunol.175.3.1636. [DOI] [PubMed] [Google Scholar]
- 33.Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdorfer B, Giese T, Endres S, Hartmann G. Quantitative expression of toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168:4531–4537. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- 34.Sivori S, Falco M, Della Chiesa M, Carlomagno S, Vitale M, Moretta L, Moretta A. CpG and double-stranded RNA trigger human NK cells by Toll-like receptors: induction of cytokine release and cytotoxicity against tumors and dendritic cells. Proc Natl Acad Sci U S A. 2004;101:10116–10121. doi: 10.1073/pnas.0403744101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 36.Meier A, Alter G, Frahm N, Sidhu H, Li B, Bagchi A, Teigen N, Streeck H, Stellbrink HJ, Hellman J, van Lunzen J, Altfeld M. MyD88-dependent immune activation mediated by human immunodeficiency virus type 1-encoded Toll-like receptor ligands. J Virol. 2007;81:8180–8191. doi: 10.1128/JVI.00421-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beignon AS, McKenna K, Skoberne M, Manches O, DaSilva I, Kavanagh DG, Larsson M, Gorelick RJ, Lifson JD, Bhardwaj N. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J Clin Invest. 2005;115:3265–3275. doi: 10.1172/JCI26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alter G, Suscovich TJ, Teigen N, Meier A, Streeck H, Brander C, Altfeld M. Single-stranded RNA derived from HIV-1 serves as a potent activator of NK cells. J Immunol. 2007;178:7658–7666. doi: 10.4049/jimmunol.178.12.7658. [DOI] [PubMed] [Google Scholar]
- 39.Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, Carson WE, Caligiuri MA. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood. 2001;97:3146–3151. doi: 10.1182/blood.v97.10.3146. [DOI] [PubMed] [Google Scholar]
- 40.Caligiuri MA, Zmuidzinas A, Manley TJ, Levine H, Smith KA, Ritz J. Functional consequences of interleukin 2 receptor expression on resting human lymphocytes. Identification of a novel natural killer cell subset with high affinity receptors. J Exp Med. 1990;171:1509–1526. doi: 10.1084/jem.171.5.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Romagnani C, Juelke K, Falco M, Morandi B, D’Agostino A, Costa R, Ratto G, Forte G, Carrega P, Lui G, Conte R, Strowig T, Moretta A, Munz C, Thiel A, Moretta L, Ferlazzo G. CD56brightCD16- killer Ig-like receptor- NK cells display longer telomeres and acquire features of CD56dim NK cells upon activation. J Immunol. 2007;178:4947–4955. doi: 10.4049/jimmunol.178.8.4947. [DOI] [PubMed] [Google Scholar]
- 42.Chan A, Hong DL, Atzberger A, Kollnberger S, Filer AD, Buckley CD, McMichael A, Enver T, Bowness P. CD56bright human NK cells differentiate into CD56dim cells: role of contact with peripheral fibroblasts. J Immunol. 2007;179:89–94. doi: 10.4049/jimmunol.179.1.89. [DOI] [PubMed] [Google Scholar]
- 43.Mavilio D, Benjamin J, Daucher M, Lombardo G, Kottilil S, Planta MA, Marcenaro E, Bottino C, Moretta L, Moretta A, Fauci AS. Natural killer cells in HIV-1 infection: dichotomous effects of viremia on inhibitory and activating receptors and their functional correlates. Proc Natl Acad Sci U S A. 2003;100:15011–15016. doi: 10.1073/pnas.2336091100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alter G, Teigen N, Davis BT, Addo MM, Suscovich TJ, Waring MT, Streeck H, Johnston MN, Staller KD, Zaman MT, Yu XG, Lichterfeld M, Basgoz N, Rosenberg ES, Altfeld M. Sequential deregulation of NK cell subset distribution and function starting in acute HIV-1 infection. Blood. 2005;106:3366–3369. doi: 10.1182/blood-2005-03-1100. [DOI] [PubMed] [Google Scholar]
- 45.Mavilio D, Lombardo G, Benjamin J, Kim D, Follman D, Marcenaro E, O’Shea MA, Kinter A, Kovacs C, Moretta A, Fauci AS. Characterization of CD56-/CD16+ natural killer (NK) cells: a highly dysfunctional NK subset expanded in HIV-infected viremic individuals. Proc Natl Acad Sci U S A. 2005;102:2886–2891. doi: 10.1073/pnas.0409872102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alter G, Martin MP, Teigen N, Carr WH, Suscovich TJ, Schneidewind A, Streeck H, Waring M, Meier A, Brander C, Lifson JD, Allen TM, Carrington M, Altfeld M. Differential natural killer cell-mediated inhibition of HIV-1 replication based on distinct KIR/HLA subtypes. J Exp Med. 2007;204:3027–3036. doi: 10.1084/jem.20070695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alter G, Rihn S, Walter K, Nolting A, Martin M, Rosenberg ES, Miller JS, Carrington M, Altfeld M. HLA class I subtype-dependent expansion of KIR3DS1+ and KIR3DL1+ NK cells during acute human immunodeficiency virus type 1 infection. J Virol. 2009;83:6798–6805. doi: 10.1128/JVI.00256-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vivian JP, Duncan RC, Berry R, O’Connor GM, Reid HH, Beddoe T, Gras S, Saunders PM, Olshina MA, Widjaja JM, Harpur CM, Lin J, Maloveste SM, Price DA, Lafont BA, McVicar DW, Clements CS, Brooks AG, Rossjohn J. Killer cell immunoglobulin-like receptor 3DL1-mediated recognition of human leukocyte antigen B. Nature. 2011;479:401–405. doi: 10.1038/nature10517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Long BR, Ndhlovu LC, Oksenberg JR, Lanier LL, Hecht FM, Nixon DF, Barbour JD. Conferral of enhanced natural killer cell function by KIR3DS1 in early human immunodeficiency virus type 1 infection. J Virol. 2008;82:4785–4792. doi: 10.1128/JVI.02449-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O’Connell KA, Han Y, Williams TM, Siliciano RF, Blankson JN. Role of natural killer cells in a cohort of elite suppressors: low frequency of the protective KIR3DS1 allele and limited inhibition of human immunodeficiency virus type 1 replication in vitro. J Virol. 2009;83:5028–5034. doi: 10.1128/JVI.02551-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gaudieri S, DeSantis D, McKinnon E, Moore C, Nolan D, Witt CS, Mallal SA, Christiansen FT. Killer immunoglobulin-like receptors and HLA act both independently and synergistically to modify HIV disease progression. Genes Immun. 2005;6:683–690. doi: 10.1038/sj.gene.6364256. [DOI] [PubMed] [Google Scholar]
- 52.Boulet S, Kleyman M, Kim JY, Kamya P, Sharafi S, Simic N, Bruneau J, Routy JP, Tsoukas CM, Bernard NF. A combined genotype of KIR3DL1 high expressing alleles and HLA-B*57 is associated with a reduced risk of HIV infection. AIDS. 2008;22:1487–1491. doi: 10.1097/QAD.0b013e3282ffde7e. [DOI] [PubMed] [Google Scholar]
- 53.Boulet S, Song R, Kamya P, Bruneau J, Shoukry NH, Tsoukas CM, Bernard NF. HIV protective KIR3DL1 and HLA-B genotypes influence NK cell function following stimulation with HLA-devoid cells. J Immunol. 2010;184:2057–2064. doi: 10.4049/jimmunol.0902621. [DOI] [PubMed] [Google Scholar]
- 54.Kamya P, Boulet S, Tsoukas CM, Routy JP, Thomas R, Cote P, Boulassel MR, Baril JG, Kovacs C, Migueles SA, Connors M, Suscovich TJ, Brander C, Tremblay CL, Bernard N. Receptor-ligand requirements for increased NK cell polyfunctional potential in slow progressors infected with HIV-1 coexpressing KIR3DL1*h/*y and HLA-B*57. J Virol. 2011;85:5949–5960. doi: 10.1128/JVI.02652-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pelak K, Need AC, Fellay J, Shianna KV, Feng S, Urban TJ, Ge D, De Luca A, Martinez-Picado J, Wolinsky SM, Martinson JJ, Jamieson BD, Bream JH, Martin MP, Borrow P, Letvin NL, McMichael AJ, Haynes BF, Telenti A, Carrington M, Goldstein DB, Alter G. Copy number variation of KIR genes influences HIV-1 control. PLoS biology. 2011;9:e1001208. doi: 10.1371/journal.pbio.1001208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scott-Algara D, Truong LX, Versmisse P, David A, Luong TT, Nguyen NV, Theodorou I, Barre-Sinoussi F, Pancino G. Cutting edge: increased NK cell activity in HIV-1-exposed but uninfected Vietnamese intravascular drug users. J Immunol. 2003;171:5663–5667. doi: 10.4049/jimmunol.171.11.5663. [DOI] [PubMed] [Google Scholar]
- 57.Tomescu C, Duh FM, Lanier MA, Kapalko A, Mounzer KC, Martin MP, Carrington M, Metzger DS, Montaner LJ. Increased plasmacytoid dendritic cell maturation and natural killer cell activation in HIV-1 exposed, uninfected intravenous drug users. AIDS. 2010;24:2151–2160. doi: 10.1097/QAD.0b013e32833dfc20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ravet S, Scott-Algara D, Bonnet E, Tran HK, Tran T, Nguyen N, Truong LX, Theodorou I, Barre-Sinoussi F, Pancino G, Paul P. Distinctive NK-cell receptor repertoires sustain high-level constitutive NK-cell activation in HIV-exposed uninfected individuals. Blood. 2007;109:4296–4305. doi: 10.1182/blood-2006-08-040238. [DOI] [PubMed] [Google Scholar]
- 59.Montoya CJ, Velilla PA, Chougnet C, Landay AL, Rugeles MT. Increased IFN-gamma production by NK and CD3+/CD56+ cells in sexually HIV-1-exposed but uninfected individuals. Clin Immunol. 2006;120:138–146. doi: 10.1016/j.clim.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 60.Parsons MS, Boulet S, Song R, Bruneau J, Shoukry NH, Routy JP, Tsoukas CM, Bernard NF. Mind the gap: lack of association between KIR3DL1*004/HLA-Bw4-induced natural killer cell function and protection from HIV infection. J Infect Dis. 2010;202(Suppl 3):S356–360. doi: 10.1086/655966. [DOI] [PubMed] [Google Scholar]
- 61.Boulet S, Sharafi S, Simic N, Bruneau J, Routy JP, Tsoukas CM, Bernard NF. Increased proportion of KIR3DS1 homozygotes in HIV-exposed uninfected individuals. AIDS. 2008;22:595–599. doi: 10.1097/QAD.0b013e3282f56b23. [DOI] [PubMed] [Google Scholar]
- 62.Jennes W, Verheyden S, Demanet C, Adje-Toure CA, Vuylsteke B, Nkengasong JN, Kestens L. Cutting edge: resistance to HIV-1 infection among African female sex workers is associated with inhibitory KIR in the absence of their HLA ligands. J Immunol. 2006;177:6588–6592. doi: 10.4049/jimmunol.177.10.6588. [DOI] [PubMed] [Google Scholar]
- 63.Iannello A, Debbeche O, Samarani S, Ahmad A. Antiviral NK cell responses in HIV infection: I. NK cell receptor genes as determinants of HIV resistance and progression to AIDS. J Leukoc Biol. 2008;84:1–26. doi: 10.1189/jlb.0907650. [DOI] [PubMed] [Google Scholar]
- 64.Brunetta E, Hudspeth KL, Mavilio D. Pathologic natural killer cell subset redistribution in HIV-1 infection: new insights in pathophysiology and clinical outcomes. J Leukoc Biol. 2010;88:1119–1130. doi: 10.1189/jlb.0410225. [DOI] [PubMed] [Google Scholar]
- 65.Eger KA, Unutmaz D. Perturbation of natural killer cell function and receptors during HIV infection. Trends Microbiol. 2004;12:301–303. doi: 10.1016/j.tim.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 66.Shah AH, Sowrirajan B, Davis ZB, Ward JP, Campbell EM, Planelles V, Barker E. Degranulation of natural killer cells following interaction with HIV-1-infected cells is hindered by downmodulation of NTB-A by Vpu. Cell Host Microbe. 2010;8:397–409. doi: 10.1016/j.chom.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ward J, Bonaparte M, Sacks J, Guterman J, Fogli M, Mavilio D, Barker E. HIV modulates the expression of ligands important in triggering natural killer cell cytotoxic responses on infected primary T-cell blasts. Blood. 2007;110:1207–1214. doi: 10.1182/blood-2006-06-028175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Parsons MS, Wren L, Isitman G, Navis M, Stratov I, Bernard NF, Kent SJ. HIV infection abrogates the functional advantage of natural killer cells educated through KIR3DL1/HLA-Bw4 interactions to mediate anti-HIV antibody-dependent cellular cytotoxicity. J Virol. 2012 doi: 10.1128/JVI.06112-11. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu Q, Sun Y, Rihn S, Nolting A, Tsoukas PN, Jost S, Cohen K, Walker B, Alter G. Matrix metalloprotease inhibitors restore impaired NK cell-mediated antibody-dependent cellular cytotoxicity in human immunodeficiency virus type 1 infection. J Virol. 2009;83:8705–8712. doi: 10.1128/JVI.02666-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chung AW, Isitman G, Navis M, Kramski M, Center RJ, Kent SJ, Stratov I. Immune escape from HIV-specific antibody-dependent cellular cytotoxicity (ADCC) pressure. Proc Natl Acad Sci U S A. 2011;108:7505–7510. doi: 10.1073/pnas.1016048108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alter G, Kavanagh D, Rihn S, Luteijn R, Brooks D, Oldstone M, van Lunzen J, Altfeld M. IL-10 induces aberrant deletion of dendritic cells by natural killer cells in the context of HIV infection. J Clin Invest. 2010;120:1905–1913. doi: 10.1172/JCI40913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Quaranta MG, Napolitano A, Sanchez M, Giordani L, Mattioli B, Viora M. HIV-1 Nef impairs the dynamic of DC/NK crosstalk: different outcome of CD56(dim) and CD56(bright) NK cell subsets. FASEB journal. 2007;21:2323–2334. doi: 10.1096/fj.06-7883com. [DOI] [PubMed] [Google Scholar]
- 73.Cohen GB, Gandhi RT, Davis DM, Mandelboim O, Chen BK, Strominger JL, Baltimore D. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity. 1999;10:661–671. doi: 10.1016/s1074-7613(00)80065-5. [DOI] [PubMed] [Google Scholar]
- 74.Collins KL, Chen BK, Kalams SA, Walker BD, Baltimore D. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature. 1998;391:397–401. doi: 10.1038/34929. [DOI] [PubMed] [Google Scholar]
- 75.Le Gall S, Erdtmann L, Benichou S, Berlioz-Torrent C, Liu L, Benarous R, Heard JM, Schwartz O. Nef interacts with the mu subunit of clathrin adaptor complexes and reveals a cryptic sorting signal in MHC I molecules. Immunity. 1998;8:483–495. doi: 10.1016/s1074-7613(00)80553-1. [DOI] [PubMed] [Google Scholar]
- 76.Guma M, Angulo A, Lopez-Botet M. NK cell receptors involved in the response to human cytomegalovirus infection. Curr Top Microbiol Immunol. 2006;298:207–223. doi: 10.1007/3-540-27743-9_11. [DOI] [PubMed] [Google Scholar]
- 77.Bonaparte MI, Barker E. Killing of human immunodeficiency virus-infected primary T-cell blasts by autologous natural killer cells is dependent on the ability of the virus to alter the expression of major histocompatibility complex class I molecules. Blood. 2004;104:2087–2094. doi: 10.1182/blood-2004-02-0696. [DOI] [PubMed] [Google Scholar]
- 78.Bonaparte MI, Barker E. Inability of natural killer cells to destroy autologous HIV-infected T lymphocytes. AIDS. 2003;17:487–494. doi: 10.1097/00002030-200303070-00003. [DOI] [PubMed] [Google Scholar]
- 79.Ward JP, Bonaparte MI, Barker E. HLA-C and HLA-E reduce antibody-dependent natural killer cell-mediated cytotoxicity of HIV-infected primary T cell blasts. AIDS. 2004;18:1769–1779. doi: 10.1097/00002030-200409030-00005. [DOI] [PubMed] [Google Scholar]
- 80.Fausther-Bovendo H, Sol-Foulon N, Candotti D, Agut H, Schwartz O, Debre P, Vieillard V. HIV escape from natural killer cytotoxicity: nef inhibits NKp44L expression on CD4+ T cells. AIDS. 2009;23:1077–1087. doi: 10.1097/QAD.0b013e32832cb26b. [DOI] [PubMed] [Google Scholar]
- 81.Vieillard V, Strominger JL, Debre P. NK cytotoxicity against CD4+ T cells during HIV-1 infection: a gp41 peptide induces the expression of an NKp44 ligand. Proc Natl Acad Sci U S A. 2005;102:10981–10986. doi: 10.1073/pnas.0504315102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Carroll IR, Wang J, Howcroft TK, Singer DS. HIV Tat represses transcription of the beta 2-microglobulin promoter. Mol Immunol. 1998;35:1171–1178. doi: 10.1016/s0161-5890(98)00107-2. [DOI] [PubMed] [Google Scholar]
- 83.Kerkau T, Bacik I, Bennink JR, Yewdell JW, Hunig T, Schimpl A, Schubert U. The human immunodeficiency virus type 1 (HIV-1) Vpu protein interferes with an early step in the biosynthesis of major histocompatibility complex (MHC) class I molecules. J Exp Med. 1997;185:1295–1305. doi: 10.1084/jem.185.7.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Martini F, Agrati C, D’Offizi G, Poccia F. HLA-E up-regulation induced by HIV infection may directly contribute to CD94-mediated impairment of NK cells. Int J Immunopathol Pharmacol. 2005;18:269–276. doi: 10.1177/039463200501800209. [DOI] [PubMed] [Google Scholar]
- 85.Nattermann J, Nischalke HD, Hofmeister V, Kupfer B, Ahlenstiel G, Feldmann G, Rockstroh J, Weiss EH, Sauerbruch T, Spengler U. HIV-1 infection leads to increased HLA-E expression resulting in impaired function of natural killer cells. Antiviral Ther. 2005;10:95–107. doi: 10.1177/135965350501000107. [DOI] [PubMed] [Google Scholar]
- 86.Derrien M, Pizzato N, Dolcini G, Menu E, Chaouat G, Lenfant F, Barre-Sinoussi F, Bouteiller PL. Human immunodeficiency virus 1 downregulates cell surface expression of the non-classical major histocompatibility class I molecule HLA-G1. J Gen Virol. 2004;85:1945–1954. doi: 10.1099/vir.0.79867-0. [DOI] [PubMed] [Google Scholar]
- 87.Lozano JM, Gonzalez R, Kindelan JM, Rouas-Freiss N, Caballos R, Dausset J, Carosella ED, Pena J. Monocytes and T lymphocytes in HIV-1-positive patients express HLA-G molecule. AIDS. 2002;16:347–351. doi: 10.1097/00002030-200202150-00005. [DOI] [PubMed] [Google Scholar]
- 88.Cabello A, Rivero A, Garcia MJ, Lozano JM, Torre-Cisneros J, Gonzalez R, Duenas G, Galiani MD, Camacho A, Santamaria M, Solana R, Montero C, Kindelan JM, Pena J. HAART induces the expression of HLA-G on peripheral monocytes in HIV-1 infected individuals. Hum Immunol. 2003;64:1045–1049. doi: 10.1016/j.humimm.2003.08.353. [DOI] [PubMed] [Google Scholar]
- 89.Champsaur M, Lanier LL. Effect of NKG2D ligand expression on host immune responses. Immunol Rev. 2010;235:267–285. doi: 10.1111/j.0105-2896.2010.00893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fogli M, Mavilio D, Brunetta E, Varchetta S, Ata K, Roby G, Kovacs C, Follmann D, Pende D, Ward J, Barker E, Marcenaro E, Moretta A, Fauci AS. Lysis of endogenously infected CD4+ T cell blasts by rIL-2 activated autologous natural killer cells from HIV-infected viremic individuals. PLoS pathog. 2008;4:e1000101. doi: 10.1371/journal.ppat.1000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Norman JM, Mashiba M, McNamara LA, Onafuwa-Nuga A, Chiari-Fort E, Shen W, Collins KL. The antiviral factor APOBEC3G enhances the recognition of HIV-infected primary T cells by natural killer cells. Nat Immunol. 2011;12:975–983. doi: 10.1038/ni.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cerboni C, Neri F, Casartelli N, Zingoni A, Cosman D, Rossi P, Santoni A, Doria M. Human immunodeficiency virus 1 Nef protein downmodulates the ligands of the activating receptor NKG2D and inhibits natural killer cell-mediated cytotoxicity. J Gen Vir. 2007;88:242–250. doi: 10.1099/vir.0.82125-0. [DOI] [PubMed] [Google Scholar]
- 93.Laguette N, Bregnard C, Benichou S, Basmaciogullari S. Human immunodeficiency virus (HIV) type-1, HIV-2 and simian immunodeficiency virus Nef proteins. Mol Aspects Med. 2010;31:418–433. doi: 10.1016/j.mam.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 94.Nolting A, Dugast AS, Rihn S, Luteijn R, Carrington MF, Kane K, Jost S, Toth I, Nagami E, Faetkenheuer G, Hartmann P, Altfeld M, Alter G. MHC class I chain-related protein A shedding in chronic HIV-1 infection is associated with profound NK cell dysfunction. Virology. 2010;406:12–20. doi: 10.1016/j.virol.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Planelles V, Barker E. Roles of Vpr and Vpx in modulating the virus-host cell relationship. Mol Aspects Med. 2010;31:398–406. doi: 10.1016/j.mam.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Richard J, Sindhu S, Pham TN, Belzile JP, Cohen EA. HIV-1 Vpr up-regulates expression of ligands for the activating NKG2D receptor and promotes NK cell-mediated killing. Blood. 2010;115:1354–1363. doi: 10.1182/blood-2009-08-237370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ward J, Davis Z, DeHart J, Zimmerman E, Bosque A, Brunetta E, Mavilio D, Planelles V, Barker E. HIV-1 Vpr triggers natural killer cell-mediated lysis of infected cells through activation of the ATR-mediated DNA damage response. PLoS pathog. 2009;5:e1000613. doi: 10.1371/journal.ppat.1000613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wissing S, Galloway NL, Greene WC. HIV-1 Vif versus the APOBEC3 cytidine deaminases: an intracellular duel between pathogen and host restriction factors. Mol Aspects Med. 2010;31:383–397. doi: 10.1016/j.mam.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436:1186–1190. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sousa MM, Krokan HE, Slupphaug G. DNA-uracil and human pathology. Mol Aspects Med. 2007;28:276–306. doi: 10.1016/j.mam.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 101.Kaufman DS, Schoon RA, Leibson PJ. Role for major histocompatibility complex class I in regulating natural killer cell-mediated killing of virus-infected cells. Proc Natl Acad Sci U S A. 1992;89:8337–8341. doi: 10.1073/pnas.89.17.8337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Malnati MS, Lusso P, Ciccone E, Moretta A, Moretta L, Long EO. Recognition of virus-infected cells by natural killer cell clones is controlled by polymorphic target cell elements. J Exp Med. 1993;178:961–969. doi: 10.1084/jem.178.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fadda L, Borhis G, Ahmed P, Cheent K, Pageon SV, Cazaly A, Stathopoulos S, Middleton D, Mulder A, Claas FH, Elliott T, Davis DM, Purbhoo MA, Khakoo SI. Peptide antagonism as a mechanism for NK cell activation. Proc Natl Acad Sci U S A. 2010;107:10160–10165. doi: 10.1073/pnas.0913745107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rajagopalan S, Long EO. The direct binding of a p58 killer cell inhibitory receptor to human histocompatibility leukocyte antigen (HLA)-Cw4 exhibits peptide selectivity. J Exp Med. 1997;185:1523–1528. doi: 10.1084/jem.185.8.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hansasuta P, Dong T, Thananchai H, Weekes M, Willberg C, Aldemir H, Rowland-Jones S, Braud VM. Recognition of HLA-A3 and HLA-A11 by KIR3DL2 is peptide-specific. Eur J Immunol. 2004;34:1673–1679. doi: 10.1002/eji.200425089. [DOI] [PubMed] [Google Scholar]
- 106.Mandelboim O, Wilson SB, Vales-Gomez M, Reyburn HT, Strominger JL. Self and viral peptides can initiate lysis by autologous natural killer cells. Proc Natl Acad Sci U S A. 1997;94:4604–4609. doi: 10.1073/pnas.94.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zappacosta F, Borrego F, Brooks AG, Parker KC, Coligan JE. Peptides isolated from HLA-Cw*0304 confer different degrees of protection from natural killer cell-mediated lysis. Proc Natl Acad Sci U S A. 1997;94:6313–6318. doi: 10.1073/pnas.94.12.6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Boyington JC, Brooks AG, Sun PD. Structure of killer cell immunoglobulin-like receptors and their recognition of the class I MHC molecules. Immunol Rev. 2001;181:66–78. doi: 10.1034/j.1600-065x.2001.1810105.x. [DOI] [PubMed] [Google Scholar]
- 109.Peruzzi M, Parker KC, Long EO, Malnati MS. Peptide sequence requirements for the recognition of HLA-B*2705 by specific natural killer cells. J Immunol. 1996;157:3350–3356. [PubMed] [Google Scholar]
- 110.Malnati MS, Peruzzi M, Parker KC, Biddison WE, Ciccone E, Moretta A, Long EO. Peptide specificity in the recognition of MHC class I by natural killer cell clones. Science. 1995;267:1016–1018. doi: 10.1126/science.7863326. [DOI] [PubMed] [Google Scholar]
- 111.Peruzzi M, Wagtmann N, Long EO. A p70 killer cell inhibitory receptor specific for several HLA-B allotypes discriminates among peptides bound to HLA-B*2705. J Exp Med. 1996;184:1585–1590. doi: 10.1084/jem.184.4.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Thananchai H, Gillespie G, Martin MP, Bashirova A, Yawata N, Yawata M, Easterbrook P, McVicar DW, Maenaka K, Parham P, Carrington M, Dong T, Rowland-Jones S. Cutting Edge: Allele-specific and peptide-dependent interactions between KIR3DL1 and HLA-A and HLA-B. J Immunol. 2007;178:33–37. doi: 10.4049/jimmunol.178.1.33. [DOI] [PubMed] [Google Scholar]
- 113.Brackenridge S, Evans EJ, Toebes M, Goonetilleke N, Liu MK, di Gleria K, Schumacher TN, Davis SJ, McMichael AJ, Gillespie GM. An early HIV mutation within an HLA-B*57-restricted T cell epitope abrogates binding to the killer inhibitory receptor 3DL1. J Virol. 2011;85:5415–5422. doi: 10.1128/JVI.00238-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fadda L, O’Connor GM, Kumar S, Piechocka-Trocha A, Gardiner CM, Carrington M, McVicar DW, Altfeld M. Common HIV-1 peptide variants mediate differential binding of KIR3DL1 to HLA-Bw4 molecules. J Virol. 2011;85:5970–5974. doi: 10.1128/JVI.00412-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lopez M, Soriano V, Benito JM. Escape mutations in HIV infection and its impact on CD8+ T cell responses. Curr Mol Med. 2007;7:446–458. doi: 10.2174/156652407781387091. [DOI] [PubMed] [Google Scholar]
- 116.Brumme ZL, Brumme CJ, Carlson J, Streeck H, John M, Eichbaum Q, Block BL, Baker B, Kadie C, Markowitz M, Jessen H, Kelleher AD, Rosenberg E, Kaldor J, Yuki Y, Carrington M, Allen TM, Mallal S, Altfeld M, Heckerman D, Walker BD. Marked epitope- and allele-specific differences in rates of mutation in human immunodeficiency type 1 (HIV-1) Gag, Pol, and Nef cytotoxic T-lymphocyte epitopes in acute/early HIV-1 infection. J Virol. 2008;82:9216–9227. doi: 10.1128/JVI.01041-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dokun AO, Kim S, Smith HR, Kang HS, Chu DT, Yokoyama WM. Specific and nonspecific NK cell activation during virus infection. Nat Immunol. 2001;2:951–956. doi: 10.1038/ni714. [DOI] [PubMed] [Google Scholar]
- 118.Gillard GO, Bivas-Benita M, Hovav AH, Grandpre LE, Panas MW, Seaman MS, Haynes BF, Letvin NL. Thy1+ NK [corrected] cells from vaccinia virus-primed mice confer protection against vaccinia virus challenge in the absence of adaptive lymphocytes. PLoS pathog. 2011;7:e1002141. doi: 10.1371/journal.ppat.1002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.O’Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7:507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- 120.Paust S, Gill HS, Wang BZ, Flynn MP, Moseman EA, Senman B, Szczepanik M, Telenti A, Askenase PW, Compans RW, von Andrian UH. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat Immunol. 2010;11:1127–1135. doi: 10.1038/ni.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]