Abstract
It is known that fluorescence, much of it caused by UVA light excitation, increases in the aging human lens, resulting in loss of sharp vision. This study used an in vivo animal model to investigate UVA-excited fluorescence in the rabbit lens, which contains a high level of the UVA chromophore NADH, existing both free and bound to λ-crystallin. Also, the ability of a Class I (senofilcon A) soft contact lens to protect against UVA-induced effects on the rabbit lens was tested. Rabbit eyes were irradiated with UVA light in vivo (100 mW/cm2 on the cornea) for 1 hour using monochromatic 365 nm light. Irradiation was conducted in the presence of either a senofilcon A contact lens, a minimally UV-absorbing lotrafilcon A contact lens, or no contact lens at all. Eyes irradiated without a contact lens showed blue 365 nm-excited fluorescence initially, but this changed to intense yellow fluorescence after 1 hour. Isolated, previously irradiated lenses exhibited yellow fluorescence originating from the lens nucleus when viewed under 365 nm light, but showed normal blue fluorescence arising from the cortex. Previously irradiated lenses also exhibited a faint yellow color when observed under visible light. The senofilcon A contact lens protected completely against the UVA-induced effects on fluorescence and lens yellowing, whereas the lotrafilcon A lens showed no protection. The UVA-exposure also produced a 53% loss of total NADH (free plus bound) in the lens nucleus, with only a 13% drop in the anterior cortex. NADH loss in the nucleus was completely prevented with use of a senofilcon A contact lens, but no significant protection was observed with a lotrafilcon A lens. Overall, the senofilcon A lens provided an average of 67% protection against UVA-induced loss of four pyridine nucleotides in four different regions of the lens. HPLC analysis with fluorescence detection indicated a nearly six-fold increase in 365 nm-excited yellow fluorescence arising from lens nuclear λ-crystallin after the in vivo UVA exposure. It is concluded that UVA-induced loss of free NADH (which fluoresces blue) may have allowed the natural yellow fluorescence of λ-crystallin and other proteins in the lens nucleus to become visible. Increased fluorescence exhibited by UVA-exposed λ-crystallin may have been the result of a UVA-induced change in the conformation of the protein occurring during the initial UVA-exposure in vivo. The results demonstrate the greater susceptibility of the lens nucleus to UVA-induced stress, and may relate to the formation of human nuclear cataract. The senofilcon A contact lens was shown to be beneficial in protecting the rabbit lens against effects of UVA light, including changes in fluorescence, increased yellowing and loss of pyridine nucleotides.
Keywords: UVA light, rabbit, in vivo, lens, yellowing, fluorescence, pyridine nucleotides, nuclear cataract
1. Introduction
The possibility that UV radiation in sunlight may be a cause of human nuclear cataract continues to be controversial. While certain epidemiological studies have failed to find a link between sunlight and nuclear cataract (Cruickshanks et al., 1992; Delcourt et al., 2000; Taylor et al., 1988; West et al., 1998), others have detected a possible connection (Hayashi et al., 2003; Mohan et al., 1989; Neale et al., 2003; Pastor-Valero et al., 2007; Wong et al., 1993; Zigman et al., 1979). An unusually high incidence (nearly 70%) of nuclear cataract has been documented for individuals in their sixth decade of life in regions with high UV exposure, including Singapore (Sasaki et al., 2002), Indonesia (Sasaki et al., 1989), India (Murthy et al., 2007), and two subtropical regions of Japan (Sasaki et al., 2002; Sasaki et al., 1995). Truscott has hypothesized that it may only be past the age of 50 that the human lens nucleus becomes vulnerable to UV-induced protein damage (Truscott, 2003). At this age in the human, a substantially decreased level of reduced glutathione in the lens nucleus can cause normally protective kynurenine UV filters to bind to lens proteins, possibly leading to toxic interactions between UV light and lens nuclear crystallins (Aquilina et al., 1997; Korlimbinis et al., 2007; Mizdrak et al., 2008; Parker et al., 2004). Once nuclear cataract begins to form in the human lens, the opacity proceeds rapidly (Sasaki et al., 2002), possibly too fast for epidemiological studies to detect a correlation with cumulative sunlight exposure.
If sunlight is indeed a cause of lens nuclear opacity, it is likely that UVA radiation (315 to 400 nm wavelength), and not UVB (290 to 315 nm), is the initiating factor. Of the total UV radiation reaching the earth (up to 10 mW/cm2), 97% of it consists of UVA wavelengths, and because of the strong absorption of UVB light by the cornea, 1000x as much UVA light reaches the human lens epithelium, compared to UVB (Zigman, 1995). In addition, almost all UVB radiation reaching the lens is absorbed in the first few layers of cells, whereas UVA light is able to enter deep into the lens nucleus and be absorbed (Balasubramanian, 2000; Gaillard et al., 2000). Animal models used to study cataract have shown that UVB radiation produces anterior subcapsular and cortical cataracts (Dong et al., 2007; Giblin et al., 2011; Pitts et al., 1977), while long-term exposure to UVA light induces increased lens nuclear light scatter and aggregation of lens nuclear crystallins (Barron et al., 1988; Bergauer, 1991; Giblin et al., 2002; Simpanya et al., 2008). The potentially damaging nature of UVA light, which is only about 20% lower in energy per photon than UVB light, has been well-documented for both non-ocular (Cadet et al., 2009; Godar et al., 1993; McMillan et al., 2008; Tyrrell, 1991) and ocular (Azzam et al., 2004; Balasubramanian, 2000; Rudy et al., 1982; Weinreb et al., 2001; Zigman, 2000) tissues. UVA radiation has been strongly implicated in photoaging of the skin (Gasparro, 2000; Hanson and Simon, 1998), and there is even evidence that signals evoked by UVA light entering the eye can accelerate skin photoaging (Hiramoto et al., 2012). Old human lenses are known to contain protein-bound UVA sensitizers that can generate UVA-induced reactive oxygen species, including substantial amounts of singlet oxygen (Linetsky and Ortwerth, 1997; Ortwerth et al., 2002). Similarly, decomposition products of ascorbic acid forming in the old human lens nucleus have been proposed to act as toxic UVA chromophores (Avila et al., 2010; Ortwerth et al., 2003). Harmful effects of UVA light in the lens nucleus may be accelerated by an increase in the partial pressure of oxygen occurring as a result of age-related vitreous humor liquefaction and posterior vitreous detachment (Giblin et al., 2009; Harocopos et al., 2004).
Lenses of diurnal species, including human, duck, frog, guinea pig, rabbit and squirrel, contain high levels of UVA chromophores, in contrast to lenses of nocturnal species, such as rat and cat (Hains et al., 2006; Wood and Truscott, 1994; Zigler and Rao, 1991). Absorption of UVA light by young human lenses is due to the presence of high concentrations of unbound 3-hydroxy kynurenine glucoside, which is replaced in the aging human lens by UVA-absorbing old yellow proteins (Gaillard et al., 2000; Korlimbinis et al., 2007). The present study involves the rabbit lens, which contains high levels of the UVA-absorbing pyridine nucleotide NADH, both free and bound to λ-crystallin (Bando et al., 2006; Giblin and Reddy, 1980; Zigler and Rao, 1991). Rabbit lens λ-crystallin is identical to the liver enzyme L-gulonate 3-dehydrogenase whose crystal structure in complex with NADH has been determined at 1.85 Å resolution (Asada et al., 2010; Ishikura et al., 2005).
It has long been known that as the human lens ages, there is a significant increase in the level of fluorescence, much of it the result of excitation by UVA wavelengths present in sunlight (Balasubramanian, 2005; Jacobs and Krohn, 1976; Lerman et al., 1978; Weale, 1985; Zigman, 1985). The fluorescence appears to be concentrated in the lens nucleus where it may be involved in the development of nuclear cataract (Bando et al., 1976). Beginning at about the age of 60, increased fluorescence in the human lens can produce an elevated level of veiling glare, resulting in substantial loss of sharp vision (Asbell and Potapova, 2005; Weale, 1985; Zuclich et al., 1992). The present study investigates the effects of an acute in vivo dose of 365 nm light on subsequent UVA-excited fluorescence in the rabbit lens, and the ability of a class I UV-blocking contact lens to prevent the fluorescence effects, as well as UVA-induced loss of lens pyridine nucleotides. Class I silicone hydrogel contact lenses contain UV-blocking materials that absorb more than 99% of incident UVB radiation and 90% of UVA (Moore and Ferreira, 2006) (ANSI Z80.20-2010 and ISO 18369-2:2006 standards). We have previously shown that these contact lenses are beneficial in protecting ocular tissues of the rabbit against harmful effects of UVB light, including photokeratitis and anterior subcapsular cataract (Giblin et al., 2011).
2. Materials and methods
2.1 Animals
Rabbits (New Zealand White, 2.5–3.0 kg) were obtained from Kuiper Rabbit Ranch (Indianapolis, IN, USA). All studies conformed to the ARVO Statement for Use of Animals in Ophthalmic and Vision Research, and were approved by the Oakland University Animal Care and Use Committee. Euthanization was conducted by first anesthetizing the animals with Xylazine and Ketamine HCl, followed by injection of an overdose of sodium pentobarbital.
2.2 UVA irradiation
Prior to UVA irradiation, rabbits were tranquilized with an intramuscular injection of Xylazine (20 mg/kg) and Ketamine HCl (5 mg/kg), and the eyes fully dilated with 1% tropicamide (Mydral, Ocusoft, Inc., Richmond, TX, USA). During irradiation, the rabbits were confined in an adjustable retaining cage which protected most of the animal, except the head, from the UVA light. UVA levels were determined with a UVX Digital Radiometer (San Gabriel, CA, USA) equipped with a UVA sensor at 365 nm (Model UVX-36). Eyes of anesthetized rabbits were exposed to UVA irradiation using a Hamamatsu UV-LED light source (Hamamatsu USA, Bridgewater, NJ, USA) at a monochromatic wavelength of 365 nm, and a distance of 7 cm from the cornea. The irradiated eye was held open with use of an eye speculum, and the corneal surface of each irradiated eye was kept moist by topical application of 0.9% saline every 5 min. The irradiance on the cornea was 100 mW/cm2 which is about 60 times the maximum exposure of the human cornea to UVA contained in sunlight (Zigman, 1995). Eyes were irradiated for 1 hr to produce a total fluence of 360 J/cm2.
Eyes were exposed to UVA radiation in the presence of either a senofilcon A contact lens (ACUVUE® OASYS®, Johnson & Johnson Vision Care, Inc., Jacksonville, FL, USA), which absorbs >99% of incident UVB and >90% of UVA (Moore and Ferreira, 2006), a lotrafilcon A contact lens (Focus Night and Day, CIBA Vision, Duluth, GA, USA), which absorbs 30% of incident UVB and 15% of UVA (Moore and Ferreira, 2006), or no contact lens at all. Contact lenses were applied to the corneas of tranquilized rabbits. A drop of saline was added to the contact lens (to prevent air being trapped between the lens and the cornea) and, using the tip of the index finger, the lens was applied to the cornea.
2.3 Lens photography
Photographs of emission of UVA-induced blue and yellow fluorescence from lenses in vivo and in vitro were taken in a dark room using a Nikon D40x digital camera with a macro lens. Photographs of isolated pale yellow lenses from UVA-exposed eyes were taken in a dark room with a Nikon D40x digital camera attached to a dissecting microscope. For this photography, each lens was placed in saline in a culture dish on a light box (Scienceware, Bel-Art Products, Pequannock, NJ, USA; catalog #37864-2000) that was used for back light illumination. The light box contained a fluorescent lamp that was color-corrected to a color temperature of 6,500 °K (daylight, overcast).
2.4 Biochemical analysis
For pyridine nucleotide analysis, lenses were isolated from the eye by posterior approach, frozen rapidly in crushed dry ice, and separated into anterior cortex (AC), posterior cortex (PC), equatorial cortex (EC) and nucleus (N) with use of a cork borer and razor blade. The percent of total lens weight for each region was 10% (AC), 10% (PC), 50% (EC) and 30% (N). Levels of pyridine nucleotides in the four regions were measured using a cycling assay as previously described (Giblin and Reddy, 1980). Levels of NADH and NADPH were also measured in certain HPLC elution fractions. Fractions from five HPLC runs were pooled and concentrated to a volume of 200 μl using an Amicon Ultra Centrifugal Filter (Millipore, Billerica, MA, USA). Protein concentration was measured by the Pierce BCA Protein Assay using bovine serum albumin as a standard (Fisher Scientific, Rockford, IL, USA). For analysis of HPLC fractions, the cycling assay was modified slightly to use double strength KOH to compensate for dilution.
2.5 HPLC analysis
For HPLC analysis, control and UVA-exposed rabbit lenses were frozen rapidly in crushed dry ice and stored for a few weeks at −80 degrees C before use. The lenses were separated into nucleus (16% of the total weight) and anterior cortex (5% of the total weight) with use of a cork borer and razor blade. The tissues were homogenized (100 mg wet weight of lens tissue per ml buffer) in a nitrogen atmosphere at 4°C in 20 mM phosphate buffer (pH 7.0) containing 1 mM EDTA. After centrifugation of the homogenate for 25 min at 15,000 rpm (20,000 ×g), concentrations of water soluble (WS) proteins were determined as described above. Nuclear and anterior cortical WS proteins were fractionated by size-exclusion chromatography on an HPLC system (Shimadzu Scientific Co., Kyoto, Japan). The column was calibrated with the following protein standards (all from Sigma-Aldrich Chemical Co., St. Louis, MO, USA): thyroglobulin (670 kDa), β-amylase (200 kDa), bovine serum albumin (66 kDa), carbonic anhydrase (29 kDa) and cytochrome C (12.4 kDa). 1.0 mg protein samples in phosphate buffer (pH 7.0) were injected and separated using a flow rate of 250 μl/min of 20 mM sodium phosphate buffer, pH 7.0, containing 1 mM EDTA. Separation of proteins was performed on a 300 × 7.8-mm column (BioSep-SEC s4000; Phenomenex, Torrance, CA, USA), having an exclusion range of 15 to 1,500 kDa. 250 μl fractions were collected from 15 to 60 min. Fluorescence was measured at 365nm excitation and 570nm emission. Absorbance was measured at 280 nm for protein and 340 nm for NADH. Areas under certain peaks were measured by first drawing a baseline, adding the microvolt readings from the beginning to the end of the baseline, and subtracting the sum of the microvolts making up the baseline.
2.6 SDS-PAGE
HPLC protein fractions from pooled peaks were analyzed by SDS-PAGE using a 10% gel after reduction of disulfides in a 4x SDS gel sample buffer as described previously (Simpanya et al., 2005). Samples were treated in a water bath at 95°C to 97°C for 5 minutes and loaded into the wells. The gel was run at a constant current of 20 mA until the bromophenol blue dye was a few millimeters from the end.
2.7 UVA-irradiation of malic dehydrogenase in vitro
The NADH-binding enzyme malic dehydrogenase (MDH) was irradiated with UVA light in vitro. MDH (bovine heart, EC 1.1.1.37, catalog #M9004, Sigma-Aldrich, St. Louis, MO, USA) was first dialyzed overnight against 20 mM sodium phosphate buffer, pH 7.0, containing 1 mM EDTA, in order to remove ammonium sulfate. The solution was centrifuged, and a 0.5 ml, 1.6 mg/ml, aliquot was irradiated with 365nm light in an open-top, plastic 1.5 ml Eppendorf tube, with shaking. Irradiation was conducted from above, striking the surface of the shaking solution. The irradiation conditions were the same as those for the in vivo experiments described above (section 2.2), i.e., 100 mW/cm2 of 365 nm light for 1 hr. HPLC analysis of irradiated and non-irradiated samples was conducted as described above in section 2.5. Fluorescence was measured at 365 nm excitation and 570 nm emission.
3. Results
3.1 UVA-excited lens fluorescence
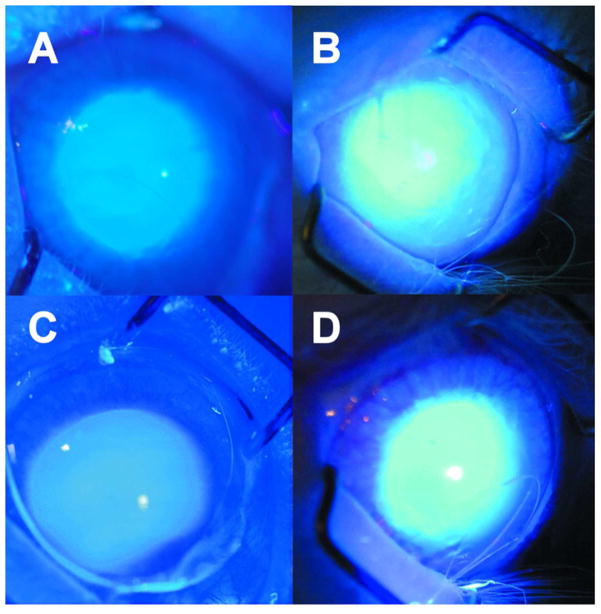
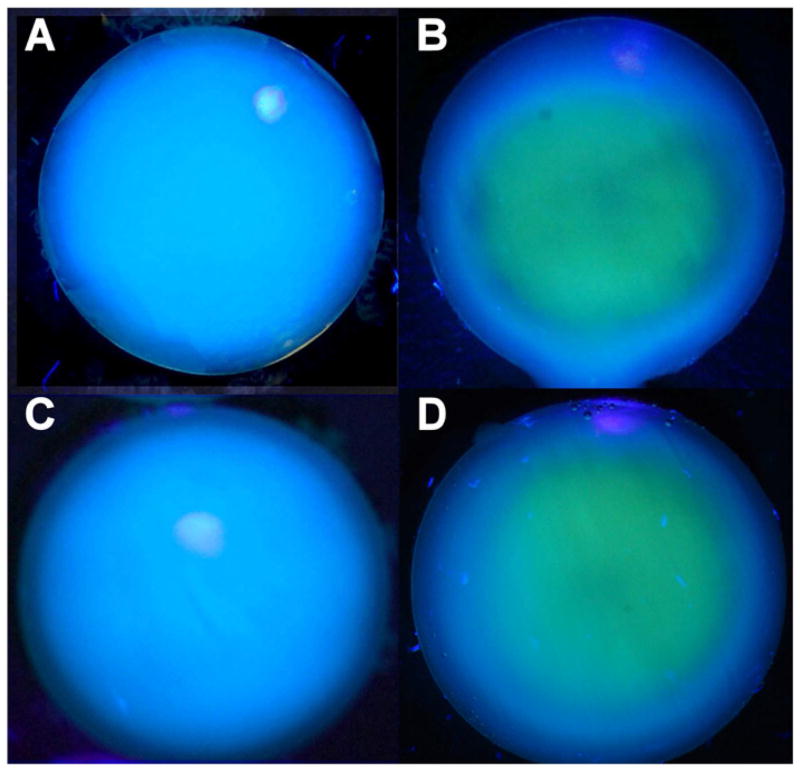
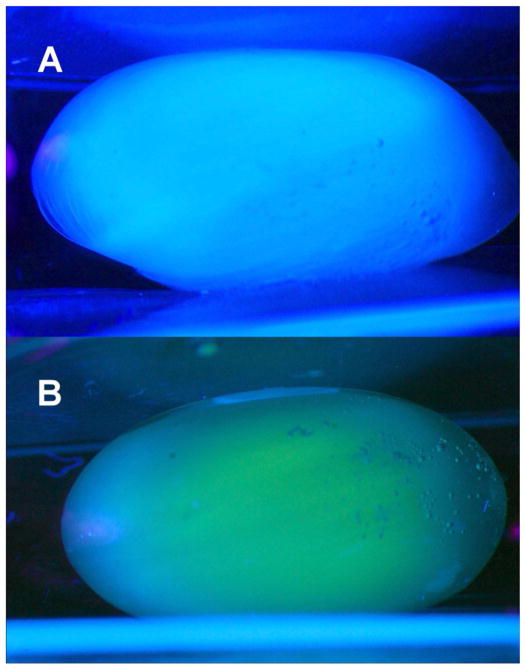
When rabbits eyes were irradiated in vivo with 365 nm UVA light (100 mW/cm2 on the cornea) without contact lens protection, a blue fluorescence was observed emanating from the lens of the eye during the first minute of exposure (Fig. 1A). After about 30 min, the blue fluorescence had changed to a yellow glow, arising primarily from the center of the lens, and after 1 hr, the yellow fluorescence had become more intense (Fig. 1B). In separate experiments, irradiation was conducted for 1 hr with a senofilcon A contact lens in place on the eye. When the contact lens was removed after 1 hr, and the eye was observed under UVA light for 1 min, the normal blue fluorescence was found to have been preserved (Fig. 1C). However, repeating the same procedure with use of a lotrafilcon A contact lens produced abnormal yellow, rather than blue fluorescence (Fig. 1D). When the rabbits of the study of Fig. 1 were euthanized, and the lenses isolated and then observed under UVA light (365 nm, 100 mW/cm2), the control lens showed the typical blue fluorescence (Fig. 2A), whereas the lens that had previously been irradiated in vivo for 1 hour with UVA light in the absence of a contact lens showed an intense yellow fluorescence emanating from the lens nucleus (Fig. 2B). Initial Irradiation in the presence of a senofilcon A lens was able to completely prevent the subsequent UVA-excited yellow fluorescence arising from the lens nucleus (Fig. 2C), while use of a lotrafilcon A lens had minimal protective effect (Fig. 2D). A side view photograph of the isolated lens of Fig. 2B confirmed that the UVA-excited yellow fluorescence emanated from the nucleus of the experimental lens (Fig. 3B), but was absent in the control (Fig. 3A).
Figure 1.
Photography taken during exposure of a rabbit eye to UVA radiation in vivo (365 nm, 100 mW/cm2 on the cornea). A. No contact lens; after 1 min of irradiation, B. No contact lens; after 1 hour of irradiation, C. senofilcon A lens; 1 hour of irradiation with the contact lens, followed by removal of the contact lens, continuation of irradiation for 1 min and photography, D. lotrafilcon A lens; 1 hour of irradiation with the contact lens, followed by removal of the contact lens, continuation of irradiation for 1 min and photography. Representative of 3–6 experiments.
Figure 2.
Photography taken during exposure of isolated rabbit lenses of Figure 1 to UVA light (365 nm, 100 mW/cm2). A. Normal lens, B. Prior exposure for 1 hr in vivo with no contact lens (Fig. 1B), C. Prior exposure for 1 hr in vivo with a senofilcon A lens (Fig. 1C), D. Prior exposure for 1 hr in vivo with a lotrafilcon A lens (Fig. 1D). Representative of 3–6 experiments.
Figure 3.
Side view photographs taken during exposure of the isolated rabbit lenses of Figure 2(A and B) to UVA light (365 nm, 100 mW/cm2). A. Normal lens (Fig. 2A), B. Prior exposure for 1 hr in vivo with no contact lens (Figs. 2B). Representative of 3–6 experiments.
To aid in identifying the source of the observed UVA-excited blue fluorescence of Fig. 1A, solutions of 1μM NADPH and 1μM NADH in phosphate buffered saline were irradiated with 365 nm light (100 mW/cm2). The irradiation produced an immediate intense blue fluorescence (emission at 470 nm) which lasted for about 10 minutes and then disappeared (results not shown). The blue fluorescence was not replaced by a yellow fluorescence, as had been observed when the rabbit eye was irradiated in vivo (Fig. 1B).
3.2 UVA-induced lens yellowing
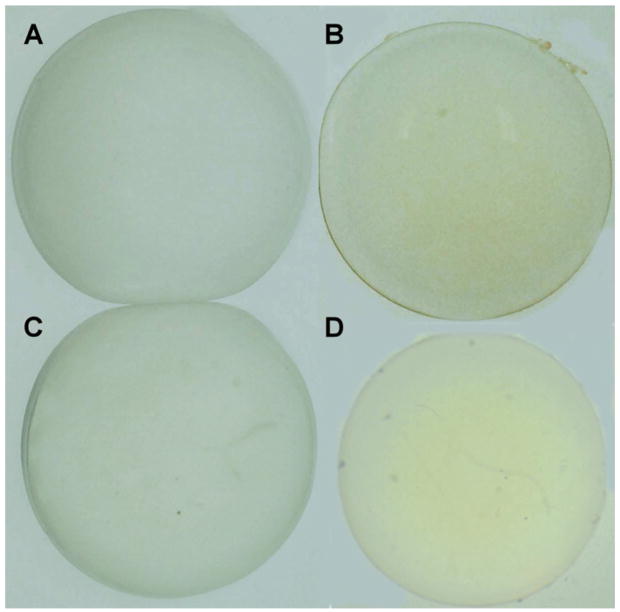
When lenses were isolated following a 1 hr exposure of rabbit eyes to 100 mW/cm2 of 365 nm UVA light in vivo without a contact lens, the lenses exhibited a distinct pale yellow color when observed under normal visible light, whereas the contralateral control lenses were clear (Figures 4A and 4B). The senofilcon A contact lens prevented the formation of the UVA-induced yellow lens color (Fig. 4C), while the lotrafilcon A lens did not (Fig. 4D).
Figure 4.
Photographs of rabbit lenses in phosphate buffered saline following a 1 hour in vivo exposure of rabbit eyes to UVA radiation (365 nm, 100 mW/cm2), with and without contact lens protection. A. Normal lens, B. No contact lens. C. senofilcon A lens, D. lotrafilcon A lens. Representative of 3–6 experiments.
3.3 UVA-induced effects on lens pyridine nucleotides
The effects of UVA light in vivo on the levels of all four pyridine nucleotides in four different regions of the rabbit lens were investigated. A 1 hr exposure to 365 nm light produced a 53% decrease in the level of NADH in the lens nucleus (N), compared to the contralateral control (Table IA). Irradiation in the presence of a senofilcon A lens completely prevented this loss of NADH in the nucleus. Levels of NADH in the other three regions of the lens, including anterior cortex (AC), equatorial cortex (EC) and posterior cortex (PC), were each affected to a lesser extent (11 to 16% losses), compared to that observed for the nucleus. Even though NAD+ does not absorb UVA radiation, exposure to 365 nm light for 1 hr was observed to produce a decrease in the level of this nucleotide in the four regions of the rabbit lens, ranging from 22 to 56% (Table IB). A senofilcon A lens provided significant protection (77 to 100%) against UVA-induced loss of NAD+ in each of the affected regions, with protection in the nucleus being at a 93% level. Concentrations of NADPH decreased by 44 to 62% in the lens AC, EC, N and PC following a 1 hr exposure to UVA light (Table IC). A senofilcon A lens showed significant protection (50 to 62%) in the AC, N and PC, but the protection was not significant in the EC. Similar to NAD+, NADP+ also does not absorb UVA light, but nonetheless the concentration of this nucleotide was substantially decreased in the AC, EC, N and PC (52 to 75%) following a 1 hr UVA exposure (Table ID). The senofilcon A lens exhibited significant protection (46 to 92%) against UVA-induced loss of NADP+ in all four lens regions. Overall, the senofilcon A lens provided an average of 67% protection against UVA-induced loss of the four pyridine nucleotides in the four regions of the lens. In contrast, the lotrafilcon A lens showed no significant protection (p>0.1) against the loss of any of the pyridine nucleotides in the four regions (Table I).
Table I.
Contact lens protection against UVA-induced loss of pyridine nucleotides in rabbit lens in vivo*
| Pyridine nucleotide concentrations (% loss, compared to control) | |||||||
|---|---|---|---|---|---|---|---|
| A | NADH | ||||||
| no lens (% loss) | senofilcon A (% loss) | protection (%) | p | lotrafilcon A (% loss) | protection (%) | p | |
| AC | 13±19 | 6±24 | 54 | >0.1 | 10±17 | 23 | >0.1 |
| EC | 11±15 | 16±18 | 0 | >0.1 | 15±11 | 0 | >0.1 |
| N | 53±13 | 0±4 | 100 | <0.0001 | 44±22 | 17 | >0.1 |
| PC | 16±17 | 0±14 | 100 | >0.05 | 23±12 | 0 | >0.1 |
| B | NAD+ | ||||||
| AC | 46±18 | 0±1 | 100 | <0.001 | 48±35 | 0 | >0.1 |
| EC | 22±14 | 5±3 | 77 | <0.05 | 24±12 | 0 | >0.1 |
| N | 56±14 | 4±17 | 93 | <0.001 | 54±11 | 4 | >0.1 |
| PC | 50±25 | 10±16 | 80 | <0.05 | 43±28 | 14 | >0.1 |
| C | NADPH | ||||||
| AC | 62±17 | 25±29 | 60 | <0.01 | 65±8 | 0 | >0.1 |
| EC | 44±23 | 34±31 | 23 | >0.1 | 58±14 | 0 | >0.1 |
| N | 52±12 | 26±10 | 50 | <0.01 | 58±15 | 0 | >0.1 |
| PC | 47±19 | 18±7 | 62 | <0.05 | 55±22 | 0 | >0.1 |
| D | NADP+ | ||||||
| AC | 75±11 | 6±41 | 92 | <0.001 | 60±7 | 20 | >0.1 |
| EC | 52±4 | 22±15 | 58 | <0.0001 | 50±18 | 4 | >0.1 |
| N | 71±8 | 38±28 | 46 | <0.01 | 74±4 | 0 | >0.1 |
| PC | 63±14 | 19±23 | 70 | <0.001 | 64±8 | 0 | >0.1 |
Eyes of anesthetized rabbits were exposed to UVA light for 1 hour (monochromatic 365 nm light, 100 mW/cm2 on the cornea) with and without the presence of a senofilcon A or lotrafilcon A contact lens. The rabbits were euthanized immediately after the exposure, and the isolated lenses frozen in dry ice and divided into anterior cortex (AC), equatorial cortex (EC), nucleus (N) and posterior cortex (PC) for analysis. In each case, the contralateral lens served as the control. Results (% loss, compared to control) are expressed as means ± S.D; n=10 for no contact lens, n=3 for a senofilcon A contact lens and n=4 for a lotrafilcon A contact lens. Percent protection is calculated as %loss (no contact lens) − %loss (contact lens) / %loss (no contact lens) × 100. Mean control values (nm/g wet wt.) for the four nucleotides ranged from 861(N) to 1384(EC) for NAD+, 384(EC) to 733(N) for NADH, 11(N) to 31(EC) for NADP+ and 6(N) to 39(EC) for NADPH.
3.4 HPLC analysis of lens nuclear WS proteins
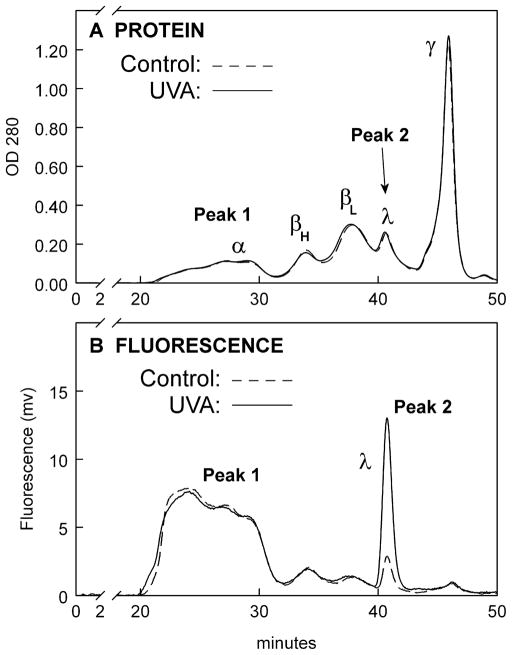
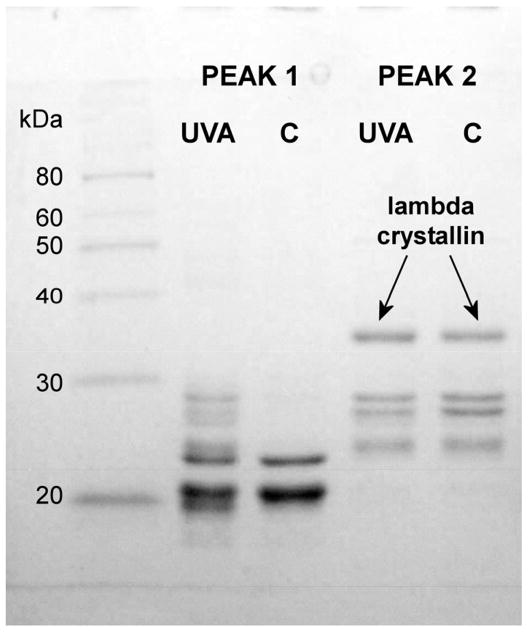
To investigate the source of the UVA-induced yellow fluorescence in the rabbit lens nucleus, size exclusion HPLC analysis was conducted of the lens nuclear water-soluble (WS) proteins using a Biosep-SEC S4000 column. The OD 280 protein elution profiles for the control and UVA-exposed samples were found to be identical (Fig. 5A). Two peaks of 365 nm-induced yellow fluorescence (Peak 1 and Peak 2, Fig. 5B) were observed for both the control and UVA samples. Peak 1 consisted mainly of α-crystallin while Peak 2 contained λ-crystallin and some low molecular weight β-crystallin (Fig. 6). Surprisingly, the level of yellow fluorescence for the UVA-exposed λ-crystallin fraction was determined to be 5.9 +/− 0.3 times higher than that of the control (Fig. 5B, measurement of the areas under Peak 2 control and experimental, p=0.001), in spite of the fact that the levels of protein for the control and UVA-exposed λ-crystallin fractions were identical (Fig. 5A).
Figure 5.
HPLC profiles of rabbit lens nuclear proteins and fluorescence following a 1 hr in vivo exposure of the eye to UVA light (365 nm, 100 mW/cm2). A BioSep-SEC-s4000 column was employed. A. Protein (OD 280nm), B. Fluorescence, excitation: 365nm, emission: 570nm. Representative of 3 experiments. Note: The area under the experimental λ-crystallin fluorescence peak is 5.9 +/− 0.3 × greater than that for the control (p=0.001).
Figure 6.
SDS-PAGE analysis of Peaks 1 and 2 of Fig. 5. UVA and control (C).
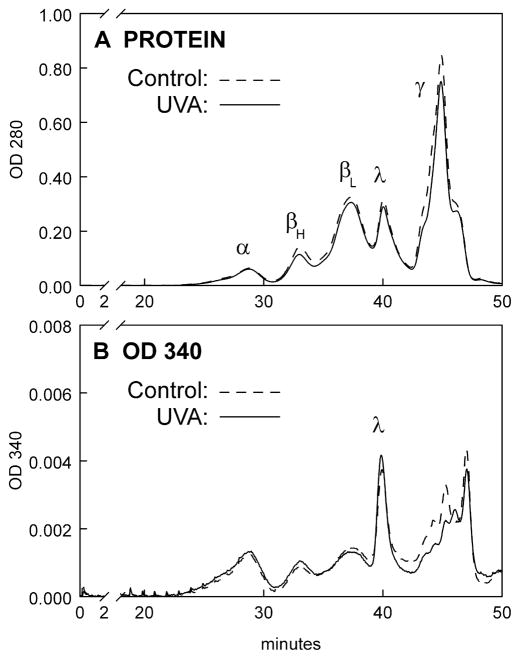
In an attempt to identify the UVA chromophores present in Peaks 1 and 2 of Fig. 5B, levels of NADH and NADPH were measured in the HPLC fractions. Peak 1 was found to contain only trace levels of NADH and NADPH for both the control and UVA samples (Fig. 7). Similarly, Peak 2 contained only trace amounts of NADPH for both sets of samples. However, as expected, the λ-crystallin fraction, Peak 2, contained substantial amounts of NADH for both control and UVA. There was some indication that the level of NADH might be higher in the UVA sample, compared to the control, but because of the scatter in the results, the results were not statistically significant (p>0.1). To confirm that levels of NADH were no different in the Fig. 5B control and experimental λ-crystallin fractions, OD 340 nm absorbance was measured for the two fractions (NADH exhibits maximum absorbance at 340 nm). The HPLC elution profiles for lens nuclear WS protein show that the levels of both protein and OD 340 nm absorbance were identical for the control and experimental λ-crystallin fractions (Figs. 8A and 8B, respectively).
Figure 7.
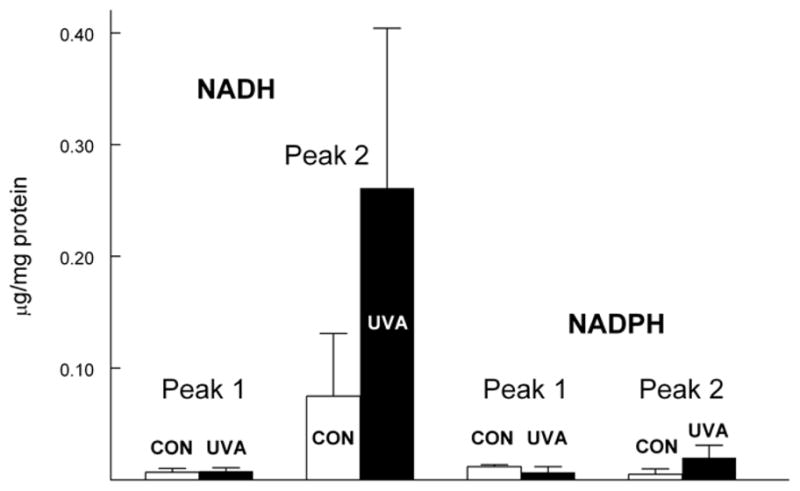
NADH and NADPH levels in Peaks 1 and 2 of Fig. 5. Control (open bars) and UVA (solid bars). Results are expressed as means +/− S.D. for 3 experiments. Note: NADH levels for Peak 2, control and UVA, were not significantly different (p>0.1).
Figure 8.
HPLC profiles of rabbit lens nuclear proteins and OD 340 nm following a 1 hr in vivo exposure of the eye to UVA light (365 nm, 100 mW/cm2). A BioSep-SEC-s4000 column was employed. A. Protein (OD 280 nm), B. OD 340 nm. Representative of 3 experiments. Note: OD 340 nm values for the control and UVA λ-crystallin fractions are nearly identical, indicating no differences in bound NADH levels.
3.5 HPLC analysis of lens cortical WS proteins
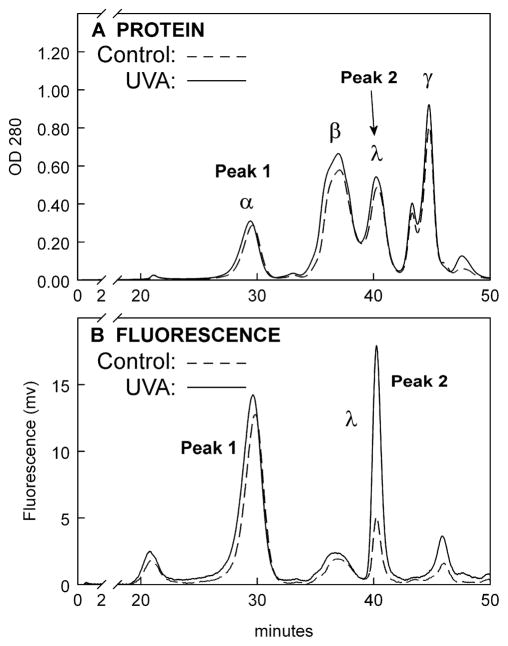
HPLC analysis was also conducted for the lens anterior cortical WS proteins. Similar to the result for the lens nuclear WS proteins (Fig. 5B), the level of yellow fluorescence for the UVA-exposed λ-crystallin fraction for the anterior cortex was determined to be 4.4 +/− 0.9 times higher than that of the control (Fig. 9B, measurement of the areas under Peak 2 control and experimental, p<0.01). The UVA/control increase for the nucleus (5.9 times, Fig. 5B) was 34% higher than that for the anterior cortex (4.4 times, p<0.05).
Figure 9.
HPLC profiles of rabbit lens cortical proteins and fluorescence following a 1 hr in vivo exposure of the eye to UVA light (365 nm, 100 mW/cm2). A BioSep-SEC-s4000 column was employed. A. Protein (OD 280nm), B. Fluorescence, excitation: 365nm, emission: 570nm. Representative of 3 experiments. Note: The area under the experimental λ-crystallin fluorescence peak is 4.4 +/− 0.9 × greater than that for the control (p<0.01).
3.6 In vitro exposure of malic dehydrogenase to UVA light
Experiments were conducted to determine whether UVA irradiation of the NADH-binding enzyme malic dehydrogenase (MDH) in vitro would produce an increase in 365 nm-excited yellow fluorescence, as had been observed for λ-crystallin in vivo (Figs. 5B and 9B). A 1.6 mg/ml solution of MDH was irradiated with 365 nm light under the same conditions used for the in vivo experiments, and then analyzed by HPLC (see Section 2.7 for details). In contrast to the in vivo results, in vitro irradiation of NADH bound to the enzyme did not produce a subsequent increase in 365 nm-excited yellow fluorescence, compared to that shown by a non-irradiated sample (data not shown).
4. Discussion
4.1 The UVA dose
This study showed that a senofilcon A contact lens was able to protect the lens against a high dose of UVA light (Figs. 1C, 2C and 4C; Table I). Assuming that absorption of the incident 100 mW/cm2 of 365 nm light by the rabbit cornea was 30% (Dillon et al., 1999), the irradiance reaching the lens epithelium, 70 mW/cm2, would have been 70 times the maximum irradiance of UVA radiation contained in sunlight striking the human lens (Zigman, 1995). The dose of 365 nm light received by the lens, 252 J/cm2, was comparable to 70 hrs of sunlight condensed into 1 hr (Zigman, 1995). It is not known what percentage of this dose was actually absorbed by the lens. The adult rabbit lens nucleus has been reported to absorb over 10 times the amount of 365 nm light compared to the cortex, indicating the presence of higher levels of UVA chromophores in the lens interior (Dillon et al., 1999). In this study, some of the UVA light was evidently able to pass through the lens nucleus since substantial losses of pyridine nucleotides were observed in the posterior cortex (Table I).
4.2 UVA-excited blue lens fluorescence
The UVA-excited blue fluorescence observed in vivo in Figs. 1A and 1C, and in vitro in Figs. 2 and 3, has been reported previously for the rabbit lens (Pitts, 1978; Tsubota et al., 1989), and cultured rabbit lens epithelial cells (Atherton et al., 1999). Based on the in vitro experiment described in the Results, the fluorescence is apparently due to absorption of 365 nm light by NADPH and NADH. Other researchers have used redox fluorometry to show that 99% of the pyridine nucleotide blue fluorescence arising from the rabbit lens is due to absorption by NADH (Tsubota et al., 1989). The rabbit lens contains unusually high levels of NADH, four and eight times higher than lenses of the rat and human, respectively (Giblin and Reddy, 1980), due in part to the presence of NADH bound to λ-crystallin (Bando et al., 2006). Presumably, the loss of blue fluorescence in the lens nucleus (Figs. 1B, 1D, 2B, 2D and 3B) was due to UVA-induced photolysis of free NADPH and NADH. Photobleaching of NADH and NADPH by UVA light has been observed before (Atherton et al., 1999; Czochralska et al., 1984).
4.3 UVA-excited yellow lens fluorescence
It is possible that the UVA-excited yellow fluorescence observed in the lens after 1 hr of UVA irradiation (Figs. 1B, 1D, 2B, 2D and 3B) was present from time zero, but became visible only after the blue fluorescence had disappeared due to photolysis of free NADPH and NADH. Indeed, HPLC analysis of control lens samples showed a substantial amount of 365 nm-excited yellow fluorescence being emitted by both Peak 1 proteins and the λ-crystallin fraction (Figs. 5B and 9B). The fact that the yellow fluorescence was visible only in the lens nucleus, and not the cortex (Figs. 2B, 2D and 3B), may be due to a greater UVA-induced loss of NADH in the nucleus, compared to the cortex. In support of this idea, UVA-induced loss of NADH was determined to be 53% in the nucleus, compared to only 13% and 16% in the anterior and posterior cortex, respectively (Table IA).
The appearance of UVA-excited yellow fluorescence in the lens nucleus but not the cortex (Figs. 2B, 2D and 3B) demonstrates the greater susceptibility of this region to effects of stress, which may be related to its lower reducing capability. For example, the concentration of GSH in the rabbit lens nucleus is 5 times lower than that in the cortex (4.6 vs. 22.4 mM) (Padgaonkar et al., 1989). Greater effects of UVA in the lens nucleus compared to the cortex have also been shown in a long-term guinea pig in vivo model (Giblin et al., 2002). Rabbit lenses treated in vitro with elevated levels of oxygen also exhibit initial oxidative effects in the nucleus, prior to the effects being observed in the cortex (Giblin et al., 1988; Padgaonkar et al., 1989). These results may have relevance to possible roles for UVA light and oxygen in the formation of human maturity-onset nuclear cataract. One other study that we are aware of showed that exposure of rabbit lenses to a low level of UVA light in vivo produced a significant increase in autofluorescence at wavelengths between 530 and 600 nm; the authors concluded that low intensity UVA exposure to the human lens should be avoided (Van Vreeswijk et al., 1993).
4.4 UVA-induced lens yellowing
It is not clear what may have caused the UVA-induced pale yellow color of the rabbit lenses seen in Figs. 4B and 4D, or whether the color is associated with the yellow fluorescence that was observed. Lenses of guinea pigs housed under a low level of UVA light for 15 months also developed a yellow color (Bergauer, 1991). Age-related yellowing of human lenses may be linked with formation of ascorbic acid oxidation products (Cheng et al., 2001; Fan et al., 2006) and/or the presence of soluble and protein-bound kynurenine compounds (Hood et al., 1999). It has recently been shown that incubation of lens proteins with ascorbic acid in the presence of UVA light can generate damaging UVA chromophores (Avila et al., 2010). Although rabbit lenses contain mM levels of ascorbic acid (Matsuda et al., 1981), they possess only μM levels of kynurenine and 3-hydroxykynurenine (Chiarugi et al., 1999).
4.5 UVA-induced loss of lens pyridine nucleotides
To our knowledge, this is the first study to investigate UVA-induced effects on lens pyridine nucleotide levels using an in vivo animal model. The results (Table I) demonstrate the susceptibility of these vital lens molecules to UVA-induced photolysis throughout the tissue. For NADH, the loss was particularly evident in the nucleus (53%, compared to only 13% in the anterior cortex; Table IA), whereas for NADPH, the loss was roughly 50% in each of the four regions analyzed (Table IC). It was surprising that levels of NAD+ and NADP+ were affected to a greater extent overall than those for NADH and NADPH (Table I), even though oxidized nucleotides do not absorb UVA light. Apparently, NADH and NADPH were not oxidized to NAD+ and NADP+, respectively, by 365 nm light in this in vivo study, as they have been shown to be in previous in vitro investigations (Czochralska et al., 1990; Czochralska et al., 1984). In the present study, reduced pyridine nucleotides may have been degraded to ADP-ribose and nicotinamide by the UVA light, and the oxidized nucleotides then depleted during regeneration of NADH and NADPH. It is known that active species of oxygen can hydrolyze pyridine nucleotides to ADP-ribose and nicotinamide (Tavazzi et al., 2000), and that absorption of UVA light by pyridine nucleotides can generate both H2O2 and superoxide anion (Cunningham et al., 1985; Czochralska et al., 1990; Czochralska et al., 1984). However, it is not clear whether these processes could have taken place in the current study since the partial pressure of oxygen in the center of the normal rabbit lens is very low, about 2 mm Hg (Giblin et al., 2009). Near UV irradiation of NADH under oxygen-poor conditions has been shown to generate adenosine 5′-diphosphoribose plus a second compound, possibly nicotinamide (Vitinius et al., 2004).
4.6 Protection by the senofilcon A contact lens
The senofilcon A contact lens provided an overall average of 67% protection against UVA-induced loss of four pyridine nucleotides in four regions of the rabbit lens (Table I). Exceptional protection was observed for NADH and NAD+ in the nucleus (100% and 93%, respectively). Complete protection for all regions of the lens was not obtained since the senofilcon A lens absorbs approximately 90% of incident UVA light, not 100%. In addition, the high irradiance of 365 nm light (70 mW/cm2 reaching the lens) used in the study must also be taken into consideration. The lotrafilcon A contact lens which absorbs only 15% of incident UVA light provided no significant protection against loss of pyridine nucleotides. We believe this to be the first study to demonstrate the ability of a UV-absorbing contact lens to protect against UVA-induced loss of lens pyridine nucleotides in vivo (Table 1), as well as changes in UVA-excited lens fluorescence (Fig. 1C and 2C), and lens yellowing (Fig. 4C). However, a previous study was able to show that a UV-absorbing tefilcon contact lens could prevent lens yellowing in guinea pigs exposed to a low UVA irradiance over a 15 month period (Bergauer, 1991).
4.7 HPLC fluorescence analysis
Substantial amounts of UVA-excited yellow fluorescence were observed for proteins present in both the control and experimental nuclear and anterior cortical HPLC fractions of Figs. 5B and 9B. For both the nucleus and cortex, the α-crystallin and λ-crystallin fractions each showed significant peaks of fluorescence. High molecular weight (HMW) fractions for the lens nucleus (Fig. 5B, 20–25 min elution time) exhibited substantially more fluorescence than the same fractions for the cortex (Fig. 9B, 20–25 min elution time). Protein fluorescence in old human lenses has also been shown to be localized mainly in the nucleus, which contains more water-insoluble protein than the cortex (Bando et al., 1976). The only protein found to be associated with either NADH or NADPH as the UVA chromophore was λ-crystallin, as a result of its bound NADH (Fig. 7). UVA chromophores associated with the α-crystallin and HMW fractions of Figs. 5B and 9B are not known.
It was surprising that levels of UVA-excited yellow fluorescence were significantly higher for the UVA-exposed λ-crystallin fractions, compared to the controls, for both the nucleus (Fig. 5B) and anterior cortex (Fig 9B), despite identical levels of control and UVA-exposed protein and NADH (Figs. 5A and 9A, and Fig. 7, respectively). A possible explanation for this is that the initial 1 hr in vivo exposure to UVA irradiation may have altered the conformation of the λ-crystallin such that subsequent exposure to 365 nm light (during HPLC separation) produced significantly more yellow fluorescence compared to that for the control unexposed protein. UVA-excitation of bound NADH of λ-crystallin, coupled with energy or electron transfer through the protein, may have oxidized tryptophan or tyrosine residues, causing a change in conformation (Pattison et al., 2012). It was of interest that the phenomenon occurred in the anterior cortex (Fig. 9B), as well as the nucleus (Fig. 5B), even though the intact lens anterior cortex did not exhibit UVA-excited yellow fluorescence (Fig. 3B). This suggests that any change in conformation of the λ-crystallin would have been independent of overall loss of NADH, which was only 13% in the anterior cortex, compared to 53% in the nucleus (Table 1).
Experiments in which the NADH-binding enzyme MDH was irradiated with UVA light in vitro (section 3.6) were unsuccessful in producing the increase in 365 nm-excited yellow fluorescence that was observed for UVA-irradiation of λ-crystallin in vivo (Figs. 5B and 9B). It may be that the three-dimensional structure of the highly concentrated mixture of proteins in the intact lens may be required during UVA-irradiation in order to obtain a subsequent effect on 365 nm-excited fluorescence. Other researchers investigating UVA-induced effects on lens proteins have found distinct differences in results for intact lenses, compared to lens supernatants, possibly because of variation in protein concentration, O2 level, antioxidant defenses, etc. (Kessel et al., 2005; Ortwerth et al., 2002)
5. Conclusions
An in vivo rabbit model has been used to investigate UVA-induced effects on the lens. A UV-absorbing senofilcon A contact lens was found to prevent most UVA-induced effects, including subsequent UVA-excited yellow fluorescence in the intact lens nucleus, lens yellowing and substantial losses of NADH and NAD+ in the lens nucleus. A minimally UV-absorbing lotrafilcon A contact lens showed no significant protective effects. Increases in UVA-excited fluorescence and lens yellowing are known effects occurring in the aging human lens (Balasubramanian, 2005; Jacobs and Krohn, 1976; Lerman et al., 1978; Weale, 1985, 1988; Zigman, 1985). It is concluded that use of a senofilcon A contact lens may be beneficial for protecting the ocular lens against effects of UVA light contained in sunlight, including aging of the lens nucleus.
Highlights.
Rabbit eyes were irradiated with 365 nm light (100mW/cm2) in vivo for 1 hr.
Isolated lenses under 365 nm light showed yellow fluorescence from the nucleus.
UVA-exposed lens λ-crystallin showed 6-fold higher yellow fluorescence than control.
The UVA caused 53% loss of NADH in the lens nucleus and 13% in the cortex.
A senofilcon A contact lens prevented UVA effects on lens fluorescence and NADH.
Acknowledgments
The authors thank Janet Schofding for care of the rabbits. The work was supported in part by National Eye Institute grants EY 02027 and EY 014893, and Vistakon, Division of Johnson & Johnson, Vision Care, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aquilina JA, Carver JA, Truscott RJ. Oxidation products of 3-hydroxykynurenine bind to lens proteins: relevance for nuclear cataract. Exp Eye Res. 1997;64:727–735. doi: 10.1006/exer.1996.0258. [DOI] [PubMed] [Google Scholar]
- Asada Y, Kuroishi C, Ukita Y, Sumii R, Endo S, Matsunaga T, Hara A, Kunishima N. Dimeric crystal structure of rabbit L-gulonate 3-dehydrogenase/lambda-crystallin: insights into the catalytic mechanism. J Mol Biol. 2010;401:906–920. doi: 10.1016/j.jmb.2010.06.069. [DOI] [PubMed] [Google Scholar]
- Asbell PA, Potapova N. Effects of topical antiglaucoma medications on the ocular surface. Ocul Surf. 2005;3:27–40. doi: 10.1016/s1542-0124(12)70120-9. [DOI] [PubMed] [Google Scholar]
- Atherton SJ, Lambert C, Schultz J, Williams N, Zigman S. Fluorescence studies of lens epithelial cells and their constituents. Photochem Photobiol. 1999;70:823–828. [PubMed] [Google Scholar]
- Avila F, Friguet B, Silva E. Simultaneous chemical and photochemical protein crosslinking induced by irradiation of eye lens proteins in the presence of ascorbate: the photosensitizing role of an UVA-visible-absorbing decomposition product of vitamin C. Photochem Photobiol Sci. 2010;9:1351–1358. doi: 10.1039/c0pp00048e. [DOI] [PubMed] [Google Scholar]
- Azzam N, Levanon D, Dovrat A. Effects of UV-A irradiation on lens morphology and optics. Exp Gerontol. 2004;39:139–146. doi: 10.1016/j.exger.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Balasubramanian D. Ultraviolet radiation and cataract. J Ocul Pharmacol Ther. 2000;16:285–297. doi: 10.1089/jop.2000.16.285. [DOI] [PubMed] [Google Scholar]
- Balasubramanian D. Photodynamics of cataract: an update on endogenous chromophores and antioxidants. Photochem Photobiol. 2005;81:498–501. doi: 10.1562/2004-11-01-RA-354. [DOI] [PubMed] [Google Scholar]
- Bando M, Ishii Y, Nakajima A. Changes in blue fluorescence intensity and coloration of human lens protein with normal lens aging and nuclear cataract. Ophthalmic Res. 1976;8:456–463. [Google Scholar]
- Bando M, Oka M, Kawai K, Obazawa H, Kobayashi S, Takehana M. NADH binding properties of rabbit lens lambda-crystallin. Mol Vis. 2006;12:692–697. [PubMed] [Google Scholar]
- Barron BC, Yu NT, Kuck JF., Jr Raman spectroscopic evaluation of aging and long-wave UV exposure in the guinea pig lens: a possible model for human aging. Exp Eye Res. 1988;46:249–258. doi: 10.1016/s0014-4835(88)80082-4. [DOI] [PubMed] [Google Scholar]
- Bergauer KL, Kuck JFR, Su KC, Yu N-T. Use of a UV-blocking contact lens in evaluation of UV-induced damage to the guinea pig lens. International Contact Lens Clinic. 1991:18, 182–186. [Google Scholar]
- Cadet J, Douki T, Ravanat JL, Di Mascio P. Sensitized formation of oxidatively generated damage to cellular DNA by UVA radiation. Photochem Photobiol Sci. 2009;8:903–911. doi: 10.1039/b905343n. [DOI] [PubMed] [Google Scholar]
- Cheng R, Lin B, Lee KW, Ortwerth BJ. Similarity of the yellow chromophores isolated from human cataracts with those from ascorbic acid-modified calf lens proteins: evidence for ascorbic acid glycation during cataract formation. Biochim Biophys Acta. 2001;1537:14–26. doi: 10.1016/s0925-4439(01)00051-5. [DOI] [PubMed] [Google Scholar]
- Chiarugi A, Rapizzi E, Moroni F. The kynurenine metabolic pathway in the eye: studies on 3-hydroxykynurenine, a putative cataractogenic compound. FEBS Lett. 1999;453:197–200. doi: 10.1016/s0014-5793(99)00724-3. [DOI] [PubMed] [Google Scholar]
- Cruickshanks KJ, Klein BE, Klein R. Ultraviolet light exposure and lens opacities: the Beaver Dam Eye Study. Am J Public Health. 1992;82:1658–1662. doi: 10.2105/ajph.82.12.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham ML, Johnson JS, Giovanazzi SM, Peak MJ. Photosensitized production of superoxide anion by monochromatic (290–405 nm) ultraviolet irradiation of NADH and NADPH coenzymes. Photochem Photobiol. 1985;42:125–128. doi: 10.1111/j.1751-1097.1985.tb01549.x. [DOI] [PubMed] [Google Scholar]
- Czochralska B, Bojarska E, Pawlicki K, Shugar D. Photochemical and enzymatic redox transformations of reduced forms of coenzyme NADP+ Photochem Photobiol. 1990;51:401–410. doi: 10.1111/j.1751-1097.1990.tb01731.x. [DOI] [PubMed] [Google Scholar]
- Czochralska B, Kawczynski W, Bartosz G, Shugar D. Oxidation of excited-state NADH and NAD dimer in aqueous medium involvement of O2- as a mediator in the presence of oxygen. Biochim Biophys Acta. 1984;801:403–409. [Google Scholar]
- Delcourt C, Carriere I, Ponton-Sanchez A, Lacroux A, Covacho MJ, Papoz L. Light exposure and the risk of cortical, nuclear, and posterior subcapsular cataracts: the Pathologies Oculaires Liees a l’Age (POLA) study. Arch Ophthalmol. 2000;118:385–392. doi: 10.1001/archopht.118.3.385. [DOI] [PubMed] [Google Scholar]
- Dillon J, Zheng L, Merriam JC, Gaillard ER. The optical properties of the anterior segment of the eye: implications for cortical cataract. Exp Eye Res. 1999;68:785–795. doi: 10.1006/exer.1999.0687. [DOI] [PubMed] [Google Scholar]
- Dong X, Lofgren S, Ayala M, Soderberg PG. Maximum tolerable dose for avoidance of cataract after repeated exposure to ultraviolet radiation in rats. Exp Eye Res. 2007;84:200–208. doi: 10.1016/j.exer.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Fan X, Reneker LW, Obrenovich ME, Strauch C, Cheng R, Jarvis SM, Ortwerth BJ, Monnier VM. Vitamin C mediates chemical aging of lens crystallins by the Maillard reaction in a humanized mouse model. Proc Natl Acad Sci U S A. 2006;103:16912–16917. doi: 10.1073/pnas.0605101103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard ER, Zheng L, Merriam JC, Dillon J. Age-related changes in the absorption characteristics of the primate lens. Invest Ophthalmol Vis Sci. 2000;41:1454–1459. [PubMed] [Google Scholar]
- Gasparro FP. Sunscreens, skin photobiology, and skin cancer: the need for UVA protection and evaluation of efficacy. Environ Health Perspect. 2000;108(Suppl 1):71–78. doi: 10.1289/ehp.00108s171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giblin FJ, Leverenz VR, Padgaonkar VA, Unakar NJ, Dang L, Lin LR, Lou MF, Reddy VN, Borchman D, Dillon JP. UVA light in vivo reaches the nucleus of the guinea pig lens and produces deleterious, oxidative effects. Exp Eye Res. 2002;75:445–458. [PMC free article] [PubMed] [Google Scholar]
- Giblin FJ, Lin LR, Leverenz VR, Dang L. A class I (Senofilcon A) soft contact lens prevents UVB-induced ocular effects, including cataract, in the rabbit in vivo. Invest Ophthalmol Vis Sci. 2011;52:3667–3675. doi: 10.1167/iovs.10-6885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giblin FJ, Quiram PA, Leverenz VR, Baker RM, Dang L, Trese MT. Enzyme-induced posterior vitreous detachment in the rat produces increased lens nuclear pO2 levels. Exp Eye Res. 2009;88:286–292. doi: 10.1016/j.exer.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giblin FJ, Reddy VN. Pyridine nucleotides in ocular tissues as determined by the cycling assay. Exp Eye Res. 1980;31:601–609. doi: 10.1016/s0014-4835(80)80019-4. [DOI] [PubMed] [Google Scholar]
- Giblin FJ, Schrimscher L, Chakrapani B, Reddy VN. Exposure of rabbit lens to hyperbaric oxygen in vitro: regional effects on GSH level. Invest Ophthalmol Vis Sci. 1988;29:1312–1319. [PubMed] [Google Scholar]
- Godar DE, Thomas DP, Miller SA, Lee W. Long-wavelength UVA radiation induces oxidative stress, cytoskeletal damage and hemolysis. Photochem Photobiol. 1993;57:1018–1026. doi: 10.1111/j.1751-1097.1993.tb02965.x. [DOI] [PubMed] [Google Scholar]
- Hains PG, Simpanya MF, Giblin F, Truscott RJ. UV filters in the lens of the thirteen lined ground squirrel (Spermophilus tridecemlineatus) Exp Eye Res. 2006;82:730–737. doi: 10.1016/j.exer.2005.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson KM, Simon JD. Epidermal trans-urocanic acid and the UV-A-induced photoaging of the skin. Proc Natl Acad Sci U S A. 1998;95:10576–10578. doi: 10.1073/pnas.95.18.10576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harocopos GJ, Shui YB, McKinnon M, Holekamp NM, Gordon MO, Beebe DC. Importance of vitreous liquefaction in age-related cataract. Invest Ophthalmol Vis Sci. 2004;45:77–85. doi: 10.1167/iovs.03-0820. [DOI] [PubMed] [Google Scholar]
- Hayashi LC, Hayashi S, Yamaoka K, Tamiya N, Chikuda M, Yano E. Ultraviolet B exposure and type of lens opacity in ophthalmic patients in Japan. Sci Total Environ. 2003;302:53–62. doi: 10.1016/s0048-9697(02)00320-0. [DOI] [PubMed] [Google Scholar]
- Hiramoto K, Yamate Y, Kobayashi H, Ishii M. Long-term ultraviolet A irradiation of the eye induces photoaging of the skin in mice. Arch Dermatol Res. 2012;304:39–45. doi: 10.1007/s00403-011-1183-3. [DOI] [PubMed] [Google Scholar]
- Hood BD, Garner B, Truscott RJ. Human lens coloration and aging. Evidence for crystallin modification by the major ultraviolet filter, 3-hydroxy-kynurenine O-beta-D-glucoside. J Biol Chem. 1999;274:32547–32550. doi: 10.1074/jbc.274.46.32547. [DOI] [PubMed] [Google Scholar]
- Ishikura S, Usami N, Araki M, Hara A. Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase. J Biochem. 2005;137:303–314. doi: 10.1093/jb/mvi033. [DOI] [PubMed] [Google Scholar]
- Jacobs R, Krohn DL. Variations in fluorescence characteristics of intact human crystalline lens segments as a function of age. J Gerontol. 1976;31:641–647. doi: 10.1093/geronj/31.6.641. [DOI] [PubMed] [Google Scholar]
- Kessel L, Kalinin S, Soroka V, Larsen M, Johansson LB. Impact of UVR-A on whole human lenses, supernatants of buffered human lens homogenates, and purified argpyrimidine and 3-OH-kynurenine. Acta Ophthalmol Scand. 2005;83:221–227. doi: 10.1111/j.1600-0420.2005.00388.x. [DOI] [PubMed] [Google Scholar]
- Korlimbinis A, Aquilina JA, Truscott RJ. Protein-bound UV filters in normal human lenses: the concentration of bound UV filters equals that of free UV filters in the center of older lenses. Invest Ophthalmol Vis Sci. 2007;48:1718–1723. doi: 10.1167/iovs.06-1134. [DOI] [PubMed] [Google Scholar]
- Lerman S, Yamanashi BS, Palmer RA, Roark JC, Borkman R. Photoacoustic, fluorescence and light transmission spectra of normal, aging and cataractous lenses. Ophthalmic Res. 1978;10:168–176. [Google Scholar]
- Linetsky M, Ortwerth BJ. Quantitation of the singlet oxygen produced by UVA irradiation of human lens proteins. Photochem Photobiol. 1997;65:522–529. doi: 10.1111/j.1751-1097.1997.tb08598.x. [DOI] [PubMed] [Google Scholar]
- Matsuda H, Giblin FJ, Reddy VN. The effect of x-irradiation on cation transport in rabbit lens. Exp Eye Res. 1981;33:253–265. doi: 10.1016/s0014-4835(81)80049-8. [DOI] [PubMed] [Google Scholar]
- McMillan TJ, Leatherman E, Ridley A, Shorrocks J, Tobi SE, Whiteside JR. Cellular effects of long wavelength UV light (UVA) in mammalian cells. J Pharm Pharmacol. 2008;60:969–976. doi: 10.1211/jpp.60.8.0004. [DOI] [PubMed] [Google Scholar]
- Mizdrak J, Hains PG, Truscott RJ, Jamie JF, Davies MJ. Tryptophan-derived ultraviolet filter compounds covalently bound to lens proteins are photosensitizers of oxidative damage. Free Radic Biol Med. 2008;44:1108–1119. doi: 10.1016/j.freeradbiomed.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Mohan M, Sperduto RD, Angra SK, Milton RC, Mathur RL, Underwood BA, Jaffery N, Pandya CB, Chhabra VK, Vajpayee RB, et al. India-US case-control study of age-related cataracts. India-US Case-Control Study Group. Arch Ophthalmol. 1989;107:670–676. doi: 10.1001/archopht.1989.01070010688028. [DOI] [PubMed] [Google Scholar]
- Moore L, Ferreira JT. Ultraviolet (UV) transmittance characteristics of daily disposable and silicone hydrogel contact lenses. Cont Lens Anterior Eye. 2006;29:115–122. doi: 10.1016/j.clae.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Murthy GV, Gupta SK, Maraini G, Camparini M, Price GM, Dherani M, John N, Chakravarthy U, Fletcher AE. Prevalence of lens opacities in North India: the INDEYE feasibility study. Invest Ophthalmol Vis Sci. 2007;48:88–95. doi: 10.1167/iovs.06-0284. [DOI] [PubMed] [Google Scholar]
- Neale RE, Purdie JL, Hirst LW, Green AC. Sun exposure as a risk factor for nuclear cataract. Epidemiology. 2003;14:707–712. doi: 10.1097/01.ede.0000086881.84657.98. [DOI] [PubMed] [Google Scholar]
- Ortwerth BJ, Chemoganskiy V, Mossine VV, Olesen PR. The effect of UVA light on the anaerobic oxidation of ascorbic acid and the glycation of lens proteins. Invest Ophthalmol Vis Sci. 2003;44:3094–3102. doi: 10.1167/iovs.02-0857. [DOI] [PubMed] [Google Scholar]
- Ortwerth BJ, Chemoganskiy V, Olesen PR. Studies on singlet oxygen formation and UVA light-mediated photobleaching of the yellow chromophores in human lenses. Exp Eye Res. 2002;74:217–229. doi: 10.1006/exer.2001.1114. [DOI] [PubMed] [Google Scholar]
- Padgaonkar V, Giblin FJ, Reddy VN. Disulfide cross-linking of urea-insoluble proteins in rabbit lenses treated with hyperbaric oxygen. Exp Eye Res. 1989;49:887–899. doi: 10.1016/s0014-4835(89)80047-8. [DOI] [PubMed] [Google Scholar]
- Parker NR, Jamie JF, Davies MJ, Truscott RJ. Protein-bound kynurenine is a photosensitizer of oxidative damage. Free Radic Biol Med. 2004;37:1479–1489. doi: 10.1016/j.freeradbiomed.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Pastor-Valero M, Fletcher AE, de Stavola BL, Chaques-Alepuz V. Years of sunlight exposure and cataract: a case-control study in a Mediterranean population. BMC Ophthalmol. 2007;7:18. doi: 10.1186/1471-2415-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattison DI, Rahmanto AS, Davies MJ. Photo-oxidation of proteins. Photochem Photobiol Sci. 2012;11:38–53. doi: 10.1039/c1pp05164d. [DOI] [PubMed] [Google Scholar]
- Pitts DG. Glenn A. Fry Award Lecture--1977. The ocular effects of ultraviolet radiation. Am J Optom Physiol Opt. 1978;55:19–35. doi: 10.1097/00006324-197801000-00004. [DOI] [PubMed] [Google Scholar]
- Pitts DG, Cullen AP, Hacker PD. Ocular effects of ultraviolet radiation from 295 to 365 nm. Invest Ophthalmol Vis Sci. 1977;16:932–939. [PubMed] [Google Scholar]
- Rudy MA, Zigman S, Girsch SJ, Schenk E. Sub-threshold near-UV radiation effects on aphakic guinea pig retinas. Curr Eye Res. 1982;2:39–45. doi: 10.3109/02713688208998378. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Jonasson F, Shui YB, Kojima M, Ono M, Katoh N, Cheng HM, Takahashi N, Sasaki K. High prevalence of nuclear cataract in the population of tropical and subtropical areas. Dev Ophthalmol. 2002;35:60–69. doi: 10.1159/000060806. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Ono M, Aoki K, Katoh N, Morine M, Nakaizumi H, Fujisawa K, Kojima M, Sakamoto Y, Hatano T. Cataract epidemiology survey in three different areas in Japan - Prevalence of cataracts and types of lens opacification. J Jpn Ophthalmol Soc. 1995;99:204–211. [PubMed] [Google Scholar]
- Sasaki K, Zainuddin D, Fujisawa K, Kojima M, Sakamoto Y. Cataract epidemiology survey in west Sumatra. Dev Ophthalmol. 1989;17:26–32. [PubMed] [Google Scholar]
- Simpanya MF, Ansari RR, Leverenz V, Giblin FJ. Measurement of lens protein aggregation in vivo using dynamic light scattering in a guinea pig/UVA model for nuclear cataract. Photochem Photobiol. 2008;84:1589–1595. doi: 10.1111/j.1751-1097.2008.00390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpanya MF, Ansari RR, Suh KI, Leverenz VR, Giblin FJ. Aggregation of lens crystallins in an in vivo hyperbaric oxygen guinea pig model of nuclear cataract: dynamic light-scattering and HPLC analysis. Invest Ophthalmol Vis Sci. 2005;46:4641–4651. doi: 10.1167/iovs.05-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavazzi B, Di Pierro D, Amorini AM, Fazzina G, Galvano M, Lupi A, Giardina B, Lazzarino G. Direct NAD(P)H hydrolysis into ADP-ribose(P) and nicotinamide induced by reactive oxygen species: a new mechanism of oxygen radical toxicity. Free Radic Res. 2000;33:1–12. doi: 10.1080/10715760000300561. [DOI] [PubMed] [Google Scholar]
- Taylor HR, West SK, Rosenthal FS, Munoz B, Newland HS, Abbey H, Emmett EA. Effect of ultraviolet radiation on cataract formation. N Engl J Med. 1988;319:1429–1433. doi: 10.1056/NEJM198812013192201. [DOI] [PubMed] [Google Scholar]
- Truscott RJ. Human cataract: the mechanisms responsible; light and butterfly eyes. Int J Biochem Cell Biol. 2003;35:1500–1504. doi: 10.1016/s1357-2725(03)00145-6. [DOI] [PubMed] [Google Scholar]
- Tsubota K, Krauss JM, Kenyon KR, Laing RA, Miglior S, Cheng HM. Lens redox fluorometry: pyridine nucleotide fluorescence and analysis of diabetic lens. Exp Eye Res. 1989;49:321–334. doi: 10.1016/0014-4835(89)90043-2. [DOI] [PubMed] [Google Scholar]
- Tyrrell R. UVA (320–380nm) radiation as an oxidative stress. In: Sies H, editor. Oxidative stress: oxidants and antioxidants. Academic Press; San Diego, CA: 1991. pp. 57–83. [Google Scholar]
- Van Vreeswijk H, Boets EP, Van Best JA. The effect of white light and UV-A on the green autofluorescence of the rabbit lens in vivo. Exp Eye Res. 1993;56:349–354. doi: 10.1006/exer.1993.1045. [DOI] [PubMed] [Google Scholar]
- Vitinius U, Schaffner K, Demuth M, Heibel M, Selbach H. New photoproducts from irradiation of NADH with near-UV light. Chem Biodivers. 2004;1:1487–1497. doi: 10.1002/cbdv.200490109. [DOI] [PubMed] [Google Scholar]
- Weale RA. Human lenticular fluorescence and transmissivity, and their effects on vision. Exp Eye Res. 1985;41:457–473. doi: 10.1016/s0014-4835(85)80004-x. [DOI] [PubMed] [Google Scholar]
- Weale RA. Age and the transmittance of the human crystalline lens. J Physiol. 1988;395:577–587. doi: 10.1113/jphysiol.1988.sp016935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreb O, Dovrat A, Dunia I, Benedetti EL, Bloemendal H. UV-A-related alterations of young and adult lens water-insoluble alpha-crystallin, plasma membranous and cytoskeletal proteins. Eur J Biochem. 2001;268:536–543. doi: 10.1046/j.1432-1327.2001.01885.x. [DOI] [PubMed] [Google Scholar]
- West SK, Duncan DD, Munoz B, Rubin GS, Fried LP, Bandeen-Roche K, Schein OD. Sunlight exposure and risk of lens opacities in a population-based study: the Salisbury Eye Evaluation project. JAMA. 1998;280:714–718. doi: 10.1001/jama.280.8.714. [DOI] [PubMed] [Google Scholar]
- Wong L, Ho SC, Coggon D, Cruddas AM, Hwang CH, Ho CP, Robertshaw AM, MacDonald DM. Sunlight exposure, antioxidant status, and cataract in Hong Kong fishermen. J Epidemiol Community Health. 1993;47:46–49. doi: 10.1136/jech.47.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood AM, Truscott RJ. Ultraviolet filter compounds in human lenses: 3-hydroxykynurenine glucoside formation. Vision Res. 1994;34:1369–1374. doi: 10.1016/0042-6989(94)90135-x. [DOI] [PubMed] [Google Scholar]
- Zigler JS, Jr, Rao PV. Enzyme/crystallins and extremely high pyridine nucleotide levels in the eye lens. FASEB J. 1991;5:223–225. doi: 10.1096/fasebj.5.2.2004667. [DOI] [PubMed] [Google Scholar]
- Zigman S. Photobiology of the lens. In: Maisel H, editor. The ocular lens: Structure, function, and pathology. Marcel Dekker, Inc; New York: 1985. pp. 301–347. [Google Scholar]
- Zigman S. Environmental near-UV radiation and cataracts. Optom Vis Sci. 1995;72:899–901. doi: 10.1097/00006324-199512000-00008. [DOI] [PubMed] [Google Scholar]
- Zigman S. Lens UVA photobiology. J Ocul Pharmacol Ther. 2000;16:161–165. doi: 10.1089/jop.2000.16.161. [DOI] [PubMed] [Google Scholar]
- Zigman S, Datiles M, Torczynski E. Sunlight and human cataracts. Invest Ophthalmol Vis Sci. 1979;18:462–467. [PubMed] [Google Scholar]
- Zuclich JA, Glickman RD, Menendez AR. In situ measurements of lens fluorescence and its interference with visual function. Invest Ophthalmol Vis Sci. 1992;33:410–415. [PubMed] [Google Scholar]