Abstract
Background
We sought to examine how expansions in insurance coverage of nonbiologic and biologic disease modifying anti-rheumatic drugs (DMARDs) impacted the access, costs and health status of older patients with rheumatoid arthritis.
Methods
We identified a nationally-representative sample of older adults with rheumatoid arthritis in the 2000–2006 Medicare Current Beneficiary Survey (unweighted n=1051). We examined changes in DMARD use, self-reported health status, functional status (activities of daily living [ADL]), and total costs and out-of-pocket costs for medical care and prescription drugs. Tests for time trends were conducted using weighted regressions.
Results
Between 2000 and 2006, the proportion of older adults with rheumatoid arthritis who received biologics tripled (4.6% vs. 13.2%, p=0.01), while the proportion of people that used a nonbiologic did not change. During the same period, the proportion of older rheumatoid arthritis patients rating their health as excellent/good significantly increased (43.0% in 2000 to 55.6% in 2006; p=0.015). Significant improvements occurred in activities of daily living measures of functional status. Total prescription drug costs (in 2006 US dollars) increased from $2645 in 2000 to $4685 in 2006, p=0.0001, while out-of-pocket prescription costs remained constant ($842 in 2000 vs. $832 in 2006; p=0.68). Total medical costs did not significantly increase ($16563 in 2000 vs. $19510 in 2006; p=0.07).
Conclusions
Receipt of biologics in older adults with rheumatoid arthritis increased over a period of time where insurance coverage was expanded without increasing patients’ out-of-pocket costs. During this time period concurrent improvements in self-reported health status and functional status suggest improved arthritis care.
Keywords: Rheumatoid Arthritis, Disease Modifying Anti-Rheumatic Drugs (DMARD), Biologic Response Modifiers, Medication, Costs
Introduction
Treatment practices for rheumatoid arthritis have changed dramatically in the last ten years to promote earlier intervention and more aggressive use of disease modifying anti-rheumatic drugs (DMARDs).1 Biologic agents such as adalimumab, etanercept, and infliximab as well as nonbiologic DMARDs have demonstrated substantial effectiveness in reducing disease activity, reducing joint erosions and improving quality of life of rheumatoid arthritis patients.1–4 However, there is some limited evidence that older adults with rheumatoid arthritis are less likely to receive DMARDs than younger adults despite similar disease activity.5–9 Possible explanations include concerns regarding the safety of DMARDs in older adults with multiple comorbid conditions as well as age bias.10
Financial constraints may also influence the use of DMARDs, particularly biologic agents which are expensive. These agents are priced much higher than traditional DMARDs since they are costly to make, have unique mechanisms of action and are not available as a generic formulation. For example, the average cost of one administration of infliximab in 2006 was $1,728.11 Biologic agents can be either self-administered, such as etanercept and adalimumab, in which case they are designated as “specialty” drugs by most drug benefit plans and not covered by Medicare prior to 2004 or can be given intravenously like infliximab in doctor’s offices or infusion clinics and covered under insurers’ medical benefit (Medicare Part B).12 Under Part B, Medicare paid drug manufacturers based on a drug’s average wholesale price resulting in higher prices than negotiated prices generally included in drug benefit plans.13 In 2003, the Medicare Modernization Act (MMA) established a temporary drug benefit for self- administered agents since prior to this no Medicare coverage existed. The goal of the MMA was to replace the need for infused drugs covered under Part B, including infliximab (called The Medicare Replacement Drug Demonstration program, which lasted from 2004 to 2005 and enrolled 14,929 low-income patients with rheumatoid arthritis). In 2006, Medicare offered prescription drug coverage (Part D) to Medicare beneficiaries and within 6 months 22.5 million seniors had enrolled in a Medicare Part D plan with 15.8 million having another source of drug coverage, and approximately 4.4 million (10 percent) with no coverage.14 For patients with rheumatoid arthritis, prescription drug plan provided access to oral and self-administered nonbiologic and biologic DMARDs. However, the specialty status of the biologic DMARDs required higher cost-sharing for rheumatoid arthritis patients enrolled in Part D plans.15
The objective of this study was to examine how expansions in insurance coverage influenced the use and costs of biologic and nonbiologic DMARDs in older patients with rheumatoid arthritis. We also tracked changes in self-reported health status and functional status. We examined these changes before and during the MMA demonstration project, and in the first year of Part D. We hypothesized that use of the DMARDs would increase, especially the most expensive biologics, as financial barriers were decreased.
Patients and Methods
Data Source
The Medicare Current Beneficiary Survey (MCBS) is a continuous face-to-face panel survey of a representative national sample of Medicare beneficiaries conducted by the Center for Medicare and Medicaid Services (CMS) in order to inform and evaluated health policies for Medicare beneficiaries.16 Since 1991, the MCBS has provided detailed longitudinal data on annual samples of Medicare beneficiaries with a current sample size of approximately 12,000 community-dwelling and institutionalized elderly and the disabled. The rich variety of measures includes demographic information, income, health status, functioning, health behaviors, health insurance coverage, drug coverage, health services utilization (including copayments, deductibles, and non-covered services), and access to medical care.
The sample for the MCBS is drawn from Medicare enrollment records according to a multi-stage sampling plan with oversampling of vulnerable subgroups such as the disabled and the oldest old. Respondents are selected in rotating 4-year panels with annual replenishments thus ensuring continued generalizability. The MCBS conducts a baseline interview between September and December covering demographics and household composition, as well as health insurance, health status, and health care utilization, including prescription drugs. This general interview is repeated yearly for the following 3 years. In addition, thrice-annual interviews collect detailed information on health care use and expenditures, with reviews of respondents’ insurance statements and receipts. Each respondent keeps a record of insurance statements, receipts, and prescription bottles, in order to enhance the accuracy of data collection. Interviews are conducted in person with computer assistance resulting in very high response rates (initially ~85%). The typical MCBS interview lasts approximately one hour.
Study Sample
The study sample included Medicare beneficiaries with rheumatoid arthritis during the years 2000 through 2006. We indentified patients as having rheumatoid arthritis if they had at least one of the following: 1) 2 or more ICD-9 diagnoses for rheumatoid arthritis (714.XX) based on claims data; 2) both self-reported rheumatoid arthritis and an ICD-9 diagnosis of rheumatoid arthritis; or 3) a dispensing of a nonbiologic or biologic DMARD (adalimumab, etanercept, hydroxychloroquine, infliximab, lefunomide, methotrexate, or sulfalazine) and either self-reported of rheumatoid arthritis or an ICD-9 diagnosis of rheumatoid arthritis. We excluded institutional respondents as prescription drug expenditures are not captured for this population, and individuals with 3 or fewer months of entitlement to ensure a reliable time frame for estimation of annual medical care utilization. For individuals with 4 to 11 months of observation, we weighted their observation to reflect the partial year contribution (e.g. a person observed 4 months received a weight of 4/12). Given that each individual with rheumatoid arthritis could have provided a maximum of 4 years of data, the total number of individuals who participated in the study was 1,055. The unweighted sample size in each year ranged from 225 to 260.
Study variables
Our main study measure was receipt of one or more prescriptions of a DMARD each year of the study, which was based on self-reported medication use and prescription drug claims in Medicare Parts B and D. DMARD use was further classified into noncytotoxic (hydroxychloroquine and sulfasalazine), cytotoxic (leflunomide and methotrexate) and biologic (adalimumab, etanercept and infliximab).
Functional status was measured based on an assessment of activities of daily living, specifically responses about how much difficulty, if any, the respondents have with: 1) bathing or showering; 2) dressing; 3) eating; 4) getting into or out of a bed or a chair; 5) walking; and 6) using the toilet.17 For the purpose of our study, we used a dichotomous outcome of difficulty versus no difficulty with each activity of daily living. Persons who reported having any difficulty or not being able to perform any of the listed activities for reasons of health were categorized as having a limitation in the activity. The range of possible scores was 0 to 6 with a higher score reflecting a greater number of limitations. General health status is a global self-rating of health ranging from excellent to poor, which we dichotomized as excellent/very good/good and fair/poor.
Health care expenditures, measured through claims and review of receipts and insurance statements, included total medical costs, total prescription costs, DMARD-specific ambulatory prescription costs, and out of pocket costs. We inflated all cost values to 2006 prices using the consumer price index.18
Other covariates of interest included age, gender, race, residence (rural versus metropolitan), and comorbidity burden. Comorbidity burden was assessed based on a count of self-reported comorbid medical conditions (0, 1 – 2, and ≥ 3). We also identified whether there was prescription drug coverage (yes or no). We also examined income in MCBS, which is underreported and therefore we inflated the self-reported income by 20% as recommended previously and converted the adjusted income to the federal poverty levels.19 Poverty status was classified based on Medicaid status and federal poverty level (FPL). Patients were stratified into 5 mutually-exclusive categories: Medicaid, 0–100% FPL, 101–150% FPL, 151–200% FPL, and 201+% FPL as others have done.20
Analysis
First we described the annual rates (and 95% confidence intervals [CIs]) of socio-demographic and health characteristics of the study population, 2000 to 2006, weighted to represent the overall rheumatoid arthritis population of community-dwelling Medicare beneficiaries. Then we calculated the annual prevalence of nonbiologic and biologic DMARDs (specifically the proportion of rheumatoid arthritis patients who received at least 1 prescription in each year) and the mean values of functional status, and health care expenditures (and 95% confidence intervals). Subsequently we tested for trends over time were using weighted regressions using ordinary least squares accounting for survey design. All analyses were conducted using SAS version 9.2. The a priori level of statistical significance was p=0.05. This study was reviewed and approved by the Institutional Review Board at the University of Massachusetts.
Results
We identified rheumatoid arthritis patients annually between 2000 and 2006, most of whom were women living in metropolitan areas (Table 1). Most patients (70%) were identified based on 2 ICD-9 diagnoses of rheumatoid arthritis within 1 calendar year. Approximately two-thirds of patients reported both a diagnosis of rheumatoid arthritis and had at least one ICD-9 code for rheumatoid arthritis. In 2000, 49% of the sample had at least one ICD-9 diagnosis of rheumatoid arthritis as well as use of a nonbiologic or biologic DMARD. This increased to 59% by 2006. The mean age was 69.1 to71.7 years. Over time there were greater numbers of Hispanic patients and fewer Whites. The proportion of patients with prescription drug coverage increased from 85.3% in 2000 to 97.1% in 2006 (p =0.0001). There were no significant changes in poverty status or comorbidity burden. However, self-reported health status of excellent, very good or good increased from 43.0% in 2000 to 55.6% in 2006.
Table 1.
Trends over time in the characteristics of RA patients enrolled in Medicare (only even years shown).
| 2000 | 2002 | 2004 | 2006 | P value | |
|---|---|---|---|---|---|
| unweighted n | 225 | 260 | 253 | 247 | |
| age in year, mean (SE) | 70.5 (0.81) | 71.7 (0.62) | 69.9 (0.75) | 69.1 (0.76) | 0.081 |
| female gender (%) | 77.0 | 75.5 | 79.6 | 79.3 | 0.237 |
| Race/ethnicity (%) | 0.016 | ||||
| Hispanic | 5.6 | 7.5 | 9.3 | 12.6 | |
| Black/non-Hispanic | 12.7 | 7.5 | 11.2 | 12.1 | |
| White | 76.4 | 82.1 | 74.4 | 71.9 | |
| Residence (%) | 0.138 | ||||
| Rural | 26.8 | 27.1 | 26.2 | 20.0 | |
| Metropolitan | 73.2 | 72.9 | 73.8 | 80.0 | |
| Prescription drug coverage (%) | 85.3 | 89.7 | 84.9 | 97.1 | 0.001 |
| Poverty status | 0.625 | ||||
| Medicaid | 14.2 | 10.7 | 15.6 | 12.7 | |
| 0–100% FPL | 17.4 | 15.8 | 19.9 | 17.4 | |
| 101–150% FPL | 17.1 | 13.2 | 15.7 | 12.7 | |
| 151–200% FPL | 17.5 | 27.5 | 18.6 | 25.8 | |
| 201+% FPL | 33.7 | 32.8 | 30.2 | 31.4 | |
| Comorbidity burden (%) | 0.105 | ||||
| 0 | 15.0 | 8.9 | 10.0 | 11.6 | |
| 1 to 2 | 41.3 | 51.6 | 47.0 | 47.3 | |
| 3 or more | 58.1 | 39.5 | 43.0 | 41.1 | |
| Self-reported health status (%) | 0.015 | ||||
| excellent/very good/good | 43.0 | 44.0 | 48.7 | 55.6 | |
| fair/poor | 57.0 | 56.0 | 51.3 | 44.4 |
As shown in Table 2, use of cytotoxic DMARDs remained stable from 2000 to 2006 (28.5% in 2000 vs. 34.1% in 2006, p=0.19), as did use of noncytotoxic DMARDs (25.2% in 2000 vs. 23.2% in 2006, p=0.83). Between 2000 and 2006, 2.2–5.5% of patients used etanercept (p=0.32) and 1.3–4.9% used infliximab (p=0.54). Adalimumab, which was approved in 2003 by the FDA, was used in 0.7 to 5.3% of patients (p=0.001). Overall use of both the noncytotoxic and cytotoxic DMARDs remained at a stable rate ranging from one-quarter to one-third of patients receiving each drug class.
Table 2.
Trends in the use of disease modifying anti-rheumatic drugs (DMARDs) (only even years shown).
| Medication | 2000 | 2002 | 2004 | 2006 | p value |
|---|---|---|---|---|---|
| Noncytotoxic dmards *(%, SE) | 25.2 (3.3) | 21.4 (2.9) | 22.0 (2.8) | 23.2 (2.7) | 0.829 |
| Cytotoxic dmards** (%, SE) | 28.5 (3.3) | 26.9 (3.0) | 32.6 (3.8) | 34.1 (3.6) | 0.193 |
| Biologic agent*** | |||||
| Adalimumab (%, SE) | 0.0 | 0.0 | 0.7 (0.4) | 5.3 (1.6) | 0.001 |
| Etanercept (%, SE) | 3.3 (1.3) | 2.2 (1.0) | 4.6 (1.3) | 3.5 (1.2) | 0.320 |
| Infliximab (%, SE) | 1.3 (0.7) | 4.9 (1.5) | 2.5 (1.0) | 4.4 (1.4) | 0.544 |
noncytotoxic: hydroxychloroquine and sulfasalazine
cytotoxic: methotrexate and leflunomide
biologic use included the anti-TNFs only
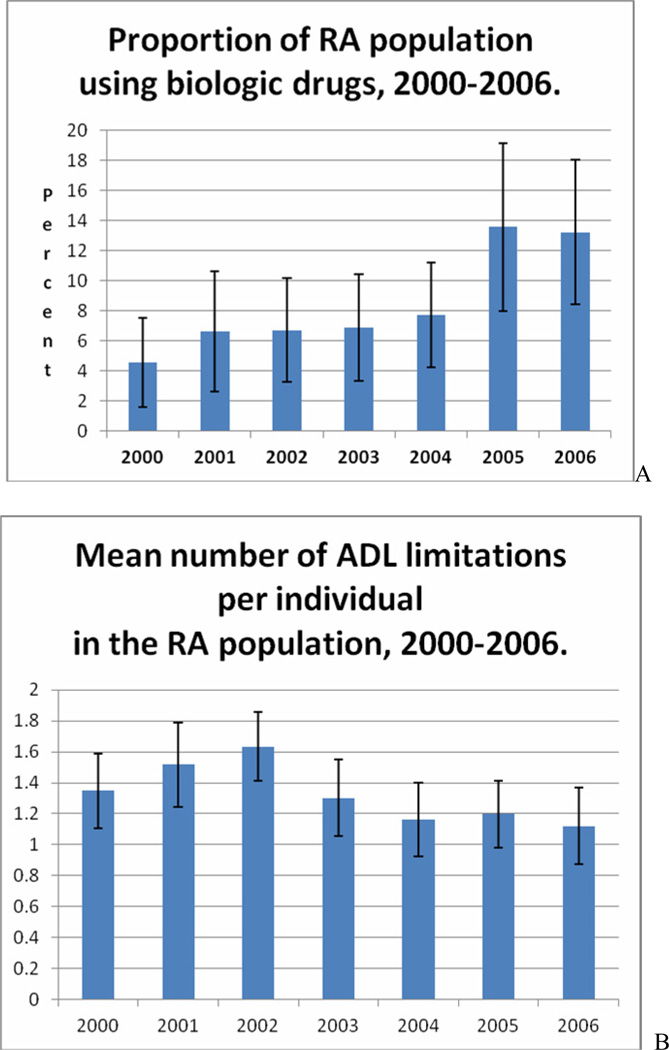
In contrast, use of biologics increased substantially (Figure 1A). Between 2000 and 2006, the proportion of biologic users increased from 4.6% in 2000 to 13.2% in 2006 (p=0.006). In addition, Figure 1 shows the adoption of the biologics and the functional status assessments over the same time period. Concurrent to the increase in the use of biologics from 2000 to 2006, we also observed a significant improvement in functional status measures (Figure 1B). The mean activities of daily living score (range 0 to 6; higher score denotes greater limitations) was 1.35 in 2000 as compared to 1.12 in 2006 (p=0.034).
Figure 1.
Time trends in the use of biologics and functional status, 2000–2006.*
*RA = Rheumatoid Arthritis; ADL = Acitivities of Daily Living
Table 3 summarizes the changes in health care expenditures during the study. Total costs for ambulatory DMARD medications increased by almost 150% ($396 in 2000 to $984 in 20006, p=0.014), while costs for all prescription medications (not just DMARDs) increased by 77% ($2645 in 2000 to $4680 in 2006, p=0.0001). However, out of pocket costs remained stable over the time period ($842 in 2000 to $832 in 2006, p=0.68). Total medical costs increased slightly but the trend was not significant (Table 3). However, there was a significant increase in the mean proportion of total medical costs attributable to prescription medications. In 2000, the mean proportion of medical costs attributable to prescription medications was 30.0% as compared to 37.5% in 2006 (p=0.001).
Table 3.
Trends in ambulatory prescription costs, out of pocket costs, total medical costs and the proportion of costs due to prescription drugs (2006 dollars) (only even years shown).
| Medical Care | 2000 | 2002 | 2004 | 2006 | P value |
|---|---|---|---|---|---|
| Ambulatory prescription costs, mean ($, SE) | 2645 (172) | 3270 (184) | 3726 (243) | 4680 (315) | 0.0001 |
| RA specific ambulatory prescription costs, mean ($, SE) | 396 (62) | 592 (98) | 547 (78) | 984 (166) | 0.014 |
| Out-of-pocket prescription costs, mean ($, SE) | 842 (82) | 854 (55) | 924 (65)1 | 832 (68) | 0.682 |
| Total medical costs, mean ($, SE) | 16563 (1714) | 18310 (1484) | 17783 (1714) | 19510 (1365) | 0.070 |
| Proportion of total medical costs due to prescription medications (%, SE) | 30.2 (1.90) | 31.9 (1.77) | 36.6 (1.97) | 37.5 (1.93) | 0.001 |
Discussion
Between 2000 and 2006, the use of biologic agents in older adults with rheumatoid arthritis increased from 4.6% in 2000 to 13.2% in 2006. This increase occurred during a period of expansion in prescription drug coverage for these agents. During the same period, we also observed statistically significant improvement in patient-reported functional status however the magnitude of the improvement is of unclear clinical significance. In addition, the proportion of patients rating their health status as excellent, very good or good increased by 30% from 43.0% in 2000 to 55.6% in 2006. While large increases in total prescription medication costs were observed, over the same time period out-of-pocket costs for prescription drug remained constant.
The increased use of biologic utilization without a concomitant rise in out-of -pocket costs suggests that recent policy changes have enabled increased access to medications without increasing drug spending for beneficiaries, which has been seen in other chronic medical conditions since the introduction of Part D.14 Medical care for rheumatoid arthritis is expensive; individuals with rheumatoid arthritis have higher medical expenditures than persons without arthritis.21 Specifically Yelin found that the amount Americans spent on arthritis medications more than doubled between 1998 and 2003.22 For elderly patients, greater medical expenses combined with limited income can result in increased out of pocket burden. Other research has shown that drug benefit generosity influences the likelihood that rheumatoid arthritis patients will initiate and continue a biologic agent.23 Based on our results, it appears that the Medicare Replacement Drug Demonstration (MRDD) program and Medicare Part D likely controlled out of pocket costs and potentially permitted increased utilization of biologics in these patients. The MRDD program was created by Congress to provide temporary drug insurance until the start of Medicare Part D. Low income vulnerable Medicare beneficiaries with select conditions, including rheumatoid arthritis, without comprehensive drug insurance coverage were targeted for MRDD enrollment. MRDD was structured to have similar patient cost-sharing arrangements as the anticipated standard Medicare Part D benefit. For rheumatoid arthritis, the program covered self-injectable medications including adalimumab and etanercept.24 This was followed by Medicare Part D in 2006, which has been successful in reducing the out of pocket costs among Medicare beneficiaries.25
While the adoption of biologic use in our population increased substantially, it is still less than what has been observed in younger rheumatoid arthritis patients.26,27 For example, in a younger population where over 60% had commercial insurance, biologic use increased 800% over the same time period resulting in 26% of the sample receiving biologic agents in 2006--double the increase among Medicare patients.28 While financial concerns may play a role, treatment decisions may be influenced by the age of the patient. Even though traditional DMARDs and biologics are both safe and efficacious in the elderly29–33, rheumatologists state they are less likely to treat these patients aggressively.10 This has been borne out in clinical practice with biologics being used less often in older rheumatoid arthritis patients as compared to younger patients taking into account disease activity, disease duration and comorbidities.34 While elderly patients may require more monitoring because of comorbidity, polypharmacy and age related changes in pharmacokinetics and dynamics, the American College of Rheumatology treatment recommendations do not suggest different rheumatoid arthritis treatment strategies based on patient age or disease duration.1 While pharmacotherapeutic decision-making in the elderly can be complex, the beneficial impact of biologics with regard to functional status and independence should be examined as older patients and providers weigh the risks and benefits of treatment.
Due to the cross-sectional nature of the study, small sample size and the low proportion of people using biologic agents, this work cannot examine whether there is a causal relationship between drug coverage and health outcomes. For example, it is possible that more aggressive treatment approaches led to simultaneous increased use of biologics and improvement in functional status. However, the concurrent improvements in self-reported health status and functional status deserve further study at the population level. Other limitations include no information on rheumatoid arthritis disease activity or disease severity thus we are unable to examine whether all patients who were candidates for biologic drugs received them. We also cannot assess whether patients declined use of biologics or whether they discontinued the agents due to clinical or financial reasons. Medication use was identified based on patient self-report of medications, thus there may be some undercounting. Given the small numbers of biologic users, our estimates of use for each of the specific agents could have been influenced by the sampling strategy. As with all studies using ICD-9 codes to identify patients, misclassification is a concern. However, studies using Medicare claims have reported a sensitivity of 65 to 90% and a high positive predictive value.35,36 To try to improve sensitivity, we used three different strategies to identify our population.
In summary, we identified a nationally-representative sample of rheumatoid arthritis patients annually from 2000 through 2006. We were able to measure use of biologic and nonbiologic DMARDs in conjunction with patient-reported functional status and health status as well as associated costs. Over those 7 years, there was a dramatic increase in the number of patients receiving biologic agents. While these agents are more than 10 times more expensive than traditional agents, patient out-of-pocket expenses did not increase suggesting recent health care policy changes have been successful in tempering the direct financial burden of medication costs for Medicare patients with rheumatoid arthritis.
Acknowledgements
Funding:
Dr. Harrold was supported by Grant Number K23AR053856 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Briesacher was supported by a Research Scientist Development Award from the National Institute on Aging (K01AG031836).
Acknowledgement of Data and Analysis:
Dr. Harrold had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the National Institute on Aging or the National Institutes of Health.
Conflict of Interest Statement:
All authors have no conflict of interest to disclose.
All authors had access to the data and a role in writing the manuscript.
References
- 1.Saag KG, Teng GG, Patkar NM, Anuntiyo J, Finney C, Curtis JR, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59(6):762–784. doi: 10.1002/art.23721. [DOI] [PubMed] [Google Scholar]
- 2.Breedveld FC, Weisman MH, Kavanaugh AF, Cohen SB, Pavelka K, van Vollenhoven R, et al. The PREMIER study: A multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54(1):26–37. doi: 10.1002/art.21519. [DOI] [PubMed] [Google Scholar]
- 3.Emery P, Breedveld FC, Hall S, Durez P, Chang DJ, Robertson D, et al. Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double-blind, parallel treatment trial. Lancet. 2008;372(9636):375–382. doi: 10.1016/S0140-6736(08)61000-4. [DOI] [PubMed] [Google Scholar]
- 4.St Clair EW, van der Heijde DM, Smolen JS, Maini RN, Bathon JM, Emery P, et al. Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: a randomized, controlled trial. Arthritis Rheum. 2004;50(11):3432–3443. doi: 10.1002/art.20568. [DOI] [PubMed] [Google Scholar]
- 5.Berard A, Solomon DH, Avorn J. Patterns of drug use in rheumatoid arthritis. J Rheumatol. 2000;27(7):1648–1655. [PubMed] [Google Scholar]
- 6.Carli C, Ehlin AG, Klareskog L, Lindblad S, Montgomery SM. Trends in disease modifying antirheumatic drug prescription in early rheumatoid arthritis are influenced more by hospital setting than patient or disease characteristics. Ann Rheum Dis. 2006;65(8):1102–1105. doi: 10.1136/ard.2004.027656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzalez-Alvaro I, Carmona L, Balsa A, Sanmarti R, Belmonte MA, Tena X. Patterns of disease modifying antirheumatic drug use in a Spanish cohort of patients with rheumatoid arthritis. J Rheumatol. 2003;30(4):697–704. [PubMed] [Google Scholar]
- 8.Schmajuk G, Schneeweiss S, Katz JN, Weinblatt ME, Setoguchi S, Avorn J, et al. Treatment of older adult patients diagnosed with rheumatoid arthritis: improved but not optimal. Arthritis Rheum. 2007;57(6):928–934. doi: 10.1002/art.22890. [DOI] [PubMed] [Google Scholar]
- 9.Tutuncu Z, Reed G, Kremer J, Kavanaugh A. Do patients with older-onset rheumatoid arthritis receive less aggressive treatment? Ann Rheum Dis. 2006;65(9):1226–1229. doi: 10.1136/ard.2005.051144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fraenkel L, Rabidou N, Dhar R. Are rheumatologists' treatment decisions influenced by patients' age? Rheumatology (Oxford) 2006;45(12):1555–1557. doi: 10.1093/rheumatology/kel144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Medicare Payment Advisory Commission. Report to the congress: impact of changes in Medicare payments for Part B drugs. 2007 URL: http://www.medpac.gove/documents/Jan07_partb_mandated_report.pdf.
- 12.Hargrave E, Hoadley J, Merrell K, Cubanski J. Washington, DC: 2009. Drugs on Specialty Tiers in Part D: Final Report. URL: http://www.medpac.gov/documents/Feb09_DrugsonSpecialtyTiers_CONTRACTOR_RS.pdf. [Google Scholar]
- 13.US Department of Health and Human Services OoIG. US Department of Health and Human Services, Office of the Inspector General. Medicare reimbursement od prescription drugs. Washington (DC): HHS; 2001. Medicare reimbursement of prescription drugs. editor. [Google Scholar]
- 14.Schneeweiss S, Patrick AR, Pedan A, Varasteh L, Levin R, Liu N, et al. The effect of Medicare Part D coverage on drug use and cost sharing among seniors without prior drug benefits. Health Aff (Millwood) 2009;28(2):w305–w316. doi: 10.1377/hlthaff.28.2.w305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polinski JM, Mohr PE, Johnson L. Impact of Medicare Part D on access to and cost sharing for specialty biologic medications for beneficiaries with rheumatoid arthritis. Arthritis Rheum. 2009;61(6):745–754. doi: 10.1002/art.24560. [DOI] [PubMed] [Google Scholar]
- 16.Adler GS. A profile of the Medicare Current Beneficiary Survey. Health Care Financ Rev. 1994;15(4):153–163. [PMC free article] [PubMed] [Google Scholar]
- 17.Nagi SZ. An epidemiology of disability among adults in the United States. Milbank Mem Fund Q Health Soc. 1976;54(4):439–467. [PubMed] [Google Scholar]
- 18.US Department of Labor Bureau of Labor Statistics: Consumer Price Indexes. 2010 URL: http://www.bls.gov/cpi/
- 19.Poisal JA. Reporting of drug expenditures in the MCBS. Health Care Financ Rev. 2003;25(2):23–36. [PMC free article] [PubMed] [Google Scholar]
- 20.U.S Census Beareau. 2010 http://www.census.gov/hhes/www/poverty/methods/definitions.html.
- 21.Lurie IZ, Dunlop DD, Manheim LM. Trends in out-of-pocket medical care expenditures for Medicare-age adults with arthritis between 1998 and 2004. Arthritis Rheum. 2008;58(8):2236–2240. doi: 10.1002/art.23731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yelin E, Murphy L, Cisternas MG, Foreman AJ, Pasta DJ, Helmick CG. Medical care expenditures and earnings losses among persons with arthritis and other rheumatic conditions in 2003, and comparisons with 1997. Arthritis Rheum. 2007;56(5):1397–1407. doi: 10.1002/art.22565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karaca-Mandic P, Joyce GF, Goldman DP, Laouri M. Cost sharing, family health care burden, and the use of specialty drugs for rheumatoid arthritis. Health Serv Res. 45(5 Pt 1):1227–1250. doi: 10.1111/j.1475-6773.2010.01117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leavitt MO. Secretary of Health and Human Services. Report to congress: evaluation of the Medicare replacement drug demonstration. 2007 URL: https://www.cms.gov/DemoProjectsEvalRpts/downloads/MMA641_RTC.pdf.
- 25.Millett C, Everett CJ, Matheson EM, Bindman AB, Mainous AG., 3rd Impact of Medicare Part D on seniors' out-of-pocket expenditures on medications. Arch Intern Med. 2010;170(15):1325–1330. doi: 10.1001/archinternmed.2010.208. [DOI] [PubMed] [Google Scholar]
- 26.Grijalva CG, Chung CP, Stein CM, Mitchel EF, Jr, Griffin MR. Changing patterns of medication use in patients with rheumatoid arthritis in a Medicaid population. Rheumatology (Oxford) 2008;47(7):1061–1064. doi: 10.1093/rheumatology/ken193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee SJ, Chang H, Yazici Y, Greenberg JD, Kremer JM, Kavanaugh A. Utilization trends of tumor necrosis factor inhibitors among patients with rheumatoid arthritis in a United States observational cohort study. J Rheumatol. 2009;36(8):1611–1617. doi: 10.3899/jrheum.080889. [DOI] [PubMed] [Google Scholar]
- 28.Yazici Y, Shi N, John A. Utilization of biologic agents in rheumatoid arthritis in the United States: analysis of prescribing patterns in 16,752 newly diagnosed patients and patients new to biologic therapy. Bull NYU Hosp Jt Dis. 2008;66(2):77–85. [PubMed] [Google Scholar]
- 29.Capell HA, Porter DR, Madhok R, Hunter JA. Second line (disease modifying) treatment in rheumatoid arthritis: which drug for which patient? Ann Rheum Dis. 1993;52(6):423–428. doi: 10.1136/ard.52.6.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gardner G, Furst DE. Disease-modifying antirheumatic drugs. Potential effects in older patients. Drugs Aging. 1995;7(6):420–437. doi: 10.2165/00002512-199507060-00003. [DOI] [PubMed] [Google Scholar]
- 31.Hirshberg B, Muszkat M, Schlesinger O, Rubinow A. Safety of low dose methotrexate in elderly patients with rheumatoid arthritis. Postgrad Med J. 2000;76(902):787–789. doi: 10.1136/pmj.76.902.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Genevay S, Finckh A, Ciurea A, Chamot AM, Kyburz D, Gabay C. Tolerance and effectiveness of anti-tumor necrosis factor alpha therapies in elderly patients with rheumatoid arthritis: a population-based cohort study. Arthritis Rheum. 2007;57(4):679–685. doi: 10.1002/art.22688. [DOI] [PubMed] [Google Scholar]
- 33.Radovits BJ, Kievit W, Laan RF. Tumour necrosis factor-alpha antagonists in the management of rheumatoid arthritis in the elderly: a review of their efficacy and safety. Drugs Aging. 2009;26(8):647–664. doi: 10.2165/11316460-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 34.Radovits BJ, Fransen J, Eijsbouts A, van Riel PL, Laan RF. Missed opportunities in the treatment of elderly patients with rheumatoid arthritis. Rheumatology (Oxford) 2009;48(8):906–910. doi: 10.1093/rheumatology/kep129. [DOI] [PubMed] [Google Scholar]
- 35.Katz JN, Barrett J, Liang MH, Bacon AM, Kaplan H, Kieval RI, et al. Sensitivity and positive predictive value of Medicare Part B physician claims for rheumatologic diagnoses and procedures. Arthritis Rheum. 1997;40(9):1594–1600. doi: 10.1002/art.1780400908. [DOI] [PubMed] [Google Scholar]
- 36.Losina E, Barrett J, Baron JA, Katz JN. Accuracy of Medicare claims data for rheumatologic diagnoses in total hip replacement recipients. J Clin Epidemiol. 2003;56(6):515–519. doi: 10.1016/s0895-4356(03)00056-8. [DOI] [PubMed] [Google Scholar]