Abstract
The enzyme-linked immunosorbent assays (ELISA) is an extremely common and powerful laboratory technique for detecting proteins by antibodies. Researchers frequently use bovine serum albumin (BSA) as a blocking agent to prevent non-specific binding of antigens and antibodies to the microtiter well. While studying the interactions of the vaccinia virus complement control protein (VCP) with complement, we found nonspecific binding of VCP to BSA and identify a BSA preparation that did not result in non-specific binding. This work draws attention to the fact that not all BSA preparations are alike. It also highlights the need to perform critical controls to ensure that ELISA reactants do not inappropriately bind to the blocking agent.
Keywords: Enzyme-Linked Immunosorbent Assay/methods*; False Positive Reactions; Serum Albumin, Bovine/immunology*; Protein Binding; Vaccinia virus complement control protein
1. Introduction
Solid phase enzyme-linked immunosorbent assay (ELISA) is a conventional method for detecting proteins or protein-protein interactions by using appropriate antibodies (Hornbeck et al., 2001). When optimized, the indirect ELISA has high sensitivity and specificity. To ensure specificity, non-specific binding of reactants (i.e., the test proteins and detecting antibodies) should be confirmed. Researchers mainly worry about nonspecific binding of reactants to wells in ELISA microtiter plates that have been poorly blocked. To eliminate the residual binding capacity of the wells, blocking agents such as bovine serum albumin (BSA), non-fat dry milk, and whole serum are commonly used. Blocking agents can also stabilize the biomolecules bound to the well surface and reduce non-specific interactions (Gibbs, 2001). Researchers should also consider the potential of non-specific binding of ELISA reactants to the blocking agent. Since BSA is a widely used blocking agent, here we describe a non-specific binding interaction that occurred between an ELISA reactant and some preparations of BSA. The work highlights the fact that not all BSA preparations are alike.
ELISAs have been used to show the interaction between complement control proteins (CCPs) and complement (Liszewski and Atkinson, 1996; Liszewski et al., 2009). Our lab has had a longstanding interest in CCPs encoded by orthopoxviruses. During an investigation of the interaction of the vaccinia virus complement control protein (VCP) to human C3b and C4b using an ELISA format, we discovered significant non-specific binding of VCP to some preparations of BSA that we were using as a blocking agent.
2. Materials and Methods
ELISA 96-well Maxisorp Immuno plates (Nunc) were coated with 5 µg/ml human C3b or C4b protein (Complement Technology) in PBS overnight at 4°C as previously described by others (Liszewski et al., 2009). As a negative control, wells were also coated with 5 µg/ml BSA. After washing the plate with low salt washing buffer (10 mM Tris (pH 7.2), 25 mM sodium chloride, 0.05% Tween 20, and 0.25% Nonidet-P40), the plate was blocked in blocking buffer (PBS with 5% BSA, 0.1% Tween 20) at 37°C for 2 hours followed by additional washes with washing buffer. Concentrated media containing VCP from vaccinia virus-infected cells was then serially diluted in low salt washing buffer containing 4% BSA and the plate was incubated at 37°C for 1 hour. After extensive washes, rabbit anti-VCP antibody (1:5000 in washing buffer containing 4% BSA) was added and incubated at 37°C for 1 h. After washes, horseradish peroxidase-conjugated donkey anti-rabbit IgG (1:8000) (ECL) was added and incubated at 37°C for 1 hour. After extensive washes, the plate was incubated with TMS substrate (KPL) at room temperature for ~20 min after which stop solution (2N H2SO4) was added and the absorbance was measured at 450 nm using ELISA plate reader.
3. Results
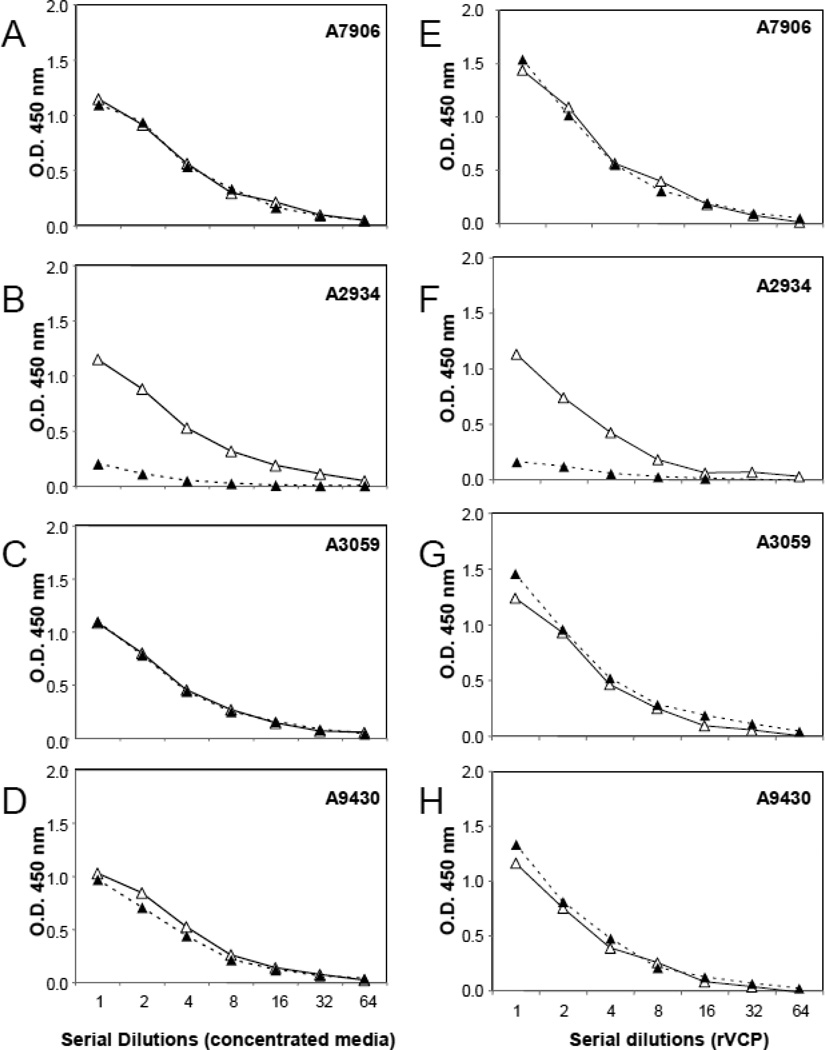
In an initial experiment, we used buffers containing BSA purchased from Sigma (A7906). To our surprise, the control wells coated and blocked with BSA-7906 showed the same O.D. value as those coated with complement protein C3b (Figure 1A) and C4b (data not shown). However, this non-specific binding of VCP to BSA did not occur when we used another BSA preparation we had in the lab (A2934, Figure 1B). As shown in Table 1, BSA-2934 is “globulin free and endotoxin low (≤1 ng/mg)”. Given the low non-specific binding we found with BSA-2934, we wondered if VCP was binding to either the globulin or endotoxin that might be present in BSA-7906. Thus, we obtained and tested additional BSA preparations from Sigma: A3059 (globulin free and protease free), A9430 (low endotoxin), and A7638 (globulin free) (Table 1). As shown in Figure 1C and 1D, these BSA preparations also gave very high non-specific binding. Because we were working with concentrated media from vaccinia virus-infected cells, we wondered if there was something in the media that was causing this non-specific binding of VCP to BSA. We thus used a bacteria-expressed recombinant VCP (rVCP) and found the same results (Figure 1E to H). Thus, only BSA-2934, which had been depleted both globulin and endotoxin, was capable of eliminating the non-specific binding of VCP to a contaminant in BSA.
Figure 1. Specific vs. non-specific binding of VCP to C3b or various BSA preparations.
ELISA results using concentrated media containing VCP from vaccinia virus-infected cells (A–D) or purified recombinant VCP (E–H). To determine specific vs. non-specific binding of VCP, microtiter wells were coated with C3b (open triangles and solid lines) or BSA and blocked with the following BSA preparations (solid triangles and dashed lines): BSA-7906 (A & E), BSA-2934 (B & F), BSA-3059 (C & G), or BSA-9430 (D & H). Data for BSA-7638 is not shown, but it gives identical results as BSA-3059, -A7906, and -A9430.
Table 1.
Sigma-Aldrich BSA preparations used in this report
| Sigma BSA catalog number |
Purity^ (based on agarose gel electrophoresis) |
Essentially globulin free^ |
Low endotoxin^ (≤1 ng/mg) |
Essentially protease free^ |
BSA used in Figure 1 |
|---|---|---|---|---|---|
| A2934 | 98% | * | * | B&F | |
| A3059 | 98% | * | * | C&G | |
| A7638 | 99% | * | - # | ||
| A7906 | 98% | A&E | |||
| A9430 | 98% | * | D&H |
Description of BSA products based on Sigma-Aldrich catalog
Data not shown, but results identical to that of A3059, A7906, and A9430
4. Discussion
BSA is one of the most commonly used blocking agents for ELISA. But since BSA is a serum protein, in certain circumstances it could cause non-specific ELISA signals. For example, in a study that was examining for the presence of human antibodies to Japanese encephalitis virus, antibodies that cross-reacted with BSA were found (Konishi et al., 2010). Another study found that human antibodies non-specifically bound to BSA (A2153 (≥ 96% pure preparation from Sigma)) (Chart et al., 1998). To prevent falsepositive results from either cross reactive antibodies or from non-specific binding of ELISA reagents to BSA, alternative blocking agents can be used. Examples include rabbit serum (Chart et al., 1998), casein (Konishi et al., 2010), heat-denaturing the blocking protein (Mauracher et al., 1991), non-protein blocking solutions like Synblock (Afrough et al., 2007) or Ficoll or polyvinylalcohol (Huber et al., 2009). Alternatively, no protein can be included in the blocking buffer (Jorgensen et al., 2005), although this too can potentially cause artifactual results (Bird et al., 1988).
In this report we show that different BSA preparations used as a blocking agent in an ELISA can give different amounts of non-specific binding of ELISA reactants. A vast majority of published articles that include BSA as a reagent do not provide specific information about the BSA. While the company from which a reagent is purchased is often identified, this still can create difficulties if the vendor sells multiple types of a reagent (e.g., the Sigma-Aldrich catalog lists forty-four BSA products). To investigate if bovine C3 was the contaminant in BSA that caused VCP binding, we directly probed the various BSA preparations with rabbit anti-bovine C3 (Cappel), as well as with goat antihuman C3b antibody (Calbiochem). We found no signal and thus do not think bovine C3 is present in BSA preparations. Given the high homology of human and bovine C3, we were not surprised to find that in control wells coated with human C3b, the anti-bovine C3 antibody recognized human C3b. It is also likely that anti-human C3b would recognize bovine C3 (Boulard, 1989). We were thus unable to easily identify the contaminating substance in the BSA preparations that VCP bound to. Since BSAs that were either globulin free or low endotoxin still bound VCP, we speculate that the process to generate a globulin free, low endotoxin BSA (i.e., BSA-2934) depletes the BSA of this substance.
In conclusion, this report serves as a reminder that not all BSA preparations are alike. While journals tend to discourage the inclusion of catalog numbers for reagents, when a specific reagent is ideally the one to use, a catalog number should be included.
Acknowledgements
We would like to thank John Atkinson (Washington University, St. Louis) for the recombinant VCP. Partial funding of this work is from NIH grants U01 AI077913 and U01 AI066333, the Middle Atlantic Regional Center of Excellence in Biodefense and Emerging Infectious Diseases (U54 AI057168), and the Philadelphia Veterans Affairs Medical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afrough B, Dwek MV, Greenwell P. Identification and elimination of false-positives in an ELISA-based system for qualitative assessment of glycoconjugate binding using a selection of plant lectins. Biotechniques. 2007;43:458, 460. doi: 10.2144/000112554. 462 passim. [DOI] [PubMed] [Google Scholar]
- Bird CR, Gearing AJ, Thorpe R. The use of Tween 20 alone as a blocking agent for immunoblotting can cause artefactual results. J Immunol Methods. 1988;106:175–179. doi: 10.1016/0022-1759(88)90194-9. [DOI] [PubMed] [Google Scholar]
- Boulard C. Degradation of bovine C3 by serine proteases from parasites Hypoderma lineatum (Diptera, Oestridae) Vet Immunol Immunopathol. 1989;20:387–398. doi: 10.1016/0165-2427(89)90083-4. [DOI] [PubMed] [Google Scholar]
- Chart H, Evans J, Chalmers RM, Salmon RL. Escherichia coli O157 serology: false-positive ELISA results caused by human antibodies binding to bovine serum albumin. Lett Appl Microbiol. 1998;27:76–78. doi: 10.1046/j.1472-765x.1998.00396.x. [DOI] [PubMed] [Google Scholar]
- Gibbs J. ELISA Tehcnical Bulletin - No 3. Corning Inc., Life Sciences; 2001. Effective Blocking Procedures. [Google Scholar]
- Hornbeck P, Winston SE, Fuller SA. Enzyme-linked immunosorbent assays (ELISA) In: Ausubel FM, editor. Curr Protoc Mol Biol. New York: J. Wiley; 2001. pp. 11.2.1–11.2.22. [DOI] [PubMed] [Google Scholar]
- Huber D, Rudolf J, Ansari P, Galler B, Fuhrer M, Hasenhindl C, Baumgartner S. Effectiveness of natural and synthetic blocking reagents and their application for detecting food allergens in enzyme-linked immunosorbent assays. Anal Bioanal Chem. 2009;394:539–548. doi: 10.1007/s00216-009-2698-8. [DOI] [PubMed] [Google Scholar]
- Jorgensen CS, Hansen KB, Jacobsen S, Halberg P, Ullman S, Hansen D, Mikkelsen TL, Weile B, Madsen MH, Wiik A, Houen G. Absence of high-affinity calreticulin autoantibodies in patients with systemic rheumatic diseases and coeliac disease. Scand J Clin Lab Invest. 2005;65:403–412. doi: 10.1080/00365510510013857. [DOI] [PubMed] [Google Scholar]
- Konishi E, Kitai Y, Nishimura K, Harada S. Antibodies to bovine serum albumin in human sera: problems and solutions with casein-based ELISA in the detection of natural Japanese encephalitis virus infections. Jpn J Infect Dis. 2010;63:296–298. [PubMed] [Google Scholar]
- Liszewski MK, Atkinson JP. Membrane cofactor protein (MCP; CD46). Isoforms differ in protection against the classical pathway of complement. J Immunol. 1996;156:4415–4421. [PubMed] [Google Scholar]
- Liszewski MK, Leung MK, Hauhart R, Fang CJ, Bertram P, Atkinson JP. Smallpox inhibitor of complement enzymes (SPICE): dissecting functional sites and abrogating activity. J Immunol. 2009;183:3150–3159. doi: 10.4049/jimmunol.0901366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauracher CA, Mitchell LA, Tingle AJ. Reduction of rubella ELISA background using heat denatured sample buffer. J Immunol Methods. 1991;145:251–254. doi: 10.1016/0022-1759(91)90334-c. [DOI] [PubMed] [Google Scholar]