Abstract
Objectives
In a well-defined sample, we sought to determine what clinical variables, some of potential nosological relevance, influence subsequent course following prospectively observed initial episodes of hypomania or mania (H/M).
Methods
We identified 108 individuals in the National Institute of Mental Health Collaborative Depression Study diagnosed with unipolar major depression at intake who subsequently developed H/M. We assessed time to repeat H/M based on whether one had been started on an antidepressant or electroconvulsive therapy within eight weeks of developing H/M, had longer episodes, or had a family history of bipolar disorder.
Results
Modeling age of onset, treatment-associated H/M, family history of bipolar disorder, duration of index H/M episode, and psychosis in Cox regression analysis, family history of bipolar disorder (n = 21) was strongly associated with repeat episodes of H/M [hazard ratio (HR) = 2.01, 95% confidence interval (CI): 1.06–3.83, p = 0.03]. Those with treatment-associated episodes (n = 12) were less likely to experience subsequent episodes of H/M, though this was not significant in the multivariate model (HR = 0.25, 95% CI: 0.06–1.05, p = 0.06). These individuals also had a later age of onset for affective illness and were more likely to be depressed. Duration of illness with a temporal resolution of one week, psychosis, and age of onset were not associated with time to repeat H/M episode.
Conclusions
Family history of bipolar disorder influences course of illness even after an initial H/M episode. In this select sample, treatment-associated H/M did not appear to convey the same risk for a course of illness characterized by recurrent H/M episodes.
Keywords: bipolar disorder, depressive disorder, antidepressants, prospective studies
The onset of a new hypomanic or manic (H/M) episode warrants a change in diagnosis from unipolar major depression to bipolar disorder. The duration of episodes and concurrent treatments are considered to have nosological relevance though there has been limited empirical study to inform contemporary diagnostic nomenclature. Are such episodes equivalent to those that develop without antidepressant provocation in indicating a lifetime bipolar diathesis? Does duration of the H/M episode matter? Are there other variables that predict course of illness? Resolution of these questions is hampered by a paucity of prospective follow-up data that describe those who develop their first H/M episode in the context of antidepressant treatment. Most definitions of antidepressant-induced H/M focus on the time between antidepressant initiation and the development of H/M. Eight weeks (1–3) has been the most common, intermediate to other definitions of four weeks (4) or 12 weeks (5–7). Some have expanded the definition to any H/M that occurs during antidepressant treatment (8–10) and others have proposed an eight-week period because it is “the customary period over which antidepressant response is engaged (1).” In fact, most reported cases have had onsets within this time frame (7, 11, 12).
Few prospective studies have linked retrospectively ascertained antidepressant-associated H/M to the subsequent development of bipolar disorder. Strober and Carlson (13) identified two cases of pharmacologically-induced hypomania, both of which developed bipolar disorder. In another report, ‘pharmacological hypomania’ was seen in only one unipolar patient who did not have spontaneous mania over three and a half years of follow-up (14). A later analysis, however, found that all of 18 individuals with pharmacological hypomania developed bipolar disorder (15). This, and other studies, compared individuals with antidepressant-induced H/M to those with spontaneous H/M episodes by family histories of H/M and none found clear differences (15–17). Akiskal et al. (17) did find those with antidepressant-associated hypomania to have a later age of onset, a greater chronicity of depressive symptoms, and higher propensity for psychosis, though no differences emerged in the specificity or quantity of hypomanic symptoms.
In this report, we used data from a long-term follow-up study to assess the course of illness and family history of patients with treatment-associated H/M in a sample with an initial diagnosis of unipolar major depression. We hypothesized that individuals with treatment-associated H/M would be at similar risk of subsequent episodes of H/M as those with unipolar major depression who developed their first, prospectively observed H/M in the absence of recent antidepressant or electroconvulsive therapy (ECT) initiation. We further hypothesized that a longer duration of illness and family history of bipolar disorder would be associated with recurrence of H/M. This is the first report to compare such groups by time to subsequent episodes of H/M following a prospectively observed episode of H/M, in a sample with an initial diagnosis of unipolar major depression confirmed by structured interview.
Methods
The National Institute of Mental Health Collaborative Depression Study (CDS) consented Caucasian (genetic hypotheses were tested), English-speaking participants from five academic centers (Massachusetts General Hospital and Harvard University, Rush Presbyterian – St. Luke’s Medical Center in Chicago, the University of Iowa, New York State Psychiatric Institute and Columbia University, and Washington University School of Medicine in St. Louis) with IRB approval at each center. Participants received treatment in the community and were not assigned to treatments through involvement in this prospective observational study.
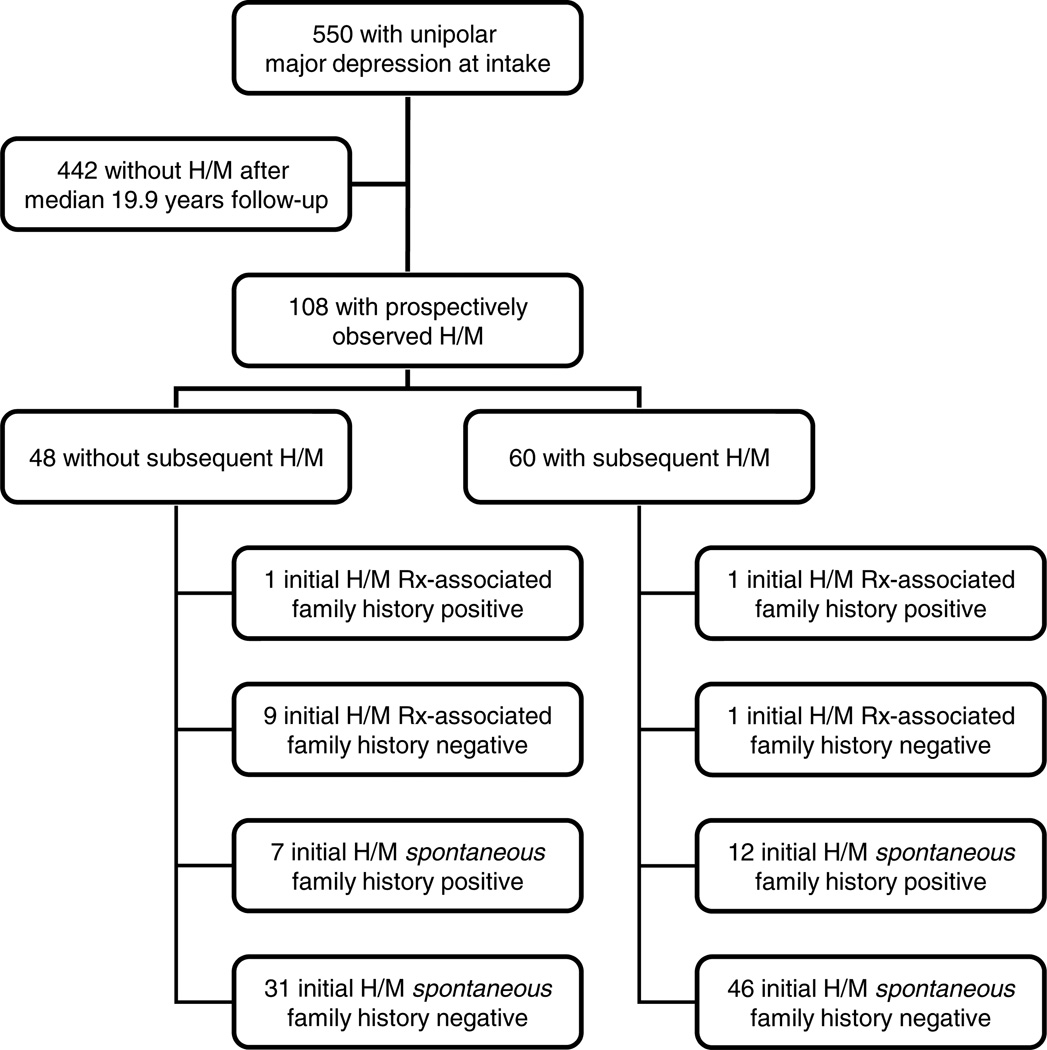
At intake, diagnoses were based upon Research Diagnostic Criteria (RDC) using data from medical records and the Schedule for Affective Disorders and Schizophrenia (SADS) (18, 19). A diagnosis of unipolar MDD was based on an intake RDC diagnosis of MDD or schizoaffective disorder, depressed, mainly affective. DSM-IV-TR criteria for MDD are very similar to these RDC. Age of onset, education, and co-occurring conditions were identified at study intake. Restricting the sample to those with at least one year of follow-up to ensure a minimum of two follow-up assessments yielded 550 participants with MDD. In a prior analysis, we determined that 108 of these 550 participants subsequently developed hypomania or mania over a mean (median; SD) follow-up of 17.5 (19.9; 9.9) and up to 31 years (20). These 108 participants served as the sample for this analysis as shown in Figure 1.
Fig. 1.
Flow-chart for cohort. Of the initial 550 National Institute of Mental Health Collaborative Depression Study (CDS) participants with unipolar major depression on intake and at least one year of follow-up, a total of 108 experienced a prospectively observed hypomania or mania (H/M) as previously reported after a median follow-up of 19.9 years (20). This manuscript reports on the subsequent follow-up of these 108 individuals after an additional follow-up for a median of 13.3 (mean of 14.4) years, during which 60 experienced a subsequent hypomania. The figure details the number of these whose initial prospectively observed hypomanic or manic (H/M) episode was associated with treatment (Rx-associated) or occurred in the setting of a positive family history of bipolar disorder.
Family history of mood disorders was determined as follows (20). For 75 individuals from this sample, family history data were derived from consensus diagnoses from 501 first-degree biological relatives, who were interviewed in person or by telephone as part of a family study. Raters blind to proband diagnosis used the lifetime version of the SADS (SADS-L) to interview all adult, first-degree relatives willing to provide information about themselves and all of their first-degree relatives using the Family History Research Diagnostic Criteria. Investigators formulated a consensus diagnosis from the SADS-L and all available sources of information to derive RDC diagnoses. For the remaining 32 individuals, consensus diagnoses for 208 first-degree relatives were based on the Family History Research Diagnostic Criteria, wherein interviews with one or more family members are used to estimate diagnoses on other relatives.
Family history was imputed as negative for one participant without family history information. Follow-up assessments were completed using the Longitudinal Interval Follow-up Evaluation (LIFE) (21) or a revised version, which was administered semiannually in the first five years and annually thereafter. The Psychiatric Status Rating (PSR) of the LIFE tracked the severity of each RDC syndrome weekly to identify the week of onset for any H/M that developed during follow-up. The presence of psychotic symptoms was tracked on participants with relevant diagnoses in the first two years and on all participants thereafter. The presence of psychotic or depressive symptoms prior to H/M onset was based on PSR data and when missing in the first two years for psychosis imputed from intake data. The onset of H/M episodes (20) was based on any week with a score of ≥ 3/6 on the PSRs for mania or schizoaffective mania or 3/3 (definite criteria) on the PSR for hypomania. The threshold for clinically significant depressive symptoms (22, 23) was similarly based as any week with a score of ≥ 3/6 on the PSRs for major depression or schizoaffective depression or 3/3 on the PSRs for minor depressive or intermittent depressive syndromes. If a participant had been started on an antidepressant, regardless of dose, after at least a week without receiving any antidepressant, or began ECT within 8 weeks of a new onset of H/M, the episode was operationally defined as a treatment-associated H/M. For clarity, any other episodes of H/M are referred to as spontaneous H/M. For the purposes of this analysis, the first prospectively observed H/M was the index episode. The first week of this episode served as the starting point for survival analysis with the individual participant as the unit of analysis. The temporal resolution of the PSR data is one week and duration of episode could be determined in only one week increments. For the primary analyses, duration of index episode is treated as a continuous variable (weeks). Follow-up analyses explore this variable at thresholds of ≥ 2 weeks and ≥ 4 weeks. Our analyses were interested in whether episode duration, association with new treatment, or family history influenced time to subsequent H/M, requiring eight weeks without H/M symptoms prior to subsequent episode.
Data analyses
Those with and without a family history of bipolar disorder and with and without treatment-associated H/M were compared using chi-square for categorical measures and the non-parametric Wilcoxon Rank Sum was used to contrast continuous measures, where the assumption of normality was violated. Time to subsequent H/M was examined using Kaplan-Meier product limit estimate with survival time reflecting the primary outcome of time from first episode of H/M to onset of a second episode of H/M. Participants were censored upon loss to follow-up, death, drop-out, or end of the study. Differences in survival were contrasted using the log-rank test. Survival analysis was also performed using Cox proportional hazards regression to include age of onset, treatment-associated mania, preceding psychotic symptoms, duration of index H/M episode, and family history of bipolar disorder in a multivariate model. For this model, we selected family history of bipolar disorder, psychosis and age of onset as covariates because they were significant predictors of H/M from a prior analysis (20). Censuring was assumed to be non-informative. Proportional hazards were assumed for all variables modeled in Cox regression. Analyses were performed using SAS 9.3 software except for Kaplan-Meier survival curves produced in SigmaPlot 12.0.
Results
The initial prospectively observed H/M for our study cohort lasted a median (mean; SD) of 4 (9.8; 26.5) weeks. From this cohort of 108 individuals with prospectively identified H/M, 60 (56%) went on to have a subsequent H/M episode. In survival analysis over a mean (SD) follow-up of 14.4 (9.0) and up to 29.6 years after index episode, participants had a cumulative probability of subsequent H/M of 71% with a median survival of 6.1 years [95% confidence interval (CI): 2.1–10.1 years]. Of the initial sample, 21 had a family history of bipolar disorder and 12 developed H/M within 8 weeks after the initiation of any antidepressant or ECT as illustrated in Figure 1. The number that developed H/M for each week after antidepressant initiation are reported in brackets as follows: week 1: [2], week 2: [1], week 3: [1], week 4: [3], week 5: [2], week 6: [2], week 7: [1], week 8: [0]. Of the antidepressant-associated episodes, 4/12 (33%) reached the threshold for mania while the remainder had only hypomania. Of the spontaneous episodes, 26/96 (27%) met criteria for mania and the remainder were hypomania.
The 21 participants with a family history of bipolar disorder and 12 individuals with episodes of treatment-associated H/M were contrasted to the remainder of the sample on a variety of sociodemographic and clinical characteristics in Table 1. Those with family history of bipolar disorder had a significantly earlier age of onset, spent significantly less time with clinically significant depressive symptoms prior to index H/M, and were also more likely to also have a family history of major depression. Individuals with treatment-associated H/M had a significantly later age of onset for mood disorder from those with spontaneous H/M. They were also more likely to have been suffering from depressive symptoms in the eight weeks prior to index H/M episode. In contrast to 58/96 (60%) of the comparison group, only 2/12 (17%) with treatment-associated H/M developed a subsequent H/M (Fisher’s exact p = 0.005). One of these twelve participants suffered mania; the other had hypomania. For one of these two cases (mania), the subsequent H/M was also antidepressant-associated. This was true for only 8/58 (14%) of the comparison group. Those with antidepressant associated H/M were no more likely than those with spontaneous mania to have an episode that lasted two or more weeks (Fisher’s Exact p = 0.69) or four or more weeks (Fisher’s Exact p = 0.21).
Table 1.
Demographic and clinical characteristics at index episode
| Demographic/clinical characteristics | Treatment- associated H/M (n = 12) |
Family history of bipolar disorder (n = 21) |
Entire sample (n = 108) |
|---|---|---|---|
| Age of onset, years, mean (SD)a | 35.4 (14.7)c | 21.7 (6.9) | 25.1 (10.8) |
| Follow-up until index H/M episode, years, mean (SD) | 4.8 (5.5) | 6.8 (8.7) | 5.8 (6.8) |
| Percentage of follow-up weeks with clinically significant depressive symptoms prior to index H/M episode, mean (SD) | 65.2 (26.1) | 40.7 (34.6)e | 54.0 (33.6) |
| Age at index H/M episode, years, mean (SD) | 47.6 (16.6) | 35.8 (13.2) | 40.2 (14.2) |
| Duration of index H/M episode, weeks, mean (SD) | 8.5 (7.8) | 5.9 (7.2) | 9.8 (26.5) |
| Follow-up after index H/M episode, years, mean (SD) | 12.7 (9.0) | 13.2 (8.4) | 14.4 (9.0) |
| Percent of follow-up weeks after index episode [mean (SD)] taking: | |||
| Antidepressants | 54.9 (35.5) | 29.5 (40.9) | 44.5 (40.7) |
| Antipsychotics | 17.3 (26.7) | 19.1 (34.7) | 23.1 (34.2) |
| Mood stabilizers | 43.6 (40.1) | 34.6 (40.6) | 30.9 (39.1) |
| Female, n (%) | 6 (50) | 14 (67) | 74 (69) |
| College graduate or greater education (intake), n (%) | 5 (42) | 8 (38) | 33 (31) |
| Family history of major depression, n (%) | 7 (58) | 17 (81)f | 53 (49) |
| Family history of bipolar disorder, n (%) | 2 (18) | – | 21 (19) |
| Bipolar I disorder | 1 (8) | – | 5 (5) |
| Bipolar II disorder | 1 (8) | – | 19 (18) |
| Treatment-associated H/M, n (%) | – | 2 (10) | 12 (11) |
| Co-occurring psychiatric diagnoses (study intake), n (%) | |||
| Alcohol abuse | 2 (17) | 9 (43) | 32 (30) |
| Drug abuse | 1 (8) | 3 (14) | 11 (10) |
| Generalized anxiety disorder | 2 (17) | 2 (10) | 9 (8) |
| Obsessive-compulsive disorder | 0 (0) | 1 (5) | 2 (2) |
| Panic disorder | 1 (8) | 3 (14) | 6 (6) |
| Phobic disorder | 1 (8) | 1 (5) | 7 (6) |
| Psychosis within 8 weeks of H/M onsetb | 0 (0) | 1 (5) | 17 (16) |
| Depressed within 8 weeks of H/M onset | 12 (100)d | 10 (48) | 74 (69) |
| H/M for 2 weeks or more | 11 (92) | 15 (71) | 89 (82) |
| On mood stabilizer during or 4 weeks following H/M | 4 (33) | 7 (33) | 36 (33) |
| On antipsychotic during or 4 weeks following H/M | 3 (25) | 5 (24) | 34 (31) |
SD = standard deviation; H/M = hypomania or mania.
Retrospectively assessed at intake.
Psychosis at intake imputed if not assessed for index episodes (H/M onset) in first two years of follow-up.
z = 3.11, p = 0.002.
Fisher's Exact p = 0.02.
z = 1.94, p = 0.052.
Fisher's Exact p = 0.001.
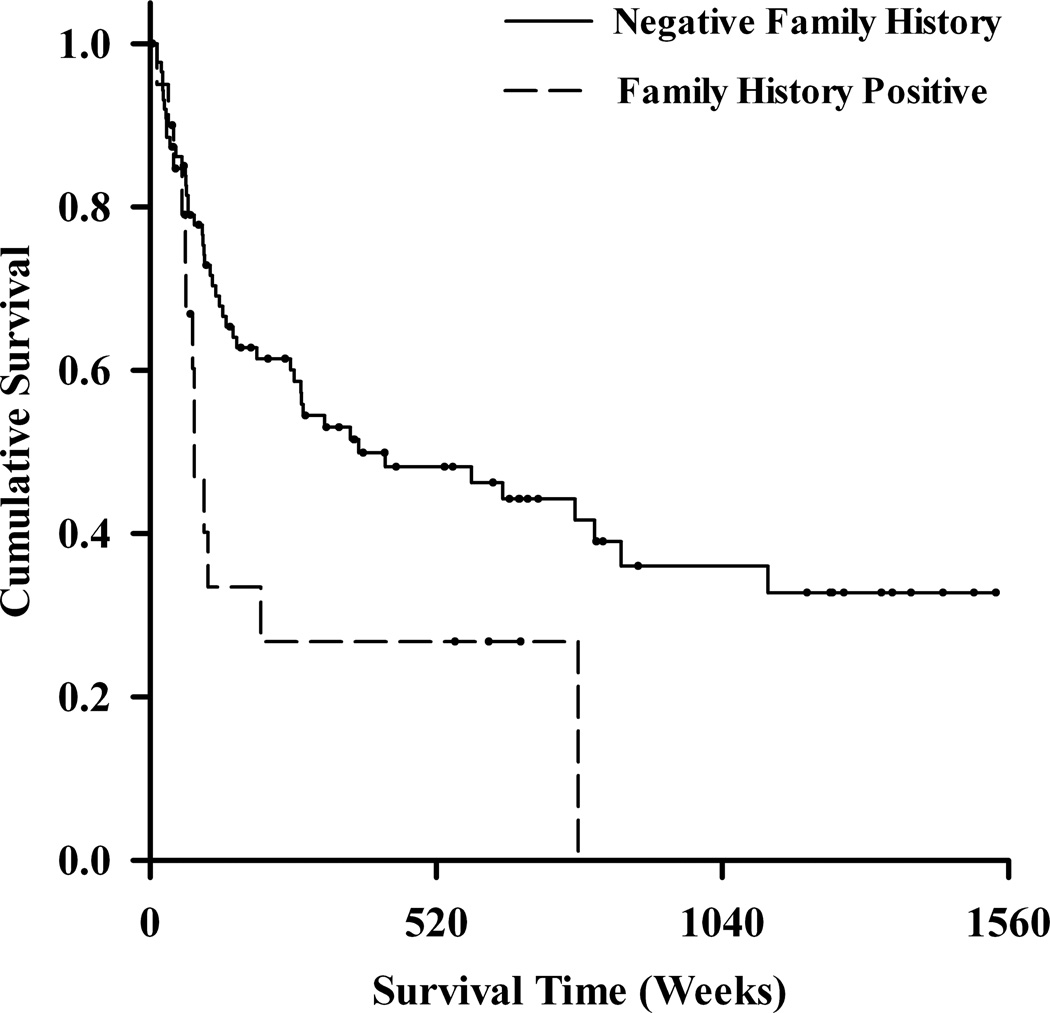
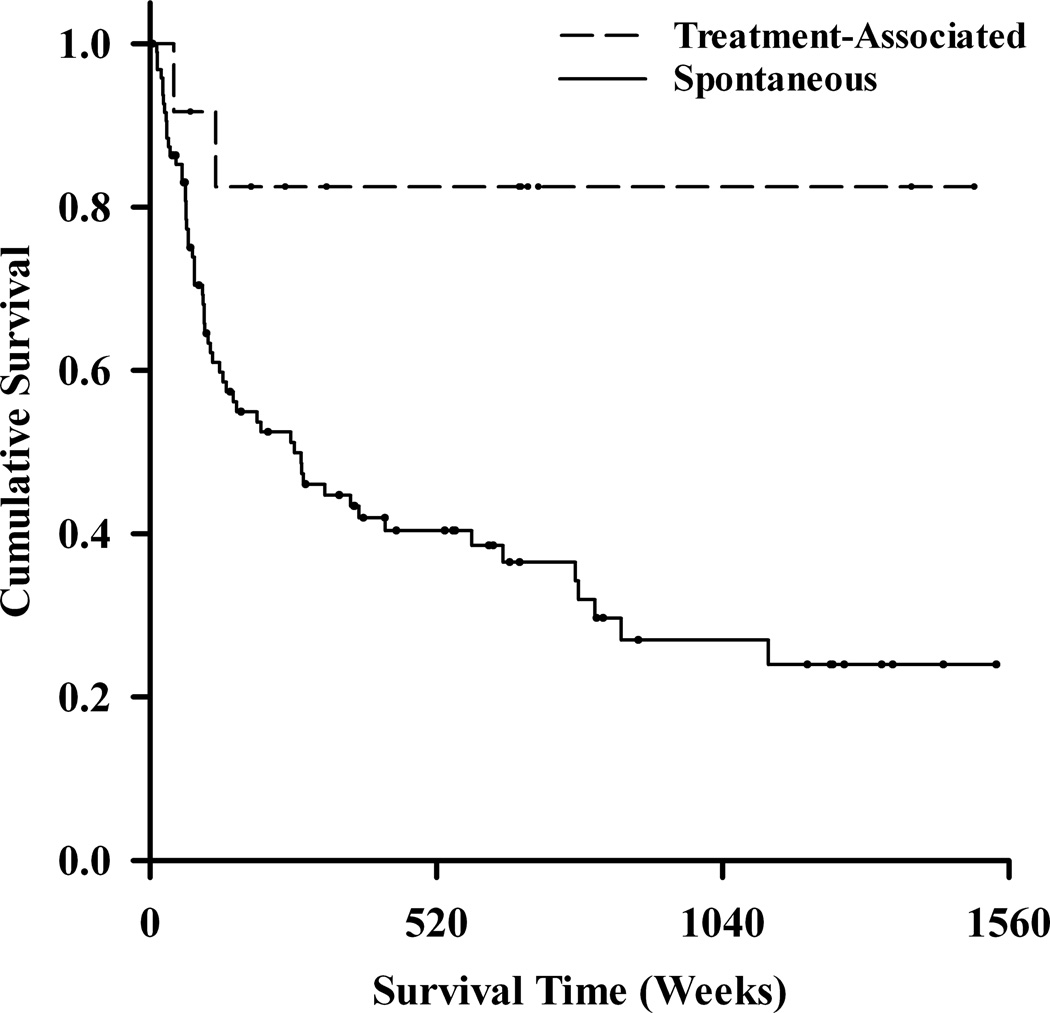
Kaplan–Meier survival curves illustrated the time to the development of any H/M following the initial prospectively observed H/M (Fig. 2). As illustrated in this figure (Fig. 2A), the cumulative probability of developing a repeat H/M was 17.5% for those with treatment-associated H/M and approximately 76% for those with spontaneous H/M. This survival difference was statistically significant using the log-rank test (χ2 = 5.9, df = 1, p = 0.01). In Cox regression, this difference was similarly significant in a univariate model [hazard ratio (HR) = 0.21, 95% CI: 0.05–0.84, p = 0.03]. A family history of bipolar disorder was associated with time to subsequent mania (log-rank χ2 = 5.3, df = 1, p = 0.02) as illustrated in Figure 2B.
Fig. 2.
Kaplan–Meier survival curves for time to second hypomania or mania. On survival analysis, those with an initial treatment-associated mania (n = 12) were less likely than those with spontaneous mania (n = 96) to develop a second episode of mania or hypomania (A). Participants with a family history of bipolar disorder (n = 21) were more likely than those without such a family history (n = 87) to experience subsequent episode of mania or hypomania (B).
In a multivariate Cox regression model (Table 2), treatment-associated H/M no longer met the threshold for significance (HR = 0.25, 95% CI: 0.06–1.05, p = 0.057) though any family history of bipolar disorder (HR = 2.01, 95% CI: 1.06–3.83, p = 0.03) remained significantly associated with subsequent H/M. Age of onset, duration of the index H/M episode, and the presence of psychotic symptoms prior to index H/M episode were not associated with repeat H/M episode in the multivariate model. Duration of the index H/M episode did not predict a repeat H/M episode when alternatively modeled categorically as ≥ 2 weeks (HR = 1.07, 95% CI: 0.53–2.14, p = 0.85) or ≥ 4 weeks (HR = 1.15, 95% CI: 0.68–1.94, p = 0.60) in multivariate models.
Table 2.
Cox proportional hazards ratio (HR) estimates for multivariate model of predictors of time to onset of second hypomania or mania (H/M)
| HR | 95% CI HR | p-value | |
|---|---|---|---|
| Dependent variablea | |||
| Age of onset (years) | 0.99 | 0.96–1.02 | 0.36 |
| Family history of bipolar disorder | 2.01 | 1.06–3.83 | 0.03 |
| Antidepressant-associated mania | 0.25 | 0.06–1.05 | 0.06 |
| Psychosis | 1.19 | 0.61–2.34 | 0.61 |
| Duration of initial H/M episode (weeks) | 0.99 | 0.98–1.01 | 0.42 |
This table illustrates the relationship between selected variables and second H/M following an initial prospectively observed H/M from an original sample with major depression. CI = confidence interval.
Time to either hypomania or mania
Discussion
In this sample of participants with prior major depressive disorder and prospectively identified first H/M episodes, there was a greater risk of subsequent H/M in those with a family history of bipolar disorder and a lower risk of subsequent H/M when the H/M episode occurred within eight weeks of initiating starting an antidepressant or ECT. This latter, unexpected finding stands in some contrast to a scant evidence base on this topic. Some studies did not include a structured baseline assessment (14, 15), and the under-recognition of earlier H/M episodes may have resulted in samples with higher likelihoods of future episodes. Retrospective assessment of treatment-associated episodes of H/M may have been more vulnerable to misclassification (13–15). In agreement with earlier findings, treatment-associated and spontaneous H/M groups did not appear to differ with regard to severity of illness or family history of bipolar disorder and those with treatment-associated H/M had a later age of illness onset (17). Not surprisingly, those with treatment-associated episodes of H/M were more likely to have a depressive syndrome during the time antidepressants were initiated.
Across the entire sample, duration of index H/M episode did not appear useful in determining risk of subsequent H/M and therefore appears to be of limited utility in identifying the prognostic relevance of a treatment-associated H/M episode. Our analysis also found that family history of bipolar disorder, an established predictor of new onset H/M in major depression, further predicts a course of illness characterized by recurrent episodes of H/M. This is consistent with a literature demonstrating individuals with bipolar disorder and a family history of bipolar disorder have more episodes than those without such a family history, as was previously reported from a CDS sample with bipolar disorder at intake (24).
There are a number of important limitations of this prospective cohort study. Only 12 cases with an initial treatment-associated H/M were prospectively identified. This limits our ability to detect significant differences between groups. Although our findings related to course of illness were statistically significant on univariate survival analysis, the potential for spurious findings in a small sample remains a concern. Our definition for antidepressant-associated H/M represented an arbitrary consensus in its focus on initiation rather than on dose increase of antidepressants and in its use of an eight-week time frame. Our groups may have also differed from earlier ones in important variables that could influence course of illness. However, it is worth noting that this analysis was not undertaken to directly address the question of whether antidepressants may cause H/M, but rather to prospectively assess the prognostic significance of a H/M episode that occurs following initiation of an antidepressant. It is not possible to ascertain whether any differences between these two groups, for which our sample is underpowered to detect, represent important confounds or, instead, reflect true differences between a hypothetical population vulnerable to antidepressant-associated H/M and those prone to spontaneous H/M. Given the older age and lack of psychosis among those with antidepressant-associated H/M, our findings should not be generalized to those with a younger age of onset of mania or the presence of psychosis, both of which have been established predictors of mania. Of note, although Akiskal et al. (17) found more psychosis in those with treatment-associated mania a more recent study similarly found an absence of psychosis in this group (25). The relationship between psychosis and antidepressant-associated mania thus remains unclear. The temporal resolution of our PSR data further limited our ability to gauge the impact of episode duration when less than one week. Nonetheless, our data suggest that duration of H/M in weeks does not have any predictive validity, nor do specific thresholds of ≥ 2 or ≥ 4 weeks.
The prospective identification of H/M episodes in a well-characterized sample with unipolar major depression and the length of follow-up are notable strengths of the study as is the availability of rigorously obtained family history data. We maximally utilized our prospective data by modeling time-to-event in survival analysis and were able to include covariates in Cox regression. In so doing, we found those with antidepressant H/M were at lower risk of subsequent episodes of H/M and that a family history of bipolar disorder predicted recurrence. Duration of index H/M episode also appeared unrelated to time to repeat H/M episode. The study’s inclusion of a comprehensive structured interview at baseline decreased the risk of individuals with bipolar disorder being misclassified as unipolar major depression at intake. We also identified treatment-associated H/M prospectively instead of retrospectively. While these methodological differences could partially account for the lower risk of subsequent H/M episodes in those with treatment-associated H/M compared to prior studies, the differences between our results and those of prior work demand prudence in interpretation of our findings, particularly given the small samples of treatment-induced H/M studied to date.
The nosological position of antidepressant-associated H/M thus remains vexing. The proposed revisions for DSM-V specify that “a full manic [or hypomanic] episode emerging during antidepressant treatment (medication, ECT, etc.) and persisting beyond the physiological effect of that treatment is sufficient evidence for a manic [or hypomanic] episode diagnosis (26).” Our findings do not support the predictive validity of treatment-associated mania and further fail to support the proposed relevance of a H/M episode ‘persisting beyond the physiological effect of that treatment.’ Nonetheless, our findings lend some support to those who distinguish treatment-associated hypomania as a distinct disorder on the bipolar spectrum (17). These and related unresolved nosological issues underscore the importance of continued support for prospective study of mood disorders.
Acknowledgements
This study was funded by NIMH grants 5R01MH025416-33 (WHC), 5R01MH023864-35 (JE), 5R01MH025478-33 (MBK), 5R01MH025430-33 (J. Rice), and 5R01MH029957-30 (W.A. Scheftner).
JGF is supported by the National Institutes of Health (1K23MH083695-01A210) and the Institute for Clinical and Translational Science at the University of Iowa (3 UL1 RR024979-03S4).
JE has received research support from the U.S. National Institute of Mental Health, Cyberonics, and Pfizer; and has served as a consultant or advisory board member to AstraZeneca, Bayer Shering, Berlex, Cyberonics, Eli Lilly & Co., Forest Laboratories, GlaxoSmithKline, Otsuka, Pfizer, and Wyeth-Ayerst. DAS serves as Deputy Editor to UpToDate.com. MBK has received grant/research support from Pfizer and Wyeth; has served as a consultant or received honoraria from CENEREX, Forest Laboratories, Medtronic, Organon, Pfizer, Sierra Neuropharmaceuticals, and Wyeth; and has served on advisory boards for CENEREX, Forest Laboratories, and Organon.
Appendix
Conducted with current participation of the following investigators: M.B. Keller, M.D. (Chairperson, Providence); W. Coryell, M.D. (Co-Chairperson, Iowa City); D.A. Solomon, M.D. (Providence); W.A. Scheftner, M.D. (Chicago); J. Endicott, Ph.D., A.C. Leon, Ph.D.,* J. Loth, M.S.W. (New York); J. Rice, Ph.D., (St. Louis). Other current contributors include: H.S. Akiskal, M.D., J. Fawcett, M.D., L.L. Judd, M.D., P.W. Lavori, Ph.D., J.D. Maser, Ph.D., T.I. Mueller, M.D.
The data for this manuscript came from the National Institute of Mental Health (NIMH) Collaborative Program on the Psychobiology of Depression-Clinical Studies (Katz and Klerman, 1979). The Collaborative Program was initiated in 1975 to investigate nosologic, genetic, family, prognostic and psychosocial issues of mood disorders, and is an ongoing, long-term multidisciplinary investigation of the course of mood and related affective disorders. The original Principal and Co-principal investigators were from five academic centers and included Gerald Klerman, M.D. * (Co-Chairperson); Martin Keller, M.D., Robert Shapiro, M.D.* (Massachusetts General Hospital, Harvard Medical School); Eli Robins, M.D.,* Paula Clayton, M.D., Theodore Reich, M.D.,* Amos Wellner, M.D.* (Washington University Medical School); Jean Endicott, Ph.D., Robert Spitzer, M.D. (Columbia University); Nancy Andreasen, M.D., Ph.D., William Coryell, M.D., George Winokur, M.D.* (University of Iowa); Jan Fawcett, M.D., William Scheftner, M.D. (Rush-Presbyterian-St. Luke’s Medical Center). The NIMH Clinical Research Branch was an active collaborator in the origin and development of the Collaborative Program with Martin M. Katz, Ph.D., Branch Chief as the Co-Chairperson and Robert Hirschfeld, M.D. as the Program Coordinator. Other past contributors include: J. Croughan, M.D., M.T. Shea, Ph.D., R. Gibbons, Ph.D., M.A. Young, Ph.D., D.C. Clark, Ph.D.
*Deceased
Footnotes
Disclosures
JGF and WHC have no potential conflicts of interest to report.
References
- 1.Goldberg JF, Truman CJ. Antidepressant-induced mania: an overview of current controversies. Bipolar Disord. 2003;5:407–420. doi: 10.1046/j.1399-5618.2003.00067.x. [DOI] [PubMed] [Google Scholar]
- 2.Joffe RT, MacQueen GM, Marriott M, Robb J, Begin H, Young LT. Induction of mania and cycle acceleration in bipolar disorder: effect of different classes of antidepressant. Acta Psychiatr Scand. 2002;105:427–430. doi: 10.1034/j.1600-0447.2002.02360.x. [DOI] [PubMed] [Google Scholar]
- 3.Altshuler LL, Post RM, Leverich GS, Mikalauskas K, Rosoff A, Ackerman L. Antidepressant-induced mania and cycle acceleration: a controversy revisited. Am J Psychiatry. 1995;152:1130–1138. doi: 10.1176/ajp.152.8.1130. [DOI] [PubMed] [Google Scholar]
- 4.Serretti A, Artioli P, Zanardi R, et al. Genetic features of antidepressant induced mania and hypo-mania in bipolar disorder. Psychopharmacology. 2004;174:504–511. doi: 10.1007/s00213-004-1948-x. [DOI] [PubMed] [Google Scholar]
- 5.Manwani SG, Pardo TB, Albanese MJ, Zablotsky B, Goodwin FK, Ghaemi SN. Substance use disorder and other predictors of antidepressant-induced mania: a retrospective chart review. J Clin Psychiatry. 2006;67:1341–1345. doi: 10.4088/jcp.v67n0903. [DOI] [PubMed] [Google Scholar]
- 6.Baumer FM, Howe M, Gallelli K, Simeonova DI, Hallmayer J, Chang KD. A pilot study of antidepressant-induced mania in pediatric bipolar disorder: Characteristics, risk factors, and the serotonin transporter gene. Biol Psychiatry. 2006;60:1005–1012. doi: 10.1016/j.biopsych.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 7.Goldberg JF, Whiteside JE. The association between substance abuse and antidepressant-induced mania in bipolar disorder: a preliminary study. J Clin Psychiatry. 2002;63:791–795. doi: 10.4088/jcp.v63n0907. [DOI] [PubMed] [Google Scholar]
- 8.Masoliver E, Menoyo A, Perez V, et al. Serotonin transporter linked promoter (polymorphism) in the serotonin transporter gene may be associated with antidepressant-induced mania in bipolar disorder. Psychiatr Genet. 2006;16:25–29. doi: 10.1097/01.ypg.0000180684.26288.d7. [DOI] [PubMed] [Google Scholar]
- 9.Mundo E, Cattaneo E, Russo M, Altamura AC. Clinical variables related to antidepressant-induced mania in bipolar disorder. J Affect Disord. 2006;92:227–230. doi: 10.1016/j.jad.2006.01.028. [DOI] [PubMed] [Google Scholar]
- 10.Rousseva A, Henry C, van den Bulke D, et al. Antidepressant-induced mania, rapid cycling and the serotonin transporter gene polymorphism. Pharmacogenomics J. 2003;3:101–104. doi: 10.1038/sj.tpj.6500156. [DOI] [PubMed] [Google Scholar]
- 11.Howland RH. Induction of mania with serotonin reuptake inhibitors. J Clin Psychopharmacol. 1996;16:425–427. doi: 10.1097/00004714-199612000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Henry C, Sorbara F, Lacoste J, Gindre C, Leboyer M. Antidepressant-induced mania in bipolar patients: identification of risk factors. J Clin Psychiatry. 2001;62:249–255. doi: 10.4088/jcp.v62n0406. [DOI] [PubMed] [Google Scholar]
- 13.Strober M, Carlson G. Bipolar illness in adolescents with major depression: clinical, genetic, and psychopharmacologic predictors in a three- to four-year prospective follow-up investigation. Arch Gen Psychiatry. 1982;39:549–555. doi: 10.1001/archpsyc.1982.04290050029007. [DOI] [PubMed] [Google Scholar]
- 14.Akiskal HS, Rosenthal RH, Rosenthal TL, Kashgarian M, Khani MK, Puzantian VR. Differentiation of primary affective illness from situational, symptomatic, and secondary depressions. Arch Gen Psychiatry. 1979;36:635–643. doi: 10.1001/archpsyc.1979.01780060025002. [DOI] [PubMed] [Google Scholar]
- 15.Akiskal HS, Walker P, Puzantian VR, King D, Rosenthal TL, Dranon M. Bipolar outcome in the course of depressive illness, Phenomenologic, familial, and pharmacologic predictors. J Affect Disord. 1983;5:115–128. doi: 10.1016/0165-0327(83)90004-6. [DOI] [PubMed] [Google Scholar]
- 16.Wada K, Sasaki T, Jitsuiki H, Yoshimura Y, Erabi H, Hada Y, et al. Manic/hypomanic switch during acute antidepressant treatment for unipolar depression. J Clin Psychopharmacol. 2006;26:512–515. doi: 10.1097/01.jcp.0000237950.65517.be. [DOI] [PubMed] [Google Scholar]
- 17.Akiskal HS, Hantouche EG, Allilaire JF, et al. Validating antidepressant-associated hypomania (bipolar III): a systematic comparison with spontaneous hypomania (bipolar II) J Affect Disord. 2003;73:65–74. doi: 10.1016/s0165-0327(02)00325-7. [DOI] [PubMed] [Google Scholar]
- 18.Endicott J, Spitzer RL. A diagnostic interview: the schedule for affective disorders and schizophrenia. Arch Gen Psychiatry. 1978;35:837–844. doi: 10.1001/archpsyc.1978.01770310043002. [DOI] [PubMed] [Google Scholar]
- 19.Endicott J, Spitzer RL. Use of the Research Diagnostic Criteria and the Schedule for Affective Disorders and Schizophrenia to study affective disorders. Am J Psychiatry. 1979;136:52–56. doi: 10.1176/ajp.136.1.52. [DOI] [PubMed] [Google Scholar]
- 20.Fiedorowicz JG, Endicott J, Leon AC, Solomon DA, Keller MB, Coryell WH. Subthreshold hypomanic symptoms in progression from unipolar major depression to bipolar disorder. Am J Psychiatry. 2011;168:40–48. doi: 10.1176/appi.ajp.2010.10030328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keller MB, Lavori PW, Friedman B, et al. The Longitudinal Interval Follow-up Evaluation. A comprehensive method for assessing outcome in prospective longitudinal studies. Arch Gen Psychiatry. 1987;44:540–548. doi: 10.1001/archpsyc.1987.01800180050009. [DOI] [PubMed] [Google Scholar]
- 22.Fiedorowicz JG, Solomon DA, Endicott J, et al. Manic/hypomanic symptom burden and cardiovascular mortality in bipolar disorder. Psychosom Med. 2009;71:598–606. doi: 10.1097/PSY.0b013e3181acee26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fiedorowicz JG, Leon AC, Keller MB, Solomon DA, Rice JP, Coryell WH. Do risk factors for suicidal behavior differ by affective disorder polarity? Psychol Med. 2009;39:763–771. doi: 10.1017/S0033291708004078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winokur G, Coryell W, Akiskal HS, Endicott J, Keller M, Mueller T. Manic-depressive (bipolar) disorder: the course in light of a prospective ten-year follow-up of 131 patients. Acta Psychiatr Scand. 1994;89:102–110. doi: 10.1111/j.1600-0447.1994.tb01495.x. [DOI] [PubMed] [Google Scholar]
- 25.Saatcioglu O, Erim R, Tomruk N, Oral T, Alpay N. Antidepressant-associated mania or hypomania: a comparison with personality and bipolarity features of bipolar I disorder. J Affect Disord. 2011;134:85–90. doi: 10.1016/j.jad.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 26.American Psychiatric Association. Proposed revision: Manic episode. http://www.dsm5.org/ProposedRevisions/Pages/proposedrevision.aspx?rid=425.