Abstract
Objective
To compare MRI-based knee cartilage T2 measurements and focal knee lesions and 36 month changes in these parameters, among knees of normal controls and knees of normal-weight, overweight, and obese subjects with risk factors for knee osteoarthritis (OA).
Methods
267 subjects aged 45–55 years from the Osteoarthritis Initiative (OAI) database were analysed in this study. 231 subjects had risk factors for knee OA, but no radiographic OA (KL-score≤1) at baseline. 36 subjects were normal controls. Subjects with OA risk factors were stratified in three groups: normal weight (n=78), overweight (n=84), and obese (n=69). All subjects underwent 3T MRI of the right knee at baseline and after 36 months. Focal knee lesions were assessed and cartilage T2 measurements (mean T2 and T2 texture analysis) were performed.
Results
The baseline prevalence and severity of meniscal and cartilage lesions were highest in obese subjects and lowest in normal controls (p<0.05). Obese subjects had the highest mean T2 values and the most heterogeneous cartilage (as assessed by T2 texture analysis), while normal controls had the lowest mean T2 values and the most homogeneous cartilage at baseline (p<0.05). Increased BMI was significantly (p<0.05) associated with greater progression of cartilage lesions and constantly elevated cartilage T2 entropy over 36 months.
Conclusion
In pre-clinical OA, increased BMI is associated with more severe cartilage degeneration as assessed by both morphological and quantitative MRI measurements.
Keywords: Osteoarthritis, BMI, MRI, WORMS, T2 relaxation time
Introduction
Nearly 27 million adults in the United States have clinically symptomatic osteoarthritis (OA), most commonly affecting the knee joint (1). Risk factors for OA include female gender, previous knee injury, repetitive knee bending activities, and overweight/obesity (2). Being overweight or obese is considered an upcoming “epidemic” and projections have suggested that 86.3% of adults in the United States will be overweight or obese by 2030 (3;4). These conditions will have grave financial implications, not only for associated life-threatening co-morbidities such as diabetes and cardiovascular disease, but also for therapeutic management of overweight/obesity associated OA (5).
Previous studies used magnetic resonance imaging (MRI) and observed that body mass index (BMI) was significantly associated with the prevalence of knee cartilage defects and bone marrow edema pattern (BMEP), and with reduced patellar cartilage volume (6–10). Furthermore cartilage defects were associated with physical disability in obese adults (11). Therefore it would be valuable to detect overweight and obese individuals in the early phase of OA, since they may benefit most from treatment or behavioural interventions prior to the occurrence of severe cartilage degeneration and clinical symptoms.
Quantitative MRI such as T2 relaxation time measurement has emerged as a potential cartilage biomarker to assess early degenerative disease (12–15). The early phase of OA is characterized by biochemical changes of the cartilage including proteoglycan loss, increased water content and deterioration of the collagen network, which can be detected by T2 measurements (16;17). Furthermore cartilage degeneration is associated with a more heterogeneous cartilage matrix, which can be analysed using grey level co-occurrence matrix (GLCM) based T2 texture analysis (18–22).
The NIH launched the Osteoarthritis Initiative (OAI), a longitudinal, observational multi-center study with 4,796 participants, to better understand the natural evolution of OA (http://www.oai.ucsf.edu/). The OAI database contains clinical data, biological samples, radiographs, and MR images including a T2 mapping sequence (23). The study population consists of subjects with symptomatic knee OA at baseline (progression cohort), those with no symptomatic knee OA, but with risk factors for OA at baseline (incidence cohort), and normal controls. The OAI currently provides the largest longitudinally acquired database with T2 relaxation time measurements.
The purpose of this study was to compare MRI-based knee cartilage T2 measurements and focal knee lesions, and changes in these parameters over 36 months, among knees of normal controls without risk factors and normal-, overweight, and obese subjects with risk factors for knee OA, but without knee pain and without radiographic knee OA. We hypothesized (i) that both focal knee lesions and cartilage T2 measurements would correlate with BMI and (ii) that cartilage T2 measurements would be more sensitive than grading of focal knee lesions (by using a modified whole organ MRI score (WORMS)) cross-sectionally and longitudinally to observe differences in osteoarthritic changes between normal controls versus normal-, overweight, and obese subjects with risk factors for knee OA.
Materials and Methods
Subjects
The study was HIPAA compliant. All subjects included in this study provided informed consent. The study protocol, amendments and informed consent documentation were reviewed and approved by the local institutional review boards.
Data used in the preparation of this article were obtained from the Osteoarthritis Initiative (OAI) database, which is available for public access at http://www.oai.ucsf.edu/. Specific OAI datasets used were baseline clinical dataset 0.2.2, baseline imaging datasets 0.E.1 and 0.C.2, 36 month follow-up clinical dataset 5.2.1, and 36 month follow-up imaging datasets 5.E.1 and 5.C.1.
We studied the right knee of 267 subjects from the OAI incidence and normal control cohorts. Subjects in the OAI normal control cohort (n=122) had no radiographic evidence of OA (defined as a definite tibiofemoral osteophyte) in either knee at baseline and had no OA risk factors at baseline. Subjects in the OAI incidence cohort (n=3284) did not have symptomatic knee OA (defined as frequent symptoms and radiographic OA in the same knee) in either knee at baseline, but had at least one of the following OA risk factors at baseline: overweight or obesity, knee symptoms (“pain, aching, or stiffness in or around the knee” in the past 12 months), history of knee injury, history of knee surgery, family history of total knee replacement, or Heberden nodes.
Specific inclusion criteria for this study at baseline were: 45–55 years of age, Western Ontario and McMaster University (WOMAC) pain score of zero in both knees and Kellgren-Lawrence (KL)-score ≤1 (based on an additional reading done for the present study) in the right knee. In addition, baseline and 36 month follow-up right knee MR images had to be available and useable. These specific inclusion criteria were applied to focus on younger and relatively asymptomatic subjects. Based on these criteria, 36 normal controls (11 males, 25 females) and 231 subjects with OA risk factors (128 males, 103 females) were eligible and included in the study. The subjects with OA risk factors were stratified by BMI category: 78 subjects (33 males, 45 females), who had normal weight (baseline BMI <25.0kg/m2), 84 subjects (58 males, 26 females), who were overweight (baseline BMI 25.0–29.9kg/m2), and 69 subjects (37 males, 32 females), who were obese (baseline BMI ≥30.0kg/m2). All included controls had normal weight (baseline BMI <25.0kg/m2). The individual subject groups were defined as group A (normal controls), group B (subjects with OA risk factors and normal weight), group C (subjects with OA risk factors and overweight), and group D (subjects with OA risk factors and obesity).
Imaging
Bilateral standing posterior-anterior fixed flexion knee radiographs were acquired at baseline and 36 month follow-up. Knees were positioned in a plexiglas frame (SynaFlexer, CCBR-Synarc, San Francisco, CA, USA) with 20°–30° flexion and 10° internal rotation of the feet. Right knee radiographs were graded by two radiologists (L.N. with 4 years of experience and W.V. with 7 years of experience) in consensus by using the Kellgren-Lawrence (KL) scoring system (24).
All subjects underwent 3T MRI (Trio, Siemens, Erlangen, Germany) of the right knee at baseline and 36 month follow-up. MR images were obtained as described in the OAI MRI protocol (23).
Grading of Focal Knee Lesions
Baseline and 36 month follow-up MR images of the right knee were transferred to picture archiving communication system (PACS) workstations (Agfa, Ridgefield Park, NJ, USA). Presence and grade of meniscal and cartilage lesions as well as bone marrow edema pattern (BMEP) were assessed using a modified whole organ MRI score (WORMS) as previously described (12;22;25–30). Meniscal lesions were graded separately in 6 regions (medial/lateral and anterior/body/posterior) using a 5-point scale. Cartilage lesions and BMEP were not assessed by using the original 15 regions, but six condensed regions (patella, trochlea, medial/lateral femur and medial/lateral tibia). Cartilage lesions were graded using an 8-point scale, BMEP using a 4-point scale. Three radiologists (L.N. with 4 years, W.V. with 7 years and T.M.L. with 22 years of experience) analyzed 40 MRI studies in consensus to calibrate thresholds for grading abnormalities. The remaining 227 MRI studies were read by two radiologists (L.N. and W.V.) independently. In case of disagreement, consensus reading was performed with the third, most experienced radiologist (T.M.L.). The radiologists were blinded to patient information while performing the WORMS grading. MR images were read with baseline and follow-up paired and in known chronological order.
A WORMS maximum score (WORMS Max) was assigned to each knee by the greatest WORMS score in any compartment. WORMS Max was used to express the severity of focal knee lesions. WORMS Max >0 in any joint structure was defined as presence of a lesion. A meniscal WORMS Max >1 indicated a non-displaced tear or worse, while a cartilage WORMS Max >1 identified subjects with at least one partial thickness defect. Cartilage WORMS Max >1 was also used to exclude lesions characterized only by signal abnormalities, i.e. grade 1 lesions.
Incident focal knee lesions over 36 months were defined on knee level basis as new lesions detected in the 36 month follow-up MR studies that occurred in a knee with a WORMS score of zero at baseline (i.e. baseline WORMS Max =0 and 36 month follow-up WORMS Max >0). Possible remission of BMEP was defined as baseline WORMS Max >0 and 36 month follow-up WORMS Max =0.
To determine progression of focal knee lesions over 36 months, WORMS summation scores (WORMS Sum) were calculated for each joint structure on knee level basis by summing up the WORMS scores of all evaluated compartments. Progression of focal knee lesions over 36 months was defined as baseline WORMS Sum >0 and ΔWORMS Sum >0 (36 month follow-up WORMS Sum – baseline WORMS Sum). Possible regression of BMEP was defined as baseline WORMS Sum >0, 36 month follow-up WORMS Sum >0, and ΔWORMS Sum <0.
Incidence and progression of focal knee lesions over 36 months had partly low counts per group (e.g. normal controls; Table 2). Therefore we analyzed incidence and progression combined as total WORMS progression, which was defined as ΔWORMS Sum >0 (36 month follow-up WORMS Sum – baseline WORMS Sum). Subjects with ΔWORMS Sum <0 for BMEP (i.e. BMEP regression or remission) were excluded for the statistical analysis of BMEP total WORMS progression.
Table 2.
Baseline prevalence and severity of focal knee lesions as well as incidence (BMEP: incidence and remission) and progression (BMEP: progression and regression) of focal knee lesions over 36 months in right knees of (A) normal controls, (B) subjects with OA risk factors and normal weight, (C) subjects with OA risk factors and overweight, and (D) subjects with OA risk factors and obesity. Results are given as n (%) or as mean ± standard deviation.
| A | B | C | D | |
|---|---|---|---|---|
| (n=36) | (n=78) | (n=84) | (n=69) | |
| Meniscus: | ||||
| baseline prevalence of lesions | 16 (44.4%) | 39 (50.0%) | 57 (67.9%) | 50 (72.5%) |
| baseline prevalence of tears | 5 (13.9%) | 23 (29.5%) | 32 (38.1%) | 21 (30.4%) |
| baseline severity of lesions | 0.64±0.87 | 1.00±1.25 | 1.31±1.25 | 1.25±1.16 |
| incidence of lesions over 36 months | 2/20 (10.0%) | 6/39 (15.4%) | 9/27 (33.3%) | 3/19 (15.8%) |
| progression of lesions over 36 months | 1/16 (6.3%) | 11/39 (28.2%) | 18/57 (31.6%) | 14/50 (28.0%) |
| Cartilage: | ||||
| baseline prevalence of lesions | 27 (75.0%) | 55 (70.5%) | 67 (79.8%) | 63 (91.3%) |
| baseline prevalence of grade 2 or higher lesions | 15 (41.7%) | 41 (52.6%) | 47 (56.0%) | 47 (68.1%) |
| baseline severity of lesions | 1.46±1.23 | 1.87±1.64 | 1.99±1.57 | 2.34±1.46 |
| incidence of lesions over 36 months | 0/9 (0.0%) | 5/23 (21.7%) | 4/17 (23.5%) | 1/6 (16.7%) |
| progression of lesions over 36 months | 3/27 (11.1%) | 15/55 (27.3%) | 23/67 (34.3%) | 31/63 (49.2%) |
| BMEP: | ||||
| baseline prevalence of lesions | 15 (41.7%) | 25 (32.1%) | 40 (47.6%) | 41 (59.4%) |
| baseline severity of lesions | 0.75±0.94 | 0.51±0.82 | 0.88±1.01 | 1.04±1.01 |
| remission of lesions over 36 months | 1/15 (6.7%) | 1/25 (4.0%) | 3/40 (7.5%) | 4/41 (9.8%) |
| incidence of lesions over 36 months | 4/21 (19.0%) | 12/53 (22.6%) | 11/44 (25.0%) | 8/28 (28.6%) |
| regression of lesions over 36 months | 0/15 (0.0%) | 0/25 (0.0%) | 2/40 (5.0%) | 6/41 (14.6%) |
| progression of lesions over 36 months | 3/15 (20.0%) | 12/25 (48.0%) | 10/40 (25.0%) | 16/41 (39.0%) |
Cartilage T2 Measurements
The multi-slice multi-echo (MSME) spin echo sequences were transferred to a SUN workstation (Sun Microsystems, Mountain View, CA, USA) and T2 maps were calculated with custom-built software on a pixel-by-pixel basis skipping the first echo and using a noise-corrected exponential fitting as outlined previously (31). Five distinct compartments (patella, medial/lateral femur and medial/lateral tibia) were segmented with in-house software based on IDL (Interactive Data Language, Research Systems, Boulder, CO, USA) directly in the T2 maps.
In order to exclude both fluid and chemical shift artifacts from the region of interest, a technique was used that allowed adjustment of the region of interest simultaneously in the T2 map and first echo of the multiecho sequence by opening separate image panels at the same time with synchronized cursor, slice number and zoom. This segmentation procedure has been used in previous studies (12;25;26;28;30;31). The patella compartment was segmented in the T2 maps of all subjects at baseline and 36 month follow-up by one operator (H.A.). The remaining compartments were segmented by a second operator (A.A.). All segmentations were supervised by a radiologist (T.B.). Segmentation of the trochlea compartment was not performed due to flow artifacts from the popliteal artery.
Mean T2 values for each compartment were calculated after segmentation. T2 texture analysis of the segmented compartments was performed on a slice-by-slice basis using grey level co-occurrence matrix (GLCM) as outlined by Haralick et al. (32). GLCM extracts information related to the spatial distribution of pixel intensities in the T2 map. One texture parameter from the orderliness group (entropy), one from the contrast group (contrast), and one from the stats group (variance) were calculated as previously reported (18–22). A pixel offset of 1 pixel was chosen and texture parameters were calculated by averaging over the four computed directions (0° - corresponding to the anterior-posterior axis, 45°, 90° - corresponding to the superior-inferior axis, and 135°). Contrast is a measure of the differences in neighboring pixel values. High T2 contrast signifies that many pixels with different T2 values are neighboring. Entropy is a measure of disorder in an image. Higher T2 entropy signifies more uniform distribution of probabilities of T2 relaxation time co-occurrences, i.e. it is more likely to find any combination of T2 relaxation time co-occurrence. Variance is a measure of the distribution of pixels about the mean. Higher T2 contrast, T2 entropy, and T2 variance were found in subjects with OA risk factors compared to normal controls (22).
Changes in T2 measurements including texture parameters over 36 months (Δ mean T2, Δ T2 entropy, Δ T2 contrast, Δ T2 variance) were computed by subtracting baseline T2 measurements from 36 month follow-up T2 measurements.
Statistical Analysis
The statistical analyses were performed with SPSS (SPSS Inc., Chicago, IL, USA) using a two-sided 0.05 level of significance.
Cross-sectional analysis
Pearson chi-square tests and ANOVA were used to compare age, BMI, and frequencies of gender, OA risk factors, and KL-scores between the subject groups.
Multivariate linear regression models were used to compare T2 measurements between the four groups. Independent variable was group and the normal control group was selected as the reference group. Dependent variable was the respective T2 measurement (mean T2, T2 contrast, T2 entropy, and T2 variance). Covariates gender, age, and OA risk factors other than BMI (i.e. knee symptoms, history of knee injury, history of knee surgery, family history of total knee replacement, and Heberden nodes) were entered into the models to obtain adjusted effects. Similar to previous studies (12;22), T2 measurements were only analyzed in the medial femur compartment and for the average over all five compartments to avoid multiple testing. The medial femur was chosen, since it is a predominant weight bearing region and has a higher incidence of OA than the lateral side (33;34).
Logistic regression models were used to assess differences in prevalence of focal knee lesions between the four groups. Independent variable was group and the normal control group was selected as the reference group. Dependent variable was WORMS prevalence (WORMS Max >0 or >1). Covariates gender, age, and OA risk factors other than BMI were entered into the models to obtain adjusted effects. Results were expressed as odds ratio with 95% confidence interval (CI) and adjusted p-value. Differences in severity of focal knee lesion between the four groups were evaluated by using multivariate linear regression models. Independent variable was group and the normal control group was selected as the reference group. Dependent variable was WORMS severity (WORMS Max). Covariates gender, age, and OA risk factors other than BMI were entered into the models to obtain adjusted effects.
Longitudinal analysis
Paired t-tests were used to determine differences between baseline and 36 month follow-up T2 measurements in each group.
Multivariate linear regression models were used to compare changes in T2 measurements over 36 months between the four groups similar to the cross-sectional analysis of T2 measurements.
Since incidence and progression of focal knee lesions over 36 months had partly low counts per group, we analyzed incidence and progression combined per group (total WORMS progression). Differences in total WORMS progression of focal knee lesions between the four groups were assessed by using logistic regression models similar to the cross-sectional analysis of prevalence of focal knee lesions.
Additional adjustment for BMI change over 36 months in the regression models did not affect the p-values of the longitudinal statistical analysis.
Reproducibility
Intra-reader reproducibility for T2 measurements of each compartment were determined in baseline T2 maps of 20 randomly selected subjects. The patella compartment was segmented three times in the T2 maps of each subject by one operator (H.A.), the remaining compartments as well three times by one operator (A.A.). Reproducibility errors for each compartment were calculated as the root mean square error coefficient of variation (35). Intra-reader reproducibility for mean T2 ranged from 0.80% to 2.95% (mean: 1.76%), for T2 contrast from 2.84% to 6.99% (mean: 4.66%), for T2 entropy from 1.16% to 2.60% (mean: 1.88%), and for T2 variance from 2.14% to 6.32% (mean: 4.29%). Highest reproducibility errors were observed in the patella, lowest reproducibility errors in the medial femur compartment. These results indicated good agreement for mean T2 and T2 entropy and moderate agreement for T2 contrast and T2 variance similar to previous studies (12;22;25;31).
To assess intra- and inter-reader reproducibility of the WORMS grading, 20 subjects were randomly selected and WORMS grading was performed two times by two readers (L.N. and W.V.) independently. Intra-class correlation coefficients (ICC) were calculated to compare the exact WORMS score for meniscal and cartilage lesions and BMEP in each compartment. An intra-reader (inter-reader) reproducibility for meniscal WORMS grading of 0.95 and 0.97 (0.97) was calculated, for cartilage WORMS grading of 0.96 and 0.98 (0.98) and for BMEP WORMS grading of 0.98 and 0.98 (0.98). These results indicated good intra- and inter-reader agreement for WORMS grading.
Results
Subject Characteristics
Mean age and BMI, frequency of gender, KL-score, and OA risk factors of the four groups are listed in Table 1. While the mean age of the four groups was not significantly different, distribution of gender differed significantly between the four groups (p<0.05). As defined by the inclusion criteria, all subjects had KL-scores≤1 at baseline in the study knee. Six subjects with OA risk factors showed radiographic OA with KL-scores=2 at 36 month follow-up. Frequency of KL-scores were not significantly different between the three BMI groups with OA risk factors at baseline and 36 month follow-up (p>0.05). Frequency of “knee symptoms in the past 12 months” was the only OA risk factor, which was significantly different between the three BMI groups with OA risk factors (p<0.05).
Table 1.
Characteristics of (A) normal controls, (B) subjects with OA risk factors and normal weight, (C) subjects with OA risk factors and overweight, and (D) subjects with OA risk factors and obesity. Results are given as n (%) or as mean ± standard deviation.
| A | B | C | D | |
|---|---|---|---|---|
| (n=36) | (n=78) | (n=84) | (n=69) | |
| baseline age [years] | 50.4±3.1 | 51.0±2.7 | 50.3±3.0 | 51.5±2.6 |
| baseline BMI [kg/m2] | 22.7±1.5 | 22.9±1.4 | 27.2±1.5 | 32.9±2.3 |
| 36 month follow-up BMI [kg/m2] | 23.4±2.1 | 23.2±1.9 | 27.2±2.1 | 33.3±2.5 |
| males | 11 (30.6%)* | 33 (42.3%)* | 58 (69.0%)* | 37 (53.6%)* |
| baseline KL-score: | ||||
| KL-score = 0 | 36 (100%) | 58 (74.4%) | 59 (70.2%) | 43 (62.3%) |
| KL-score = 1 | 0 (0%) | 20 (25.6%) | 25 (29.8%) | 26 (37.7%) |
| 36 month follow-up KL-score: | ||||
| KL-score = 0 | 34 (94.4%) | 57 (73.1%) | 52 (61.9%) | 38 (55.1%) |
| KL-score = 1 | 2 (5.6%) | 18 (23.1%) | 30 (35.7%) | 30 (43.5%) |
| KL-score = 2 | 0 (0%) | 3 (3.8%) | 2 (2.4%) | 1 (1.4%) |
| baseline OA risk factors other than | ||||
| overweight/obesity (self-reported): | ||||
| knee symptoms in the past 12 months | 0 (0%) | 68 (87.2%)* | 78 (92.9%)* | 50 (72.5%)* |
| history of knee injury | 0 (0%) | 38 (48.7%) | 49 (58.3%) | 30 (43.5%) |
| history of knee surgery | 0 (0%) | 18 (23.1%) | 17 (20.2%) | 9 (13.0%) |
| family history of knee replacement | ||||
| surgery | 0 (0%) | 16 (20.8%) | 16 (19.3%) | 8 (11.6%) |
| Heberden nodes | 0 (0%) | 15 (19.2%) | 19 (22.6%) | 10 (14.7%) |
indicates statistically significant differences between the groups (p<0.05).
Focal Knee Lesions
Baseline prevalence and severity of focal knee lesions as well as incidence (BMEP: incidence and remission) and progression (BMEP: progression and regression) of focal knee lesions over 36 months are listed for all four groups in Table 2.
The greatest baseline prevalence of meniscal lesions (WORMS Max >0) and tears (WORMS Max >1) was found in group C (subjects with OA risk factors and overweight) and group D (subjects with OA risk factors and obesity), the lowest in group A (normal controls) (Table 2). Significant differences in baseline prevalence of meniscal lesions and tears were only found for prevalence of meniscal lesions between group D versus group A with an odds ratio (95% CI) of 2.52 (1.06–5.97) (Table 3). The greatest baseline prevalence of cartilage lesions (WORMS Max >0) and of grade 2 or higher cartilage lesions (WORMS Max >1) was observed in group D, while the lowest was found in group A (Table 2). Similar to meniscal lesions, significant differences in baseline prevalence of cartilage lesions were only observed between group D versus group A with an odds ratio (95% CI) of 3.58 (1.16–11.07) (Table 3).
Table 3.
Comparison of baseline prevalence and severity of focal knee lesions and total WORMS progression over 36 months in right knees of (A) normal controls versus (B) subjects with OA risk factors and normal weight, (C) subjects with OA risk factors and overweight, and (D) subjects with OA risk factors and obesity, respectively. Odds ratios and 95% confidence intervals (CI) were calculated for differences in baseline prevalence and total WORMS progression over 36 months between the respective groups by using logistic regression models, adjusting for gender, age, and OA risk factors other than BMI. Differences in baseline severity of focal knee lesions between the respective groups were assessed with multivariate regression models, adjusting for gender, age, and OA risk factors other than BMI. Values printed in bold indicate statistically significant differences between the respective groups (p<0.05).
| B vs. A | C vs. A | D vs. A | |
|---|---|---|---|
| given as odds ratio (95% CI; adjusted p-value) | |||
| Meniscus: | |||
| baseline prevalence of lesions | 0.94 (0.41–2.16; p=0.883) | 1.85 (0.79–4.31; p=0.155) | 2.52 (1.06–5.97; p=0.037) |
| baseline prevalence of tears | 1.64 (0.40–6.71; p=0.495) | 2.13 (0.51–8.95; p=0.304) | 1.77 (0.47–6.63; p=0.399) |
| total WORMS progression | |||
| over 36 months | 2.18 (0.44–10.76; p=0.340) | 4.48 (0.89–22.53; p=0.069) | 2.87 (0.63–13.05; p=0.173) |
| Cartilage: | |||
| baseline prevalence of lesions | 0.87 (0.35–2.14; p=0.754) | 1.34 (0.53–3.40; p=0.532) | 3.58 (1.16–11.07; p=0.027) |
| baseline prevalence of grade | |||
| 2 or higher lesions | 1.10 (0.34–3.58; p=0.872) | 1.46 (0.43–4.94; p=0.540) | 2.31 (0.77–6.92; p=0.136) |
| total WORMS progression over 36 months | 3.26 (0.89–11.94; p=0.075) | 4.39 (1.22–15.79; p=0.024) | 8.74 (2.43–31.45; p=0.001) |
| BMEP: | |||
| baseline prevalence of lesions | 0.65 (0.20–2.12; p=0.477) | 1.38 (0.41–4.59; p=0.605) | 2.11 (0.71–6.25; p=0.180) |
| total WORMS progression over 36 months | 1.24 (0.33–4.72; p=0.751) | 1.14 (0.28–4.60; p=0.852) | 2.27 (0.64–8.06; p=0.204) |
| B vs. A | C vs. A | D vs. A | |
| given as adjusted p-value | |||
| Meniscus: baseline severity of | |||
| Lesions | 0.858 | 0.382 | 0.318 |
| Cartilage: baseline severity | |||
| of lesions | 0.427 | 0.225 | 0.049 |
| BMEP: baseline severity of lesions | 0.732 | 0.286 | 0.102 |
The greatest prevalence of BMEP (WORMS Max >0) was found in group D, the lowest in group A (Table 2). However, differences in baseline prevalence of BMEP between the groups were not statistically significant (p>0.05; Table 3).
Group C and D showed the greatest severity of focal knee lesions at baseline (Table 2). Differences in severity of focal knee lesions at baseline reached only statistical significance (p<0.05) for cartilage lesions between group D versus group A (Table 3).
Since incidence and progression of focal knee lesions over 36 months had partly low counts per group (Table 2), we analyzed incidence and progression combined per group. Total WORMS progression of meniscal lesions and BMEP were not significantly different between the four groups (p>0.05; Table 3). However, group C and group D showed a significantly greater total WORMS progression of cartilage lesions over 36 months, compared to group A (Table 3). Odds ratio (95% CI) of group C versus group A amounted 4.39 (1.22–15.79), and of group D versus group A 8.74 (2.43–31.45).
T2 Measurements
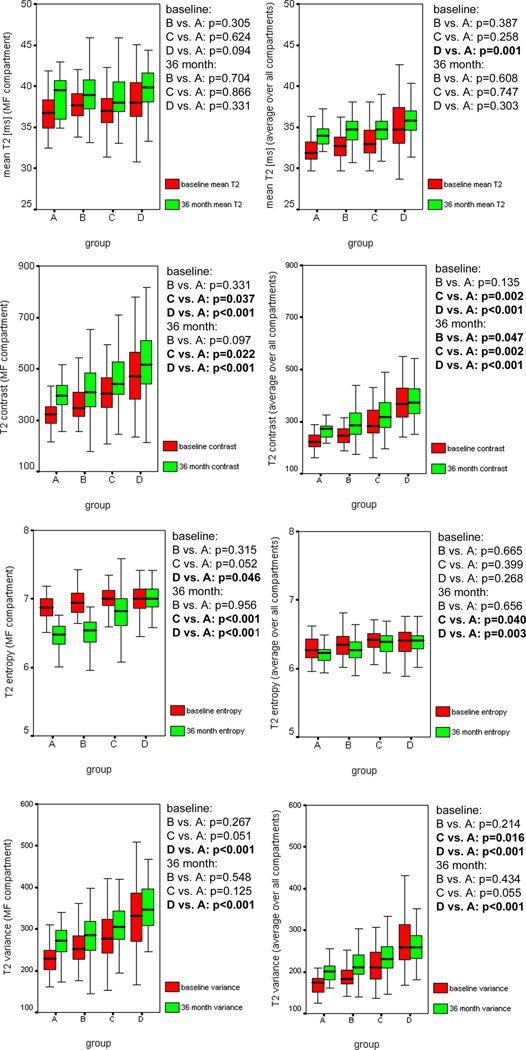
In the medial femur compartment and averaged over all five compartments, highest mean T2, T2 contrast, T2 entropy, and T2 variance at baseline were observed in group C and group D, while group A showed the lowest values (Figure 1 and Figure 2). Out of all baseline T2 measurements, T2 contrast and T2 variance showed the strongest statistically significant differences between group C versus group A, and group D versus group A, respectively (p<0.05; Figure 2).
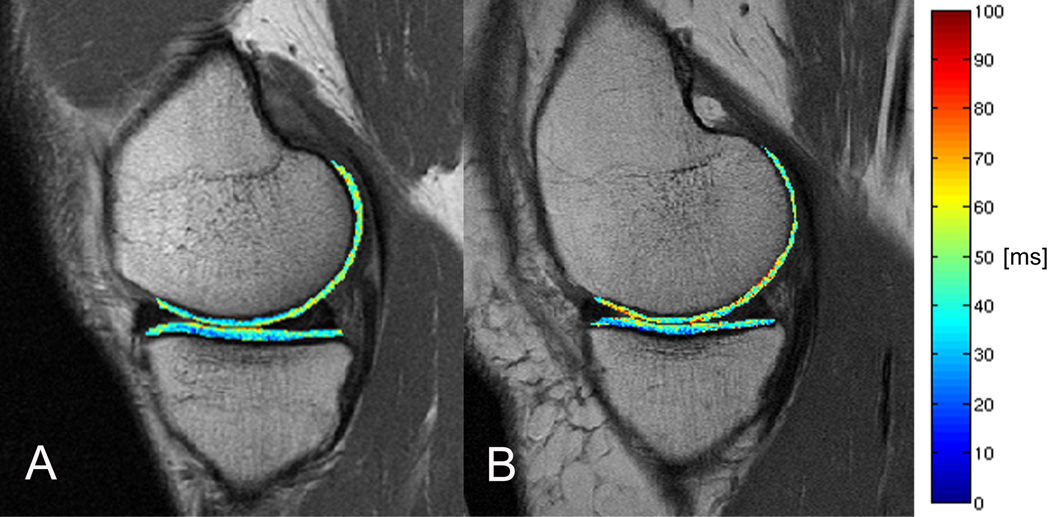
Figure 1.
T2 color maps of the medial femur and medial tibia compartments of the right knee overlaid with the first-echo images of MSME sequence. (A) Representative normal control and (B) representative subject with OA risk factors and obesity. Blue color indicates low, red color high cartilage T2 values. The subject with OA risk factors and obesity showed elevated T2 values compared to the normal control in the medial femur compartment.
Figure 2.
The boxplots show knee cartilage T2 measurements at baseline and 36 month follow-up for the medial femur (MF) compartment and the average over all compartments in (A) normal controls, (B) subjects with OA risk factors and normal weight, (C) subjects with OA risk factors and overweight, and (D) subjects with OA risk factors and obesity. Differences in T2 measurements of group A versus group B, C, and D, respectively were assessed with multivariate regression models, adjusting for gender, age, and OA risk factors other than BMI. P-values printed in bold indicate statistically significant differences between the respective groups (p<0.05).
While T2 entropy decreased, mean T2, T2 contrast, and T2 variance increased over 36 months in the four subject groups in the medial femur compartment (Figure 2, Table 4). Similar results were found for the average over all five compartments with the exception that T2 contrast and T2 variance did not increase, but decreased in group D (Figure 2, Table 4). The smallest, mostly non-significant changes in T2 measurements over 36 months were observed in group D (Table 4). Consequently, differences in changes of T2 measurements over 36 months between group D versus group A were statistically significant (p<0.05) for T2 parameters as outlined in Table 4. However, differences in T2 variance at 36 month follow-up remained statistically significant (p<0.05) between group A versus group D as well as differences in T2 contrast between group A versus group B, C, and D, respectively (Figure 2). Interestingly, T2 entropy decreased considerably in group A and B despite mean T2 increase (Table 4). These changes resulted in statistically significant (p<0.05) differences in T2 entropy at 36 month follow-up between group D versus group A, and group C versus group A, respectively (Figure 2).
Table 4.
Changes in knee cartilage T2 measurements over 36 months for the medial femur compartment and the average over all compartments in (A) normal controls, (B) subjects with OA risk factors and normal weight, (C) subjects with OA risk factors and overweight, and (D) subjects with OA risk factors and obesity. Results are given as mean ± standard deviation. Differences in changes in knee cartilage T2 measurements between the respective groups were assessed with multivariate regression models, adjusting for gender, age, and OA risk factors other than BMI. P-values printed in bold indicate statistically significant differences between the respective groups (p<0.05).
| Changes in T2 measurements over 36 months | Multivariate regression models | ||||||
|---|---|---|---|---|---|---|---|
| A | B | C | D | B vs. A | C vs. A | D vs. A | |
| (n=36) | (n=78) | (n=84) | (n=69) | p-value* | p-value* | p-value* | |
| Medial Femur compartment: | |||||||
| Δ mean T2 | 1.9±1.7* | 1.2±2.6* | 1.9±3.4* | 1.7±5.7* | 0.244 | 0.556 | 0.582 |
| Δ T2 contrast | 71±69* | 75±138* | 68±137* | 46±138* | 0.455 | 0.677 | 0.857 |
| Δ T2 entropy | −0.45±0.20* | −0.47±0.28* | −0.18±0.28* | −0.01±0.15 | 0.577 | 0.002 | <0.001 |
| Δ T2 variance | 42±41* | 36±83* | 37±78* | 8±97 | 0.629 | 0.612 | 0.097 |
| average over all five compartments: | |||||||
| Δ mean T2 | 2.1±1.0* | 1.6±5.1* | 2.1±3.9* | 0.4±4.3 | 0.281 | 0.522 | 0.042 |
| Δ T2 contrast | 43±39* | 58±90* | 36±110* | −27±155 | 0.915 | 0.573 | 0.013 |
| Δ T2 entropy | −0.08±0.14* | −0.08±0.18* | −0.00±0.19 | −0.01±0.12 | 0.803 | 0.098 | 0.068 |
| Δ T2 variance | 30±25* | 31±74* | 19±81* | −35±118* | 0.515 | 0.292 | 0.001 |
statistically significant (p<0.05) change of respective T2 measurement over 36 months (paired t-test).
Discussion
Obese subjects with OA risk factors, but no radiographic OA showed a higher prevalence and severity of meniscal and cartilage lesions, compared to normal controls. Furthermore overweight and obese subjects had a greater progression of cartilage lesions over 36 months. Being overweight or obese was associated with more heterogeneous cartilage according to T2 texture analysis. T2 entropy remained constantly elevated in overweight and obese subjects over 36 months, in contrast to normal controls and subjects with OA risk factors and normal weight, who showed a considerable decrease in T2 entropy despite mean T2 increase.
We found a higher prevalence and severity of meniscal and cartilage lesions in obese subjects than in normal controls. Furthermore overweight and obese subjects showed a significantly greater total WORMS progression of cartilage lesions over 36 months, compared to normal controls. These findings suggest an advanced degenerative joint disease in subjects with high BMI and are consistent with previous studies (6–10;27;36). However, most of these studies have been limited to assessing subjects with clinically symptomatic and/or radiographic knee OA, while the findings in our study were observed in subjects without pain and without radiographic OA.
Previous cross-sectional studies have reported elevated cartilage mean T2 relaxation times and higher values of T2 texture parameters contrast, entropy, and variance in subjects with OA risk factors and mild OA, compared to normal controls (18;21;22). High T2 contrast signifies that many pixels with different T2 values are neighboring. Higher T2 entropy means that it is more likely to find any combination of T2 relaxation time co-occurrence. High T2 variance signifies a high dispersion of co-occurrences of T2 relaxation times. In this study we analyzed normal controls and normal-, overweight, and obese subjects with OA risk factors and found the highest values of mean T2, T2 contrast, T2 entropy, and T2 variance in overweight/obese subjects at baseline and 36 month follow-up. These findings suggest that elevated BMI is associated with advanced cartilage matrix degeneration. Mean T2 was only significantly different between normal controls versus obese subjects for the average over all five compartments. T2 texture parameters revealed multiple statistically significant differences between normal controls versus normal-, overweight, and obese subjects, respectively. Thus, T2 texture parameters may be more sensitive compared to mean T2 values to detect BMI associated cartilage matrix degeneration. While WORMS grading showed cross-sectionally only statistically significant differences between normal controls versus obese subjects, T2 texture measurements demonstrated statistically significant differences not only between normal controls versus obese subjects, but also between normal controls and overweight subjects. Therefore T2 measurements may be useful, since they offer interesting insights in the condition of cartilage matrix beyond morphologically detectable focal knee lesions.
The smallest, mostly non-significant changes in T2 measurements over 36 months were observed in the obese subjects, suggesting a ceiling effect for cartilage T2 measurements. Little is known about the kind of T2 changes over time. Linear and non-linear increases as well as temporary and permanent ceiling effects are possible. Future studies with even longer follow-up times are needed to fully understand the significance of our findings, e.g. studies using the 48 month follow-up data from the OAI. The non-obese subject groups showed significant increases of mean T2, T2 contrast, and T2 variance over 36 months. These results are consistent with a recent study by Baum et al. (12). They reported significant mean T2 increases over 24 months in normal controls and non-obese subjects (BMI<27kg/m2) with OA risk factors. Interestingly, T2 entropy considerably decreased over 36 months in normal controls and subjects with OA risk factors and normal weight despite increasing mean T2 values. This leads to significant differences in T2 entropy between normal controls versus overweight and obese subjects at 36 month follow-up. Higher T2 entropy means more uniform distribution of probabilities of T2 relaxation time co-occurrences, i.e. it is more likely to find any combination of T2 relaxation time co-occurrence. Thus, we observed a relatively homogenous cartilage T2 increase over 36 months in normal controls and subjects with OA risk factors and normal weight, while T2 entropy remained constantly elevated in overweight and obese subjects. Since elevated T2 entropy is associated with osteoarthritic changes (18–21), our findings suggest advanced cartilage matrix degeneration in overweight and obese subjects, which is also reflected by the significantly greater progression of cartilage lesions as assessed by WORMS grading.
Delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) is used to assess the relative distribution of glycosaminoglycans in cartilage (37). Glycosaminoglycans are lost in the early phase of cartilage degeneration. The negatively charged contrast agent gadopentate dimeglumine (Gd-DTPA2−) distributes within cartilage matrix in an inverse relationship to the concentration of negatively charged glycosaminoglycans. Thus, concentration of gadopentate dimeglumine reflects the content of glycosaminoglycans of cartilage. Previous studies have demonstrated a relationship between BMI and dGEMRIC (11;38;39). Knee dGEMRIC index was associated with clinical knee OA in obese adults (38). Tiderius et al. reported a negative correlation between dGEMRIC and BMI in the knees of subjects with OA, but no correlation in asymptomatic knees (39). While these studies have been limited to assessing subjects with clinically symptomatic knee OA, the findings in our study were observed in subjects without pain and without radiographic OA. Thus, the results of our study suggest a high sensitivity of T2 relaxation times as biomarker for early cartilage matrix degeneration. Furthermore T2 measurements have the advantage to be less time consuming than dGEMRIC, which requires the intra-venous injection of gadopentate dimeglumine and a penetration time into cartilage of about 90min.
This study had some limitations. Firstly, incidence and progression of focal knee lesions had partly low counts per group. This may result from limitations with the WORMS grading system to measure incidence and progression of focal knee lesions (40–42). There is on ongoing discussion on how to measure incidence and progression of focal knee lesions best. For this study, we combined incidence and progression for the statistical analysis, since the partly low counts of incidence and progression of focal knee lesions per group limited a reliable separate analysis of incidence and progression of focal knee lesions. However, the statistically significant odds ratios of total WORMS progression (Table 3) had relatively large 95% confidence intervals, which seems to result from still low counts per group and consequently limits the informative value of these analyses. Secondly, malalignment is a known OA risk factor (43;44). Malalignment significantly affects prevalence and severity of focal knee lesions and cartilage T2 measurements. However, the baseline data collected by the OAI only included goniometer alignment readings, which have been found to be inaccurate (45). Therefore we did not adjust for malalignment in the statistical analysis, which is a limitation of our study. Thirdly, T2 texture parameters contrast and variance showed relatively high reproducibility errors. This may limit the validity of these parameters. Lastly, the comparison of baseline and 36 month follow-up T2 measurements requires reliable and accurate MR imaging, which is challenging. However, in the OAI rigorous quality assurance methods were established to allow a high quality of cartilage T2 measurements over 36 months (46).
In conclusion, increased BMI is associated with more severe cartilage degeneration as assessed by both morphological and quantitative MRI measurements in the pre-clinical phase of OA. Cartilage T2 relaxation time measurements may be a sensitive biomarker for monitoring cartilage quality in subjects at risk for developing knee OA, since they offer interesting insights in the condition of cartilage matrix beyond morphologically detectable focal knee lesions.
Supplementary Material
Significance and Innovations.
Increased BMI is associated with more severely degenerated cartilage as demonstrated by elevated mean and more heterogeneous cartilage T2 in subjects with risk factors for knee OA, but without knee pain and without radiographic knee OA.
In pre-clinical OA, overweight and obese subjects showed a higher prevalence and severity of meniscal and cartilage lesions and a greater progression of cartilage lesions over 36 months.
Cartilage T2 relaxation time measurements may be a sensitive biomarker for monitoring cartilage quality in subjects at risk for developing knee OA, since they offer interesting insights in the condition of cartilage matrix beyond morphologically detectable focal knee lesions.
Acknowledgement
This study was supported by NIH U01AR059507-01, P50 (project 3, AR060752-01), and F32AR059478-01.
The OAI is a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health.
This manuscript has received the approval of the OAI Publications Committee based on a review of its scientific content and data interpretation.
Footnotes
Financial interests / Conflict of interest: The authors have no financial interests and no conflict of interest with regard to the work.
Reference List
- 1.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med. 2010;26(3):355–369. doi: 10.1016/j.cger.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y, Beydoun MA. The obesity epidemic in the United States—gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev. 2007;29:6–28. doi: 10.1093/epirev/mxm007. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Beydoun MA, Liang L, Caballero B, Kumanyika SK. Will all Americans become overweight or obese? estimating the progression and cost of the US obesity epidemic. Obesity (Silver Spring) 2008;16(10):2323–2330. doi: 10.1038/oby.2008.351. [DOI] [PubMed] [Google Scholar]
- 5.Russell GV, Pierce CW, Nunley L. Financial implications of obesity. Orthop Clin North Am. 2011;42(1):123–127. doi: 10.1016/j.ocl.2010.09.003. vii. [DOI] [PubMed] [Google Scholar]
- 6.Brennan SL, Cicuttini FM, Pasco JA, Henry MJ, Wang Y, Kotowicz MA, et al. Does an increase in body mass index over 10 years affect knee structure in a population-based cohort study of adult women? Arthritis Res Ther. 2010;12(4):R139. doi: 10.1186/ar3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding C, Cicuttini F, Scott F, Cooley H, Jones G. Knee structural alteration and BMI: a cross-sectional study. Obes Res. 2005;13(2):350–361. doi: 10.1038/oby.2005.47. [DOI] [PubMed] [Google Scholar]
- 8.Hanna FS, Bell RJ, Davis SR, Wluka AE, Teichtahl AJ, O'Sullivan R, et al. Factors affecting patella cartilage and bone in middle-aged women. Arthritis Rheum. 2007;57(2):272–278. doi: 10.1002/art.22535. [DOI] [PubMed] [Google Scholar]
- 9.Teichtahl AJ, Wang Y, Wluka AE, Szramka M, English DR, Giles GG, et al. The longitudinal relationship between body composition and patella cartilage in healthy adults. Obesity (Silver Spring) 2008;16(2):421–427. doi: 10.1038/oby.2007.37. [DOI] [PubMed] [Google Scholar]
- 10.Teichtahl AJ, Wluka AE, Wang Y, Hanna F, English DR, Giles GG, et al. Obesity and adiposity are associated with the rate of patella cartilage volume loss over 2 years in adults without knee osteoarthritis. Ann Rheum Dis. 2009;68(6):909–913. doi: 10.1136/ard.2008.093310. [DOI] [PubMed] [Google Scholar]
- 11.Anandacoomarasamy A, Smith G, Leibman S, Caterson I, Giuffre B, Fransen M, et al. Cartilage defects are associated with physical disability in obese adults. Rheumatology (Oxford) 2009;48(10):1290–1293. doi: 10.1093/rheumatology/kep246. [DOI] [PubMed] [Google Scholar]
- 12.Baum T, Stehling C, Joseph GB, Carballido-Gamio J, Schwaiger BJ, Muller-Hocker C, et al. Changes in knee cartilage T2 values over 24 months in subjects with and without risk factors for knee osteoarthritis and their association with focal knee lesions at baseline: Data from the osteoarthritis initiative. J Magn Reson Imaging. 2012;35(2):370–378. doi: 10.1002/jmri.22834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blumenkrantz G, Majumdar S. Quantitative magnetic resonance imaging of articular cartilage in osteoarthritis. Eur Cell Mater. 2007;13:76–86. doi: 10.22203/ecm.v013a08. [DOI] [PubMed] [Google Scholar]
- 14.Liess C, Lusse S, Karger N, Heller M, Gluer CC. Detection of changes in cartilage water content using MRI T2-mapping in vivo. Osteoarthritis Cartilage. 2002;10(12):907–913. doi: 10.1053/joca.2002.0847. [DOI] [PubMed] [Google Scholar]
- 15.Mosher TJ, Dardzinski BJ, Smith MB. Human articular cartilage: influence of aging and early symptomatic degeneration on the spatial variation of T2--preliminary findings at 3 T. Radiology. 2000;214(1):259–266. doi: 10.1148/radiology.214.1.r00ja15259. [DOI] [PubMed] [Google Scholar]
- 16.Dunn TC, Lu Y, Jin H, Ries MD, Majumdar S. T2 relaxation time of cartilage at MR imaging: comparison with severity of knee osteoarthritis. Radiology. 2004;232(2):592–598. doi: 10.1148/radiol.2322030976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X, Benjamin MC, Link TM, Castillo DD, Blumenkrantz G, Lozano J, et al. In vivo T(1rho) and T(2) mapping of articular cartilage in osteoarthritis of the knee using 3 T MRI. Osteoarthritis Cartilage. 2007;15(7):789–797. doi: 10.1016/j.joca.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carballido-Gamio J, Stahl R, Blumenkrantz G, Romero A, Majumdar S, Link TM. Spatial analysis of magnetic resonance T1rho and T2 relaxation times improves classification between subjects with and without osteoarthritis. Med Phys. 2009;36(9):4059–4067. doi: 10.1118/1.3187228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carballido-Gamio J, Joseph GB, Lynch JA, Link TM, Majumdar S. Longitudinal analysis of MRI T2 knee cartilage laminar organization in a subset of patients from the osteoarthritis initiative: a texture approach. Magn Reson Med. 2011;65(4):1184–1194. doi: 10.1002/mrm.22693. [DOI] [PubMed] [Google Scholar]
- 20.Blumenkrantz G, Stahl R, Carballido-Gamio J, Zhao S, Lu Y, Munoz T, et al. The feasibility of characterizing the spatial distribution of cartilage T(2) using texture analysis. Osteoarthritis Cartilage. 2008;16(5):584–590. doi: 10.1016/j.joca.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, Pai A, Blumenkrantz G, Carballido-Gamio J, Link T, Ma B, et al. Spatial distribution and relationship of T1rho and T2 relaxation times in knee cartilage with osteoarthritis. Magn Reson Med. 2009;61(6):1310–1318. doi: 10.1002/mrm.21877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joseph GB, Baum T, Carballido-Gamio J, Nardo L, Virayavanich W, Alizai H, et al. Texture analysis of cartilage T2 maps: individuals with risk factors for OA have higher and more heterogeneous knee cartilage MR T2 compared to normal controls - data from the osteoarthritis initiative. Arthritis Res Ther. 2011;13(5):R153. doi: 10.1186/ar3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage. 2008;16(12):1433–1441. doi: 10.1016/j.joca.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kellgren J, Lawrence J. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baum T, Joseph GB, Arulanandan A, Nardo L, Virayavanich W, Carballido-Gamio J, et al. Association of magnetic resonance imaging-based knee cartilage T2 measurements and focal knee lesions with knee pain: data from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken) 2012;64(2):248–255. doi: 10.1002/acr.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hovis KK, Stehling C, Souza RB, Haughom BD, Baum T, Nevitt M, et al. Physical Activity Is Associated With Magnetic Resonance Imaging-Based Knee Cartilage T2 Measurements in Asymptomatic Subjects With and Those Without Osteoarthritis Risk Factors. Arthritis and Rheumatism. 2011;63(8):2248–2256. doi: 10.1002/art.30419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laberge MA, Baum T, Virayavanich W, Nardo L, Nevitt MC, Lynch J, et al. Obesity increases the prevalence and severity of focal knee abnormalities diagnosed using 3T MRI in middle-aged subjects-data from the Osteoarthritis Initiative. Skeletal Radiology. 2011 doi: 10.1007/s00256-011-1259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan J, Stehling C, Muller-Hocker C, Schwaiger BJ, Lynch J, McCulloch CE, et al. Vastus lateralis/vastus medialis cross-sectional area ratio impacts presence and degree of knee joint abnormalities and cartilage T2 determined with 3T. Osteoarthritis Cartilage. 2011;19(1):65–73. doi: 10.1016/j.joca.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stehling C, Lane NE, Nevitt MC, Lynch J, McCulloch CE, Link TM. Subjects with higher physical activity levels have more severe focal knee lesions diagnosed with 3T MRI: analysis of a non-symptomatic cohort of the osteoarthritis initiative. Osteoarthritis Cartilage. 2010;18(6):776–786. doi: 10.1016/j.joca.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stehling C, Liebl H, Krug R, Lane NE, Nevitt MC, Lynch J, et al. Patellar cartilage: T2 values and morphologic abnormalities at 3.0-T MR imaging in relation to physical activity in asymptomatic subjects from the osteoarthritis initiative. Radiology. 2010;254(2):509–520. doi: 10.1148/radiol.09090596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stehling C, Baum T, Mueller-Hoecker C, Liebl H, Carballido-Gamio J, Joseph GB, et al. A novel fast knee cartilage segmentation technique for T(2) measurements at MR imaging - data from the Osteoarthritis Initiative. Osteoarthritis and Cartilage. 2011;19(8):984–989. doi: 10.1016/j.joca.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haralick R, Shanmugam K, Dinstein I. Textural features for image classification. IEEE Trans Syst Man Cybern. 1973;SMC-3(6):610e8. [Google Scholar]
- 33.Schipplein OD, Andriacchi TP. Interaction between active and passive knee stabilizers during level walking. J Orthop Res. 1991;9(1):113–119. doi: 10.1002/jor.1100090114. [DOI] [PubMed] [Google Scholar]
- 34.Winby CR, Lloyd DG, Besier TF, Kirk TB. Muscle and external load contribution to knee joint contact loads during normal gait. J Biomech. 2009;42(14):2294–2300. doi: 10.1016/j.jbiomech.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 35.Gluer CC, Blake G, Lu Y, Blunt BA, Jergas M, Genant HK. Accurate assessment of precision errors: how to measure the reproducibility of bone densitometry techniques. Osteoporos Int. 1995;5(4):262–270. doi: 10.1007/BF01774016. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Wluka AE, English DR, Teichtahl AJ, Giles GG, O'Sullivan R, et al. Body composition and knee cartilage properties in healthy, community-based adults. Ann Rheum Dis. 2007;66(9):1244–1248. doi: 10.1136/ard.2006.064352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burstein D, Velyvis J, Scott KT, Stock KW, Kim YJ, Jaramillo D, et al. Protocol issues for delayed Gd(DTPA)(2-)-enhanced MRI (dGEMRIC) for clinical evaluation of articular cartilage. Magn Reson Med. 2001;45(1):36–41. doi: 10.1002/1522-2594(200101)45:1<36::aid-mrm1006>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 38.Anandacoomarasamy A, Giuffre BM, Leibman S, Caterson ID, Smith GS, Fransen M, et al. Delayed gadolinium-enhanced magnetic resonance imaging of cartilage: clinical associations in obese adults. J Rheumatol. 2009;36(5):1056–1062. doi: 10.3899/jrheum.080997. [DOI] [PubMed] [Google Scholar]
- 39.Tiderius C, Hori M, Williams A, Sharma L, Prasad PV, Finnell M, et al. dGEMRIC as a function of BMI. Osteoarthritis Cartilage. 2006;14(11):1091–1097. doi: 10.1016/j.joca.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y, Niu J, Felson D, Choi H, Nevitt M, Neogi T. Methodologic challenges in studying risk factors for progression of knee osteoarthritis. Arthritis Care Res (Hoboken) 2010;62(11):1527–1532. doi: 10.1002/acr.20287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Felson DT, Lynch J, Guermazi A, Roemer FW, Niu J, McAlindon T, et al. Comparison of BLOKS and WORMS scoring systems part II. Longitudinal assessment of knee MRIs for osteoarthritis and suggested approach based on their performance: data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2010;18(11):1402–1407. doi: 10.1016/j.joca.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lynch JA, Roemer FW, Nevitt MC, Felson DT, Niu J, Eaton CB, et al. Comparison of BLOKS and WORMS scoring systems part I. Cross sectional comparison of methods to assess cartilage morphology, meniscal damage and bone marrow lesions on knee MRI: data from the osteoarthritis initiative. Osteoarthritis Cartilage. 2010;18(11):1393–1401. doi: 10.1016/j.joca.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Friedrich KM, Shepard T, Chang G, Wang L, Babb JS, Schweitzer M, et al. Does joint alignment affect the T2 values of cartilage in patients with knee osteoarthritis? Eur Radiol. 2010;20(6):1532–1538. doi: 10.1007/s00330-009-1689-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hunter DJ, Sharma L, Skaife T. Alignment and Osteoarthritis of the Knee. Journal of Bone and Joint Surgery-American Volume. 2009;91A:85–89. doi: 10.2106/JBJS.H.01409. [DOI] [PubMed] [Google Scholar]
- 45.Hinman RS, May RL, Crossley KM. Is there an alternative to the full-leg radiograph for determining knee joint alignment in osteoarthritis? Arthritis & Rheumatism-Arthritis Care & Research. 2006;55(2):306–313. doi: 10.1002/art.21836. [DOI] [PubMed] [Google Scholar]
- 46.Schneider E, NessAiver M, White D, Purdy D, Martin L, Fanella L, et al. The osteoarthritis initiative (OAI) magnetic resonance imaging quality assurance methods and results. Osteoarthritis Cartilage. 2008;16(9):994–1004. doi: 10.1016/j.joca.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.