Abstract
The PIVENS [Pioglitazone versus Vitamin E versus Placebo for the Treatment of Non-diabetic Patients with Nonalcoholic Steatohepatitis (NASH)] trial demonstrated that pioglitazone and vitamin E improved liver histology to varying degrees but mechanisms are unknown. We conducted a study to examine the changes in adipose tissue insulin resistance (Adipo-IR) during the PIVENS trial and its relationship to histological end points. Adipo-IR [fasting non-esterified fatty acids (NEFA) × fasting insulin] was calculated at baseline and after 16 and 96 weeks of therapy. Compared to placebo, the baseline Adipo-IR was not different in either vitamin E group (p=0.34) or pioglitazone group (p=0.29).). Baseline Adipo-IR was significantly associated with fibrosis score (p=0.017) but not with other histological features or NAFLD activity score (NAS). After 16 weeks, compared to placebo, the pioglitazone group had significant reduction in Adipo-IR (−15.7 vs. −1.91, p=0.02) but this effect did not persist at 96 weeks (−3.25 vs. −4.28, p=0.31). Compared to placebo, Adipo-IR in the vitamin E group did not change significantly either after 16 weeks (p=0.70) or after 96 weeks (p=0.85). Change in Adipo-IR at week 16 was not associated with changes in any histological parameters at week 96, but improvement in Adipo-IR at week 96 was significantly associated with improvement in ballooning (p=0.02), fibrosis (p=0.004), and NAFLD activity score (p=0.01).
Conclusion
Vitamin E improved liver histology independent of changes in Adipo-IR, and pioglitazone treatment acutely improved Adipo-IR but this was not sustained. Changes in Adipo-IR were associated with changes in liver histology, including fibrosis.
Keywords: Nonalcoholic steatohepatitis, vitamin E, pioglitazone, insulin resistance, adipose tissue
Introduction
Despite the increasing incidence of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH), there are no therapies currently approved for treatment of these common liver disorders. Recently published results from the Pioglitazone versus Vitamin E versus Placebo for the Treatment of Nondiabetic Patients with Nonalcoholic Steatohepatitis (PIVENS) clinical trial showed that pioglitazone and vitamin E improved liver histology to varying degrees (1). Of particular interest is the fact that pioglitazone and vitamin E may target different mechanistic pathways (insulin sensitivity and oxidative stress, respectively) implicated in the pathogenesis of NASH (2–4), but liver histology improved in both treatment groups. Furthermore, although insulin resistance is thought to be directly involved in the development of NASH, liver histology improved in patients taking vitamin E despite no change in general insulin sensitivity as measured by Homeostasis Model Assessment for Insulin Resistance (HOMA-IR) index (1). Delineation of mechanisms involved in the development, progression, and improvement of NASH are clearly needed to better understand natural history and treatment options for this increasingly prevalent disease.
While hepatic and muscle insulin resistance are established hallmark features of NAFLD and NASH (2, 3, 5–9), a report by Gastaldelli et al. highlighted an additional component, adipose tissue insulin resistance (Adipo-IR), as an important contributor to the pathogenesis and treatment of NASH (10). In this study, patients with NASH had elevated fasting concentrations of non-esterified fatty acids (NEFA) compared to control subjects, and an index of Adipo-IR, calculated as the product of fasting NEFA×fasting insulin (7, 10), was significantly elevated in NASH patients independent of the degree of obesity. Interestingly, treatment with pioglitazone decreased NEFA concentrations, resulting in a significant reduction in Adipo-IR, and decreases in Adipo-IR were correlated with improvements in hepatic steatosis and necroinflammation. Overall, this study suggests a central role for reduction of adipose tissue insulin resistance in pioglitazone-induced improvement of liver histology in patients with NASH.
The intriguing relationship between changes in adipose tissue insulin resistance and improvements in liver histology (10) led us to investigate Adipo-IR in patients who participated in the PIVENS clinical trial. Specifically, the aims of this study were to: (1) examine the predictors of Adipo-IR at baseline and after 96 weeks, (2) assess changes in Adipo-IR in the placebo, pioglitazone, and vitamin E groups after 16 and 96 weeks of therapy, and (3) determine if early (after 16 weeks) or long term (after 96 weeks) changes in Adipo-IR correlate with changes in liver histology.
Patients and Methods
Study Design
Data included in this analysis were obtained from patients who participated in the PIVENS trial, an adult treatment trial conducted by the Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) (1). PIVENS study design has been described previously (11) and the Clinical Trials.gov identifier is NCT00063622. Briefly, this study investigated the efficacy of pioglitazone or vitamin E for improving liver histology in patients with NASH. Patients enrolled in the PIVENS trial had to be ≥18 years of age and have a histological diagnosis of NASH without cirrhosis (liver biopsy within 6 months of randomization was required). Patients were excluded if they had diabetes, consumed >20 g alcohol/day if female or >30 g alcohol/day if male, had any other form of chronic liver disease besides NASH, took medications known to possibly cause or affect NASH (for example, methotrexate or tamoxifen), used fluctuating doses of lipid-lowering medications, had alanine aminotransferase (ALT) levels >300 U/L or serum creatinine levels ≥2.0 mg/dl, or were females of childbearing age and were pregnant, nursing, or unwilling to use birth control.
The study protocol and informed consent statements were approved by the NASH CRN Steering Committee and by the Institutional Review Boards at each participating site. Patients who met all eligibility criteria gave written informed consent prior to participation in the study, and were randomized to receive pioglitazone (30 mg Actos® once daily), vitamin E (800 IU 100% natural RRR-α-tocopherol once daily) or placebo for 96 weeks. Patients underwent an end-of-treatment liver biopsy after 96 weeks of therapy.
Evaluation of Liver Histology
Baseline and 96-week liver biopsy specimens were formalin-fixed, paraffin-embedded, and unstained slides were cut from tissue blocks and sent to the NASH CRN repository for preparation by a central laboratory and review by the NASH CRN Pathology Committee (10 hepatopathologists blinded to all clinical and group assignment data). Scoring of biopsies was done by consensus according to the NAFLD activity score (NAS) and fibrosis score previously published by the NASH CRN (12).
Laboratory Analyses and Calculation of Indices
Blood was collected into serum separator tubes, allowed to clot for at least 30 min at room temperature, and centrifuged at 1800 × g for 15 min at 4°C. Serum was aliquoted (0.5 ml) and immediately frozen at −70°C. All processing was completed within two hours and samples were free of hemolysis. Routine measurement of fasting insulin and glucose concentrations was carried out at each study site. Fasting serum nonesterified fatty acid (NEFA) concentrations were measured by colorimetric assay (Free Fatty Acids Half Micro Test, Roche Diagnostics Corporation, Indianapolis, IN). Adipo-IR index was calculated as: [fasting NEFA (mM) × fasting insulin (pM)] and HOMA-IR index was calculated as: [fasting glucose (mg/dl) × fasting insulin (µU/ml)] ÷ 405.
Statistical Analyses
Means and standard deviations (SD) were used to show variations across treatment and other subgroups in Adipo-IR, insulin, and NEFA at baseline and follow-up visits. Multiple linear regression models for the outcome measure, log-transformed Adipo-IR index included two indicator variables to represent the separate effects of vitamin E and pioglitazone versus placebo together with other potential confounders: gender, ethnicity (Hispanic vs. non-Hispanic), age at randomization, BMI, waist circumference, total body fat, AST, ALT, glucose, triglycerides, total cholesterol, HDL cholesterol, and histological scores (steatosis, lobular inflammation, hepatocyte ballooning, fibrosis, and the NAFLD Activity Score [NAS]) (12). The log-transform was necessary to correct for the right-skewness in the Adipo-IR index. These regression models were used to assess cross-sectional correlates of the Adipo-IR index at baseline and after 96-weeks of follow-up. Regression models were also used to examine changes in the Adipo-IR index at 16 and 96 weeks in relation to vitamin E and pioglitazone and other potential correlates of change. Two multiple linear regression models of log-transformed ratios of the Adipo-IR index at 16 weeks vs. baseline and at 96 weeks vs. baseline were used to assess the direction, magnitude, and statistical significance of associations of changes in the Adipo-IR index at each of the two time points with corresponding changes in liver histology at 96 weeks, BMI (16 and 96 weeks), and ALT (16 and 96 weeks), controlling for baseline Adipo-IR, ethnicity, and baseline BMI. Changes in Adipo-IR index from baseline to 16 and 96 weeks were expressed as ratios (rather than absolute change) and analyzed on a log scale to improve normality of the outcome measures used in the linear regression models. Consequently, we reported exponentiated regression coefficients, which are more easily interpretable as ratios of geometric means of the Adipo-IR index per unit change in a given independent variable in the model for otherwise similar patients. All analyses were carried out using SAS 9.2 (SAS Institute, Cary, NC) and Stata 11 (Stata Corp., College Station, TX). Nominal, two-sided p-values were used and were considered statistically significant if p<0.05.
Results
Study Population
As reported previously, all patients included in this study were previously characterized in the original publication of the PIVENS clinical trial (1). Patients were well-matched across the placebo, pioglitazone, and vitamin E groups at baseline, as there were no significant differences in demographics/anthropometric factors, liver biochemistries, metabolic and lipid profile parameters, or histological features. Effects of pioglitazone and vitamin E therapy on liver histology [as assessed by a composite of standardized scores for histological features (primary outcome) as well as scores for individual components], liver biochemistries, lipid panels, and metabolic factors, compared to placebo, have been previously described in detail (1). Briefly, vitamin E (43% of patients met the primary outcome; p=0.001) but not pioglitazone (34% of patients met the primary outcome; p=0.04) was superior to placebo (19% of patients met the primary outcome) for treatment of NASH; however, both pioglitazone and vitamin E significantly improved steatosis and lobular inflammation (both p≤0.02) and significantly reduced serum ALT compared to placebo (p<0.001). Neither pioglitazone nor vitamin E improved fibrosis scores. Although pioglitazone was beneficial for metabolic endpoints, patients gained significantly more weight (p<0.001) compared to the placebo group. As expected, 96 weeks of pioglitazone treatment significantly improved insulin resistance (p=0.03; as assessed by HOMA-IR), but improvements in liver histology induced by vitamin E treatment were independent of changes in HOMA-IR.
Changes in Adipo-IR during the PIVENS Clinical Trial
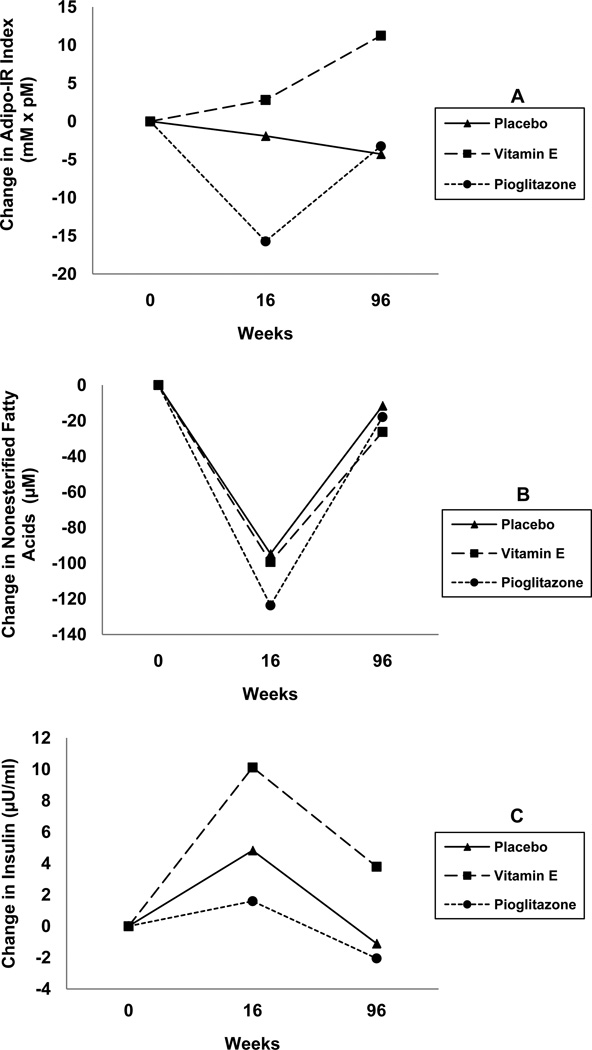
Table 1 shows Adipo-IR index in 3 treatment groups at baseline and at 16 and 96 weeks. Compared to placebo, the baseline Adipo-IR was not different in either vitamin E group (p=0.34) or pioglitazone group (p=0.29). After 16 weeks, compared to placebo, the pioglitazone group had significant reduction in Adipo-IR (−15.7 vs. −1.91, p=0.02). However, this effect did not persist at 96 weeks as mean Adipo-IR (placebo 61.5 ± 81.5 vs. pioglitazone 48.2 ± 49.0, p=0.25) or its mean change from baseline (placebo −4.28 vs. pioglitazone −3.25, p=0.31) (Figure 1; Panel A). Compared to placebo, Adipo-IR in the vitamin E group did not change significantly either after 16 weeks or after 96 weeks (Table 1, Figure 1). Baseline NEFA and fasting insulin concentrations as well as their values after 16 and 96 weeks are shown in Table 1.
Table 1.
Adipo-IR, Insulin, and NEFA at Baseline, 16 and 96 Weeks of Therapy with Vitamin E, Pioglitazone or Placebo
| p | |||||
|---|---|---|---|---|---|
| Placebo | Vit E | Pioglitazone | Vit E vs. Placebo |
Pioglitazone vs. Placebo |
|
| Baseline* | |||||
| Number | 82 | 84 | 80 | ||
| Adipo-IR (mM × pM) | 64.0 ± 85.5 | 54.1 ± 39.8 | 52.9 ± 38.2 | 0.34 | 0.29 |
| Insulin (µU/ml)§ | 23.3 ± 21.8 | 21.5 ± 15.8 | 21.4 ± 14.9 | 0.55 | 0.51 |
| NEFA (µM) | 456.6 ± 199.6 | 452.0 ± 182.9 | 436.5 ± 193.7 | 0.88 | 0.52 |
| After 16 weeks therapy** | |||||
| Adipo-IR | |||||
| Number | 72 | 73 | 63 | ||
| Mean± s.d. | 59.4 ± 62.6 | 56.0 ± 43.1 | 37.0 ± 37.3 | 0.70 | 0.01 |
| Mean change from baseline± s.d. | −1.91±110.7 | 2.81±53.5 | −15.7±35.6 | 0.71 | 0.02 |
| Insulin(µU/ml) | |||||
| Number | 72 | 74 | 64 | ||
| Mean± s.d. | 27.2 ± 22.2 | 31.6 ± 28.8 | 22.7 ± 19.0 | 0.30 | 0.21 |
| Mean change from baseline± s.d. | 4.83±30.1 | 10.1±29.8 | 1.60±17.6 | 0.30 | 0.23 |
| NEFA(µM) | |||||
| Number | 78 | 80 | 72 | ||
| Mean± s.d. | 366.3 ± 190.9 | 342.2 ± 199.6 | 303.1 ± 181.6 | 0.44 | 0.04 |
| Mean change from baseline± s.d. | −94.7±272.9 | −99.3±217.4 | −123.7±222.6 | 0.56 | 0.08 |
| After 96 weeks therapy | |||||
| Adipo-IR | |||||
| Number | 74 | 69 | 66 | ||
| Mean± s.d. | 61.5 ± 81.5 | 64.8 ± 119.2 | 48.2 ± 49.0 | 0.85 | 0.25 |
| Mean change from baseline± s.d. | −4.28±120.3 | 11.2±120.9 | −3.25±45.4 | 0.8 | 0.31 |
| Insulin(µU/ml) | |||||
| Number | 74 | 73 | 69 | ||
| Mean± s.d. | 22.4 ± 16.6 | 25.8 ± 37.8 | 19.2 ± 19.1 | 0.49 | 0.29 |
| Mean change from baseline± s.d. | −1.11±26.3 | 3.80±37.7 | 2.06±19.1 | 0.37 | 0.56 |
| NEFA(µM) | |||||
| Number | 74 | 74 | 67 | ||
| Mean± s.d | 449.7 ± 228.5 | 409.0 ± 205.8 | 407.1 ± 218.9 | 0.26 | 0.26 |
| Mean change from baseline± s.d. | −11.6±249.8 | −26.3±235.2 | −17.9±217.8 | 0.39 | 0.43 |
Plus-minus values are mean ± SD. Abbreviations: NEFA, non-esterified fatty acids; Adipo-IR, adipose tissue insulin resistance.
Conversion factor used for calculation of Adipo-IR: 1 µU/ml insulin = 6 pM.
For the means of outcome measures, p values were derived from multiple linear regression models with two indicator variables for the effect of treatment versus placebo.
For the mean change in scores, p values were calculated with multiple linear regression models with two indicator variables for the effect of treatment versus placebo, adjusting for the baseline value of the outcome.
Figure 1. Changes in Adipo-IR throughout the PIVENS clinical trial.
Adipo-IR index was calculated [fasting NEFA (mM) × fasting insulin (pM)] at baseline and after 16 and 96 weeks of placebo, pioglitazone, or vitamin E therapy. Throughout the 96 week treatment period, Adipo-IR was unchanged in patients receiving placebo or vitamin E therapy (Panel A). In contrast, pioglitazone significantly reduced Adipo-IR after 16 weeks compared to the placebo and vitamin E groups (p=0.005), but this effect was not maintained out to 96 weeks (Panel A). Changes in the individual components used to calculate Adipo-IR index, fasting NEFA concentrations (Panel B) and fasting insulin levels (Panel C), are also shown. Note that conversion of NEFA concentration units (from µM to mM) and insulin concentration units (from µU/ml to pM) was necessary for calculation of Adipo-IR index. Refer to Table 1 for the standard deviations of the measures and the p values for the comparisons between the treatment group and placebo at each time point presented in the panels.
Cross-sectional Correlates of Adipo-IR
The correlates of baseline Adipo-IR from the multiple linear regression analysis are shown in Table 2. Higher BMI, fibrosis score ≥ 3, and higher serum glucose had significant positive association with baseline Adipo-IR. The cross-sectional relationship between Adipo-IR at 96 weeks and demographic, anthropometric, laboratory, and histological variables are shown in Table 2. Higher BMI, female gender, and higher cholesterol levels had significant positive association with Adipo-IR at week 96.
Table 2.
Cross-sectional Correlates of Adipo-IR at Baseline and at 96 weeks
| Correlates at baseline or 96 weeks | Baseline Adipo-IR | Adipo-IR at 96 weeks | ||
|---|---|---|---|---|
| Estimated ratio of Adipo-IR (95% CI) |
P* | Estimated ratio of Adipo-IR (95% CI) |
P‡ | |
| Demographics | ||||
| Female vs. Male | 1.13 (0.79, 1.61) | 0.50 | 2.06 (1.40, 3.03) | <0.001 |
| Hispanic vs. other ethnicities | 1.16 (0.89, 1.50) | 0.27 | 1.09 (0.80, 1.50) | 0.56 |
| Age at Randomization | 1.00 (0.99, 1.01) | 0.68 | 0.999 (0.988, 1.010) | 0.89 |
| Anthropometrics | ||||
| BMI (kg/m2) | 1.06 (1.02, 1.09) | 0.001 | 1.05 (1.01, 1.08) | 0.004 |
| Waist circumference (cm) | 0.99 (0.98, 1.00) | 0.12 | 1.01 (1.00, 1.02) | 0.05 |
| Total body fat (%) | 1.02 (0.99, 1.04) | 0.16 | 0.98 (0.96, 1.00) | 0.12 |
| Laboratory measures | ||||
| AST (U/L) | 0.998 (0.993, 1.003) | 0.46 | 1.00 (0.99, 1.01) | 0.92 |
| ALT (U/L) | 1.001 (0.998, 1.004) | 0.39 | 1.004 (0.999, 1.008) | 0.08 |
| Glucose (mg/dl) | 1.01 (1.00, 1.02) | 0.001 | 1.01 (1.00, 1.02) | 0.001 |
| Triglycerides (mg/dl) | 1.00 (1.00, 1.00) | 0.62 | 1.00 (1.00, 1.00) | 0.96 |
| Total cholesterol (mg/dl) | 1.002 (0.999, 1.005) | 0.17 | 1.004 (1.001, 1.008) | 0.03 |
| HDL cholesterol (mg/dl) | 0.997 (0.988, 1.006) | 0.46 | 0.995 (0.981, 1.004) | 0.29 |
| Histology | ||||
| Fibrosis score: 3–4 vs. <3 | 1.33 (1.05, 1.69) | 0.02 | 1.03 (0.74, 1.41) | 0.88 |
| Steatosis Grade 2–3 vs. 0–1 | 1.21 (0.96, 1.51) | 0.10 | 0.99 (0.75, 1.31) | 0.93 |
| Lobular inflammation >2 foci vs. 0–2 foci | 1.02 (0.80, 1.28) | 0.89 | 1.08 (0.78, 1.50) | 0.63 |
| Ballooning: Many vs. None/Few | 1.16 (0.93, 1.45) | 0.20 | 0.99 (0.72, 1.37) | 0.97 |
| NAS>=5 vs. <5 | 0.91 (0.68, 1.21) | 0.52 | 1.00 (0.66, 1.52) | 1.00 |
| Resolution of NASH | - | - | 0.74 (0.56, 0.98) | 0.03 |
P values were from multiple linear regression model, regressing the logarithm of Adipo-IR at baseline on the listed correlates at baseline.
P values were from multiple linear regression model, regressing the logarithm of Adipo-IR at 96 weeks on the listed correlates at 96 weeks.
Abbreviations: BMI, body mass index; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDL, low-density lipoprotein; Adipo-IR, adipose tissue insulin resistance; NAS, NAFLD activity score; NASH, nonalcoholic steatohepatitis.
Relationship between Changes in Adipo-IR and Changes in Liver Histology
In the multiple linear regression analysis of log-transformed Adipo-IR, after adjusting for baseline BMI, baseline Adipo-IR, treatment, and ethnicity, there were no significant relationships between change in Adipo-IR at 16 weeks and changes in liver histology between baseline and 96 weeks or change in ALT either at 16 or 96 weeks (Table 3). However, Table 3 also shows that, while there was no significant association between change in Adipo-IR after 96 weeks and the primary histological outcome of the PIVENS trial or change in ALT, there were significant associations between improvement in Adipo-IR after 96 weeks and histologic measures: improvement in ballooning (p=0.03), NAS (p=0.01), and fibrosis (p=0.004). There was also a strong association between increase in BMI and increase in Adipo-IR from baseline to week 96.
Table 3.
Relationship between change in Adipo-IR and improvements in histologic features at 96 weeks, change in BMI, and change in ALT at 16 or 96 weeks of treatment
| Changes in Histologic and Other Features |
16 weeks | 96 weeks | ||
|---|---|---|---|---|
| Ratio of Adipo-IR Geometric Means* (95% CI) |
p‡ | Ratio of Adipo-IR Geometric Means* (95% CI) |
p¶ | |
|
Histologic Improvement at 96 wk∥ |
0.89 (0.63–1.26) | 0.50 | 1.18 (0.81–1.72) | 0.39 |
|
Improvement in individual Histologic Features at 96 wk† |
||||
| Steatosis | 0.89 (0.65–1.22) | 0.46 | 0.88 (0.62–1.24) | 0.47 |
| Lobular Inflammation | 1.02 (0.76–1.35) | 0.91 | 1.25 (0.90–1.72) | 0.18 |
| Hepatocellular Ballooning | 1.16 (0.81–1.65) | 0.42 | 0.63 (0.41–0.94) | 0.03 |
| Fibrosis | 0.91 (0.67–1.25) | 0.57 | 0.59 (0.41–0.84) | 0.004 |
| NAS | 1.01 (0.67–1.54) | 0.95 | 0.52 (0.33–0.82) | 0.01 |
| ∆BMI (kg/m2) at 16 wk | 1.06 (0.97–1.16) | 0.20 | ||
| ∆BMI (kg/m2) at 96 wk | 1.11 (1.04–1.18) | 0.002 | ||
| ∆ALT (U/L) at 16 wk | 0.998 (0.998–1.000) | 0.35 | ||
| ∆ALT (U/L) at 96 wk | 0.999 (0.999–1.000) | 0.73 | ||
Histologic Improvement required improvement by 1 or more points in the hepatocellular ballooning score; no increase in the fibrosis score; and either a decrease in the activity score for nonalcoholic fatty liver disease to a score of 3 points or less or a decrease in the activity score of at least 2 points, with at least a 1-point decrease in either the lobular inflammation or steatosis score.
The binary change variables for histology scores are defined as improvement versus no improvement. For each histology score, improvement is: at least a 1-point decrease in the Steatosis score; at least a 1-point decrease in the lobular inflammation score; improvement of 1 or more points in the hepatocellular ballooning score; no increase in the fibrosis score; and at least 2 points decrease in the NAS score, respectively.
Geometric means estimated as exponentiated regression coefficients from the multiple linear regression model for log-transformed Adipo-IR (see Methods).
P values are from multiple linear regression models, regressing the log of the ratio between the outcome variables at 16 weeks and baseline on the five indicator variables for improvement in the histologic features at 96 weeks, the two indicator variables for treatment, the deltas for BMI, and ALT at 16 weeks, adjusting for baseline Adipo-IR, ethnicity, and baseline BMI. N=191; R2=0.39; RMSE=0.81.
P values are from multiple linear regression models, regressing the log of the ratio between the outcome variables at 96 weeks and baseline on the five indicator variables for improvement in the histologic features at 96 weeks, the two indicator variables for treatment, the deltas for BMI, and ALT at 96 weeks, adjusting for baseline Adipo-IR, ethnicity, and baseline BMI. N=205; R2=0.29; RMSE=0.93.
Abbreviations: Adipo-IR, adipose tissue insulin resistance; NAS, NAFLD activity score; BMI, body mass index; ALT, alanine aminotransferase; NEFA, nonesterified fatty acids; CI, confidence intervals.
Discussion
Our study adds significantly to the growing body of literature investigating the significance of Adipo-IR in patients with NAFLD and NASH. Gastaldelli et al. initially described the characteristics of Adipo-IR in 47 subjects with NASH and 20 non-diabetic controls who participated in a randomized controlled trial that demonstrated the efficacy of pioglitazone administered for 6 months in improving liver histology in NASH (10). In this study, there was a strong relationship between Adipo-IR and NASH that was independent of degree of obesity and pioglitazone reduced Adipo-IR significantly in participants with NASH. Interestingly, improvement in Adipo-IR was closely associated with an improvement in steatosis and necroinflammation, suggesting that pioglitazone improves NASH through its effect on dysfunctional adipose tissue. More recently, Lomonaco et al. measured Adipo-IR in 207 individuals with NAFLD and 20 controls without NAFLD (13). This study not only confirmed the strong association between severe Adipo-IR and NAFLD, but showed a strong relationship between Adipo-IR and advanced liver fibrosis. Individuals with Adipo-IR in top two quartiles had more severe liver fibrosis compared to individuals with Adipo-IR in bottom two quartiles. Our study differed from these two studies in that it consisted of a larger sample size which was followed for a much longer treatment period and also examined the characteristics of Adipo-IR in vitamin E treated patients who had robust histological response.
The main observations of our study are (a) there was an early improvement in Adipo-IR with pioglitazone therapy, but it was not maintained throughout the 96 week treatment period, (b) vitamin E treatment had no effect on Adipo-IR despite its benefits on histology, and (c) improvement in Adipo-IR at week 96 correlated with significantly with improvement in ballooning, fibrosis, and NAS, but not with primary histological endpoint of the PIVENS trial. Furthermore, improvement in Adipo-IR at 96 weeks was associated with resolution of NASH at week 96 weeks, but this association was of borderline statistical significance.
In the current study, we observed an initial improvement in Adipo-IR in pioglitazone treated patients but this reduction was not persistent at week 96. The mechanism behind this rebound in Adipo-IR is not entirely clear, but it is possibly related to the weight gained by the pioglitazone treated patients. As shown in Table 3 there was a strong association between increase in Adipo-IR and increase in BMI over the 96-week treatment period (p=0.005) , and 82% of patients in the pioglitazone group experienced an increase in BMI during the treatment period. Additional measurements of Adipo-IR between weeks 16 and 96 would have helped to further define this rebound in Adipo-IR, but due to sample constraints, we could not perform these interval Adipo-IR measurements.
As Gastaldelli et al. observed a significant relationship between changes in Adipo-IR and changes in steatosis and ballooning in their study, we were interested to explore if early or long term changes in Adipo-IR would predict histological changes observed at week 96. We also observed that change in Adipo-IR at week 96 was significantly associated with changes in many histological parameters in the entire cohort irrespective of the treatment group. But it was surprising that change in Adipo-IR in the pioglitazone treated subjects between baseline and week 96 did not correlate with changes in any histological parameters and we suspect this is because of the rebound in Adipo-IR that we observed between week 16 and week 96.
Of particular interest is the relationship between Adipo-IR and liver fibrosis that we observed in this study. Subjects with F3/F4 had an average 33% higher Adipo-IR than individuals with F1/F2 (p=0.017) and improvement in fibrosis at week 96 was associated with significantly decreased Adipo-IR over the treatment period. Considering that Lomonaco et al. also observed a strong relationship between Adipo-IR and fibrosis, we believe that this relationship deserves further investigation. The mechanistic basis for this relationship is unclear. Advanced cirrhosis has been associated with decreased hepatic extraction of insulin which may give rise to higher Adipo-IR (14,15), but none of the patients with PIVENS had advanced cirrhosis. Therefore we do not believe that the relationship observed between fibrosis and Adipo-IR can be explained by abnormal hepatic extraction of insulin. Our observations raise the intriguing possibility that Adipo-IR may serve as a biomarker for predicting changes in liver histology in patients with NASH.
In conclusion, pioglitazone therapy improved Adipo-IR in the short-term but this effect was not sustained, whereas vitamin E treatment had no short- or long-term effects on Adipo-IR. The rebound in Adipo-IR in pioglitazone treated individuals is likely related to weight gain that is common in individuals receiving pioglitazone. The intriguing relationship between Adipo-IR and fibrosis noted in this study as well as the study by Lomonaco et al. requires further investigation.
Acknowledgments
Financial Support: Supported in part by K24 DK072101 (to NC). PIVENS was supported by grants from the National Institutes of Health to the NASH Clinical Research Network (U01DK61718, U01DK61728, U01DK61731, U01DK61732, U01DK61734, U01DK61737, U01DK61738, U01DK61730, U01DK61713) and, in part, by the NIH intramural program, National Cancer Institute. Other grant support includes the following National Institutes of Health General Clinical Research Centers or Clinical and Translational Science Awards: UL1RR024989, UL1RR024128, M01RR000750, UL1RR024131, M01RR000827, UL1RR025014, M01RR000065.
Abbreviations
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- PIVENS
Pioglitazone versus Vitamin E versus Placebo for the Treatment of Nondiabetic Patients with Nonalcoholic Steatohepatitis
- NASH CRN
Nonalcoholic Steatohepatitis Clinical Research Network
- HOMA-IR
Homeostasis Model Assessment for insulin resistance
- Adipo-IR
adipose tissue insulin resistance
- NEFA
nonesterified fatty acids
- BMI
body mass index
- ALT
alanine aminotransferase
- NAS
NAFLD activity score
- IQR
interquartile range
- SD
standard deviation
- PPARγ
peroxisome proliferator activated nuclear receptor γ; TGF-β, transforming growth factor-β
Appendix
Members of the Nonalcoholic Steatohepatitis Clinical Research Network:
Members of the Nonalcoholic Steatohepatitis Clinical Research Network Adult Clinical Centers
Case Western Reserve University Clinical Centers:
MetroHealth Medical Center, Cleveland, OH: Arthur J. McCullough, MD; Patricia Brandt; Diane Bringman, RN (2004–2008); Srinivasan Dasarathy, MD; Jaividhya Dasarathy, MD; Carol Hawkins, RN; Yao-Chang Liu, MD (2004–2009); Nicholette Rogers, PhD, PA-C (2004–2008)
Cleveland Clinic Foundation, Cleveland, OH: Arthur J. McCullough, MD; Srinivasan Dasarathy, MD; Mangesh Pagadala, MD; Ruth Sargent, LPN; Lisa Yerian, MD; Claudia Zein, MD
California Pacific Medical Center, San Francisco, CA: Raphael Merriman, MD; Anthony Nguyen
Columbia University, New York, NY: Joel E. Lavine, MD, PhD
Duke University Medical Center, Durham, NC: Manal F. Abdelmalek, MD; Stephanie Buie; Anna Mae Diehl, MD; Marcia Gottfried, MD (2004–2008); Cynthia Guy, MD; Meryt Hanna (2010); Christopher Kigongo; Paul Killenberg, MD (2004–2008); Samantha Kwan, MS (2006–2009); Yi-Ping Pan; Dawn Piercy, FNP; Melissa Smith (2007–2010); Savita Srivastava, MD
Indiana University School of Medicine, Indianapolis, IN: Naga Chalasani, MD; Oscar W. Cummings, MD; Marwan Ghabril, MD; Ann Klipsch, RN; Linda Ragozzino, RN; Girish Subbarao, MD; Sweta Tandra, MD; Raj Vuppalanchi, MD
Saint Louis University, St Louis, MO: Debra King, RN; Andrea Morris; Joan Siegner, RN; Susan Stewart, RN; Brent A. Neuschwander-Tetri, MD; Judy Thompson, RN
University of California San Diego, San Diego, CA: Cynthia Behling, MD, PhD; Jennifer Collins; Janis Durelle; Tarek Hassanein, MD (2004–2009); Joel E. Lavine, MD, PhD (2002–2010); Rohit Loomba, MD; Anya Morgan (2009–2010); Thu Nguyen; Heather Patton, MD; Melissa Paiz; Jeffrey B. Schwimmer, MD; Claude Sirlin, MD
University of California San Francisco, San Francisco, CA: Bradley Aouizerat, PhD; Kiran Bambha, MD (2006–2010); Marissa Bass; Nathan M. Bass, MD, PhD; Linda D. Ferrell, MD; Bo Gu (2009–2010); Bilal Hameed, MD; Mark Pabst; Monique Rosenthal (2005–2010); Tessa Steel (2006–2008)
University of Washington Medical Center, Seattle, WA: Matthew Yeh, MD, PhD
Virginia Commonwealth University, Richmond, VA: Sherry Boyett, RN, BSN; Melissa J. Contos, MD; Michael Fuchs, MD; Amy Jones; Velimir AC Luketic, MD; Puneet Puri, MD; Bimalijit Sandhu, MD (2007–2009); Arun J. Sanyal, MD; Carol Sargeant, RN, BSN, MPH; Kimberly Noble; Melanie White, RN, BSN (2006–2009)
Virginia Mason Medical Center, Seattle, WA: Sarah Ackermann; Kris V. Kowdley, MD; Jane Park; Tracey Pierce; Jody Mooney, MS; James Nelson, PhD; Cheryl Shaw, MPH; Alice Stead; Chia Wang, MD
Washington University, St. Louis, MO: Elizabeth M. Brunt, MD
Resource Centers
National Cancer Institute, Bethesda, MD: David E. Kleiner, MD, PhD
National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD: Edward C. Doo, MD; Jay H. Hoofnagle, MD; Patricia R. Robuck, PhD, MPH; Averell Sherker, MD
Johns Hopkins University, Bloomberg School of Public Health (Data Coordinating Center), Baltimore, MD: Patricia Belt, BS; Frederick L. Brancati, MD, MHS (2003–2009); Jeanne M. Clark, MD, MPH; Ryan Colvin, MPH (2004–2010); Michele Donithan, MHS; Mika Green, MA; Rosemary Hollick (2003–2005); Milana Isaacson, BS; Wana K. Jin, BS; Alison Lydecker, MPH (2006–2008), Pamela Mann, MPH (2008–2009); Kevin P. May, MS; Laura Miriel, BS; Alice Sternberg, ScM; James Tonascia, PhD; Aynur Ünalp-Arida, MD, PhD; Mark Van Natta, MHS; Ivana Vaughn, MPH; Laura Wilson, ScM; Katherine Yates, ScM
Footnotes
Potential Conflicts of Interest: Potential conflicts of interests for all authors were fully disclosed in the original PIVENS publication (1). There are no additional conflicts of interests to disclose for this report.
References
- 1.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, McCullough AJ, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844–1850. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 3.Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, Luketic VA, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–1192. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 4.McClain CJ, Mokshagundam SP, Barve SS, Song Z, Hill DB, Chen T, Deaciuc I. Mechanisms of non-alcoholic steatohepatitis. Alcohol. 2004;34:67–79. doi: 10.1016/j.alcohol.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Seppala-Lindroos A, Vehkavaara S, Hakkinen AM, Goto T, Westerbacka J, Sovijarvi A, Halavaara J, et al. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab. 2002;87:3023–3028. doi: 10.1210/jcem.87.7.8638. [DOI] [PubMed] [Google Scholar]
- 6.Bugianesi E, Gastaldelli A, Vanni E, Gambino R, Cassader M, Baldi S, Ponti V, et al. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms. Diabetologia. 2005;48:634–642. doi: 10.1007/s00125-005-1682-x. [DOI] [PubMed] [Google Scholar]
- 7.Gastaldelli A, Cusi K, Pettiti M, Hardies J, Miyazaki Y, Berria R, Buzzigoli E, et al. Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology. 2007;133:496–506. doi: 10.1053/j.gastro.2007.04.068. [DOI] [PubMed] [Google Scholar]
- 8.Korenblat KM, Fabbrini E, Mohammed BS, Klein S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology. 2008;134:1369–1375. doi: 10.1053/j.gastro.2008.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, Balas B, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297–2307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- 10.Gastaldelli A, Harrison SA, Belfort-Aguilar R, Hardies LJ, Balas B, Schenker S, Cusi K. Importance of changes in adipose tissue insulin resistance to histological response during thiazolidinedione treatment of patients with nonalcoholic steatohepatitis. Hepatology. 2009;50:1087–1093. doi: 10.1002/hep.23116. [DOI] [PubMed] [Google Scholar]
- 11.Chalasani NP, Sanyal AJ, Kowdley KV, Robuck PR, Hoofnagle J, Kleiner DE, Unalp A, et al. Pioglitazone versus vitamin E versus placebo for the treatment of non-diabetic patients with non-alcoholic steatohepatitis: PIVENS trial design. Contemp Clin Trials. 2009;30:88–96. doi: 10.1016/j.cct.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 13.Lomonaco R, Ortiz-Lopez C, Orsak B, Webb A, Hardies J, Darland C, Finch J, Gastaldelli A, Harrison S, Tio F, Cusi K. Effect of adipose tissue insulin resistance on metabolic parameters and liver histology in obese patients with NAFLD. Hepatology. 2011 Dec 20; doi: 10.1002/hep.25539. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Marchesini G, Pacini G, Bianchi G, Patrono D, Cobelli C. Glucose disposal, beta-cell secretion, and hepatic insulin extraction in cirrhosis: a minimal model assessment. Gastroenterology. 1990;99:1715–1722. doi: 10.1016/0016-5085(90)90478-j. [DOI] [PubMed] [Google Scholar]
- 15.Greco AV, Mingrone G, Mari A, Capristo E, Manco M, Gasbarrini G. Mechanisms of hyperinsulinemia in Child's Disease grade B liver cirrhosis investigated in free liver conditions. Gut. 2002;51:870–875. doi: 10.1136/gut.51.6.870. [DOI] [PMC free article] [PubMed] [Google Scholar]