Abstract
Autism spectrum disorders are a heterogeneous group of behaviorally defined disorders having complex etiologies. We previously reported a direct correlation between lower plasma levels of the immunoglobulins (Ig) IgG and IgM and increased severity of behavioral symptoms in children with autism. Our current objective was to determine if these reduced plasma levels of IgG and IgM are the result of defective B cell development, activation, or function. Results suggest no differences in the B cell parameters measured, indicating that decreased Ig in autism is not a result of B cell dysfunction and other immune cells might be involved.
Keywords: Autism, B cell, Immunoglobulin, Immune system
1. Introduction
Autism spectrum disorders (ASD) are a heterogeneous group of behaviorally defined disorders that are widely considered to be genetic in origin on account of the high rates of heritability. However, only a minority of ASD cases have been directly associated with a genetic change including chromosomal abnormalities, copy number variations (CNV), and single-gene disorders (Miles, 2011). Moreover, the etiological significance of these changes has yet to be fully determined. It is likely that the risk associated with genetic perturbations is manifested by either a direct impact on the nervous system, or indirectly through the disruption of peripheral organ systems that negatively affect nervous system development and/or function. The immune system is one such network that has been shown to be very important during neurodevelopment as well as in adult brain homeostasis (Marques-Deak et al., 2005; Mehler and Kessler, 1998), and perturbations of which have been linked to autism.
Previous studies have reported changes in several aspects of the immune system in children with autism. These include differences in immune cell numbers, differences in immune cell phenotype, the presence of autoantibodies, altered cytokine profiles, immune pathology in the gut, altered levels of complement proteins, and altered levels of immunoglobulins (Ashwood et al., 2006). Of these immune differences, changes in levels of immunoglobulins are commonly reported. However, these reports are often variable and no clear consensus has emerged. For example, IgG and IgM have been reported to be both increased (Croonenberghs et al., 2002; Trajkovski et al., 2004) and decreased (Ashwood et al., 2003; Gupta et al., 1996) in children with ASD compared to typically developing controls. Differences that are most likely attributed to small sample sizes (range 18–40 autism subjects), variations in types of controls (siblings vs. age-matched general population controls), associated medical conditions (such as gastrointestinal dysfunction), age, and method of detection. Previously, our laboratory conducted a large study including 143 children with an autism spectrum disorder aged 2–5 years as well as 96 aged matched general population controls and 32 age matched developmentally delayed controls. We reported lower levels of immunoglobulin (IgG and IgM) in children with autism, which significantly correlated with severity of behavioral symptoms (Heuer et al., 2008). Following this publication, a study of the same population of children reported an increase in IgG4, a subclass of IgG, but no change in the remaining subclasses IgG1, IgG2, and IgG3 using a different method of detection (Enstrom et al., 2009), adding to the uncertainty of immunoglobulin production as a predictor of autism risk.
Immunoglobulin production is the end result of B cell activation, and is generated during an immune response. We hypothesized that altered levels of total immunoglobulin were indicative of a defect in cellular function within the B cell fraction, and that discovery of the mechanism responsible would provide evidence for an immune mediated etiology in autism. B cells are stimulated upon encounter with their specific antigen and begin to divide. Once activated, the B cells present the antigen to specific T cells, which further instruct them to switch from producing IgM to producing IgG, IgA, or IgE. Some of these mature B cells will then be directed to become either memory B cells, or plasma cells that migrate to the bone marrow and produce mass quantities of antigen specific immunoglobulin. In this study, to determine if there was a functional defect in B cells within the autism population, we examined B cell subpopulations within the circulation, as well as the in vitro B cell response to antigenic stimulation.
2. Methods
2.1 Sample Collection
All children (n=73) originally participated in the Childhood Autism Risks from Genetics and the Environment (CHARGE) study (Hertz-Picciotto et al.). The children enrolled in the current study (CHARGE-back), met the following criteria: a) they were between the ages of 48 and 108 months at the time of blood collection b) lived with at least one biologic parent, c) had an English or Spanish speaking parent, d) were born in California, and e) resided in the catchment areas of Regional Centers in Northern California. Children with ASD were first identified through Californian Regional Center referral, which provides case management services to eligible children with developmental disorders across socioeconomic levels and racial/ethnic groups. Families from the original CHARGE study were contacted and recruited to participate in CHARGE-back.
This study protocol followed the ethical guidelines of the most recent Declaration of Helsinki (Edinburgh, 2000) and was approved by the institutional review boards at the University of California, Davis and The State of California Department of Developmental Services. Informed consent was obtained prior to participation.
2.2 Measures and Procedures for Original CHARGE Participants
The diagnosis of autism (AU, n=32), autism spectrum disorder (ASD, n=10), or typically developing (TD, n=31) was confirmed in all children in the original CHARGE study using the Autism Diagnostic Interview–Revised (ADI-R) (Lord et al., 1997) and the Autism Diagnostic Observation Schedule modules (Mercade et al.) 1 or 2 (DiLavore et al., 1995; Joseph et al., 2002; Lord et al., 2001; Owley et al., 2001). All CHARGE study clinical assessment personnel have attained research reliability on the ADI-R and the ADOS and include bilingual and bicultural staff, which administered assessment measures in Spanish for Spanish-speaking families unable to participate in English. Final autism case status was defined as meeting criteria on the communication, social interaction, and repetitive behavior domains of the ADI-R with onset prior to 36 months and scoring at or above the social + communication cutoff for autism on the ADOS module 1 or 2. Children classified with autism spectrum disorders (ASD sample) did not meet full criteria for autism on either or both the ADI-R and ADOS instruments, but did meet criteria on either the communication or the social interaction domain of the ADI-R prior to 36 months, were within 2 points of the cut-off on the other domain, and were above the social + communication cutoff for ASD on the ADOS module 1 or 2. The sample designation AU-ASD refers to the combined AU and ASD subgroups. The Social Communication Questionnaire was used to screen for behavioral and developmental characteristics of autism among the TD controls; children who scored above the screening cut-off score of 15 were fully assessed using the ADI-R and ADOS. Controls who did not meet criteria for AU-ASD, or for a developmental delay based on the Vineland Scales of Adaptive Behavior (Sparrow et al., 1984) and the Mullen Scales of Early Learning (Mullen, 1995)(scores for cognitive and adaptive function below 70), were classified as TD. Subjects were not included if they were currently on intravenous IgG therapy.
2.3 Cell Culture
Whole blood was collected from subjects through venipuncture into 8.5ml citrate containing tubes (BD Biosciences, San Diego CA). One hundred micro liters of whole blood was aliquoted for cellular phenotypic analysis by flow cytometry. The remaining blood was centrifuged at 900g for 10 minutes and the plasma removed. The cell pellet was re-suspended in HBSS and layered over Histopaque density gradient (Sigma, St. Louis, MO) followed by centrifugation at 600g for 30min. The peripheral blood mononuclear cell (PBMC) layer was removed, washed, and centrifuged twice more at which point cells were counted with a hemocytometer and plated into three wells (in triplicate) of a 96 well plate at 500,000 cells per well. Triplicate wells were then treated with either media alone, 10ug/ml Staphylococcus aureus Cowan (Sigma, St. Louis, MO), 10ug/ml Pokeweed Mitogen (Sigma, St. Louis, MO), 5% PHA leukocyte conditioned medium (Stem cell technologies, Vancouver, Canada), or 10ug/ml ODN 2006 CpG (Invivogen, San Diego, CA). After 72 hours in culture, cells were pulsed with 10uM BrdU for 24 hours (BD Biosciences, San Diego, CA).
2.4 Whole Blood Phenotyping
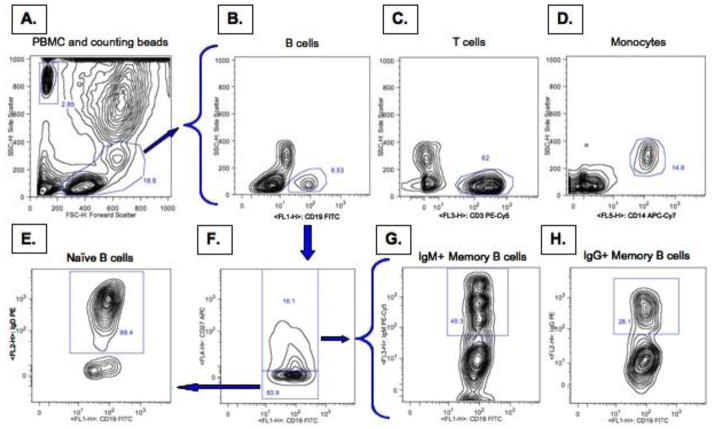
Four panels were developed to assess B cell populations by flow cytometry. One hundred micro liters of whole blood was aliquoted into each of four 15 ml tubes for phenotypic analysis of cell populations present in the circulation. Cells were washed twice with 10 ml FACS buffer and centrifuged at 900g for 10 min. The cells were then treated with FcR block according to manufactures instructions for 30 min (Miltenyi biotech, Auburn, CA). Anti-human fluorochrome labeled antibodies (BD Biosciences, San Diego, CA) were then added at optimal concentrations in four different panels. Panel one consisted of anti-CD19-FITC, anti-IgD-PE, anti-CD3-PE-Cy5, anti-CD27-APC, and anti-CD14-APC-Cy7. Panel two consisted of anti-CD19-FITC, anti-IgG-PE, anti-IgM-PE-Cy5, and anti-CD27APC. Panel three (anti-CD19-FITC, anti-IgD-PE, anti-CD3-PE-Cy5, and anti-CD14-APC-Cy7) and four (anti-CD19-FITC, anti-IgG-PE, and anti-CD27APC) were identical to panel two minus anti-CD27APC (panel 3) or anti-IgM-PE-Cy5 (panel 4) in order to optimize gating strategy (Fluorescence minus one). Cells were then incubated for 30 min at room temp followed by addition of RBC lysis solution for 12 min (BD Biosciences, San Diego, CA). Lysis solution was neutralized with 5 ml FACS buffer and then 50 μl of counting beads (containing 50,000 beads) were added to allow quantification of absolute numbers of the B cell populations (Invitrogen, Carlsbad, CA). Cells and beads were then centrifuged at 900g, the supernatant aspirated, and 300 μl of 1% paraformaldehyde was added. Samples were run immediately on a five-color FACScan flow cytometer (BD Biosciences, San Diego, CA). Prior to sample analysis, the instrument was calibrated using compensation beads (BD Biosciences, San Diego, CA). Figure 1 is a representative gating strategy and analysis of the cell populations examined in this study. Analysis was performed using FlowJo flow cytometry analysis software.
Figure 1. Flow Cytometry gating strategy.
Whole blood was collected and stained for phenotypic surface markers to assess peripheral blood cell populations. Peripheral blood mononuclear cells were gated on in forward and side scatter to exclude cell debris and polymorphonuclear cells (A). Counting beads were also gated on based on size and fluorescence (A). B cells were assed based on the staining of CD19-FITC (B), T cells on staining of CD3-PE-Cy5 (C), and monocytes based on staining of CD14 APC-Cy7 (D). In the second panel B cells were gated on by the presence of CD19 and then separated based on the presence of CD27-APC (F). Naïve B cells were characterized by the absence of CD27-APC and presence of IgD-PE (E). The CD27-APC positive memory B cells were further split into IgM-PE-Cy5 positive (G) and IgG-PE positive (H) cells.
2.5 Cell proliferation assay
After 96 hours in culture, cells were re-suspended and a 20 μl aliquot was taken for absolute quantitation as described above. The remaining cells were centrifuged at 900g for 4 minutes, and the supernatant aspirated and saved for the determination of IgG levels by ELISA. The cells were then washed with 1ml FACS and treated with FcR block according to manufactures instructions for 30 min (Miltenyi biotech, Auburn, CA). While still in the presence of FcR block, antibodies directed against surface proteins were added, including anti-CD19-FITC, as well as both anti-IgG and anti-IgM un-conjugated antibodies to block fluorochrome associated antibodies from binding surface IgG and IgM. After 30 min incubation at 4°C, the cells were washed and then treated with fix/perm according to manufacturers instructions (BD Biosciences, San Diego, CA). In order to visualize BrdU uptake into the DNA of proliferating cells, 50 ul DNase (300ug/ml) was added for 1hr at 37°C. The remaining antibodies were then added including anti-IgG-PE, anti-IgM-PE-Cy5, and anti-BrdU-APC, for 30 min at room temperature. Cells were then washed, and 300 ul 1%PFA were added prior to analysis on a BD FACScan flow cytometer.
2.6 ELISA
Levels of total IgG and IgM in culture supernatants and plasma were analyzed using commercially available assay kits designed to determine IgG and IgM levels (Immunology Consultants, Newberg, Oregon). The kits were run according to the manufacturer’s instructions. Briefly, samples were added to 96-well plates pre-coated with capture antibody. After a one-hour incubation and subsequent washing, HRP conjugated detection antibodies were added and TMB/peroxide substrate used for visualization of reactivity. Data is reported as mean ng/ml of total IgG or IgM in the cell supernatant.
2.7 Statistical analysis
All analyses were carried out using PASW Statistics GradPack 17.0. Data was natural log-transformed and assessed for potential confounders using covariates considered to be a direct or proxy determinant of the outcome. Covariates selected included age (mother, father, child), sex, race (mother, father, child), socioeconomic factors (mother/father education, payment method for delivery), and immune status (any allergy, ever had asthma, ever had eczema, had MMR vaccine). A Pearson correlation was used to examine associations between each of the covariates with both the individual predictors of interest (markers of B cell function), as well as the outcome variable (diagnostic group). None of the covariates examined exhibited a correlation coefficient with either predictor or outcome that was greater than 0.4, a predetermined value signifying a weak correlation. Covariates were therefore excluded from the statistical model. Independent-samples t-test was used to compare each B cell parameter between diagnostic groups. The relationship between CHARGE and CHARGE-back immunoglobulin levels, with each other as well as with each of the B cell parameters, was analyzed using linear regression.
3. Results
3.1 Longitudinal analysis of total IgG plasma levels for subjects enrolled in both CHARGE and CHARGE-back
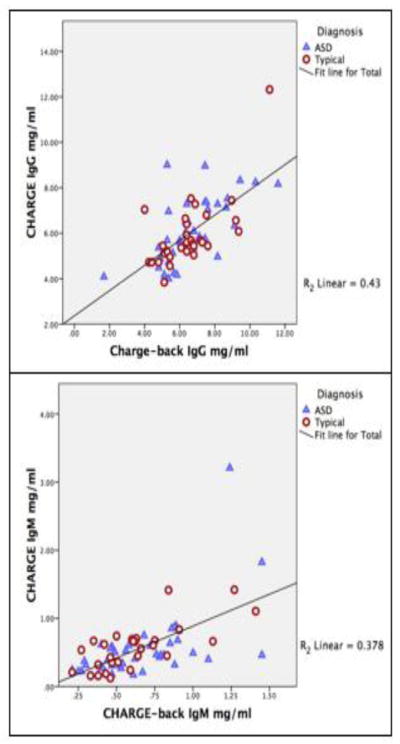
Total levels of immunoglobulin develop over time in the adolescent child, producing little to no IgG of their own at birth, and reaching adult levels between the ages of 6–8 years. In data from the CHARGE study (age 2–5 years), we previously reported differences in plasma IgG and IgM levels in children with autism compared to typically developing controls, showing the greatest difference for IgG. In the current study, we brought back a subpopulation of the original subjects (CHARGE-back ages 4–9 years) to determine plasma immunoglobulin levels at a later stage in development, and to test whether IgG production correlated with B cell function. Due to the manner in which subjects were selected for this study, the differences between IgG levels in AU-ASD (median 6.4 mg/ml; interquartile range 2.04 mg/mL), and TD (6.4 mg/ml; 2.08mg/mL) were not significant between groups in the CHARGE-back study. This is further exemplified when analysis of the original CHARGE data revealed that these same subjects also did not show a significant difference between groups (AU-ASD (5.8; 2.06 mg/mL) and TD (5.56; 1.48 mg/mL)). A similar trend was observed for IgM levels in both CHARGE samples AU- ASD (0.43; .32mg/ml) and TD (0.58; .36mg/ml); and CHARGE-back AU-ASD (0.57; .898 mg/ml), TD (0.6; 0.33 mg/ml). However, we did find that there was a significant positive correlation between the levels of IgG (p<0.0001, B=0.785) and IgM (p<0.0001, B=0.462) present in the original CHARGE study and the corresponding levels for the same individual in the CHARGE-back study (Fig. 2). These data indicate that subjects exhibiting low levels of immunoglobulin in the original study continued to have lower levels over time.
Figure 2. Immunoglobulin levels over time.
Plasma collected from AU-ASD and TD subjects in CHARGE-back were analyzed for total IgG and IgM levels by ELISA. These were then correlated to the levels of IgG and IgM assayed from plasma of the same subjects that was collected during their participation in the original CHARGE study. A significant correlation was observed between the original levels seen in the CHARGE study and the levels seen at a later time point in the CHARGE-back study for both IgG (p<0.0001, B=0.785) and IgM (p<0.0001, B=0.462).
3.2 Quantification of peripheral blood mononuclear cell subsets in children with autism and controls
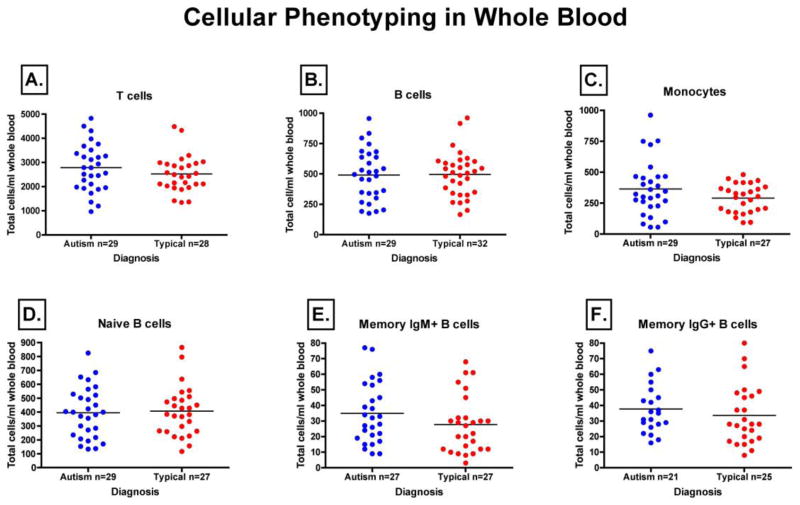
Immunoglobulin production is dependent upon the proper development and activation of B cells. Therefore, we sought to evaluate B cell development, as well as the cells that contribute to B cell activation, by performing a quantitative assessment of their absolute numbers in the peripheral blood of children with AU-ASD and TD controls. As determined by flow cytometry, Figure 3 represents total cell number for each of the cell populations defined. Total number of B cells (CD19+) (Fig 3A) [AU-ASD (median 495, IQR 325), TD (493, 252)] that are ultimately responsible for immunoglobulin production, T cells (CD3+) (Fig 3B) [AU-ASD (2781, 1530), TD (2446, 957)] that provide help to increase immunoglobulin production, and monocytes (CD14+) (Fig 3C) [AU-ASD (323, 237), TD (300, 191)] that affect immunoglobulin production through cytokine secretion, showed no difference between AU-ASD and TD controls.
Figure 3. Median quantities of immune cell subsets in peripheral blood as determined by flow cytometry.
Whole blood from both autism and typically developing controls were stained using fluorochrome-conjugated antibodies directed against cell surface phenotypic markers. Absolute quantification of peripheral blood immune cell populations was then carried out by flow cytometry using counting beads. We found no significant differences in the absolute quantity of CD19+ B cells (A), CD3+ T cells (B), CD14+ Monocytes (C), CD19+CD27−IgD+ Naïve B cells (D), CD19+CD27+IgM+ Memory B cells (E), or CD19+CD27+IgG+ Memory B cells (F).
B cell subpopulations were further analyzed based upon their stage in development. The number of naïve B cells (CD19+IgD+IgM+CD27−), which might be indicative of any dysfunction in early B cell development, did not differ between children with AU-ASD (median 400, IQ 293) and TD controls (384, 214) (Fig. 3D). In addition, the number of IgM+ memory B cells (CD19+IgD−CD27+IgM+) in AU-ASD (32, 33) compared with TD controls (24, 23), and the number of IgG+ memory B cells (CD19+IgD−CD27+IgG+) in AU-ASD (35, 22) and TD controls (27, 30), also showed no differences. Changes in this B cell subpopulation would have been indicative of a problem in the response to antigenic challenge (Fig. 3E,F). We observed no statistical difference between AU-ASD children and TD control children in any of the immune cell subsets that we examined.
3.3 B cell function following ex vivo challenge in children with autism and controls
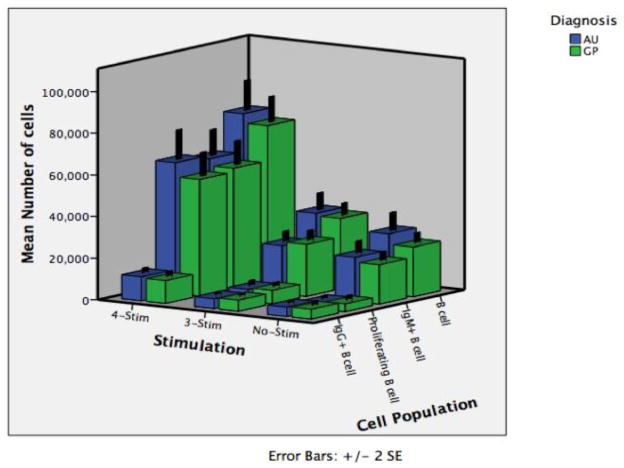
In order to evaluate the functional response of B cells in children with autism we isolated peripheral blood immune cells, stimulated them ex vivo, and measured the proliferative response of specific B cell subsets. B cells require four distinct signals for optimal activation. The first signal is ligation of the B cell receptor (membrane bound Ig), which we accomplished through the application of Staphylococcus aureus C protein, which acts as a B cell superantigen. The second and third signals come from T cell help in the form of both CD40 ligation and stimulation through cytokine production. We mimicked this signal process through the application of pokeweed mitogen (activates a CD40 MAP kinase), and activated T cell supernatant (contains necessary cytokines), respectively. The fourth signal required for optimal activation involves toll-like receptor (TLR) activation, which we achieved through addition of the TLR9 agonist CpG (unmethylated DNA). TLR9 ligation was of particular interest in that previous studies had indicated a lower TLR9 response in children with autism and controls (Enstrom et al.). For this reason, cells were stimulated with either vehicle (No-Stim), the first three stimulants (SAC, Pokeweed mitogen, T cell supernatant) referred to as 3-Stim, or all four stimulants together (3-Stim plus CpG) referred to as 4-Stim. The experimental outcome was measured by quantitatively assessing the total number of B cells (CD19+), the total number of proliferating B cells (CD19+BrdU+), the total number of IgG secreting B cells (CD19+, intracellular IgG+), and the total number of IgM secreting B cells (CD19+ intracellular IgM+) by flow cytometry (Figure 4). Median cell numbers, interquartile range, and p-values are reported in Table 2. While the data indicate a dramatic effect on B cell responsiveness with the addition of stimulation, the varying degrees of stimulation show no statistical difference between the AU and TD groups.
Figure 4. B cell proliferation in response to ex-vivo challenge.
To quantitatively assess B cell function, PBMC from autism and typically developing controls were either unstimulated (No-Stim) or stimulated with B cell specific antigens. Staphylococcus aureus C protein, Pokeweed mitogen, and T cell conditioned medium were included in the 3-Stim treatment group while the 4-Stim treatment group included all reagents in 3-Stim in as well as CpG. After 96 hours cells were assessed by flow cytometry for absolute number of total B cells (CD19+), IgM producing B cells (CD19+, intracellular IgM+), actively proliferating B cells (CD19+BrdU+), and IgG producing B cells (CD19+, intracellular IgG+). The data indicate a dramatic effect on B cell responsiveness with the addition of stimulation. However, cellular responses to the varying degrees of stimulation show no statistical difference between behavioral groups.
Table 2. Descriptive statistics for both cell proliferation assays and supernatant immunoglobulin levels for AU-ASD and Typically Developing children.
Treatment groups were either unstimulated (No-Stim) or stimulated with B cell specific antigens. Staphylococcus aureus C protein, Pokeweed mitogen, and T cell conditioned medium were included in the 3-Stim treatment group while the 4-Stim treatment group included all reagents in 3-Stim in as well as CpG.
| Assay | Treatment | Diagnosis | N | Median | ta | p-value |
|---|---|---|---|---|---|---|
| Cell Proliferation (Total cells per well) |
No-Stim B cell | AU-ASD | 29 | 19,087 | 27,420 | 0.491 |
| TD | 28 | 23,000 | 13,704 | |||
| No-Stim IgM+ B cell | AU-ASD | 29 | 16,677 | 15,153 | 0.446 | |
| TD | 28 | 17,055 | 13,923 | |||
| No-Stim Proliferating B cell | AU-ASD | 29 | 1,770 | 2,140 | 0.761 | |
| TD | 28 | 1,689 | 3,107 | |||
| No-Stim IgG+ B cell | AU-ASD | 29 | 3,665 | 3,283 | 0.858 | |
| TD | 28 | 3,555 | 4,017 | |||
| 3-Stim B cell | AU-ASD | 29 | 27,635 | 25,868 | 0.997 | |
| TD | 28 | 30,670 | 19,950 | |||
| 3-Stim IgM+ B cell | AU-ASD | 29 | 17,892 | 15,920 | 0.672 | |
| TD | 28 | 21,319 | 17,566 | |||
| 3-Stim Proliferating B cell | AU-ASD | 29 | 3,191 | 5,525 | 0.349 | |
| TD | 28 | 3,810 | 7,298 | |||
| 3-Stim IgG+ B cell | AU-ASD | 29 | 3,775 | 4,364 | 0.213 | |
| TD | 28 | 5,455 | 4,727 | |||
| 4-Stim B cell | AU-ASD | 29 | 72,349 | 41,279 | 0.386 | |
| TD | 28 | 67,418 | 43,618 | |||
| 4-Stim IgM+ B cell | AU-ASD | 29 | 53,859 | 41,742 | 0.348 | |
| TD | 28 | 46,376 | 42,361 | |||
| 4-Stim Proliferating B cell | AU-ASD | 29 | 53,648 | 48,912 | 0.201 | |
| TD | 28 | 50,518 | 40,102 | |||
| 4-Stim IgG+ B cell | AU-ASD | 29 | 10,329 | 7,997 | 0.99 | |
| TD | 28 | 10,205 | 10,876 | |||
| Supernatants (ng/ml Ig) | No-Stim IgG | AU-ASD | 35 | 56.89 | 26.28 | 0.971 |
| TD | 29 | 51.76 | 27.95 | |||
| No-Stim IgM | AU-ASD | 30 | 5.09 | 7.83 | 0.254 | |
| TD | 28 | 5.02 | 3.12 | |||
| 3-Stim IgG | AU-ASD | 35 | 36.62 | 24.44 | 0.594 | |
| TD | 30 | 32.65 | 20.69 | |||
| 3-Stim IgM | AU-ASD | 30 | 7.49 | 6.84 | 0.321 | |
| TD | 28 | 7.85 | 5.35 | |||
| 4-Stim IgG | AU-ASD | 35 | 40.28 | 19.6 | 0.133 | |
| TD | 30 | 32.96 | 19.52 | |||
| 4-Stim IgM | AU-ASD | 30 | 22.75 | 36.92 | 0.512 | |
| TD | 28 | 23.77 | 29.19 |
3.4 Ex-vivo production of immunoglobulin in response to stimulation
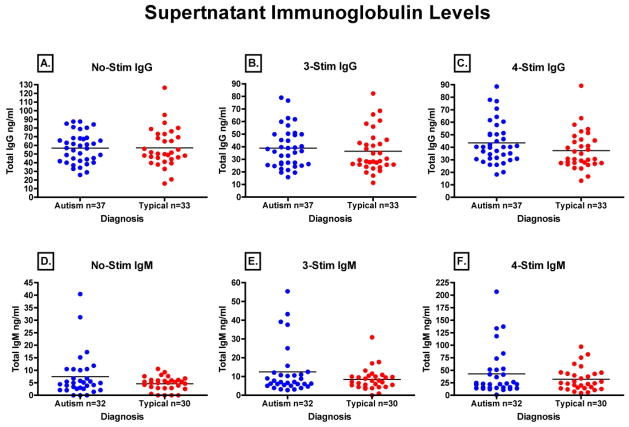
Our final question was whether or not there was a difference in immunoglobulin production between AU-ASD and TD children’s B cells under optimal in-vitro conditions. To do so, we measured the total levels of immunoglobulin produced by stimulated B cells in their cell culture supernatants after four days of stimulation. Figure 5 represents the total IgG (Fig. 5A, B, and C) and IgM (Fig. 5D,E, and F) produced in response to the different levels of stimulation. Median, interquartile range, and p-values are reported in Table 2. While there were differences in the total level of immunoglobulin produced by B cells depending on the amount of stimulation, there were no statistical differences between the behavioral groups. The lack of any difference between AU-ASD and TD children in any of the B cell parameters measured led us to evaluate the relationship between ex-vivo B cell function and plasma levels of IgG and IgM. In a linear regression model there were no significant correlations between any of the B cell parameters measured and plasma levels of either IgG or IgM (data not shown). Thus, for those children with lower IgG, there were no defects noted in B cell function for any parameter tested. For example, one subject had plasma IgG concentration of less than 2.0 mg/ml, which is considered clinically low. However, the functional status of this subject’s B cells was within one standard deviation both above and below the mean. This would indicate that any difference in plasma immunoglobulin levels between AU-ASD and TD children is not likely the result of defective B cell function.
Figure 5. Immunoglobulin production in response to ex vivo challenge.
To qualitatively asses the B cell response in children with autism and typically developing controls, PBMC from both groups were either un-stimulated or stimulated with B cell specific antigens followed by measurement of total immunoglobulin production in culture supernatants. Neither baseline (No-Stim) (A,D), 3-Stim (Staphylococcus aureus C protein, Pokeweed mitogen, and T cell conditioned medium) (B,E), or 4-Stim (3-stim plus CpG) treatments (C,F) showed any significant difference in IgG or IgM production between AU-ASD children and controls.
3.5 Clinical features of both CHARGE and CHARGE-back study populations with respect to IgG levels
3.5.1 Parent report of GI symptoms
Parent report of six categories of GI symptoms were selected to determine GI status at the time of the CHARGE visit—1) frequent diarrhea, 2) frequent constipation, 3) frequent abdominal pain, 4) frequent gaseousness/bloating, 5) frequent vomiting, 6) frequent pain on stooling. Parents used a Likert scale to rate the child’s symptoms in the past three months with 0=Never, 1=Rarely, 2=Sometimes, 3=Frequently, 4=Always. For the initial CHARGE profile, 1/73 subjects was missing a CHARGE GI History, but no GI problems for that subject were reported in the Child Medical History. Seven children in this subset of 72 were reported to have at least one of the six symptoms frequently (none reported having any of the symptoms always). All seven of the children with reported GI symptoms were in the AU/ASD group. There was no relationship between GI symptoms and plasma IgG concentration (mean IgG in AU/ASD children with GI symptoms was 6.72 mg/ml vs. 6.00 mg/ml in AU/ASD children without GI symptoms (p=0.24)). Two children were reported to have four of the six symptoms frequently and their IgG measures were 8.16 mg/ml and 5.36 mg/ml, which are within normal range for their age.
For the CHARGE-back visit, 3/73 subjects were missing a GI History. Two children were reported as diagnosed with malabsorption, and one with a dairy allergy. Fourteen children in this subset of 70 were reported to have at least one of the six GI symptoms frequently. Twelve of the 14 children with reported frequent GI symptoms were in the AU/ASD group. Mean plasma IgG concentration in AU/ASD children with GI symptoms was slightly higher at 7.36 mg/ml vs. 6.24 mg/ml in AU/ASD children without frequent GI symptoms (p=0.06).
3.5.2 Parent report of restricted diets and use of multivitamins
For the CHARGE study profile, 17 of the children were reported in the GI History to be on some type of restricted diet. Fourteen of the children on restricted diets were in the AU/ASD group while 3 were in the TD group. Reasons given for the AU/ASD food restrictions were as follows: 1) to improve behavior, 2) sensory issues such as aversions (or attractions) to certain textures, colors, or 3) because children would not try new foods. Several parents described their child as a “picky” or “very picky” eater. Of the 3 TD children on a restricted diet, one was for religious purposes and two gave no explanation. Mean IgG in children who were on a restricted diet irrespective of diagnostic group was 5.80 mg/ml vs. 6.01 mg/ml in children who were not on a restricted diet (p=0.57). Sixty four percent of children in this sample were reported to be taking a multivitamin. Of the 17 children on a restricted diet, 13 were taking a multivitamin while 4 were not, with plasma IgG levels of 5.76 and 5.86 mg/ml respectively. When comparing IgG levels in children who did and did not take a multivitamin, irrespective of diet restrictions and diagnostic group, those children who took a multivitamin tended to have slightly lower IgG than those children who did not take a multivitamin, 5.83 mg/ml and 6.41 mg/ml respectively (p=0.11).
For those subjects evaluated in CHARGE-back, 17 children were reported to be on a restricted diet within the three months prior to participation in CHARGE-Back. Of these, 5 were on a gluten-free/casein-free diet, 4 were on a dairy-free diet, 2 were on a wheat-free diet, 1 was on an egg-free diet, and 2 were restricted from eating nuts. Reasons given for food restriction were: 1) sensory issues or strong preference/aversion (n=4), 2) behavior (n=5), and 3) GI symptoms (n=9). Eight of the children on a restricted diet were reported as having food allergies by the parent. Approximately 50% of children in the CHARGE-back sample were reported to be taking a multivitamin. Fifty-five percent of AU/ASD children were reportedly taking multivitamins versus 42% of TD children, but this difference was not statistically significant. Comparing IgG levels in children who did and did not take a multivitamin, irrespective of diet restrictions and diagnostic group, those children who took a multivitamin (n=37) had a mean IgG level of 6.58 mg/ml and those children who did not take a multivitamin (n=36) had the same mean IgG level of 6.58 mg/ml. Similarly, there was no difference when comparing AU/ASD children who were and were not taking a multivitamin (6.53 mg/ml vs. 6.77 mg/ml, p=0.66).
3.5.3 Parent report of current medications
For the original CHARGE evaluation, none of the children in this sample set were taking neuropsychiatric medications or stimulants at the time of blood collection. Supplementary Table 1 shows other current medications reported in one of the following forms: Child Medical History, Services and Treatments Interview, or Blood Draw Information Form, and the number of children taking each medication. The child getting IV IgG was following the DAN! protocol during that period and had an IgG value of 5.36 mg/ml at the time of blood collection.
For the CHARGE-back sample collection period, medications being taken at the time of blood draw as reported in the Blood Draw Information form, and the number of children taking each medication (does not include over-the-counter cough and cold remedies, antipyretics/analgesics, probiotics and other supplements) are listed in Supplementary Table 2. Six children out of 73 had reportedly taken neuropsychiatric medications during the month prior to sample collection. Plasma IgG levels of children taking neuropsychiatric medications were not significantly different than children not taking this type of medication (6.6 mg/ml vs. 6.3 mg/ml respectively).
3.5.4 Recurrent Infections
Complete medical evaluations were administered for the study subjects at the time of the initial CHARGE visit but not for the secondary CHARGE-back visit. A medical history of frequent infection was broken down into 5 categories represented in Supplementary Table 3: 1) frequent otitis, 2) frequent pneumonia, 3) recurrent infection (defined as >2 pneunomia or sinus infections per year or >8 ear infections or abscesses per year), 4) skin infection, or 5) urinary tract infection (UTI). Subjects within the AU/ASD group with a history of any recurrent infection (n=7) had a significantly increased plasma level of total IgG when compared to those children in the AU/ASD group that did not have a history of recurrent infection (n=33) (7.56 mg/ml vs 5.80 mg/ml, p=0.0005). In contrast, TD children with a history of recurrent infection (n=4) did not significantly differ from TD children with no history of recurrent infection with respect to total IgG levels (5.7 mg/ml vs 5.96 mg/ml, p=0.62).
4. Discussion
In our original study, we observed a decrease in the total levels of both IgG and IgM in the peripheral blood of autism children compared to typically developing controls (Heuer et al., 2008). In addition, these lower levels were shown to correlate with more severe scores on the aberrant behavior checklist (ABC). Our aim in the current study was to determine if reduced plasma levels of IgG and IgM in children with autism are the result of defective B cell development, activation, or function. We were unable to discern any significant difference in the total number of naïve, memory IgG, or memory IgM B cell populations in the peripheral blood. This would indicate that there was no dysfunction in B cell development at the level of maturation from bone marrow, or in the transition from naïve B cell to a class-switched memory B cell. The functional response of B cells to antigenic challenge as measured through proliferation was also unchanged between groups. This would indicate that there was no difference in the signaling pathways necessary for B cell activation between autism and controls. Finally, we did not observe a difference in the amount of immunoglobulin produced by B cells between groups in response to in-vitro stimulation, providing further evidence that the decrease in plasma levels of immunoglobulin originally reported is not likely due to an intrinsic defect in B cell function. This is in contrast to a recent study describing an increase in the number of total B cells in autism compared to typically developing controls (Ashwood et al., 2011). However, in comparing the two studies; the subjects were from different study populations (differing median age), had different experimental approaches (SurroScan™ vs flow cytometry) and used different cellular differentiation markers (CD20, CD38, CD5, vs CD19, CD27, IgD, IgG, and IgM in this study). Finally, the Ashwood study was soley a cell phenotyping study whereas in the current study we were able to analyze B cell function following very specific stimuli.
Limitations of this study were two-fold. First, we were unable to include a significant proportion of the subjects who had exhibited clinically low levels of immunoglobulin from the original CHARGE study as planned as these subjects were undergoing immunotherapy. These subjects were of particular interest as we had predicted that any defect would have been most pronounced in this population. However, based on our findings that B cell function does not correlate with plasma levels of immunoglobulin, it is unlikely that these subjects would have skewed our data towards significance. Secondly, in an attempt to bring back subjects with clinically low levels that were not receiving IV IgG therapy, as well as the need for a larger quantity of blood for functional studies, the subjects had aged considerably from a median age of 39 months in CHARGE to a median age of 78 months in CHARGE-back. A valid argument would suggest that as the immune system matures, immunoglobulin levels could normalize between groups over time. However, the goal of this study was to determine if children with autism had an intrinsic defect in B cell function, a condition that should not be affected by age.
Based on evidence presented in this manuscript, it is unlikely that a defect in B cell function is responsible for the lower levels of immunoglobulin previously reported in autism. This leaves us with two other possible explanations for the reduced Ig levels previously reported: 1) Either there is a defect in another immune cell type that contributes to immunoglobulin production, or 2) there is a difference in the development of the immune system in some children with autism for which the environment and age could be important factors. In addition, while not a comprehensive study of potential environmental factors, we did have access to limited data on; GI symptoms, diet and nutritional supplementation, medication history, and history of recurrent infections. Interestingly, children with GI symptoms at the time of blood draw or a history of recurrent infection within the AU/ASD population had a significant increase in IgG compared to the typically developing controls. This would indicate that immune challenge at an early age is not contributing to the lower immunoglobulin levels reported, but rather those children with a history of recurrent infection may be artificially skewing our data in the opposite direction. These data may reflect increased levels due to response to infection.
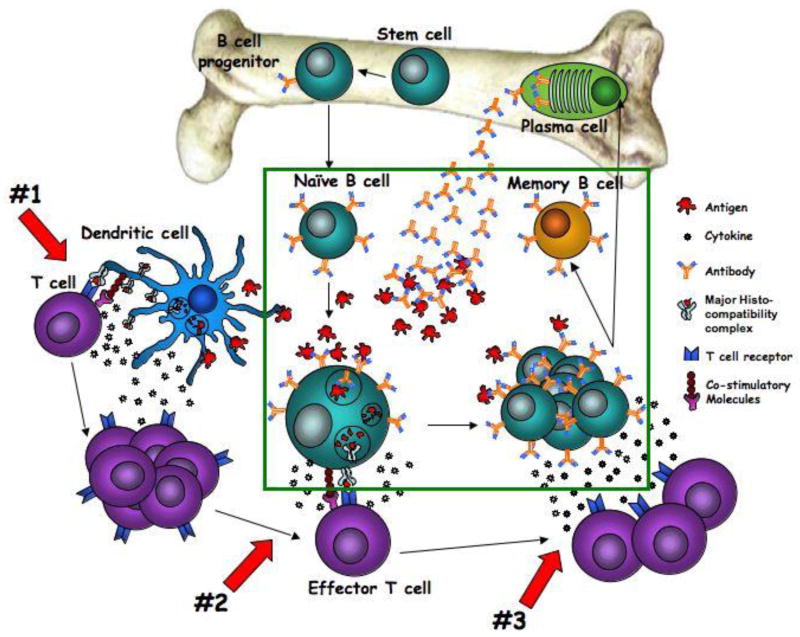
While immunoglobulins are produced directly by B cells, these cells are only one component of a very complex immune system. In the case of immunoglobulin production, both antigen presenting cells and T cells play a significant role in determining the strength and type of immunoglobulin produced. In this study, we simulated these interactions through artificial means in vitro, in order to elucidate whether B cells were responsible for the deficiency. However, in vivo there are multiple cellular interactions that contribute to the humoral immune response, and it is perhaps a defect in either the innate or T cell response to antigenic stimulation that is ultimately responsible for the reduced levels of immunoglobulin. Figure 6 provides a schematic overview of the immune pathway that leads to IgG and IgM production to better envision the major cell types and factors involved in this complex process.
Figure 6. Schematic overview of the immune pathway that leads to IgG and IgM production.
The green box represents cells tested and proven to be functional in this manuscript. Red arrows indicate potential mechanisms of dysfunction not yet tested: #1 indicates the interaction between antigen presenting cells and T cells, #2 indicates the interaction between effector T cells and B cells during antigen recognition, and #3 indicates T cell-B cell interaction at the proliferation/differentiation stage.
Previous studies have implicated defects in both antigen presenting cells and T cells in children with autism. Dysfunction in antigen presenting cells has been shown both within the brain (Vargas et al., 2005), and in the periphery (Enstrom et al.; Jyonouchi et al., 2008). In addition, animal models of autism are currently being developed that rely on the activation of the innate immune system (Fortier et al., 2007; Gilmore et al., 2005; Meyer et al., 2007; Smith et al., 2007). T cells are another critical cell type involved in the production of antibodies by providing important signals to antibody-producing B cells. There have been numerous reports showing T cell dysfunction in children with autism including changes in lymphocyte subsets (Denney et al., 1996), decreased proliferative response to mitogen challenge (Stubbs and Crawford, 1977), altered activation states in the periphery (Ashwood et al.; Plioplys et al., 1994; Warren et al., 1995), and altered cytokine profiles (Ashwood et al., 2004; Ashwood and Wakefield, 2006; Gupta et al., 1998; Jyonouchi et al., 2001; Molloy et al., 2006). While these studies have provided evidence for antigen presenting cell and T cell dysfunction, to date there have been no studies examining the consequences of this dysfunction on the production of immunoglobulin.
In addition to the possibility that other cellular fractions are contributing to the decrease in Ig levels, a second possibility is that the immune system of children with autism may be slower to develop to immune competency. In this study we show that there is no inherent defect in B cell function but there still exists the possibility that that the immune system as a whole reacts differently to challenge at a younger age in children with autism (as is evident in decreased Ig levels), especially during critical periods of neurodevelopment. Further investigation is needed to address potential dysfunction in the supporting immune cells that are critical for immunoglobulin production, and while there are significant limitations to conducting experiments in infants, future studies on immune dysregulation should take age into consideration.
Supplementary Material
Table 1.
Age, sex, and ethnicity distribution for children with AU-ASD, and typically developing control children.
| Ethnicity | N | Median Age | Range | Male (N) | Female (N) | |
|---|---|---|---|---|---|---|
| AU-ASD | White | 28 | 85.5 months | 64–102 | 27 | 1 |
| Black | 1 | 73 | na | 1 | 0 | |
| Hispanic | 13 | 77 | 61–108 | 9 | 4 | |
| Total | 42 | 82 | 61–108 | 37 | 5 | |
| TD | White | 17 | 81 | 59–108 | 15 | 2 |
| Hispanic | 7 | 66 | 48–78 | 5 | 2 | |
| Multiracial | 7 | 64 | 49–106 | 4 | 3 | |
| Total | 31 | 73 | 48–108 | 24 | 7 |
Acknowledgments
Sponsor: NIEHS1P01ES11269-01, U.S. EPA Grant R829388.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashwood P, Anthony A, Pellicer AA, Torrente F, Walker-Smith JA, Wakefield AJ. Intestinal lymphocyte populations in children with regressive autism: evidence for extensive mucosal immunopathology. Journal Clinical Immunology. 2003;23:504–517. doi: 10.1023/b:joci.0000010427.05143.bb. [DOI] [PubMed] [Google Scholar]
- Ashwood P, Anthony A, Torrente F, Wakefield AJ. Spontaneous mucosal lymphocyte cytokine profiles in children with autism and gastrointestinal symptoms: mucosal immune activation and reduced counter regulatory interleukin-10. J Clin Immunol. 2004;24:664–673. doi: 10.1007/s10875-004-6241-6. [DOI] [PubMed] [Google Scholar]
- Ashwood P, Corbett BA, Kantor A, Schulman H, Van de Water J, Amaral DG. In search of cellular immunophenotypes in the blood of children with autism. PLoS One. 2011;6:e19299. doi: 10.1371/journal.pone.0019299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah IN, Van de Water J. Altered T cell responses in children with autism. Brain Behav Immun. doi: 10.1016/j.bbi.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Wakefield AJ. Immune activation of peripheral blood and mucosal CD3+ lymphocyte cytokine profiles in children with autism and gastrointestinal symptoms. J Neuroimmunol. 2006;173:126–134. doi: 10.1016/j.jneuroim.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Ashwood P, Wills S, Van de Water J. The immune response in autism: a new frontier for autism research. J Leukoc Biol. 2006;80:1–15. doi: 10.1189/jlb.1205707. [DOI] [PubMed] [Google Scholar]
- Croonenberghs J, Wauters A, Devreese K, Verkerk R, Scharpe S, Bosmans E, Egyed B, Deboutte D, Maes M. Increased serum albumin, gamma globulin, immunoglobulin IgG, and IgG2 and IgG4 in autism. Psychol Med. 2002;32:1457–1463. doi: 10.1017/s0033291702006037. [DOI] [PubMed] [Google Scholar]
- Denney DR, Frei BW, Gaffney GR. Lymphocyte subsets and interleukin-2 receptors in autistic children. J Autism Dev Disord. 1996;26:87–97. doi: 10.1007/BF02276236. [DOI] [PubMed] [Google Scholar]
- DiLavore PC, Lord C, Rutter M. The pre-linguistic autism diagnostic observation schedule. J Autism Dev Disord. 1995;25:355–379. doi: 10.1007/BF02179373. [DOI] [PubMed] [Google Scholar]
- Edinburgh 2000 [Google Scholar]
- Enstrom A, Krakowiak P, Onore C, Pessah IN, Hertz-Picciotto I, Hansen RL, Van De Water JA, Ashwood P. Increased IgG4 levels in children with autism disorder. Brain, Behavior, and Immunity. 2009;23:389–395. doi: 10.1016/j.bbi.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enstrom AM, Onore CE, Van de Water JA, Ashwood P. Differential monocyte responses to TLR ligands in children with autism spectrum disorders. Brain Behav Immun. 24:64–71. doi: 10.1016/j.bbi.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier ME, Luheshi GN, Boksa P. Effects of prenatal infection on prepulse inhibition in the rat depend on the nature of the infectious agent and the stage of pregnancy. Behav Brain Res. 2007;181:270–277. doi: 10.1016/j.bbr.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Gilmore JH, Jarskog LF, Vadlamudi S. Maternal poly I:C exposure during pregnancy regulates TNF alpha, BDNF, and NGF expression in neonatal brain and the maternal-fetal unit of the rat. J Neuroimmunol. 2005;159:106–112. doi: 10.1016/j.jneuroim.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Gupta S, Aggarwal S, Heads C. Dysregulated immune system in children with autism: beneficial effects of intravenous immune globulin on autistic characteristics. J Autism Dev Disord. 1996;26:439–452. doi: 10.1007/BF02172828. [DOI] [PubMed] [Google Scholar]
- Gupta S, Aggarwal S, Rashanravan B, Lee T. Th1- and Th2-like cytokines in CD4+ and CD8+ T cells in autism. J Neuroimmunol. 1998;85:106–109. doi: 10.1016/s0165-5728(98)00021-6. [DOI] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Croen LA, Hansen R, Jones CR, van de Water J, Pessah IN. The CHARGE study: an epidemiologic investigation of genetic and environmental factors contributing to autism. Environ Health Perspect. 2006;114:1119–1125. doi: 10.1289/ehp.8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer L, Ashwood P, Schauer J, Goines P, Krakowiak P, Hertz-Picciotto I, Hansen R, Croen LA, Pessah IN, Van de Water J. Reduced levels of immunoglobulin in children with autism correlates with behavioral symptoms. Autism Res. 2008;1:275–283. doi: 10.1002/aur.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph RM, Tager-Flusberg H, Lord C. Cognitive profiles and social-communicative functioning in children with autism spectrum disorder. J Child Psychol Psychiatry. 2002;43:807–821. doi: 10.1111/1469-7610.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jyonouchi H, Geng L, Cushing-Ruby A, Quraishi H. Impact of innate immunity in a subset of children with autism spectrum disorders: a case control study. J Neuroinflammation. 2008;5:52. doi: 10.1186/1742-2094-5-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jyonouchi H, Sun S, Le H. Proinflammatory and regulatory cytokine production associated with innate and adaptive immune responses in children with autism spectrum disorders and developmental regression. J Neuroimmunol. 2001;120:170–179. doi: 10.1016/s0165-5728(01)00421-0. [DOI] [PubMed] [Google Scholar]
- Lord C, Leventhal BL, Cook EH., Jr Quantifying the phenotype in autism spectrum disorders. Am J Med Genet. 2001;105:36–38. [PubMed] [Google Scholar]
- Lord C, Pickles A, McLennan J, Rutter M, Bregman J, Folstein S, Fombonne E, Leboyer M, Minshew N. Diagnosing autism: analyses of data from the Autism Diagnostic Interview. J Autism Dev Disord. 1997;27:501–517. doi: 10.1023/a:1025873925661. [DOI] [PubMed] [Google Scholar]
- Marques-Deak A, Cizza G, Sternberg E. Brain-immune interactions and disease susceptibility. Mol Psychiatry. 2005;10:239–250. doi: 10.1038/sj.mp.4001643. [DOI] [PubMed] [Google Scholar]
- Mehler MF, Kessler JA. Cytokines in brain development and function. Adv Protein Chem. 1998;52:223–251. doi: 10.1016/s0065-3233(08)60437-4. [DOI] [PubMed] [Google Scholar]
- Mercade J, Espinosa A, Adsuara JE, Adrados R, Segura J, Maes T. Orymold: ontology based gene expression data integration and analysis tool applied to rice. BMC Bioinformatics. 2009;10:158. doi: 10.1186/1471-2105-10-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Yee BK, Feldon J. The neurodevelopmental impact of prenatal infections at different times of pregnancy: the earlier the worse? Neuroscientist. 2007;13:241–256. doi: 10.1177/1073858406296401. [DOI] [PubMed] [Google Scholar]
- Miles JH. Autism spectrum disorders--a genetics review. Genet Med. 2011;13:278–294. doi: 10.1097/GIM.0b013e3181ff67ba. [DOI] [PubMed] [Google Scholar]
- Molloy CA, Morrow AL, Meinzen-Derr J, Schleifer K, Dienger K, Manning-Courtney P, Altaye M, Wills-Karp M. Elevated cytokine levels in children with autism spectrum disorder. J Neuroimmunol. 2006;172:198–205. doi: 10.1016/j.jneuroim.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Mullen E. The Mullen Scales of Early Learning. American Guidance Service; Circle Pines, MN: 1995. [Google Scholar]
- Owley T, McMahon W, Cook EH, Laulhere T, South M, Mays LZ, Shernoff ES, Lainhart J, Modahl CB, Corsello C, Ozonoff S, Risi S, Lord C, Leventhal BL, Filipek PA. Multisite, double-blind, placebo-controlled trial of porcine secretin in autism. J Am Acad Child Adolesc Psychiatry. 2001;40:1293–1299. doi: 10.1097/00004583-200111000-00009. [DOI] [PubMed] [Google Scholar]
- Plioplys AV, Greaves A, Kazemi K, Silverman E. Lymphocyte function in autism and Rett syndrome. Neuropsychobiology. 1994;29:12–16. doi: 10.1159/000119056. [DOI] [PubMed] [Google Scholar]
- Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow SS, Balla DA, Cicchetti DV. Vineland adaptive behavior scales. American Guidance Service; Circle Pines, MN: 1984. [Google Scholar]
- Stubbs EG, Crawford ML. Depressed lymphocyte responsiveness in autistic children. J Autism Child Schizophr. 1977;7:49–55. doi: 10.1007/BF01531114. [DOI] [PubMed] [Google Scholar]
- Trajkovski V, Ajdinski L, Spiroski M. Plasma concentration of immunoglobulin classes and subclasses in children with autism in the Republic of Macedonia: retrospective study. Croatian Medical Journal. 2004;45:746–749. [PubMed] [Google Scholar]
- Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57:67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- Warren RP, Yonk J, Burger RW, Odell D, Warren WL. DR-positive T cells in autism: association with decreased plasma levels of the complement C4B protein. Neuropsychobiology. 1995;31:53–57. doi: 10.1159/000119172. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.