Abstract
Epithelial membrane protein 2 (EMP2) regulates collagen gel contraction by the retinal pigment epithelium cell line ARPE-19 by modulating FAK activation. Collagen gel contraction is one in vitro model for an aberrant wound healing response, proliferative vitreoretinopathy (PVR), which occurs as a complication of severe ocular trauma. The purpose of this study is to investigate whether EMP2 specific recombinant diabody decreases activation of FAK and collagen gel contraction in ARPE-19. Anti-EMP2 diabody was recombinantly constructed from a human phage library-derived clone selected for reactivity against an extracellular domain of human EMP2. ARPE-19 cells were exposed to an anti-EMP2 or control diabody, and toxicity, adhesion, and migration were assessed respectively through toluidine blue exclusion, binding to collagen type 1, and a migration assay. Collagen gel contraction was assessed using an in vitro assay. FAK activation was evaluated using Western blot. Exposure to anti-EMP2 diabody resulted in a 75% reduction in EMP2 protein levels at 4 hours. No significant toxicity was observed with anti-EMP2 diabody at levels that maximally reduced EMP2. Anti-EMP2 diabody, but not control diabody significantly reduced collagen gel contraction (p<0.001), and was not secondary to changes in adhesion or migration. Concordantly, anti-EMP2 diabody as compared to a control diabody reduced collagen stimulated FAK activation (p=0.01). Anti-EMP2 diabody decreases EMP2 protein levels, FAK activation, and collagen gel contraction by ARPE-19 cells without an adverse effect on cell survival. Modulation of EMP2 using anti-EMP2 diabody could be a new approach for targeting EMP2 and pathologic consequences associated with EMP2.
INTRODUCTION
The most common reason for failure of surgical repair of retinal detachment or open globe injuries is the proliferation and contraction of cellular membranes that form in the vitreous cavity, termed proliferative vitreoretinopathy (PVR) (Colyer et al., 2007; Kirchhof, 2004; Sobaci et al., 2000). It is estimated that PVR occurs as a complication in up to 5–20% of cases of rhegmatogenous retinal detachment (Al-Khairi et al., 2008; Asaria and Gregor, 2002; Campochiaro, 1997; Cardillo et al., 1997; Charteris et al., 2002; Glaser et al., 1987; Hinton et al., 2002; James et al., 2007; Kirchhof, 2004; Kruger et al., 2002). In ocular trauma, the risk of PVR is very high. A review of veterans who had suffered ocular trauma with intraocular foreign bodies in Iraq during the years 2003–2005 revealed PVR as the cause for poor vision in 21% of the patients (Colyer et al., 2007). Other studies have reported PVR as the cause for up to 38% of severe vision loss associated with combat or trauma (Bonnet and Fleury, 1991; Sobaci et al., 2005; Sobaci et al., 2000). Surgical therapy for PVR is not always successful. An effective surgical adjuvant to help prevent or treat PVR development may be of potential therapeutic benefit.
The retinal pigment epithelium (RPE) is believed to be one of the critical cell types implicated in PVR. PVR pathogenesis is complex; however it is likely that, following trauma or retinal detachment, RPE cells migrate from their normal anatomic compartment, proliferate, dedifferentiate, undergo an epithelial to mesenchymal transformation (EMT), respond to many stimuli, and help create an intravitreal membrane (Al-Khairi et al., 2008; Asaria and Gregor, 2002; Campochiaro, 1997; Cardillo et al., 1997; Glaser et al., 1987; Kruger et al., 2002; Shimada et al., 2006). It is likely that the RPE cells produce membrane contraction that generates tractional force leading to retinal detachment (Hinton et al., 2002; Jin et al., 2000; Mukherjee and Guidry, 2007; Sakamoto et al., 1994). Control of RPE cellular functions relevant to the pathobiology of PVR, such as membrane contraction, is a primary goal in developing a therapy for PVR.
Epithelial membrane protein-2 (EMP2) is highly expressed in RPE (Wadehra et al., 2003b). EMP2 is a member of the growth arrest specific gene 3/peripheral myelin protein 22 (GAS3/PMP22) group of the tetraspan protein superfamily (Forbes et al., 2007; Wadehra et al., 2004; Wadehra et al., 2002; Wadehra et al., 2008; Wadehra et al., 2003a; Wang et al., 2001). EMP2 has been shown to regulate trafficking, intracellular compartmentalization, and surface display of selected receptors and glycolipids by facilitating transfer of molecules from post-Golgi endosomal compartments to appropriate plasma membrane locations (Wadehra et al., 2004). We have previously shown that EMP2 physically associates with and regulates activity of integrin-FAK signaling complexes (Forbes et al., 2007; Morales et al., 2009b; Wadehra et al., 2005). The FAK signal transduction pathway is a key signaling pathway regulating cellular contractile capacity (Morales et al., 2009a; Morales et al., 2007; Morales et al., 2009b). Increased expression of EMP2 in ARPE-19 cells increased cellular contractile capacity, leading to a more rapid and further development of disease in an in vivo rabbit model of PVR, concomitantly, decreased EMP2 levels reduced PVR severity (Telander et al., 2011). Blockade of EMP2 with anti-human polyclonal EMP2 antibody significantly decreased PVR severity (Telander et al., 2011). These findings, concordant with the role of other members of the tetraspan family, suggest that EMP2 curates molecules at the cell surface that are brought into engagement with specific signaling complexes therefore controlling downstream biologic responses.
Although the polyclonal anti-EMP2 antibody was effective both in vitro and in vivo, a goal was to create a monoclonal antibody reagent that could be effective in blocking the activities of EMP2. A recombinant anti-EMP2 diabody, created in our laboratory, was previously reported to induce cell death and caspase-3 cleavage in human endometrial and ovarian cancer cell lines (Fu et al.; Shimazaki et al., 2008). This response correlated to cellular EMP2 expression. In vivo, treatment of subcutaneous human xenografts of HEC-1A cell lines with anti-EMP2 diabodies suppressed tumor growth and induced cell death in the xenograft (Fu et al., 2010). These studies observed that anti-EMP2 diabody is toxic to malignant cells. In the present paper, the question of efficacy of controlling collagen gel contraction and toxicity of the anti-EMP2 diabody in ARPE-19 cells was tested. The experiments confirmed that anti-EMP2 diabody decreased FAK activation and collagen gel contraction by ARPE-19 without inducing cell death.
METHODS
Cell Line
ARPE-19, a spontaneously arising retinal pigment epithelial (RPE) cell line, which expresses the RPE-specific markers CRALBP and RPE-65, was obtained from the American Type Culture Collection (CRL-2302, ATCC, Manassas, VA). ARPE-19/EMP2, an EMP2 overexpressing cell line, was produced through stable infection of an EMP2 over expressing retrovirus construct (Morales et al., 2007). ARPE-19 cells were cultured in DMEM-F12 medium, supplemented with 10% fetal bovine serum (FBS) (ATCC, Manassas, VA) at 37°C in a humidified chamber with 5% CO2.
Diabody Construction
Diabody construction was performed as previously described (Shimazaki et al., 2008). Briefly, anti-EMP2 diabodies binding to the second extracellular loop of human EMP2 were isolated from a human tonsil antibody phage display library. Preparations of diabody were expressed and purified according to published protocol (Marks and Bradbury, 2004).
Toxicity
ARPE-19 cells were pretreated with 20 μg/ml of anti-EMP2 or control diabody for 2 hours. The cells were then incubated for 48 hours in a 6-well plate in 1ml of serum free media containing 20 μg/ml of diabody. As a positive control for apoptosis ARPE-19 cells were incubated with 4μM Camptothecin (CPT) for 48 hours. Camptothecin inhibits DNA topoisomerase I leading to the induction of apoptosis (Hueber et al., 1998; Nair et al., 2005). Cells death was analyzed by cell count using toluidine blue exclusion.
Adhesion Assay
Adhesion assay was performed as previously described (Morales et al., 2009a). Briefly, ARPE-19 and ARPE-19/EMP2 cells were pretreated with 20 μg/ml of anti-EMP2 or control diabody for 2 hours. The cells were then plated onto a 24-well collagen coated plate (BD Biosciences) at a concentration of 2×105 cells per well. The cells were incubated at 37°C in a humidified chamber with 5% CO2 for 2 hours. The plate was then washed 3 times with PBS to remove any unattached cells. Bound cells were stained with 0.2% crystal violet solution in 10% ethanol. The cell-bound stain was completely solubilized and absorbance measured at a wavelength of 595nm by a Bio-Rad microplate reader 550 (Hercules, CA). Each experiment included at least eight replicates, and at least three independent experiments were performed with comparable results. A Student’s t-test (unpaired, two-tailed) was used and a P<0.05 was judged to be statistically significant.
Migration
Migration assay was performed as previously described (Morales et al., 2009b). Briefly, ARPE-19 cells were seeded onto a 24-well plate and incubated for 3 days until cells reached confluency. The cells were washed with PBS, serum free media was added, and the cells were incubated overnight. A 10μl pipette tip was used to make a scratch in the monolayer and the media was removed and replaced with serum free media that contained 20 μg/ml of anti-EMP2 or control diabody. Pictures of the wound were taken at various time points and percent closure of the scratch was quantified using NIH Image J software. The area of the scratch was measured immediately after the wound is created. Over time, there is migration of cells into the cleared area, however a gap was still visible after 24 hours. The gap size was measured, divided by the original scratch size, and this value was expressed as percent closure.
Collagen Gel Contraction
Collagen gel contraction assays were performed as previously reported (Morales et al., 2007). Briefly, collagen gels were prepared by combining collagen type I (BD Biosciences, San Diego, CA) 10X DMEM, and DMEM/F12. The final concentration of the collagen type I mixture was 2.5mg/ml. 500μl of the collagen solution was added to each well of a 24 well plate and incubated at 37°C in 5% CO2 for 1 hour. Cultured ARPE-19 and ARPE-19/EMP2 cells were harvested and resuspended at a final concentration of 5×105/ml in serum-free DMEM/F12 containing 2, 7, or 20 μg/ml anti-EMP2 or control diabody. The cells were pretreated with diabody for 2 hours and then seeded onto the collagen gel at a concentration of 2.5×105 cells per well and the percent contraction was measured at 24 hours. The area of the each gel was obtained by taking a picture of the gel using image capture (Gel Doc) and quantified using NIH Image J software. To measure the area of the gel the oval measuring tool was used to outline each gel. The area of the gel at time zero was compared to the area of the gel after 24 hours, generating a percent contraction for each sample. Each experiment included at least six replicates, and at least three independent experiments were performed with comparable results. A Student’s t-test (unpaired, two-tailed) was used and a P<0.05 was judged to be statistically significant.
Western blot analysis
Cultured ARPE-19 cells were harvested and resuspended in serum-free DMEM/F12 containing 20 μg/ml anti-EMP2 diabody. The cells were plated onto 6-well plate at a concentration of 1×106 cells per well. The cells were incubated on the plate for various times and then protein was isolated for western blot analysis. Cell protein was isolated using RIPA buffer containing protease and phosphatase inhibitors (Upstate, Charlottesville, VA) and the protein concentration determined with BCA Protein Assay (Bio Rad, Hercules, CA). A total of 10 μg of protein was loaded in each lane and the proteins fractionated by 4–20% SDS-PAGE gradient gel under reducing conditions. Proteins were transferred to nitrocellulose membranes (Amersham Life Sciences, Buckinghamshire, UK) and the adequacy of transfer confirmed using Ponceau S red staining (Sigma Chemical Co., St. Louis, MO). The membrane was then blocked with nonfat milk in TBS Tween (TBST; Upstate, Charlottesville, VA). Blots were incubated for 1 hour with primary antibody at a dilution of 1:200 for p-FAK (Tyr 576/577), 1:1000 for EMP2, and 1:5000 for β-actin. Horseradish peroxidase–conjugated goat anti-rabbit or horseradish peroxidase–conjugated goat anti-mouse was exposed to the blots at a 1:2000 dilution. Blots were then developed with ECL to visualize bound antibody (Pierce, Rockford, IL) and quantified using β-actin as an internal control. The blots were quantified using the NIH program Image J, digitized using a flatbed scanner and the band density measured. To account for loading variability β-actin was used to normalize each sample. At least three independent experiments were performed and, where indicated, the results were evaluated for statistical significance using a Student’s t-test (unpaired, two-tailed). A level of P<0.05 was considered to be statistically significant.
Immunofluorescence
ARPE-19 cells were plated overnight onto glass coverslips (Fisher Scientific, Pittsburgh, PA). The following day the media was changed to serum-free DMEM/F12 containing 20 μg/ml of anti-EMP2 diabody. The cells were incubated for various times and then the cells were fixed with 4% paraformaldehyde for 20 minutes. Cells that were permeabilized were incubated for 15 minutes with 0.075% saponin. Cells were blocked with 10% normal donkey serum for 30 minutes. Our data suggests that EMP2 is internalized with the anti-EMP2 antibody and both are degraded. Therefore the second overnight incubation at 4°C in a humidified chamber with the primary antibody is needed to view newly produced EMP2 protein. The cells are then washed three to four times with PBS plus 0.01% Triton X-100 (PBST). Cells were incubated for 1 hour with anti-Myc FITC or Texas Red-conjugated donkey anti-goat at room temperature in a humidified chamber. Cells were washed with PBST, rinsed briefly with double-distilled H2O, and mounted onto microscope slides with mounting medium (Vectashield; Vector Laboratories, Burlingame, CA).
A laser scanning confocal microscope (LSM 510; Zeiss, Thornwood, NY) was used to assess the distribution of protein. To detect FITC-labeled or Texas Red–labeled cells, samples were excited with argon and krypton lasers at 488 and 568 nm, respectively. LSM software was used for controlling the microscope, scanning and laser modules, image recording, and analysis of image data. At least six fields were randomly chosen for analysis for each sample. In all experiments, cells were observed using a 60x oil immersion objective. Each experiment was repeated at least four times.
RESULTS
Diabody toxicity
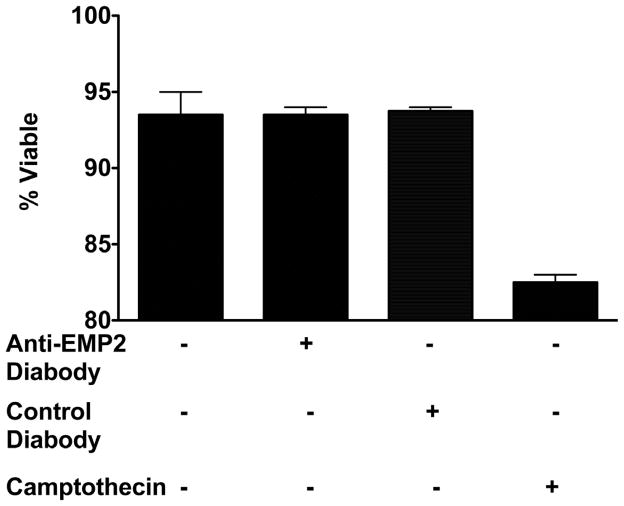
Toxicity of the anti-EMP2 diabody was determined using ARPE-19 cells. These cells were treated with anti-EMP2 or control diabody at a concentration of 20 μg/ml and incubated for 48 hours. This dose was chosen based on the maximum toxicity effect in our prior report using endometrial cells (Fu et al.; Shimazaki et al., 2008). Following incubation cell viability was analyzed by toluidine blue exclusion. Anti-EMP2 diabody, at this dose, did not show any significant toxicity in ARPE-19 cells (Figure 1).
Figure 1.
Anti-EMP2 diabody is not toxic to ARPE-19 cells. ARPE-19 cells were pretreated with 20 μg/ml of anti-EMP2 or control diabody for 2 hours. As a positve control for apoptosis 4μM of camptothecin was used. The cells were then incubated for 48 hours in a 6-well plate in 1ml of serum free media containing 20 μg/ml of diabody. Cells death was analyzed by cell count using toluidine blue exclusion.
Diabody Effect on EMP2 Protein Expression
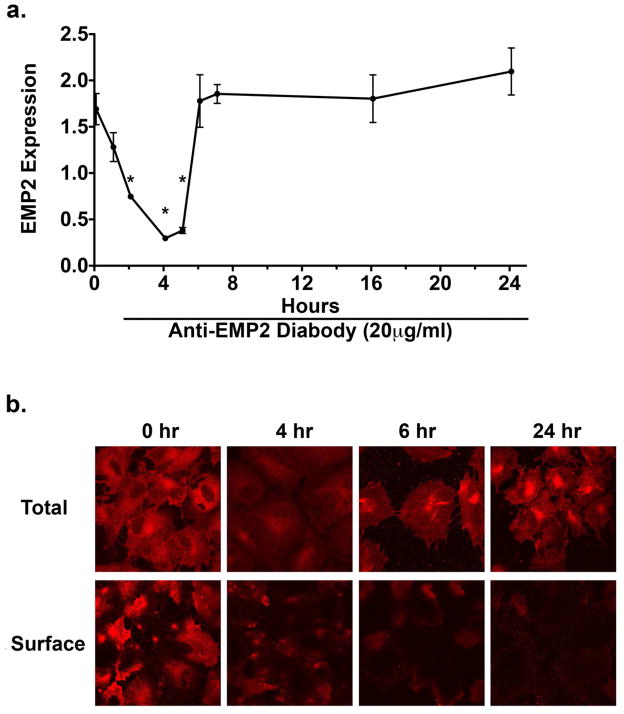
We previously reported that EMP2 regulates cellular contractile capacity by modulating FAK activation (Morales et al., 2009a; Morales et al., 2007; Morales et al., 2009b). We also have found that the anti-EMP2 diabody specifically decreases EMP2 in the human endometrial adenocarcinoma cell line, HEC-1A (Shimazaki et al., 2008). The efficacy of the EMP2-specific recombinant diabody in decreasing EMP2 and its associated cellular consequences, FAK activation and collagen gel contraction, was tested. To address this issue ARPE-19 cells were pretreated with 20 μg/ml of anti-EMP2 diabody for various times (1 to 24 hours) followed by western blot analysis to determine EMP2 protein levels (Figure 2a). Anti-EMP2 diabody treatment reduced EMP2 protein levels in ARPE-19 cells by 75% (P=0.03) following exposure to diabody for 4 hours. EMP2 protein levels began rising by 8 hours and were equal to untreated cells by 16 and 24 hours. To examine the localization of EMP2 protein expression, cell surface and total expression were evaluated by immunofluorescence (Figure 2b). This indicated that although the total EMP2 protein levels return to normal after 24 hours, functional EMP2 expression on the cell surface is significantly delayed, rendering the EMP2 biologically unavailable.
Figure 2.
Anti-EMP2 diabody decreases EMP2 expression. ARPE-19 cells were plated on a 6-well plate and treated with 20 μg/ml of anti-EMP2 diabody and examined after 0–24 hours. (a) EMP2 protein levels were determined by western blot analysis. Band density was quantitated, normalized to the β-actin loading control. Anti-EMP2 diabody treatment reduced EMP2 protein levels in ARPE-19 cells by 75% (*P=0.03) following a 4-hour treatment. EMP2 total protein levels began to return to normal by 8 hours and were equal to untreated cells by 16 and 24 hours. (b) EMP2 expression was analyzed by immunofluorescence in permeabilized (total expression) and non-permeabilized (surface expression) ARPE-19 cells. Anti-EMP2 diabody rapidly reduces EMP2 expression. Although total EMP2 protein levels return to normal after 24 hours, its expression in the biologically active location on the cell surface is significantly delayed. At least three independent experiments were performed and, where indicated, the results were evaluated for statistical significance using a Student’s t-test (unpaired, two-tailed). A level of P<0.05 was considered to be statistically significant.
Diabody effect on collagen gel contraction
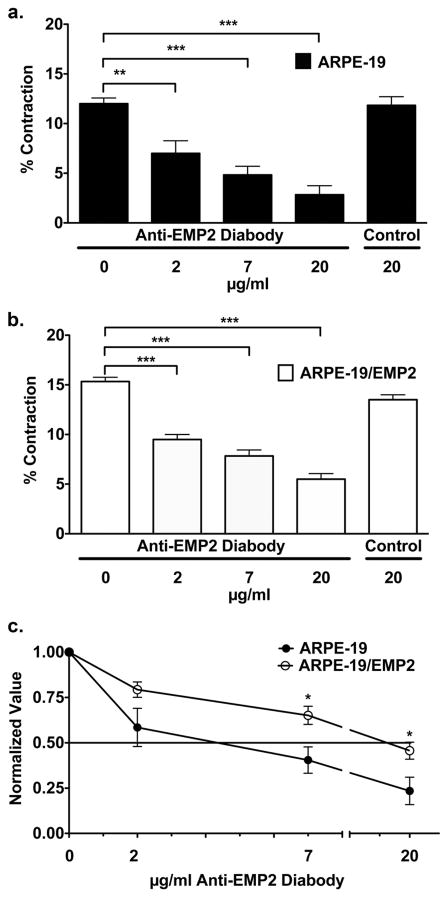
The foregoing showed that cells exposed to anti-EMP2 diabody decreased EMP2 expression, but otherwise were spared evidence of cellular toxicity. To address the functional significance of anti-EMP2 diabody treatment, collagen gel contraction was examined in cells grown in the presence or absence of anti-EMP2 diabody. ARPE-19 and ARPE-19/EMP2 cells were exposed to various concentrations of diabody starting 2 hours prior to use in a collagen gel contraction assay. Percent contraction was determined after 24 hours. ARPE-19 cells showed a dose dependent response to anti-EMP2 treatment (Figure 3a). The low dose of 2 μg/ml of anti-EMP2 diabody reduced contraction by 41% (P=0.005). The medium dose of 7 μg/ml reduced contraction by 57% (P<0.0001). The high dosage of 20 μg/ml reduced contraction by 75% (P<0.0001). Control diabody did not alter contraction. The EMP2 overexpressing ARPE-19/EMP2 cells showed a similar, but reduced dose response to anti-EMP2 diabody treatment (Figure 3b). Treatment using 2, 7, and 20 μg/ml resulted in 40% (P<0.0001), 47% (P<0.0001), and 60% (P<0.0001) inhibition of contraction respectively. The percentage inhibition of contraction, as normalized to the untreated cells, was determined for each cell line at the different concentrations of anti-EMP2 diabody treatment. The concentration of anti-EMP2 diabody required to achieve 50% reduction was lower in ARPE-19 cells as compared to ARPE-19/EMP2 cells (Figure 3c). Less than 7μg/ml was required to reach 50% reduction in collagen gel contraction in ARPE-19 cells and nearly 20 μg/ml was required in ARPE-19/EMP2 cells.
Figure 3.
Anti-EMP2 diabody decreases collagen gel contraction. (a) ARPE-19 and (b) ARPE-19/EMP2 cells were pretreated with various concentrations, ranging from 2–20 μg/ml, of anti-EMP2 or control diabody for 2 hours. Percent contraction was determined after 24 hours. ARPE-19 and ARPE-19/EMP2 cells showed a statistically significant (*P≤0.05, **P≤0.01, ***P≤0.001) dose dependent response to anti-EMP2 treatment. Control diabody did not alter contraction. (c) The percent inhibition of contraction, as normalized to the untreated cells, was determined for each cell line at the different concentrations of anti-EMP2 diabody treatment. The concentration of anti-EMP2 diabody required to achieve 50% reduction was lower in ARPE-19 cells as compared to ARPE-19/EMP2 cells. Each experiment included at least six replicates, and at least three independent experiments were performed with comparable results. A Student’s t-test (unpaired, two-tailed) was used and a P<0.05 was judged to be statistically significant.
Migration and adhesion
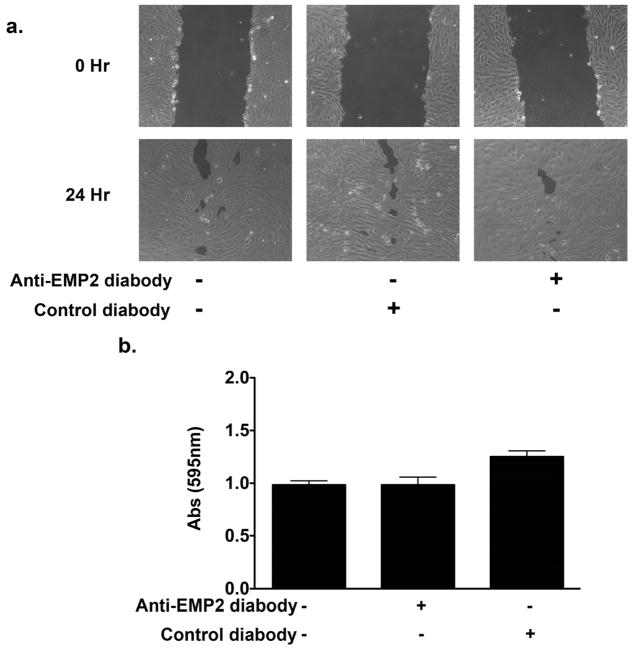
Migration and adhesion to collagen are key factors in the pathobiology of PVR. To investigate the possible mechanism of reduced collagen gel contraction following diabody treatment migratory and adhesive capacity were assessed in ARPE-19 cells following diabody treatment. A migration assay was performed with ARPE-19 cells in the presence or absence of 20 μg/ml of anti-EMP2 or control diabody. The ARPE-19 cells were grown to confluency and scratch was made in the monolayer. The denuded area was measured and percent closure was calculated. Diabody treatment did not significantly alter migratory capacity of the ARPE-19 cells (Figure 4a). To evaluate adhesive capacity an adhesion assay was performed on ARPE-19 cells pretreated with 20 μg/ml of diabody for 2 hours. The cells were then plated onto a collagen coated plate and incubated for 2 hours. Unattached cells were washed away and bound cells were analyzed for crystal violet uptake. Anti-EMP2 antibody did not affect adhesion in the ARPE-19 cells (Figure 4b). Control diabody did show a numeric increase in adhesion but this increase was not statistically significant.
Figure 4.
Migratory and adhesive capacities are not significantly altered by anti-EMP2 diabody. (a) ARPE-19 cells were grown in the presence or absence of 20 μg/ml of anti-EMP2 or control diabody. The ARPE-19 cells were grown to confluency and scratch was made in the monolayer. The denuded area was measured and percent closure was calculated. Diabody treatment did not significantly alter migratory capacity of the ARPE-19 cells. (b) Adhesive capacity was measured on ARPE-19 cells pretreated with 20 μg/ml of diabody for 2 hours. The cells were then plated onto a collagen coated plate and incubated for 2 hours. Unattached cells were washed away and bound cells were analyzed for crystal violet uptake. Anti-EMP2 antibody did not affect adhesion of the ARPE-19 cells. Control diabody did show a numeric increase in adhesion but this increase was not statistically significant. A Student’s t-test (unpaired, two-tailed) was used and a P<0.05 was judged to be statistically significant.
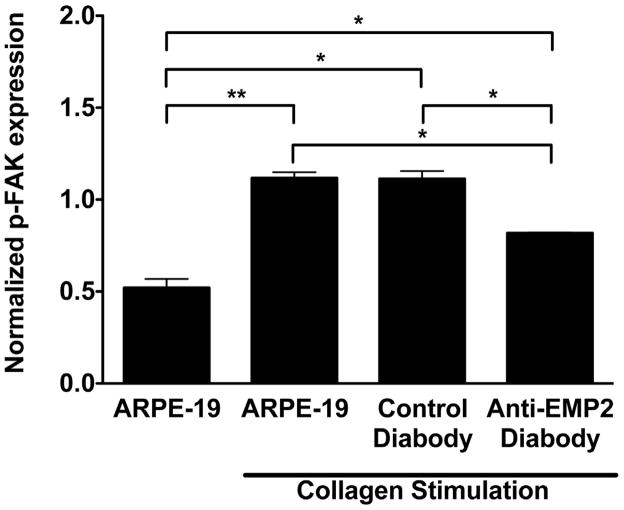
Collagen Stimulated FAK Phosphorylation
FAK activation is a key step in initiating the cellular events necessary to generate contractile force. EMP2 facilitates FAK activation, but other factors are also important in the FAK activation cascade. A key component in this cascade is integrin engagement to collagen, which leads to clustering followed by FAK activation. Given that diabody treatment inhibits collagen gel contraction and that FAK activation is a key component in generating contractile cellular forces, collagen stimulated FAK activation following diabody treatment was examined in the ARPE-19 cell line. ARPE-19 cells were incubated with 20 μg/ml of anti-EMP2 diabody for 2 hours prior to exposure to collagen (Figure 5). The cells were then plated onto collagen coated plates and incubated for an additional 2 hours. Collagen stimulation increased FAK phosphorylation by 172% (P=0.009) in the untreated cells. Exposure of the cells to the anti-EMP2 diabody significantly reduced the collagen stimulated FAK activation (P=0.01).
Figure 5.
Collagen stimulated FAK activation is decreased following anti-EMP2 diabody treatment. ARPE-19 cells were pretreated with 20 μg/ml of anti-EMP2 diabody for 2 hours. The cells were then plated onto collagen coated plates and incubated for 2 hours. Collagen stimulation increased FAK phosphorylation by 172% (**P≤0.01) in the untreated cells. The anti-EMP2 diabody reduced the collagen stimulated FAK activation by more than a quarter (*P≤0.05). At least three independent experiments were performed and, where indicated, the results were evaluated for statistical significance using a Student’s t-test (unpaired, two-tailed). A level of P<0.05 was considered to be statistically significant.
DISCUSSION
In this study, we described the safety and efficacy of an EMP2 specific recombinant diabody in decreasing biologically available EMP2, and its associated FAK activation and collagen gel contraction.
We have previously demonstrated that EMP2 expression positively modulates collagen gel contraction by ARPE-19 cells through increased FAK activation (Morales et al., 2009a; Morales et al., 2007; Morales et al., 2009b). Increased expression of EMP2 in ARPE-19 cells increased cellular contractile capacity, leading to a more rapid and further development of disease in an in vivo rabbit model of PVR. Decreased EMP2 levels inhibited PVR progression, while blockage of EMP2 with anti-human EMP2 polyclonal antibody significantly decreased PVR severity (Telander et al., 2011). These findings led to the hypothesis that EMP2 may serve as a novel therapeutic target in reducing cellular contractile capacity of the RPE cells in conditions such as PVR. To further develop anti-EMP2 therapy a monoclonal diabody system was tested. Diabody technology has several key advantages over standard polyclonal antibodies, first the diabody is a small bivalent biospecific antibody fragment with high avidity and specificity. Secondly, the diabody offers a high signal to noise ratio due to increased specificity allowing for direct specific therapeutic targeting of a specific antigen (Holliger et al., 1993; Olafsen et al., 2004). RPE cells are important at maintaining homeostasis within the retina and, in turn, play a key role in responding to pathologic alterations in the microenvironment (Sparrow et al., 2010). Dysfunction of the RPE leads to many clinical disorders resulting in vision loss and blindness (Sparrow et al., 2010). Therapeutically targeting EMP2 would need to be well tolerated and nontoxic to the RPE in order to avoid detrimental side effects.
A concern regarding use of the anti-EMP2 diabodies is their observed toxicity in malignant cell models, necessitating a careful evaluation of function in ARPE-19 cells (Fu et al.; Shimazaki et al., 2008). Malignant cells have many phenotypic and functional differences as compared to non-malignant cells, any of which may result in selective toxicity to various agents. In ARPE-19, there was no toxicity in response to anti-EMP2 diabody treatment at levels that maximally decreased collagen gel contraction. In addition, incubation with the diabody led to rapid degradation of EMP2 protein followed by recovery of EMP2 protein in a non-functional location within 24 hours. Anti-EMP2 diabody reduced collagen stimulated FAK activation in the ARPE-19 cells. Concordant with decreased EMP2 and FAK activation levels, ARPE-19 cells exhibited reduced collagen gel contraction following anti-EMP2 diabody treatment. The findings in this current study support our previous work that demonstrated a regulatory relationship between EMP2 expression, FAK activation, and cellular contractile capacity. EMP2 expression positively modulates collagen gel contraction by ARPE-19 cells through increased FAK activation (Morales et al., 2009a; Morales et al., 2007; Morales et al., 2009b). In this report we show that EMP2 diabody treatment reduced EMP2 expression, FAK activation, and collagen gel contraction. An interesting finding was the rapid decrease in EMP2 expression followed by the recovery after 24 hours in the anti-EMP2 diabody treated cells. Despite the recovery of EMP2 the anti-EMP2 diabody was still exerting a functionally significant cellular response in the ARPE-19 cells beyond 24 hours, as measured by decreased collagen gel contraction. Careful examination revealed that surface expression of EMP2 was absent at 24 hours. The recovery of EMP2 was intracellular but biologically unavailable, rendering EMP2 related mechanisms nonfunctional. An important mechanism for FAK activation is integrin receptor–mediated clustering of FAK, resulting in autophosphorylation, Src recruitment, and FAK phosphorylation at multiple sites (Calalb et al., 1995; Schlaepfer et al., 1999; Schlaepfer and Hunter, 1998). We have previously demonstrated that EMP2 associates with FAK, leading to the increased activation of FAK by enhancing focal adhesion formation (Morales et al., 2009a). EMP2 acts as a molecular adaptor between integrin ligation and FAK activation, which occurs at or near the cell surface.
A goal of this study was to lay the foundation for the development of a novel therapeutic reagent for the treatment and prevention of PVR. Contraction of PVR membranes and the traction they produce on the retina is the cause of recurrent retinal detachments and vision loss (Grierson et al., 1996). Collagen gel contraction is an important model used as an in vitro correlate for the contractile phase of PVR. Selectively targeting EMP2 provides a novel mechanism to reduce cellular contractile capacity. We hypothesize that targeting EMP2, through the use of an anti-EMP2 diabody, could be used as a therapeutic reagent to regulate cellular functions important in PVR. Additional studies using primary RPE cells as well as in vivo studies will need to be completed in order to validate these findings.
Tetraspan proteins, including EMP2, have been shown to contribute to the formation of diverse complexes to form the tetraspan web (Levy and Shoham, 2005a, b), which is the creation of scaffolds and membrane domains that regulate signaling and sorting processes (Hemler, 2005; Levy and Shoham, 2005a). These complexes can regulate trafficking, signaling, and structural characteristics of their membrane protein constituents. Specifically targeting components of the tetraspan web could potentially lead to novel therapeutic targets, as demonstrated by anti-EMP2 diabody treatment.
Highlights.
Exposure to anti-EMP2 diabody resulted in a 75% reduction in EMP2 protein
No significant toxicity was observed with anti-EMP2 diabody
Anti-EMP2 diabody significantly reduced collagen gel contraction
Anti-EMP2 diabody reduced collagen stimulated FAK activation
Acknowledgments
Grant Information: This work was supported by NIH RO1 EY019909 Grant (LKG), American Health Assistance Foundation (LKG), A. P. Giannini Foundation (S.A.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Khairi AM, Al-Kahtani E, Kangave D, Abu El-Asrar AM. Prognostic factors associated with outcomes after giant retinal tear management using perfluorocarbon liquids. Eur J Ophthalmol. 2008;18:270–277. doi: 10.1177/112067210801800216. [DOI] [PubMed] [Google Scholar]
- Asaria RH, Gregor ZJ. Simple retinal detachments: identifying the at-risk case. Eye. 2002;16:404–410. doi: 10.1038/sj.eye.6700189. [DOI] [PubMed] [Google Scholar]
- Bonnet M, Fleury J. Management of retinal detachment after penetrating eye injury. Graefes Arch Clin Exp Ophthalmol. 1991;229:539–542. doi: 10.1007/BF00203318. [DOI] [PubMed] [Google Scholar]
- Calalb MB, Polte TR, Hanks SK. Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for Src family kinases. Mol Cell Biol. 1995;15:954–963. doi: 10.1128/mcb.15.2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campochiaro PA. Pathogenic mechanisms in proliferative vitreoretinopathy. Arch Ophthalmol. 1997;115:237–241. doi: 10.1001/archopht.1997.01100150239014. [DOI] [PubMed] [Google Scholar]
- Cardillo JA, Stout JT, LaBree L, Azen SP, Omphroy L, Cui JZ, Kimura H, Hinton DR, Ryan SJ. Post-traumatic proliferative vitreoretinopathy. The epidemiologic profile, onset, risk factors, and visual outcome. Ophthalmology. 1997;104:1166–1173. doi: 10.1016/s0161-6420(97)30167-5. [DOI] [PubMed] [Google Scholar]
- Charteris DG, Sethi CS, Lewis GP, Fisher SK. Proliferative vitreoretinopathy-developments in adjunctive treatment and retinal pathology. Eye. 2002;16:369–374. doi: 10.1038/sj.eye.6700194. [DOI] [PubMed] [Google Scholar]
- Colyer MH, Weber ED, Weichel ED, Dick JS, Bower KS, Ward TP, Haller JA. Delayed intraocular foreign body removal without endophthalmitis during Operations Iraqi Freedom and Enduring Freedom. Ophthalmology. 2007;114:1439–1447. doi: 10.1016/j.ophtha.2006.10.052. [DOI] [PubMed] [Google Scholar]
- Forbes A, Wadehra M, Mareninov S, Morales S, Shimazaki K, Gordon LK, Braun J. The tetraspan protein EMP2 regulates expression of caveolin-1. J Biol Chem. 2007;282:26542–26551. doi: 10.1074/jbc.M702117200. [DOI] [PubMed] [Google Scholar]
- Fu M, Maresh EL, Soslow RA, Alavi M, Mah V, Zhou Q, Iasonos A, Goodglick L, Gordon LK, Braun J, Wadehra M. Epithelial membrane protein-2 is a novel therapeutic target in ovarian cancer. Clin Cancer Res. 2010;16:3954–3963. doi: 10.1158/1078-0432.CCR-10-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser BM, Cardin A, Biscoe B. Proliferative vitreoretinopathy. The mechanism of development of vitreoretinal traction. Ophthalmology. 1987;94:327–332. doi: 10.1016/s0161-6420(87)33443-8. [DOI] [PubMed] [Google Scholar]
- Grierson I, Mazure A, Hogg P, Hiscott P, Sheridan C, Wong D. Non-vascular vitreoretinopathy: the cells and the cellular basis of contraction. Eye. 1996;10 ( Pt 6):671–684. doi: 10.1038/eye.1996.160. [DOI] [PubMed] [Google Scholar]
- Hemler ME. Tetraspanin functions and associated microdomains. Nat Rev Mol Cell Biol. 2005;6:801–811. doi: 10.1038/nrm1736. [DOI] [PubMed] [Google Scholar]
- Hinton DR, He S, Jin ML, Barron E, Ryan SJ. Novel growth factors involved in the pathogenesis of proliferative vitreoretinopathy. Eye. 2002;16:422–428. doi: 10.1038/sj.eye.6700190. [DOI] [PubMed] [Google Scholar]
- Holliger P, Prospero T, Winter G. “Diabodies”: small bivalent and bispecific antibody fragments. Proc Natl Acad Sci U S A. 1993;90:6444–6448. doi: 10.1073/pnas.90.14.6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hueber A, Esser P, Heimann K, Kociok N, Winter S, Weller M. The topoisomerase I inhibitors, camptothecin and beta-lapachone, induce apoptosis of human retinal pigment epithelial cells. Exp Eye Res. 1998;67:525–530. doi: 10.1006/exer.1998.0544. [DOI] [PubMed] [Google Scholar]
- James M, O’Doherty M, Beatty S. The prognostic influence of chronicity of rhegmatogenous retinal detachment on anatomic success after reattachment surgery. Am J Ophthalmol. 2007;143:1032–1034. doi: 10.1016/j.ajo.2007.01.057. [DOI] [PubMed] [Google Scholar]
- Jin M, He S, Worpel V, Ryan SJ, Hinton DR. Promotion of adhesion and migration of RPE cells to provisional extracellular matrices by TNF-alpha. Invest Ophthalmol Vis Sci. 2000;41:4324–4332. [PubMed] [Google Scholar]
- Kirchhof B. Strategies to influence PVR development. Graefes Arch Clin Exp Ophthalmol. 2004;242:699–703. doi: 10.1007/s00417-004-0978-8. [DOI] [PubMed] [Google Scholar]
- Kruger EF, Nguyen QD, Ramos-Lopez M, Lashkari K. Proliferative vitreoretinopathy after trauma. Int Ophthalmol Clin. 2002;42:129–143. doi: 10.1097/00004397-200207000-00015. [DOI] [PubMed] [Google Scholar]
- Levy S, Shoham T. Protein-protein interactions in the tetraspanin web. Physiology (Bethesda) 2005a;20:218–224. doi: 10.1152/physiol.00015.2005. [DOI] [PubMed] [Google Scholar]
- Levy S, Shoham T. The tetraspanin web modulates immune-signalling complexes. Nat Rev Immunol. 2005b;5:136–148. doi: 10.1038/nri1548. [DOI] [PubMed] [Google Scholar]
- Marks JD, Bradbury A. Selection of human antibodies from phage display libraries. Methods Mol Biol. 2004;248:161–176. doi: 10.1385/1-59259-666-5:161. [DOI] [PubMed] [Google Scholar]
- Morales SA, Mareninov S, Coulam P, Wadehra M, Goodglick L, Braun J, Gordon LK. Functional consequences of interactions between FAK and epithelial membrane protein 2 (EMP2) Invest Ophthalmol Vis Sci. 2009a;50:4949–4956. doi: 10.1167/iovs.08-3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales SA, Mareninov S, Prasad P, Wadehra M, Braun J, Gordon LK. Collagen gel contraction by ARPE-19 cells is mediated by a FAK-Src dependent pathway. Exp Eye Res. 2007;85:790–798. doi: 10.1016/j.exer.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Morales SA, Mareninov S, Wadehra M, Zhang L, Goodglick L, Braun J, Gordon LK. FAK activation and the role of epithelial membrane protein 2 (EMP2) in collagen gel contraction. Invest Ophthalmol Vis Sci. 2009b;50:462–469. doi: 10.1167/iovs.07-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Guidry C. The insulin-like growth factor system modulates retinal pigment epithelial cell tractional force generation. Invest Ophthalmol Vis Sci. 2007;48:1892–1899. doi: 10.1167/iovs.06-1095. [DOI] [PubMed] [Google Scholar]
- Nair AR, Schliekelman M, Thomas MB, Wakefield J, Jurgensen S, Ramabhadran R. Inhibition of p53 by lentiviral mediated shRNA abrogates G1 arrest and apoptosis in retinal pigmented epithelial cell line. Cell Cycle. 2005;4:697–703. doi: 10.4161/cc.4.5.1672. [DOI] [PubMed] [Google Scholar]
- Olafsen T, Cheung CW, Yazaki PJ, Li L, Sundaresan G, Gambhir SS, Sherman MA, Williams LE, Shively JE, Raubitschek AA, Wu AM. Covalent disulfide-linked anti-CEA diabody allows site-specific conjugation and radiolabeling for tumor targeting applications. Protein Eng Des Sel. 2004;17:21–27. doi: 10.1093/protein/gzh009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto T, Hinton DR, Sakamoto H, Oganesian A, Kohen L, Gopalakrishna R, Ryan SJ. Collagen gel contraction induced by retinal pigment epithelial cells and choroidal fibroblasts involves the protein kinase C pathway. Curr Eye Res. 1994;13:451–459. doi: 10.3109/02713689408999873. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Hauck CR, Sieg DJ. Signaling through focal adhesion kinase. Prog Biophys Mol Biol. 1999;71:435–478. doi: 10.1016/s0079-6107(98)00052-2. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Hunter T. Integrin signalling and tyrosine phosphorylation: just the FAKs? Trends Cell Biol. 1998;8:151–157. doi: 10.1016/s0962-8924(97)01172-0. [DOI] [PubMed] [Google Scholar]
- Shimada H, Fujita K, Matsumoto Y, Mori R, Yuzuwa M. Preoperative factors influencing visual outcome following surgical excision of subfoveal choroidal. Eur J Ophthalmol. 2006;16:287–294. doi: 10.1177/112067210601600215. [DOI] [PubMed] [Google Scholar]
- Shimazaki K, Lepin EJ, Wei B, Nagy AK, Coulam CP, Mareninov S, Fu M, Wu AM, Marks JD, Braun J, Gordon LK, Wadehra M. Diabodies targeting epithelial membrane protein 2 reduce tumorigenicity of human endometrial cancer cell lines. Clin Cancer Res. 2008;14:7367–7377. doi: 10.1158/1078-0432.CCR-08-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobaci G, Akyn T, Mutlu FM, Karagul S, Bayraktar MZ. Terror-related open-globe injuries: a 10-year review. Am J Ophthalmol. 2005;139:937–939. doi: 10.1016/j.ajo.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Sobaci G, Mutlu FM, Bayer A, Karagul S, Yildirim E. Deadly weapon-related open-globe injuries: outcome assessment by the ocular trauma classification system. Am J Ophthalmol. 2000;129:47–53. doi: 10.1016/s0002-9394(99)00254-8. [DOI] [PubMed] [Google Scholar]
- Sparrow JR, Hicks D, Hamel CP. The retinal pigment epithelium in health and disease. Curr Mol Med. 2010;10:802–823. doi: 10.2174/156652410793937813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telander DG, Morales SA, Mareninov S, Forward K, Gordon LK. Epithelial membrane protein-2 (EMP2) and experimental proliferative vitreoretinopathy (PVR) Curr Eye Res. 2011;36:546–552. doi: 10.3109/02713683.2011.561468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadehra M, Forbes A, Pushkarna N, Goodglick L, Gordon LK, Williams CJ, Braun J. Epithelial membrane protein-2 regulates surface expression of alphavbeta3 integrin in the endometrium. Dev Biol. 2005;287:336–345. doi: 10.1016/j.ydbio.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Wadehra M, Goodglick L, Braun J. The tetraspan protein EMP2 modulates the surface expression of caveolins and glycosylphosphatidyl inositol-linked proteins. Mol Biol Cell. 2004;15:2073–2083. doi: 10.1091/mbc.E03-07-0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadehra M, Iyer R, Goodglick L, Braun J. The tetraspan protein epithelial membrane protein-2 interacts with beta1 integrins and regulates adhesion. J Biol Chem. 2002;277:41094–41100. doi: 10.1074/jbc.M206868200. [DOI] [PubMed] [Google Scholar]
- Wadehra M, Mainigi M, Morales SA, Rao RG, Gordon LK, Williams CJ, Braun J. Steroid hormone regulation of EMP2 expression and localization in the endometrium. Reprod Biol Endocrinol. 2008;6:15. doi: 10.1186/1477-7827-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadehra M, Su H, Gordon LK, Goodglick L, Braun J. The tetraspan protein EMP2 increases surface expression of class I major histocompatibility complex proteins and susceptibility to CTL-mediated cell death. Clin Immunol. 2003a;107:129–136. doi: 10.1016/s1521-6616(03)00048-2. [DOI] [PubMed] [Google Scholar]
- Wadehra M, Sulur GG, Braun J, Gordon LK, Goodglick L. Epithelial membrane protein-2 is expressed in discrete anatomical regions of the eye. Exp Mol Pathol. 2003b;74:106–112. doi: 10.1016/s0014-4800(03)00009-1. [DOI] [PubMed] [Google Scholar]
- Wang CX, Wadehra M, Fisk BC, Goodglick L, Braun J. Epithelial membrane protein 2, a 4-transmembrane protein that suppresses B-cell lymphoma tumorigenicity. Blood. 2001;97:3890–3895. doi: 10.1182/blood.v97.12.3890. [DOI] [PubMed] [Google Scholar]