Abstract
Various water-soluble L-valine-, L-glutamate-, and glycine ester prodrugs of two 3-Carboranyl Thymidine Analogues (3-CTAs), designated N5 and N5-2OH, were synthesized for Boron Neutron Capture Therapy (BNCT) of brain tumors since the water solubilities of the parental compounds proved to be insufficient in preclinical studies. The amino acid ester prodrugs were prepared and stored as hydrochloride salts. The water solubilities of these amino acid ester prodrugs, evaluated in phosphate buffered saline (PBS) at pH 5, pH 6 and pH 7.4, improved 48 to 6600 times compared with parental N5 and N5-2OH. The stability of the amino acid ester prodrugs was evaluated in PBS at pH 7.4, Bovine serum, and Bovine cerebrospinal fluid (CSF). The rate of the hydrolysis in all three incubation media depended primarily on the amino acid promoiety and, to a lesser extend, on the site of esterification at the deoxyribose portion of the 3-CTAs. In general, 3'-amino acid ester prodrugs were less sensitive to chemical and enzymatic hydrolysis than 5'-amino acid ester prodrugs and the stabilities of the latter decreased in the following order: 5'-valine > 5'-glutamate > 5'-glycine. The rate of the hydrolysis of the 5'-amino acid ester prodrugs in Bovine CSF was overall higher than in PBS and somewhat lower than in Bovine serum. Overall, 5'-glutamate ester prodrug of N5 and the 5'-glycine ester prodrugs of N5 and N5-2OH appeared to be the most promising candidates for preclinical BNCT studies.
Keywords: 3-Carboranyl thymidine analogues, Amino acid ester prodrugs, Boron neutron capture therapy, Glioblastoma multiforme
1. Introduction
Boron Neutron Capture Therapy (BNCT) is a binary cancer treatment modality that is based on the irradiation of boron-10 (10B), a stable isotope, with low energy (thermal) neutrons. The resulting capture reaction produces cytocidal high linear energy transfer (LET) lithium- and helium nuclei (α-particles). These have ranges of < 10 µm in biological tissue, which approximates one cell diameter. Thus, lethal damage caused by BNCT can be restricted largely to cancerous tissue by selective delivery of 10B to cancer cells. Approximately 20 µg of 10B/g tumor and tumor-to-normal tissue ratios larger than 3:1 at the time of neutron irradiation are required for successful BNCT.[1]
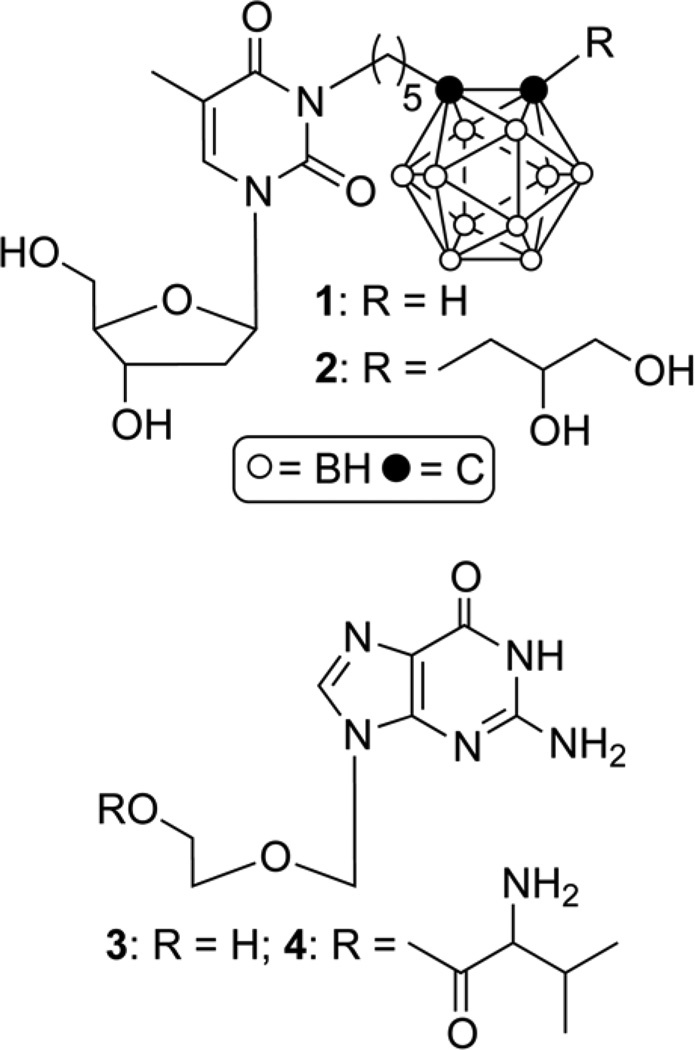
In recent years, 3-Carboranyl Thymidine Analogues (3-CTAs), such as N5 and N5-2OH, (compounds 1 & 2, Fig. 1), have been a major focus of compound development for BNCT of high-grade brain tumors, such as glioblastoma multiforme (GBM).[2–5] Human thymidine kinase 1 (hTK1), an enzyme of the DNA salvage pathway synthesis that is predominantly active in proliferating cells,[6] catalyzes the 5'-monophosphorylation of these agents. Apparently, this causes the selective accumulation of 3-CTAs in cancer cells by a process that has been referred to as Kinase Mediated Trapping (KMT).[5, 7] The in vitro uptake of 1 and 2 in TK1 (+) L929 cells was 5–10 higher than in TK1 (−) L929 cells following 24 h incubation in compound containing medium. In addition, both compounds were retained only in the former cell line when incubation was continued for 12 h in compound free medium.[2] After intratumoral (i.t.) injection of 2 into subcutaneous TK1 (+)- or TK1 (−) L929 tumors implanted in nude mice, boron concentrations were ~ 3 times higher in the former tumor.[3] Survival times of rats bearing intracranial RG2 glioma that had received 2 via intracranial (i.c.) injection prior to BNCT were about 2 times longer compared with rats that received BNCT without administration of 2.[3] Compared with i.c. and i.t. injections, intravenous (i.v.) administration of 2 appeared to be less effective.[3]
Fig. 1.
Structures of N5 (1), N5-2OH (2), acyclovir (3), and valacyclovir (4).
These biological studies clearly established the therapeutic potential of 1 and 2 but they also revealed a major limitation. Both compounds are very lipophilic because of the presence of the carborane cluster[4] and the absence any functional groups that can be ionized under physiological conditions. Thus, both agents are almost insoluble in water and aqueous solutions containing 30%-70% dimethyl sulfoxide (DMSO) had to be used to solubilize 2 for i.t. and i.c. injections into tumor-bearing rodents.[3] Although no significant pathologic alterations were observed in rat brains following i.c. injections,[3] administration of 2 in DMSO via i.c. route to the brains of human patients with intracranial tumors has the potential to cause damage to healthy brain tissue. Even if such injections should prove to be acceptable for humans, it may significantly complicate approval of clinical trials by regulatory agencies.
A successful approach to overcome water-insolubility of drugs has been the preparation of carrier-linked prodrugs.[8] For the design and synthesis of water-soluble amino acid ester prodrugs of 1 and 2, three important factors have to be considered: 1) the water solubilities of the amino acid ester prodrugs of 1 and 2 should be in the range of those of the parental compounds in aqueous DMSO solutions. 2) The tumor selective uptake of 1 and 2 depends on TK1 depended KMT. Thus, cleavage of any promoiety attached to 1 and 2 should be rapid, as additional structural modification of these structures, not only at the 5'-position, will most likely abolish their TK1 substrate characteristics.[9] 3) The protein (enzyme) concentration in the interstitial fluid (ISF) of the brain appears to be significantly lower than that in normal serum.[10] The increased molecular weight and hydrophilicity of prodrugs of 1 and 2 may prevent their effective passage through cell membranes and the subsequent intracellular enzymatic removal of the promoiety. Therefore, cleavage of such prodrugs following i.c. administration may depend primarily on chemical rather than enzymatic hydrolysis in brain ISF.
Consequently, the ideal water-solubilizing promoieties for 1 and 2 should be rapidly cleavable by chemical hydrolysis. Phosphate-, carbohydrate-, or sulfate esters are probably not suitable as promoieties of 1 and 2 because they proved to be sensitive to enzymatic cleavage but were fairly resistant towards chemical hydrolysis when used for other types of agents.[11–13] In contrast, amino acid ester prodrugs of established drugs, such as the antibiotic/antiprotozoal metronidazole,[14] the anticancer nucleoside analogues gemcitabine, floxuridine and cytarabine,[15–18] and the widely used antiviral nucleoside analogue acyclovir[19–20] (compound 3, Fig. 1) proved to be sensitive to both enzymatic and chemical hydrolysis. In addition, valacyclovir (compound 4, Fig. 1), the established valine ester prodrug of acyclovir, is about 130 times better water-soluble than its parental nucleoside analogue.[21]
This paper describes the design, synthesis, and evaluation of water-soluble amino acid ester prodrugs of 1 and 2. Based on the aforementioned studies with metronidazole, gemcitabine, floxuridine, cytarabine, and acyclovir, glycine-, L-valine-, and L-glutamate were selected as promoieties. The chemical and enzymatic stabilities of the synthesized amino acid ester prodrugs were evaluated in phosphate buffered saline (PBS), Bovine serum, and Bovine cerebrospinal fluid (CSF), which has a similar protein concentration as brain ISF.[10] The solubilities of the synthesized prodrugs were evaluated in PBS at pH 5, pH 6, and pH 7.4.
2. Results and discussion
2.1. Chemistry
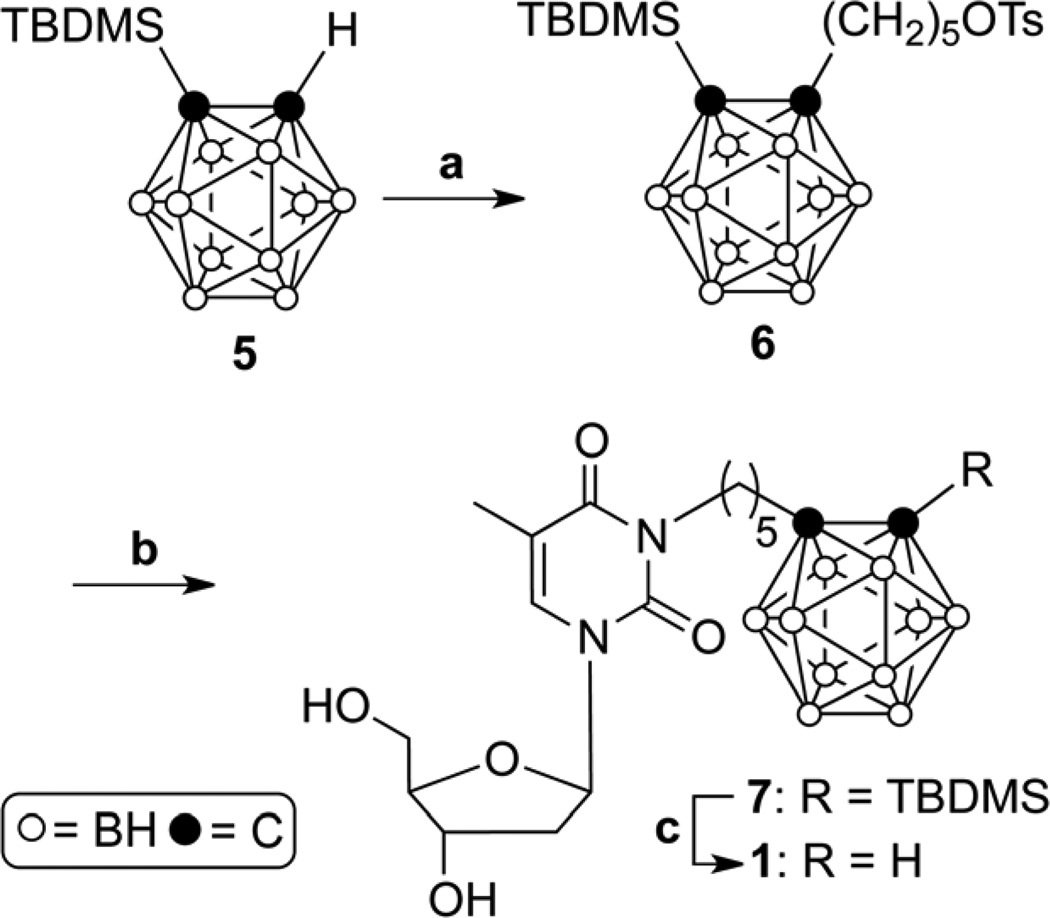
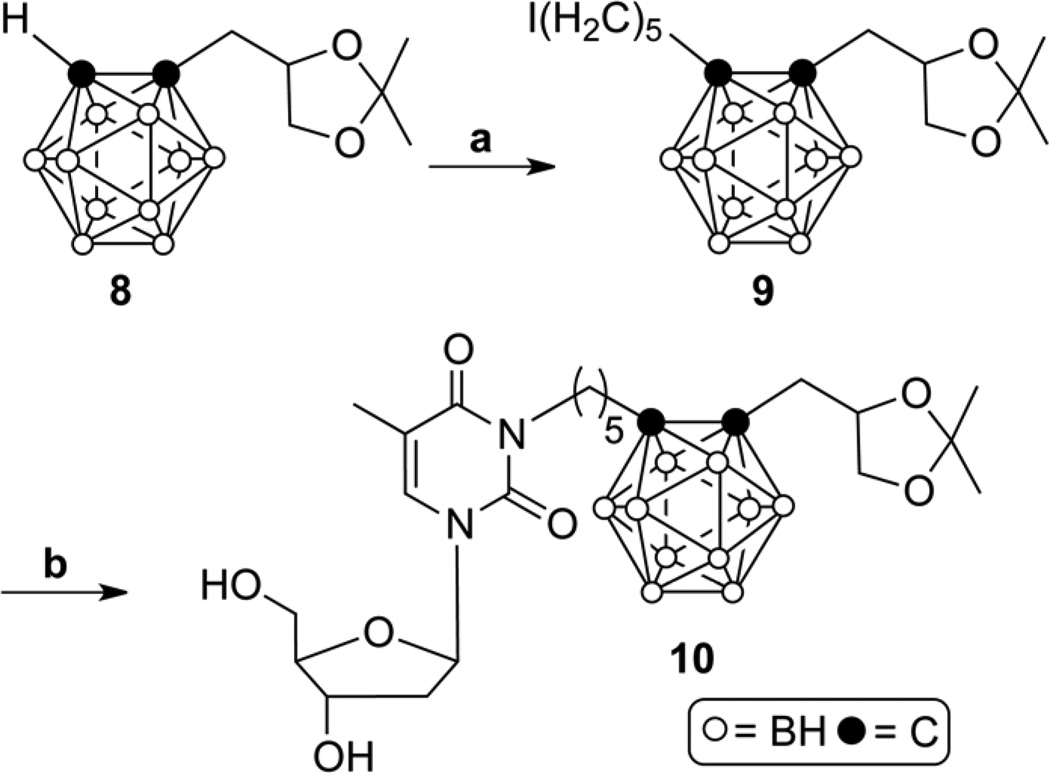
The syntheses of N5 (1) and acetonide-protected N5-2OH (10) are shown in Schemes 1 and 2. Both compounds served as starting materials in the synthesis of the amino acid ester prodrugs of 1 and N5-2OH (2), as shown in Schemes 3.
Scheme 1.
Reagents and conditions: (a) n-BuLi, THF, 1,5-pentandiol ditosylate, 5 h, 80 °C; (b) Thd, K2CO3, DMF/acetone (1/1), 6 h, 50 °C; (c) TBAF, THF, 0.5 h, rt.
Scheme 2.
Reagents and conditions: (a) n-BuLi, THF, 1,5-diiodopentane, 6 h, 80 °C; (b) Thd, K2CO3, DMF/acetone (1/1), overnight, 50 °C.
Scheme 3.
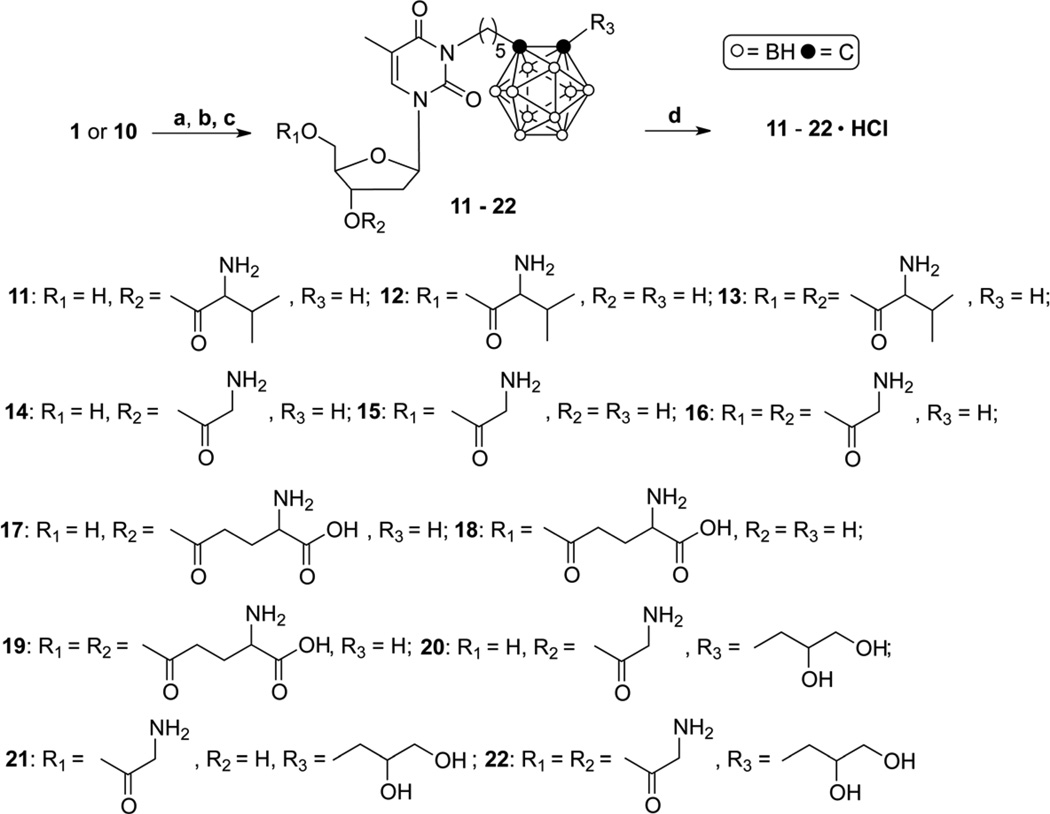
Reagents and conditions: (a) N-Boc-L-amino acids, DCC, DMAP, 24 h, rt; (b) Partial column chromatographic purification (ethyl acetate/hexanes, 8/5) of 3'-N-Boc-, 5'-N-Boc-, and 3',5'-di-N-Boc-amino acid esters of 1 and 10; (c) TFA:DCM, 1:1, (2–4) h, rt; (d) 1 M HCl in diethyl ether, THF, overnight, rt.
Previously reported syntheses for 1 utilized highly toxic and explosive decaborane as the starting material.[22–23] Therefore, an alternative synthesis was developed, which is shown in Scheme 1. In this route, tert-butyldimethylsilyl (TBDMS)-o-carborane (5) is used as the staring material, which is prepared from less toxic, stable, and commercially-available o-carborane.[24] The reaction of 5 with n-BuLi in THF followed by addition of 1,5-pentandiol ditosylate[25] afforded compound 6 in 36 % yield. Use of readily available commercial 1,5-diiodopentane rather than 1,5-pentanediol ditosylate resulted in complex reaction mixtures from which the desired product could not be separated effectively. Reacting 6 with thymidine (Thd) in the presence of potassium carbonate in a DMF/acetone (1/1) mixture at 50 °C gave compound 7 in 65 % yield. Removal of the TBDMS protective groups of compound 7 was achieved with tetrabutylammonium fluoride (TBAF) in THF at room temperature for 0.5 h to give target compound 1 in 58 % yield.
Compound 10 was prepared according to Scheme 2. In this reaction sequence, treatment of compound 8[25] with n-BuLi followed by addition of 1,5-diiodopentane rather than 1,5-pentanediol ditosylate, as described previously by Byun et al.,[25] was feasible yielding 9 in 41 % yield. The reaction of 9 with Thd in the presence of potassium carbonate in a DMF/acetone mixture at 50 °C produced 10 in 48 % yield.
Compound 1 was treated with commercially available N-Boc protected amino acids (N-Boc-L-valine, N-Boc-glycine, or N-Boc-L-glutamic acid α-tert.-butyl ester). As reported by Anand et al.,[19] and Katragadda et al.[20] for studies with acyclovir, glutamic acid was attached to the 5′-position of 1 via the γ-carboxylic function. For reasons discussed in section 2.2., compound 10 was only treated with N-Boc-glycine. All reactions were carried out in the presence of dicyclohexylcarbodiimide (DCC) and a catalytic amount of 4-dimethylaminopyridine (DMAP) in DMF for 24 h at room temperature to give mixtures of 3'-N-Boc-, 5'-N-Boc-, and 3',5'-di-N-Boc-amino acid ester prodrugs of 1 and 10 (Scheme 3). Progress of the reactions was monitored by TLC and separation of the Boc-protected amino acid ester prodrugs was possible by column chromatography. Unfortunately, all of these isolated intermediates remained contaminated with DCC (see supplementary material for 1H-NMR data and Rf values). Thus, removal of the Boc groups from these partially purified intermediates was achieved with a 50 % solution of trifluoroacetic acid (TFA) in dichloromethane at room temperature for 2 h to give the 3'-monosubstituted amino acid ester prodrugs 11, 14, 17, and 20 (Scheme 3) in yields ranging from 1% to 5%, the 5'-monosubstituted amino acid ester prodrugs 12, 15, 18, and 21 (Scheme 3) in yields ranging from 7% to 22%, and the 3',5'-disubstituted amino acid ester prodrugs 13, 16, 19, and 22 (Scheme 3) in yields ranging from 13% to 30%.
In the case of the glutamate ester prodrugs of N5 (17–19), the reaction was carried for 4 h to assure removal of both the Boc- and the α-tert.-butyl protective group. All amino acid ester prodrugs were obtained with wax-like consistency following purification by preparative RP18-HPLC (Table 3) and were analyzed and identified by 1H-NMR, 13CNMR, and MS. The latter was most useful to distinguish between monosubstituted- and disubstituted amino acid ester prodrugs, whereas 1H-NMR spectroscopy was helpful to distinguish between 3'-monosubstituted- and 5'-monosubstituted amino acid ester prodrugs based on the chemical shifts (δ) of the 3'- and 5'-protons. In general, amino acid ester substitution at the 3'-hydroxyl group caused a downfield shift for the signal of the 3'-Hs from 3.88 ppm-3.95 ppm in the case of 1 and 10 to 5.04 ppm-5.54 ppm in the case of 11, 14, 17, and 20 whereas 5'-subsititution caused a downfield shift for the signal of the 5'-Hs from 3.74 ppm-3.81 ppm in the case of 1 and 10 to 4.30 ppm-4.74 ppm in the case of 12, 15, 18, and 21.
Table 3.
HPLC methods and retention times of compounds 1-4 and 11–22.
| Retention time (min) |
||||
|---|---|---|---|---|
| Compds | Method1 | 3' | 5' | 3', 5' |
| 11–13 | A1 – 65:35 to 30:70 (H2O:MeCN) over 27 min | 18.82 | 18.09 | 13.91 |
| 14–16 | A2 – 70:30 to 30:70 (H2O:MeCN) over 27 min | 18.43 | 17.57 | 14.67 |
| 17–19 | A3 – 60:40 to 30:70 (H2O:MeCN) over 27 min | 13.99 | 13.69 | 8.92 |
| 20–22 | A2 – 70:30 to 30:70 (H2O:MeCN) over 27 min | 14.00 | 13.51 | 10.55 |
| 11–13 | B1 – 65:35 to 0:100 (H2O:MeOH) over 40 min | 33.03 | 31.29 | 27.63 |
| 14–16 | B2 – 70:30 to 0:100 (H2O:MeOH) over 40 min | 30.61 | 30.40 | 26.45 |
| 17–19 | B3 – 60:40 to 0:100 (H2O:MeOH) over 40 min | 32.05 | 31.64 | 26.50 |
| 20–22 | B2 – 70:30 to 0:100 (H2O:MeOH) over 40 min | 28.30 | 28.18 | 23.52 |
| Retention time (min) | ||||
| 1 | A1 – 65:35 to 30:70 (H2O:MeCN) over 27 min | 25.45 | ||
| 2 | A2 – 70:30 to 30:70 (H2O:MeCN) over 27 min | 18.72 | ||
| 3 | A4 – 100:0 to 85:15 (H2O:MeCN) over 20 min | 14.35 | ||
| 4 | A4 – 100:0 to 85:15 (H2O:MeCN) over 20 min | 19.43 | ||
Solvent systems contained 0.1 % TFA.
The low overall yields starting from 1 or 10 are a shortcoming of the applied synthetic route. However, low yields are inherent to this specific synthetic route and have also been reported previously in the synthesis of various gemcitabine amino acid ester prodrugs.[15] On the other hand, introduction of ester functions at different hydroxyl groups is desirable to explore which ester site is most susceptible to chemical or enzymatic cleavage (see section 2.2.). Once the optimal esterification site has been identified, appropriate protective group strategies could be applied to improve yields.
Treatment of 11–22 (Scheme 3) with commercially available anhydrous 1 M HCl gas in diethyl ether followed by stirring overnight at room temperature gave the corresponding hydrochloride salts 11•HCl–22•HCl (Scheme 3) in yields ranging from 50% to 70%. The formation of 11•HCl–22•HCl was confirmed by elemental analysis except for the 3'-glycine ester prodrug of N5 (14) since the low synthetic yield for this compound limited its availability.
These hydrochloride salts were synthesized for two reasons. 1) They had crystalline consistency and were much easier to handle than the wax-like free bases. 2) Various amino acid ester prodrugs of acyclovir (3), AZT, and gemcitabine exhibited significant stability in aqueous solution in the pH-range of 3–5 for several days whereas fast chemical cleavage was observed in phosphate buffer at pH 7.4,[15, 19, 26–27] which indicated preferential basic hydrolysis of the ester bond. Thus, the free bases are expected to be rather sensitive towards traces of water during storage and handling since the pH environment should be fairly neutral. In contrast, the hydrochloride salts should generate a more acidic environment in the presence of water traces and remain more stable.
2.2. Chemical and enzymatic stability studies
The stabilities of the synthesized amino acid ester prodrugs of N5 (11–19) and the 5'-glycine prodrug of N5-2OH (21) towards hydrolysis were evaluated in three different media: 1) PBS to examine their chemical hydrolysis, 2) Bovine serum to evaluate their enzymatic hydrolysis, and 3) Bovine CSF. The protein composition of the latter medium is similar to that of the ISF of the brain.[10, 28] Thus, it seems likely that the hydrolysis pattern of amino acid ester prodrugs under conditions similar to those they may encounter following i.c. injection can be established in CSF. Valacyclovir (4) was evaluated as a reference compound in all three media.
2.2.1. Chemical (non-enzymatic) hydrolysis in PBS at pH 7.4
The hydrolytic half-lives (t1/2) of the prodrugs, measured by using the linear regression of pseudo-first-order plots of the remaining prodrug concentrations versus the hydrolysis times, are shown in Table 1. The obtained results clearly indicate that the hydrolysis rates of the amino acid ester prodrugs primarily depend on the amino acid promoieties. The glycine ester prodrugs of N5 (14–16) and the 5′-glycine ester prodrug of N5-2OH (21) are 20–25 times more sensitive to chemical hydrolysis than the corresponding glutamate- (17- 19) and valine ester prodrugs (11–13) of N5. The sensitivity of 14–16 and 21 may be due to the presence of the small glycine promoiety, which is sterically not interfering with the nucleophilic hydrolytic attack at the ester bond.
Table 1.
Prodrug half-lives (t1/2) in PBS (pH 7.4), Bovine serum, and Bovine CSF.
| Compds | t1/2 (h) |
||
|---|---|---|---|
| PBS | Bovine serum | Bovine CSF | |
| 4 | 13.60 ± 0.49 | 6.20 ± 0.17 | 7.59 ± 0.12 |
| 11 | 29.79 ± 0.42 | ND | ND |
| 12 | 25.59 ± 0.61 | 22.21 ± 0.52 | 25.47 ± 0.10 |
| 13 | 12.91 ± 0.17 | ND | ND |
| 14 | 1.51 ± 0.11 | ND | ND |
| 15 | 0.70 ± 0.04 | 0.69 ± 0.02 | 0.96 ± 0.04 |
| 16 | 0.22 ± 0.03 | ND | ND |
| 17 | 26.91 ± 0.71 | ND | ND |
| 18 | 11.16 ± 0.37 | 0.85 ± 0.12 | 1.85 ± 0.11 |
| 19 | 3.42 ± 0.10 | ND | ND |
| 21 | 2.21 ± 0.15 | 1.51 ± 0.04 | 2.11 ± 0.14 |
Standard deviations are based on three experiments;
ND = not determined.
Although 4, 11, and 12 are all monovaline esters, the half-live of 4 is ~ 2 times lower than those of 11 and 12. This difference may be due to the presence of the lipophilic carborane cage in 11 and 12, which reduces their solubility, and hence, limits their accessibility to chemical hydrolysis. Song et al.[15] reported a half-life of 17.15 h for the chemical hydrolysis of 4, which is somewhat higher than the value we found in our studies (13.6 h), but still comparable.
Compounds 17–19 appear to have intermediate stability towards chemical hydrolysis. In previous studies,[19] the γ-glutamate ester prodrug of acyclovir exhibited significant chemical stability (t1/2: 28.2 h) in PBS at pH 7.4, which is more than 2 times higher than the value we found for 18 in our studies.
The site of esterification also influences the rate of the hydrolysis of the amino acid ester prodrugs. Generally, 3'-monosubstituted amino acid ester prodrugs were more stable to chemical hydrolysis than the corresponding 5'-monosubstituted amino acid ester prodrugs, which is consistent with reported data for floxuridine amino acid ester prodrugs,[29] but in contrast to those found for amino acid ester prodrugs of gemcitabine.[15] The preference for the cleavage of the 5'-promoieties may be due to steric effects since the 3'-position is generally more crowded by the ribofuranose ring thereby interfering with the rate of the hydrolysis.
In general, the 3',5'-disubstituted amino acid ester prodrugs of N5 showed the shortest half-lives (Table 1). This result is consistent with previously reported data for the amino acid ester prodrugs of floxuridine[29] and gemcitabine.[15] This finding seems plausible as their half-lives not only depend on the hydrolysis to the parental compounds, but also on the conversion into the corresponding 3'- and 5'-monosubstituted amino acid ester prodrugs. On the other hand, the presence of two promoieties in theses structures decreases, of course, the rate of production of parental compounds by a factor of ~ 2 compared with 5'-monosubstituted amino acid ester prodrugs (data not shown). In the case of 17–19, the half-life of the 3',5'-diglutamate ester prodrug of N5 (19) is significantly smaller than those of the corresponding 3'- (17) and 5'-glutamate (18) esters. This may be due to the observed high solubility of 19 in PBS, which may increase access of the molecule to hydrolyzing conditions.
Compound 12, as a representative of the very stable prodrugs, 18, as a representative of the medium stable prodrugs, and 15, as an example of the more sensitive prodrugs, were chosen for further studies in Bovine serum and Bovine CSF. Low synthetic yields of the 3'-monosubstituted amino acid ester prodrugs (~2 times lower than the corresponding 5'-monosubstituted amino acid ester prodrugs) and the special characteristics of the 3',5'-disubstituted amino acid ester prodrugs were major reasons for their exclusion from further stability assays. The same observations and the fact that 14–16 were generally most sensitive to chemical hydrolysis provided the rationale for limiting preparative efforts to the synthesis of the 3'-mono- (20), 5'-mono- (21), and 3',5'-diglycine (22) ester prodrugs of N5-2OH (section 2.1.) and evaluating exclusively the stability of 21 in all three test media.
2.2.2. Enzymatic stability studies
2.2.2.1. Stability studies in Bovine serum
The in vitro enzymatic hydrolysis pattern of the amino acid ester prodrugs was studied in Bovine serum at 37 °C. The hydrolytic half-lives of the prodrugs are shown in Table 1. The hydrolysis rates of 4, 12, 18, and 21 in Bovine serum are 2.2-, 1.2-, 13.1- and 1.5 times, respectively, faster than the corresponding rates in PBS. These findings are consistent with previously reported results for other amino acid ester prodrugs.[14–15, 19] They clearly indicate that the hydrolysis of the amino acid ester prodrugs is generally facilitated by enzymatic catalysis or protein-aided hydrolysis. However, the hydrolysis pattern of 15 in PBS did not change in serum. This may be due to the fact that chemical hydrolysis is already so fast that the contribution of enzyme activity becomes negligible.
The rate of the hydrolysis of the amino acid ester prodrugs in serum was also influenced by the amino acid promoieties. In particular 18 appears to depend significantly stronger on enzymatic (protein-aided) cleavage than the other prodrugs. The enzymatic cleavage of 4 is 2.6 times more rapid than that of 12. Song et al. [15] reported a half-life of 5.2 h for the hydrolysis of 4 in human plasma, which is comparable with the half-life of 6.2 h found in our studies with Bovine serum. The difference between the half-lives of 12 in PBS and Bovine serum (25.59 ± 0.61 vs. 22.21 ± 0.52) is relatively small indicating that it is in contrast to 4 not a good substrate for any degrading enzymes.
2.2.2.2. Stability studies in CSF
The hydrolysis patterns of 4, 12, 15, 18, and 21 were also studied in Bovine CSF at 37 °C for various time intervals and the data are presented in Table 1. It appears that the rate of the hydrolysis of the amino acid ester prodrugs in Bovine CSF is overall higher than in PBS and somewhat lower than in Bovine serum. The protein content in the Bovine CSF (obtained from Biochemed Services, Winchester, Virginia) was determined by the Bradford protein assay (see section 4.4.) and was found to be 150 times lower than the protein content in Bovine serum, which is somewhat higher than previously reported (260 times lower).[10] Although a low protein (enzyme) concentration is present in the CSF, enzymatic degradation may be possible causing an increased rate of the hydrolysis in the CSF compared with PBS. This is especially the case for the hydrolysis rate of 18, which is 6 times higher than in PBS. The dramatic increase in the rate of the hydrolysis of 18 in the CSF may be due to the presence of glutamate-specific enzymes in the CSF, as glutamate is the most common neurotransmitters in the brain.[30]
2.2.3. Solubility studies
The solubilities of 1, 2, and 4 and the hydrochloride salts of 12, 15, 18, and 21 were determined in PBS at pH 5, pH 6 and pH 7.4 at 37 °C (Table 2). The pHs of these PBS solutions were controlled before and after the addition of the amino acid ester prodrugs with pH-indicator strips (color pH ast®, Associate of Merck KGaA 64271 Darmstadt, Germany) and no changes were observed. Overall, the obtained data are in agreement with the estimated pKa values for the amino acid ester prodrugs (Table 2). Compound 12 is ~ 1–7 times more soluble than compounds 15 and 21, as the former has a slightly higher estimated pKa value. On the other hand, 4 is ~ 1.5–3 times more soluble than 12. The estimated water solubilities for 4 were 84 g/L at pH 5, 12 g/L at pH 6, and 1.4 g/L at pH 7.4 (Advanced Chemistry Development [ACD/Labs] software within Scifinder Scholar®), which are almost identical to those we measured in our study. On the other hand, Beauchamp et al.[21] determined the solubility of 4 in aqueous solution with 174 mg/mL. Although 12 has a higher estimated pKa value than 4 (7.85 vs 7.47, Table 2), the latter showed better solubility. The differences in the solubility between 4 and 12 may be due to the presence of the lipophilic carborane cage[31–34] in the latter compound.
Table 2.
pKa Values and compound solubilities in PBS at pH 5, pH 6, and pH 7.4 at 37 °C.
| Compds | pKa Values1 | PBS pH 5 | PBS pH 6 | PBS pH 7.4 |
|---|---|---|---|---|
| 1 | - | 0.003–0.005 g/L | 0.003–0.005 g/L | 0.003–0.005 g/L |
| 2 | - | 0.007–0.01 g/L | 0.007–0.01 g/L | 0.007–0.01 g/L |
| 4 | 1.90, 7.47 & 9.432 | 72.70 ± 0.40 g/L | 13.43 ± 0.26 g/L | 1.91 ± 0.06 g/L |
| 12 | ~ 7.85 | 26.30 ± 0.90 g/L | 3.70 ± 0.31 g/L | 1.31 ± 0.05 g/L |
| 15 | ~ 7.30 | 10.99 ± 0.20 g/L | 2.06 ± 0.11 g/L | 0.19 ± 0.01 g/L |
| 18 | ~ 2.20 & ~ 9.25 | 1.16 ± 0.04 g/L | 1.13 ± 0.06 g/L | 1.77 ± 0.08 g/L |
| 21 | ~7.30 | 11.18 ± 0.21 g/L | 3.60 ± 0.12 g/L | 0.62 ± 0.03 g/L |
Standard deviations are based on three experiments;
The pKa values for 12, 15, 18, and 21 are estimated based on calculations using Advanced Chemistry Development (ACD/Labs) software within ScifinderScholar® for the corresponding benzyl esters;
Measured values from reference[36].
Compound 21 showed slightly higher solubilities than 15, which was expected due to the presence of two additional hydroxyl groups in the former. The measured solubilities for 18 were moderate at all three pH levels and increased only slightly from 1.13 g/L at pH 5 to 1.77 g/L at pH 7.4. This is probably due to the presence of the additional carboxylic function with an estimated pKa of 2.20 (Table 2), and thus, zwitterions formation in the pH range of 5–7.4. Zwitterions can form strong intramolecular ion-ion interactions that interfere with the formation of ion-dipole interactions with water molecules, thereby reducing water-solubility.[35]
All evaluated prodrugs showed significantly increased solubilities compared with their parental drugs. Depending on the pH level in the test media, 12 is 327 to 6600 times more soluble than 1, 18 is 282 to 443 times more soluble than 1, and 15 and 21 are 48 to 2748 and 78 to 1400 times more soluble than 1 and 2, respectively.
In preclinical BNCT studies with rats bearing intracerebral glioma,[3] 200 µL of a solution of 2.5 g of 2 in one liter of a mixture of 35% DMSO and 65% of water was injected i.c. The solubility data shown in Table 2 demonstrate that similar concentrations can be achieved successfully with all tested prodrugs under aqueous conditions. Compound 12 showed the highest solubility, in particular at pH 5. Unfortunately, this amino acid ester prodrug does not seem to be a suitable candidate for further consideration because of its stability to chemical and enzymatic hydrolysis. Compounds 15 and 21 showed adequate solubilities at pH 5 and pH 6, whereas 18 had its highest solubility at pH 7.4. This may allow for the formulation of the latter amino acid ester prodrug at physiological pH for i.c. administration because it is fairly stable to chemical hydrolysis at pH 7.4 (see Table 1). In contrast, 15 and 21 require slightly acidic pH for appropriate solubilization. The possibility of administration at physiological pH may be an advantage of 18 compared with 15 and 21 because it may reduce toxic side effects.
3. Summary and Conclusions
Novel improved strategies were developed for the synthesis of 1 and 10. All proposed amino acid ester prodrugs of N5 (11–19) and N5-2OH (20–22) were successfully prepared. Notable are the inherently low overall yields for the synthesized prodrugs, in particular those for the 3'-monosubstituted amino acid ester prodrugs.
The rate of the hydrolysis of the amino acid ester prodrugs of 1 and 2 in the three incubation media depends primarily on the amino acid promoiety, and to lesser extend, on the site of esterification at the deoxyribose portion. Generally, 3'-monosubstituted amino acid ester prodrugs were more stable to chemical hydrolysis than 5'-monosubstituted amino acid ester prodrugs. The 5′-valine ester prodrug 12 is the most stable both to enzymatic and non-enzymatic hydrolysis, the 5′-glycine acid ester prodrugs 15 and 21 are the most sensitive under both conditions, whereas the 5′-glutamate ester prodrug 18 shows intermediate stability to chemical hydrolysis and high sensitivity to cleavage in serum and CSF. The high sensitivity of 15 and 21 and the stability of the 12 to hydrolysis are in agreement with the results of previous stability studies with prodrugs of metronidazole[14] and gemcitabine.[15] In all three incubation media, the hydrolysis of valacyclovir (4) was faster than that of 12. In contrast to 4, however, 12 is notably less susceptible to enzymatic or protein-aided cleavage, indicating that it is not a good substrate for any degrading enzymes. Compounds 15 and 21 appeared to be least affected by enzymatic or protein-aided cleavage, as their half-lives did not vary significantly in all three media. Overall, the observed solubilities for all tested amino acid ester prodrugs at pH 5, 6, and 7.4 are significantly higher than those of 1 and 2 and appeared to be suitable for i.c. injection. As discussed earlier, the fundamental prerequisite for amino acid ester prodrugs of 1 and 2 must be rapid cleavage to enable 5'-monophosphorylation. This requirement is fulfilled in the cases of 15 and 21. Also, 18 seems to be a suitable candidate for preclinical BNCT studies because it showed rapid cleavage in CSF combined with moderate stability in PBS at pH 7.4.
4. Experimental section
4.1. Chemistry
NMR spectra were obtained on a Bruker Avance 400 at The Ohio State University College of Pharmacy (400 MHz for 1H and 100 MHz for 13C). Chemical shifts are reported in parts per million (ppm). The coupling constants are reported in Hertz (Hz). High Resolution-Electrospray Ionization (HR-ESI) mass spectra were recorded on a Micromass LCT Electrospray mass spectrometer at The Ohio State University Campus Chemical Instrumentation Center (OSU-CCIC). For all carborane-containing compounds, the mass of the most intensive peak of the isotopic pattern was reported for an 80% boron-11 to 20% boron-10 distribution. Compound visualization on Silica Gel 60 F254 precoated TLC plates (0.25 mm layer thickness) (Merck, Darmstadt, Germany) was attained by UV light and KMnO4 spray. Carborane-containing compounds were selectively visualized by spraying a solution of 0.06% PdCl2 /1% aqueous HCl on TLC plates and subsequent heating to ~ 120 °C. Reagent grade solvents were used for column chromatography using Silica gel 60, particle size 63–200 µm (Dynamic Adsorbents, Inc, Georgia). Standard reagent grade chemicals were obtained from commercial vendors and used as such. o-Carborane was purchased from Katchem, Ltd, Prague, Czech Republic. Valacyclovir was purchased from Toronto Research Chemicals, Inc. Canada. Solvents were dried prior to use following standard procedures. All chemical reactions were carried out under argon atmosphere. Bovine serum and phosphate buffered saline (PBS) were obtained from Sigma-Aldrich. Bovine cerebrospinal fluid (CSF) was obtained from Biochemed Services, Winchester (Virginia). Filters (Corning, Germany) used in the stability studies had 0.2 µm pore size. The colorimetric measurement of protein concentrations in Bovine serum and Bovine CSF was carried out by using a Spectramax Plus Spectrophotometer from Molecular Devices (Sunnyvale, California). The obtained data were analyzed using SoftMax Pro 3.1.2 software.
Preparative HPLC was performed on a Gemini 5µ C18 column (21.20 mm × 250 mm, 5 µm particle size) from Phenomenex Inc. CA, USA using a Hitachi HPLC system (L-2130) with a Windows based data acquisition and a Hitachi Diode array detector (L-2455). Purification was accomplished using a water (0.1% TFA)/methanol gradient at 7 mL/min flow rate (100:0 to 50:50 over 15 min then 50:50 to 15:85 [H2O:MeOH] over 70 min followed by 15:85 to 0:100 over 15 min).
Analytical HPLC for purity confirmation and stability analysis was carried out with a Gemini 5µ C18 110A Column (250 × 4.6 mm) from Phenomenex Inc. CA, USA using the HPLC system above. Two different solvent systems were used at 1 mL/min flow rate: A = water (0.1% TFA)/acetonitrile (0.1% TFA); B = water (0.1% TFA)/methanol (0.1% TFA). For each solvent system, different gradients were used for compound analysis (Table 3, A1-A4, B1-B3). For all Thd derivatives, a wavelength of 265 nm was used for detection, whereas valacyclovir/acyclovir were detected at 254 nm.
4.1.1. 5-{2-(tert.-Butyldimethylsilyl)-o-carboran-1-yl}pentyl 4-methylbenzenesulfonate (6)
To a solution of 5[24] (0.52 g, 2 mmol) in THF (50 mL) was added n-BuLi (0.9 mL, 2.36 mmol, 2.5 M solution in hexanes) at −78 °C over a period of 10 min. The solution was gradually warmed to rt and then stirred for 1 h. Subsequently, the reaction mixture was cooled to 0 °C and 1,5-pentandiol ditosylate (1.1 g, 2.6 mmol) was added dropwise. The mixture was refluxed at 80 °C for 5 h. Distilled water (20 mL) was added and excess THF was removed under reduced pressure. The residue was extracted with ethyl acetate (30 mL × 2). The combined organic layers were washed with diluted HCl solution (2%, 30 mL) and brine (30 mL), and dried over anhydrous MgSO4. After filtration and evaporation, the residue was purified by silica gel column chromatography using hexanes/ dichloromethane, 8:2, as the eluent to give compound 6 (0.35 g, 36 %). Rf 0.70; 1H-NMR (CDCl3): δ 0.28 (s, 6H, (CH3)2Si), 1.01 (s, 9H, (CH3)3C), 1.18–1.30 (m, 2H, C2H4CH2), 1.36–1.46 (m, 2H, CH2CH2-Ccarborane), 1.56–1.64 (m, 2H, OCH2CH2), 2.09 (t, J = 7.7 Hz, 2H, CH2-Ccarborane), 2.42 (s, 3H, CH3), 3.96 (t, J = 6.1 Hz, 2H, OCH2), 7.32 (d, J = 7.9 Hz, 2H, Ph), 7.74 (d, J = 7.9 Hz, 2H, Ph); 13C-NMR (CDCl3): δ -1.53 ((CH3)2Si), 21.24 ((CH3)3C), 22.59 (CH3), 25.96 (C2H4CH2), 28.45 ((CH3)3C), 29.34 (CH2CH2-Ccarborane), 30.38 (OCH2CH2), 38.55 (CH2-Ccarborane), 70.82 (CH2O), 77.12, 82.10 (Ccarborane), 128.76, 130.83, 133.84, 145.38 (Ph); MS (HR-ESI) calcd for C20H42B10O3SSiNa (M+Na)+: 521.3525, found: 521.3434.
4.1.2. 3-(5-{2-[tert.-Butyldimethylsilyl]-o-carboran-1-yl}pentan-1-yl)thymidine (7)
To a solution of compound 6 (0.35 g, 0.71 mmol) in a mixture of DMF and acetone (30 mL, 1:1) were added Thd (0.86 g, 3.55 mmol) and potassium carbonate (0.59 g, 4,26 mmol) and stirred at 50 °C for 6 h. The reaction mixture was filtered and the filtrate was concentrated under reduced pressure. The residue was purified by silica gel column chromatography using ethyl acetate/methanol, 20:1, as the eluent to give compound 7 (0.26 g, 65 %). Rf 0.5; 1H-NMR (CD3OD): δ 0.37 (s, 6H, Si(CH3)2), 1.09 (s, 9H, C(CH3)3), 1.27–1.37 (m, 2H, NC2H4CH2), 1.54–1.65 (m, 4H, NCH2CH2, and CH2CH2-Ccarborane), 1.91 (s, 3H, CH3), 2.16–2.34 (m, 4H, CH2-Ccarborane, H-2" and H-2'), 3.75 (dd, J = 11.8, and 3.3 Hz, 1H, H-5"), 3.82 (dd, J = 11.8, and 3.3 Hz, 1H, H-5'), 3.87–3.96 (m, 3H, CH2N, and H-3'), 4.39–4.45 (m, 1H, H-4'), 6.31 (t, J = 6.8 Hz, 1H, H-1'), 7.85 (s, 1H, H-6), 13C-NMR (CD3OD): δ-2.94 (Si(CH3)2), 12.49 (CH3), 20.38 ((CH3)3C), 26.47 (NC2H4CH2), 27.25 ((CH3)3C), 30.14 (CH2CH2-Ccarborane, NCH2CH2), 38.01 (CH2-Ccarborane), 40.50 (CH2N), 41.02 (C-2'), 61.82 (C-5'), 71.15 (C-3'), 76.88, 82.24 (Ccarborane), 86.10 (C-1'), 87.87 (C-4'), 109.70 (C-5), 135.48 (C-6), 151.24 (C-2), 164.30 (C-4); MS (HR-ESI) calcd for C23H48B10N2O5SiNa (M+Na)+: 591.4233, found: 591.4304.
4.1.3. 3-(5-{o-Carboran-1-yl}pentan-1-yl)thymidine (1) [22–23]
To a solution of compound 7 (0.35 g, 0.62 mmol) in THF (30 mL) was added a 1 M solution of TBAF (1.0 mL, 1 mmol) in THF at −78 °C. The reaction mixture was stirred at rt for 0.5 h. Distilled water (10 mL) was added and excess THF was removed under reduced pressure. The residue was extracted with ethyl acetate (50 mL × 2), the combined organic layers were washed with brine (10 mL) and dried over anhydrous MgSO4. After filtration and evaporation, the residue was subjected to silica gel column chromatography using ethyl acetate/methanol, 20:1, as the eluent to give compound 1 (0.17 g, 58 %). Rf 0.6; 1H-NMR (CD3OD) δ 1.28–1.37 (m, 2H, NC2H4CH2), 1.49–1.66 (m, 4H, NCH2CH2, and CH2CH2-Ccarborane), 1.92 (s, 3H, CH3), 2.17–2.33 (m, 4H, CH2-Ccarborane, H-2", and H-2'), 3.74 (dd, J = 11.8, and 3.3 Hz, 1H, H-5"), 3.81 (dd, J = 11.8, and 3.3 Hz, 1H, H-5'), 3.88–3.95 (m, 3H, CH2N, and H-3'), 4.39–4.44 (m, 1H, H-4'), 4.53 (s, 1H, carboranyl-CH), 6.31 (t, J = 6.4 Hz, 1H, H-1'), 7.85 (s, 1H, H-6); MS (HR-ESI) calcd for C17H34B10N2O5Na (M+Na)+: 477.3369, found: 477.3546.
4.1.4. 4-(2-{5-Iodopentyl}-o-carboran-1-yl)methyl-2,2-dimethyl-1,3-dioxolane (9)
To a solution of compound 8[25] (2.58 g, 10 mmol) in THF (50 mL) was added a solution of n-BuLi (4.8 mL, 12 mmol, 2.5 M solution in hexanes) at −78 °C. The reaction mixture was allowed to warm up to 0 °C and 1,5-diiodopentane (5.83 g, 18 mmol) was added dropwise in rapid fashion. The reaction mixture was refluxed at 80 °C for 6 h. Distilled water (30 mL) was added and excess THF was removed under reduced pressure. The residue was extracted with ethyl acetate (50 mL × 2), and the combined organic layers were washed with brine (10 mL) and dried over anhydrous MgSO4. After filtration and evaporation, the residue was purified by silica gel column chromatography using hexanes/dichloromethane, 7:4, as the eluent to give compound 9 (1.85 g, 41 %). Rf 0.3; 1H-NMR ((CD3)2CO): δ 1.31–1.45 (m, 8H, C(CH3)2, NC2H4CH2), 1.50–1.62 (m, 2H, CH2CH2-Ccarborane), 1.78–1.87 (m, 2H, ICH2CH2), 2.16–2.33 (m, 2H, CH2-Ccarborane), 2.39 (dd, J = 11.9, and 3.4 Hz, 1H, CHCH2-Ccarborane), 2.42 (dd, J = 11.9, and 3.4 Hz, 1H, CHCH2-Ccarborane), 3.17 (t, J = 6.8 Hz, 2H, ICH2), 3.55 (dd, J = 11.9 and 3.4 Hz, 1H, CH2O), 4.13 (dd, J = 11.9 and 3.6 Hz, 1H, CHCH2O), 4.21–4.30 (m, 1H, CH2CHO); 13C-NMR ((CD3)2CO): δ 6.92 (CH2I), 25.80 (CH3), 27.35 (CH3), 29.07 (C2H4CH2), 30.52 (CH2CH2-Ccarborane), 33.20 (ICH2CH2), 35.32 (CH2-Ccarborane), 39.92 (CH2-Ccarborane), 69.51 (CH2O), 74.87 (CHO),77.44, 80.22 (Ccarborane), 110.02 (C(CH3)2); MS (HR-ESI) calcd for C13H31B10IO2Na (M+Na)+: 477.2270, found: 477.2257.
4.1.5. 3-(5-{2-[(2,2-Dimethyl-1,3-dioxolane-4-yl)methyl]-o-carboran-1-yl}pentan-1-yl)thymidine (10).[25]
To a solution of compound 9 (1.67 g, 3.68 mmol) in a mixture of DMF and acetone (50 mL, 1:1) were added Thd (4.45 g, 18.4 mmol) and potassium carbonate (3.05 g, 22.1 mmol) and stirred at 50 °C overnight. The reaction mixture was filtered and the filtrate was concentrated under reduced pressure. The residue was purified by silica gel column chromatography using ethyl acetate/methanol, 20:1, as the eluent to give compound 10 (1.0 g, 48 %). Rf 0.29; 1H-NMR (CD3OD): δ 1.29–1.42 (m, 8H, (CH3)2C, NC2H4CH2), 1.57–1.69 (m, 4H, NCH2CH2, and CH2CH2-Ccarborane), 1.92 (s, 3H, CH3), 2.17–2.40 (m, 4H, CH2-Ccarborane, H-2", and H-2'), 2.54 (dd, J = 11.9, and 3.4 Hz, 1H, CHCH2-Ccarborane), 2.61 (dd, J = 11.9, and 3.4 Hz, 1H, CHCH2-Ccarborane), 3.56 (t, J = 6.8 Hz, 1H, CH2O), 3.74 (dd, J = 11.9, and 3.4 Hz, 1H, H-5"), 3.82 (dd, J = 11.9, and 3.4 Hz, 1H, H-5'), 3.88–3.96 (m, 3H, CH2N, and H-3'), 4.06–4.14 (m, 1H, CH2O), 4.20–4.32 (m, 1H, CH2CHO), 4.38–4.44 (m, 1H, H-4'), 6.32 (t, J = 6.8 Hz, 1H, H-1'), 7.86 (s, 1H, H-6).
4.1.6.a. General procedure for the synthesis of the amino acid ester prodrugs of N5 (11–19) and the glycine ester prodrugs of N5-2OH (20–22)
Compound 1 (0.22 mmol) or 10 (0.18 mmol), N-Boc-amino acid (Boc-L-valine, Bocglycine, or Boc-L-glutamic acid α-tert-butyl ester) (0.44/0.36 mmol), DCC (0.44/0.36 mmol) and DMAP (0.044/0.036 mmol) were dissolved in 10 mL of anhydrous DMF. The reaction mixture was stirred at rt for 24 h. The progress of the reaction was monitored by TLC (ethyl acetate:hexanes, 8:5). The reaction yielded three products as determined by TLC. After 24 h, the reaction mixture was filtered and DMF was removed in vacuum. The residue was dissolved in ethyl acetate (30 mL) and washed with water (20 mL), 0.1 N HCl (20 mL), saturated NaHCO3 (20 mL), and brine (20 mL). The organic layer was dried over anhydrous MgSO4. The three intermediates observed by TLC were separated and purified partially by column chromatography (ethyl acetate: hexane, 8:5, see supplementary material for 1H-NMR data and Rf values for Boc-protected intermediates). Individual Boc-substituted amino acid ester prodrugs were added to 10 mL of a mixture of trifluoroacetic acid (TFA) and dichloromethane (1:1) and stirred at rt for 2 h (Boc-L-glutamic acid 5-tert.-butyl esters were stirred for 4 h). The solvent was removed and the amino acid ester prodrugs were purified by preparative HPLC to give products 11–22 as wax-like compounds.
4.1.6.b. General procedure for the synthesis of the hydrochloride salts of the amino acid ester prodrugs 11–13 and 15–22
To a solution of the amino acid ester prodrug (11–13 and 15–22 [0.2 mmol]) in diethyl ether/THF (1/1, 2 mL) was added 1 mL of a 1 M solution of HCl gas in diethyl ether at 0 °C and stirred overnight at rt. The solvent was removed under reduced pressure and the oily residue was solidified by adding 5 mL of a 0.1 M solution of HCl gas in diethyl ether. The obtained solid was washed 3–4 times with a 0.1 M solution of HCl gas in diethyl ether to give product (11–13•HCl and 15–22•HCl) in 50% to 70% yield.
4.1.6.1. 3'-L-Valyl-3-(5-{o-carboran-1-yl}pentan-1-yl)thymidine (11)
Yield: 6 mg, 5%; 1H-NMR (CD3OD): δ 1.11 (d, J = 4.6 Hz, 6H, CH(CH3)2), 1.28–1.38 (m, 2H, NC2H4CH2), 1.48–1.68 (m, 4H, NCH2CH2, and CH2CH2-Ccarborane), 1.92 (s, 3H, CH3), 2.21–2.50 (m, 5H, CH2-Ccarborane, H-2", H-2', and CH(CH3)2), 3.82–3.85 (m, 2H, H-5", and H-5'), 3.90 (t, J = 6.8 Hz, 2H, CH2N), 4.09 (d, J = 4.6 Hz, 1H, CHNH2), 4.14–4.18 (m, 1H, H-4'), 4.52 (s, 1H, carboranyl-CH), 5.48–5.52 (m, 1H, H-3'), 6.34 (t, J = 6.8 Hz, 1H, H-1'), 7.86 (s, 1H, H-6); 13C-NMR (CD3OD): δ 12.69 (CH3), 17.87 (CH(CH3)2), 26.59 (NC2H4CH2), 27.39 (CH2CH2-Ccarborane), 29.42 (NCH2CH2), 30.54 (CH(CH3)2), 37.89 (CH2-Ccarborane), 38.04 (CH2N), 41.42 (C-2'), 58.81 (CHNH2), 62.29 (Ccarborane), 63.14 (C-5'), 76.75 (Ccarborane), 78.24 (C-3'), 85.78 (C-1'), 86.52 (C-4'), 110.69 (C-5), 135.56 (C-6), 151.86 (C-2), 164.76 (C-4), 169.20 (COO); MS (HR-ESI) calcd for C22H44B10N3O6 (M+H)+: 554.4233, found: 554.4145; 11•HCl: Anal. (C22H44B10ClN3O6) C, H, N.
4.1.6.2. 5'-L-Valyl-3-(5-{o-carboran-1-yl}pentan-1-yl)thymidine (12)
Yield: 12 mg, 10%; 1H-NMR (CD3OD): δ 1.04–1.09 (m, 6H, CH(CH3)2), 1.25–1.34 (m, 2H, NC2H4CH2), 1.46–1.63 (m, 4H, NCH2CH2, and CH2CH2-Ccarborane), 1.91 (s, 3H, CH3), 2.23–2.36 (m, 5H, CH2-Ccarborane, H-2", H-2', and CH(CH3)2), 3.89 (t, J = 7.2 Hz, 2H, CH2N), 3.97 (d, J = 4.4 Hz, 1H, CHNH2), 4.06–4.13 (m, 1H, H-3'), 4.32–4.37 (m, 1H, H-4'), 4.41 (dd, J = 11.8, and 3.3 Hz, 1H, H-5"), 4.52 (s, 1H, carboranyl-CH), 4.56–4.64 (m, 1H, H-5'), 6.27 (t, J = 6.8 Hz, 1H, H-1'), 7.46 (s, 1H, H-6); 13C-NMR (CD3OD): δ 12.68 (CH3), 17.65, 17.76 (CH(CH3)2), 26.62 (NC2H4CH2), 27.41 (CH2CH2-Ccarborane), 29.44 (NCH2CH2), 30.55 (CH(CH3)2), 38.05 (CH2-Ccarborane), 39.59 (CH2N), 41.42 (C-2'), 58.86 (CHNH2), 63.16 (Ccarborane), 66.56 (C-5'), 71.61 (C-3'), 76.76 (Ccarborane), 84.64 (C-1'), 87.37 (C-4'), 110.58 (C-5), 135.92 (C-6), 151.66 (C-2), 164.75 (C-4), 169.55 (COO); MS (HR-ESI) calcd for C22H44B10N3O6 (M+H)+: 554.4233, found: 554.4227, calcd for C22H43B10N3O6Na (M+Na)+ 576.4053 (M+Na)+, found 576.4076; 12•HCl: Anal. (C22H44B10ClN3O6) C, H, N.
4.1.6.3. 3',5'-Di-L-valyl-3-(5-{o-carboran-1-yl}pentan-1-yl)thymidine (13)
Yield: 18 mg, 13%; 1H-NMR (CD3OD): δ 1.05 (d, J = 4.4 Hz, 6H, CH(CH3)2), 1.10 (d, J = 4.4 Hz, 6H, CH(CH3)2), 1.26–1.35 (m, 2H, NC2H4CH2), 1.47–1.63 (m, 4H, NCH2CH2, and CH2CH2-Ccarborane), 1.92 (s, 3H, CH3), 2.23–2.38 (m, 4H, CH2-Ccarborane, H-2" and H-2'), 2.50–2.57 (m, 1H, CH(CH3)2), 2.61–2.70 (m, 1H, CH(CH3)2), 3.89 (t, J = 6.8 Hz, 2H, CH2N), 3.98 (d, J = 4.4 Hz, 1H, CHNH2), 4.02 (d, J = 4.4 Hz, 1H, CHNH2), 4.33–4.39 (m, 1H, H-4'), 4.45–4.55 (m, 2H, carboranyl-CH and H-5"), 4.68–4.74 (m, 1H, H-5'), 5.46–5.50 (m, 1H, H-3'), 6.23 (t, J = 6.8 Hz, 1H, H-1'), 7.47 (s, 1H, H-6); 13C-NMR (CD3OD) δ 12.16 (CH3), 17.17, 17.24, 17.36, 17.39 (2CH(CH3)2), 26.09 (NC2H4CH2), 26.89 (CH2CH2-Ccarborane), 28.91 (NCH2CH2), 30.04 (2CH(CH3)2), 35.96 (CH2-Ccarborane), 37.55 (CH2N), 40.91 (C-2'), 58.32 (2CHNH2), 62.69 (Ccarborane), 65.51 (C-5'), 72.28 (Ccarborane), 76.31 (C-3'), 81.89 (C-1'), 87.71 (C-4'), 110.32 (C-5), 135.72 (C-6), 151.14 (C-2), 164.13 (C-4), 168.83, 169.07 (2COO); MS (HR-ESI) calcd for C27H53B10N4O7 (M+H)+: 653.4917, found: 653.4932; 13•HCl: Anal. (C27H54B10Cl2N4O7) C, H, N.
4.1.6.4. 3'-Glycinyl-3-(5-{o-carboran-1-yl}pentan-1-yl)thymidine (14)
Yield: 1 mg, 1%; 1H-NMR (CD3OD): δ 1.22–1.34 (m, 2H, NC2H4CH2), 1.44–1.63 (m, 4H, NCH2CH2, and CH2CH2-Ccarborane), 1.89 (s, 3H, CH3), 2.20–2.48 (m, 4H, CH2-Ccarborane, H-2" and H-2'), 3.87–3.94 (m, 6H, CH2N, CH2NH2, H-5" and H-5'), 4.12–4.17 (m, 1H, H-4'), 4.49 (s, 1H, carboranyl-CH, and), 5.45–5.55 (m, 1H, H-3'), 6.32 (t, J = 6.7 Hz, 1H, H-1'), 7.83 (s, 1H, H-6); 13C-NMR (CD3OD): δ 13.07 (CH3), 26.99 (NC2H4CH2), 27.78 (CH2CH2-Ccarborane), 29.82 (NCH2CH2), 38.27 (CH2-Ccarborane), 38.43 (NCH2), 41.02 (CH2NH2), 41.82 (C-2'), 62.71 (Ccarborane), 63.53 (C-5'), 77.13 (Ccarborane), 78.49 (C-3'), 86.22 (C-1'), 86.82 (C-4'), 111.08 (C-5), 135.92 (C-6), 152.25 (C-2), 165.13 (C-4), 168.17 (COO); MS (HR-ESI) calcd for C19H38B10N3O6 (M+H)+: 512.3764, found: 512.5839.
4.1.6.5. 5'-Glycinyl-3-(5-{o-carboran-1-yl}pentan-1-yl)thymidine (15)
Yield: 8 mg, 7%; 1H-NMR (CD3OD): δ 1.25–1.35 (m, 2H, NC2H4CH2), 1.44–1.64 (m, 4H, NCH2CH2, and CH2CH2-Ccarborane), 1.92 (s, 3H, CH3), 2.23–2.35 (m, 4H, CH2-Ccarborane, H-2" and H-2'), 3.84–3.95 (m, 4H, CH2N, and CH2NH2), 4.04–4.10 (m, 1H, H-3'), 4.36–4.40 (m, 1H, H-4'), 4.44 (dd, J = 11.7, and 3.6 Hz, 1H, H-5"), 4.48–4.55 (m, 2H, carboranyl-CH, and H-5'), 6.26 (t, J = 6.7 Hz, 1H, H-1'), 7.46 (s, 1H, H-6); 13C-NMR (CD3OD): 12.20 (CH3), 26.13 (NC2H4CH2), 26.92 (CH2CH2-Ccarborane), 28.94 (NCH2CH2), 37.56 (CH2-Ccarborane), 39.17 (NCH2), 39.96 (CH2NH2), 40.94 (C-2'), 62.65 (Ccarborane), 65.81 (C-5'), 71.05 (C-3'), 76.25 (Ccarborane), 84.12 (C-1'), 86.63 (C-4'), 110.11 (C-5), 135.34 (C-6), 151.19 (C-2), 164.27 (C-4), 167.47 (COO); MS (HR-ESI) calcd for C19H38B10N3O6 (M+H)+: 512.3764, found: 512.3798; 15•HCl: Anal. (C19H38B10ClN3O6) C, H, N.
4.1.6.6. 3',5'-Di-glycinyl-3-(5-{o-carboran-1-yl}pentan-1-yl)thymidine (16)
Yield: 19 mg, 15%; 1H-NMR (CD3OD): δ 1.24–1.36 (m, 2H, NC2H4CH2), 1.48–1.64 (m, 4H, NCH2CH2, and CH2CH2-Ccarborane), 1.92 (s, 3H, CH3), 2.26 (t, J=6.8 Hz, 2H, CH2-Ccarborane), 2.50–2.62 (m, 2H, H-2" and H-2'), 3.89–3.95 (m, 6H, CH2N, and 2CH2NH2), 4.32–4.38 (m, 1H, H-4'), 4.48–4.55 (m, 2H, carboranyl-CH, and, H-5"), 4.60 (dd, J = 11.7, and 3.6 Hz, 1H, H-5'), 5.46–5.49 (m, 1H, H-3'), 6.25 (t, J = 6.7 Hz, 1H, H-1'), 7.48 (s, 1H, H-6); 13C-NMR (CD3OD): δ 13.33 (CH3), 27.52 (NC2H4CH2), 28.04 (CH2CH2-Ccarborane), 30.08 (NCH2CH2), 37.17 (CH2-Ccarborane), 38.68 (NCH2), 41.11, 41.26 (2CH2NH2), 42.09 (C-2'), 63.80 (Ccarborane), 66.45 (C-5'), 77.39 (Ccarborane), 77.49 (C-3'), 82.93 (C-1'), 88.20 (C-4'), 111.45 (C-5), 136.52 (C-6), 152.28 (C-2), 165.33 (C-4), 168.52, 168.58 (2COO); MS (HR-ESI) calcd for C21H41B10N4O7 (M+H)+: 569.3978, found: 569.3975; 16•HCl: Anal. (C21H42B10Cl2N4O7) C, H, N.
4.1.6.7. 3'-L-Glutam-5-yl-3-(5-{o-carboran-1-yl}pentan-1-yl)thymidine (17)
Yield: 3 mg, 2%; 1H-NMR (CD3OD): δ 1.28–1.35 (m, 2H, NC2H4CH2), 1.47–1.64 (m, 4H, NCH2CH2, and CH2CH2-Ccarborane), 1.92 (s, 3H, CH3), 2.15–2.40 (m, 6H, CH2-Ccarborane, CH2CHNH2, H-2" and H-2'), 2.64–2.69 (m, 2H, CH2COO), 3.81–3.83 (m, 2H, H-5" and H-5'), 3.90 (t, J = 6.8 Hz, 2H, CH2N), 4.05 (t, J = 6.8 Hz, 1H, CHNH2), 4.10–4.13 (m, 1H, H-4'), 4.52 (s,1H, carboranyl-CH), 5.34–5.39 (m, 1H, H-3'), 6.31 (t, J = 6.8 Hz, 1H, H-1'), 7.86 (s, 1H, H-6); 13C-NMR (CD3OD): δ 12.19 (CH3), 25.45 (CH2CHNH2), 26.12 (NC2H4CH2), 26.91 (CH2CH2-Ccarborane), 28.94 (NCH2CH2), 29.50 (CH2COO), 37.51 (CH2-Ccarborane), 37.55 (NCH2), 40.94 (C-2'), 52.00 (CHNH2), 61.90 (C-5'), 62.66 (Ccarborane), 75.97 (C-3'), 77.50 (Ccarborane), 85.65 (C-1'), 85.98 (C-4'), 110.17 (C-5), 135.12 (C-6), 151.38 (C-2), 164.28 (C-4), 170.37 (COOCH2), 172.15 (COOH). MS (HR-ESI) calcd for C22H41B10N3O8Na (M+Na)+: 606.3795, found: 606.3813 (M+Na)+; 17•HCl: Anal. (C22H42B10ClN3O8) calcd: C, 42.61; H, 6.83; N, 6.78, found: C, 41.95; H, 6.54; N, 6.62.
4.1.6.8. 5'-L-Glutam-5-yl-3-(5-{o-carboran-1-yl}pentan-1-yl)thymidine (18)
Yield: 17 mg, 13%; 1H-NMR (CD3OD): δ 1.28–1.35 (m, 2H, NC2H4CH2), 1.46–1.64 (m, 4H, NCH2CH2, and CH2CH2-Ccarborane), 1.92 (s, 3H, CH3), 2.12–2.35 (m, 6H, CH2-Ccarborane, CH2CHNH2, H-2" and H-2'), 2.66 (t, J = 6.9 Hz, 2H, CH2COO), 3.89 (t, J = 6.9 Hz, 2H, CH2N), 4.01–4.11 (m, 2H, CHNH2, and H-3'), 4.30 (dd, J = 11.9, and 3.4 Hz, 1H, H-5"), 4.33–4.38 (m, 1H, H-4'), 4.41 (dd, J = 11.9, and 3.4 Hz, 1H, H-5'), 4.51 (s,1H, carboranyl-CH), 6.28 (t, J = 6.8 Hz, 1H, H-1'), 7.49 (s, 1H, H-6); 13C-NMR (CD3OD): δ 12.29 (CH3), 25.56 (CH2CHNH2), 26.13 (NC2H4CH2), 26.91 (CH2CH2-Ccarborane), 28.94 (NCH2CH2), 29.48 (CH2COO), 37.55 (CH2-Ccarborane), 39.57 (NCH2), 40.93 (C-2'), 51.99 (CHNH2), 62.64 (C-5'), 64.56 (Ccarborane), 71.22 (C-3'), 76.26 (Ccarborane), 84.65 (C-1'), 86.47 (C-4'), 110.00 (C-5), 135.00 (C-6), 151.18 (C-2), 164.28 (C-4), 170.29 (COOCH2), 172.32 (COOH); MS (HR-ESI) calcd for C22H41B10N3O8Na (M+Na)+ : 606.3795, found: 606.3793. 18•HCl: Anal. (C22H42B10ClN3O8) calcd: C, 42.61; H, 6.83; N, 6.78, found: C, 42.12; H, 6.74; N, 6.74.
4.1.6.9. 3',5'-Di-L-glutam-5-yl-3-(5-{o-carboran-1-yl}pentan-1-yl)thymidine (19)
Yield: 48 mg; 1H-NMR (CD3OD): δ 1.28–1.35 (m, 2H, NC2H4CH2), 1.45–1.64 (m, 4H, NCH2CH2, and CH2CH2-Ccarborane), 1.91 (s, 3H, CH3), 2.10–2.28 (m, 4H, CH2-Ccarborane, H-2", and H-2'), 2.35–2.50 (m, 4H, 2CH2CHNH2), 2.62–2.69 (m, 4H, 2CH2COO), 3.89 (t, J = 6.8 Hz, 2H, CH2N), 3.93–4.01 (m, 2H, 2CHNH2), 4.26–4.31 (m, 1H, H-4'), 4.34 (dd, J = 11.9, and 3.4 Hz, 1H, H-5"), 4.47 (dd, J = 11.9, and 3.4 Hz, 1H, H-5'), 4.51 (s,1H, carboranyl-CH), 5.29–5.33 (m, 1H, H-3'), 6.24 (t, J = 6.8 Hz, 1H, H-1'), 7.49 (s, 1H, H-6), 13C-NMR (CD3OD): δ 12.32 (CH3), 25.40, 25.54 (2CH2CHNH2), 26.12 (NC2H4CH2), 26.90 (CH2CH2-Ccarborane), 28.94 (NCH2CH2), 29.34, 29.52 (2CH2COO), 36.66 (CH2-Ccarborane), 37.55 (NCH2), 40.97 (C-2'), 52.00 (2CHNH2), 62.66 (C-5'), 64.36 (Ccarborane), 76.20 (C-3'), 77.25 (Ccarborane), 82.65 (C-1'), 86.59 (C-4'), 110.29 (C-5), 134.77 (C-6), 151.20 (C-2), 164.21 (C-4), 170.34, 170.38 (2COOCH2), 172.17, 172.31 (2COOH); MS (HR-ESI) calcd for C27H48B10N4O11Na, (M+Na)+, 735.4220, found: 735.4203; 19•HCl: Anal. (C27H50B10Cl2N4O11) C, H, N.
4.1.6.10. 3'-Glycinyl-3-[5-(2-{2,3-dihydroxyprop-1-yl}-o-carboran-1-yl)pentan-1-yl]thymidine (20)
Yield: 4 mg, 4%; 1H-NMR (CD3OD): δ 1.28–1.38 (m, 2H, NC2H4CH2), 1.52–1.66 (m, 4H, NCH2CH2, and CH2CH2-Ccarborane), 1.89 (s, 3H, CH3), 2.17–2.58 (m, 6H, CH2-Ccarborane, CHCH2-Ccarborane, H-2" and H-2'), 3.33–3.49 (m, 2H, CH2OH), 3.71–3.96 (m, 7H, CH2N, CH2NH2, H-5", H-5' and CH2CHO), 4.12–4.17 (m, 1H, H-4'), 5.44–5.49 (m, 1H, H-3'), 6.31 (t, J = 6.7 Hz, 1H, H-1'), 7.83 (s, 1H, H-6), 13C-NMR (CD3OD): δ 13.37 (CH3), 27.38 (NC2H4CH2), 28.00 (CH2CH2-Ccarborane), 30.49 (NCH2CH2), 35.93 (CH2-Ccarborane), 38.55 (CH2-Ccarborane), 40.03 (NCH2), 41.32 (CH2NH2), 42.09 (C-2'), 62.98 (C-5'), 67.06 (CH2OH), 72.35 (CHOH), 78.75 (C-3'), 80.57 (Ccarborane), 82.00 (Ccarborane), 86.47 (C-1'), 87.08 (C-4'), 111.34 (C-5), 136.16 (C-6), 152.51 (C-2), 165.40 (C-4), 16.46 (COO); MS (HR-ESI) calcd for C22H44B10N3O8 (M+H)+: 586.4132, found: 586.4059; 20•HCl: Anal. (C22H44B10ClN3O8) C, H, N.
4.1.6.11. 5'-Glycinyl-3-[5-(2-{2,3-dihydroxyprop-1-yl}-o-carboran-1-yl)pentan-1-yl]thymidine (21)
Yield: 23 mg, 22%; 1H-NMR (CD3OD): δ 1.24–1.37 (m, 2H, NC2H4CH2), 1.51–1.63 (m, 4H, NCH2CH2, and CH2CH2-Ccarborane), 1.90 (s, 3H, CH3), 2.18–2.38 (m, 4H, CH2-Ccarborane, H-2" and H-2'), 2.52 (d, J = 4.4 Hz, 2H, CHCH2-Ccarborane), 3.27–3.36 (m, 1H, CH2OH), 3.45 (dd, J = 11.7, and 3.6 Hz, 1H, CH2OH), 3.72–3.80 (m, 1H, H-3'), 3.82–3.94 (m, 4H, CH2N, and CH2NH2), 4.02–4.08 (m, 1H, CH2CHO), 4.31–4.37 (m, 1H, H-4'), 4.41 (dd, J = 11.7, and 3.6 Hz, 1H, H-5"), 4.49 (dd, J = 11.7, and 3.6 Hz, 1H, H-5'), 6.24 (t, J = 6.7 Hz, 1H, H-1'), 7.44 (s, 1H, H-6); 13C-NMR (CD3OD): δ 12.23 (CH3), 26.29 (NC2H4CH2), 26.91 (CH2CH2-Ccarborane), 29.38 (NCH2CH2), 34.82 (CH2-Ccarborane), 38.91 (CH2-Ccarborane), 39.19 (NCH2), 39.99 (CH2NH2), 40.99 (C-2'), 65.84 (C-5'), 65.93 (CH2OH), 71.07 (CHOH), 71.22 (C-3'), 79.45 (Ccarborane), 80.89 (Ccarborane), 84.14 (C-1'), 86.67 (C-4'), 110.12 (C-5), 135.35 (C-6), 151.12 (C-2), 164.28 (C-4), 167.46 (COO); MS (HR-ESI) calcd C22H44B10N3O8 (M+H)+: 586.4132, found: 586.4107; 21•HCl: Anal. (C22H44B10ClN3O8) C, H, N.
4.1.6.12. 3',5'-Di-Glycinyl-3-[5-(2-{2,3-dihydroxyprop-1-yl}-o-carboran-1-yl)pentan-1-yl]-thymidine (22)
Yield: 24 mg, 21%; 1H-NMR (CD3OD): δ 1.25–1.38 (m, 2H, NC2H4CH2), 1.53–1.64 (m, 4H, NCH2CH2, and CH2CH2-Ccarborane), 1.91 (s, 3H, CH3), 2.20–2.39 (m, 4H, CH2-Ccarborane, H-2" and H-2'), 2.46–2.61 (m, 2H, CHCH2-Ccarborane), 3.45 (dd, J = 11.7, and 3.6 Hz, 1H, CH2OH), 3.71–3.80 (m, 1H, CH2OH), 3.82–3.97 (m, 7H, CH2N, 2CH2NH2 and CH2CHO), 4.32–4.37 (m, 1H, H-4'), 4.49 (dd, J = 11.7, and 3.6 Hz, 1H, H-5"), 4.59 (dd, J = 11.7, and 3.6 Hz, 1H, H-5'), 5.42–5.47 (m, 1H, H-3'), 6.23 (t, J = 6.7 Hz, 1H, H-1'), 7.46 (s, 1H, H-6);13C-NMR (CD3OD): δ 12.21 (CH3), 26.25 (NC2H4CH2), 26.86 (CH2CH2-Ccarborane), 29.35 (NCH2CH2), 34.79 (CH2-Ccarborane), 36.09 (CH2-Ccarborane), 38.89 (NCH2), 39.97, 40.15 (2CH2NH2), 40.79 (C-2'), 65.41 (C-5'), 65.93 (CH2OH), 71.21 (CHOH), 76.39 (C-3'), 79.44 (Ccarborane), 80.86 (Ccarborane), 81.83 (C-1'), 87.16 (C-4'), 110.33 (C-5), 135.39 (C-6), 151.19 (C-2), 164.18 (C-4), 167.41, 167.47 (2COO); MS (HR-ESI) calcd for C24H47B10N4O9 (M+H)+: 643.4346, found: 643.4391; 22•HCl: Anal. (C24H47B10ClN4O9) calcd: C, 40.28; H, 6.76; N, 7.83, found: C, 40.36; H, 6.27; N, 7.53.
4.2.1. Stability studies in PBS at pH 7.4
Solutions of amino acid ester prodrugs 4, 11–19, and 21 in PBS (2 mM, 2.7 mL, pH 7.4) were incubated at 37 °C for 6–48 h depending on the degradation time of the prodrug. Aliquots of 200 µL from the incubated solutions were withdrawn at various time intervals and mixed with ice-cold TFA in acetonitrile solution (10%, 400 µL). The mixtures were passed through 0.2 µm filters and analyzed by HPLC (Table 3) to determine the areas under the curves (AUCs) that corresponded to the prodrugs and their degradation products.
4.2.2. Stability studies in Bovine serum
Solutions of amino acid ester prodrugs 4, 12, 15, 18, and 21 in Bovine serum (2 mM, 2.7 mL) were incubated at 37 °C for 6–48 h depending on the degradation profiles of the prodrugs. Aliquots of 200 µL from the incubation media were taken at various time points and added to ice-cold TFA in acetonitrile solutions (10%, 400 µL). The samples were centrifuged for 10 min at 3000 rcf, the supernatants were passed through 0.2 µm filters, and the filtrates were analyzed by HPLC (Table 3) to obtain AUCs.
4.2.3. Stability studies in Bovine CSF
Solutions of amino acid ester prodrugs 4, 12, 15, 18, and 21 in CSF (2 mM, 2.7 mL) were incubated at 37 °C for 6–48 h depending on the prodrug degradation profiles. Aliquots of 200 µL from each experiment were taken at various time intervals and mixed with ice-cold TFA in acetonitrile solution (10%, 400 µL). The samples were passed through 0.2 µm filters and analyzed by HPLC (Table 3) to give the corresponding AUCs.
4.2.4. Calculation of the degradation half-lives (t½) of the amino acid ester prodrugs
The hydrolytic half-lives (t½) of the amino acid ester prodrugs 4, 11–19, and 21 were calculated by utilizing the integrated AUCs from analytical HPLC data. The AUCs were converted into the remaining concentrations of the amino acid ester prodrugs using generated standard curves for the amino acid ester prodrugs. Standard curves for the amino acid ester prodrugs were generated by plotting different concentrations of the amino acid ester prodrug vs. the corresponding AUCs values. The apparent first-order degradation rate constants of the amino acid ester prodrugs 4, 11–19, and 21 were determined by plotting the logarithm of the remaining concentrations of the amino acid ester prodrug vs. time. The concentration-time curves were analyzed by a computerized a curve-stripping program (R Strip; Micromath Scientific Software, Salt Lake City, UT, USA). The slopes of these plots were related to the rate constant k given by the following relation:
K = 2.303 * slope (log C vs. time)[15]
The hydrolytic half-lives were then determined by the following equation:
t½ = 0.693/k[15]
4.3. Solubility studies
Quantities of 1 mg of the amino acid ester prodrugs 4, 12, 15, 18, and 21 or their corresponding parental drugs 1 and 2 were added to micro centrifuge tubes containing either PBS (100 µL, pH 5), PBS (100 µL, pH 6) or PBS (100 µL, pH 7.4). The micro centrifuge tubes were placed in a shaker for 4 h followed by centrifugation at 3000 rcf for 10 min. The solutions were passed through 0.2 µm filters and analyzed by HPLC (Table 3) to obtain the AUC corresponding to amount of the drug soluble in the each buffer. Standard curves for the each amino acid ester prodrug and its parental compound were generated and utilized to calculate the amino acid ester prodrug concentration corresponding to each AUC.
4.4. Determination of the protein content of PBS, Bovine serum and CSF using the Bradford protein assay
Seven dilutions of the standard protein (Bovine serum albumin, BSA) were required to set up a standard curve. Ten µL of each BSA concentration and 10 µL of each sample (PBS, serum, CSF) were added into separate plate wells of a 96 well plate. The quantity of 500 µL of 5-fold diluted Bradford reagent (BioRAD, Philadelphia) was added to each well. The plate was incubated in the dark at room temperature for 5 min. Following incubation, the absorbances of the different BSA concentrations and the samples were measured at 595 nm. A standard curve was generated by plotting the seven BSA concentrations vs. the corresponding absorbances. The standard curve was utilized to calculate the protein concentrations corresponding to the measured absorbance of each test sample.
Supplementary Material
Highlights.
L-Val-, L-Glu- & Gly-prodrugs of carboranyl thymidine analogues were synthesized.
Stability studies conducted in PBS, Bovine serum, and Bovine cerebrospinal fluid.
Solubility studies conducted in PBS at pH 5, 6 & 7.4.
5'-Gly-N5, 5'-Gly-N5-2OH & 5'-Glu-N5 selected for boron neutron capture therapy.
Acknowledgments
This work was supported by funds from The Ohio State University College of Pharmacy, NIH Grant R01CA127935, and a scholarship from the Scholar Exchange Program of the Egyptian Education Ministry. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- 10B
boron-10
- 3-CTAs
3-carboranyl thymidine analogues
- AUCs
areas under the curves
- BNCT
boron neutron capture therapy
- Boc
butoxycarbonyl
- BSA
Bovine serum albumin
- BuLi
butylithium
- CSF
cerebrospinal fluid
- DCC
dicyclohexylcarboiimide
- DCM
dichloromethane
- DMAP
4-dimethylaminopyridine
- DMF
dimethyl formamide
- DMSO
dimethyl sulfoxide
- GBM
glioblastoma multiforme
- HR-ESI
high resolution-electrospray ionization
- hTK1
human thymidine kinase 1
- ISF
interstitial fluid
- i.c.
intracranial
- i.t.
intratumoral
- i.v.
intravenous
- KMT
kinase mediated trapping
- LET
linear energy transfer
- OSU-CCIC
The Ohio State University Campus Chemical Instrumentation Center
- PBS
phosphate buffered saline
- RP-18
reversed phase 18
- rt
room temperature
- TBAF
tetrabutylammonium fluoride
- TBDMS
tert.-butyldimethylsilyl
- TFA
trifluoroacetic acid
- Thd
thymidine
- THF
tetrahydrofurane
- TK1
thymidine kinase
Appendix A. Supplementary material
Supplementary data related to this article can be found online in the online version, at ……………... Data include original MS-, 1H-NMR-, and 13C-NMR spectra, HPLC chromatograms, additional NMR data, and figures related to stability studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barth RF, Coderre JA, Vicente M, Blue TE. Boron neutron capture therapy of cancer: Current status and future prospects. Clin. Cancer Res. 2005;11:3987–4002. doi: 10.1158/1078-0432.CCR-05-0035. [DOI] [PubMed] [Google Scholar]
- 2.Barth RF, Yang W, Al-Madhoun AS, Johnsamuel J, Byun Y, Chandra S, Smith DR, Tjarks W, Eriksson S. Boron-containing nucleosides as potential delivery agents for neutron capture therapy of brain tumors. Cancer Res. 2004;64:6287–6295. doi: 10.1158/0008-5472.CAN-04-0437. [DOI] [PubMed] [Google Scholar]
- 3.Barth RF, Yang W, Wu G, Swindall M, Byun Y, Narayanasamy S, Tjarks W, Tordoff K, Moeschberger ML, Eriksson S, Binns PJ, Riley KJ. Thymidine kinase 1 as a molecular target for boron neutron capture therapy of brain tumors. Proc. Nat. Acad. Sci. U.S.A. 2008;105:17493–17497. doi: 10.1073/pnas.0809569105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Madhoun AS, Johnsamuel J, Barth RF, Tjarks W, Eriksson S. Evaluation of human thymidine kinase 1 substrates as new candidates for boron neutron capture therapy. Cancer Res. 2004;64:6280–6286. doi: 10.1158/0008-5472.CAN-04-0197. [DOI] [PubMed] [Google Scholar]
- 5.Tjarks W, Tiwari R, Byun Y, Narayanasamy S, Barth RF. Carboranyl thymidine analogues for neutron capture therapy. Chem. Commun. 2007;47:4978–4991. doi: 10.1039/b707257k. [DOI] [PubMed] [Google Scholar]
- 6.Arner ES, Eriksson S. Mammalian deoxyribonuclease kinases. Pharmacol. Ther. 1995;67:155–186. doi: 10.1016/0163-7258(95)00015-9. [DOI] [PubMed] [Google Scholar]
- 7.Aspland SE, Ballatore C, Castillo R, Desharnais J, Eustaquio T, Goelet P, Guo Z, Li Q, Nelson D, Sun C, Castellino AJ, Newman MJ. Kinase-mediated trapping of bi-functional conjugates of paclitaxel or vinblastine with thymidine in cancer cells. Bioorg. Med. Chem. Lett. 2006;16:5194–5198. doi: 10.1016/j.bmcl.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Silverman RB. The Organic Chemistry of Drug Design and Drug Action. second ed. San Diego: Elsevier Academic Press; 2004. [Google Scholar]
- 9.Al-Madhoun AS, Tjarks W, Eriksson S. The role of thymidine kinases in the activation of pyrimidine nucleoside analogues. Mini-Rev. Med. Chem. 2004;4:341–350. doi: 10.2174/1389557043403963. [DOI] [PubMed] [Google Scholar]
- 10.Davson H, Segal MB. Physiology of the CSF and Blood-Brain Barriers. Boca Raton: CRC Press; 1996. [Google Scholar]
- 11.Bonina FP, Arenare L, Palagiano F, Saija A, Nava F, Trombetta D, Caprariis PD. Synthesis, stability, and pharmacological evaluation of nipecotic acid prodrugs. J. Pharm. Sci. 1999;88:561–567. doi: 10.1021/js980302n. [DOI] [PubMed] [Google Scholar]
- 12.Williams DB, Varia SA, Stella VJ, Pitman IH. Evaluation of the prodrug potential of the sulfate esters of acetaminophen and 3-hydroxymethyl-phenytoin. Int. J. Pharm. 1983;14:113–120. [Google Scholar]
- 13.Flynn GL, Lamb DJ. Factors influencing solvolysis of corticosteroid-21-phosphate esters. J. Pharm. Sci. 1970;59:1433–1438. doi: 10.1002/jps.2600591014. [DOI] [PubMed] [Google Scholar]
- 14.Bundgaard H, Larsen C, Thorbek P. Prodrugs as drug delivery systems XXVI. Preparation and enzymatic hydrolysis of various water-soluble amino acid esters of metronidazole. Int. J. Pharm. 1984;18:67–77. [Google Scholar]
- 15.Song X, Lorenzi PL, Landowski CP, Vig BS, Hilfinger JM, Amidon GL. Amino acid ester prodrugs of the anticancer agent gemcitabine: synthesis, bioconversion, metabolic bioevasion, and hPEPT1-mediated transport. Mol. Pharm. 2005;2:157–167. doi: 10.1021/mp049888e. [DOI] [PubMed] [Google Scholar]
- 16.Tsume Y, Vig BS, Sun J, Landowski CP, Hilfinger JM, Ramachandran C, Amidon GL. Enhanced absorption and growth inhibition with amino acid monoester prodrugs of floxuridine by targeting hPEPT1 transporters. Molecules. 2008;13:1441–1454. doi: 10.3390/molecules13071441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vig BS, Lorenzi PJ, Mittal S, Landowski CP, Shin H, Mosberg HI, Hilfinger John M, Amidon GL. Amino acid ester prodrugs of floxuridine: Synthesis and effects of structure, stereochemistry, and site of esterification on the rate of hydrolysis. Pharm. Res. 2003;20:1381–1388. doi: 10.1023/a:1025745824632. [DOI] [PubMed] [Google Scholar]
- 18.Sun Y, Sun J, Jing SY, Yin S, Chen Y, Li G, Xu Y, He Z. Synthesis, transport and pharmacokinetics of 5'-amino acid ester prodrugs of 1-β-D-arabinofuranosylcytosine. Mol. Pharm. 2009;6:315–325. doi: 10.1021/mp800200a. [DOI] [PubMed] [Google Scholar]
- 19.Anand B, Katragadda S, Nashed Y, Mitra A. Amino acid prodrugs of acyclovir as possible antiviral agents against ocular HSV-1 infections: Interactions with the neutral and cationic amino acid transporter on the corneal epithelium. Curr. Eye Res. 2004;29:153–166. doi: 10.1080/02713680490504614. [DOI] [PubMed] [Google Scholar]
- 20.Katragadda S, Jain R, Kwatra D, Hariharan S, Mitra A. Pharmacokinetics of amino acid ester prodrugs of acyclovir after oral administration: Interaction with the transporters on Caco-2 cells. Int. J. Pharm. 2008;362:93–101. doi: 10.1016/j.ijpharm.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beauchamp LM, Orr GF, De Miranda P, Burnette T, Krenitsky TA. Amino acid ester prodrugs of acyclovir. Antiviral Chem. Chemother. 1992;3:157–164. [Google Scholar]
- 22.Lunato AJ, Wang J, Woollard JE, Anisuzzaman AKM, Ji W, Rong FG, Ikeda S, Soloway AH, Eriksson S, Ives DH, Blue TE, Tjarks W. Synthesis of 5-(carboranylalkylmercapto)-2'-deoxyuridines and 3-(carboranylalkyl)thymidines and their evaluation as substrates for human thymidine kinases 1 and 2. J. Med. Chem. 1999;42:3378–3389. doi: 10.1021/jm990125i. [DOI] [PubMed] [Google Scholar]
- 23.Tiwari R, Toppino A, Agarwal HK, Huo T, Byun Y, Gallucci J, Hasabelnaby S, Khalil A, Goudah A, Baiocchi RA, Darby MV, Barth RF, Tjarks W. Synthesis, biological evaluation, and radioiodination of halogenated closo-carboranylthymidine analogues. Inorg. Chem. 2012;51:629–639. doi: 10.1021/ic202150b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gomez FA, Hawthorne MF. A simple route to C-monosubstituted carborane derivatives. J. Org. Chem. 1992;5:1384–1390. [Google Scholar]
- 25.Byun Y, Thirumamagal BTS, Yang W, Eriksson S, Barth RF, Tjarks W. Preparation and biological evaluation of 10B-enriched 3-[5-{2-(2,3-dihydroxyprop-1-yl)-o-carboran-1-yl}pentan-1-yl]thymidine (N5-2OH), a new boron delivery agent for boron neutron capture therapy of brain tumors. J. Med. Chem. 2006;49:5513–5523. doi: 10.1021/jm060413w. [DOI] [PubMed] [Google Scholar]
- 26.Colla L, De Clercq E, Busson R, Vanderhaeghe H. Synthesis and antiviral activity of water-soluble esters of acyclovir [9-[(2-hydroxyethoxy)methyl]guanine] J. Med. Chem. 1983;26:602–604. doi: 10.1021/jm00358a029. [DOI] [PubMed] [Google Scholar]
- 27.Thomsen AE, Friedrichsen GM, Sørensen AH, Andersen R, Nielsen CU, Brodin B, Begtrup M, Frokjaer S, Steffansen B. Prodrugs of purine and pyrimidine analogues for the intestinal di/tri-peptide transporter PepT1: Affinity for hPepT1 in Caco-2 cells, drug release in aqueous media and in vitro metabolism. J. Control. Release. 2003;86:279–292. doi: 10.1016/s0168-3659(02)00413-3. [DOI] [PubMed] [Google Scholar]
- 28.Abbott NJ. Evidence for bulk flow of brain interstitial fluid: significance for physiology and pathology. Neurochem. Int. 2004;45:545–552. doi: 10.1016/j.neuint.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Landowski CP, Lorenzi PL, Song X, Amidon GL. Nucleoside ester prodrug substrate specificity of liver carboxylesterase. J. Pharmacol. Exp. Ther. 2006;316:572–580. doi: 10.1124/jpet.105.092726. [DOI] [PubMed] [Google Scholar]
- 30.Nedergaard M, Takano T, Hansen AJ. Beyond the role of glutamate as a neurotransmitter. Nature Rev. Neurosci. 2002;3:748–755. doi: 10.1038/nrn916. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto K, Endo Y. Bioorg. Utility of boron clusters for drug design. Hansch-fujita hydrophobic parameters π of dicarba-closo-dodecaboranyl groups. Med. Chem. Lett. 2001;11:2389–2392. doi: 10.1016/s0960-894x(01)00438-3. [DOI] [PubMed] [Google Scholar]
- 32.Thirumamagal BTS, Zhao XB, Bandyopadhyaya AK, Johnsamuel J, Tiwari R, Golightly DW, Patel V, Jehning BT, Backer MV, Barth RF, Lee RJ, Backer JM, Tjarks W. Receptor-targeted liposomal delivery of boron-containing cholesterol mimics for boron neutron capture therapy (BNCT) Bioconjugate Chem. 2006;17:1141–1150. doi: 10.1021/bc060075d. [DOI] [PubMed] [Google Scholar]
- 33.Fauchere JL, Quang Do K, Jow PYC, Hansch C. Unusually strong lipophilicity of 'fat' or 'super' amino-acids, including a new reference value for glycine. Experientia. 1980;36:1203–1204. doi: 10.1007/BF01976129. [DOI] [PubMed] [Google Scholar]
- 34.Narayanasamy S, Thirumamagal BTS, Johnsamuel J, Byun Y, Al-Madhoun AS, Usova E, Cosquer GY, Yan J, Bandyopadhyaya AK, Tiwari R, Eriksson S, Tjarks W. Hydrophilically enhanced 3-carboranyl thymidine analogues (3CTAs) for boron neutron capture therapy (BNCT) of cancer. Bioorg. Med. Chem. 2006;14:6886–6899. doi: 10.1016/j.bmc.2006.06.039. [DOI] [PubMed] [Google Scholar]
- 35.Lemke TL, Williams DA. Foye's Principles of Medicinal Chemistry. sixth ed. Philadelphia: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 36.Granero GE, Amidon GL. Stability of valacyclovir: Implications for its oral bioavailability. Int. J. Pharm. 2006;317:14–18. doi: 10.1016/j.ijpharm.2006.01.050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.