Abstract
Long-standing type 1 diabetes (T1D) is associated with an absolute loss of endogenous insulin secretion (circulating C-peptide is undetectable) and a related defect in glucose counterregulation that is often complicated by hypoglycemia unawareness, markedly increasing the risk for severe hypoglycemia. Both the transplantation of isolated islets and a whole pancreas can restore β-cell secretory capacity, improve glucose counterregulation, and return hypoglycemia awareness, thus alleviating severe hypoglycemia. The transplantation of islets may require more than one donor pancreas, and the recovery of endocrine function for now appears more durable with a whole pancreas; however, islet transplantation outcomes are steadily improving. Because not all patients with T1D experiencing severe hypoglycemia are candidates to receive a whole pancreas, and since not all pancreata are technically suitable for whole organ transplantation, islet and pancreas transplantation are evolving as complementary approaches for the recovery of endocrine function in patients with the most problematic T1D.
Keywords: Type 1 diabetes, C-peptide response, functional β-cell mass, β-cell secretory capacity, insulin secretion, insulin sensitivity, proinsulin secretion, glucagon secretion, glucose counterregulation, hypoglycemia unawareness, immunosuppression drugs, prednisone, tacrolimus, sirolimus, mycophenolate, Endocrine function, Islet and pancreas transplantation
Introduction
The transplantation of isolated islets and a whole pancreas are both potential therapies for the treatment of type 1 diabetes (T1D), particularly when complicated by recurrent episodes of severe hypoglycemia (1). Both approaches can restore insulin secretion, but the transplantation of islets isolated from more than one donor pancreas is often required to achieve insulin independence. The durability of insulin independence is superior following whole pancreas transplantation (2), especially when a pancreas is transplanted at the same time as a kidney (simultaneous pancreas-kidney or SPK (3)). The majority of islet recipients will return to requiring some insulin therapy by three years following transplantation, but they can expect continued amelioration from episodes of severe hypoglycemia for the duration of graft function that is currently retained in 90% of recipients at four years (4). With more durable insulin independent graft function, severe hypoglycemia episodes may be eliminated in the majority of SPK recipients for more than a decade (5;6).
Presently, islets are transplanted either alone into patients with T1D who are experiencing severe problems with hypoglycemia or into patients who have already received a kidney transplant and so are already committed to immunosuppressive therapy. A whole pancreas is usually transplanted as a SPK because of superior long-term graft function when compared to the transplant of a pancreas alone (3), and because this approach limits the risk of additional surgery. Thus, the transplantation of isolated islets and whole pancreata are evolving as complementary approaches for patients with T1D who are experiencing recurrent severe hypoglycemia or requiring a concomitant kidney allograft. This review will focus on the endocrine defects responsible for the development of severe hypoglycemia in T1D and the physiologic recovery from those defects currently afforded by islet or pancreas transplantation.
Functional β-cell mass in type 1 diabetes
T1D results from autoimmune destruction of the insulin-producing β-cells in the endocrine pancreatic islets of Langerhans. The endocrine pancreas normally contains ~ 1 million islets that comprise 2 - 3% of the total pancreatic mass. After a variable period of months to years of autoimmune destruction, clinically overt diabetes is diagnosed when the functional β-cell mass has been reduced to that nearly sufficient to meet daily insulin needs. Functional β-cell mass is best estimated from the β-cell secretory capacity, a measure that correlates with calculated β-cell mass in animal models of β-cell reduction (7), with resection (8) and transplantation (9-11) of a hemi-pancreas in humans, and with transplanted islet mass in successful human islet autotransplantation (12). The β-cell secretory capacity is derived from glucose-potentiation of insulin or C-peptide release in response to injection of a non-glucose secretagogue such as arginine or glucagon. Glucose-potentiation involves the creation of controlled hyperglycemia that serves to prime the β-cells by inducing the recruitment of secretory granules to a readily releasable pool that is subsequently released in response to membrane depolarization induced by arginine or glucagon.
In one study of preclinical T1D the mean β-cell secretory capacity was 25% of normal (13), and in another study of new-onset T1D the median β-cell secretory capacity was 25% of normal (14), together suggesting this as the minimal functional β-cell mass required to avoid overt diabetes. Many patients will maintain endogenous insulin secretion, as estimated from levels of C-peptide, for up to 5 years, and the institution of intensive insulin therapy at the time of diagnosis has been shown to slow the rate of β-cell loss (15;16). Nevertheless, the majority of patients with T1D will lose all β-cell function by 10 - 15 years from diagnosis and become C-peptide “negative” (reviewed in (17)).
The maintenance of low levels of endogenous insulin secretion in T1D is clinically important. In the Diabetes Control and Complications Trial (DCCT) a 90 minute mixed-meal stimulated C-peptide >0.6 ng/ml was associated with reduced incidence of retinopathy and nephropathy, and a decreased prevalence of severe hypoglycemia; all effects were more pronounced in those receiving intensive insulin therapy (18). Conversely, DCCT participants who had undetectable C-peptide were at the greatest risk for severe hypoglycemia regardless of treatment intensity (19). The protection from severe hypoglycemia is best explained by the presence of residual islet β-cells maintaining the paracrine signal for islet α-cell glucagon secretion in response to declining blood glucose (20). Presently available assays for C-peptide have a lower limit of detection of 0.05 – 0.1 ng/ml, but may falsely detect C-peptide up to 0.2 ng/ml. In the Clinical Islet Transplantation (CIT) Consortium, “C-peptide negative” has been defined as <0.3 ng/ml 90 minutes after mixed-meal (Boost® High Protein) stimulation (21). When the fasting C-peptide alone is taken into account, an undetectable level is almost assuredly “negative” as long as the concomitantly measured fasting glucose is not low. The selection of C-peptide negative patients for islet or pancreas transplantation not only helps to identify those in whom restoration of endogenous insulin secretion is most likely to benefit in terms of alleviation from severe hypoglycemia, but is also useful for monitoring transplanted islet graft function by paired levels of C-peptide and glucose (22).
Recovery of functional β-cell mass by islet transplantation
The transplantation of islets isolated from a deceased donor pancreas is accomplished in humans via portal vein infusion accessed either by a percutaneous transhepatic or a minilaparotomy approach with subsequent intrahepatic islet engraftment (23). To date, the liver is the only site that has enabled sufficient survival of transplanted islets to consistently reverse diabetes and achieve insulin independence in large animal models (24) and humans, an attribute best explained by the provision of oxygenation via the portal circulation until revascularization occurs from the hepatic arterial system (25). As intrahepatic islets degranulate in the hours following intraportal infusion (26) and revascularization may not be complete before a month in non-human primates ((27) and so presumably humans), complete functional recovery of transplanted islets should not be expected for several weeks. During this engraftment period intensive insulin therapy should be maintained to avoid glucotoxicity (28) and reduce β-cell demand during this critical time of relative hypoxia. Reduced insulin requirements to avoid hypoglycemia together with detectable C-peptide indicate post-transplant islet survival. In the CIT Consortium, subjects at least 2 months post-transplant who are able to taper off insulin therapy and at day 75 maintain a fasting glucose < 126 mg/dl and a 90 minute mixed-meal stimulated glucose < 180 mg/dl (among other criteria (21)) may be considered insulin-independent, whereas insufficient glycemic control is an indication for resumed insulin management and consideration of a second (or rarely third) islet infusion.
The Edmonton protocol for islet transplantation established that under glucocorticoid-free immunosuppression a subsequent islet infusion from a second donor pancreas could reproducibly achieve a sufficient engrafted islet β-cell mass to render the recipient insulin-independent (29). A multi-center trial subsequently demonstrated that this approach resulted in insulin-independence in 60% of recipients, although the majority of these patients returned to insulin therapy by 2 years post-transplant (30). Nonetheless, 80% of recipients maintained islet graft function as indicated by a reduction in insulin requirements and C-peptide production for the 2 years of follow-up (30), that in Edmonton was maintained for 5 years with associated protection from severe hypoglycemia (31). Using the Edmonton approach, we (32) and Paty and colleagues (33) have shown that after infusion of a mean total 860,984 and 618,000 islet equivalents, respectively, insulin-independent recipients had a β-cell secretory capacity only ~ 25% of normal. Using another glucocorticoid-free regimen with islets transplanted from pooled donors to achieve insulin-independence, Keymeulen and colleagues (34) also demonstrated a β-cell secretory capacity of ~ 25% of normal in islet recipients. The lower functional islet β-cell mass for the numbers transplanted suggests early loss of transplanted islets before engraftment due to nonspecific inflammatory and thrombotic mechanisms (35). Collectively, these results indicate a markedly reduced engrafted islet β-cell mass in transplant recipients that is just at the margin of what is required to avoid hyperglycemia, and so helps to explain the eventual return to insulin therapy experienced by the majority of recipients.
Important for a low functional islet β-cell mass, insulin sensitivity derived from the minimal model in islet recipients transplanted according to the Edmonton protocol is improved when compared to T1D pre-transplant and not different from normal assessed both cross-sectionally (36) and prospectively (37). Both studies demonstrated enhanced insulin-mediated glucose disposal and free fatty acid suppression, suggesting correction of hyperglycemia and elevated free fatty acids in T1D as mechanisms for the improved insulin sensitivity following islet transplantation. The finding of improved insulin sensitivity in human islet recipients is also noteworthy since the maintenance immunosuppression agents tacrolimus and sirolimus used in the Edmonton protocol have been implicated in the development of insulin resistance in a rat model of islet transplantation (38). As will be discussed below for pancreas transplantation, modern dosing of the calcineurin-inhibitor (CNI) tacrolimus does not appear to cause a significant impairment in insulin sensitivity (39), and the use of the mammalian target of rapamycin (mTOR) inhibitor sirolimus did not impair insulin sensitivity derived from the minimal model in a non-human primate model of islet transplantation (40).
Excessive secretion of proinsulin relative to insulin, resulting in an elevated molar ratio of proinsulin-to-insulin, can accompany increased β-cell demand with recruitment of immature secretory granules containing an abundance of incompletely processed proinsulin (41). In two studies involving insulin-independent (39;42) and dependent (42) islet transplant recipients where the mean HbA1c was ~ 6.0%, fasting proinsulin-to-insulin ratios were normal, as were proinsulin secretory ratios in response to glucose-potentiated arginine (39). In contrast, another study of islet transplant recipients with a mean HbA1c level of 7.1% exhibited elevated fasting proinsulin-to-insulin ratios (43). Because hyperglycemia increases β-cell recruitment of immature secretory granules leading to relative hyperproinsulinemia (44), these reports suggest that the maintenance of near-normal glycemia (HbA1c < 6.5%) with insulin therapy when appropriate may protect islet recipients from secreting incompletely processed proinsulin (reviewed in (45)). Moreover, the absence of relative hyperproinsulinemia in islet transplant recipients with near-normal glycemic control provides additional evidence against a clinically important increase in metabolic demand imposed by tacrolimus and sirolimus immunotherapy.
Additional evidence for the importance of avoiding hyperglycemia in islet recipients comes from the finding of amyloid deposition in intrahepatic islets of 3/4 recipients on postmortem examination ~ 2 - 5 years post-transplant (46;47). Islet amyloid is composed of islet amyloid polypeptide (IAPP or amylin) fibrils deposited within and surrounding β-cells, where they exhibit direct toxicity (48). IAPP is co-secreted from the β-cell with insulin (49), but normally is inhibited from forming amyloid by appropriate proportions of insulin and other factors in the β-cell (50). We demonstrated disproportionately increased IAPP-to-insulin secretion during glucose-potentiated arginine testing in insulin-independent recipients (51), suggesting that hyperglycemia may disturb regulation of insulin and IAPP within transplanted islets and facilitate the development of islet amyloid. Recently, we showed in a non-human primate model that transplanted islets develop amyloid deposits coincident with animal growth increasing the β-cell secretory demand, and that the presence of amyloid predated a decline in β-cell secretory capacity and subsequent recurrence of hyperglycemia (52). From these observations it follows that avoiding hyperglycemia and reducing metabolic demand through the provision of exogenous insulin or increasing the engrafted islet β-cell mass should improve long-term functional outcomes for islet recipients.
There is indirect evidence for a long-term benefit of establishing a functional reserve capacity, i.e. > 25% of a functional β-cell mass, following islet transplantation in humans. In the Collaborative Islet Transplant Registry (CITR) report of transplants conducted from 1999 to 2006, of those recipients who ever achieved insulin independence 75% maintained islet graft function at 3 years whereas of those who never achieved insulin independence only 25% had persistent function (53). In the most recent update from CITR, among recipients transplanted from 2007 to 2010, 95% of those who ever achieved insulin independence retained islet graft function at 3 years compared to 70% for those who never achieved insulin independence (4). These data support, in the absence of evidence for alloimmune rejection or recurrent autoimmunity, the practice of performing a second islet infusion when needed to establish insulin independence. Also noteworthy, in this most recent period of CITR data less than half of recipients received a second islet infusion compared to two-thirds of prior recipients (4), likely reflecting improved islet engraftment with transplantation protocols developed since the Edmonton protocol.
New induction immunosuppression protocols from Minneapolis incorporating as well peri-transplant anti-inflammatory and anti-thrombotic (54) therapy with similar low-dose calcineurin inhibitor and mTOR inhibitor maintenance therapy as in the Edmonton protocol appear to have improved rates of insulin-independence occurring more frequently with islets isolated from a single donor (55) and being sustaining for a longer duration (56;57). One regimen based on the polyclonal T cell depleting antibody rabbit anti-thymocyte globulin (rATG) and the tumor necrosis factor alpha (TNFα) inhibitor etanercept at induction and low-dose tacrolimus and sirolimus for maintenance is presently being evaluated as part of the multicenter CIT Consortium Protocol CIT07 (21). Our preliminary results using the CIT07 protocol indicate a β-cell secretory capacity > 40% of normal at 75 days post-transplant which is a significant improvement in engrafted islet β-cell mass over our experience with the Edmonton protocol, particularly since the CIT07 subjects had more often received islets from a single donor resulting in significantly less islet equivalents per kg recipient body weight transplanted (58). In addition to improving the initially engrafted islet β-cell mass to promote prolonged transplant function, other groups have advocated the use of supplemental islet infusions at the time of recurrent hyperglycemia in the absence of evidence of immune activation to restore the islet β-cell reserve and return to insulin independence (59).
While there may be benefit to the peri-transplant use of glucagon-like peptide-1 (GLP-1) agonists for an anti-apoptotic effect on human islet β-cells (60), clinical studies adding the GLP-1 agonist exenatide have also added etanercept and so attribution of benefit cannot be ascribed to exenatide alone (59;61). In islet recipients with graft dysfunction the use of exenatide does not improve glycemic control over the use of insulin (62;63). Because in islet recipients GLP-1 increases proinsulin secretory ratios in response to glucose-potentiated arginine (64) and exenatide disproportionately increases IAPP secretion following mixed-meal stimulation (63), we worry that GLP-1 agonists increasing metabolic demand on a marginal islet β-cell mass may be detrimental for long-term function, and so prefer insulin as needed to maintain near-normal glycemic control (reviewed in (45)).
Recovery of functional β-cell mass by pancreas transplantation
The transplantation of a whole pancreas from a deceased donor is accomplished in humans via pancreaticoduodenal grafting with exocrine drainage either by duodenojejunostomy (enteric) or duodenocystostomy (bladder) and venous drainage into either the systemic or portal circulation (5). This results in the transplantation of 100% of an islet β-cell mass that is immediately vascularized, albeit at ~ 10% risk for immediate technical failure due to vascular thrombosis or pancreatitis that often necessitates graft removal (3). While the endocrine compartment of the pancreas graft is often expected to function completely from the outset, as many as a third of pancreas transplants can be complicated by delayed graft function (65) as occurs with the transplantation of other solid organs. Moreover, although glucocorticoid-free immunosuppression regimens are being used for pancreas transplantation, most centers continue to employ at least short-term high dose glucocorticoid therapy that induces significant insulin resistance markedly increasing the metabolic demand placed on the pancreas in the early post-transplant period. Because most delayed pancreatic graft function resolves by 3 months post-transplant (65), we advocate for continued insulin therapy in the early post-transplant period to “rest” the islet β-cells until recovery, particularly as long as glucocorticoid doses remain supraphysiologic.
Historically, the assessment of β-cell secretory capacity has been confounded by the presence of hyperinsulinemia due to both glucocorticoid-induced insulin resistance and systemic venous drainage that bypasses first-pass hepatic extraction of insulin secreted from the pancreas graft (66). Moreover, as the pancreas recipients studied most often have simultaneously or previously received a kidney transplant, renal clearance of both insulin and C-peptide are reduced relative to healthy controls because of the decreased functional renal mass present. In order to normalize elevated levels of insulin and C-peptide, some reports have divided the insulin and C-peptide responses by their basal levels (67), a practice that will always lower the adjusted responses in a pancreas transplant group. But importantly, the magnitude of the β-cell secretory capacity responses following whole pancreas transplantation appear normal, and may be sustained for more than a decade despite ongoing immunosuppression drug exposure (10;11). Moreover, in the absence of immunologic graft loss, the β-cell secretory capacity can remain stable for years during longitudinal follow-up, while first-phase insulin response to glucose may decrease coincident with lessening of glucocorticoid doses and improvement in insulin sensitivity indicating appropriate functional adaptation of the secretory reserve of the transplanted pancreas (11;68).
Insulin sensitivity in non-diabetic kidney transplant recipients is normal when receiving no more than physiologic doses of glucocorticoid (≤ 5 mg prednisone) together with calcineurin-inhibitors as assessed by euglycemic (69) or hyperglycemic (39;70) clamps, and physiologic hepatic extraction of insulin can be achieved via portal venous drainage of the pancreas graft (66). Thus, we evaluated insulin-independent recipients of whole pancreas transplantation with portal venous drainage receiving 5 mg of prednisone with modern dosing of tacrolimus (12 hour blood trough levels 6 – 10 μg/L) plus mycophenolate and found the β-cell secretory capacity was 100% of that in a control group of healthy kidney donors selected to account for renal clearance of insulin and C-peptide (39). Importantly, the incremental insulin and C-peptide responses to glucose-potentiated arginine in the pancreas transplant recipients that were equivalent to the kidney donor control group were also identical to those seen in a normal control group (39), supporting that direct comparison of incremental responses across groups with different pre-stimulus levels of insulin and C-peptide is preferred over adjustment for basal levels. Finally, fasting proinsulin-to-insulin and proinsulin secretory ratios in response to glucose-potentiated arginine are normal in whole pancreas transplant recipients (39), providing additional evidence against a clinically important increase in metabolic demand imposed by modern tacrolimus-based immunosuppression.
Using the same immunosuppression regimen in whole pancreas transplantation with systemic venous drainage, Gillard and colleagues (70) demonstrated in insulin-independent recipients a β-cell secretory capacity of 63% of normal, and reasoned that peri-transplant graft injury and post-transplant allo- and autoimmune insults likely explained the reduced function β-cell mass. We agree that this best explains the slightly different findings in our studies as the normal β-cell secretory capacity in nondiabetic kidney transplant recipients receiving the same immunosuppression regimen reported by us (39) and Gillard and colleagues (70) also indicates that modern tacrolimus-based immunosuppression does not impose significant β-cell toxicity. Therefore, the β-cell secretory capacity may be useful as a sensitive indicator of changes in functional islet β-cell mass post-transplant to identify alloimmune rejection or autoimmune recurrence before there is substantial β-cell loss and recurrence of hyperglycemia. Also, given that some pancreas recipients may have an injury-induced reduction in β-cell secretory capacity, prudence dictates counseling and monitoring against the development of overweight/obesity (BMI ≥ 27 kg/m2) that has been associated with the recurrence of hyperglycemia post-transplant of a hemi-pancreas with ~ 50% β-cell secretory capacity (71).
Glucose counterregulation in type 1 diabetes
The risk of experiencing a severe hypoglycemic episode increases with the duration of T1D, such that the risk is 3 times greater with more than 15 years compared to less than 5 years of disease duration (72). This increasing risk occurs with the progressive development of compromised physiologic defense mechanisms against a falling plasma glucose concentration in the setting of therapeutic hyperinsulinemia. By 15 years of T1D essentially all patients have developed a near total loss of insulin-producing β-cells (17), which removes any autoregulatory capability to reduce excessive insulin. Furthermore, the loss of endogenous insulin secretion produces an associated defect in glucagon secretion from α-cells (73), whereby the absence of an intraislet decrement in insulin secretion abolishes the paracrine activation of glucagon secretion during hypoglycemia (74). This α-cell dysfunction is specific for hypoglycemia since T1D patients release glucagon normally in response to other stimuli such as arginine (73). Thus, T1D patients with established disease (i.e. C-peptide negative) have lost the primary defenses against hypoglycemia, namely inhibition of endogenous insulin secretion and activation of glucagon secretion, which together normally serve to increase hepatic glucose production and prevent or correct low blood glucose.
In the absence of intact islet cell responses to hypoglycemia in T1D, additional sympathoadrenal (epinephrine secretion and autonomic symptom generation) and pituitary-adrenal (growth hormone and cortisol secretion) counterregulatory responses become necessary to increase hepatic glucose production, decrease peripheral glucose utilization, and promote food ingestion, which together may correct low blood glucose (75). Unfortunately, both the glycemic threshold (i.e. the glucose level that elicits the response) and magnitude of these hormonal and symptom responses are impaired by recurrent episodes of hypoglycemia leading to a syndrome of hypoglycemia unawareness that increases the risk of life-threatening hypoglycemia twenty-fold in T1D (76). The shifting of glycemic thresholds for counterregulatory responses to lower plasma glucose concentrations is best explained by the hypothesis of hypoglycemia-associated autonomic failure (HAAF) (77), which posits recurrent episodes of hypoglycemia blunt subsequent sympathoadrenal and pituitary-adrenal responses to hypoglycemia via central adaptation to low blood glucose. While strict hypoglycemia avoidance can normalize the glycemic thresholds for counterregulatory epinephrine, autonomic symptoms, and growth hormone responses and consequently reestablish awareness of hypoglycemia (78-80), the majority of patients studied to date have had rather short disease duration, and the reversibility of HAAF in patients with long-standing (> 15 years) diabetes has only been demonstrated after islet or pancreas transplantation.
Recovery of glucose counterregulation by islet transplantation
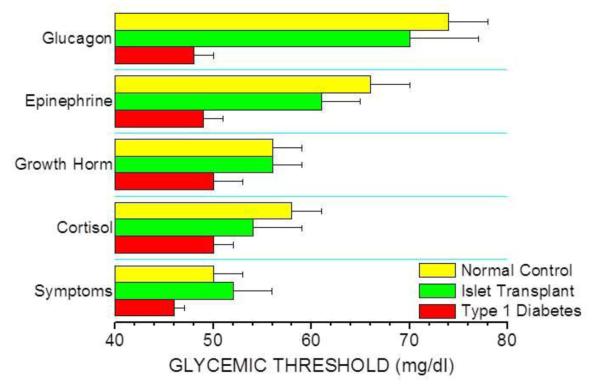
In T1D recipients of intrahepatic islet transplants there is recovery of the physiologic islet cell hormonal responses to insulin-induced hypoglycemia whereby endogenous insulin secretion is appropriately suppressed and glucagon secretion is partially restored (81). Curiously, earlier studies reported that the glucagon response to hypoglycemia was not improved by islet transplantation, but these reports lacked either T1D control subjects (82) or euglycemic control experiments (82;83). Controlling for the inhibitory effect of hyperinsulinemia (used experimentally to induce hypoglycemia) on glucagon secretion (84) is important since a lack of glucagon suppression may be misinterpreted as an absent response when in fact representing activation quite distinct from that present in T1D subjects or during hyperinsulinemia but euglycemia. Indeed, our work demonstrates that islet transplant recipients secrete more glucagon during insulin-induced hypoglycemia than T1D subjects, as well as more glucagon during hypoglycemic compared to euglycemic clamps (81). That this islet α-cell response may only be partial is not unexpected given the engrafted islet β-cell mass may only be ~ 25% of normal. Importantly, the glucagon response to hypoglycemia is activated at a normal glycemic threshold (Figure 1; (85)), with the normal decrease in endogenous insulin secretion supporting that the transplanted β-cells provide the required intraislet signal to the transplanted α-cells to release glucagon.
Fig. 1.
Glycemic thresholds for the activation of counterregulatory hormonal and symptom responses are restored in islet transplant recipients in normal hierarchical fashion (glucagon > epinephrine > growth hormone ≥ cortisol > autonomic symptoms) while impaired in long-standing T1D complicated by hypoglycemia unawareness. (Data from Rickels et al. [85].)
Studies using continuous glucose monitoring systems in islet transplant recipients have demonstrated significant decreases (in insulin requiring subjects) to abolition (in insulin independent subjects) of time spent in the hypoglycemic range (< 60 mg/dl) (86). Because even moderate hypoglycemia between 50 – 58 mg/dl has been shown experimentally in humans to impair subsequent counterregulatory responses to hypoglycemia (87), the avoidance of hypoglycemia after islet transplantation would be expected to ameliorate HAAF. Indeed, in addition to normalizing the glycemic threshold for counterregulatory glucagon secretion, we have demonstrated in islet transplant recipients normalization of the glycemic thresholds for counterregulatory epinephrine, autonomic symptom, and growth hormone responses, and here occurring in patients with ~ 30 years of T1D (Figure 1; (85)). However, the magnitude of the epinephrine response remained less than normal, and complete recovery of this response has only been reported in a third of islet transplant recipients (81;83;88). Nevertheless, the normal glycemic threshold for epinephrine, as well as normal magnitude of the autonomic symptom and growth hormone responses, evidences recovery of central recognition of hypoglycemia that is the underlying defect in HAAF. More recent work from our group using paired hyperinsulinemic hypoglycemic and euglycemic clamps with stable isotope tracers in islet transplant recipients has preliminarily shown the recovery of intact islet cell and sympathoadrenal responses is associated with a restored endogenous (primarily hepatic) glucose production response that is what is ultimately required to protect patients from the development of low blood glucose (89).
Recovery of glucose counterregulation by pancreas transplantation
In T1D recipients of whole pancreas transplants there is also recovery of the physiologic islet cell hormonal responses to insulin-induced hypoglycemia whereby endogenous insulin secretion is partially suppressed and glucagon secretion is normalized (90;91). That endogenous insulin secretion is not completed suppressed during insulin-induced hypoglycemia may be explained by the absent innervation of the transplanted pancreas (92). Insulin, thought to mediate suppression of its own secretion through the sympathetic nervous system, fails to suppress endogenous insulin secretion during euglycemic clamps (90;93). This is different from intrahepatic transplanted islets where hyperinsulinemia does partially suppress endogenous insulin secretion under euglycemic conditions (Rickels and colleagues, unpublished data), and where complete suppression occurs with hypoglycemia (81). Re-innervation of intrahepatic islets by the sympathetic nervous system has been demonstrated in rodents (94) and likely accounts for the more appropriate suppression of endogenous insulin secretion by transplanted islets in humans. Nevertheless, the suppression of endogenous insulin secretion during hypoglycemia in whole pancreas transplant recipients is sufficient to enable a normal glucagon response from the transplanted pancreas (90;95). In support of the argument above that transplanted islet mass is important for recovery of the glucagon response to hypoglycemia, recipients of pancreas segments release less glucagon during hypoglycemia than normal (96), a finding similar to that in recipients of intrahepatic islets (81).
Similar evidence for the reversibility of HAAF in long-standing T1D as described above for islet transplantation had been previously reported following pancreas transplantation. Kendall and colleagues (91) showed that pancreas transplant recipients with ~ 25 years of T1D exhibited normal glycemic thresholds for activation of epinephrine and autonomic symptom responses to hypoglycemia. Again, despite the normal magnitude of the symptom responses (and of the growth hormone response documented elsewhere (96)) evidencing amelioration of HAAF, the magnitude of the epinephrine response, while greater than in T1D, remained less than normal (91). This group subsequently showed that even after a decade of successful pancreas transplant function the improved epinephrine response to hypoglycemia was only partially restored (97). It has been shown that T1D patients with documented autonomic neuropathy secrete less epinephrine in response to insulin-induced hypoglycemia than those without autonomic neuropathy (98;99), and so structural deterioration in the autonomic innervation of the adrenal medulla affecting patients with 25-30 years of T1D likely explains the residual impairment in the magnitude of epinephrine secretion. Most importantly, whole pancreas transplantation has been shown to normalize the endogenous glucose production response to insulin-induced hypoglycemia, thereby affording recipients protection and recovery from low blood glucose (100).
Conclusions
The loss of functional islet β-cell mass in T1D leads to both insulin-dependence and an associated defect in islet α-cell responsiveness to hypoglycemia with subsequent defective glucose counterregulation eventually limiting the effectiveness of insulin therapy without risk for severe hypoglycemia, particularly in patients with long-standing C-peptide negative disease and hypoglycemia unawareness. In such patients the transplantation of isolated islets or a whole pancreas restores both islet β-cell secretory capacity that may be associated with insulin-independence, and islet α-cell responses to insulin-induced hypoglycemia that may be associated with reversal of HAAF and restoration of glucose counterregulation. To date, the magnitude and durability of endocrine recovery is superior following whole pancreas compared to islet transplantation; however, evidence of improved islet engraftment and functional survival with current protocols suggests that islets should now be considered along with whole pancreas transplantation in patients not presently requiring a kidney transplant. While simultaneous pancreas-kidney (SPK) transplantation remains the “gold-standard” approach for T1D patients in need of a kidney transplant (5), there are many patients desperate for endocrine recovery not eligible for SPK and many recovered pancreata technically unsuited for whole organ transplantation that support the complimentary development of both islet and pancreas transplantation as crucial therapeutic options for T1D patients experiencing the most difficulty with this disease.
Acknowledgments
This work was supported in part by Public Health Service grants R01-DK091331 (to M.R.R.) and U01-DK070430 (Penn Clinical Islet Transplantation Center) from the National Institutes of Health, the Pennsylvania State Department of Health (Penn Center of Excellence for Regenerative Medicine), and the Schiffrin Award for research in autoimmune disorders at the University of Pennsylvania (to M.R.R.).
Footnotes
Disclosure
Conflicts of interest: M.R. Rickels: was a Scientific Advisory Board Member, 2011, for Schulze Diabetes Institute, Univ. of Minnesota.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance; •• Of outstanding importance.
- 1.Ludwig B, Ludwig S, Steffen A, Saeger HD, Bornstein SR. Islet versus pancreas transplantation in type 1 diabetes: competitive or complementary? Curr Diab Rep. 2010;10:506–511. doi: 10.1007/s11892-010-0146-y. This review discusses the current indications for islet and pancreas transplantation as treatments for T1D.
- 2.Frank A, Deng SP, Huang XL, et al. Transplantation for type 1 diabetes - Comparison of vascularized whole-organ pancreas with isolated pancreatic islets. Annals of Surgery. 2004;240:631–640. doi: 10.1097/01.sla.0000140754.26575.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gruessner AC, Sutherland DE. Pancreas transplant outcomes for United States (US) and non-US cases as reported to the United Network for Organ Sharing (UNOS) and the International Pancreas Transplant Registry (IPTR) as of June 2004. Clin Transplant. 2005;19:433–455. doi: 10.1111/j.1399-0012.2005.00378.x. [DOI] [PubMed] [Google Scholar]
- 4.Barton FB, Rickels MR, Alejandro R, et al. Improvement in outcomes of clinical islet transplantation: 1999-2010. Diabetes Care. doi: 10.2337/dc12-0063. In press. • This most recent report from the Collaborative Islet Transplant Registry describes the progress in achieving key metabolic outcomes balanced against safety over the last decade.
- 5.Sollinger HW, Odorico JS, Becker YT, D’Alessandro AM, Pirsch JD. One thousand simultaneous pancreas-kidney transplants at a single center with 22-year follow-up. Ann Surg. 2009;250:618–630. doi: 10.1097/SLA.0b013e3181b76d2b. An impressive accounting of the “gold-standard” approach to the recovery of endocrine function in advanced T1D complicated by end-stage nephropathy.
- 6.Gruessner AC, Sutherland DE, Gruessner RW. Long-term outcome after pancreas transplantation. Curr Opin Organ Transplant. 2012;17:100–105. doi: 10.1097/MOT.0b013e32834ee700. This most recent report from the International Pancreas Transplant Registry provides 10 year outcomes for the various approaches to whole pancreas transplantation and highlights the importance of donor organ selection for achieving long-term benefit.
- 7.Larsen MO, Rolin B, Sturis J, et al. Measurements of insulin responses as predictive markers of pancreatic beta-cell mass in normal and beta-cell-reduced lean and obese Gottingen minipigs in vivo. Am J Physiol Endocrinol Metab. 2006;290:E670–E677. doi: 10.1152/ajpendo.00251.2005. [DOI] [PubMed] [Google Scholar]
- 8.Seaquist ER, Robertson RP. Effects of hemipancreatectomy on pancreatic alpha and beta cell function in healthy human donors. J Clin Invest. 1992;89:1761–1766. doi: 10.1172/JCI115779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christiansen E, Tibell A, Volund A, et al. Pancreatic endocrine function in recipients of segmental and whole pancreas transplantation. J Clin Endocrinol Metab. 1996;81:3972–3979. doi: 10.1210/jcem.81.11.8923846. [DOI] [PubMed] [Google Scholar]
- 10.Robertson RP, Sutherland DE, Lanz KJ. Normoglycemia and preserved insulin secretory reserve in diabetic patients 10-18 years after pancreas transplantation. Diabetes. 1999;48:1737–1740. doi: 10.2337/diabetes.48.9.1737. [DOI] [PubMed] [Google Scholar]
- 11.Robertson RP. Consequences on beta-cell function and reserve after long-term pancreas transplantation. Diabetes. 2004;53:633–644. doi: 10.2337/diabetes.53.3.633. [DOI] [PubMed] [Google Scholar]
- 12.Teuscher AU, Kendall DM, Smets YF, Leone JP, Sutherland DE, Robertson RP. Successful islet autotransplantation in humans: functional insulin secretory reserve as an estimate of surviving islet cell mass. Diabetes. 1998;47:324–330. doi: 10.2337/diabetes.47.3.324. [DOI] [PubMed] [Google Scholar]
- 13.Greenbaum CJ, Prigeon RL, D’Alessio DA. Impaired beta-cell function, incretin effect, and glucagon suppression in patients with type 1 diabetes who have normal fasting glucose. Diabetes. 2002;51:951–957. doi: 10.2337/diabetes.51.4.951. [DOI] [PubMed] [Google Scholar]
- 14.Keymeulen B, Vandemeulebroucke E, Ziegler AG, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med. 2005;352:2598–2608. doi: 10.1056/NEJMoa043980. [DOI] [PubMed] [Google Scholar]
- 15.The DCCT Research Group Effects of age, duration and treatment of insulin-dependent diabetes mellitus on residual beta-cell function: observations during eligibility testing for the Diabetes Control and Complications Trial (DCCT) J Clin Endocrinol Metab. 1987;65:30–36. doi: 10.1210/jcem-65-1-30. [DOI] [PubMed] [Google Scholar]
- 16.The Diabetes Control and Complications Trial Research Group Effect of intensive therapy on residual beta-cell function in patients with type 1 diabetes in the diabetes control and complications trial. A randomized, controlled trial. Ann Intern Med. 1998;128:517–523. doi: 10.7326/0003-4819-128-7-199804010-00001. [DOI] [PubMed] [Google Scholar]
- 17.Tsai EB, Sherry NA, Palmer JP, Herold KC. The rise and fall of insulin secretion in type 1 diabetes mellitus. Diabetologia. 2006;49:261–270. doi: 10.1007/s00125-005-0100-8. [DOI] [PubMed] [Google Scholar]
- 18.Steffes MW, Sibley S, Jackson M, Thomas W. Beta-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care. 2003;26:832–836. doi: 10.2337/diacare.26.3.832. [DOI] [PubMed] [Google Scholar]
- 19.The Diabetes Control and Complications Trial Research Group Hypoglycemia in the diabetes control and complications trial. Diabetes. 1997;46:271–286. [PubMed] [Google Scholar]
- 20.Fukuda M, Tanaka A, Tahara Y, et al. Correlation between minimal secretory capacity of pancreatic beta-cells and stability of diabetic control. Diabetes. 1988;37:81–88. doi: 10.2337/diab.37.1.81. [DOI] [PubMed] [Google Scholar]
- 21. [Accessed May 2012];Clinical Islet Transplantation Study. Available at http://www.isletstudy.org/
- 22.Faradji RN, Monroy K, Messinger S, et al. Simple measures to monitor beta-cell mass and assess islet graft dysfunction. Am J Transplant. 2007;7:303–308. doi: 10.1111/j.1600-6143.2006.01620.x. [DOI] [PubMed] [Google Scholar]
- 23.Shapiro AM. State of the art of clinical islet transplantation and novel protocols of immunosuppression. Curr Diab Rep. 2011;11:345–354. doi: 10.1007/s11892-011-0217-8. This review discusses the current approach to clinical islet transplantation including the use of novel immunosuppression protocols aimed at increasing the rates of insulin-independence with islets isolated from a single donor.
- 24.Kaufman DB, Morel P, Field MJ, Munn SR, Sutherland DER. Purified canine islet autografts - functional outcome as influenced by islet number and implantation site. Transplantation. 1990;50:385–391. [PubMed] [Google Scholar]
- 25.Andersson A, Korsgren O, Jansson L. Intraportally transplanted pancreatic-islets revascularized from hepatic arterial system. Diabetes. 1989;38:192–195. doi: 10.2337/diab.38.1.s192. [DOI] [PubMed] [Google Scholar]
- 26.Faradji RN, Monroy K, Cure P, et al. C-peptide and glucose values in the peritransplant period after intraportal islet infusions in type 1 diabetes. Transplant Proc. 2005;37:3433–3434. doi: 10.1016/j.transproceed.2005.09.090. [DOI] [PubMed] [Google Scholar]
- 27.Hirshberg B, Mog S, Patterson N, Leconte J, Harlan DM. Histopathological study of intrahepatic islets transplanted in the nonhuman primate model using Edmonton protocol immunosuppression. J Clin Endocrinol Metab. 2002;87:5424–5429. doi: 10.1210/jc.2002-020684. [DOI] [PubMed] [Google Scholar]
- 28.Davalli AM, Ricordi C, Socci C, et al. Abnormal sensitivity to glucose of human islets cultured in a high glucose medium: partial reversibility after an additional culture in a normal glucose medium. J Clin Endocrinol Metab. 1991;72:202–208. doi: 10.1210/jcem-72-1-202. [DOI] [PubMed] [Google Scholar]
- 29.Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 30.Shapiro AMJ, Ricordi C, Hering BJ, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355:1318–1330. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 31.Ryan EA, Shandro T, Green K, et al. Assessment of the severity of hypoglycemia and glycemic lability in type 1 diabetic subjects undergoing islet transplantation. Diabetes. 2004;53:955–962. doi: 10.2337/diabetes.53.4.955. [DOI] [PubMed] [Google Scholar]
- 32.Rickels MR, Schutta MH, Markmann JF, Barker CF, Naji A, Teff KL. Beta-cell function following human islet transplantation for type 1 diabetes. Diabetes. 2005;54:100–106. doi: 10.2337/diabetes.54.1.100. [DOI] [PubMed] [Google Scholar]
- 33.Paty BW, Imes S, Ryan EA, Senior PA, Robertson RP, Shapiro AMJ. Insulin secretory reserve as a measure of functional islet mass following islet allotransplantation in type 1 diabetes [abstract] Diabetes. 2004;53(Suppl 2):A35–A36. [Google Scholar]
- 34.Keymeulen B, Gillard P, Mathieu C, et al. Correlation between beta cell mass and glycemic control in type 1 diabetic recipients of islet cell graft. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:17444–17449. doi: 10.1073/pnas.0608141103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eriksson O, Eich T, Sundin A, et al. Positron emission tomography in clinical islet transplantation. Am J Transplant. 2009;9:2816–2824. doi: 10.1111/j.1600-6143.2009.02844.x. • Using 18F-fluorodeoxyglucose labeled islets in clinical transplantation, this study demonstrates by PET/CT the heterogeneous distribution of intraportally infused islets within the liver and estimates ~ 25% early post-transplant islet loss.
- 36.Rickels MR, Naji A, Teff KL. Insulin sensitivity, glucose effectiveness, and free fatty acid dynamics after human islet transplantation for type 1 diabetes. J Clin Endocrinol Metab. 2006;91:2138–2144. doi: 10.1210/jc.2005-2519. [DOI] [PubMed] [Google Scholar]
- 37.Hirsch D, Odorico J, Radke N, et al. Correction of insulin sensitivity and glucose disposal after pancreatic islet transplantation: preliminary results. Diabetes Obes Metab. 2010;12:994–1003. doi: 10.1111/j.1463-1326.2010.01290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lopez-Talavera JC, Garcia-Ocana A, Sipula I, Takane KK, Cozar-Castellano I, Stewart AF. Hepatocyte growth factor gene therapy for pancreatic islets in diabetes: Reducing the minimal islet transplant mass required in a glucocorticoid-free rat model of allogeneic portal vein islet transplantation. Endocrinology. 2004;145:467–474. doi: 10.1210/en.2003-1070. [DOI] [PubMed] [Google Scholar]
- 39.Rickels MR, Mueller R, Teff KL, Naji A. Beta-cell secretory capacity and demand in recipients of islet, pancreas, and kidney transplants. J Clin Endocrinol Metab. 2010;95:1238–1246. doi: 10.1210/jc.2009-2289. • Using glucose-potentiation of arginine-induced insulin secretion to measure β-cell secretory capacity and demand in islet, pancreas-kidney and kidney transplant groups compared to carefully selected normal and kidney donor control groups, this study evidences that the impaired β-cell secretory capacity seen in islet and not pancreas recipients is best explained by a low engrafted β-cell mass and not by a deleterious effect of tacrolimus.
- 40.Soleimanpour SA, Hirshberg B, Bunnell DJ, et al. Metabolic function of a suboptimal transplanted islet mass in non-human primates on rapamycin monotherapy. Cell Transplant. doi: 10.3727/096368911X603620. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seaquist ER, Kahn SE, Clark PM, Hales CN, Porte D, Robertson RP. Hyperproinsulinemia is associated with increased beta cell demand after hemipancreatectomy in humans. J Clin Invest. 1996;97:455–460. doi: 10.1172/JCI118435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McDonald CG, Ryan EA, Paty BW, et al. Cross-sectional and prospective association between proinsulin secretion and graft function after clinical islet transplantation. Transplantation. 2004;78:934–937. doi: 10.1097/01.tp.0000134973.77057.39. [DOI] [PubMed] [Google Scholar]
- 43.Klimek AM, Soukhatcheva G, Thompson DM, et al. Impaired proinsulin processing is a characteristic of transplanted islets. Am J Transplant. 2009;9:2119–2125. doi: 10.1111/j.1600-6143.2009.02740.x. [DOI] [PubMed] [Google Scholar]
- 44.Hostens K, Ling ZD, Van Schravendijk C, Pipeleers D. Prolonged exposure of human beta-cells to high glucose increases their release of proinsulin during acute stimulation with glucose or arginine. J Clin Endocrinol Metab. 1999;84:1386–1390. doi: 10.1210/jcem.84.4.5621. [DOI] [PubMed] [Google Scholar]
- 45.Rickels MR, Naji A. Proinsulin processing and transplanted islets. Am J Transplant. 2010;10(6):1495. doi: 10.1111/j.1600-6143.2010.03069.x. [DOI] [PubMed] [Google Scholar]
- 46.Westermark GT, Westermark P, Berne C, Korsgren O. Widespread amyloid deposition in transplanted human pancreatic islets. N Engl J Med. 2008;359:977–979. doi: 10.1056/NEJMc0802893. [DOI] [PubMed] [Google Scholar]
- 47.Westermark GT, Davalli AM, Secchi A, et al. Further evidence for amyloid deposition in clinical pancreatic islet grafts. Transplantation. 2012;93:219–223. doi: 10.1097/TP.0b013e31823e46ef. This follow-up autopsy study to the initial case report now documents intrahepatic islet amyloid affecting 3 out of 4 transplant recipients, and so suggests islet amyloidosis as a potential contributor to non-immunologic clinical islet graft loss.
- 48.Ritzel RA, Meier JJ, Lin CY, Veldhuis JD, Butler PC. Human islet amyloid polypeptide oligomers disrupt cell coupling, induce apoptosis, and impair insulin secretion in isolated human islets. Diabetes. 2007;56:65–71. doi: 10.2337/db06-0734. [DOI] [PubMed] [Google Scholar]
- 49.Kahn SE, Dalessio DA, Schwartz MW, et al. Evidence of cosecretion of islet amyloid polypeptide and insulin by beta-cells. Diabetes. 1990;39:634–638. doi: 10.2337/diab.39.5.634. [DOI] [PubMed] [Google Scholar]
- 50.Westermark P, Li ZC, Westermark GT, Leckstrom A, Steiner DF. Effects of beta cell granule components on human islet amyloid polypeptide fibril formation. FEBS Lett. 1996;379:203–206. doi: 10.1016/0014-5793(95)01512-4. [DOI] [PubMed] [Google Scholar]
- 51.Rickels MR, Collins HW, Naji A. Amyloid and transplanted islets. N Engl J Med. 2008;359:2729–2731. doi: 10.1056/NEJMc082011. This letter provides data that islet transplant recipients may release disproportionately increased islet amyloid polypeptide relative to insulin during hyperglycemia, providing a potential mechanism for the reports of observed amyloid deposition.
- 52.Liu C, Koeberlein B, Feldman MD, et al. Accumulation of intrahepatic islet amyloid in a nonhuman primate transplant model. Endocrinology. 2012;153:1673–1683. doi: 10.1210/en.2011-1560. This study involving a non-human primate model of islet transplantation documents the accumulation of intraheptic islet amyloid over time as a non-immunologic mechanism of islet β-cell loss predating the recurrence of hyperglycemia.
- 53.The CITR Research Group 2007 update on allogeneic islet transplantation from the Collaborative Islet Transplant Registry (CITR) Cell Transplant. 2009;18:753–767. doi: 10.3727/096368909X470874. [DOI] [PubMed] [Google Scholar]
- 54.Koh A, Senior P, Salam A, et al. Insulin-heparin infusions peritransplant substantially improve single-donor clinical islet transplant success. Transplantation. 2010;89:465–471. doi: 10.1097/TP.0b013e3181c478fd. By multivariate analysis, this retrospective study identified the introduction of the practice of peri-transplant glycemic and anti-thrombotic therapy with infusions of insulin and heparin as significantly associated with the achievement of insulin-independence following transplantation of islets isolated from a single donor.
- 55.Hering BJ, Kandaswamy R, Ansite JD, et al. Single-donor, marginal-dose islet transplantation in patients with type 1 diabetes. JAMA. 2005;293:830–835. doi: 10.1001/jama.293.7.830. [DOI] [PubMed] [Google Scholar]
- 56.Bellin MD, Kandaswamy R, Parkey J, et al. Prolonged insulin independence after islet allotransplants in recipients with type 1 diabetes. Am J Transplant. 2008;8:2463–2470. doi: 10.1111/j.1600-6143.2008.02404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bellin MD, Barton FB, Heitman A, et al. Potent induction immunotherapy promotes long-term insulin independence after islet transplantation in type 1 diabetes. Am J Transplant. doi: 10.1111/j.1600-6143.2011.03977.x. In press. • Using data both from Minneapolis and the Collaborative Islet Transplant Registry, this analysis indicates that potent T-cell induction therapy with anti-CD3 or a depleting antibody (anti-thymocyte globulin or alemtuzumab) is associated with insulin-independence rate of 50% at 5 years, significantly more than that seen with use of an IL-2 receptor antagonist (as in the Edmonton protocol) and similar to that presently achieved by pancreas alone transplantation. Importantly, the addition of a TNF-α inhibitor (e.g. etanercept) was necessary with use of a depleting antibody to have a beneficial effect.
- 58.Rickels MR, Chiou AJ, Fuller C, et al. Beta-cell secretory capacity after human islet transplantation by the CIT07 vs. Edmonton protocol: preliminary results [abstract] Am J Transplant. 2011;11(Suppl 2):79–80. [Google Scholar]
- 59.Faradji RN, Tharavanij T, Messinger S, et al. Long-term insulin independence and improvement in insulin secretion after supplemental islet infusion under exenatide and etanercept. Transplantation. 2008;86:1658–1665. doi: 10.1097/TP.0b013e31818fe448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Farilla L, Bulotta A, Hirshberg B, et al. Glucagon-like peptide 1 inhibits cell apoptosis and improves glucose responsiveness of freshly isolated human islets. Endocrinology. 2003;144:5149–5158. doi: 10.1210/en.2003-0323. [DOI] [PubMed] [Google Scholar]
- 61.Gangemi A, Salehi P, Hatipoglu B, et al. Islet transplantation for brittle type 1 diabetes: The UIC Protocol. Am J Transplant. 2008;8:1–12. doi: 10.1111/j.1600-6143.2008.02234.x. [DOI] [PubMed] [Google Scholar]
- 62.Al Ghofaili K, Fung M, Ao ZL, et al. Effect of exenatide on beta cell function after islet transplantation in type 1 diabetes. Transplantation. 2007;83:24–28. doi: 10.1097/01.tp.0000251379.46596.2d. [DOI] [PubMed] [Google Scholar]
- 63.Froud T, Faradji RN, Pileggi A, et al. The use of exenatide in islet transplant recipients with chronic allograft dysfunction: Safety, efficacy, and metabolic effects. Transplantation. 2008;86:36–45. doi: 10.1097/TP.0b013e31817c4ab3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rickels MR, Mueller R, Markman JF, Naji A. Effect of GLP-1 on beta- and alpha-cell function in isolated islet and whole pancreas transplant recipients. J Clin Endocrinol Metab. 2009;94:181–189. doi: 10.1210/jc.2008-1806. This study demonstrated that infusion of the incretin hormone glucagon-like peptide-1 (GLP-1) induced enhancement of glucose-dependent insulin secretion in islet and pancreas transplant recipients, an effect that was dependent on the functional β-cell mass, and also was associated with increased proinsulin secretory ratios that may be associated with depletion of mature β-cell granules.
- 65.Tan M, Kandaswamy R, Sutherland DE, Gruessner RW, Gruessner AC, Humar A. Risk factors and impact of delayed graft function after pancreas transplants. Am J Transplant. 2004;4:758–762. doi: 10.1111/j.1600-6143.2004.00408.x. [DOI] [PubMed] [Google Scholar]
- 66.Diem P, Abid M, Redmon JB, Sutherland DER, Robertson RP. Systemic venous drainage of pancreas allografts as independent cause of hyperinsulinemia in type-I diabetic recipients. Diabetes. 1990;39:534–540. doi: 10.2337/diab.39.5.534. [DOI] [PubMed] [Google Scholar]
- 67.Teuscher AU, Seaquist ER, Robertson RP. Diminished insulin secretory reserve in diabetic pancreas transplant and nondiabetic kidney transplant recipients. Diabetes. 1994;43:593–598. doi: 10.2337/diab.43.4.593. [DOI] [PubMed] [Google Scholar]
- 68.Cottrell DA. Normalization of insulin sensitivity and glucose homeostasis in type I diabetic pancreas transplant recipients: A 48-month cross-sectional study - A clinical research center study. J Clin Endocrinol Metab. 1996;81:3513–3519. doi: 10.1210/jcem.81.10.8855794. [DOI] [PubMed] [Google Scholar]
- 69.Midtvedt K, Hjelmesaeth J, Hartmann A, et al. Insulin resistance after renal transplantation: The effect of steroid dose reduction and withdrawal. J Am Soc Nephrol. 2004;15:3233–3239. doi: 10.1097/01.ASN.0000145435.80005.1E. [DOI] [PubMed] [Google Scholar]
- 70.Gillard P, Vandemeulebroucke E, Keymeulen B, et al. Functional beta-cell mass and insulin sensitivity is decreased in insulin-independent pancreas-kidney recipients. Transplantation. 2009;87:402–407. doi: 10.1097/TP.0b013e3181928a1c. [DOI] [PubMed] [Google Scholar]
- 71.Robertson RP, Lanz KJ, Sutherland DE, Seaquist ER. Relationship between diabetes and obesity 9 to 18 years after hemipancreatectomy and transplantation in donors and recipients. Transplantation. 2002;73:736–741. doi: 10.1097/00007890-200203150-00013. [DOI] [PubMed] [Google Scholar]
- 72.Heller SR, Choudhary P, Davies C, et al. Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia. 2007;50:1140–1147. doi: 10.1007/s00125-007-0599-y. [DOI] [PubMed] [Google Scholar]
- 73.Gerich JE, Langlois M, Noacco C, Karam JH, Forsham PH. Lack of glucagon response to hypoglycemia in diabetes - evidence for an intrinsic pancreatic alpha cell defect. Science. 1973;182:171–173. doi: 10.1126/science.182.4108.171. [DOI] [PubMed] [Google Scholar]
- 74.Cooperberg BA, Cryer PE. Insulin reciprocally regulates glucagon secretion in humans. Diabetes. 2010;59:2936–2940. doi: 10.2337/db10-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care. 2003;26:1902–1912. doi: 10.2337/diacare.26.6.1902. [DOI] [PubMed] [Google Scholar]
- 76.Pedersen-Bjergaard U, Pramming S, Heller SR, et al. Severe hypoglycaemia in 1076 adult patients with type 1 diabetes: influence of risk markers and selection. Diabetes Metab Res Rev. 2004;20:479–486. doi: 10.1002/dmrr.482. [DOI] [PubMed] [Google Scholar]
- 77.Cryer PE. Mechanisms of hypoglycemia-associated autonomic failure and its component syndromes in diabetes. Diabetes. 2005;54:3592–3601. doi: 10.2337/diabetes.54.12.3592. [DOI] [PubMed] [Google Scholar]
- 78.Fanelli CG, Epifano L, Rambotti AM, et al. Meticulous prevention of hypoglycemia normalizes the glycemic thresholds and magnitude of most of neuroendocrine responses to, symptoms of, and cognitive function during hypoglycemia in intensively treated patients with short-term IDDM. Diabetes. 1993;42:1683–1689. doi: 10.2337/diab.42.11.1683. [DOI] [PubMed] [Google Scholar]
- 79.Cranston I, Lomas J, Maran A, Macdonald I, Amiel SA. Restoration of hypoglycemia awareness in patients with long-duration insulin-dependent diabetes. Lancet. 1994;344:283–287. doi: 10.1016/s0140-6736(94)91336-6. [DOI] [PubMed] [Google Scholar]
- 80.Dagogojack S, Rattarasarn C, Cryer PE. Reversal of hypoglycemia unawareness, but not defective glucose counterregulation, in IDDM. Diabetes. 1994;43:1426–1434. doi: 10.2337/diab.43.12.1426. [DOI] [PubMed] [Google Scholar]
- 81.Rickels MR, Schutta MH, Mueller R, et al. Islet cell hormonal responses to hypoglycemia after human islet transplantation for type 1 diabetes. Diabetes. 2005;54:3205–3211. doi: 10.2337/diabetes.54.11.3205. [DOI] [PubMed] [Google Scholar]
- 82.Kendall DM, Teuscher AU, Robertson RP. Defective glucagon secretion during sustained hypoglycemia following successful islet allo- and autotransplantation in humans. Diabetes. 1997;46:23–27. doi: 10.2337/diab.46.1.23. [DOI] [PubMed] [Google Scholar]
- 83.Paty BW, Ryan EA, Shapiro AMJ, Lakey JRT, Robertson RP. Intrahepatic islet transplantation in type 1 diabetic patients does not restore hypoglycemic hormonal counterregulation or symptom recognition after insulin independence. Diabetes. 2002;51:3428–3434. doi: 10.2337/diabetes.51.12.3428. [DOI] [PubMed] [Google Scholar]
- 84.Diamond MP, Hallarman L, Starickzych K, et al. Suppression of counterregulatory hormone response to hypoglycemia by insulin per se. J Clin Endocrinol Metab. 1991;72:1388–1390. doi: 10.1210/jcem-72-6-1388. [DOI] [PubMed] [Google Scholar]
- 85.Rickels MR, Schutta MH, Mueller R, et al. Glycemic thresholds for activation of counterregulatory hormone and symptom responses in islet transplant recipients. J Clin Endocrinol Metab. 2007;92:873–879. doi: 10.1210/jc.2006-2426. [DOI] [PubMed] [Google Scholar]
- 86.Paty BW, Senior PA, Lakey JR, Shapiro AM, Ryan EA. Assessment of glycemic control after islet transplantation using the continuous glucose monitor in insulin-independent versus insulin-requiring type 1 diabetes subjects. Diabetes Technol Ther. 2006;8:165–173. doi: 10.1089/dia.2006.8.165. [DOI] [PubMed] [Google Scholar]
- 87.Heller SR, Cryer PE. Reduced neuroendocrine and symptomatic responses to subsequent hypoglycemia after 1 episode of hypoglycemia in nondiabetic humans. Diabetes. 1991;40:223–226. doi: 10.2337/diab.40.2.223. [DOI] [PubMed] [Google Scholar]
- 88.Meyer C, Hering BJ, Grossmann R, et al. Improved glucose counterregulation and autonomic symptoms after intraportal islet transplants alone in patients with long-standing type I diabetes mellitus. Transplantation. 1998;66:233–240. doi: 10.1097/00007890-199807270-00017. [DOI] [PubMed] [Google Scholar]
- 89.Rickels MR, Cullison K, Fuller C, et al. Improvement of glucose counterregulation following human islet transplantation in long-standing type 1 diabetes: preliminary results [abstract] Diabetes. 2011;60(Suppl 1):293. OR. [Google Scholar]
- 90.Luzi L, Battezzati A, Perseghin G, et al. Lack of Feedback Inhibition of Insulin-Secretion in Denervated Human Pancreas. Diabetes. 1992;41:1632–1639. doi: 10.2337/diab.41.12.1632. [DOI] [PubMed] [Google Scholar]
- 91.Kendall DM, Rooney DP, Smets YFC, Bolding LS, Robertson RP. Pancreas transplantation restores epinephrine response and symptom recognition during hypoglycemia in patients with long-standing type I diabetes and autonomic neuropathy. Diabetes. 1997;46:249–257. doi: 10.2337/diab.46.2.249. [DOI] [PubMed] [Google Scholar]
- 92.Bolinder J, Wahrenberg H, Persson A, et al. Effect of pancreas transplantation on glucose counterregulation in insulin-dependent diabetic-patients prone to severe hypoglycemia. J Intern Med. 1991;230:527–533. doi: 10.1111/j.1365-2796.1991.tb00484.x. [DOI] [PubMed] [Google Scholar]
- 93.Boden G, Chen XH, Desantis R, Kolaczynski J, Morris M. Evidence that suppression of insulin-secretion by insulin itself is neurally-mediated. Metab Clin Exp. 1993;42:786–789. doi: 10.1016/0026-0495(93)90250-r. [DOI] [PubMed] [Google Scholar]
- 94.Gardemann A, Jungermann K, Grosse V, et al. Intraportal transplantation of pancreatic-islets into livers of diabetic rats - reinnervation of islets and regulation of insulin-secretion by the hepatic sympathetic-nerves. Diabetes. 1994;43:1345–1352. doi: 10.2337/diab.43.11.1345. [DOI] [PubMed] [Google Scholar]
- 95.Diem P, Redmon JB, Abid M, et al. Glucagon, catecholamine and pancreatic-polypeptide secretion in type-I diabetic recipients of pancreas allografts. J Clin Invest. 1990;86:2008–2013. doi: 10.1172/JCI114936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Battezzati A, Luzi L, Perseghin G, et al. Persistence of counter-regulatory abnormalities in insulin-dependent diabetes-mellitus after pancreas transplantation. Eur J Clin Invest. 1994;24:751–758. doi: 10.1111/j.1365-2362.1994.tb01072.x. [DOI] [PubMed] [Google Scholar]
- 97.Paty BW, Lanz K, Kendall DM, Sutherland DER, Robertson RP. Restored hypoglycemic counterregulation is stable in successful pancreas transplant recipients for up to 19 years after transplantation. Transplantation. 2001;72:1103–1107. doi: 10.1097/00007890-200109270-00021. [DOI] [PubMed] [Google Scholar]
- 98.Hoeldtke RD, Boden G, Shuman CR, Owen OE. Reduced epinephrine secretion and hypoglycemia unawareness in diabetic autonomic neuropathy. Ann Intern Med. 1982;96:459–462. doi: 10.7326/0003-4819-96-4-459. [DOI] [PubMed] [Google Scholar]
- 99.Meyer C, Grossmann R, Mitrakou A, et al. Effects of autonomic neuropathy on counterregulation and awareness at hypoglycemia in type 1 diabetic patients. Diabetes Care. 1998;21:1960–1966. doi: 10.2337/diacare.21.11.1960. [DOI] [PubMed] [Google Scholar]
- 100.Barrou Z, Seaquist ER, Robertson RP. Pancreas transplantation in diabetic humans normalizes hepatic glucose-production during hypoglycemia. Diabetes. 1994;43:661–666. doi: 10.2337/diab.43.5.661. [DOI] [PubMed] [Google Scholar]