Summary
Cogent evidence highlights a key role of neurosteroids and androgens in schizophrenia. We recently reported that inhibition of steroid 5α-reductase (5αR), the rate-limiting enzyme in neurosteroid synthesis and androgen metabolism, elicits antipsychotic-like effects in humans and animal models, without inducing extrapyramidal side effects. To elucidate the anatomical substrates mediating these effects, we investigated the contribution of peripheral and neural structures to the behavioral effects of the 5αR inhibitor finasteride (FIN) on the prepulse inhibition (PPI) of the acoustic startle reflex (ASR), a rat paradigm that dependably simulates the sensorimotor gating impairments observed in schizophrenia and other neuropsychiatric disorders. The potential effect of drug-induced ASR modifications on PPI was excluded by measuring this index both as percent (%PPI) and absolute values (ΔPPI). In both orchidectomized and sham-operated rats, FIN prevented the %PPI deficits induced by the dopamine (DA) receptor agonists apomorphine (APO, 0.25 mg/kg, SC) and d-amphetamine (AMPH, 2.5 mg/kg, SC), although the latter effect was not corroborated by ΔPPI analysis. Conversely, APO-induced PPI deficits were countered by FIN infusions in the brain ventricles (10 μg/1 μl) and in the nucleus accumbens (NAc) shell and core (0.5 μg/0.5 μl/side). No significant PPI-ameliorating effect was observed following FIN injections in other brain regions, including dorsal caudate, basolateral amygdala, ventral hippocampus and medial prefrontal cortex, although a statistical trend was observed for the latter region. The efflux of DA in NAc was increased by systemic, but not intracerebral FIN administration. Taken together, these findings suggest that the role of 5αR in gating regulation is based on post-synaptic mechanisms in the NAc, and is not directly related to alterations in DA efflux in this region.
Keywords: 5α-reductase, Finasteride, Sensorimotor gating, Prepulse inhibition, Schizophrenia, Dopamine, Nucleus accumbens
1. Introduction
Several lines of evidence document that neuroactive steroids (NSs) play a critical role in the pathophysiology of psychotic disorders. While numerous studies have highlighted alterations in NS levels in schizophrenia (Goyal et al., 2004; Taherianfard and Shariaty, 2004; Huber et al., 2005; Marx et al., 2006), the functional significance of these changes remains elusive, in view of the pleiotropic activity of NSs in behavioral regulation. Sex hormones are posited to modulate the course of schizophrenia (Häfner, 2003; Halari et al., 2004; Elias and Kumar, 2007) and may account for the earlier onset and higher severity of this disorder in males (Andreasen et al., 1990; Tamminga, 1997; Gur et al., 2000; Goldstein et al., 2002; Häfner, 2003; Seeman, 2004). Another neuro-steroid, allopregnanolone (AP, 3α,5α-tetrahydroprogester-one), has been linked to the pathophysiology of schizophrenia (Barbaccia et al., 2001; Gizerian et al., 2004; Grobin et al., 2006; Marx et al., 2006), in view of its key involvement in the response to psychosocial stress (Purdy et al., 1991; Barbaccia et al., 1997; Dong et al., 2001), a key risk factor for psychosis (van Winkel et al., 2008).
Capitalizing on these premises, our group has recently investigated the role of steroid 5α-reductase (5αR), a key rate-limiting enzyme in NS synthesis and metabolism, in psychosis and related conditions. 5αR synthesizes the androgen 5α-dihydrotestosterone (DHT) from testosterone, and catalyzes the conversion of progesterone and deoxycorticosterone into AP and 3α,5α-tetrahydro-deoxycorticosterone, respectively (Martini et al., 1993; Paba et al., 2011).
We recently found that 5αR blockade elicits antipsychotic-like effects in several rat models of behavioral perturbations relevant to schizophrenia, such as the deficits in prepulse inhibition (PPI) of the acoustic startle reflex (ASR) induced by dopamine (DA) receptor agonists (Bortolato et al., 2008). This endophenotype is deemed to carry a high validity in modeling the gating impairments observed in psychosis (Braff et al., 1992) and other disorders related to DAergic hyper-activity, such as Tourette syndrome (Castellanos et al., 1996; Swerdlow et al., 2001a) mania (Perry et al., 2001) and pathological gambling (Stojanov et al., 2003); accordingly, DAergic activation induces a robust PPI disruption in rodents, which is efficiently reversed by antipsychotic drugs (Geyer et al., 2001). In line with our preclinical results, our preliminary clinical studies suggest that the prototypical 5αR inhibitor finasteride (FIN) may exert therapeutic properties in patients affected by chronic schizophrenia (Koethe et al., 2008), levodopa-induced pathological gambling (Bortolato et al., in press) and Tourette syndrome (Bortolato et al., 2007; Muroni et al., 2011). These findings, albeit partially anecdotal and not yet confirmed by double-blinded, placebo-controlled studies, point to 5αR as a potentially interesting therapeutic target for schizophrenia and other neuropsychiatric disorders (Paba et al., 2011).
Interestingly, FIN elicited no antipsychotic-like action on the gating deficits induced by other non-DAergic agents (Bortolato et al., 2008), suggesting that its actions may specifically involve DA neurotransmission. In the present study, we sought to identify the anatomical substrates of FIN-mediated antipsychotic-like effects, using the rat model of PPI deficits induced by DA receptor agonists. Furthermore, we explored whether the effects of FIN may be paralleled by brain-regional changes in DA efflux, as measured by microdialysis.
2. Materials and methods
2.1. Animals
A total of 448 male Sprague—Dawley albino rats (Harlan, San Pietro al Natisone, Italy) weighing 225—300 g were kept on a 12/12-h reverse light/dark cycle (with lights off from 7 AM to 7 PM), with food and water available ad libitum. All experimental protocols were accepted by the Ethics Committee at the University of Cagliari and carried out in strict accordance with the Italian Ministry of Health regulation for the care and use of laboratory animals (DL 11692). Each animal was used only once throughout the study, with the exception of 8 gonadectomized and 12 sham-operated rats, which were treated with vehicle + saline in the first PPI experiment and subsequently (7 days after) injected with either vehicle + saline or vehicle + amphetamine for the second PPI experiment.
2.2. Drugs
For systemic injections, FIN (Sigma—Aldrich, St. Louis, MO) was suspended in a vehicle (VEH) solution of Tween 80 in distilled water (1:9 v:v). For intracerebroventriculal infusions, FIN was dissolved in DMSO—Ringer solution (final concentration, 1:1 v:v). Intracerebral regional infusions were performed with a solution of FIN in cyclodextrine/Ringer solution (final concentration, 1:5 v:v). Apomorphine (APO; Sigma—Aldrich) was dissolved in a solution containing 0.9% saline (SAL) with 0.1 mg/ml ascorbic acid to prevent oxidization. d-Amphetamine (AMPH, Sigma—Aldrich) was dissolved in saline. All systemic administrations were performed in an injection volume of 1 (subcutaneous, SC) or 2 ml/kg body weight (intraperitoneal, IP). Doses of FIN (both systemic and intracerebral), APO and AMPH were based on preliminary studies.
2.3. Orchidectomy
Gonadectomy and sham surgeries were performed under aseptic conditions using Equithesin (0.97 g pentobarbital, 2.1 g MgSO4, 4.25 g chloral hydrate, 42.8 ml propylene glycol, 11.5 ml 90% ethanol, distilled water up to 100 ml, 5 ml/kg, IP) for anesthesia. For both operations, the sac of the scrotum and underlying tunica were incised; orchidectomy was performed by bilateral ligation of the vas deferens and removal of the testes. Incisions were closed using sterile surgical staples.
2.4. Stereotaxic surgery
Rats were anaesthetised with Equithesin and placed in a stereotaxic apparatus (Kopf, Tujunga, CA), with blunt ear bars to avoid damage of the tympanic membranes. Under aseptic conditions, rats were shaved and their scalp was retracted. Bilateral craniotomies were performed above the target sites, and stainless steel 22-G guide-cannulae (Plastics One, Roanoke, VA) were lowered slowly into place and implanted using dental cement and two skull screws. The lengths of the cannulae were selected so as to end 1 mm above the targeted areas with the corresponding injector projecting 1 mm beyond guide tip. Cannulae were plugged with wire stylets, and wounds were closed with surgical staples. The target locations for cannulation from bregma were: lateral ventricles (monolaterally; AP = 1 mm, ML = ± 1 mm; DV = −3 mm); medial prefrontal cortex (mPFC) (AP = +3.0 mm, ML = ± 0.5 mm; DV = −3 mm from the skull surface); nucleus accumbens (NAc) core (AP = +1.2 mm, ML = ±2 mm; DV = −7 mm from the skull surface); NAc shell (AP = +1.7 mm, ML = ± 0.8 mm; DV = −7.4 mm from the skull surface), dorsal caudate nucleus (AP = +0.5 mm, L = ±3.0 mm; DV = −4 mm from the skull surface), basolateral amygdala (AP = −2.6 mm, L = ±4.8 mm; DV = −7 mm from the skull surface), and ventral hippocampus (AP = −5.0 mm, L = ±5.0 mm; DV = −6 mm from the skull surface). These locations were selected based on their well-characterized role in the regulation of sensorimotor gating (Swerdlow et al., 2001b).
Coordinates were taken from bregma, according to the stereotaxic brain atlas (Paxinos and Watson, 1998). Bilateral guides were used for mPFC and NAc shell, with center-tocenter distances between the stainless steel tubing of 1 and 1.6 mm, respectively. Rats were given antibiotic therapy for 5 days (enrofloxacin, Bayer HealthCare, Shawnee Mission, KS) and allowed to recover in their home cages for 10–15 days before testing.
For microdialysis, animals were implanted with a vertical microdialysis probe (membrane AN 69-HF, Hospal-Dasco, Bologna, Italy; cut-off 40,000 Da) as previously described (Devoto et al., 2008). For intracerebral administration, microdialysis probes were coupled to a 26-G injection cannula, whose tip terminated just above the dialyzing portion of the probe and 1 mm lateral. These modified probes were placed in the mPFC (AP = +3.0 mm, L = ±0.6 mm, V = 6.5 mm from bregma), or in the NAc shell (AP = +1.9 mm, L = ±0.7 mm, V = −8.3 mm from bregma), according to the rat stereotaxic atlas by Paxinos and Watson (1998). The probes were oriented to set dialysis and injection cannulae along the antero-posterior axis. The dialyzing membrane length was 3 mm in mPFC and 2 mm in NAc shell.
On completion of testing, rats were sacrificed and the locations of cannula tips and dialysis probes were histologically verified by trained operators blind to behavioral results. Rats with errant locations of either device or damage of the targeted areas were excluded from analysis.
2.5. Startle reflex and PPI
Startle and PPI testing were performed as previously described (Bortolato et al., 2008). All animals used in these studies were tested between 10 AM and 3 PM. Each session was performed with a 70-dB white noise background and consisted of a 5-min acclimatization, followed by five 115-dB pulse-alone trials and a pseudo-random sequence of trials, including: 17 pulse-alone trials; 20 prepulse + pulse trials, in which the same acoustic bursts were preceded by 74, 78 or 82 dB prestimuli; 8 no-stimulus trials (with only background noise). Sound levels were assessed using an A Scale setting. All experiments were performed with a between-subject design.
The first series of experiments was performed on orchidectomized (ORX) rats or their sham-operated (SHAM) controls (N = 109), to verify whether gonadectomy may either reproduce or limit FIN-induced behavioral effects by reducing plasma levels of testosterone and its 5α-reduced metabolite DHT. Fourteen days after castration, rats were injected with FIN (50—100 mg/kg, IP) or its vehicle. Forty minutes later, each group received either APO (0.25 mg/kg, SC) or saline. After 5 min, all animals were placed in the testing cages. In a second experiment (N = 64), we injected ORX and SHAM rats with FIN (100 mg/kg, IP) followed by AMPH (2.5 mg/kg, SC). The time interval between AMPH administration and testing lasted 10 min.
The second group of experiments (N = 53) was aimed at the evaluation of the intracerebroventricular (ICV) effects of FIN (1—10 μg/1 μl) or its vehicle (DMSO/Ringer solution, 1:1, v:v) in relation to the PPI deficits induced by subcutaneous APO (0.25 mg/kg) or saline. Immediately after APO injection, rats were subjected to administration of FIN or DMSO/Ringer solution through 33-gauge internal cannulae (Plastics One) connected to a 10-μl syringe (Hamilton, Reno, NV, USA) by PE tubing (Intramedic, New York, NY, USA). The rate of infusion (0.5 μl/min) was controlled by microinjection pumps (CMA Microdialysis, Stockholm, Sweden). Injections were confirmed by monitoring movement of liquid in the tubing via a small air bubble. The injectors were left in place for 2 min after infusion, to allow diffusion of fluid. PPI testing took place immediately after completion of infusion.
The third set of experiments mirrored the previous one, but targeted six brain areas (mPFC, NAc core and shell, dorsal caudate, basolateral amygdala and ventral hippocampus) in bilaterally cannulated rats (N = 216: 8—10 rats/treatment group/region). Following APO (0.25 mg/kg, SC) or saline, rats immediately received either intracerebral FIN (0.5 μg/0.5 μl/side) or vehicle (cyclodextrine/Ringer solution, 1:5, v:v) with the aforementioned infusion conditions, and were then tested for startle and PPI.
2.6. Microdialysis
Experiments were performed as previously described (Devoto et al., 2008). The day after probe implantation, an artificial cerebrospinal fluid (147 mM NaCl, 4 mM KCl, 1.5 mM CaCl2, pH 6—6.5) was pumped through the dialysis probes at a constant rate of 2.2 μl/min via a CMA/100 microinjection pump (CMA Microdialysis, Stockholm, Sweden). Samples were collected every 20 min, and DA and DOPAC simultaneously evaluated in real time by HPLC with electrochemical detection (ESA Coulochem II detectors, Chelmford, MA, USA).
In the first experiment (N = 15), we tested the effects of FIN (100 mg/kg, IP) on extracellular DA and DOPAC values. When a stable baseline was obtained, FIN was injected and changes in DA and DOPAC levels were calculated as percent of mean basal value obtained from three consecutive samples with a variance not exceeding 15%.
In the second series of experiments (N = 27), we tested the effects of intracerebral FIN injections (0.5 μg/0.5 μl for each side) in either mPFC or NAc shell on the local DA and DOPAC concentrations. When a stable baseline was obtained (with variations ≤15% over three consecutive time points), 33-G injection cannulae connected to pump-operated Hamilton syringes were inserted into the guide cannulae, and either FIN (0.5 μg/0.5 μl for each side) or its vehicle (DMSO—Ringer solution) were bilaterally infused. Then, two further samples were collected and analysed.
The third series of experiments (N = 28) was aimed at ascertaining whether the combined action of intracerebral FIN and systemic APO (with the same regimen and treatment schedule used to test PPI effects) may affect DA extracellular concentrations in the areas of infusion (mPFC and NAc shell). Testing was performed with the same procedure as in the previous one, but intracerebral infusions of FIN were administered 5 min following systemic injection of APO (0.25 mg/ kg, SC).
2.7. Data analysis
Normality and homoscedasticity of data distribution were verified by using the Kolmogorov—Smirnov and Bartlett’s tests. For PPI analysis, data relative to different prepulse levels were collapsed, as no interaction between prepulse and any other factor was found in any experiment. Percent PPI was calculated according to the formula: %PPI = [100 × ], with and indicating the mean startle amplitudes for all pre-pulse + pulse and pulse-alone trials, respectively. The first 5 pulse-alone trials were excluded from the calculation. In consideration of FIN’s ability to reduce startle magnitude (Bortolato et al., 2008), we envisioned the possibility of drug-induced artefacts in %PPI calculation due to “floor” effects; to avoid this occurrence, the results of %PPI analyses were always confirmed using DPPI values, calculated with the formula: (Bortolato et al., 2004). Furthermore, an activity index (AI), defined as the ratio of and mean activity during no-stimulus trials was calculated and analysed for each animal.
Analyses were performed by multiple-way ANOVAs (with repeated measures for microdialysis data), as appropriate, followed by Tukey’s test (with Spjøtvoll—Stoline correction for unequal N whenever required) for post hoc comparisons of the means. Significance threshold was set at 0.05.
3. Results
Throughout all PPI experiments, AIs were always between the values of 8 and 12. Furthermore, this parameter was never affected significantly by any treatment combination; thus, its analysis will not be presented here.
3.1. Effects of FIN on the behavioral performances of orchidectomized rats
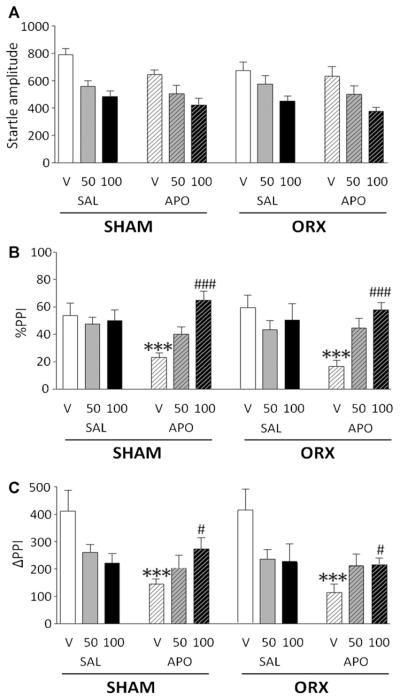
The effects of FIN and APO on the magnitude of startle reflex in ORX rats were compared with those observed in shamoperated controls (Fig. 1A). Values were analysed by a 3-way ANOVA, with post-surgical conditions (ORX vs SHAM), pre-treatment (FIN doses vs VEH) and treatment (APO vs SAL) as factors. Startle amplitude was not affected by orchidectomy [F(1,97) = 1.29, NS], but was reduced by FIN [F(2,97) = 26.85, P < 0.001; P < 0.01 VEH vs FIN 100, Tukey’s] and APO [F(1,97) = 6.45, P < 0.05]. No specific interactions among factors were detected [F(2,97) = 0.49, NS], indicating that orchidectomy did not significantly affect the decrement in startle produced by FIN or APO.
Figure 1.
Effects of systemic finasteride (FIN, 50—100 mg/kg, IP) and apomorphine (APO, 0.25 mg/kg, SC) on startle reflex (A), %PPI (B) and ΔPPI (C) in sham-operated (SHAM) and orchidectomized (ORX) rats. FIN doses are indicated in mg/kg (IP). VEH, vehicle of FIN; SAL, saline. Values are expressed as mean ± S.E.M. N = 8—12/group. ***P < 0.001 vs rats treated with vehicle and saline (pre-treatment × treatment interaction); ###P < 0.05; ###P < 0.001 vs rats treated with vehicle and APO. Main statistical effects are not indicated. For further details, see text.
The %PPI analysis, performed with the same design as the one employed for startle amplitude values (Fig. 1B), detected that ORX rats did not exhibit any change in PPI parameters [F(1,97) = 1.29, NS]. Conversely, main effects were found for both pretreatment [F(2,97) = 6.04,P < 0.01] and treatment [F(1,97) = 4.80,P < 0.05]. A highly significant pre-treatment × treatment interaction was found [F(2,97) = 11.38,P < 0.001]. Post hoc comparisons revealed that APO significantly disrupted PPI (VEH + APO vs VEH + SAL,P < 0.001, Tukey’s) and that this effect was prevented by FIN (FIN 50 + SAL vs FIN 50 + APO, NS; FIN 100 + SAL vs FIN 100 + APO, NS) specifically, while only a statistical trend was found for the 50 mg/kg dose of FIN (VEH + APO vs FIN 50 + APO,P < 0.10, Tukey’s), the 100 mg/kg dose fully countered the gating deficits induced by APO (VEH + APO vs FIN 100 + APO,P < 0.001, Tukey’s). FIN did not affect the baseline %PPI in comparison to vehicle-treated animals. The effects of FIN and APO on %PPI were not influenced by surgical castration, as indicated by the lack of interactions among the three factors [F(2,97) = 0.52, NS].
The analysis of DPPI values (Fig. 1C) did not confirm any main effect for pre-treatment [F(2,97) = 0.41, NS], but revealed significant main effects for treatment [F(1,97) = 13.60,P < 0.001]. Orchidectomy did not appear to affect DPPI [F(1,97) = 0.57, NS]. In parallel to the previous set of results, ANOVA identified a significant pre-treatment × treatment interaction [F(2,97) = 11.68,P < 0.001]. Tukey’s test revealed significant DPPI differences between VEH + APO and VEH + SAL (P < 0.001) and between VEH + APO and FIN 100-APO (P < 0.05).
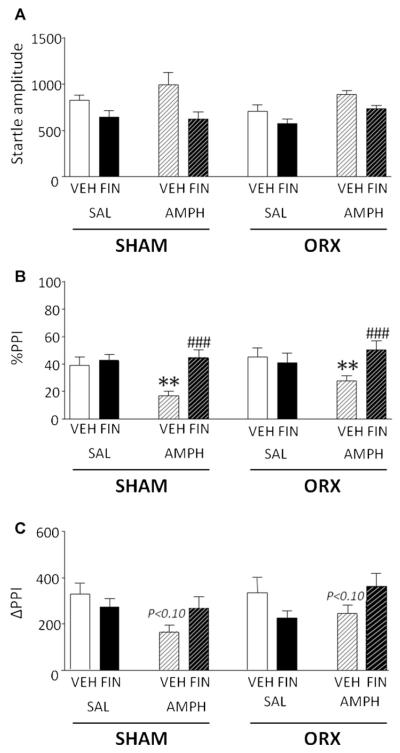
In a second set of experiments, we tested the effect of FIN (100 mg/kg, IP) on the PPI deficits induced by AMPH (2.5 mg/kg, SC) (Fig. 2A) in ORX and sham-operated rats. ANOVA revealed that, while orchidectomy did not induce changes in startle amplitude [F(1,56) = 0.79, NS], this parameter was reduced by FIN [F(1,56) = 17.72,P < 0.001] and enhanced by AMPH [F(1,56) = 5.84,P < 0.05]. No interactions among factors were found.
Figure 2.
Effects of systemic finasteride (FIN, 50—100 mg/kg, IP) and d-amphetamine (AMPH, 2.5 mg/kg, SC) on startle reflex (A), %PPI (B) and ΔPPI (C) in sham-operated (SHAM) and orchidectomized (ORX) rats. VEH, vehicle of FIN; SAL, saline. Values are expressed as mean ± S.E.M. N = 8/group. *P < 0.05; **P < 0.01 vs rats treated with vehicle and saline (pre-treatment treatment interaction); ###P < 0.001 vs rats treated with vehicle and AMPH. Statistical trends (P < 0.10) for comparisons with rats treated with vehicle and SAL (both SHAM and ORX) are indicated on the respective columns. Main statistical effects are not indicated. For further details, see text.
In line with the results obtained on APO, we found that AMPH induced a deficit in %PPI [F(1,56) = 9.76, P < 0.01], while neither orchidectomy [F(1,56) = 1.95, NS] nor FIN pre-treatment [F(1,56) = 3.32 NS] had any significant effect on this index (Fig. 2B). A significant pretreatment × treatment interaction was found [F(1,56) = 10.69, P < 0.01]. Post hoc analyses revealed that the latter effect depended on the %PPI-disrupting effects of AMPH (VEH + SAL vs VEH + AMPH, P < 0.01, Tukey’s), which were reversed by FIN (VEH + AMPH vs FIN + AMPH, P < 0.001, Tukey’s). The evaluation of ΔPPI did not reveal any main effect {orchidectomy: [F(1,56) = 0.91, NS]; pre-treatment [F(1,56) = 0.18, NS]; treatment [F(1,56) = 0.30, NS]}, but confirmed a significant pre-treatment × treatment interaction [F(1,56) = 7.60, P < 0.01]. In contrast with the results on %PPI, Tukey’s test identified no significant difference among treatment groups, although a statistical trend (P < 0.10) was found for the comparison between VEH + SAL and VEH + AMPH.
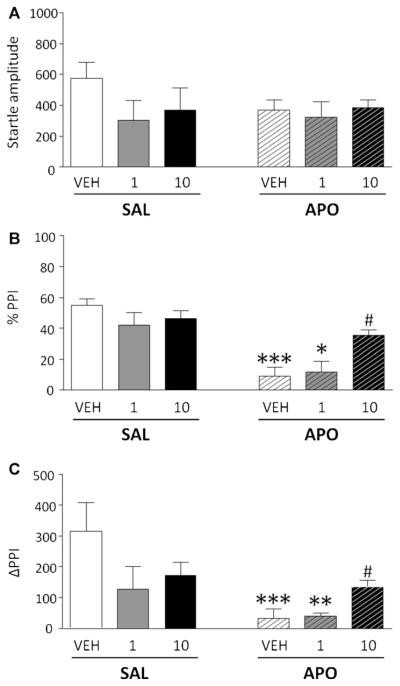
3.2. Effects of intracerebroventricular FIN injections on apomorphine-induced PPI disruption
To verify whether the effects of FIN reflect central mechanisms, we next measured the effects of ICV injections of FIN (1.10 μg/1 μl) in countering the PPI disruption mediated by subcutaneous APO (0.25 mg/kg). While no significant change was observed in startle amplitude (Fig. 3A), the analysis of %PPI revealed a main effect of APO [F(1,47) = 32.77; P < 0.001], and a significant interaction of APO and FIN [F(2,47) = 4.74; P < 0.05]. Post hoc analyses revealed that rats treated with DMSO/Ringer solution + APO and FIN (1 μg) + APO manifested a significant %PPI deficit in comparison with their saline-treated counterparts (P < 0.001 and P < 0.05, respectively). Conversely, no significant difference was found between animals treated with FIN (10 μg) + APO and FIN (10 μg) + SAL. Moreover, the highest ICV FIN dose was found to significantly prevent APO-mediated PPI disruption, as revealed by the significant enhancement of PPI in the FIN (10 μg) + APO group as compared to the rats treated with DMSO/Ringer solution and APO (P < 0.05) (Fig. 3B). These results were further borne out by the examination of ΔPPI values [FIN × APO interaction: F(2,47) = 4.92, P < 0.05] (Fig. 3C).
Figure 3.
Effects of intracerebroventricular finasteride (FIN) on startle reflex (A), %PPI (B) and ΔPPI (C) in relation to the gating deficits induced by systemic apomorphine (APO, 0.25 mg/kg, SC). Finasteride doses are indicated in μg (in 1 μL of solution). VEH, vehicle of finasteride (DMSO—Ringer solution, v:v = 1:1). Values are expressed as mea N ± S.E.M. N = 6—14/group. *P < 0.05; **P < 0.01; ***P < 0.001 vs rats treated with DMSO—Ringer solution (or FIN1) and saline (pre-treatment treatment interaction); #P < 0.05 vs rats treated with DMSO—Ringer Solution and APO. For further details, see text.
3.3. Effects of local FIN injections on apomorphine-induced PPI disruption
The next series of experiments was aimed at the identification of the brain structures involved in the antipsychotic-like effects of FIN. Thus, we tested the effects of FIN injections (0.5 μg/0.5 μl/side) in different forebrain regions, in combination with systemic APO (0.25 mg/kg, SC), on startle and PPI parameters.
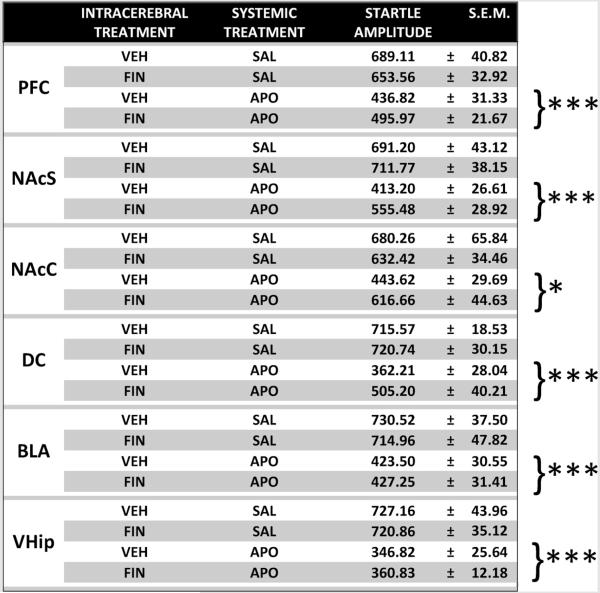
While APO significantly reduced startle amplitude in all experiments [Main effects for APO: mPFC: F(1,36) = 19.91, P < 0.001; NAc shell: F(1,36) = 17.66, P < 0.001; NAc core: F(1,36) = 5.20, P < 0.05; dorsal caudate: F(1,28) = 30.59, P < 0.001; basolateral amygdala: F(1,28) = 40.79, P < 0.001; ventral hippocampus: F(1,28) = 61.00, P < 0.001], locally administered FIN failed to elicit any significant alterations in startle amplitude or to elicit significant interactions with APO on this parameter, irrespective of the region of infusion (Table 1).
Table 1.
Effects of intracerebral finasteride (FIN; 0.5 μg/0.5 μl/side, bilaterally) on startle reflex across medial prefrontal cortex (mPFC), nucleus accumbens shell (NAcS), nucleus accumbens core (NAcS), dorsal caudate (DC), basolateral amygdala (BLA) and ventral hippocampus (VHip). APO, apomorphine (0.25 mg/kg, SC). SAL, saline; VEH, vehicle of finasteride (cyclodextrine–Ringer solution). Braces indicate main effect for APO vs SAL. Values are expressed in arbitrary units, as mean ± S.E.M. N = 8–10/group.
|
P < 0.05
P < 0.001 vs SAL. For further details, see text.
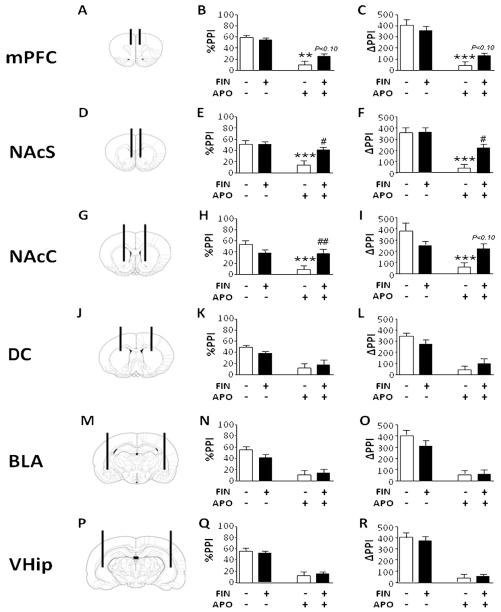
The analysis of %PPI parameters in mPFC (Fig. 4A and B) revealed a significant main effect for APO [F(1,36) = 66.50, P < 0.001], but not for FIN [F(1,36) = 1.40, NS]. The interaction between the two treatments was also significant [F(1,36) = 4.70, P < 0.05]; post hoc calculations revealed that this effect was due to significant differences between the VEH + SAL and the VEH + APO groups (P < 0.001) and between the FIN + SAL and the FIN + APO groups (P < 0.001); furthermore, the difference between VEH + APO and FIN + APO, although not significant, was found to be associated to a statistical trend (P < 0.10, Tukey’s test;Fig. 4B). The examination of ΔPPI (Fig. 4C) by ANOVA showed a main effect for APO [F(1,36) = 83.85, P < 0.001], but not for FIN [F(1,36) = 0.29, NS]. Similarly to the %PPI analysis, FIN × APO interaction was found to be significant [F(1,36) = 4.42, P < 0.05], due to the difference between VEH + SAL and VEH + APO (P < 0.001, Tukey’s). Again, a statistical trend was identified for the comparison between VEH + APO and FIN + APO showed a statistical trend (P < 0.10) (Fig. 4C).
Figure 4.
Topographical schematizations of cannulae placements and effects of local finasteride (FIN, 0.5 μg/0.5 μl/side, bilaterally) on %PPI and ΔPPI across medial prefrontal cortex (mPFC, AP = + 3.0, ML = ± 0.5; DV = ±3) (A—C), nucleus accumbens shell (NAcS, AP = + 1.7, ML = ± 0.8; DV = ±7.4) (D—F), nucleus accumbens core (NAcC, AP = + 1.2, ML = ± 2; DV = ±7) (G—I), dorsal caudate (DC, AP = + 0.5, L = ± 3.0; DV = −4) (J–L), basolateral amygdala (BLA, AP = −2.6, L = ± 4.8; DV = −7) (M–O) and ventral hippocampus (VHip, AP = −5.0, L = ± 5.0; DV = −6) (P–R). APO, apomorphine (0.25 mg/kg, SC). The black lines represent the injectors placements while the signs (+) and (−) refer to the presence of a drug or its vehicle (Ringer solution–cyclodextrine for FIN, saline for APO). Values are expressed as mean ± S.E.M. N = 8–10/group **P < 0.01; ***P < 0.001 vs rats treated with Ringer solution–cyclodextrine and saline (pre-treatment × treatment interaction); #P < 0.05; ##P < 0.01 vs rats treated with vehicle and APO. Statistical trends (P < 0.10) for comparisons with rats treatedwith vehicle andAPO are indicated on therespective columns. Significant main effects are not indicated. For furtherdetails, see text.
In the NAc shell (Fig. 4D—F), FIN restored the %PPI disruption induced by APO (Fig. 4E) [main treatment effect for APO: F(1,36) = 14.97, P < 0.001; interaction between treatments: F(1,36) = 11.71, P < 0.01; P < 0.001 for comparisons between VEH + SAL and VEH + APO and P < 0.05 for comparison between VEH + APO vs FIN + APO]. In the subsequent analyses on ΔPPI values (Fig. 4F), ANOVA detected main effects for FIN [F(1,36) = 8.21, P < 0.01] and APO [F(1,36) = 40.52, P < 0.001], and a significant FIN APO interaction [F(1,36) = 6.08, P < 0.05]; post hoc comparisons showed significant differences between VEH + SAL and VEH + APO (P < 0.001) as well as VEH + APO and FIN + APO (P < 0.05).
In the analysis of %PPI values related to the experiments in the NAc core (Fig. 4G—I), ANOVA disclosed significant main effects for both FIN [F(1,36) = 7.10, P < 0.05] and APO [F(1,36) = 21.20, P < 0.001], as well as a significant interaction between the treatments [F(1,36) = 6.13, P < 0.05]. Post hoc scrutiny of this effect revealed that FIN reversed the PPI decrement produced by subcutaneous APO (Fig. 4H) (VEH + SAL vs VEH + APO, P < 0.001; VEH + APO vs FIN + APO, P < 0.01, Tukey’s]. ΔPPI analysis (Fig. 4I) revealed a significant main effect for APO [F(1,36) = 15.68, P < 0.001], but not FIN [F(1,36) = 0.21, NS]. A significant FIN APO interaction was also found [F(1,36) = 10.35, P < ± 0.01], which was found to depend on significant differences between VEH + SAL and VEH + APO (P < 0.001; Tukey’s test). The comparison between VEH + APO and FIN + APO, albeit not significant, was associated with a statistical trend (P < 0.10).
Administration of FIN in dorsal caudate (Fig. 4J—L), basolateral amygdala (Fig. 4M—O) and ventral hippocampus (Fig. 4P—R) did not elicit any significant effect on either startle or PPI, and failed to reverse the %PPI deficit caused by systemic APO administration [main effects for APO: dorsal caudate: F(1,28) = 26.95, P < 0.001; basolateral amygdala: F(1,28) = 34.20, P < 0.001; ventral hippocampus: F(1,28) = 65.49, P < 0.001]. Analyses of ΔPPI values confirmed these results [main effects for APO: dorsal caudate: F(1,28) = 64.77, P < 0.001; basolateral amygdala: F(1,28) = 63.97, P < 0.001; ventral hippocampus: F(1,28) = 117.80, P < 0.001].
3.4. Effects of systemic and local FIN injections on DA extracellular concentrations
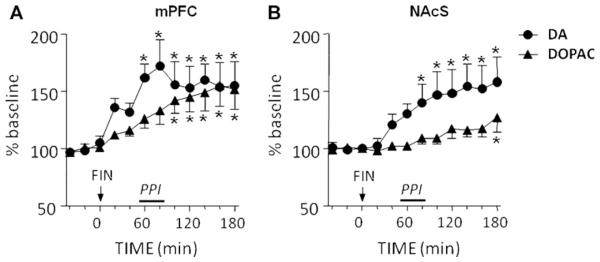
We then tested whether the effects of systemic FIN (100 mg/ kg, IP) in PPI may be accompanied by changes in DA levels in the two regions that mediated its antipsychotic-like effects, mPFC and NAc shell. In the mPFC (mean baseline values ± S.E.M.: DA = 1.2 ± 0.1 pg/40 μl dialysate; DOPAC = 162.4 30.2 pg/40 μl dialysate), FIN induced a significant increase in DA extracellular concentrations [F(11,83) = 4.33, P < 0.001], which started at 40 min after FIN injection and lasted for the whole duration (3 h) of the experiment (Fig. 5A). Likewise, DOPAC concentrations were significantly enhanced [F(11,83) = 6.53, P < 0.001] in a time-dependent fashion, from 100 min after FIN injection onwards (Fig. 5A). In the NAc shell (mean baseline values ± S.E.M.: DA = 6.0 1.6 pg/ 40 μl dialysate; DOPAC = 3046.7 681.9 pg/40 μl dialysate). FIN also induced a significant enhancement in DA [F(11,95) = 5.66, P < 0.001] and DOPAC [F(11,95) = 4.05, P < 0.001], starting by 80 and 180 min after FIN injection, respectively (Fig. 5B).
Figure 5.
Time-related effects of systemic finasteride (FIN, 100 mg/kg, IP) on extracellular concentrations of dopamine (DA) and 3,4-dihydroxyphenylacetic acid (DOPAC) in medial prefrontal cortex (mPFC) (A) and nucleus accumbens shell (NAcS) (B). Arrows represent injection time of FIN or its vehicle. The period corresponding to PPI testing is indicated by a bar alongside the time axis. Values are expressed as mean percent of the baseline (average values of the three first samples) ± S.E.M. N = 7—8/group. *P < 0.05; **P < 0.01, ***P < 0.001 in comparison to baseline values. For further details, see text.
In contrast with these results, intracerebral FIN injections failed to induce alterations of local levels of DA (Fig. 6) and DOPAC (data not shown) in either mPFC [F(2,22) = 0.11, NS] (Fig. 6A) or NAc shell [F(2,28) = 1.57, NS] (Fig. 6B). As expected (Shilliam and Heidbreder, 2003; Devoto and Flore, 2006), APO time-dependently decreased extracellular DA levels in the Nac shell [2-way, repeated measure ANOVA: main effect for time: F(2,20) = 23.59, P < 0.001; APO 0′ vs APO 40′, P < 0.01, Tukey’s test], but not in the mPFC [F(2,28) = 4.09, NS]; notably, no significant treatment × time interaction was found, indicating that these effects were equivalent for FIN- and vehicle-treated animals (Fig. 6C and D).
Figure 6.
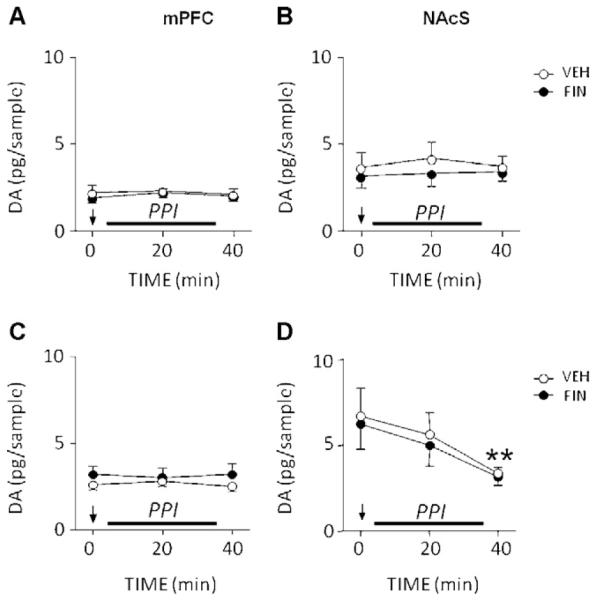
Time-related effects of local infusions of finasteride (FIN, 0.5 μg/0.5 μl/side) in the medial prefrontal cortex (mPFC; left column) and nucleus accumbens shell (NAcS; right column) on local dopamine (DA) concentrations, by itself (A and B) or in combination with apomorphine (APO, 0.25 mg/kg, SC) (B and C). VEH, vehicle of finasteride. Arrows represent the injection time for FIN/VEH (A and B) or APO + FIN/APO + VEH (with APO injection immediately preceding FIN/VEH administration) (C and D). The period corresponding to PPI testing is indicated by a bar alongside the time axis. Each time point represents the content of DA in the sample relative to the baseline. N = 6—7/group. **P < 0.05 vs baseline (Main time effect; APO 0′ vs APO 40′). Values are expressed as mean ± S.E.M. For further details, see text.
4. Discussion
The major finding of the present study is that PPI deficits induced by DAergic stimulation were dose-dependently reversed by systemic and intracerebral administration of the 5αR inhibitor FIN. This phenomenon was mainly observed in the NAc (particularly in its shell subdivision), the key terminal of the DAergic mesolimbic pathway; additionally, our data indicated a statistical trend for the involvement of the mPFC in FIN’s role on PPI regulation. Conversely, we found that the same dose of FIN did not affect DAergic modulation of PPI when infused in other forebrain regions, such as the dorsal caudate, basolateral amygdala and ventral hippocampus. In addition, orchidectomy did not modify the effects of FIN on PPI, suggesting that the mechanisms mediating these effects are not directly dependent on plasma levels of androgens.
These results extend and complement our previous reports on the antipsychotic-like profile of 5αR inhibitors in rats (Bortolato et al., 2008) and in patients affected by schizophrenia (Koethe et al., 2008), Tourette syndrome (Bortolato et al., 2007; Muroni et al., 2011) and levodopa-induced pathological gambling (Bortolato et al., in press). In addition, these findings delineate a preliminary topographical assessment of the neural underpinnings of 5αR’s role in the regulation of sensorimotor gating and DAergic signaling. The implication of NAc in the 5αR-mediated modulation of DA signaling is supported by recent studies from our group and others, documenting the expression of 5αR isoforms and their products in this region (Saalmann et al., 2007; Bortolato et al., 2011). Furthermore, both the core and the shell of this area have been implicated in the behavioral actions of FIN and 5αR products (Rhodes and Frye, 2001; Frye et al., 2002; Molina-Hernández et al., 2005; Engin and Treit, 2007) and are pivotal substrates for the DAergic regulation of PPI (reviewed in Swerdlow et al., 2001b). In particular, the activation of accumbal DA receptors induces a remarkable PPI disruption in rats (Swerdlow et al., 1992; Wan et al., 1994, 1996; Kretschmer and Koch, 1998). Notably, Hart et al. (1998) showed that the PPI deficits induced by systemic APO administration was significantly prevented by infusions of the typical antipsychotic haloperidol in mPFC, NAc core, ventral tegmental area and ventral subiculum, but not in NAc shell.
Although the role of both compartments of NAc, core and shell, was tested in our study, it should be noted that, in consideration of the lipid nature of FIN and the extremely rapid rate of diffusion of steroids throughout biological membranes (Oren et al., 2004), it is extremely likely that the effect of the 5αR inhibitor in each subdivision of NAc may have been affected by the contribution of the other area or other adjacent structures. Future studies with non-lipophilic 5αR inhibitors (with lower levels of diffusion) will be required to ultimately define the potential contribution of NAc core and shell on the control of DAergic modulation of sensorimotor gating.
Notably, FIN injection in other brain regions, such as amygdala, hippocampus and caudate, failed to counter apomorphine-induced PPI deficits, suggesting that the involvement of these areas in the 5αR-mediated gating regulation may be less critical than NAc (and possibly mPFC). Nevertheless, the participation of those structures in the systemic effects of FIN in PPI cannot be excluded; in fact, each experiment was performed following intracerebral administration of only one dose of the 5αR inhibitor in one individual region, raising the possibility that either higher doses of the compound or the concomitant engagement of different regions (with multiple simultaneous stereotaxic injections) could still attenuate the PPI deficits induced by DA receptor activation.
In agreement with previous data, we showed that the reduction of plasma androgen levels in ORX rats did not elicit any significant effect on startle and PPI responses at 2 weeks after surgery (Gogos and van den Buuse, 2003; Turvin et al., 2007); additionally, gonadectomy failed to change the effects of DAergic agonists (Wong et al., 2002) and FIN on PPI responses. These results strongly suggest that peripheral sex hormones may play only a negligible role on gating functions. This possibility is also confirmed by our observation that FIN exerts significant antipsychotic-like effects also in female rats, irrespective of the phase of estrous cycle (unpublished data).
Similarly to our previous results (Bortolato et al., 2008), intraperitoneal FIN administration induced a significant reduction in startle amplitude, irrespective of gonadectomy; in contrast, intracerebral FIN injections that reduced the PPI-disruptive action of APO in NAc and mPFC did not significantly affect startle magnitude, indicating a dissociation between the outcomes of FIN on startle reflex and gating regulation. Notably, the reduction in startle amplitude elicited by systemic administration of the 5αR inhibitor was accompanied by hypolocomotion (Bortolato et al., 2007), as well as overt muscle relaxation (unpublished data); this last outcome was also observed in orchidectomized rats, but not in animals subjected to intracerebral FIN infusion. These phenomena could be due to non-androgenic 5αR substrates, such as progesterone; the blockade of 5αR mediated by FIN is likely to enhance the concentrations of this hormone by inhibiting its conversion into its 5α-reduced metabolites, 5α-dihydro-progesterone and AP. Interestingly, progesterone has been found to reduce locomotor activity and startle response in rats (Rupprecht et al., 1999); the apparent lack of involvement of forebrain regions may indicate that the hormone may induce myorelaxation via the brainstem nuclei (which also regulate startle reflex) or by reducing the respiratory function of skeletal muscles (Gras et al., 2007).
Throughout the study, startle amplitude was significantly reduced by APO and increased by AMPH. Previous evidence showed that, while indirect DAergic activators are generally conducive to a potentiation of startle reactivity (Kehne and Sorenson, 1978; Davis, 1980; Johansson et al., 1995), the effects of APO on this parameter vary in relation to the dose, with low dosages typically exerting a depressing influence on startle amplitude (likely due to the preferential activation of presynaptic autoreceptors) and high doses increasing it (Davis and Aghajanian, 1976). In addition, other factors have been shown to govern the effect of APO on startle responsiveness in rats, including: genetic background (Swerdlow et al., 2000a; Conti et al., 2006); duration of the time interval between treatment and testing (Davis and Aghaja-nian, 1976; Young et al., 1991); functional integrity of specific brain areas, such as the hippocampus and PFC (Swerdlow et al., 1995, 2000b). Based on these premises, the opposite effects of APO and AMPH on startle amplitude may reflect differential degrees of activation of DAergic receptors, with respect to their synaptic and brain-regional location.
Irrespective of this issue, the finding that both systemic FIN and DAergic activators induced significant changes in Spa introduces potential confounds in the interpretation of %PPI data (Mansbach et al., 1988; Davis et al., 1990; Swerdlow and Geyer, 1993; Kinney et al., 1999; Swerdlow et al., 2000a). Because %PPI is defined as a function of Spp and Spa, its alterations can be unequivocally assessed when the reduction of the startle reflex induced by pre-pulses are not accompanied by significant changes in overall startle reactivity. A major caveat in %PPI computation is that increases or reductions in startle magnitude can respectively lead to artefactual changes of this index, due to “ceiling” or “floor” effects (Davis et al., 1990; Swerdlow et al., 2000a). Floor effects consequent to marked reductions of Spa are particularly problematic, since even small variations of Spp or general activity may lead to substantial changes in %PPI. Thus, in the event of significant modifications of Spa, alternative strategies are required to rule out false-positive results due to computational issues. In our study, two complementary approaches were adopted to ascertain this eventuality. First, we verified that AI (the activity index, defined as the ratio) was always comprised between 8 and 12 for all animals; in fact, preliminary tests conducted in our laboratory found that AI values in this range robustly predict against floor and ceiling effects (unpublished observations); furthermore, statistical analyses showed that AI was not significantly affected by any treatment combination (data not shown). Second, we controlled for computational false positives by counter-analyzing the results of each experiment using ΔPPI values; this double assessment is a stringent and dependable method to exclude potential floor effects in %PPI calculations due to overall reductions in startle magnitude (Bortolato et al., 2004). The majority of significant effects in the analysis of %PPI were matched by similar results in the statistical computation of ΔPPI, albeit with generally lower Ps or, in the case of FIN infusion in NAc core, only a statistical trend.
Collectively, these convergent results indicate that, across most experiments, the impact of FIN and/or DAergic agonists on PPI did not reflect substantial modifications of startle amplitude. This conclusion was also confirmed by separate analyses on Spp values, which showed that, unlike Spa, this parameter was not diminished by VEH + APO treatment in comparison with VEH + SAL controls; conversely, the combination of systemic FIN with either SAL or APO produced consensual reductions of both indices (data not shown).
The main discrepancy between %PPI and cognate ΔPPI values was found in the post hoc analysis of the significant interactions between FIN and AMPH. The possibility that the effects on PPI of the two drugs may be spurious is challenged by several observations. Both APO and AMPH are posited to disrupt PPI by activating postsynaptic DAergic receptors in NAc (Swerdlow et al., 1990), in a fashion unrelated to variations in startle amplitude (Kinney et al., 1999); thus, it appears unlikely that FIN’s ability to counter the PPI impairment caused by APO is not matched by analogous properties in the presence of AMPH. Additionally, we previously reported that FIN significantly reversed the %PPI deficits induced by 5 mg/kg (s.c.) of AMPH (Bortolato et al., 2008) — a result that was further corroborated by ΔPPI analyses (unpublished data). Notably, the effects of direct DA agonists in PPI are consistently more pronounced than those elicited by AMPH, due to the latter’s dependence on endogenous mesolimbic DA levels to exert its effects on sensorimotor gating (Geyer et al., 2001; Swerdlow et al., 2001b). Given the statistical strictness of ΔPPI analysis, this parameter appears only sensitive to profound gating impairments, and higher doses of AMPH may therefore be needed to elicit significant reductions of ΔPPI and capture FIN’s antipsychotic-like effects with this computational approach.
In keeping with previous evidence, we found that the antipsychotic-like effects of systemic FIN treatment were paralleled by a time-dependent increase in extracellular DA and DOPAC levels in both NAc and mPFC (Dazzi et al., 2002). These neurochemical effects, however, were dissociated from the antipsychotic-like actions of FIN and did not reflect the blockade of 5αR in either region, as shown by the lack of effects on DA concentrations following intracerebral FIN treatment under the same conditions that elicited PPI-restorative effects.
The divergence between the neurochemical effects of intraperitoneal and intracerebral injections suggests that the increase in accumbal and medial prefrontal DA levels induced by systemic FIN may reflect the modulation of its release due to a direct action of 5αR on the somata of DA neurons in the ventral tegmental area or on other regions, rather than alterations in DA reuptake in the presynaptic boutons. Notably, the administration of 5αR substrate pro-gesterone has been shown to induce DA release in striatum (Petitclerc et al., 1995), while its metabolite AP has been reported to induce a reduction of DA release in NAc (Motzo et al., 1996; but see Rougé-Pont et al., 2002 for contrasting evidence). Testosterone and its metabolite β-estradiol have also been shown to affect DA release (Thompson and Moss, 1994; Putnam et al., 2003).
Although the subcutaneous doses of APO used in this study are arguably consistent with activation of DA autoreceptors and post-synaptic receptors, the observed effects of intra-cerebral FIN injections suggest that the antipsychotic-like actions of this drug are likely supported by post-synaptic processes, as they were not accompanied by significant alterations in DA concentrations. Furthermore, previous studies suggest that the gating impairments induced by DA direct agonists are mainly mediated by post-synaptic mechanisms (Swerdlow et al., 2001b). Interestingly, both prefrontal pyramidal cells and accumbal medium spiny neurons, which receive most DAergic projections in mPFC and NAc, respectively (Meredith et al., 1992; Santana et al., 2009), feature high levels of 5αR (Agís-Balboa et al., 2006). In these neurons, 5αR substrates and products are known to modulate the signaling of several targets, such as GABAA, AMPA, NMDA, 5-HT3 and s1 receptors (Rupprecht and Holsboer, 1999), which may in turn interact with — and modify — the downstream cascade of DA receptors. In line with this possibility, preliminary studies have provided functional and anatomical evidence on a modulatory role of NSs on cortical and striatal DAergic functions (Beatty et al., 1982; Bitar et al., 1991; Dluzen et al., 1986; Engel et al., 1979; Fabre-Nys, 1998; Hernandez et al., 1994; Menniti and Baum, 1981; Savageau and Beatty, 1981). Since 5αR isoforms catalyze an irreversible reaction on the metabolism of several ketosteroids, including progestagens, androgens and glucocorticoids, its blockade is likely to induce profound modifications of the steroidal profile in the targeted neurons.
Several limitations of the present study need to be acknowledged. First, we did not analyse the specific impact of FIN on steroid concentrations in each brain region; nevertheless, it should be noted that the content of several neurosteroids in rat mPFC and NAc may be below the detection limit of currently available methods, such as radioimmunoassay or HPLC/MS (Donatella Caruso, personal communication). Second, our study did not include the analyses of full intracerebral dose—response curves for FIN and omitted several important brain structures implicated in gating regulation, such as the ventral tegmental area, dorsal subiculum and globus pallidus. Third, the high affinity of FIN for both 5αR1 and 5αR2 isozymes in rats (Thigpen and Russell, 1992) does not allow us to elucidate the specific contribution of each isoenzyme to the antipsychotic-like effects of 5αR inhibition. Fourth, our behavioral analyses were mainly focused on startle reflex and PPI; however, the reduction of this index is currently regarded as one of the endophenotypes with highest relevance to schizophrenia-associated perturbations, in view of its high degree of isomorphism with the alterations featured in psychotic disorders (Braff et al., 2001a; Cadenhead et al., 1993) and its sensitivity to antipsychotic agents (Braff et al., 2001b; Kumari et al., 2007; Leumann et al., 2002). Whereas further research is needed to address these limitations, our findings highlight the critical role of 5αR in the pathophysiology of gating deficits and psychosis, and point to both NAc subdivisions as the key substrates implicated in the link between neurosteroids and a number of disorders featuring gating deficits and alterations of DAergic signaling. In particular, given the growing evidence collected by our group in support of a potential therapeutic efficacy of FIN in Tourette syndrome (Bortolato et al., 2007; Muroni et al., 2011), the current results may help elucidate the neurobiological underpinnings of the well-characterized male predominance of this condition (Tanner and Goldman, 1997) as well as the mechanism of action of 5αR inhibitors in this and other related neuropsychiatric disorders (see Paba et al., 2011 for an overview of the issue).
Acknowledgments
We would like to thank Marco Orru’, Alberto Casti and Silvia Paba for their technical assistance.
Role of the funding sources
This work was supported by grants from National Institute of Health (R21 HD070611, to M.B.), Tourette Syndrome Association (to M.B. and P.D.), USC Zumberge Research Grant (to M.B.) and from Bank of Sardinia Foundation (to P.D.). None of these institutions had any further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Conflicts of interest
None declared.
Contributors: PD designed the experiments, monitored data collection, analysed microdialysis data and performed statistical analyses, drafted and revised the manuscript. RF executed microsurgery, designed and performed behavioral tests and statistics, drafted and revised the manuscript. VB and GP executed microsurgery and performed behavioral tests. PS and GF performed microsurgery and microdialysis experiments. MC analysed behavioral data, discussed and revised the manuscript. FM discussed and revised the paper. MB supervised the experimental design and execution, monitored data collection, performed statistical analyses, wrote and revised the manuscript.
References
- Agís-Balboa RC, Pinna G, Zhubi A, Maloku E, Veldic M, Costa E, Guidotti A. Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proc. Natl. Acad. Sci. U.S.A. 2006;103:14602–14607. doi: 10.1073/pnas.0606544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, Swayze VW, 2nd, Flaum M, Yates WR, Arndt S, McChesney C. Ventricular enlargement in schizophrenia evaluated with computed tomographic scanning. Effects of gender, age, and stage of illness. Arch. Gen. Psychiatry. 1990;47:1008–1015. doi: 10.1001/archpsyc.1990.01810230024005. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Roscetti G, Trabucchi M, Purdy RH, Mostallino MC, Concas A, Biggio G. The effects of inhibitors of GABAergic transmission and stress on brain and plasma allopregnanolone concentrations. Br. J. Pharmacol. 1997;120:1582–1588. doi: 10.1038/sj.bjp.0701046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbaccia ML, Affricano D, Purdy RH, Maciocco E, Spiga F, Biggio G. Clozapine, but not haloperidol, increases brain concentrations of neuroactive steroids in the rat. Neuropsychopharmacology. 2001;25:489–497. doi: 10.1016/S0893-133X(01)00254-8. [DOI] [PubMed] [Google Scholar]
- Beatty WW, Dodge AM, Traylor KL. Stereotyped behavior elicited by amphetamine in the rat: influences of the testes. Pharmacol. Biochem. Behav. 1982;16:565–568. doi: 10.1016/0091-3057(82)90416-6. [DOI] [PubMed] [Google Scholar]
- Bitar MS, Ota M, Linnoila M, Shapiro BH. Modification of gonadectomy-induced increases in brain monoamine metabolism by steroid hormones in male and female rats. Psychoneuroendo-crinology. 1991;16:547–557. doi: 10.1016/0306-4530(91)90038-u. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Frau R, Aru GN, Orru’ M, Gessa GL. Baclofen reverses the reduction in prepulse inhibition of the acoustic startle response induced by dizocilpine, but not by apomorphine. Psychopharmacology. 2004;171:322–330. doi: 10.1007/s00213-003-1589-5. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Muroni A, Marrosu F. Treatment of Tourette’s syndrome with finasteride. Am. J. Psychiatry. 2007;164:1914–1915. doi: 10.1176/appi.ajp.2007.07060978. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Frau R, Orru’ M, Bourov Y, Marrosu F, Mereu G, Devoto P, Gessa GL. Antipsychotic-like properties of 5-alpha-reductase inhibitors. Neuropsychopharmacology. 2008;33:3146–3156. doi: 10.1038/npp.2008.39. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Cannas A, Solla P, Bini V, Puligheddu M, Marrosu F. Finasteride attenuates pathological gambling in Parkinson’s disease patients. J. Clin. Psychopharmacol. doi: 10.1097/JCP.0b013e3182549c2a. in press. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Devoto P, Roncada P, Frau R, Flore G, Saba P, Pistritto G, Soggiu A, Pisanu S, Zappala’ A, Ristaldi MS, Tattoli M, Cuomo V, Marrosu F, Barbaccia ML. Isolation rearing-induced reduction of brain 5α-reductase expression: relevance to dopaminergic impairments. Neuropharmacology. 2011;60:1301–1308. doi: 10.1016/j.neuropharm.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Braff DL, Grillon C, Geyer MA. Gating and habituation of the startle reflex in schizophrenic patients. Arch. Gen. Psychiatry. 1992;49:206–215. doi: 10.1001/archpsyc.1992.01820030038005. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Light GA, Sprock J, Perry W, Cadenhead KS, Swerdlow NR. Impact of prepulse characteristics on the detection of sensorimotor gating deficits in schizophrenia. Schizophr. Res. 2001a;49:171–178. doi: 10.1016/s0920-9964(00)00139-0. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology. 2001b;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Cadenhead KS, Geyer MA, Braff DL. Impaired startle prepulse inhibition and habituation in patients with schizotypal personality disorder. Am. J. Psychiatry. 1993;150:1862–1867. doi: 10.1176/ajp.150.12.1862. [DOI] [PubMed] [Google Scholar]
- Hallett M. Sensorimotor gating in boys with Tourette’s syndrome and ADHD: preliminary results. Biol. Psychiatry. 1996;39:33–41. doi: 10.1016/0006-3223(95)00101-8. [DOI] [PubMed] [Google Scholar]
- Conti LH, Berridge CW, Tayler JE. Both corticotropin-releasing factor and apomorphine reduce prepulse inhibition following repeated central infusion of corticotropin-releasing factor. Pharmacol. Biochem. Behav. 2006;85:261–272. doi: 10.1016/j.pbb.2006.08.009. 2006. [DOI] [PubMed] [Google Scholar]
- Davis M, Aghajanian GK. Effects of apomorphine and haloperidol on the acoustic startle response in rats. Psychophar-macology. 1976;47:217–223. doi: 10.1007/BF00427605. [DOI] [PubMed] [Google Scholar]
- Davis M. Neurochemical modulation of sensory-motor reactivity: acoustic and tactile startle reflexes. Neurosci. Biobehav. Rev. 1980;4:241–263. doi: 10.1016/0149-7634(80)90016-0. [DOI] [PubMed] [Google Scholar]
- Davis M, Mansbach RS, Swerdlow NR, Braff DL, Geyer MA. Apomorphine disrupts the inhibition of acoustic startle induced by weak prepulses in rats. Psychopharmacology. 1990;102:1–4. doi: 10.1007/BF02245735. [DOI] [PubMed] [Google Scholar]
- Dazzi L, Serra M, Seu E, Cherchi G, Pisu MG, Purdy RH, Biggio G. Progesterone enhances ethanol-induced modulation of mesocortical dopamine neurons: antagonism by finasteride. J. Neurochem. 2002;83:1103–1109. doi: 10.1046/j.1471-4159.2002.01218.x. [DOI] [PubMed] [Google Scholar]
- Devoto P, Flore G. On the origin of cortical dopamine: is it a co-transmitter in noradrenergic neurons? Curr. Neuropharmacol. 2006;4:115–125. doi: 10.2174/157015906776359559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoto P, Flore G, Saba P, Castelli MP, Piras AP, Luesu W, Viaggi MC, Ennas MG, Gessa GL. 6-Hydroxydopamine lesion in the ventral tegmental area fails to reduce extracellular dopamine in the cerebral cortex. J. Neurosci. Res. 2008;86:1647–1658. doi: 10.1002/jnr.21611. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, Green MA, Ramirez VD. The effect of hormonal condition on dose-dependent amphetamine-stimulated behaviors in the male rat. Horm. Behav. 1986;20:1–6. doi: 10.1016/0018-506x(86)90024-3. [DOI] [PubMed] [Google Scholar]
- Dong E, Matsumoto K, Uzunov V, Sugaya I, Takahata H, Nomura H. Brain 5alpha-dihydroprogesterone and allopregnanolone synthesis in a mouse model of protracted social isolation. Proc. Natl. Acad. Sci. U.S.A. 2001;98:2849–2854. doi: 10.1073/pnas.051628598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias A, Kumar A. Testosterone for schizophrenia. Cochrane Database Syst. Rev. 2007;3:CD006197. doi: 10.1002/14651858.CD006197.pub2. [DOI] [PubMed] [Google Scholar]
- Engel J, Ahlenius S, Almgren O, Carlsson A, Larsson K, Södersten P. Effects of gonadectomy and hormone replacement on brain monoamine synthesis in male rats. Pharmacol. Biochem. Behav. 1979;10:149–154. doi: 10.1016/0091-3057(79)90181-3. [DOI] [PubMed] [Google Scholar]
- Engin E, Treit D. The anxiolytic-like effects of allopregnanolone vary as a function of intracerebral microinfusion site: the amygdala, medial prefrontal cortex, or hippocampus. Behav. Pharmacol. 2007;18:461–470. doi: 10.1097/FBP.0b013e3282d28f6f. [DOI] [PubMed] [Google Scholar]
- Fabre-Nys C. Steroid control of monoamines in relation to sexual behaviour. Rev. Reprod. 1998;3:31–41. doi: 10.1530/ror.0.0030031. [DOI] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME, Rosellini R, Svare B. The nucleus accumbens as a site of action for rewarding properties of testosterone and its 5alpha-reduced metabolites. Pharmacol. Biochem. Behav. 2002;74:119–127. doi: 10.1016/s0091-3057(02)00968-1. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology. 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Gizerian SS, Morrow AL, Lieberman JA, Grobin AC. Neonatal neurosteroid administration alters parvalbumin expression and neuron number in medial dorsal thalamus of adult rats. Brain Res. 2004;25:66–74. doi: 10.1016/j.brainres.2004.03.049. [DOI] [PubMed] [Google Scholar]
- Gogos A, van den Buuse M. Castration reduces the effect of serotonin-1A receptor stimulation on prepulse inhibition in rats. Behav. Neurosci. 2003;117:1407–1415. doi: 10.1037/0735-7044.117.6.1407. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, O’Brien LM, Horton NJ, Kennedy DN, Makris N, Caviness VS, Jr., Faraone V, Tsuang MT. Impact of normal sexual dimorphisms on sex differences in structural brain abnormalities in schizophrenia assessed by magnetic resonance imaging. Arch. Gen. Psychiatry. 2002;59:154–164. doi: 10.1001/archpsyc.59.2.154. [DOI] [PubMed] [Google Scholar]
- Goyal RO, Sagar R, Ammini AC, Khurana ML, Alias AG. Negative correlation between negative symptoms of schizophrenia and testosterone levels. Ann. N.Y. Acad. Sci. 2004;1032:291–294. doi: 10.1196/annals.1314.042. [DOI] [PubMed] [Google Scholar]
- Gras F, Brunmair B, Quarré L, Szöcs Z, Waldhaäusl W, Fürnsinn C. Progesterone impairs cell respiration and suppresses a compensatory increase in glucose transport in isolated rat skeletal muscle: a non-genomic mechanism contributing to metabolic adaptation to late pregnancy? Diabetologia. 2007;50:2544–2552. doi: 10.1007/s00125-007-0836-4. [DOI] [PubMed] [Google Scholar]
- Grobin AC, Gizerian S, Lieberman JA, Morrow AL. Perinatal allopregnanolone influences prefrontal cortex structure, connectivity and behavior in adult rats. Neuroscience. 2006;138:809–819. doi: 10.1016/j.neuroscience.2005.12.026. [DOI] [PubMed] [Google Scholar]
- Gur RE, Turetsky BI, Cowell PE, Finkelman C, Maany V, Grossman RI, Arnold SE, Bilker WB, Gur RC. Temporolimbic volume reductions in schizophrenia. Arch. Gen. Psychiatry. 2000;57:769–775. doi: 10.1001/archpsyc.57.8.769. [DOI] [PubMed] [Google Scholar]
- Haäfner H. Gender differences in schizophrenia. Psychoneur-oendocrinology. 2003;28:17–54. doi: 10.1016/s0306-4530(02)00125-7. [DOI] [PubMed] [Google Scholar]
- Halari R, Kumari V, Mehrotra R, Wheeler M, Hines M, Sharma T. The relationship of sex hormones and cortisol with cognitive functioning in schizophrenia. J. Psychopharmacol. 2004;18:366–374. doi: 10.1177/026988110401800307. [DOI] [PubMed] [Google Scholar]
- Hart S, Zreik M, Carper R, Swerdlow NR. Localizing haloperidol effects on sensorimotor gating in a predictive model of antipsychotic potency. Pharmacol. Biochem. Behav. 1998;61:113–119. doi: 10.1016/s0091-3057(98)00079-3. [DOI] [PubMed] [Google Scholar]
- Hernandez L, Gonzalez L, Murzi E, Paez X, Gottberg E, Baptista T. Testosterone modulates mesolimbic dopaminergic activity in male rats. Neurosci. Lett. 1994;71:172–174. doi: 10.1016/0304-3940(94)90632-7. [DOI] [PubMed] [Google Scholar]
- Huber TJ, Tettenborn C, Leifke E, Emrich HM. Sex hormones in psychotic men. Psychoneuroendocrinology. 2005;30:111–114. doi: 10.1016/j.psyneuen.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Johansson C, Jackson DM, Zhang J, Svensson L. Prepulse inhibition of acoustic startle, a measure of sensorimotor gating: effects of antipsychotics and other agents in rats. Pharmacol. Biochem. Behav. 1995;52:649–654. doi: 10.1016/0091-3057(95)00160-x. [DOI] [PubMed] [Google Scholar]
- Kehne JH, Sorenson CA. The effects of pimozide and phenoxybenzamine pretreatments on amphetamine and apomorphine potentiation of the acoustic startle response in rats. Psychopharmacology. 1978;58:137–144. doi: 10.1007/BF00426896. [DOI] [PubMed] [Google Scholar]
- Kinney GG, Wilkinson LO, Saywell KL, Tricklebank MD. Rat strain differences in the ability to disrupt sensorimotor gating are limited to the dopaminergic system, specific to prepulse inhibition, and unrelated to changes in startle amplitude or nucleus accumbens dopamine receptor sensitivity. J. Neurosci. 1999;19:5644–5653. doi: 10.1523/JNEUROSCI.19-13-05644.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koethe D, Bortolato M, Piomelli D, Leweke FM. Improvement of general symptoms in a chronic psychotic patient treated with finasteride: case report. Pharmacopsychiatry. 2008;41:115–116. doi: 10.1055/s-2008-1058110. [DOI] [PubMed] [Google Scholar]
- Kretschmer BD, Koch M. The ventral pallidum mediates disruption of prepulse inhibition of the acoustic startle response induced by dopamine agonists, but not by NMDA antagonists. Brain Res. 1998;798:204–210. doi: 10.1016/s0006-8993(98)00424-7. [DOI] [PubMed] [Google Scholar]
- Kumari V, Antonova E, Geyer MA, Ffytche D, Williams SC, Sharma T. A fMRI investigation of startle gating deficits in schizophrenia patients treated with typical or atypical antipsychotics. Int. J. Neuropsychopharmacol. 2007;10:463–477. doi: 10.1017/S1461145706007139. [DOI] [PubMed] [Google Scholar]
- Leumann L, Feldon J, Vollenweider FX, Ludewig K. Effects of typical and atypical antipsychotics on prepulse inhibition and latent inhibition in chronic schizophrenia. Biol. Psychiatry. 2002;52:729–739. doi: 10.1016/s0006-3223(02)01344-6. [DOI] [PubMed] [Google Scholar]
- Mansbach R, Geyer MA, Braff DL. Dopaminergic stimulation disrupts sensorimotor gating in the rat. Psychopharmacology. 1988;94:507–514. doi: 10.1007/BF00212846. [DOI] [PubMed] [Google Scholar]
- Martini L, Melcangi RC, Maggi R. Androgen and progesterone metabolism in the central and peripheral nervous system. J. Steroid Biochem. Mol. Biol. 1993;47:195–205. doi: 10.1016/0960-0760(93)90075-8. [DOI] [PubMed] [Google Scholar]
- Marx CE, Stevens RD, Shampine LJ, Uzunova V, Trost WT, Butterfield MI, Massing MW, Hamer RM, Morrow AL, Lieberman JA. Neuroactive steroids are altered in schizophrenia and bipolar disorder: relevance to pathophysiology and therapeutics. Neuropsychopharmacology. 2006;31:1249–1263. doi: 10.1038/sj.npp.1300952. [DOI] [PubMed] [Google Scholar]
- Menniti FS, Baum MJ. Differential effects of estrogen and androgen on locomotor activity induced in castrated male rats by amphetamine, a novel environment, or apomorphine. Brain Res. 1981;216:89–107. doi: 10.1016/0006-8993(81)91280-4. [DOI] [PubMed] [Google Scholar]
- Meredith GE, Agolia R, Arts MP, Groenewegen HJ, Zahm DS. Morphological differences between projection neurons of the core and shell in the nucleus accumbens of the rat. Neuroscience. 1992;50:149–162. doi: 10.1016/0306-4522(92)90389-j. [DOI] [PubMed] [Google Scholar]
- Molina-Hemández M, Tellez-Alcántara NP, García JP, Lopez JI, Jaramillo MT. Antidepressant-like actions of intraaccumbens infusions of allopregnanolone in ovariectomized Wistar rats. Pharmacol. Biochem. Behav. 2005;80:401–409. doi: 10.1016/j.pbb.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Motzo C, Porceddu ML, Maira G, Flore G, Concas A, Dazzi L, Biggio G. Inhibition of basal and stress-induced dopamine release in the cerebral cortex and nucleus accumbens of freely moving rats by the neurosteroid allopregnanolone. J. Psychopharmacol. 1996;10:266–272. doi: 10.1177/026988119601000402. [DOI] [PubMed] [Google Scholar]
- Muroni A, Paba S, Puligheddu M, Marrosu F, Bortolato M. A preliminary study of finasteride in Tourette syndrome. Mov. Disord. 2011;26:2146–2147. doi: 10.1002/mds.23810. [DOI] [PubMed] [Google Scholar]
- Oren I, Fleishman SJ, Kessel A, Ben-Tal N. Free diffusion of steroid hormones across biomembranes: a simplex search with implicit solvent model calculations. Biophys. J. 2004;87:768–779. doi: 10.1529/biophysj.103.035527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paba S, Frau R, Godar SC, Devoto P, Marrosu F, Bortolato M. Steroid 5α-reductase as a novel therapeutic target for schizophrenia and other neuropsychiatric disorders. Curr. Pharm. Des. 2011;17:151–167. doi: 10.2174/138161211795049589. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 1998. [Google Scholar]
- Perry W, Minassian A, Feifel D, Braff DL. Sensorimotor gating deficits in bipolar disorder patients with acute psychotic mania. Biol. Psychiatry. 2001;50:418–424. doi: 10.1016/s0006-3223(01)01184-2. [DOI] [PubMed] [Google Scholar]
- Petitclerc M, Bedard PJ, Di Paolo T. Progesterone releases dopamine in male and female rat striatum: a behavioral and microdialysis study. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1995;19:491–497. doi: 10.1016/0278-5846(95)00029-u. [DOI] [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Moore PH, Jr., Paul SM. Stress-induced elevations of gamma-aminobutyric acid type A receptoractive steroids in the rat brain. Proc. Natl. Acad. Sci. U.S.A. 1991;15:4553–4557. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam SK, Sato S, Hull EM. Effects of testosterone metabolites on copulation and medial preoptic dopamine release in castrated male rats. Horm. Behav. 2003;44:419–426. doi: 10.1016/j.yhbeh.2003.06.006. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Frye CA. Inhibiting progesterone metabolism in the hippocampus of rats in behavioral estrus decreases anxiolytic behaviors and enhances exploratory and antinociceptive behaviors. Cogn. Affect. Behav. Neurosci. 2001;1:287–296. doi: 10.3758/cabn.1.3.287. [DOI] [PubMed] [Google Scholar]
- Rougé-Pont F, Mayo W, Marinelli M, Gingras M, Le Moal M, Piazza PV. The neurosteroid allopregnanolone increases dopamine release and dopaminergic response to morphine in the rat nucleus accumbens. Eur. J. Neurosci. 2002;16:169–173. doi: 10.1046/j.1460-9568.2002.02084.x. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Holsboer F. Neuroactive steroids: mechanisms of action and neuropsychopharmacological perspectives. Trends Neurosci. 1999;22:410–416. doi: 10.1016/s0166-2236(99)01399-5. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Koch M, Montkowski A, Lancel M, Faulhaber J, Harting J, Spanagel R. Assessment of neuroleptic-like properties of progesterone. Psychopharmacology. 1999;143:29–38. doi: 10.1007/s002130050916. [DOI] [PubMed] [Google Scholar]
- Saalmann YB, Kirkcaldie MT, Waldron S, Calford MB. Cellular distribution of the GABA-A receptor-modulating 3alpha-hydroxy, 5alpha-reduced pregnane steroids in the adult rat brain. J. Neuroendocrinol. 2007;19:272–284. doi: 10.1111/j.1365-2826.2006.01527.x. [DOI] [PubMed] [Google Scholar]
- Santana N, Mengod G, Artigas F. Quantitative analysis of the expression of dopamine D1 and D2 receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cereb. Cortex. 2009;19:849–860. doi: 10.1093/cercor/bhn134. [DOI] [PubMed] [Google Scholar]
- Savageau MM, Beatty WW. Gonadectomy and sex differences in the behavioral responses to amphetamine and apomorphine of rats. Pharmacol. Biochem. Behav. 1981;14:17–21. doi: 10.1016/0091-3057(81)90097-6. [DOI] [PubMed] [Google Scholar]
- Shilliam CS, Heidbreder CA. Gradient of dopamine responsiveness to dopamine receptor agonists in subregions of the rat nucleus accumbens Eur. J. Pharmacol. 2003;477:113–122. doi: 10.1016/j.ejphar.2003.08.019. [DOI] [PubMed] [Google Scholar]
- Seeman MV. Gender differences in the prescribing of antipsychotic drugs. Am. J. Psychiatry. 2004;161:1324–1333. doi: 10.1176/appi.ajp.161.8.1324. [DOI] [PubMed] [Google Scholar]
- Stojanov W, Karayanidis F, Johnston P, Bailey A, Carr V, Schall U. Disrupted sensory gating in pathological gambling. Biol. Psychiatry. 2003;54:474–484. doi: 10.1016/s0006-3223(02)01745-6. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Lehmann-Masten V, Geyer MA. Schizophrenic-like sensorimotor gating abnormalities in rats following dopamine infusion into the nucleus accumbens. Psychopharmacology. 1990;101:414–420. doi: 10.1007/BF02244063. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Caine SB, Geyer MA. Regionally selective effects of intracerebral dopamine infusion on sensorimotor gating of the startle reflex in rats. Psychopharmacology. 1992;108:189–195. doi: 10.1007/BF02245306. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA. Clozapine and haloperidol in an animal model of sensorimotor gating deficits in schizophrenia. Pharmacol. Biochem. Behav. 1993;44:741–744. doi: 10.1016/0091-3057(93)90193-w. [DOI] [PubMed] [Google Scholar]
- Swerdlow NW, Lipska BK, Jaskin GE, Geyer MA. Increased sensitivity to the sensorimotor gating-disruptive effects of apomorphine after lesions of medial prefrontal cortex or ventral hippocampus in adult rats. Psychopharmacology. 1995;122:27–34. doi: 10.1007/BF02246438. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Martinez ZA, Hanlon FM, Platten A, Farid M, Auerbach P, Braff DL, Geyer MA. Toward understanding the biology of a complex phenotype: rat strain and substrain differences in the sensorimotor gating-disruptive effects of dopamine agonists. J. Neurosci. 2000a;20:4325–4336. doi: 10.1523/JNEUROSCI.20-11-04325.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Taaid N, Halim N, Randolph E, Kim YK, Auerbach P. Hippocampal lesions enhance startle gating-disruptive effects of apomorphine in rats: a parametric assessment. Neuroscience. 2000b;96:523–536. doi: 10.1016/s0306-4522(99)00528-x. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Platten A, Kim YK, Gaudet I, Shoemaker J, Pitcher L, Auerbach P. Sensitivity to the dopaminergic regulation of prepulse inhibition in rats: evidence for genetic, but not environmental determinants. Pharmacol. Biochem. Behav. 2001a;70:219–226. doi: 10.1016/s0091-3057(01)00598-6. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology. 2001b;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- Taherianfard M, Shariaty M. Evaluation of serum steroid hormones in schizophrenic patients. Indian J. Med. Sci. 2004;58:3–9. [PubMed] [Google Scholar]
- Tamminga CA. Gender and schizophrenia. J. Clin. Psychiatry. 1997;58:33–37. [PubMed] [Google Scholar]
- Tanner CM, Goldman SM. Epidemiology of Tourette syndrome. Neurol. Clin. 1997;15:395–402. doi: 10.1016/s0733-8619(05)70320-0. [DOI] [PubMed] [Google Scholar]
- Thigpen AE, Russell DW. Four-amino acid segment in steroid 5 alpha-reductase 1 confers sensitivity to finasteride, a competitive inhibitor. J. Biol. Chem. 1992;267:8577–8583. [PubMed] [Google Scholar]
- Thompson TL, Moss RL. Estrogen regulation of dopamine release in the nucleus accumbens: genomic- and nongenomic-mediated effects. J. Neurochem. 1994;62:1750–1756. doi: 10.1046/j.1471-4159.1994.62051750.x. [DOI] [PubMed] [Google Scholar]
- Turvin JC, Messer WS, Jr., Kritzer MF. On again, off again effects of gonadectomy on the acoustic startle reflex in adult male rats. Physiol. Behav. 2007;90:473–482. doi: 10.1016/j.physbeh.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Winkel R, Stefanis NC, Myin-Germeys I. Psychosocial stress and psychosis. A review of the neurobiological mechanisms and the evidence for gene-stress interaction. Schizophr. Bull. 2008;34:1095–1105. doi: 10.1093/schbul/sbn101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan FJ, Geyer MA, Swerdlow NR. Accumbens D2 modulation of sensorimotor gating in rats: assessing anatomical localization. Pharmacol. Biochem. Behav. 1994;49:155–163. doi: 10.1016/0091-3057(94)90470-7. [DOI] [PubMed] [Google Scholar]
- Wan FJ, Taaid N, Swerdlow NR. Do D1/D2 interactions regulate prepulse inhibition in rats? Neuropsychopharmacology. 1996;14:265–274. doi: 10.1016/0893-133X(95)00133-X. [DOI] [PubMed] [Google Scholar]
- Wong J, Shoemaker JM, Platten A, Martinez ZA, Pitcher L, Hakimzada F, Auerbach P, Swerdlow NR. Atypical antipsychotic effects on startle gating in adult rats: lack of sex difference and impact of prepubertal castration. Abstr. Biol. Psychiatry. 2002;51:120S. [Google Scholar]
- Young KA, Randall PK, Wilcox RE. Dose and time response analysis of apomorphine’s effect on prepulse inhibition of acoustic startle. Behav. Brain Res. 1991;42:43–48. doi: 10.1016/s0166-4328(05)80038-5. [DOI] [PubMed] [Google Scholar]