Abstract
In animal and plant cells, a wide range of key cellular processes that require the establishment of cell polarity are governed by Rho-GTPases. In contrast to animals and yeast, however, plants possess a single Rho-GTPase subfamily called ROP (Rho-like GTPases from plants). This raises the question of how plants achieve the high level of regulation required for polar cellular processes. It is becoming evident that plants have evolved specific regulators, including ROP-Guanine Exchange Factors (GEFs) and the Rop-interactive CRIB motif–containing proteins (RIC) effectors. Recent research shows that the spatiotemporal dynamics of ROPs, the cytoskeleton, endocytosis and exocytosis are intertwined. This review focuses on the proposed self-organizing nature of ROPs in plants and how ROP-mediated cellular mechanisms compare with those responsible for cell polarity in animals and yeast.
The ROP monopoly
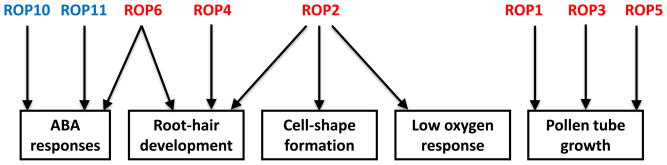
Rho GTPases are switch-like proteins that cycle between the inactive and cytosolic GDP-bound state and the active membrane-bound GTP-state. The Rho family of small GTPases is conserved in all eukaryotic cells and regulates many fundamental cellular processes, including cell division, polarization, morphogenesis, and directionality. Most of these processes require the formation of cell polarity, which is typically associated with the cytoskeleton and vesicular trafficking. Due to the plethora of Rho-mediated processes, animals and fungi have evolved Rho-GTPase subfamilies such as Cdc42, Rac, and Rho [1, 2]. In contrast, plants possess only a single unique subfamily of closely related members known as ROPs (Rho-like GTPases from plants) [3, 4], which are sometimes called RACs due to the protein sequence similarities with human Rac GTPases [5]. Within the ROP family the individual members may have different functions. Some of these functions will be overlapping with other ROPs within the same family making some group members functionally redundant. However, some of the ROPs within a family will have specific nonoverlapping functions (Figure 1).
FIGURE 1. The functions of ROPs.
The ROP GTPase family members have numerous functions. The Arabidopsis genome encodes for 11 ROPs organized into four phylogenetic groups: group I (ROP8), group II (ROP9–ROP11, shown in blue text), group III (ROP7) and group IV (ROP1–ROP6, shown in red text). Evidence suggests that different groups have distinct functions. The function of an individual ROP can be redundant or overlapping, distinct and/or multiple.
The existence of a single plant subfamily of Rho-GTPases poses key questions. How do plants use only a limited number of ROPs to regulate a broad array of cytoskeleton- and vesicular trafficking-based processes? Studies of Rho-based signaling in plants lag behind those in animals and yeast, but there have been important advances in recent years that have at least partially elucidated how plants regulate so many fundamental cellular processes with only a few ROPs. At the same time, studying ROPs has introduced new paradigms for regulatory mechanisms underlying fundamental cellular processes, which may be applicable to animals and fungi. The knowledge accrued in recent years has offered important insights into both conserved and plant-specific cellular processes.
Here, we review current knowledge on the functional diversification of ROPs, ROP signaling at the plasma membrane and the spatial regulation of ROPs, ROP activation by RopGEFs and discuss the possibility that ROP may act as a self-organizing system.
Functional diversification of ROP members
In plants, members of the ROP subfamily have diverged to regulate distinct cellular functions, particularly in higher plants [6, 7]. The genome of the moss Physcomitrella patens, an ancestral plant, encodes four nearly identical ROPs [8], which are most likely redundant, whilst the higher plant Arabidopsis thaliana genome encodes for 11 ROPs (Figure 1). A nuclear function for Rho GTPases has been reported in mammalian cells, where for example Cdc42 localizes to the nucleus to regulate epigenetic centromere maintenance [9]. Examples of nuclear function for ROPs in plants include the tobacco ROPs implicated in regulating the formation of nuclear bodies containing 26S proteasome, which in turn participates in the auxin- dependent induction of gene expression.
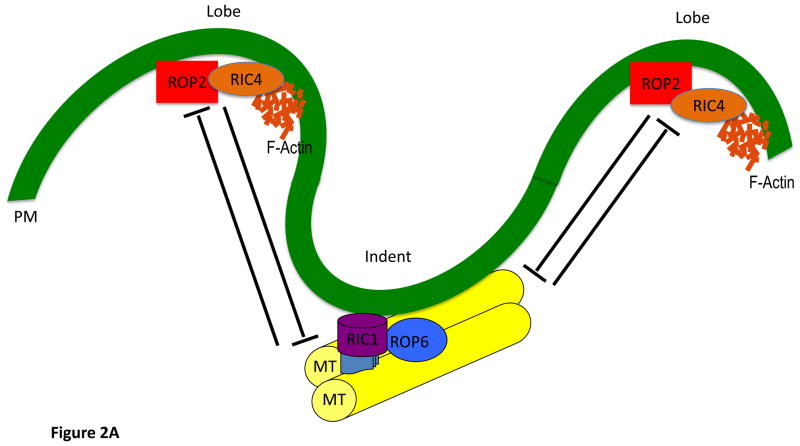
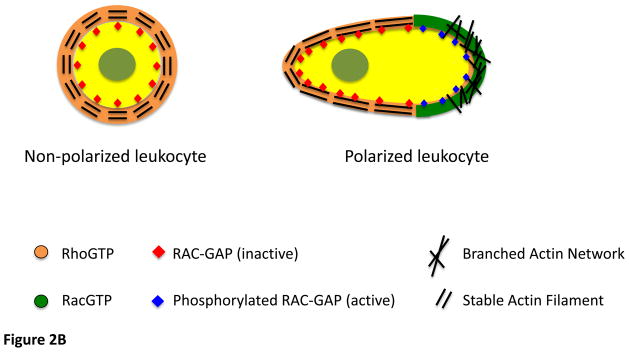
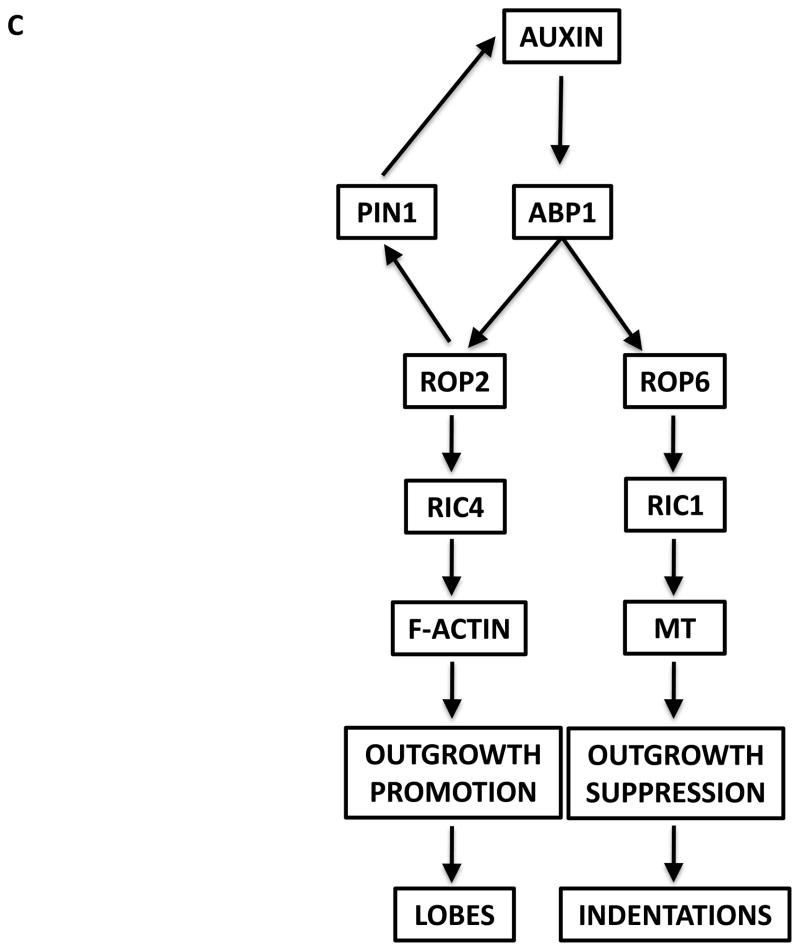
ROP1 through ROP6 retain the conserved Rho-GTPase function of spatially controlling a variety of cellular processes by regulating the cytoskeleton and vesicular trafficking. ROP1, ROP3 and ROP5 may act redundantly in the modulation of tip growth in Arabidopsis pollen tubes [10–12]. Polar cell growth and cell polarity in vegetative cells have also been shown to be modulated by ROP2, ROP4, and ROP6. ROP2 and ROP4 modulate tip growth in root hairs [13–17]. Surprisingly, ROP6 is highly similar in sequence to ROP2 (94%) but is functionally distinct from both ROP2 and ROP4. During the generation of the jigsaw-shaped Arabidopsis leaf pavement cells, ROP2 and ROP4 have overlapping functions in promoting lobe formation via the actin cytoskeleton, whereas ROP6 promotes indentation associated with cortical microtubules arrangements (Figure 2A). Interestingly, these ROP2, ROP4-and ROP6-dependent pathways are antagonistic to mutually exclude each other for the generation of lobe and indentation domains. This mechanism is analogous to the mammalian Rac and Rho regulation of front and back polarization and consequent protrusion observed in leukocytes (Figure 2B). Here, the polarized distribution of active Rho has been reported to restrict Rac-dependent protrusion to the cell front [18, 19].
FIGURE 2. Comparison of ROP2-ROP6 antagonism in plants with that of Rac-Rho in mammals.
2 A
The control of interdigitating growth in PCs.
In Arabidopsis leaf PCs, interdigitating growth is controlled by a ROP GTPase signaling network with two pathways with opposing actions on localized cell expansion: the ROP2-RIC4 pathway promotes lobe outgrowth via a fine cortical MF network, while the ROP6-RIC1 pathway suppresses outgrowth via well-organized cortical MTs.
During the initiation of lobes and indentations, these two pathways are locally activated and spatially separated. Counter signaling establishes borders that separate outgrowth-promoting from outgrowth-suppressing domains of the cell cortex.
2 B
A model indicating how RAC GAP and active Rho may restrict cell protrusion to the front in leukocytes.
High Rho activity at the rear and sides causes the activation of Rac GAP with a subsequent depression of Rac in this region.
ROPs: signaling at the plasma membrane and spatial regulation
ROP GTPase signaling is functionally conserved between plants and animals in terms of its regulation of cell polarity, polar growth, and morphogenesis via the targeting of the cytoskeleton and vesicular trafficking. However, homologs of the effector proteins of Rho-family GTPases discovered in yeast and animals are either absent from plants or do not appear to be ROP effectors [20, 21]. Instead, two families of novel proteins, RICs and ICR1/RIP1 (interactor of constitutively active ROP1 and ROP Interactive Partner 1), function as ROP effectors [22–24]. These plant-specific ROP effector proteins are linked to the regulation of polar processes via cytoskeletal dynamics and reorganization as well as membrane trafficking [25–31].
The pollen tube tip region is composed of four defined regions: an apical zone that contains exocytic vesicles that accumulate in a V-shape to facilitate tip targeted exocytosis, a sub-apical organelle rich zone, a nuclear zone, and a vacuolated zone that can extend toward the grain [32].
Actin microfilaments
In Arabidopsis pollen tubes, evidence suggests that RIC4 and RIC3 act as ROP1 effectors to promote apical F-actin dynamics. RIC4 promotes the polymerization or stabilization of apical F-actin via an unknown mechanism, whereas RIC3 promotes actin depolymerization via calcium (Figure 5A) [33, 34]. Thus a single ROP GTPase coordinates two antagonistic downstream pathways that control tightly regulated actin dynamics that are essential for polarized tip growth in pollen tubes. Such tight regulation of actin dynamics is a common feature of numerous cellular processes in eukaryotic cells including fungal hyphal growth and neuronal cell growth. It is of key importance to identify whether the same processes regulating hyphal growth and neuronal growth are operating in plants.
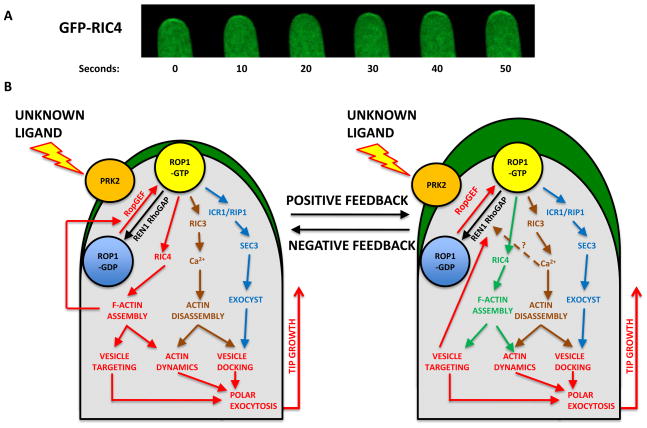
Figure 5. Self-organizing ROP1 signaling controls pollen tube tip growth.
5 A
A time course of the dynamic apical cap of active ROP1 visualized using ΔRIC4-GFP.
To understand the spatial regulation of ROP1, a GFP reporter was developed (ΔRIC4-GFP) in which the CRIB domain of the RIC4, which is a ROP1 effector, is fused to GFP. ΔRIC4-GFP localizes to the PM in the apical cap region in a manner reminiscent of active ROP1 [33]. ΔRIC4-GFP thus enables the distribution and oscillation of active ROP1 to be monitored.
5 B
The ROP1 signaling network controls pollen tube tip growth.
This network is made up of different pathways that coordinate and promote tip-targeted exocytosis and positive and negative feedback loops. It is likely that these balance each other so that an apical ROP1 cap of a certain size is maintained. This cap defines the tip growth domain and may allow oscillation of ROP1 activity. ROP1 is locally activated in the PM to determine the site of exocytosis and this activates multiple pathways leading to polar exocytosis. The RIC4 pathway promotes F-actin assembly and induces the accumulation of exocytic vesicles to the tip. It also promotes positive feedback loops to increase the area of active ROP1 possibly through the targeting of ROP1 upstream components including RopGEFs and RLKs. Positive feedback and diffusion quickly generate the apical cap of active ROP1 and this is the defining feature of the tip growth domain.
ROP1 also activates the RIC3–calcium pathway. RIC3-dependent Ca2+ promotes tip F-actin disassembly and facilitates exocytosis. Polar exocytosis is also promoted by another likely ROP1 effector, RIP1/ICR1, which subsequently interacts with the SEC3 exocyst subunit that mediates the tethering of exocytic vesicles on the PM. Polarized exocytosis causes the migration of the RhoGAP REN1 to the apical PM, which deactivates PM-localized active ROP1. Thus, REN1-based negative-feedback globally inhibits ROP1, prevents excess ROP1 activation in the apical PM, and restricts enlargement of the apical cap to the tip growth domain. The red arrows indicate the key events involved in tip growth. The key pathways of the signaling network have been assigned different colored arrows for clarity. The broken arrow indicates a supposed event.
Cortical microtubules
Cortical microtubules (MT), including interphase arrays and preprophase bands, are unique to plant cells and are critical for cell morphogenesis, anisotropic cell growth, and positioning of cell division plates [35–37]. These cortical MTs uniquely generate branches through γ-tubulin complex-dependent nucleation and undergo rapid dynamics by sustained treadmilling [38, 39]. The function of these cortical MTs depends on their dynamics and specific arrangements, which are regulated by developmental, hormonal and environmental cues [25, 29, 40]. The study of ROP effectors has led to the initial understandings of the signaling mechanisms underlying the organization of cortical MTs. RIC1, a microtubule-associated protein (MAP), acts as a ROP6 effector that promotes the organization of transversely orientated cortical MTs [25, 41]. Only upon activation by ROP6 does RIC1 become associated with cortical MTs, visualized as punctate structures by laser scanning confocal microscopy [41]. This is particularly evident at branch points and leads to the reorganization of branching MTs in a parallel manner. Parallel MTs are found in the indenting regions of leaf pavement cells and promote the formation of indents [41]. In other plant cell types, parallel MTs are typically orientated perpendicular to the axis of cell elongation to promote anisotropic cell growth [25]. Interestingly, the ROP6-RIC1 pathway is activated by auxin through the putative auxin receptor, auxin binding protein 1 (ABP1) [42]. This ROP6-RIC1 pathway represents the only well-characterized signaling pathway that leads to the organization of cortical MTs and MT-mediated cell morphogenesis in plants.
Polar exocytosis
Polar exocytosis is a conserved target of Rho GTPase signaling in fungi, animals and plants. It is critical for the establishment and/or maintenance of cell polarity, polar growth and cell morphogenesis [43–47]. One of the best-characterized signaling mechanisms underlying this fundamental process is the yeast exocyst subunit SEC3, a direct effector of the Rho GTPase Cdc42 [48, 49]. In plants, polar exocytosis enables tip growth in root hairs and pollen tubes through the provision of membrane and cell wall materials. Loss of function mutant studies showed that the absence of exocyst subunits that control the polar docking of vesicles to the PM, SEC3 and SEC8, leads to the termination of tip growth [50].
In plants, the ROP effector ICR1/RIP1 interacts with the Arabidopsis homolog of SEC3 and is required for polar localization of the auxin efflux carrier PIN1and auxin-dependent processes such as maintenance of root apical meristem [51]. PIN1 polarization is thought to involve both polar inhibition of endocytosis (see below) and polar recycling of the endosomal PIN1 protein [52, 53]. Interestingly ICR1 is specifically involved in the promotion of PIN1 recycling but not in the inhibition of endocytosis [51]. These findings suggest that ICR1 may function as an adaptor protein linking activated ROPs to the exocyst complex subunit SEC3 [24]. It is this polar recruitment of the exocyst that activates exocytosis of PIN1 recycling vesicles. Although further studies are needed to test this hypothesis, Rho effector proteins acting as a linker between activated Rho GTPases and the exocyst may be a common mechanism for Rho regulation of exocytosis in both plants and animals [54].
Polar exocytosis in pollen tubes is regulated by ROP1 signaling via two downstream antagonistic pathways. The ROP1 effector RIC4 promotes assembly of apical F-actin, which then allows the apical accumulation of exocytic vesicles, but prevents fusion of these vesicles to the PM. The disassembly of apical F-actin, which is induced by the signaling pathway activated by RIC3, the ROP1 effector, is a prerequisite for the fusion of apical exocytic vesicles. High levels of apical F-actin are thought to block docking to the PM. In addition, ICR1/RIP1 localizes to the apical cortex [23], and probably plays an active role in the docking of apical exocytic vesicles to the PM.
Polar inhibition of clathrin-dependent endocytosis
Endocytosis has emerged as another important target of Rho-GTPase signaling to regulate cell polarity in yeast, mammalian cells, and animal model systems [55]. Rho GTPase-mediated promotion of endocytosis is commonly linked to cell polarization [56]. Interestingly, Cdc42/Par6-mediated asymmetric inhibition of clathrin-dependent endocytosis is sufficient for the generation of apical polarity of cultured epithelial cells from rat and neuroectodermal epithelial Drosophila cells [56–58].
A very recent study shows that this mechanism is employed not only by animal cells but also by plant cells [59]. It was recently reported that interfering with ROP function leads to the inhibition of PIN1 endocytosis [59]. The phytohormone auxin is known to promote the formation of interdigitated cell patterning in pavement cells in a spatiotemporally regulated manner. High auxin levels correspond to the formation of lobes [42]. ROP regulation of PIN1 polarity [60] therefore appears to be a mechanism linked to auxin, introducing the question of whether auxin-ROP signaling is a common mechanism for the generation of cell polarity in plants [59].
The polarization of the PIN1 auxin efflux carrier to the pavement cell lobe is critical to the formation of the puzzle-piece shape typical of this cell type. Clathrin-dependent PIN1 endocytosis is inhibited in the lobe region where the ROP2-RIC4 pathway is activated to promote lobe formation and expansion [41](Figure 3A). The localized inhibition of PIN1 endocytosis can at least partially explain how the ROP2 pathway promotes PIN1 polarization [61], because PIN1 endocytosis is observed in the indenting region, but not the lobe region. The ROP2-dependent localized inhibition of PIN1 endocytosis is similar to Cdc42 and PAR6 in yeast and C. elegans (Figure 3B) [56]. The effects of Cdc42 in animals and ROP2/4 in PCs appear to be similar. The Rho-GTPase inhibition of endocytosis leads to polarized patterning in both the animal and plant kingdom.
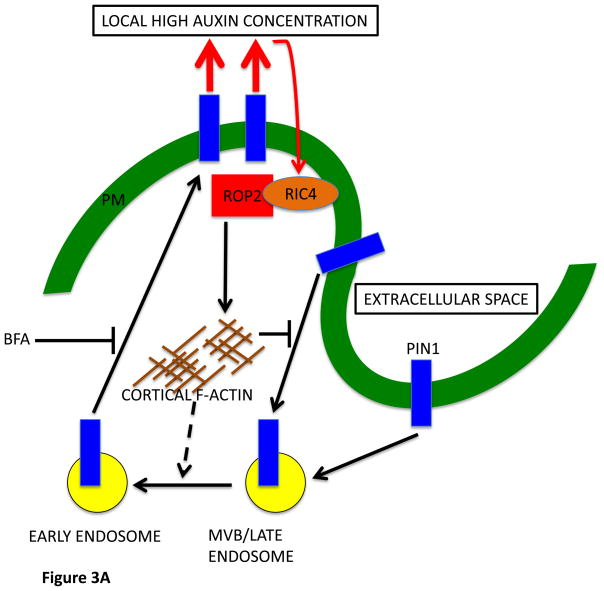
FIGURE 3. ROP2/RIC4 inhibition of PIN1 endocytosis is analogous to Cdc42/Par inhibition of endocytosis in Drosophila.
3 A
A model for PIN1 polarization to the lobe region of PCs via a ROP signaling-based feed-forward mechanism.
ROP2 in the lobing region is activated by extracellular auxin, and the activated ROP2-RIC4 pathway leads to inhibition of PIN1 internalization through RIC4-dependent cortical F-actin, causing PIN1 polarization to the lobing region. The PIN1-based export of auxin causes additional ROP2 activation to complete this feed-forward cycle. Recently published data implies a role for the activated ROP2-RIC4 pathway in the promotion of endosomal trafficking from Ara7-marked endosomes to recycling endosomes [41]. Ara7 is a Rab5 homolog and resides in an endosomal compartment from which many internalized proteins, including PIN1, are sorted for targeting to the vacuole or recycling to the PM.
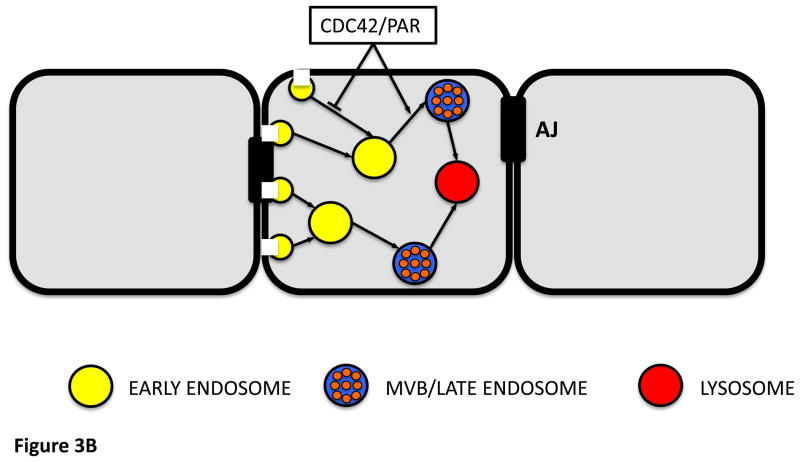
3 B
A model for the regulatory function of the Cdc42-Par complex in the apical endocytic pathway.
Recent data [97] indicate that Cdc42 and the Par complex cooperate in regulating two distinct steps in the endocytosis of apical membrane components. Endocytosis from the plasma membrane is inhibited by the Cdc42–Par complex, which in turn promotes the progression of early endosomes (EE) to the multivesicular bodies/late endosomes (MVBs/LEs). Apical and basolateral proteins, including adherens junction (AJ) proteins, follow distinct endocytotic routes.
RIC4-mediated accumulation of fine cortical actin is crucial for the ROP2/RIC4-dependent inhibition of PIN1 endocytosis [61]. This may seem surprising, because evidence indicates that Cdc42/Arp2/3-dependent F-actin patches are required for the early step of clathrin-dependent endocytosis by providing force for membrane invagination and scission in yeast and animals cells [62]. However, the dynamics of F-actin (e.g., depolymerization of filaments) are also necessary for clathrin-dependent endocytosis [62–64]. If this is also the case in pavement cells, RIC4-mediated actin stabilization could explain why RIC4-dependent actin inhibits PIN1 endocytosis. Cdc42/Rac-mediated inhibition of endocytosis is also associated with its activation of actin filaments [57]. Nevertheless, it will be interesting to determine the mechanism by which Rho-dependent F-actin inhibits endocytosis and whether the Rho-actin signaling module provides a common mechanism for localized inhibition of endocytosis and its associated polarity formation.
Novel posttranslational modifications
The precise regulation of ROP signaling activities is necessitated by the variety of responses in plants that are ROP-mediated. As in other Rho family GTPases from other species, ROP activities are regulated by GEFs, GTPase-activiting proteins (GAPs) and guanine GDP-dissociation inhibitors (GDIs), [65–70]. Isoprenylation of ROPs is required for their membrane association and activation [10, 71]. In addition, recent studies have uncovered novel posttranslational modifications that play a critical role in ROP regulation.
A recent publication found that a particular serine residue conserved in all plant ROPs can be phosphorylated. Lack of phosphorylation prevents the binding of the upstream activator, the plant-specific ROP nucleotide exchanger (PRONE)-domain-containing RopGEF (guanine nucleotide exchange factor) protein [72]. This mutation in Medicago ROP6 and Arabidopsis ROP4 precluded binding to the PRONE-domain-containing RopGEF protein (see below), which prevented phosphorylation in vitro [72]. Structural evidence supports the theory that phosphorylation of S74 restricts PRONE-domain binding to the adjacent conserved arginine residue (R76), which is thought to be part of the recognition site (S74YR) for serine/threonine kinases in ROPs [20, 73].
Posttranslational lipid modifications of ROP proteins that affect their membrane attachment are thought to be important in regulating their activity. Prenylation and S-acylation of ROPs affect their subcellular distribution, membrane interaction dynamics and function. ROPs fall into two groups in terms of the types of lipid modifications they receive on the hyper-variable C-terminal regions: type I ROPs are prenylated by geranylgeranyltransferase, and this is necessary for membrane attachment, whereas type II ROPs undergo S-acylation [74–76]. Recent work suggests that S-acylation occurs in the G-domain of activated ROPs and is important for their association with lipid rafts [77, 78].
The RopGEF family
GEFs catalyze the exchange of GDP for GTP. Two types of RhoGEFs are well characterized in various animal systems: DH-type with a dbl homology (DH) domain as the catalytic domain, and DHR2 type with dock-homology region 2 (DHR2) domain as the catalytic domain [79, 80]. Both types of RhoGEFs are apparently present in plants [67, 81], but plant ROPs seem to predominately use plant-specific GEFs, RopGEFs, for their activation [65, 66]. RopGEFs have a conserved plant-specific ROP nucleotide exchanger (PRONE) domain for GEF catalytic activity but the N terminal and C terminal regions are variable.
The Arabidopsis genome encodes 14 RopGEFs. Overexpression analysis of RopGEFs in tobacco and Arabidopsis pollen tubes indicates that a specific subset are involved in the regulation of tip growth [66, 82, 83]. However, their biological roles in development and growth are not well understood.
Recent work on the mechanism of pollen tube growth and root hair development suggests a role for RopGEFs in receptor-like kinase (RLK)-mediated signaling. More recently, a yeast two-hybrid screen identified members of the Catharanthus roseus RLK-related family as upstream regulators of RopGEFs, [84, 85]. Loss of the CrRLK, FERONIA, leads to reduced levels of active RAC/ROPs and severe root hair defects [16]. The Arabidopsis genome encodes more than 400 RLKs. This abundance is probably due to a lack of RTKs and scarcity of GPCRs in plants and the need to transmit specific signals to specific ROP GEFs. It is tempting to speculate that RLK-mediated signaling could be a common mechanism linking cell surface signaling to ROP signaling (Figure 4). A recent study in maize has demonstrated the key role that ROPs have in the polarization of plant cell division and cell growth [86]. Importantly it is also established that the correct localization of ROP requires the RLK protein PANGLOSS1 (PAN1), which in turn supports the conclusion that a ROP-RLK interaction is important during cell polarity formation in plants [86].
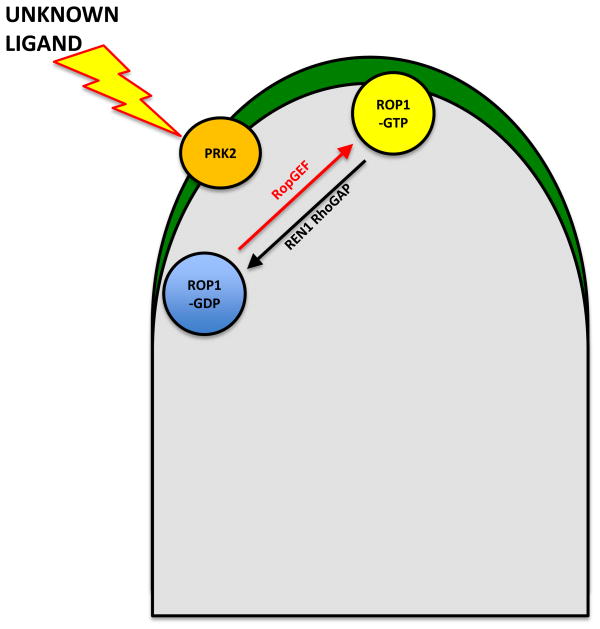
FIGURE 4. The interaction of a receptor like kinase (RLK) with a RopGEF in pollen tubes.
A pollen-expressed RLK interacts directly with RopGEFs to control polar tip growth. The localization of active ROP1 to the apical PM region indicates the future direction of pollen tube turning. In addition, it is highly probable that guidance signal-sensing RLKs interact with RopGEFs to activate ROP1 signaling. In response to these guidance signals there is a consequent modulation of the spatial distribution of ROP1 signaling and directing pollen tube growth.
Recent work also demonstrates that RNAi knockdown of RopGEF7 expression leads to progeny with defective root meristems, as RopGEF7 regulates the levels of PLETHORA1 and PLETHORA2, transcription factors that regulate patterning of the root stem cell niche [87]. RopGEF7 also interacts with ROP1 and overexpression of C-terminally truncated constitutively active RopGEF7 activates ROP GTPases [87].
ROP-based self-organizing systems
Self-organizing ROP GTPase signalling networks have emerged over the last decade as a common theme underlying polarized cell expansion in plants. The networks are composed of multiple coordinated pathways and feedback loops, and provide a robust molecular linkage between the cytoskeleton, vesicular trafficking and polarity formation [33, 34, 55, 65, 69, 88, 89]. Here, we discuss examples of ROP-based self-organizing centres in pollen tubes and pavement cells (PCs) (Figure 5A and Figure 5B). Pollen tubes are a single cell system where cell polarity regulation is more similar to the apical-basal polarity in yeast and other fungal systems. In contrast, cell polarization in leaf PCs involves cell-cell coordination in tissues, a system more similar to planar morphogenesis in animals. A comparison between these systems may provide insights into common themes and specific requirements for self-organization design principles in unicellular and multicellular systems.
Unicellular
ROP1 is a key regulator of pollen tip growth [10, 11, 33] and is activated in the apical cap, which defines the apical region where exocytosis takes place and drives tip growth [10, 12, 32, 33, 71, 88]. Evidence suggests that apical ROP1 signaling is regulated by a self-organizing center. Active ROP1 oscillates, peaking just prior to peak growth and then falling to a minimum followed by growth rate decreases [33]. The apical ROP1 cap is formed through the positive feedback-based lateral propagation of localized ROP1 activity through ROP1-dependent actin assembly and is maintained by negative feedback through alterations in Ca2+ levels [33, 69, 88, 90, 91]. The RIC4-mediated increase in apical F-actin is thought to induce the lateral propagation. RIC4 overexpression-induced stabilization of F-actin leads to an enlargement of the cap area where ROP1 is active and results in ballooned pollen tube tips [33, 69, 90, 91] (Figure 5B). The mechanism by which apical F-actin activates ROP1 is not understood, but actin-dependent exocytosis is expected to target PM-localized or extracellular upstream activators of ROP1, such as PRK2. An increase in Ca2+ levels or actin depolymerization reduces apical ROP1 activity [11, 90, 91].
REN1, a RhoGAP that was identified from an elegant forward-genetic screen for depolarized pollen tube growth, is a global inhibitor of ROP1 [69]. A global inhibitor displays an apical or uniform localization in contrast to a lateral inhibitor, which would be localized and active in the subapical PM region of pollen tubes. REN1 accumulates in exocytic vesicles and at the site of ROP1 activation, which is the apical PM. REN1 restricts active ROP1 to the apical cap in Arabidopsis pollen tubes [69]; this is in contrast to RhoGAP-mediated spatial restriction of Rho GTPase activation in several animal systems, in which RhoGAPs act as lateral inhibitors [18, 92–95]. The REN1-based global inhibition may provide a mechanism for efficient and effective negative feedback, which may be particularly important for cells undergoing rapid tip growth such as pollen tubes and fungi [32].
The increase in cytosolic Ca2+ and the tip-localized assembly of F-actin, both of which are regulated by ROP1, target REN1-containing exocytic vesicles to the tip of pollen tubes [34, 91]. An initial Ca2+ increase may induce actin disassembly and exocytic vesicle fusion to the apical PM [34, 91]. PM-localized REN1 may not be active until a Ca2+ threshold level is surpassed, which may then trigger REN1 activation on the PM. This hypothesis fits with previous observations that surpassing a cytosolic Ca2+ threshold inhibits pollen tube growth and that Ca2+ levels below this threshold stimulate growth [10, 91].
Multicellular
In contrast to unicellular pollen tubes where ROP-based self-organizing signaling does not require a signal or factor from another cell, self-organizing centers in multicellular systems, such as the interdigitation of PCs in the leaf epidermis, involve cell-to-cell signaling. PCs coordinately develop multi-polarities to define interdigitated regions for the formation of complementary lobes and indents (Figure 6A and 6B). Lobes in one cell expand into indents in neighboring cells, akin to puzzle pieces. The ROP-based signaling network that controls PC interdigitation centers on the generation of a self-organizing signal that acts both as a self-cell signal and cross-cell signal (Figure 6C and 6D).
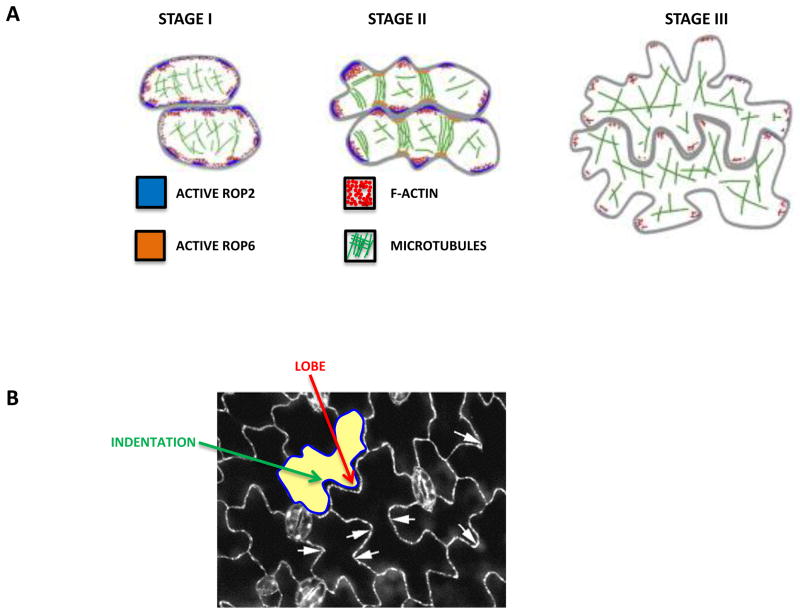
Figure 6. A self-organizing ROP signaling network controls cellular interdigitation.
6 A
The formation of the jigsaw puzzle appearance of Arabidopsis leaf PCs
The development of PCs is separated into three stages and is associated with cortical F-actin and MT.
Stage I: An almost square PC elongates slightly and forms nearly rectangular cells.
Stage II: The PC produces alternating small bumps and indentations, generating cells with multiple shallow lobes and indentations.
Stage III: Reiterative outgrowing and indenting continues, which produces highly lobed interlocking PCs that often contain secondary lobes.
FIGURE 6B
Arabidopsis PC with both lobes and indentations.
The additional white arrows highlight lobes.
6 C and D
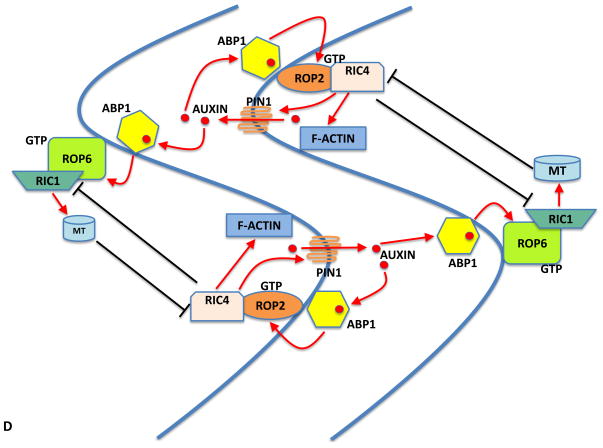
Model for Auxin Control of Interdigitated Cell Growth
(C) Schematic for the coordination of two ROP signaling pathways triggered by localized extracellular auxin, which is due to PIN1-mediated positive feedback loop. (D) Model for auxin control of interdigitated growth through both inter- and intracellular coordination of the ROP2 and ROP6 pathways. It is thought that PC interdigitated growth is organized through an auxin-dependent self-organizing mechanism.
Localized extracellular auxin is generated by self-activation via the auxin→ROP2→PIN1→auxin feedback loop. The self-maintenance of lobing and indentations occurs via ROP2 andROP6 pathways in two adjacent cells. These pathways are spatially segregated within each cell, which allow for the formation of alternating lobes and indentations.
Auxin is proposed to be a self-organizing signal. The formation of lobes and indentations is compromised in leaves that are deficient in auxin biosynthesis [42]. Recent work demonstrated that the detection of auxin by ABP1 leads to the activation of both ROP2 and ROP6 pathways [42]. These pathways are thought to be activated at opposite sides of the cell wall in a mutually exclusive manner (Figure 6C and 6D) [25, 29, 41]. Therefore when the cell is at steady state the activation of ROP2 and ROP6 must be at opposing sides of the cell wall but not at exactly the same location to form indents and lobes. PIN1-exported auxin is also proposed to act as a cross-cell signal to activate ROP6. The downstream target of ROP6 is RIC1, which suppresses outgrowth and creates indentations through its effects on MTs. The mechanism employed by pollen tubes and PCs to define regions for out-growth is similar. In pollen tubes, ROP1 establishes the tip-growth region via RIC4-mediated F-actin assembly and the same RIC4-mediated F-actin pathway is utilized by ROP2 for determining the lobe region in PCs. However, pollen tubes and PCs employ different mechanisms for limiting the growth regions. Increases in cytosolic Ca2+ levels caused by RIC3 in pollen tubes, which may act through the global inhibitor REN1, act globally to limit the lateral propagation of the apical active ROP1. In contrast, RIC1-mediated microtubules suppress the activation of ROP2, limiting ROP2 activity to the lobe regions in PCs.
These findings demonstrate the positive feedback effect of ROPs in pollen tube tip growth and lobe development. In both cases, typifying the self-organizing nature of these systems, negative feedback operates to restrict the positive feedback amplification of ROP activation.
Concluding remarks
Studies of ROPs/Racs and their plant-specific interactors have uncovered new mechanisms and principles for Rho GTPase signaling, including ROP activation of two opposing pathways for regulation of actin dynamics, regulation of ROP activity by novel protein modifications, spatial restriction of ROP activity by global inhibitor-based negative feedback, ROP-based generation of self-organizing signals that coordinate self activation and cross-cell regulation. Given the conservation of Rho GTPases and the fundamental processes that they regulate, these new mechanisms and principles observed in plants may eventually be identified in non-plant systems and other subfamilies of Rho GTPases.
Our current understanding of ROP signaling probably represents the tip of iceberg. To date, only a few ROPs and several dozens of ROP interactors have been functionally characterized, and further research in this area is expected to produce insights into many other signaling pathways and processes in plants. A major challenge is the complex functionality of these ROP signaling proteins. These include members of a gene family that can be functionally redundant and at the same time can regulate multiple processes, explaining why forward-genetic approaches have identified few ROP signaling proteins and making it difficult to use reverse-genetic approaches to investigate ROP signaling. Cell- or tissue-specific down-regulation of functionally redundant gene members may provide a way to expand our understanding of ROP signaling.
The self-organizing ROP signaling frameworks that involve counteracting but coordinating pathways shed important light into the mechanisms underlying polarized tip growth in single cells and interdigitated cell growth in multi-cellular tissues. In addition, it remains to be established if ER-associated ROP signaling is functionally linked to ROP-dependent cell polarization and morphogenesis. A possible linkage between polarity signaling and Cdc42-mediated Golgi mobility/trafficking has been proposed. These frameworks lay a ground for elucidating detailed and quantitative mechanisms for polar cell growth in plants and may also provide a guiding light for illuminating other polarity systems in plants such as zygote polarization and PIN protein polarization.
Of significant interest in both plants and animals is the nucleotide-binding domain and leucine-rich repeat-containing (NLR) protein family, which induce immune responses upon being triggered by pathogen-derived molecules. Recently, it has been shown that a rice NLR protein that confers resistance to the rice blast fungus Pit, directly interacts with the OsRac1 ROP at the PM in rice [96].
The participation in such diverse and numerous cellular processes by ROPs make these master regulators of great significance to answer fundamental biological questions.
Acknowledgments
We thank the past and present members of the Yang laboratory for stimulating discussions and the generation of data that made this review possible. The work is supported by funding from NIH-NIGMS (R01GM081451 and R01GM100130) and DOE (DE-FG02-04ER15555) to ZY.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chant J, Stowers L. GTPase cascades choreographing cellular behavior: movement, morphogenesis, and more. Cell. 1995;81:1–4. doi: 10.1016/0092-8674(95)90363-1. [DOI] [PubMed] [Google Scholar]
- 2.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 3.Yang Z, Watson JC. Molecular cloning and characterization of rho, a ras-related small GTP-binding protein from the garden pea. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:8732–8736. doi: 10.1073/pnas.90.18.8732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li H, et al. Arabidopsis Rho-related GTPases: differential gene expression in pollen and polar localization in fission yeast. Plant physiology. 1998;118:407–417. doi: 10.1104/pp.118.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winge P, et al. Cloning and characterization of rac-like cDNAs from Arabidopsis thaliana. Plant molecular biology. 1997;35:483–495. doi: 10.1023/a:1005804508902. [DOI] [PubMed] [Google Scholar]
- 6.Vernoud V, et al. Analysis of the small GTPase gene superfamily of Arabidopsis. Plant physiology. 2003;131:1191–1208. doi: 10.1104/pp.013052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang Z. Small GTPases: versatile signaling switches in plants. The Plant cell. 2002;14(Suppl):S375–388. doi: 10.1105/tpc.001065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eklund DM, et al. Physcomitrella patens: a model to investigate the role of RAC/ROP GTPase signalling in tip growth. Journal of experimental botany. 2010;61:1917–1937. doi: 10.1093/jxb/erq080. [DOI] [PubMed] [Google Scholar]
- 9.Lagana A, et al. A small GTPase molecular switch regulates epigenetic centromere maintenance by stabilizing newly incorporated CENP-A. Nature cell biology. 2010;12:1186–1193. doi: 10.1038/ncb2129. [DOI] [PubMed] [Google Scholar]
- 10.Li H, et al. Control of pollen tube tip growth by a Rop GTPase-dependent pathway that leads to tip-localized calcium influx. The Plant cell. 1999;11:1731–1742. doi: 10.1105/tpc.11.9.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kost B, et al. Rac homologues and compartmentalized phosphatidylinositol 4, 5-bisphosphate act in a common pathway to regulate polar pollen tube growth. The Journal of cell biology. 1999;145:317–330. doi: 10.1083/jcb.145.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu Y, et al. ROP GTPase regulation of pollen tube growth through the dynamics of tip-localized F-actin. Journal of experimental botany. 2003;54:93–101. doi: 10.1093/jxb/erg035. [DOI] [PubMed] [Google Scholar]
- 13.Yang G, et al. A mutation in MRH2 kinesin enhances the root hair tip growth defect caused by constitutively activated ROP2 small GTPase in Arabidopsis. PloS one. 2007;2:e1074. doi: 10.1371/journal.pone.0001074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Molendijk AJ, et al. Arabidopsis thaliana Rop GTPases are localized to tips of root hairs and control polar growth. The EMBO journal. 2001;20:2779–2788. doi: 10.1093/emboj/20.11.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones MA, et al. NADPH oxidase-dependent reactive oxygen species formation required for root hair growth depends on ROP GTPase. Journal of experimental botany. 2007;58:1261–1270. doi: 10.1093/jxb/erl279. [DOI] [PubMed] [Google Scholar]
- 16.Duan Q, et al. FERONIA receptor-like kinase regulates RHO GTPase signaling of root hair development. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:17821–17826. doi: 10.1073/pnas.1005366107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoo CM, et al. AGD1, a class 1 ARF-GAP, acts in common signaling pathways with phosphoinositide metabolism and the actin cytoskeleton in controlling Arabidopsis root hair polarity. The Plant journal: for cell and molecular biology. 2012;69:1064–1076. doi: 10.1111/j.1365-313X.2011.04856.x. [DOI] [PubMed] [Google Scholar]
- 18.Ohta Y, et al. FilGAP, a Rho- and ROCK-regulated GAP for Rac binds filamin A to control actin remodelling. Nature cell biology. 2006;8:803–814. doi: 10.1038/ncb1437. [DOI] [PubMed] [Google Scholar]
- 19.Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nature reviews Molecular cell biology. 2008;9:690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- 20.Berken A. ROPs in the spotlight of plant signal transduction. Cellular and molecular life sciences: CMLS. 2006;63:2446–2459. doi: 10.1007/s00018-006-6197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brembu T, et al. A RHOse by any other name: a comparative analysis of animal and plant Rho GTPases. Cell research. 2006;16:435–445. doi: 10.1038/sj.cr.7310055. [DOI] [PubMed] [Google Scholar]
- 22.Wu G, et al. A genome-wide analysis of Arabidopsis Rop-interactive CRIB motif-containing proteins that act as Rop GTPase targets. The Plant cell. 2001;13:2841–2856. doi: 10.1105/tpc.010218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li S, et al. RIP1 (ROP Interactive Partner 1)/ICR1 marks pollen germination sites and may act in the ROP1 pathway in the control of polarized pollen growth. Molecular plant. 2008;1:1021–1035. doi: 10.1093/mp/ssn051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lavy M, et al. A Novel ROP/RAC effector links cell polarity, root-meristem maintenance, and vesicle trafficking. Current biology: CB. 2007;17:947–952. doi: 10.1016/j.cub.2007.04.038. [DOI] [PubMed] [Google Scholar]
- 25.Fu Y, et al. A ROP GTPase signaling pathway controls cortical microtubule ordering and cell expansion in Arabidopsis. Current biology: CB. 2009;19:1827–1832. doi: 10.1016/j.cub.2009.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ehrhardt DW, Shaw SL. Microtubule dynamics and organization in the plant cortical array. Annual review of plant biology. 2006;57:859–875. doi: 10.1146/annurev.arplant.57.032905.105329. [DOI] [PubMed] [Google Scholar]
- 27.Staiger CJ, Blanchoin L. Actin dynamics: old friends with new stories. Current opinion in plant biology. 2006;9:554–562. doi: 10.1016/j.pbi.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 28.Staiger CJ, et al. Regulation of actin dynamics by actin-binding proteins in pollen. Journal of experimental botany. 2010;61:1969–1986. doi: 10.1093/jxb/erq012. [DOI] [PubMed] [Google Scholar]
- 29.Yang Z. Cell polarity signaling in Arabidopsis. Annual review of cell and developmental biology. 2008;24:551–575. doi: 10.1146/annurev.cellbio.23.090506.123233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee YJ, Yang Z. Tip growth: signaling in the apical dome. Current opinion in plant biology. 2008;11:662–671. doi: 10.1016/j.pbi.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mucha E, et al. Rho proteins of plants--functional cycle and regulation of cytoskeletal dynamics. European journal of cell biology. 2011;90:934–943. doi: 10.1016/j.ejcb.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 32.Qin Y, Yang Z. Rapid tip growth: insights from pollen tubes. Seminars in cell & developmental biology. 2011;22:816–824. doi: 10.1016/j.semcdb.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hwang JU, et al. Oscillatory ROP GTPase activation leads the oscillatory polarized growth of pollen tubes. Molecular biology of the cell. 2005;16:5385–5399. doi: 10.1091/mbc.E05-05-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee YJ, et al. Rho-GTPase-dependent filamentous actin dynamics coordinate vesicle targeting and exocytosis during tip growth. The Journal of cell biology. 2008;181:1155–1168. doi: 10.1083/jcb.200801086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fuchs U, et al. Microtubules are dispensable for the initial pathogenic development but required for long-distance hyphal growth in the corn smut fungus Ustilago maydis. Molecular biology of the cell. 2005;16:2746–2758. doi: 10.1091/mbc.E05-03-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mourino-Perez RR, et al. Microtubule dynamics and organization during hyphal growth and branching in Neurospora crassa. Fungal genetics and biology: FG & B. 2006;43:389–400. doi: 10.1016/j.fgb.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 37.Raudaskoski M, et al. Microtubule cytoskeleton in hyphal growth. Response to nocodazole in a sensitive and a tolerant strain of the homobasidiomycete Schizophyllum commune. European journal of cell biology. 1994;64:131–141. [PubMed] [Google Scholar]
- 38.Nakamura M, et al. Arabidopsis GCP3-INTERACTING PROTEIN 1/MOZART1 is an integral component of the gamma-tubulin-containing microtubule nucleating complex. The Plant journal: for cell and molecular biology. 2012 doi: 10.1111/j.1365-313X.2012.04988.x. [DOI] [PubMed] [Google Scholar]
- 39.Shaw SL, et al. Sustained microtubule treadmilling in Arabidopsis cortical arrays. Science. 2003;300:1715–1718. doi: 10.1126/science.1083529. [DOI] [PubMed] [Google Scholar]
- 40.Wasteneys GO, Ambrose JC. Spatial organization of plant cortical microtubules: close encounters of the 2D kind. Trends in cell biology. 2009;19:62–71. doi: 10.1016/j.tcb.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 41.Fu Y, et al. Arabidopsis interdigitating cell growth requires two antagonistic pathways with opposing action on cell morphogenesis. Cell. 2005;120:687–700. doi: 10.1016/j.cell.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 42.Xu T, et al. Cell surface- and rho GTPase-based auxin signaling controls cellular interdigitation in Arabidopsis. Cell. 2010;143:99–110. doi: 10.1016/j.cell.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brennwald P, Rossi G. Spatial regulation of exocytosis and cell polarity: yeast as a model for animal cells. FEBS letters. 2007;581:2119–2124. doi: 10.1016/j.febslet.2007.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.France YE, et al. The polarity-establishment component Bem1p interacts with the exocyst complex through the Sec15p subunit. Journal of cell science. 2006;119:876–888. doi: 10.1242/jcs.02849. [DOI] [PubMed] [Google Scholar]
- 45.Mehta SQ, et al. Mutations in Drosophila sec15 reveal a function in neuronal targeting for a subset of exocyst components. Neuron. 2005;46:219–232. doi: 10.1016/j.neuron.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 46.Wedlich-Soldner R, et al. Robust cell polarity is a dynamic state established by coupling transport and GTPase signaling. The Journal of cell biology. 2004;166:889–900. doi: 10.1083/jcb.200405061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zajac A, et al. Cyclical regulation of the exocyst and cell polarity determinants for polarized cell growth. Molecular biology of the cell. 2005;16:1500–1512. doi: 10.1091/mbc.E04-10-0896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guo W, et al. Spatial regulation of the exocyst complex by Rho1 GTPase. Nature cell biology. 2001;3:353–360. doi: 10.1038/35070029. [DOI] [PubMed] [Google Scholar]
- 49.Zhang X, et al. Cdc42 interacts with the exocyst and regulates polarized secretion. The Journal of biological chemistry. 2001;276:46745–46750. doi: 10.1074/jbc.M107464200. [DOI] [PubMed] [Google Scholar]
- 50.Cole RA, Fowler JE. Polarized growth: maintaining focus on the tip. Current opinion in plant biology. 2006;9:579–588. doi: 10.1016/j.pbi.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 51.Hazak O, et al. A rho scaffold integrates the secretory system with feedback mechanisms in regulation of auxin distribution. PLoS biology. 2010;8:e1000282. doi: 10.1371/journal.pbio.1000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mravec J, et al. Cell plate restricted association of DRP1A and PIN proteins is required for cell polarity establishment in Arabidopsis. Current biology: CB. 2011;21:1055–1060. doi: 10.1016/j.cub.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 53.Richter S, et al. Polarized cell growth in Arabidopsis requires endosomal recycling mediated by GBF1-related ARF exchange factors. Nature cell biology. 2012;14:80–86. doi: 10.1038/ncb2389. [DOI] [PubMed] [Google Scholar]
- 54.Nagawa S, et al. RHO GTPase in plants: Conservation and invention of regulators and effectors. Small GTPases. 2010;1:78–88. doi: 10.4161/sgtp.1.2.14544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao Y, et al. Phosphoinositides regulate clathrin-dependent endocytosis at the tip of pollen tubes in Arabidopsis and tobacco. The Plant cell. 2010;22:4031–4044. doi: 10.1105/tpc.110.076760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shivas JM, et al. Polarity and endocytosis: reciprocal regulation. Trends in cell biology. 2010;20:445–452. doi: 10.1016/j.tcb.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Izumi G, et al. Endocytosis of E-cadherin regulated by Rac and Cdc42 small G proteins through IQGAP1 and actin filaments. The Journal of cell biology. 2004;166:237–248. doi: 10.1083/jcb.200401078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harris KP, Tepass U. Cdc42 and Par proteins stabilize dynamic adherens junctions in the Drosophila neuroectoderm through regulation of apical endocytosis. The Journal of cell biology. 2008;183:1129–1143. doi: 10.1083/jcb.200807020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nagawa S, et al. ROP GTPase-dependent actin microfilaments promote PIN1 polarization by localized inhibition of clathrin-dependent endocytosis. PLoS biology. 2012 doi: 10.1371/journal.pbio.1001299. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paciorek T, et al. Auxin inhibits endocytosis and promotes its own efflux from cells. Nature. 2005;435:1251–1256. doi: 10.1038/nature03633. [DOI] [PubMed] [Google Scholar]
- 61.Xu T, et al. Uniform auxin triggers the Rho GTPase-dependent formation of interdigitation patterns in pavement cells. Small GTPases. 2011;2:227–232. doi: 10.4161/sgtp.2.4.16702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaksonen M, et al. Harnessing actin dynamics for clathrin-mediated endocytosis. Nature reviews Molecular cell biology. 2006;7:404–414. doi: 10.1038/nrm1940. [DOI] [PubMed] [Google Scholar]
- 63.Yarar D, et al. A dynamic actin cytoskeleton functions at multiple stages of clathrin-mediated endocytosis. Molecular biology of the cell. 2005;16:964–975. doi: 10.1091/mbc.E04-09-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Anitei M, Hoflack B. Bridging membrane and cytoskeleton dynamics in the secretory and endocytic pathways. Nature cell biology. 2012;14:11–19. doi: 10.1038/ncb2409. [DOI] [PubMed] [Google Scholar]
- 65.Berken A, et al. A new family of RhoGEFs activates the Rop molecular switch in plants. Nature. 2005;436:1176–1180. doi: 10.1038/nature03883. [DOI] [PubMed] [Google Scholar]
- 66.Gu Y, et al. Members of a novel class of Arabidopsis Rho guanine nucleotide exchange factors control Rho GTPase-dependent polar growth. The Plant cell. 2006;18:366–381. doi: 10.1105/tpc.105.036434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Basu D, et al. A SPIKE1 signaling complex controls actin-dependent cell morphogenesis through the heteromeric WAVE and ARP2/3 complexes. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:4044–4049. doi: 10.1073/pnas.0710294105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu G, et al. Arabidopsis RopGAPs are a novel family of rho GTPase-activating proteins that require the Cdc42/Rac-interactive binding motif for rop-specific GTPase stimulation. Plant physiology. 2000;124:1625–1636. doi: 10.1104/pp.124.4.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hwang JU, et al. A tip-localized RhoGAP controls cell polarity by globally inhibiting Rho GTPase at the cell apex. Current biology: CB. 2008;18:1907–1916. doi: 10.1016/j.cub.2008.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Klahre U, et al. Nt-RhoGDI2 regulates Rac/Rop signaling and polar cell growth in tobacco pollen tubes. The Plant journal: for cell and molecular biology. 2006;46:1018–1031. doi: 10.1111/j.1365-313X.2006.02757.x. [DOI] [PubMed] [Google Scholar]
- 71.Lin Y, et al. Localization of a Rho GTPase Implies a Role in Tip Growth and Movement of the Generative Cell in Pollen Tubes. The Plant cell. 1996;8:293–303. doi: 10.1105/tpc.8.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fodor-Dunai C, et al. The phosphomimetic mutation of an evolutionarily conserved serine residue affects the signaling properties of Rho of plants (ROPs) The Plant journal: for cell and molecular biology. 2011;66:669–679. doi: 10.1111/j.1365-313X.2011.04528.x. [DOI] [PubMed] [Google Scholar]
- 73.Berken A, Wittinghofer A. Structure and function of Rho-type molecular switches in plants. Plant physiology and biochemistry: PPB/Societe francaise de physiologie vegetale. 2008;46:380–393. doi: 10.1016/j.plaphy.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 74.Ivanchenko M, et al. Maize ROP7 GTPase contains a unique, CaaX box-independent plasma membrane targeting signal. The Plant journal: for cell and molecular biology. 2000;24:79–90. doi: 10.1046/j.1365-313x.2000.00855.x. [DOI] [PubMed] [Google Scholar]
- 75.Lavy M, et al. A cell-specific, prenylation-independent mechanism regulates targeting of type II RACs. The Plant cell. 2002;14:2431–2450. doi: 10.1105/tpc.005561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lavy M, Yalovsky S. Association of Arabidopsis type-II ROPs with the plasma membrane requires a conserved C-terminal sequence motif and a proximal polybasic domain. The Plant journal: for cell and molecular biology. 2006;46:934–947. doi: 10.1111/j.1365-313X.2006.02749.x. [DOI] [PubMed] [Google Scholar]
- 77.Sorek N, et al. Activation status-coupled transient S acylation determines membrane partitioning of a plant Rho-related GTPase. Molecular and cellular biology. 2007;27:2144–2154. doi: 10.1128/MCB.02347-06. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 78.Sorek N, et al. An S-acylation switch of conserved G domain cysteines is required for polarity signaling by ROP GTPases. Current biology: CB. 2010;20:914–920. doi: 10.1016/j.cub.2010.03.057. [DOI] [PubMed] [Google Scholar]
- 79.Brugnera E, et al. Unconventional Rac-GEF activity is mediated through the Dock180-ELMO complex. Nature cell biology. 2002;4:574–582. doi: 10.1038/ncb824. [DOI] [PubMed] [Google Scholar]
- 80.Meller N, et al. Zizimin1, a novel Cdc42 activator, reveals a new GEF domain for Rho proteins. Nature cell biology. 2002;4:639–647. doi: 10.1038/ncb835. [DOI] [PubMed] [Google Scholar]
- 81.Yamaguchi K, et al. SWAP70 functions as a Rac/Rop guanine nucleotide-exchange factor in rice. Plant Journal. 2011 doi: 10.1111/j.1365-313X.2011.04874.x. [DOI] [PubMed] [Google Scholar]
- 82.Zhang Y, McCormick S. A distinct mechanism regulating a pollen-specific guanine nucleotide exchange factor for the small GTPase Rop in Arabidopsis thaliana. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:18830–18835. doi: 10.1073/pnas.0705874104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cheung AY, et al. The dynamic pollen tube cytoskeleton: live cell studies using actin-binding and microtubule-binding reporter proteins. Molecular plant. 2008;1:686–702. doi: 10.1093/mp/ssn026. [DOI] [PubMed] [Google Scholar]
- 84.Nibau C, Cheung AY. New insights into the functional roles of CrRLKs in the control of plant cell growth and development. Plant signaling & behavior. 2011;6:655–659. doi: 10.4161/psb.6.5.14951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kessler SA, et al. Conserved molecular components for pollen tube reception and fungal invasion. Science. 2010;330:968–971. doi: 10.1126/science.1195211. [DOI] [PubMed] [Google Scholar]
- 86.Humphries JA, et al. ROP GTPases act with the receptor-like protein PAN1 to polarize asymmetric cell division in maize. The Plant cell. 2011;23:2273–2284. doi: 10.1105/tpc.111.085597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen M, et al. RopGEF7 regulates PLETHORA-dependent maintenance of the root stem cell niche in Arabidopsis. The Plant cell. 2011;23:2880–2894. doi: 10.1105/tpc.111.085514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hwang JU, et al. Pollen-tube tip growth requires a balance of lateral propagation and global inhibition of Rho-family GTPase activity. Journal of cell science. 2010;123:340–350. doi: 10.1242/jcs.039180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Myers C, et al. Calcium-dependent protein kinases regulate polarized tip growth in pollen tubes. The Plant journal: for cell and molecular biology. 2009;59:528–539. doi: 10.1111/j.1365-313X.2009.03894.x. [DOI] [PubMed] [Google Scholar]
- 90.Yan A, et al. Calcium participates in feedback regulation of the oscillating ROP1 Rho GTPase in pollen tubes. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:22002–22007. doi: 10.1073/pnas.0910811106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gu Y, et al. A Rho family GTPase controls actin dynamics and tip growth via two counteracting downstream pathways in pollen tubes. The Journal of cell biology. 2005;169:127–138. doi: 10.1083/jcb.200409140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jenkins N, et al. CYK-4/GAP provides a localized cue to initiate anteroposterior polarity upon fertilization. Science. 2006;313:1298–1301. doi: 10.1126/science.1130291. [DOI] [PubMed] [Google Scholar]
- 93.Saito K, et al. Transbilayer phospholipid flipping regulates Cdc42p signaling during polarized cell growth via Rga GTPase-activating proteins. Developmental cell. 2007;13:743–751. doi: 10.1016/j.devcel.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 94.Anderson DC, et al. Polarization of the C. elegans embryo by RhoGAP- mediated exclusion of PAR-6 from cell contacts. Science. 2008;320:1771–1774. doi: 10.1126/science.1156063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Klahre U, Kost B. Tobacco RhoGTPase ACTIVATING PROTEIN1 spatially restricts signaling of RAC/Rop to the apex of pollen tubes. The Plant cell. 2006;18:3033–3046. doi: 10.1105/tpc.106.045336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kawano Y, et al. Activation of a Rac GTPase by the NLR family disease resistance protein Pit plays a critical role in rice innate immunity. Cell host & microbe. 2010;7:362–375. doi: 10.1016/j.chom.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 97.Harris KP, Tepass U. Cdc42 and vesicle trafficking in polarized cells. Traffic. 2010;11:1272–1279. doi: 10.1111/j.1600-0854.2010.01102.x. [DOI] [PubMed] [Google Scholar]