Abstract
Non-small cell lung cancer (NSCLC) is the leading cause of cancer-related deaths worldwide and results from a complex interaction between carcinogen exposure and inherent susceptibility. Despite its prevalence genetic factors that predispose to the development of lung cancer remain elusive. Inbred mouse models offer a unique and clinically relevant tool to study genetic factors that contribute to lung carcinogenesis due to the development of tumors that resemble human adenocarcinoma and broad strain-specific variation in cancer incidence after carcinogen administration. Here we set out to investigate whether strain-specific variability in tumor immunosurveillance contributes to differences in lung cancer. Using bone marrow transplantation we determined that hematopoietic cells from lung cancer-resistant mice could significantly impede the development of cancer in a susceptible strain. Furthermore, we show that this is not due to differences in tumor-promoting inflammatory changes or variability in immunosurveillance by the adaptive immune system, but results from strain-specific differences in natural killer cell (NK) cytotoxicity. Using a newly discovered congenic strain of mice we demonstrate a previously unrecognized role for strain-specific polymorphisms in the natural killer gene complex (NKC) in immunosurveillance for carcinogen-induced lung cancer. Since polymorphisms in the NKC are highly prevalent in man, our data may explain why certain individuals without obvious risk factors develop lung cancer while others remain resistant to the disease despite heavy environmental carcinogen exposure.
Introduction
Investigations of mouse and human models of cancer have significantly advanced our understanding of the innate and adaptive immune responses to malignancies (1). Despite the fact that NSCLC remains the leading cause of cancer-related deaths, only limited progress has been made in understanding mechanisms regulating its immunosurveillance. Quantitative trait loci (QTL) mapping using genome-wide association studies and linkage analysis of lung cancer-susceptible and -resistant strains of mice and families with hereditary lung cancer has led to the identification of several polymorphisms that contribute to this disease (2–5). Such a “global approach”, however, has not led to the identification of genetic differences responsible for immunologic susceptibility to lung cancer. In fact, almost all genes identified by QTL-mapping function in a tumor cell-intrinsic manner and can suppress or promote growth of stromal cells both in vitro and in vivo (6). Thus, the study of immunologic processes that contribute to lung cancer susceptibility and resistance may facilitate the design of rational immunotherapy strategies for this disease.
In this communication we demonstrate that bone marrow-derived cells of lung cancer resistant mice impede the development of urethane-induced tumors. Unlike the case for other malignancies (7), we show that NK cells rather than T lymphocytes play a critical role in immunosurveillance for urethane-induced lung cancer. We further define that strain-specific differences in the NK gene complex (NKC) contribute to resistance to NSCLC.
Methods
Animals and Reagents
Mice consisted of 6–12 week old males purchased from Jackson Laboratories (Bar Harbor, MA) except for 129/SvEv, which were purchased from Taconic (German Town, NY). 129/SvEv.B6-NKC Rag2−/− mice were identified and maintained at Washington University. Microsatellite typing was performed as previously described(8). Urethane (Sigma, St. Louis, MO) was injected intraperitoneally at 3mg/g in three separate injections over the course of six days. Mice were euthanized 4 months after injection of urethane.
Immunodepletion was accomplished with antibodies for CD4 (GK1.5), CD8 (YTS169.4), NK1.1 (PK136) or isotype controls at an initial dose of 500μg i.p. and a weekly dose of 250μg i.p. (BioXcell, West Lebanon, NH.). Such treatment depletes respective cell populations in both lungs and secondary lymphoid organs by flow cytometry (data not shown)(9). Lungs were digested in type II collagenase and stained in saturating concentrations of fluorochrome-conjugated antibodies (eBioscience, San Diego, Ca) for 30 minutes on ice.
Bone marrow chimeras were created by injecting 1×107 T cell-depleted donor bone marrow cells intravenously after lethal irradiation of the host with 10 Gy. These chimeras were injected with urethane three months after bone marrow transplantation and are designated as: donor genotype→recipient genotype.
Tumor Analysis
Lungs were fixed in 10% formalin followed by 70% ETOH. Tumors were counted and measured by a co-author (X.L.), blinded to the experimental condition. The individual tumor volume was calculated as (mm3)=4/3πr3. Tumors were graded on a four stage grading system on H&E slides with typical adenomatous hyperplasia as Grade 1, tumors with round to ovoid uniform nuclei as Grade 2, moderately pleomorphic nuclei with occasional mitotic figure as Grade 3 and markedly pleomorphic nuclei with nucleoli, increased mitoses, with or without necrosis and with or without glandular architecture as Grade 4 (10).
In Vitro Cytotoxicity Assays
51Chromium release was performed by incubating the target cells with 100μCi sodium-51-chromate (PerkinElmer, Wellesely, MA) for 1 hour. Effector cells were isolated from secondary lymphoid organs for NK cells and bone marrow for macrophages, monocytes and neutrophils (Miltenyi Biotec, Bergisch Gladbach, Germany). Specific lysis was expressed as: (experimental release-spontaneous release)/(maximum release-spontaneous release) ×100% with 0% specific lysis as lowest expressed value. Blockade of NKC receptors was accomplished using antibodies manufactured in house (by WMY) at 30 ug/ml.
Statistical analysis
Analysis was performed by T test, Mann-Whitney U test, ANOVA and Chi-square analysis as appropriate based on consultation with statistician Dr. Kathryn Trinkas.
Results and Discussion
Bone Marrow-Derived Cells Contribute to Strain-Specific Susceptibility to Lung Cancer
Urethane, or ethyl carbamate, is found in most if not all fermented foods and is considered by the International Agency for Research on Cancer as a group 2A human carcinogen for lung cancer (11). Consistent with previous reports (6) we found that the incidence, number, size, and overall burden of tumor vary in the highly susceptible AJ, moderately susceptible 129/SvEv, and resistant C57BL/6 (B6) mice following urethane injection (Figure 1A). In order to determine if bone marrow-derived cells contribute to this process we next created bone marrow chimeras between the highly susceptible AJ and resistant B6 mice and injected them with urethane. We postulated that the drastic differences in tumor burden between these two strain combinations would more accurately allow us to determine whether hematopoietic cells contribute to strain-specific susceptibility to carcinogenesis. We noted that the tumor burden was significantly lower in AJ mice receiving B6 bone marrow compared to syngeneic transplants (Figure 1B). Similar albeit less pronounced differences were evident in B6 recipients of AJ bone marrow (Figure 1C). The fact that AJ hematopoietic cells did not completely revert B6 mice to the susceptible phenotype extends our previous reports that strain-specific differences in non-hematopoietic stromal cells contributes to lung cancer susceptibility (2). Our data, however, now identify a previously unknown contribution of hematopoietic cells to lung carcinogenesis.
Figure 1. Bone marrow-derived cells contribute to strain-specific differences in urethane-induced lung cancer.
A) Tumor comparison between AJ, 129/SvEv and B6 mice. B) Comparison of tumors in AJ mice transplanted with B6 bone marrow compared to syngeneic transplants. C) Comparison of tumors in B6 mice transplanted with AJ bone marrow compared to syngeneic transplants. Data represented as a low power H&E slide in the left panel followed by graphs representing the number of tumors, diameter of tumors and total tumor burden per mouse (ns=p> .05).
Minimal Inflammatory Changes are Evident after Treatment with Urethane
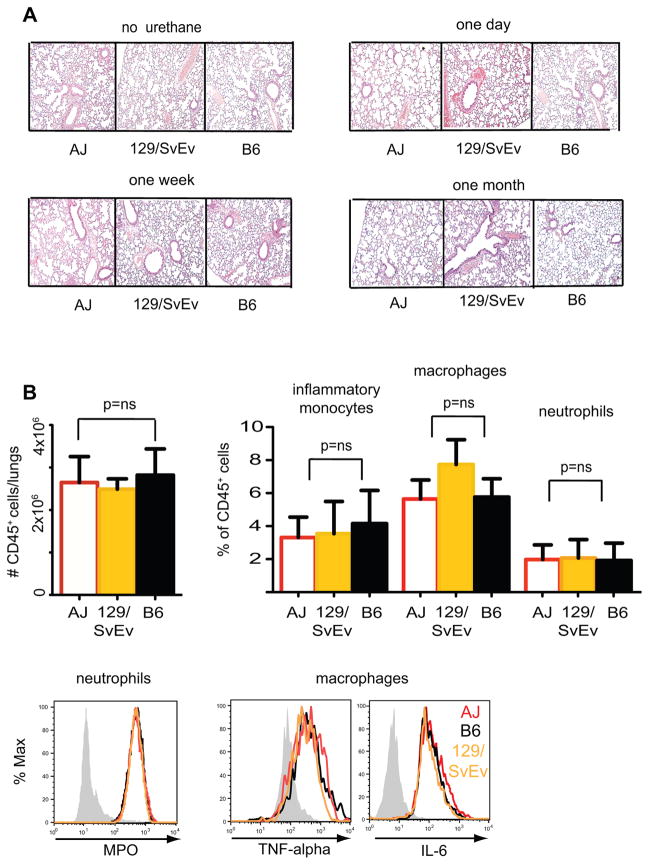
Inflammation can enhance the development of lung cancer (12). No evidence of pulmonary inflammation, however, was evident after urethane injection by H&E staining (Figure 2A) or flow cytometric analysis of lung-resident CD45+ hematopoietic cells in AJ, 129/SvEv, or B6 strains of mice (Figure 2B). No differences in accumulation or activation of neutrophils, macrophages, or inflammatory monocytes were evident as well (Figure 2B). These data support the notion that urethane can lead to the promotion and induction of lung cancer independent of inflammation. Based on these data we considered it unlikely that differences in susceptibility to urethane-induced lung cancer were due to tumor-promoting inflammatory changes.
Figure 2. Minimal Inflammatory Changes are Evident after Treatment with Urethane.
A) Histologic and flow cytometric evaluation of pulmonary inflammation in B6, 129SvEv or AJ mice one day, one week and one month after treatment with urethane. B) Quantitative analysis of total leucocytes (defined as # of CD45+ cells/lungs), inflammatory monocytes (defined as CD45+Ly6chiCD11b+), macrophages (defined as CD45+F4/80+CD11b+) and neutrophils (defined as CD45+Ly6g+Gr1+CD11b+) as well as activation, based on myeloperoxidase (MPO) production by neutrophils and macrophage TNF-α or IL-6 secretion after PMA+ionomycin stimulation. Representative of at least three animals per group with flow cytometry performed two weeks after treatment with urethane.
The Innate Rather than the Adaptive Immune System Plays a Critical Role in Immunosurveillance for Urethane-Induced Lung Cancer
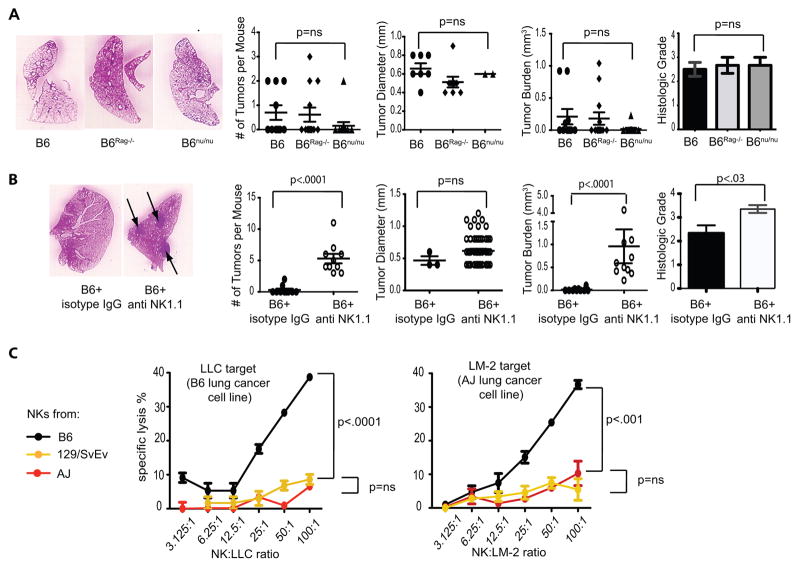
Based on reports demonstrating the critical role of T lymphocytes in eliminating both chemically-induced and spontaneous tumors (1, 7), we next set out to determine if the adaptive immune system of resistant mice can prevent the development of lung cancer. To this end we injected wild-type B6, B6Rag−/− and B6 athymic nude mice with urethane and analyzed them four months later for lung cancer. Surprisingly, we saw no differences in cancer incidence between wild-type and Rag−/− mice (4/10 vs. 4/13 mice, respectively; p=0.68) with similar numbers of tumors per mouse and identical overall tumor burden. B6 nude mice seemed especially resistant to the development of lung cancer with only one out of 13 mice developing tumors (Figure 3A). To evaluate the possibility that the adaptive immune response may control malignant progression rather than the incidence or tumor burden, individual tumors were histologically graded on a four-stage scale as previously described (10). No differences in tumor grade were evident between immunocompetent and immunodeficient animals (Figure 3A). Similarly, T cell depletion did not impact tumor burden in susceptible AJ mice (Supplemental Figure 1). Taken together, these data indicate that components of the adaptive immune system, such as T and B lymphocytes, play essentially no role in strain-specific differences in urethane-induced lung cancer.
Figure 3. NK cell control of Lung cancer.
A) Comparison of lung cancer in urethane-treated wild-type, Rag−/−, or nude (nu/nu) mice on a B6 background. B) Comparison of lung cancer in B6 mice treated with anti-NK1.1 or isotype control. C) Lysis of LLC and LM-2 lung cancer cell lines by freshly isolated B6, 129/SvEv and AJ NK cells (representative of three experiments).
Our findings are in stark contrast to certain other models of primary carcinogenesis, such as 3-methylcholanthrene(3-MCA)-induced fibrosarcoma, where both innate and adaptive immunity play a key role in controlling the growth and shaping the immunogenicity of cancer(7, 9). Such discrepancies may be due to the unique tolerogenic properties of the lung, as direct antigen administration into this organ can lead to systemic tolerance rather than priming of the adaptive immune response(13).
In order to investigate the role of the innate immune system we next depleted NK cells in a cohort of B6 mice by weekly injection with anti-NK1.1 antibody for four months after urethane injection. Mice treated in this fashion demonstrated 100% tumor incidence with a significantly larger tumor burden and a more advanced tumor grade compared to controls (Figure 3B). Tumors developing in anti-NK1.1-treated mice had a similar size to those treated with control IgG. This finding is consistent with the notion that NK cells are able to eliminate malignant cells early in their transformation, but not once a large tumor has developed. While we recognize that PK136 treatment also depletes other NK1.1+ cells, such as NKT cells, B6 nude and Rag−/− mice that are deficient in NKT cells remain relatively resistant to urethane-induced lung cancer (Figure 3A). Nevertheless, in order to corroborate our findings in an in vitro model, we isolated NK cells from AJ, 129/SvEv, and B6 mice and tested lung-cancer directed cytotoxicity in a 51Chromium release assay. B6 NK cells were significantly more efficient in lysing both Lewis Lung Carcinoma (LLC), derived from the B6 strain, and LM-2 lung cancer cell line, derived from a urethane-induced AJ tumor, when compared to AJ and 129/SvEv NK cells (Figure 3C). Little to no lung cancer-specific cytotoxicity was evident when using other cells of the innate immune system, such as neutrophils or macrophages and monocytes (Supplemental Figure 2). Collectively our in vitro and in vivo findings support the notion that resistance to lung cancer in B6 mice is at least partially due to NK-mediated immunosurveillance.
Strain-Specific Differences in Chromosome 6-linked Natural Killer Gene Complex Contribute to Immunosurveillance for Lung Cancer
The fact that NK cells from highly susceptible AJ and moderately susceptible 129/SvEv mice (Figure 1A) demonstrate an identical level of lung cancer-specific cytotoxicity (Figure 3C) indicates that factors other than NK cells contribute to urethane-induced lung cancer development as well. At the same time these data reaffirm that, when compared to B6 mice, defects in NK cytotoxicity may contribute to the development of lung cancer in AJ and 129/SvEv strains. The relationship between AJ and 129/SvEv mice is difficult to infer as these two strains are virtually unrelated, possess different major histocompatibility antigens and immunologic characteristics(14). Furthermore, AJ mice demonstrate several NK cell-specific defects in peripheral function(15) and are highly susceptible to lymphotoxic substances including urethane(16). NK cells from 129 mice, on the other hand, demonstrate only limited defects and are able to lyse other targets, such as YAC-1 (17), better then AJ mice (Supplemental Figure 3). Taken together, these data suggest that another factor may account for the deficiency of lung cancer-specific cytotoxicity in 129/SvEv and AJ NK cells.
NK cell function is regulated by inhibitory and activating receptors that bind target cell ligands to stimulate (or not) NK cell killing. The natural killer gene complex (NKC) on distal chromosome 6 contains several clusters of genes encoding the Nkrp1, Ly49, CD94 and NKG2 families of receptors (18). We have recently demonstrated using array-based comparative genomic hybridization and sequence-tagged site analysis by microsatellite markers that different strains of mice can be grouped based on their NKC (8,19). It is in this region of their genome that the AJ and 129 strains demonstrate similarity and differ from B6. In order to evaluate this further we compared lung cancer-specific NK cytotoxicity of Balb/c mice, which share NKC homology to AJ and 129, and C57BL/10 mice, which share homology to the B6 strain. Similar differences in lung cancer-specific cytotoxicity were evident between these strains (Supplemental Figure 4) further implicating strain-specific polymorphisms in the NKC locus and lung cancer-specific cytotoxicity (19). Nevertheless, as these mice differ in other parts of their genome as well a more rigorous model is required to link the NKC locus to lung cancer susceptibility.
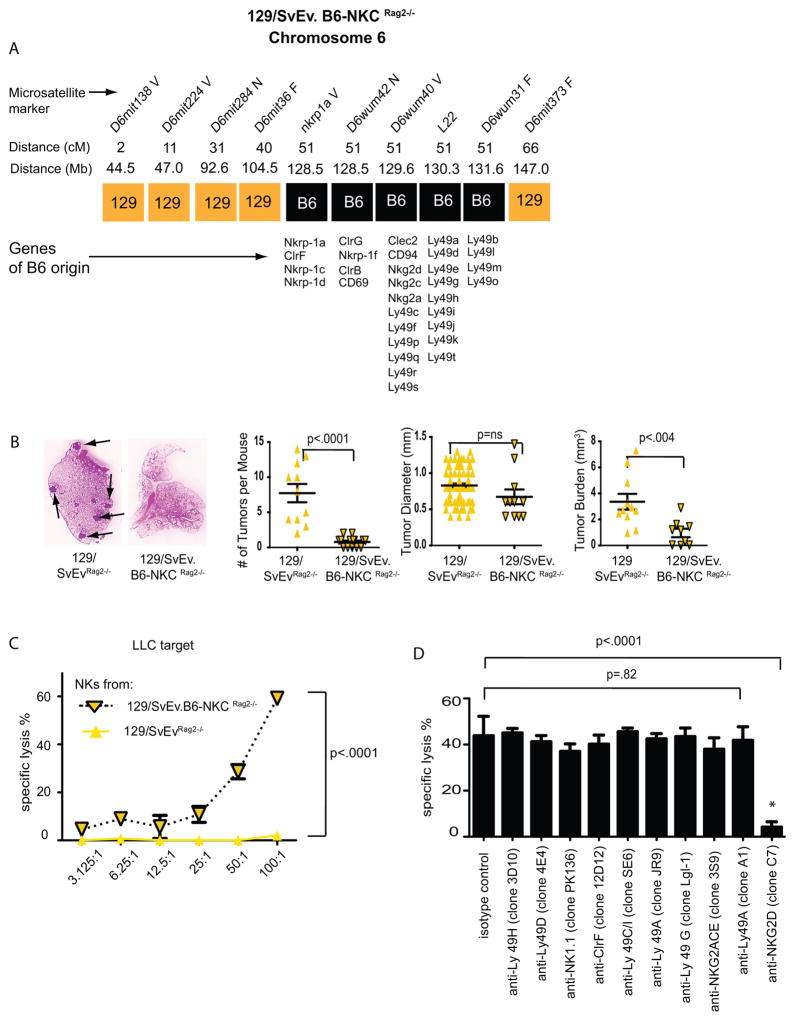
Recombination activating gene deficient (Rag2−/−) mice, originally generated from a 129/SvEv-strain ES cell (20), have been backcrossed to both B6 and 129/SvEv by Taconic Farms. Recently we discovered that one sub-strain of a Rag2−/− mouse on the 129/SvEv background was incompletely backcrossed. Using microsatellite analysis we determined that this sub-strain was congenic with B6 for the NKC genomic region while otherwise containing the near complete genome of the 129/SvEv strain (Figure 4A)(Supplemental Figure 5). The Pas-1 locus, which contains Kras, is also located on chromosome 6 and is therefore genetically linked to the NKC. By both microsatellite and SNP analysis, however, the Pas-1 locus of this mouse was of 129 origin (Supplemental Figures 5,6). The microsatellite data demonstrating B6 origin of the NKC was confirmed by FACS analysis using NK1.1 as a B6-specific antigen and polymerase chain reaction to amplify the 129/SvEv and B6 alleles of the Nkrp1c gene (data not shown). This mouse, designated as 129/SvEv.B6-NKC Rag2−/−, thus represents a unique animal model containing the genome derived from the 129 strain of mice, which are susceptible to lung cancer(6), but the NKC derived from the resistant B6 strain. Importantly both 129/SvEv and 129/SvEv.B6-NKC Rag2−/− mice display identically high susceptibility to 3-MCA tumorigenesis and 3-MCA-induced fibrosarcomas from both strains display identically high levels of immunogenicity (7)(unpublished observations R.D.S.).
Figure 4. Polymorphisms in the NKC contribute to lung cancer susceptibility and in vitro cytotoxicity of NK cells.
A) Structure of chromosome 6 in the 129/SvEv.B6-NKC Rag2−/−mouse. B) Comparison of lung cancer in urethane-treated wild-type 129/SvEvRag2−/− and 129/SvEv.B6-NKCRag2−/− mice. C) Lysis of LLC lung cancer cell line by freshly isolated 129/SvEvRag2−/− and 129/SvEv.B6-NKCRag2−/− NK cells. Graph representative of three separate 51Cr release experiments demonstrating increased cytotoxicity of NK cells from 129/SvEv.B6-NKCRag2−/− mice compared to those isolated from 129/SvEv Rag2−/− mice. D) In vitro blockade of LLC lysis by B6 NK cells using NKC-specific antibodies (representative of three experiments).
In order to investigate the impact of NKC polymorphism on urethane-induced lung cancer we injected fully back-crossed 129/SvEvRag2−/− and 129/SvEv.B6-NKC Rag2−/− mice with urethane and evaluated lung cancer by necropsy 4 months later. Similar to the finding in the B6 strain (Figure 3A), no differences in the number of tumors or total tumor burden were detectable between wild-type and Rag2−/− mice on a pure 129 background (3.9±0.7 mm3 vs. 3.4±0.6 mm3 respectively; p=.54)(Figure 1A vs. 4B). In stark contrast, 129/SvEv.B6-NKC Rag2−/− mice had a significantly lower tumor burden compared to mice with 129/SvEv NKC (Figure 4B). NK cells from 129/SvEv.B6-NKC Rag2−/− mice also demonstrated superior lung-cancer specific cytotoxicity in vitro compared to NK cells from 129/SvEvRag2−/− mice (Figure 4C). These data further indicate that NK cells from the resistant B6 strain of mice, and specifically the B6 NKC alleles, form an efficient barrier to the development of lung cancer. In order to evaluate what receptors in the B6 NKC are critical for eradication of lung cancer we repeated the in vitro chromium release experiments using a panel of blocking antibodies. Blockade of NKG2D led to near complete inhibition of cytotoxicity (Figure 4D) while blockade of other receptors had little to no effect on cytotoxicity. Such data also suggest that NKG2D plays a critical role in lung cancer recognition and implies that polymorphisms in this activating receptor, or other inhibitory receptors that may prevent NKG2D signaling, may contribute to strain-specific differences in lung cancer cytotoxicity.
In contrast to the adaptive immune system, receptors of the innate immune system are encoded in the germ-line. Such receptors, especially those in the NKC locus, manifest extreme genetic polymorphism that includes both allelic variations within a single gene and structural genomic variation of total gene content(19). While such genetic diversity is most likely shaped by evolutionary pressure brought on by infectious diseases, the impact of this genetic diversity on cancer is unknown. We now demonstrate that NK cells, rather than cells of the adaptive immune system, play a critical role in immunosurveillance for urethane-induced lung cancer and show, for the first time, that allelic variation in the NKC of otherwise immunocompetent animals accounts for susceptibility or resistance to lung cancer. Our data may explain why certain individuals develop lung cancer despite minimal risk factors or carcinogen exposure.
Supplementary Material
No difference in tumor number, size or incidence was evident between AJ mice depleted of T lymphocytes with anti CD4 and CD8 antibodies (500 μg of GK1.5 and YTS169.4 i.p. followed by 250μg of each weekly for 4 months after urethane treatment) vs. isotype control-treated mice.
Only minimal cytotoxcity was evident by neutrophils (isolated using the “no touch” technique of biotinylated antibodies followed by magnetic cell depletion) or monocytes and macrophages (isolated by CD115+ selection from the bone marrow) with no differences between AJ, 129SvEv or B6 mice.
Unlike the case for lung cancer cell lines, 129/SvEv NK cells are able to lyse YAC-1 cells at similar levels to those isolated from B6 mice while AJ NK cells demonstrate a virtual absence of YAC-1 cytotoxicity. Data demonstrates 100:1 effector:target ratio and is representative of two separate experiments.
C57BL/10 mice, which share the NKC with the B6 strain, are able to lyse LLC cells more efficiently than NK cells from Balb/c mice, which have a NKC locus similar to 129 and AJ strains.
The 129/SvEv.B6-NKC Rag2−/− mouse contains B6 alleles only in chromosome 6 in the NKC region (detailed in Figure 4). The indicated mice were genotyped for 138 microsatellites, as indicated.
SNP rs13459098, located at 145123190 bp, was analyzed and was determined to be of the 129 strain (identical results obtained using 129SvEv and 129 Ola mice).
Acknowledgments
supported by ATS/Lungevity Foundation, the Alvin Siteman Cancer Center Internal Research Grant by The American Cancer Society, NIH (KO8CA131097) and Biostatistics Core (P30 Ca091842), the Rheumatic Diseases Core Center NIH (P30 AR48335), NIH 1R01HL094601, the Barnes Jewish Research Foundation, The American Association for Thoracic Surgery, Advancing a Healthier Wisconsin, Thoracic Surgery Foundation for Research and Education and the generous support of Charlotte and Sheldon Rudnick, RDS is supported by NIH/NCI CA43059, CA141541 and the Cancer Research Institute, WMY is an investigator of the Howard Hughes Medical Institute
we kindly thank Sanjay Murala, Jessica Archambault and Michelle Becker-Hapak for their expert technical assistance
References
- 1.Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–71. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Z, Futamura M, Vikis HG, Wang M, Li J, Wang Y, et al. Positional cloning of the major quantitative trait locus underlying lung tumor susceptibility in mice. Proc Natl Acad Sci U S A. 2003;100:12642–7. doi: 10.1073/pnas.2133947100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang M, Zhang Z, Vikis H, Yan Y, Wang Y, You M. Fine mapping and candidate gene analyses of pulmonary adenoma resistance 1, a major genetic determinant of mouse lung adenoma resistance. Cancer Res. 2007;67:2508–16. doi: 10.1158/0008-5472.CAN-06-3157. [DOI] [PubMed] [Google Scholar]
- 4.Wang D, You M. Five loci, SLT1 to SLT5, controlling the susceptibility to spontaneously occurring lung cancer in mice. Cancer Res. 2005;65:8158–65. doi: 10.1158/0008-5472.CAN-05-1508. [DOI] [PubMed] [Google Scholar]
- 5.Hung RJ, McKay JD, Gaborieau V, Boffetta P, Hashibe M, Zaridze D, et al. A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature. 2008;452:633–7. doi: 10.1038/nature06885. [DOI] [PubMed] [Google Scholar]
- 6.Liu P, Wang Y, Vikis H, Maciag A, Wang D, Lu Y, et al. Candidate lung tumor susceptibility genes identified through whole-genome association analyses in inbred mice. Nat Genet. 2006;38:888–95. doi: 10.1038/ng1849. [DOI] [PubMed] [Google Scholar]
- 7.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–11. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 8.Brown MG, Scalzo AA, Stone LR, Clark PY, Du Y, Palanca B, et al. Natural killer gene complex (Nkc) allelic variability in inbred mice: evidence for Nkc haplotypes. Immunogenetics. 2001;53:584–91. doi: 10.1007/s002510100365. [DOI] [PubMed] [Google Scholar]
- 9.Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–7. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 10.DuPage M, Dooley AL, Jacks T. Conditional mouse lung cancer models using adenoviral or lentiviral delivery of Cre recombinase. Nat Protoc. 2009;4:1064–72. doi: 10.1038/nprot.2009.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.IARC. Overall Evaluation of Carcinogenicity; Cancer IAfRo. IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Humans. Lyon, France: 1987. p. 440. [Google Scholar]
- 12.Malkinson AM. Role of inflammation in mouse lung tumorigenesis: a review. Exp Lung Res. 2005;31:57–82. doi: 10.1080/01902140490495020. [DOI] [PubMed] [Google Scholar]
- 13.Derbyshire K, Addey C, Coe D, Stuckey DW, Muezzin H, Bubier JA, et al. Molecular mechanisms of induction of antigen-specific allograft tolerance by intranasal peptide administration. J Immunol. 2011;186:5719–28. doi: 10.4049/jimmunol.1002444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beck JA, Lloyd S, Hafezparast M, Lennon-Pierce M, Eppig JT, Festing MF, et al. Genealogies of mouse inbred strains. Nat Genet. 2000;24:23–5. doi: 10.1038/71641. [DOI] [PubMed] [Google Scholar]
- 15.Whyte AL, Miller SC. Strain differences in natural killer cell-mediated immunity among mice: a possible mechanism for the low natural killer cell activity of A/J mice. Immunobiology. 1998;199:23–38. doi: 10.1016/S0171-2985(98)80061-2. [DOI] [PubMed] [Google Scholar]
- 16.Gorelik E, Herberman RB. Susceptibility of various strains of mice to urethan-induced lung tumors and depressed natural killer cell activity. J Natl Cancer Inst. 1981;67:1317–22. [PubMed] [Google Scholar]
- 17.McVicar DW, Winkler-Pickett R, Taylor LS, Makrigiannis A, Bennett M, Anderson SK, et al. Aberrant DAP12 signaling in the 129 strain of mice: implications for the analysis of gene-targeted mice. J Immunol. 2002;169:1721–8. doi: 10.4049/jimmunol.169.4.1721. [DOI] [PubMed] [Google Scholar]
- 18.Yokoyama WM, Plougastel BF. Immune functions encoded by the natural killer gene complex. Nat Rev Immunol. 2003;3:304–16. doi: 10.1038/nri1055. [DOI] [PubMed] [Google Scholar]
- 19.Higuchi DA, Cahan P, Gao J, Ferris ST, Poursine-Laurent J, Graubert TA, et al. Structural variation of the mouse natural killer gene complex. Genes Immun. 2010;11:637–48. doi: 10.1038/gene.2010.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, Mendelsohn M, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–67. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
No difference in tumor number, size or incidence was evident between AJ mice depleted of T lymphocytes with anti CD4 and CD8 antibodies (500 μg of GK1.5 and YTS169.4 i.p. followed by 250μg of each weekly for 4 months after urethane treatment) vs. isotype control-treated mice.
Only minimal cytotoxcity was evident by neutrophils (isolated using the “no touch” technique of biotinylated antibodies followed by magnetic cell depletion) or monocytes and macrophages (isolated by CD115+ selection from the bone marrow) with no differences between AJ, 129SvEv or B6 mice.
Unlike the case for lung cancer cell lines, 129/SvEv NK cells are able to lyse YAC-1 cells at similar levels to those isolated from B6 mice while AJ NK cells demonstrate a virtual absence of YAC-1 cytotoxicity. Data demonstrates 100:1 effector:target ratio and is representative of two separate experiments.
C57BL/10 mice, which share the NKC with the B6 strain, are able to lyse LLC cells more efficiently than NK cells from Balb/c mice, which have a NKC locus similar to 129 and AJ strains.
The 129/SvEv.B6-NKC Rag2−/− mouse contains B6 alleles only in chromosome 6 in the NKC region (detailed in Figure 4). The indicated mice were genotyped for 138 microsatellites, as indicated.
SNP rs13459098, located at 145123190 bp, was analyzed and was determined to be of the 129 strain (identical results obtained using 129SvEv and 129 Ola mice).